Introduction
Melanoma is an aggressive cutaneous malignancy
accounting for just 4% of skin cancers but resulting in 80% of all
skin-cancer related deaths (1).
Melanoma may be induced by continuous proliferation of the
melanocytes and dysregulation of the epidermal melanin unit
(2). Melanocyte growth is
controlled by the surrounding keratinocytes by intercellular
communication through cell-cell adhesion molecules, cell-matrix
adhesion, and gap junctional intercellular communication (3). A previous study has confirmed that
downregulation of programmed cell death 4 (PDCD4) leads to
the downregulation of E-cadherin and urokinase-type plasminogen
activator receptor, which leads to degradation of extracellular
matrix and invasion (4).
PDCD4 was first identified by differential
display that is upregulated upon in apoptosis-induced murine cell
lines (5). The human PDCD4
gene is localized in chromosome 10q24 (6). PDCD4 protein can suppress tumor
promotion and progression to carcinomas in cultured cells and
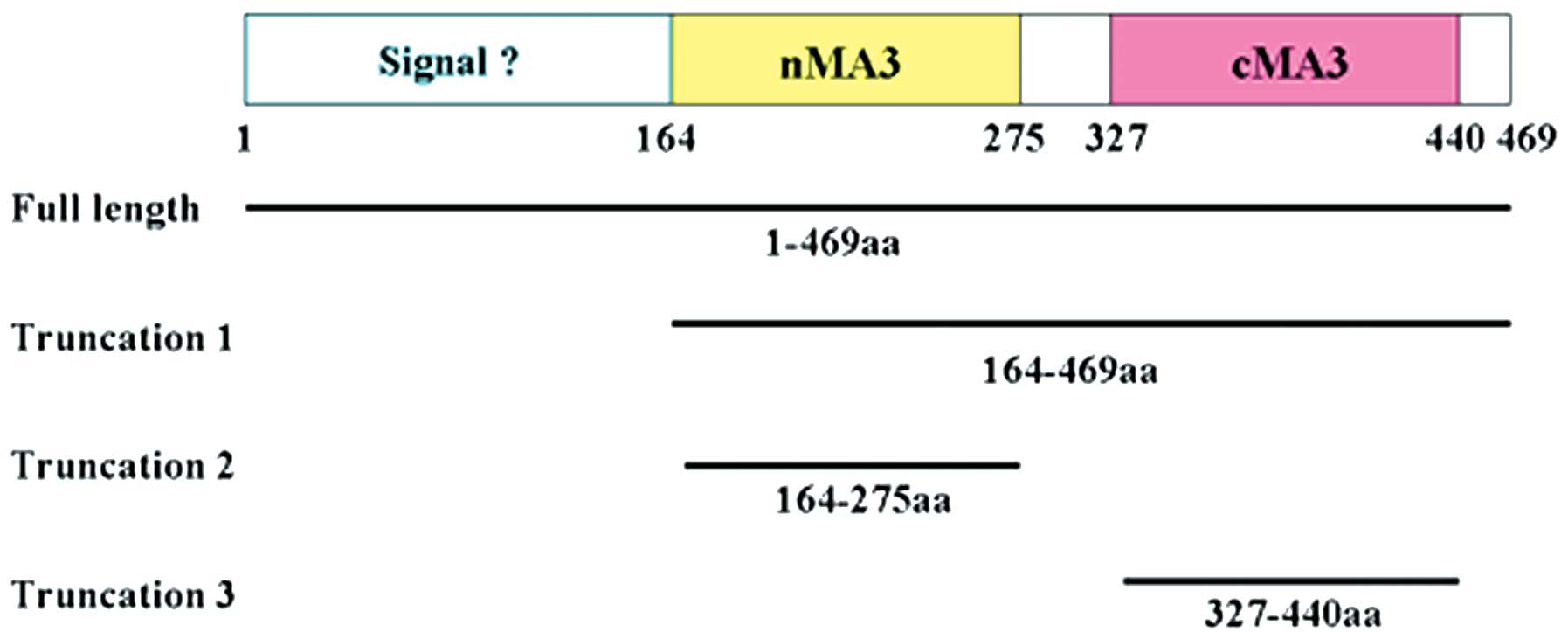
transgenic mice (7,8). As shown in Fig. 1, full length human PDCD4 contains
an N-terminal putative RNA-binding domain (residues 1–140) and two
tandem MA3 domains, designated as nMA3 (residues 164-275) and cMA3
(residues 327–440) (9). The two
MA3 domains have very similar structure and eIF4A-binding surfaces
(10). The molecular basis for
PDCD4-mediated suppression has been linked to its high affinity
MA-3 domains (11,12). NMR binding analysis has shown that
both MA3 domains of PDCD4 interacted with eIF4A and prevented
translation (10,11). However, another study has
demonstrated that the cMA3 domain alone is sufficient for the
inhibition of RNA helicase and translation (13). PDCD4 expression is significantly
downregulated in various human cancers such as lung cancer
(14), hepatocellular carcinoma
(15), breast carcinoma (16), colorectal cancer (17) and gastric cancer (18). PDCD4 can induce tumor cell to
apoptosis, inhibit tumor angiogenesis and increase sensitivity of
tumor cells to antitumor drugs or radiotherapy (19,20).
Recent studies showed the indirect relationship between PDCD4 and
melanoma cells (21–23), however, to our knowledge, little is
known yet about the individual role of cMA3 or nMA3 of PDCD4 in B16
melanoma cells.
In our study, we showed that cMA3 domain of PDCD4
caused profound suppression of cell proliferation in B16 melanoma
cells. In contrast to the role of cMA3, our findings showed that
the antitumor effects of nMA3 were weak relatively in B16 melanoma
cells. The cMA3 domain of PDCD4 may serve as an effective drug
independently.
Materials and methods
Cell lines and culture
The human melanoma cell line, B16, was obtained from
the American Type Culture Collection (ATCC, Bethesda, MD). Cell
lines were grown in Dulbecco’s modified Eagle’s medium (Hyclone,
Logan, UT) supplemented with 10% fetal bovine serum and antibiotics
(100 U/ml penicillin and 100 μg/ml streptomycin) and
maintained in a humidified incubator with 5% CO2 at
37°C.
Subjects
Total specimens of 21 patients with melanoma were
obtained from the Department of Plastic Surgery, The First
Affiliated Hospital of China Medical University (Jan. 2001 to Dec.
2010). None of the patients underwent radiotherapy or chemotherapy
before operation. This study was in compliance with the Helsinki
Declaration, all patients gave written informed consent for
participation and the procedure was approved by Our University
Ethics Committee.
Immunohistochemical staining
All tissues were routinely fixed in 10% buffered
formalin and embedded in paraffin. Sections (4 μm) were
deparaffinized in xylene. Endogenous peroxidase was blocked with 3%
hydrogen peroxide in deionized water for 20 min. Antigen retrieval
was carried out in citrate buffer (10 mM, pH 6.0) for 30 min at
95°C. Section were immunostained with polyclone antibody to PDCD4
(sc-27123; Santa Cruz Biotechnology, Santa Cruz, CA) at 1:200
dilution or CXCR4 (PA1237; Boster Biological Technology, Fremont,
CA) at 1:50 dilution for 60 min at 37°C, followed by biotinylated
secondary antibody for 30 min, subsequently reacted with by HRP for
30 min. For visualization, hydrogen peroxide-activated diamino
benzidine (DAB) was applied. Washes (50-min) in PBS were carried
out between each step. Tissue sections were lightly counterstained
with hematoxylin, dehydrated through graded alcohols, cleared with
xylene and mounted in mounting medium. Normal tissue was used as a
control. Sections treated without primary antibodies were used as
negative controls.
SDS-PAGE and immunoblotting
Proteins (45 μg per lane) were separated by
10% SDS-polyacrylamide gel electrophoresis and transferred to PVDF
membranes (Millipore Corp., Billerica, MA). Western blotting was
performed using primary antibodies: PDCD4 (sc-27123; Santa Cruz
Biotechnology), CXCR4 (PA1237; Boster Biological Technology) and
β-actin (sc-47778; Santa Cruz Biotechnology). Incubation with
antibodies was performed in 1.5% BSA in TBS, 0.1% Tween. Detection
of the immune complexes was performed with the ECL western blotting
detection system (Amersham Biosciences, Piscataway, NJ).
Construction of PDCD4 cDNA expression
vector
For the preparation of truncated PDCD4 proteins, the
relevant sequences were amplified from full-length PDCD4
(GenBank accession no. NM_014456.3) by PCR using primers that
included designed restriction sites (Table I). PDCD4 and the truncated forms
created in this study are presented in Fig. 1. cDNAs of the truncated PDCD4 forms
were obtained by PCR, then digested with the relevant restriction
enzymes and ligated into pcDNA3.1. Transfection of plasmids into
B16 cells was performed using Lipofectamine™ 2000 (Invitrogen,
Carlsbad, CA) according to the manufacturer’s instructions.
 | Table IPrimers used to generate intact and
truncated forms of PDCD4. |
Table I
Primers used to generate intact and
truncated forms of PDCD4.
| Region of PDCD4
amplified | Primers
(5′-3′) | Product (bp) |
|---|
| aa 1–469
(intact) | F: AAGCTT
TACCTATATCTTTTACTCGT
R: GAATTC
CTAGTAGCTCTCAGGTTTAA | 1,407 |
| aa 164–469
(truncated 1) | F: AAGCTT
GTTCGTTTTCGGTTT
R: GAATTC
CTAGTAGCTCTCAGGTTTAA | 918 |
| aa 164–275
(truncated 2) | F: AAGCTT
GTTCGTTTTCGGTTT
R: GAATTC
ACATAAGATTCCATC TCCAA | 336 |
| aa 327–440
(truncated 3) | F: AAGCTT
ACAATTTCTCTAACTATACG
R: GAATTC
GGAAATTATTCCAGCCTGAA | 339 |
Real-time PCR and RT-PCR
Total tissue and cellular RNA was isolated using
TRIzol reagent (Invitrogen). cDNA was then synthesized from 1
μg of total RNA using SuperScript II reverse transcriptase
(Invitrogen) according to the manufacturer’s protocol. The level of
tissular PDCD4 mRNA was quantitated by real-time PCR (ABI
PRISM 7500) using power SYBR® Green PCR Master Mix
(Takara Dalian, Dalian, China) and specific primers for
PDCD4 and GAPDH. The following primer sets were used:
PDCD4 sense, 5′-GTATGAT GTGGAGGAGGTGGAT-3′; antisense:
5′-CCCTCCAATGCT AAGGATACTG-3′; GAPDH sense,
5′-GAAGGTGAAGGTCG GAGT-3′; antisense: 5′-CATGGGTGGAATCATATTGGAA-3′.
The real-time PCR conditions were as follows: one cycle at 95°C for
10 min followed by 40 cycles at 95°C for 15 sec and at 60°C for 1
min. Each reaction was repeated independently three times in
triplicate. Results for the quantity of mRNA were expressed as the
number of copies of PDCD4 mRNA per copy of GAPDH
mRNA.
PCR amplification of cellular cDNA was performed in
15-μl mixtures. The primers are summarized in Table I. The RT-PCR conditions were: one
cycle at 94°C for 5 min, followed by 35 cycles of 94°C for 30 sec,
72°C for 45 sec, 58°C for 45 sec and final extension at 72°C for 10
min. Finally, products were resolved by 1% agarose gel
electrophoresis and visualized by ethidium bromide staining and a
UV imaging system (UVP, Upland, CA).
Immunofluorescence
Transfected cells were washed with PBS, fixed in 4%
paraformaldehyde, permeabilized in 1% Triton X-100 for 5 min and
blocked with 5% bovine serum albumin in PBS containing 0.5% Triton
X-100 for 1 h. Intact PDCD4 (aa 1–469), truncated 1 (aa 164–469)
and truncated 3 (aa 327–440) were detected using anti-PDCD4
(sc-27123; Santa Cruz Biotechnology). Truncated 2 (aa 164–275) was
detected using anti-PDCD4 (sc-376430; Santa Cruz Biotechnology).
Cells were washed with PBS and incubated with appropriate secondary
fluorophore-conjugated antibody for 1 h at room temperature, washed
with PBS and mounted using Prolong Anti-fade (Sigma-Aldrich,
Carlsbad, CA). Secondary antibody used for detection of intact
PDCD4, truncated 1 and truncated 3 was Alexa Fluor® 488
donkey anti-goat IgG (H+L). Alexa Fluor® 488 goat
anti-mouse IgG (H+L) was used to detect truncated 2. Cells were
examined and captured using an Olympus CX71 fluorescence microscope
(Olympus, Tokyo, Japan).
Cell viability assay
Viability of transfected cells was determined using
the 3-(4,5-dimethylthiazolyl)-2,5-diphenyltetrazolium-bromide (MTT)
assay (Sigma). Cells were plated in 96-well plates (1,500 cells per
well) and incubated under normal culture conditions. After 24 h,
the cells were treated with 0.5 mg/ml MTT for 4 h and lysed with
dimethyl sulfoxide (DMSO). Absorbance rates were measured at
550–560 nm using a microplate reader (Bio-Rad, Hercules, CA).
Measurement of apoptotic cell death
Annexin V-FITC/PI double staining assays were
performed to detect apoptosis. Following the manufacturer’s
instructions (Apoptosis Detection kit, KeyGEN, Nanjing, China),
cells were trypsinized, washed twice with cold PBS, then
resuspended in 200 μl binding buffer. Annexin V-FITC was
added to a final concentration of 0.5 μg/ml. In addition
samples were incubated at room temperature in the dark. After 20
min, 300 μl binding buffer containing 0.5 μg/ml PI
was added and samples were immediately analyzed on a FACSCalibur
flow cytometer (Becton-Dickinson Medical Devices, Shanghai, China).
Cells in the stage of early apoptosis were defined as
FITC+/PI− cells.
Migration assay
Migration was assessed in Boyden chambers containing
polycarbonate filters with 8-μm pore size (Costar,
Bodenheim, Germany). The lower compartment was filled in DMEM with
10% FBS used as a chemoattractant and the filter was placed above.
Cells were harvested by trypsinization and resuspended in DMEM
without FBS. Cell suspensions (600 μl) at a density of
3×104 cells/ml were placed in the upper compartment of
the chambers. After incubation at 37°C for 4 h filters were
removed, cells were fixed, stained and counted. Each condition was
assayed in triplicate and assays were repeated at least twice.
Gelatin zymography
Fifty micrograms of protein was applied to 10%
polyacrylamide gels with 1% gelatin incorporated as a substrate for
gelatinolytic proteases. After running the gel the SDS was removed
by washing twice in 2.5% Triton X-100 for 30 min. The gels were
incubated overnight in zymography development buffer containing 50
mM Tris-HCl (pH 7.4), 2 mM NaN3 and 5 mM
CaCl2. After development the gels were stained for 3 h
in 45% methanol/10% glacial acetic acid containing 1% (w/v).
Coomassie Blue R-250 and subsequently partially destained with the
same solution without dye. The gelatinolytic activity of each MMP
was qualitatively evaluated as a clear band against the blue
stained gelatin background.
Flow cytometry analysis
The CXCR4 or CXCR7 cell surface expression was
measured and quantified using the fluorescent-antibody (24). Cells were labeled with
APC-anti-CXCR4 monoclonal antibody (#555976, BD Pharmingen™,
Baltimore, MD). Mouse anti-CXCR7 (AF4227, R&D Systems China,
Shanghai, China) and PE-conjugated goat anti-mouse antibody
(#115-116-146, Jackson ImmunoResearch Laboratories, Inc., West
Grove, PA) was used for the detection of CXCR7. Expression was
measured by flow cytometry using a FACSCalibur machine (BD
Biosciences). Receptor expression was evaluated by the mean channel
fluorescence values.
Statistical analysis
Data are presented as the mean ± standard deviation
(SD). Differences between groups were analyzed using Student’s
t-test for continuous variables. Statistical analysis was performed
using Statistical Package for the Social Sciences (SPSS, version
17.0; SPSS, Inc.) and significance was established at
P<0.05.
Results
PDCD4 expression in human melanoma
specimens
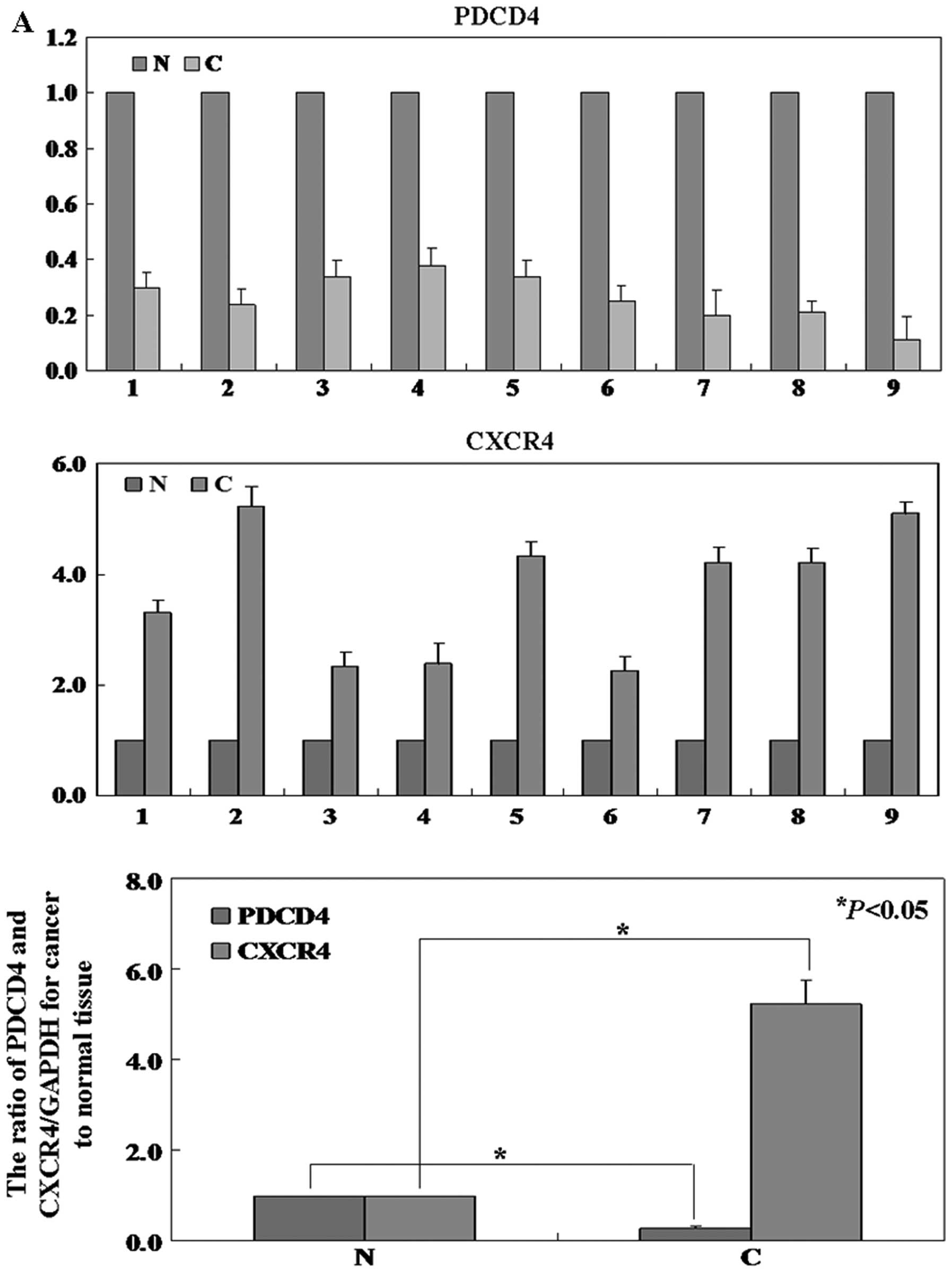
The level of PDCD4 protein in cancer tissue was
significantly lower than that in normal tissue (P<0.05, Fig. 2B). To determine whether
PDCD4 mRNA was also reduced, real-time PCR analysis was
performed. Results show that the level of PDCD4 mRNA expression was
coincident with the level of protein expression (P<0.05,
Fig. 2A). Furthermore, the
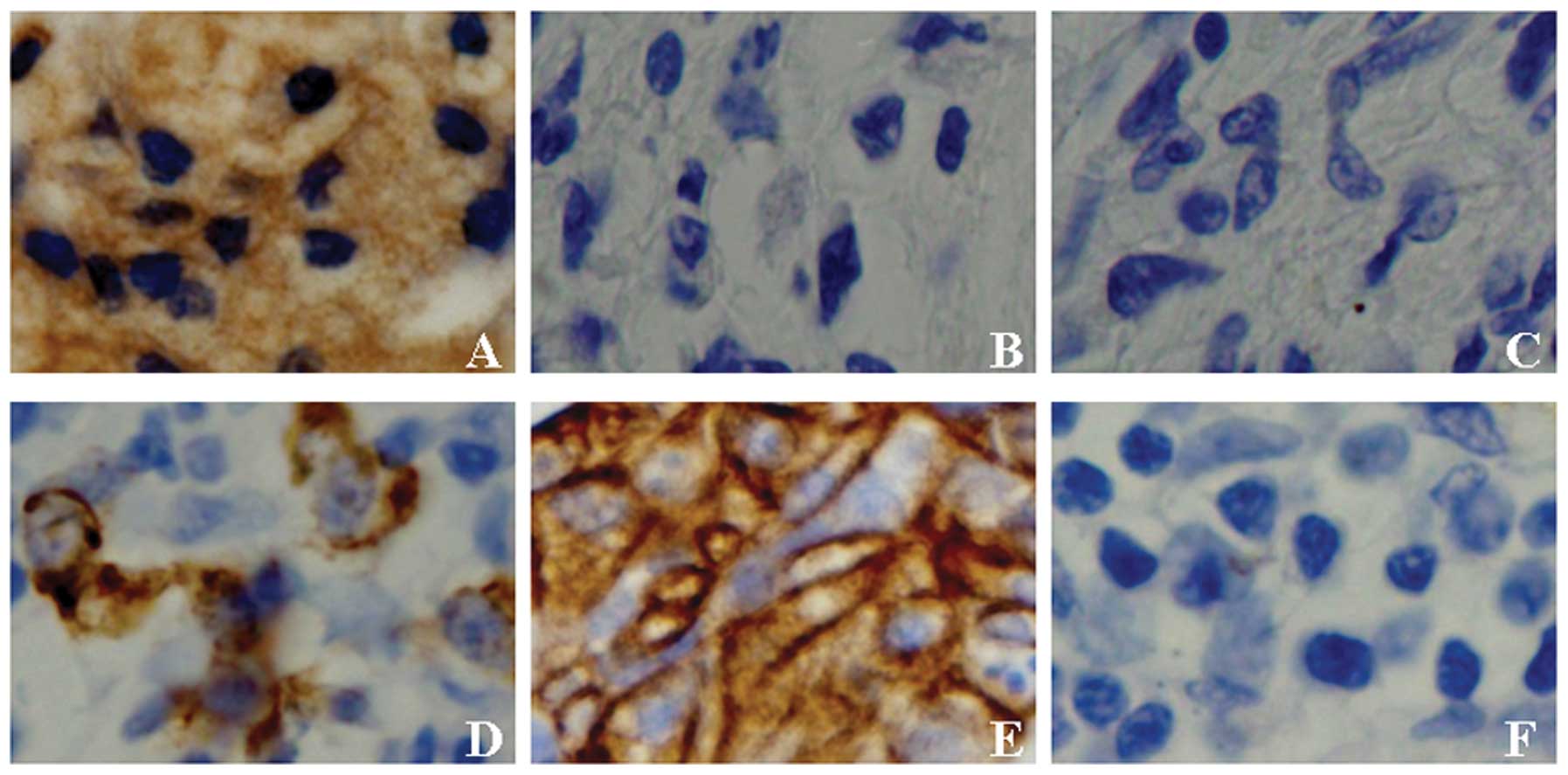
immunohistochemical staining results showed PDCD4 proteins were
located both in nucleus and cytoplasm (Fig. 3A).
Evaluation of the mRNA and protein levels
of different PDCD4 fragments in B16 cells
B16 cells were transfected with PDCD4 fragments that
included: intact (aa 1–469), truncated 1 (aa 164–469), truncated 2
(aa 164–275) and truncated 3 (aa 327–440). Levels of PDCD4
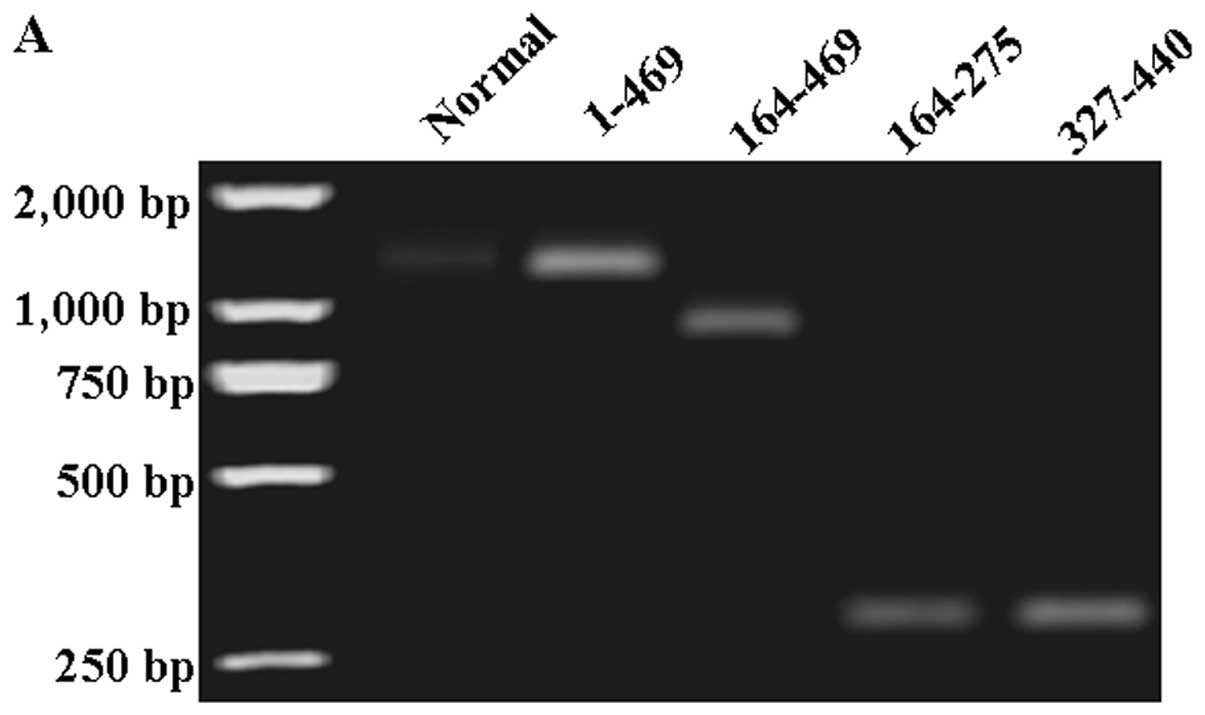
fragments were detected using immunofluorescence and RT-PCR assays.
In RT-PCR assay, levels of PDCD4 mRNA were lower in B16
cells than in treated cells (Fig.
4A). In the immunofluorescence assay, levels of PDCD4 proteins
were lower in untransfected B16 cells compared with transfected B16
cells (Fig. 4B). The results
showed that intact PDCD4 proteins were able to shuttle between
nucleus and cytoplasm. Under normal growth conditions, intact PDCD4
proteins were located predominantly in the nucleus. However,
truncated PDCD4 fragments were observed to localize in the
cytoplasm (Fig. 4B).
Upregulation of PDCD4 expression in B16
cell line results in decreased proliferation, increased apoptosis
and diminished migration
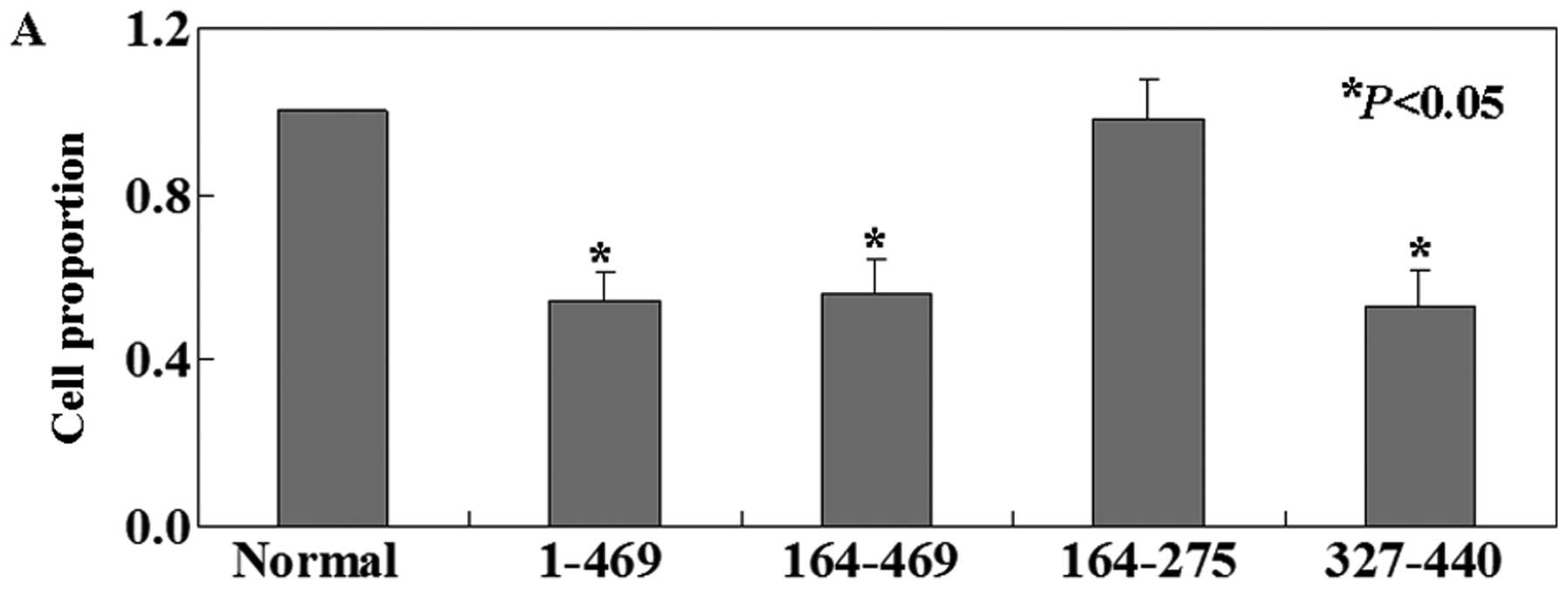
We then investigated the functions of PDCD4
fragments in B16 cell line. The MTT assay showed that the
proliferation ratio of intact PDCD4-expressing B16 cells was
decreased compared with untransfected cells (P<0.05, Fig. 5A). Truncated 1 (aa 164–469) and
truncated 3 (aa 327–440) had similar effects with intact PDCD4 on
B16 cells. However, truncated 2 (aa 164–275) showed no effects on
B16 cells. We determined that the percentage of apoptosis by using
Annexin V and PI double-staining in untreated B16 cells was 0.34%,
significantly lower compared to intact PDCD4 (2.71%), truncated 1
(2.68%) and truncated 3 (3.25%) cells (P<0.05, Fig. 5B). Truncated 2 cells (0.41%) showed
no significant changes compared with untreated ones (P>0.05,
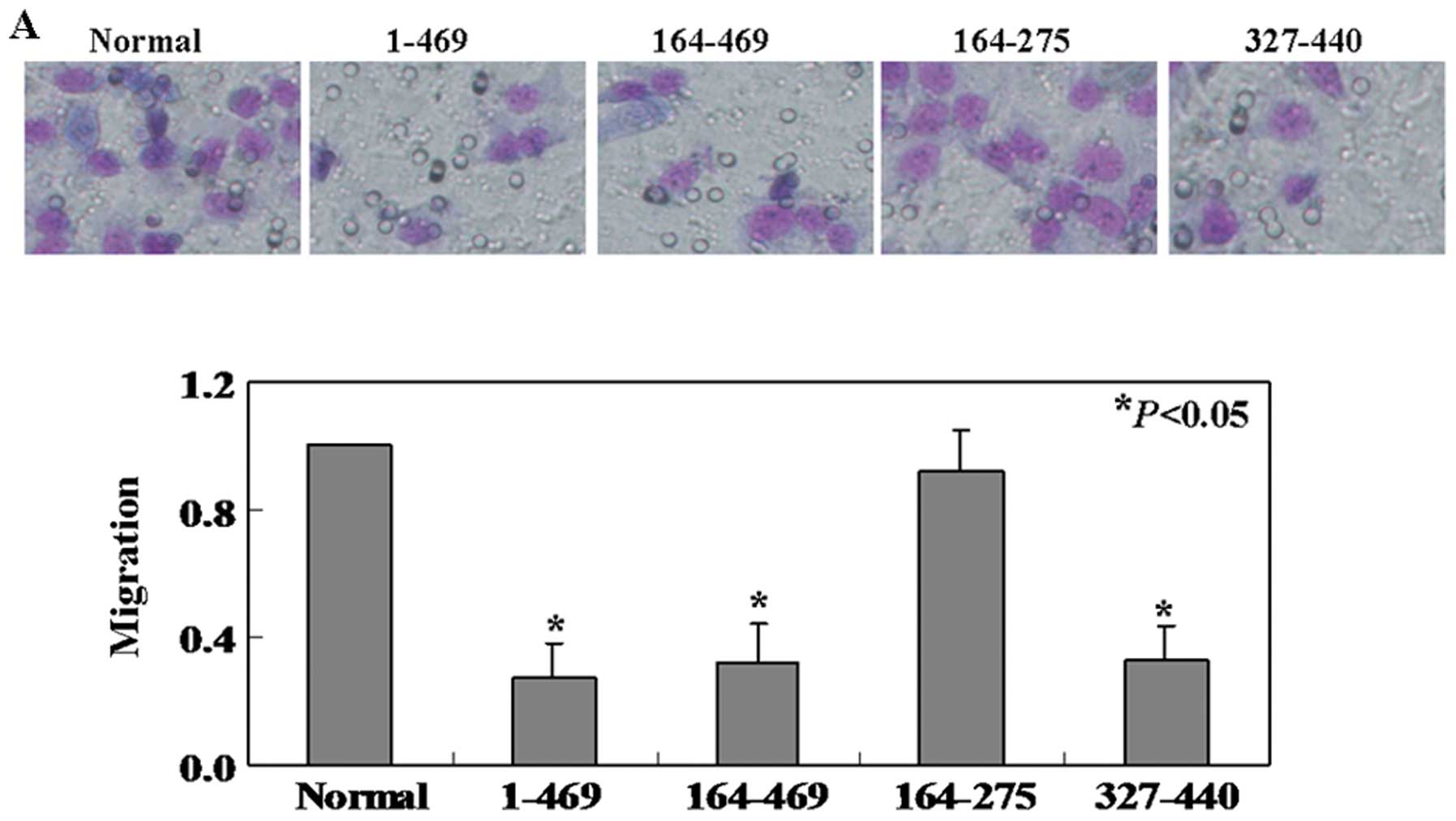
Fig. 5B). To quantitatively assess
the cell migration rate, transwell migration assay was applied. The
cells in intact PDCD4, truncated 1 and truncated 3 groups showed
54, 56 and 53% less migration, respectively, than the untreated
ones (P<0.05, Fig. 6A). We
found that no significantly less of the truncated 2 cells migrated
to the lower membrane compared to control cells (P>0.05,
Fig. 6A). In addition, we
evaluated expression of MMPs and observed the levels of MMP-2 in
intact PDCD4, truncated 1 and truncated 3 cell lines were lower
than that in untreated and truncated 2 cell lines (Fig. 6B).
Cell surface expression of CXCR4 on human
melanoma specimens and B16 cell lines
In order to determine the level of CXCR4 protein and
mRNA in human melanoma specimens, western blotting, IHC and
real-time PCR were performed respectively. The results showed both
CXCR4 protein and mRNA were decreased in cancer tissues compared
with normal tissues (Fig. 2). The
immunohistochemical staining results showed CXCR4 proteins were
located in cytomembrane (Fig. 3D and
E). B16 cells with PDCD4 different fragment treatment expressed
different relative levels of the cell surface receptor CXCR4. We
observed that untreated B16 (55.8%) and truncated 2 (67.2%) cell
lines stained highly positive for CXCR4 (Fig. 6C). In contrast, CXCR4 was expressed
in intact PDCD4 (4.4%), truncated 1 (4.2%) and truncated 3 (6.6%)
cell lines at lower levels (Fig.
6C). As shown in Fig. 6C, this
is not in striking difference to CXCR7 expression, which was barely
expressed on the cell lines.
Discussion
PDCD4 has been characterised as a new tumor
suppressor gene, which is downregulated in several human
malignancies (14–18). Consistent with our results, we also
found the levels of PDCD4 protein and mRNA were lower in cancer
tissues than that in normal tissues. Yang et al(22) found that microRNA-21 (miR-21)
regulates the metastatic behavior of B16 melanoma cells by
promoting cell proliferation, survival and migration/invasion.
MicroRNA (miR) target prediction databases suggest that PDCD4 is
regulated by miR-21 (25,26). The expression of PDCD4 is increased
during apoptosis (27,28) and decreased during human and mouse
carcinogenesis (29,30). Overexpression of PDCD4 inhibits
tumorigenesis and tumor progression in a transgenic-mouse model and
inhibits tumor cell invasion in vitro(27–31).
We also confirmed the antitumor activities of PDCD4 in B16 cells.
These results provided the first direct evidence for an essential
role of the PDCD4 in melanoma.
Furthermore, the main purpose of our study was to
identify the roles of PDCD4 protein distinct domains. We designed
three deletion mutants, PDCD4Δ164-469, PDCD4Δ164-275 and
PDCD4Δ327-440. Residues 1–140 of human PDCD4 protein were an
RNA-binding domain, residues 164–275 were nMA3 and residues 327–440
were cMA3 (9). The localization of
PDCD4 has been reported to vary in a cell type-dependent manner
(27). One explanation for this
variability is that PDCD4 cycles between the nucleus and cytoplasm
(32). However, a nuclear
localization signal has not been formally identified. Interesting,
we found only intact PDCD4 protein was able to shuttle between the
nucleus and the cytoplasm. The results indicated that the nuclear
localization signal of PDCD4 may be included in residues 1–140. The
only direct molecular function identified for PDCD4 is bound to and
inhibits the function of the mammalian mRNA initiation factor eIF4A
(11,33). As noted in the introduction,
mutational analysis and NMR binding analysis have shown that PDCD4
uses both MA3 domains to interact with eIF4A (10,11).
However, another study also demonstrated that the cMA3 domain alone
is sufficient for the inhibition of RNA helicase and translation
(13). Consistent with previous
studies, our results showed that PDCD4Δ164-469 and PDCD4Δ327-440
had similar effects with intact PDCD4 on B16 cells. However,
PDCD4Δ164-275 showed no biological activity.
Melanoma is an extremely aggressive disease with
high metastatic potential (34).
MMP-2 (gelatinase A) and MMP-9 (gelatinase B) are the major MMPs
secreted by activated inflammatory macrophages (35,36).
MMP-2 is released as a latent enzyme and must be activated to
degrade the matrix (37). In our
study, intact PDCD4, PDCD4Δ164-469 and PDCD4Δ327-440 were able to
reduce the gelatinolytic activity of MMP-2. Previous studies have
proven that CXCR4 is a major chemokine receptor expressed in many
types of cancer cells (38,39).
CXCR4/SDF-1 axis plays a major role in migration and adhesion of
various tumor cells, including colon cancer (40), breast cancer (41,42)
and melanoma (43,44). Consistent with previous studies, we
found that CXCR4 was highly expressed in melanoma tissue compared
to normal tissue by real-time PCR, western blotting and IHC.
Interesting, we confirmed that CXCR4 was decreased in B16 cells
after treatment with intact PDCD4, PDCD4Δ164-469 or PDCD4Δ327-440.
The biological significance of CXCR7 receptor expression on B16
cells was not found. Our data presented from this study provide
evidence that PDCD4 could inhibit mobility of B16 cells by
regulating CXCR4 expression. However, the mechanism remains
unclear.
Above all, our study demonstrated that: i) the
levels of PDCD4 mRNA and protein were deficient in melanoma,
however, CXCR4 showed a higher level in cancer tissues than normal
tissues; ii) the tumor suppressor activity of PDCD4 and its
fragments in vitro. Especially, PDCD4 inhibited mobility of
B16 cells by suppressing MMP-2 activity and CXCR4 expression. This
is the first report to demonstrate that different fragments of
PDCD4 protein played distinct roles in B16 cells. This report may
provide novel information for melanoma treatment.
Acknowledgements
We thank Dr Zhang Jin for pCDNA3.1
plasmid and Dr Yu Miao for technical assistance.
References
|
1.
|
Jemal A, Bray F, Center MM, et al: Global
cancer statistics. CA Cancer J Clin. 61:69–90. 2011. View Article : Google Scholar
|
|
2.
|
Haass NK and Herlyn M: Normal human
melanocyte homeostasis as a paradigm for understanding melanoma. J
Investig Dermatol Symp Proc. 10:153–163. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Haass NK, Smalley KS and Herlyn M: The
role of altered cell– cell communication in melanoma progression. J
Mol Histol. 35:309–318. 2004.
|
|
4.
|
Wang Q, Sun ZX, Allgayer H, et al:
Downregulation of E-cadherin is an essential event in activating
beta-catenin/Tcf-dependent transcription and expression of its
target genes in Pdcd4 knockdown cells. Oncogene. 29:128–138. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Shibahara K, Asano M, Ishida Y, et al:
Isolation of a novel mouse gene MA-3 that is induced upon
programmed cell death. Gene. 166:297–301. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Jansen AP, Camalier CE and Colburn NH:
Epidermal expression of the translation inhibitor programmed cell
death 4 suppresses tumorigenesis. Cancer Res. 65:6034–6041. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Soejima H, Miyoshi O, Yoshinaga H, et al:
Assignment of the programmed cell death 4 gene (PDCD4) to human
chromosome band 10q24 by in situ hybridization. Cytogenet Cell
Genet. 87:113–114. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Cmarik JL, Min H, Hegamyer G, et al:
Differentially expressed protein PDCD4 inhibits tumor
promoter-induced neoplastic transformation. Proc Natl Acad Sci USA.
96:14037–14042. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Chang JH, Cho YH, Sohn SY, et al: Crystal
structure of the eIF4A-PDCD4 complex. Proc Natl Acad Sci USA.
106:3148–3153. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Suzuki C, Garces RG, Edmonds KA, et al:
PDCD4 inhibits translation initiation by binding to eIF4A using
both its MA3 domains. Proc Natl Acad Sci USA. 105:3274–3279. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Yang HS, Cho MH, Zakowicz H, et al: A
novel function of the MA-3 domains in transformation and
translation suppressor Pdcd4 is essential for its binding to
eukaryotic translation initiation factor 4A. Mol Cell Biol.
24:3894–3906. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Zakowicz H, Yang HS, Stark C, et al:
Mutational analysis of the DEAD-box RNA helicase eIF4AII
characterizes its interaction with transformation suppressor Pdcd4
and eIF4GI. RNA. 11:261–274. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
LaRonde-LeBlanc N, Santhanam AN, Baker AR,
et al: Structural basis for inhibition of translation by the tumor
suppressor Pdcd4. Mol Cell Biol. 27:147–156. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Chen Y, Knosel T, Kristiansen G, et al:
Loss of PDCD4 expression in human lung cancer correlates with
tumour progression and prognosis. J Pathol. 200:640–646. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Zhang H, Ozaki I, Mizuta T, et al:
Involvement of programmed cell death 4 in transforming growth
factor-beta1-induced apoptosis in human hepatocellular carcinoma.
Oncogene. 25:6101–6112. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Afonja O, Juste D, Das S, et al: Induction
of PDCD4 tumor suppressor gene expression by RAR agonists,
antiestrogen and HER-2/neu antagonist in breast cancer cells.
Evidence for a role in apoptosis. Oncogene. 23:8135–8145. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Mudduluru G, Medved F, Grobholz R, et al:
Loss of programmed cell death 4 expression marks adenoma-carcinoma
transition, correlates inversely with phosphorylated protein kinase
B, and is an independent prognostic factor in resected colorectal
cancer. Cancer. 110:1697–1707. 2007. View Article : Google Scholar
|
|
18.
|
Motoyama K, Inoue H, Mimori K, et al:
Clinicopathological and prognostic significance of PDCD4 and
microRNA-21 in human gastric cancer. Int J Oncol. 36:1089–1095.
2010.PubMed/NCBI
|
|
19.
|
Jin H, Kim TH, Hwang SK, et al: Aerosol
delivery of urocanic acid-modified chitosan/programmed cell death 4
complex regulated apoptosis, cell cycle, and angiogenesis in lungs
of K-ras null mice. Mol Cancer Ther. 5:1041–1049. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Jansen AP, Camalier CE, Stark C, et al:
Characterization of programmed cell death 4 in multiple human
cancers reveals a novel enhancer of drug sensitivity. Mol Cancer
Ther. 3:103–110. 2004.PubMed/NCBI
|
|
21.
|
Caporali S, Alvino E, Levati L, et al:
Down-regulation of the PTTG1 proto-oncogene contributes to the
melanoma suppressive effects of the cyclin-dependent kinase
inhibitor PHA-848125. Biochem Pharmacol. 84:598–611. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Yang CH, Yue J, Pfeffer SR, et al:
MicroRNA miR-21 regulates the metastatic behavior of B16 melanoma
cells. J Biol Chem. 286:39172–39178. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Rothhammer T, Hahne JC, Florin A, et al:
The Ets-1 transcription factor is involved in the development and
invasion of malignant melanoma. Cell Mol Life Sci. 61:118–128.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Amara A, Gall SL, Schwartz O, et al: HIV
coreceptor down-regulation as antiviral principle:
SDF-1alpha-dependent internalization of the chemokine receptor
CXCR4 contributes to inhibition of HIV replication. J Exp Med.
186:139–146. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
25.
|
Griffiths-Jones S, Grocock RJ, van Dongen
S, et al: miRBase: microRNA sequences, targets and gene
nomenclature. Nucleic Acids Res. 34:D140–D144. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26.
|
Betel D, Wilson M, Gabow A, et al: The
microRNA. org resource: targets and expression Nucleic Acids Res.
36:D149–D153. 2008.PubMed/NCBI
|
|
27.
|
Lankat-Buttgereit B and Goke R: The tumour
suppressor Pdcd4: Recent advances in the elucidation of function
and regulation. Biol Cell. 101:309–317. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28.
|
Allgayer H: Pdcd4, a colon cancer
prognostic that is regulated by a microRNA. Crit Rev Oncol Hematol.
73:185–191. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29.
|
Yang HS, Jansen AP, Nair R, et al: A novel
transformation suppressor, Pdcd4, inhibits AP-1 transactivation but
not NF-κB or ODC transactivation. Oncogene. 20:669–676.
2001.PubMed/NCBI
|
|
30.
|
Schmid T, Jansen AP, Baker AR, et al:
Translation inhibitor Pdcd4 is targeted for degradation during
tumor promotion. Cancer Res. 68:1254–1260. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
31.
|
Leupold JH, Yang HS, Colburn NH, et al:
Tumor suppressor Pdcd4 inhibits invasion/intravasation and
regulates urokinase receptor (u-PAR) gene expression via
Sp-transcription factors. Oncogene. 26:4550–4562. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
32.
|
Böhm M, Sawicka K, Siebrasse JP, et al:
The transformation suppressor protein Pdcd4 shuttles between
nucleus and cytoplasm and binds RNA. Oncogene. 22:4905–4910.
2003.PubMed/NCBI
|
|
33.
|
Yang HS, Jansen AP, Komar AA, et al: The
transformation suppressor Pdcd4 is a novel eukaryotic translation
initiation factor 4A binding protein that inhibits translation. Mol
Cell Biol. 23:26–37. 2003. View Article : Google Scholar
|
|
34.
|
Tawbi HA and Kirkwood JM: Management of
metastatic melanoma. Semin Oncol. 34:532–545. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
35.
|
Gibbs DF, Warner RL, Weiss SJ, et al:
Characterization of matrix metalloproteinases produced by rat
alveolar macrophages. Am J Respir Cell Mol Biol. 20:1136–1144.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
36.
|
Shah PK, Falk E, Badimon JJ, et al: Human
monocyte-derived macrophages induce collagen breakdown in fibrous
caps of atherosclerotic plaques. Potential role of matrix-degrading
metal-loproteinases and implications for plaque rupture.
Circulation. 92:1565–1569. 1995.
|
|
37.
|
Jackson C, Nguyen M, Arkell J, et al:
Selective matrix metalloproteinase (MMP) inhibition in rheumatoid
arthritis-targetting gelatinase A activation. Inflamm Res.
50:183–186. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
38.
|
Balkwill F: The significance of cancer
cell expression of the chemokine receptor CXCR4. Semin Cancer Biol.
14:171–179. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
39.
|
Kucia M, Jankowski K, Reca R, et al:
CXCR4-SDF-1 signalling, locomotion, chemotaxis and adhesion. J Mol
Histol. 35:233–245. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
40.
|
Schimanski CC, Schwald S, Simiantonaki N,
et al: Effect of chemokine receptors CXCR4 and CCR7 on the
metastatic behavior of human colorectal cancer. Clin Cancer Res.
11:1743–1750. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
41.
|
Harvey JR, Mellor P, Eldaly H, et al:
Inhibition of CXCR4-mediated breast cancer metastasis: a potential
role for heparinoids? Clin Cancer Res. 13:1562–1570. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
42.
|
Zhou W, Jiang Z, Liu N, et al:
Down-regulation of CXCL12 mRNA expression by promoter
hypermethylation and its association with metastatic progression in
human breast carcinomas. J Cancer Res Clin Oncol. 135:91–102. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
43.
|
Turner RR, Ye X, Bilchik AJ, et al:
Chemokine receptor CXCR4 expression in patients with melanoma and
colorectal cancer liver metastases and the association with disease
outcome. Ann Surg. 244:113–120. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
44.
|
Scala S, Giuliano P, Ascierto PA, et al:
Human melanoma metastases express functional CXCR4. Clin Cancer
Res. 12:2427–2433. 2006. View Article : Google Scholar : PubMed/NCBI
|