Introduction
Colorectal cancer is a leading malignancy worldwide
with more than 1,233,000 new cases and 608,000 deaths in recent
years (1). In current
chemotherapeutic treatment for CRC, irinotecan (CPT-11), a
semisynthetic derivative of camptothecin, is one of the key
cytotoxic drugs with a 30% single agent response rate and 50%
response rate when combined with other agents such as
5-Fu/leucovorin (LV) (2). However,
more than 40% of patients treated with this drug have severe
toxicities, such as diarrhea and neutropenia that limit its
efficacy (3), and there remain
many patients treated with CPT-11 who become resistant and exhibit
further tumor progression despite their initial response (4). Thus, it remains important to search
for novel agents for new combination treatments to enhance
efficiency, reduce toxicities and avoid the progression of
colorectal tumors.
As a key factor contributing to tumor survival and
progression during colorectal cancer therapy (5,6),
NF-κB can be activated by CPT-11, it is already upregulated in most
colorectal cancers during early treatment (7) and could be a potential
chemo-resistance mechanism in malignant cells (8,9) that
will reduced chemosensitivity. NF-κB activation is regulated by
proteasomes, which are an attractive target for cancer therapy, as
the proteasome plays a central role in the regulation of proteins
that modulate cell cycle progression, cell survival, migration and
direct induction of apoptosis (10,11).
There are various kinds of proteasome inhibitors which can be
classified by different structures and reaction mechanisms
(12), such as bortezomib
(PS-341), carfilzomib (CFZ), NPI0052, MLN-9807 and CEP-18770, it is
encouraging that all these proteasome inhibitors block activation
of NF-κB that is one of the most important mechanisms for killing
transformed tumor cells and is the foundation of rational
combination therapy (13). In the
study by Tamatani et al, PS-341 enhanced radiosensitivity
through inhibiting radiation-induced NF-κB activity and suppressed
oral tumor growth (14). Moreover,
PS-341 and NPI0052 can enhance chemosensitivity and the tumoricidal
response to CPT-11 in colorectal cancer by blocking
chemotherapy-induced NF-κB activation and expression of genes
involved in cancer cell survival (15–18).
In addition, proteasome inhibition also augments the cancer cell
response to chemotherapy and radiation by modulating other NF-κB
related proteasome-dependent regulatory proteins involved in
treatment resistance such as Bcl2, p53, the caspases and stress
response molecules like SAPK/JNK, as well as accumulation of
misfolded proteins and anti-angiogenic effects (10,13).
Carfilzomib (CFZ) is an epoxyketone proteasome
inhibitor that irreversibly inhibits the 26S proteasome, and has
high specificity for inhibiting chymotrypsin-like activity
(19–21). In preclinical studies, CFZ has
shown single-agent activity against hematopoietic malignancies and
some solid tumors, such as head and neck cancer, through inhibiting
NF-κB activation by preventing ubiquitination and proteasome
degradation of IκBα as well as through other NF-κB related
biological mechanisms (22–25).
This novel second-generation proteasome inhibitor was approved by
the FDA in July of 2012 and is in clinical use for the treatment of
relapsed and refractory multiple myeloma (MM), it can be used
safely and effectively in place of PS-341 combination therapy,
especially in patients with PS-341 resistant MM (26), and CFZ has no effect on normal skin
or normal umbilical vein cells and is less toxic to normal
peripheral blood mononuclear cells from healthy individuals than to
tumor cells (19,25). CFZ is drawing increasing attention,
but little is known about its activity against CRC and it has not
yet been fully evaluated. In addition, the combination effect of
CFZ and CPT-11 in colorectal cancer treatment is not known.
These results led us to hypothesize that CFZ, which
is more efficacious and less toxic to patient with haematological
malignancies than earlier proteasome inhibitors, could block CPT-11
induced NF-κB activation and mediate apoptosis pathways to improve
the effectiveness of CPT-11, leading to a dramatic augmentation of
chemosensitivity. In the present study, we examine the therapeutic
ability of CFZ combined with CPT-11 in vitro and in
vivo by evaluating the effect on CRC tumor growth, cell
proliferation, cell cycle progression, apoptosis, migration and
invasion, as well as on NF-κB regulated pathways. Our results
indicate that CFZ and CPT-11 interact synergistically in SW620
cells in vitro and in vivo through a process that
involves NF-κB inhibition that is related to the apoptotic
response.
Materials and methods
Cell lines and culture
Human colorectal cancer cell lines, SW620 and HCT8
were obtained from the Cell Bank of the Type Culture Collection of
the Chinese Academy of Sciences (Shanghai, China). SW620 was
cultured in L-15 medium and HCT8 was maintained in RPMI-1640
medium, both nutrient media (Gibco, USA) were supplemented with 10%
fetal bovine serum (Gibco). Cells were grown at 37°C with
saturating humidity.
Drugs and antibodies
Carfilzomib was purchased from Biorbyt Ltd.
(Cambridge, UK) and CPT-11 from Tocris Bioscience (Bristol, UK).
Both agents were maintained in dimethyl sulfoxide for in
vitro studies, CFZ was in 10% captisol
(sulfobutylether-β-cyclodextrin) in 10 mmol/l citrate buffer pH 3.5
and CPT-11 was dissolved in sterile water for in vivo
studies. Antibodies against TRAF6, BCL10, IKKs, phospho-IκBα/IκBα,
NF-κB (p65/p52/p50), phospho-NF-κB p65, MEK, phospho-MEK
(Ser217/221), ERK1/2, phospho-ERK1/2 (p44/42 MAP kinase,
Thr202/Tyr204), SAPK/JNK, phospho-SAPK/JNK (Thr183/Tyr185), PI3K,
phospho-PI3 kinase p85 (Tyr458)/p55 (Tyr199), AKT, phospho-AKT
(Ser473), PCNA, survivin, Stat5, phospho-Stat5 (Tyr694), Stat3,
phospho-Stat3 (Tyr705), p53 and β-tubulin were from Cell Signaling
Technology Inc. (Beverly, MA). Antibodies against β-catenin,
cdc25c, cyclin D1 (M20), cyclin B1 (H20), cyclin A (C-19), Cdk1
(C-19), phospho-Cdk1 (Thr14/Thr15), Cdk2 (M2), phospho-Cdk2
(Thr160), p21 (WAF1/CIP), PARP, p38, phospho-p38 (Thr180/Tyr182),
ATF3, MMP1, MMP2, MMP9, TIMP1, Egr1 and β-actin were from Santa
Cruz Biotechnology (Santa Cruz, CA, USA). Anti-MKP-1 was from Merck
Millipore (Bedford, MA, USA).
WST-1 test for cell proliferation
assay
The cytotoxicity of CFZ and CPT-11 on SW620 and HCT8
cells was tested using the WST-1 cell proliferation assay (27). Cells (1×l04 cells per
well) were plated overnight in 96-well microplates (Costar,
Corning, NY, USA) with 100 μl culture medium and then treated with
CFZ or CPT-11 at various concentrations. After various periods of
incubation, 10 μl of WST-1 reagent (Roche, Germany) was added to
each well and incubated with cells at 37°C for 4 h, and plates were
read on a microplate reader (Bio-Rad, model 550) at 450 nm with a
reference wavelength at 630 nm after being shaken thoroughly, as
described previously (28).
Clonogenic assay
A clonogenic assay was performed with SW620 cells,
500 cells per well were plated in 6-well plates in L-15 medium
supplemented with 10% fetal bovine serum. The cells were treated
with CFZ and CPT-11. The number of colonies (>50 cells) was
counted after 14 days incubation at 37°C.
Cell cycle analysis and apoptosis assay
by flow cytometry (FACS)
The CycleTESTy Plus DNA reagent kit from
Becton-Dickinson Immunocytometry Systems was used to test cell
cycle distribution. According to the manufacturer’s instructions,
the cells were treated with trypsin buffer, trypsin inhibitor,
RNase buffer and propidium iodide (PI) stain solution. The cells
were evaluated on a FACSCalibur (BD Biosciences) and results
analysed with Cell Quest and ModiFit software; analysis of
phosphatidyl serine (PS) was performed as described in the Annexin
V apoptosis detection kit (BD Biosciences). Briefly, SW620 cells
treated with different concentrations of drugs were harvested,
labelled with Annexin V and PI, and analyzed with a FACSCalibur
flow cytometer. For caspase 3 expression, SW620 cells were treated
with permeabilizing solution and incubated with FITC anti-caspase 3
antibody. CD95 expression was detected by direct labelling with
anti-CD95 antibody.
Terminal
deoxynucleotidyltransferase-mediated TMR red-dUTP nick end
labelling (TUNEL) experiment
TUNEL assays were performed according to the
manufacturer’s protocol with the In Situ Cell Death Detection Kit
(TMR red; Roche, Germany). For the in vitro cell assay,
after fixing with 4% paraformaldehyde/PBS, cells were incubated
with permeabilisation solution (freshly prepared; 0.1% Triton X-100
in 0.1% sodium citrate) on ice (2–8°C). Cells were washed twice
with PBS, and resuspended in the TUNEL reaction mixture (terminal
deoxynucleotidyl transferase enzyme with digoxigenin-nucleotide),
and incubated for 1 h at 37°C. The incorporation of nucleotides
into 3′-DNA through cleavage of DNA during apoptosis was detected
by a TMR red staining system. The cells were analyzed by
fluorescence microscopy; for paraffin-embedded tissue, after
dewaxing and rehydration, tissue slices were incubated with
permeabilization solution and rinsed twice with PBS, and then
processed using the protocol described for cells according to the
manufacturer’s instructions.
NF-κB activity assay - electrophoretic
mobility shift assay (EMSA)
Nuclear proteins from in vitro treated cells
were extracted with the Norvagen NucBuster protein extraction kit
(EMD Biosciences, affiliate of Merck KgaA, Germany). For release of
nuclei, cell pellets were suspended in NucBuster extraction reagent
I, 50 μl of packed cell volume suspended in 150 μl of reagent I on
ice, then centrifuged to remove the cytoplasmic fraction by washing
with ice-cold 1X PBS (137 mM NaCl, 43 mM
Na2HPO4, 27 mM KCl, 15 mM
KH2PO4, pH 7.3). The nuclei were harvested
and suspended in 50 μl of NucBuster extraction reagent II on ice
and centrifuged at 16,000 × g for 5 min at 4°C in order to separate
nuclear extracts. Nuclear proteins from in vivo treatment of
colorectal tumor tissues were extracted using the protocols from
the NE-PER Nuclear and Cytoplasmic Extraction kit (Pierce Thermo
Scientific, USA). A total of 7 μg of protein extract was subjected
to electrophoretic mobility shift assay for NF-κB/DNA binding using
a DIG label as per the protocol in the DIG Gel Shift kit (Roche,
Germany). Oct-1 or Oct-2A was used as a loading control in
EMSA.
Western blot analysis
Cells were lysed in modified RIPA buffer (Roche) for
30 min at 4°C, then the cell lysates were cleared of debris by
centrifugation and they were mixed in an equal volume of sample
buffer (0.2% bromophenol blue, 20% glycerol, 125 mM Tris-HCl, 640
mM βME, 4% SDS), then boiled for 10 min. The Bradford assay was
used to determine the protein concentration, and bovine serum
albumin (BSA) (Sigma) was used as the standard. Protein samples, 25
μg, were separated on 8 or 10% SDS-PAGE and then the proteins were
transferred onto nitrocellulose membranes (Amersham, UK). Membranes
were blocked at room temperature for 1 h and incubated overnight
with the appropriate antibody at 4°C, followed by three washings
with Tris-buffered saline containing 0.1% Tween-20 (TBS-T).
Membranes were incubated for 2 h with secondary antibody
(anti-rabbit or anti-mouse; diluted 1:10,000) at room temperature.
Tubulin or actin was assayed as protein loading controls.
Chemiluminescence reagent (ECL Western Blotting Detection System,
Amersham, UK) was used to detect the expression of proteins.
Wound-healing assay
SW620 cells were seeded into 12-well plates, a line
wound was scraped in the confluent adherent cells using a pipette
tip in each well and the plate washed with PBS. The cells were then
cultured with serum-free medium. After treatment with the indicated
drugs, the scraped line was observed and photographed in three
randomly selected views in each treatment well. The reduction of
the scraped area was considered as wound-healing induced by cell
migration.
Transwell cell migration/Matrigel
penetration assays
Transwells with 8 μm pore polycarbonate membranes
(Costar, Corning) were left uncoated or were coated with matrix
before use in simple migration assays and invasion assays,
respectively. Matrigel (BD Biosciences) were added to the Transwell
inserts and polymerised at 37°C, then SW620 cells in 1%
serum-containing medium were seeded into the upper wells of the
transwell, and 20% serum-containing medium was added to the outer
wells. In order to verify that cells did not proliferate during the
assay, cells were also seeded into tissue culture dishes under the
same condition. Following treatment with the indicated drugs for 48
h, the migrating or invading cells were fixed and stained with
crystal violet, then photographed and counted in three randomly
selected views using a microscope.
Animal studies
Five to six weeks old female athymic BALB/c nude
mice were purchased from the Vital River Laboratory Animal
Technology Co., Ltd. (Beijing, China). SW620 cells,
10×106, were inoculated into the backs of mice and
treatment began when the diameter of tumor reached 7 mm. Mice were
randomly grouped into four sets with five mice in each set: i)
control group, treated with vehicle alone, 10% Captisol in 10
mmol/l citrate buffer, (i.v. twice weekly for three weeks on days 1
and 2); ii) CFZ group, 2.0 mg/kg (i.v. twice weekly for three weeks
on days 1 and 2); iii) CPT-11 group, 33 mg/kg (i.p. once weekly for
four weeks on day 1); and iv) combined group, CFZ in combination
with CPT-11. Tumor size was measured twice per week and the volume
calculated as follows: V=(a × b2)/2, where ‘a’ and ‘b’
are the largest and smallest diameters, respectively. After the
final treatment, mice were sacrificed and tumor were excised and
weighed. All experimental procedures and protocols were approved by
the Animal Ethics Committee of Fuzhou General Hospital.
Statistical analysis
The comparison of differences between control and
treated groups was performed using a 2-tailed Student’s t-test
carried out with SPSS 11.0 statistical software (SPSS, Inc.,
Chicago, IL, USA). P<0.05 was considered significant.
Synergistic and antagonistic interactions were defined by median
dose effect analysis with a commercially available software
(CompuSyn) (29). Combination
index (CI) scores of <0.7, 0.7–0.85, 0.85–0.90, 0.90–1.10 and
>1.10 indicate synergism, moderate synergism, slight synergism,
additive effect and antagonism, respectively.
Results
Carfilzomib interacts synergistically
with CPT-11 on SW620 cells
To verify the cytotoxicity of CFZ on CRC cells,
SW620 cells, in which NF-κB is constitutively activated, and HCT8
cells, in which NF-κB is not constitutively activated, were exposed
to increasing concentrations for various times and assessed by the
WST-1 assay. The results demonstrated that CFZ reduced cell
viability in a concentration-dependent and time-dependent manner
(Fig. 1A). The IC50 of
CFZ for inhibiting SW620 proliferation at 24, 48 and 72 h were
201.06±4.38, 50.26±1.38 and 27.06±0.31 nM, respectively. The
IC50 of CFZ inhibition of HCT8 cells was more than 2,500
nM at 24, 48 and 72 h. At concentrations of 50, 100 and 600 nM, the
inhibition of SW620 cell proliferation by CFZ was significantly
higher than inhibition of HCT8 cells (Fig. 1B).
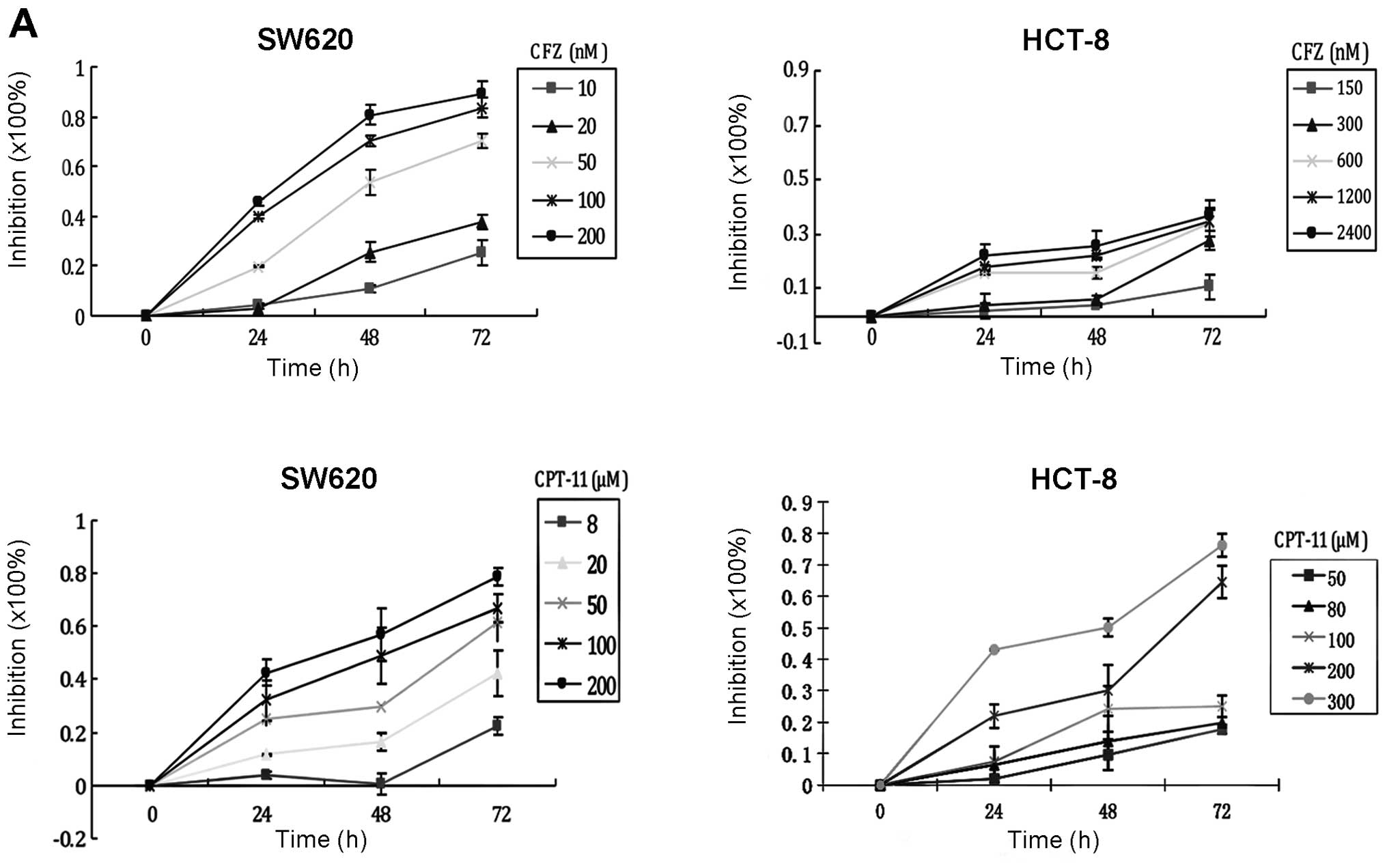 | Figure 1CFZ interacts synergistically with
CPT-11 on CRC cells. (A) Inhibitory effect of CFZ on human
colorectal cancer cell proliferation. SW620 cells were treated with
10, 20, 50, 100 and 200 nM of CFZ and 8, 20, 50, 100 and 200 μM of
CPT-11 for 24, 48 and 72 h. HCT8 cells were treated with 150, 300,
600, 1,200 and 2,400 nM of CFZ and 50, 80, 100, 200 and 300 μM of
CPT-11 for 24, 48 and 72 h. The WST-1 assay determined cell
proliferation. (B) Using the same concentration of CFZ (50, 100 and
600 nM) on SW620 cells and HCT8 cells, we find that SW620 cells
were more sensitive to CFZ than HCT8. NF-κB was detected by EMSA.
(C) CFZ and CPT-11 exhibit synergistic cytotoxicity on SW620 cells.
SW620 cells were treated with CFZ and CPT-11 at the indicated
concentrations for 48 h. Each CFZ concentration was combined with
50, 100 and 200 μM of CPT-11 as showed in Table 1. Cell viability was measured using
the WST-1 assay. Effects of CFZ and CPT-11 on the colony formation
of SW620 cells. The colonies (>50 cells) were scored after 14
days. (d-1) SW620 cells were treated with 0.5, 1, 1.5, 2 and 2.5 nM
of CFZ, colony number decreased as the concentration of CFZ
increased. (d-2) SW620 cells were treated with 0.5 or 1 nM of CFZ
and with 2 μM CPT-11. The number of colonies significantly
decreased compared to CFZ or CPT-11 treatment alone. All the above
data shown represent the mean ± SEM (n=3). |
To determine whether treatment with CFZ would
enhance the anticancer effects of CPT-11 chemotherapy, human SW620
cells were exposed to various concentrations of CFZ, CPT-11 or
combinations of the two drugs. As shown in Table I, when we give a concentration of
10 nM CFZ in combination with 50 μM CPT-11, inhibition reaches
52.29±3.17%, which is equal to or better than the effect of 100 μM
CPT-11 alone (48.19±1.84%), and much better than single-drug effect
of 50 μM CPT-11 (26.87±3.28%). Similarly, if we add 20 nM CFZ to
CPT-11 treatment of SW620 cells, we can reduce the CPT-11 dosage to
100 μM and achieve a better inhibition rate, 85.39±8.11%, than a
200 μM CPT-11 treatment alone, 71.63±2.91%. In order to achieve
equivalent inhibition to 200 μM CPT-11 (71.63±2.91%), while
reducing the dose of drugs in a combination regimen as much as
possible, we found that 50 nM CFZ combined with 50 μM CPT-11 will
produce a 75.94±2.57% inhibition rate. When we increased the
concentration of CPT-11 to 100 μM with 50 nM CFZ, single drug
concentrations that produced about a 50% inhibition rate, the
inhibition effects of the combination regimen reached almost the
best, and higher doses produced a plateau of inhibition. When CFZ
was used as a single-agent treating SW620 cells at 10, 20, 50 and
100 nM for 48 h, we recorded 10.62±2.78, 28.16±3.75, 54.09±1.55 and
66.98±1.99% growth inhibition, respectively. When CPT-11 was
combined with CFZ, the growth inhibition was improved
significantly, compared with single agent CPT-11; a synergistic
action was observed with all dose of CFZ added to 50 and 100 μM of
CPT-11 (CI<0.9). An additive effect was seen with the 200 μM
dose of CPT-11 with all doses of CFZ except 50 nM
(0.90<CI<1.10; Fig. 1C).
These data show that the inhibition of proliferation of SW620 cells
by CPT-11 is greater when combined with CFZ.
 | Table IThe combined effects of CFZ and
CPT-11 on SW620 cells (mean ± SEM, n=3). |
Table I
The combined effects of CFZ and
CPT-11 on SW620 cells (mean ± SEM, n=3).
| CFZ (nM) | CPT-11 (μM) | Inhibition rate
(%) | CI-value |
|---|
| 0 | 0 | 0 | |
| 50 | 26.87±3.28 | |
| 100 | 48.19±1.84 | |
| 200 | 71.63±2.91 | |
| 10 | 0 | 10.62±2.78 | |
| 50 | 52.29±3.17 | 0.64 |
| 100 | 71.26±2.76 | 0.60 |
| 200 | 75.85±5.92 | 0.93 |
| 20 | 0 | 28.16±3.75 | |
| 50 | 59.03±2.33 | 0.67 |
| 100 | 85.39±8.11 | 0.37 |
| 200 | 75.54±5.93 | 1.02 |
| 50 | 0 | 54.09±1.55 | |
| 50 | 75.94±2.57 | 0.61 |
| 100 | 84.25±2.56 | 0.55 |
| 200 | 91.63±6.52 | 0.49 |
| 100 | 0 | 66.98±1.99 | |
| 50 | 80.95±5.79 | 0.79 |
| 100 | 89.23±1.90 | 0.57 |
| 200 | 86.88±2.31 | 0.93 |
Similar effects were observed in the clonogenic
assay with SW620 cells (Fig. 1D).
Co-administration of 0.5–1.0 nM of CFZ with 2 μM of CPT-11 sharply
decreased colony formation of SW620 cells. These results
demonstrate an inhibitory effect of CFZ on proliferation and colony
formation of SW620 cells, and that the cell growth inhibition of
CPT-11 was enhanced by CFZ.
Inhibition of NF-κB activation plays a
key role in CFZ enhanced chemosensitivity of SW620 cells to
CPT-11
SW620 cells, in which NF-κB is activated, were used
to assess the functional role of NF-κB activity during CFZ/CPT-11
inhibition of CRC cell growth. As shown in Fig. 1B the SW620 cells were more
sensitive to CFZ than were HCT8 cells, in which NF-κB was shown by
EMSA to be not activated. This suggests that NF-κB may play a
significant role in CFZ induced lethality.
An EMSA and western blot analysis assay showed that
CPT-11 may induce slightly the NF-κB activation in SW620 cells
(Fig. 2A and 2D). Treatment with
CFZ alone or combined with CPT-11 resulted in a decrease of NF-κB
by blocking the degradation of IκBα (Fig. 2A, B and D), although there is
significantly less of the phosphorylated form of IκBα and total
IKKα was slightly upregulated, IκBα protein was not completely
degraded, and may still bind to NF-κB. There was no effect on IκB
upstream genes, such as TRAF6, BCL10 or other IκB kinase complexes
(IKKs) (Fig. 2C). Thus, we
conclude that CFZ co-administration reduced NF-κB DNA binding in
CPT-11-treated SW620 cells.
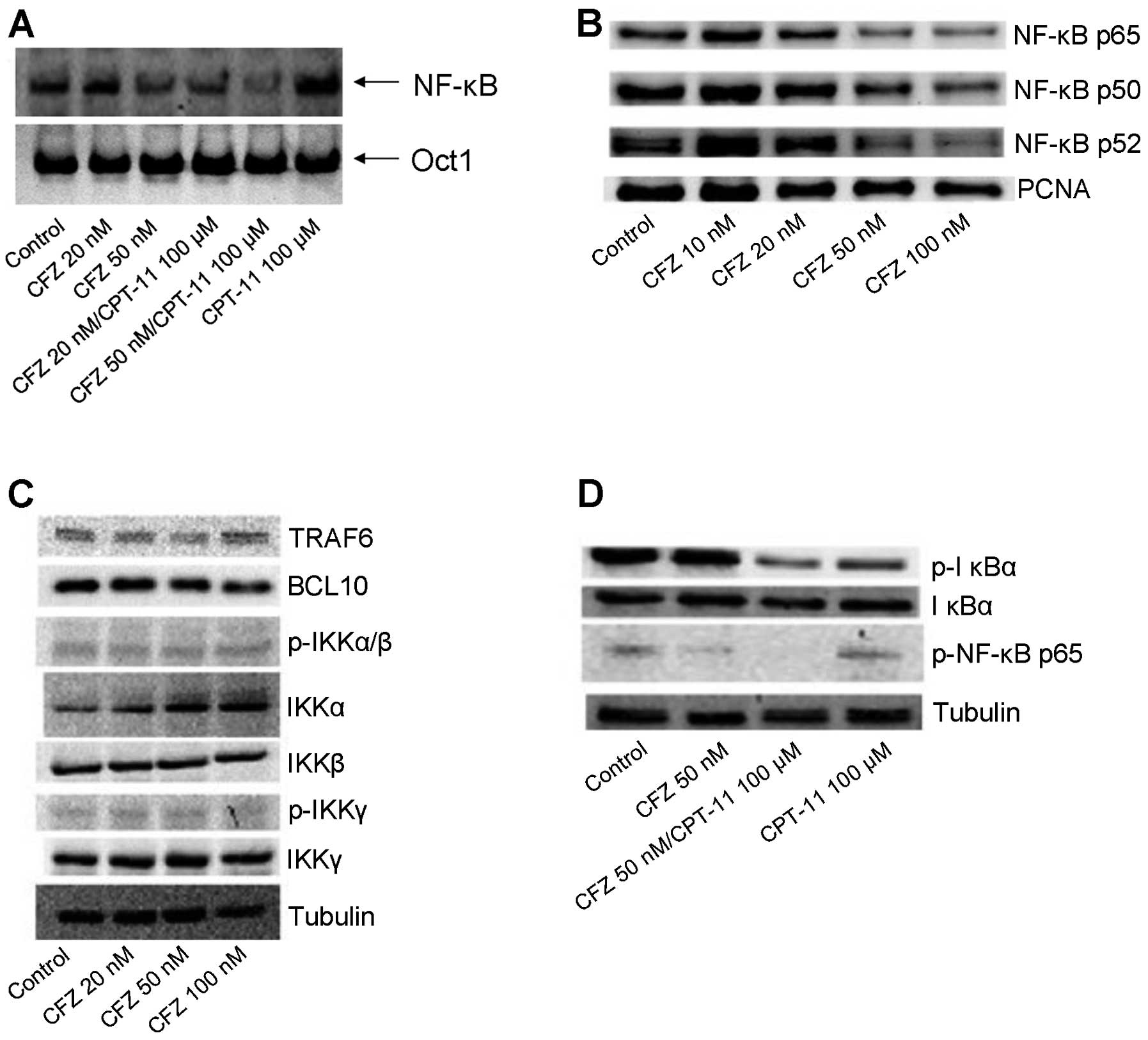 | Figure 2CFZ alone or combined with CPT-11
inhibits NF-κB activation in SW620 cells. (A) EMSA was used to
evaluate the effect of CFZ (20 and 50 nM) ± 100 μM CPT-11 treatment
in SW620 cells. Cells were harvested and the nuclear proteins of
the cells were extracted and assayed for nuclear translocation of
NF-κB at 48 h after of chemotherapy treatment. An Oct1 probe was
used as a control for EMSA. (B) The nuclear proteins from SW620
cells were extracted after treatment with 10, 20, 50 and 100 nM of
CFZ for 48 h. NF-κB activation was analyzed by western blot
analysis. PCNA was used as the internal control for nuclear
protein. With increasing concentrations of CFZ, the nuclear protein
expression of NF-κB p65, p50 and p52 were gradually reduced. (C)
SW620 cells were incubated with 20, 50 and 100 nM of CFZ for 48 h,
whole cell lysates were collected and subjected to western blot
analysis. IKKα was moderately upregulated but TRAF6, BCL10,
p-IKKα/β, IKKβ, p-IKKγ and IKKγ were not changed. (D) After 48 h of
chemotherapy treatment, we probed total protein preparations by
immunoblot analysis using anti-phospho-IκB, anti-IκB and
anti-phospho-NF-κB p65. Tubulin was used as an internal control.
CPT-11 (100 μM) combined treatment with CFZ (50 nM) in SW620 cells
sharply decrease p-NF-κB p65 activation, and also decrease p-IκB
activation but had no effect on the total IκB protein. |
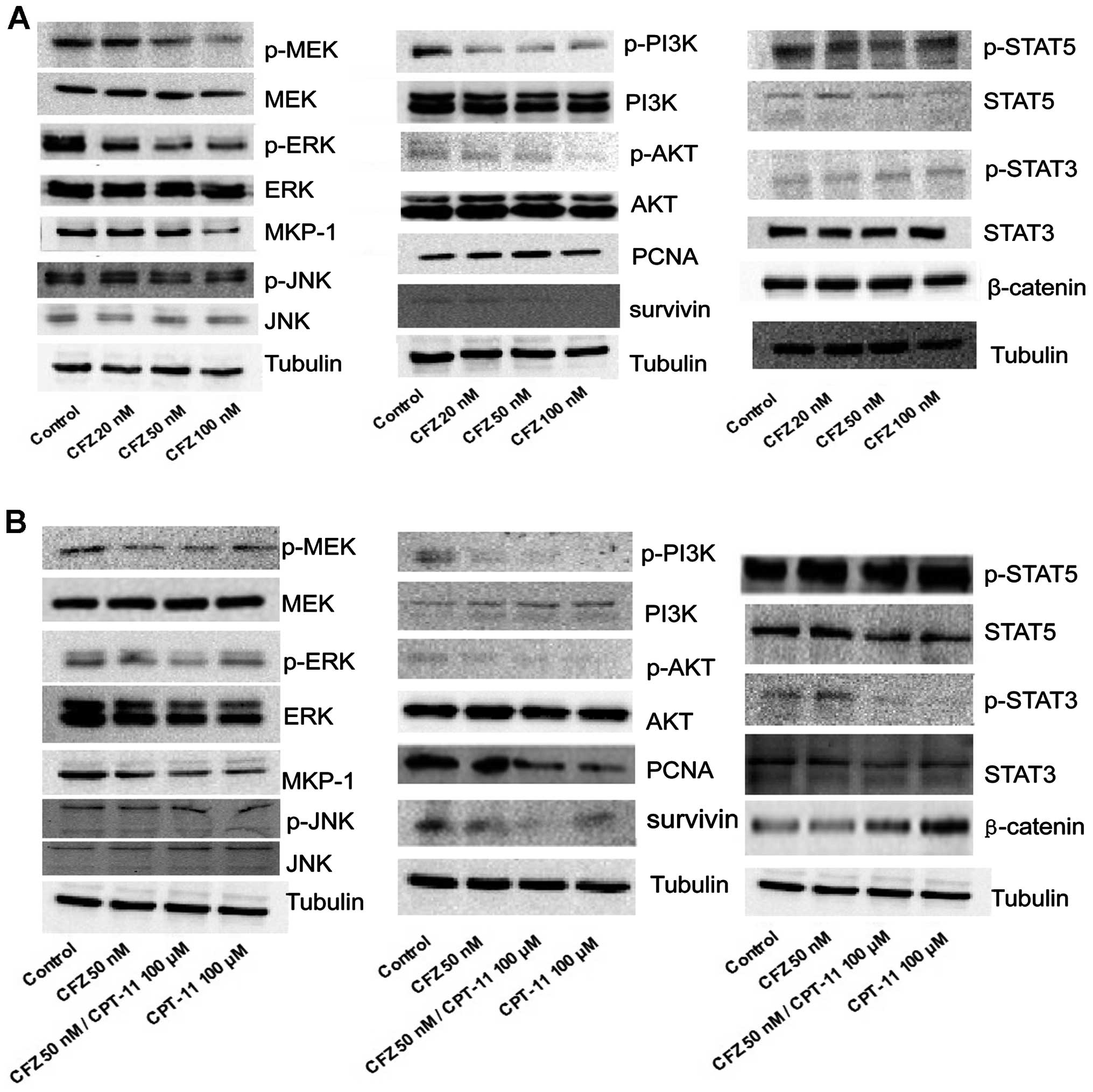
CFZ combined with CPT-11 attenuates the
MEK/ERK pathway, accompanied by PI3K/AKT pathway dephosphorylation
and survivin downregulation in SW620 cells
To determine whether the combination chemotherapy or
CFZ alone induced growth inhibition of SW620 cells was associated
with multiple mechanisms, such as blocking other survival pathways,
CFZ at 20, 50 and 100 nM, and 100 μM of CPT-11 combined with 50 nM
of CFZ was used to treat SW620 cells for 48 h. In the
stress-related MAP kinase signalling modules, 100 μM of CPT-11
alone had little effect, while exposure of cells for 48 h to CFZ,
with or without CPT-11, reduced phospho-MEK and phospho-ERK
expression as well as that of MKP-1. Levels of total MEK and total
ERK and JNK pathway related proteins were unchanged. Perturbations
were also observed in the cytoprotective-related PI3K/AKT pathway.
Administration of CFZ alone to SW620 cells decreased phospho-PI3K
and phospho-AKT, and decreased the expression of survivin. Combined
treatment with CPT-11 also resulted in a marked decrease in
phosphorylation of PI3K and AKT, as well as in expression of
downstream survivin. Total PI3K and AKT levels were unchanged under
various concentration of CFZ treatment and there was little effect
from administration of individual agents. CFZ alone exerted a
minimal effect and CPT-11 contributed more to the combined effect
in lowering the expression of STAT5, phospho-STAT3, STAT3, PCNA and
enhancing the expression of β-catenin, and treatments failed to
produce changes in other STAT-related pathway molecules (Fig. 3A and B).
Together, these findings indicate that
administration of CFZ and CPT-11 or CFZ alone leads to MEK/ERK
pathway inactivation and MKP-1 downregulation, accompanied by
PI3K/AKT pathway dephosphorylation and survivin inhibition.
Combined CFZ with CPT-11 or CFZ alone
induces G2/M arrest
To further elucidate the mechanism of growth
inhibition and whether the cell cycle changed under CFZ and
combined regimens, SW620 cells were treated with 50 nM CFZ for 24,
48 and 72 h, and exposed to various concentrations of CFZ (20, 50
and 100 nM) for 48 h. Both resulted in a change in the cell cycle
profile. CFZ alone induced G2/M arrest and CFZ combined with CPT-11
also resulted in a pronounced accumulation of cells in G2/M
(Fig. 4A). Before treatment 7.4%
of cells were in G2/M. After 72-h treatment with 50 nM CFZ, 46.08%
of cells were in G2/M. The percentage of cells in G2/M phase
increased in a dose-dependent manner from 6.68 to 50.48% after
treatment with CFZ for 48 h. The percentage of cells in G0/G1
decreased from 54.8% in untreated cells to 33.87% for cells treated
with 100 nM CFZ. CFZ treatment led to significant
concentration-dependent and time-dependent G2/M arrest without a
change in S phase. In the combined regimen there was also a shift
toward G2/M arrest compared with the untreated controls, 6.95 vs.
31.35%. These results demonstrate that the inhibition of SW620 cell
proliferation proceeds via G2/M-phase arrest. To determine what
effect combined exposure of SW620 cells to CFZ and CPT-11 would
have on various cell cycle regulatory proteins, cells were exposed
for 48 h to various concentrations of CFZ and to 50 nM CFZ combined
with 100 μM CPT-11, after which expression of cyclins, CDKs, CKIs
and cdc25c were monitored by western blot analysis. Both CFZ
treatment alone or combined with CPT-11 resulted in reduction in
the levels of cdc25c, cyclin D1 and cyclin B1, as well as cdk1 and
mutant p53 (Fig. 4B). There was an
increase in the levels of cyclin A, p-cdk1Thr14/Thr15
and p21WAF1/CIP (Fig.
4B), which may explain this shift toward G2/M arrest.
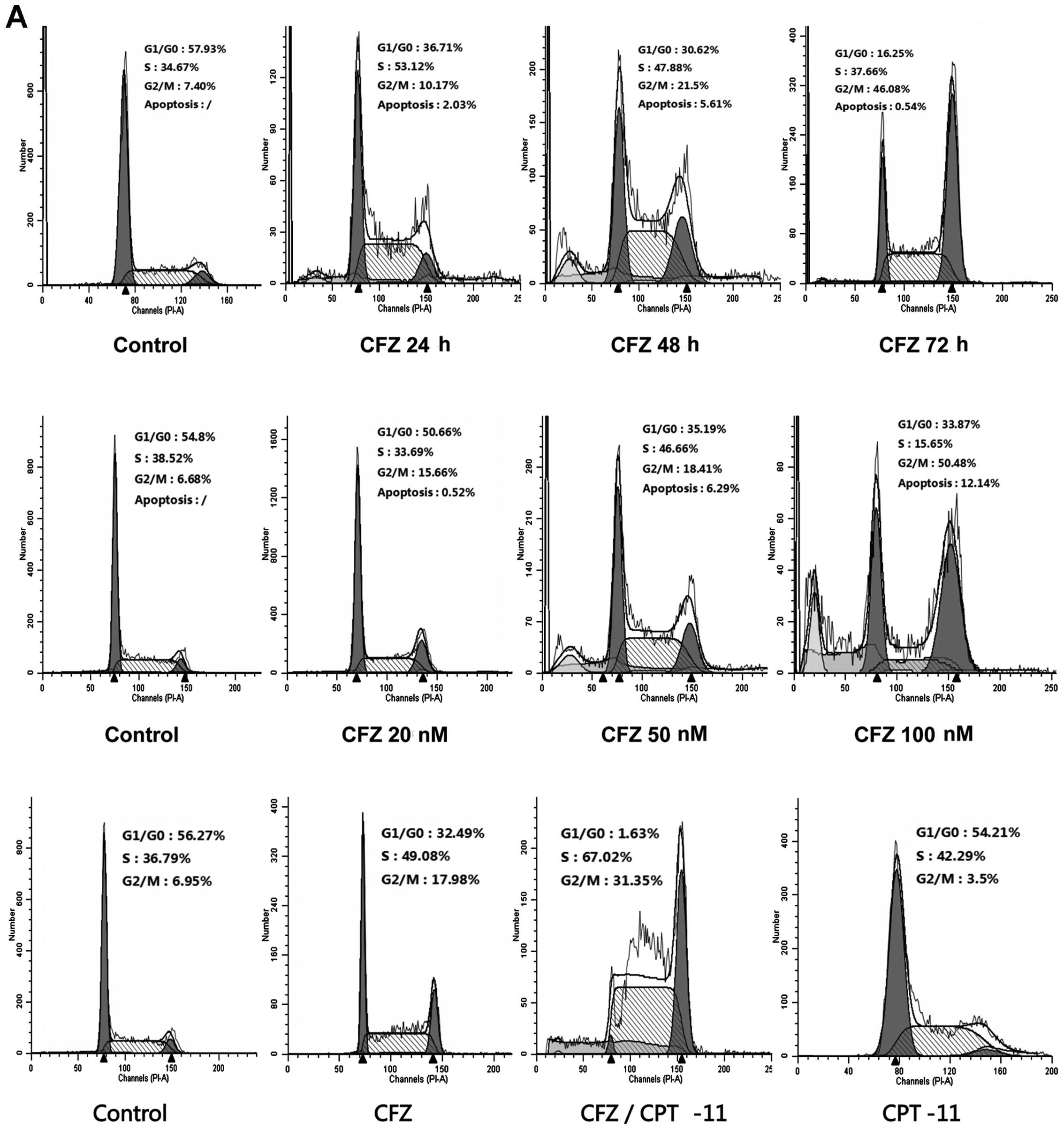 | Figure 4Combined CFZ with CPT-11 or CFZ alone
induces G2/M arrest. (A) Cell cycle analyzed by FCM of SW620 cells.
Cells were treated with 50 nM of CFZ for 24, 48 and 72 h, and
varying doses of CFZ (20, 50 and 100 nM) for 48 h. In the combined
regimen, cells were exposed to CFZ (50 nM) and CPT-11 (100 μM) for
48 h. (B) Expression of cell cycle related proteins in SW620 cells.
Cells were treated with various doses of CFZ, or with CFZ ± CPT-11
for 48 h, then the total protein were extracted for western blot
analysis. Both CFZ alone or in combination with CPT-11 decreased
the expression of cdc25c, cyclin D1, cyclin B1, cdk1 and mutant
p53, and increased the expression of cyclin A,
phospho-cdk1Thr14/Thr15 and p21WAF1/CIP, but
had no notable effect on the level of phospho-cdk2 or cdk2. |
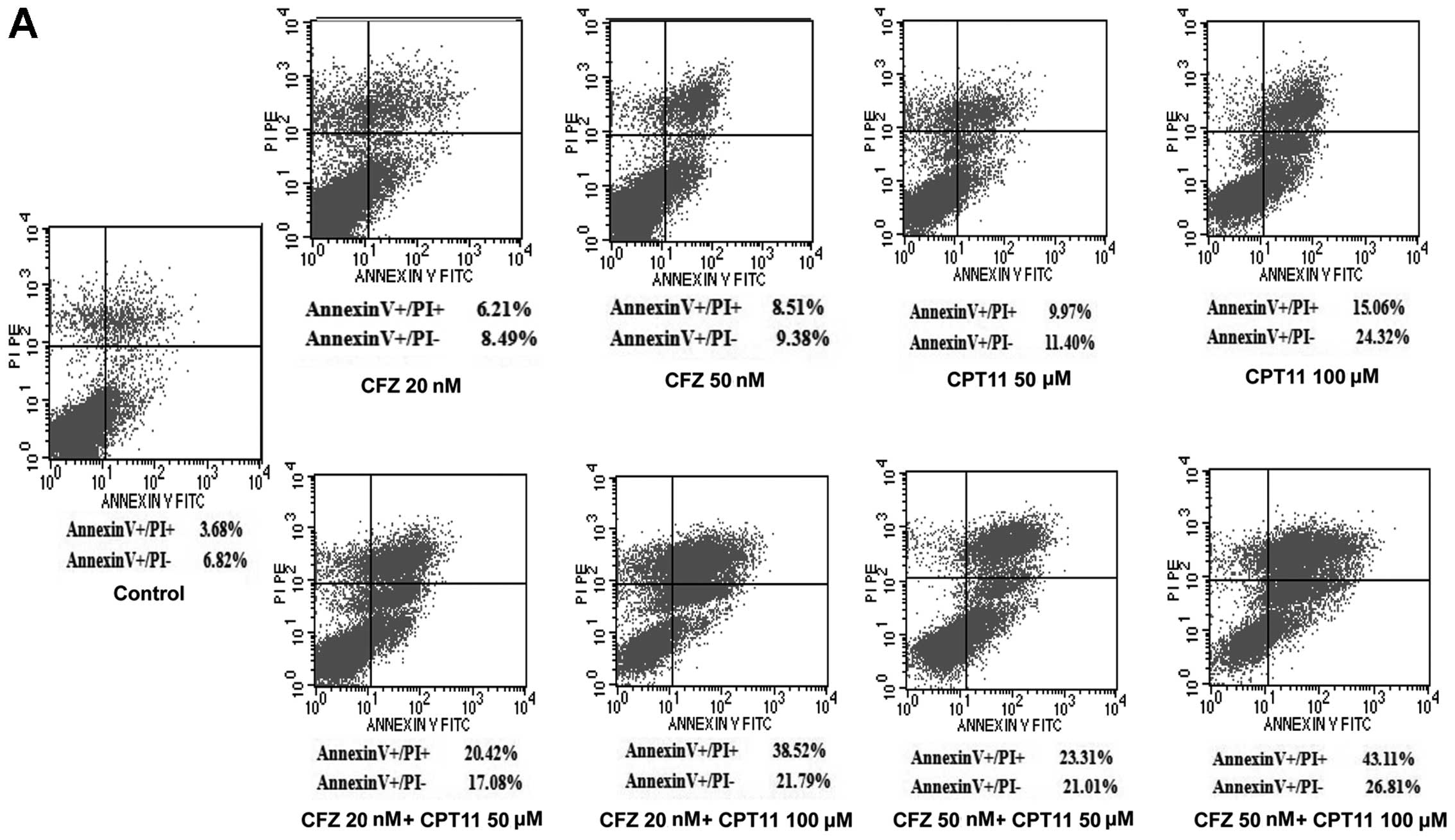
CFZ increases apoptosis and necrosis
induced by CPT-11 in SW620 cells by inducing caspase 3 and CD95
upregulation, as well as p-p38 and ATF3 activation
To investigate whether CFZ was able to reduce cell
viability by apoptosis, Annexin V/PI analysis and TUNEL were
performed to determine whether apoptosis could be responsible for
the synergistic effect of the combined treatment of SW620 cells.
Notably, the Annexin V/PI test showed that administration of
low-middle doses of CFZ (20 and 50 nM) and CPT-11 (50 and 100 μM)
resulted in pronounced apoptosis in SW620 cells (Fig. 5A). The average percent of apoptotic
cells increased significantly after exposure to single agents
compared with the control (P<0.05). The combination treatment
produced the greatest increase in both necrotic and apoptotic SW620
cells. Apoptosis induced by combination treatment was
1.62–2.30-fold higher in SW620 cells, compared with CPT-11 alone
(Fig. 5B). The TUNEL assay of
SW620 cells revealed that, whereas CPT-11 had little effect and CFZ
alone modestly increased TUNEL labelling, combined treatment
resulted in a pronounced increase in TUNEL labelling (Fig. 5C). Western blot analysis revealed
that combined treatment as well as CFZ treatment also resulted in a
striking increase in PARP cleavage, which is indicative of
apoptosis (Fig. 5E).
To elucidate the molecular mechanism by which CFZ or
the combination regimen induces apoptosis in SW620 cells, we
assessed caspase 3 activity and CD95 expression. CFZ combined with
CPT-11 resulted in a greater increase in the caspase 3 activity and
CD95 expression (Fig. 5D),
accompanied by marked increases in p-p38 and ATF3 activation,
without changes in total p38 (Fig.
5E). This indicates that the p-p38/ATF3 pathway, as well as
caspase 3 activation and CD95 upregulation, may play a significant
functional role in CFZ/CPT-11 lethality in SW620 cells.
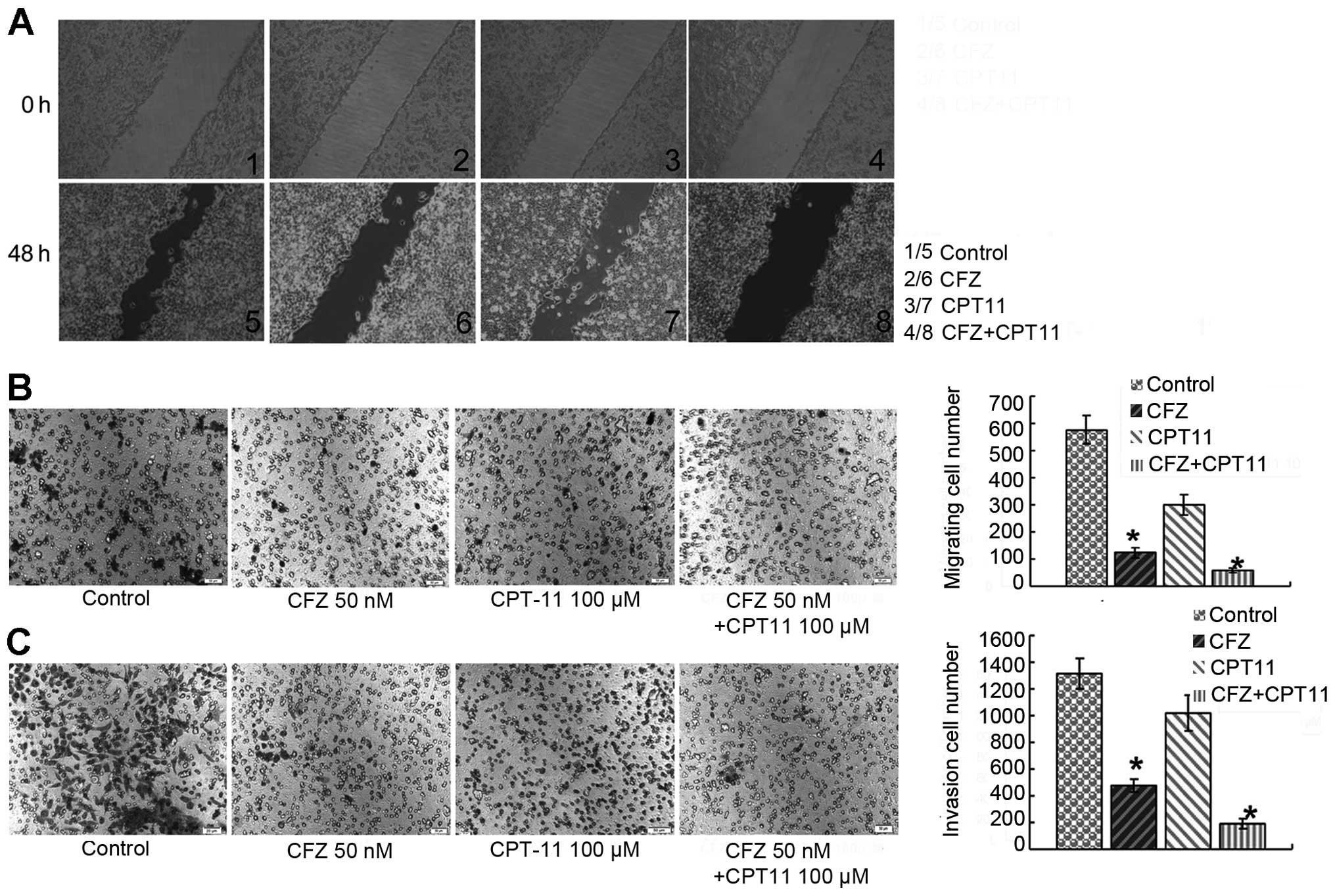
The invasion and migration inhibitory
effects of CFZ or CFZ/CPT-11 in vitro
To assess the inhibition of invasion and migration
of CFZ ± CPT-11 in vitro, its effects on the motility of
SW620 cells were evaluated in the wound-healing assay. After 48 h,
untreated SW620 cells migrated into the wounded area on the plate,
whereas the migration of cells under 50 nM CFZ treatment was
inhibited. Combined treatment with 50 nM CFZ and 100 μM CPT-11
resulted in a significant decrease in migration of SW620 cells
compared to CFZ or CPT-11 alone (Fig.
6A). The effect of CFZ on SW620 cell migration across the
transwell membrane and invasion through a Matrigel was also
assayed. The combination of 50 nM CFZ and 100 μM CPT-11 led to a
decrease in cell migration (Fig.
6B) and cell invasion (Fig.
6C), which was significantly greater than the decrease with
either CFZ or CPT-11 alone.
To further investigate the underlying mechanism of
CFZ invasion and migration inhibitory activity, the effect of CFZ
with and without CPT-11 on MMPs, TIMPs and Egr1 in SW620 cells was
investigated. The results of the western blot analysis showed that
both CFZ alone and combination treatment reduced MMP1 and MMP9
protein expression and increased TIMP1 protein in SW620 cells. The
downregulation of MMP2 was observed only in combination treatment,
but there was little change in Egr1 expression (Fig. 6D).
CFZ combination of CPT-11 suppresses
colorectal cancer growth in vivo
To evaluate the in vivo anti-colorectal
cancer effects of the CFZ/CPT-11 combination regimen, a SW620 cell
xenograft model was used. As shown in Fig. 7A, CFZ alone or CFZ/CPT-11
co-administration suppressed tumor growth and reduced tumor size.
The volume of tumors in mice receiving combination treatment was
less compared with single-agent treatment, especially after day 21
(P<0.05). This regimen also significantly abrogated tumor
weight; CFZ combined with CPT-11 reduced the tumor weight to
0.53±0.12 g in the final treatment, which is significant less
compared with single-agent treatment groups, 1.03±0.11 g in the CFZ
group and 1.22±0.04 g in the CPT-11 group (P<0.05). The H&E
staining of tissue from xenografted tumors confirmed that they were
malignant colorectal cancer cells (Fig. 7B). In the TUNEL staining of tissue
sections, CFZ combined with CPT-11 also increased apoptosis of
tumor cells (Fig. 7C). Further,
excised tumor tissue assayed in the EMSA showed that CFZ alone
resulted in NF-κB downregulation and combined treatment also
inhibited NF-κB activation induced by CPT-11 (Fig. 7D). These findings indicate that CFZ
increases the in vivo antitumor activity of CPT-11 in
colorectal cancer by blocking NF-κB activity.
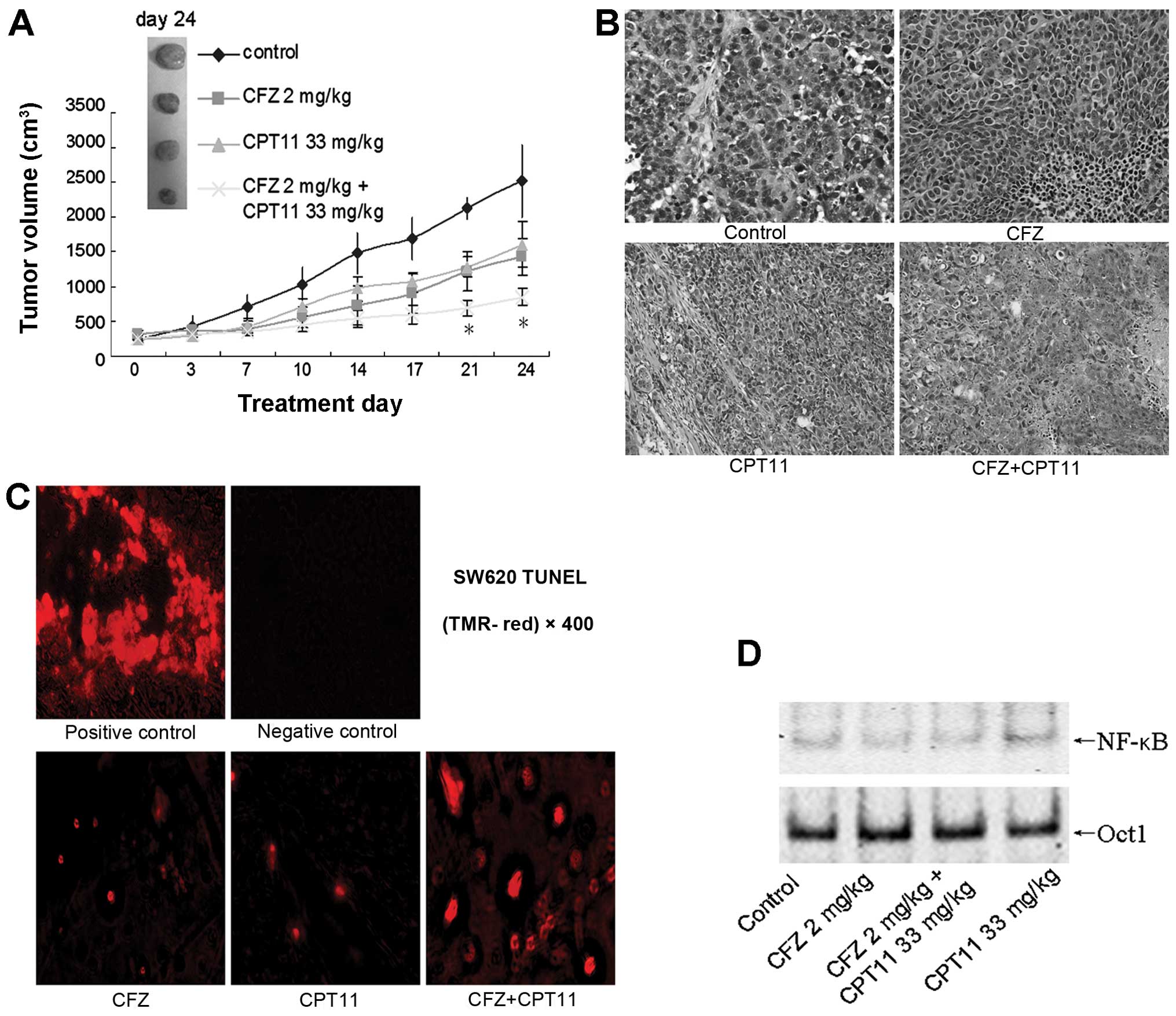 | Figure 7Combination effects of CFZ and CPT-11
in a SW620 xenograft model. (A) BALB/c nude mice were
subcutaneously inoculated in the back with 10×106 SW620
cells. Once the tumor diameter reached 7 mm, mice were treated with
2.0 mg/kg carfilzomib (i.v., twice weekly for 3 weeks) ± 33 mg/kg
CPT-11 (i.p., once weekly for 4 weeks). Tumor volumes were measured
twice weekly, and mean tumor volumes were plotted against days of
treatment. Bars, SD. *P<0.05. (B) H&E staining of
tumor tissues from SW620 xenografts after indicated treatment.
Magnification, ×200. (C) Representative photomicrographs
demonstrating TUNEL staining of SW620 tumor sections after
treatment with 2.0 mg/kg CFZ, 33 mg/kg CPT-11, and combination CFZ
and CPT-11. Tumors were removed from xenografts and processed
according the protocol of the In situ Cell Death Detection Kit, a
fluorescent microscope was used to detect TUNEL positive
(fluorescent red) cells. (D) After various indicated treatments,
the tumors were excised and nuclear protein was extracted. EMSA was
carried out for NF-κB activity assay. Oct-1 was used as a loading
control. |
Discussion
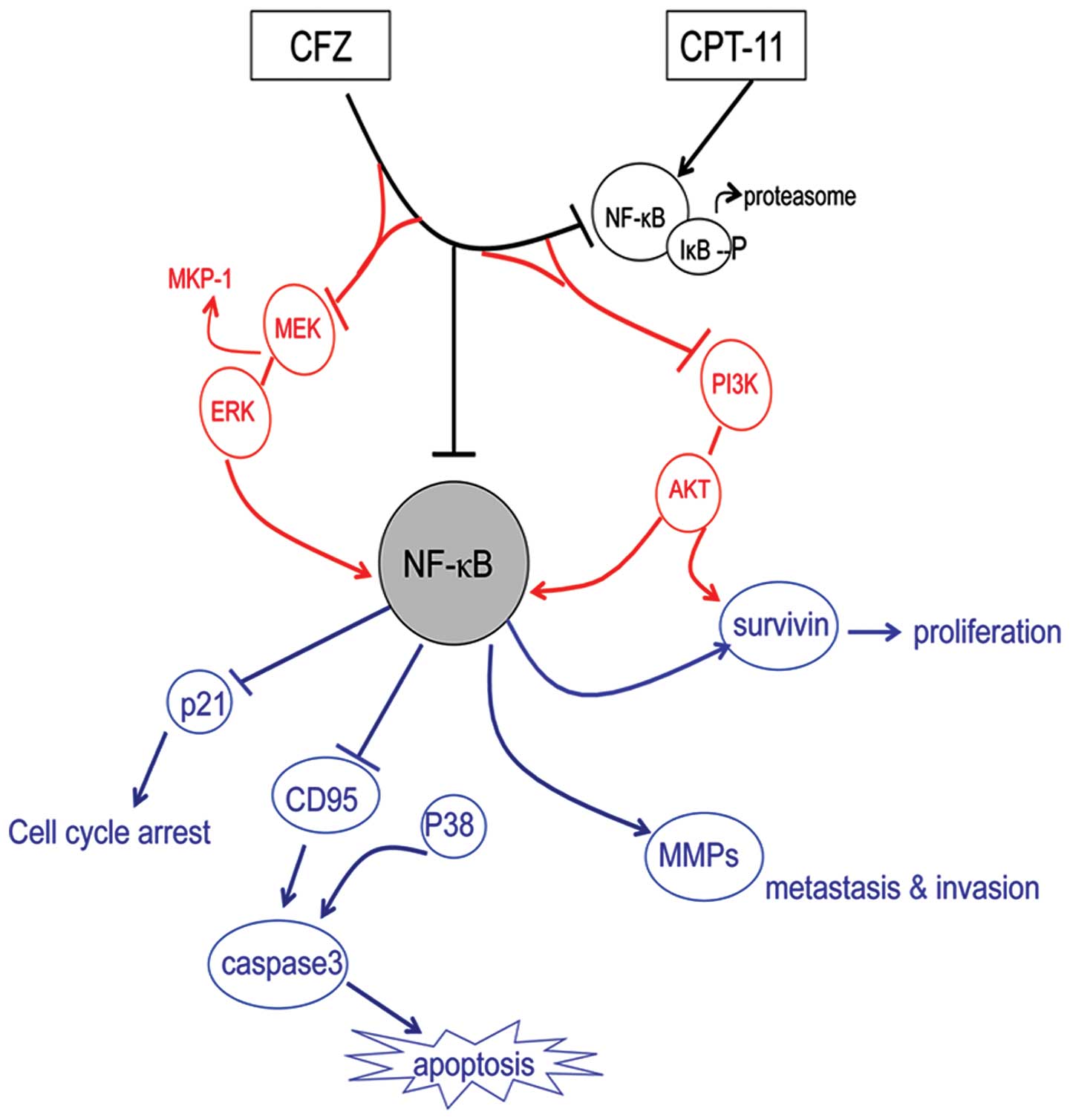
In this study, we determined the combined
therapeutic effects of carfilzomib with CPT-11 in SW620 cells and
in a xenograft model, as well as investigated the mechanism of
action. The results of this study indicate that CFZ significantly
potentiated CPT-11 activity against SW620 cells, suppressed
proliferation, inducing apoptosis, G2/M arrest, and inhibition of
aggresome formation through multiple mechanisms, including NF-κB
inhibition, MEK/ERK and PI3K/AKT phosphorylation modulation,
survivin downregulation, and the CFZ/CPT-11 combination regimen
also displays significant in vivo activity in a SW620
xenograft model. The schema of the proposed CFZ combination with
CPT-11 is shown in Fig. 8. To the
best of our knowledge, this is the first report that the
second-generation proteasome inhibitor, carfilzomib, which is more
selective with better effects and lower toxicity than first
generation proteasome inhibitors, is an effective antitumor agent
that significantly enhanced the CPT-11 chemosensitivity of
colorectal cancer through inhibition of multiple NF-κB related
mechanisms, and could be a potential novel therapeutic for treating
colorectal cancer patients.
CFZ interacts synergistically with CPT-11
to induce growth inhibition and apoptosis in colorectal cancer
cells
Proteasome inhibitors have been used as effective
antineoplastic agents, although mainly in hematologic patients
(30,31). They have been shown to inhibit
growth of many other solid tumors, such as ovarian carcinoma,
prostate tumor and colorectal cancer (19,32–34),
and have an increased anticancer potency for solid tumor treatment
when combined with some conventional anticancer agents (35,36).
Carfilzomib is a novel ‘second-generation’ proteasome inhibitor
with a greater potency than bortezomib, and it was given an
accelerated approval by the FDA and can be a safe and effective
replacement for bortezomib for MM therapy (19,25,26).
However, little is known about its activity against CRC. In our
study, CFZ given alone induced the inhibition of growth of CRC cell
lines in a concentration-dependent and time-dependent manner, and
decreased the colony formation from above 400 colonies to under 100
colonies when we increased the CFZ concentration. It suppressed
tumor growth along with tumor volume and tumor weights, as well as
induced apoptosis both in SW620 cells and in xenografted tumors,
exhibiting cytotoxic effects in vitro and in vivo.
Addition of CFZ to CPT-11 greatly potentiated inhibitory effects
and increased apoptosis in SW620 cells and in the xenograft model.
Single-agents replaced with the combination regimen was more
efficacious and the dose of CPT-11 can be reduced more than 2-fold
to a less toxic dose without any loss in efficiency. The
synergistic interaction was greatest between low-middle doses of
CPT-11 and CFZ. Similar synergistic effects were reflected in
clonogenic assays, where administration of CFZ and CPT-11 also
sharply decreased colony formation of SW620 cells. The enhanced
apoptosis-inducing effects of CFZ in combination with CPT-11 in
SW620 cells was 1.62–2.30-fold higher compared to treatment with
single agents, and the anticancer effect of the combined regimen on
SW620 tumors appears to be partly due to enhanced apoptosis. These
results demonstrate that CFZ not only displays a single-agent
activity but also enhanced chemosensitivity to CPT-11 in CRC
cells.
CFZ applied alone or combined with CPT-11
led to G2/M arrest and further enhanced apoptosis in CRC cells
In our study, the inhibition of SW620 cell growth by
combinations of CFZ with CPT-11 or by CFZ alone was accompanied by
a time-dependent and concentration-dependent cell cycle arrest in
G2/M. Induction of cell cycle arrest in the G2/M-phase may induce
cell apoptosis if damage is extensive (37). It is noteworthy that SW620 cells
exhibit abnormalities in components of DNA damage checkpoints, with
reductions of cdc25c and mutant p53, which may contribute to the
enhanced G2/M arrest and further the apoptotic response (38,39).
As reported by McConkey and Zhu (40), in the presence of proteasome
inhibition, expression of the cell cycle regulators, including
cdc25a, cdc25c, KIP1, INKs and cyclins, were destabilized, which
will make cells more susceptible to apoptosis. One possible
explanation for this dramatic shift toward G2/M arrest was
identified in the western blot analysis which showed there were
decreases in cyclin D1 that regulates G1/S transition, cyclin
B1/cdk1 that regulates transition in G2/M, cdc25c that functions as
a cyclin B1/cdk1 phosphatase, as well as of mutant p53. There were
increases in the levels of cyclin A that promote cell cycle
transition from the G1 phase to the S phase and from the S phase to
the G2 phase, phosphorylation of cdk1 at Thr14/Thr15, the sites
associated with inhibition, and of the cell cycle negative
regulator p21. Consistent with the effect on SW620 cells, it was
reported that the G2/M arrest is induced along with apoptosis
(41). In our study, after a
moderate shift in the cell population toward G2/M arrest by
addition of CFZ to CPT-11, there was a further increase of
apoptosis of SW620 cells. This suggests that combining CFZ and
CPT-11 induced cell cycle arrest that may play a role in mediating
cell death. As shown in our results, treatment of SW620 cells with
CFZ and CPT-11 resulted in the activation of caspase 3 and
CD95-dependent apoptotic pathways, as well as of proteins
associated with pro-apoptotic effects; this included p-p38 and ATF3
which have been shown to play an important regulatory role in the
apoptosis of tumor cells (42).
For these reasons, activation of caspase 3 and CD95, as well as
p-p38 and ATF3, both mobilized by combined treatment, are thought
to be involved in CFZ-mediated lethality and may participate in the
increased apoptosis induced by CPT-11.
Inhibition of CPT-11-induced NF-κB
activation plays a significant role in the chemosensitivity of the
SW620 cells
It has been demonstrated that CRC has high
constitutive NF-κB expression (7).
The NF-κB pathway plays a crucial role in cancer cell survival
(10,43), persistent activity of NF-κB is
associated with tumor formation, growth and metastasis, as well as
drug resistance in many cancers (44–46).
Since the proteasome pathway is important for activating NF-κB
(25), the ability of a proteasome
inhibitor to inhibit the NF-κB pathway by blocking proteasome
degradation of IκB plays a key role in the activity of this agent
against tumor cells (47–49). Zanotto-Filho et al reported
that inhibition of the NF-κB pathway is one of the major effects by
which the proteasome inhibitor induces selective apoptosis in
glioblastoma cells (50). In
addition, CFZ induced growth inhibition and apoptosis through
inhibiting the NF-κB signaling pathways in mantle cell lymphoma
(25), and co-administration
abrogated NF-κB activity in vorinostat-treated granta cells and
HF-4B cells (23). Moreover,
synergistic anticancer activity was reported when chemotherapeutic
agents such as CPT-11, were combined with NF-κB inhibitors,
including proteasome inhibitors (15,18,51).
Studies of NF-κB inhibition by proteasome inhibitors, such as
NPI-0052, have demonstrated a synergistic response with
chemotherapeutic drugs in a colon cancer model (18). Studies using the proteasome
inhibitor bortezomib (PS-341), that also blocks NF-κB activation,
have also demonstrated increased chemosensitivity of CRC cells to
CPT-11 toxicity (16), and an
increased cytotoxic effect of CPT-11 on glioma cells (52). In the study by Park et al,
simvastatin inhibiting of the proteasome also abrogated NF-κB
activation and sensitized NSCLC cells to CPT-11 induced apoptosis
(53). These reports indicate that
inhibition of NF-κB with proteasome inhibitors may be an important
therapeutic approach to improve the effect of chemotherapy.
Consistent with these results, our findings show
that CFZ is cytotoxic to human SW620 CRC cells and CFZ blocks NF-κB
activation in a dose-dependent manner. We also report that combined
treatment of SW620 cells with CFZ/CPT-11 will inhibit NF-κB
activation that was induced by CPT-11 by blocking the degradation
of IκB, and the inhibition of NF-κB was also seen when CFZ/CPT-11
was administered in vivo. It is possible that this is a
mechanism that contributes to growth inhibition and apoptosis
induced by this regimen. Moreover, we have demonstrated that SW620
cells, which have high levels of constitutively active NF-κB, as
shown in the EMSA assay, are sensitive to CFZ-induced cell death,
whereas HCT8 cells, that have significantly lower levels of
constitutively active NF-κB, are considerably less sensitive to
CFZ-induced cell death. Thus, the state of NF-κB activation
correlates with CFZ sensitivity in these colorectal cancer cells.
Combined with the results from EMSA and western blot analysis, this
suggests that regulation of NF-κB plays an essential role in CFZ
lethality.
CFZ-enhanced anticancer effect of CPT-11
is also attributable to MEK/ERK and PI3K/AKT dephosphorylation, and
survivin downregulation disrupting multiple cytoprotective
signaling pathways
It is reported that proteasome inhibitors target
various genes and pathways which regulate growth and survival of
transformed cells; targets such as cell cycle, NF-κB, aggresome
formation and stress pathways that can modulate tumor development
(10). NF-κB regulation is pivotal
in the therapeutic function of these proteasome inhibitors
(21,50,54),
it is involved in tumor progression through regulation of
transcription of various genes that regulate cell proliferation,
apoptosis, invasion and metastasis; including NF-κB target genes
such as cyclin D1 and survivin, ERK, STATs, caspases and MMP9
(55–59). This suggests that the action of
proteasome inhibitors is not limited to NF-κB inhibition but that
other related mechanisms may play a role in the antitumor activity.
In the report by Ye et al, MEK/ERK and PI3K/AKT pathways are
often activated in CRC, and they cooperate to regulate survivin
expression that is associated with metastatic progression and poor
survival of CRC (60). Our study
indicated that CFZ alone inhibited the phosphorylation of MEK, ERK,
PI3K and AKT, and it decreased MKP-1 and survivin. Exposure of
SW620 cells to combined CFZ and CPT-11 also attenuated the MEK/ERK
pathway and reduced MKP-1, as well as inducing PI3K/AKT pathway
dephosphorylation and survivin downregulation. All these effects
may play a significant role in the enhanced lethality of CPT-11.
These results also support the role of MKP-1 as a possible mediator
between CFZ/CPT-11 treatment and induction of apoptosis. Thus, the
potential of CPT-11 as an antineoplastic drug may be significantly
enhanced with CFZ, not only through inhibiting NF-κB, but also by
modifying factors downstream from NF-κB or survival signaling
pathways dependent on proteasome function. Taken together the
collective data is consistent with CFZ potentiating CPT-11
lethality by disrupting multiple cytoprotective signaling
pathways.
CFZ or combination treatment also
inhibits invasion and migration of SW620 cells, with decreased MMPs
and increased TIMPs
Using a wound-healing migration assay, combined with
Transwell migration and Matrigel invasion assays, cell migration
and invasion were found to be markedly decreased with the
combination regimen, indicating that CFZ may attenuate colorectal
cancer cell motility. MMPs, members of the neutral endopeptidase
family that can catalyze the degradation of ECM components, are
thought to play an important role in tumor invasion and metastasis
in colon carcinoma and many other human cancers, and their action
in tumors is inhibited by specific tissue inhibitors (TIMPs)
(61,62). In our present western blot analysis
results, CFZ alone or combined with CPT-11 diminished MMP1 and MMP9
protein expression, while it increased the TIMP1 protein levels in
SW620 cells, which was correlated with the invasion and migration
inhibition function of CFZ. The downregulation of MMP2 was observed
only in combination treatment, showing that CPT-11 contributed more
to these effects. This suggests that this combination regimen may
act through reducing MMPs and increasing TIMPs to reduce their
metastatic and invasive capability.
Using CFZ in combination with CPT-11
might be a promising strategy for treating colorectal cancer
In summary, our data support the notion that the
second-generation proteasome inhibitor CFZ should be considered a
potential antitumor agent when combined with CPT-11 for the
treatment of CRC. It is highly synergistic for potentiating the
cytotoxic effects of CPT-11 on SW620 colorectal cancer cells and in
a tumor xenograft model. It actively mediates multiple mechanisms,
including NF-κB inhibition, MEK/ERK pathway blocking, PI3K/AKT
pathway dephosphorylation, and survivin downregulation, and these
events are accompanied by cell cycle arrest, increased apoptosis,
as well as inhibition of cell migration and invasion. Thus, this
regimen warrants consideration as a therapeutic strategy for CRC
and merits further clinical studies.
Acknowledgements
This study was partially supported by a grant from
the National Natural Science Foundation of China (grant no.
81250002), and Fujian Provincial Natural Science Foundation (grant
no. 2012J01327). We gratefully thank Professor Xin Lin for
providing invaluable assistance.
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar
|
|
2
|
Douillard JY, Cunningham D, Roth AD, et
al: Irinotecan combined with fluorouracil compared with
fluorouracil alone as first-line treatment for metastatic
colorectal cancer: a multicentre randomised trial. Lancet.
355:1041–1047. 2000. View Article : Google Scholar
|
|
3
|
Ikeguchi M, Arai Y, Maeta Y, Ashida K,
Katano K and Wakatsuki T: Topoisomerase I expression in tumors as a
biological marker for CPT-11 chemosensitivity in patients with
colorectal cancer. Surg Today. 41:1196–1199. 2011. View Article : Google Scholar
|
|
4
|
Calvo E, Cortes J, Rodriguez J, et al:
Irinotecan, oxaliplatin, and 5-fluorouracil/leucovorin combination
chemotherapy in advanced colorectal carcinoma: a phase II study.
Clin Colorectal Cancer. 2:104–110. 2002. View Article : Google Scholar
|
|
5
|
Tamatani T, Azuma M, Aota K, Yamashita T,
Bando T and Sato M: Enhanced IkappaB kinase activity is responsible
for the augmented activity of NF-kappaB in human head and neck
carcinoma cells. Cancer Lett. 171:165–172. 2001. View Article : Google Scholar
|
|
6
|
Tamatani T, Azuma M, Ashida Y, et al:
Enhanced radiosensitization and chemosensitization in
NF-kappaB-suppressed human oral cancer cells via the inhibition of
gamma-irradiation- and 5-FU-induced production of IL-6 and IL-8.
Int J Cancer. 108:912–921. 2004. View Article : Google Scholar
|
|
7
|
Lind DS, Hochwald SN, Malaty J, et al:
Nuclear factor-kappaB is upregulated in colorectal cancer. Surgery.
130:363–369. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Xu Y and Villalona-Calero MA: Irinotecan:
mechanisms of tumor resistance and novel strategies for modulating
its activity. Ann Oncol. 13:1841–1851. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Janssens S and Tschopp J: Signals from
within: the DNA-damage-induced NF-kappaB response. Cell Death
Differ. 13:773–784. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Adams J: The proteasome: a suitable
antineoplastic target. Nat Rev Cancer. 4:349–360. 2004. View Article : Google Scholar
|
|
11
|
Adams J: Preclinical and clinical
evaluation of proteasome inhibitor PS-341 for the treatment of
cancer. Curr Opin Chem Biol. 6:493–500. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Pevzner Y, Metcalf R, Kantor M, Sagaro D
and Daniel K: Recent advances in proteasome inhibitor discovery.
Expert Opin Drug Discov. 8:537–568. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Holkova B and Grant S: Proteasome
inhibitors in mantle cell lymphoma. Best Pract Res Clin Haematol.
25:133–141. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tamatani T, Takamaru N, Hara K, et al:
Bortezomib-enhanced radiosensitization through the suppression of
radiation-induced nuclear factor-κB activity in human oral cancer
cells. Int J Oncol. 42:935–944. 2013.PubMed/NCBI
|
|
15
|
Cusack JC, Liu R and Baldwin AS: NF-kappa
B and chemoresistance: potentiation of cancer drugs via inhibition
of NF-kappa B. Drug Resist Updat. 2:271–273. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Cusack JC Jr, Liu R, Houston M, et al:
Enhanced chemosensitivity to CPT-11 with proteasome inhibitor
PS-341: implications for systemic nuclear factor-kappaB inhibition.
Cancer Res. 61:3535–3540. 2001.PubMed/NCBI
|
|
17
|
Cusack JC Jr, Liu R and Baldwin AS Jr:
Inducible chemoresistance to
7-ethyl-10-[4-(1-piperidino)-1-piperidino]-carbonyloxycamptothe cin
(CPT-11) in colorectal cancer cells and a xenograft model is
overcome by inhibition of nuclear factor-kappaB activation. Cancer
Res. 60:2323–2330. 2000.PubMed/NCBI
|
|
18
|
Cusack JC Jr, Liu R, Xia L, et al:
NPI-0052 enhances tumoricidal response to conventional cancer
therapy in a colon cancer model. Clin Cancer Res. 12:6758–6764.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Demo SD, Kirk CJ, Aujay MA, et al:
Antitumor activity of PR-171, a novel irreversible inhibitor of the
proteasome. Cancer Res. 67:6383–6391. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kuhn DJ, Chen Q, Voorhees PM, et al:
Potent activity of carfilzomib, a novel, irreversible inhibitor of
the ubiquitin-proteasome pathway, against preclinical models of
multiple myeloma. Blood. 110:3281–3290. 2007. View Article : Google Scholar
|
|
21
|
Adams J: Proteasome inhibition in cancer:
development of PS-341. Semin Oncol. 28:613–619. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kuhn DJ, Orlowski RZ and Bjorklund CC:
Second generation proteasome inhibitors: carfilzomib and
immunoproteasome-specific inhibitors (IPSIs). Curr Cancer Drug
Targets. 11:285–295. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Dasmahapatra G, Lembersky D, Son MP, et
al: Carfilzomib interacts synergistically with histone deacetylase
inhibitors in mantle cell lymphoma cells in vitro and in vivo. Mol
Cancer Ther. 10:1686–1697. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zang Y, Thomas SM, Chan ET, et al:
Carfilzomib and ONX 0912 inhibit cell survival and tumor growth of
head and neck cancer and their activities are enhanced by
suppression of Mcl-1 or autophagy. Clin Cancer Res. 18:5639–5649.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhang L, Pham LV, Newberry KJ, et al: In
vitro and in vivo therapeutic efficacy of carfilzomib in mantle
cell lymphoma: targeting the immunoproteasome. Mol Cancer Ther.
29:292013.PubMed/NCBI
|
|
26
|
Berenson JR, Hilger JD, Yellin O, et al:
Replacement of bortezomib with carfilzomib for multiple myeloma
patients progressing from bortezomib combination therapy. Leukemia.
16:272014.
|
|
27
|
Ishiyama M, Tominaga H, Shiga M, Sasamoto
K, Ohkura Y and Ueno K: A combined assay of cell viability and in
vitro cytotoxicity with a highly water-soluble tetrazolium salt,
neutral red and crystal violet. Biol Pharm Bull. 19:1518–1520.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ye YB, Lin JY, Chen Q, et al: The
cytotoxicity of a Grb2-SH3 inhibitor in Bcr-Abl positive K562
cells. Biochem Pharmacol. 75:2080–2091. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chou TC and Talalay P: Quantitative
analysis of dose-effect relationships: the combined effects of
multiple drugs or enzyme inhibitors. Adv Enzyme Regul. 22:27–55.
1984. View Article : Google Scholar
|
|
30
|
Richardson P: Clinical update: proteasome
inhibitors in hematologic malignancies. Cancer Treat Rev. 1:33–39.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
O’Connor OA, Stewart AK, Vallone M, et al:
A phase 1 dose escalation study of the safety and pharmacokinetics
of the novel proteasome inhibitor carfilzomib (PR-171) in patients
with hematologic malignancies. Clin Cancer Res. 15:7085–7091.
2009.
|
|
32
|
Frankel A, Man S, Elliott P, Adams J and
Kerbel RS: Lack of multicellular drug resistance observed in human
ovarian and prostate carcinoma treated with the proteasome
inhibitor PS-341. Clin Cancer Res. 6:3719–3728. 2000.PubMed/NCBI
|
|
33
|
Qiao D, Gaitonde SV, Qi W and Martinez JD:
Deoxycholic acid suppresses p53 by stimulating proteasome-mediated
p53 protein degradation. Carcinogenesis. 22:957–964. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Pasquini L, Petronelli A, Petrucci E, et
al: Primary ovarian cancer cells are sensitive to the proaptotic
effects of proteasome inhibitors. Int J Oncol. 36:707–713.
2010.
|
|
35
|
Yang H, Zonder JA and Dou QP: Clinical
development of novel proteasome inhibitors for cancer treatment.
Expert Opin Investig Drugs. 18:957–971. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Abaza MS, Bahman AM and Al-Attiyah R:
Superior antimitogenic and chemosensitization activities of the
combination treatment of the histone deacetylase inhibitor apicidin
and proteasome inhibitors on human colorectal cancer cells. Int J
Oncol. 44:105–128. 2014.
|
|
37
|
Rosato RR, Almenara JA, Maggio SC, et al:
Role of histone deacetylase inhibitor-induced reactive oxygen
species and DNA damage in LAQ-824/fludarabine antileukemic
interactions. Mol Cancer Ther. 7:3285–3297. 2008. View Article : Google Scholar
|
|
38
|
Jares P, Colomer D and Campo E: Genetic
and molecular pathogenesis of mantle cell lymphoma: perspectives
for new targeted therapeutics. Nat Rev Cancer. 7:750–762. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Perdiguero E and Nebreda AR: Regulation of
Cdc25C activity during the meiotic G2/M transition. Cell Cycle.
3:733–737. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
McConkey DJ and Zhu K: Mechanisms of
proteasome inhibitor action and resistance in cancer. Drug Resist
Updat. 11:164–179. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Heerdt BG, Houston MA, Mariadason JM and
Augenlicht LH: Dissociation of staurosporine-induced apoptosis from
G2-M arrest in SW620 human colonic carcinoma cells: initiation of
the apoptotic cascade is associated with elevation of the
mitochondrial membrane potential (deltapsim). Cancer Res.
60:6704–6713. 2000.
|
|
42
|
Lu D, Chen J and Hai T: The regulation of
ATF3 gene expression by mitogen-activated protein kinases. Biochem
J. 401:559–567. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Blonska M and Lin X: NF-kappaB signaling
pathways regulated by CARMA family of scaffold proteins. Cell Res.
21:55–70. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Belardo G, Piva R and Santoro MG: Heat
stress triggers apoptosis by impairing NF-kappaB survival signaling
in malignant B cells. Leukemia. 24:187–196. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Milhollen MA, Traore T, Adams-Duffy J, et
al: MLN4924, a NEDD8-activating enzyme inhibitor, is active in
diffuse large B-cell lymphoma models: rationale for treatment of
NF-{kappa}B-dependent lymphoma. Blood. 116:1515–1523. 2010.
View Article : Google Scholar
|
|
46
|
Ben-Neriah Y and Karin M: Inflammation
meets cancer, with NF-kappaB as the matchmaker. Nat Immunol.
12:715–723. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Ling YH, Liebes L, Zou Y and Perez-Soler
R: Reactive oxygen species generation and mitochondrial dysfunction
in the apoptotic response to Bortezomib, a novel proteasome
inhibitor, in human H460 non-small cell lung cancer cells. J Biol
Chem. 278:33714–33723. 2003. View Article : Google Scholar
|
|
48
|
Fribley A, Zeng Q and Wang CY: Proteasome
inhibitor PS-341 induces apoptosis through induction of endoplasmic
reticulum stress-reactive oxygen species in head and neck squamous
cell carcinoma cells. Mol Cell Biol. 24:9695–9704. 2004. View Article : Google Scholar
|
|
49
|
Hideshima T, Chauhan D, Richardson P, et
al: NF-kappa B as a therapeutic target in multiple myeloma. J Biol
Chem. 277:16639–16647. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Zanotto-Filho A, Braganhol E, Battastini
AM and Moreira JC: Proteasome inhibitor MG132 induces selective
apoptosis in glioblastoma cells through inhibition of PI3K/Akt and
NFkappaB pathways, mitochondrial dysfunction, and activation of
p38-JNK1/2 signaling. Invest New Drugs. 30:2252–2262. 2012.
View Article : Google Scholar
|
|
51
|
Jani TS, DeVecchio J, Mazumdar T, Agyeman
A and Houghton JA: Inhibition of NF-kappaB signaling by quinacrine
is cytotoxic to human colon carcinoma cell lines and is synergistic
in combination with tumor necrosis factor-related
apoptosis-inducing ligand (TRAIL) or oxaliplatin. J Biol Chem.
285:19162–19172. 2010. View Article : Google Scholar
|
|
52
|
Weaver KD, Yeyeodu S, Cusack JC Jr,
Baldwin AS Jr and Ewend MG: Potentiation of chemotherapeutic agents
following antagonism of nuclear factor kappa B in human gliomas. J
Neurooncol. 61:187–196. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Park IH, Kim JY, Choi JY and Han JY:
Simvastatin enhances irinotecan-induced apoptosis in human
non-small cell lung cancer cells by inhibition of proteasome
activity. Invest New Drugs. 29:883–890. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Adams J: The development of proteasome
inhibitors as anticancer drugs. Cancer Cell. 5:417–421. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Li X and Stark GR: NFkappaB-dependent
signaling pathways. Exp Hematol. 30:285–296. 2002. View Article : Google Scholar
|
|
56
|
Sakamoto K, Maeda S, Hikiba Y, et al:
Constitutive NF-kappaB activation in colorectal carcinoma plays a
key role in angiogenesis, promoting tumor growth. Clin Cancer Res.
15:2248–2258. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Huang S, DeGuzman A, Bucana CD and Fidler
IJ: Nuclear factor-kappaB activity correlates with growth,
angiogenesis, and metastasis of human melanoma cells in nude mice.
Clin Cancer Res. 6:2573–2581. 2000.
|
|
58
|
Garg A and Aggarwal BB: Nuclear
transcription factor-kappaB as a target for cancer drug
development. Leukemia. 16:1053–1068. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Wu JT and Kral JG: The NF-kappaB/IkappaB
signaling system: a molecular target in breast cancer therapy. J
Surg Res. 123:158–169. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Ye Q, Cai W, Zheng Y, Evers BM and She QB:
ERK and AKT signaling cooperate to translationally regulate
survivin expression for metastatic progression of colorectal
cancer. Oncogene. 29:1222013.PubMed/NCBI
|
|
61
|
Yip D, Ahmad A, Karapetis CS, Hawkins CA
and Harper PG: Matrix metalloproteinase inhibitors: applications in
oncology. Invest New Drugs. 17:387–399. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Kolli-Bouhafs K, Boukhari A, Abusnina A,
et al: Thymoquinone reduces migration and invasion of human
glioblastoma cells associated with FAK, MMP-2 and MMP-9
down-regulation. Invest New Drugs. 30:2121–2131. 2012. View Article : Google Scholar : PubMed/NCBI
|