Introduction
Currently, lung cancer is the leading cause of
cancer-related mortality throughout the world (1). Although there have been significant
advances in cancer treatments, this malignancy remains poorly
responsive to conventional therapy. Hence, it is urgent to
determine the survival mechanism of carcinoma cells to develop more
efficient therapies for patients.
Human mesenchymal stem cells (hMSCs) are pluripotent
progenitor cells that reside within the adult bone marrow. They
have self-renewal capacity, long-term viability and can
differentiate into the adipocytic, chondrocytic or osteocytic
lineages (2–4). Although hMSCs reside predominantly in
the bone marrow, they are also distributed throughout many other
tissues, where they are thought to function as local sources of
dormant stem cells (5). After
injury or chronic inflammation, the wounded tissue would release
specific endocrine signals that are then transmitted to the bone
marrow, leading to the mobilization of multi-potent hMSCs and their
subsequent recruitment to the damage site (6). Moreover, recent evidence has
indicated that hMSCs are recruited and incorporated within the
connective tissue stroma of tumors (7–10).
In the tumor microenvironment, hMSCs can secrete several tumor
growth-promoting factors, favoring tumor growth, enhancing tumor
vessel formation, promoting cancer metastasis and creating tumor
stem cell niches (11–14). The ability of hMSCs to home to
sites of injury and tumors has encouraged investigation of these
cells as potential therapeutic tools.
Nutrient deprivation and oxygen deficiency are
representative characteristics of the solid tumor microenvironment
during cancer development (15,16).
Autophagy is a well-established mechanism for degrading cytoplasmic
proteins, macromolecules, and organelles to provide a nutrient
source to promote the survival of cells that are under metabolic
stress (17,18). Starvation increases the number and
size of autophagosomes in many tissues, suggesting that autophagy
is a critical component of the body’s response to nutrient
deprivation and amino acid/fuel homeostasis. Autophagy has been
implicated in a number of different physiological and pathological
conditions, including development, differentiation, immunity, aging
and cell death (19,20). In addition, accumulating evidence
demonstrates interesting links between autophagy and tumorigenesis,
tumor progress and chemoresistance (21,22).
In particular, the regulation of autophagy in carcinoma cells is
complex because it can enhance tumor cell survival in response to
certain stresses.
Because stromal cells play an important role in
solid tumor development, there is a significant gap in our
understanding of the relationship between stromal cells and
carcinoma cells under stressful conditions, such as hypoxia or
nutrient deprivation. In this study, we investigated the influence
of hMSCs on A549 and SPC-1 cells under nutrient deprivation and the
role of autophagy in this context, in lung carcinoma cells. Our
study demonstrates that hMSCs can protect carcinoma cells from
nutrient deprivation-induced apoptosis, and interestingly,
autophagy plays an important role in this protection. Further, we
found that hMSCs promoted earlier tumor initiation and growth in
vivo. This result indicated that protection by stromal cells is
a factor that helps lung carcinoma cells survive and proliferate
continually in the ischemic microenvironment, even under extreme
nutrient limitation.
Materials and methods
Cell culture and reagents
The human lung carcinoma cell lines A549 and SPC-1
were purchased from the American Type Culture Collection (Manassas,
VA) and maintained in Dulbecco’s modified Eagle’s medium: Nutrient
Mixture F-12 (DMEM/F12) (Gibco, Invitrogen), containing 10% fetal
bovine serum (FBS) (Gibco, Invitrogen), 100 U/ml penicillin and 100
μg/ml streptomycin in a humidified incubator under 95% air
and 5% CO2 at 37°C. 3-MA and DMSO were purchased from
Sigma-Aldrich. Both DMSO and 3-MA were used at 5 mM.
HMSCs were isolated from hip aspirates of two male
healthy donors with locally approved informed consent. The marrow
was diluted twice with phosphate buffered saline (PBS) and then
isolated by Percoll (Sigma-Aldrich) density-gradient centrifugation
(specific gravity 1.073). Primary cells were collected and
incubated in DMEM/F12 containing 10% FBS, 0.2 mmol/l glutamine, 100
U/ml penicillin and 100 μg/ml streptomycin under 95% air and
5% CO2 at 37°C. After 48 h, the medium was replaced and
non-adherent cells were discarded. After 3–5 passages, the cells
met the minimal criteria for defining multi-potent mesenchymal
stromal cells with typical CD45−, CD34−, CD14−, CD19−, HLA-DR−,
CD73+, CD90+ and CD105+ expression (23), as identified by flow cytometry
(data not shown). Passages three to five hMSCs were used in this
study.
Cell co-culture or hMSC SD-conditioned
medium treatment
Co-culture systems were established using 6-well
transwell (0.4 μm pore, Corning) plates. hMSCs were plated
at 1×105 cells per insert and A549 or SPC-1 cell
suspensions 2×105 cells per well were placed in the
lower compartment of the culture well. The ratio of carcinoma cells
to hMSCs (2:1 ratio) was selected according to previously optimized
conditions for high tumor implantation rate (24). After 6 h, both the inserts and
lower culture wells were washed three times with PBS and then the
medium was switched to DF-12 without FBS for 24 h.
Serum-deprived hMSC conditioned medium (hMSC
SD-conditioned medium) were also used as indirect co-culture
medium. hMSCs were cultured until they reached 80% confluence,
washed three times with PBS and incubated in DF-12 medium without
FBS for 24 h. Then, SD-conditioned media were harvested by 0.22
μm filtration.
Serum-deprived co-culture groups (SD+co-culture)
grew in the presence of hMSCs in DF-12 media without FBS for 24 h;
SD-conditioned medium groups (SD-conditioned) were grown in the
presence of only hMSCs SD-conditioned media for 24 h;
serum-deprived control groups (SD+control) were grown in DF-12
media without FBS; and control groups (control) were grown in DF-12
media with FBS for 24 h. Every group had three wells.
Cell Counting Kit-8
The measurement of viable cell mass was performed
with a Cell Counting Kit-8 (CCK8, Beyotime, Jiangsu, China) assay.
Cells were first seeded in 24-well flat-bottomed plates for 6 h.
Next, the wells were prepared for the four different culture
groups, and the cells were grown for 24 h. Cell proliferation was
measured according to the manufacturer’s instructions.
Cell apoptosis assay
Apoptotic cells were analyzed by flow cytometry.
Four groups were cultured in different culture media for 24 h.
Approximately 2×105 cells were incubated with the Cell
Apoptosis Assay according to the manufacturer’s instructions
(Beyotime). Briefly, cells were resuspended in 195 μl 1X
binding buffer containing 5 μl Annexin V for 10 min at room
temperature in the dark, and then mixed with another 190 μl
1X binding buffer containing 10 μl PI and incubated in an
ice bath for another 10 min. After incubation, at least 10,000
cells were measured on a Beckman Coulter flow cytometer. Cells
undergoing an early stage apoptosis are stained with Annexin V-FITC
only, while cells at a late stage of apoptosis and necrotic cells
are stained with both Annexin V-FITC and propidium iodide.
Transfection
GFP-tagged microtubule-associated protein 1 light
chain 3 (GFP-MAP1LC3) was used to monitor autophagy through direct
fluorescence microscopy. Cells undergoing autophagy were observed
to have significant numbers of punctate GFP, while normal cells
showed a primarily diffuse GFP signal. The carcinoma cells were
seeded (2×105 cells/well) in 6-well plates overnight.
The cells were transiently transfected using Lipofectamine 2000
Plus GFP-MAP1LC3 (Yrgene, China) according to the manufacturer’s
protocol. The cells were cultured for 24 h to ensure the expression
of GFP-MAP1LC3, divided into the four experimental groups, and
incubated for 24 h. Then, the cells were fixed in 4%
paraformaldehyde for 10 min, stained with DAPI and analyzed under a
Leica laser confocal microscope to measure the cells with
GFP-MAP1LC3-positive dots.
Transmission electron microscopy
(TEM)
After treatment with different culture media for 24
h, cells were fixed in ice-cold 2.5% glutaraldehyde acid in 0.1 M
PBS buffer for 2 h or longer, rinsed with PBS, postfixed in 1%
osmium tetroxide with 0.1% potassium ferricyanide, dehydrated in a
graded series of ethanol (30–90%) and embedded in Epon resin. Thin
sections of 50–60 nm were cut and picked up on copper grids,
post-stained with uranyl acetate and lead citrate and then observed
using a Philips EM420 transmission electron microscopy.
Western blot analysis
At the end of the treatments, cells were lysed in
cell lysis buffer (Beyotime) with 1 mM phenylmethylsulfonyl
fluoride (PMSF), and the protein concentration of the lysate was
quantified using a BCA protein assay kit (Beyotime). Equal amounts
of protein for each sample were electrophoresed in SDS-PAGE and
transferred to a polyvinylidene fluoride (PVDF) membrane. After
blocking with 5% non-fat milk, the membranes were incubated with
primary antibodies against MAP1LC3, Beclin-1, B-cell
lymphoma/leukemia-2 (Bcl-2) (Abcam Inc.) and β-actin (Beyotime)
overnight at 4°C. Then, the membranes were incubated with a
horseradish peroxidase-conjugated goat anti-rabbit IgG secondary
antibody (Beyotime) for 2 h. Bands were detected using ECL
(Beyotime). Protein levels were quantitated by densitometry using
Quantity One software (Bio-Rad Laboratories, Munich, Germany).
Animal studies
Male NOD/SCID mice aged 4–6 weeks were used in our
study. All study protocols were approved by the Third Military
Medical University Animal Care and Use Committee (Chongqing, China;
no. 2011-11). All procedures were carried out in accordance with
the advice and permission of the Institutional Ethics Committee.
Two lines of carcinoma cells were treated with or without hMSCs for
24 h under serum-deprived medium and then resuspended as
single-cell type suspensions (1×106) or mixed with hMSCs
(5×105) in 0.1 ml PBS. Cells were injected
subcutaneously into the left flank of the mice. The mice were
examined each week and palpable tumors at the injection sites with
a size of more than 3 mm in diameters were considered tumors. After
30 days, the mice were sacrificed and tumor volume was calculated
according to the formula V=π/6 (length × width × height). Each
group included 10 NOD/SCID mice.
Statistical analysis
All experiments were repeated at least three times.
The statistical analyses were performed using SPSS v.13.0. The data
were expressed as the means ± SD and analyzed using the Student’s
t-test. The criterion for statistical significance was taken as
p<0.05.
Results
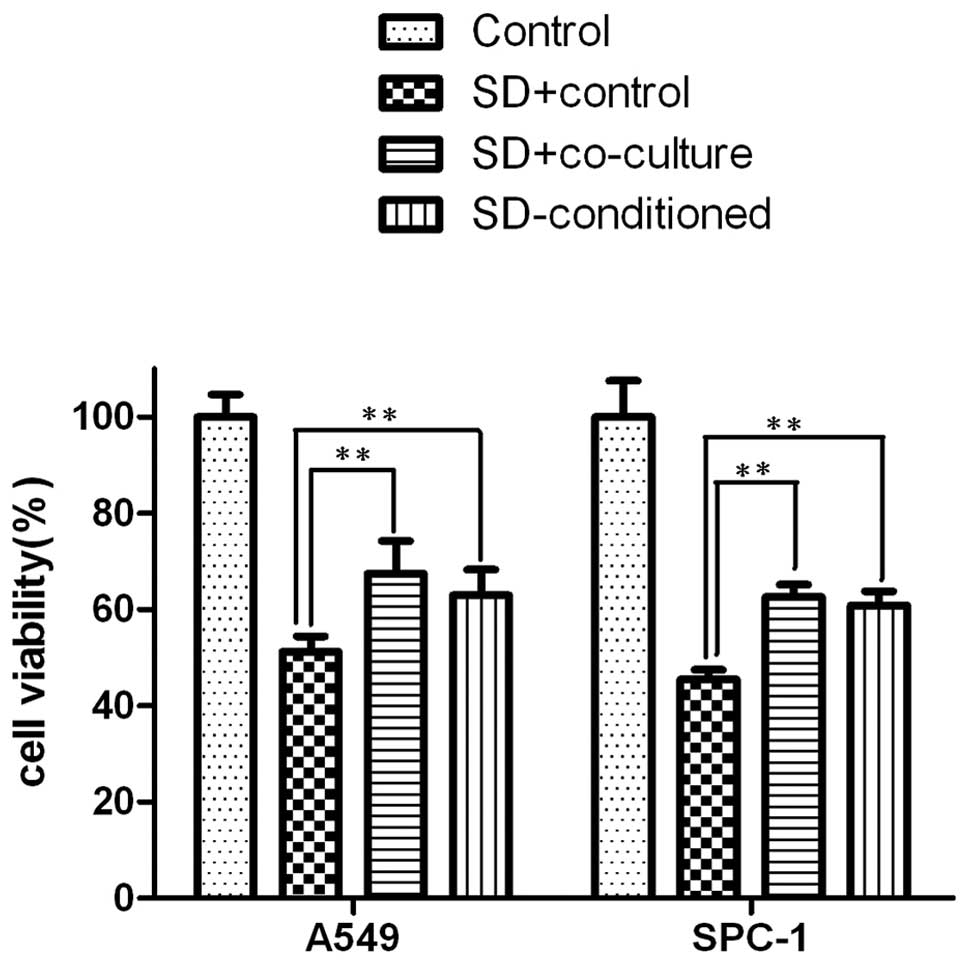
Lung carcinoma cells have better
tolerance with co-culture or hMSCs SD-conditioned media in
serum-deprived conditions
There is evidence showing that hMSCs provide
sufficient stromal support for tumor cells. Solid tumors are
typically characterized by nutrient deprivation and an ischemic
micro-environment as they grow. To determine whether hMSCs
influence lung carcinoma cell survival, the lung cancer cell lines
A549 and SPC-1 were used. The two cell lines were cultured in
DMEM/F12 with or without hMSCs under serum-deprived conditions or
with hMSC SD-conditioned media for 24 h. At the end of the
treatment, cell viability analysis showed that hMSCs helped
maintain the viability of A549 and SPC-1 under serum deprived
conditions. The viability levels of A549 and SPC-1 cells in the
SD+co-culture groups were higher than those in the SD+control
groups (p<0.01) and similar to those of the SD-conditioned
groups (p<0.01). Moreover, the viability of SD+co-culture was
similar to that of the SD-conditioned groups (Fig. 1).
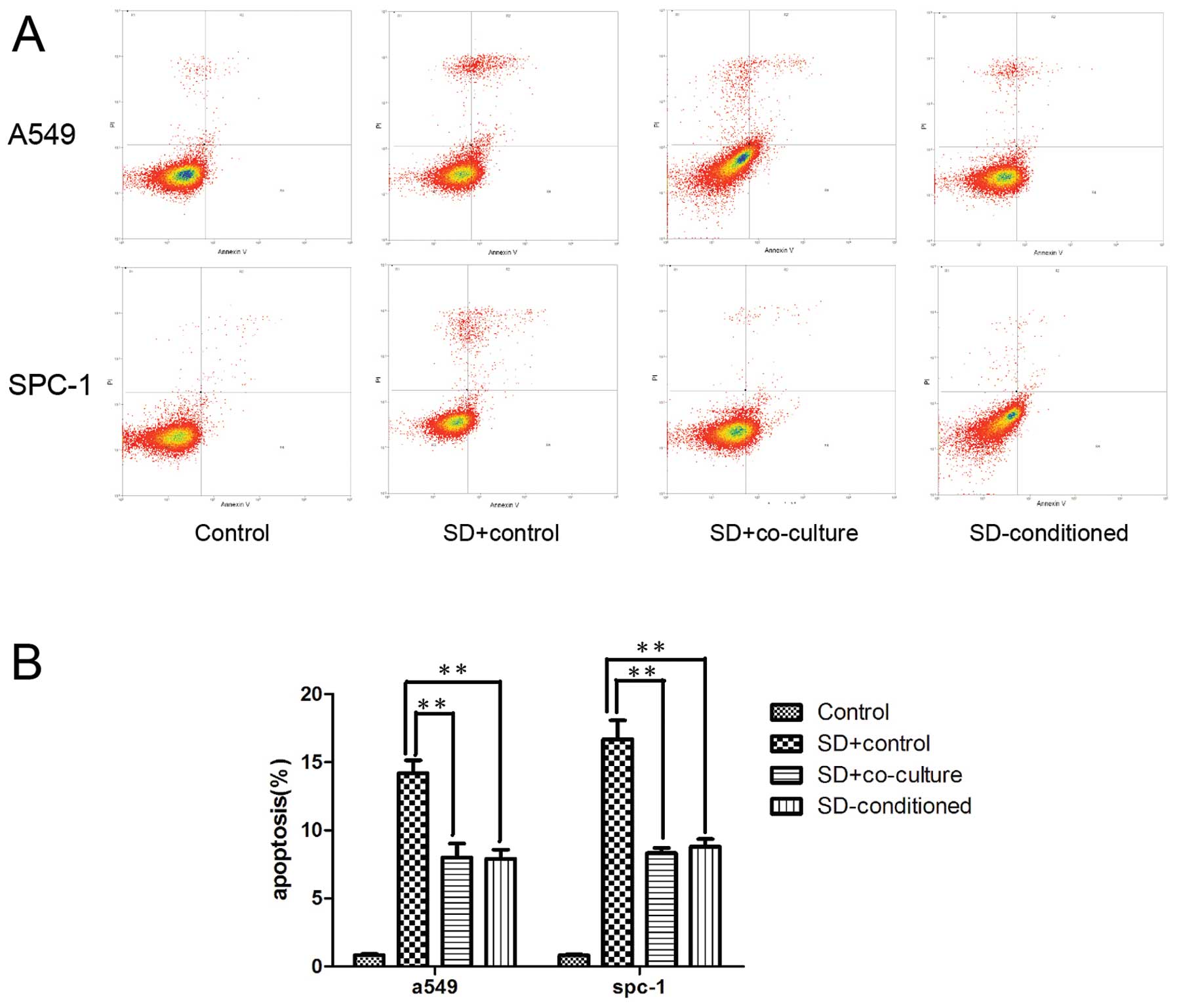
The survival of lung carcinoma cells is
attributed to the decrease in apoptosis induced by hMSCs under
serum deprivation
As higher viability was observed for lung carcinoma
cells in the co-cultured and SD-conditioned groups, we investigated
the effects of hMSCs on the apoptosis of tumor cells, which occurs
in stressful conditions. The Annexin V/PI assay was used to detect
apoptosis by flow cytometry. Our findings showed that the apoptotic
rate of A549 and SPC-1 cells was obviously reduced in the
SD+co-culture and SD-conditioned groups compared with SD+control
(p<0.01). Likewise, the apoptotic rate of A549 and SPC-1
SD+co-culture resembled that of the SD-conditioned groups (Fig. 2) (p>0.05).
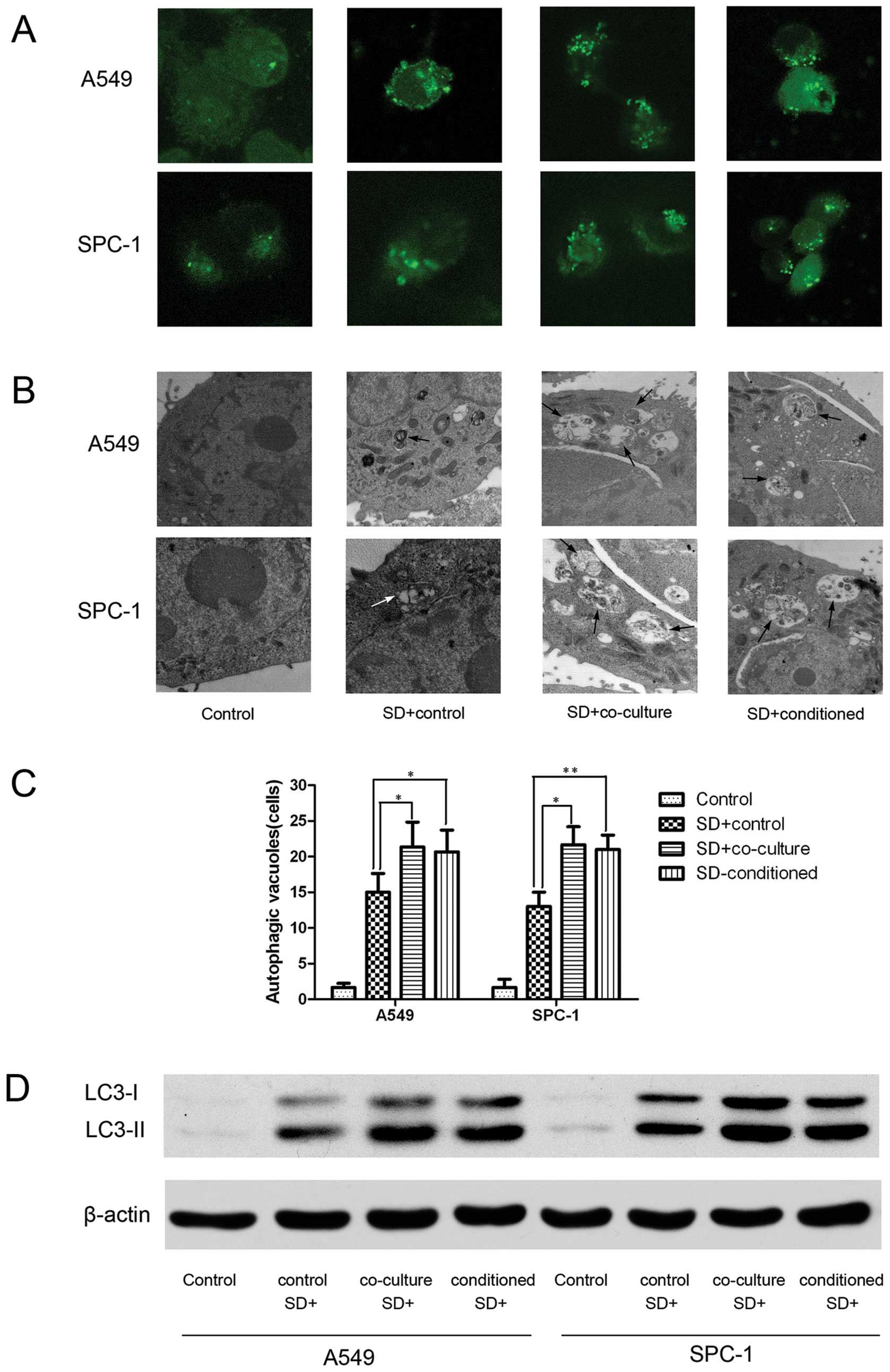
Autophagy is activated by hMSCs in lung
carcinoma cells under serum deprivation conditions
To determine whether autophagy is involved in the
hMSC-mediated increase in lung carcinoma cell survival upon
nutrient deprivation, we examined the accumulation of
autophagosomes (25). After
transient transfection with GFP-MAP1LC3 plasmids, A549 and SPC-1
cells were incubated in the four different experimental media for
24 h, and we then observed GFP-MAP1LC3 dots under a fluorescence
microscope (Fig. 3A). Transmission
electron microscopy was used to examine autophagic vacuoles
(Fig. 3B). The A549 and SPC-1
cells in the SD+co-culture and SD-conditioned groups obviously have
more autophagosomes than SD+control, and the SD+co-culture
resembled the SD-conditioned groups (Fig. 3C). We also evaluated MAP1LC3-I and
MAP1LC3-II protein levels using western blot analysis (Fig. 3D).
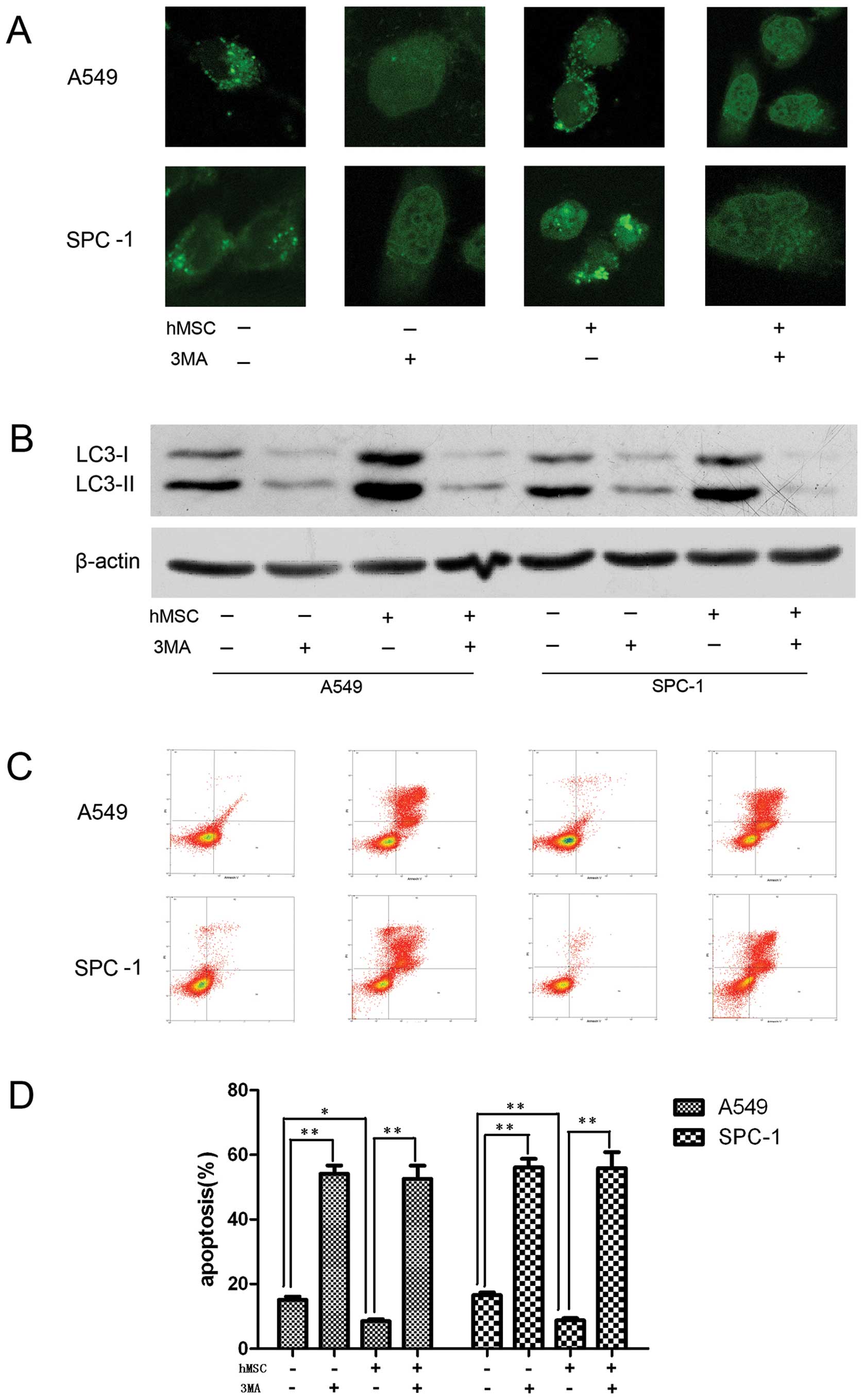
Autophagy activated by hMSCs is involved
in the tolerance of lung carcinoma cells to serum deprivation
To analyze whether autophagy is involved in the
observed decrease in apoptosis, the effect of autophagy inhibition
on cell viability was observed. The autophagy inhibitor
3-methyladenine (3-MA) (26) was
used to block autophagy. SD+control group and SD-conditioned group
were treated with DMSO or 3-MA, respectively. A549 and SPC-1 cells
incubated with or without hMSC in serum deprivation lacked
autophagic activity when treated with 3-MA (Fig. 4A and B). Moreover, greater numbers
of apoptotic cells were observed in the 3-MA treatment groups
compared to the non-3-MA-treated groups (Fig. 4C and D).
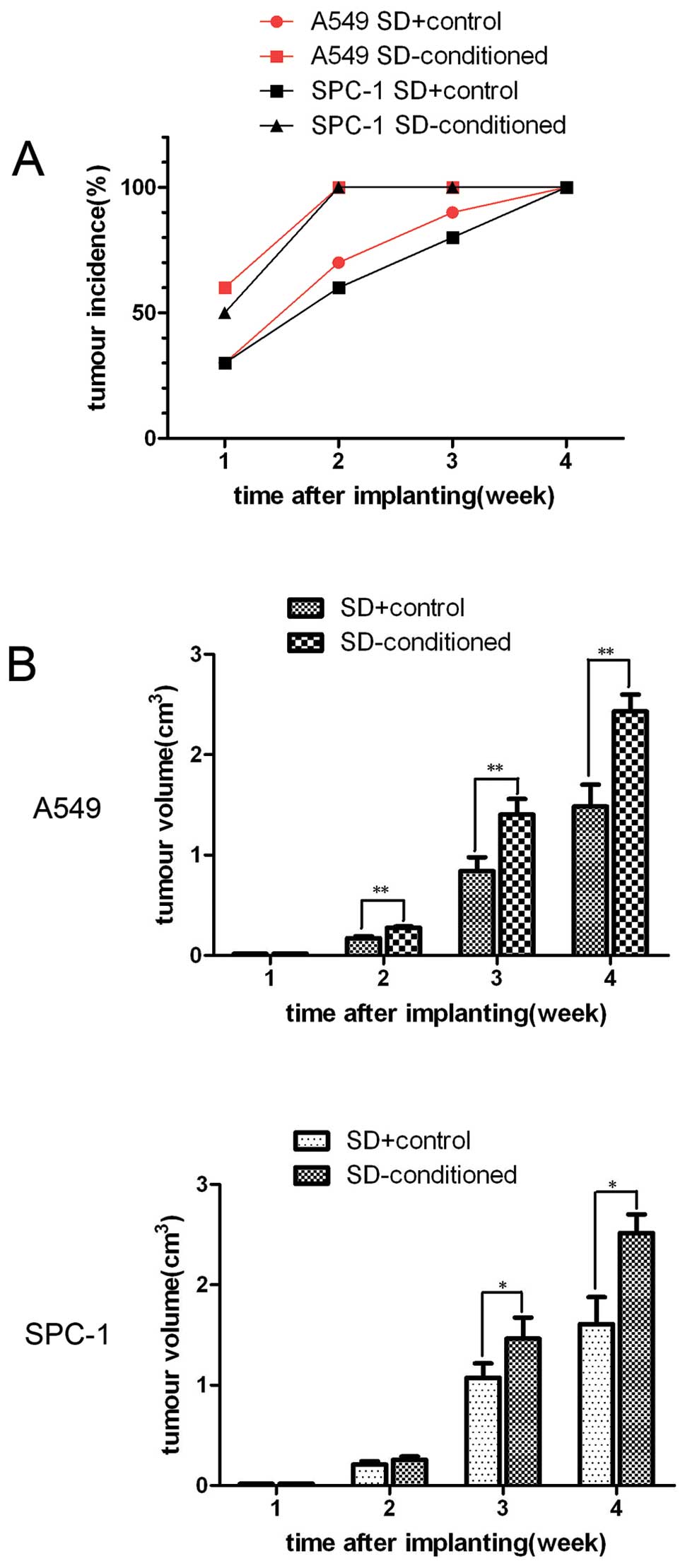
hMSCs favor tumorigenesis and growth
after starvation in vivo
To investigate the effects of hMSCs on the growth of
A549 cells and SPC-1 cells after starvation in vivo, we
assessed the growth of tumor cells in NOD/SCID mice. After 2 weeks,
all mice transplanted with carcinoma cells and hMSCs have palpable
tumor nodules, compared to only 70 and 60% of the mice injected
with carcinoma cells alone (Fig.
5A). The mean volume of tumors of the mice co-injected with
hMSCs and tumor cells was dramatically larger than that of the
control groups (Fig. 5B).
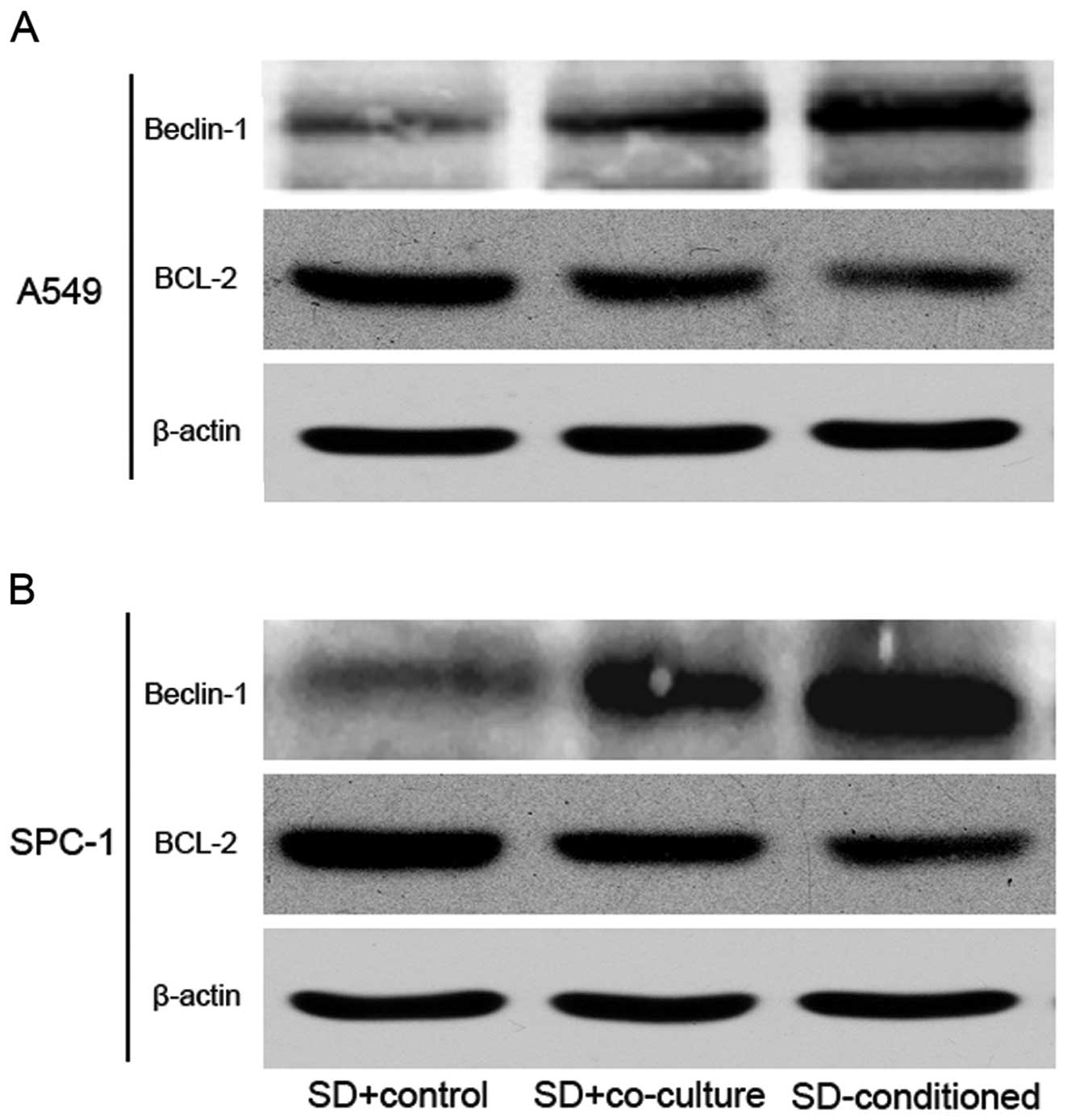
Protein expression changes in the tumor
cells induced by the SD-hMSC-conditioned medium
To elucidate the molecular mechanism underlying the
protective effects of hMSCs on tumor cells in vitro, we
investigated the expression of Beclin-1 and Bcl-2 in tumor cells by
western blot analysis. The results showed that the presence of MSCs
during serum deprivation increased the levels of Beclin-1, a
mammalian autophagy protein, in A549 and SPC-1 cells. In contrast,
the expression of Bcl-2, an anti-apoptotic and anti-autophagy
protein, was clearly reduced in the SD-conditioned groups. Both
Beclin-1 and Bcl-2 had statistically significant differences in
expression in the SD-conditioned groups and SD+control groups
(Fig. 6A and B).
Discussion
Solid tumors are composed of tumor cells and
supportive non-tumor components known as tumor stroma. There is a
niche that is enriched with carcinoma cells and a subpopulation of
closely associated stromal cells that control the activity of the
carcinoma cells. hMSCs can be recruited to these niches and
subsequently secrete various cytokines and growth factors (7). When tumors grow beyond 2 mm in
diameter, carcinoma cells and stromal cells could undergo
starvation due to lack of sufficient nutrients in the niche
(27). Because of the close
relationship between hMSCs and carcinoma cells, it is unclear how
hMSCs influence the fate of carcinoma cells under nutrient
deprivation conditions. In the present study, we used an SD
co-culture system and SD-hMSC-conditioned medium to explore the
effects of hMSCs on lung carcinoma cell lines A549 and SPC-1. This
is the first time the role of hMSCs in lung carcinoma cells under
serum deprivation has been addressed. We found that the viability
of carcinoma cells grown with hMSCs is higher than that of cells
grown without hMSCs after serum starvation for 24 h. Moreover, a
marked decrease in apoptosis was observed in lung carcinoma cells
that were co-cultured with hMSCs, supporting the protective role of
hMSCs against apoptosis.
Autophagy is a well-established mechanism to degrade
cytoplasmic proteins, macromolecules, and organelles and to provide
a nutrient source to promote the survival of cells in metabolic
distress. Accumulating evidence supports a role for autophagy in
maintaining tumor cell survival in response to metabolic stress and
hypoxia (28–31). Autophagy has been considered an
indispensable physiological reaction for sustaining cell viability
during starvation. A549 and SPC-1 cells were co-cultured with hMSCs
during nutrient deprivation showed more autophagic cells than were
observed in SD-condition groups. Less apoptosis was observed in the
co-culture system. After the addition of 3-MA, a commonly used
inhibitor of starvation or rapamycin-induced autophagy, apoptosis
increased, concomitant with a decrease in autophagy. Moreover,
cells at late stage apoptosis and necrotic cells were found in the
3-MA groups, supporting the protective role of hMSCs against
apoptosis under nutrient starvation conditions. Similarly, 3-MA
treatment also led to a dramatic decrease in the survival of single
culture group under starvation conditions. This is similar to
findings in starved HeLa cells, in which it was shown that the
suppression of autophagy could promote apoptosis and caspase-3
activation (32). The indirect
system used in this study indicated that this protective mechanism
may involve cytokines such as growth factors, anti-apoptotic
factors and TGF-β, which have been found in conditioned medium from
SD-hMSCs (27).
In vivo, subcutaneous injection experiments
showed that starved lung carcinoma cells that were co-injected with
hMSCs exhibited stronger tumor initiation and growth than carcinoma
cells that were injected alone. There are several possible
explanations for this observation. First, tumor initiation and
growth may be promoted by the protective role of hMSCs on lung
carcinoma cell viability and apoptosis, as reported in this study.
Further, there is evidence that hMSCs may differentiate into tumor
stromal fibroblasts, forming a niche that favors tumor growth
(33). Furthermore, hMSCs enhanced
vascular endothelial growth factor (VEGF) expression in tumor cells
(34). Importantly, as the tumor
grows, the stromal cells and tumor could undergo starvation and
stromal cells may secrete growth factors and anti-apoptotic factors
that protect carcinoma cells (27). Overall, we hypothesize that
SD-hMSCs may provide a protective niche for lung carcinoma cells.
During tumor growth, the growth factors and anti-apoptotic factors
secreted by hMSCs protect lung carcinoma cells and allow them to
survive the nutrient deficient situation.
Beclin-1 is identified as mammalian autophagy gene
that plays a major role in the formation of the autophagosome
(35). It also interacts with
anti-apoptotic multidomain proteins Bcl-2 family members, in
particular Bcl-2 and its homologue Bcl-XL, whose overexpression
would inhibit the autophagy-inducing activity of Beclin-1 (36–38).
Blocking the interaction between Beclin-1 and Bcl-2 has been
reported to enhance autophagy (39). We determined that the expression
levels of Beclin-1 and Bcl-2 proteins in A549 and SPC-1 cells after
inoculation. Under normal conditions, A549 and SPC-1 cells
exhibited a basal expression of Beclin-1 and Bcl-2; under serum
deprivation conditions, the expression of Bcl-2 was decreased and
the expression of Beclin-1 increased. The addition of hMSCs
resulted in a dramatic decrease in the levels of Bcl-2 and an
increase in Beclin-1, which triggers autophagy as protective
mechanism. Hence, we hypothesize that hMSCs could trigger autophagy
by Beclin-1 without the inhibition of Bcl-2.
According to our studies, we conclude that hMSCs
could promote tumor cell proliferation and reduce tumor cell
apoptosis and that autophagy plays an important role in the
survival of carcinoma cells. In vivo, earlier tumor
initiation and growth were observed when tumor cells were
subcutaneously injected along with hMSCs. The disruption of the
Bcl-2 and Beclin-1 interaction may be involved in this autophagy
protective mechanism. Taken together, this study provides
preliminary exploratory research results for understanding stromal
protection in the survival mechanism of lung carcinoma cells and
show that the promotion of autophagy by hMSCs play an important
role in tumor cell survival.
References
|
1
|
Siegel R, Desantis C, Virgo K, et al:
Cancer treatment and survivorship statistics, 2012. CA Cancer J
Clin. 62:220–241. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Prockop DJ: Marrow stromal cells as stem
cells for nonhematopoietic tissues. Science. 276:71–74. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Young HE, Steele TA, Bray RA, et al: Human
reserve pluripotent mesenchymal stem cells are present in the
connective tissues of skeletal muscle and dermis derived from
fetal, adult, and geriatric donors. Anat Rec (Hoboken). 264:51–62.
2001. View
Article : Google Scholar
|
|
4
|
Pittenger MF, Mackay AM, Beck SC, et al:
Multilineage potential of adult human mesenchymal stem cells.
Science. 284:143–147. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Young HE, Duplaa C, Young TM, et al:
Clonogenic analysis reveals reserve stem cells in postnatal
mammals: I. Pluripotent mesenchymal stem cells. Anat Rec (Hoboken).
263:350–360. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Fox JM, Chamberlain G, Ashton BA and
Middleton J: Recent advances into the understanding of mesenchymal
stem cell trafficking. Br J Haematol. 137:491–502. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Karnoub AE, Dash AB, Vo AP, et al:
Mesenchymal stem cells within tumour stroma promote breast cancer
metastasis. Nature. 449:557–563. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mishra PJ, Humeniuk R, Medina DJ, et al:
Carcinoma-associated fibroblast-like differentiation of human
mesenchymal stem cells. Cancer Res. 68:4331–4339. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Studeny M, Marini FC, Champlin RE,
Zompetta C, Fidler IJ and Andreeff M: Bone marrow-derived
mesenchymal stem cells as vehicles for interferon-beta delivery
into tumors. Cancer Res. 62:3603–3608. 2002.PubMed/NCBI
|
|
10
|
Xin H, Kanehira M, Mizuguchi H, et al:
Targeted delivery of CX3CL1 to multiple lung tumors by mesenchymal
stem cells. Stem Cells. 25:1618–1626. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Stagg J: Mesenchymal stem cells in cancer.
Stem Cell Rev. 4:119–124. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhang W: Mesenchymal stem cells in cancer:
friends or foes. Cancer Biol Ther. 7:252–254. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bian ZY, Fan QM, Li G, Xu WT and Tang TT:
Human mesenchymal stem cells promote growth of osteosarcoma:
involvement of interleukin-6 in the interaction between human
mesenchymal stem cells and Saos-2. Cancer Sci. 101:2554–2560. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bagley RG, Weber W, Rouleau C, et al:
Human mesenchymal stem cells from bone marrow express tumor
endothelial and stromal markers. Int J Oncol. 34:619–627. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kato K, Ogura T, Kishimoto A, et al:
Critical roles of AMP-activated protein kinase in constitutive
tolerance of cancer cells to nutrient deprivation and tumor
formation. Oncogene. 21:6082–6090. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Li Y, Iglehart JD, Richardson AL and Wang
ZC: The amplified cancer gene LAPTM4B promotes tumor growth and
tolerance to stress through the induction of autophagy. Autophagy.
8:273–274. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kelekar A: Introduction to the review
series Autophagy in Higher Eukaryotes--a matter of survival or
death. Autophagy. 4:555–556. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yin L, Kharbanda S and Kufe D: MUC1
oncoprotein promotes autophagy in a survival response to glucose
deprivation. Int J Oncol. 34:1691–1699. 2009.PubMed/NCBI
|
|
19
|
Carew JS, Medina EC, Esquivel JA II, et
al: Autophagy inhibition enhances vorinostat-induced apoptosis via
ubiquitinated protein accumulation. J Cell Mol Med. 14:2448–2459.
2010. View Article : Google Scholar
|
|
20
|
Kang C, You YJ and Avery L: Dual roles of
autophagy in the survival of Caenorhabditis elegans during
starvation. Genes Dev. 21:2161–2171. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Mathew R, Karantza-Wadsworth V and White
E: Role of autophagy in cancer. Nature reviews Cancer. 7:961–967.
2007. View
Article : Google Scholar
|
|
22
|
Fujii S, Mitsunaga S, Yamazaki M, et al:
Autophagy is activated in pancreatic cancer cells and correlates
with poor patient outcome. Cancer Sci. 99:1813–1819.
2008.PubMed/NCBI
|
|
23
|
Dominici M, Le Blanc K, Mueller I, et al:
Minimal criteria for defining multipotent mesenchymal stromal
cells. The International Society for Cellular Therapy position
statement. Cytotherapy. 8:315–317. 2006. View Article : Google Scholar
|
|
24
|
Rhodes LV, Muir SE, Elliott S, et al:
Adult human mesenchymal stem cells enhance breast tumorigenesis and
promote hormone independence. Breast Cancer Res Treat. 121:293–300.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Klionsky DJ, Abeliovich H, Agostinis P, et
al: Guidelines for the use and interpretation of assays for
monitoring autophagy in higher eukaryotes. Autophagy. 4:151–175.
2008. View Article : Google Scholar
|
|
26
|
Seglen PO and Gordon PB: 3-Methyladenine:
specific inhibitor of autophagic/lysosomal protein degradation in
isolated rat hepatocytes. Proc Natl Acad Sci USA. 79:1889–1892.
1982. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Sanchez CG, Penfornis P, Oskowitz AZ, et
al: Activation of autophagy in mesenchymal stem cells provides
tumor stromal support. Carcinogenesis. 32:964–972. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Degenhardt K, Mathew R, Beaudoin B, et al:
Autophagy promotes tumor cell survival and restricts necrosis,
inflammation, and tumorigenesis. Cancer Cell. 10:51–64. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Karantza-Wadsworth V and White E: Role of
autophagy in breast cancer. Autophagy. 3:610–613. 2007. View Article : Google Scholar
|
|
30
|
Lum JJ, Bauer DE, Kong M, et al: Growth
factor regulation of autophagy and cell survival in the absence of
apoptosis. Cell. 120:237–248. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Karantza-Wadsworth V, Patel S, Kravchuk O,
et al: Autophagy mitigates metabolic stress and genome damage in
mammary tumorigenesis. Genes Dev. 21:1621–1635. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Boya P, Gonzalez-Polo RA, Casares N, et
al: Inhibition of macro-autophagy triggers apoptosis. Mol Cell
Biol. 25:1025–1040. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Roorda BD, ter Elst A, Kamps WA and de
Bont ES: Bone marrow-derived cells and tumor growth: contribution
of bone marrow-derived cells to tumor micro-environments with
special focus on mesenchymal stem cells. Crit Rev Oncol Hematol.
69:187–198. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Tian LL, Yue W, Zhu F, Li S and Li W:
Human mesenchymal stem cells play a dual role on tumor cell growth
in vitro and in vivo. J Cell Physiol. 226:1860–1867. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Sinha S and Levine B: The autophagy
effector Beclin 1: a novel BH3-only protein. Oncogene. 27(Suppl 1):
S137–S148. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Maiuri MC, Criollo A, Tasdemir E, et al:
BH3-only proteins and BH3 mimetics induce autophagy by
competitively disrupting the interaction between Beclin 1 and
Bcl-2/Bcl-X(L). Autophagy. 3:374–376. 2007. View Article : Google Scholar
|
|
37
|
Pattingre S, Tassa A, Qu X, et al: Bcl-2
antiapoptotic proteins inhibit Beclin 1-dependent autophagy. Cell.
122:927–939. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Chang NC, Nguyen M, Germain M and Shore
GC: Antagonism of Beclin 1-dependent autophagy by BCL-2 at the
endoplasmic reticulum requires NAF-1. EMBO J. 29:606–618. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Lian J, Wu X, He F, et al: A natural BH3
mimetic induces autophagy in apoptosis-resistant prostate cancer
via modulating Bcl-2-Beclin1 interaction at endoplasmic reticulum.
Cell Death Differ. 18:60–71. 2011. View Article : Google Scholar : PubMed/NCBI
|