Introduction
Gastric cancer is one of the most common types of
cancer with approximately 989,600 new cases and 738,000 deaths
worldwide in 2008 (1). Its
mortality remains high as most patients are diagnosed at an
advanced stage when the tumor is irresectable or metastatic.
Although chemotherapy has made progress in the remission of this
disease, drug resistance in the course of treatment has become more
common. Therefore, new cytotoxic agents, especially natural
compounds, or novel therapeutic strategies still need to be
explored.
Matrine, one of the main alkaloid components
extracted from a traditional Chinese herb, Sophora flavescens Ait,
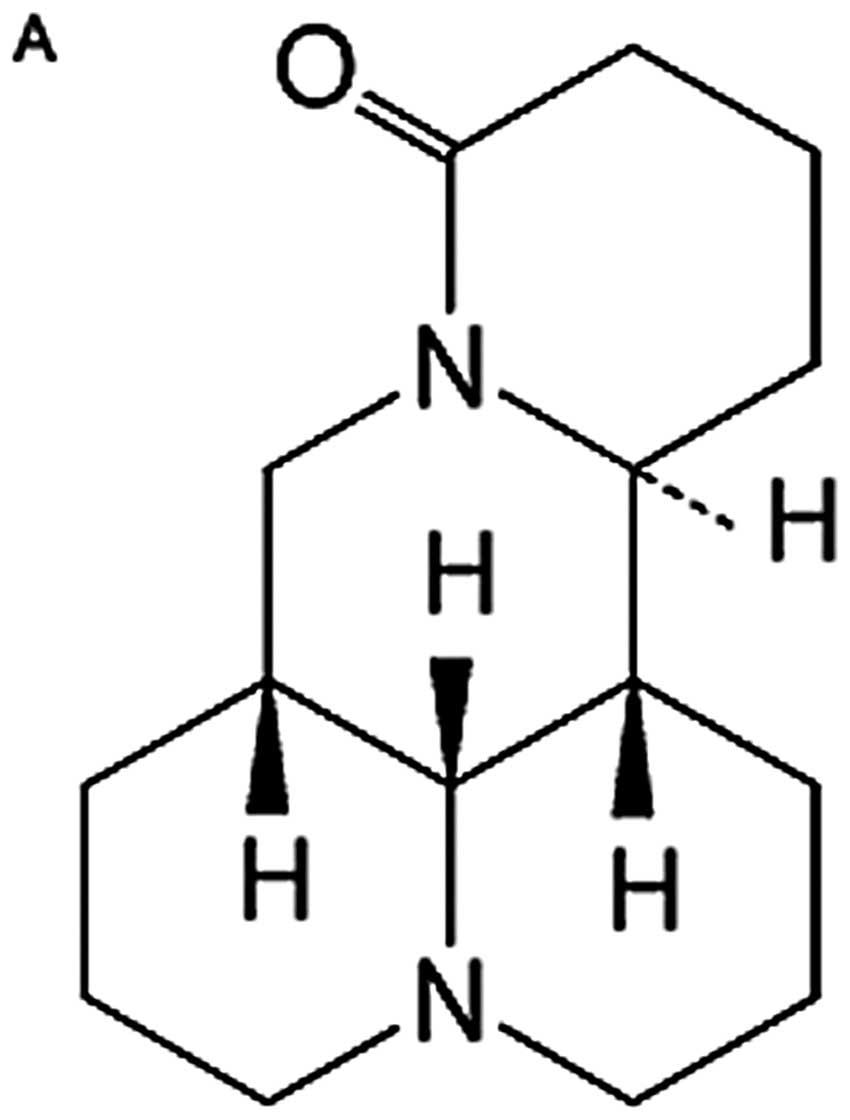
with a molecular formula of
C15H24N2O (Fig. 1A), has been shown to possess
various biological properties including anti-inflammatory (2–4),
antiviral (5,6), antifibrotic (7,8),
analgesic (9) and antiarrhythmic
(10,11). Recent evidence indicates that
matrine plays an important role in the treatment of tumors, without
obvious toxicity or side-effects. Matrine also attenuates cancer
cachexia-related symptoms in colon-26 tumor-bearing mice (12), and enhances patient immune
functions and thus improves the quality of life (13). Our previous studies showed that
matrine exhibited potent anticancer effects in gastric cancer and
hepatocellular carcinoma cells (14,15).
The mechanisms underlying the antineoplastic activity of matrine
may stem from cell cycle arrest, inhibition of cell proliferation
and induction of apoptosis, perhaps through the modulation of
apoptosis- and/or proliferation-related genes and proteins, such as
proliferating cell nuclear antigen, c-myc, apoptosis
protein-activating factor, Bcl-2 family members, caspases, and
Fas/FasL (13,16–21),
indicating matrine-induced cell apoptosis can be triggered by the
death receptor extrinsic pathway and/or the mitochondrial intrinsic
pathway. Also, our previous research demonstrated that autophagy is
involved in the antitumor effects of matrine on SGC-7901 human
gastric cancer cells (14).
Autophagy is an intracellular lysosome-dependent
catabolic process that is essential for maintaining cellular
homeostasis through the turnover and elimination of defective or
redundant proteins and damaged or aged organelles (22). Evidence suggests that autophagy may
be important in the regulation of cancer development and
progression, which is a double-edged sword in oncology (23–27).
In addition, a large body of findings indicates that various
anticancer therapies induce autophagy in different cancer cells
(28,29). However, whether autophagy in
response to therapies results in cell death or instead protects
cancer cells from death is controversial. Under physiological
conditions, autophagy is constitutively at low basal levels in
every cell. A variety of environmental stresses, such as nutrient
starvation, or anti-cancer drug treatment, can trigger dramatic
enhancement of the level of autophagy acting as a cytoprotective
response, resulting in adaptation and survival; however, autophagy
may become a cell death mechanism if the amplitude of autophagy
increases above a threshold level (30,31).
In several cases, it is agreed that autophagic cell death, also
defined as type II programmed cell death, is an important cell
death process distinct from apoptosis (type I programmed cell
death) (32,33). In our previous study, we found that
both autophagy and apoptosis were activated during the
matrine-induced death of SGC-7901 cells. Since the role of
autophagy in anticancer treatment may depend on the nature of the
cancer, the drug, or both (34),
it is necessary to elucidate whether matrine-induced autophagy
itself is responsible for cell killing, or protects cancer cells
against matrine treatment by blocking the apoptosis. In addition,
the exact mechanism by which matrine induces autophagy remains
unclear. There are a number of signaling pathways involved in the
regulation of autophagy. The class I phosphatidylinositol 3-kinase
(PI3K)/Akt/mammalian target of rapamycin (mTOR)/p70 ribosomal
protein S6 kinase (p70S6K) signaling pathway has been studied
extensively (35,36), and several anticancer compounds
have been reported to induce autophagy through inhibiting the
pathway (37–41). Therefore, in order to make matrine
therapy for gastric cancer more efficacious and less toxic, we
further clarified whether the signaling pathway is involved in the
induction of autophagy triggered by matrine.
Materials and methods
Reagents and antibodies
Reagents used included fetal bovine serum (Hyclone),
dimethyl sulfoxide (DMSO) (Sigma, D2650), 3-methyladenine (3-MA)
(Sigma, M9281), bafilomycin A1 (Sigma, B1793), rapamycin (Sigma,
R8781), Z-Val-Ala-Asp fluoromethylketone (zVAD-fmk) (Sigma, C2105),
matrine (Tianyuan Biologics Plant, Xi’an, China), Annexin V-FITC
apoptosis detection kit (Invitrogen, V13242), PhosSTOP Phosphatase
Inhibitor Cocktail (Roche, 04906845001), and SuperSignal West Pico
Chemiluminescent Substrate (Pierce, NCI5080). Antibodies were
obtained from the following sources: microtubule-associated protein
1 light chain 3 (LC3) (Abcam, ab51520), Akt (Cell Signaling
Technology, 9272), phospho-Akt (Ser473) (Cell Signaling Technology,
4060), mTOR (Cell Signaling Technology, 2972), phospho-mTOR
(Ser2448) (Cell Signaling Technology, 2971), p70S6K (Cell Signaling
Technology, 9202), phospho-p70S6K (Thr389) (Cell Signaling
Technology, 9205), glyceraldehyde-3-phosphate dehydrogenase (GAPDH)
(Hangzhou Goodhere Biotech Co., AB-P-R 001), horseradish peroxidase
(HRP)-conjugated goat anti-rabbit secondary antibody (Zhongshan
Goldenbridge Biotech Co., ZB-2301).
Cell culture and treatments
The human gastric cancer cell line SGC-7901 was
obtained from the Cell Collection of the Chinese Academy of
Sciences (Shanghai, China). Cells were maintained in RPMI-1640
medium supplemented with 10% fetal bovine serum, 100 U/ml of
penicillin and 100 μg/ml of streptomycin at 37°C in a 5%
CO2 incubator. The cells in mid-log phase were used in
the experiments. Matrine (purity >99%) was dissolved in sterile
double distilled water at a stock concentration of 40 mg/ml, and
then diluted in RPMI-1640 medium to obtain the desired
concentration. zVAD-fmk and bafilomycin A1 were dissolved in DMSO
and control cells were similarly treated with DMSO to a maximum
final concentration of 0.2%. This concentration of DMSO did not
cause any adverse morphologic response. zVAD-fmk, rapamycin and
bafilomycin A1 were added 1 h before matrine treatment. 3-MA was
dissolved in heated sterile double distilled water to make a 100-mM
stock solution and then added to the medium for a final
concentration of 5 mM. After 3 h, matrine was added for
treatment.
Western blot analysis
SGC-7901 cells were seeded into tissue culture
flasks, allowed to attach 24 h and then exposed to matrine with or
without specific inhibitors 3-MA, zVAD-fmk, rapamycin or
bafilomycin A1. After the end of specified treatment periods, cells
were lysed on ice with RIPA buffer for 20 min in the presence of
PhosSTOP and then centrifuged at 12,000 rpm for 15 min at 4°C. The
supernatant was collected and stored in aliquots at −80°C until
analysis by western blotting. Fifty micrograms protein were loaded
into each well and separated by electrophoresis through 8, 10 or
12% sodium dodecyl sulfate-polyacrylamide gel (SDS-PAGE) and then
transferred onto polyvinylidene difluoride membranes (Millipore)
using Trans-Blot Semi-Dry Cell or Mini Trans-Blot Cell apparatus
(Bio-Rad). After blocking with 5% non-fat dry milk or bovine serum
albumin in 1X TBST (20 mM Tris-HCl, 150 mM NaCl and 0.05% Tween-20)
for 1 h at room temperature, the membranes were incubated with
primary antibodies overnight at 4°C followed by incubation with
HRP-conjugated goat anti-rabbit secondary antibodies for 1 h at
room temperature. The specific protein bands were developed using
SuperSignal West Pico Chemiluminescent Substrate and imaged using a
VersaDoc imaging system (Bio-Rad).
Propidium iodide staining assay
Cells were trypsinized with 0.25% trypsin, collected
and resuspended in 100 μl of precooled phosphate-buffered
saline (PBS). After adding 1 μl of propidium iodide (PI)
staining solution (100 μg/ml), cells were incubated for 20
min in the dark at room temperature, supplemented with 400
μl of binding buffer and analyzed using a flow cytometer
(Beckman Coulter, USA).
Transmission electron microscopy
After 24 h of treatment, SGC-7901 cells were
harvested by trypsinization, washed twice with PBS and fixed in
2.5% glutaraldehyde for 90 min at room temperature, and post-fixed
in 1% osmium tetraoxide for 30 min. After washing with PBS, the
cells were progressively dehydrated in ascending grades of ethanol
solutions (50, 70, 95 and 100%), and embedded in Epon 812 resin.
The blocks were cut into ultra-thin sections with a microtome, and
were then stained with saturated uranyl acetate and lead citrate.
The ultrastructure of the cells was then examined in a transmission
electron microscope (JEM-1230, Jeol, Japan).
Annexin V-FITC/PI staining assay
Following drug treatment, floating cells were
collected and combined with adherent cells that were detached from
culture dishes by treating with trypsin. Total cells were then
washed with cold PBS twice and resuspended in 1X Annexin V binding
buffer at a concentration of 1×106 cells/ml. A
single-cell suspension (100 μl) was stained with 5 μl
Annexin V-FITC and 1 μl of PI for 15 min at room temperature
in the dark. After the incubation period, 400 μl of 1X
Annexin V binding buffer were added to each tube, followed by
cytometric analysis (EPICS XL, Beckman Coulter). The extent of
apoptosis was quantified as a percentage of Annexin V-positive
cells, and all experiments were performed in triplicate.
Sulphorhodamine B colorimetric assay
To determine cell viability, the sulphorhodamine B
(SRB) colorimetric assay, which estimates cell number indirectly by
staining total cellular protein with the dye SRB, was used
(42). SGC-7901 cells were seeded
in 96-well flat bottom microtiter plates at a density of
5×103 cells per well. Following treatment, the cells
were fixed with 10% (w/v) trichloroacetic acid at 4°C for 1 h, and
then stained at room temperature for 20 min with 0.4% (w/v) SRB
solution. The cells were subsequently washed with 1% acetic acid
five times and dissolved in 150 μl of 10 mmol/l Tris base
solution (pH 10.5). The absorbance value per well at 510 nM was
read using an automatic multiwell spectrophotometer (PowerWave x,
Bio-Tek Instruments Inc., USA). All SRB assays were repeated three
times. Cell viability was calculated according to the formula: Cell
viability (%) = A510 (sample)/A510 (control) × 100.
Statistical analysis
All data are expressed as the means ± standard
deviation (SD). Statistical analysis was performed using the SPSS
16.0, and p<0.05 was considered to indicate statistically
significant differences. The data were analyzed by ANOVA followed
by Bonferroni t-test for multiple comparisons.
Results
Matrine induces LC3-II accumulation
We have reported that matrine induces autophagy in
SGC-7901 cells which relies on the observation of cell
ultrastructural changes by transmission electron microscopy and the
visualization of monodansylcadaverine-labeled autophagic vacuoles
by fluorescence microscopy. Here, we further examined the
expression levels of LC3 which exists in cells in two forms, LC3-I
and LC3-II. LC3-I residing in the cytoplasm is conjugated to
phosphatidylethanolamine to form LC3-II, which is closely
associated with autophagosome membranes and serves as a reliable
marker to monitor autophagy. Western blot analysis of proteins from
matrine-treated cells revealed the presence of two bands (Fig. 1B). Weak bands corresponding to
LC3-I were found in the untreated cells whereas LC3-II was
undetectable. When the cells were treated with matrine at the
concentration of 1.0 mg/ml, the expression levels of LC3-II
significantly enhanced in a time-dependent manner. Furthermore,
LC3-II was observed as early as 3 h posttreatment with matrine.
Exposure of SGC-7901 cells to matrine also resulted in a
concentration-dependent increase of LC3-II protein levels at each
of the treatment points indicated (Fig. 1C).
Effects of 3-MA and bafilomycin A1 on
autophagy during matrine treatment
The increase in LC3-II expression could reflect
either increased formation of autophagosomes due to increases in
autophagic activity, or reduced turnover of autophagosomes
(43). To address the issue, we
assessed the influence of matrine on LC3-II levels in the presence
of bafilomycin A1 or 3-MA. Bafilomycin A1 is an inhibitor of the
vacuolar-type ATPase that alters the pH of acidic compartments,
which blocks the fusion of autophagosomes with lysosomes, thus
preventing the autophagic degradation including that of LC3-II
(44). 3-MA can inhibit autophagy
due to suppression of class III phosphatidylinositol 3-kinase which
is essential for the initiation of the early stages of autophagy
(45). The addition of 3-MA was
shown to decrease LC3-II levels in matrine-treated cells, while
bafilomycin A1 had their LC3-II further accumulated during matrine
treatment (Fig. 1D and E). The
opposite effects of 3-MA and bafilomycin A1 on LC3 are due to the
two inhibitors blocking autophagy at different stages; 3-MA
inhibited autophagosome formation from the beginning, whereas
bafilomycin A1 prevented degradation of LC3 in autophagolysosomes
and in turn increased LC3 levels (46). These findings demonstrated that the
LC3-II accumulation induced by matrine was a consequence of
increased autophagosome formation, indicating that matrine
increased autophagic flux, rather than prevented autophagic
degradation, in SGC-7901 cells.
Matrine induces cell death in a
dose-dependent manner
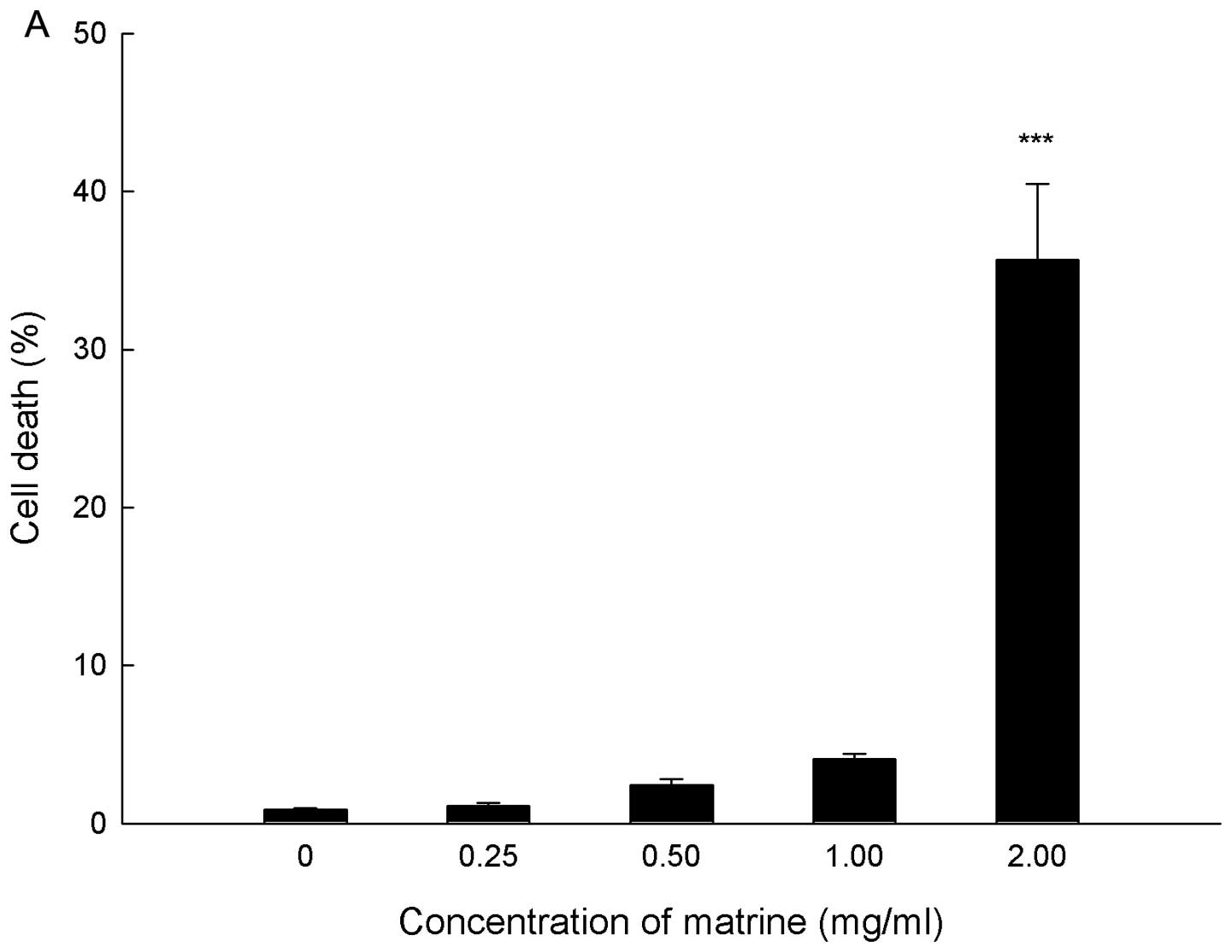
Cell death in response to matrine was quantified by
PI staining and flow cytometry, and PI positive cells were counted
as dead cells. SGC-7901 cells were treated with matrine at
concentrations ranging from 0 to 2.0 mg/ml for 24 h. As shown in
Fig. 2A, matrine killed gastric
cancer cells in a dose-dependent manner. At the concentration of
2.0 mg/ml matrine, the cell death rate reached 35.67±4.82%.
Inhibition of autophagy enhances
matrine-induced cyto toxicity
The above results showed that exposure of gastric
cancer cells to matrine resulted in the extent of autophagy
increasing in a dose- and time-dependent manner, which is
positively correlated with the cytotoxic effect of matrine; hence,
we further investigated whether matrine-induced cell death was
mediated by autophagy. Furthermore, our previous study demonstrated
that both apoptosis and autophagy were activated during matrine
treatment (14), therefore it is
necessary to further explore the interconnection between
matrine-induced autophagy and apoptosis. To address these
questions, we first determined whether inhibition of autophagy by
3-MA affects the cytotoxicity of matrine. The result showed that
the cell death by flow cytometry in the gastric cells treated with
matrine plus 3-MA sharply increased compared with matrine alone
(Fig. 2B). At the same time, we
used another inhibitor of autophagy at a late stage, bafilomycin
A1, to further establish the role of autophagy in cell death
induced by matrine. As shown in Fig.
2C, although bafilomycin A1 treatment alone had little effect
on SGC-7901 cells, this agent similarly enhanced the cytotoxicity
of matrine. Also, the combination of matrine with 3-MA acted
cooperatively to decrease the viability of SGC-7901 cells when
compared with matrine alone, measured by SRB assay (Fig. 2D). Markedly, when 3-MA inhibited
matrine-induced autophagy, inspection of the cells co-treated with
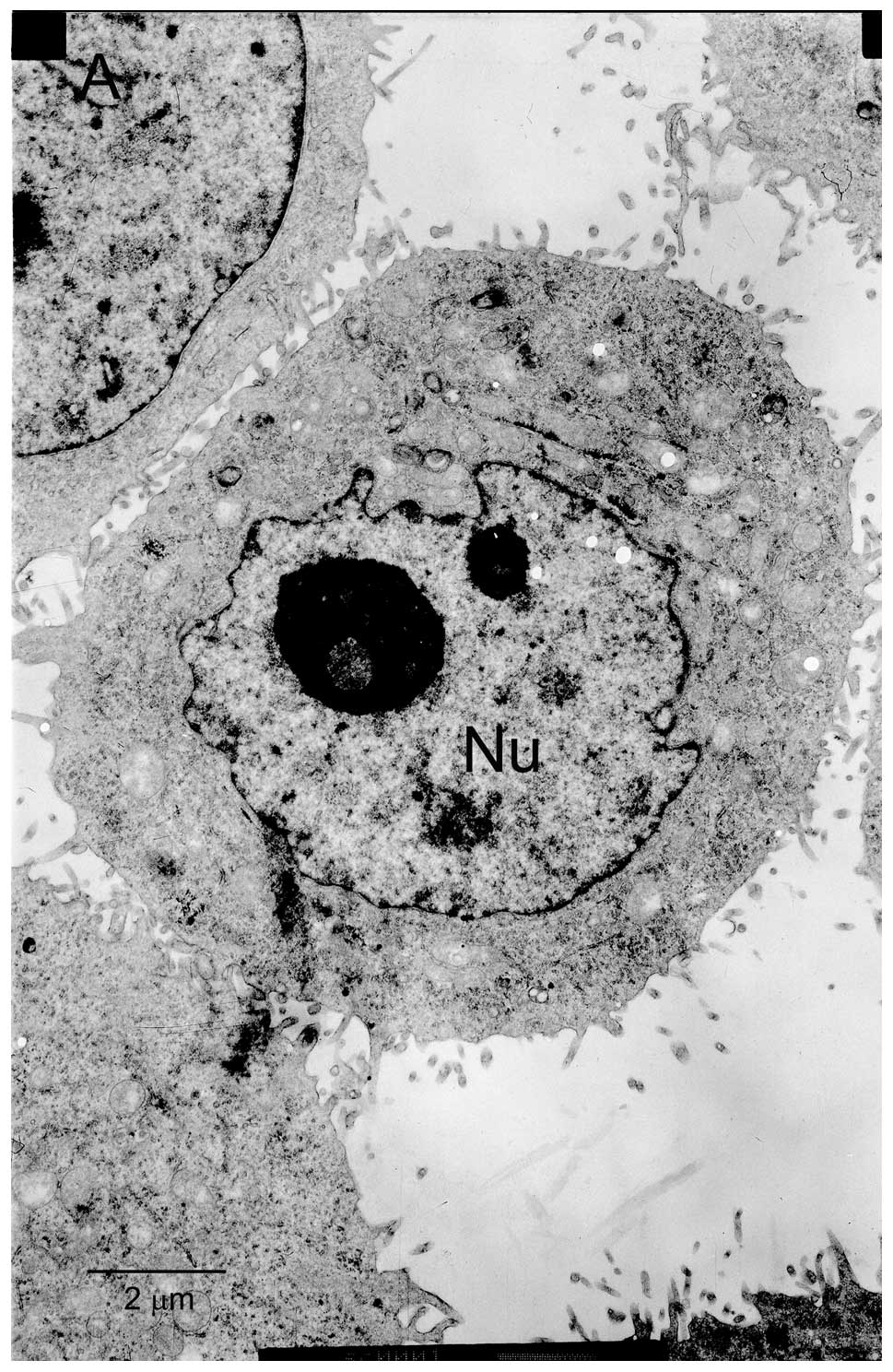
matrine and 3-MA using transmission electron microscopy revealed
prototypical characteristics of apoptosis. As shown in Fig. 3, control cells without matrine
exposure exhibited normal ultrastructural morphology. The cells
treated with 1 mg/ml matrine alone showed presence of abundant
autophagic vacuoles sequestrating cytoplasm and organelles and
absence of chromatin changes. By contrast, after incubation with
matrine in the presence of 3-MA for 24 h, the number of autophagic
vacuoles decreased in the cells and some of these cells underwent
apoptosis, which is characterized by cell shrinkage, plasma
membrane blebbing, and chromatin condensation with margination of
chromatin to the nuclear membrane. Similar effects were further
confirmed by Annexin V-FITC/PI double staining that addition of
3-MA augmented matrine-induced apoptosis of gastric cancer cells
compared to matrine treatment alone (Fig. 3E), indicating that matrine-induced
autophagy may constitute a pro-survival compensatory mechanism, the
inhibition of which drives more cells to die of apoptosis.
Consistent with these observations, other studies also reported
that matrine induced apoptosis in gastric cancer SGC-7901 cells
(21) and MKN45 cells (20) via activation of crucial caspases
such as caspase-3. Collectively, the results suggested that the
cytotoxic effect of matrine on SGC-7901 cells is caused by
apoptosis but not by autophagy which is a protective response by
preventing cells from undergoing apoptosis, and autophagy
inhibition could accelerate cell death induced by matrine.
Blockade of apoptosis reduces
matrine-induced cell death
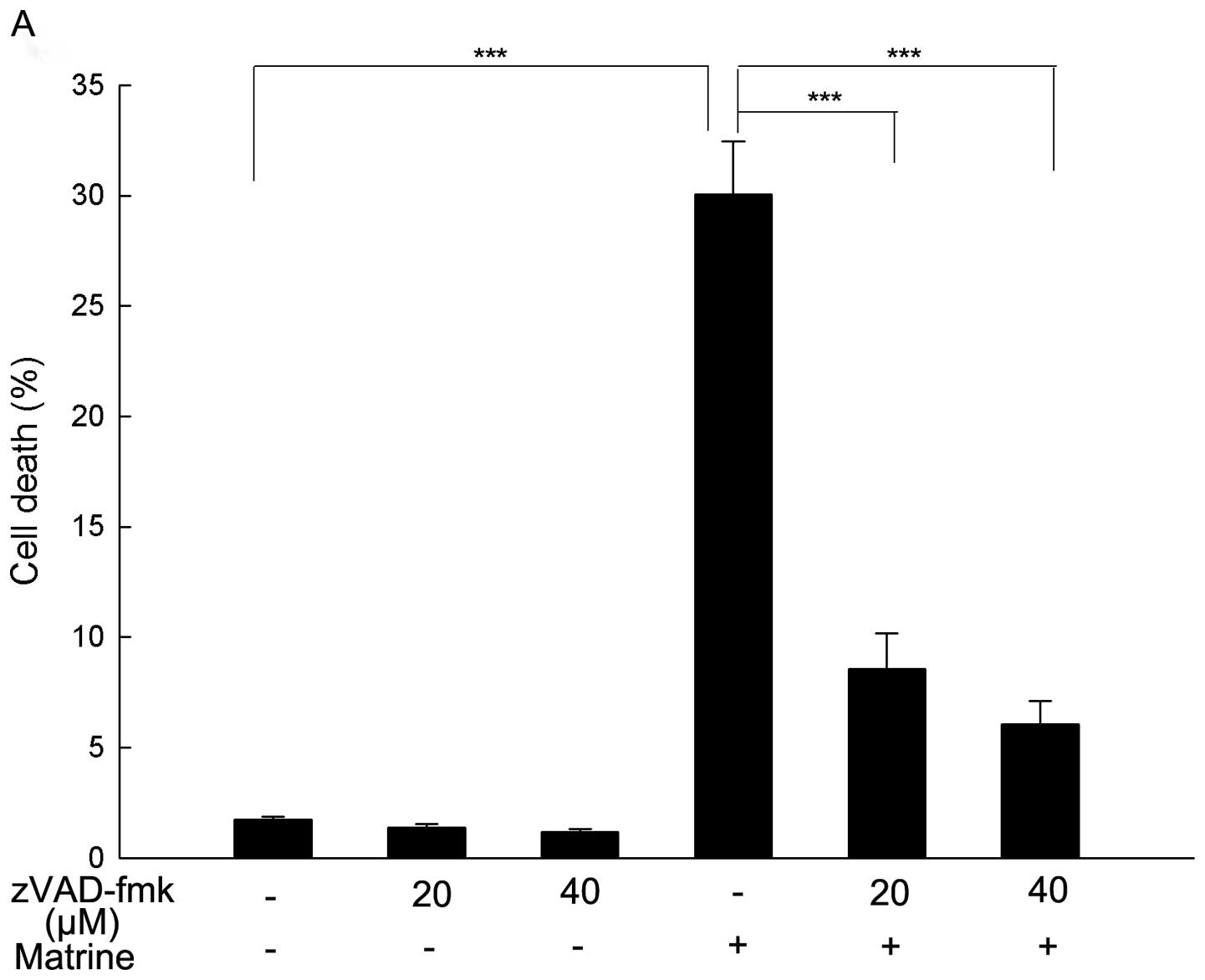
To further explore if matrine-induced cell death is
apoptotic, the cells were pretreated with a pan-caspase inhibitor
zVAD-fmk for 1 h followed by exposure to matrine for 24 h. zVAD-fmk
is a widely used inhibitor in characterizing apoptotic cell death
as it binds irreversibly to the active site of activated caspases
and inhibits caspase-mediated apoptosis (47). As shown in Fig. 4A, matrine-induced cell death was
markedly reversed by zVAD-fmk, which further demonstrated apoptosis
was the major form of cell death induced by matrine and may be
caspase-dependent.
Enhancement of autophagy decreases
matrine-induced cell death
It has been suggested that excessive autophagy
ultimately induces a type of cell death called autophagic cell
death (32,48–50).
Therefore, we hypothesized that if the cell death induced by
matrine is indeed mediated by autophagy, the cytotoxicity of
matrine is significantly enhanced after co-treatment with matrine
and rapamycin which is known to be a potent autophagy inducer. As
shown in Fig. 4B, however,
rapamycin did not cause an additional increase of cell death
quantified by flow cytometry, but instead attenuated the cell
death.
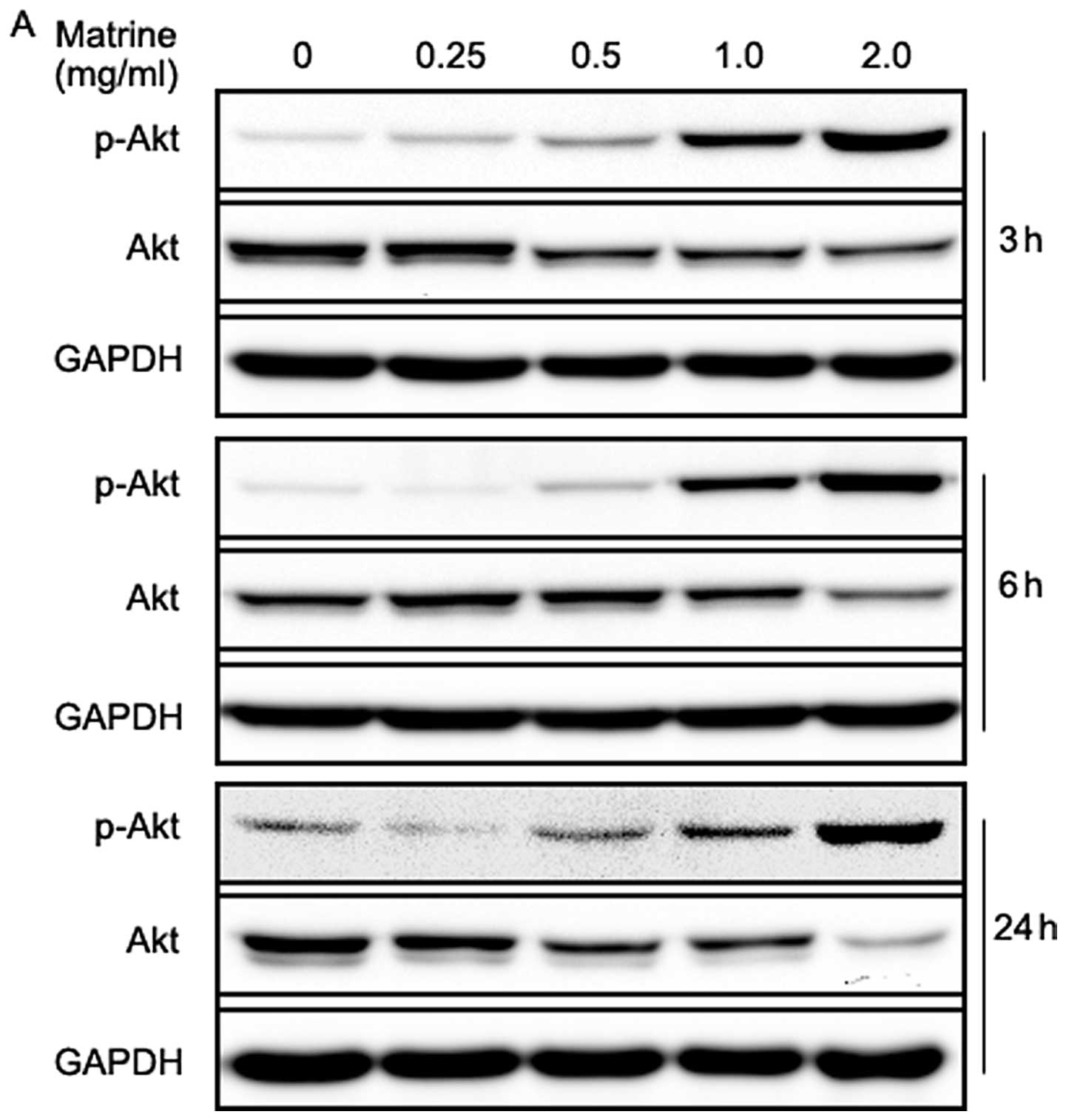
Matrine decreases total Akt level but
increases Akt phosphorylation in gastric cancer cells
The PI3K/Akt/mTOR/p70S6K signaling pathways are
well-known pathways involved in the regulation of autophagy, and
some anticancer agents are reported to block the pathways (37–41).
Therefore, we examined the effect of matrine on the pathways, using
western blotting. Akt is a downstream target of PI3K, which is
phosphorylated at Tyr308 by phosphoinositide-dependent kinase 1 and
at Ser473 by the mTOR complex 2 (mTORC2), resulting in its full
activation (51). As shown in
Fig. 5A, matrine-treated cells
exhibited a dramatic increase in Akt phosphorylation at Ser473 in a
dose-dependent manner. However, total Akt levels decreased in a
dose-dependent manner after treatment with matrine at 3, 6 and 24
h, respectively, especially in the cells exposed to 2.0 mg/ml of
matrine for 24 h. This was unexpected, as Akt activation is well
known to suppress autophagy in mammalian cells. On the other hand,
the increased Akt phosphorylation might, in part, limit clinical
anticancer efficacy of matrine due to its suppression of
apoptosis.
It has been reported that mTOR inhibitors such as
rapamycin induce increased levels of phospho-Akt via negative
feedback regulation of insulin receptor substrate-1 (52). As expected, rapamycin increased
levels of phospho-Akt; notably, co-treatment with matrine and
rapamycin was able to augment this counterproductive increase
(Fig. 5B). The activation of Akt
may be responsible for the finding that addition of rapamycin
contributed to the decrease of the cell death induced by matrine
(Fig. 4B).
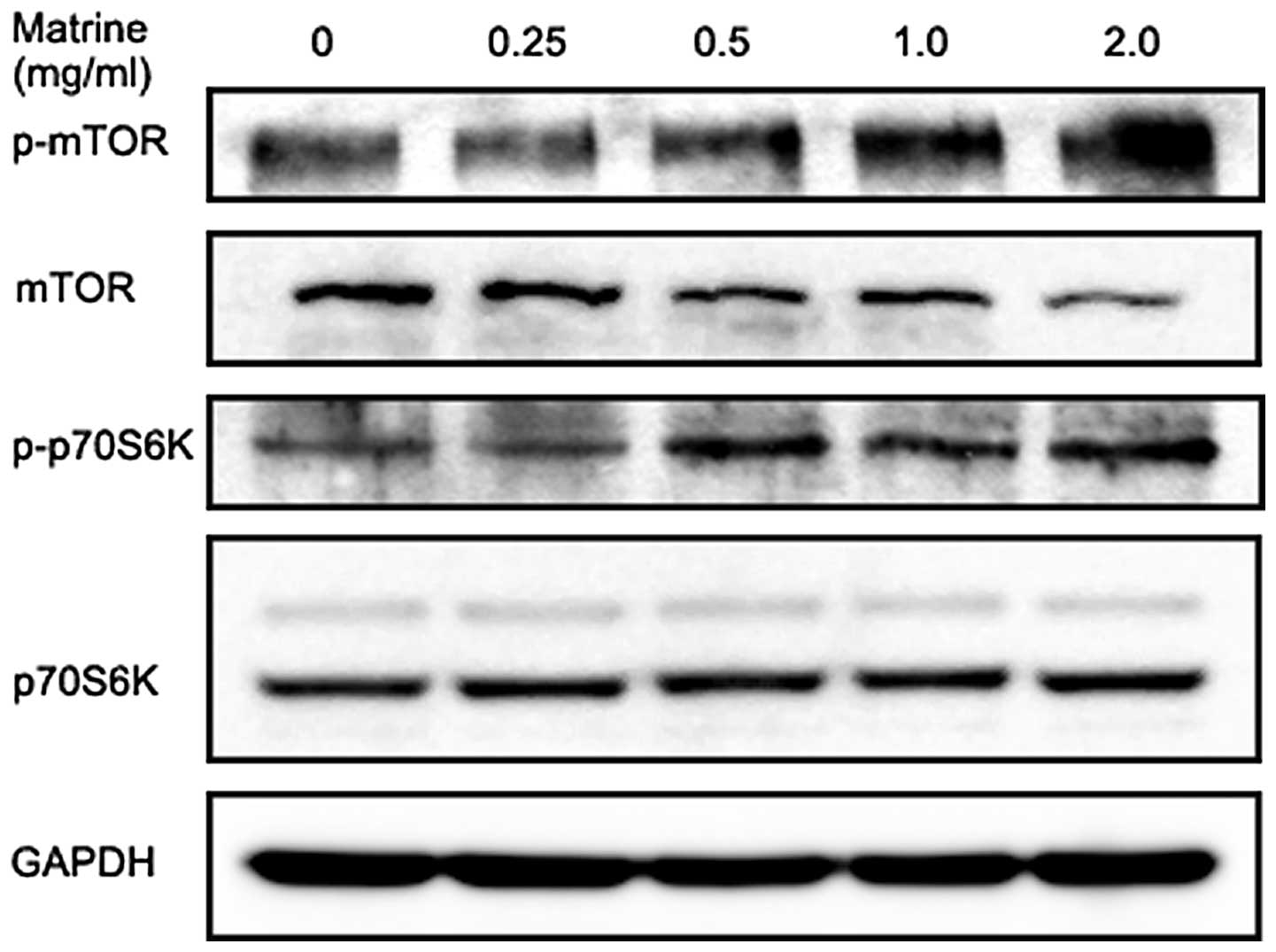
Matrine induces a slight increase in
phosphorylation of mTOR and p70S6K in gastric cancer cells
The serine/threonine kinase mTOR is a major
downstream substrate of Akt and is at the core of two distinct
multiprotein kinase complexes, mTOR complex 1 (mTORC1) and mTORC2.
mTORC1 activation by the PI3K/Akt pathway results in
phosphorylation and activation of p70S6K at Thr389 and mTORC2 has a
role in activating Akt through Ser473 phosphorylation (53). As shown in Fig. 6, matrine slightly increased
phosphorylation of mTOR at Ser2448 in gastric cancer cells at 6 h,
which was accompanied by a slight decrease in total mTOR protein in
a dose-dependent manner. We also observed matrine treatment for 6 h
modestly increased p70S6K at Thr389 phosphorylation and without any
effect on the total p70S6K level. These results were
counterproductive, as activated mTOR is generally considered to be
involved in the negative control of mammalian autophagy.
Discussion
Sophora flavescens Ait is listed in Chinese
Pharmacopoeia and has been widely used in China for medicinal
purposes. Our previous study showed that matrine purified from
Sophora flavescens Ait has potent antitumor activity against
gastric cancer cells, and is a strong inducer of autophagy as well
as of apoptosis (14). In the
present study, we further demonstrated that the degree of autophagy
in gastric cancer cells by detecting LC3 increased in a dose- and
time-dependent manner after matrine treatment. From these results,
we considered two possibilities: matrine-induced autophagy may be a
mechanism of the antitumor effect of matrine, resulting in
autophagic cell death, or a stress adaptation leading to survival
of tumor cells during matrine treatment. Here, we employed several
pharmacological inhibitors of the autophagic or apoptotic process
to determine the significance of autophagy in antineoplastic
properties of matrine. The data demonstrated that the presence of
autophagic inhibitors such as 3-MA or bafilomycin A1 enhances
lethality of matrine against gastric cancer cells. Notably, after
pretreatment with 3-MA, some of the cells exposed to matrine
displayed classic apoptotic morphology. Accordingly, 3-MA increased
matrine-induced apoptosis evidenced by flow cytometry using Annexin
V-FITC/PI staining, suggesting that matrine-induced autophagy
protects cells from apoptosis and could be a target for enhancing
its antitumor effects. Moreover, co-treatment with matrine plus
3-MA significantly enhanced the antiproliferative effect on
SGC-7901 cells in comparison with matrine alone. In addition,
administration of the pancaspase inhibitor zVAD-fmk decreased the
cell death rate in matrine-treated cells, indicating that apoptosis
is the major form of cell death induced by matrine, consistent with
other studies that matrine induces apoptosis in gallbladder
carcinoma (13), retinoblastoma
(54), multiple myeloma (55), osteosarcoma (56), pancreatic cancer (16), hepatocellular carcinoma (57), and gastric cancer cells (20,21).
Autophagy appears to function as a prosurvival mechanism perhaps
through the degradation and recycling of the damaged cellular
proteins and organelles caused by matrine treatment, which protects
cancer cells from matrine-mediated apoptosis and causes resistance
to matrine therapy. Therefore, inhibition of autophagy potentiates
the apoptosis-inducing and anticancer activity of matrine,
suggesting a strategy in the clinic to augment the antineoplastic
efficacy of matrine by combining matrine with an autophagic
inhibitor.
Increasing evidence indicates that various
anticancer therapies induce autophagy in different cancer cells.
Furthermore, autophagic cell death can be activated in cancer cell
lines in response to various agents used in cancer treatment, such
as Rhabdastrellic acid-A (58),
arsenic trioxide (59), silibinin
(60), zoledronic acid (61), berberine (37), α-mangostin (62), dasatinib (38), resveratrol (63), fisetin (64), 5-fluorouracil (65) and triptolide (39). However, despite these examples, we
did not find that matrine-treated cell death is executed by
autophagy; we found that cell death is accompanied by features of
autophagy which acts primordially as a cytoprotective mechanism
during matrine treatment, consistent with the notion that
autophagy, in most cases, constitutes an attempt of dying cells to
cope with lethal stress rather than a mechanism to execute cell
demise (66). A recent study
clearly showed that autophagic cell death may be unlikely to exist
as a phenomenon since a large collection of clinically used or
experimental anticancer agents (∼1400 compounds) did not contain a
single compound that would trigger cell death through induction of
autophagy (67). In addition,
there is a growing body of literature supporting the idea that
autophagy is activated in tumor cells as a prosurvival mechanism
against cytotoxic agents and may therefore favor chemoresistance.
For instance, compound C (40),
resveratrol (68), quercetin
(41) timosaponin A-III (69), and celecoxib (70) induce protective autophagy in
various cancer cells, and acquired cisplatin resistance in human
lung adenocarcinoma cells is associated with enhanced autophagy
(71). Therefore, the term
‘autophagic cell death’ is considered to be a misnomer in that it
describes a reality in which cells die with autophagy but not by
autophagy (66,72). Since the term ‘autophagic cell
death’ is highly prone to misinterpretation, the Nomenclature
Committee of Cell Death in 2012 suggested that the term should only
be used in the rare occasions where definite proof can be provided
that cell death is mediated by autophagy (73).
Although there is extensive knowledge on the
mechanisms of induction of apoptosis by matrine (13,16,19–21),
very little is known about the specific mechanisms of
matrine-induced autophagy. Diverse signaling pathways have been
reported in the regulation of autophagy in mammalian cells in
response to multiple forms of cellular stress including starvation,
hypoxia, radiation or chemical insults (74). Of these, the Akt/mTOR pathway is
considered a typical negative regulator for the initiation of
autophagy (41). Accumulating
anti-cancer agents have been documented to trigger the cellular
autophagic process by suppressing the pathway, such as berberine
(37), dasatinib (38), triptolide (39), compound C (40) and quercetin (41). Therefore, we sought to determine
the potential involvement of the Akt/mTOR pathway in the
matrine-induced autophagic process. However, the results showed
that matrine treatment did not inhibit the phosphorylation of Akt
(Ser473) and its downstream effectors mTOR (Ser2448) as well as
p70S6K (Thr389), although the levels of the total Akt and mTOR were
decreased. Accordingly, it appeared that the Akt/mTOR pathway was
not involved in the induction of autophagy in the matrine-treated
SGC-7901 cells. Therefore, another main pathway might be involved
in the autophagy induction process. For example, autophagy can also
be activated by the Raf/mitogen-activated protein kinase kinase
(MEK)/extra cellular signal-related kinase (ERK) pathway (30,75).
It has been reported that autophagy induced by triptolide or
curcumin is associated with the activation of the pathway (39,76).
Also, arsenic-induced autophagy appears to require activation of
the MEK/ERK pathway but not the Akt/mTOR pathways (77). Hence, further investigations are
required to gain insights into the potential involvement of other
signaling pathways and the precise underlying mechanism of
matrine-induced autophagy. In addition, matrine treatment increased
the phosphorylation of Akt at Ser473 in a dose-dependent manner,
which may contribute to the resistance of gastric cancer cells to
matrine, thus attenuating their potential antitumor activity. In
line with the unexpected result, treatment with arsenic trioxide or
sorafenib also enhanced the levels of the phosphorylation of Akt in
several types of cancer cells (77–79).
Despite inducing Akt phosphorylation, matrine significantly
suppressed the proliferation of gastric cancer cells in our
previous and present study (14).
It is possible that combinations of matrine with Akt inhibitor may
provide a potential approach to augment the antitumor properties of
matrine.
In conclusion, the present study suggests that
matrine-induced autophagy in gastric cancer cells is an adaptive
response that delays the eventual cell death, and blockade of
autophagy could be a promising strategy to improve the ability of
matrine to kill gastric cancer cells. In addition, the
Akt/mTOR/p70S6K signaling pathway might not be involved in the
induction of autophagy in the matrine-treated SGC-7901 cells.
Acknowledgements
This study was supported by the
National Natural Science Foundation of China (grant no. 30870364)
and the Science and Technology Support Program of Gansu province,
China (grant no. 0708NKCA129).
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar
|
|
2
|
Zhang B, Liu ZY, Li YY, et al:
Antiinflammatory effects of matrine in LPS-induced acute lung
injury in mice. Eur J Pharm Sci. 44:573–579. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Liu JY, Hu JH, Zhu QG, Li FQ, Wang J and
Sun HJ: Effect of matrine on the expression of substance P receptor
and inflammatory cytokines production in human skin keratinocytes
and fibroblasts. Int Immunopharmacol. 7:816–823. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Suo Z, Liu Y, Ferreri M, et al: Impact of
matrine on inflammation related factors in rat intestinal
microvascular endothelial cells. J Ethnopharmacol. 125:404–409.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Li CQ, Zhu YT, Zhang FX, et al: Anti-HBV
effect of liposome-encapsulated matrine in vitro and in vivo. World
J Gastroenterol. 11:426–428. 2005.PubMed/NCBI
|
|
6
|
Long Y, Lin XT, Zeng KL and Zhang L:
Efficacy of intramuscular matrine in the treatment of chronic
hepatitis B. Hepatobiliary Pancreat Dis Int. 3:69–72.
2004.PubMed/NCBI
|
|
7
|
Zhang JP, Zhang M, Zhou JP, et al:
Antifibrotic effects of matrine on in vitro and in vivo models of
liver fibrosis in rats. Acta Pharmacol Sin. 22:183–186.
2001.PubMed/NCBI
|
|
8
|
Zhang JP, Zhang M, Jin C, et al: Matrine
inhibits production and actions of fibrogenic cytokines released by
mouse peritoneal macrophages. Acta Pharmacol Sin. 22:765–768.
2001.PubMed/NCBI
|
|
9
|
Yin LL and Zhu XZ: The involvement of
central cholinergic system in (+)-matrine-induced antinociception
in mice. Pharmacol Biochem Behav. 80:419–425. 2005.
|
|
10
|
Li X, Chu W, Liu J, et al: Antiarrhythmic
properties of long-term treatment with matrine in arrhythmic rat
induced by coronary ligation. Biol Pharm Bull. 32:1521–1526. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhou Y, Xu W, Han R, et al: Matrine
inhibits pacing induced atrial fibrillation by modulating I(KM3)
and I(Ca-L). Int J Biol Sci. 8:150–158. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhang Y, Wang S, Li Y, Xiao Z, Hu Z and
Zhang J: Sophocarpine and matrine inhibit the production of
TNF-alpha and IL-6 in murine macrophages and prevent
cachexia-related symptoms induced by colon26 adenocarcinoma in
mice. Int Immunopharmacol. 8:1767–1772. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhang Z, Wang X, Wu W, et al: Effects of
matrine on proliferation and apoptosis in gallbladder carcinoma
cells (GBC-SD). Phytother Res. 26:932–937. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhang J, Li Y, Chen X, et al: Autophagy is
involved in anticancer effects of matrine on SGC-7901 human gastric
cancer cells. Oncol Rep. 26:115–124. 2011.PubMed/NCBI
|
|
15
|
Zhang JQ, Li YM, Liu T, et al: Antitumor
effect of matrine in human hepatoma G2 cells by inducing apoptosis
and autophagy. World J Gastroenterol. 16:4281–4290. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Liu T, Song Y, Chen H, Pan S and Sun X:
Matrine inhibits proliferation and induces apoptosis of pancreatic
cancer cells in vitro and in vivo. Biol Pharm Bull. 33:1740–1745.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Qin XG, Hua Z, Shuang W, Wang YH and Cui
YD: Effects of matrine on HepG2 cell proliferation and expression
of tumor relevant proteins in vitro. Pharm Biol. 48:275–281. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhang LP, Jiang JK, Tam JW, et al: Effects
of Matrine on proliferation and differentiation in K-562 cells.
Leuk Res. 25:793–800. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Jiang H, Hou C, Zhang S, et al: Matrine
upregulates the cell cycle protein E2F-1 and triggers apoptosis via
the mitochondrial pathway in K562 cells. Eur J Pharmacol.
559:98–108. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Luo C, Zhu Y, Jiang T, et al: Matrine
induced gastric cancer MKN45 cells apoptosis via increasing
pro-apoptotic molecules of Bcl-2 family. Toxicology. 229:245–252.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Dai ZJ, Gao J, Ji ZZ, et al: Matrine
induces apoptosis in gastric carcinoma cells via alteration of
Fas/FasL and activation of caspase-3. J Ethnopharmacol. 123:91–96.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Li Y, Zhang J, Chen X, et al: Molecular
machinery of autophagy and its implication in cancer. Am J Med Sci.
343:155–161. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Apel A, Zentgraf H, Buchler MW and Herr I:
Autophagy-A double-edged sword in oncology. Int J Cancer.
125:991–995. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hippert MM, O’Toole PS and Thorburn A:
Autophagy in cancer: good, bad, or both? Cancer Res. 66:9349–9351.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Mathew R, Kongara S, Beaudoin B, et al:
Autophagy suppresses tumor progression by limiting chromosomal
instability. Genes Dev. 21:1367–1381. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Degenhardt K, Mathew R, Beaudoin B, et al:
Autophagy promotes tumor cell survival and restricts necrosis,
inflammation, and tumorigenesis. Cancer Cell. 10:51–64. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Mathew R, Karantza-Wadsworth V and White
E: Role of autophagy in cancer. Nat Rev Cancer. 7:961–967. 2007.
View Article : Google Scholar
|
|
28
|
Kondo Y and Kondo S: Autophagy and cancer
therapy. Autophagy. 2:85–90. 2006. View Article : Google Scholar
|
|
29
|
Eisenberg-Lerner A and Kimchi A: The
paradox of autophagy and its implication in cancer etiology and
therapy. Apoptosis. 14:376–391. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yang Z and Klionsky DJ: Mammalian
autophagy: core molecular machinery and signaling regulation. Curr
Opin Cell Biol. 22:124–131. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Levine B and Kroemer G: Autophagy in the
pathogenesis of disease. Cell. 132:27–42. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Gozuacik D and Kimchi A: Autophagy and
cell death. Curr Top Dev Biol. 78:217–245. 2007. View Article : Google Scholar
|
|
33
|
Sun Y and Peng ZL: Programmed cell death
and cancer. Postgrad Med J. 85:134–140. 2009. View Article : Google Scholar
|
|
34
|
Marx J: Autophagy: is it cancer’s friend
or foe? Science. 312:1160–1161. 2006.
|
|
35
|
Yang Z and Klionsky DJ: An overview of the
molecular mechanism of autophagy. Curr Top Microbiol Immunol.
335:1–32. 2009.PubMed/NCBI
|
|
36
|
Glick D, Barth S and Macleod KF:
Autophagy: cellular and molecular mechanisms. J Pathol. 221:3–12.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Wang N, Feng Y, Zhu M, et al: Berberine
induces autophagic cell death and mitochondrial apoptosis in liver
cancer cells: the cellular mechanism. J Cell Biochem.
111:1426–1436. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Le XF, Mao W, Lu Z, Carter BZ and Bast RC
Jr: Dasatinib induces autophagic cell death in human ovarian
cancer. Cancer. 116:4980–4990. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Mujumdar N, Mackenzie TN, Dudeja V, et al:
Triptolide induces cell death in pancreatic cancer cells by
apoptotic and autophagic pathways. Gastroenterology. 139:598–608.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Vucicevic L, Misirkic M, Janjetovic K, et
al: Compound C induces protective autophagy in cancer cells through
AMPK inhibition-independent blockade of Akt/mTOR pathway.
Autophagy. 7:40–50. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Wang K, Liu R, Li J, et al: Quercetin
induces protective autophagy in gastric cancer cells: involvement
of Akt-mTOR- and hypoxia-induced factor 1alpha-mediated signaling.
Autophagy. 7:966–978. 2011. View Article : Google Scholar
|
|
42
|
Skehan P, Storeng R, Scudiero D, et al:
New colorimetric cytotoxicity assay for anticancer-drug screening.
J Natl Cancer Inst. 82:1107–1112. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Klionsky DJ, Abeliovich H, Agostinis P, et
al: Guidelines for the use and interpretation of assays for
monitoring autophagy in higher eukaryotes. Autophagy. 4:151–175.
2008. View Article : Google Scholar
|
|
44
|
Klionsky DJ, Baehrecke EH, Brumell JH, et
al: A comprehensive glossary of autophagy-related molecules and
processes (2nd edition). Autophagy. 7:1273–1294. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Wu YT, Tan HL, Shui G, et al: Dual role of
3-methyladenine in modulation of autophagy via different temporal
patterns of inhibition on class I and III phosphoinositide
3-kinase. J Biol Chem. 285:10850–10861. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Periyasamy-Thandavan S, Jiang M, Wei Q,
Smith R, Yin XM and Dong Z: Autophagy is cytoprotective during
cisplatin injury of renal proximal tubular cells. Kidney Int.
74:631–640. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Chen SY, Chiu LY, Maa MC, Wang JS, Chien
CL and Lin WW: zVAD-induced autophagic cell death requires
c-Src-dependent ERK and JNK activation and reactive oxygen species
generation. Autophagy. 7:217–228. 2011. View Article : Google Scholar
|
|
48
|
Hotchkiss RS, Strasser A, McDunn JE and
Swanson PE: Cell death. N Engl J Med. 361:1570–1583. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Scarlatti F, Granata R, Meijer AJ and
Codogno P: Does autophagy have a license to kill mammalian cells?
Cell Death Differ. 16:12–20. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Levine B and Yuan J: Autophagy in cell
death: an innocent convict? J Clin Invest. 115:2679–2688. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Coughlin CM, Johnston DS, Strahs A, et al:
Approaches and limitations of phosphatidylinositol-3-kinase pathway
activation status as a predictive biomarker in the clinical
development of targeted therapy. Breast Cancer Res Treat. 124:1–11.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
O’Reilly KE, Rojo F, She QB, et al: mTOR
inhibition induces upstream receptor tyrosine kinase signaling and
activates Akt. Cancer Res. 66:1500–1508. 2006.PubMed/NCBI
|
|
53
|
Guertin DA and Sabatini DM: Defining the
role of mTOR in cancer. Cancer Cell. 12:9–22. 2007. View Article : Google Scholar
|
|
54
|
Zhao B, Li B, Bai S, et al: Effects of
matrine on proliferation and apoptosis of cultured retinoblastoma
cells. Graefes Arch Clin Exp Ophthalmol. 250:897–905. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Han Y, Zhang S, Wu J, et al: Matrine
induces apoptosis of human multiple myeloma cells via activation of
the mitochondrial pathway. Leuk Lymphoma. 51:1337–1346. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Liang CZ, Zhang JK, Shi Z, Liu B, Shen CQ
and Tao HM: Matrine induces caspase-dependent apoptosis in human
osteosarcoma cells in vitro and in vivo through the upregulation of
Bax and Fas/FasL and downregulation of Bcl-2. Cancer Chemother
Pharmacol. 69:317–331. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Ma L, Wen S, Zhan Y, He Y, Liu X and Jiang
J: Anticancer effects of the Chinese medicine matrine on murine
hepatocellular carcinoma cells. Planta Med. 74:245–251. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Li DD, Guo JF, Huang JJ, et al:
Rhabdastrellic acid-A induced autophagy-associated cell death
through blocking Akt pathway in human cancer cells. PLoS One.
5:e121762010. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Kanzawa T, Zhang L, Xiao L, Germano IM,
Kondo Y and Kondo S: Arsenic trioxide induces autophagic cell death
in malignant glioma cells by upregulation of mitochondrial cell
death protein BNIP3. Oncogene. 24:980–991. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Duan WJ, Li QS, Xia MY, Tashiro S, Onodera
S and Ikejima T: Silibinin activated p53 and induced autophagic
death in human fibrosarcoma HT1080 cells via reactive oxygen
species-p38 and c-Jun N-terminal kinase pathways. Biol Pharm Bull.
34:47–53. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Lin JF, Lin YC, Lin YH, et al: Zoledronic
acid induces autophagic cell death in human prostate cancer cells.
J Urol. 185:1490–1496. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Chao AC, Hsu YL, Liu CK and Kuo PL:
α-Mangostin, a dietary xanthone, induces autophagic cell death by
activating the AMP-activated protein kinase pathway in glioblastoma
cells. J Agric Food Chem. 59:2086–2096. 2011.
|
|
63
|
Puissant A, Robert G, Fenouille N, et al:
Resveratrol promotes autophagic cell death in chronic myelogenous
leukemia cells via JNK-mediated p62/SQSTM1 expression and AMPK
activation. Cancer Res. 70:1042–1052. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Suh Y, Afaq F, Khan N, Johnson JJ, Khusro
FH and Mukhtar H: Fisetin induces autophagic cell death through
suppression of mTOR signaling pathway in prostate cancer cells.
Carcinogenesis. 31:1424–1433. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Xiong HY, Guo XL, Bu XX, et al: Autophagic
cell death induced by 5-FU in Bax or PUMA deficient human colon
cancer cell. Cancer Lett. 288:68–74. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Shen S, Kepp O and Kroemer G: The end of
autophagic cell death? Autophagy. 8:1–3. 2012. View Article : Google Scholar
|
|
67
|
Shen S, Kepp O, Michaud M, et al:
Association and dissociation of autophagy, apoptosis and necrosis
by systematic chemical study. Oncogene. 30:4544–4556. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Li J, Qin Z and Liang Z: The prosurvival
role of autophagy in resveratrol-induced cytotoxicity in human U251
glioma cells. BMC Cancer. 9:2152009. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Sy LK, Yan SC, Lok CN, Man RY and Che CM:
Timosaponin A-III induces autophagy preceding mitochondria-mediated
apoptosis in HeLa cancer cells. Cancer Res. 68:10229–10237. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Huang S and Sinicrope FA:
Celecoxib-induced apoptosis is enhanced by ABT-737 and by
inhibition of autophagy in human colorectal cancer cells.
Autophagy. 6:256–269. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Ren JH, He WS, Nong L, et al: Acquired
cisplatin resistance in human lung adenocarcinoma cells is
associated with enhanced autophagy. Cancer Biother Radiopharm.
25:75–80. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Kroemer G and Levine B: Autophagic cell
death: the story of a misnomer. Nat Rev Mol Cell Biol. 9:1004–1010.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Galluzzi L, Vitale I, Abrams JM, et al:
Molecular definitions of cell death subroutines: recommendations of
the Nomenclature Committee on Cell Death 2012. Cell Death Differ.
19:107–120. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Kroemer G, Marino G and Levine B:
Autophagy and the integrated stress response. Mol Cell. 40:280–293.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Pattingre S, Bauvy C and Codogno P: Amino
acids interfere with the ERK1/2-dependent control of macroautophagy
by controlling the activation of Raf-1 in human colon cancer HT-29
cells. J Biol Chem. 278:16667–16674. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Aoki H, Takada Y, Kondo S, Sawaya R,
Aggarwal BB and Kondo Y: Evidence that curcumin suppresses the
growth of malignant gliomas in vitro and in vivo through induction
of autophagy: role of Akt and extracellular signal-regulated kinase
signaling pathways. Mol Pharmacol. 72:29–39. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Goussetis DJ, Altman JK, Glaser H, McNeer
JL, Tallman MS and Platanias LC: Autophagy is a critical mechanism
for the induction of the antileukemic effects of arsenic trioxide.
J Biol Chem. 285:29989–29997. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Qian W, Liu J, Jin J, Ni W and Xu W:
Arsenic trioxide induces not only apoptosis but also autophagic
cell death in leukemia cell lines via up-regulation of Beclin-1.
Leuk Res. 31:329–339. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Shimizu S, Takehara T, Hikita H, et al:
Inhibition of autophagy potentiates the antitumor effect of the
multikinase inhibitor sorafenib in hepatocellular carcinoma. Int J
Cancer. 131:548–557. 2012. View Article : Google Scholar : PubMed/NCBI
|