Introduction
Hepatocellular carcinoma (HCC) is the fifth most
common solid tumor worldwide and the third most common cause of
cancer-related death (1),
resulting in almost 700,000 deaths in 2008. Clinically, HCC is
often diagnosed at the late stage and medical treatments including
chemotherapy, chemoembolization, ablation and proton beam therapy
remain disappointing. There is an urgent need for new therapies for
this aggressive disease. Recently, HCC progression has been thought
to be driven by a small subset of cells, namely liver cancer stem
cells (LCSC), through their capacity for self-renewal, production
of heterogeneous progeny, resistance to chemotherapy and limitless
proliferation. Many research groups in leukemia and several solid
tumors supported the existence of such a subpopulation, which was
successfully isolated and manifested marked tumorigenic capacity
assessed by NOD/SCID mouse xenograft assay (2–8).
CD133 (AC133) is a highly conserved antigen as the
human homologue of mouse Prominin-1, which was originally
identified as a 5-transmembrane cell surface glycoprotein expressed
in a subpopulation of the CD34 hematopoietic stem and progenitor
cells derived from human fetal liver and bone marrow (9,10).
Notably, CD133 was expressed in most types of cancer stem cells
within colon, breast, prostate, glioblastoma, medulloblastoma (MB)
(11), and hepatocellular
carcinoma (12,13). In the past few years, compelling
evidence has emerged in support of the notion that CD133 is a
surface marker for LCSCs in human liver cancer cell lines and
clinical samples and that CD133+ LCSCs are associated
with a hypoxic marker in clinical HCC samples, suggesting that
CD133+ LCSCs have a critical role in tumor growth and
resistance to anticancer therapy in liver cancers (14). CD44 is also regarded as an
important marker for LCSC (15).
In all HCC cell lines studied, CD133-positive cells showed higher
cell migration activity and upregulated invasion- and
EMT-associated genes including Twist (16).
The Twist1 gene encodes a transcription factor
containing a basic helix-loop-helix (bHLH) domain (17) and an aminoacid motif present in a
protein family involved in the regulation of organogenesis
(18–20). Recently, a number of studies have
revealed that Twist plays essential roles not only in the
development of multiple organs and systems, but also in cancer
metastasis (21–23). It has been reported that Twist1
overexpression correlates positively with HCC metastasis (24). Further study on different HCC cell
lines revealed that HCC cells with higher levels of Twist1 have
higher metastatic ability. This suggests that Twist1 induces EMT
changes, which are partially responsible for the increased HCC cell
invasiveness (24). β-catenin,
encoded by the CTNNB1 gene, has multiple functions, including
mediation of cell adhesion and signal transduction. It combines
with a variety of proteins to regulate cell proliferation and
differentiation, which is critical for embryonic development and
tumorigenesis. In addition, β-catenin has been shown to be
accumulated within 67% of HCC tissues and is closely related to the
clinicopathological features of HCC (25–27).
8-bromo-7-methoxychrysin (BrMC) is a novel synthetic
analogue of chrysin (5,7-dihydroxyflavone, ChR), which is a natural
and biologically active flavone extracted from many plants, honey
and bee propolis and has been shown to inhibit cell proliferation
and induce apoptotic cell death in a variety of cancer cells
(28–34). Because of the poor oral
bioavailability, chrysin may not be successful when used as a
dietary flavonoid for cancer chemotherapeutics (35). It has been reported that ChR
halogenated derivatives had stronger bioactivities than the lead
compound (36). Our previous study
showed that the effect of BrMC on the inhibition of proliferation
and induction of apoptosis in the colon cancer cell line HT-29 and
the gastric cancer cell line SGC-7901, was stronger than that of
ChR (37,38). BrMC also induces apoptosis of HCC
cells by ROS generation and sustained JNK activation (39). Recently, our laboratory reported
that BrMC affected the number of glioma stem-like cells (GSLCs)
derived tumor spheres by MTT assay (40).
In this study, we investigated the possible
functions of BrMC in inhibiting the characteristics of
CD133+ sphere-forming cells (SFC) derived from SMMC-7721
cell line in vitro and in vivo and explored the
potential mechanisms.
Materials and methods
Cell culture and reagents
The hepatoma cell line SMMC-7721 and the
immortalized embryo liver cell line L-02 were obtained from the
Chinese Academy of Sciences (Shanghai, China). SMMC-7721 cells were
maintained in Dulbecco’s modified Eagle’s medium (DMEM)
supplemented with 10% fetal bovine serum, 100 U/ml penicillin and
100 μg/ml streptomycin (Invitrogen Life Technologies) in an
incubator containing 5% CO2 at 37°C. BrMC was
synthesized as described previously (37). Methyl thiazolyl tetrazolium (MTT)
was purchased from Sigma (St. Louis, MO, USA). Fetal bovine serum
was purchased from Hyclone (Thermo Scientific, USA). Trypsin and
dimethyl sulfoxide (DMSO) were from Amersco Co. (USA).
Immunomagnetic separation of CD133
hepatoma cells
Cells were suspended with PBE incubation solution
(0.5% bovine serum albumin, 0.08% EDTA in PBS, pH 7.2) to a final
concentration of 1×108 cells in 0.5 ml, then incubated
with anti-CD133 antibody (final concentration 20 μg/ml) at
4°C for 30 min and incubated with antibody-coated superfine
magnetic beads (Miltenyi Biotec GmbH, Bergisch Gladbach, Germany)
at 10°C for 15 min and suspended in 20 times the total volume of
PBE solution. The separation column was installed into a magnetic
field and pretreated with 0.5 ml PBE which was naturally eluted due
to gravity. The incubated cell suspension was added to the
separation column and naturally eluted. The column was rinsed twice
and then separated from the magnetic field and subsequently
inserted into a new tube, followed by administration of 1–2 ml PBE
along the needle core to remove the CD133-positive cells.
Simultaneously, negative cells were collected and the two types of
cells were rinsed with medium.
Flow cytometry (FCM)
The freshly isolated cells from each fraction were
prepared at a concentration of 105 cells/ml using
William’s E medium (containing 20% FBS) and incubated for 15–30 min
at room temperature to block non-specific sites. These cells were
then washed twice with PBS and re-suspended in 990 μl PBS.
Subsequently, 10 μl of antibodies, including CD133
(PE-conjugated, Biolegend, USA) and isotype control IgG2b
(PE-conjugated, Biolegend), were added to each cell suspension.
After 30 min of incubation at 4°C in the dark, the cells were
washed twice with PBS, fixed in 0.1% formaldehyde and analyzed
using the FACS Calibur™ system (BD Immunocytometry Systems, San
Jose, CA, USA).
Spheroid formation and self-renewal
assay
Parental cells were plated at a density of 2,000
cells/well in low adherence plates (6 wells) with serum-free stem
cell conditional medium that containing DMEM/F12 (Gibco Invitrogen)
plus 20 ng/ml EGF (Peprotech, NJ, USA), 10 ng/ml bFGF (Peprotech),
1X B27 (Invitrogen, CA, USA) and 0.4 μg/ml insulin
(Peprotech). After 4 days of culture, sphere forming cells (SFCs)
were visualized in a microscope and the number of cells after
trypsin-EDTA digestion was counted.
To investigate self-renewal capacity of liver cancer
sphere, single cell suspension prepared from the SFCs was diluted
to 500 cells/ml. Single cell suspension (2 μl) was plated in
96-well ultra-low plates containing 150 μl serum-free medium
per well. Wells containing about one or two cells were included and
those with single cells were marked and monitored daily under a
microscope (Nikon Eclipse TE2000-S) for 6 days. Then, the colonies
were counted.
To analyze the effects of BrMC on self-renewal of
SFCs, single cell suspension of SFCs was plated at a density of
2,000 cells/well in 6-well ultra-low plates. In addition, different
concentrations of BrMC (0.1, 0.3 and 1.0 μM) were added to
medium. After culturing for 6 days, the colonies were counted under
a microscope.
Cell viability assay
Cell growth was measured by the MTT assay (Sigma).
Briefly, SMMC-7721 cells were plated at a density of
5×103 cells/well in 96-well plates and allowed to attach
for 24 h for each conditions, resulting in log phase growth at the
time of drug treatment. BrMC (0.1, 0.3, 1.0 and 3.0 μmol/l)
was added to the wells for 48 h. After treatment, MTT reagent was
added to each well at 5 mg/ml in a 20-μl volume and the
reaction was incubated for another 4 h. The formazan crystals
formed by viable cells were subsequently solubilized in DMSO.
Absorbance was measured at 550 nm using an automated microplate
reader (Bio-Rad 550). Cell viability was expressed as a percentage
of the value for control cultures. The cytotoxic effects of BrMC on
SMMC-7721 sorted or non-sorted cells were expressed as
IC50 values (the drug concentration that reduced the
absorbance of treated cells by 50% compared to untreated cells),
which was plotted using Graph Pad Prism 5 (GraphPad Software, San
Diego, CA, USA). All experiments were carried out in
triplicate.
Matrigel invasion assay
The invasion chamber has 24 cell culture inserts,
each of which contains a polyethylene terepthalate membrane with
8-μm pores (Corning Inc., Lowell, MA, USA) coated with
Matrigel. Serum-free DMEM (1 ml) was added to the apical side of an
insert and then 1 ml of DMEM plus 10% fetal calf serum was added to
the basal side of the insert as the chemoattractant. A total of
2,000 sorted or non-sorted SMMC-7721 cells were plated in the top
chamber of the transwell and treated with BrMC (0.1, 0.3, 1.0 and
3.0 μmol/l) for 24 h. The cells that had not invaded through
the pores of the insert were scraped off the apical side of the
inserts with a sterile cotton swab and discarded. Cells invaded to
the lower chamber were fixed with methanol, stained with crystal
violet and counted.
Experimental studies by a xenograft model
in nude mice
Eight-week-old BALB/c mice of either sex were
purchased from Hunan Agricultural University [SCXK (Xiang
2002-003)], maintained in Hunan Research Center for Safety
Evaluation of Drug (Experimental Animal Center of Hunan Province)
and then bred under specific pathogen-free conditions. Mice were
kept in ventilated and filtered cages, fed an irradiated diet and
housed on irradiated bedding. Food and water were supplied ad
libitum. All animal experiments were performed in compliance
with the guidelines of the Chinese government approved by the
College BioResources Ethics Review Board. The area between the
right leg and abdominal cavity in the nude mice was disinfected
with iodine and a single cell suspension was injected into the mice
subcutaneously using a 100-μl micro syringe. The needle was
held in place for 1 min and then gradually withdrawn to prevent
liquid return. After inoculation, mice were housed in a sterile
barrier system at constant temperature (25±2°C) and humidity
(45–50%). Tumor formation and growth were observed daily. At the
end of the experiment, the nude mice were sacrificed and the tumors
were peered and fixed in fresh 4% paraformaldehyde, then embedded
and H&E stained.
RNA interference and pcDNA3-Twist1
transfection
Twist1 siRNA (5′-GAU GGC AAG CUG CAG CUA UTT-3′,
5′-AUA GCU GCA GCU UGC CAU CTT-3′) and a non-silencing control
siRNA (5′-UUC UCC GAA CGU GUC ACG UTT-3′, 5′-ACG UGA CAC GUU CGG
AGA ATT-3′) were synthesized by GenePharma (Shanghai, China). SiRNA
transfections were performed according to the manufacturer’s
instructions. Briefly, Twist1 siRNA or negative control siRNA of
100 pmol was diluted in 250 μl of Opti-MEM I medium. Next, 5
μl Lipofectamine 2000 was diluted in 250 μl of
Opti-MEM I Medium. After 5-min incubation, the diluted siRNA was
mixed with diluted Lipofectamine 2000 gently and incubated for 20
min at room temperature. The oligomer-lipofectamine complexes were
applied to the subconfluent cells which were seeded in a 6-well
plate 24 h before the experiment. Forty-eight hours after
transfection, the Twist1 protein levels were assessed by western
blotting.
pcDNA3-Twist1 was purchased from GenePharma. To
generate Twist1-expressing stable transfectants, L-02 and SMMC-7721
cells and SFCs of SMMC-7721 cell line were transfected with
pcDNA3-Twist1 and stable clones were selected with 1000
μg/ml of G418 (Calbiochem) for 4 weeks.
Western blotting
Cells were washed once in pre-cold PBS and lysed in
RIPA buffer [50 mM Tris-HCl (pH 7.2), 150 mM NaCl, 1% (v/v) Triton
X-100, 1% (w/v) sodium deoxycholate, 0.1% (w/v) SDS and protease
inhibitors]. Sample proteins were separated by 10% SDS-PAGE gel,
after electrophoresis, proteins were transferred to PVDF
(polyvinylidene difluoride) membrane (Bio-Rad, Richmond, CA, USA)
at 4°C for 2 h at 100 mA. The membranes were detected by rabbit
polyclonal antibodies against ZO-1 (Abcam), Twist (Cell Signaling)
or mouse monoclonal antibodies against N-cadherin (Upstate),
Vimentin Ab-2 (Neo Markers), E-cadherin (BD Transduction),
β-catenin (Cell Signaling), β-actin (Sigma), respectively.
Statistical analysis
Data were presented as the mean ± SD. Comparisons of
experimental values between BrMC-treated cells and untreated
controls were conducted using analysis of variance or the
Kruskal-Wallis rank test. Statistical significance was defined as
P<0.05.
Results
LCSCs exist in hepatoma carcinoma cell
line SMMC-7721 and in sorted CD133+ cells
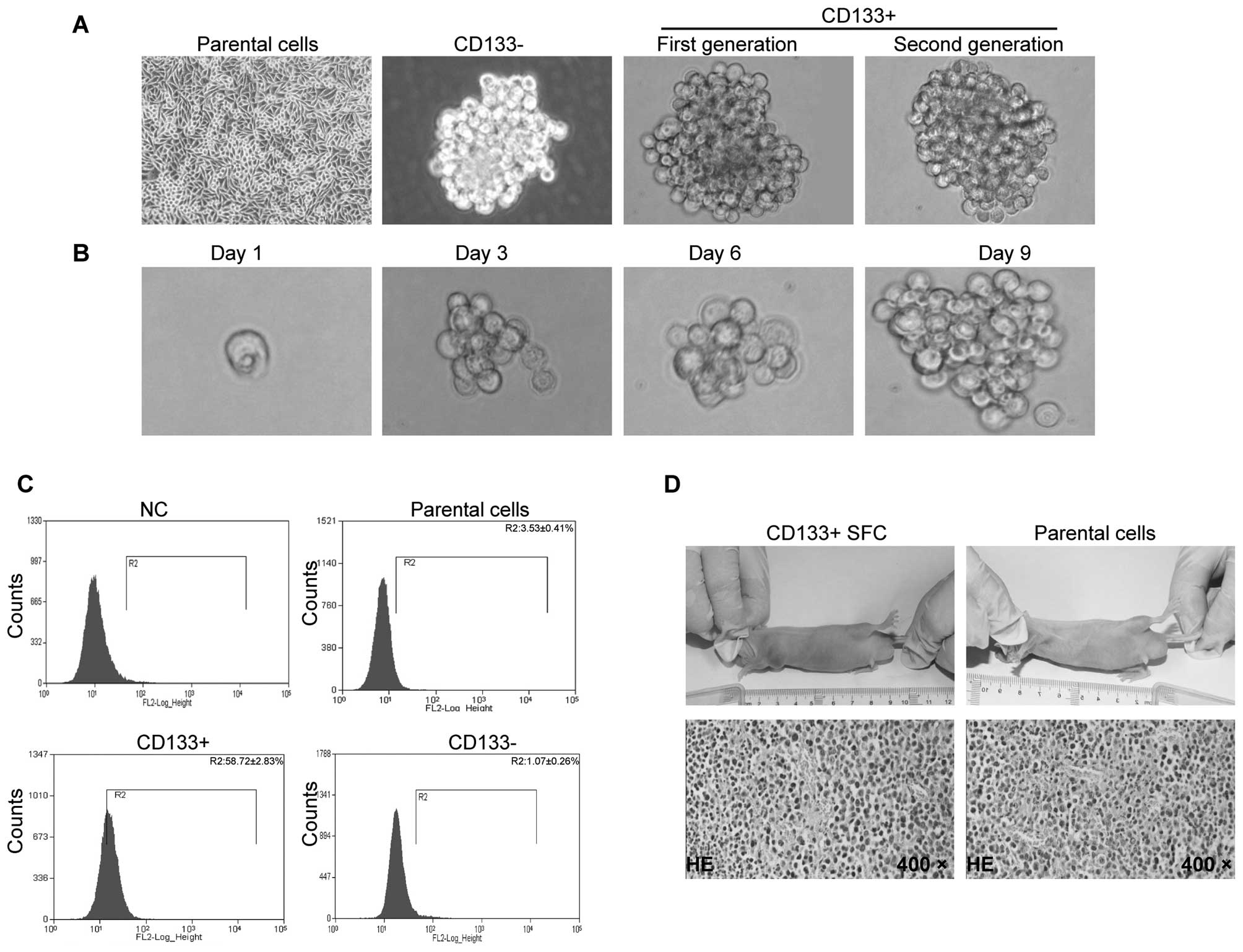
Human hepatoma carcinoma cell line SMMC-7721 was
cultured in vitro as normal condition and the cells adhered
to the culture slides (Fig. 1A,
parental cells). In order to isolate LCSCs, SMMC-7721 cells were
enzymatically dispersed into single-cell suspensions and subjected
to immunostaining with an anti-CD133 antibody (Miltenyi Biotec
Inc.) and FACS analysis. FACS results showed that the percentage of
CD133+ population was ∼58.72% (Fig. 1C), which is much higher than that
of CD133− population (1.07%) or non-sorted cells
(3.53%), indicating our immunomagnetic separation system is
efficient. On the other hand, a major characteristic of CSC cells
is their capacity to form three-dimensional structures, or spheres.
In the case of inoculation of 2,000 cells per well, there were many
more spheres formed in the group of CD133+ cells
(Fig. 1A and Table I), while at the same time-point of
sphere formation, the sphere volume of CD133+ cells was
much greater than that of parental cells both for the first
generation and for the second generation (Fig. 1A).
 | Table I.Comparison of sphere-forming ability
between CD133+ cells and parental cells. |
Table I.
Comparison of sphere-forming ability
between CD133+ cells and parental cells.
| No. of spheres per
2,000 cells | Sphere volume
(μm3) |
|---|
|
|
|---|
| Cell line | Parental cells | CD133+
cells | Parental cells | CD133+
cells |
|---|
| SMMC-7721 | 83±21 | 232±45a | 314±34 | 986±52a |
One of the cancer stem cell characteristics is
self-renewal. To test this, one SMMC-7721 cell per well was plated
to a 96-well plate and the wells with one cell were visualized
daily. Fig. 1B shows the process
of single SMMC-7721 cell forming a sphere. The result showed that
the SMMC-7721 derived CD133+ cells have the ability to
form spheres, even a single cell became a new hepatoma cancer
sphere (Fig. 1B). These data
indicate that the SMMC-7721 derived CD133+ cell
population has the capacity of self-renewal.
Xenotransplantation is the gold standard for
evaluating tumorigenicity of tumor cells. We tested whether our
sorted CD133+ LCSCs have more tumor initiating
capability. We injected different cell numbers of sphere-formed
SMMC-7721 CD133+ LCSCs, or parent cells into Balb/c-nu
mice to get the minimum number of seeded cells in vivo
tumorigenicity, respectively.
As shown in Table
II and Fig. 1D, as few as
10,000 cells from the SMMC-7721 CD133+ SFC were able to
grow into tumors (4/4, 100%) when subcutaneously injected into
Blab/c-nu mice, while 1×106 parental cells were needed
for tumor formation (4/4, 100%). There was also a great difference
in the time needed for tumor formation, ∼17 days for
CD133+ sphere cells compared to ∼35 days for the
parental cells (Table II). These
results demonstrate that CD133+ SFCs derived from
SMMC-7721 cell line was highly tumorigenic and characteristic of
CSC.
 | Table II.Xenotransplantation of human hepatoma
carcinoma cell line SMMC-7721 and its LCSCs into Balb/c-nu
immunodeficient mice. |
Table II.
Xenotransplantation of human hepatoma
carcinoma cell line SMMC-7721 and its LCSCs into Balb/c-nu
immunodeficient mice.
| Cell | Inoculum size | Tumor
incidencea | Latency period
(days)b |
|---|
| Parental cell |
1×104 | 0/4 | - |
|
1×105 | 1/4 | 47 |
|
1×106 | 4/4 | 35 |
| CD133+
sphere cells |
2×103 | 3/4 | 36 |
|
1×104 | 4/4 | 17 |
|
1×105 | 4/4 | 9 |
BrMC suppresses proliferation,
self-renewal and invasion of LCSCs
Previously, our laboratory reported that the BrMC
inhibits proliferation and induces apoptosis of HCC cells (39). Here, we performed MTT assay to
analyze the influence of BrMC to CD133+ SFCs that
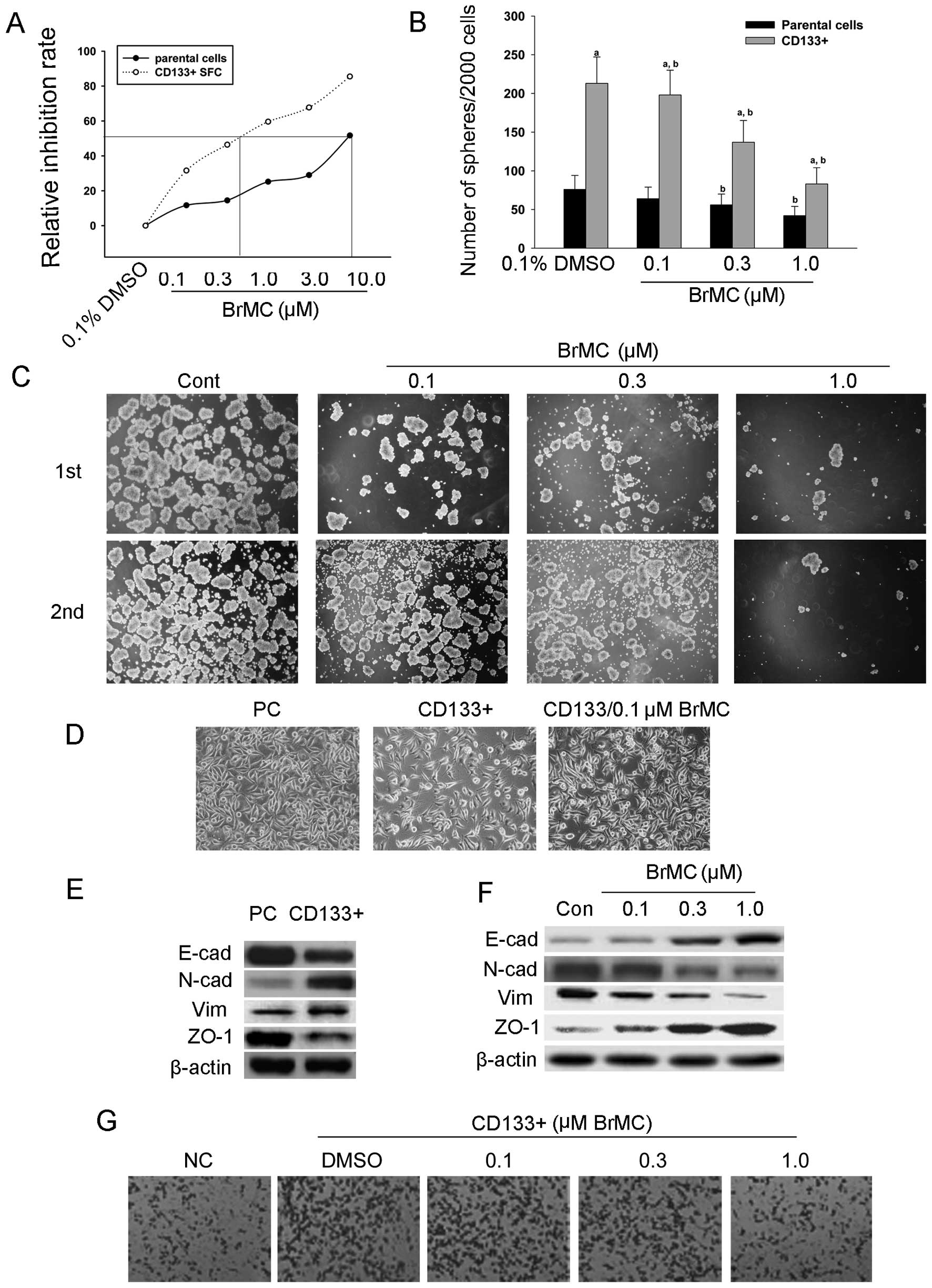
derived from the SMMC-7721 cell line. As shown in Fig. 2A, as compared to negative control
(0.1% DMSO), the IC50 of BrMC to CD133+
sphere cells is 0.5 μmol/l, which is much lower than that of
parental cells (13.1 μmol/l), suggesting that BrMC
preferentially inhibits the proliferation of CD133+
sphere cells.
In order to observe whether BrMC efficiently
inhibits the self-renewal of our sorted SMMC-7721 CD133+
sphere cells, we treated the spheres seeded in 6-well low adherence
plates with different concentrations of BrMC. Forty-eight hours
after treatment, the spheres were passaged for second sphere
formation without treatment, after 6-day culture, at which
time-point, the second spheres were counted under a microscope. As
depicted in Fig. 2B and C, BrMC
inhibited both the size and the numbers of the first or second
passaged spheres in a dose-dependent manner.
Epithelial-mesenchymal transition (EMT) is a
critical process providing tumor cells with the ability to migrate
and escape from the primary tumor and metastasize to distant sites.
Recently, EMT has been shown to be associated with the cancer stem
cell (CSC) phenotype in hepatoma cancer (2,41–43).
Based on above mentioned results, we further examined whether BrMC
affects the EMT process of CD133+ SFCs derived from the
SMMC-7721 cell line. As shown in Fig.
2D, the parental cells exhibited epithelial cell morphology.
When the SMMC-7721 cell line derived CD133+ sphere cells
were cultured in 10% FBS and allowed to adhere to plates, the cells
presented fusiform morphology, which is the mesenchymal cell
phenotype. However, after treatment with 0.1 μM BrMC, the
cell morphology tended to change to epithelial phenotype. The
experiments showed that SMMC-7721-derived CD133+ sphere
cells possess mesenchymal cell morphology and BrMC induced
mesenchymal to epithelial transformation.
We also performed western blot analysis to check the
variety of EMT biomarkers in CD133+ sphere cells,
parental cells and the cell populations treated with the indicated
concentration of BrMC (0.1, 0.3 and 1.0 μM). From Fig. 2C–F we can see that the
CD133+ sphere cells highly expressed mesenchymal cell
biomarker N-cadherin and Vimentin and epithelial biomarkers
E-cadherin and ZO-1 at low levels. While the treatment of BrMC
leads to downregulation of N-cadherin and Vimentin and upregulation
of E-cadherin and ZO-1 in CD133+ sphere cells. Together,
our data suggest that BrMC can effectively inhibit EMT in
LCSCs.
We next performed a transwell assay to demonstrate
whether BrMC affects invasion of LCSC. As described in Materials
and methods, a total of 2,000 parental or CD133+ cells
derived from SMMC-7721 cells were treated with BrMC (0.1, 0.3, 1.0
and 3.0 μmol/l) for 24 h and then plated in the top chamber
of the transwell, cells that invaded the lower chamber were
counted. As shown in Fig. 2G,
sorted CD133+ sphere cells were statistically
significantly more invasive than parental SMMC-7721 cells. BrMC
inhibited the invasion of CD133+ sphere cells in a
dose-dependent manner.
BrMC downregulates the expression of LCSC
biomarkers CD133 and CD44
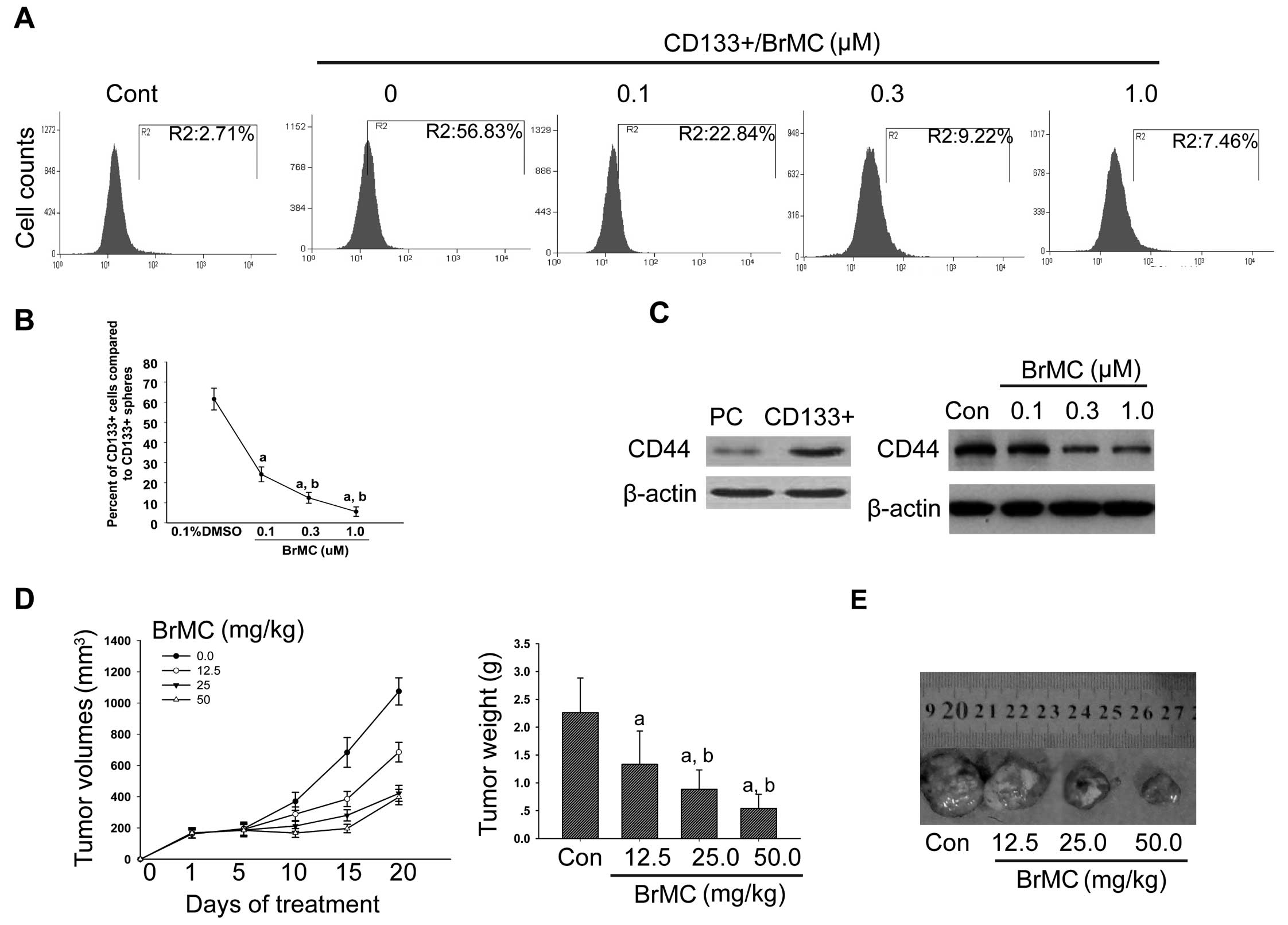
In our study, we have shown that the
CD133+ sphere cells derived from SMMC-7721 cell line had
the property of self-renewal, EMT and were highly tumorigenic. To
better understand the influence of BrMC on the biomarkers of LCSCs,
we performed FACS and western blotting to analyze the expression of
CD133 and CD44. The results provided evidence that in
CD133+ sphere cells derived from SMMC-7721 cell line,
BrMC downregulated the expression of CD133 (Fig. 3A and B) and CD44 in a
dose-dependent manner (Fig.
3C).
BrMC suppresses tumor growth in vivo
To determine whether BrMC targeted the inhibition of
growth of LCSCs in vivo, we transplanted human
SMMC-7721-originated CD133+ spheres subcutaneously to
Balb/c-nu mice for a xenograft nude mouse model. Two weeks after
transplantation, mice were randomized to four groups and received
daily gavage of indicated different dosage of BrMC (0, 12.5, 25 and
50 mg/kg). After treatment of 20 days, the volume of tumors that
were treated by high doses for 25 and 50 mg/kg reduced to half size
of that of model controls (Fig.
3D). To further confirm this result, we dissected these tumors
in mice and re-seeded them subcutaneously to different nude mice.
In order to avoid the possible changes caused by heterogeneity, we
seeded 50,000 tumor cells from the model control in the side of
forelimb, seeded another 50,000 tumor cells from xenograft nude
mice that were treated by high dose of BrMC (50 mg/kg) into the
other side of forelimb. Interestingly, we can see from Table III, the tumor cells from the
control group grew very fast and the final volume reached 567–686
mm3. However, the tumor cells originated from xenograft
nude mice that were treated with BrMC did not grow until day 33
after transplantation. Among the 12 mice that received the BrMC
treated tumor cells, only one mouse formed a small tumor (37
mm3). In the control group, however, as early as the
sixth day of tumor cell injection, the tumors emerged and all
tumors appeared on the 15th day. These results suggest that BrMC is
able to eliminate LCSCs in the initial transplanted tumors, thereby
inhibiting tumor re-growth of the secondary inoculated mice,
hinting that BrMC could eradicate LCSCs in vivo.
 | Table III.Effects of BrMC on the secondary
tumor formation ability in BALB/c nu mice for SMMC-7721 derived
CD133+ SFCs. |
Table III.
Effects of BrMC on the secondary
tumor formation ability in BALB/c nu mice for SMMC-7721 derived
CD133+ SFCs.
| Secondary tumor
incidencea | Secondary tumor
volumes (mm3) |
|---|
|
|
|---|
| Days | Con | BrMC | Con | BrMC |
|---|
| 1 | 0/12 | 0/12 | - | - |
| 3 | 0/12 | 0/12 | - | - |
| 6 | 3/12 | 0/12 | 19±2.7 | - |
| 12 | 8/12 | 0/12 | 92±27.6 | - |
| 15 | 12/12 | 1/12 | 258±89.1 | 14 |
| 18 | 12/12 | 1/12 | 599±152 | 18 |
| 21 | 8/8 | 1/8 | 394±64 | 29 |
| 24 | 8/8 | 1/8 | 638±168 | 37 |
| 27 | 4/4 | 0/4 | 227±82 | - |
| 30 | 4/4 | 0/4 | 493±91 | - |
| 33 | 4/4 | 0/4 | 686±187 | - |
BrMC downregulates the expression of
Twist and β-catenin in LCSCs
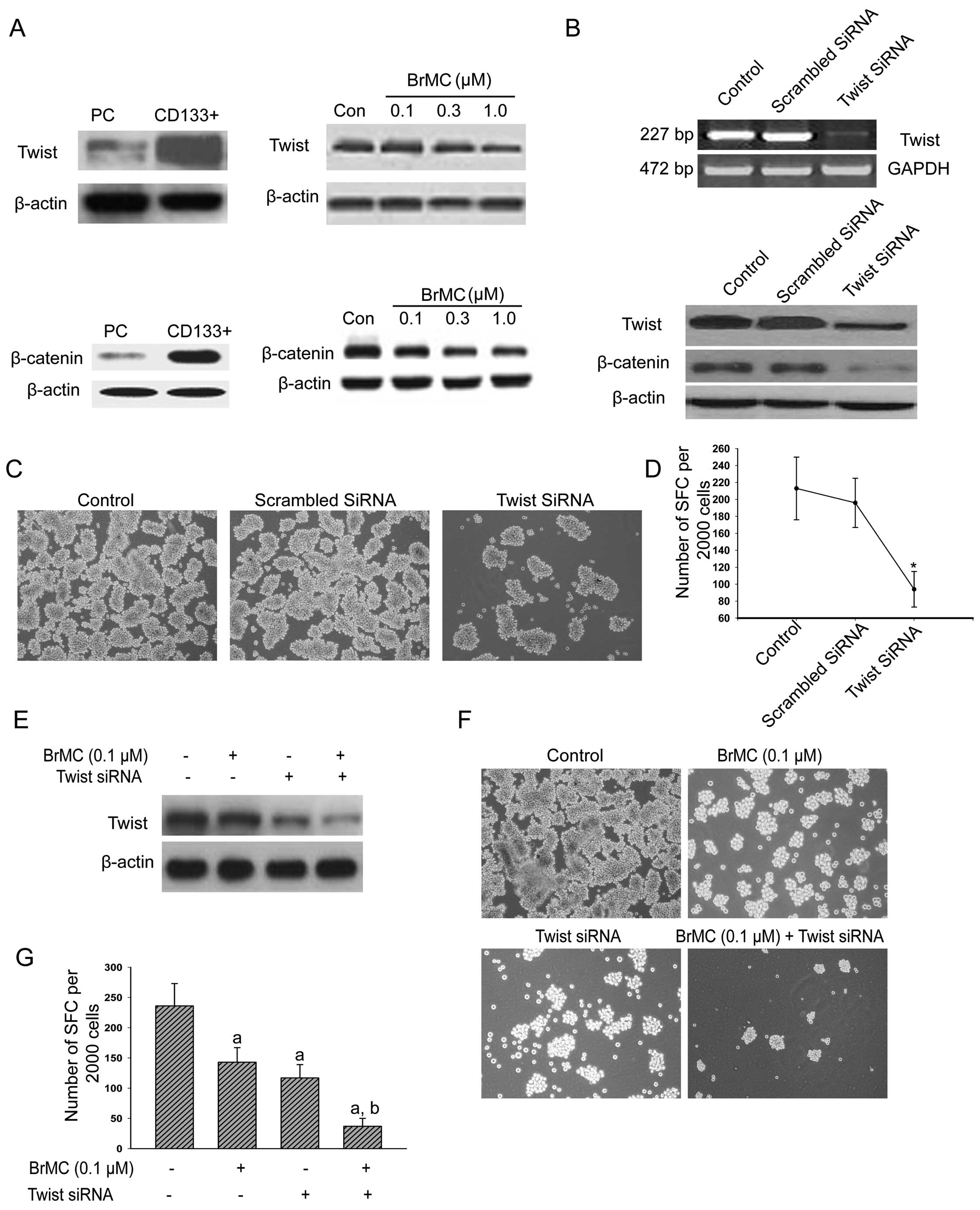
Transcription factor Twist and β-catenin were proved
to be the critical epithelial-mesenchymal transitional molecules
(44–47). In cancer cells, the nuclear
translocation of CD44 causes stimulation of Twist transcription,
which mediates the MSC-triggered epithelial-to-mesenchymal
transition (EMT) of carcinoma cells (44). Based on the results that BrMC
downregulates the expression of CD133 and CD44 and inhibits EMT in
LCSCs, we further investigated the effects of BrMC on the
expression of Twist and β-catenin, as reported by other groups
(45,48,49),
Twist and β-catenin were highly expressed in our sorted
CD133+ sphere cells (Fig.
4A). Western blot analysis indicated that the protein levels
were downregulated after these CD133+ SFCs were treated
by the indicated concentration of BrMC (0.1, 0.3 and 1.0 μM)
(Fig. 4A).
Synergistic inhibition of self-renewal of
LCSCs by BrMC and Twist silencing
To further explore the biological functions of Twist
in CD133+ SFCs and the maintenance of LCSC
characteristics, we silenced the expression of Twist by RNA
interference in CD133+ SFCs of the SMMC-7721 cells. The
mRNA levels and protein expression of Twist decreased significantly
after transfection with Twist siRNA (Fig. 4B). Interestingly, as the protein
levels of Twist decreased, the β-catenin expression was reduced
(Fig. 4B). Furthermore, the
decreased expression of Twist also downregulated the sphere
formation capacity of CD133+ SFCs of SMMC-7721 cell line
(Fig. 4C and D). As BrMC inhibits
proliferation and self-renewal of CD133+ sphere-forming
cells derived from the SMMC-7721 cell line, to confirm the results,
we treated CD133+ SFCs of the SMMC-7721 cells that were
transfected with Twist siRNA with 0.1 μM of BrMC again.
Fig. 4E shows the cells that were
transfected with Twist siRNA, the addition of BrMC further
decreased the protein levels of Twist and the sphere-forming
capacity in this group also reduced more by the co-treatment with
Twist siRNA and 0.1 μM of BrMC (Fig. 4F and G), indicating synergistic
inhibition of self-renewal of LCSCs.
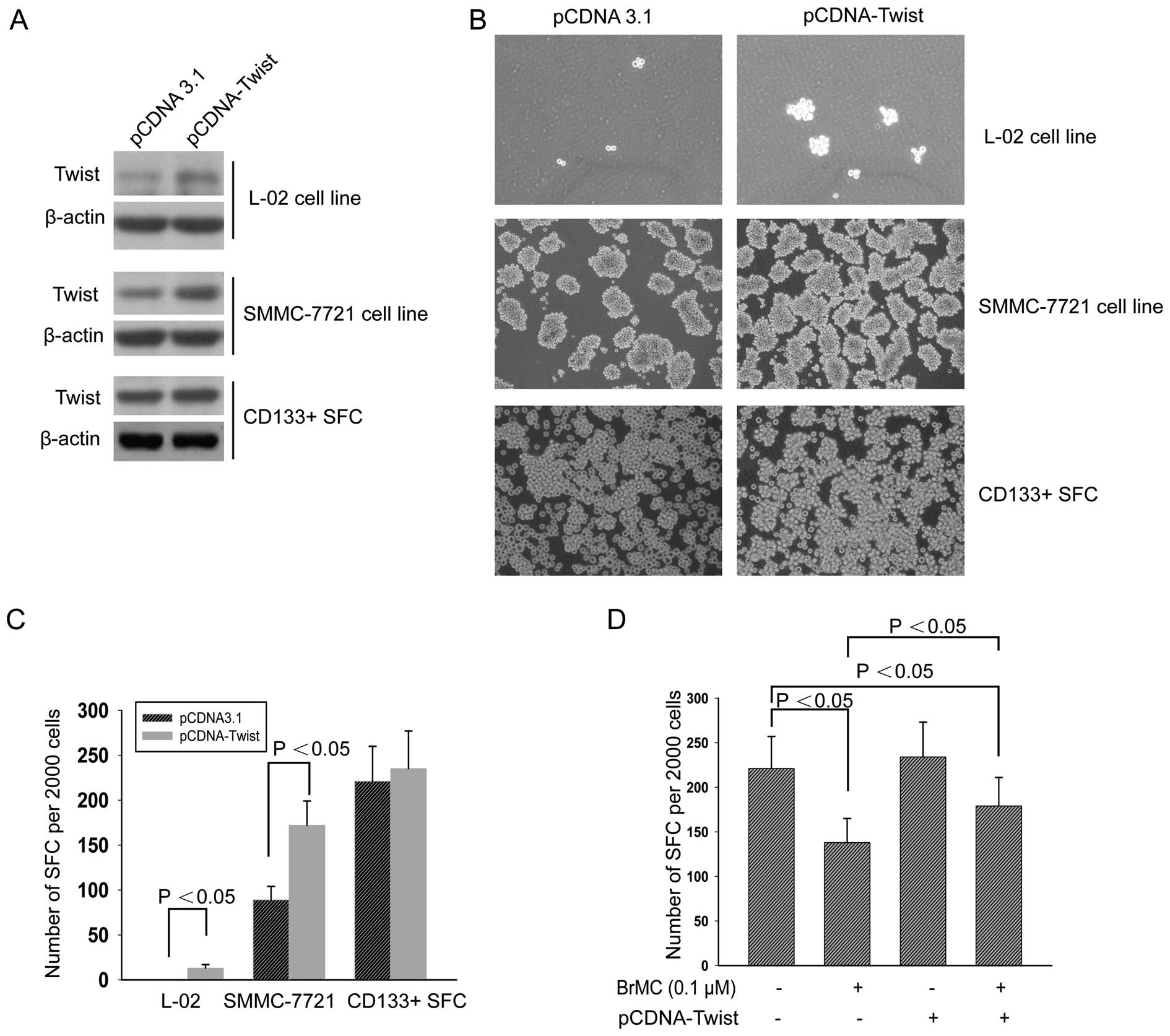
Overexpression of Twist attenuates
inhibition of LCSC self-renewal by BrMC
To corroborate whether Twist rescued BrMC, we
transfected the plasmid pcDNA-Twist and negative control pcDNA3.1
to the human embryonic liver cell line L-02, HCC cell line
SMMC-7721 and CD133+ sphere cells derived from SMMC-7721
cells, respectively. As shown in Fig.
5A, compared to negative control vector pcDNA3.1, the three
cell lines significantly overexpressed Twist. Tumor sphere forming
experiment showed that the ectopic expression of Twist in these
cell lines promoted the tumor sphere formation (Fig. 5B and C). We also treated
CD133+ SFCs of SMMC-7721 cells that were transfected
with pcDNA-Twist followed by treatment with 0.1 μM of BrMC.
After addition of BrMC, the number of spheres formed in this group
was relatively more than that of the control groups (Fig. 5D). These results suggest that Twist
overexpression could partly reduce the inhibitory effect of BrMC on
self-renewal of LCSCs.
Discussion
Cancer stem cell research is becoming a growing and
exciting field. In fact, it appears that most cancer types contain
populations of cells that exhibit stem-cell properties. CSCs have
the ability to renew indefinitely, which can drive tumor
development and metastatic invasion. As these cells are classically
resistant to conventional chemotherapy and to radiation therapy,
they may contribute to treatment failure and relapse. Most cancer
research experts focused on isolation and targeted killing of CSCs
and the development of novel strategies for antitumor therapy
relies on the use of biomarkers to identify, enrich and/or isolate
the cell population(s) of interest. Magnetic activated cell sorting
(MACS) is one of the specific methods to separate CSCs, which are
based on cell surface markers. The proposed markers for liver CSCs
include CD133, CD90, CD44, CD13, EpCAM and OV6, on the basis of the
hypothesis that CSCs are originated from somatic stem cells and
accordingly express the same surface markers (50,51).
Prominin-1 (CD133) is generally regarded as one of the most
important molecular markers for stem cells, cancer stem and
stem-like cells in tumors originating from colon cancer (52), glioblastoma multiforme (GBM) cell
line (53), HCC (12,54),
pancreatic cancer (55), gastric
cancer (56), and lung cancer
(57) have been reported.
Although the cell surface expression of the human
CD133 antigen, in particular of the AC133 epitope, is among those
that have been most frequently studied in solid cancers, no
mechanism has yet been proposed to link CD133 expression with the
CSC phenotype (58). In our study,
we quantified percentage of the isolated CD133+ cells
from the SMMC-7721 cell line by flow cytometry. There was no
detectable CD133+ cell in the CD133−
population (1.07%), while the percentage of CD133+
population was ∼58.72%, indicating that CD133 is highly expressed
in LCSCs derived from SMMC-7721, which was in agreement with the
study by Yin et al, who also performed flow cytometry, for
purity, before and after MACS sorting from SMMC-7721 cell line and
the CD133+ groups ranged from 60.2 to 91.2% compared to
non-sorted SMMC-7721 cells (0.1–2%) (3,59).
Sphere formation experiment indicates high levels of
CD133 were associated with increased spheroid forming capacity both
for the first passage and for the second passage in vitro.
Next, the ability of tumorigenicity was measured in BALB/c nu mice
to verify CD133+ sphere cells derived from SMMC-7721 is
LCSCs. As expected, CD133+ sphere-forming cell
population exhibited stronger tumorigenicity than others, even
10,000 cells were enough to form tumors, showing slight difference
compared to results by De Hert et al (60), who used as few as 500 cells from
the PLC/PRF/5 spheres to form a tumor when subcutaneously injected
into NOD/SCID mice, while 2×105 parental cells were
needed.
During EMT, epithelial cells lose their
characteristics and gain mesenchymal features. It has been
suggested that transformed epithelial cells can activate embryonic
programs of epithelial plasticity and switch from a sessile,
epithelial phenotype to a motile, mesenchymal phenotype. Induction
of EMT can, therefore, lead to invasion of surrounding stroma,
intravasation, dissemination and colonization of distant sites.
According to the cancer stem cell hypothesis, sustained metastatic
growth requires the dissemination of a CSC from the primary tumor
followed by its re-establishment in a secondary site. SNAI, ZEB and
TWIST family members repress the CDH1 gene to induce EMT, but also
regulate the transcription of other target genes. TWIST1 is
upregulated in human breast cancer, gastric cancer, esophageal
cancer and prostate cancer (61).
The activation of Twist caused translocation of β-catenin into the
nucleus and elevated Wnt/β-catenin signaling promotes EMT
transition (62,63). In this study, the knockdown of
Twist leads to reduced β-catenin expression (Fig. 4B), hinting that β-catenin is the
downstream target gene of Twist, which is in agreement with a
previous report that activation of β-catenin pathway by Twist is
critical for the maintenance of EMT associated CSC-like
characteristics (64).
BrMC is a novel ChR analogue synthesized by our
laboratory. We previously showed that the effect of BrMC on the
inhibition of proliferation and induction of apoptosis in the colon
cancer cell line HT-29, breast cancer and in the gastric cancer
cell line SGC-7901 was stronger than that of ChR (37,65,66).
BrMC has been shown to induce apoptosis of HCC cell line in a
dose-dependent manner, but have little effect on human embryo liver
L-02 cells. In addition, BrMC also inhibits self-renewal of glioma
stem-like cells (GSLCs) (39,40).
In this study, we found that BrMC inhibited formation of primary
and secondary spheroids in suspension and cell viability in those
spheroids, inhibited self-renewal, EMT, cell invasion of
CD133+ sphere-forming cells from SMMC-7721 cell line
in vitro. Furthermore, BrMC suppressed tumorigenicity in
BALB/c nu mouse xenograft model. As the molecular mechanism, BrMC
dose-dependently inhibited the expression of CD133 and CD44, which
was related to LCSC characteristics and also reduced protein levels
of EMT-associated crucial protein Twist and β-catenin.
In conclusion, we present supportive evidence for
the first time that BrMC, a novel synthetic chrysin analogue, was
able to target LCSCs both in vitro and in vivo.
Furthermore, our study identified the blockage of Twist signaling
pathway by BrMC as one of the possible mechanisms for this
efficacy. This study supports the use of BrMC for HCC
chemoprevention or chemotherapy.
Acknowledgements
This study was supported in part by
National Natural Science Foundation of China (General Program, no.
81172375), by program for excellent talents in Hunan Normal
University (no. ET13107), by the Construct Program of the Key
Discipline of Basic Medicine in Hunan Province and Research Fund
for the Doctoral Program of Hunan Normal University (no.
110656).
References
|
1.
|
el-Serag HB: Epidemiology of
hepatocellular carcinoma. Clin Liver Dis. 5:87–107. 2001.
View Article : Google Scholar
|
|
2.
|
Stebbing J, Filipovic A and Giamas G:
Claudin-1 as a promoter of EMT in hepatocellular carcinoma.
Oncogene. doi:10.1038. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Wang F, He L, Dai WQ, et al: Salinomycin
inhibits proliferation and induces apoptosis of human
hepatocellular carcinoma cells in vitro and in vivo. PLoS One.
7:e506382012. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Wang Y, Liu YH, Jiang JS and Cui HB: A new
method for purification and identification of hepatocellular
carcinoma stem cell of SMMC-7721. Zhonghua Yi Xue Za Zhi.
92:3434–3437. 2012.(In Chinese).
|
|
5.
|
Keung EZ, Nelson PJ and Conrad C: Concise
review: genetically engineered stem cell therapy targeting
angiogenesis and tumor stroma in gastrointestinal malignancy. Stem
Cells. 31:227–235. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Vu NB, Nguyen TT, Tran LC, et al:
Doxorubicin and 5-fluorouracil resistant hepatic cancer cells
demonstrate stem-like properties. Cytotechnology. 65:491–503.
2012.PubMed/NCBI
|
|
7.
|
Song K, Wu J and Jiang C: Dysregulation of
signaling pathways and putative biomarkers in liver cancer stem
cells (Review). Oncol Rep. 29:3–12. 2013.PubMed/NCBI
|
|
8.
|
Lapidot T, Sirard C, Vormoor J, et al: A
cell initiating human acute myeloid leukaemia after transplantation
into SCID mice. Nature. 367:645–648. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Yin AH, Miraglia S, Zanjani ED, et al:
AC133, a novel marker for human hematopoietic stem and progenitor
cells. Blood. 90:5002–5012. 1997.PubMed/NCBI
|
|
10.
|
Miraglia S, Godfrey W, Yin AH, et al: A
novel five-transmembrane hematopoietic stem cell antigen:
isolation, characterization and molecular cloning. Blood.
90:5013–5021. 1997.PubMed/NCBI
|
|
11.
|
de Antonellis P, Liguori L, Falanga A, et
al: MicroRNA 199b-5p delivery through stable nucleic acid lipid
particles (SNALPs) in tumorigenic cell lines. Naunyn Schmiedebergs
Arch Pharmacol. 386:287–302. 2013.PubMed/NCBI
|
|
12.
|
Ma S: Biology and clinical implications of
CD133(+) liver cancer stem cells. Exp Cell Res. 319:126–132.
2013.
|
|
13.
|
Chen Y, Yu D, Zhang H, et al:
CD133(+)EpCAM(+) phenotype possesses more characteristics of tumor
initiating cells in hepatocellular carcinoma Huh7 cells. Int J Biol
Sci. 8:992–1004. 2012.
|
|
14.
|
Nagano H, Ishii H, Marubashi S, et al:
Novel therapeutic target for cancer stem cells in hepatocellular
carcinoma. J Hepatobiliary Pancreat Sci. 19:600–605. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Pang RW and Poon RT: Cancer stem cell as a
potential therapeutic target in hepatocellular carcinoma. Curr
Cancer Drug Targets. 12:1081–1094. 2012.PubMed/NCBI
|
|
16.
|
Na DC, Lee JE, Yoo JE, Oh BK, Choi GH and
Park YN: Invasion and EMT-associated genes are up-regulated in B
viral hepatocellular carcinoma with high expression of CD133-human
and cell culture study. Exp Mol Pathol. 90:66–73. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Thisse B, Stoetzel C, Gorostiza-Thisse C
and Perrin-Schmitt F: Sequence of the twist gene and nuclear
localization of its protein in endomesodermal cells of early
Drosophila embryos. EMBO J. 7:2175–2183. 1988.PubMed/NCBI
|
|
18.
|
Murre C, McCaw PS, Vaessin H, et al:
Interactions between heterologous helix-loop-helix proteins
generate complexes that bind specifically to a common DNA sequence.
Cell. 58:537–544. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Jan YN and Jan LY: HLH proteins, fly
neurogenesis and vertebrate myogenesis. Cell. 75:827–830. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Kadesch T: Consequences of heteromeric
interactions among helix-loop-helix proteins. Cell Growth Differ.
4:49–55. 1993.PubMed/NCBI
|
|
21.
|
Yang J, Mani SA, Donaher JL, et al: Twist,
a master regulator of morphogenesis, plays an essential role in
tumor metastasis. Cell. 117:927–939. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Kwok WK, Ling MT, Lee TW, et al:
Up-regulation of TWIST in prostate cancer and its implication as a
therapeutic target. Cancer Res. 65:5153–5162. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Fu J, Qin L, He T, et al: The
TWIST/Mi2/NuRD protein complex and its essential role in cancer
metastasis. Cell Res. 21:275–289. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Lee TK, Poon RT, Yuen AP, et al: Twist
overexpression correlates with hepatocellular carcinoma metastasis
through induction of epithelial-mesenchymal transition. Clin Cancer
Res. 12:5369–5376. 2006. View Article : Google Scholar
|
|
25.
|
Devereux TR, Stern MC, Flake GP, et al:
CTNNB1 mutations and beta-catenin protein accumulation in human
hepatocellular carcinomas associated with high exposure to
aflatoxin B1. Mol Carcinog. 31:68–73. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
26.
|
Hsu HC, Jeng YM, Mao TL, Chu JS, Lai PL
and Peng SY: Beta-catenin mutations are associated with a subset of
low-stage hepatocellular carcinoma negative for hepatitis B virus
and with favorable prognosis. Am J Pathol. 157:763–770. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
27.
|
Wong CM, Fan ST and Ng IO: beta-catenin
mutation and over-expression in hepatocellular carcinoma:
clinicopathologic and prognostic significance. Cancer. 92:136–145.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
28.
|
Zhang T, Chen X, Qu L, Wu J, Cui R and
Zhao Y: Chrysin and its phosphate ester inhibit cell proliferation
and induce apoptosis in HeLa cells. Bioorg Med Chem. 12:6097–6105.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
29.
|
Woo KJ, Jeong YJ, Park JW and Kwon TK:
Chrysin-induced apoptosis is mediated through caspase activation
and Akt inactivation in U937 leukemia cells. Biochem Biophys Res
Commun. 325:1215–1222. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
30.
|
Lee SJ, Yoon JH and Song KS: Chrysin
inhibited stem cell factor (SCF)/c-Kit complex-induced cell
proliferation in human myeloid leukemia cells. Biochem Pharmacol.
74:215–225. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
31.
|
Ramos AM and Aller P: Quercetin decreases
intracellular GSH content and potentiates the apoptotic action of
the antileukemic drug arsenic trioxide in human leukemia cell
lines. Biochem Pharmacol. 75:1912–1923. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
32.
|
Wang W, VanAlstyne PC, Irons KA, Chen S,
Stewart JW and Birt DF: Individual and interactive effects of
apigenin analogs on G2/M cell-cycle arrest in human colon carcinoma
cell lines. Nutr Cancer. 48:106–114. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
33.
|
Zhang Q, Zhao XH and Wang ZJ: Flavones and
flavonols exert cytotoxic effects on a human oesophageal
adenocarcinoma cell line (OE33) by causing G2/M arrest and inducing
apoptosis. Food Chem Toxicol. 46:2042–2053. 2008. View Article : Google Scholar
|
|
34.
|
Kachadourian R, Leitner HM and Day BJ:
Selected flavonoids potentiate the toxicity of cisplatin in human
lung adenocarcinoma cells: a role for glutathione depletion. Int J
Oncol. 31:161–168. 2007.PubMed/NCBI
|
|
35.
|
Walle T, Otake Y, Brubaker JA, Walle UK
and Halushka PV: Disposition and metabolism of the flavonoid
chrysin in normal volunteers. Br J Clin Pharmacol. 51:143–146.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
36.
|
Park H, Dao TT and Kim HP: Synthesis and
inhibition of PGE2 production of 6,8-disubstituted chrysin
derivatives. Eur J Med Chem. 40:943–948. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
37.
|
Zheng X, Meng WD, Xu YY, Cao JG and Qing
FL: Synthesis and anticancer effect of chrysin derivatives. Bioorg
Med Chem Lett. 13:881–884. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
38.
|
Ai XH, Zheng X, Tang XQ, et al: Induction
of apoptosis of human gastric carcinoma SGC-7901 cell line by 5,
7-dihydroxy-8-nitrochrysin in vitro. World J Gastroenterol.
13:3824–3828. 2007.PubMed/NCBI
|
|
39.
|
Yang XH, Zheng X, Cao JG, Xiang HL, Liu F
and Lv Y: 8-Bromo-7-methoxychrysin-induced apoptosis of
hepatocellular carcinoma cells involves ROS and JNK. World J
Gastroenterol. 16:3385–3393. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
40.
|
Feng X, Zhou Q, Liu C and Tao ML: Drug
screening study using glioma stem-like cells. Mol Med Rep.
6:1117–1120. 2012.PubMed/NCBI
|
|
41.
|
Li Q, Gu X, Weng H, et al: Bone
morphogenetic protein-9 (BMP-9) induces epithelial to mesenchymal
transition (EMT) in hepatocellular carcinoma cells. Cancer Sci.
104:398–408. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
42.
|
Liu FY, Deng YL, Li Y, et al:
Down-regulated KLF17 expression is associated with tumor invasion
and poor prognosis in hepatocellular carcinoma. Med Oncol.
30:4252013. View Article : Google Scholar : PubMed/NCBI
|
|
43.
|
Tanaka S, Shiraha H, Nakanishi Y, et al:
Runt-related transcription factor 3 reverses epithelial-mesenchymal
transition in hepatocellular carcinoma. Int J Cancer.
131:2537–2546. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
44.
|
El-Haibi CP, Bell GW, Zhang J, et al:
Critical role for lysyl oxidase in mesenchymal stem cell-driven
breast cancer malignancy. Proc Natl Acad Sci USA. 109:17460–17465.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
45.
|
Singh N, Liu G and Chakrabarty S:
Isolation and characterization of calcium sensing receptor null
cells: a highly malignant and drug resistant phenotype of colon
cancer. Int J Cancer. 132:1996–2005. 2013. View Article : Google Scholar
|
|
46.
|
Yang MH, Chen CL, Chau GY, et al:
Comprehensive analysis of the independent effect of twist and snail
in promoting metastasis of hepatocellular carcinoma. Hepatology.
50:1464–1474. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
47.
|
Barr MP, Gray SG, Hoffmann AC, et al:
Generation and characterisation of cisplatin-resistant non-small
cell lung cancer cell lines displaying a stem-like signature. PLoS
One. 8:e541932013. View Article : Google Scholar : PubMed/NCBI
|
|
48.
|
Meng F, Glaser SS, Francis H, et al:
Functional analysis of microRNAs in human hepatocellular cancer
stem cells. J Cell Mol Med. 16:160–173. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
49.
|
Ji J and Wang XW: Clinical implications of
cancer stem cell biology in hepatocellular carcinoma. Semin Oncol.
39:461–472. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
50.
|
Pardal R, Clarke MF and Morrison SJ:
Applying the principles of stem-cell biology to cancer. Nat Rev
Cancer. 3:895–902. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
51.
|
Marx J: Cancer research. Mutant stem cells
may seed cancer. Science. 301:1308–1310. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
52.
|
Swindall AF, Londono-Joshi AI, Schultz MJ,
Fineberg N, Buchsbaum DJ and Bellis SL: ST6Gal-I protein expression
is upregulated in human epithelial tumors and correlates with stem
cell markers in normal tissues and colon cancer cell lines. Cancer
Res. 73:2368–2378. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
53.
|
Lehnus KS, Donovan LK, Huang X, et al:
CD133 glycosylation is enhanced by hypoxia in cultured glioma stem
cells. Int J Oncol. 42:1011–1017. 2013.PubMed/NCBI
|
|
54.
|
Zeng Z, Ren J, O’Neil M, et al: Impact of
stem cell marker expression on recurrence of TACE-treated
hepatocellular carcinoma post liver transplantation. BMC Cancer.
12:5842012. View Article : Google Scholar : PubMed/NCBI
|
|
55.
|
Hori Y: Prominin-1 (CD133) reveals new
faces of pancreatic progenitor cells and cancer stem cells: current
knowledge and therapeutic rerspectives. Adv Exp Med Biol.
777:185–196. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
56.
|
Lee HH, Seo KJ, An CH, Kim JS and Jeon HM:
CD133 expression is correlated with chemoresistance and early
recurrence of gastric cancer. J Surg Oncol. 106:999–1004. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
57.
|
Yi H, Cho HJ, Cho SM, et al: Blockade of
interleukin-6 receptor suppresses the proliferation of H460 lung
cancer stem cells. Int J Oncol. 41:310–316. 2012.PubMed/NCBI
|
|
58.
|
Grosse-Gehling P, Fargeas CA, Dittfeld C,
et al: CD133 as a biomarker for putative cancer stem cells in solid
tumours: limitations, problems and challenges. J Pathol.
229:355–378. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
59.
|
Yin S, Li J, Hu C, et al: CD133 positive
hepatocellular carcinoma cells possess high capacity for
tumorigenicity. Int J Cancer. 120:1444–1450. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
60.
|
De Hert M, Dockx L, Bernagie C, et al:
Prevalence and severity of antipsychotic related constipation in
patients with schizophrenia: a retrospective descriptive study. BMC
Gastroenterol. 11:172011.PubMed/NCBI
|
|
61.
|
Qu F, Zhai W, Chen H, Zhu LH and Morris
TJ: Cloning, characterization and transient expression of the gene
encoding a rice U3 small nuclear RNA. Gene. 172:217–220. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
62.
|
Wu Y, Ginther C, Kim J, et al: Expression
of Wnt3 activates Wnt/β-catenin pathway and promotes EMT-like
phenotype in trastuzumab-resistant HER2-overexpressing breast
cancer cells. Mol Cancer Res. 10:1597–1606. 2012.
|
|
63.
|
Grosse-Steffen T, Giese T, Giese N, et al:
Epithelial-to-mesenchymal transition in pancreatic ductal
adenocarcinoma and pancreatic tumor cell lines: the role of
neutrophils and neutrophil-derived elastase. Clin Dev Immunol.
2012:720–768. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
64.
|
Li J and Zhou BP: Activation of
beta-catenin and Akt pathways by Twist are critical for the
maintenance of EMT associated cancer stem cell-like characters. BMC
Cancer. 11:492011. View Article : Google Scholar : PubMed/NCBI
|
|
65.
|
Xiang HL, Zheng X and Cao JG: Induction of
apoptosis of human gastric carcinoma SGC-790 cell line by
8-bromo-7-mehoxychrysin. Zhongguo Yaolixue Tongbao. 24:1370–1372.
2008.
|
|
66.
|
Zhao XC, Tian L, Cao JG and Liu F:
Induction of apoptosis by 5,7-dihydroxy-8-nitrochrysin in breast
cancer cells: the role of reactive oxygen species and Akt. Int J
Oncol. 37:1345–1352. 2010.PubMed/NCBI
|