Introduction
Although advances in science and technology have
replaced raw herbs and/or herbal compounds with powerful synthetic
drugs including molecular target-based drugs in cancer therapy,
still the issue of concern is resistance, disease relapse and side
effects of drugs in a clinical setting. These cancer-related
deficits have led many cancer patients to look for potential cures
outside the mainstream of Western medical treatments in order to
maintain their quality of life (1,2). In
this regard, herbal and other botanical remedies have been
historically used by cancer patients in efforts to control their
disease progression or to manage symptoms associated with cancer
and cancer treatments (1,2). Mixtures of compounds interacting each
other produced by plants have provided important combination
therapies that affect multiple pharmacological targets
simultaneously, resulting in substantial clinical efficacy beyond
the reach of single compound-based drugs (2,3). The
ideology of wanting to assign a specific biological activity to a
specific compound has slowed acceptance of the idea of
multicomponent therapies in Western medicine (2). Therefore, rediscovering the
mechanisms underlying the efficacy of herbal drugs or extracts will
not only have important implications for appropriate clinical use
of these traditional medicines, but also provide novel insight into
the interaction between these traditional medicines and
conventional drugs due to the fact that they may be
counterproductive when used by patients on chemotherapy or on other
prescription medications (1,2,4).
We have investigated the antiproliferative activity
of naturally derived compounds against the growth of various types
of cancer cells. Of these, Vitex, an extract from the ripe fruit of
Vitex agnus-castus, has attracted great attention (5–10).
V. agnus-castus is a shrub of the Lamiaceae family
(previously known as Verbenaceae family) and found naturally
in the Middle East and Southern Europe and China. Ripe fruit of
V. agnus-castus has traditionally been used to treat
patients with moderate to severe premenstrual syndrome in Europe as
well as in China, and demonstrated to be well tolerated and
effective (11,12). In addition, we have previously
demonstrated that Vitex exhibits cytotoxic activities against
various types of solid tumor cells, such as KATO-III (a human
gastric signet ring carcinoma cell line), COLO 201 (a human colon
adenocarcinoma cell line) and MCF-7 (a human breast carcinoma cell
line) (9,10). We further demonstrated that the
levels of cytotoxic activity of Vitex were attributed to growth
rate of the respective cells, the cells with faster growth rate
were more susceptible to Vitex cytotoxicity (9). More importantly, no apparent
cytotoxicity was observed in non-tumor cells, such as human uterine
cervical canal fibroblast (HCF), human embryo fibroblast (HE-21)
and peripheral blood mononuclear cells (PBMNCs) from healthy
volunteers when treated with concentrations showing significant
cytotoxicity in tumor cells (6,9,13).
We recently demonstrated that cytotoxicity of Vitex correlated with
differentiation status in leukemia cell lines (13), however, to date, the effects of
Vitex on hematopoietic cell line have not been characterized in
detail.
We have previously demonstrated that oxidative
stress is implicated in KATO-III cell apoptosis induced by Vitex
(10), but not in COLO 201 cells
(7). We recently also demonstrated
that cytotoxicity induced by casticin, a major component of Vitex,
in HL-60 cells was independent of reactive oxygen species (ROS)
generation (14). Therefore, there
is much interest in the alteration of intracellular ROS levels in
HL-60 cells exposed to Vitex, since ROS have been widely believed
to play a pivotal role in a wide variety of cellular functions,
including cell proliferation, differentiation and apoptosis, in
both normal and cancer cells (15,16).
Indeed, it has been demonstrated that nicotinamide adenine
dinucleotide phosphate (NADPH) oxidase, which was described for the
first time in professional phagocytes responsible for generation a
large burst of superoxide to kill the ingested pathogens (16–18),
plays a critical role in the survival of HL-60 cells via ROS
generation (19).
In the current study, we first investigated the
effects of Vitex on the human promyelocytic cell line HL-60, in
view of cell viability, apoptosis induction and cell cycle arrest.
Since we recently demonstrated that casticin induced cytotoxicity
in HL-60 cells through p38 mitogen-activated protein kinase (MAPK)
signaling pathway (14), the
details of whether p38 MAPK is implicated in Vitex-mediated
cytocidal effects in the cells were also investigated using its
specific inhibitor, SB203580. We further investigated the
alteration of intracellular ROS levels in Vitex-treated HL-60
cells. We also investigated whether NADPH oxidase and heme
oxygenase-1 (HO-1), a stress-responsive gene closely correlated
with cellular redox status, are involved in the regulation of
intracellular redox status along with cytotoxicity in Vetix-treated
HL-60 cells using their respective inhibitor.
Materials and methods
Materials
Casticin was obtained from ChromaDex (Irvine, CA,
USA). Fetal bovine serum (FBS) was purchased from Nichirei
Biosciences (Tokyo, Japan). RPMI-1640 medium, phenazine
methosulfate (PMS) and an RNA extraction kit, Isogen were obtained
from Wako Pure Chemical Industries (Osaka, Japan). Moloney murine
leukemia virus reverse transcriptase (M-MLV RT) and
dichlorofluorescin diacetate (DCFH-DA) were purchased from and
Invitrogen (Carlsbad, CA, USA). Random primer was purchased from
Takara Bio, (Shiga, Japan). GoTaq DNA polymerase was purchased from
Promega (Madison, WI, USA). Propidium iodide (PI) and
2,3-bis(2-methoxy-4-
nitro-5-sulfophenyl)-5-[(phenylamino)carbonyl]-2H-tetrazolium
hydroxide (XTT), were purchased from Sigma-Aldrich (St. Louis, MO,
USA). Both SB203580, a specific inhibitor of p38 MAPK, and its
negative control SB202474 were purchased from Calbiochem (La Jolla,
CA, USA). Tin protoporphyrin IX dichloride (SnPP), an inhibitor
specific for HO-1, was purchased from Alexis Biochemicals (San
Diego, CA, USA).
Preparation of an ethanol extract from
dried ripened V. agnus-castus fruits (Vitex)
Preparation of Vitex was carried out according to
the methods described previously (9,13).
Briefly, dried ripened V. agnus-castus fruit from Israel was
gently triturated. The extract was prepared from 1 g of the
triturate with 10 ml of ethanol under reflux for 2 h. The extract
was then cooled, filtered, evaporated and dried in a vacuum
desiccator, a product of which was designated as Vitex. The yield
of Vitex was 0.08–0.1 g from 1 g of dried fruit.
Cell culture and treatment
HL-60 cells were obtained from the Health Science
Research Resources Bank (Tokyo, Japan). Cells were cultured in
RPMI-1640 medium supplemented with 10% heat-inactivated FBS and 100
U/ml of penicillin and 100 μg/ml of streptomycin at 37°C in a
humidified atmosphere (5% CO2 in air). Cells were
treated with SB203580, a specific inhibitor of p38 MAPK, and its
negative control SB202474 at the indicated concentrations for 1 h
prior to treatment with Vitex in the presence or absence of these
reagents. In order to investigate whether HO-1 is involved in
Vitex-mediated cytotoxicity, cells were also treated with Vitex in
the presence or absence of SnPP.
Cell viability assay
After treatment with Vitex (10, 30 and 50 μg/ml) for
24 or 48 h, cell viability was measured by the XTT assay as
described previously (5,13). Briefly, cells were exposed to
Vitex, followed by washing with PBS twice and resuspension in
appropriate volume of PBS. An aliquot (0.2 ml) of cell suspension
was inoculated into 96-well microplates followed by the addition of
50 μl XTT/PMS mixed solution (1.5 mM XTT and 0.025 mM PMS). After
incubation at 37°C for 4 h, plates were mixed on a mechanical plate
shaker, and absorbance at 450 nm was measured with a microplate
reader (Safire, Tecan, Switzerland). The relative cell viability
was expressed as the ratio of the absorbance of each treatment
group against those of the corresponding untreated control group.
The IC50 value of Vitex was calculated from the cell
proliferation inhibition curve after 24- and 48-h treatment,
respectively.
DNA fragmentation analysis
After treatment with Vitex (10, 20, 30, 40 and 50
μg/ml) for 24 h, DNA extraction and DNA fragmentation analysis were
carried out according to a method described previously (20). Briefly, extracted DNA was dissolved
in TE buffer (10 mM Tris-HCl, pH 7.8, 1 mM EDTA). These DNA samples
(approximately 20 μg DNA/20 μl TE buffer) and 100 bp DNA Ladder
were electrophoresed, respectively, on a 2% agarose X gel (Nippon
Gene, Tokyo, Japan), and visualized by ethidium bromide staining,
followed by viewing under UV Light Printgraph (ATTO Corp., Tokyo,
Japan).
Cell cycle analysis
After treatment with 20 μg/ml of Vitex in the
presence or absence of SB203580 or SB202474 for 12 h, cell cycle
analysis was performed using a FACSCanto flow cytometer
(Becton-Dickinson, CA, USA) according to a method reported
previously (14,21). A total of 10,000 events were
acquired and cell cycle distribution (sub-G1,
G0/G1, S and G2/M phase fraction)
was analyzed by using FlowJo software (Ver. 7.6.5, TreeStar, USA)
on the basis of ‘Watson Pragmatic’ algorithm.
Western blot analysis
Protein samples were separated on SDS-PAGE, followed
by transferring to a nitrocellulose membrane as described
previously (22). Protein bands
were detected using the following primary antibodies: mouse
anti-human β-actin (1:5,000 dilution, Sigma-Aldrich); rabbit
anti-human phospho-p38 MAPK (Thr180/Tyr182) (1:1,000 dilution) and
rabbit anti-human p38 MAPK (1:1,000 dilution) (Cell Signaling
Technology, MA, USA). Blotted protein bands were detected with
respective horseradish peroxidase-conjugated secondary antibody and
an enhanced chemiluminescence (ECL) western blot analysis system
(Amersham Pharmacia Biotech, Buckinghamshire, UK). Relative amounts
of the immunoreactive proteins were calculated from the density of
the gray level on a digitized image using a program, Multi Gauge
Ver. 3.0 (Fujifilm, Tokyo, Japan).
Detection of phosphorylation of histone
H3
After treatment with 20 μg/ml of Vitex in the
presence or absence of SB203580 or SB202474 (20 μM, respectively)
for 12 h, the expression levels of phosphorylation of histone H3
were analyzed using a FACSCanto flow cytometer according to a
method reported previously (14).
A total of 10,000 events were acquired and analyzed using Diva
software.
Measurement of intracellular ATP
levels
ATP levels were determined using a ‘Cellno’ ATP
assay reagent (Toyo B-Net, Tokyo, Japan) according to the
manufacturer’s instructions. Briefly, after treatment with 20 μg/ml
of Vitex for 6 h, cells (2×105 cells) were washed with
PBS twice and suspended in 100 μl of PBS. Cell suspension was
inoculated into 96-well microtiter plates followed by the addition
of 100 μl of ‘Cellno’ ATP assay reagent. After shaking for 1 min
and incubating for 10 min at room temperature, luminescence was
measured in a luminometer as described in our previous report
(14).
Measurement of intracellular ROS
levels
Intracellular ROS levels were analyzed using DCFH-DA
as a ROS-reactive fluorescence probe as described previously
(14). In brief, after treatment
with 20 μg/ml of Vitex for 3 or 12 h in the presence or absence of
20 μM of SnPP, cells (1×106 cells) were suspended in 1
ml of PBS with 5 mM DCFH-DA and incubated for 20 min at 37°C. Next,
cells were washed with PBS twice, and resuspended in 500 μl of 2
μg/ml PI/PBS. A total of 30,000 events were acquired for flow
cytometry analysis using a FACSCanto flow cytometer and Diva
software.
Reverse transcription-polymerase chain
reaction (RT-PCR) analysis
Total RNA isolation and complementary DNA were
prepared according to methods described previously with
modifications (23). Total RNA was
extracted from cells using an RNA extraction kit, Isogen.
Complementary DNA was synthesized from 1 μg of RNA using 100 pmol
random primer and 50 U M-MLV RT in a total volume of 20 μl,
according to the manufacturer’s instructions. PCR was performed
according to the methods described previously (23). DNA primers for RT-PCR were
purchased from Amersham Pharmacia Biotech (Piscataway, NJ, USA);
sense primer (5′-GAA TGG GGA AAA ATA AAG GAA TG-3′) and antisense
primer (5′-ACC CCT TCT TCT TCA TCT GTA GC-3′) for
gp91phox mRNA (24);
sense primer (5′-CGC TTC ACC CAG TGG TAC TT-3′) and antisense
primer (5′-GAG AGC AGG AGA TGC AGG AC-3′) for p22phox
mRNA (24); and sense primer
(5′-CCT TCC TGG GCA TGG AGT CCT G-3′) and antisense primer (5′-GGA
GCA ATG ATC TTG ATC TTC-3′) for β-actin mRNA (23). PCR was carried out 36, 27 and 21
cycles for gp91phox, p22phox and β-actin
mRNA, respectively (45 sec at 94°C for denaturation, 45 sec at 60°C
for annealing and 2 min at 72°C for extension) using a Takara PCR
Thermal Cycler Dice (Takara Bio). PCR products and a Tracklt™ 100
bp DNA Ladder as a DNA size marker were electrophoresed on a 2%
UltraPure™ agarose gel (Invitrogen), respectively, and visualized
by ethidium bromide staining, followed by viewing under UV Light
Printgraph. The relative expression levels of gp91phox
or p22phox/β-actin gene were calculated as the ratios
against those at 0-time using ImageJ 1.46m (Wayne Rasband,
USA).
Statistical analysis
Data were analyzed using Student’s t-test and
p<0.05 was considered as statistically significant.
Results
Contribution of apoptosis induction and
cell cycle arrest to Vitex-induced cytotoxicity in HL-60 cells
Since both apoptosis and cell cycle arrest have been
demonstrated to contribute to cytocidal effects of substances
derived from natural product (25), alteration of cell viability and
induction of apoptosis along with cell cycle arrest were
investigated in HL-60 cells after treatment with Vitex ranging from
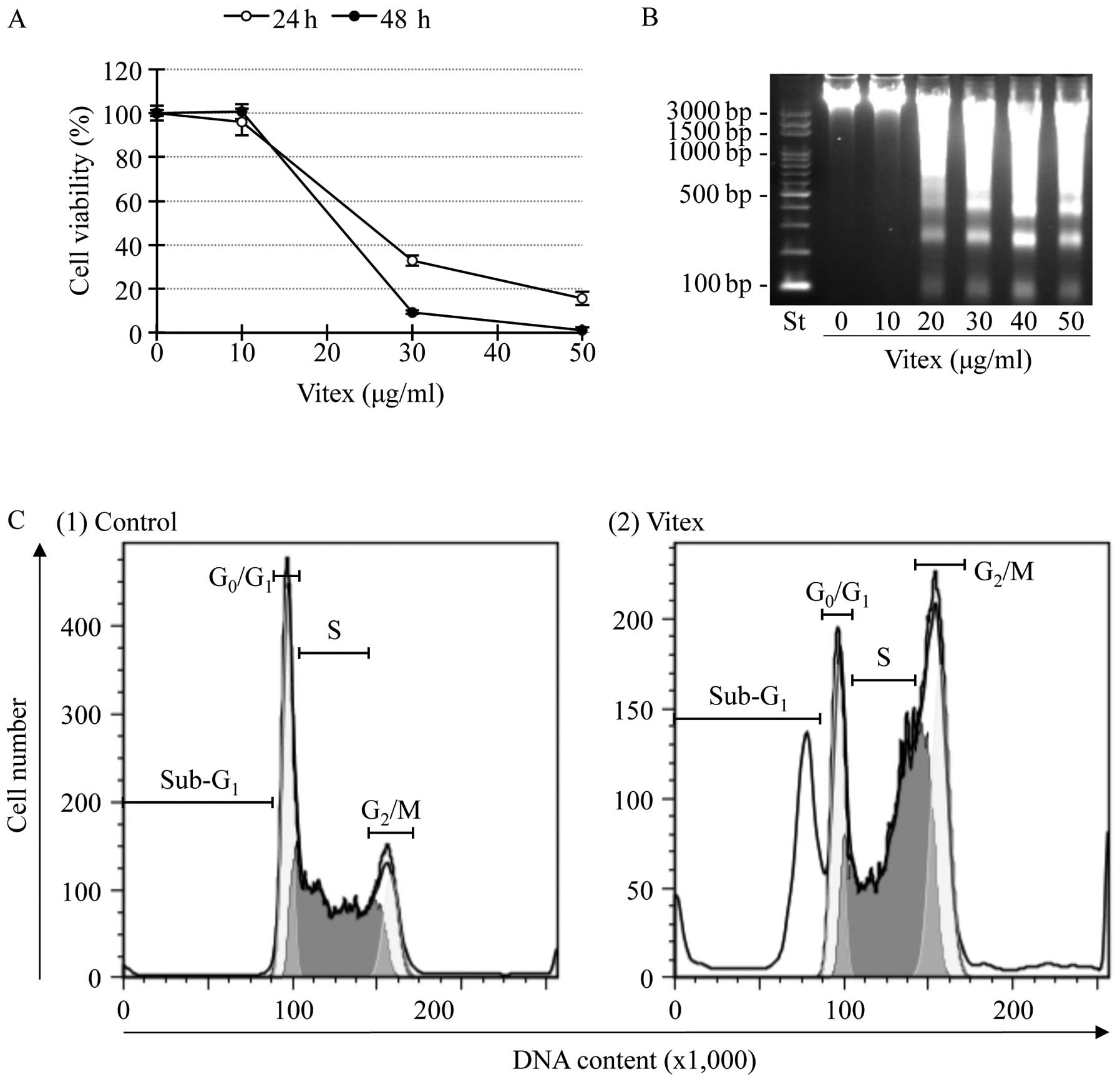
10 to 50 μg/ml for the indicated time. A significant decrease in
cell viability was observed in a dose- and time-dependent manner
(Fig. 1A). The IC50
values were 20.2±0.92 and 18.4±0.38 μg/ml for 24- and 48-h
treatment, respectively. A significant difference of the
IC50 values was observed between two time points
(p<0.01). DNA fragmentation was observed at concentrations
starting from 20 μg/ml of Vitex at 24 h (Fig. 1B). Flow cytometric analysis also
showed a significant accumulation of cells in sub-G1
phase after treatment with the IC50 value of Vitex at 20
μg/ml for 12 h (Fig. 1C), in
agreement with results in Fig. 1B.
Moreover, G2/M cell cycle arrest along with a
significant decrease in the number of cells in both
G0/G1 and S phase was observed simultaneously
(Fig. 1C).
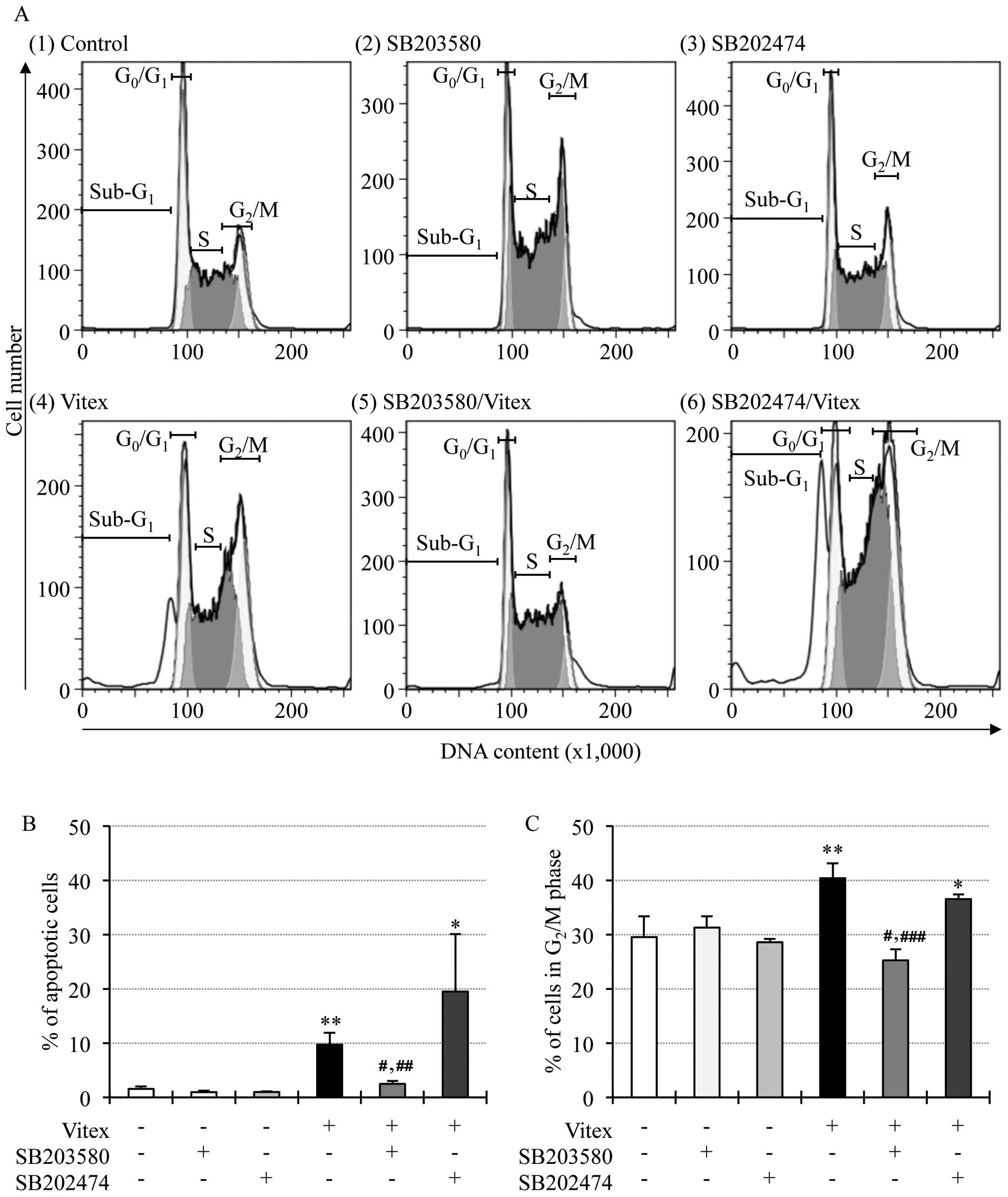
Effects of p38 MAPK inhibitor on
Vitex-induced apoptosis and cell cycle arrest in HL-60 cells
We recently demonstrated that casticin, one of major
components of Vitex, induced cytocidal effects in HL-60 cells
through p38 MAPK pathway (14). In
order to investigate whether the pathway is primarily involved in
the effects of Vitex, alteration of apoptosis induction and cell
cycle arrest were first determined in the cells after treatment
with 20 μg/ml of Vitex in the presence or absence of 20 μM SB203580
for 12 h. As shown in Fig. 2A
(panel 4), B and C, the apoptosis induction and cell cycle arrest
were reconfirmed by the increase in the number of cells in
sub-G1, G2/M phase and the decrease in the
number of cells in both G0/G1 and S phase,
respectively. The addition of SB203580 not only significantly
suppressed the apoptosis induction, but also corrected cell cycle
arrest at G2/M phase, concomitant with an increase in
the number of cells in both G0/G1 and S phase
[Fig. 2A (panel 5), B and C]. A
similar phenomenon was not observed in the presence of SB202474
[Fig. 2A (panel 6), B and C].
SB203580 and SB202474 per se showed no influence on
apoptosis and cell cycle arrest in HL-60 cells [Fig. 2A (panels 2 and 3), B and C].
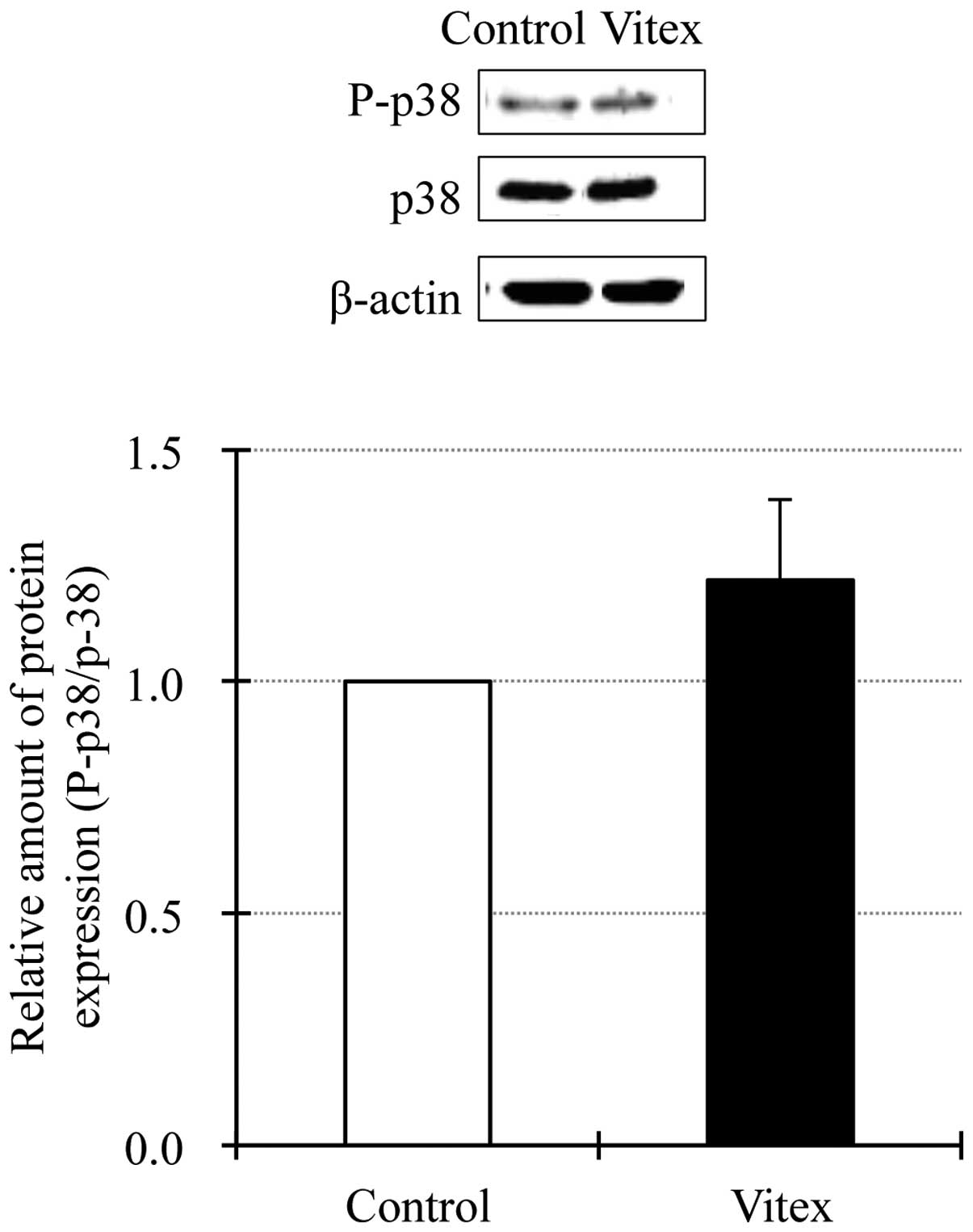
Detection of phosphorylation of p38 MAPK
in Vitex-treated HL-60 cells
In order to further investigate the details of
involvement of p38 MAPK signaling pathway in Vitex-mediated
cytotoxicity in HL-60 cells, the activation of p38 MAPK was
assessed by western blot analysis. Similar to our previous results
(14,26), endogenous p38 MAPK activation was
observed in untreated HL-60 cells based on the detection of a
distinct protein band corresponding to phospho-p38 MAPK (Fig. 3). Unexpectedly, although a trend
towards increased expression level of phospho-p38 MAPK was observed
in 20 μg/ml of Vitex-treated HL-60 cells, there was no significant
difference in its expression levels between Vitex-treated and
untreated cells, indicating that exposure to Vitex did not affect
the degree of p38 MAPK activation (Fig. 3). Moreover, no change in the
expression levels of total p38 MAPK was observed in untreated or
treated cells.
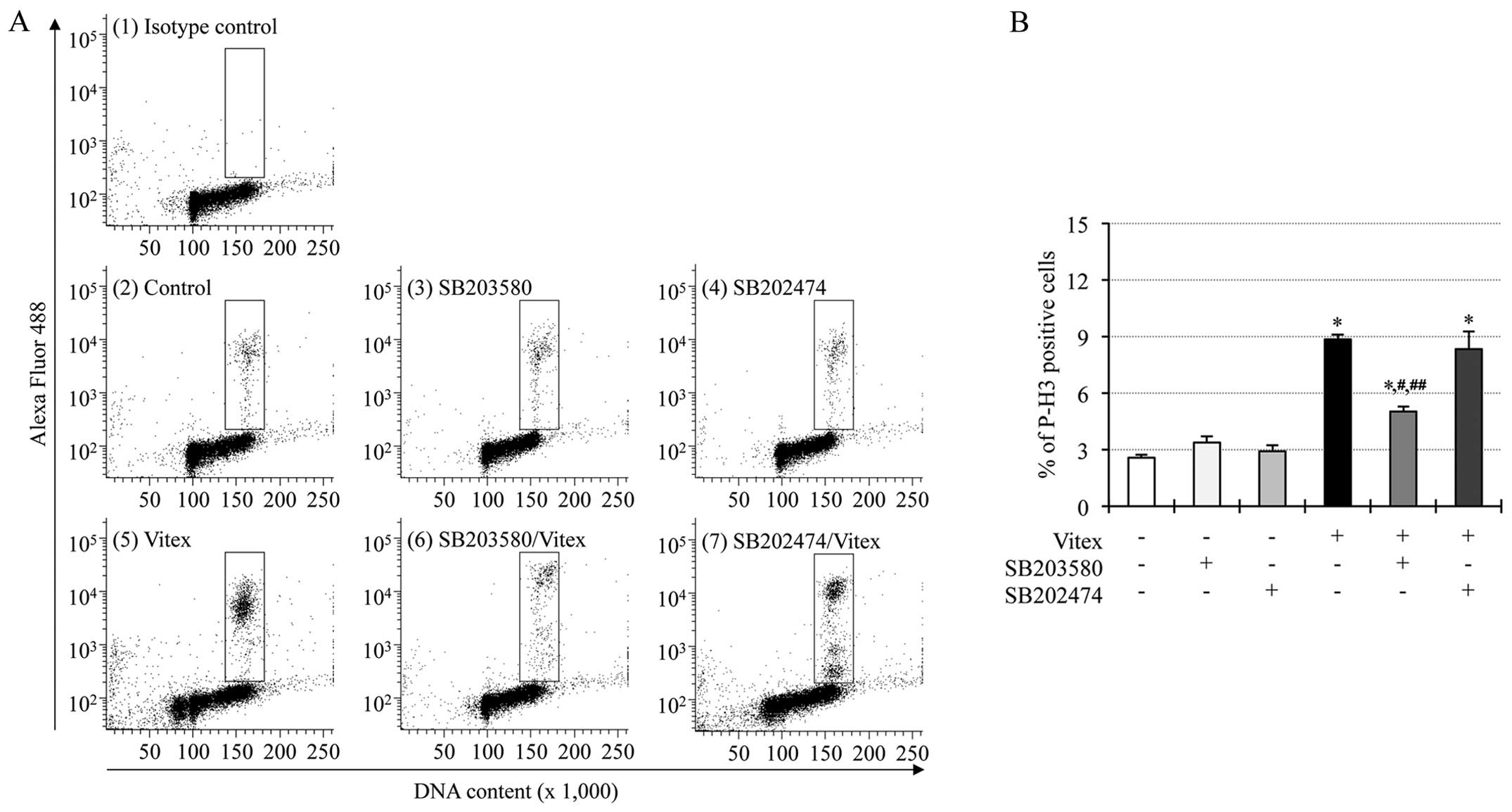
Suppression of Vitex-induced
phosphorylation of histone H3 by p38 MAPK inhibitor in HL-60
cells
We recently demonstrated that phosphorylation of
histone H3 was observed in casticin-treated HL-60 cells, and
suppressed by the addition of SB203580, suggesting that
phosphorylation of histone H3 associated with the activation of p38
MAPK plays a critical role in casticin-mediated cytotoxicity in the
cells (14). Therefore,
phosphorylation of histone H3, and its correlation with p38 MAPK
signaling pathway were also investigated in Vitex-treated HL-60
cells. Just like the phenomena observed in casticin-treated HL-60
cells (14), a substantial
increase in the expression levels of phospho-histone H3 over the
endogenous levels was detected in HL-60 cells exposed to 20 μg/ml
of Vitex for 12 h [Fig. 4A (panels
2 and 5) and B)]. Furthermore, the addition of 20 μM SB203580, but
not SB202474, significantly suppressed Vitex-mediated
phosphorylation of histone H3 [Fig.
4A (panels 6 and 7) and B]. SB203580 and SB202474 per se
showed no influence on the expression levels of phosphorylation of
histone H3 in HL-60 cells [Fig. 4A
(panels 3 and 4), B and C].
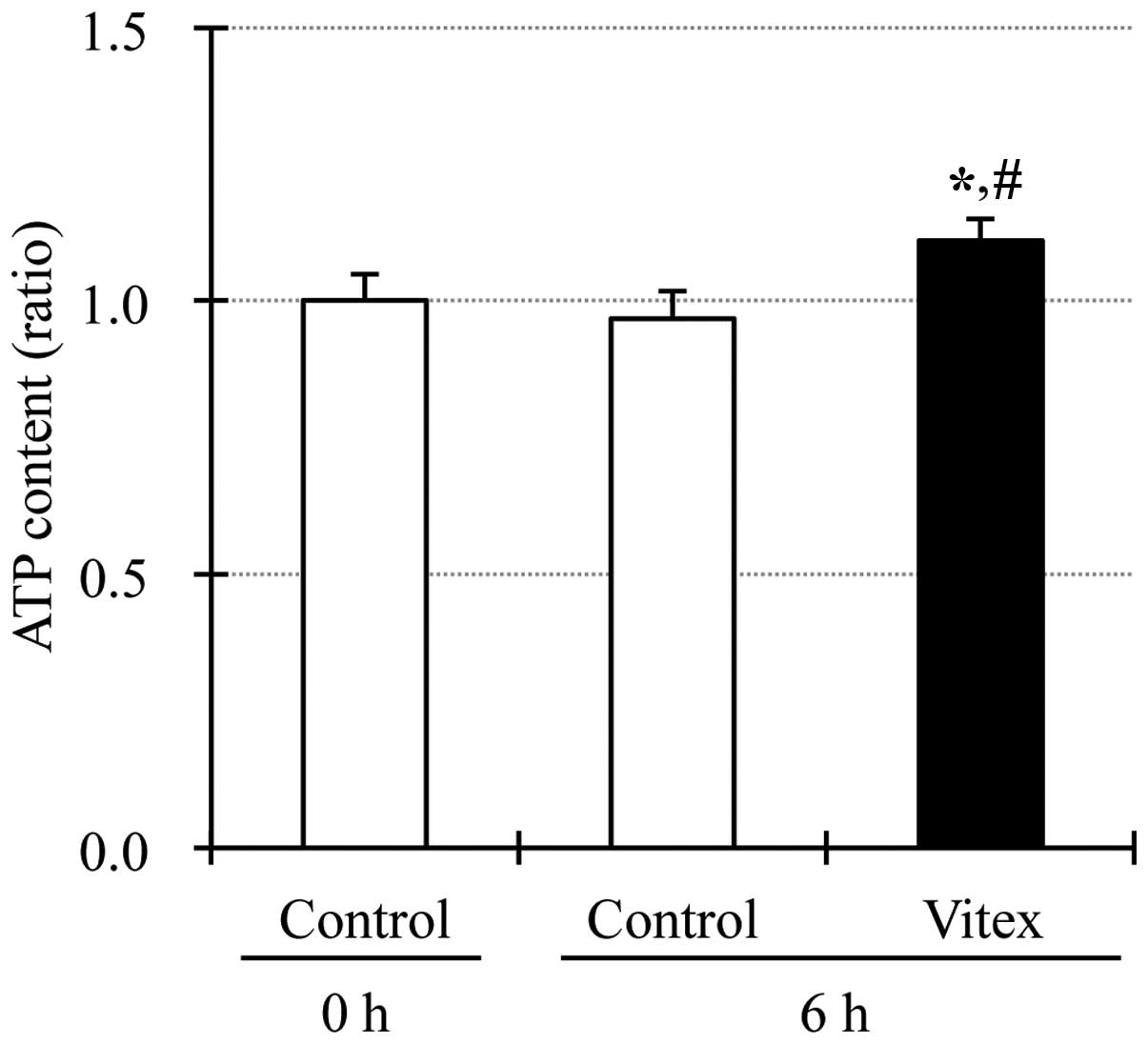
Upregulation of intracellular ATP level
in Vitex-treated HL-60 cells
Since binding of ATP to its binding pocket inside
the activated p38 MAPK has been reported to be required for the
activation of downstream molecules of p38 MAPK (27, 28), the alteration of intracellular ATP
level was determined in HL-60 cells after treatment with 20 μg/ml
of Vitex for 6 h. As shown in Fig.
5, intercellular ATP level was significantly upregulated in
Vitex-treated cells when compared to that in untreated cells,
similar to the phenomena observed in casticin-treated HL-60 cells
(14).
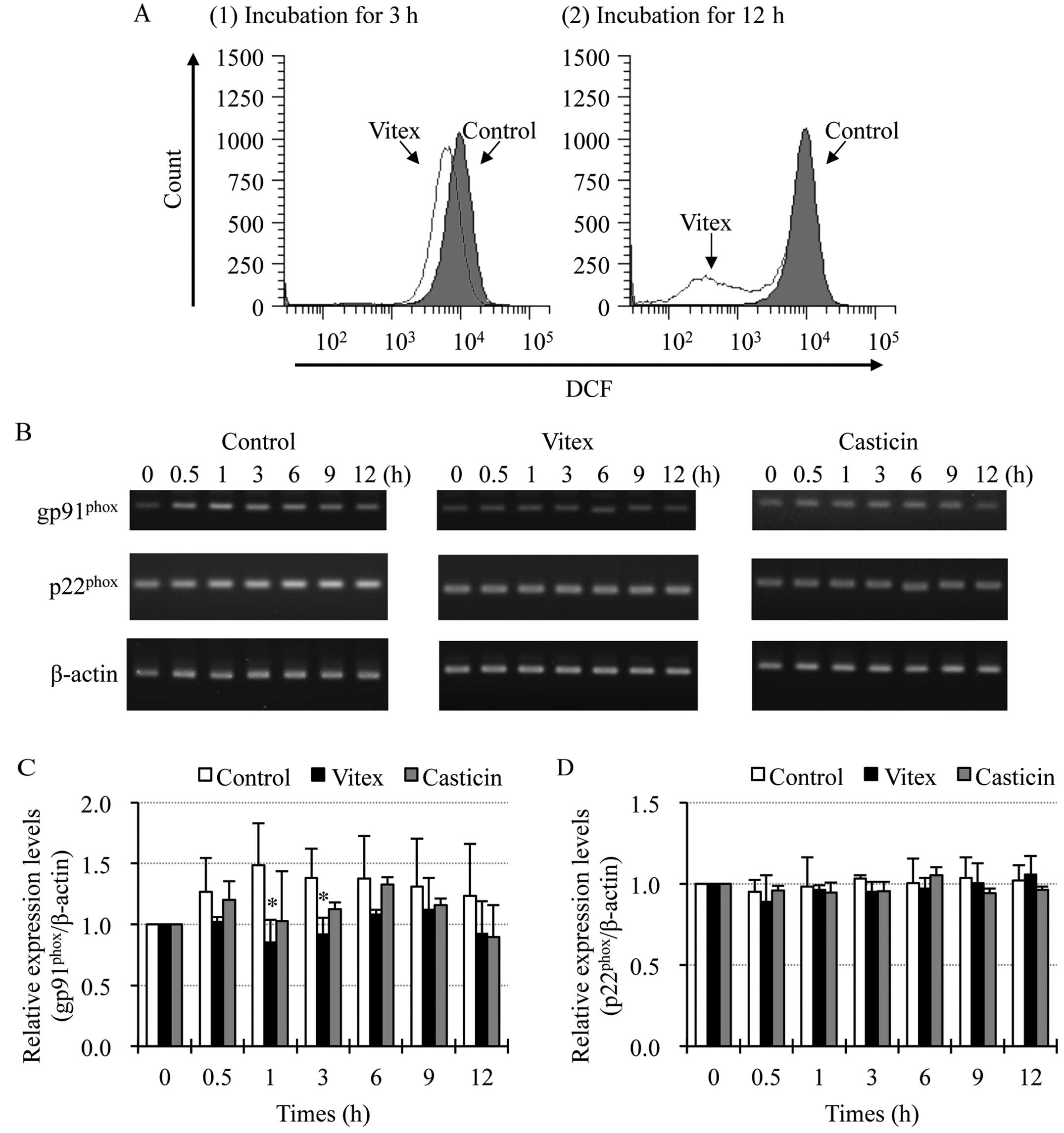
Involvement of gp91phox in
Vitex-mediated reduction of intracellular ROS levels in HL-60
cells
FACS analysis using DCFH-DA as a ROS-reactive
fluorescence probe showed a significant time-dependent decrease in
the intracellular ROS levels after treatment with 20 μg/ml of Vitex
for 3 and 12 h (Fig. 6A). In this
regard, it is interesting to note that NADPH oxidase exists in
undifferentiated HL-60 cells, and that its mediated generation of
ROS is critically required for survival of the cells (19). Therefore, the expression levelsof
gp91phox and p22phox, important subunits of
NADPH oxidase, were investigated in HL-60 cells when treated with
20 μg/ml of Vitex for indicated time. Compared with control groups,
a trend towards reduced expression level of gp91phox,
but not p22phox mRNA was observed in Vitex-treated HL-60
cells throughout the periods of treatment (Fig. 6B–D). Notably, a significant
decrease in the expression level of gp91phox mRNA was
observed at 1- and 3-h post-treatment (Fig. 6B and C). In contrast, as shown
Fig. 6B and D, similar phenomena
were not observed in the cells treated with 0.3 μg/ml of casticin,
the IC50 value of casticin after 24-h treatment as
described in our previous report (13).
Enhancement of Vitex-induced cytotoxicity
by tin protoporphyrin IX dichloride (SnPP), a HO-1 specific
inhibitor, in HL-60 cells
HO-1, a stress-associated gene, is well known to
play a critical role in the regulation of intracellular redox
status through its biologically active products, such as iron ion,
carbon monoxide and biliverdin (29,30).
Therefore, whether HO-1 is involved in the regulation of redox
status along with cytotoxicity in Vitex-treated HL-60 cells was
evaluated. After treatment with 20 μg/ml of Vitex and 20 μM of
SnPP, alone or combination, for indicated time, alteration of the
intracellular ROS levels and cell viability were investigated,
respectively. As shown in Fig. 7A,
compared to control groups, a significant decrease in intracellular
ROS levels was reconfirmed in HL-60 cells treated with Vitex alone
for 3 and 12 h, and further enhanced by the addition of SnPP at 3-h
post-treatment. A small but not significant enhanced decrease in
ROS levels was also observed at 12-h post-treatment. These results
indicate that biological activity of HO-1 contributes to ROS
production in HL-60 cells. Furthermore, the addition of SnPP
significantly enhanced the apoptosis induced by Vitex when compared
to treatment with Vitex alone for 24 h, indicating the
cytoprotective activity of HO-1 (Fig.
7B).
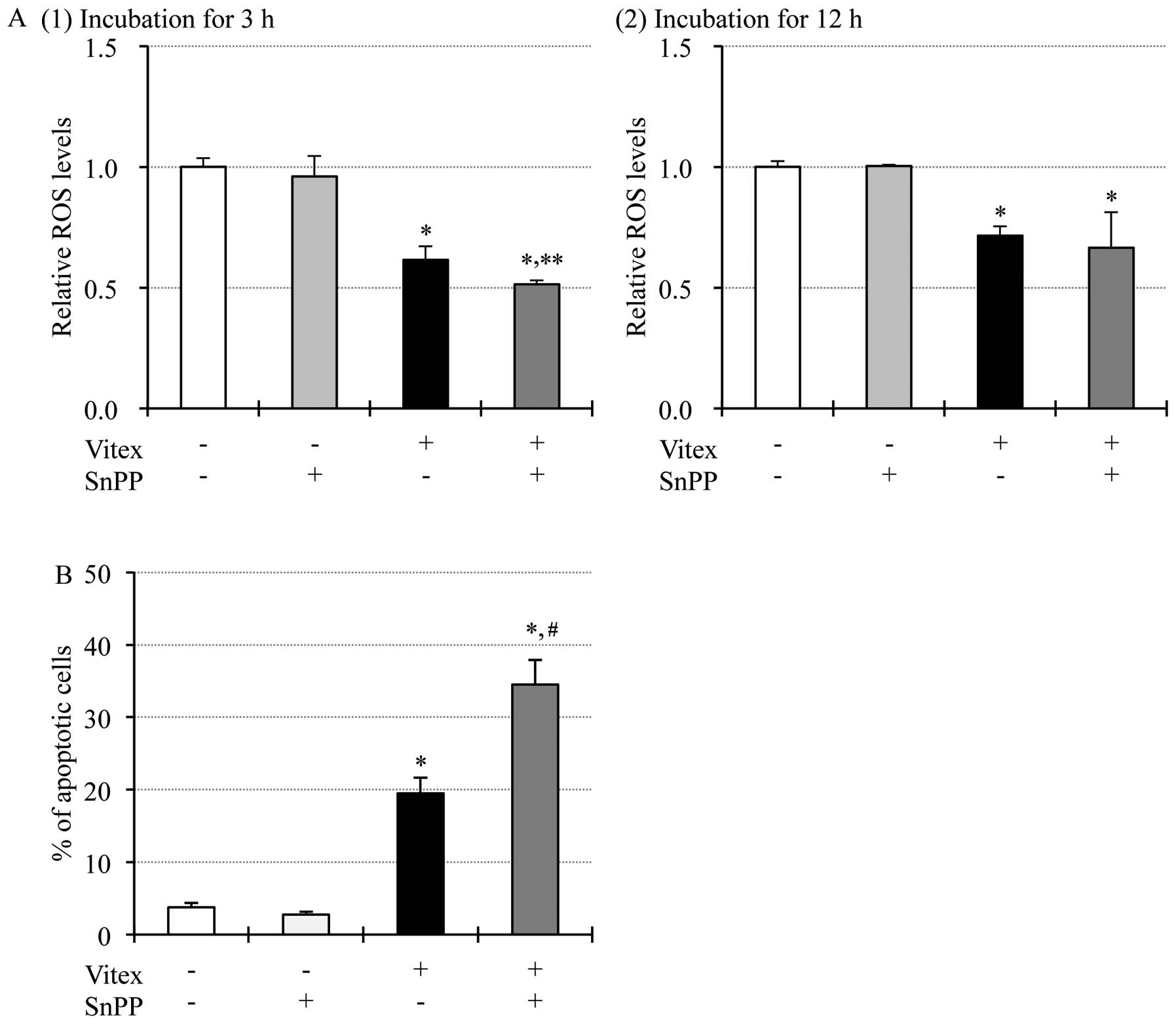 | Figure 7Enhancement of Vitex-induced
cytotoxicity by tin protoporphyrin IX dichloride (SnPP), a HO-1
specific inhibitor, in HL-60 cells. (A) After the treatment with
Vitex (20 μg/ml) and SnPP (20 μM), alone or combination, for 3 or
12 h, alteration of the intracellular ROS levels were investigated
as described in Fig. 6A. Relative
ROS levels were calculated as the ratios of the mean fluorescence
intensity (MFI) of DCF in treatment group and control group at each
time-point, as described in our previous report (26). (B) After treatment with Vitex (20
μg/ml) and SnPP (20 μM), alone or combination, for 24 h, percentage
of apoptotic cells, represented by the accumulation of cells in
sub-G1 phase, were calculated as described in Fig. 1. Experiments were carried out in
triplicate and results are shown as mean ± SD from three
independent experiments. *p<0.01 vs. control;
**p<0.05 and #p<0.01 vs. Vitex
alone. |
Discussion
Vitex has traditionally been used in obstetrics and
gynecology in Europe as well as in China, and was demonstrated to
be well tolerated and effective (11,12).
Besides these current clinical uses, we have demonstrated that
Vitex exhibits cytotoxic activities against various types of solid
tumor cells (9,10). Furthermore, we and others have
demonstrated that no apparent cytotoxicity of Vitex and its major
component, casticin, was observed in non-tumor cells, PBMNCs and
embryo fibroblasts, when treated with concentrations showing
significant cytotoxicity in tumors cells (9,13,31).
Therefore, we suggest that Vitex/casticin possesses selective
cytotoxic activity against tumor cells. More recently, we
investigated the cytocidal effects of Vitex/casticin, against
leukemia cell lines with a different degree of differentiation, and
demonstrated that their cytotoxicity correlated with
differentiation status in these cells (13). As a traditional medicine and a
promising anticancer candidate, further detailed studies aimed at
characterizing the effects of Vitex against tumor cells are eagerly
awaited, since the continuous efforts to explore the mechanisms
underlying the efficacy of Vitex will provide a molecular rationale
for clinical development in anticancer therapy.
In the current study, we demonstrated that
Vitex-mediated reduction in cell viability of HL-60 cells was
observed in a dose- and time-dependent manner. Furthermore, a
dose-dependent apoptosis induction, along with an accumulation of
cells in G2/M phase and a decrease in the number of
cells both in G0/G1 and S phase, was observed
in the Vitex-treated HL-60 cells. All these phenomena are similar
to observations in casticin-treated HL-60 cells previous reported
by us (14), suggesting that as a
major component of Vitex, casticin plays a primary role in
Vitex-mediated cytocidal effects against HL-60 cells.
Our experimental results regarding the cytotoxicity
of casticin against HL-60 cells suggested that the cytotoxicity
associated with apoptosis and cell arrestwas mediated by p38 MAPK
pathway, in which intracellular ATP levels and phosphorylation of
histone H3 played critical roles (14). In this regard, it is interesting to
note that the addition of SB203580, an inhibitor for p38 MAPK,
clearly not only corrected Vitex-mediated cell cycle arrest but
also efficiently suppressed apoptosis induction, reconfirming that
casticin primarily contributes the cytocidal effects induced by
Vitex in HL-60 cells. Very similar to that in casticin-treated
HL-60 cells (14), although almost
no alteration of the expression level of phospho-p38 MAPK was
observed in the cells treated with Vitex, a substantial increase in
the expression levels of phospho-histone H3 over the endogenous
levels was detected. It should be noted that histone H3
phosphorylation is implicated in arsenic trioxide-induced apoptosis
in human acute promyelocytic leukemia NB4 cells and
gliotoxin-induced apoptosis in mouse thymocytes, respectively
(32,33). Furthermore, the addition of
SB203580 significantly suppressed Vitex-induced phosphorylation of
histone H3, suggesting that histone H3 is one of downstream
molecules of p38 MAPK pathway. In fact, it has been demonstrated
that in response to various stimuli, activated p38 MAPK regulates
the immediate early gene expression and other cellular responses by
phosphorylating various substrates, including chromatin proteins,
and transcription factors (34,35).
We further demonstrated that intracellular ATP levels were
significantly upregulated in Vitex-treated HL-60 cells when
compared to those in untreated cells. Based on the fact that
SB203580 has been reported to compete with ATP for binding to the
active form of p38 MAPK, and consequently blocks the p38 MAPK
activity in downstream molecules (27,28),
we suggest that upregulation of intracellular ATP levels and
phosphorylation of histone H3 are closely associated with the
activation of p38 MAPK signaling pathway, consequently contributing
to Vitex-mediated cytocidal effects against HL-60 cells.
Collectively, these findings demonstrated that in view of apoptosis
induction and cell cycle arrest, the experimental data obtained in
Vitex- and casticin-treated HL-60 cells showed a close similarity,
strongly suggesting that casticin plays a primary role in apoptosis
induction and cell cycle arrest induced by Vitex in HL-60
cells.
We further demonstrated a significant time-dependent
decrease in the intracellular ROS levels in Vitex-treated HL-60
cells. Intriguingly, this result is the opposite to our recent
report showing that increased intracellular ROS production was
observed in casticin-treated HL-60 cells, although casticin-induced
cytotoxicity in the cells is independent of ROS generation
(14). These results thus
suggested that other phytochemicals, rather than casticin,
contributed to the decline in the intracellular ROS levels. Indeed,
flavonoids isolated from V. agnus-castus, such as
isoorientin, 2″-O-trans-caffeoylisoorientin,
6″-O-trans-caffeoylisoorientin and luteolin
7-O-glucoside, have been demonstrated to possess free
radical scavenging activity (36).
Concomitantly, the expression level of gp91phox, an
important component of NADPH oxidase, was also suppressed by the
treatment with Vitex. It has been demonstrated that NADPH
oxidase-mediated generation of ROS is critically required for
survival of undifferentiated HL-60 cells (19). Similar to the previous report, our
experimental data also demonstrated that treatment with
diphenyleneiodonium (an inhibitor of NADPH oxidase) alone resulted
in a significant and dose-dependent decline in the survival of
HL-60 cells (data not shown). Collectively, these results suggest
that Vitex exhibits its cytocidal effects via downregulation of
intracellular ROS levels, which is at least partially attributed to
the suppression of NADPH oxidase. It is of interest to note that a
greater decline in the ROS levels along with enhanced induction of
apoptosis was observed in HL-60 cells treated with Vitex in
combination with SnPP, an inhibitor specific for HO-1. HO-1 has
been well recognized as a proproliferative molecule in various
types of tumor cells (37),
although its antiproliferative effects of HO-1 in tumor cells have
also been conducted (38). Taking
these previous results and our observations into account, we
suggest that HO-1 exhibits cytoprotective activity through keeping
ROS levels required for survival of HL-60 cells. Since iron ion is
well known to be responsible for the generation of ROS, iron ion,
one of biologically active products of HO-1, is thus proposed to
contribute to maintain the balance of ROS levels in the cells.
Further investigation of the molecular details for the maintenance
is ongoing.
In conclusion, we demonstrated that apoptosis and
cell cycle arrest are involved in Vitex-induced cytocidal effects
in HL-60 cells, and that histone H3 phosphorylation via the
activation of p38 MAPK pathway is implicated in the cytocidal
effects. Based on our recent study on the cytotoxicity of casticin
(14), we further confirmed that
casticin, as a major component of Vitex, plays a primary role in
Vitex-mediated cytotoxicity in HL-60 cells. Intriguingly, we
demonstrated that Vitex significantly downregulated the
intracellular ROS levels, which appeared to be related to the
suppression of NADPH oxidase. Furthermore, treatment with Vitex in
combination with SnPP resulted in a greater decline in the ROS
levels along with enhanced induction of apoptosis. Therefore, these
results suggest that intracellular redox status is a critical
factor in determining the fate of Vitex-treated cells. It has been
reported that p38 MAPK is considered to be a sensor of moderate
oxidative stress, and was activated in a ROS-dependent manner
(39). Based on the downregulation
of intracellular ROS levels and upregulation of intracellular ATP
levels, we suggest that activation of p38 MAPK pathway might be
dependent on the alterations of intracellular ATP levels, rather
than that of intracellular ROS levels, details of investigation
into the mechanism is ongoing. Furthermore, these results may have
important implications for appropriate clinical use of Vitex and
provide novel insight into the interaction between Vitex and other
conventional drugs capable of affecting intracellular redox
status.
Acknowledgements
This study was supported in part by grants from the
Ministry of Education, Culture, Sports, Science and Technology and
by the Promotion and Mutual Aid Corporation for Private Schools of
Japan.
Abbreviations:
|
MAPK
|
mitogen-activated protein kinase
|
|
ROS
|
reactive oxygen species
|
|
NADPH oxidase
|
nicotinamide adenine dinucleotide
phosphate oxidase
|
|
SnPP
|
tin protoporphyrin IX dichloride
|
|
HO-1
|
heme oxygenase-1
|
References
|
1
|
Cassileth B, Yeung KS and Gubili J: Herbs
and other botanicals in cancer patient care. Curr Treat Options
Oncol. 9:109–116. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Schmidt BM, Ribnicky DM, Lipsky PE and
Raskin I: Revisiting the ancient concept of botanical therapeutics.
Nat Chem Biol. 3:360–366. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wang L, Zhou GB, Liu P, Song JH, Liang Y,
Yan XJ, Xu F, Wang BS, Mao JH, Shen ZX, Chen SJ and Chen Z:
Dissection of mechanisms of Chinese medicinal formula
Realgar-Indigo naturalis as an effective treatment for
promyelocytic leukemia. Proc Natl Acad Sci USA. 105:4826–4831.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
HemaIswarya S and Doble M: Potential
synergism of natural products in the treatment of cancer. Phytother
Res. 20:239–249. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Imai M, Kikuchi H, Denda T, Ohyama K,
Hirobe C and Toyoda H: Cytotoxic effects of flavonoids against a
human colon cancer derived cell line, COLO 201: a potential natural
anti-cancer substance. Cancer Lett. 276:74–80. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Imai M, Kikuchi H, Yuan B, Aihara Y,
Mizokuchi A, Ohyama K, Hirobe C and Toyoda H: Enhanced growth
inhibitory effect of 5-fluorouracil in combination with Vitex
agnus-castus fruits extract against a human colon
adenocarcinoma cell line, COLO 201. J Chin Clin Med. 6:14–19.
2011.
|
|
7
|
Imai M, Yuan B, Kikuchi H, Saito M, Ohyama
K, Hirobe C, Oshima T, Hosoya T, Morita H and Toyoda H: Growth
inhibition of a human colon carcinoma cell, COLO 201, by a natural
product, Vitex agnus-castus fruits extract, in vivo and in
vitro. Adv Biol Chem. 2:20–28. 2012. View Article : Google Scholar
|
|
8
|
Yuan B, Imai M, Kikuchi H, Fukushima S,
Hazama S, Akaike T, Yoshino Y, Ohyama K, Hu X, Pei X and Toyoda H:
Cytocidal effects of polyphenolic compounds, alone or in
combination with, anticancer drugs against cancer cells: potential
future application of the combinatory therapy. Apoptosis and
Medicine. Ntuli TM: InTech; Croatia: pp. 155–174. 2012
|
|
9
|
Ohyama K, Akaike T, Hirobe C and Yamakawa
T: Cytotoxicity and apoptotic inducibility of Vitex
agnus-castus fruit extract in cultured human normal and cancer
cells and effect on growth. Biol Pharm Bull. 26:10–18.
2003.PubMed/NCBI
|
|
10
|
Ohyama K, Akaike T, Imai M, Toyoda H,
Hirobe C and Bessho T: Human gastric signet ring carcinoma
(KATO-III) cell apoptosis induced by Vitex agnus-castus
fruit extract through intracellular oxidative stress. Int J Biochem
Cell Biol. 37:1496–1510. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ma L, Lin S, Chen R and Wang X: Treatment
of moderate to severe premenstrual syndrome with Vitex agnus castus
(BNO 1095) in Chinese women. Gynecol Endocrinol. 26:612–616. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Schellenberg R: Treatment for the
premenstrual syndrome with agnuscastus fruit extract: prospective,
randomised, placebo controlled study. BMJ. 322:134–137. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kikuchi H, Yuan B, Nishimura Y, Imai M,
Furutani R, Kamoi S, Seno M, Fukushima S, Hazama S, Hirobe C,
Ohyama K, Hu XM, Takagi N, Hirano T and Toyoda H: Cytotoxicity of
Vitex agnus-castus fruit extract and its major component,
casticin, correlates with differentiation status in leukemia cell
lines. Int J Oncol. 43:1976–1984. 2013.
|
|
14
|
Kikuchi H, Yuan B, Yuhara E, Takagi N and
Toyoda H: Involvement of histone H3 phosphorylation through p38
MAPK pathway activation in casticin-induced cytocidal effects
against the human promyelocytic cell line HL-60. Int J Oncol.
43:2046–2056. 2013.
|
|
15
|
Schumacker PT: Reactive oxygen species in
cancer cells: live by the sword, die by the sword. Cancer Cell.
10:175–176. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Trachootham D, Alexandre J and Huang P:
Targeting cancer cells by ROS-mediated mechanisms: a radical
therapeutic approach? Nat Rev Drug Discov. 8:579–591. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang J and Yi J: Cancer cell killing via
ROS: to increase or decrease, that is the question. Cancer Biol
Ther. 7:1875–1884. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yuan B, Yoshino Y, Kaise T and Toyoda H:
Application of arsenic trioxide therapy for patients with leukemia.
Biological Chemistry of Arsenic, Antimony and Bismuth. Sun H: John
Wiley and Sons, Ltd; Chichester: pp. 263–292. 2011
|
|
19
|
Dong JM, Zhao SG, Huang GY and Liu Q:
NADPH oxidase-mediated generation of reactive oxygen species is
critically required for survival of undifferentiated human
promyelocytic leukemia cell line HL-60. Free Radic Res. 38:629–637.
2004. View Article : Google Scholar
|
|
20
|
Yuan B, Ohyama K, Bessho T, Uchide N and
Toyoda H: Imbalance between ROS production and elimination results
in apoptosis induction in primary smooth chorion trophoblast cells
prepared from human fetal membrane tissues. Life Sci. 82:623–630.
2008. View Article : Google Scholar
|
|
21
|
Kon A, Yuan B, Hanazawa T, Kikuchi H, Sato
M, Furutani R, Takagi N and Toyoda H: Contribution of membrane
progesterone receptor α to the induction of progesterone-mediated
apoptosis associated with mitochondrial membrane disruption and
caspase cascade activation in Jurkat cell lines. Oncol Rep.
30:1965–1970. 2013.
|
|
22
|
Yuan B, Ohyama K, Takeichi M and Toyoda H:
Direct contribution of inducible nitric oxide synthase expression
to apoptosis induction in primary smooth chorion trophoblast cells
of human fetal membrane tissues. Int J Biochem Cell Biol.
41:1062–1069. 2009. View Article : Google Scholar
|
|
23
|
Yuan B, Ohyama K, Bessho T and Toyoda H:
Contribution of inducible nitric oxide synthase and
cyclooxygenase-2 to apoptosis induction in smooth chorion
trophoblast cells of human fetal membrane tissues. Biochem Biophys
Res Commun. 341:822–827. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Perner A, Andresen L, Pedersen G and
Rask-Madsen J: Superoxide production and expression of NAD(P)H
oxidases by transformed and primary human colonic epithelial cells.
Gut. 52:231–236. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Reddy L, Odhav B and Bhoola KD: Natural
products for cancer prevention: a global perspective. Pharmacol
Ther. 99:1–13. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hu XM, Yuan B, Tanaka S, Zhou Q, Onda K,
Toyoda H and Hirano T: Involvement of oxidative stress associated
with glutathione depletion and p38 mitogen-activated protein kinase
activation in arsenic disulfide-induced differentiation in HL-60
cells. Leuk Lymphoma. 55:392–404. 2014. View Article : Google Scholar
|
|
27
|
Frantz B, Klatt T, Pang M, Parsons J,
Rolando A, Williams H, Tocci MJ, O’Keefe SJ and O’Neill EA: The
activation state of p38 mitogen-activated protein kinase determines
the efficiency of ATP competition for pyridinylimidazole inhibitor
binding. Biochemistry. 37:13846–13853. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Young PR, McLaughlin MM, Kumar S, Kassis
S, Doyle ML, McNulty D, Gallagher TF, Fisher S, McDonnell PC, Carr
SA, Huddleston MJ, Seibel G, Porter TG, Livi GP, Adams JL and Lee
JC: Pyridinyl imidazole inhibitors of p38 mitogen-activated protein
kinase bind in the ATP site. J Biol Chem. 272:12116–12121. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Keyse SM and Tyrrell RM: Hemeoxygenase is
the major 32-kDa stress protein induced in human skin fibroblasts
by UVA radiation, hydrogen peroxide, and sodium arsenite. Proc Natl
Acad Sci USA. 86:99–103. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lau AT, Wang Y and Chiu JF: Reactive
oxygen species: current knowledge and applications in cancer
research and therapeutic. J Cell Biochem. 104:657–667. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kobayakawa J, Sato-Nishimori F, Moriyasu M
and Matsukawa Y: G2-M arrest and antimitotic activity mediated by
casticin, a flavonoid isolated from Viticis Fructus (Vitex
rotundifolia Linne fil.). Cancer Lett. 208:59–64. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Li J, Chen P, Sinogeeva N, Gorospe M,
Wersto RP, Chrest FJ, Barnes J and Liu Y: Arsenic trioxide promotes
histone H3 phosphoacetylation at the chromatin of CASPASE-10 in
acute promyelocytic leukemia cells. J Biol Chem. 277:49504–49510.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Waring P, Khan T and Sjaarda A: Apoptosis
induced by gliotoxin is preceded by phosphorylation of histone H3
and enhanced sensitivity of chromatin to nuclease digestion. J Biol
Chem. 272:17929–17936. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Clayton AL and Mahadevan LC: MAP
kinase-mediated phosphoacetylation of histone H3 and inducible gene
regulation. FEBS Lett. 546:51–58. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Lee YJ and Shukla SD: Histone H3
phosphorylation at serine 10 and serine 28 is mediated by p38 MAPK
in rat hepatocytes exposed to ethanol and acetaldehyde. Eur J
Pharmacol. 573:29–38. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Kuruüzüm-Uz A, Güvenalp Z, Ströch K,
Demirezer LO and Zeeck A: Antioxidant potency of flavonoids from
Vitex agnus-castus L. growing in Turkey. FABAD J Pharm Sci.
33:11–16. 2008.
|
|
37
|
Jozkowicz A, Was H and Dulak J: Heme
oxygenase-1 in tumors: is it a false friend? Antioxid Redox Signal.
9:2099–2117. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Hill M, Pereira V, Chauveau C, Zagani R,
Remy S, Tesson L, Mazal D, Ubillos L, Brion R, Asghar K, Mashreghi
MF, Kotsch K, Moffett J, Doebis C, Seifert M, Boczkowski J, Osinaga
E and Anegon I: Heme oxygenase-1 inhibits rat and human breast
cancer cell proliferation: mutual cross inhibition with indoleamine
2,3-dioxygenase. FASEB J. 19:1957–1968. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Sánchez Y, Amrán D, Fernández C, de Blas E
and Aller P: Genistein selectively potentiates arsenic
trioxide-induced apoptosis in human leukemia cells via reactive
oxygen species generation and activation of reactive oxygen
species-inducible protein kinases (p38-MAPK, AMPK). Int J Cancer.
123:1205–1214. 2008.
|