Introduction
Multiple myeloma (MM) is a fatal plasma cell (PC)
malignancy characterized by an accumulation of malignant PCs within
the bone marrow (1). MM is
responsible for 15,000 new cases per year both in Europe and in the
US (2) and represents 10–15% of
hematological malignancies in Caucasian and 20% in the
Afro-American populations. Several risk factors are associated with
this malignant disease, such as male gender, black race, positive
family history and monoclonal gammopathy of undetermined
significance (MGUS), another clinical picture that often precedes
multiple myeloma (3).
The introduction of several novel and active
treatments and improvements in the supportive care of myeloma
patients has resulted in the prolongation of the survival of these
patients, although some patients may be cured with an allogeneic
stem cell transplantation, and the median survival remains around
3–4 years (4). In the past decade,
there have been major advances in the treatment of MM. The
proteasome inhibitor bortezomib has emerged as a highly active
agent in the treatment of MM (5).
The response rates with relapsed diseases are approximately 50%
with the combination of bortezomib with thalidomide and steroids
and 65% with a 3-drug combination of thalidomide, steroids and
cyclophosphamide (6). Patients who
are not transplant candidates are treated with standard alkylating
agents therapy, namely, melphalan, prednisone and thalidomide
(MPT); melphalan, prednisone and bortezomib (MPB); and melphalan,
prednisone and lenalidomide (MPR).
Although the initial clinical responses to drug
therapies are achieved, a significant percentage of MM patients
relapses and no longer responds to single or combined treatments
(7). Therefore, the resistance of
MM to current therapeutic regimens remains an unsolved problem in
the management of patients with MM. The main problem in MM patient
treatments is the development of cross-resistance to conventional
therapies and this resistance causes this disease to remain
incurable. Therefore, the identification of novel biomarkers for
unresponsiveness is urgently needed in order to predict the
outcomes of various treatment regimens of MM and to apply an
appropriate customized and more beneficial treatment regimen.
Ying Yan 1 (YY1) is a ubiquitous and multifunctional
transcription factor that can act as a transcriptional repressor,
activator, or initiator element-binding protein, depending on the
context in which it acts (8). We
and others have reported of the role of YY1 as a prognostic marker
in different cancer diseases, such as prostate cancer and cervical
carcinoma (9,10). Recent studies suggest that elevated
YY1 expression and/or its transcriptional activity might contribute
to tumor formation and/or progression (11–13).
In addition, YY1 has been identified as a potential repressor
factor for several genes involved in immuno- and chemo-resistance.
We reported that YY1 can act as a transcription repressor for both
the Fas and the TRAIL DR5 receptors in prostate carcinoma and
lymphoma cell lines (14,15). YY1 also mediates the regulation of
tumor cell resistance to cytotoxic chemotherapy (16).
The objective of the present study was to identify a
biomarker of prognostic significance in MM and investigate its
clinical significance. We hypothesized that high YY1 expression in
MM might be directly associated in both the pathogenesis of MM and
its resistance to cytotoxic chemotherapy. To test this hypothesis,
we have investigated the following: i) the expression levels of YY1
in MM cell lines and bone marrow-derived tissues from MM patients;
ii) the association between the cell frequency and the intensity of
YY1 expression as a function of disease progression; iii)
bioinformatic analyses of YY1 transcript levels in publicly
available datasets containing gene expression profiles of MM and
normal B cells and correlation with both clinical parameters and
patient’s outcome; and iv) the role of YY1 in the regulation of MM
resistance to cytotoxic drugs such as bortezomib. The findings
reported herein supported the above hypothesis.
Materials and methods
Cells and culture conditions
The human MM cell lines (MM1s, 8266, IM-9 and U266)
were obtained from the American Type Culture Collection (ATCC,
Manassas, VA, USA). Cells were maintained in culture dishes in
RPMI-1640 (Life Technologies, Bethesda, MD, USA), supplemented with
5% heat-inactivated fetal bovine serum (FBS) (Life Technologies)
(to ensure the absence of complement), 1% (v/v) penicillin (100
U/ml), 1% (v/v) streptomycin (100 U/ml), 1% (v/v) L-glutamine, 1%
(v/v) pyruvate, and 1% non-essential amino acids (Invitrogen Life
Technologies, Carlsbad, CA, USA). The cell cultures were incubated
at 37°C and 5% CO.
Reagents
The anti-β-actin and the anti-tubulin monoclonal
antibodies were purchased from Biosource International (Camarrillo,
CA, USA) and from Calbiochem (San Francisco, CA, USA),
respectively. Anti-YY1 antibody was purchased from Santa Cruz
Biotechnology, Inc. (Santa Cruz, CA, USA). Phycoerythrin
(PE)-conjugated anti-active caspase 3 and PE-conjugated IgG were
purchased from BD Pharmingen (San Diego, CA, USA). SureSilencing™
siRNA kits were purchased from SuperArray Bioscience Corporation
(Frederick, MD, USA). Melphalan was purchased from Sigma-Aldrich
(St. Louis, MO, USA). Bortezomib was available commercially
(Millennium Pharmaceuticals, Inc, Cambridge, MA, USA).
Patient population
Eligible patients had a prior confirmed diagnosis of
MM based on the Durie criteria (17). All patients had been pretreated,
showing either relapsed disease at any point in time following
stabilization or a response to at least one prior anti-myeloma
regimen or refractory disease (progressed while receiving an
anti-myeloma treatment) (Table
I).
 | Table IThe patient cohort. |
Table I
The patient cohort.
| No of patient | Stage | Treatment
response | Disease |
|---|
| 1011 | IA/I | PR |
Non-progressive |
| 1020 | IA/II | PR |
Non-progressive |
| 1022 | IIIA/II | NR | Progressive |
| 1029 | NA | NR | Progressive |
| 1033 | IIA/II | PR |
Non-progressive |
| 1034 | IIA/II | PR |
Non-progressive |
| 1036 | IIIA | NR | Progressive |
| 1058 | IA/I | NR |
Non-progressive |
| 1059 | IA/I | NR |
Non-progressive |
| 1101 | IA/I | NR | Progressive |
| 1111 | NA | PR |
Non-progressive |
| 1115 | IA/I | NR | Progressive |
| 1185 | IA/I | PR |
Non-progressive |
| 3494 | IA | NR | Progressive |
| 6581 | IIIA | NA | Progressive |
| 7720 | IA | PR |
Non-progressive |
| 8480 | NA | NR | Progressive |
| 8579 | IIIA | NR | Progressive |
| 9662 | IA | NR | Progressive |
| 11110 | IA | NR | Progressive |
Tissues used for analysis
Peripheral blood (PB) and bone marrow (BM) aspirates
were obtained from patients with MM and age and gender-matched
healthy control subjects. The study was approved by the
Institutional Review Board (Western IRB BIO 001) and informed
consent was obtained in accordance with the Declaration of
Helsinki. Patients were defined as having indolent MM or
symptomatic disease. The treated patients were determined by
showing a progressive or a responsive disease [partial response
(PR), very good (VG), or complete response (CR)] according to the
International Myeloma Working Group (IMWG) criteria (18). Individual patients with multiple
myeloma were analyzed from the time of their first assessment. PB
and BM aspirates were collected in heparinized tubes and
mononuclear cells (MCs) were isolated using density-gradient
centrifugation with Histopaque-1077 (Sigma-Aldrich). Cells were
cultured in RPMI-1640 medium (Omega Scientific, Tarzana, CA, USA)
supplemented with 10% fetal bovine serum, non-essential amino
acids, 2 mmol/l glutamine, 1 mmol/l sodium pyruvate, 25 mmol/l
HEPES, 200 U/ml penicillin and streptomycin at 37°C and 5% CO.
Cell treatment
The log-phase culture of the U266 cell line was
seeded into 6-well plates at approximately 6×105
cells/ml and grown in 1 ml of medium, as described above in 5% FBS
for 24 h to approximately 70% confluence. The U266 cells were
synchronized by treatment with 1% FBS for 18 h prior to each
experiment.
Semiquantitative reverse
transcription-polymerase chain reaction (RT-PCR)
Total RNA was extracted and purified from
1×106 cells by a single-step monophasic solution of
phenol and guanidine isothiocyanate-chloroform using
TRIzol® reagent (Life Technologies) as previously
described (19).
Western blot analysis
MM cells were cultured at a low FBS concentration
(1%) for 18 h prior to each treatment and then lysates were
prepared and analyzed by western blot analysis as described
(20).
Immunocytochemistry
The expression level of YY1 was determined using the
antibody directed against YY1 in MM cell lines or bone marrow
samples derived from MM patients. Analysis and quantification were
done as previously described (21).
Scoring of immunocytochemistry of bone
marrow cells derived from MM patients
A semiquantitative assessment of antibody staining
on the bone marrow cells derived from MM patients was done by a
study pathologist (M.C.-M.) blinded to the clinopathologic
variables. The stained slides were checked by a second pathologist
for consistency of scoring. YY1 cytoplasmic and nuclear expressions
were scored based on the positive staining in the nucleus and
cytoplasm or both. Data are presented as either positively stained
target cells per 200 cells (range 10–100% positive), per region on
each spot (4 regions in each spot), or density (quantitative
assessment), where four 100-μm2 regions per spot were
selected randomly and the density of the staining intensity in each
region was analyzed using the Image Pro-plus 6.4 software (Media
Cybernetics, Bethesda, MD, USA). To represent expression within
cases, the mean pooled integrated intensity of the bone marrow
cells from MM patients or normal bone marrow was used.
Determination of apoptosis
After each treatment, the cells were recovered
following centrifugation at 1,800 rpm for 8 min. The cells were
washed and resuspended in 100 μl of the cytofix/cytoperm solution
(BD Pharmigen) for 20 min. Thereafter, the samples were washed and
were stained with PE-labeled anti-active caspase 3 mAb for 30 min.
The samples were subsequently washed and analyzed on a flow
cytometer EPICSR XL-MCL (Beckman Coulter, Co., Miami, FL, USA),
with the System II™ Software and the percent positive cells was
recorded. As a negative control, the cells were stained with the
isotype control (PE-IgG) under the conditions described above.
siRNA transfection
U266 cells were cultured in 1 ml of RPMI medium
supplemented with 5% FBS. Transfection was performed by using the
Lipofectamine 2000 CD Reagent supplied by Invitrogen (Invitrogen
Life Technologies) and the SureSilencin siRNA kit supplied by
SuperArray Bioscience Corporation according to the manufacturer’s
instructions. To determine the expression of YY1, the cells were
harvested 48 h after transfection and RT-PCR and western blot
analyses were performed. To determine the MM sensitization to
bortezomib-mediated apoptosis, 48 h after transfection the cells
were treated for 18 h with bortezomib (2.5 and 5 nM) or melphalan
(5, 10 and 20 μM) and then levels of apoptosis were evaluated.
Oncomine data analysis
The web-based human cancer microarray database
Oncomine (https://www.oncomine.com) was used to
analyze the mRNA expression associated with MM (22,23).
Details of standardized normalization techniques and statistical
calculations can be found on the Oncomine website (https://www.oncomine.com).
Study description
A total of 74 multiple myeloma samples, 7 multiple
myeloma cell lines, 5 monoclonal gammopathy of undetermined
significance samples, and 45 normal samples were analyzed on
Affymetrix HumanGeneFL microarrays.
Statistical analysis
The experimental values were expressed as the mean ±
SD for the number of separate experiments indicated in each case.
One-way ANOVA was used to compare variance within and among
different groups. When necessary, Student’s t-test was used for
comparison between two groups. Significant differences were
considered for probabilities <5% (p<0.05).
Results
Overexpression of YY1 in MM BM cells
Given the reported role of YY1 as a negative
regulator of apoptosis in various tumors including prostate,
ovarian carcinoma and NHL (15,24,25),
we have hypothesized that YY1 may also be playing an important role
in the resistance of MM to chemotherapy-induced apoptosis. We
tested this hypothesis by examining first the expression levels of
YY1 in four different MM cell lines and bone marrow-derived MM
tissues from MM patients by western blot and immunocytochemistry
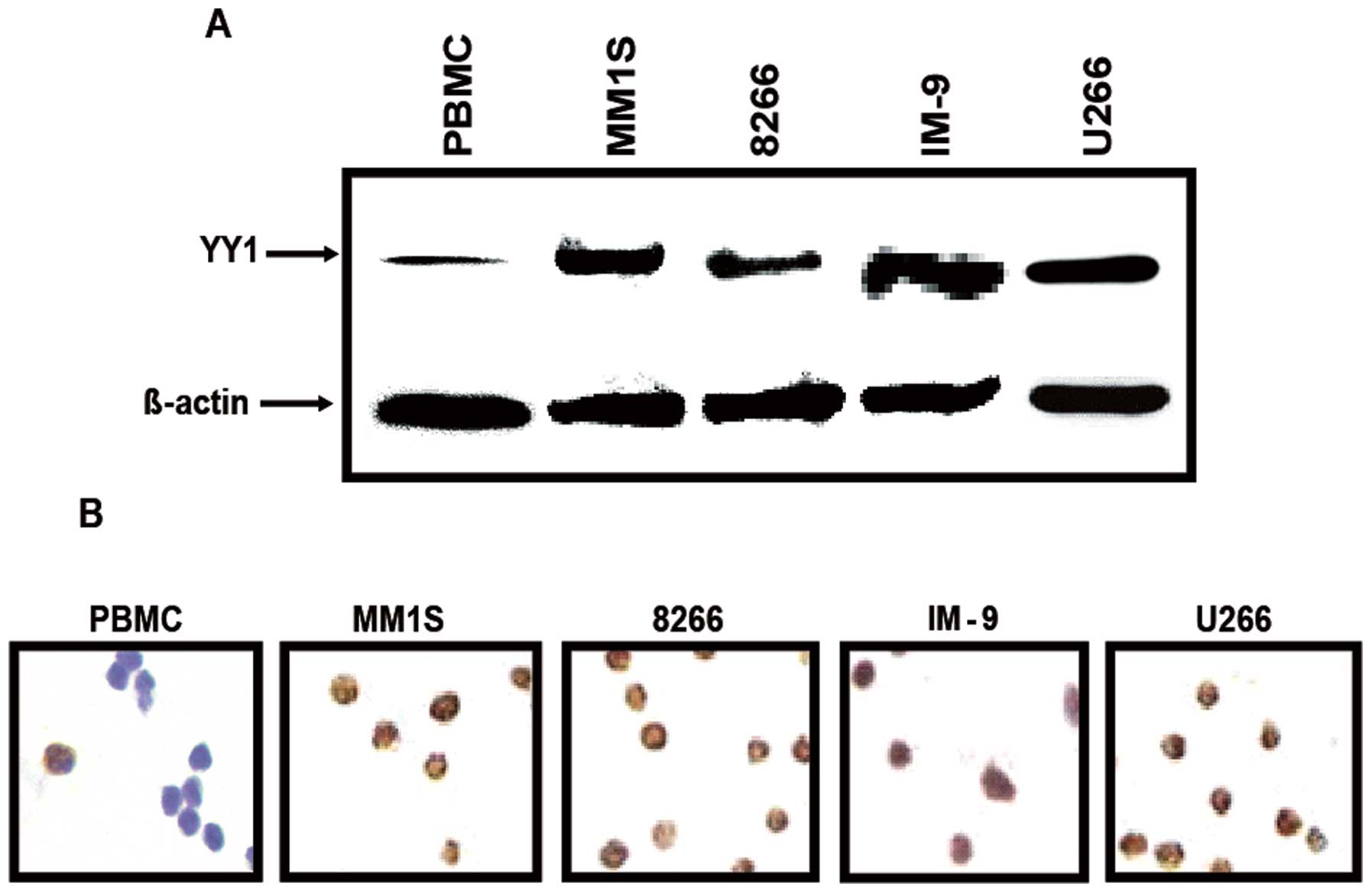
analyses. All of the tested MM cell lines, MM1.S, RPMI-8826, IM9
and U266, expressed significantly higher levels of YY1 compared to
PBMC cells used as a normal control (Fig. 1A). These findings were further
verified using immunocytochemistry whereby the expression of YY1
was significantly higher in the entire MM cell lines tested and was
predominantly expressed in the nucleus. In contrast, the expression
of YY1 in PBMC was very low and predominantly in the cytoplasm
(Fig. 1B).
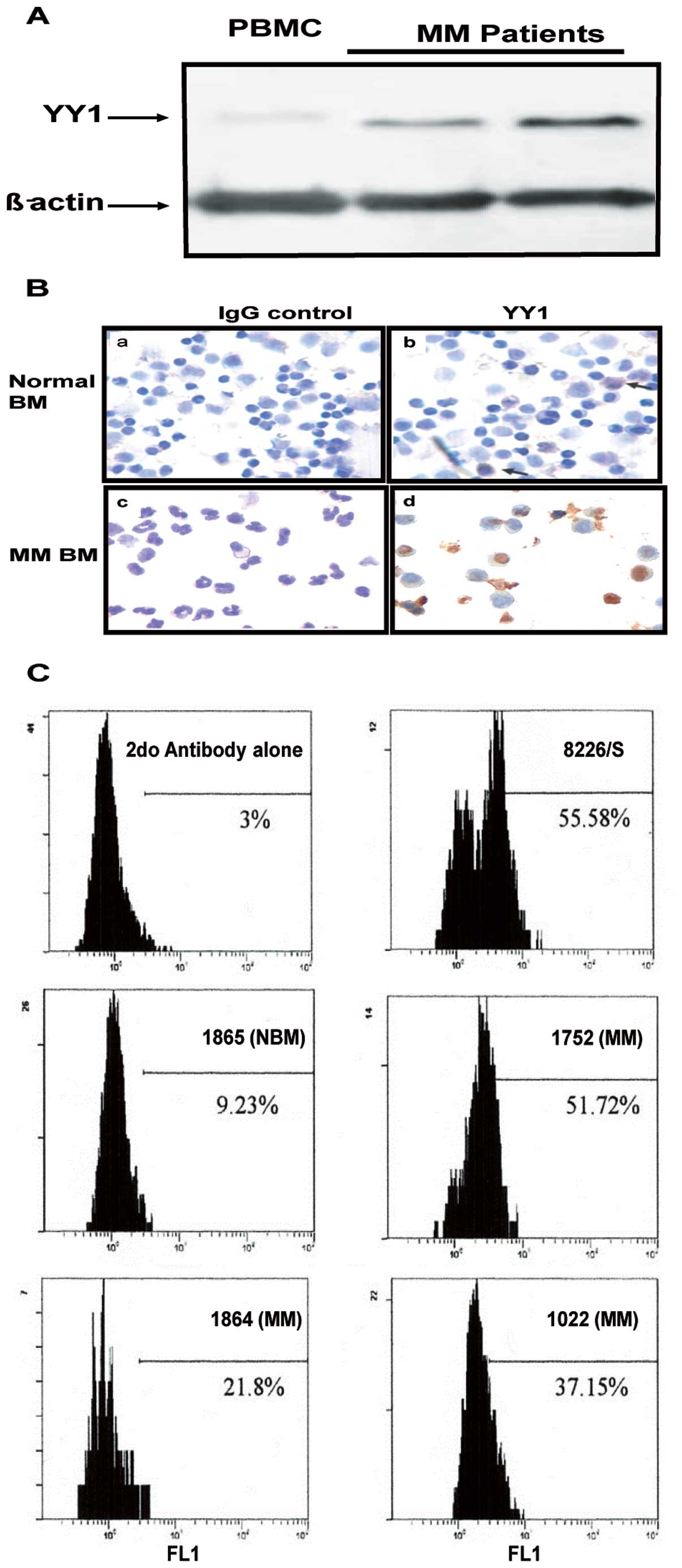
The above findings in cell lines were corroborated
in bone marrow-derived MM from patients. Fig. 2A shows the western blot analysis
results of two representative MM samples from two patients whereby
the expression of YY1 was significantly higher than the expression
in a normal PBMC. These findings were further verified using
immunocytochemistry staining which demonstrated an increased
expression of YY1 in MM compared to a normal BM sample (Fig. 2B). Similar findings were observed
when the YY1 expression in a few representative MM samples was
analyzed by flow cytometry. Fig.
2C shows a significantly higher expression of YY1 in BM-derived
MM samples from three patients as compared to normal BM. The MM
8226 cell line was used as a positive control for YY1 staining. The
frequency of cells stained for YY1 in BM-derived MM tissues varied
from one patient to another. Altogether, the above findings
demonstrated that MM cells (cell lines and patient samples)
expressed higher levels of YY1 in comparison with normal BM or
PBMC.
The overexpression of YY1 in MM is
associated with progressive disease
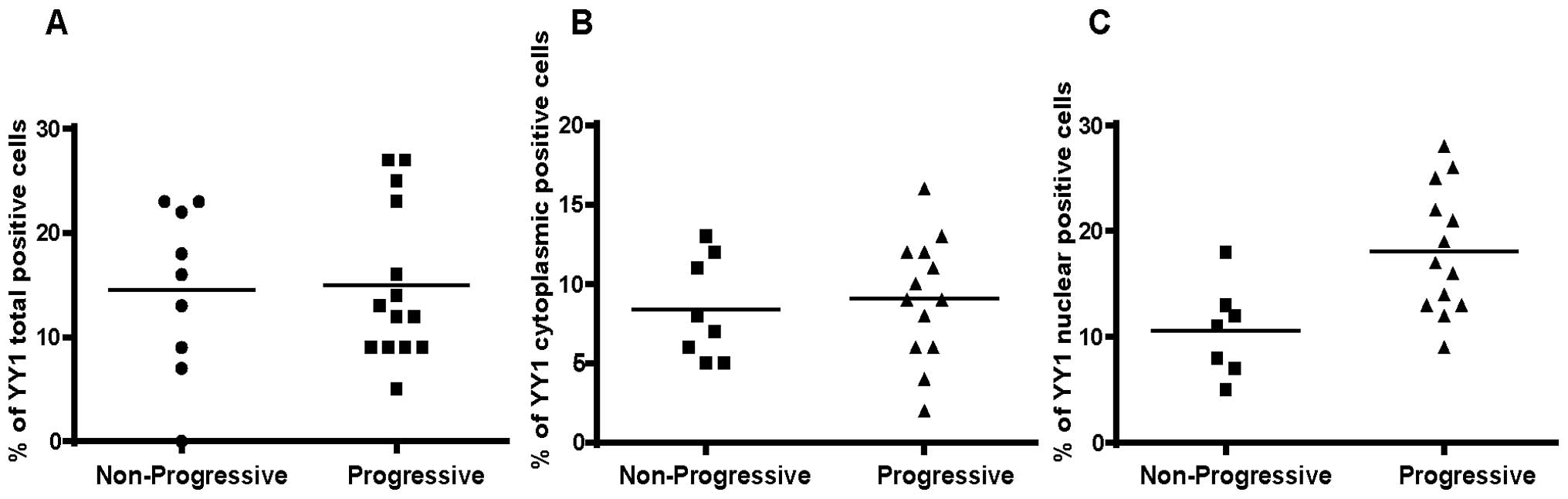
Analysis by immunocytochemistry
We examined a total of 20 BM-derived MM tissue
samples and separated the non-progressive from the progressive
patients (Table I). There were no
differences on the total density of YY1 between the two groups
(data not shown). Analysis of the cell frequencies for total
expression (cytoplasmic and nuclear) of YY1 did not demonstrate any
significant differences between the progressive and non-progressive
groups (Fig. 3A). Likewise, there
were no significant differences for the cytoplasmic expression of
YY1 between the two groups (Fig.
3B). However, in contrast, there was a significant
overexpression of nuclear YY1 in the progressive group versus the
non-progressive group (Fig. 3C)
(p=0.035). These findings suggested that the overexpression of YY1
in the nucleus (presumably it is transcriptionally active)
correlated with a poor prognosis.
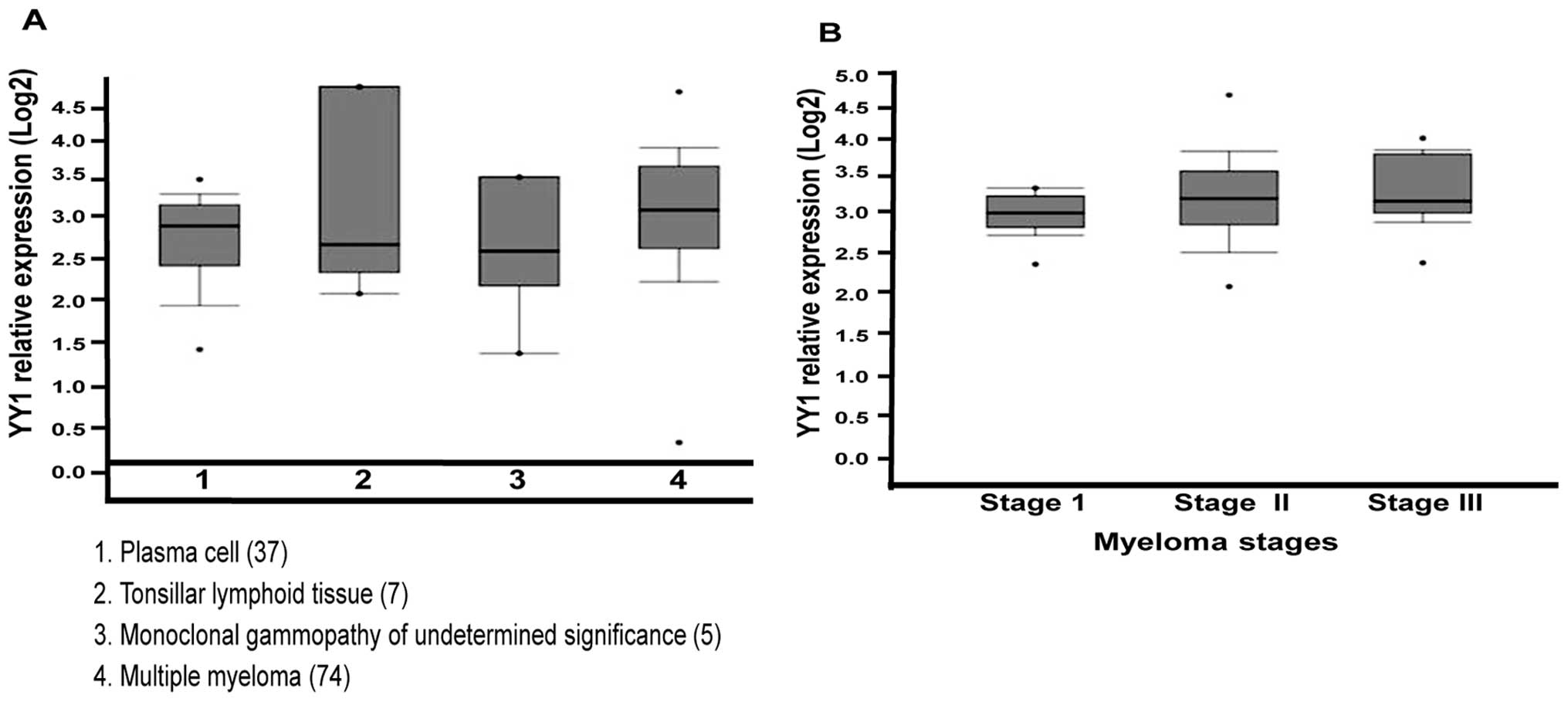
Bioinformatic analyses
In order to corroborate the above findings, we used
computational analysis of YY1 mRNA levels in different groups of MM
patients, as described in Materials and methods, and the findings
are summarized in Fig. 4. In
Fig. 4A, we show the Oncomine
analysis of YY1 mRNA expression in MM. Box plot diagrams were
analyzed to compare the YY1 mRNA levels in normal plasma cells,
tonsillar lymphoid tumors and monoclonal gammopathy. Analysis of
studies reported by Zhan et al (22,23)
revealed that the YY1 expression was higher in MM than in normal
plasma cells, tonsillar lymphoid tissue and monoclonal gammopathy,
suggesting that YY1 may play a crucial role in the pathogenesis of
MM development. The vertical axis represents the log2 median value.
The upper (75%) and lower (25%) quartiles are represented by the
upper and lower borders of the boxes, respectively (p<0.05). In
Fig. 4B, YY1 gene expression is
shown as a function of clinical stages and shows that YY1 is
significantly overexpressed in stage II and stage III as compared
with stage I (p<0.001).
The direct role of YY1 expression in an
MM cell line in the regulation of resistance to bortezomib-induced
apoptosis
The above findings demonstrated that the
overexpressed YY1 in BM correlated with poor prognosis. We have
recently reported that YY1 regulates chemoresistance in solid tumor
cells (25). Thus, we hypothesized
that the overexpression of YY1 in MM may be involved in the
chemoresistance to cytotoxic chemotherapeutic drugs. The MM cell
line U266 was used as a representative cell line for analysis.
Transfection of U266 cells with YY1 siRNA or control siRNA was
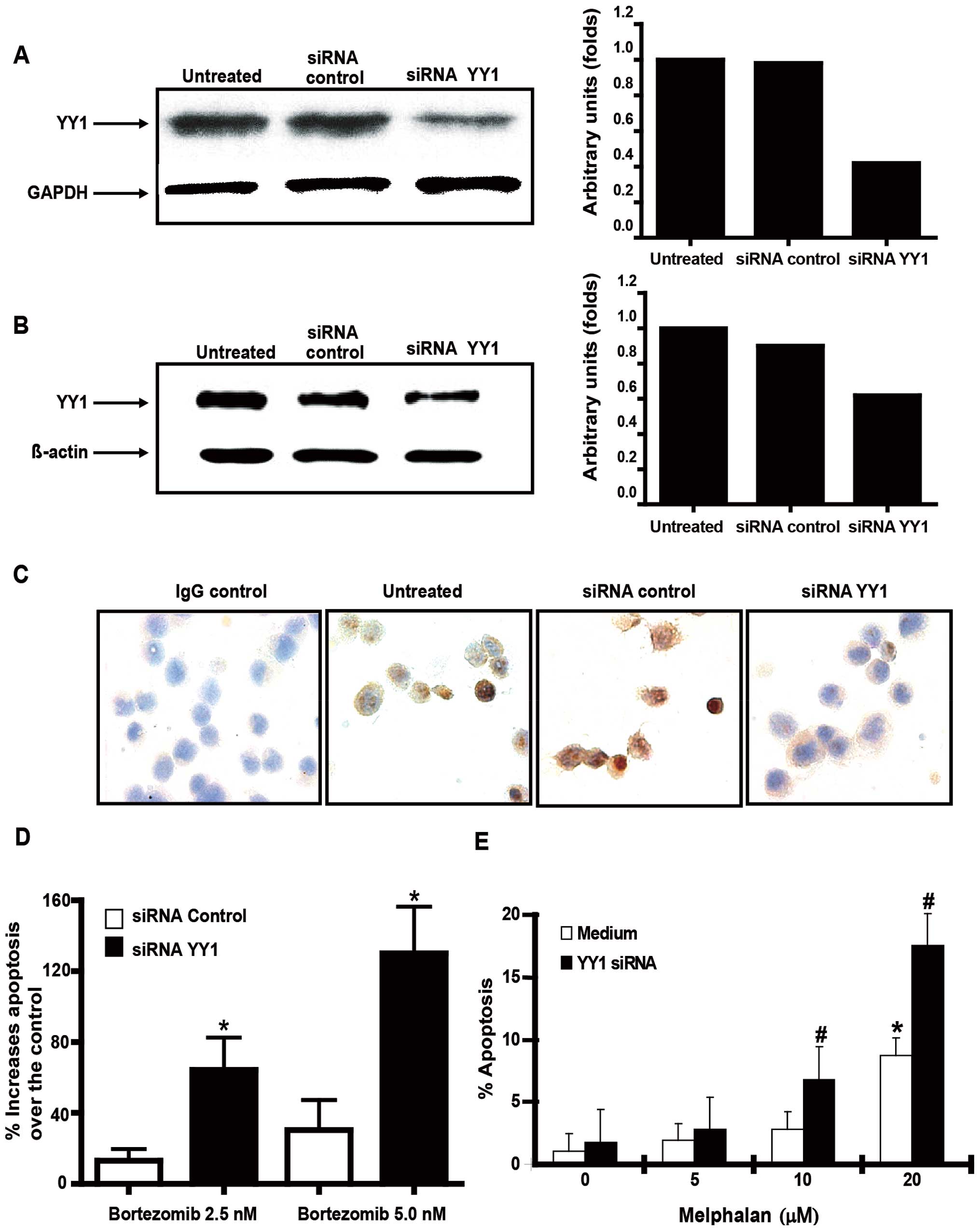
performed as described in Materials and methods. Fig. 5A demonstrates that treatment of
U266 cells with YY1 siRNA, but not control siRNA, inhibited the
expression of mRNA and protein for YY1 as assessed by both western
blot analysis (Fig. 5B) and
immunocytochemistry (Fig. 5C). The
YY1 siRNA transfected cells or control siRNA transfected cells were
treated with two different concentrations of bortezomib (2.5 and 5
nM) or different concentrations of melphalan (5, 10 and 20 μM) for
18 h and analyzed for apoptosis as described in Materials and
methods. There was significant apoptosis in YY1 siRNA-transfected
cells treated with both concentrations of bortezomib as compared
with control siRNA-transfected cells (Fig. 5D). Similar results were found when
YY1 siRNA-transfected cells were treated with different
concentrations of melphalan (Fig.
5E). The above findings demonstrated the participation of YY1
in the regulation of resistance of MM to drug-induced
apoptosis.
Discussion
The present findings revealed that the expression of
the transcription factor YY1 is upregulated in MM cell lines and in
patients bone marrow-derived MM tissues as compared to its
expression in normal blood cells and normal bone marrow-derived
tissues. The total expression of YY1 (cytoplasmic and nuclear) was
the same in patients who experienced progressive and
non-progressive diseases. However, patients who experienced a
progressive disease showed a significantly higher nuclear
expression of YY1 as compared to patients who experienced a
non-progressive disease. Bioinformatic analysis of public mRNA data
sets corroborated the recent findings and revealed that the
expression of YY1 in MM was significantly elevated compared to the
expression in plasma cells and monoclonal gammopathy of
undetermined significance. Further, the expression of YY1 was
increased as a function of disease stage. Since we have reported
that YY1 regulates resistance of different tumors to
chemotherapeutic drugs, we examined its role in the resistance of
MM cells. Treatment of MM cells that are resistant to
bortezomib-induced cytotoxicity with siRNA YY1 sensitized the MM
cells to apoptosis by bortezomib. The above findings demonstrated
that the nuclear expression of YY1 in MM may be considered as a
novel prognostic biomarker for disease progression in a subset of
patients and these patients may benefit from different treatment
regimens. We also suggest that YY1 is a potential therapeutic
target.
Analysis of MM cell lines for YY1 expression
revealed a significant overexpression in both the cytoplasm and
nucleus when compared to its expression in normal PBMC and normal
BM tissues. The overexpression of YY1 was shown by various methods,
namely, IHC, RT-PCR, flow cytometry and western blot analyses. The
findings observed in MM cell lines were corroborated in patients
bone marrow-derived MM tissues as assessed by both IHC and western
blot analyses. The clinical history of the examined MM patients
consisted of one subset who experienced disease progression and one
subset who did not experience disease progression. Analysis of the
IHC staining data with patient-derived MM tissues revealed that the
frequency of YY1 positively stained MM cells as well as the
frequency of positively stained cytoplasmic YY1 in cells was not
significantly different between the progressive and non-progressive
subsets. However, noteworthy, when the frequency of YY1 positively
stained nuclei in the cells was analyzed, there was a significantly
higher frequency in the progressive subset as compared to the
frequency in the non-progressive subset. These findings suggest
that in the progressive subset, the translocation of YY1 in the
nucleus, whereby its transcriptional activity takes place, may be
functionally consistent and by being involved in the regulation of
gene products that regulate disease progression and
unresponsiveness to treatment (26). These preliminary findings, however,
need to be validated in a larger cohort of MM patients.
Our findings in MM were also observed in lymphoma
and the prognostic role of YY1 in lymphoma was reported by various
investigators. Sakhinia et al (27) reported the upregulation of YY1 mRNA
in neoplastic lymph nodes in patients with follicular or DLBCL
compared with reactive lymph nodes. A high level of YY1 was
associated with poor outcome in both FL and DLBCL. In contrast,
Naidoo et al (28) reported
the association of high protein expression levels of YY1 with
improved survival. These results were opposite to the YY1 mRNA
levels. Both investigators used the same cohort of patients and,
therefore, suggesting the presence of a negative feedback loop
controlling YY1 mRNA and protein levels. The role of YY1 in the
development of DLBCL in public microarray data was analyzed for the
possible association of YY1 with other genes. The positive
expression of Bcl-6 protein in tumors was associated significantly
with high levels of YY1 gene transcripts in DLBCL (29). Further analysis of data sets for
DLBCL identified the transcription factor paired box (PAX)-5
amongst the top 50 gene proteins correlating with YY1. These data
suggested the involvement of YY1 in B cell transformation and
resulting in the development of high grade lymphoma. PAX-5
downregulates p53 expression while YY1 overexpression promotes p53
degradation. YY1 and PAX-5 may, therefore, act to transform B cells
via upregulation of the cell cycle.
Conflicting data also exist on the survival outcome
and their relationship to YY1 expression. In some cases, high YY1
expression correlated with poor prognosis of prostate, breast and
bone cancers (30,31). In other cases, YY1 expression
correlated with positive outcomes (ovarian cancer, colon cancer and
follicular lymphoma) (28,32–34).
The mechanism by which YY1 is overexpressed in MM
cells is not clear. Different mechanisms have been shown to
regulate the transcription of YY1 (35), YY1 regulates multiple genes
including itself. Kim et al (36) reported that YY1 is able to regulate
through its own DNA-binding sites in its first intron. Exogenous
YY1 inhibits the expression of endogenous YY1 gene suggesting a
feedback negative loop. YY1 expression is enhanced by NF-κB which
directly binds the YY1 promoter through its heterodimer subunit
p50/p65 (37). YY1 regulates
post-translationally the modification of multiple proteins. YY1 is
a signal modified with phosphorylation, acetylation, sumoylation
and ubiquitination (35). YY1 is
also regulated by growth-factors such as insulin-like growth factor
1 (38). YY1 can be suppressed by
miRs. For example, miR-29 targets the U′-UTR of the YY1 mRNA and
blocks its translation (39). AKT
and PTEN also regulate YY1 expression (40). PTEN antagonizes PI3K/AKT signaling
leading to YY1 downregulation.
Overall, the present findings demonstrated that YY1
expression is upregulated in MM cell lines and patient-derived MM
in bone marrow tissues. The findings were corroborated by
bioinformatic analysis. The role of YY1 in the progression of MM
was suggested by comparing the nuclear expression of YY1 in the
various MM tumor tissues. A correlation was found between the
nuclear expression of YY1 and disease progression. The disease
progression was, in part, the result of patients unresponsiveness
to drug therapy and correlated with the overexpression of YY1 and
its role in drug resistance. We suggest that patients with MM
tumors that overexpress YY1 in the nucleus may benefit from
different treatment regimens. We also suggest that YY1 may be
considered as a novel prognostic biomarker and a therapeutic target
for intervention. Clearly, the validation of our present findings
should be examined with a large cohort of MM patients.
Acknowledgements
The authors would like to thank ‘Miembros del
Patronato del HIM’ (S.H.-Y.) for ther support to provide equipment.
The authors also acknowledge the assistance of the Jonsson
Comprehensive Cancer Center at UCLA. The authors thank Melissa Cao
for the preparation of the manuscript.
References
|
1
|
Bataille R and Harousseau JL: Multiple
myeloma. N Engl J Med. 336:1657–1664. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Mahtouk K, Hose D, De VJ, et al: Input of
DNA microarrays to identify novel mechanisms in multiple myeloma
biology and therapeutic applications. Clin Cancer Res.
13:7289–7295. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Alexander DD, Mink PJ, Adami HO, et al:
Multiple myeloma: a review of the epidemiologic literature. Int J
Cancer. 120(Suppl 12): 40–61. 2007. View Article : Google Scholar
|
|
4
|
Reece DE: Management of multiple myeloma:
the changing landscape. Blood Rev. 21:301–314. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Richardson PG: A review of the proteasome
inhibitor bortezomib in multiple myeloma. Expert Opin Pharmacother.
5:1321–1331. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Dimopoulos MA, Beksac M, Benboubker L, et
al: Phase II study of bortezomib-dexamethasone alone or with added
cyclophosphamide or lenalidomide for sub-optimal response as
second-line treatment for patients with multiple myeloma.
Haematologica. 98:1264–1272. 2013. View Article : Google Scholar
|
|
7
|
Kastritis E, Palumbo A and Dimopoulos MA:
Treatment of relapsed/refractory multiple myeloma. Semin Hematol.
46:143–157. 2009. View Article : Google Scholar
|
|
8
|
Gordon S, Akopyan G, Garban H and Bonavida
B: Transcription factor YY1: structure, function, and therapeutic
implications in cancer biology. Oncogene. 25:1125–1142. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Baritaki S, Sifakis S, Huerta-Yepez S, et
al: Overexpression of VEGF and TGF-β1 mRNA in Pap smears correlates
with progression of cervical intraepithelial neoplasia to cancer:
Implication of YY1 in cervical tumorigenesis and HPV infection. Int
J Oncol. 31:69–79. 2007.
|
|
10
|
Seligson D, Horvath S, Huerta-Yepez S, et
al: Expression of transcription factor Yin Yang 1 in prostate
cancer. Int J Oncol. 27:131–141. 2005.PubMed/NCBI
|
|
11
|
Begon DY, Delacroix L, Vernimmen D,
Jackers P and Winkler R: Yin Yang 1 cooperates with activator
protein 2 to stimulate ERBB2 gene expression in mammary cancer
cells. J Biol Chem. 280:24428–24434. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Brankin B, Skaar TC, Brotzman M, Trock B
and Clarke R: Autoantibodies to the nuclear phosphoprotein
nucleophosmin in breast cancer patients. Cancer Epidemiol
Biomarkers Prev. 7:1109–1115. 1998.PubMed/NCBI
|
|
13
|
Erkeland SJ, Valkhof M,
Heijmans-Antonissen C, et al: The gene encoding the transcriptional
regulator Yin Yang 1 (YY1) is a myeloid transforming gene
interfering with neutrophilic differentiation. Blood.
101:1111–1117. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Vega MI, Huerta-Yepez S, Jazirehi AR,
Garban H and Bonavida B: Rituximab (chimeric anti-CD20) sensitizes
B-NHL cell lines to Fas-induced apoptosis. Oncogene. 24:8114–8127.
2005.PubMed/NCBI
|
|
15
|
Vega MI, Jazirehi AR, Huerta-Yepez S and
Bonavida B: Rituximab-induced inhibition of YY1 and Bcl-xL
expression in Ramos non-Hodgkin’s lymphoma cell line via inhibition
of NF-kappa B activity: role of YY1 and Bcl-xL in Fas resistance
and chemoresistance, respectively. J Immunol. 175:2174–2183.
2005.PubMed/NCBI
|
|
16
|
Baritaki S, Huerta-Yepez S, Sakai T,
Spandidos DA and Bonavida B: Chemotherapeutic drugs sensitize
cancer cells to TRAIL-mediated apoptosis: up-regulation of DR5 and
inhibition of Yin Yang 1. Mol Cancer Ther. 6:1387–1399. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Durie BG: Staging and kinetics of multiple
myeloma. Semin Oncol. 13:300–309. 1986.PubMed/NCBI
|
|
18
|
Rajkumar SV, Harousseau JL, Durie B, et
al: Consensus recommendations for the uniform reporting of clinical
trials: report of the International Myeloma Workshop Consensus
Panel 1. Blood. 117:4691–4695. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Huerta-Yepez S, Vega M, Jazirehi A, et al:
Nitric oxide sensitizes prostate carcinoma cell lines to
TRAIL-mediated apoptosis via inactivation of NF-kappa B and
inhibition of Bcl-xl expression. Oncogene. 23:4993–5003. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Vega MI, Huerta-Yepez S, Martinez-Paniagua
M, et al: Rituximab-mediated cell signaling and
chemo/immuno-sensitization of drug-resistant B-NHL is independent
of its Fc functions. Clin Cancer Res. 15:6582–6594. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Huerta-Yepez S, Vega M, Escoto-Chavez SE,
et al: Nitric oxide sensitizes tumor cells to TRAIL-induced
apoptosis via inhibition of the DR5 transcription repressor Yin
Yang 1. Nitric Oxide. 20:39–52. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhan F, Hardin J, Kordsmeier B, et al:
Global gene expression profiling of multiple myeloma, monoclonal
gammopathy of undetermined significance, and normal bone marrow
plasma cells. Blood. 99:1745–1757. 2002. View Article : Google Scholar
|
|
23
|
Zhan F, Barlogie B, Arzoumanian V, et al:
Gene-expression signature of benign monoclonal gammopathy evident
in multiple myeloma is linked to good prognosis. Blood.
109:1692–1700. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Garban HJ and Bonavida B: Nitric oxide
inhibits the transcription repressor Yin-Yang 1 binding activity at
the silencer region of the Fas promoter: a pivotal role for nitric
oxide in the up-regulation of Fas gene expression in human tumor
cells. J Immunol. 167:75–81. 2001. View Article : Google Scholar
|
|
25
|
Huerta-Yepez S, Baritaki S, Baay-Guzman G,
et al: Contribution of either YY1 or BclxL-induced inhibition by
the NO-donor DETANONOate in the reversal of drug resistance, both
in vitro and in vivo. YY1 and BclXL are overexpressed in prostate
cancer. Nitric Oxide. 29:17–24. 2013. View Article : Google Scholar
|
|
26
|
Liu H, Schmidt-Supprian M, Shi Y, et al:
Yin Yang 1 is a critical regulator of B-cell development. Genes
Dev. 21:1179–1189. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Sakhinia E, Glennie C, Hoyland JA, et al:
Clinical quantitation of diagnostic and predictive gene expression
levels in follicular and diffuse large B-cell lymphoma by RT-PCR
gene expression profiling. Blood. 109:3922–3928. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Naidoo K, Clay V, Hoyland JA, et al: YY1
expression predicts favourable outcome in follicular lymphoma. J
Clin Pathol. 64:125–129. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Castellano G, Torrisi E, Ligresti G, et
al: Yin Yang 1 overexpression in diffuse large B-cell lymphoma is
associated with B-cell transformation and tumor progression. Cell
Cycle. 9:557–563. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Allouche A, Nolens G, Tancredi A, et al:
The combined immunodetection of AP-2alpha and YY1 transcription
factors is associated with ERBB2 gene overexpression in primary
breast tumors. Breast Cancer Res. 10:R92008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
De Nigris F, Rossiello R, Schiano C, et
al: Deletion of Yin Yang 1 protein in osteosarcoma cells on cell
invasion and CXCR4/angiogenesis and metastasis. Cancer Res.
68:1797–1808. 2008.PubMed/NCBI
|
|
32
|
Berchuck A, Iversen ES, Lancaster JM, et
al: Patterns of gene expression that characterize long-term
survival in advanced stage serous ovarian cancers. Clin Cancer Res.
11:3686–3696. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Chinnappan D, Xiao D, Ratnasari A, Andry
C, King TC and Weber HC: Transcription factor YY1 expression in
human gastrointestinal cancer cells. Int J Oncol. 34:1417–1423.
2009.PubMed/NCBI
|
|
34
|
Matsumura N, Huang Z, Baba T, et al: Yin
Yang 1 modulates taxane response in epithelial ovarian cancer. Mol
Cancer Res. 7:210–220. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zhang Q, Stovall DB, Inoue K and Sui G:
The oncogenic role of Yin Yang 1. Crit Rev Oncog. 16:163–197. 2011.
View Article : Google Scholar
|
|
36
|
Kim JD, Yu S and Kim J: YY1 is
autoregulated through its own DNA-binding sites. BMC Mol Biol.
10:852009. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Wang H, Hertlein E, Bakkar N, et al:
NF-kappaB regulation of YY1 inhibits skeletal myogenesis through
transcriptional silencing of myofibrillar genes. Mol Cell Biol.
27:4374–4387. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Flanagan JR: Autologous stimulation of YY1
transcription factor expression: role of an insulin-like growth
factor. Cell Growth Differ. 6:185–190. 1995.PubMed/NCBI
|
|
39
|
Li Y, Wang F, Xu J, et al: Progressive
miRNA expression profiles in cervical carcinogenesis and
identification of HPV-related target genes for miR-29. J Pathol.
224:484–495. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Petrella BL and Brinckerhoff CE: PTEN
suppression of YY1 induces HIF-2 activity in von-Hippel-Lindau-null
renal-cell carcinoma. Cancer Biol Ther. 8:1389–1401. 2009.
View Article : Google Scholar : PubMed/NCBI
|