Introduction
Although its incidence rate has steadily declined in
recent decades, gastric cancer remains a global health problem.
Gastric carcinoma is the fourth most common malignancy in the
world, with an estimated 989,000 new cases and 738,000 deaths
reported in 2008. The depth of invasion and the presence of lymph
node metastases are considered to be the most important prognostic
factors in gastric cancer (1,2).
However, investigating molecular biomarkers not only can provide
indications for clinic prognosis, but also can identify potential
targets for clinical therapy.
SOX [sex-determining region Y (Sry) box containing]
factors are a family of structurally-related transcription factors
with potent effects on cellular phenotypes. SOX11 belongs to group
C of mammalian SOX proteins. It has two functional domains: a
Sry-related HMG box (SOX) DNA-binding domain, located in the
N-terminal half of the protein, and a transactivation domain (TAD)
located at the C-terminus (3). The
HMG box domain alone fulfills the functions of DNA binding, DNA
bending, protein interactions and nuclear import or export. The SOX
HMG box domain contains two nuclear localization domains that are
independent of each other and that have been highly conserved in
all SOX proteins (4). The Sox EHMG
box domain also features a nuclear export signal (5,6).
To date, the main function of SOX11 in non-malignant
tissues is in neural development and organogenesis (7) during fetal development. SOX11 is
present during gastrulation and early postgastrulation development
throughout the embryo (8). Later
during development, SOX11 is prominently expressed in the
developing nervous system in both glial and neuronal lineages and
at many sites throughout the embryo where epithelial-mesenchymal
interactions occur (8). At sites
of such epithelial-mesenchymal interactions, SOX11 can be found in
the mesenchymal or epithelial compartment, and it has been
postulated to be involved in inductive remodeling (8). SOX11-depletion can cause death at
birth and other severe developmental defects (9). Among which, abdominal wall closure
defects, asplenia, and stomach hypoplasia are major phenotypes of
SOX11-deficiency in gut development (9).
SOX11 was firstly reported in hematopoietic
malignancies such as lymphoma (10). SOX11 is considered as a diagnostic
and prognostic antigen in B cell lymphomas (11,12)
and has been demonstrated to have tumor suppressor functions
(10). Recently, SOX11 has been
studied in other solid tumors. The presence of SOX11 is associated
with improved recurrence-free survival (RFS) in ovarian cancer
(13). However, the studies on the
expression and significance of SOX11 in malignant tumors other than
lymphoma are very limited.
In this study, we examined the role of SOX11 in
gastric cancer using in vitro and in vivo models.
Immunohistochemistry was used to investigate the expression pattern
of SOX11. The correlations of SOX11 with patient
clinicopathological features were analyzed and the prognostic
significance of SOX11 was evaluated.
Materials and methods
Patients and specimens
Gastric cancer tissues, confirmed by pathological
diagnosis, were obtained from 151 patients who underwent radical
resection for gastric cancer between 2006 and 2008 at the
Department of Surgery, Ruijin Hospital, Shanghai, China. The
corresponding non-tumor gastric tissue was obtained at least 6 cm
from the tumor. All tissue samples were formalin-fixed and
paraffin-embedded. Clinicopathological and survival data of all
patients were collected. TNM staging was classified based on the
criteria of American Joint Committee on Cancer (AJCC, 7th edition)
for gastric cancer. The mean age of the patients at initial surgery
was 65 years (range, 34–84 years); 104 men and 47 women were
included in this study. The mean duration of follow-up was 39
months (range, 1–73 months). The AJCC tumor stage distribution and
vital status of the patients are shown in Table I. The study was approved by the
Shanghai Jiao Tong University Medical School institutional review
board.
 | Table I.Clinicopathological characteristics of
SOX11 in gastric cancer. |
Table I.
Clinicopathological characteristics of
SOX11 in gastric cancer.
| SOX11
| |
|---|
| Clinicopathological
characteristics | Negative (%) | Positive (%) | P-value |
|---|
| Gender | | | |
| Male | 54 (51.9) | 50 (48.1) | 0.191 |
| Female | 19 (40.4) | 28 (59.6) | |
| Age | | | |
| ≤60 | 29 (55.8) | 23 (44.2) | 0.186 |
| >60 | 44 (44.4) | 55 (55.6) | |
| Tumor invasion | | | |
| T1–T2 | 9 (37.5) | 15 (62.5) | 0.246 |
| T3–T4 | 64 (50.4) | 63 (49.6) | |
| Lymph node
metastasis | | | |
| Negative | 12 (34.3) | 23 (65.7) | 0.058 |
| Positive | 61 (52.6) | 55 (47.4) | |
| Distant
metastasis | | | |
| Negative | 70 (49.6) | 71 (50.4) | 0.382 |
| Positive | 3 (30.0) | 7 (70.0) | |
| TNM staging | | | |
| I–II | 23 (37.7) | 38 (62.3) | 0.031 |
| III–IV | 50 (55.6) | 40 (44.4) | |
| Lauren’s type | | | |
| Intestinal | 27 (29.3) | 65 (70.7) | <0.001 |
| Diffuse | 46 (78.0) | 13 (22.0) | |
|
Differentiation | | | |
| High | 6 (22.2) | 21 (77.8) | <0.001 |
| Median | 13 (25.0) | 39 (75.0) | |
| Low | 54 (75.0) | 18 (25.0) | |
Immunohistochemistry staining
Immunohistochemistry (IHC) staining was performed
using a highly sensitive streptavidin-biotin-peroxidase detection
system with gastric cancer tissue microarrays. Mouse monoclonal
anti-SOX11 at a dilution of 1:100 (Cell Marque, CA, USA) was used
as previously reported (14).
Mayer’s haematoxylin was used for counterstain. The slides were
evaluated by a single board-certified pathologist (RRT) who
remained blinded to the clinical data using standard light
microscopy.
Immunohistochemistry assessment
Expression status of SOX11 was determined by using a
semi-quantitative scoring system based on percentage and staining
intensity of positive cells. The percentage of positive cells was
divided into five grades (percentage scores): <10% (0), 10–25%
(1), 25–50% (2), 50–75% (3) and >75% (4). The staining intensity
was divided into four grades (intensity scores): no staining (0),
weak staining (1), moderate staining (2) and strong staining (3).
SOX11 staining status was determined by the following formula:
overall score = percentage score x intensity score. The overall
score ≤3 was defined as negative, and >3 was defined as
positive.
Gastric cancer cell lines and cell
culture
Gastric cancer cell lines AGS, N87, MKN45, MKN28,
SGC-7901, KATOIII, BGC823 and Hs746T were preserved in the
institute. The cells were grown in RPMI-1640 medium containing 10%
fetal bovine serum (FBS), penicillin and streptomycin (Gibco BRL,
Gaithersburgh, MD, USA). Plasmid containing Myc-DDK-tagged ORF
clone of Homo sapiens SOX11 was purchased from Origene
(Origene Technologies, MD, USA). Cell proliferation was assessed by
the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium (MTT)
(Sigma, St. Louis, MO, USA) assay. Soft agar colony formation assay
was performed by using 0.3% agar in complete medium with cells as
the feeder layer and 0.6% agar in complete medium as the bottom
layer. 3D Matrigel culture was performed using Matrigel matrix (BD
Biosciences, San Jose, CA, USA).
Quantitative real-time PCR (qRT-PCR)
Total RNA was isolated from cultured cells using the
RNeasy mini kit (Qiagen) and cDNA was synthesized with oligo(dT)
primers by using a SuperScript first-strand cDNA synthesis kit
(Invitrogen) according to the manufacturer’s protocols. Gene
expression was assessed by qRT-PCR using an Applied Biosystems 7500
Fast Sequence Detection System (Life Technologies Corp., CA, USA).
The PCR reaction mixture consisted of QuantiTect SYBR Green PCR
master mix (2X QuantiTect SYBR Green kit, contains HotStart
Taq® DNA polymerase, QuantiTect SYGB Green PCR buffer,
dNTP mix, SYGB I, Rox passive reference dye and 5 mM
MgCl2) (Qiagen), 0.5 μmol/l of each primer and
cDNA. The transcript of the housekeeping gene,
glyceraldehyde-3-phosphate dehydrogenase (GAPDH) gene was used as
endogenous control to normalize expression data. Primers used for
qRT-PCR analysis of SOX11 expression are
5′-GGTGGATAAGGATTTGGATTCG-3′ (forward) and 5′-GCTCCGGCGTGCAGTAG
T-3′ (reverse). Primers used for analysis of GAPDH are
5′-TTGGCATCGTTGAGGGTCT-3′ (forward) and 5′-CAGTGGGAACACGGAAAGC-3′
(reverse). The comparative Ct (threshold cycle) method was used to
calculate the relative changes in gene expression.
Western blotting
Whole cell lysates were harvested using RIPA cell
lysis buffer supplemented with a protease inhibitor cocktail
(Sigma). The nuclear and cytosol extracts were isolated using the
nuclear extract kit (Active Motif, Carlsbad, CA, USA) according to
the manufacturer’s instructions. Rabbit monoclonal antibody against
SOX11 (Epitomics) were used at 1:1,000 dilutions. The signals were
visualized using Li-COR Odyssey-Sa model 9260 (Li-COR Corp., USA),
and images were taken and managed using Odyssey Sa Infrared Image
System (Li-COR Corp.).
Flow cytometry
For cell cycle analysis, single cells were fixed in
70% ice-cold ethanol at 4˚C overnight, washed with PBS, incubated
with 100 μg/ml RNase at 37˚C for 20 min. After staining with
propidium iodide (50 μg/ml), the cells were subjected to
fluorescence activated cell sorting (FACS). For apoptosis analysis,
Annexin V/PI (BD Biosciences) double staining was used and followed
by FACS analysis. FACSCalibur was used in flow cytometry and the
data were analyzed by Cell Quest software (BD Biosciences).
Immunofluorescence staining
Cells plated on glass coverslips were washed with
PBS and fixed in 4% paraformaldehyde for 15 min at room
temperature. Monolayers were washed with PBS, blocked with 5% BSA
for 30 min and incubated with the primary antibody diluted in
blocking solution for 2 h at room temperature. After washing, the
cover slips were incubated with cyanine-3- and/or
cyanine-2-conjugated secondary antibodies for 30 min, washed three
times in PBS and mounted. DAPI (4′,6-diamidino-2-phenylindole)
staining [1:5,000 (vol/vol) of a 5 mg/ml stock] was used to
visualize DNA. Immunofluorescence staining was visualized using
Olympus BX50 microscope (Olympus Opticol Co., Japan), images were
taken using Nikon Digital Sight DS-U2 (Nikon, Japan) and NIS
elements F3.0 software was used (Nikon).
Cell migration and invasion assays
Cell migration was analyzed by a transwell chamber
assay. Cell invasion assays were performed using BD
BioCoat™ Matrigel™ Invasion Chambers. FBS
(10%) was used as the chemoattractant. Cells on the lower surface
of the insert were fixed and stained followed by counting under a
light microscope. Cells were visualized using Olympus BX50
microscope (Olympus Opticol Co.), images were taken using Nikon
Digital Sight DS-U2 (Nikon, Japan) and NIS elements F3.0 software
was used (Nikon).
In vivo tumorigenesis and metastasis
Male BALB/c nu/nu nude mice (Institute of Zoology
Chinese Academy of Sciences, Shanghai, China), were housed at a
specific pathogen-free environment in the Animal Laboratory Unit,
School of Medicine, Shanghai Jiao Tong University, China. Mice
received humane care and the study protocols comply with the
Institution’s guideline and animal research laws. Cells
(1×106) were subcutaneously injected into 4-week-old
male BALB/c mice. The growth of primary tumors was monitored every
3 days by measuring tumor diameters. Tumor length (L) and width (W)
were measured and tumor volume was calculated by the equation:
volume = (W2 × L)/2 (15). Mice were sacrificed 28 days after
injection under anesthesia. Eight mice were used in each group.
To produce peritoneal spreading experimental
metastasis, 2×106 cells were injected into 5-week-old
male BALB/c nude mice intraperitoneally. After 6 weeks, the mice
were sacrificed under anesthesia. Ten mice were used in each group.
Macrometastatics were visualized and counted.
Statistical analyses
The differences in clinicopathological features
between different groups were determined using Pearson’s
χ2 test. The survival curves of each group were
estimated by Kaplan-Meier survival analyses, and the curves were
compared using log-rank tests. In multivariate analysis, a Cox’s
proportional hazard model was applied to determine whether a factor
was an independent predictor of survival. The statistical analyses
were performed using SPSS 13.0 software (SPSS Inc., Chicago, IL,
USA). Values are presented as the mean ± standard deviation (SD) of
samples measured in triplicate. Each experiment was repeated three
times, unless otherwise indicated. The significance of differences
between experimental groups was analyzed using the Student’s t test
and two-tailed distribution.
Results
Database analysis of SOX11 mRNA
expression in human gastric cancer
We first investigated the SOX11 mRNA levels in human
gastric cancer using the datasets from the publicly available
Oncomine database (www.oncomine.org). SOX11 mRNA was
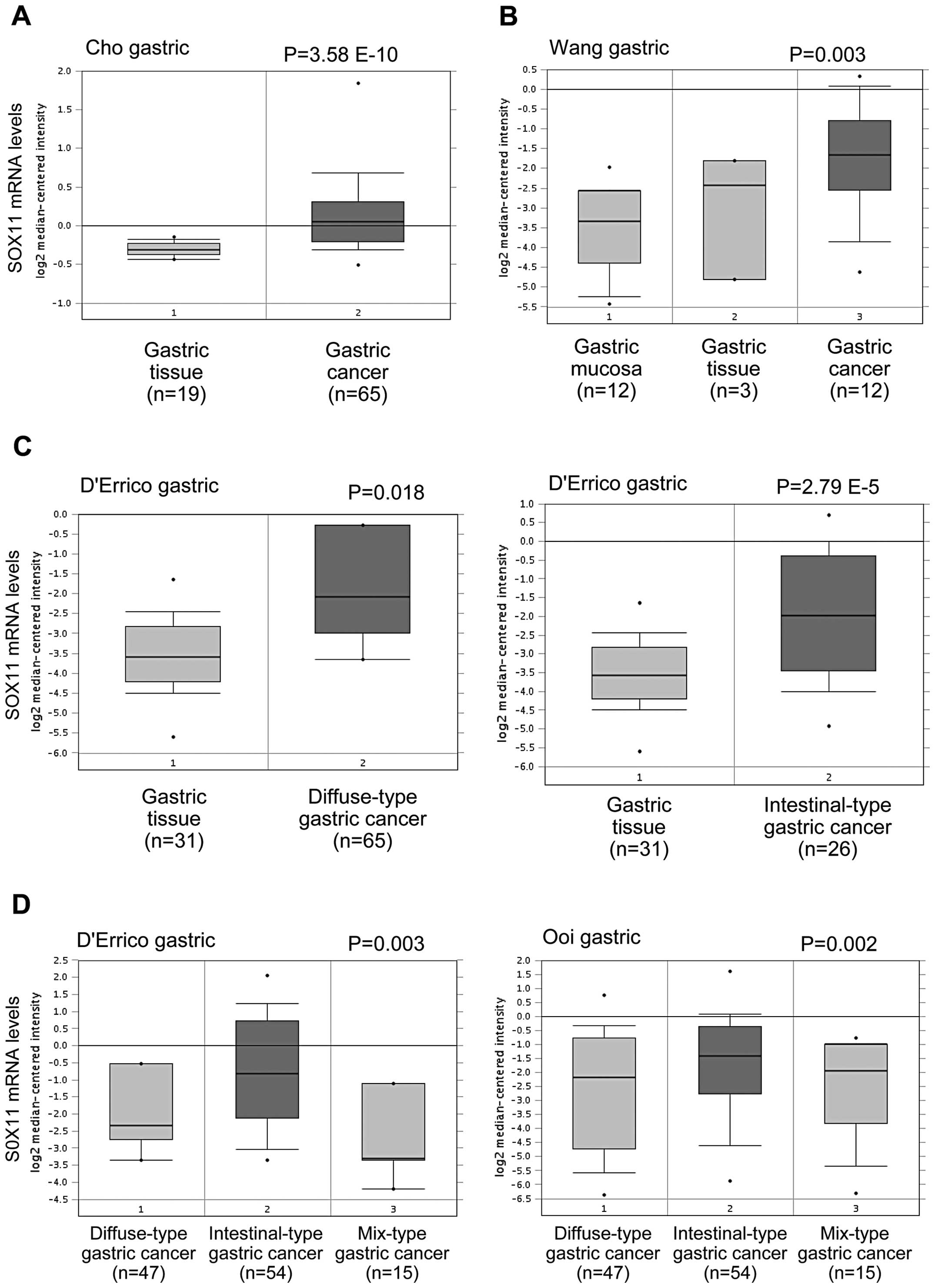
significantly elevated in human gastric cancer tissues compared
with normal tissues in the Cho et al (16), Wang et al (17) and D’Errico et al (18) datasets from the Oncomine database
(Fig. 1A–C). Furthermore, SOX11
mRNA levels were significantly higher in gastric cancer of
intestinal-type than other types in Ooi et al (19) and D’Errico et al (18) datasets (Fig. 1D). These data suggest that SOX11
mRNA level is upregulated in human gastric cancer.
Expression of SOX11 in gastric cancer
cell lines
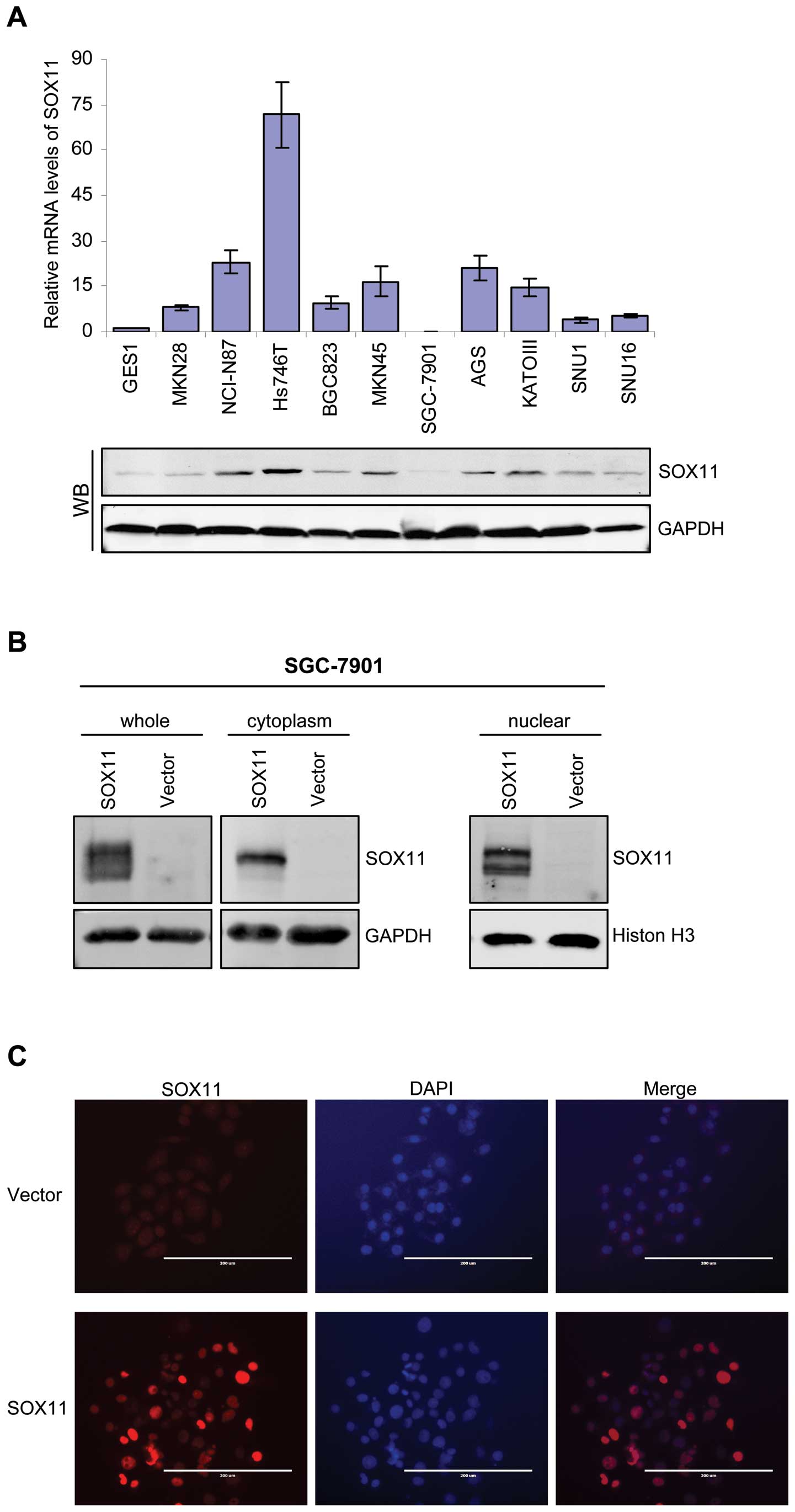
We then analyzed the SOX11 expression in human
gastric cancer cell lines and the immortalized normal gastric
epithelial cell line GES-1. We performed qRT-PCR and immunoblotting
to analyze the SOX11 mRNA and protein levels in gastric cancer cell
lines and GES-1. Consistent with database analysis, we showed that
SOX11 mRNA and protein levels were highly expressed in all gastric
cancer cell lines compared to GES-1. Notably, five gastric cancer
cell lines (NCI-N87, Hs746T, AGS, KATO-III) showed higher and five
cell lines (MKN28, BGC823, SGC-7901, SNU1, SNU16) showed lower
SOX11 protein levels (Fig. 2A).
These data demonstrate that SOX11 is highly expressed in some
gastric cancer cell lines.
Ectopic overexpression of SOX11 in
gastric cancer cells does not affect growth in vitro and in
vivo
We generated SOX11 overexpression models using
SGC-7901 (Fig. 2B) and MKN45 cell
line (data not shown), which did not express or expressed weak
levels of SOX11. Immunofluorescence staining also revealed strong
nuclear signaling in SOX11-overexpressing SGC-7901 cells (Fig. 2C). To investigate the role of SOX11
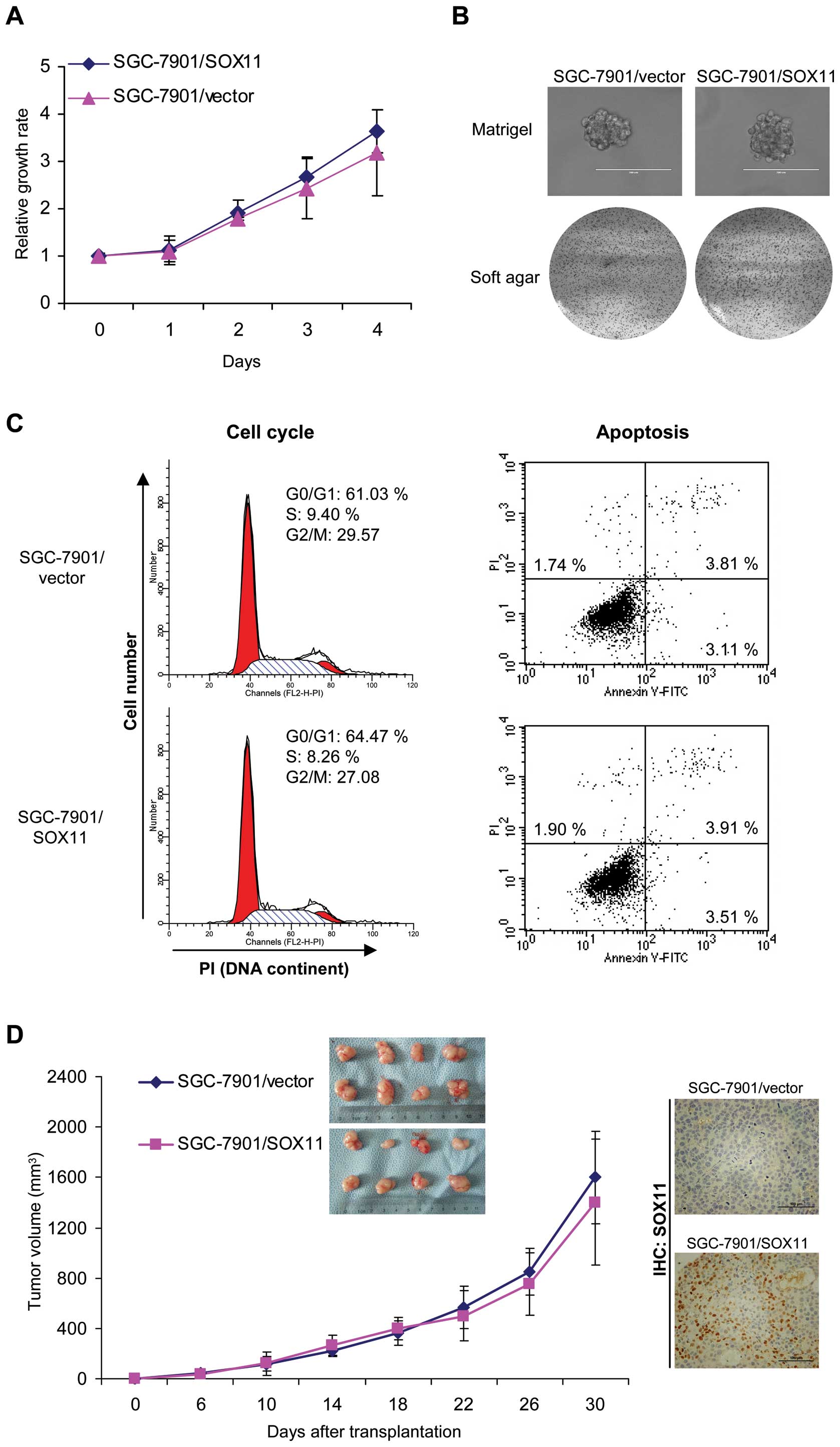
in the growth of gastric cancer cells, we first examined the cell
proliferation in monolayer culture. As shown in Fig. 3A, SOX11 overexpression did not
affect cell growth in monolayer culture. We then performed soft
agar and Matrigel 3D culture. We found that SOX11 overexpression
did not suppress the growth of SGC-7901 (Fig. 3A) and MKN45 (data not shown)
gastric cancer cells in soft agar and Matrigel (Fig. 3B). We then analyzed cell cycle
division and apoptosis by flow cytometry. Cell cycle distribution
and apoptotic rate did not show significant difference between
vector-control and SOX11-overexpressing cells (Fig. 3C).
In order to examine the effects of SOX11 on the
in vivo growth of gastric cancer cells, we employed two
experimental models. Control and SOX11-overexpressing SGC-7901
cells were injected orthotopically into nude mice and tumor growth
was examined. Mice injected with vector-control (SGC-7901/vector)
and SOX11-overexpressing (SGC-7901/SOX11) cells formed similar size
tumors within 37 days (Fig. 3D,
left). The nuclear SOX11 was observed in tumors formed by
SOX11-overexpressing cells but not in that formed by vector-control
cells (Fig. 3D, right). These data
suggested that SOX11 overexpression did not affect gastric cancer
cell growth.
Ectopic overexpression of SOX11 in
gastric cancer cells inhibits invasion in vitro and in vivo
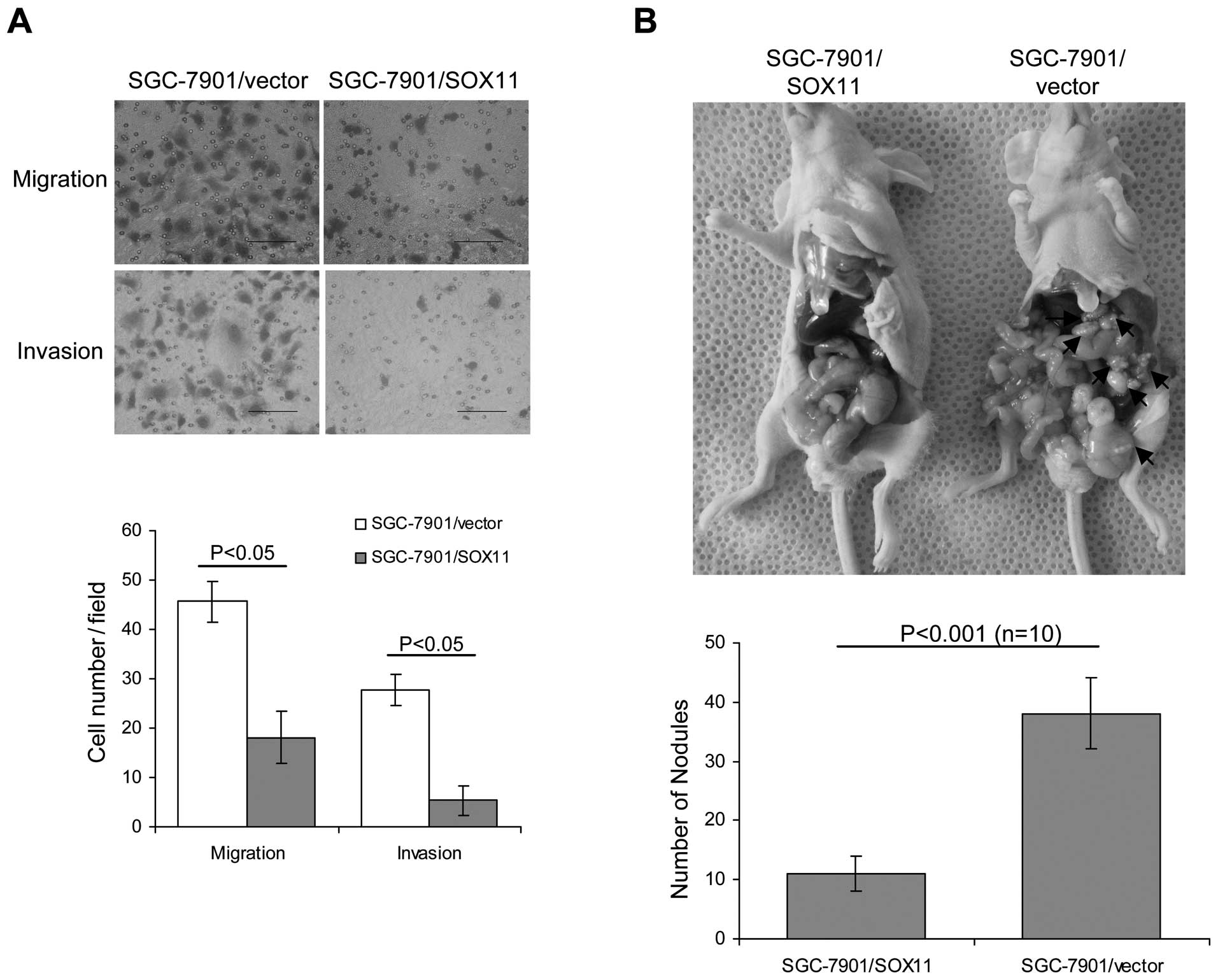
We next asked whether overexpression of SOX11
affected gastric cancer migration and invasion. Boydon chamber
assays were used to investigate the in vitro ability of
migration and invasion in SOX11-overexpression and vector-control
cells. As expected, SOX11 overexpression suppressed cell migration
and invasion (Fig. 4A).
As peritoneal spreading and metastasis are common in
gastric cancer and are pivotal factors for its poor prognosis, we
used a nude mouse model to investigate the influence of SOX11
levels on peritoneal metastasis. Consistent with in vitro
observations, we found that SOX11-overexpressing cells formed less
metastatic nodules than vector-control cells (Fig. 4B). Our data suggest that SOX11
overexpression suppresses cell migration and invasion.
SOX11 protein expression in gastric
cancer tissues and non-tumor tissues
As in vitro and in vivo cell model
analyses strongly suggested a tumor-suppressor role of SOX11 in
gastric cancer cells, we asked whether the expression pattern of
SOX11 human gastric cancer tissues agreed with the cell study. We
performed immunohistochemistry staining to examine the SOX11
protein expression in gastric cancer tissues. Gastric tumor and
paired non-tumor tissues from 151 patients were stained. Of the
gastric cancer tissues 51.7% (78 of 151) showed positive SOX11
staining (IHC score: 4–12), and 48.3% (73 of 151) of the samples
showed negative staining (IHC score: 0–3). A significant
correlation was found between positive expression of SOX11 and TNM
stage (I+II, P=0.031), Lauren’s classification (intestinal type,
P<0.001) and differentiation status (high and medium,
P<0.001) (Table I). Of note,
the SOX11 positive percentile was higher in lymph node metastasis
negative and stage (T1+T2) tumors than lymph node metastasis
positive and stage (T3+T4) tumors, although this was not
statistically significant (Table
I). These data suggest that SOX11 is highly expressed in a
subset of human gastric cancer, which shows less malignant
characteristics according to clinicopathological features.
SOX11 expression is an independent
prognostic factor and associated with better survival in gastric
cancer patients
SOX11 was recently discovered to be a prognostic
factor in lymphoma and ovarian cancer (11,20).
To investigate the prognostic significance of SOX11 in gastric
cancer, we analyzed the correlation of SOX11 with survival of
patients using Kaplan-Meier analysis. We discovered that patients
in the SOX11-positive group showed longer overall survival than
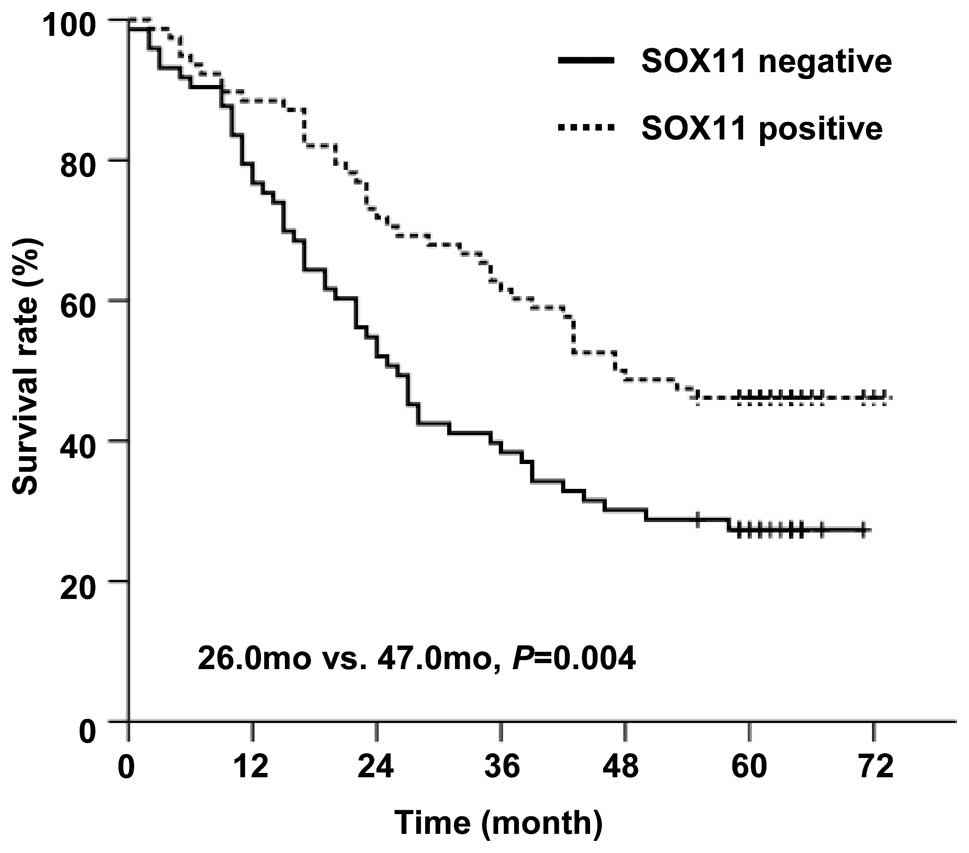
those in SOX11-negative group (medium survival: 47.0 months vs.
26.0 months, P= 0.004, Fig. 5).
Our data suggested an association between SOX11 expression and
improved overall survival in gastric cancer patients.
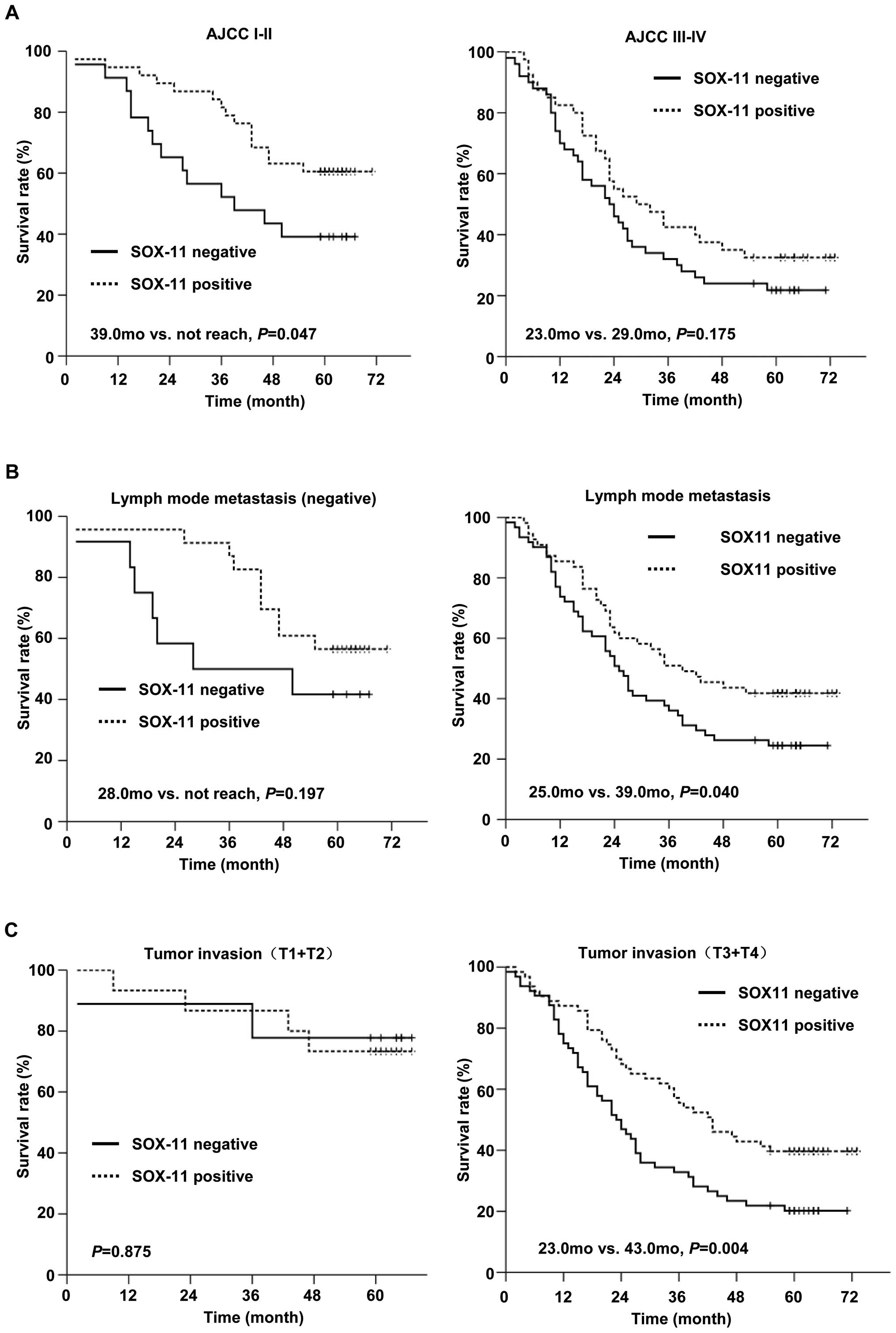
Cases with negative lymph node status and early
tumor invasion stage tended to have a higher SOX11 positive rate.
We stratified the patients with TNM stage, and further stratified
by N and T stage to evaluate the prognostic value of SOX11. In 61
patients whose TNM stage was I and II, SOX11-positive group had
longer survival than SOX11-negative group (P=0.047, Fig. 6A, right). In patients whose TNM
stage was III and IV, SOX11-positive group showed a trend of longer
survival than SOX11-negative group, but not statistical
significance (Fig. 6A, left). In
116 patients with lymph node metastasis, 55 cases (47.4%) were
SOX11-positive and 61 cases (52.6%) were SOX11-negative.
Kaplan-Meier analysis showed that patients in SOX11-positive group
had improved survival compared to SOX11-negative group (medium
survival: 39.0 months vs. 25.0 months, P=0.04, Fig. 6B, right). Similarly, in a group of
127 patients at late tumor invasion stage (T3+T4), 63 cases (49.6%)
of the SOX11-positive group showed longer medium survival (43.0
months) than the other 64 cases (50.4%) of the SOX11-negative group
(23.0 months) patients (P=0.004, Fig.
6C, right). SOX11 was not significantly correlated with
survival in patients without lymph node metastasis (Fig. 6B, left) and at early tumor invasion
stage (T1+T2) (Fig. 6C, left).
These data suggest that the expression of SOX11 is a prognostic
factor for improved survival in patients with lymph node metastasis
and at advanced tumor invasion stage.
Further multivariate analysis showed that tumor
invasion (relative risk = 2.155, 95% CI, 1.531–3.032, P<0.001),
lymph node metastasis (relative risk = 1.345, 95% CI, 1.099–1.647,
P=0.004), SOX11 (relative risk = 0.604, 95% CI, 0.391–0.9340,
P=0.023), and age (relative risk = 1.913, 95% CI, 1.213–3.019,
P=0.005) were independent prognostic factors for the survival rate
of gastric cancer patients (Table
II). These data demonstrate that SOX11 expression is an
independent positive prognostic factor in gastric cancer.
 | Table II.Multivariate Cox regression analysis
of the association of SOX11 expression with gastric cancer patient
survival. |
Table II.
Multivariate Cox regression analysis
of the association of SOX11 expression with gastric cancer patient
survival.
| Parameter | HR (95% CI) | P-value |
|---|
| Tumor invasion | 2.155
(1.531–3.032) | <0.001 |
| Lymph node
metastasis | 1.345
(1.099–1.647) | 0.004 |
| SOX11 | 0.604
(0.391–0.934) | 0.023 |
| Age | 1.913
(1.213–3.019) | 0.005 |
Discussion
SOX11 exhibits a wide and highly dynamic expression
during embryogenesis (9). SOX11
has been previously studied in lymphoma and ovarian cancers and
showed correlations to patients’ survival. We investigated the role
of SOX11 overexpression on gastric cancer malignant behavior. SOX11
overexpression suppressed migration and invasion ability of gastric
cancer cells in vitro and in vivo. We further
examined the expression and clinical significance of SOX11 in
gastric cancer. We uncovered SOX11 as a prognostic factor for
improved survival in gastric cancer patients.
SOX11-deficient mice exhibited severe defects in
several organ systems which all express SOX11 at times of extensive
remodeling. SOX11-deficience causes stomach hypoplasia in embryos
(9). However, the function of
SOX11 in gastric cancer is unclear. From the Oncomine database, we
found that SOX11 was overexpressed in intestinal-type gastric
cancer. This finding suggested that overexpressin of SOX11 might be
intestinal-type related and suggest a favorable outcome.
SOX11-overexpressing gastric cancer cells showed suppressed
migration and invasion malignant behavior. In embryogenesis, SOX11
expression accompanies the activation of several signal
transduction pathways, including Wnt, transforming growth factor β
(TGF-β), bone morphogenetic protein (BMP), fibroblast growth factor
(FGF) and Hedgehog signaling (21). However, the role of SOX11 in
tumorigenesis is poorly understood. In hematopoietic malignancies,
SOX11 knockdown and overexpression were found to be correlated with
increased and decreased cell proliferation, respectively (10). Rb-E2F growth regulatory pathway was
involved in this growth regulatory role of SOX11 by signaling
pathway analysis (10). However,
we showed that SOX11 levels did not affect gastric cancer cell
growth. The cell cycle and apoptosis analysis also showed no
difference between SOX11-overexpressing and vector-control cells.
These results suggested that SOX11 did not suppress gastric
tumorigenesis through regulating cell growth. We further discovered
that SOX11 overexpression strongly inhibited in vitro cell
migration and invasion as well as in vivo peritoneal
metastasis. SOX11 overexpression may inhibit gastric tumorigenesis
through suppressing cancer cell motility.
SOX11 has been studied in various
lymphoproliferative diseases. However, the prognostic relevance of
SOX11 remains unclear since it is found to be associated with both
improved and reduced survival. Consistent with previous finding
which showed SOX11 as a tumor-suppressor in ovarian cancer
(20), we found SOX11 was
correlated with less malignant features in gastric cancer.
Specifically, SOX11 is expressed in early stage, differentiated and
intestinal-type gastric cancer. Furthermore, SOX11 expression was
correlated with improved survival in gastric cancer patients. Cox
regression multivariate analysis also reveals that SOX11 is an
independent prognostic factor for predicting survival of patients.
It is noteworthy that SOX11 is a correlated with longer survival in
patients with lymph node metastasis and deep tumor invasion,
suggesting that SOX11 is a predictor of patient survival even in
advanced stage. As gastric cancer patients are often diagnosed at
advanced stage, the prognostic value of SOX11 in advanced stage
patients could be of great importance in predicting patient
survival. These findings were also consistent with the results from
SOX11-overexpression cell models, which suggested a
tumor-suppressor role of SOX11 in gastric cancer. Together, our
study reveals the correlation of SOX11 with clinicopathological
features and survival of patients.
In concusion, our findings demonstrate that SOX11
suppresses gastric cancer migration and invasion in vitro
and in vivo. SOX11 may serve as a marker for a subgroup of
gastric cancer which shows less aggressive features and better
prognosis.
Acknowledgements
This study was supported by grants
from National Natural Science Foundation of China (nos. 81172324,
81101585 and 91229106), Science and Technology Commission of
Shanghai Municipality (nos. 812XD1403700 and 12PJ1406300), Key
Projects in the National Science & Technology Pillar Program of
China (no. 2011BA203191), Doctoral Innovation fund of the Ministry
of Health of China (20110073110071), National Institutes of Health
(CA151610) and the Avon Foundation (02-2010-068).
References
|
1.
|
Hohenberger P and Gretschel S: Gastric
cancer. Lancet. 362:305–315. 2003. View Article : Google Scholar
|
|
2.
|
Smith DD, Schwarz RR and Schwarz RE:
Impact of total lymph node count on staging and survival after
gastrectomy for gastric cancer: data from a large US-population
database. J Clin Oncol. 23:7114–7124. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Dy P, Penzo-Mendez A, Wang H, Pedraza CE,
Macklin WB and Lefebvre V: The three SoxC proteins - Sox4, Sox11
and Sox12 - exhibit overlapping expression patterns and molecular
properties. Nucleic Acids Res. 36:3101–3117. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Sudbeck P and Scherer G: Two independent
nuclear localization signals are present in the DNA-binding
high-mobility group domains of SRY and SOX9. J Biol Chem.
272:27848–27852. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Gasca S, Canizares J, De Santa Barbara P,
et al: A nuclear export signal within the high mobility group
domain regulates the nucleocytoplasmic translocation of SOX9 during
sexual determination. Proc Natl Acad Sci USA. 99:11199–11204. 2002.
View Article : Google Scholar
|
|
6.
|
Rehberg S, Lischka P, Glaser G, Stamminger
T, Wegner M and Rosorius O: Sox10 is an active nucleocytoplasmic
shuttle protein, and shuttling is crucial for Sox10-mediated
transactivation. Mol Cell Biol. 22:5826–5834. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Prior HM and Walter MA: SOX genes:
architects of development. Mol Med. 2:405–412. 1996.PubMed/NCBI
|
|
8.
|
Hargrave M, Wright E, Kun J, Emery J,
Cooper L and Koopman P: Expression of the Sox11 gene in mouse
embryos suggests roles in neuronal maturation and
epithelio-mesenchymal induction. Dev Dyn. 210:79–86. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Sock E, Rettig SD, Enderich J, Bosl MR,
Tamm ER and Wegner M: Gene targeting reveals a widespread role for
the high-mobility-group transcription factor Sox11 in tissue
remodeling. Mol Cell Biol. 24:6635–6644. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Gustavsson E, Sernbo S, Andersson E, et
al: SOX11 expression correlates to promoter methylation and
regulates tumor growth in hematopoietic malignancies. Mol Cancer.
9:1872010. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Wang X, Asplund AC, Porwit A, et al: The
subcellular Sox11 distribution pattern identifies subsets of mantle
cell lymphoma: correlation to overall survival. Br J Haematol.
143:248–252. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Mozos A, Royo C, Hartmann E, et al: SOX11
expression is highly specific for mantle cell lymphoma and
identifies the cyclin D1-negative subtype. Haematologica.
94:1555–1562. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Brennan DJ, Ek S, Doyle E, et al: The
transcription factor Sox11 is a prognostic factor for improved
recurrence-free survival in epithelial ovarian cancer. Eur J
Cancer. 45:1510–1517. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Zeng W, Fu K, Quintanilla-Fend L, Lim M,
Ondrejka S and Hsi ED: Cyclin D1-negative blastoid mantle cell
lymphoma identified by SOX11 expression. Am J Surg Pathol.
36:214–219. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Safina A, Vandette E and Bakin AV: ALK5
promotes tumor angiogenesis by upregulating matrix
metalloproteinase-9 in tumor cells. Oncogene. 26:2407–2422. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Cho JY, Lim JY, Cheong JH, et al: Gene
expression signature-based prognostic risk score in gastric cancer.
Clin Cancer Res. 17:1850–1857. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Wang Q, Wen YG, Li DP, et al: Upregulated
INHBA expression is associated with poor survival in gastric
cancer. Med Oncol. 29:77–83. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
D’Errico M, de Rinaldis E, Blasi MF, et
al: Genome-wide expression profile of sporadic gastric cancers with
microsatellite instability. Eur J Cancer. 45:461–469.
2009.PubMed/NCBI
|
|
19.
|
Ooi CH, Ivanova T and Wu J: Oncogenic
pathway combinations predict clinical prognosis in gastric cancer.
PLoS Genet. 5:e10006762009. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Sernbo S, Gustavsson E, Brennan DJ, et al:
The tumour suppressor SOX11 is associated with improved survival
among high grade epithelial ovarian cancers and is regulated by
reversible promoter methylation. BMC Cancer. 11:4052011. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Delot EC, Bahamonde ME, Zhao M and Lyons
KM: BMP signaling is required for septation of the outflow tract of
the mammalian heart. Development. 130:209–220. 2003. View Article : Google Scholar : PubMed/NCBI
|