Introduction
Gastric cancer is the fourth most common cancer
worldwide and is the second most common cause of cancer-related
deaths. Gastric cancer has a poor prognosis (1). Although the 5-year survival rate in
patients with early-stage disease is ∼90%, since the vast majority
present with distant metastasis, the overall 5-year survival rate
is typically <20% (2). The
5-year survival rate has also been significantly correlated with
the degree of tumor invasion, the presence of lymph node and/or
distant metastases and the TNM stage (3,4).
However, very limited number of molecules that have
clinicopathological significance in gastric cancer has been
discovered.
Claudin 1 (CLDN1) is one of the integral membrane
proteins that constitute tight junctions. Tight junctions are
essential for the tight sealing of cellular sheets and maintaining
homeostasis (5). Absence of tight
junctions or defective tight junctions is associated with the
development of the neoplastic phenotype in epithelial cells
(6). Thus it is accepted that the
disruption of tight junctions leads to loss of cohesion,
invasiveness and the lack of differentiation, thereby promoting
tumorigenesis. CLDN1 is found to regulate intestinal epithelial
homeostasis through the modulation of Notch-signaling (7). Many chemicals and nutrition can
regulate CLDN1 expression through different pathways (8,9) to
maintain the integrity of intestinal barrier function.
The complexity of CLDN1 was proposed because its
dual role as a tumor suppressor and promoter, as well as a positive
and negative prognostic factor in different cancers, including
breast (10,11), gastric (12), ovarian (13), lung (14) and colon (15–17).
Decreased expression of CLDN1 is correlated with recurrence status
in breast cancer (18). Similarly,
studies on lung cancer also revealed CLDN1 expression is correlated
with better survival (14,19). Opposite findings suggest that CLDN1
is a negative prognostic factor. Alteration of CLDN expression may
affect permeability at tight junction, possibly increasing the
diffusion of nutrients and other extracellular growth factors to
promote cancer cell growth, survival and motility in gastric cancer
(20). Claudin 1 induces
epithelial-mesenchymal transition through activation of the
c-Abl-ERK signaling pathway (21).
CLDN1 is involved in many cellular functions
including cell proliferation, survival and metastasis. CLDN1
knockdown in breast cancer cells significantly decreased cell
migration and the expression EMT markers (11). CLDN1 expression was correlated to
anoikis resistance in colon cancer (22). CLDN1 upregulates the repressor
ZEB-1 to reduce expression of E-cadherin in colon cancer cells,
increasing their invasive activity and reducing anoikis (23). CLDN1 mediates TNFα-induced gene
expression and cell migration in human lung carcinoma cells
(24). The CLDN1 expression was
correlated with subtypes in breast (25,26)
and lung (27) cancer.
Overexpression of CLDN1 in lung cancer cells inhibited cancer cell
dissociation, and suppressed cancer cell migration, invasion and
metastasis (14). CLDN1 expression
can be regulated by many other transcriptional factors or signaling
pathways. CLDN1 is involved in the β-catenin-T-cell factor/lymphoid
enhancing factor (TCF/LEF) signaling pathway (28). More recently, the expression of
CLDN1 was found to be transcriptionally regulated by Cdx1, -2 and
GATA4 (29). CLDN1 was regulated
by Smad4 in a TGFβ signaling independent manner (30). Cdx2 can cooperate with Wnt pathway
to regulate CLDN1 expression in colon cancer cells (29). The CLDN1 mRNA stability can also be
regulated HDAC-dependently (31,32).
To our knowledge, the immuno-profiles of CLDN1 and
its association with β-catenin have not previously been reported in
gastric cancer. Herein, we examined CLDN1 expression and
correlations with clinicopathological characteristics, as well as
its association with β-catenin in gastric cancer.
Materials and methods
Immunohistochemistry
Gastric cancer tissues, confirmed by pathological
diagnosis, were obtained from 173 patients who underwent radical
resection for gastric cancer at the Department of Surgery, Ruijin
Hospital, Shanghai, China. The corresponding non-tumor gastric
tissue was obtained at least 6 cm from the tumor. All tissue
samples were formalin-fixed and paraffin-embedded. TNM staging was
classified based on the criteria of American Joint Committee on
Cancer (AJCC, 7th edition) for gastric cancer. The study was
approved by the Shanghai Jiaotong University Medical School
institutional review board.
Immunohistochemistry staining was performed by using
a highly sensitive streptavidin-biotin-peroxidase detection system
with gastric cancer tissue microarrays. Rabbit monoclonal
anti-CLDN1 (working dilution 1:100) was purchased from Zymed
(Invitrogen, USA) and rabbit anti-β-catenin (working dilution
1:100) was purchased from Cell Signaling (Danvers, MA, USA).
Immunolabeling was conducted using Dako Envision + Rabbit Polymer
(catalog no. K4003) from Dako (Carpinteria, CA, USA). The slides
were counterstained with hematoxylin and coverslipped.
Immunohistochemistry scoring
The histology of the samples was examined by two
histopathologists independently without knowing the
clinicopathologic information. We scored the slides as previously
described (33). The percentage of
positive tumor cells was assigned to 5 categories: ≤5% (0); 5–25%
(1); 25–50% (2); 50–75% (3); and ≥75% (4). ≤5% positive cells were used as the
cutoff to define negative tumors. The intensity of immunostaining
was scored as: weak (1); moderate
(2); and strong (3). The percentage of positivity of tumor
cells and staining intensity were multiplied to produce a weighted
score for each tumor specimen. The intensity scores were grouped as
low (including scores 0 to +4) and high (including scores +6 to
+12).
Cell culture
Human gastric cancer cell lines SGC-7901, MKN-28,
MKN-45, BGC-823 and immortalized human gastric epithelial cell line
GES-1 were obtained from Shanghai Institute of Cell Biology,
Chinese Academy of Sciences. KATOIII, SNU-1, NCI-N87, HS-746T and
AGS were obtained from American Type Culture Collection. The cells
were grown in RPMI-1640 medium containing 10% fetal bovine serum
(FBS), penicillin and streptomycin (Gibco BRL, Gaithersburgh, MD,
USA).
Western blotting
Whole cell lysates were harvested using RIPA cell
lysis buffer supplemented with a protease inhibitor cocktail
(Sigma-Aldrich, USA). Protein (50 μg) was separated by SDS
polyacrylamide gel electrophoresis and blotted onto 0.22-μm
polyvinylidene difluoride membranes (Millipore, MA, USA).
Antibodies against CLDN1 (Zymed, USA) were used at 1:250 dilution.
Antibodies against GAPDH (Sigma, St. Louis, MO, USA) were used at a
1:5,000 dilution. The signals were visualized using Li-COR
Odyssey-Sa model 9260 (Li-COR Corp., USA) and images were taken and
managed using Odyssey Sa Infrared Image System (Li-COR Corp.).
Relative density of CLDN1 or β-catenin was measured by the
following equation: Relative density = density of CLDN1 or
β-catenin band/density of GAPDH.
β-catenin knockdown and
overexpression
After examining the expression of CLDN1 and
β-catenin in nine gastric cancer cell lines (AGS, SNU-1, SGC-7901,
MKN45, MKN28, KATO-III, HS-746T, BGC823 and NCI-N87) and one
immortalized normal gastric epithelial cell line GES-1, we selected
HS-746T as a model for β-catenin knockdown and overexpression
assays to further investigate the relationship between β-catenin
and CLDN1 because this cell line showed both membrane and nuclei
expression of β-catenin. Short hairpin RNA (shRNA) lentiviral
transduction particles for the β-catenin knockdown experiment was
purchased from Genepharma (Shanghai, China). One β-catenin specific
shRNA construct (shRNA sequence targeting β-catenin:
GTGCTATCTGTCTGCTCTA) and one ‘non-target’ construct were transduced
into HS-746T cells. The non-target construct contained an shRNA
sequence (ACTACCGTTGTTATAGGTG) without targeting any known human
gene and served as a scrambled negative control. After stable
transfection into gastric cancer cell line HS-746T, cell clones of
β-catenin knockdown (HS-746T/sh) and negative control (HS-746T/NC)
were selected with puromycin (Sigma-Aldrich) at 10 μg/ml and
screened by western blot analysis and immunofluorescence detection.
To detect whether ectopic overexpression of β-catenin could restore
the CLDN1 expression, the construct of LV5-EF1a-GFP/β-catenin
lentiviral transduction particles for ectopic overexpression of
β-catenin was also purchased from Genepharma and transfected into
HS-746T/sh cells. Stable transfected cell clone of
HS-746T/sh/β-catenin was selected with puromycin and screened by
western blot analysis and immunofluorescence detection.
Immunofluorescence staining
Cells were fixed with 4% formaldehyde and then
permeabilized with PBS containing 0.2% Triton X-100. Slides were
blocked by 5% BSA and incubated with a primary antibody at room
temperature for 1 h followed by TRITC-labeled goat anti-rabbit IgG
(Sigma) for additional 1 h. Nucleus were counterstained using DAPI
(Molecular Probes). Slides were washed by PBS, mounted and observed
under a microscope. Immunofluorescence staining was visualized
using Olympus BX50 microscope (Olympus Opticol Co., Japan), images
were taken using Nikon Digital Sight DS-U2 (Nikon, Japan), and NIS
elements F3.0 software was used (Nikon).
Statistical analysis
For IHC staining, the differences in
clinicopathologic features between the different groups were
determined using Pearson’s χ2 test. P<0.05 was
considered to be statistically significant. The survival curves of
each group were estimated by Kaplan-Meier survival analyses, and
the curves were compared using log-rank tests. Statistical Package
for the Social Sciences version 13.0 (SPSS, Inc., Chicago, IL, USA)
was used for all statistical analyses.
Results
CLDN1 and β-catenin are overexpressed in
gastric cancer tissues
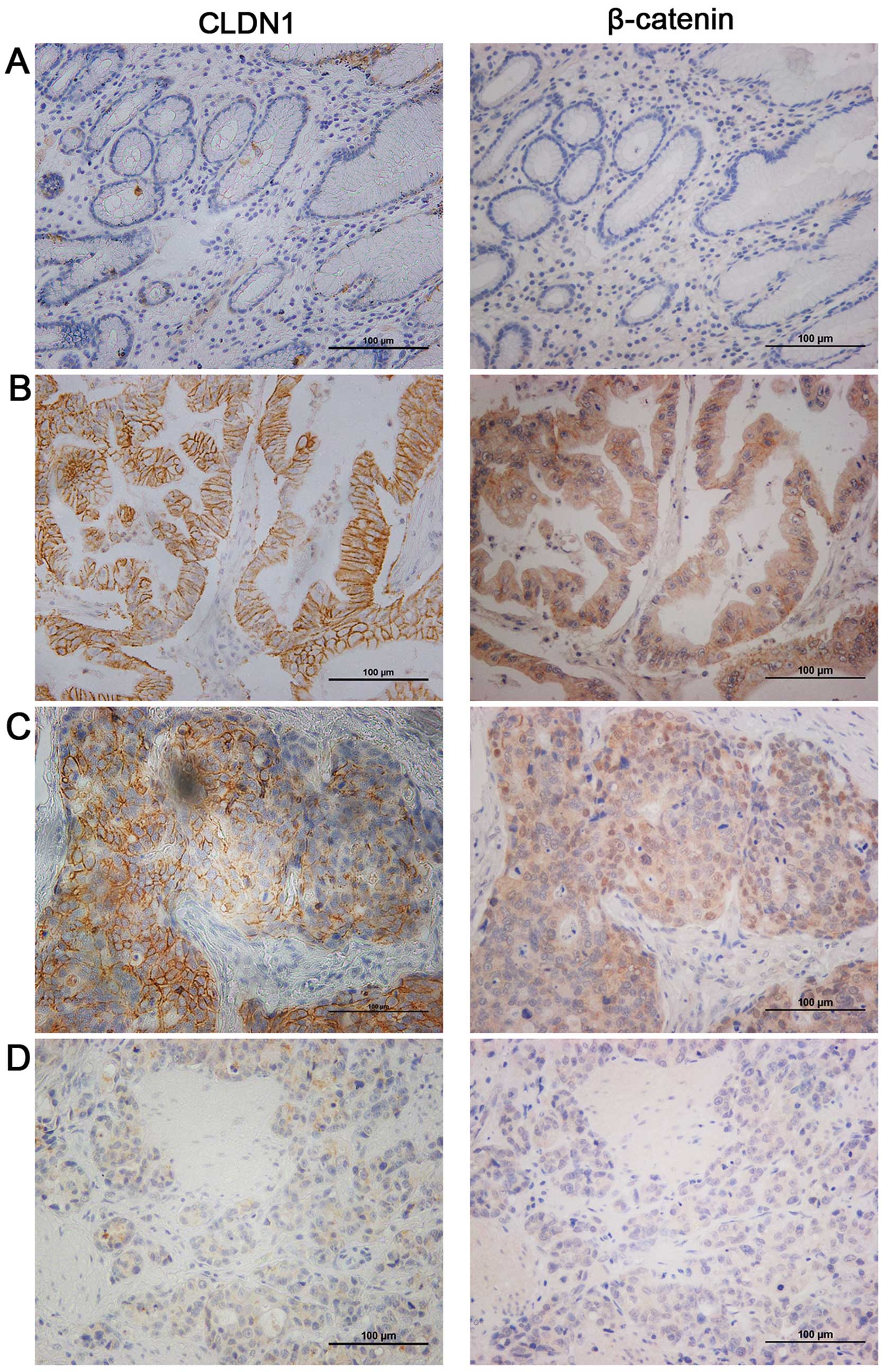
Immunohistochemistry revealed that CLDN1 positive
staining was localized in the membrane of tumor cells in gastric
cancer. To investigate the correlations between CLDN1 and
β-catenin, we performed IHC staining using the same cohort of tumor
and non-tumor gastric specimens for β-catenin staining. In 173
gastric cancer tissues, 36.4% (63 of 173) of cases were
CLDN1-negative (including no staining and cells stained ≤5% and
staining intensity scored 1), while the remaining 63.6% (110 of
173) showed variable levels of CLDN1 expression, with a
medium-score at 8.5. Likewise, 38.7% (67 of 173) of cases were
β-catenin-negative, while the remaining 61.3% (106 of 173) showed
variable levels of β-catenin expression. In contrast, CLDN1 and
β-catenin did not express, or were weakly expressed in normal
gastric epithelial cells. As shown in Table I, CLDN1 was expressed in 47.4% of
non-tumor tissues and β-catenin was expressed in 50.3% of non-tumor
tissues. In summary, CLDN1 (P=0.002) and β-catenin (P=0.040) were
highly expressed in gastric cancer tissues.
 | Table I.The results of CLDN1 and β-catenin
expression in immunohistochemical staining. |
Table I.
The results of CLDN1 and β-catenin
expression in immunohistochemical staining.
| CLDN1
| P-value | β-catenin
| P-value |
|---|
| − | + | − | + |
|---|
| Normal tissues | 91 | 82 | 0.002 | 86 | 87 | 0.040 |
| Tumor tissues | 63 | 110 | | 67 | 106 | |
Characteristics of the expression profile
of CLDN1 in gastric cancer tissues
We then investigated the clinicopathologic features
of CLDN1 protein in human gastric cancer tissues. As shown in
Table II, CLDN1 level decreased
from well-moderately differentiated to poorly differentiated
gastric cancer tissues (P=0.002). CLDN1 levels was higher in
intestinal-type gastric cancer than that in diffuse-type gastric
cancer (P=0.001). Higher CLDN1 expression levels were correlated
with lymph node metastasis (P=0.042) and advanced TNM stage (stage
III and IV) (P=0.030). CLDN1 expression was not significantly
affected by gender (P=0.053), age (P=0.256), tumor size (P=0.966),
T stage (P=0.386) and M stage (P=0.936). These data suggested that
CLDN1 was highly expressed in gastric cancer tissues with advanced
clinicopathological features.
 | Table II.Clinicopathological associations of
CLDN1 and β-catenin expression in gastric cancer. |
Table II.
Clinicopathological associations of
CLDN1 and β-catenin expression in gastric cancer.
| CLDN1
| P-value | β-catenin
| P-value |
|---|
| − | + | − | + |
|---|
| Gender | | | | | | |
| Male | 50 | 72 | 0.053 | 49 | 73 | 0.549 |
| Female | 13 | 38 | | 18 | 33 | |
| Age | | | | | | |
| ≤65 | 36 | 53 | 0.256 | 43 | 46 | 0.008 |
| >65 | 27 | 57 | | 24 | 60 | |
| Tumor size
(cm) | | | | | | |
| ≤5 | 34 | 59 | 0.966 | 31 | 62 | 0.116 |
| >5 | 29 | 51 | | 36 | 44 | |
|
Differentiation | | | | | | |
| Well +
moderate | 22 | 65 | 0.002 | 19 | 68 | 0.000 |
| Poor | 41 | 45 | | 48 | 38 | |
| Lauren
classification | | | | | | |
| Intestinal | 34 | 85 | 0.001 | 31 | 90 | 0.000 |
| Diffuse | 29 | 25 | | 36 | 16 | |
| T stage | | | | | | |
| T1+T2 | 13 | 17 | 0.386 | 14 | 16 | 0.326 |
| T3+T4 | 50 | 93 | | 53 | 90 | |
| TNM stage | | | | | | |
| I+II | 28 | 31 | 0.030 | 29 | 30 | 0.043 |
| III+IV | 35 | 79 | | 38 | 76 | |
| Lymph node
metastasis | | | | | | |
| N0 | 20 | 20 | 0.042 | 22 | 18 | 0.016 |
| N1+N2+N3 | 43 | 90 | | 45 | 88 | |
| Distance
metastasis | | | | | | |
| M0 | 59 | 102 | 0.936 | 63 | 98 | 0.928 |
| M1 | 4 | 8 | | 4 | 8 | |
Characteristics of the expression profile
of β-catenin in gastric cancer tissues
We next investigated the clinicopathologic features
of β-catenin protein in human gastric cancer tissues. As shown in
Table II, β-catenin level was
correlated with older age (P=0.008), differentiation (P<0.0001)
and intestinal-type gastric cancer (P<0.0001). Higher β-catenin
expression levels were correlated with lymph node metastasis
(P=0.016) and advanced TNM stage (stage III and IV) (P=0.043).
β-catenin expression was not significantly affected by gender
(P=0.549), tumor size (P=0.116), T stage (P=0.326) and M stage
(P=0.928). These data suggested that β-catenin was highly expressed
in gastric cancer tissues with advanced clinicopathological
features.
Correlation between CLDN1 with β-catenin
levels in gastric cancer tissues
Previous studies have shown that CLDN1 can be
regulated by β-catenin (28). To
investigate the association of CLDN1 and β-catenin, we performed
IHC staining using the same cohort of specimens as we used for
CLDN1 staining. As expected, we observed positive correlation
between the expression levels of CLDN1 and β-catenin in gastric
cancer analyzed by IHC staining. As presented in Fig. 1B–D, one specimen with high CLDN1
level also showed high β-catenin level, while another specimen with
very weak CLDN1 expression also showed nearly negative β-catenin
level. The correlation of CLDN1 and β-catenin was further analyzed
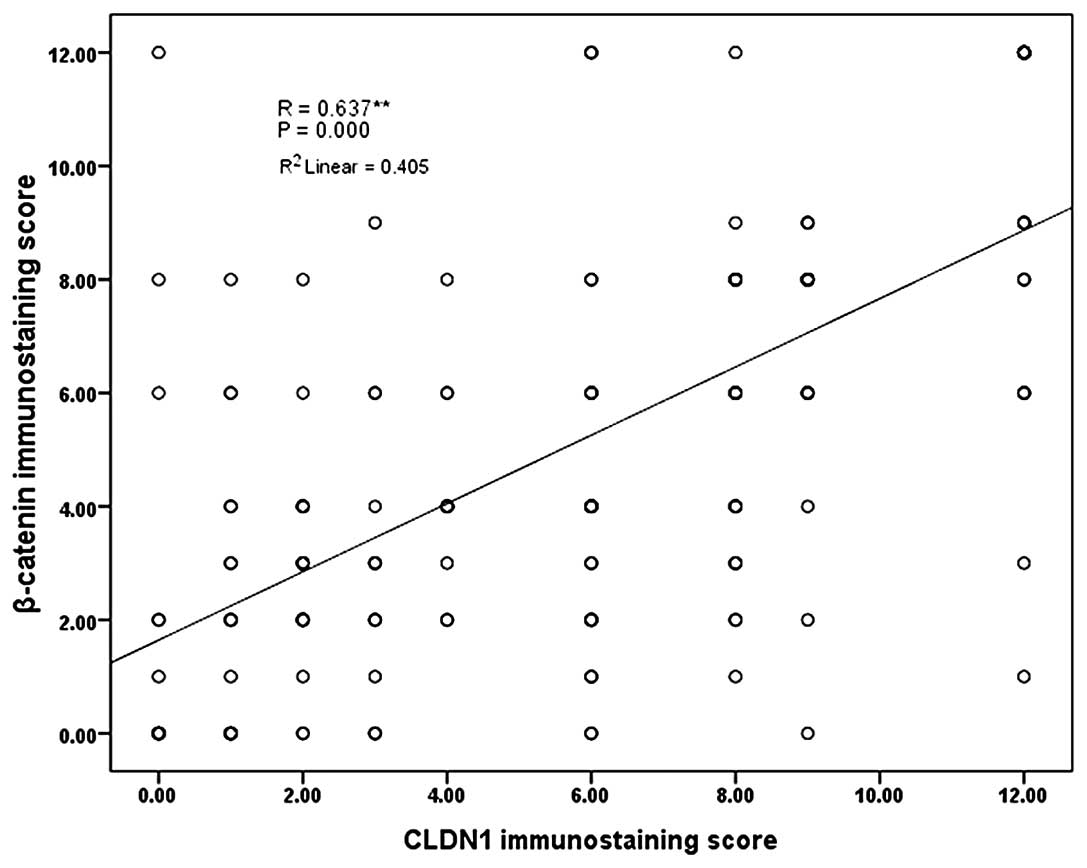
using the Person correlation. As shown in Fig. 2, y-axis presents the IHC scores of
β-catenin and x-axis presents the CLDN1 IHC scores using the same
cohort of gastric cancer specimens. Linear trend line showed CLDN1
levels were positively correlated to β-catenin levels. Person
correlation analysis showed correlation coefficient of 0.637
(P<0.0001). These data suggested that CLDN1 expression levels
were positively correlated with β-catenin levels.
Interestingly, we also established that CLDN1 was
correlated with the subcellular location of β-catenin, which might
suggest the activation of β-catenin related signaling pathway.
Among these 106 cases of β-catenin positive staining, 72 cases
displayed membrane staining of β-catenin, while 34 cases showed
nuclear β-catenin. As shown in Fig.
1C, strong CLDN1 expression was observed in an gastric cancer
specimen (left), and nuclei localized β-catenin was also observed
in the same case (right).
CLDN1 and β-catenin levels predict
survival in gastric cancer patients
To investigate the prognostic significance of CLDN1
and β-catenin in gastric cancer, we analyzed the correlation of
CLDN1 and β-catenin with survival of the patients using
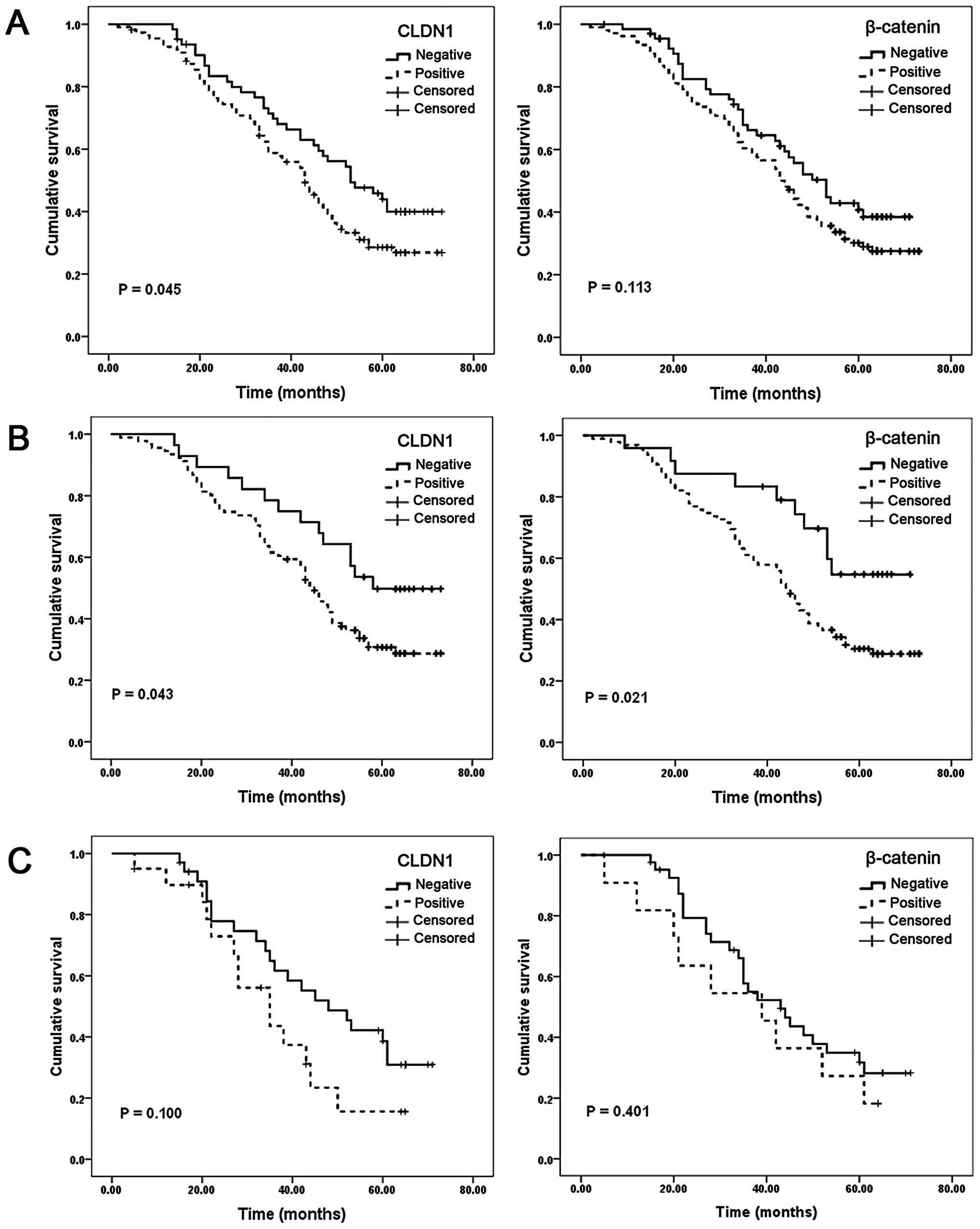
Kaplan-Meier analysis. Patients in the CLDN1-positive group showed
shorter overall survival than those in CLDN1-negative group (medium
survival: 44.1 vs. 51.5 months, P=0.045, Fig. 3A, left). However,
β-catenin-positive patients showed a trend of shorter overall
survival (medium survival: 44.6 vs. 49.6 months, P=0.113, Fig. 3A, right). CLDN1 and β-catenin
levels were significantly associated with Lauren classification
(Table II), therefore, we next
stratified patients with intestinal-and diffuse-type to investigate
the prognostic value of CLDN1 and β-catenin. In intestinal-type
patients, both CLDN1-positive group (medium survival: 45.4 vs. 55.3
months, P=0.043, Fig. 3B, left)
and β-catenin-positive group (medium survival: 45.3 vs. 56.4
months, P=0.021, Fig. 3B, right)
were significantly correlated with shorter overall survival.
However, in diffuse-type patients, no statistically significant
difference was found (Fig. 3C, left
and right). Our data suggested the expression of CLDN1 and
β-catenin was significantly associated with shorter overall
survival in intestinal-type gastric cancer patients.
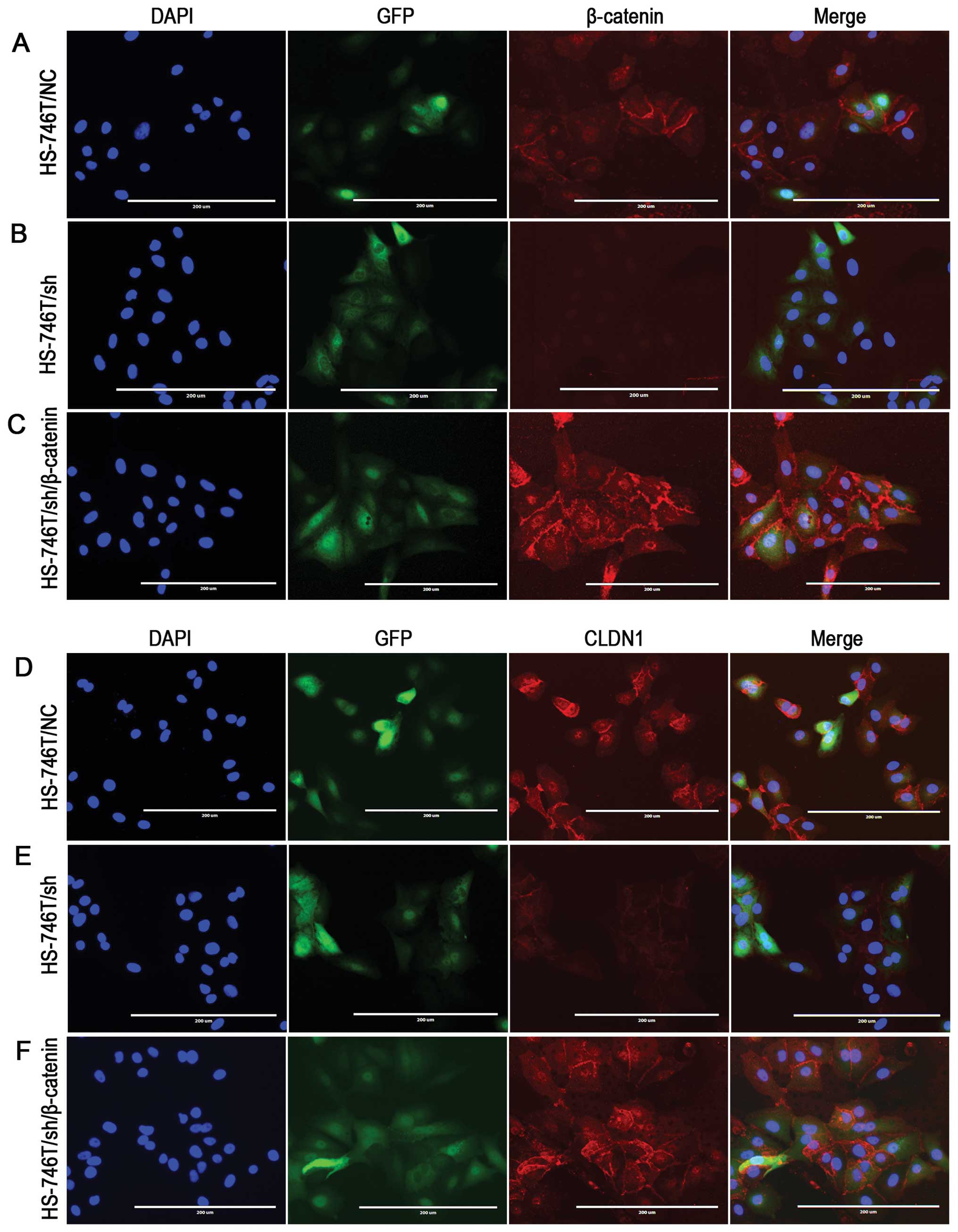
β-catenin knockdown or ectopic
overexpression regulates CLDN1 levels
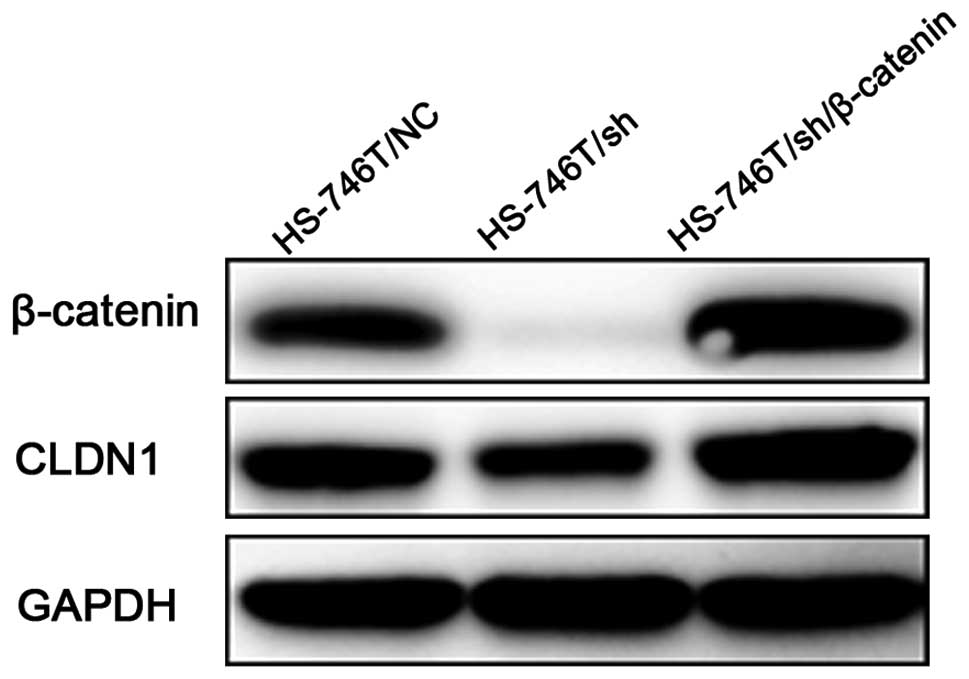
As previous studies suggested that β-catenin could
regulate CLDN1 expression, we then performed β-catenin knockdown
using pre-designed shRNA to examine the CLDN1 level. We employed
HS-746T as a model because this cell line has shown both membrane
and nuclear location of β-catenin, which suggested a potential
activation of β-catenin transcriptional activity. Immunoblotting
and immunofluorescence staining was used to observe the expression
level and subcellular location of β-catenin and CLDN1 in
control-shRNA (HS-746T/NC) and β-catenin-shRNA (HS-746T/sh)
transfected cells using anti-CLDN1 and anti-β-catenin antibodies.
β-catenin was barely observed after β-catenin-shRNA transfection
(Figs. 4 and 5B), and the CLDN1 level was dramatically
decreassed (Figs. 4 and 5E). We next ask whether ectopic
overexpression of β-catenin could restore the CLDN1 expression. To
do so, we transfected β-catenin full-length cDNA into HS-746T/sh
cells. As shown in Figs. 4 and
5C and F, CLDN1 expression was
significantly increased when HS-746T/sh cells were transfected with
β-catenin construct. These data strongly suggested that CLDN1 level
was regulated by β-catenin. Nuclei localized β-catenin showed
moderate staining in HS-746T/NC (Fig.
5A) and strong staining in HS-746T/sh/β-catenin (Fig. 5C) which might be an activation of
transcriptional activity contributing to the regulation of CLDN1
expression.
Discussion
CLDN1 can positively and negatively regulate
tumorigenesis in different human cancer types. β-catenin, a
multifunctional protein, is found to be critical in tumor
development and progression. We showed CLDN1 and β-catenin levels
were positively correlated. The expression of both CLDN1 and
β-catenin were associated with tumor differentiation, Lauren
classification, TNM stage and lymph node metastasis. Furthermore,
CLDN1 and β-catenin were predictive factors for shorter overall
survival in gastric patients. Finally, CLDN1 levels were regulated
by β-catenin.
CLDN1 is a member of tight junction protein which
generally plays a role in maintaining the integrity of the barrier
function in normal epithelial cells (34). However, in tumor cells, CLDN1 was
discovered to be either a promoter or an inhibitor. Because
CLDN1-induced acquisition of the malignant EMT phenotype, it was
exploited as a biomarker for metastasis in liver cancer (21,35,36).
Increased CLDN1 protein expression was related to metastasis
capacity of colorectal cancer (16,30).
Chang et al discovered that CLDN1 had tumor suppressive
activity and was a direct transcriptional target of RUNX3 in
gastric cancer cells (12). They
also found that knockdown of claudin 1 increased the tumorigenicity
of human gastric cancer cells (12). However, study also found that
overexpression of other members of claudin (CLDN6, 7 and 9) in
gastric cancer cells increased the invasiveness, migration and
proliferation (37).
We showed that CLDN1 was highly expressed in gastric
cancer tissues that were intestinal-type, differentiated, in
advanced TNM stage and with lymph node metastasis, furthermore,
CLDN1 showed its prognostic significance especially in
intestinal-type of gastric cancer. Our result is consistent with
earlier findings which showed loss of CLDN1 in diffuse-type of
gastric cancer in patients from Finland and United States (38,39).
However, the prognostic significance of CLDN1 in our study was the
first observation. This may be caused by the difference of patient
nationality, because Chinese mainland patients were enrolled in our
study. Our finding was thus supported by another Chinese group
which showed that CLDN1 expression was correlated with
differentiation, invasiveness and metastasis of gastric carcinoma
(40).
Accumulation of β-catenin in cytoplasm and nuclei is
frequently observed in a wide variety of tumors which reflects the
stabilization and activation of β-catenin (41,42).
The subsequent activation of Wnt/β-catenin signaling pathway is
common in majority of gastric cancers (43). CLDN1 is a known target of
β-catenin/TCF/LEF-dependent transcription regulation (28). Our findings that β-catenin levels
were associated with differentiation, TNM stage, lymph node
metastasis shared similar pattern with CLDN1. Both CLDN1 and
β-catenin predicted shorter survival in intestinal-type gastric
cancer patients. Protein levels of CLDN1 and β-catenin were
positively correlated in gastric cancer. These findings strongly
suggested a connection between these two markers. However, CLDN1
was a predictive factor for all patients suggesting it as a better
prognostic factor compared with β-catenin. We also found that the
CLDN1 level was regulated by β-catenin. CLDN1 levels decreased with
knockdown of β-catenin, while CLDN1 level was elevated when
β-catenin level was increased by overexpression. These findings
were consistent with other studies and our present study which
suggested a positively correlation of CLDN1 and β-catenin.
In conclusion, our study reveals CLDN1 as a negative
prognostic factor which predicts shorter overall survival in
gastric cancer patients. Clinicopathologic features of CLDN1 were
similar to those of β-catenin in gastric cancer. CLDN1 levels in
gastric cancer tissues were positively correlated with β-catenin
levels. Furthermore, CLDN1 levels were regulated by β-catenin.
However, CLDN1 severs as a better prognostic factor than β-catenin
in gastric cancer patients.
References
|
1.
|
Brenner H, Rothenbacher D and Arndt V:
Epidemiology of stomach cancer. Methods Mol Biol. 472:467–477.
2009. View Article : Google Scholar
|
|
2.
|
Du C, Zhou Y, Cai H, Zhao G, Fu H and Shi
YQ: Poor prognostic factors in patients with stage I gastric cancer
according to the seventh edition TNM classification: a comparative
analysis of three subgroups. J Surg Oncol. 105:323–328. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Lazar D, Taban S, Sporea I, et al: Gastric
cancer: correlation between clinicopathological factors and
survival of patients (III). Rom J Morphol Embryol. 50:369–379.
2009.PubMed/NCBI
|
|
4.
|
Lazar D, Taban S, Sporea I, et al: Gastric
cancer: correlation between clinicopathological factors and
survival of patients. II. Rom J Morphol Embryol. 50:185–194.
2009.PubMed/NCBI
|
|
5.
|
Tsukita S and Furuse M: Claudin-based
barrier in simple and stratified cellular sheets. Curr Opin Cell
Biol. 14:531–536. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Tobioka H, Isomura H, Kokai Y, Tokunaga Y,
Yamaguchi J and Sawada N: Occludin expression decreases with the
progression of human endometrial carcinoma. Hum Pathol. 35:159–164.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Pope JL, Bhat AA, Sharma A, et al:
Claudin-1 regulates intestinal epithelial homeostasis through the
modulation of Notch-signalling. Gut. Jun 13–2013.Epub ahead of
print.
|
|
8.
|
Noda S, Tanabe S and Suzuki T: Naringenin
enhances intestinal barrier function through the expression and
cytoskeletal association of tight junction proteins in Caco-2
cells. Mol Nutr Food Res. 57:2019–2028. 2013. View Article : Google Scholar
|
|
9.
|
Iraha A, Chinen H, Hokama A, et al:
Fucoidan enhances intestinal barrier function by upregulating the
expression of claudin-1. World J Gastroenterol. 19:5500–5507. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Myal Y, Leygue E and Blanchard AA: Claudin
1 in breast tumorigenesis: revelation of a possible novel ‘claudin
high’ subset of breast cancers. J Biomed Biotechnol.
2010:9568972010.PubMed/NCBI
|
|
11.
|
Blanchard AA, Ma X, Dueck KJ, et al:
Claudin 1 expression in basal-like breast cancer is related to
patient age. BMC Cancer. 13:2682013. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Chang TL, Ito K, Ko TK, et al: Claudin-1
has tumor suppressive activity and is a direct target of RUNX3 in
gastric epithelial cells. Gastroenterology. 138:255–265. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Qin W, Ren Q, Liu T, Huang Y and Wang J:
MicroRNA-155 is a novel suppressor of ovarian cancer-initiating
cells that targets CLDN1. FEBS Lett. 587:1434–1439. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Chao YC, Pan SH, Yang SC, et al: Claudin-1
is a metastasis suppressor and correlates with clinical outcome in
lung adenocarcinoma. Am J Respir Crit Care Med. 179:123–133. 2009.
View Article : Google Scholar
|
|
15.
|
Dhawan P, Singh AB, Deane NG, et al:
Claudin-1 regulates cellular transformation and metastatic behavior
in colon cancer. J Clin Invest. 115:1765–1776. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Kinugasa T, Akagi Y, Ochi T, et al:
Increased claudin-1 protein expression in hepatic metastatic
lesions of colorectal cancer. Anticancer Res. 32:2309–2314.
2012.PubMed/NCBI
|
|
17.
|
Ersoz S, Mungan S, Cobanoglu U, Turgutalp
H and Ozoran Y: Prognostic importance of Claudin-1 and Claudin-4
expression in colon carcinomas. Pathol Res Pract. 207:285–289.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Morohashi S, Kusumi T, Sato F, et al:
Decreased expression of claudin-1 correlates with recurrence status
in breast cancer. Int J Mol Med. 20:139–143. 2007.PubMed/NCBI
|
|
19.
|
Zhang Z, Wang A, Sun B, Zhan Z, Chen K and
Wang C: Expression of CLDN1 and CLDN10 in lung adenocarcinoma in
situ and invasive lepidic predominant adenocarcinoma. J
Cardiothorac Surg. 8:952013. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Matsuda Y, Semba S, Ueda J, et al: Gastric
and intestinal claudin expression at the invasive front of gastric
carcinoma. Cancer Sci. 98:1014–1019. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Suh Y, Yoon CH, Kim RK, et al: Claudin-1
induces epithelialmesenchymal transition through activation of the
c-Abl-ERK signaling pathway in human liver cells. Oncogene.
32:4873–4882. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Singh AB, Sharma A and Dhawan P: Claudin-1
expression confers resistance to anoikis in colon cancer cells in a
Src-dependent manner. Carcinogenesis. 33:2538–2547. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Singh AB, Sharma A, Smith JJ, et al:
Claudin-1 up-regulates the repressor ZEB-1 to inhibit E-cadherin
expression in colon cancer cells. Gastroenterology. 141:2140–2153.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Shiozaki A, Bai XH, Shen-Tu G, et al:
Claudin 1 mediates TNFalpha-induced gene expression and cell
migration in human lung carcinoma cells. PLoS One. 7:e380492012.
View Article : Google Scholar
|
|
25.
|
Di Cello F, Cope L, Li H, et al:
Methylation of the claudin 1 promoter is associated with loss of
expression in estrogen receptor positive breast cancer. PLoS One.
8:e686302013.PubMed/NCBI
|
|
26.
|
Blanchard AA, Skliris GP, Watson PH, et
al: Claudins 1, 3, and 4 protein expression in ER negative breast
cancer correlates with markers of the basal phenotype. Virchows
Arch. 454:647–656. 2009. View Article : Google Scholar
|
|
27.
|
Moldvay J, Jackel M, Paska C, Soltesz I,
Schaff Z and Kiss A: Distinct claudin expression profile in
histologic subtypes of lung cancer. Lung Cancer. 57:159–167. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
28.
|
Miwa N, Furuse M, Tsukita S, Niikawa N,
Nakamura Y and Furukawa Y: Involvement of claudin-1 in the
beta-catenin/Tcf signaling pathway and its frequent upregulation in
human colorectal cancers. Oncol Res. 12:469–476. 2001. View Article : Google Scholar
|
|
29.
|
Bhat AA, Sharma A, Pope J, et al: Caudal
homeobox protein Cdx-2 cooperates with Wnt pathway to regulate
claudin-1 expression in colon cancer cells. PLoS One. 7:e371742012.
View Article : Google Scholar : PubMed/NCBI
|
|
30.
|
Shiou SR, Singh AB, Moorthy K, et al:
Smad4 regulates claudin-1 expression in a transforming growth
factor-beta-independent manner in colon cancer cells. Cancer Res.
67:1571–1579. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
31.
|
Sharma A, Bhat AA, Krishnan M, Singh AB
and Dhawan P: Trichostatin-A modulates claudin-1 mRNA stability
through the modulation of Hu antigen R and tristetraprolin in colon
cancer cells. Carcinogenesis. 34:2610–2621. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32.
|
Krishnan M, Singh AB, Smith JJ, et al:
HDAC inhibitors regulate claudin-1 expression in colon cancer cells
through modulation of mRNA stability. Oncogene. 29:305–312. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
33.
|
Sinicrope FA, Ruan SB, Cleary KR, Stephens
LC, Lee JJ and Levin B: bcl-2 and p53 oncoprotein expression during
colorectal tumorigenesis. Cancer Res. 55:237–241. 1995.PubMed/NCBI
|
|
34.
|
Mrsny RJ, Brown GT, Gerner-Smidt K, et al:
A key claudin extra-cellular loop domain is critical for epithelial
barrier integrity. Am J Pathol. 172:905–915. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
35.
|
Stebbing J, Filipovic A and Giamas G:
Claudin-1 as a promoter of EMT in hepatocellular carcinoma.
Oncogene. 32:4871–4872. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
36.
|
Yoon CH, Kim MJ, Park MJ, et al: Claudin-1
acts through c-Abl-protein kinase Cdelta (PKCdelta) signaling and
has a causal role in the acquisition of invasive capacity in human
liver cells. J Biol Chem. 285:226–233. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
37.
|
Zavala-Zendejas VE, Torres-Martinez AC,
Salas-Morales B, Fortoul TI, Montano LF and Rendon-Huerta EP:
Claudin-6, 7, or 9 overexpression in the human gastric
adenocarcinoma cell line AGS increases its invasiveness, migration,
and proliferation rate. Cancer Invest. 29:1–11. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
38.
|
Soini Y, Tommola S, Helin H and
Martikainen P: Claudins 1, 3, 4 and 5 in gastric carcinoma, loss of
claudin expression associates with the diffuse subtype. Virchows
Arch. 448:52–58. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
39.
|
Resnick MB, Gavilanez M, Newton E, et al:
Claudin expression in gastric adenocarcinomas: a tissue microarray
study with prognostic correlation. Hum Pathol. 36:886–892. 2005.
View Article : Google Scholar
|
|
40.
|
Wu YL, Zhang S, Wang GR and Chen YP:
Expression transformation of claudin-1 in the process of gastric
adenocarcinoma invasion. World J Gastroenterol. 14:4943–4948. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
41.
|
Demir R, Dimmler A, Naschberger E, et al:
Malignant progression of invasive tumour cells seen in hypoxia
present an accumulation of beta-catenin in the nucleus at the
tumour front. Exp Mol Pathol. 87:109–116. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
42.
|
Li YP, Wu CC, Chen WT, Huang YC and Chai
CY: The expression and significance of WWOX and beta-catenin in
hepatocellular carcinoma. APMIS. 121:120–126. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
43.
|
Ooi CH, Ivanova T, Wu J, et al: Oncogenic
pathway combinations predict clinical prognosis in gastric cancer.
PLoS Genet. 5:e10006762009. View Article : Google Scholar : PubMed/NCBI
|