Introduction
Hepatocellular carcinoma (HCC) is the most common
aggressive carcinoma of liver and ranks as the sixth most common
malignancy with an annual incidence of >560,000 deaths (1,2).
Despite recent advances made in the diagnosis and clinical
treatment of this tumor, the global mortality rates of HCC is still
high and the prognosis of patients is very poor, with a dismal 10%
5-year overall survival rate (2,3).
Substantial research has been performed to investigate the etiology
of hepatoma; however, the accurate molecular mechanism underlying
the pathogenesis and progression of HCC remains undefined.
Therefore, to better understand the mechanism associated with
hepatoma progression is vital for explore novel therapeutic
strategies for hepatoma patients.
MicroRNAs (miRNAs) are known to be highly conserved,
small non-coding RNA molecules with 18–24 nucleotides in length. As
a new class of gene regulators, they can inversely regulate gene
transcription or translation by interacting with the
3′-untranslated region (3′-UTR) of a target gene (4). Emerging evidence has confirmed the
abnormal expression of miRNAs in various cancers, including glioma,
non-small cell lung cancer, colon cancer and pancreatic cancer
(5–7). It has been reported that miRNAs can
regulate the multiple biological processes from cell proliferation,
apoptosis, invasion to metastasis and survival (8,9).
When downregulated, miRNAs may act as oncogenes by the targeted
inhibition of tumor-suppressor gene expression, while their
increase ranks as tumor suppressor affect in tumorigenesis
(10). Among them, miRNA-451
(miR-451) has attracted increasing attention due to its critical
roles in the development and progression of several types of
tumors, such as nasopharyngeal carcinoma, esophageal carcinoma and
glioma (11–13). MiR-451 gene is located on human
chromosome 17 at 17q11.2, and its dysregulated expression has been
widely confirmed in malignancies. In nasopharyngeal cancer, miR-451
expression levels are significantly decreased in nasopharyngeal
cells and tissues; while its upregulation inhibited cell viability,
migration, invasion and therefore results in the suppression of
xenograft tumor growth, indicating a pivotal role of miR-451 in the
initiation and progression of nasopharyngeal cancer (11). Furthermore, increased expression of
miR-451 inhibits glioblastoma cell proliferation and induces cell
apoptosis (14). However, the
functions and mechanism of miR-451 in hepatoma development and
progression is still unclear.
In this study, we found that miR-451 was
downregulated in various hepatoma cells, and it as a negative
regulator of cell growth and invasion. Moreover, the underlying
molecular mechanism was also explored in the present
investigation.
Materials and methods
Reagents and antibodies
The general caspase inhibitor (z-VAD-fmk) was
obtained from Beyotime Institute of Technology (Shanghai, China).
The polyclonal antibodies against human caspase-3 were from Santa
Cruz Biotechnology (Santa Cruz, CA, USA). Rabbit anti-human MMP-9
antibodies were from Chemicon (Temecula, CA, USA).
Cell culture
Human liver cancer cell lines HepG2, HCCLM3 and
SNU449 were obtained from the American Type Culture Collection
(ATCC, Rockville, MD, USA). HepG2 and HCCLM3 cells were cultured
according to the recommendations in Dulbecco’s modified Eagle’s
medium (DMEM) containing 10% fetal bovine serum, 100 μg/ml
streptomycin and penicillin. SNU449 and immortalized normal human
liver cell lines L02 (ATCC) were maintained in complete RPMI-1640
medium supplemented with 10% fetal calf serum. L02 cells were used
as ‘normal’ controls for HCC analysis. All the cells were cultured
in a humidified atmosphere at 37°C with 5% CO2.
Oligonucleotide transfection
To specifically induce miR-451 expression in HCCLM3
and SNU449, the miRIDIAN™ miR-451 mimics were introduced. The
miR-451 mimics and scrambled control microRNA sequences were used
as previously described (14) and
obtained from GenePharma (Shanghai, China). For transfection,
1×106 cells were seeded into 24-well plates and grown
overnight until 50–80% confluence. After washing, 0.4 nmol microRNA
mimics were mixed with 15 μl Geneporter 2 Transfection reagent
(GTS, San Diego, CA, USA), followed by the transfection into cells
for 6 h. The medium was replaced with fresh medium for 48 h; the
expression of miR-451 was then confirmed by quantitative PCR.
Real-time PCR
Total RNA from cells was extracted using the RNAiso
plus kit (Roche Diagnostics, Mannheim, Germany) according to the
manufacturer’s instructions. Then, the isolated RNA was
reverse-transcribed to synthesize the first strand cDNA with the
oligo(dT)18 primer using the cDNA synthesis kit (Fermentas). To
assess the expression levels of miR-451 in hepatoma cells, the
obtained cDNA was used as a template to perform PCR amplification
using SYBR® Premix Ex Taq™ II kit (Takara). The specific
primers for miR-451, U6 and MMP-9 were used as described previously
(14,15). Each 20 μl reaction system comprised
2 μl of cDNA, 10 μl SYBR Premix Ex Taq II, 10 μmol/l of both sense
and antisense primers. For normalization, β-actin and U6 were used
to normalize mRNA and miRNA, respectively. Each experiment
contained at least three replicates, and the results were
calculated according to the method 2−ΔΔCt.
MTT assays
To evaluate cell proliferation, the MTT assay was
performed. Briefly, cells were seeded into 96-well plates at a
density of 1×105 cells/well. After treatment with
miR-451 and miR-control, ~20 μl MTT reagent (5 mg/ml) was added
into each well and then incubated for a further 6 h at 37°C. Then,
the supernatant was replaced with 200 μl isopropanol to dissolve
formazan production. Cell viability was assessed by measuring the
absorbance of MTT at 590 nm using a micro-ELISA reader (Bio-Rad).
All samples were performed in triplicate and the results are
presented as the percentage of growth inhibition.
Quantification analysis of live and dead
cells
After transfection with miR-451 mimics or control
microRNA, cells were collected and rinsed with PBS three times.
Then, cells were incubated with PBS solution consisting of 2 μM
calcein AM and 4 μM PI in the dark for 20 min. Followed by washing,
PBS was added to resuspend cells. The fluorescence of calcein AM
and PI was analyzed by flow cytometry (Becton-Dickinson), and
separately represented as the viable cells and dead cells.
Flow cytometry analysis of apoptosis
To quantitatively evaluate the rate of apoptosis,
Annexin V-propidium iodide (AV-PI) staining was performed. Briefly,
after pretreatment with caspase inhibitor z-VAD-fmk, the
transfected cells were harvested and washed three times with PBS.
Then, cells were centrifuged for 10 min, followed by resuspension
in 500 μl of binding buffer including 5 μl FITC-conjugated Annexin
V. Following incubation for 10 min in the dark, 5 μl of PI was
added. Ultimately, all specimens were assessed by flow cytometry
with a FACSCalibur using the CellQuest software (BDIS), and all the
results are shown as a percentage of total cells counted.
DNA fragmentation assay
Following stable transfection with miR-451 mimics or
control microRNA, cells were collected by centrifugation at 1500
rpm for 5 min. Then, cells were rinsed with PBS for three times,
followed by the fixation with 4% paraformaldehyde for 30 min. After
washing with PBS, the DNA-specific fluorescent dye Hoechst 33258
(Molecular Probes, Inc., Eugene, OR, USA) was added at 37°C for 0.5
h to highlight the characteristic morphological changes of
apoptosis in HCC cells. After centrifugation, cells were
resuspended in PBS to observe the nuclear morphology under a
fluorescence microscope.
Invasion assay
Invasiveness of HCC cells were measured by a
modified Boyden chamber (BD Bioscience, Bedford, MA, USA). Cells
overexpressing miR-451 were treated with antibodies against MMP-9
or control for 4 h, and then were seeded in the upper compartment.
The medium including 10% fetal bovine serum was added into the
lower compartment. Forty-eight hours later, cells that passed
through the lower side of the membrane were stained with
hematoxylin and eosin (Sigma) and quantified by counting six
high-powered fields in the center of each well.
Western blotting
Total protein from HCC cells were extracted by RIPA
lysis buffer (Beyotime, Nantong, China) and quantified using the
BCA assay (Pierce, Rockford, IL, USA). After electrophoresis with
9% SDS-PAGE, the targeted proteins were transferred to a
polyvinylidene difluoride (PVDF) membrane (Millipore). Following
blocking with buffer containing 5% non-fat dry milk in
Tris-buffered saline with Tween, the membranes were incubated with
anti-caspase-3 and MMP-9 antibodies for 1 h. Then, cells were
washed with TBST and cultured with the HRP-conjugated secondary
antibodies for 1 h. The LumiGLo reagent (Pierce) was introduced to
visualize the bound antibodies. The protein expression levels were
normalized by β-actin.
Statistical analysis
All the results are presented as mean ± SD. The
Student’s t-test was used to assess the statistical significance of
differences between groups. The data were analyzed by SPSS 11.0.
P<0.05 was considered statistically significant.
Results
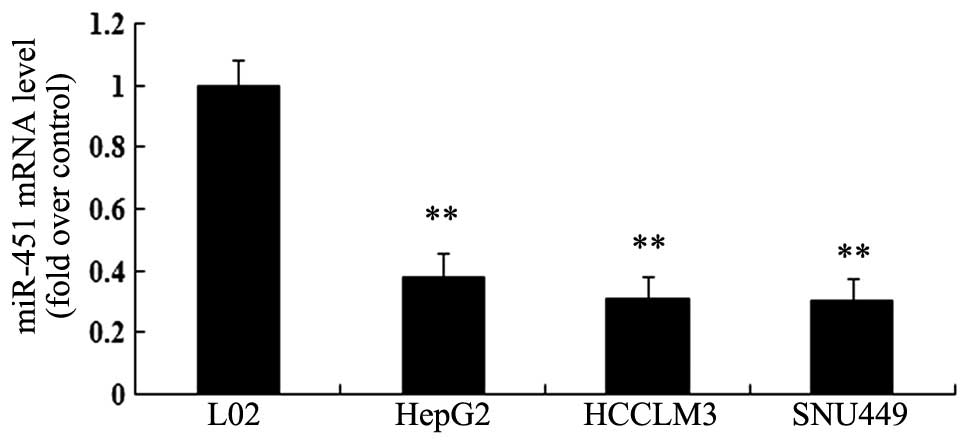
Expression of miR-451 is decreased in HCC
cell lines
Expression of miR-451 is significantly downregulated
in several tumors and exerts a vital role in the progression of
cancer. However, the related research on miR-451 in hepatoma
remains poorly understood. In this study, we examined miR-451
expression in three hepatoma cell lines (HepG2, HCCLM3 and SNU44).
As shown in Fig. 1, the expression
levels of miR-451 were obviously lower in three HCC cell lines than
in the immortalized normal human liver cell line L02. Collectively,
these results suggested a dramatical downregulation of miR-451 in
HCC cells.
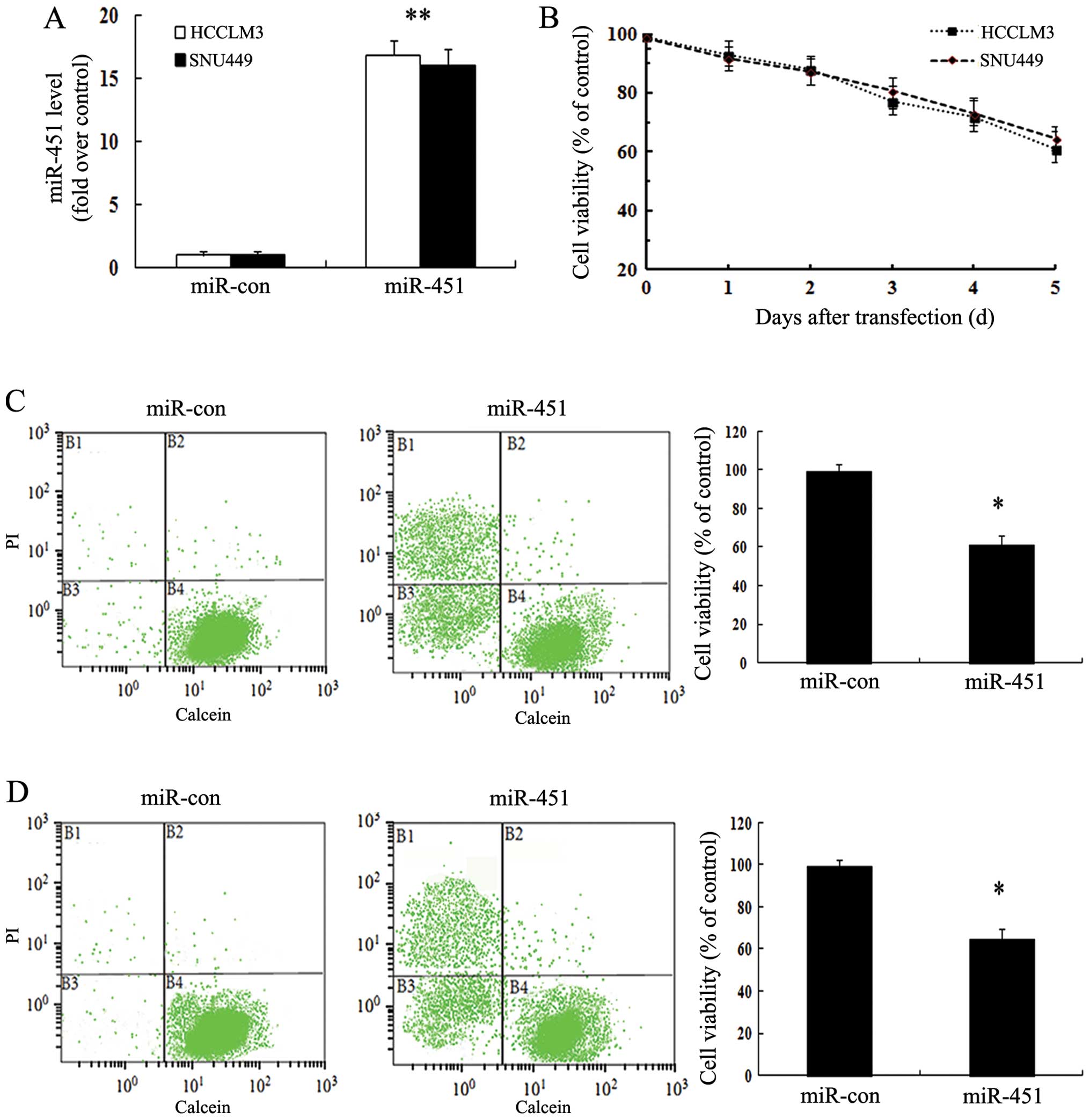
Overexpression of miR-451 abrogates
SNU449 cell viability
Though research has been performed to explore the
function of miR-451 in cancer, its effect on hepatoma remains
undefined. To investigate the effect of miR-451 on the progression
of hepatoma, we successfully upregulated the expression levels of
miR-45 in HCCLM3 and SNU449 cells by the transfection with miR-21
mimics as detecting by RT-PCR (Fig.
2A). Further MTT analysis confirmed that miR-451 overexpression
attenuated the growth of HCCLM3 and SNU449 (Fig. 2B). Moreover, cell growth inhibitory
effect was obviously increased with the gradually increasing
transfection times. To further corroborate the above results, we
introduced the fluorescent probes calcein AM and PI to analyze the
live/dead cells. Compared with the control group, the increased
fluorescent signals of PI in regions B1 and B3 and reduced calcein
AM signals in region B4 were examined, indicating that fewer live
cells were observed after miR-451 upregulation in HCCLM3 (Fig. 2C). Consistently, a similar
inhibitory effect of miR-451 in cell viability was also validated
in SNU449 (Fig. 2D). Together,
these data indicated that miR-451 overexpression attenuated HCC
cell viability.
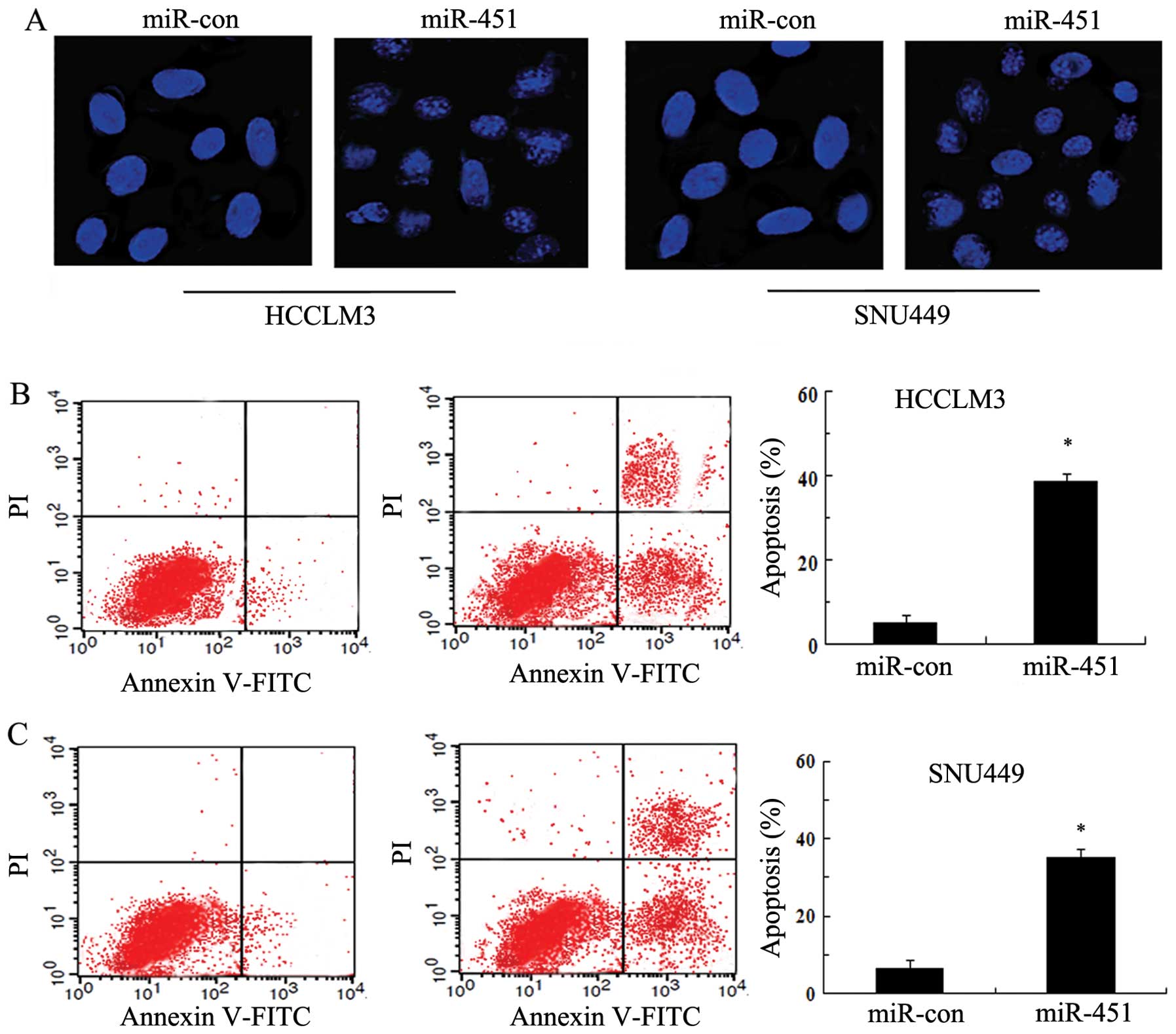
MiR-451 enhances cell apoptosis in SNU449
cells
It is known that DNA fragmentation and loss of
plasma membrane asymmetry are characteristics of cell death
(13). To explore whether cell
apoptosis is associated with HCC cell growth inhibition induced by
miR-451, we observed the morphological changes in the nuclei by
Hoechst 33258 staining. As shown in Fig. 3A, miR-451 overexpression triggered
a significant increase in nuclear shrinkage and DNA fragmentation,
implying a critical role of miR-451 in hepatoma cell apoptosis by
disrupting the nuclear morphology. Furthermore, the induction of
apoptosis was further manifested by Annexin V-FITC and PI staining.
After transfection with miR-451 mimics, a dramatical upregulation
in the number of apoptotic cells was observed; and the apoptotic
rate was 38.21% in HCCLM3 (Fig.
3B) and 34.69% in SNU449 (Fig.
3C), respectively. Therefore, these results revealed that
miR-451 upregulation induced HCC cell apoptosis.
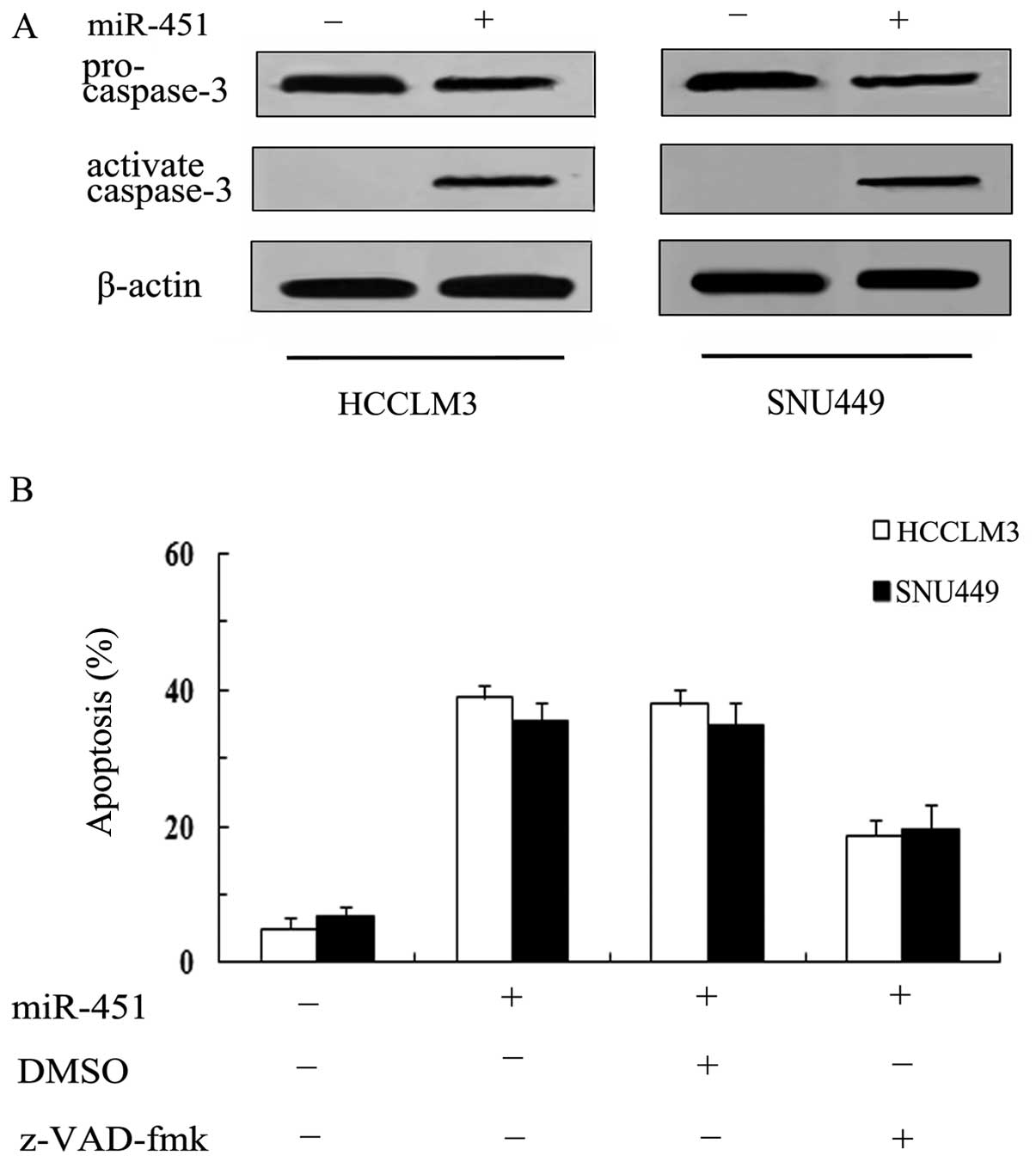
Caspase-3 is responsible for
miR-451-induced cell apoptosis
Caspase-3 has been reported to be the major effector
in apoptotic pathways, and its cleavage is a characteristic of cell
death. The fact that miR-451 can induce hepatoma cell apoptosis is
corroborated above. However, the accurate molecular mechanism of
action remains unclear. Therefore, we investigated the effect of
caspase-3. As shown in Fig. 4A,
miR-451 transfection strikingly induced the expression of cleaved
caspase-3 levels, compared with control group. To further confirm
the involvement of caspase-3 in miR-451-induced apoptosis in HCC
cells, we blocked the expression of caspase-3 with a general
caspase inhibitor z-VAD-fmk, after which we observed a remarkable
decrease in miR-451-induced apoptotic rate from 38.21 to 18.72% in
HCCLM3 and from 34.98 to 19.6% in SNU449 (Fig. 4B), suggesting that miR-451
overexpression may induce hepatoma cell apoptosis in
caspase-3-dependent manner.
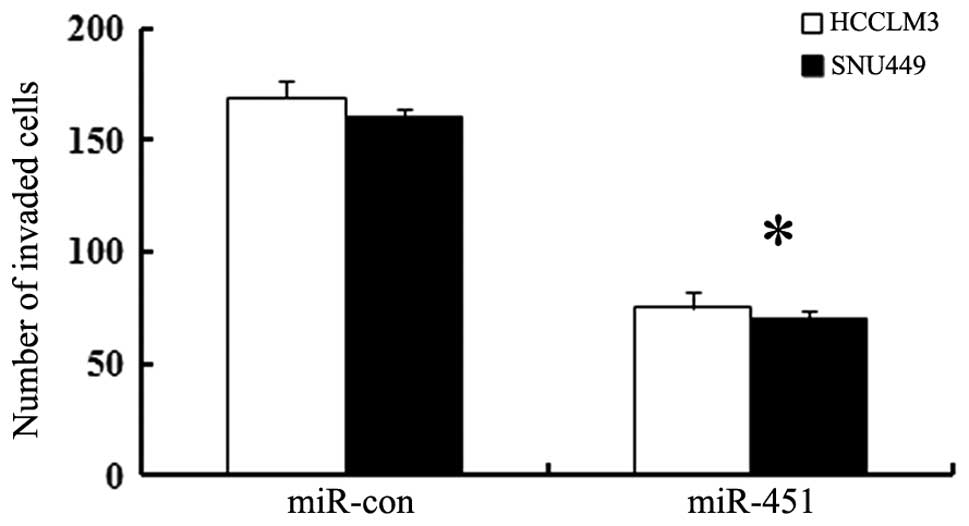
Cell invasive ability was impeded by
miR-451 mimic transfection
To investigate whether miR-451 is associated with
hepatoma cell invasion, we transfected HCCLM3 and SNU449 cell lines
with miR-451 oligonucleotide mimics or control. Approximately 48 h
later, cell invasive ability was analyzed by Transwell assay. As
shown in Fig. 5, the number of
HCCLM3 cells invading through the Matrigel after miR-451
transfection with mimics was remarkablely attenuated from 169 to
75. Similar reduction in cell invasive activity was observed in
miR-451-overexpressed SNU449 cells. Hence, these data indicated a
potential negative regulatory effect of miR-451 on HCC cell
invasion.
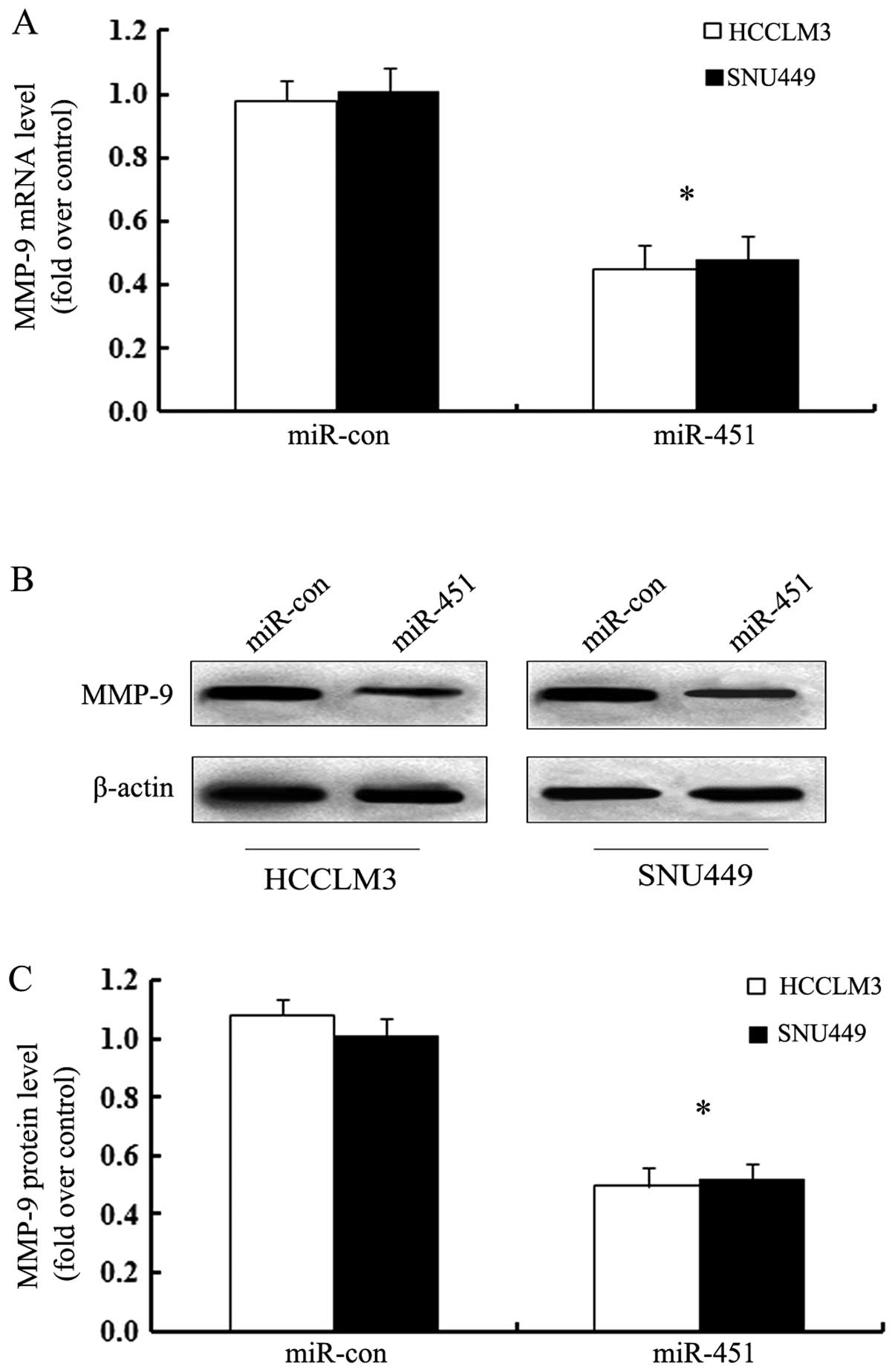
MiR-451 transfection attenuates cell
invasion by MMP-9
The extracellular matrix metalloproteinase MMP-9 is
overexpressed in various cancers and critical for the invasive
potential of tumors, including hepatoma (16,17).
To further address the molecular mechanism involved in
miR-451-inhibited HCC cell invasion, we explored MMP-9 as a
potential target. As shown in Fig.
6A, obvious downregulation of MMP-9 mRNA was identified in HCC
cell when transfected with miR-451 mimics. Simultaneously, the
corresponding decrease in MMP-9 protein levels was also confirmed
by western blotting (Fig. 6B).
Quantitative analysis suggested that overexpression of miR-451
resulted in 0.495-fold decrease of MMP-9 in HCCLM3 and 0.52-fold in
SNU449 than in the control (Fig.
6C). These data suggested that miR-451 may inhibit hepatoma
cell invasion by MMP-9 expression.
Discussion
Hepatocellular carcinoma (HCC) ranks as the most
common type of primary lethal neoplasm of liver cancer and is
considered to be the third leading cause of cancer-related deaths
worldwide (1). The high rate of
invasion and metastasis results in >560,000 deaths worldwide
each year (3). Therefore, to
explore the mechanisms related to the progression and new
biomarkers for the malignant potential of hepatocellular cancer are
urgently needed.
MicroRNAs are known as a group of small non-coding
RNAs possessing critical roles in multiple physiological processes
including cell proliferation, differentiation, apoptosis and
development (18,19). During the past decade, accumulating
evidence has demonstrated a prominent role of miRNAs in the
development and progression of various cancers by acting as
oncogenes and tumor suppressor genes (10,20).
It has been reported that many tumor suppressor miRNAs, such as
miR-302b, exert a reverse response to cancer cell proliferation,
growth, angiogenesis, invasion and metastasis signaling (21). Among them, miR-451 has received
increasing attention due to its important function in the
development of several cancer, including nasopharyngeal carcinoma,
esophageal carcinoma and glioma (11,13,14).
In this study, our results revealed a notable downregulation of
miR-451 in HCC cells, consistent with its abnormal expression in
nasopharyngeal carcinoma and glioma cells. Moreover, overexpression
of miR-451 effectively decreased HCC cell viability, indicating
that miR-451 upregulation induced cell death. According to these
results miR-451 might be as a novel tumor suppressor regulating the
progression of HCC.
It is widely accepted that many antitumor agents
influence growth inhibition effect on malignant cells through
inducing apoptosis, which is characterized with DNA fragmentation
and loss of plasma membrane asymmetry (22–24).
In the present study, transfection of miR-451 mimics promoted
significantly DNA fragmentation and morphological changes in HCC
cells. Moreover, the elevated miR-451 expression enhanced the
apoptotic rates, implying that cell apoptosis was involved in
miR-451-induced hepatoma cell growth inhibition. However, how
miR-451 induces cell apoptosis remains to be further explored.
Caspase-3 is a downstream effector of
cysteine-aspartic acid protease participating in programmed cell
death, and its sequential activation plays an important role in the
execution-phase of cell apoptosis (25,26).
Numerous studies have demonstrated that caspase-3 is overexpressed
in diverse malignancies, including HCC (27). Therefore, we speculate that miR-451
could induce cell apoptosis by caspase-3 signaling. To address this
hypothesis, we analyzed the expression levels of caspase-3 in
miR-451-transfected HCC cells. As expected, miR-451 overexpression
significantly induced the expression of activated caspase-3. When
blocking its activity with the specific caspase inhibitor
z-VAD-fmk, the miR-451-triggered cell apoptotic rate was
dramatically abrogated. Therefore, we can conclude that miR-451
induced cell death via caspase-3-regulated pro-apoptotic
pathways.
miR-451 has been confirmed to be frequently
downregulated in various cancers, including breast cancer,
nasopharyngeal carcinoma and lung cancer (7,12,14).
Importantly, its dysregulation is associated with tumor
progression, including cell proliferation, growth, migration and
invasion (11,28). It is known that the higher invasion
and metastasis is the main cause of death in HCC patients.
Therefore, to better understanding the role of miR-451 in the
development of hepatoma, we further analyzed its function in HCC
cell invasion. In this study, overexpression of miR-451
dramatically impeded the invasive ability of HCC cells, indicating
a vital role of miR-451 in the progression of HCC. However, its
underlying mechanism is still undefined.
Proteases of the matrix metalloproteinase (MMP) such
as MMP-9 execute the pivotal function in the breakdown of collagen
IV in basement membrane and extracellular matrix (ECM), a crucial
step in tumor invasion and metastasis (29,30).
Accumulating evidence has shown that MMP-9 is elevated in several
types of human cancers including breast cancer, non-small cell lung
cancer and gastric cancer (31–33).
Furthermore, its high expression is also observed in hepatoma
(16). MMP-9 is known as a key
regulator of ECM remodeling and related to the poor prognosis due
to its important function in tumor invasion and metastasis
(16,34). The fact that miR-451 could
attenuate HCC cell invasion has been confirmed herein. To further
clarify the underlying mechanism, we investigated MMP-9 in
miR-451-regulated hepatoma cell invasion. After transfection with
miR-451 mimics, the expression levels of MMP-9 mRNA and protein
were notably reduced, implying that miR-451 may inhibit HCC cell
invasion in MMP-9-dependent manner.
In conclusion, our study showed obvious
downregulation of miR-451 in human hepatocarcinoma cells. High
expression levels of miR-451 inhibited hepatoma cell growth by
suppressing cell proliferation and enhancing cell apoptosis in
caspase-3-dependent manner. Additionally, miR-451 overexpression
attenuated HCC cell invasion by MMP-9 expression. Accordingly, our
study illustrates a potential role of miR-451 in the development
and progression of hepatoma, and supports this promising
therapeutic agent for future development in anti-hepatoma
therapy.
References
|
1
|
Yang JD and Roberts LR: Hepatocellular
carcinoma: a global view. Nat Rev Gastroenterol Hepatol. 7:448–458.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Altekruse SF, McGlynn KA and Reichman ME:
Hepatocellular carcinoma incidence, mortality, and survival trends
in the United States from 1975 to 2005. J Clin Oncol. 27:1485–1491.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jelic S and Sotiropoulos G: Hepatocellular
carcinoma: ESMO Clinical Practice Guidelines for diagnosis,
treatment and follow-up. Ann Oncol. 21:v59–v64. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lu J, Getz G, Miska EA, et al: MicroRNA
expression profiles classify human cancers. Nature. 435:834–838.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Croce C, Calin G and Volinia S:
MicroRNA-based methods and compositions for the diagnosis and
treatment of solid cancers Patent CN101389770A. 2012
|
|
6
|
Li A, Yu J, Kim H, et al: Serum miR-1290
as a marker of pancreatic cancer - response. Clin Cancer Res.
19:5252–5253. 2013. View Article : Google Scholar
|
|
7
|
Yuan Y, Shen Y, Xue L and Fan H: miR-140
suppresses tumor growth and metastasis of non-small cell lung
cancer by targeting insulin-like growth factor 1 receptor. PloS
One. 8:e736042013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gregory RI and Shiekhattar R: MicroRNA
biogenesis and cancer. Cancer Res. 65:3509–3512. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lynam-Lennon N, Maher SG and Reynolds JV:
The roles of microRNA in cancer and apoptosis. Biol Rev. 84:55–71.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Li M, Li J, Ding X, He M and Cheng S-Y:
microRNA and cancer. AAPS J. 12:309–317. 2010. View Article : Google Scholar
|
|
11
|
Liu N, Jiang N, Guo R, et al: MiR-451
inhibits cell growth and invasion by targeting MIF and is
associated with survival in nasopharyngeal carcinoma. Mol Cancer.
12:1232013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang T, Zang W-Q, Li M, Wang N, Zheng Y-l
and Zhao G-Q: Effect of miR-451 on the biological behavior of the
esophageal carcinoma cell line EC9706. Dig Dis Sci. 58:706–714.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Tian Y, Nan Y, Han L, et al: MicroRNA
miR-451 downregulates the PI3K/AKT pathway through CAB39 in human
glioma. Int J Oncol. 40:1105–1112. 2012.PubMed/NCBI
|
|
14
|
Nan Y, Han L, Zhang A, et al: MiRNA-451
plays a role as tumor suppressor in human glioma cells. Brain Res.
1359:14–21. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sakamoto Y, Mafune K, Mori M, et al:
Overexpression of MMP-9 correlates with growth of small
hepatocellular carcinoma. Int J Oncol. 17:237–243. 2000.PubMed/NCBI
|
|
16
|
Chen R, Cui J, Xu C, Xue T, Guo K, Gao D,
Liu Y, Ye S and Ren Z: The significance of MMP-9 over MMP-2 in HCC
invasiveness and recurrence of hepatocellular carcinoma after
curative resection. Ann Surg Oncol. 19(Suppl): S375–S384. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lakka SS, Gondi CS, Yanamandra N, et al:
Inhibition of cathepsin B and MMP-9 gene expression in glioblastoma
cell line via RNA interference reduces tumor cell invasion, tumor
growth and angiogenesis. Oncogene. 23:4681–4689. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Bushati N and Cohen SM: microRNA
functions. Annu Rev Cell Dev Biol. 23:175–205. 2007. View Article : Google Scholar
|
|
19
|
Alvarez-Garcia I and Miska EA: MicroRNA
functions in animal development and human disease. Development.
132:4653–4662. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Meng F, Henson R, Wehbe-Janek H, Ghoshal
K, Jacob ST and Patel T: MicroRNA-21 regulates expression of the
PTEN tumor suppressor gene in human hepatocellular cancer.
Gastroenterology. 133:647–658. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wang L, Yao J, Shi X, et al: MicroRNA-302b
suppresses cell proliferation by targeting EGFR in human
hepatocellular carcinoma SMMC-7721 cells. BMC Cancer. 13:4482013.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Nautiyal J, Banerjee S, Kanwar SS, et al:
Curcumin enhances dasatinib-induced inhibition of growth and
transformation of colon cancer cells. Int J Oncol. 128:951–961.
2011.PubMed/NCBI
|
|
23
|
Liu Z-H, He Y-P and Qin H: The
growth-inhibition effect of tamoxifen in the combination
chemotherapeutics on the human cholangiocarcinoma cell line QBC939.
Mol Biol Rep. 37:2693–2701. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Liu E, Kuang Y, He W, Xing X and Gu J:
Casticin induces human glioma cell death through apoptosis and
mitotic arrest. Cell Physiol Biochem. 31:805–814. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
D’Amelio M, Sheng M and Cecconi F:
Caspase-3 in the central nervous system: beyond apoptosis. Trends
Neurosci. 35:700–709. 2012.
|
|
26
|
Ying TH, Yang SF, Tsai SJ, et al: Fisetin
induces apoptosis in human cervical cancer HeLa cells through
ERK1/2-mediated activation of caspase-8-/caspase-3-dependent
pathway. Arch Toxicol. 86:263–273. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Persad R, Liu C, Wu T-T, et al:
Overexpression of caspase-3 in hepatocellular carcinomas. Mod
Pathol. 17:861–867. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Bandres E, Bitarte N, Arias F, et al:
microRNA-451 regulates macrophage migration inhibitory factor
production and proliferation of gastrointestinal cancer cells. Clin
Cancer Res. 15:2281–2290. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kessenbrock K, Plaks V and Werb Z: Matrix
metalloproteinases: regulators of the tumor microenvironment. Cell.
141:52–67. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Gialeli C, Theocharis AD and Karamanos NK:
Roles of matrix metalloproteinases in cancer progression and their
pharmacological targeting. FEBS J. 278:16–27. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Leifler KS, Svensson S, Abrahamsson A, et
al: Inflammation induced by MMP-9 enhances tumor regression of
experimental breast cancer. J Immunol. 190:4420–4430. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lee L-Y, Wu C-M, Wang C-C, et al:
Expression of matrix metalloproteinases MMP-2 and MMP-9 in gastric
cancer and their relation to claudin-4 expression. Histol
Histopathol. 23:515–521. 2008.PubMed/NCBI
|
|
33
|
Roomi M, Monterrey J, Kalinovsky T, Rath M
and Niedzwiecki A: Patterns of MMP-2 and MMP-9 expression in human
cancer cell lines. Oncol Rep. 21:1323–1333. 2009.PubMed/NCBI
|
|
34
|
Piao S, Zhao S, Guo F, et al: Increased
expression of CD147 and MMP-9 is correlated with poor prognosis of
salivary duct carcinoma. J Cancer Res Clin Oncol. 138:627–635.
2012. View Article : Google Scholar : PubMed/NCBI
|