Introduction
Glioblastoma is the most common and lethal type of
primary brain tumours, accounting for approximately 52% of primary
intracranial tumours. Glioblastoma is characterised by strong
chemotherapy resistance and post-operative recurrence with a median
survival of less than 12 months. Despite many advances in
therapeutics over the past several years, the prognosis for
patients with glioblastoma remains dismal (1–5).
In the past decade, accumulating evidence has
indicated that glioblastoma stem-like cells (GSCs, also known as
glioblastoma initiating cells) are at the root of tumour
development, recurrence and drug resistance (6). GSCs are a small population (1–10%) of
cells with some neural stem cell (NSC) properties. GSCs have
limitless self-renewal capacity and express some stem cell
hallmarks, such as CD133, ATP-binding cassette subfamily G member 2
(ABCG2) and nestin. Additionally, GSCs are multipotent and can
differentiate into neurons, astroglial cells and oligodendroglial
cells to thus sculpt a resulting tumour that is composed of a
mixture of different cell types. GSCs also have the ability to
initiate and drive tumour progression in animal models (7,8).
These characteristics have been used to identify GSCs in many
studies (9–11).
GSCs frequently show strong radioresistance and
chemoresistance. It has been suggested that conventional
post-operative chemotherapy and radiotherapy will kill most
remaining cancer cells but will leave GSCs intact, resulting in the
recurrence of glioblastoma. It has been reported that the
radioresistance and chemoresistance of tumour stem cells (TSCs) is
markedly decreased after their differentiation into normal tumour
cells that display certain differentiation hallmarks (12,13).
Thus, finding effective ways to promote GSC differentiation will
hopefully limit the radiotherapy and chemotherapy resistance of
GSCs and notably enhance therapeutic efforts.
For use as an effective antitumour medicine, elemene
is extracted from Curcuma wenyujin as an essential oil mixture of
β-, γ- and δ-elemene (14).
β-elemene (molecular formula C15H24,
molecular weight 204.34), the major active anticancer component in
the elemene mixture, has strong anti-proliferative activity and
induces apoptosis in various tumours, such as glioma, breast
carcinoma and leukaemia (15–17).
We previously found that β-elemene can inhibit the proliferation of
glioblastoma cells and induce cell apoptosis in vitro and
in vivo (17–21). In addition, β-elemene can promote
the differentiation of Tca8113 human tongue squamous cancer cells
and PLA801D human pulmonary giant cell carcinoma cells in
vitro (22,23). Therefore, it was essential to
illustrate the effects of β-elemene on GSC proliferation,
differentiation and chemoresistance.
In this study, we illustrated the effect of
β-elemene on some stem cell phenotypes of GSCs. We found that
CD133+ cells were not only assembled in some vascular
walls but were also sparsely localized to other zones of
glioblastoma tissues. β-elemene inhibited the proliferation of GSCs
in vitro and in vivo, decreased the formation of GSC
spheres, dispersed GSC spheres and even resulted in the
fragmentation and death of some sphere cells. β-elemene
significantly downregulated the expression levels of CD133 and
ABCG2 and increased the expression of the astroglial cell marker
glial fibrillary acidic protein (GFAP) in vitro and in
vivo. Additionally, treatment with β-elemene sensitized GSCs to
temozolomide (TMZ). These results suggested that β-elemene could
impair the stemness of GSCs and promote their differentiation,
which may make it a useful tool to enhance the effects of
radiotherapy and chemotherapy.
Materials and methods
Reagents and antibodies
β-elemene (98% purity) was obtained from Jingang
Pharmaceutical Co. (Dalian, China). TMZ and
4′,6-diamidino-2-phenylindole (DAPI) were obtained from
Sigma-Aldrich Co., LLC. (St. Louis, MO, USA). The antibodies
against CD133, ABCG2, GFAP, neuron specific enolase (NSE), and
myelin basic protein (MBP), used in western blots and
immunofluorescence assays, were purchased from Boster Co., Ltd.
(Wuhan, China). Epidermal growth factor (EGF), basic fibroblast
growth factor (bFGF), leukaemia inhibitory factor (LIF), N2,
Accutase Cell Dissociation Reagent and Alexa Fluor 594 goat
anti-rabbit IgG were from Invitrogen Corp. (Carlsbad, CA, USA). The
antibody against GAPDH was from Santa Cruz Biotechnology, Inc.
(Santa Cruz, CA, USA). Anti-CD133 and IgG1 isotype control
antibodies used in flow cytometry analysis were purchased from
Miltenyi Biotec (Bergish Gladbach, Germany). The Cell Counting
Kit-8 (CCK-8) was obtained from Dojindo Molecular Technologies,
Inc. (Japan). Nude mice were provided by the Experimental Animal
Center of The Academy of Military Medical Sciences. Primary
glioblastoma cells were maintained in Dulbecco’s modified Eagle’s
medium (DMEM)/F12 (Hyclone, UT, USA) supplemented with 10% foetal
calf serum, 50 IU/ml penicillin and 50 mg/ml streptomycin and grown
at 37°C in a humidified atmosphere with 5% CO2.
Tumour specimens and primary cell
cultures
Tumour tissues originated from 20 patients with
glioblastoma (10 men and 10 women, 42±11.7 years, World Health
Organization/WHO grade III–IV) undergoing surgical resection in the
Department of Neurosurgery of the General Hospital of Shenyang
Military. The cells of two of these patients (case 1, WHO grade
III; and case 2, WHO grade IV) were cultured as primary
glioblastoma cells. The study was approved by the Ethics Committee
of the General Hospital of Shenyang Military and abided by the
Declaration of Helsinki, with informed consent obtained from all
study participants. Tumour samples were stored in sterile
serum-free DMEM/F12 and processed within 0.5 h after resection.
Tissues were cut into 1-mm2 pieces, washed with PBS and
digested with 0.25% trypsin at 37°C for 15 min. Cells were cultured
in serum-containing DMEM/F12 after passage through a 70-μm strainer
(BD Pharmingen, San Diego, CA, USA). Primary glioblastoma cells
were resuspended in NSC medium (NSCM) containing DMEM/F12 medium,
20 ng/ml EGF, 20 ng/ml bFGF, 10 ng/ml LIF and N2 (1:100) for
culturing GSCs.
Immunohistochemistry
Paraffin sections of glioblastoma tissues were
obtained and deparaffinized. Antigen retrieval was performed with
citrate buffer, pH 6.0 (Invitrogen). Non-specific sites were
blocked by incubating sections with 5% BSA in a humidified chamber
for 1 h at room temperature. The samples were incubated with 0.3%
H2O2 for 15 min to block endogenous
peroxidase activity and then labelled with different primary
antibodies at proper concentrations (1:100 for CD133, ABCG2 and
GFAP) for 1 h at room temperature. In subsequent steps, we used the
Vectastain ABC kit (Vector Laboratories, Burlingame, CA, USA) and
DAB as a chromogen (Changdao Biotech, China). The nuclear
counterstaining of sections was performed with hematoxylin. In
every tissue, additional staining without primary antibody was
performed in parallel as a negative control. The integrated optical
density (IOD) was used to semi-quantitatively estimate the
expression of CD133, ABCG2 and GFAP in Image-Pro Plus 6.0.
Determining the CD133 expression by flow
cytometry analysis
Cells were digested into single cells with Accutase
Cell Dissociation Reagent and labelled with anti-CD133 and control
IgG isotype monoclonal antibodies (Miltenyi Biotec, San Diego, CA,
USA). Flow cytometry analysis was conducted on a FACSCalibur flow
cytometer (Becton-Dickinson, San Jose, CA, USA). Data acquisition
and analysis were performed with CellQuest software
(Becton-Dickinson).
GSC culture and passage
Primary glioblastoma cells were cultured in NSCM and
formed cell spheres after 3 days. After forming spheres of at least
100 cells (culturing for approximately 5 days), cell spheres were
digested to single cell suspension with Accutase Cell Dissociation
Reagent and subcultured with NSCM in ultra-low attachment culture
flasks (Corning, Inc., NY, USA).
Immunofluorescence and DAPI staining
In each well of 24-well plates, 2×105
dissociated cells or 20 cell spheres were cultured on
poly-L-lysine-coated cover slips for various times. Cells on cover
slips were washed with PBS, fixed with methanol and then treated
with 0.1% Triton X-100. After blocking with 5% BSA, cells were
incubated with proper dilutions of anti-CD133, GFAP, MBP or NSE
antibodies at 4°C overnight. The primary antibodies were detected
with Alexa Fluor 594 goat anti-rabbit IgG (dilution 1:500) for 40
min at 37°C in the dark. Cell nuclei were stained with DAPI, and
immunofluorescent images were examined under a fluorescent
microscope (Leica Microsystems, Germany).
Cell proliferation assay
Cell viability was evaluated with the CCK-8 assay
after cells in exponential growth were cultured in a 96-well plate.
Trypan blue staining confirmed >80% viability, and cells were
treated according to the study design. Then, 10 μl of CCK-8 was
added to each well, and the mixture was incubated for 4 h at 37°C.
The optical density (OD) of each well was measured at 450 nm using
a spectrophotometric microplate reader (BioTek Instruments, Inc.,
Winooski, VT, USA). Five replicate wells were used for each cell
sample.
Western blotting
The cells were lysed with RIPA buffer [50 mM
Tris-HCl (pH 7.4), 1.0% NP-40, 0.25% Na-deoxycholate, 1 mM EDTA,
150 mM NaCl, 1 mM aprotinin, 1 mg/ml PMSF, 1 μg/ml pepstatin and 1
μg/ml leupeptin]. The concentrations of total protein in the
cellular extracts were measured using the BCA assay kit from Keygen
Biotech, Co., Ltd. (Nanjing, China). After separation in 10–12%
sodium dodecyl sulphate polyacrylamide (SDS-PAGE) gels, the
proteins were transferred to nitrocellulose filter membranes
(Bio-Rad, Hercules, CA, USA). The membranes were blocked with 5%
BSA in Tris-buffered saline with Tween-20 at 4°C overnight and
probed with various primary antibodies at 4°C overnight, followed
by incubation with horseradish peroxidase-conjugated secondary
antibodies at 37°C for 3 h. The membranes were exposed to an ECL
system (Amersham, Sweden), and fluorescence was detected by
exposing the membrane to X-ray film. The results were scanned with
the Image Quant 5.2 software (Amersham).
Transplantation of GSCs into nude mice
and treatment of animals
GSC spheres were digested into single cells, and
1000 cells/animal (suspending in 0.1 ml DMEM/F12) were
subcutaneously injected into the right shoulder region of
4-week-old female nude mice. The use of animals was approved by the
Institutional Animal Care and Use Committee. Beginning with the 7th
day after transplantation, various drugs were intraperitoneally
injected. The volumes of tumours were measured every 3 days and
calculated according to the formula: V = 1/2 × largest diameter ×
smallest diameter2 (21). Tumours were weighed on the 21st day
after transplantation.
Statistical analysis
The values are reported as the means ± standard
deviation (SD) of at least three independent experiments.
Statistical analysis was performed using Student’s t-test.
Statistical significance was accepted at the level of P<0.05
between different groups, and P<0.01 was considered highly
significant. Statistical analyses were performed with SPSS software
(SPSS, Inc., Chicago, IL, USA).
Results
The expression of CD133 in human
glioblastoma tissues and the proportion of CD133+ cells
in primary glioblastoma cells
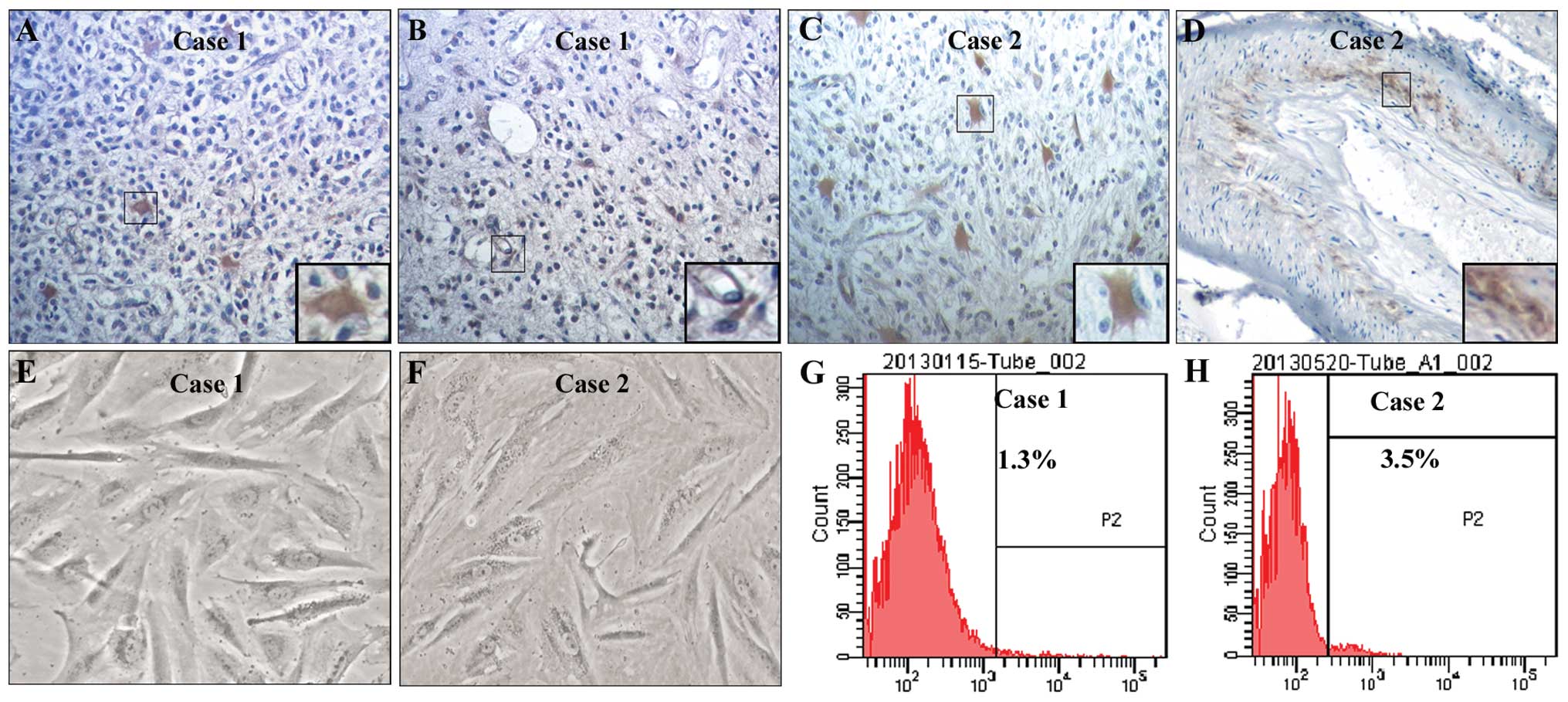
To investigate the distribution of CD133+
cells in human glioblastoma tissues, immunohistochemistry was
performed with an anti-CD133 antibody. To examine the proportion of
CD133+ primary glioblastoma cells, we cultured these
cells from fresh glioblastoma tissues and performed flow cytometry
using an anti-CD133 antibody. We found that CD133+ cells
were not only assembled in some vascular walls but were also
sparsely localized in other zones of glioblastoma tissues (Fig. 1A–D). Primary glioblastoma cells
obtained from two glioblastoma samples (case 1 and case 2) had
obvious cellular atypia and pathological nuclear fission (Fig. 1E and F). The proportions of
CD133+ cells were 1.3% in case 1 (Fig. 1G) and 3.5% in case 2 (Fig. 1H).
The formation of second-generation GSC
spheres
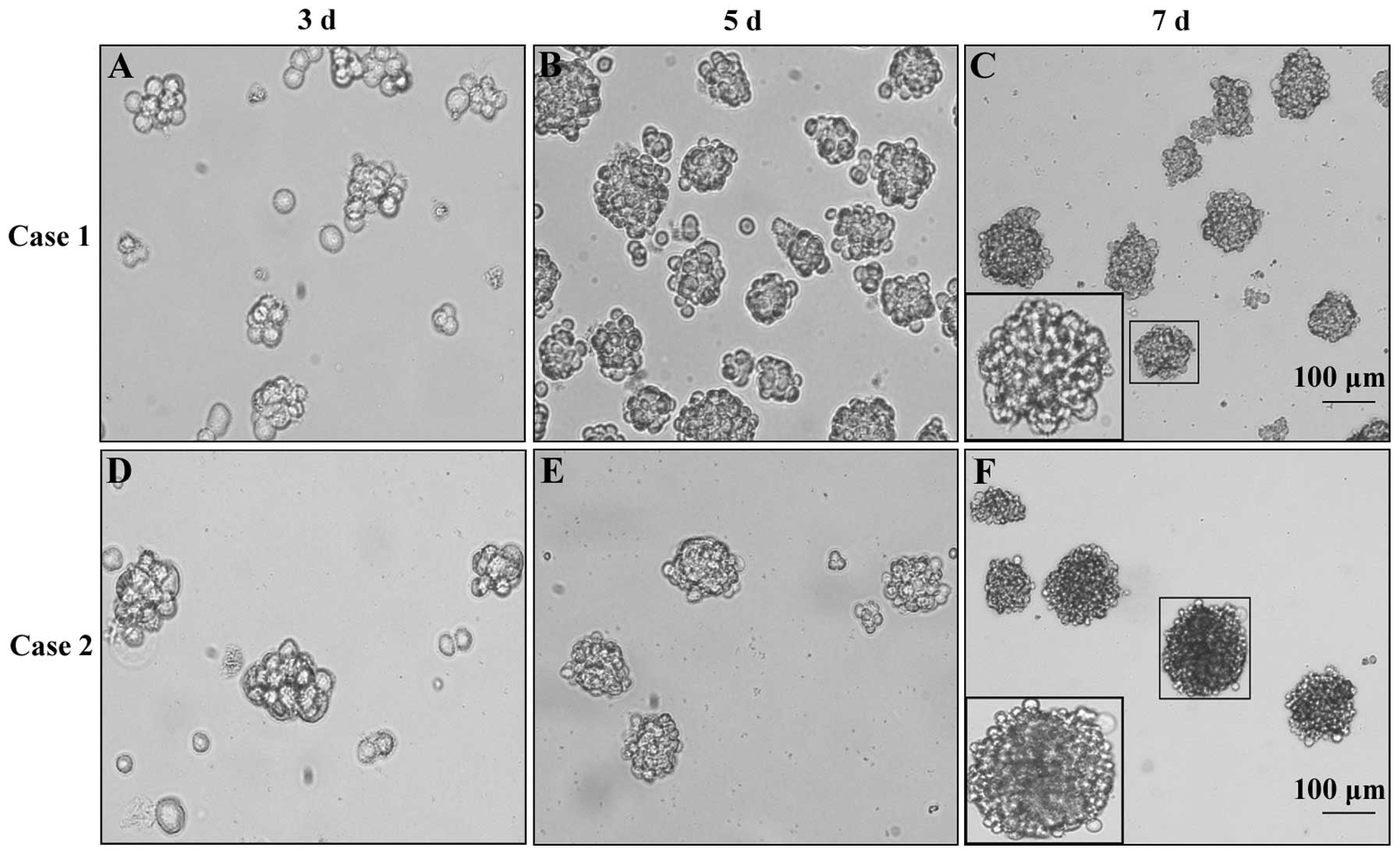
To obtain GSC spheres, we cultured primary
glioblastoma cells in NSCM and observed cell sphere formation with
an inverted microscope. The results showed that primary
glioblastoma cells derived from case 1 and case 2 could form GSC
spheres in NSCM. The second-generation GSC spheres formed on the
3rd day after passage and then grew gradually with a denser
structure and higher refractivity (Fig. 2).
GSC spheres strongly express the NSC
marker CD133 and show multipotent differentiation capacity
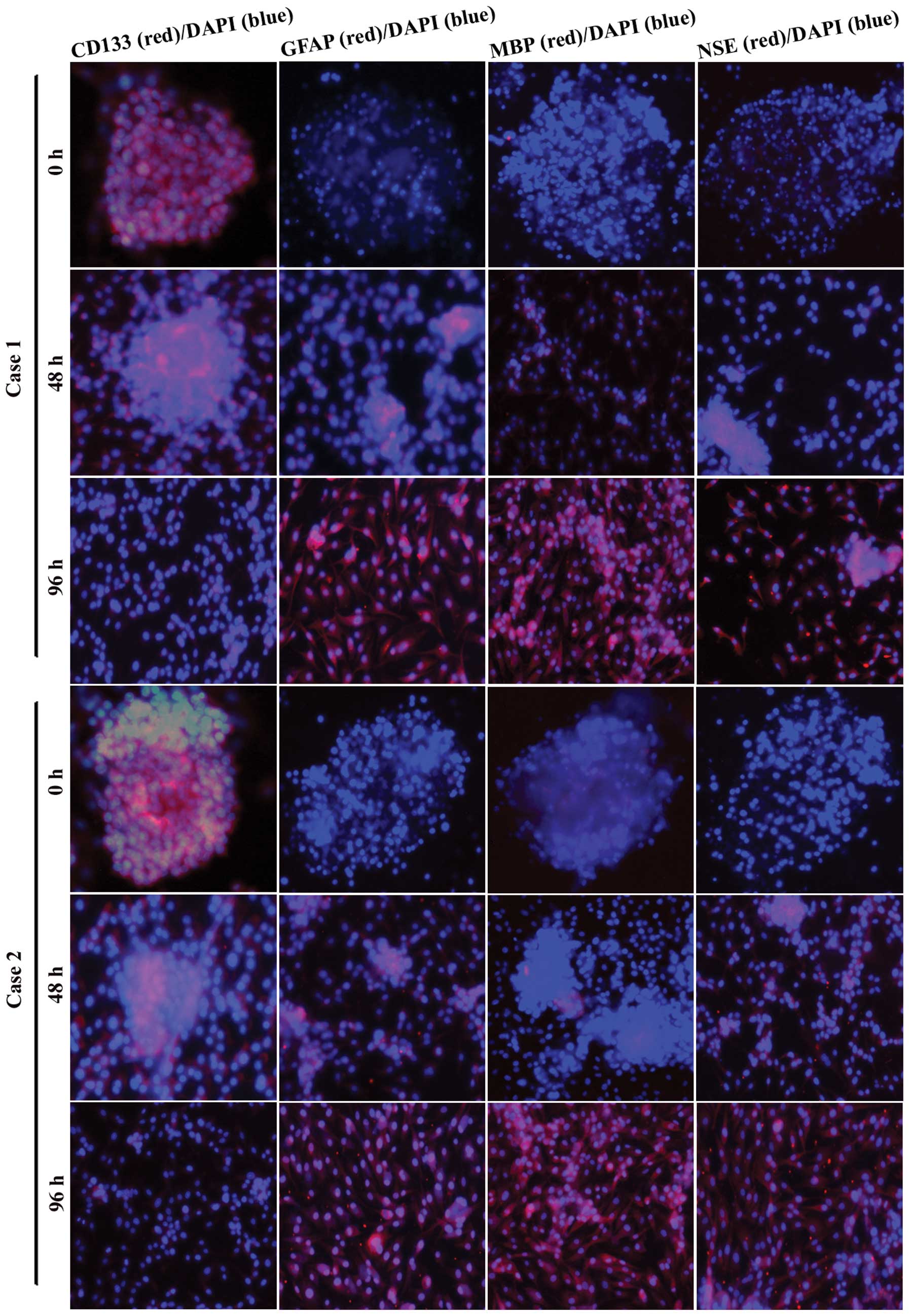
To test the expression of CD133 and the multipotent
differentiation capacity in the second-generation GSC spheres, we
cultured GSC spheres in DMEM/F12 with 10% foetal calf serum and
examined CD133, GFAP (astrocyte marker), MBP (oligodendrocyte
marker) and NSE (neuron marker) expression by immunofluorescence
assays 0, 48 and 96 h after plating. We found that the
second-generation GSC spheres strongly expressed CD133 with little
expression of GFAP, MBP and NSE (Fig.
3, 0 h). During 48 h of adherent growth conditions in
serum-containing DMEM/F12, GSC spheres gradually spread out. The
expression of CD133 decreased, whereas the expression of GFAP, MBP
and NSE increased with time. After 96 h, GFAP, MBP and NSE showed
strong expression (Fig. 3). These
results suggest that the GSC spheres we obtained possessed stem
cell phenotypes and multipotent differentiation potential.
β-elemene inhibits the formation of GSC
spheres, decreases the proliferation of GSCs and disperses cell
spheres
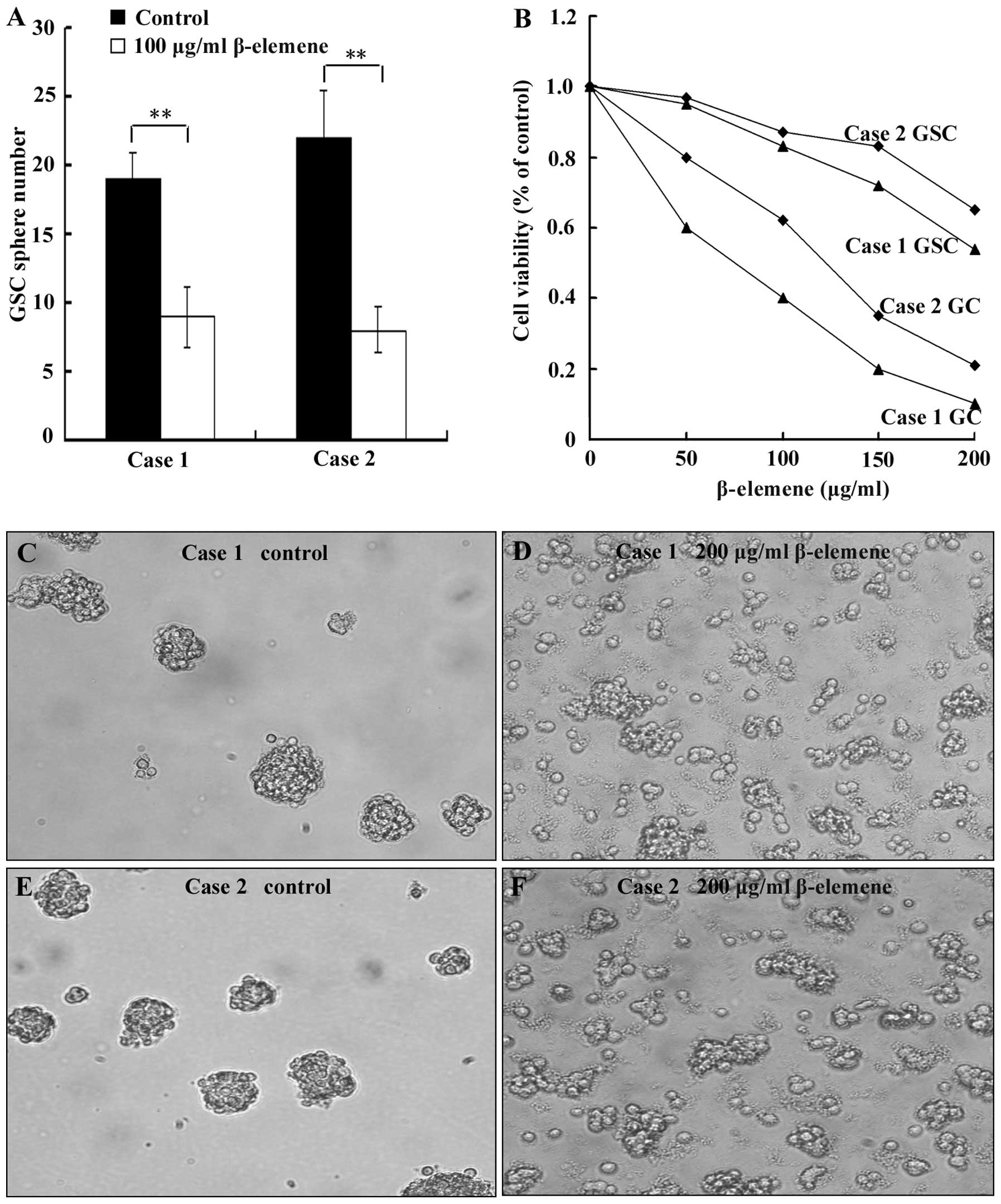
To evaluate the effect of β-elemene on GSC sphere
formation, cell spheres were digested into single cells. The cells
were then plated at a density of 100 cells/well in 96-well plates
and simultaneously treated with 100 μg/ml β-elemene for 72 h
(untreated cells as control group). Finally, the number of cell
spheres (containing at least 10 cells/sphere) was counted. To
examine the effect of β-elemene on proliferation, 1000 GSCs and
10000 primary glioblastoma cells per well were independently plated
in 96-well plates. These cells were then treated with 0 (control
group), 50, 100, 150 or 200 μg/ml β-elemene for 24 h, and the CCK-8
assay was used to examine cell viability. To test the ability of
β-elemene to dissociate GSC spheres, we added 200 μg/ml β-elemene
to cell spheres for 12 h and observed sphere dispersion with an
inverted microscope. The results showed that the number of GSC
spheres that formed in the β-elemene group was less than in the
control groups in both cases (Fig.
4A, P<0.01). The proliferation of both GSCs and primary
glioblastoma cells (GC) (Fig. 4B)
decreased dose-dependently in the presence of β-elemene. The degree
of this viability loss was less for GSCs than for primary
glioblastoma cells (Fig. 4B),
which suggested that GSCs possessed stronger resistance to
β-elemene than glioblastoma cells. β-elemene can disperse GSC
spheres, and this can result in the fragmentation and death of some
cells (Fig. 4C–F).
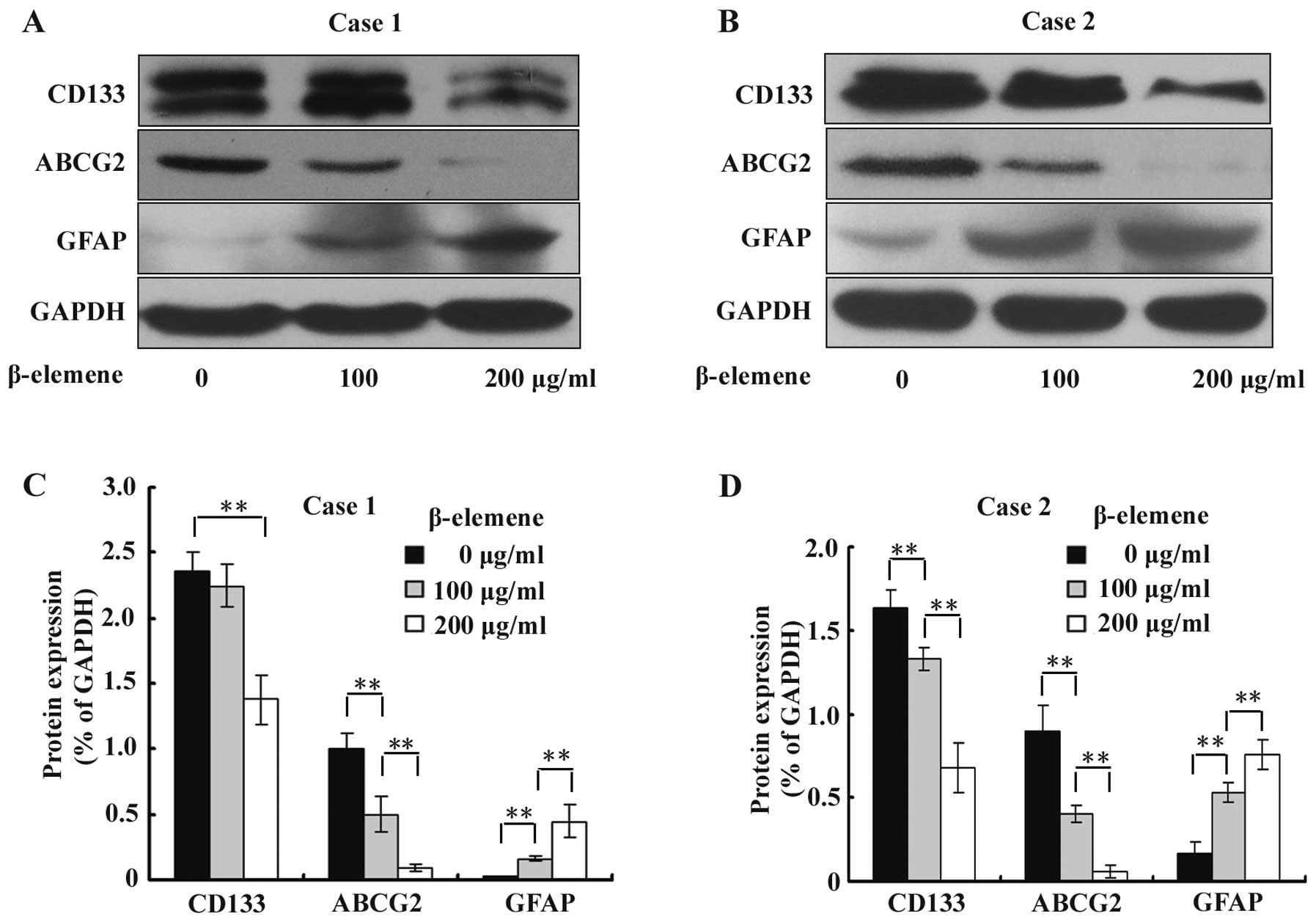
β-elemene decreases the expression of
stem cell markers CD133 and ABCG2 and increases the expression of
the differentiation marker GFAP in GSC spheres
To investigate the effect of β-elemene on the
expression of GSC markers CD133 and ABCG2 and differentiation
marker GFAP, GSC spheres were treated with 100 and 200 μg/ml
β-elemene for 24 h, and western blot analysis was performed using
specific antibodies. The results were semi-quantitatively estimated
using Gel-Pro analyzer 4.0 software and illustrated graphically. We
found that the expression levels of CD133 and ABCG2 were
significantly downregulated, while the expression of GFAP was
increased by β-elemene in a dose-dependent manner (Fig. 5). These results suggested that
β-elemene impairs the stemness of GSC spheres and promotes their
differentiation.
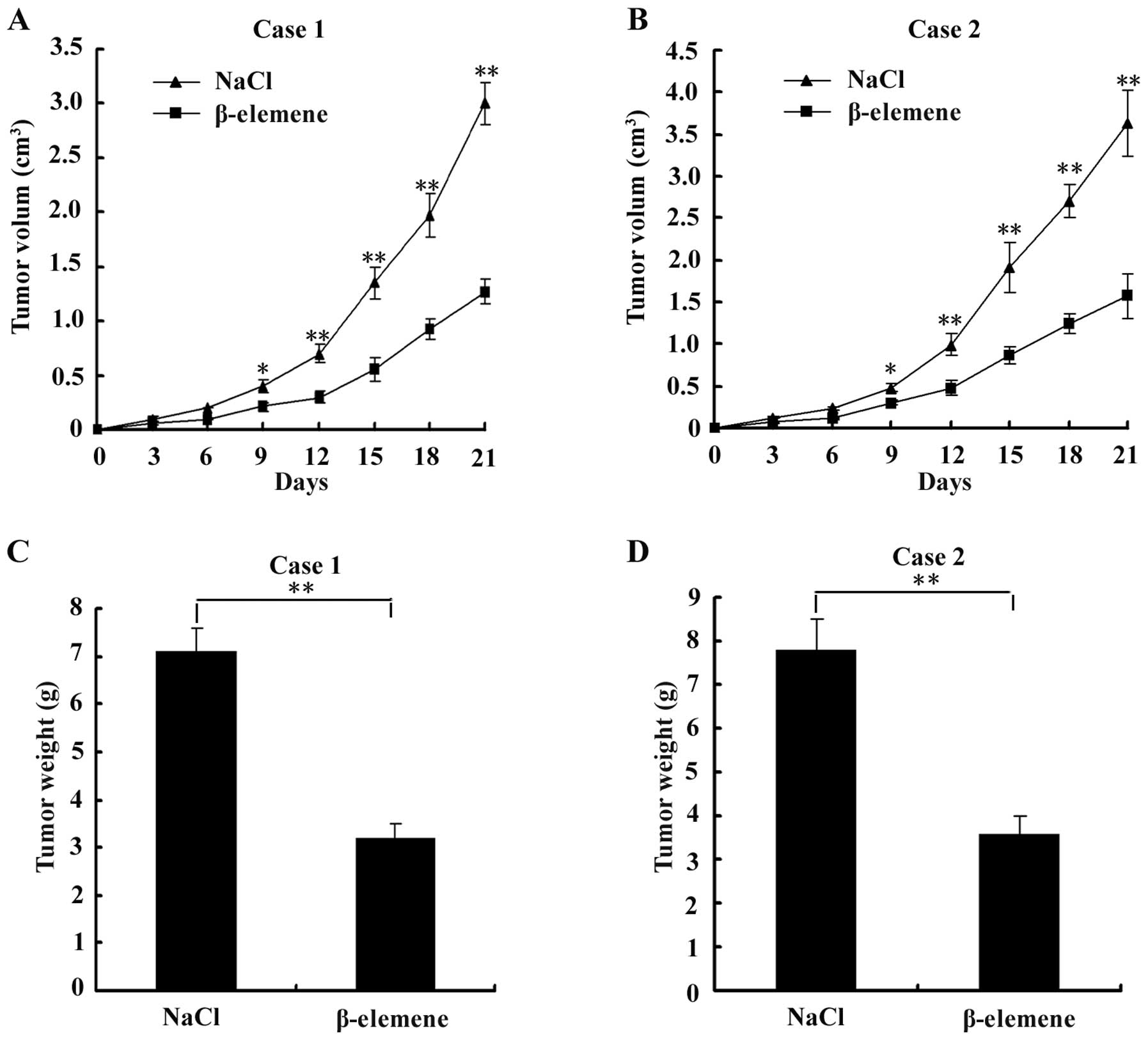
β-elemene suppresses the development of
tumours in nude mice transplanted with GSCs
To evaluate the effect of β-elemene on GSC tumours
in vivo, we subcutaneously injected GSCs into the flank of
nude mice and then intraperitoneally injected NaCl or 50 mg/kg
β-elemene for 1 week. The tumour volumes were measured every 3
days. Points are the means ± SD of tumour volume in each group
(n=6). The results showed that a significant inhibition of tumour
volume was observed in the β-elemene-treated group compared with
the control group from the 9th day after transplantation (the 3rd
day after intraperitoneal injection) to the 21st day, as shown in
Fig. 6A and B. On the 21st day,
tumours were dissected and measured. The tumour weights of the
β-elemene group were significantly lower than those of the control
group, as shown in Fig. 6C and D.
These results suggested that the development of tumours was
suppressed by β-elemene in GSC-bearing nude mice.
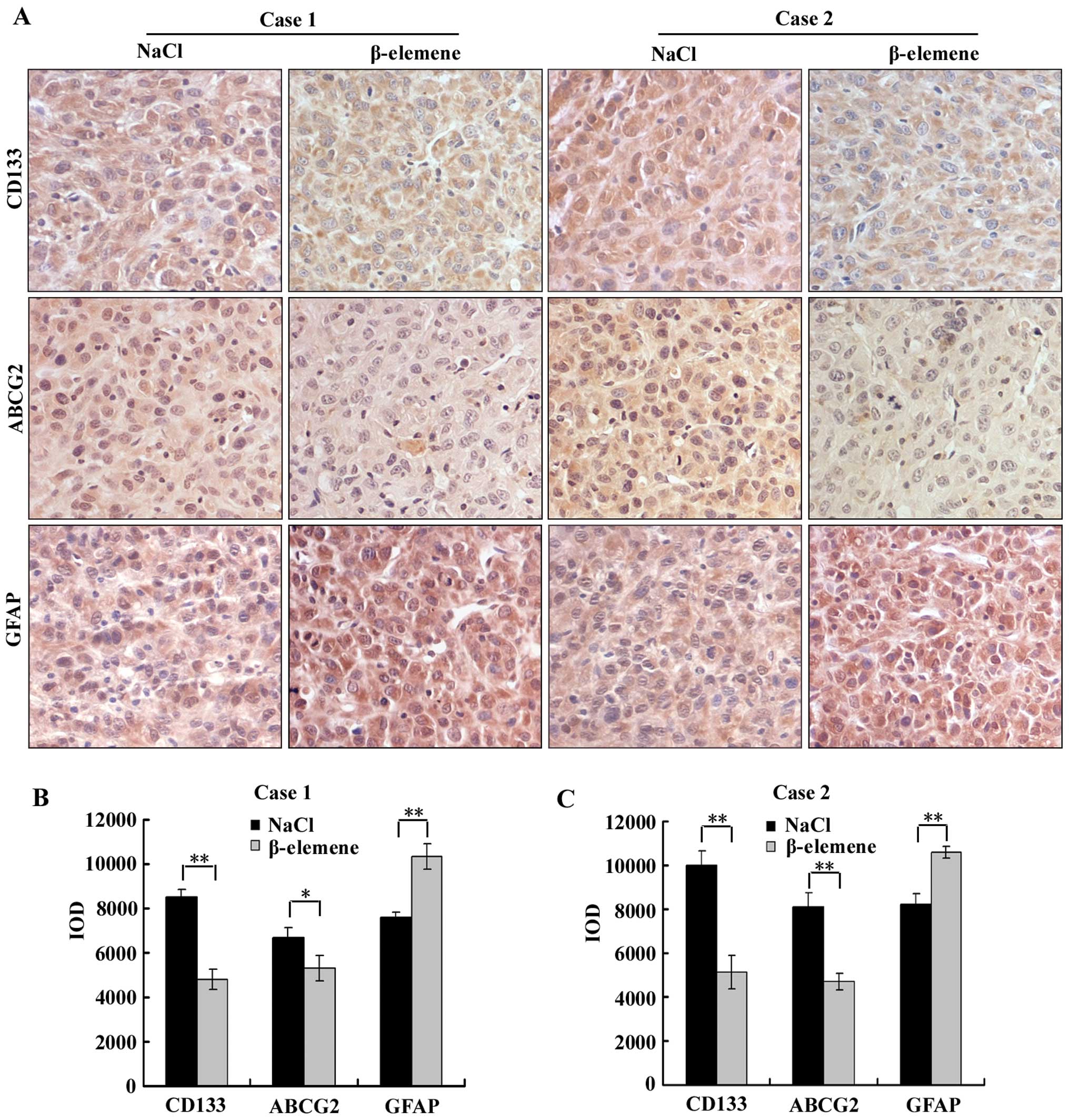
β-elemene downregulates the expression of
CD133 and ABCG2 and upregulates the expression of GFAP in
GSC-transplanted nude mice
To evaluate the effect of β-elemene on the
expression of stem cell markers and differentiation markers in
vivo, immunohistochemistry was performed using antibodies
against CD133, ABCG2 and GFAP on the aforementioned tumour tissues
of GSC-transplanted nude mice in β-elemene and NaCl groups
(Fig. 7A). Image-Pro Plus 6.0
software was applied to calculate the IOD of each group (the same
selected areas and magnifications in all images), and then
statistical analysis was performed. The IOD of each group is
illustrated in a vertical bar chart (Fig. 7B and C). We found in this assay
that the expression levels of CD133 and ABCG2 in the β-elemene
group were significantly lower than in the NaCl group, and the
expression level of GFAP in the β-elemene group was higher than in
the NaCl group in both cases 1 and 2. These results suggest that
β-elemene decreased the expression of CD133 and ABCG2 but increased
the expression of GFAP in vivo.
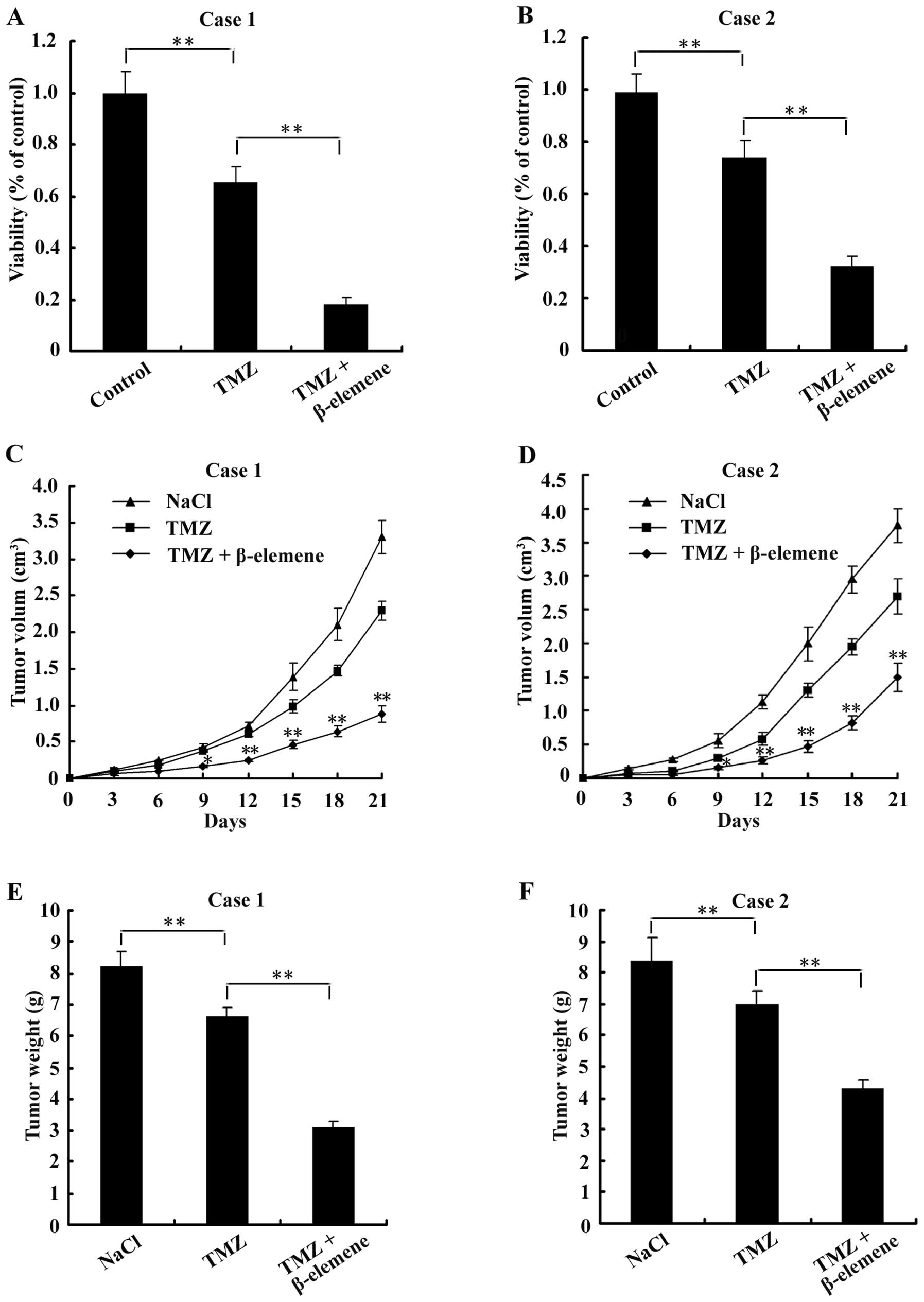
Treatment with β-elemene sensitizes GSCs
to TMZ-induced cytotoxicity
As reported, GSCs were usually more resistant to TMZ
cytotoxicity than normal glioblastoma cells (21). To determine whether treatment with
β-elemene sensitizes GSCs to TMZ, GSC spheres were digested into
single cells and then plated at a density of 5×103
cells/well in 96-well plates. Cells were organized into the
following three groups: control (untreated), TMZ (treated with 6
μg/ml TMZ for 24 h) and combination (treated with 6 μg/ml TMZ and
100 μg/ml β-elemene for 24 h). After treatment with the drugs,
CCK-8 assays were performed to examine cell viability.
Additionally, 18 nude mice were injected with GSCs to create
tumours and divided into the following three groups (6 mice/group):
NaCl (negative control, intraperitoneally injected with 50 mg/kg
NaCl for 1 week), TMZ (intraperitoneally injected with 33 mg/kg TMZ
for 1 week) and combination (intraperitoneally injected with 33
mg/kg TMZ and 50 mg/kg β-elemene for 1 week). The volumes of
tumours were measured and calculated according to the formula: V =
1/2 × largest diameter × smallest diameter2, every 3
days, and tumours were weighed on the 21st day. The results showed
that cell viability was lower in the combination group than either
the control or the TMZ groups (Fig. 8A
and B). In the in vivo experiments, we found that both
the volumes (Fig. 8C and D) and
the weights (Fig. 8E and F) of
tumours were less in the combination group than in either the
control or TMZ groups. These findings suggest that β-elemene
increased the sensitivity of GSCs to TMZ-induced cytotoxicity.
Discussion
It was reported that CD133+ cells tend to
distribute around microvessels in glioblastoma tissues (24,25).
In this study, we found that CD133+ cells were not only
assembled in some vascular walls (smaller cells) but also sparsely
localized in other parts of glioblastoma tissues (larger cells).
This difference in the cell distribution may be due to individual
variability between the samples.
It has been suggested that GSCs are the source of
malignant tumour development, recurrence and chemoresistance.
Conventional chemotherapy will only kill the bulk of normal
glioblastoma cells in a tumour but will leave the drug-resistant
GSCs intact, resulting in tumour recurrence. A representative
property of GSCs is the ability to form neurospheres in special
NSCM. Thus, the effects of drugs on GSCs can be conveniently
evaluated by measuring cell proliferation, the ability to form
neurospheres and the degree of neurosphere dispersion (26–29).
We previously found that β-elemene can significantly reduce the
size of tumours and prolong the life-span of patients without
serious side effects and that it can inhibit glioblastoma growth
through the activation of GMFβ-MKK3/6-p38 and the downregulation of
phosphorylated ERK1/2 in vitro and in vivo (17–21).
In this study, we found that β-elemene inhibited the proliferation
of GSCs in vitro and in vivo, decreased the formation
of GSC spheres, and dispersed GSC spheres, which resulted in the
fragmentation and death of some sphere cells. β-elemene also
suppressed the ability of GSCs to form xenografts in nude mice.
This is the first report of the effects of β-elemene on GSCs.
Human CD133 (prominin-1), a 120-kDa cell-surface
protein, has been accepted as the standard marker for the
identification of GSCs, although it may not be exclusive or the
most ideal marker for this cell type (30–32).
ATP-binding cassette subfamily G (ABCG) is a membrane pump that
consumes ATP to excrete endogenous small molecules, such as
cholesterol, ions and peptides, across cell membranes. ABCG also
plays a pivotal part in detoxification and protection against
xenobiotics by expelling drugs from the cell. NSCs and TSCs have
been shown to express high levels of ABCG, which is normally
inactive in more mature cells. It was shown that a major member of
the ABCG family, ABCG2 (also known as BCRP1), is an important
resistance-related molecule and GSC marker (8,33–36).
The capacity for multipotent differentiation is a representative
characteristic of GSCs. The expression of the astroglial cell
marker GFAP was low in NSCs and GSCs and gradually increased during
the cellular differentiation process. Therefore, the increase in
the expression of GFAP generally indicated an enhancement of GSC
differentiation. In this study, we found that the expression levels
of CD133 and ABCG2 were significantly downregulated, whereas the
expression of GFAP was increased by β-elemene in both GSC spheres
in vitro and in nude mouse xenografts in vivo. These
results suggested that β-elemene could impair the stemness of GSC
spheres and promote their differentiation into normal glioblastoma
cells.
TMZ is the most commonly used chemotherapeutic agent
in glioblastoma treatment. It achieves its antitumour effect
primarily by methylating the O6 position of guanine. TMZ can kill
most glioblastoma cells and improves the overall survival and the
progression-free survival of patients with glioblastoma undergoing
surgical resection and radiotherapy (37–40).
However, GSCs in glioblastoma tissues show incredible
chemoresistance to TMZ treatment. It was reported that GSCs can
survive even in the presence of 200 μM TMZ (13,41,42).
The maximum TMZ concentrations in the plasma of patients are
between 27 and 50 μM and are only 0.5–10 μM in cerebrospinal fluid
(21,43,44),
which suggests that it is virtually impossible to use TMZ to
eliminate GSCs. Therefore, enhancing the sensitivity of GSCs to
chemotherapeutic drugs is crucial for decreasing neoplasm
recurrence and improving long-term prognosis. β-elemene is not only
an antitumour agent but is also a chemosensitizer in the treatment
against glioblastoma. β-elemene could partially reverse the
multidrug resistance to adriamycin in SGC7901/Adr human gastric
carcinoma cells (45). Similarly,
the synergistic anticancer effects of β-elemene and cisplatin were
observed in human laryngeal carcinoma-bearing nude mice and ovarian
carcinoma cells (46,47). We previously found that β-elemene
can sensitize U87 human glioblastoma cells to cisplatin in
vitro (19). In this study, we
conducted experiments in vitro and in vivo and found
that β-elemene sensitized GSC spheres to TMZ-induced cytotoxicity
and enhanced the anti-proliferative effects of TMZ on nude mouse
xenografts.
However, the chemosensitization mechanism of
β-elemene remains unclear. We speculate that the following aspects
may be involved. First, the differentiation of GSCs into normal
glioblastoma cells induced by β-elemene improved the sensitivity of
GSCs to antitumour drugs. Second, β-elemene downregulated the
expression of the resistance-related protein ABCG2, which was
likely to increase the intracellular accumulation of
chemotherapeutics. Finally, the p38 mitogen-activated protein
kinase (MAPK) and extracellular signal-regulated kinases 1 and 2
(ERK1/2)/BCL-2 signalling pathways are closely related to the drug
resistance of various tumours (17–21).
We previously reported that β-elemene could activate the
GMFβ-MKK3/6-p38 pathway and inhibit the ERK1/2/BCL-2 pathway, and
this may also be a potential molecular mechanism underlying
chemosensitization with β-elemene.
In conclusion, β-elemene impaired the stemness of
GSC spheres, promoted their differentiation and sensitized GSCs to
TMZ-induced cytotoxicity in vitro and in vivo.
β-elemene will hopefully become a valuable agent to enhance the
effects of radiotherapy and chemotherapy and improve the long-term
prognosis of patients with glioblastoma.
Acknowledgments
This research was supported by the Post-doctoral
Science Foundation of China (No. 2012M521921) and the Liaoning
Province Science and Technology Key Projects (No. 2013225089). We
also thank all the colleagues in our research group for their
generous support.
References
|
1
|
Wen PY and Kesari S: Malignant gliomas in
adults. N Engl J Med. 359:492–507. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Van Meir EG, Hadjipanayis CG, Norden AD,
Shu HK, Wen PY and Olson JJ: Exciting new advances in
neuro-oncology: the avenue to a cure for malignant glioma. CA
Cancer J Clin. 60:166–193. 2010.PubMed/NCBI
|
|
3
|
Omuro A and DeAngelis LM: Glioblastoma and
other malignant gliomas: a clinical review. JAMA. 310:1842–1850.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Shah U and Morrison T: A review of the
symptomatic management of malignant gliomas in adults. J Natl Compr
Cancer Netw. 11:424–429. 2013.PubMed/NCBI
|
|
5
|
Eyüpoglu IY, Buchfelder M and Savaskan NE:
Surgical resection of malignant gliomas-role in optimizing patient
outcome. Nat Rev Neurol. 9:141–151. 2013.PubMed/NCBI
|
|
6
|
Grimm SA and Chamberlain MC: Brainstem
glioma: a review. Curr Neurol Neurosci Rep. 13:3462013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
He J, Liu Y and Lubman DM: Targeting
glioblastoma stem cells: cell surface markers. Curr Med Chem.
19:6050–6055. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ding XW, Wu JH and Jiang CP: ABCG2: a
potential marker of stem cells and novel target in stem cell and
cancer therapy. Life Sci. 86:631–637. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Singh SK, Hawkins C, Clarke ID, et al:
Identification of human brain tumour initiating cells. Nature.
432:396–401. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yuan X, Curtin J, Xiong Y, et al:
Isolation of cancer stem cells from adult glioblastoma multiforme.
Oncogene. 23:9392–9400. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bidlingmaier S, Zhu X and Liu B: The
utility and limitations of glycosylated human CD133 epitopes in
defining cancer stem cells. J Mol Med (Berl). 86:1025–1032. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Beier D, Schulz JB and Beier CP:
Chemoresistance of glioblastoma cancer stem cells - much more
complex than expected. Mol Cancer. 10:1282011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Liu G, Yuan X, Zeng Z, et al: Analysis of
gene expression and chemoresistance of CD133+ cancer
stem cells in glioblastoma. Mol Cancer. 5:672006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chen SL, You J and Wang GJ: Supercritical
fluid extraction of β-elemene under lower pressure. Se Pu.
19:179–181. 2001.(In Chinese).
|
|
15
|
Zhang X, Zhang Y and Li Y: β-elemene
decreases cell invasion by upregulating E-cadherin expression in
MCF-7 human breast cancer cells. Oncol Rep. 30:745–750. 2013.
|
|
16
|
Bao F, Qiu J and Zhang H: Potential role
of β-elemene on histone H1 in the H22 ascites hepatoma cell line.
Mol Med Rep. 6:185–190. 2012.
|
|
17
|
Zhu T, Zhao Y, Zhang J, Li L, Zou L, Yao Y
and Xu Y: β-Elemene inhibits proliferation of human glioblastoma
cells and causes cell-cycle G0/G1 arrest via mutually compensatory
activation of MKK3 and MKK6. Int J Oncol. 38:419–426. 2011.
|
|
18
|
Yao YQ, Ding X, Jia YC, Huang CX, Wang YZ
and Xu YH: Anti-tumor effect of beta-elemene in glioblastoma cells
depends on p38 MAPK activation. Cancer Lett. 264:127–134. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhu T, Xu Y, Dong B, Zhang J, Wei Z, Xu Y
and Yao Y: β-elemene inhibits proliferation of human glioblastoma
cells through the activation of gliamaturation factor β and induces
sensitization to cisplatin. Oncol Rep. 26:405–413. 2011.
|
|
20
|
Yao YQ, Xu YH, Lu J, Zhou HY and Wang YZ:
Effect of p38 MAPK on elemene-induced cell cycle arrest in C6
glioblastoma cells. Zhonghua Yi Xue Za Zhi. 88:56–58. 2008.(In
Chinese).
|
|
21
|
Zhao YS, Zhu TZ, Yao YQ, et al: β-elemene
inhibits Hsp90/Raf-1 molecular complex inducing apoptosis of
glioblastoma cells. J Neurooncol. 107:307–314. 2012.
|
|
22
|
Zhang L and Zhang RH: Investigation of
Tca8113 cell lines differentiation induced by β-elemene. J Oral Sci
Res. 18:307–309. 2002.(In Chinese).
|
|
23
|
Fang Z, Chen HS, Chen HB and Liu YM:
β-Elemene induced differentiation of PLA801D cell lines. Chin J
Gerontol. 28:768–769. 2008.(In Chinese).
|
|
24
|
Wang HM, Yang XJ, Dong XT, et al:
Correlation between the distribution of CD133-positive cells and
the proliferation of microvessels in glioblastoma multiforme.
Zhonghua Yi Xue Za Zhi. 91:781–785. 2011.(In Chinese).
|
|
25
|
He H, Niu CS and Li MW: Correlation
between glioblastoma stem-like cells and tumor vascularization.
Oncol Rep. 27:45–50. 2012.PubMed/NCBI
|
|
26
|
Carrasco-Garcia E, Sampron N, Aldaz P, et
al: Therapeutic strategies targeting glioblastoma stem cells.
Recent Pat Anticancer Drug Discov. 8:216–227. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Gal H, Makovitzki A, Amariglio N, Rechavi
G, Ram Z and Givol D: A rapid assay for drug sensitivity of
glioblastoma stem cells. Biochem Biophys Res Commun. 358:908–913.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Sampetrean O and Saya H: Characteristics
of glioma stem cells. Brain Tumor Pathol. 30:209–214. 2013.
View Article : Google Scholar
|
|
29
|
Salmaggi A, Boiardi A, Gelati M, et al:
Glioblastoma-derived tumorospheres identify a population of tumor
stem-like cells with angiogenic potential and enhanced multidrug
resistance phenotype. Glia. 54:850–860. 2006. View Article : Google Scholar
|
|
30
|
Choy W, Nagasawa DT, Trang A, Thill K,
Spasic M and Yang I: CD133 as a marker for regulation and potential
for targeted therapies in glioblastoma multiforme. Neurosurg Clin N
Am. 23:391–405. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhang B, Sun J, Yu SP, et al:
Rac1+ cells distributed in accordance with
CD133+ cells in glioblastomas and the elevated
invasiveness of CD133+ glioma cells with higher Rac1
activity. Chin Med J (Engl). 125:4344–4348. 2012.
|
|
32
|
Singh SK, Clarke ID, Terasaki M, Bonn VE,
Hawkins C, Squire J and Dirks PB: Identification of a cancer stem
cell in human brain tumors. Cancer Res. 63:5821–5828.
2003.PubMed/NCBI
|
|
33
|
Dean M, Fojo T and Bates S: Tumour stem
cells and drug resistance. Nat Rev Cancer. 5:275–284. 2005.
View Article : Google Scholar
|
|
34
|
Bleau AM, Huse JT and Holland EC: The
ABCG2 resistance network of glioblastoma. Cell Cycle. 8:2936–2944.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Scharenberg CW, Harkey MA and Torok-Storb
B: The ABCG2 transporter is an efficient Hoechst 33342 efflux pump
and is preferentially expressed by immature human hematopoietic
progenitors. Blood. 99:507–512. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Nakanishi T and Ross DD: Breast cancer
resistance protein (BCRP/ABCG2): its role in multidrug resistance
and regulation of its gene expression. Chin J Cancer. 31:73–99.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Stupp R, Mason WP, van den Bent MJ, et al:
Radiotherapy plus concomitant and adjuvant temozolomide for
glioblastoma. N Engl J Med. 352:987–996. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Zhang L, Wu X, Xu T, Luo C, Qian J and Lu
Y: Chemotherapy plus radiotherapy versus radiotherapy alone in
patients with anaplastic glioma: a systematic review and
meta-analysis. J Cancer Res Clin Oncol. 139:719–726. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Hau P, Koch D, Hundsberger T, et al:
Safety and feasibility of long-term temozolomide treatment in
patients with high-grade glioma. Neurology. 68:688–690. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Hart MG, Garside R, Rogers G, Stein K and
Grant R: Temozolomide for high grade glioma. Cochrane Database Syst
Rev. 4:CD0074152013.
|
|
41
|
Eramo A, Ricci-Vitiani L, Zeuner A, et al:
Chemotherapy resistance of glioblastoma stem cells. Cell Death
Differ. 13:1238–1241. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Clement V, Sanchez P, de Tribolet N,
Radovanovic I and Ruiz i Altaba A: HEDGEHOG-GLI1 signaling
regulates human glioma growth, cancer stem cell self-renewal, and
tumorigenicity. Curr Biol. 17:165–172. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Brada M, Judson I, Beale P, et al: Phase I
dose-escalation and pharmacokinetic study of temozolomide (SCH
52365) for refractory or relapsing malignancies. Br J Cancer.
81:1022–1030. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Ostermann S, Csajka C, Buclin T, Leyvraz
S, Lejeune F, Decosterd LA and Stupp R: Plasma and cerebrospinal
fluid population pharmacokinetics of temozolomide in malignant
glioma patients. Clin Cancer Res. 10:3728–3736. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Zhang H, Song N, Liu YP, Qu XJ, Hou KZ,
Teng YE and Zhang JD: Reversal of resistance to adriamycin in human
gastric cancer by β-elemene. J Chian Med Univ. 40:968–993. 2011.(In
Chinese).
|
|
46
|
Li X, Wang G, Zhao J, et al:
Antiproliferative effect of beta-elemene in chemoresistant ovarian
carcinoma cells is mediated through arrest of the cell cycle at the
G2-M phase. Cell Mol Life Sci. 62:894–904. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Tao L, Zhou L, Zheng LY and Yao M:
Inhibition of eIF families expression and angiogenesis for human
laryngeal carcinoma by elemene administration. Zhonghua Er Bi Yan
Hou Tou Jing Wai Ke Za Zhi. 40:840–845. 2005.(In Chinese).
|