Introduction
Gastric cancer is one of the most common
malignancies worldwide (1). The
5-year survival rates for gastric cancer remain poor throughout the
world (1,2), especially in patients with advanced
disease or metastasis, as gastric cancer is highly aggressive and
resistant to anticancer drugs. Though the molecular mechanisms
regulating the development of gastric cancer are not yet fully
elucidated, increased cell migration and invasion are closely
related to carcinogenesis and poor prognosis in gastric cancer
(3). Therefore, understanding the
mechanisms which regulate these biological changes in gastric
cancer cells may help to reduce the incidence of gastric cancer and
lead to the identification of novel methods to protect against or
treat gastric cancer.
Activation of extracellular signal-regulated kinase
(ERK), a major member of the mitogen-activated protein kinase
(MAPK) superfamily, can increase migration and invasion in many
cancer cells (4,5). Six mammalian ERK homologs have been
identified to date, termed ERK1, ERK2, ERK3, ERK5, ERK7 and ERK8
(6,7). It is well established that MAP3K
kinases can activate MAP2K kinases, which in turn can activate
MAPKs. Multiple MAP3Ks and MAP2Ks have been verified to activate
the ERK signaling pathway; including MAP3Ks such as Raf and MEKK3,
and MAP2Ks such as MAPK/ERK kinase 1 (MEK1) and MEK2. ERK1/2 is a
direct substrate of MEK1 and MEK2 (8). ERK1 and ERK2 are two of the most
essential regulators of cell growth, proliferation,
differentiation, migration and invasion (9,10).
Activation of ERK1/2 is observed in many human cancers and is
closely related to cancer cell progression and poor prognosis.
Therefore, the ERK1/2 signaling pathways are regarded as
potentially useful targets for the treatment of cancer (11).
A variety of growth factors including epidermal
growth factor (EGF) can activate ERK1/2 and lead to increased cell
growth, differentiation, migration and survival. Although it is
well known that the EGF/Raf/MEK1/2/ERK1/2 pathway is closely
associated with cancer cell metastasis, and activation of ERK1/2 is
capable of promoting the growth of gastric cancer cells (12), the effect of ERK1/2 signaling on
the metastasis of gastric adenocarcinoma cancer (GA) cells remains
to be determined.
Inflammation induced by cytokines plays important
roles in cancer carcinogenesis and progression, especially in
gastric cancer. A lot of studies have demonstrated that gastric
cancer may be an ‘inflammatory disease’, due to induction by
Helicobacter pylori (HP) infection. One of the most
important characterizations of HP infection is elevated interleukin
(IL)-1β level in location gastric tissue, which may cause
inflammation-associated gastric carcinogenesis. However, the
underlying molecular mechanism by which the IL-β signaling is
regulated during gastric cancer carcinogenesis is still not
understood. Accumulating evidence demonstrates that the tumor
microenvironment exerts a variety of pleiotropic effects during
malignant processes, and plays an important role in carcinogenesis,
malignant transformation and tumor growth, metastasis and survival
(13,14). Cytokines induced by inflammation or
secreted by tumor cells make up the important ingredients of the
tumor microenvironment. Previous findings demonstrate that IL-1β is
capable of activating ERK1/2 and the transcription factor activator
protein (AP)-1 in several cell types, which is believed to promote
inflammation-associated carcinogenesis and plays a crucial role in
cancer metastasis (15,16). However, it is not unclear whether
IL-1β can activate ERK1/2 and regulate ERK1/2-mediated metastasis
in GA. Moreover, little is known about the additive effects of EGF
and IL-1β on the metastasis of GA.
In this study, we determined the additive ability of
EGF and IL-1β to activate ERK1/2 in GA cells, and characterized the
molecular mechanisms regulating additive effects of EGF plus
IL-1β-induced ERK1/2-mediated metastasis in GA cells; furthermore,
we investigated the relationship between the expression of EGF plus
IL-1β, and the activation of ERK1/2 and the clinicopathological
features in GA tissues.
Materials and methods
Cell culture and transfection with
siRNA
AGS and MKN-45 cells (American Type Culture
Collection, Manassas, VA and Japanese Cancer Research Bank,
respectively) were grown in F12 and DMEM medium, respectively
(Invitrogen, Carlsbad, CA, USA) containing 10% fetal bovine serum
(FBS) at 37°C in an incubator containing 5% CO2. siRNA
against ERK1/2 (Cell Signaling Co., Danvers, MA, USA) (50–200 nM)
was transfected into cells with Lipofectamine 2000 according to the
manufacturer’s instructions.
Western blot analysis for ERK1/2
The western blotting for the expression of ERK1/2
and p-ERK1/2 in AGS and MKN-45 cells used the methods described by
us previously (17,18). The rabbit anti-human ERK1/2 or
p-ERK1/2 antibody was 1:1000 or 500 diluted (Cell Signaling Co.).
Anti-β-actin (1:6000 dilution, Sigma, St. Louis, MO, USA) was used
as a control for the western blots.
Cell migration and invasion assay
For AGS and MKN-45 cell invasion assays, we used
methods described by Sumida et al (19). Millicell Hanging Cell Invasion
Chambers with 8-μm pore filter (Millipore Corp., Billerica,
MA) were coated with 12 μl of ice-cold Matrigel
(Becton-Dickinson Labware, Bedfore, MA). AGS or MKN-45 cells
(5×104/well) were added to the upper chamber of these
Matrigel chambers in 200 μl serum-free F12 or DMEM medium
with 50 ng/ml human epidermal growth factor (EGF) or 20 ng/ml IL-1β
or both (R&D Systems, Minneapolis, MN) or not, which were then
put into 24-well plates in F12 or DMEM medium containing 10% FBS.
To evaluate the role of inhibitor U0126, cells were pre-treated
with the reagent for 3 h, and then performed the stimulations. To
evaluate the role of ERK1/2 siRNA in cell migration and invasion,
AGS and MKN-45 cells were transfected with scramble siRNA or ERK1/2
siRNA for 36 h, and followed the transfection, the cells were
seeded at a density of 5×104/well and then in 200
μl of serum-free medium for the stimulation as mentioned
above. Following a 20-h incubation, cells were fixed with methanol
and were then stained with crystal violet or Giemsa. Cotton tips
were used to remove the cells that remained in the Matrigel or
attached to the upper side of the filter. Light microscopy was used
to count the cells on the lower side of the filter. The assays were
performed in duplicate, and the results were averaged. The methods
used for migration assay was almost the same as invasion assay
mentioned above except no Matrigel was used for coating the well
and the time of incubation was 15 h.
Confocal microscopy assay
The relationship between the expression of p-ERK1/2,
and MMP-9 in response to EGF, or IL-1β or both in AGS and MKN-45
cells were detected by confocal microscopy using methods described
by us previously with anti-p-ERK1/2 (Cell Signaling Co.) and
anti-MMP-9 antibodies (Abcam Co., Cambridge, MA, USA) (18).
AP-1 luciferase reporter gene assay
AGS and MKN-45 cells were transfected with AP-1 luc
vector (1 μg) or co-transfected with AP-1 luc vector plus
scramble siRNA or ERK1/2 siRNA (50–200 nM) with Lipofectamine 2000.
B-gal plasmid which contains galactosidase reporter gene was
co-transfected with AP-1 luc vector to serve as control for
transfection efficiency. Thirty-six hours after transfection, the
cells were left untreated or treated with EGF (50 ng/ml) or IL-1β
(20 ng/ml) or both for 12 h, luciferase assay (for AP-1) and enzyme
assay (for B-gal) were then carried out according to the
instructions of kit purchased from Promega Corp. (Madison, WI,
USA).
Tissue samples
105 cases of paraffin-embedded gastric
adenocarcinoma (GA) tissue samples were obtained from Fuzhou
General Hospital (Fuzhou, Fujian). The tissue samples were used
with consent of the patients. This study was approved by the Ethics
Committees of Fuzhou General Hospital.
Immunohistochemistry for phosphorylation
of ERK1/2, EGF, IL-1β, EGF plus IL-1β, MMP-9 and c-fos
For phosphorylation of ERK1/2 (p-ERK1/2)
immunohistochemical detection (IHC) in the 105 cases of GA tissue
samples, we used methods previously described for transgelin
(20), using an anti-p-ERK1/2
antibody (1:100 dilution, Cell Signaling Co.) instead of an
anti-transgelin antibody. The staining results were assessed on a
four-tier scale based on Ju et al (21) and Ebert et al (22). To assess the levels of EGF, IL-1β,
EGF plus IL-1β, MMP-9 and c-fos in GA tissues by IHC, we also used
the method described above. Anti-MMP-9, and c-fos antibodies used
for IHC were from Abcam Co.; anti-human IL-1β antibody was from
Santa Cruz Biotechnology, Inc. (Santa Cruz, CA, USA).
Statistical analysis
Statistical significance of immunohistochemistry for
p-ERK1/2 was analyzed by the Wilcoxon signed-ranks test, the
χ2 test, the Fisher’s exact test and t-test. Spearman’
method was applied to evaluate the correlation in expression levels
of P-ERK1/2 with IL-1β, EGF, EGF plus IL-1β, MMP-9 and c-fos in GA
tissue samples. For other experiments, values are expressed as
means ± SD, and independent-sample T test was performed to
determine the difference among the groups. P-values <0.05 were
considered statistically significant.
Results
EGF activates ERK1/2 and increases cell
migration and invasion in GA cells
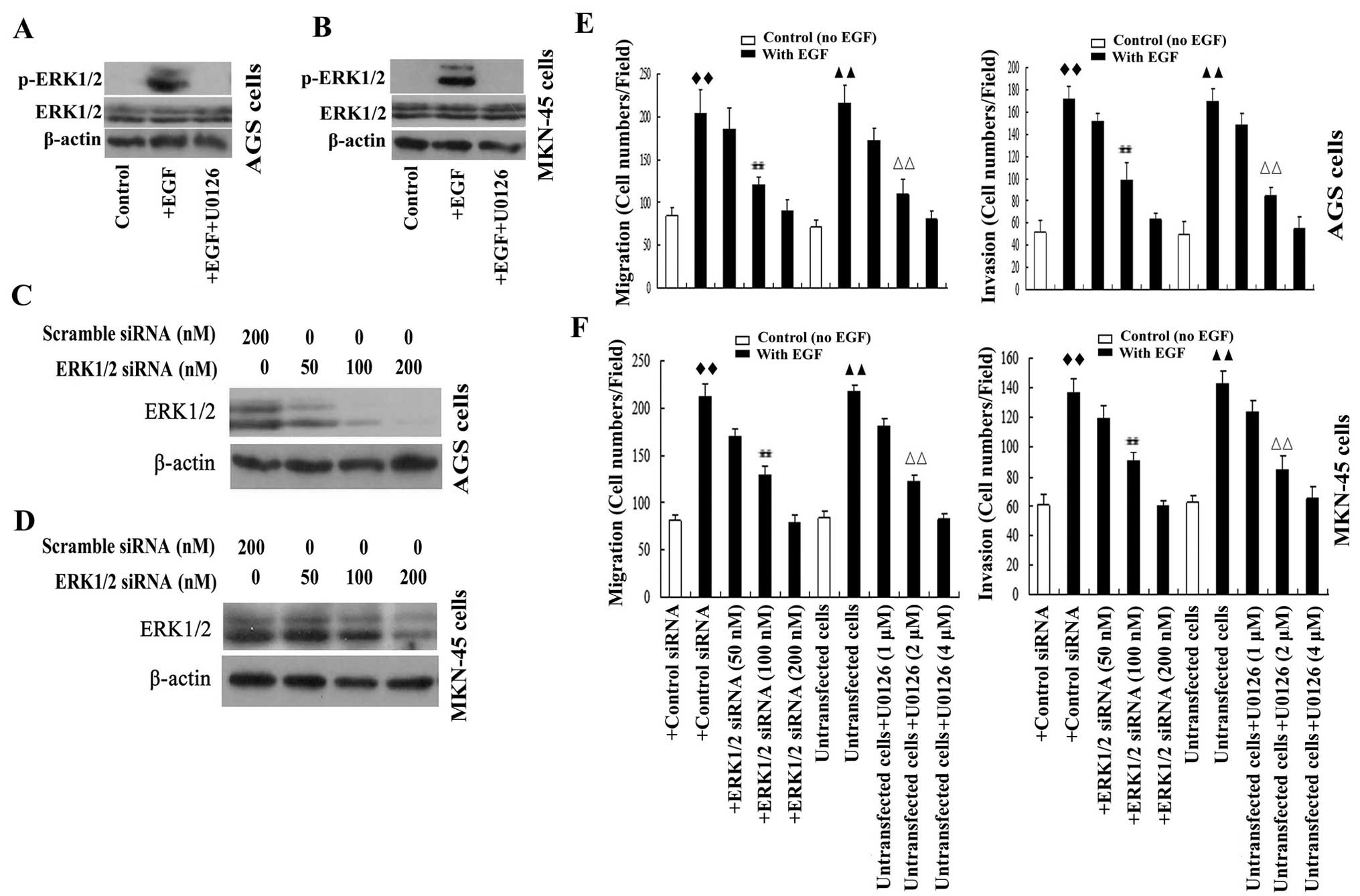
The ability of the representative growth factor EGF
to activate ERK1/2 signaling was investigated in GA cells. As
expected, expression of p-ERK1/2 was detected in both AGS and
MKN-45 cells after stimulation with EGF for 30 min, and EGF-induced
expression of p-ERK1/2 could be inhibited by the MEK/ERK pathway
inhibitor U0126 (Fig. 1A and
B).
To investigate whether EGF induced the migration and
invasion of GA cells were mediated by ERK1/2 signaling activation,
AGS and MKN-45 cells were stimulated with EGF in the presence or
absence of U0126 or transfected with ERK1/2 siRNA. The
results from Transwell assays demonstrated that EGF elevated the
migration and invasion of AGS and MKN-45 cells. EGF-induced AGS and
MKN-45 cell migration and invasion were significantly and
dose-dependently attenuated by knockdown of ERK1/2 using
50–200 nM ERK1/2 siRNA (Fig.
1C–H). In a similar manner, U0126 significantly and
dose-dependently inhibited EGF-induced cell migration and invasion
(Fig. 1E–H). The results
demonstrated that ERK1/2 played an essential role in growth
factor-induced cell migration and invasion in GA cells, and
demonstrated that the ability of ERK1/2 to stimulate GA cell
migration and invasion were mediated by MEK1/2.
IL-1β activates ERK1/2 and increases cell
migration and invasion in GA cells
Recently, increased attention has been paid to
inflammatory microenvironment signaling, which has been
demonstrated to play an important role in the progression of
cancer, including cancer metastasis (23). The role of ERK1/2 in growth
factor-induced metastasis is well characterized (24,25);
however, proinflammatory factors can also activate ERK1/2. IL-1β
can activate ERK1/2 in several types of cells, including cancer
cells (26,27). To characterize whether or not IL-1β
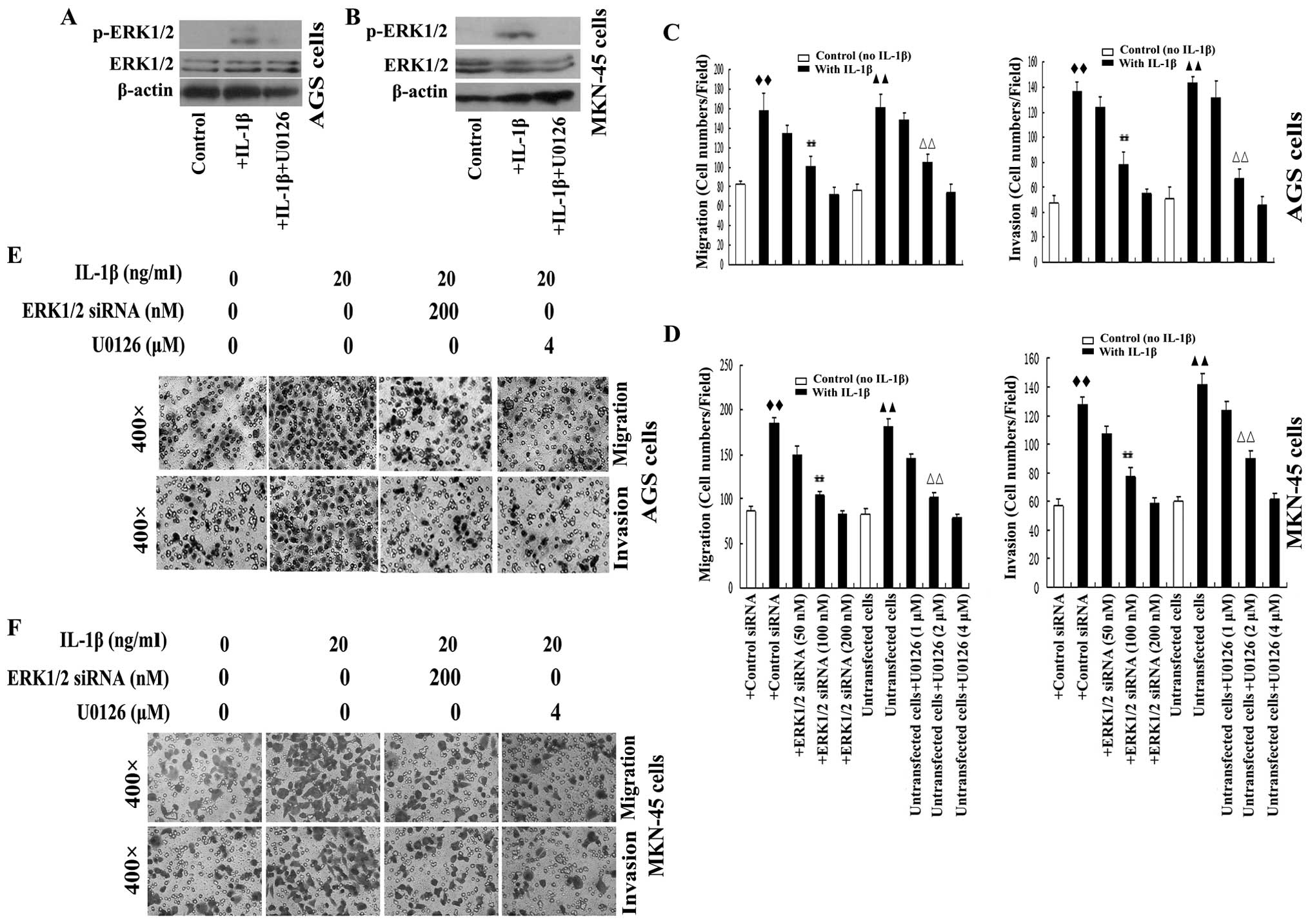
also participates in ERK1/2 mediated metastasis in GA, we treated
AGS and MKN-45 cells with IL-1β. As expected, IL-1β activated
ERK1/2 in AGS and MKN-45 cells, as expression of p-ERK1/2 was
detected after 30 min stimulation with IL-1β (Fig. 2A and B).
The effect of IL-1β on cell migration and invasion
in AGS and MKN-45 cells were further examined. AGS and MKN-45 cells
were treated with IL-1β. IL-1β increased the cell migration and
invasion of the cells. Transwell assays demonstrated that
IL-1β-induced cell migration and invasion were attenuated in a
dose-dependent manner by siRNA-mediated knockdown of ERK1/2
(Fig. 2C–F). Additionally, the
MEK/ERK inhibitor U0126 significantly suppressed IL-1β-induced AGS
and MKN-45 cell migration and invasion (Fig. 2C–F). Taken together, these results
demonstrated that IL-1β-induced GA cell metastasis were mediated by
the MEK/ERK signaling pathway, and also suggested that ERK1/2
signaling activation may play an important role in inflammatory
factor-associated GA cell migration and invasion.
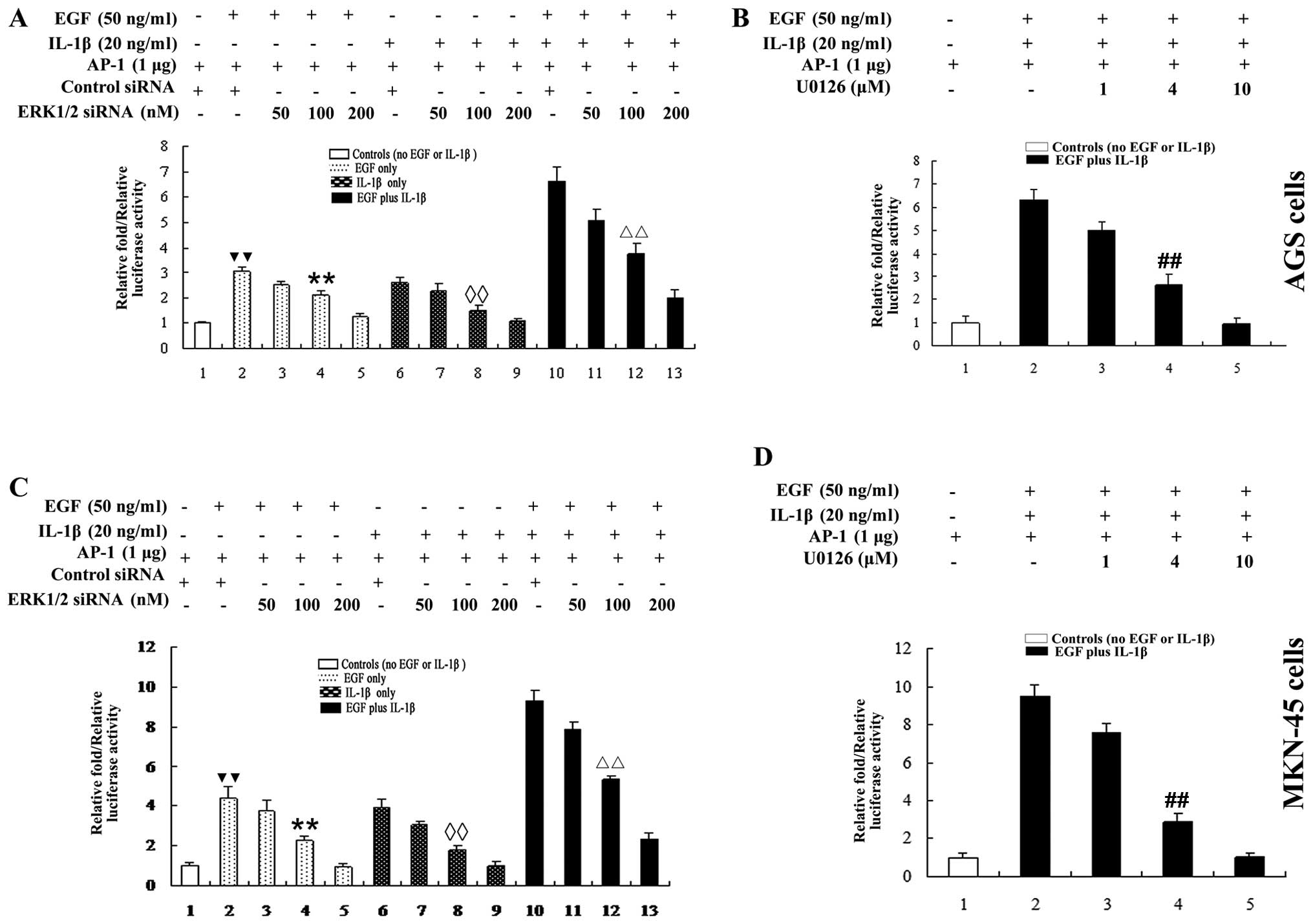
EGF and IL-1β additively increases
ERK1/2-mediated-GA cell migration and invasion
Next, we investigated whether growth and
inflammatory factors could additively affect GA cell migration and
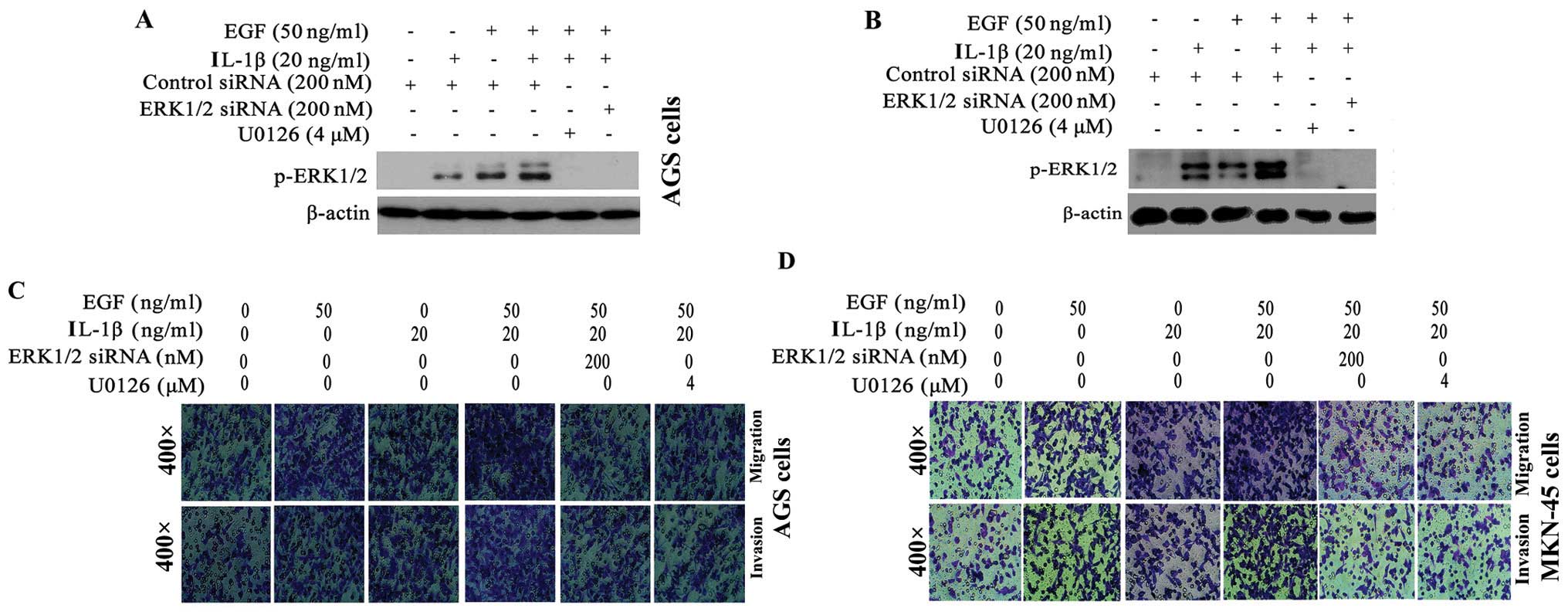
invasion. AGS and MKN-45 cells were stimulated with EGF plus IL-1β.
As shown in Fig. 3A and B, an
approximately 2-fold increase in p-ERK1/2 expression was observed
in AGS (Fig. 3A) and MKN-45
(Fig. 3B) cells treated with EGF
plus IL-1β, compared cells treated with EGF or IL-1β alone. The
ability of EGF plus IL-1β to activate ERK1/2 was almost completely
blocked by ERK1/2 siRNA or U0126. Additionally,
co-stimulation with EGF plus IL-1β additively elevated the
migration and invasion of AGS and MKN-45 cells >2-fold, compared
to cells treated with either EGF or IL-1β alone (Fig. 3C, E and F) (AGS cells) and
(Fig. 3D, G and H) (MKN-45 cells).
Therefore, growth and inflammatory factors additively promote
ERK1/2-mediated GA cell migration and invasion.
EGF and IL-1β additively upregulates
expression of MMP-9 in GA cells via activation of the ERK1/2
signaling pathway
Increased expression of MMP-9 is associated with
cancer cell migration and invasion (28,29).
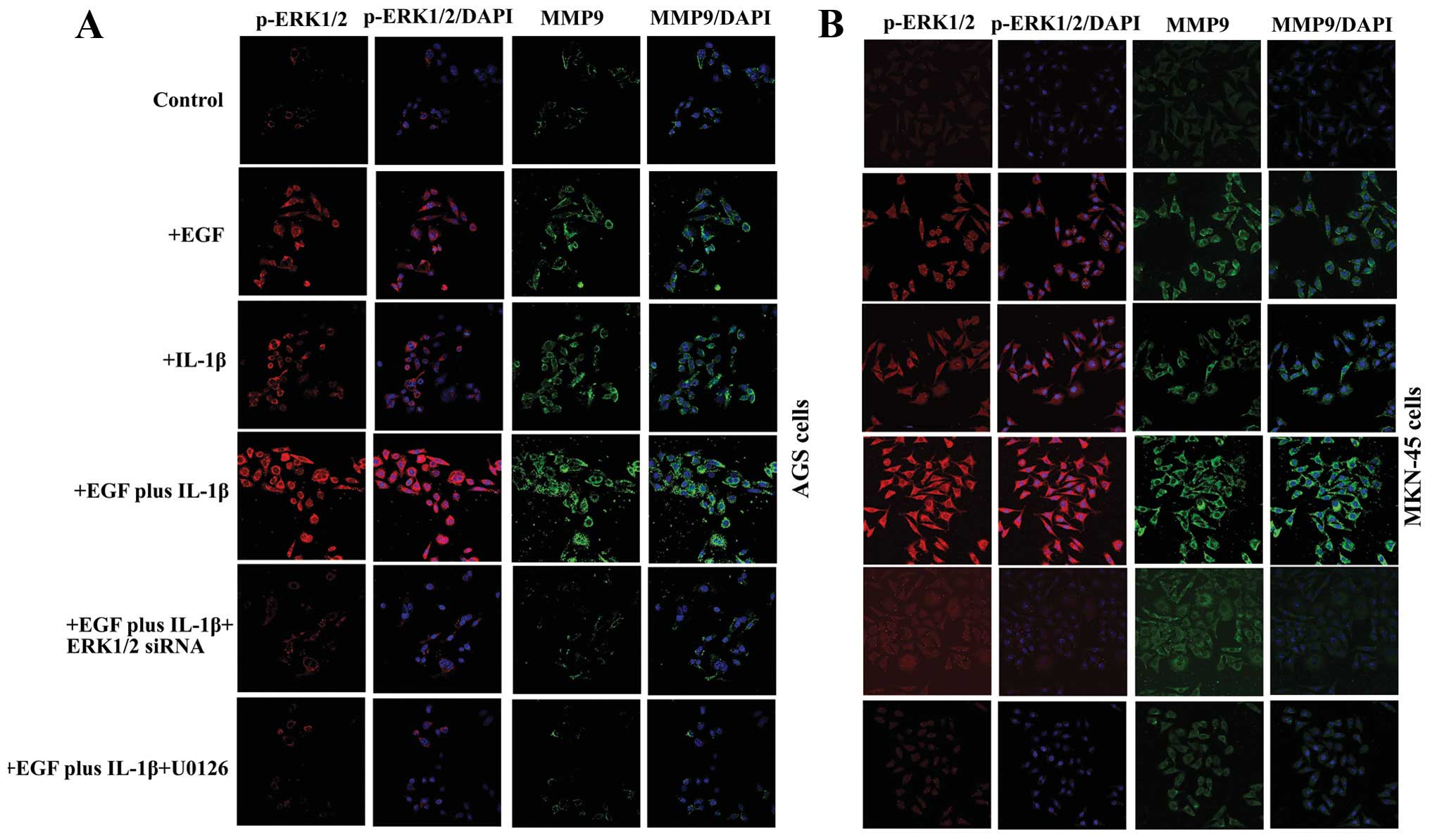
To investigate whether MMP-9 contributes to EGF and IL-1β-induced
ERK1/2-mediated metastasis, the activation of ERK1/2 and the
expression of MMP-9 were examined by confocal microscopy with a
p-ERK1/2 antibody (red fluorescently labeled), and MMP-9 antibody
(green fluorescently labeled). As shown in Fig. 4, there was only a baseline activity
of p-ERK1/2, and the expression of MMP-9 was very weak in AGS and
MKN-45 cells, without EGF or IL-1β treatment. Whereas, after
treated by EGF or IL-1β or both, the expression of p-ERK1/2 and
MMP-9 were visibly increased (Fig.
4). The most obvious elevation of the p-ERK1/2 and MMP-9 had
been observed in the cells treated with EGF plus IL-1β (Fig. 4). Pre-treatment of the cells with
pathway inhibitor U0126 or transfection of the cells with ERK1/2
siRNA, significantly suppressed EGF plus IL-1β induced ERK1/2
activation and MMP-9 upregulation (Fig. 4).
EGF and IL-1β additively activate AP-1 in
GA cells via the ERK1/2 signaling pathway
The transcription factor AP-1 regulates the
expression of MMP-9 (30) and
ERK1/2 can regulate the activation of AP-1 (31,32).
Our data revealed that both EGF and IL-1β upregulated expression of
MMP-9 in AGS (Fig. 4A) and MKN-45
(Fig. 4B) cells. In order to
understand whether AP-1 was required for the EGF and IL-1β-induced
ERK1/2-mediated upregulation of MMP-9, transcriptional activation
of AP-1 was examined in AGS and MKN-45 cells using an AP-1
luciferase reporter gene assay. As shown in Fig. 5, both EGF and IL-1β increased AP-1
reporter gene luciferase activity in AGS and MKN-45 cells; however,
co-stimulation with EGF plus IL-1β additively increased AP-1
activity >2-fold, compared to cells treated with either EGF or
IL-1β alone (Fig. 5). Inhibition
of ERK1/2 using ERK1/2 siRNA or U0126 dose-dependently
reduced AP-1 reporter gene activity in cells treated with EGF or
IL-1β alone or both EGF plus IL-1β (Fig. 5).
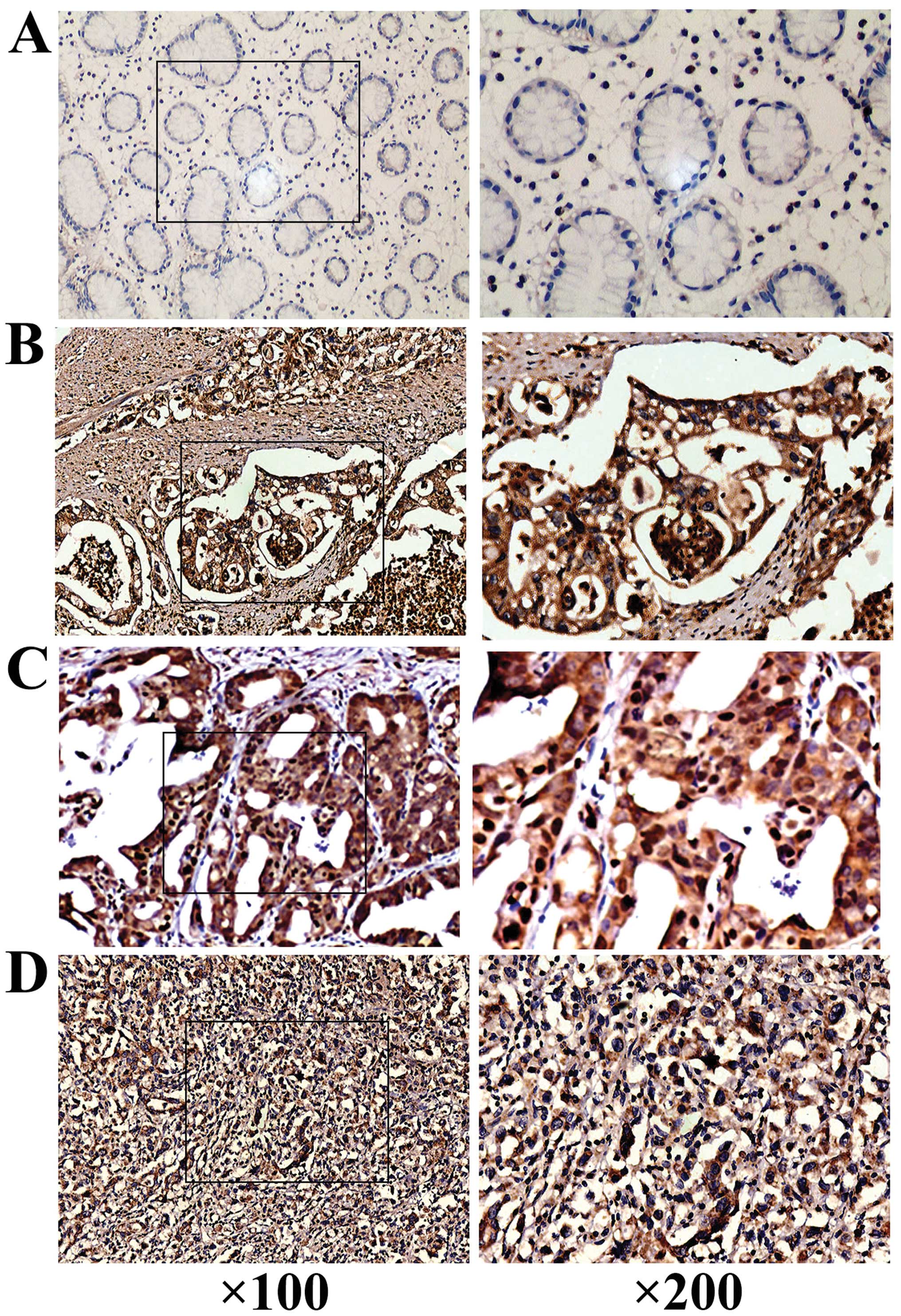
The clinicopathological features of
gastric adenocarcinoma and relationship between expression of EGF
plus IL-1β and phosphorylated ERK1/2, MMP-9, and AP-1
The expression of p-ERK1/2 with the
clinicopathological features of GA was analyzed by IHC assay. As
shown in Fig. 6A–D, elevated
levels of p-ERK1/2 were detected in GA: p-ERK1/2 was expressed or
overexpressed in 57 of the 105 GA tissues (54.29%) compared to 19
of the 105 (18.10%) non-neoplastic tissues (P= 0.006). Positive
p-ERK1/2 expression was significantly associated with higher TNM
stage, lymph node metastasis, and invasion beyond the serosa, but
not with patient age, gender, tumor size, histological type or
differentiation grade in GA (Table
I). Expression or overexpression of p-ERK1/2 had no significant
relationship with tumor size (≥3 vs. <3 cm; P=0.306), patient
age (≥50 vs. <50 years; P=0.793) or gender (male vs. female;
P=0.304). In addition, no significant difference in p-ERK
expression was observed in tumors with different histological types
(P=0.238) or differentiation grades (P=0.428). Expression of
P-ERK1/2 was detected in all four TNM stages; however, p-ERK1/2 was
more frequent in stage T4 and T3 than T2 and T1. The expression of
p-ERK1/2 was significantly different in patients with and without
lymph node metastasis (P= 0.028) and patients with and without
cancer invasion beyond serosa (P=0.020).
 | Table I.Association of p-ERK1/2 with
clinicopathological features in gastric adenocarcinoma. |
Table I.
Association of p-ERK1/2 with
clinicopathological features in gastric adenocarcinoma.
| Factor | n | Negative/unchanged
n (%) |
Positive/overexpressing n (%) | P-value |
|---|
| Total | | | | |
| GA | 105 | 48 (45.71) | 57 (54.29) | 0.006 |
| Non-neoplastic
tissues | 105 | 86 (81.90) | 19 (18.10) | |
| Age (years) | | | | |
| <50 | 25 | 12 (48.00) | 13 (52.00) | 0.793 |
| ≥50 | 80 | 36 (45.00) | 44 (55.00) | |
| Histological
type | | | | |
| Intestinal | 51 | 26 (50.98) | 25 (49.02) | 0.238 |
| Diffuse | 48 | 18 (37.50) | 30 (62.50) | |
| Unknownc | 6 | 4 (66.67) | 2 (33.33) | |
| TNM stage | | | | |
| T1 | 20 | 16 (80.00) | 4 (20.00) | 0.000 |
| T2 | 32 | 17 (53.13) | 15 (46.88) | |
| T3 | 30 | 8 (26.67) | 22 (73.33) | |
| T4 | 15 | 2 (13.33) | 13 (86.67) | |
| Unknownc | 8 | 5 (62.50) | 3 (37.50) | |
| Differentiation
grade | | | | |
| High (H) | 21 | 13 (61.90) | 8 (38.10) | 0.428 |
| Moderate (M) | 28 | 12 (42.86) | 16 (57.14) | |
| Poor (Pr) | 37 | 15 (40.54) | 22 (59.46) | |
| Signet-ring
(Sr) | 7 | 2 (28.57) | 5 (71.43) | |
| Mucinous
(Mu) | 4 | 1 (25.00) | 3 (75.00) | |
| Unknownc | 8 | 5 (62.50) | 3 (37.50) | |
| Gender | | | | |
| Male | 71 | 30 (42.25) | 41 (57.75) | 0.304 |
| Female | 34 | 18 (52.94) | 16 (47.06) | |
| Tumor size
(cm)a | | | | |
| ≥3 | 69 | 29 (42.03) | 40 (57.97) | 0.306 |
| <3 | 35 | 19 (54.29) | 16 (45.71) | |
| Unknown | 1 | 0 (0.00) | 1 (100.00) | |
| Lymph node
metastasisb | | | | |
| Positive | 56 | 20 (35.71) | 36 (64.29) | 0.028 |
| Negative | 49 | 28 (57.14) | 21 (42.86) | |
| Invasion beyond
serosa | | | | |
| Positive | 34 | 10 (29.41) | 24 (70.59) | 0.020 |
| Negative | 71 | 38 (53.52) | 33 (46.48) | |
IHC assay was also used to detect the expression of
p-ERK1/2 and EGF plus IL-1β. The expression of p-ERK1/2 exhibited
correlation with the levels of EGF alone (r=0.604, P<0.01) or
IL-β alone (r=0.502, P<0.05), but the good correlation was
determined between the levels of p-ERK1/2 with the levels of EGF
plus IL-1β in GA tissues (Fig.
6E). Strong positive staining of p-ERK1/2 was detected in the
GA samples with higher levels of EGF plus IL-1β; whereas, weak
ERK1/2 expression was detected in lower levels of EGF plus IL-1β
samples. When analyzed by Spearman’s method, a correlation
(r=0.792, P<0.001) between the expression of p-ERK1/2 and EGF
plus IL-1β was obtained.
The in vivo correlation of the expression of
IL-1β plus EGF and p-ERK1/2 with MMP-9 and AP-1 (c-fos) in GA
tissue samples was also detected by IHC. The expression of p-ERK1/2
correlated well with the levels of MMP-9 and c-fos in addition to
EGF plus IL-1β (Fig. 6E). The
results from statistical analyses showed that elevated p-ERK1/2
expression significantly correlated with the elevated expression of
EGF plus IL-1β, MMP-9 and c-fos in GA tissue samples (r=0.792,
P<0.001; r= 0.713, P<0.001; r= 0.704, P<0.001;
respectively). In vivo data further confirmed that EGF with
IL-1β might additively activate ERK1/2, which in turn upregulated
MMP-9 and c-fos expression in GA tissues.
Discussion
The EGF receptor is widely expressed in a variety of
different cells, including cancer cells (33). Extensive research has been
performed to characterize the role of EGF in cancer cell growth,
metastasis and invasion (34);
however, little is known about the effects of EGF on metastasis in
GA. In accordance with previous research in other cancer cell lines
(35,36), we observed that EGF could increase
AGS and MKN-45 cell migration, and invasion via a mechanism
mediated by ERK1/2.
It is well established that gastric cancer
carcinogenesis is closely related with inflammation. Cytokines,
especially IL-1β plays crucial roles in gastric inflammatory
reaction, and more and more evidence has suggested that
inflammatory signaling also plays important roles in cancer
progression (37). However, the
effects of inflammatory factors on the signaling pathways which
regulate metastasis are poorly characterized in GA cells, and the
combined action of EGF and IL-1β in GA cells has not been detected
previously. This study provides the first evidence that GA cell
migration and invasion can by induced by IL-1β via activation of
ERK1/2. More important, our data also demonstrated for the first
time that EGF and IL-1β additively increased GA cell migration and
invasion via activation of the ERK1/2 signaling pathway. It has
been well documented that ERK1/2 plays a crucial role in the
regulation of cancer cell metastasis (9,10);
however, the activation of ERK1/2 induced by growth factor plus
inflammatory factor and the molecular mechanisms regulating ERK1/2
signaling in GA are still unclear. Here, we also demonstrated that
ERK1/2 signaling can be activated by EGF plus IL-1β which was
significantly and dose-dependently inhibited by the MEK1/2
inhibitor U0126 or ERK1/2 siRNA. Thus, the facts further
confirmed the involvement of the ERK1/2 signaling pathway in the
ability of EGF, IL-1β or EGF plus IL-1β to increase GA cell
migration and invasion. Therefore, ERK1/2 signaling plays an
important role in both growth factor and inflammatory
factor-associated migration, and invasion in GA.
To investigate the mechanism by which EGF plus IL-1β
promoted the migration and invasion of GA cells, we investigated
the expression of MMP-9 in GA cells. The breakdown of extracellular
matrix (ECM) is an initial, critical step during cancer cell
metastasis (38), and the
involvement of MMPs in ECM degradation has been widely documented
(38,39). MMP-9 is closely associated with
cancer invasion and metastasis (30,40),
as it degrades collagen in the basement membrane, allowing cancer
cells to pass through the ECM and spread to the surrounding
tissues. Our results revealed that EGF and IL-1β additively induced
GA cell migration and invasion via activation of ERK1/2, which in
turn elevated MMP-9 expression. Increased MMP-9 expression and
activity have previously been identified as one of the major
mechanisms by which ERK1/2 mediates cancer cell metastasis
(30,39). As a target of ERK1/2 signaling, the
transcription factor AP-1 can regulate the expression of MMP-9
(41). EGF and IL-1β additively
increased AP-1 reporter gene luciferase activity, and this effect
could be inhibited by ERK1/2 siRNA and U0126, confirming
that the ability of EGF and/or IL-1β to activate AP-1 and
upregulate MMP-9 were dependent on ERK1/2 signaling.
In order to verify the above results in vivo,
the expression of p-ERK1/2, EGF plus IL-1β, MMP-9 and AP-1 (c-fos)
in GA tissues were analyzed. The data exhibited that the expression
of p-ERK1/2 correlated well with the levels of EGF plus IL-1β,
MMP-9 and AP-1 in GA tissue, and the expression of p-ERK1/2 was
detected in more than 50% of the human GA tissues tested, which was
closely related to high TNM stage, tumor invasion beyond the serosa
and lymph node metastasis. Therefore, both in vitro and
in vivo data demonstrated that growth and inflammatory
factors additively promote metastasis in GA, by increasing the
migration and invasion of GA cells via activation of ERK1/2
signaling. Taken together, our data demonstrate that growth factor
and inflammatory factor-induced activation of ERK1/2 may promote
cancer cell metastasis in GA.
In conclusion, the results of this study demonstrate
that overexpression of p-ERK1/2 is closely associated with
metastasis in GA, which correlated well with EGF plus IL-1β. EGF
and IL-1β additively increased GA cell migration and invasion via
activation of the ERK1/2 signaling pathway, leading to increased
AP-1 transcriptional activity and upregulation of MMP-9.
ERK1/2/EGF/IL-1β pathways may be closely associated with GA
progression.
Acknowledgements
This study was supported by the
National Natural Science Foundation of China (Grant No. 81372788),
the Medical Scientific Research Key Foundation of Nanjing Command
(No. 11Z032), the Army Clinical High and New Technology Major
Project (No. 2010 gxjs026) and the Medical Scientific Research
Foundation of Nanjing Command (No. 11MA107).
References
|
1.
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar
|
|
2.
|
Ajani JA: Optimizing docetaxel
chemotherapy in patients with cancer of the gastric and
gastroesophageal junction evolution of the docetaxel, cisplatin,
and 5-fluorouracil regimen. Cancer. 113:945–955. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Yoo YA, Kang MH, Lee HJ, et al: Sonic
hedgehog pathway promotes metastasis and lymphangiogenesis via
activation of Akt, EMT, and MMP-9 pathway in gastric cancer. Cancer
Res. 71:7061–7070. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Mebratu Y and Tesfaigzi Y: How ERK1/2
activation controls cell proliferation and cell death: Is
subcellular localization the answer? Cell Cycle. 8:1168–1175. 2009.
View Article : Google Scholar
|
|
5.
|
Balmanno K and Cook SJ: Tumour cell
survival signalling by the ERK1/2 pathway. Cell Death Differ.
16:368–377. 2009. View Article : Google Scholar
|
|
6.
|
Abe MK, Saelzler MP, Espinosa R III, et
al: ERK8, a new member of the mitogen-activated protein kinase
family. J Biol Chem. 277:16733–16743. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Xu YM, Zhu F, Cho YY, et al: Extracellular
signal-regulated kinase 8-mediated c-Jun phosphorylation increases
tumorigenesis of human colon cancer. Cancer Res. 70:3218–3227.
2010. View Article : Google Scholar
|
|
8.
|
Adams DG, Coffee RL Jr, Zhang H, et al:
Positive regulation of Raf1-MEK1/2-ERK1/2 signaling by protein
serine/threonine phosphatase 2A holoenzymes. J Biol Chem.
280:42644–42654. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Bai Y, Luo Y, Liu S, et al: PRL-1 protein
promotes ERK1/2 and RhoA protein activation through a non-canonical
interaction with the Src homology 3 domain of p115 Rho
GTPase-activating protein. J Biol Chem. 286:42316–42324. 2011.
View Article : Google Scholar
|
|
10.
|
Guturi KK, Mandal T, Chatterjee A, et al:
Mechanism of β-catenin-mediated transcriptional regulation of
epidermal growth factor receptor expression in glycogen synthase
kinase 3 β-inactivated prostate cancer cells. J Biol Chem.
287:18287–18296. 2012.
|
|
11.
|
Takeuchi A, Eto M, Shiota M, Tatsugami K,
et al: Sunitinib enhances antitumor effects against
chemotherapy-resistant bladder cancer through suppression of ERK1/2
phosphorylation. Int J Oncol. 40:1691–1696. 2012.
|
|
12.
|
Nakata W, Hayakawa Y, Nakagawa H, et al:
Anti-tumor activity of the proteasome inhibitor bortezomib in
gastric cancer. Int J Oncol. 39:1529–1536. 2011.PubMed/NCBI
|
|
13.
|
Leonardi GC, Candido S, Cervello M, et al:
The tumor micro-environment in hepatocellular carcinoma. Int J
Oncol. 40:1733–1747. 2012.PubMed/NCBI
|
|
14.
|
Soria G, Ofri-Shahak M, Haas I, et al:
Inflammatory mediators in breast cancer: coordinated expression of
TNFα and IL-1β with CCL2 and CCL5 and effects on
epithelial-to-mesenchymal transition. BMC Cancer.
11:1302011.PubMed/NCBI
|
|
15.
|
Yang HT, Cohen P and Rousseau S:
IL-1beta-stimulated activation of ERK1/2 and p38 alpha MAPK
mediates the transcriptional up-regulation of IL-6, IL-8 and
GRO-alpha in HeLa cells. Cell Signal. 20:375–380. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Tsai CY, Lee TS, Kou YR, et al:
Glucosamine inhibits IL-1beta-mediated IL-8 production in prostate
cancer cells by MAPK attenuation. J Cell Biochem. 108:489–498.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Huang Q, Yang J, Lin Y, et al:
Differential regulation of interleukin 1 receptor and Toll-like
receptor signaling by MEKK3. Nat Imm. 5:98–103. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Huang Q, Lan F, Zheng Z, et al: Akt2
suppresses GAPDH mediated-apoptosis in ovarian cancer cells via
phosphorylating gapdh at threonine 237 and decreasing its nuclear
translocation. J Biol Chem. 286:42211–42220. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Sumida T, Itahana Y, Hamakawa H, et al:
Reduction of human metastatic breast cancer cell aggressiveness on
introduction of either form A or B of the progesterone receptor and
then treatment with progestins. Cancer Res. 64:7886–7892. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Huang QJ, Huang QL, Chen WN, et al:
Identification of transgelin as a potential novel biomarker for
gastric adenocarcinoma based on proteomics technology. J Cancer Res
Clin Oncol. 134:1219–1227. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Ebert M, Yokoyama M, Kobrin MS, et al:
Induction and expression of amphiregulin in human pancreatic
cancer. Cancer Res. 54:3959–3962. 1994.PubMed/NCBI
|
|
22.
|
Ju XZ, Yang JM, Zhou XY, et al: Emmprin
expression as a prognostic factor in radiotherapy. Clin Cancer Res.
12:494–501. 2008.
|
|
23.
|
Chen MF, Lu MS, Chen PT, et al: Role of
interleukin 1 beta in esophageal squamous cell carcinoma. J Mol Med
(Berl). 90:89–100. 2012. View Article : Google Scholar
|
|
24.
|
Park S, Jung HH, Park YH, et al: ERK/MAPK
pathways play critical roles in EGFR ligands-induced MMP1
expression. Biochem Biophys Res Commun. 407:680–686. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
25.
|
Komurov K, Padron D, Cheng T, et al:
Comprehensive mapping of the human kinome to epidermal growth
factor receptor signal. J Biol Chem. 285:21134–21142. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
26.
|
Angst E, Reber HA, Hines OJ, et al:
Mononuclear cell-derived interleukin-1 beta confers chemoresistance
in pancreatic cancer cells by upregulation of cyclooxygenase-2.
Surgery. 144:57–65. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27.
|
Arakawa T, Hayashi H, Itoh S, et al:
IL-1-induced ERK1/2 activation up-regulates p21(Waf1/Cip1) protein
by inhibition of degradation via ubiquitin-independent pathway in
human melanoma cells A375. Biochem Biophys Res Commun. 392:369–372.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
28.
|
Deryugina EI and Quigley JP: Matrix
metalloproteinases and tumor metastasis. Cancer Metastasis Rev.
25:9–34. 2006. View Article : Google Scholar
|
|
29.
|
Yadav VR, Prasad S, Gupta SC, et al:
3-Formylchromone interacts with cysteine 38 in p65 protein and with
cysteine 179 in IκBα kinase, leading to down-regulation of nuclear
factor-κB (NF-κB)-regulated gene products and sensitization of
tumor cells. J Biol Chem. 287:245–256. 2012.PubMed/NCBI
|
|
30.
|
Han H, Du B, Pan X, et al: CADPE inhibits
PMA-stimulated gastric carcinoma cell invasion and matrix
metalloproteinase-9 expression by FAK/MEK/ERK mediated AP-1
activation. Mol Cancer Res. 8:1477–1488. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31.
|
Babykutty S, Suboj P, Srinivas P, et al:
Insidious role of nitric oxide in migration/invasion of colon
cancer cells by upregulating MMP-2/9 via activation of cGMP-PKG-ERK
signaling pathways. Clin Exp Metastasis. 29:471–492. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
32.
|
Fujisawa T, Joshi BH and Puri RK: IL-13
regulates cancer invasion and metastasis through IL-13Rα2 via
ERK/AP-1 pathway in mouse model of human ovarian cancer. Int J
Cancer. 131:344–356. 2012.PubMed/NCBI
|
|
33.
|
Han W and Lo HW: Landscape of EGFR
signaling network in human cancers: biology and therapeutic
response in relation to receptor subcellular locations. Cancer
Lett. 318:124–134. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34.
|
Yotsumoto F, Sanui A, Fukami T, et al:
Efficacy of ligand-based targeting for the EGF system in cancer.
Anticancer Res. 29:4879–4885. 2009.PubMed/NCBI
|
|
35.
|
Schäfer B, Gschwind A and Ullrich A:
Multiple G-protein-coupled receptor signals converge on the
epidermal growth factor receptor to promote migration and invasion.
Oncogene. 23:991–999. 2004.PubMed/NCBI
|
|
36.
|
Cragg MS, Kuroda J, Puthalakath H, et al:
Gefitinib-induced killing of NSCLC cell lines expressing mutant
EGFR requires BIM and can be enhanced by BH3 mimetics. Plos Med.
4:1681–1689. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
37.
|
Kaler P, Godasi BN, Augenlicht L, et al:
The NF-kappaB/AKT-dependent induction of Wnt signaling in colon
cancer cells by macrophages and IL-1beta. Cancer Microenviron.
2:69–80. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
38.
|
Rucci N, Sanità P and Angelucci A: Roles
of metalloproteases in metastatic niche. Curr Mol Med. 11:609–622.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
39.
|
Luo Y, Liang F and Zhang ZY: PRL1 promotes
cell migration and invasion by increasing MMP2 and MMP9 expression
through Src and ERK1/2 pathways. Biochemistry. 48:1838–1846. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
40.
|
Deryugina EI and Quigley JP: Matrix
metalloproteinases and tumor metastasis. Cancer Metastasis Rev.
25:9–34. 2006. View Article : Google Scholar
|
|
41.
|
Huang C, Ma WY and Dong Z: The
extracellular-signal-regulated protein kinases (Erks) are required
for UV-induced AP-1 activation in JB6 cells. Oncogene.
18:2828–2835. 1999. View Article : Google Scholar : PubMed/NCBI
|