Introduction
Synovial sarcoma (SS) is a high-grade malignant
tumor that accounts for almost 10% of all adult soft tissue
sarcomas (1). It can occur at any
age and anatomical site, but is most commonly found in the
para-articular regions in adolescents and young adults (2). SS is characterized by the presence of
a chromosomal translocation t(X;18) (p11.2;q11.2) between
chromosomes X and 18 that leads to the formation of a fusion
protein (3).
Currently, SS is classified as a miscellaneous tumor
of uncertain histological origin (4), which is thought to arise from
undifferentiated mesenchymal cells. A recent report indicates that
neural crest cells, which can differentiate into both ectoderm and
mesoderm, are potential progenitors of SS (5). However, the cellular origin of SS has
not been definitively resolved.
Clinically, SS appears as deep-seated slowly growing
mass, with more than half of patients developing metastases, mainly
to the lungs and sometimes to the lymph nodes and bone marrow
(6). The 5- and 10-year survival
rates are as low as 36 and 20%, respectively (7). Thus, SS remains a cancer associated
with high morbidity and therefore, improved therapies are
necessary.
The cancer stem-like cell (CSC) hypothesis holds
that tumor cells are hierarchically organized according to their
potential to initiate and sustain tumor growth. Only a small
population of tumor cells, defined as CSCs or tumor-initiating
cells, can form tumors in serial xenotransplantation assays, are
resistant to chemotherapy and have the ability to re-establish the
hierarchical cell organization and heterogeneity of the parental
tumor at each passage in vivo(8,9).
These cells were first described in acute myeloid leukemia
(10) and subsequently in breast
cancer (11), prostate cancer
(12), glioblastoma (13), melanoma (14) and other types of cancer (15–18).
Thus, the identification of the CSCs has significantly improved our
understanding of tumor biology and may lead to more effective tumor
therapies.
Recent technical advances have enabled the
identification of CSCs by specific surface markers that are
selectively expressed on these cells, but not on the majority of
tumor cells. Of these, CD133 is a pentaspan membrane glycoprotein
that was first described as a surface antigen specific to human
hematopoietic stem and progenitor cells (19), but has also recently been
recognized as a stem cell marker in brain (20), prostate (21), pancreatic (22), lung (23) and ovarian cancers (24). CD133 expression has also been
identified in various sarcomas, including osteosarcoma (25) and Ewing’s sarcoma (16). CD133+ Ewing’s sarcoma
cells express significantly higher levels of the stemness genes
Nanog and Oct3/4 than their CD133−
counterparts. Recently, the presence of CD133+ cell
populations was reported in SS (26). However, until now it was unknown
whether CD133+ cells with CSC properties could be
isolated from the human SS cell line SW982.
Therefore, in the present study we used CD133 as a
marker to identify CSCs within the SW982 cell population. We then
examined the self-renewal, differentiation capacity and
tumorigenicity of the SW982 CD133+ cell population using
a spheroid formation assay and xenograft model in nude BALB/c mice.
We also compared chemoresistance and examined expression of the
specific drug transporter, ABCG2 and stemness genes in
different subpopulations of SW982 cells. Our results allowed us to
define the phenotype of SW982 CSCs, which may contribute to the
development of more effective therapies for SS.
Materials and methods
Cell culture
The human SS cell line SW982 was obtained from the
American Type Culture Collection (USA) and maintained in
Leibovitz-15 medium (Gibco BRL, Grand Island, NY, USA) supplemented
with 10% fetal bovine serum (FBS, Hyclone, UT, USA) at 37°C in a
humidified atmosphere containing 5% CO2. The culture
medium was changed every 3–4 days and cells were trypsinized and
replated when 85% confluency was reached.
Magnetic- and fluorescence-activated cell
sorting
CD133+ and CD133− populations
were isolated from SW982 cell cultures by magnetic bead sorting
using a magnetic-activated cell sorting (MACS) CD133 cell isolation
kit. Briefly, SW982 cells were resuspended in MACS buffer
[phosphate-buffered saline (PBS) without Ca2+ and
Mg2+, supplemented with 0.5% BSA and 2 mM EDTA] at a
concentration of 3.3×108 cells/ml. Single-cell
suspensions (300 μl, containing 1×108 cells) were
incubated with 100 μl FcR blocking reagent and 100 μl
CD133 microbeads for 30 min at 4°C. After washing, cell populations
were separated by passing through LS columns, which retain
CD133+ cells, according to the manufacturer’s
instructions. Before and after separation, cell samples were
analyzed by fluorescence-activated cell sorting (FACS) using
anti-CD133/2 phycoerythrin (293C3) and isotype control mouse IgG2b
phycoerythrin antibodies using a FACSCalibur flow cytometer (BD
Biosciences, San Jose, CA, USA). All reagents were purchased from
Miltenyi Biotec.
Spheroid formation assay
Spheroid formation assays were performed as
described by Fujii et al(27) and Gibbs et al(28) with some modifications, using
single-cell SW982 suspensions (dissociated using 0.25%
trypsin/0.05% EDTA at ∼70% confluency) and both CD133+
and CD133− cell populations isolated by MACS. Briefly,
cells were resuspended in B27-supplemented Leibovitz-15/1%
methylcellulose medium without serum and containing human
recombinant epidermal growth factor (EGF; 10 ng/ml) and basic
fibroblast growth factor (bFGF; 10 ng/ml; PeproTech, Rocky Hill,
NY, USA) and plated at 6×104 cells/well into ultra-low
attachment 6-well plates (Corning Inc., Corning, NY, USA). B27 was
purchased from Invitrogen. Fresh aliquots of EGF and bFGF were
added every second day. After 10–14 days, colonies containing
>50 cells were counted using inverted phase contrast microscopy
(Olympus CKX41, Shinjuku, Tokyo, Japan). Spheroid formation was
investigated through at least five cycles of dissociation and
re-culturing in anchorage-independent, serum-starved condition on
96-well ultra-low attachment plates.
Chemosensitivity assays
To assess the effects of cisplatin (CDDP) and
doxorubicin (DXR) (Sigma), which are frequently used for sarcoma
chemotherapy, on the CD133+ and CD133− cell
populations, the cells were dissociated, resuspended in
Leibovitz-15/10% FBS at a concentration of 2×104
cells/ml, inoculated into 96-well microtiter plates (Corning Inc.)
at 100 μl/well and incubated at 37°C. After 24 h, cells were
exposed to 0–10 μM CDDP or DXR for 48 h. The viability of
treated and control cells was measured by the MTS assay using the
CellTiter 96 AQueous One Solution Cell Proliferation Assay kit
(Promega, Madison, WI, USA), according to the manufacturer’s
instructions.
For a more detailed characterization of the stemness
properties of the CD133+ cell population, we compared
drug resistance in CD133+ spheroids and adherent cells.
CD133+ adherent cells were grown in medium containing
serum, as previously described. CD133+ spheroids
(5×104 cells/ml) were resuspended in B27-supplemented
Leibovitz-15/1% methyl-cellulose medium, plated into 96-well
ultra-low attachment microplates at 100 μl/well and
incubated at 37°C for 14 days to induce spheroid formation.
CD133+ spheroids and adherent cells were then treated
with 0–10 μM CDDP or DXR and cell viability was measured by
the MTS assay after 48 h. Triplicate wells were used for each
treatment group.
Western blotting
Western blotting was performed as described
previously, with some modifications (28). Briefly, cells were washed twice
with cold PBS and lysed in buffer containing 50 mM Tris-HCl (pH
7.4), 150 mM NaCl, 1 mM EDTA, 1% NP-40, 0.1% SDS, 1% Na
deoxycholate, 1 mM Na vanadate and protease inhibitors (5 mg/ml
pepstatin, 1 mM PMSF, 10 mg/ml leupeptin and 1 mM NaF; Sigma) for 1
h on ice. Lysates were clarified by centrifugation at 13,000 g for
10 min at 4°C and protein concentrations were measured using the
BCA Protein Assay kit (Pierce, Rockford, IL, USA). Samples (30
μg total protein) were separated using 10% SDS
polyacrylamide gels and transferred onto polyvinylidene difluoride
membranes (Sigma). After blocking with 5% skimmed milk for 1 h at
room temperature, membranes were incubated overnight at 4°C with
primary antibody diluted in Tris-buffered saline (TBS) containing
0.1% Tween-20 (Bio-Rad Laboratories, Hercules, CA, USA) and 5%
bovine albumin (Sigma). After washing three times in TBS with 0.1%
Tween-20, blots were incubated with the appropriate horseradish
peroxidaseconjugated secondary antibody (Cell Signaling Technology,
Beverly, MA, USA; Jackson ImmunoResearch Laboratories, West Grove,
PA, USA). Immunoreactive bands were visualized using ECL Plus
SuperSignal West Pico (Thermo Scientific, Rockford, IL, USA).
Protein levels were normalized to GAPDH. Primary antibodies used
are as follows: anti-ABCG2 (1:1000), anti-Bmi1 (1:1000), anti-c-Myc
(1:1000), anti-Nanog (1:1000), anti-Oct3/4 (1:1000) and anti-Sox2
(1:1000) were purchased from Cell Signaling Technology. GAPDH
(1:5000) was purchased from Santa Cruz Biotechnology.
Real-time PCR
Total RNA was extracted from the CD133+
and CD133− cell populations using TRIzol reagent
(Invitrogen, Carlsbad, CA, USA) and reverse transcribed into cDNA
using M-MLV reverse transcriptase (Sigma). Real-time PCR was
performed according to the manufacturer’s instructions using an ABI
PRISM 7900HT sequence detection system (Applied Biosystems); mRNA
expression was normalized to GAPDH. The primer sequences are listed
in Table I.
 | Table IThe primer sequences of the tested
genes. |
Table I
The primer sequences of the tested
genes.
| Gene | Forward (5′-3) | Reverse
(5′-3′) |
|---|
| ABCG2 |
GATAAAGTGGCAGACTCCAAGGT |
CCAATAAGGTGAGGCTATCAAACA |
| Bmi1 |
CTCCACCTCTTCTTGTTTGC |
GATGACCCATTTACTGATGATTT |
| c-Myc |
GCATACATCCTGTCCGTCCA |
CAAGAGTTCCGTAGCTGTTCAAG |
| Nanog |
AGGCAAACAACCCACTTCT |
TCACACCATTGCTATTCTTCG |
| Oct 3/4 |
TATTCAGCCAAACGACCATCT |
TCAGCTTCCTCCACCCACTT |
| Sox2 |
ATCACCCACAGCAAATGACA |
CAAAGCTCCTACCGTACCACTA |
| GAPDH |
GGGAAACTGTGGCGTGAT |
GAGTGGGTGTCGCTGTTGA |
Xenograft experiments
To evaluate the tumorigenic potential of the
CD133+ and CD133− cell populations,
1×103–1×106 cells were subcutaneously
injected into the right side of the back of 6–8-week-old female
BALB/c nude mice, obtained from the Animal Research Center, Harbin
Medical University, China. All surgical procedures and animal
treatments were performed in accordance with institute guidelines.
Mice were monitored daily for tumor growth. Twelve weeks after
injection, all mice were euthanized, tumors were aseptically
excised and samples were processed for histopathological and
immunohistochemical analysis. In addition, tumor samples were
digested using collagenase II (Sigma) and the CD133+
cells were isolated and immediately re-injected into mice to
generate second-round tumors. Data were obtained from at least
three independent experiments.
Histopathological and immunohistochemical
analysis of xenografts
Excised xenograft tumors derived from
CD133+ and CD133− cells were fixed in 10%
formalin and embedded in paraffin. Sections were cut and stained
with hematoxylin and eosin (HE) using a standard protocol to assess
tumor type. Immunohistochemistry was performed using an anti-CD133
antibody (Abcam, Cambridge, UK), according to standard
immunohistochemical procedures. All microscopy images were
captured.
Statistical analysis
Statistical software SPSS19.0 was used in data
processing and analyzing. Data are expressed as mean ± SD,
comparison between experimental groups of real-time PCR analysis
were tested by one-way ANOVA. The results of the chemosensitivity
assay was calculated with the survival rates and compared by using
the χ2 test. A p<0.05 was regarded as statistically
significant.
Results
Isolation of SW982 CD133+ and
CD133− cell populations
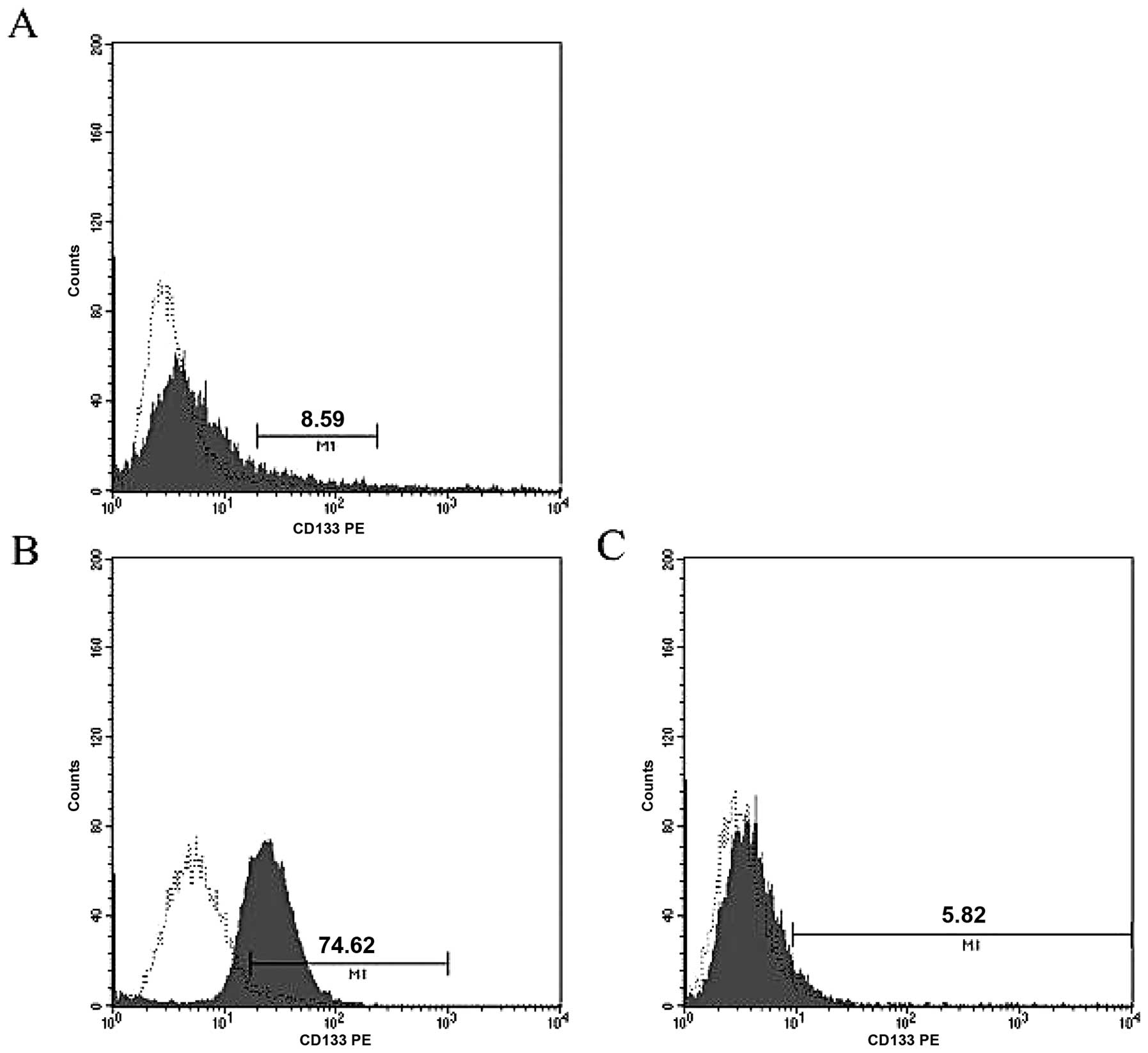
We assessed CD133 expression in human SS SW982 cells
using FACS analysis and found that CD133+ cells
comprised 8.59% of the total population (Fig. 1A). We then isolated enriched
CD133+ and CD133− cell populations using
MACS. Using this technique, the CD133+ cell population
could be enriched by ∼13-fold over CD133− cells (74.62
vs. 5.82%; Fig. 1B and C).
SW982 CD133+ cells form
spheroids in suspension culture
Suspension culture in serum-free medium was
initially developed as a method to select neural stem cells through
neurosphere formation, but has been widely adapted as a general
tumor-initiating cell selection method. Both normal cells and CSCs
from epithelial organs can be grown as spherical clones in
serum-free EGF/bFGF-supplemented medium that favors the
proliferation of undifferentiated cells (18).
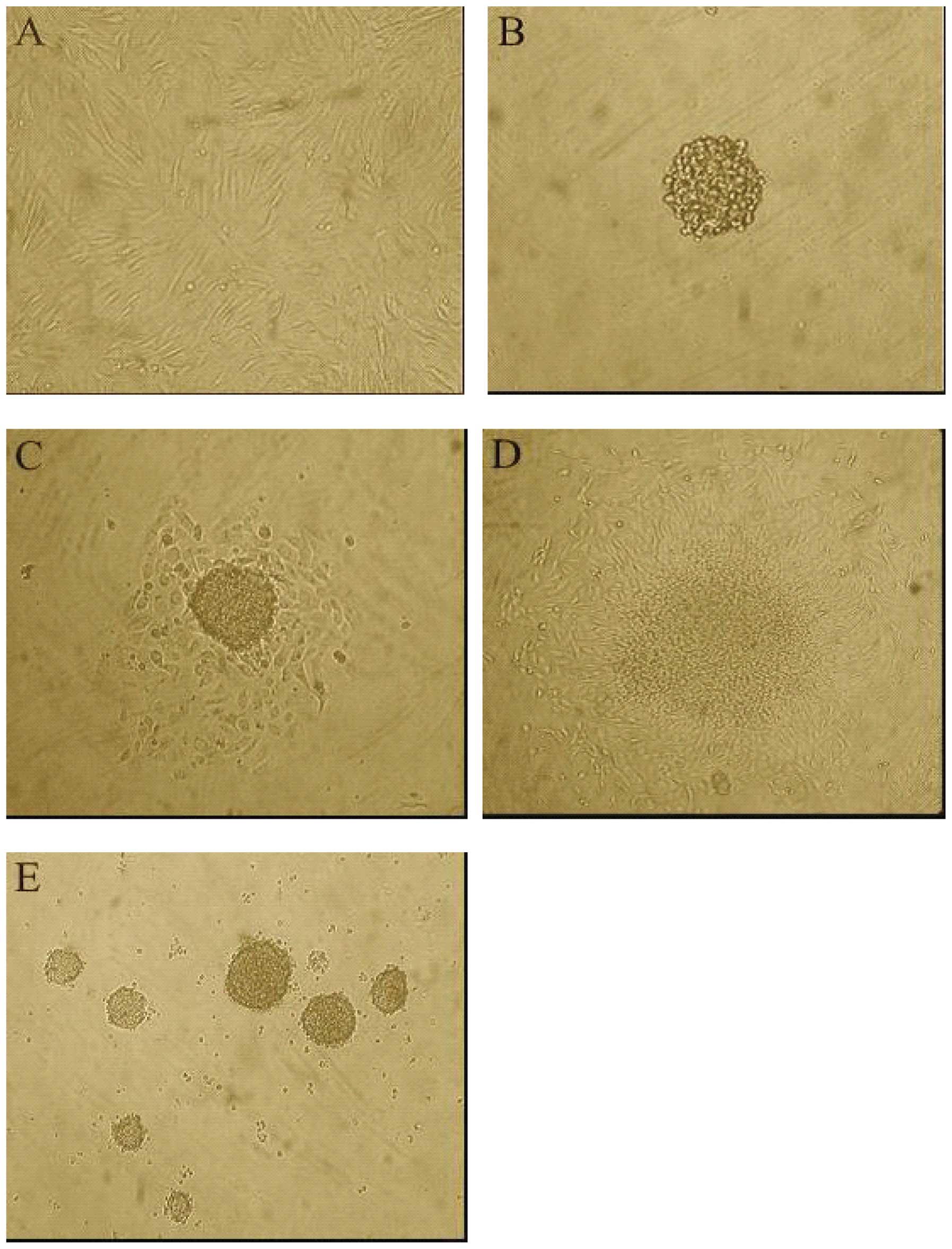
We therefore evaluated SW982 cell self-renewal and
ability to generate spherical clones under serum-starved culture
conditions. Our results indicated that the CD133+
subpopulation proliferated as floating spheres (Fig. 2B), while the CD133−
subpopulation did not form spheres. When CD133+
spheroids were seeded into serum containing Leibovitz-15 medium,
they developed the spindle-like features of the original cell
culture (Fig. 2A, C and D).
Primary CD133+ spheroid cells that were enzymatically
disaggregated after 10–14 days of culture and replated as
single-cell suspension went on to generate second passage spheres
(Fig. 2E). The ability to form
spheres was maintained over five serial passages using this
procedure.
CD133+ SW982 subpopulations
are resistant to chemotherapeutic agents
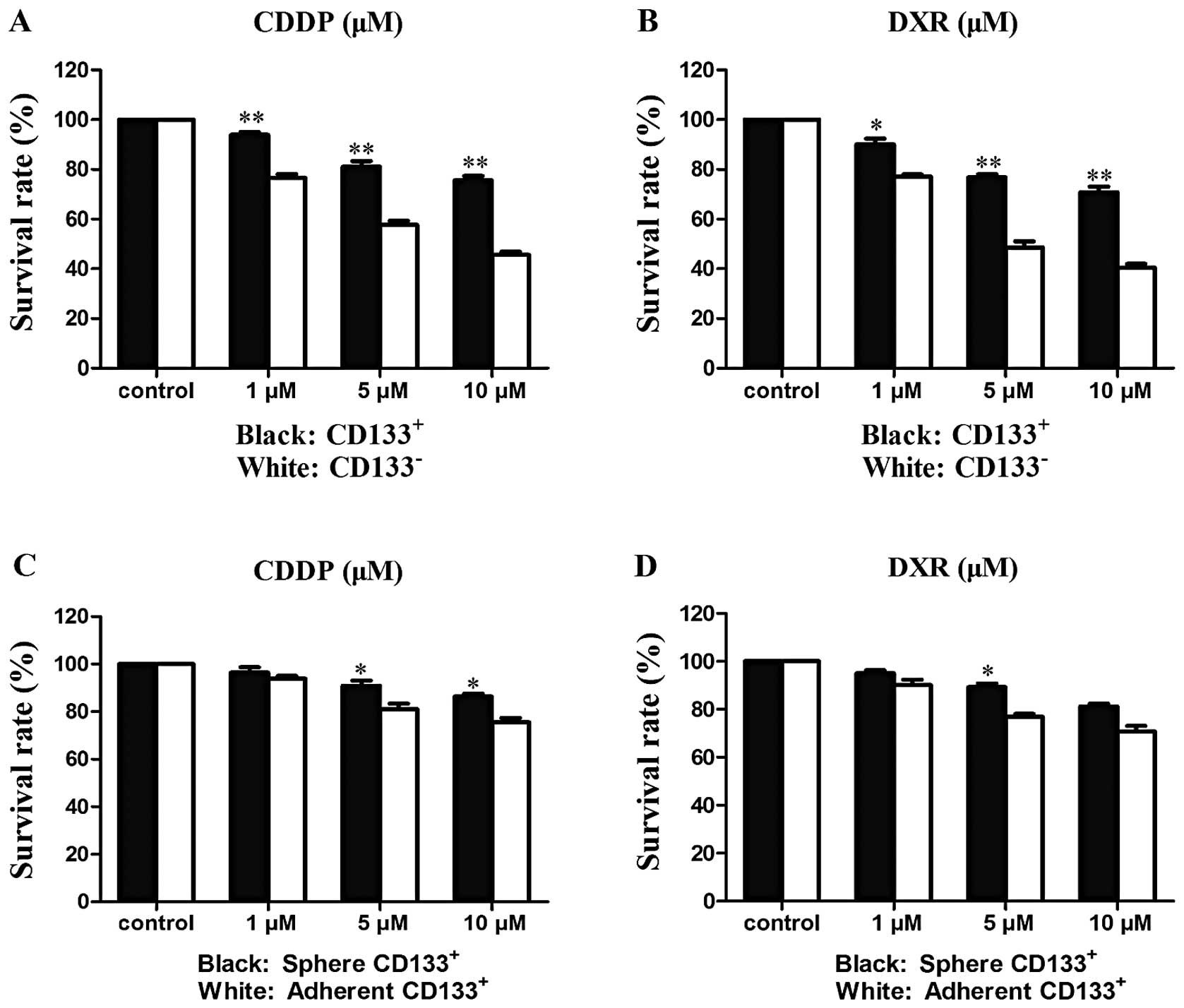
We found that CDDP and DXR inhibited the
proliferation of both CD133+ and CD133− SW982
cell populations in a dose-dependent manner. The survival rates of
CD133+ and CD133− cells after 48-h drug
treatment were measured (Tables II
and III; Fig. 3) and CD133+ cells were
found to be significantly more resistant to both CDDP and DXR than
CD133− cells (Fig. 3A and
B): 10 μM CDDP inhibited CD133− and
CD133+ proliferation by ≤54.4 and 24.5%, respectively;
10 μM DXR inhibited CD133− and CD133+
proliferation by ≤59.5 and 30.2%, respectively. These results
indicate that CD133+ cells are resistant to both CDDP
and DXR, the chemotherapeutic drugs most commonly used for
sarcomas. We also compared the drug response in CD133+
spheroids and adherent cells. Maximal growth inhibition of
CD133+ spheroids was 13.7% for CDDP and 19% for DXR
(Fig. 3C and D), demonstrating
spheroid cells were more resistant than adherent cells to both
drugs.
 | Table IICell survival rates (%) after 48 h
CDDP and DXR treatments of CD133+ and CD133−
cells. |
Table II
Cell survival rates (%) after 48 h
CDDP and DXR treatments of CD133+ and CD133−
cells.
| CDDP | DXR |
|---|
|
|
|---|
| 1 μM | 5 μM | 10 μM | 1 μM | 5 μM | 10 μM |
|---|
|
CD133+ | 93.80±1.23 | 81.10±2.26 | 75.54±1.83 | 90.10±2.21 | 76.87±1.23 | 70.87±2.21 |
|
CD133− | 76.50±1.59 | 57.63±1.64 | 45.63±1.30 | 77.13±0.85 | 48.53±2.52 | 40.50±1.44 |
| χ2 | 11.84 | 12.87 | 18.71 | 6.16 | 17.11 | 18.60 |
| p-value | 0.001 | 0.000 | 0.000 | 0.013 | 0.000 | 0.000 |
 | Table IIICell survival rates (%) after 48 h
CDDP and DXR treatments of sphere and adherent CD133+
cells. |
Table III
Cell survival rates (%) after 48 h
CDDP and DXR treatments of sphere and adherent CD133+
cells.
| CDDP | DXR |
|---|
|
|
|---|
| 1 μM | 5 μM | 10 μM | 1 μM | 5 μM | 10 μM |
|---|
| Sphere
CD133+ | 96.42±2.20 | 90.91±2.17 | 86.30±1.20 | 95.00±1.35 | 89.33±1.41 | 81.10±1.20 |
| Adherent
CD133+ | 93.80±1.23 | 81.10±2.26 | 75.54±1.83 | 90.10±2.21 | 76.87±1.23 | 70.87±2.21 |
| χ2 | 0.73 | 4.06 | 3.774 | 1.74 | 5.55 | 2.84 |
| p-value | 0.394 | 0.044 | 0.049 | 0.187 | 0.018 | 0.092 |
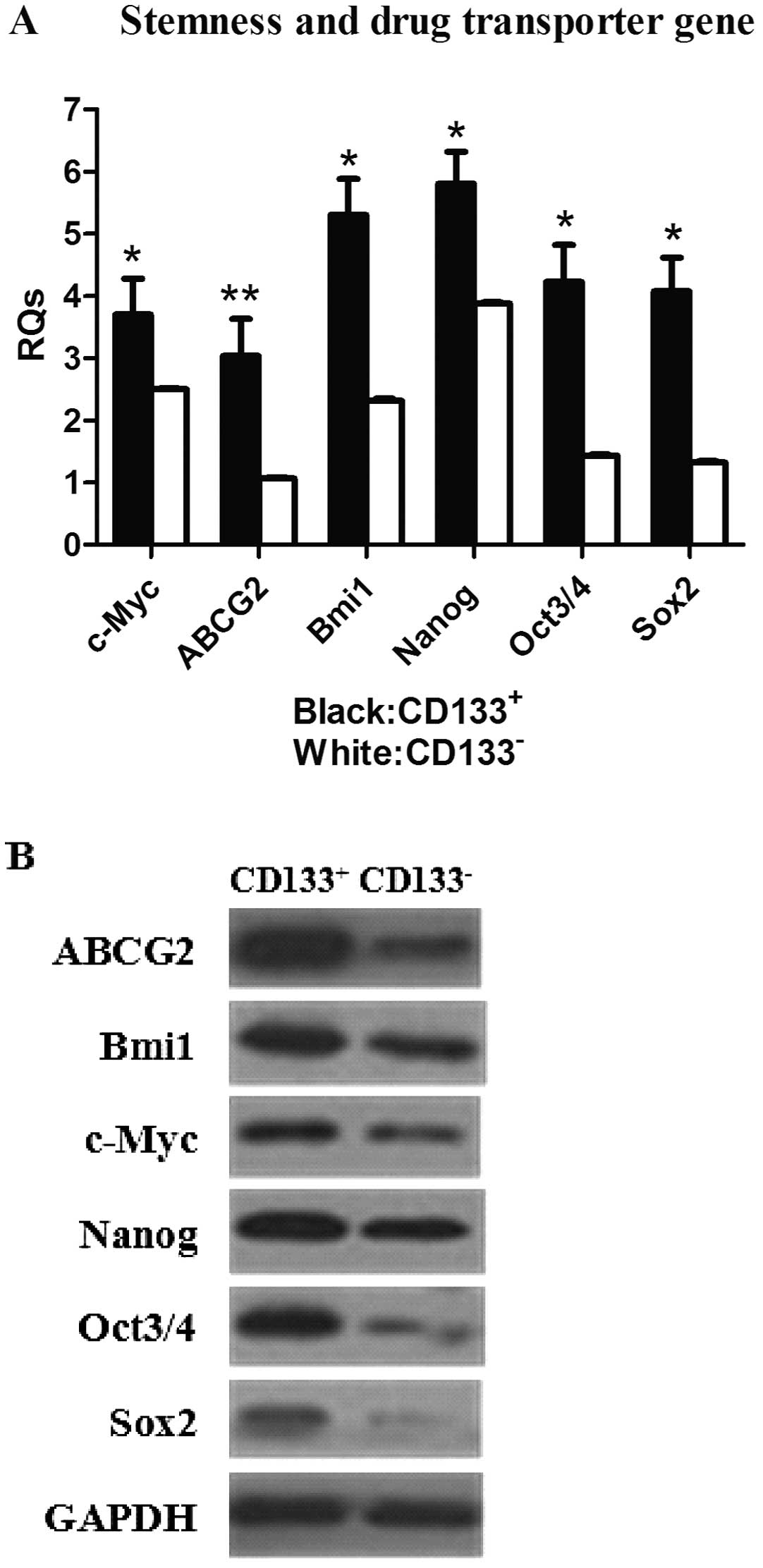
Increased expression of stemness and drug
transporter genes in SW982 CD133+ subpopulations
Expression levels of stemness and drug transporter
genes were examined in both the CD133+ and
CD133− subpopulations by real-time PCR and western
blotting. We observed that the expression of genes that play a
prominent role in stem cell maintenance and nuclear reprogramming,
including Bmi1, c-Myc, Nanog, Oct3/4 and
Sox2(29–31) were consistently higher in the
CD133+ subpopulation compared with their
CD133− counterparts (Fig.
4A). However, c-Myc and Bmi1 protein expression
was not significantly increased in CD133+ cells in
comparison with CD133− cells (Fig. 4B). In addition, both gene and
protein expression of the ABCG2 drug transporter was
significantly increased in CD133+ cells compared with
CD133− cells.
Increased cancer initiation by
CD133+ cells in vivo
To investigate the tumorigenic potential of
CD133+ and CD133− cells, both subpopulations
of SW982 cells were injected into BALB/c nude mice to generate
xenografts. A difference in tumorigenicity was observed between the
CD133+ and CD133− cell populations (Table IV). Tumor formation was observed
after the injection of as few as 5×103 CD133+
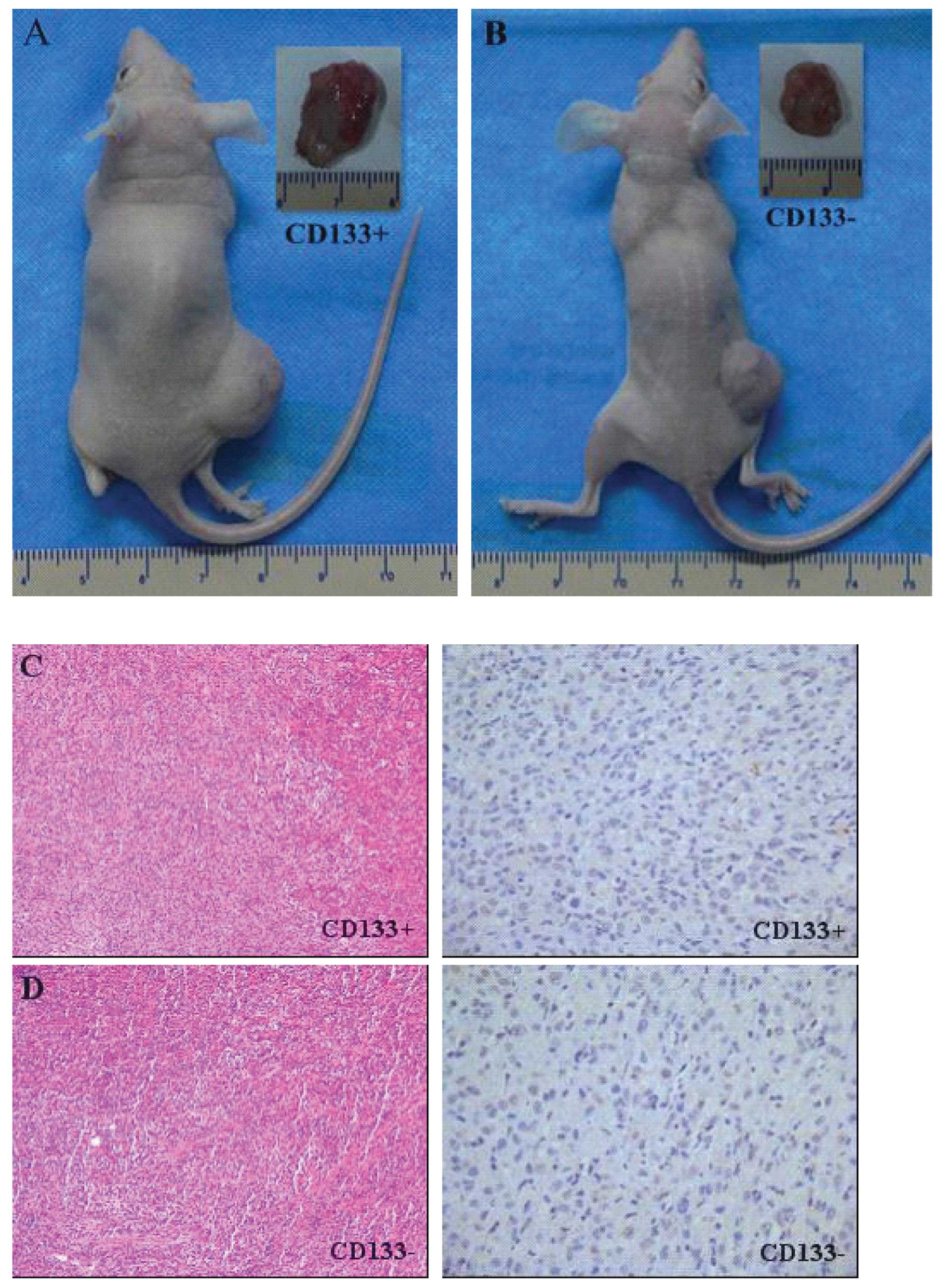
cells (Fig. 5A and B). In
contrast, only one out of five mice injected with 1×105
CD133− cells and none of the mice injected with
≤1×104 CD133− cells developed tumors.
CD133− cells (≥1×105) were required to
initiate a tumor, suggesting that the in vivo tumorigenicity
of CD133− cells is lower than that of CD133+
cells. In addition, CD133+ cells initiated both
secondary and tertiary tumors, whereas CD133− cells did
not.
 | Table IVTumor initiating capacity of
CD133+ and CD133− subpopulations. |
Table IV
Tumor initiating capacity of
CD133+ and CD133− subpopulations.
| Cell number for
injection
|
|---|
|
1×103 |
5×103 |
1×104 |
1×105 |
1×106 |
|---|
|
CD133+ | 0/5 | 2/5 | 4/5 | 5/5 | |
|
CD133− | | 0/5 | 0/5 | 1/5 | 5/5 |
We performed HE staining and immunohistochemistry to
demonstrate that the xenografts were derived from the injected
CD133+ and CD133− cells. However, both
CD133+ and CD133− tumors had similar
histological characteristics and levels of CD133 expression
(Fig. 5C and D).
Discussion
CSCs are present in numerous types of cancer
(15) and are capable of
self-renewal and differentiation and spheroid formation and have a
high tumorigenicity and resistance to current treatments. In many
cases, cancer therapy failure may be caused by a lack of effect of
current therapies upon CSCs, which are able to survive such
treatments and regenerate the tumor. It is therefore necessary to
be able to detect and characterize CSCs, in order to develop new
CSC-targeted therapeutic strategies. The major problems involved in
isolating CSCs are their rarity and the absence of specific markers
to enable their purification.
In early investigations, the side population (SP),
defined by Hoechst dye exclusion, was identified as a distinct
subset of cells; these cells were subsequently isolated and
designated CSCs, possess stemness characteristics and are
responsible for tumorigenesis in several cancer types (32–35).
Some SS have been shown to contain SP with enhanced tumorigenic
potential (36). We originally
tried to identify SP cells in the human SS cell line SW982, but
failed, possibly because of inappropriate culture conditions or
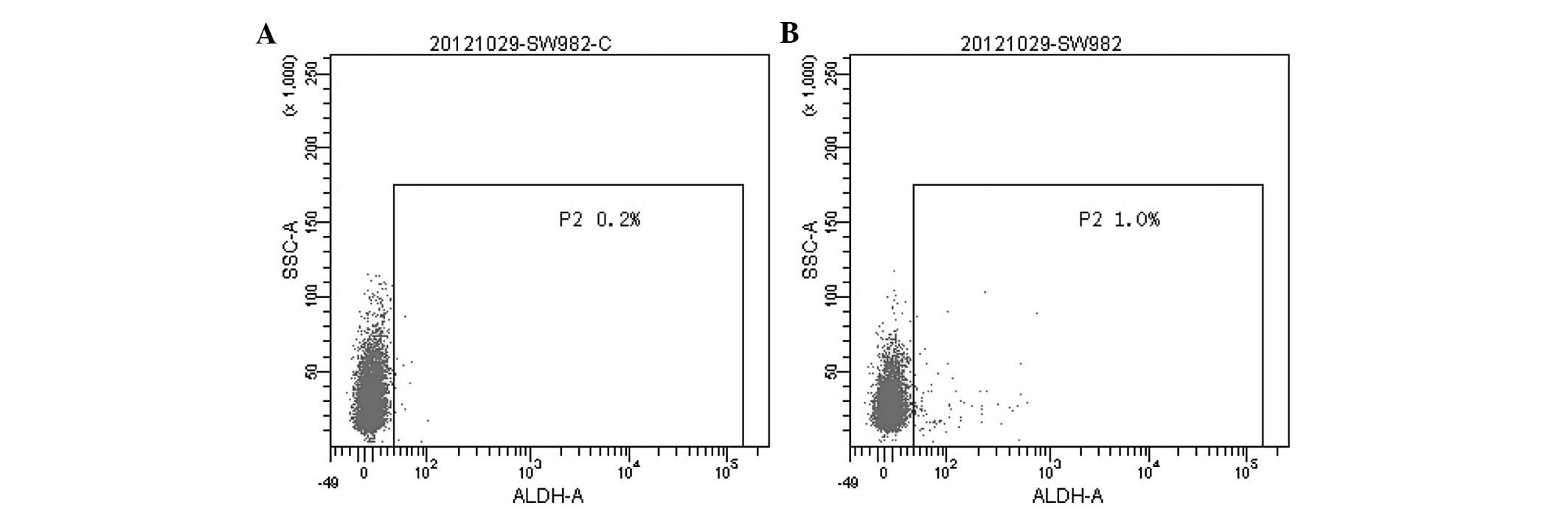
lack of a stem cell population in this cell line. Aldehyde
dehydrogenase (ALDH) functions as a detoxifying enzyme, has been
proposed as a marker of both normal and cancer stem cells (37) and has been successfully employed as
a stem cell marker in breast (38), prostate (39), colon (40) and lung cancer (41). We demonstrated that SW982 cells
contain a subpopulation with high ALDH activity, comprising ∼0.8%
of the total cell population (Fig.
6). We also found that the CD133+ subpopulation
comprises 8.59% of SW982 cells (Fig.
1A). Therefore, the CD133 antigen is potential CSC marker in
SW982 cells; using this marker, we were able to isolate a
population containing a high proportion of CD133+ cells.
To our knowledge, this is the first report of the analysis of CD133
expression in SW982 cells.
Two SS cell lines have been reported to exhibit
sarco spheres (42). In the
present study, we identified many differences between the
CD133+ and CD133− subpopulations; for
example, CD133+ cells were better at forming spherical
colonies and self-renewal in anchorage-independent, serum-starved
culture conditions. Moreover, the CD133+ subpopulation
expressed high levels of stemness genes. We compared the expression
of genes that play a prominent role in stem cell maintenance,
self-renewal and nuclear reprogramming, including Bmi1, c-Myc,
Nanog, Oct3/4 and Sox2, in the CD133+ and
CD133− subpopulations. The Oct3/4 transcription
factor is critically involved in self-renewal and the maintenance
of pluripotency in undifferentiated embryonic stem (ES) cells
(43). The Sox family of
transcription factors plays an essential role in cell
differentiation and development. Sox2 was originally thought
to be the only Sox protein expressed in ES cells (44) and associating with Oct3/4 to
maintain ES cell self-renewal. C-Myc is a dominant-acting
oncogene that encodes a transcription factor thought to regulate
the G0-G1 cell cycle transition. Bmi1 is a member of the
mammalian polycomb group (Pc-G) gene family (45), which contributes to the
proliferative capacity and self-renewal of both normal and
malignant stem cells (46).
Bmi1 recently emerged as a Myc-cooperating oncogene
(47). As both genes play an
important role in stem cell maintenance, Bmi1 and
c-Myc expression may correlate with self-renewal and
anti-apoptotic mechanisms in CSCs. Another highly expressed gene in
CD133+ cells is Nanog, which encodes a recently
identified divergent homeoprotein that maintains ES cell
self-renewal (48). All five
stemness genes were consistently and significantly overexpressed in
the CD133+ subpopulation by real-time PCR. Although we
did not demonstrate a relationship between expression of the
encoded proteins and the genesis of SS, these data provide
compelling evidence that a CD133+ subpopulation with
stem-like properties exists in SW982 cells.
CD133+ subpopulations were more resistant
to the cytotoxic effects of both CDDP and DXR, showing improved
survival compared to CD133− subpopulations. In addition,
CD133+ subpopulation-derived spheroids were more
drug-resistant than adherent cells. Increased expression of ABC
trans porter proteins, in particular ABCG2, is reported to
significantly contribute to the CSC phenotype, strongly correlates
with drug-resistance and is associated with a poor clinical outcome
(49,50). In our study, we revealed that
ABCG2 mRNA and protein expression was elevated in
CD133+ subpopulations; this may be responsible for high
levels of CSC multi-drug resistance and therefore constitutes a
good target for clinical cancer therapy.
In addition, using a BALB/c xenograft model we
observed that the CD133+ subpopulation had a higher
tumorigenic potential in vivo than the CD133−
subpopulation. Furthermore, slower tumor formation was observed by
the CD133− subpopulation, despite injection of more
cells. This observation suggests the existence of other cell
populations with tumor-initiating potential, although we cannot
exclude the possibility of CD133+ cell contamination in
the CD133− subpopulation during cell sorting
procedures.
In conclusion, this study is the first to
successfully isolate the CD133+ subpopulation from the
human SS cell line SW982 and reveals that this CD133+
subpopulation exhibits several characteristic CSC properties,
including high clonogenicity and self-renewal, increased
chemotherapeutic drug resistance, elevated expression of stemness
and drug transporter genes and high tumorigenic potential.
Therefore, CD133 may represent a new stem cell marker for SS and
may lead to novel approaches to develop therapies that selectively
attack CSCs without affecting normal tissue stem cells and thus
improve the prognosis for patients with SS.
Acknowledgements
This study was supported by a grant
from the National Natural Science Foundation of China (no.
81072192).
References
|
1
|
Eilber FC, Rosen G, Nelson S, Selch M,
Dorey F, Eckardt J and Eilber FR: High-grade extremity soft tissue
sarcomas: factors predictive of local recurrence and its effect on
morbidity and mortality. Ann Surg. 237:218–226. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Fisher C: Synovial sarcoma. Ann Diagn
Pathol. 2:401–421. 1998. View Article : Google Scholar
|
|
3
|
Amary MF, Berisha F, Bernardi Fdel C,
Herbert A, James M, Reis-Filho JS, Fisher C, Nicholson AG,
Tirabosco R, Diss TC and Flanagan AM: Detection of SS18-SSX fusion
transcripts in formalin-fixed paraffin-embedded neoplasms: analysis
of conventional RT-PCR, qRT-PCR and dual color FISH as diagnostic
tools for synovial sarcoma. Mod Pathol. 20:482–496. 2007.
View Article : Google Scholar
|
|
4
|
Fletcher CDM, Unni KK and Mertens F:
Tumours of Soft Tissue and Bone. IARC Press; Lyon: pp. 200–204.
2002
|
|
5
|
Nagayama S, Katagiri T, Tsunoda T, Hosaka
T, Nakashima Y, Araki N, Kusuzaki K, Nakayama T, Tsuboyama T,
Nakamura T, Imamura M, Nakamura Y and Toguchida J: Genome-wide
analysis of gene expression in synovial sarcomas using a cDNA
microarray. Cancer Res. 62:5859–5866. 2002.PubMed/NCBI
|
|
6
|
Weiss SW and Goldblum JR: Enzinger and
Weiss’s Soft Tissue Tumors. 5th edition. Mosby Inc.; St. Louis, MO:
pp. 1161–1182. 2008
|
|
7
|
Haldar M, Randall RL and Capecchi MR:
Synovial sarcoma: from genetics to genetic-based animal modeling.
Clin Orthop Relat Res. 466:2156–2167. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Reya T, Morrison SJ, Clarcke MF and
Weissman IL: Stem cells, cancer and cancer stem cells. Nature.
414:105–111. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Milas L and Hittelman WN: Cancer stem
cells and tumor response to therapy: current problems and future
prospects. Semin Radiat Oncol. 19:96–105. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bonnet D and Dick JE: Human acute myeloid
leukemia is organized as a hierarchy that originates from a
primitive hematopoietic cell. Nat Med. 3:730–737. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ponti D, Costa A, Zaffaroni N, Pratesi G,
Petrangolini G, Coradini D, Pilotti S, Pierotti MA and Daidone MG:
Isolation and in vitro propagation of tumorigenic breast cancer
cells with stem/progenitor cell properties. Cancer Res.
65:5506–5511. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Miki J, Furusato B, Li H, Gu Y, Takahashi
H, Egawa S, Sesterhenn IA, McLeod DG, Srivastava S and Rhim JS:
Identification of putative stem cell markers, CD133 and CXCR4, in
hTERT-immortalized primary nonmalignant and malignant tumor-derived
human prostate epithelial cell lines and in prostate cancer
specimens. Cancer Res. 67:3153–3161. 2007. View Article : Google Scholar
|
|
13
|
Singh SK, Hawkins C, Clarke ID, Squire JA,
Bayani J, Hide T, Henkelman RM, Cusimano MD and Dirks PB:
Identification of human brain tumour initiating cells. Nature.
432:396–401. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Schatton T, Murphy GF, Frank NY, Yamaura
K, Waaga-Gasser AM, Gasser M, Zhan Q, Jordan S, Duncan LM,
Weishaupt C, Fuhlbrigge RC, Kupper TS, Sayegh MH and Frank MH:
Identification of cells initiating human melanomas. Nature.
451:345–349. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Li C, Lee CJ and Simeone DM:
Identification of human pancreatic cancer stem cells. Methods Mol
Biol. 568:161–173. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Suvà ML, Riggi N, Stehle JC, Baumer K,
Tercier S, Joseph JM, Suvà D, Clément V, Provero P, Cironi L,
Osterheld MC, Guillou L and Stamenkovic I: Identification of cancer
stem cells in Ewing’s sarcoma. Cancer Res. 69:1776–1781. 2009.
|
|
17
|
O’Brien CA, Pollett A, Gallinger S and
Dick JE: A human colon cancer cell capable of initiating tumour
growth in immunodeficient mice. Nature. 445:106–110.
2007.PubMed/NCBI
|
|
18
|
Ricci-Vitiani L, Lombardi DG, Pilozzi E,
Biffoni M, Todaro M, Peschle C and De Maria R: Identification and
expansion of human colon-cancer-initiating cells. Nature.
445:111–115. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Mizrak D, Brittan M and Alison MR: CD133:
molecule of the moment. J Pathol. 214:3–9. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Singh SK, Clarke ID, Terasaki M, Bonn VE,
Hawkins C, Squire J and Dirks PB: Identification of a cancer stem
cell in human brain tumors. Cancer Res. 63:5821–5828.
2003.PubMed/NCBI
|
|
21
|
Vander Griend DJ, Karthaus WL, Dalrymple
S, Meeker A, DeMarzo AM and Isaacs JT: The role of CD133 in normal
human prostate stem cells and malignant cancer-initiating cells.
Cancer Res. 68:9703–9711. 2008.PubMed/NCBI
|
|
22
|
Hermann PC, Huber SL, Herrler T, Aicher A,
Ellwart JW, Guba M, Bruns CJ and Heeschen C: Distinct populations
of cancer stem cells determine tumor growth and metastatic activity
in human pancreatic cancer. Cell Stem Cell. 1:313–323. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Eramo A, Lotti F, Sette G, Pilozzi E,
Biffoni M, Di Virgilio A, Conticello C, Ruco L, Peschle C and De
Maria R: Identification and expansion of the tumorigenic lung
cancer stem cell population. Cell Death Differ. 15:504–514. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Curley MD, Therrien VA, Cummings CL,
Sergent PA, Koulouris CR, Friel AM, Roberts DJ, Seiden MV, Scadden
DT, Rueda BR and Foster R: CD133 expression define s a tumor
initiating cell population in primary human ovarian cancer. Stem
Cells. 27:2875–2883. 2009.PubMed/NCBI
|
|
25
|
Tirino V, Desiderio V, d’Aquino R, De
Francesco F, Pirozzi G, Graziano A, Galderisi U, Cavaliere C, De
Rosa A, Papaccio G and Giordano A: Detection and characterization
of CD133+ cancer stem cells in human solid tumors. PLoS
One. 3:e37692008. View Article : Google Scholar
|
|
26
|
Terry J and Nielsen T: Expression of CD133
in synovial sarcoma. Appl Immunohistochem Mol Morphol. 18:159–165.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Fujii H, Honoki K, Tsujiuchi T, Kido A,
Yoshitani K and Takakura Y: Sphere-forming stem-like cell
populations with drug resistance in human sarcoma cell lines. Int J
Oncol. 34:1381–1386. 2009.PubMed/NCBI
|
|
28
|
Gibbs CP, Kukekov VG, Reith JD,
Tchigrinova O, Suslov ON, Scott EW, Ghivizzani SC, Ignatova TN and
Steindler DA: Stem-like cells in bone sarcomas: implications for
tumorigenesis. Neoplasia. 7:967–976. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Okita K, Ichisaka T and Yamanaka S:
Generation of germline-competent induced pluripotent stem cells.
Nature. 448:313–317. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Takahashi K and Yamanaka S: Induction of
pluripotent stem cells from mouse embryonic and adult fibroblast
cultures by defined factors. Cell. 126:663–676. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wernig M, Meissner A, Foreman R, Brambrink
T, Ku M, Hochedlinger K, Bernstein BE and Jaenisch R: In vitro
reprogramming of fibroblasts into a pluripotent ES-cell-like state.
Nature. 448:318–324. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kondo T, Setoguchi T and Taga T:
Persistence of a small subpopulation of cancer stem-like cells in
the C6 glioma cell line. Proc Natl Acad Sci USA. 101:781–786. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Haraguchi N, Utsunomiya T, Inoue H, Tanaka
F, Mimori K, Barnard GF and Mori M: Characterization of a side
population of cancer cells from human gastrointestinal system. Stem
Cells. 24:506–513. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Chiba T, Kita K, Zheng YW, Yokosuka O,
Saisho H, Iwama A, Nakauchi H and Taniguchi H: Side population
purified from hepatocellular carcinoma cells harbors cancer stem
cell-like properties. Hepatology. 44:240–251. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Szotek PP, Pieretti-Vanmarcke R, Masiakos
PT, Dinulescu DM, Connolly D, Foster R, Dombkowski D, Preffer F,
Maclaughlin DT and Donahoe PK: Ovarian cancer side population
defines cells with stem cell-like characteristics and Mullerian
inhibiting substance responsiveness. Proc Natl Acad Sci USA.
103:11154–11159. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Wu C, Wei Q, Utomo V, Nadesan P, Whetstone
H, Kandel R, Wunder JS and Alman BA: Side population cells isolated
from mesenchymal neoplasms have tumor initiating potential. Cancer
Res. 67:8216–8222. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Ginestier C, Hur MH, Charafe-Jauffret E,
Monville F, Dutcher J, Brown M, Jacquemier J, Viens P, Kleer CG,
Liu S, Schott A, Hayes D, Birnbaum D, Wicha MS and Dontu G: ALDH1
is a marker of normal and malignant human mammary stem cells and a
predictor of poor outcome. Cell Stem Cell. 1:555–567. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Charafe-Jauffret E, Ginestier C, Iovino F,
Wicinski J, Cervera N, Finetti P, Hur MH, Diebel ME, Monville F,
Dutcher J, Brown M, Viens P, Xerri L, Bertucci F, Stassi G, Dontu
G, Birnbaum D and Wicha MS: Breast cancer cell lines contain
functional cancer stem cells with metastatic capacity and a
distinct molecular signature. Cancer Res. 69:1302–1313. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Kim H, Lapointe J, Kaygusuz G, Ong DE, Li
C, van de Rijn M, Brooks JD and Pollack JR: The retinoic acid
synthesis gene ALDH1a2 is a candidate tumor suppressor in prostate
cancer. Cancer Res. 65:8118–8124. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Huang EH, Hynes MJ, Zhang T, Ginestier C,
Dontu G, Appelman H, Fields JZ, Wicha MS and Boman BM: Aldehyde
dehydrogenase 1 is a marker for normal and malignant human colonic
stem cells (SC) and tracks SC overpopulation during colon
tumorigenesis. Cancer Res. 69:3382–3389. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Jiang F, Qiu Q, Khanna A, Todd NW, Deepak
J, Xing L, Wang H, Liu Z, Su Y, Stass SA and Katz RL: Aldehyde
dehydrogenase 1 is a tumor stem cell-associated marker in lung
cancer. Mol Cancer Res. 7:330–338. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Naka N, Takenaka S, Araki N, Miwa T,
Hashimoto N, Yoshioka K, Joyama S, Hamada K, Tsukamoto Y, Tomita Y,
Ueda T, Yoshikawa H and Itoh K: Synovial sarcoma is a stem cell
malignancy. Stem Cells. 28:1119–1131. 2010.PubMed/NCBI
|
|
43
|
Tokuzawa Y, Kaiho E, Maruyama M, Takahashi
K, Mitsui K, Maeda M, Niwa H and Yamanaka S: Fbx15 is a novel
target of Oct3/4 but is dispensable for embryonic stem cell
self-renewal and mouse development. Mol Cell Biol. 23:2699–2708.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Maruyama M, Ichisaka T, Nakagawa M and
Yamanaka S: Differential roles for Sox15 and Sox2 in
transcriptional control in mouse embryonic stem cells. J Biol Chem.
280:24371–24379. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Brunk BP, Martin EC and Adler PN:
Drosophila genes posterior sex combs and suppressor two of zeste
encodes proteins with homology to the murine bmi-1 oncogene.
Nature. 353:351–353. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Lessard J and Sauvageau G: Bmi-1
determines the proliferative capacity of normal and leukaemic stem
cells. Nature. 423:255–260. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Haupt Y, Alexander WS, Barri G, Klinken SP
and Adams JM: Novel zinc finger gene implicated as myc collaborator
by retrovirally accelerated lymphomagenesis in E mu-myc transgenic
mice. Cell. 65:753–763. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Mitsui K, Tokuzawa Y, Itoh H, Segawa K,
Murakami M, Takahashi K, Maruyama M, Maeda M and Yamanaka S: The
homeoprotein Nanog is required for maintenance of pluripotency in
mouse epiblast and ES cells. Cell. 113:631–642. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Ho MM, Ng AV, Lam S and Hung JY: Side
population in human lung cancer cell lines and tumors is enriched
with stem-like cancer cells. Cancer Res. 67:4827–4833. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Ding XW, Wu JH and Jiang CP: ABCG2: a
potential marker of stem cells and novel target in stem cell and
cancer therapy. Life Sci. 86:631–637. 2010. View Article : Google Scholar : PubMed/NCBI
|