Introduction
Hepatocellular carcinoma (HCC) is the most common
carcinoma worldwide (1). Unlike
most solid cancers, both the incidence and mortality rate for HCC
patients are expected to increase substantially in many countries
over the next 20 years, mostly as a result of infection with
hepatitis C virus (HCV), in Japan, however, the incidence of non-B
and non-C HCC has recently tended to increase (2,3).
Treatment methods for HCC vary depending on the disease stage and
liver function, and they include surgical resection (SR), liver
transplantation, radiofrequency thermal ablation (RFA),
percutaneous ethanol injection therapy, transcatheter arterial
chemotherapy with or without embolization, systemic treatment with
molecular-targeted therapy (MTT) such as sorafenib therapy and
radiation therapy (RT) (4–7). HCC also carries a considerable risk
of tumor recurrence even when curative treatment was performed at
the initial therapy, with the tumor characteristics and any
underlying liver disease important predictive factors affecting the
risk of HCC recurrence (1,2,4,6).
Branched-chain amino acids (BCAAs) are a group of
essential amino acids comprising valine, leucine, and isoleucine. A
low plasma level ratio of BCAAs to aromatic amino acids suggests
liver cirrhosis (LC) physiologically, and BCAA supplementation was
originally developed in order to normalize the patient’s amino acid
profile and nutritional status (8–11).
Most HCC patients have underlying various stages of LC including
compensated or decompensated stages. LC patients with decreased
plasma BCAA level can develop protein-energy malnutrition (PEM)
with increased catabolism (12).
PEM is associated with a high morbidity and mortality due to an
increased risk of life-threatening complications, resulting in poor
clinical outcome and deteriorated quality of life (QOL) (9). PEM in LC patients is already observed
in the compensated phase with serum albumin level, which is a
useful indicator of liver functional reserve, ≥3.6 g/dl (13). Supplementation with BCAA for the
treatment of patients with liver disorder has been attracting
attention. BCAA has a variety of pharmacological effects. BCAA
treatment can correct malnutrition associated with LC (14,15)
and long-term nutritional BCAA supplementation may be effective for
increasing plasma BCAA levels, albumin synthesis and prevention of
hepatic failure while it also improves surrogate markers in
patients with advanced LC (16,17).
On the other hand, dietary supplementation alone does not affect
plasma BCAA levels in patients with LC (18).
BCAA granules (Livact; Ajinomoto Pharmaceutical,
Tokyo, Japan) have been approved for its use in LC patients since
1996 in Japan, and BCAA granules are generally administered in LC
patients with a serum albumin level ≤3.5 g/dl. Clinical evidence
regarding the effect of this therapy is being accumulated (19–23).
Thus, the 2008 Japanese guidelines for the treatment of patients
with chronic liver diseases recommend that treatment with BCAA
granules should be performed for decompensated LC with a serum
albumin level ≤3.5 g/dl (19). In
Japan, BCAA granules are widely used in clinical practice without
serious adverse effects. In addition, we have previously
demonstrated that BCAA treatment may improve OS and recurrence-free
survival after RFA in patients with HCV-related HCC ≤3 cm in
diameter with ≤3 nodules and a serum albumin level before RFA ≤3.5
g/dl (24). On the other hand,
several investigators reported that for patients with chronic liver
diseases including LC, it might be beneficial to initiate BCAA
therapy in the compensatory stage or even earlier, which means
‘early intervention using BCAA therapy’, rather than starting BCAA
therapy in the decompensatory stage (25–27).
Thus, BCAA supplementation may be effective in improving clinical
outcome in cirrhotic patients regardless of disease stage.
However, to our knowledge, whether this early
intervention using BCAA granules in patients with HCC can
contribute to prolongation of survival remains unclear. The
objectives of the present study were to examine whether
supplementation of BCAA granules in an early stage of underlying
liver disease (pretreatment serum albumin level ≥3.6 g/dl) can
improve overall survival (OS) after HCC therapy. For reducing
selection biases, we used propensity score matching analysis.
Patients and methods
Patients
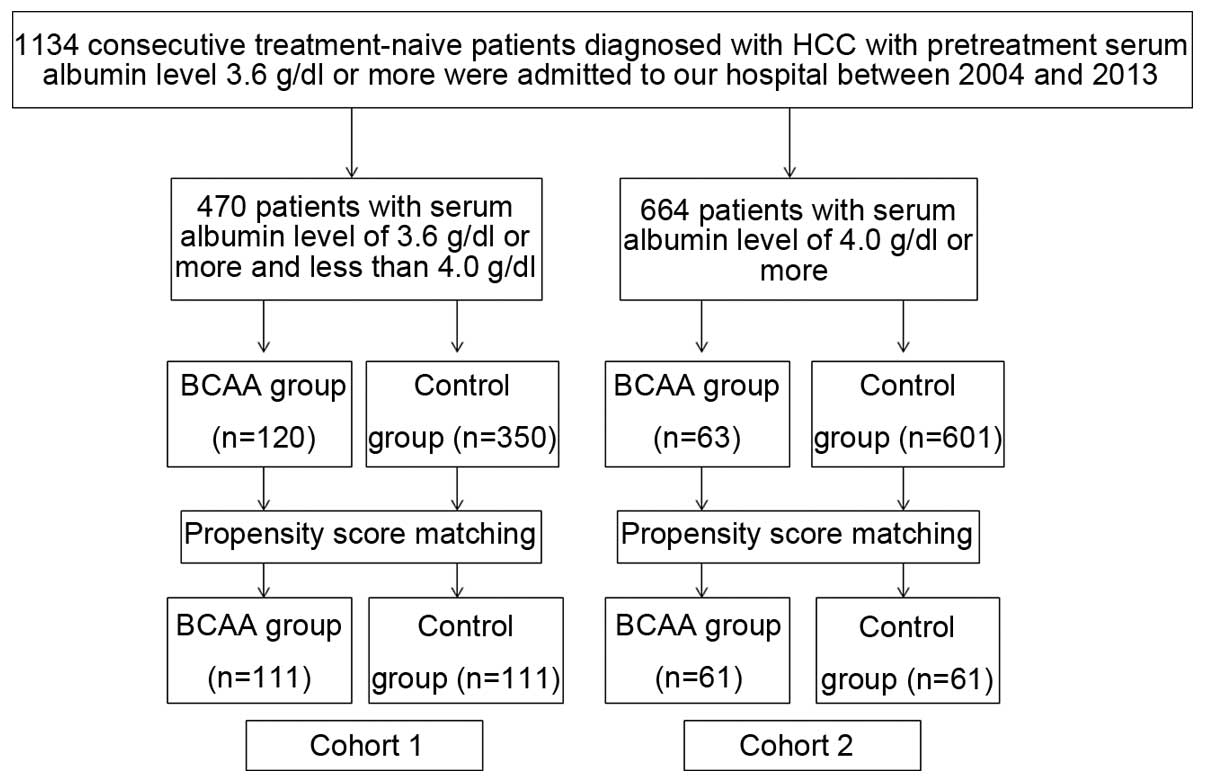
A total of 1,134 consecutive treatment-naïve
patients diagnosed with HCC with pretreatment serum albumin level
≥3.6 g/dl were admitted to the Department of Gastroenterology and
Hepatology, Osaka Red Cross Hospital, Japan, between 2004 and 2013.
We divided these patients into two groups according to pretreatment
serum albumin level (cut-off serum albumin level, 4.0 g/dl). There
were 470 patients with pretreatment serum albumin level ≥3.6 g/dl
and <4.0 g/dl at the initial treatment for HCC. Of these, BCAA
granules were prescribed in 120 patients at the initial therapy for
HCC and the remaining 350 patients did not receive such therapy at
the initial therapy for HCC. Since this study was a retrospective
observational study, covariate adjustment using the propensity
score was performed. One hundred and eleven pairs were thus
selected for analysis in this cohort (cohort 1) (Fig. 1). Similarly, there were 664
patients with pretreatment serum albumin level ≥4.0 g/dl at the
initial treatment for HCC. Of these, BCAA granules were prescribed
in 63 patients at the initial therapy for HCC and the remaining 601
patients did not receive such therapy at the initial therapy for
HCC. After using the propensity score matching, 61 pairs were
selected for analysis in this cohort (cohort 2) (Fig. 1). We compared the OS rate between
the BCAA group and the control group in each cohort.
Prior to therapy for HCC, written informed consent
was obtained from all patients. The ethics committee of our
department approved the protocol for HCC therapy. The present study
comprised a retrospective analysis of patients’ medical records in
our database and all treatments were performed in an open-label
manner.
Diagnosis of HCC and HCC therapy
HCC was diagnosed based on the results from
abdominal ultrasound and dynamic computed tomography (CT) scan
(hyperattenuation during the arterial phase in the entire or part
of the tumor, and hypoattenuation in the portal-venous phase)
and/or magnetic resonance imaging mainly as recommended by the
American Association for the Study of Liver Diseases (28). Arterial and portal phase dynamic CT
images were obtained ~30 and 120 sec after injection of contrast
material. For all patients, abdominal angiography combined with CT
(angio-CT) was performed before therapy for HCC after obtaining
informed consent from them for performing abdominal angiography.
This was performed based on the fact that this technique was useful
for detecting small satellite nodules as reported by Yamasaki et
al (29). Then, we confirmed
HCC using CT during hepatic arteriography (CTHA) and CT during
arterial-portography (CTAP). As for HCC therapy, the most
appropriate treatment modality for each patient was selected
through discussion with surgeons, hepatologists and radiologists
(30,31). In the present analysis, there was
no patient treated with liver transplantation and there was no
treatment related death.
BCAA granule treatment
The patient’s attending physician determined whether
treatment with BCAA granules would be performed in individual
patients considering their intent to receive the treatment after
providing sufficient information regarding BCAA treatment to them.
In the BCAA group, BCAA granules, containing 952 mg of
L-isoleucine, 1,904 mg of L-leucine and 1,144 mg of L-valine per
sachet, were orally administered to subjects at a dose of one
sachet three times daily after meals ≥1 month after initial therapy
for HCC. We confirmed in our database that patients in the BCAA
group were prescribed BCAA granules regularly.
Follow-up after initial therapy for
HCC
Follow-up observation consisted of regular blood
tests and monitoring of tumor markers, including α-fetoprotein
(AFP) and des-γ-carboxy prothrombin (DCP), which was measured using
a chemiluminescent enzyme immunoassay (Lumipulse PIVKAII Eisai,
Eisai, Tokyo, Japan). Dynamic CT scan was performed every 3–4
months after initial therapy for HCC. In particular, for patients
in the BCAA group, we confirmed that BCAA granules were taken
properly at every hospital visit. When HCC recurrence or disease
progression was detected based on radiologic findings, most
appropriate therapy was performed in each patient.
Statistical analysis
The primary end-point is OS. Continuous variables
were compared by unpaired t-test, and categorical variables were
compared by Fisher’s exact test. Data were analyzed using
univariate and multivariate analyses. The cumulative OS rate was
calculated by Kaplan-Meier method and tested by log-rank test. A
Cox proportional hazard model was used for multivariate analyses of
factors with P<0.1 in univariate analysis. These statistical
methods were used to estimate the interval from each initial
therapy for HCC. Data were analyzed using SPSS software (SPSS Inc.,
Chicago, IL, USA) for Microsoft Windows. Data are expressed as mean
± standard deviation. A P-value <0.05 was considered to be
statistically significant.
Propensity score analysis
To compare the OS between BCAA group patients and
control group patients, a propensity score model was used with an
attempt to reduce potential biases in survival analysis (32,33).
Possible variables associated with long-term survival of HCC
patients, including age, sex, HCC stage, maximum tumor size, cause
of liver disease, serum albumin level, aspartate aminotransferase
(AST) value and alanine aminotransferase (ALT) value were included
comprehensively for propensity score generation. With these
selected variables, a logistic regression was applied to generate a
continuous propensity score from 0 to 1. One-to-one matches between
BCAA group patients and control group patients were introduced into
the subsequent analysis.
Results
Patient demographic characteristics and
survival (cohort 1)
Baseline demographic characteristics of patients in
cohort 1 are shown in Table I.
There were 111 patients in the BCAA group and 111 patients in the
control group, respectively. In terms of baseline demographic
characteristics, no significant differences were noted between the
BCAA group and the control group, showing that balance of baseline
characteristics in the two groups was obtained in the matched
sample. As an initial therapy for HCC, in the BCAA group, SR was
performed in 35 patients, percutaneous ablative therapies in 52,
transcatheter arterial chemotherapy with or without embolization in
22, MTT in one and RT in one, whereas in the control group, SR was
performed in 23 patients, percutaneous ablative therapies in 64,
transcatheter arterial chemotherapy with or without embolization in
21 and MTT in three (P=0.201).
 | Table IBaseline characteristics between the
BCAA group and the control group in HCC patients with pretreatment
serum albumin level ≥3.6g/dl and <4.0 g/dl after propensity
score matching. |
Table I
Baseline characteristics between the
BCAA group and the control group in HCC patients with pretreatment
serum albumin level ≥3.6g/dl and <4.0 g/dl after propensity
score matching.
| Variables | BCAA group
(n=111) | Control group
(n=111) | P-value |
|---|
| Age (years) | 67.8±9.3 | 69.8±9.6 | 0.144a |
| Gender,
male/female | 77/34 | 75/36 | 0.885b |
| Causes of liver
disease |
| B/C/non-B non-C/B
and C | 7/71/32/1 | 9/79/23/0 | 0.348b |
| HCC stage
I/II/III/IV | 27/47/31/6 | 26/51/23/11 | 0.417b |
| Maximum tumor size
(cm) | 3.2±2.3 | 3.4±2.7 | 0.665a |
| Initial therapy for
HCC |
|
SR/ablation/transcatheter arterial
chemotherapy/MTT/RT | 35/52/22/1/1 | 23/64/21/3/0 | 0.201b |
| AST (IU/l) | 57.1±28.3 | 63.8±41.6 | 0.170a |
| ALT (IU/l) | 48.5±31.5 | 56.4±51.2 | 0.164a |
| ALP (IU/l) | 383.8±165.3 | 406.5±331.5 | 0.520a |
| GGT (IU/l) | 96.9±86.8 | 119.3±152.5 | 0.158a |
| Serum albumin
(g/dl) | 3.8±0.1 | 3.7±0.1 | 0.684a |
| Total bilirubin
(mg/dl) | 1.0±0.5 | 1.0±0.6 | 0.419a |
| Prothrombin time
(%) | 81.8±12.9 | 84.1±13.9 | 0.180a |
| Platelets
(x104/mm3) | 10.8±5.8 | 11.4±4.1 | 0.194a |
| AFP (ng/ml) | 1,548±12,111 | 2,918±25,034 | 0.604a |
| DCP
(mAU/ml)c | 7,570±49,443 | 3,372±12,614 | 0.387a |
The median follow-up period was 2.9 years (range,
0.5–7.0 years) in the BCAA group and 2.6 years (range, 0.1–7.6
years) in the control group. Thirty-nine patients (35.2%) in the
BCAA group died during the follow-up period. The causes of death
were HCC progression (24 patients), liver failure (12 patients) and
miscellaneous (3 patients). Sixty patients (54.1%) in the control
group died during the follow-up period. The causes of death were
HCC progression (40 patients), liver failure (14 patients) and
miscellaneous (6 patients).
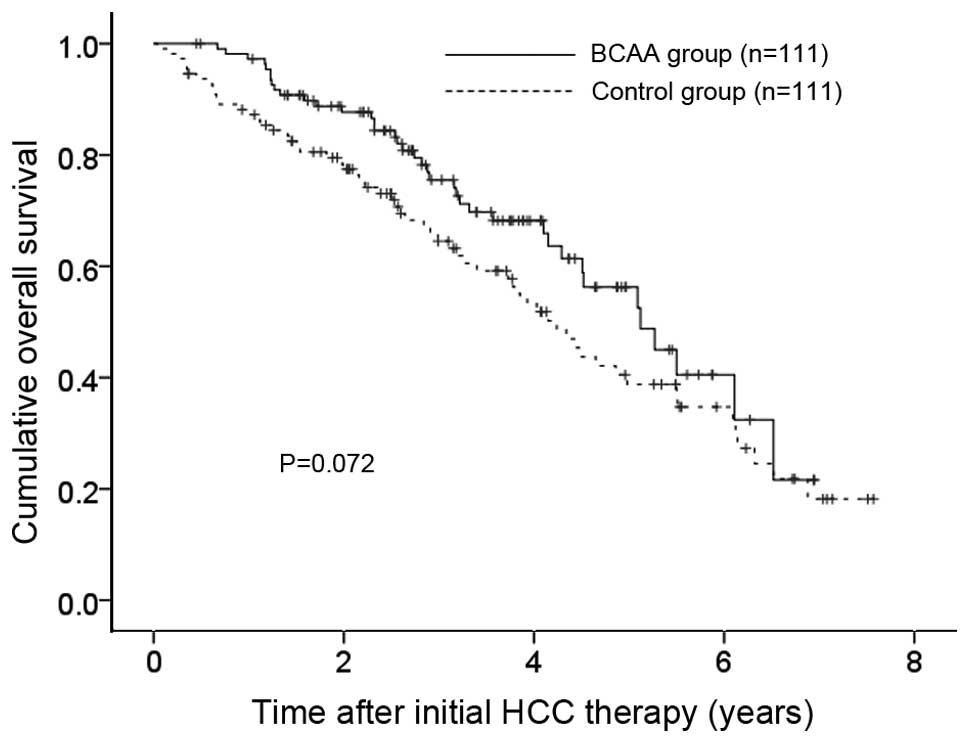
The 1-, 3- and 5-year OS rates after each initial
therapy for HCC were 97.2, 75.5 and 56.3%, respectively, in the
BCAA group and 87.2, 64.5 and 38.8%, respectively, in the control
group (P=0.072) (Fig. 2),
indicating that the OS rate in the BCAA group tended to be higher
compared to that in the control group.
Univariate and multivariate analysis of
factors contributing to OS (cohort 1)
In patients with pretreatment serum albumin level of
≥3.6 and <4.0 g/dl, using univariate analyses of factors
contributing to OS, HCC stage (P<0.001), maximum tumor size ≥2.5
cm (P=0.004), AST ≥50 IU/l (P=0.004), ALT ≥40 IU/l (P=0.018),
alkaline phosphatase (ALP) ≥330 IU/l (P=0.013), γ glutamyl
transpeptidase (GGT) ≥70 IU/l (P=0.005), AFP ≥100 ng/ml (P=0.001)
and DCP ≥100 mAU/ml (P<0.001) were found to be significant
factors (Table II). The
multivariate analyses involving nine factors with P<0.1 in the
univariate analysis showed that only HCC stage was a significant
independent predictor linked to OS (P=0.001). The hazard ratios
(HRs), 95% confidence interval (CI) and P-value for nine factors
are detailed in Table II.
 | Table IIUnivariate and multivariate analysis
of factors contributing to overall survival in HCC patients with
pretreatment serum albumin level ≥3.6 and <4.0 g/dl (cohort
1). |
Table II
Univariate and multivariate analysis
of factors contributing to overall survival in HCC patients with
pretreatment serum albumin level ≥3.6 and <4.0 g/dl (cohort
1).
| | | Multivariate
analysis |
|---|
| | |
|
|---|
| Variables | n | Univariate analysis
P-valuea | Hazard ratio (95%
CI) | P-valueb |
|---|
| Gender, male vs.
female | 152/70 | 0.558 | | |
| Age (years), ≥70
vs. <70 | 111/111 | 0.739 | | |
| BCAA vs.
control | 111/111 | 0.072 | 1.372
(0.887–2.123) | 0.155 |
| HCC stage I or II
vs. III or IV | 151/71 | <0.001 | 0.376
(0.214–0.659) | 0.001 |
| Maximum tumor size
(cm), ≥2.5 vs. <2.5 | 105/117 | 0.004 | 0.964
(0.575–1.616) | 0.889 |
| Cause of liver
disease, viral vs. non-viral | 167/55 | 0.523 | | |
| AST (IU/l), ≥50 vs.
<50 | 116/106 | 0.004 | 0.731
(0.387–1.380) | 0.333 |
| ALT (IU/l), ≥40 vs.
<40 | 111/111 | 0.018 | 0.962
(0.520–1.780) | 0.903 |
| ALP (IU/l), ≥330
vs. <330 | 116/106 | 0.013 | 0.804
(0.515–1.254) | 0.336 |
| GGT (IU/l), ≥70 vs.
<70 | 111/111 | 0.005 | 0.884
(0.563–1.389) | 0.593 |
| Serum albumin level
(g/dl), ≥3.8 vs. <3.8 | 108/114 | 0.374 | | |
| Total bilirubin
(mg/dl), ≥1.0 vs. <1.0 | 90/132 | 0.117 | | |
| Platelet count
(x104/mm3), ≥10 vs. <10 | 106/116 | 0.472 | | |
| Prothrombin time
(%), ≥70 vs. <70 | 187/35 | 0.687 | | |
| Serum AFP (ng/ml),
≥100 vs. <100 | 62/160 | 0.001 | 0.788
(0.500–1.242) | 0.305 |
| DCP (mAU/ml), ≥100
vs. <100c | 78/143 | <0.001 | 0.767
(0.458–1.282) | 0.311 |
Patient demographic characteristics and
survival (cohort 2)
Baseline demographic characteristics of patients in
cohort 2 are shown in Table III.
They included 61 patients in the BCAA group and 61 patients in the
control group, respectively. In terms of baseline demographic
characteristics, no significant differences were noted between the
BCAA group and the control group, demonstrating that balance of
baseline characteristics in the two groups was obtained in the
matched sample. As an initial therapy for HCC, in the BCAA group,
SR was performed in 31 patients, percutaneous ablative therapies in
15 and transcatheter arterial chemotherapy with or without
embolization in 15, whereas in the control group, SR was performed
in 20 patients, percutaneous ablative therapies in 19,
transcatheter arterial chemotherapy with or without embolization in
21 and MTT in one (P=0.160).
 | Table IIIBaseline characteristics between the
BCAA group and the control group in HCC patients with pretreatment
serum albumin level ≥4.0 g/dl after propensity score matching. |
Table III
Baseline characteristics between the
BCAA group and the control group in HCC patients with pretreatment
serum albumin level ≥4.0 g/dl after propensity score matching.
| Variables | BCAA group
(n=61) | Control group
(n=61) | P-value |
|---|
| Age (years) | 69.8±10.0 | 69.9±9.7 | 0.985a |
| Gender,
male/female | 43/18 | 42/19 | >0.999b |
| Causes of liver
disease |
| B/C/non-B non-C/B
and C | 10/29/21/1 | 8/31/21/1 | 0.954b |
| HCC stage
I/II/III/IV | 6/26/20/9 | 7/27/23/4 | 0.555b |
| Maximum tumor size
(cm) | 4.9±2.9 | 4.7±3.3 | 0.686a |
| Initial therapy for
HCC |
|
SR/ablation/transcatheter arterial
chemotherapy/MMT | 31/15/15/0 | 20/19/21/1 | 0.160b |
| AST (IU/l) | 67.0±54.7 | 59.3±36.4 | 0.183a |
| ALT (IU/l) | 55.0±37.2 | 54.1±47.4 | 0.905a |
| ALP (IU/l) | 365.5±204.7 | 320.6±131.5 | 0.139a |
| GGT (IU/l) | 128.9±151.7 | 133.0±145.3 | 0.879a |
| Serum albumin
(g/dl) | 4.2±0.2 | 4.3±0.2 | 0.344a |
| Total bilirubin
(mg/dl) | 1.0±0.4 | 0.8±0.3 | 0.167a |
| Prothrombin time
(%) | 89.2±11.8 | 93.4±15.1 | 0.209a |
| Platelets
(x104/mm3) | 14.7±8.7 | 14.1±3.9 | 0.627a |
| AFP (ng/ml) | 15,065±82,251 | 3,018±18,567 | 0.267a |
| DCP
(mAU/m)c | 7,011±28,784 | 7,926±24,508 | 0.853a |
The median follow-up period was 2.3 years (range,
0.2–7.8 years) in the BCAA group and 2.9 years (range, 0.3–8.4
years) in the control group. Twenty-four patients (39.3%) in the
BCAA group died during the follow-up period. The causes of death
were HCC progression (22 patients) and liver failure (2 patients).
Twenty-eight patients (45.9%) in the control group died during the
follow-up period. The causes of death were HCC progression (24
patients), liver failure (2 patients) and miscellaneous (2
patients).
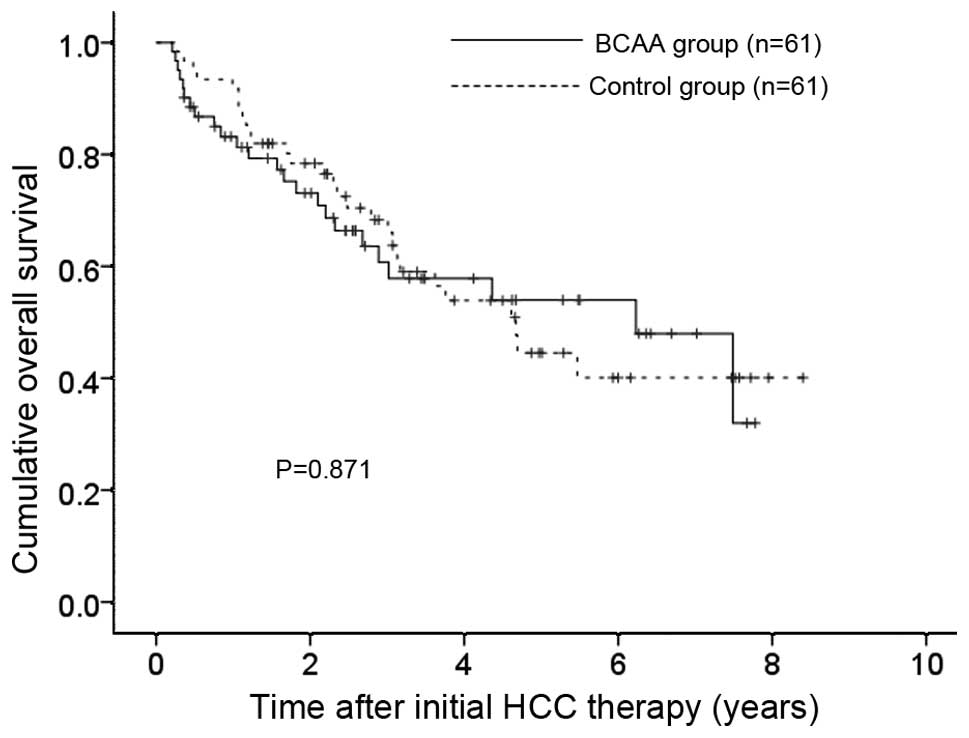
The 1-, 3- and 5-year OS rates after each initial
therapy for HCC were 83.2, 60.7 and 54.0%, respectively, in the
BCAA group and 91.8, 66.0 and 44.5%, respectively, in the control
group (P=0.871) (Fig. 3).
Univariate and multivariate analysis of
factors contributing to OS (cohort 2)
In patients with serum albumin level of ≥4.0 g/dl,
using univariate analyses of factors contributing to OS, HCC stage
(P<0.001), maximum tumor size ≥4.0 cm (P<0.001), AST ≥50 IU/l
(P=0.024), ALP ≥300 IU/l (P<0.001), GGT ≥70 IU/l (P=0.009),
total bilirubin ≥1.0 mg/dl (P=0.045), AFP ≥100 ng/ml (P=0.013) and
DCP ≥100 mAU/ml (P<0.001) were found to be significant factors
(Table IV). The multivariate
analyses involving nine factors with P<0.1 in the univariate
analysis showed that HCC stage (P=0.019), ALP ≥300 IU/l (P=0.023)
and AFP ≥100 ng/ml (P=0.019) were significant independent
predictors linked to OS. The HRs, 95% CI and P-value for nine
factors are detailed in Table
IV.
 | Table IVUnivariate and multivariate analysis
of factors contributing to overall survival in HCC patients with
pretreatment serum albumin level ≥4.0 g/dl (cohort 2). |
Table IV
Univariate and multivariate analysis
of factors contributing to overall survival in HCC patients with
pretreatment serum albumin level ≥4.0 g/dl (cohort 2).
| | | Multivariate
analysis |
|---|
| | |
|
|---|
| Variables | n | Univariate analysis
P-valuea | Hazard ratio (95%
CI) | P-valueb |
|---|
| Gender, male vs.
female | 85/37 | 0.422 | | |
| Age (years), ≥72
vs. <72 | 60/62 | 0.880 | | |
| BCAA vs.
control | 61/61 | 0.871 | | |
| HCC stage I or II
vs. III or IV | 66/56 | <0.001 | 0.437
(0.219–0.872) | 0.019 |
| Maximum tumor size
(cm), ≥4.0 vs. <4.0 | 58/64 | <0.001 | 0.610
(0.307–1.213) | 0.159 |
| Cause of liver
disease, viral vs. non-viral | 80/42 | 0.517 | | |
| AST (IU/l), ≥50 vs.
<50 | 61/61 | 0.024 | 0.679
(0.307–1.502) | 0.339 |
| ALT (IU/l), ≥40 vs.
<40 | 65/57 | 0.058 | 0.486
(0.210–1.127) | 0.093 |
| ALP (IU/l), ≥300
vs. <300 | 62/60 | <0.001 | 0.466
(0.241–0.902) | 0.023 |
| GGT (IU/l), ≥80 vs.
<80 | 61/61 | 0.009 | 0.579
(0.299–1.122) | 0.105 |
| Serum albumin level
(g/dl), ≥4.3 vs. <4.3 | 49/73 | 0.456 | | |
| Total bilirubin
(mg/dl), ≥1.0 vs. <1.0 | 40/82 | 0.045 | 0.897
(0.465–1.730) | 0.746 |
| Platelet count
(x104/mm3), ≥13 vs. <13 | 65/57 | 0.372 | | |
| Prothrombin time
(%), ≥80 vs. <80 | 102/20 | 0.937 | | |
| Serum AFP (ng/ml),
≥100 vs. <100 | 48/74 | 0.013 | 0.481
(0.260–0.889) | 0.019 |
| DCP (mAU/ml), ≥100
vs. <100c | 73/46 | <0.001 | 0.560
(0.250–1.254) | 0.158 |
Subgroup analysis according to HCC
stage
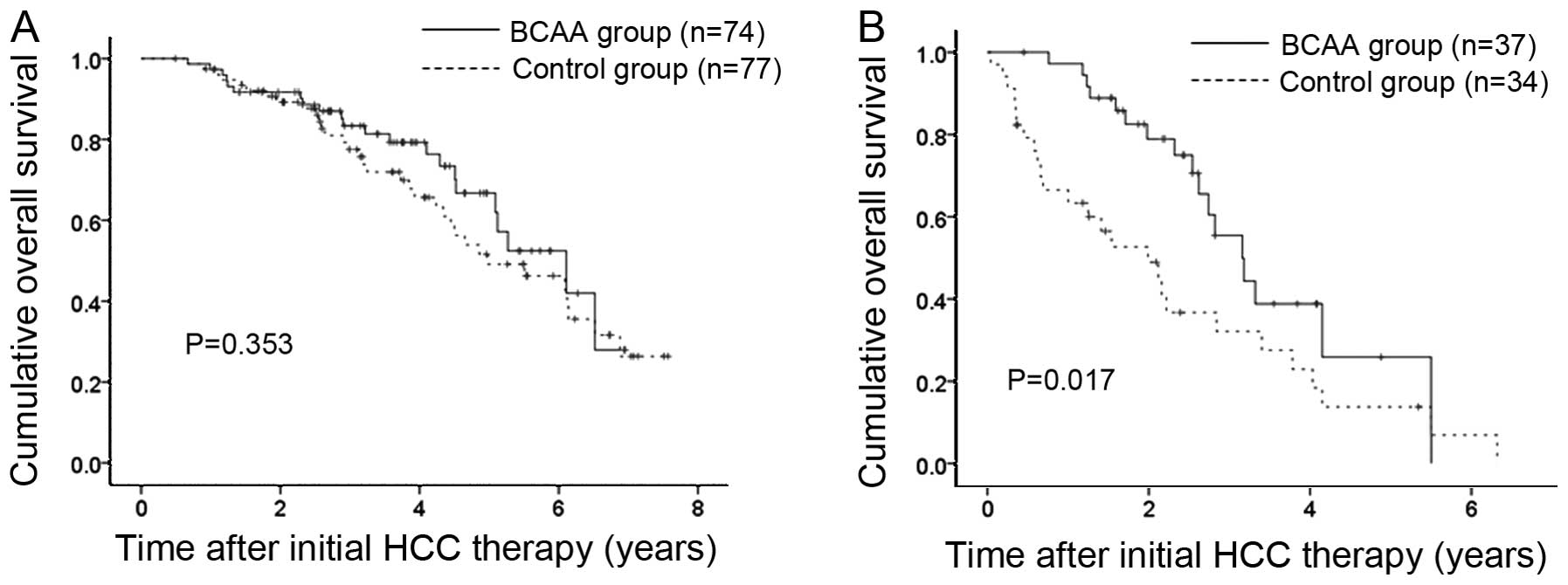
Since HCC stage was an independent predictor
associated with OS in both cohort 1 and 2, we further performed
subgroup analyses according to HCC stage. In cohort 1 patients,
there were 74 patients with HCC stage I or II in the BCAA group and
77 patients with HCC stage I or II in the control group. In terms
of OS, no significant difference was observed in the two groups
(P=0.353) (Fig. 4A). In cohort 1
patients, there were 37 patients with HCC stage III or IV in the
BCAA group and 34 patients with HCC stage III or IV in the control
group. In terms of OS, the difference in the two groups reached
significance (P=0.017) (Fig. 4B).
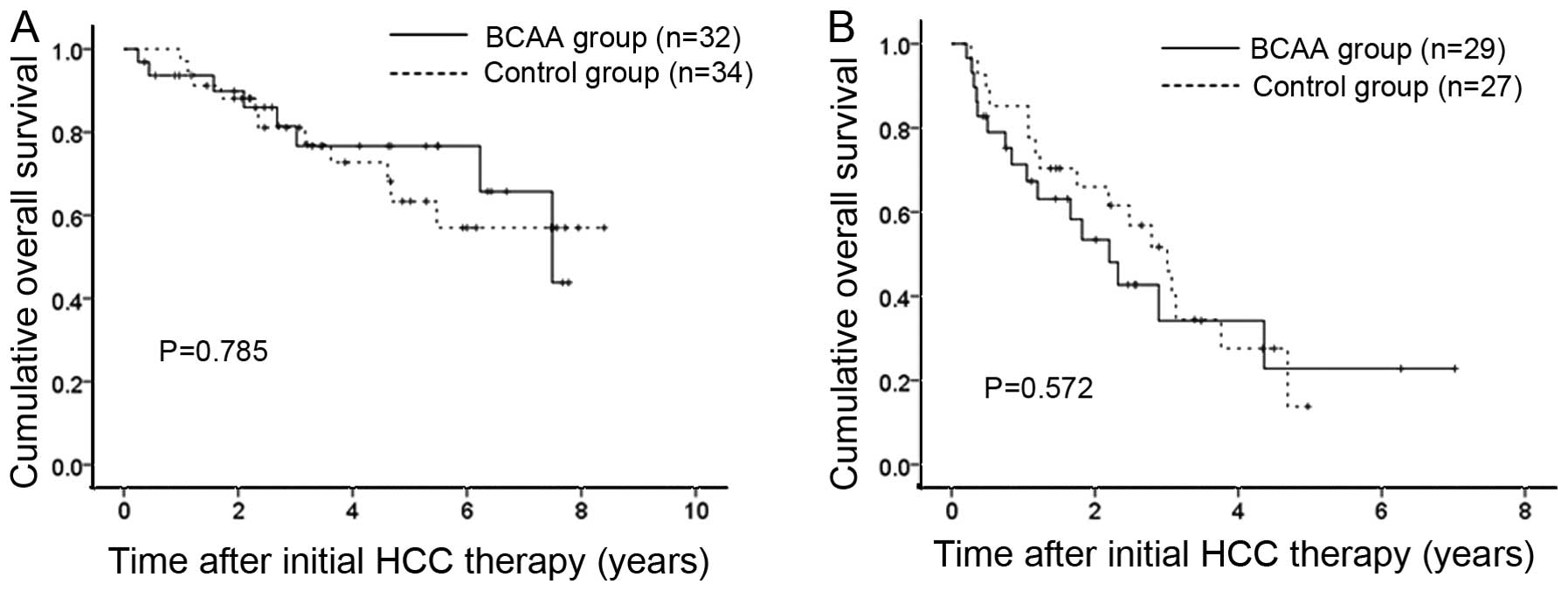
On the other hand, cohort 2 patients included 32 and 29 patients
with HCC stage I or II and III or IV in the BCAA group and 34 and
27 patients with HCC stage I or II and III or IV in the control
group. Regardless of HCC stage, no significant difference was found
in terms of OS (P=0.785 for HCC stage I or II and P=0.572 for HCC
stage III or IV) (Fig. 5).
Discussion
BCAA granules have a variety of pharmacological
effects. Kawaguchi et al (8,35)
and Kawaguchi and Sata (34)
showed that BCAA granules can improve albumin synthesis, insulin
resistance, immune function and patients’ QOL while they can reduce
liver related complications and occurrence of HCC. In addition,
BCAA supplementation can help in the management of HCC patients
since most HCC patients have underlying LC (24,27,36,37).
However, whether early interventional therapy using BCAA granules
in patients with HCC can improve survival remains unclear. An
interesting issue is when is the optimal timing of nutritional
support such as BCAA granules in patients with HCC. Hence, we
conducted these comparative studies.
In our results, in cohort 1 (patients with
pretreatment serum albumin level ≥3.6 and <4.0 g/dl), the OS
rate in the BCAA group tended to be higher compared to that in the
control group and in subgroup analysis in patients with HCC stage
III or IV, which means advanced stage of HCC, the OS rate in the
BCAA group was significantly higher compared to that in the control
group, although in other analyses, no significant difference in the
two groups was found. These results suggest that early
interventional therapy using BCAA granules can be a treatment
option for some selected patients.
In general, in patients with advanced stage HCC,
curative therapies are difficult to perform due to tumor
characteristics. Hence, repeated therapies for HCC will be needed
in these patients. However, these repeated therapies can lead to
deterioration of liver functional reserve as reflected by
hypoalbuminemia (24,27). BCAA supplementation actually
improves hypoalbuminemia. Moreover, Kawaguchi et al reported
that BCAA granules may suppress hepatic neovascularization and
hepatocarcinogenic activity and Yoshiji et al demonstrated
that BCAA therapy significantly suppressed glucose- and
insulin-induced angiogenesis in the presence of vascular
endothelial growth factor (VEGF) (8,22).
Angiogenesis is a key process in tumor growth and VEGF, which
stimulates angiogenesis, appears to be essential for HCC
progression (8,22). Our present results may be
associated with these observations. On the other hand, advanced
malignancy can result in muscle wasting and systemic catabolism,
with BCAA treatment having the potential to improve these poor
conditions (36).
As described above, in cohort 2 patients
(pretreatment serum albumin level ≥4.0 g/d), the difference in the
BCAA and control groups did not reach significance in the analyses
in terms of OS. Very early intervention using BCAA granules might
not be beneficial for improving OS. Curative treatment at the
initial therapy for HCC, close surveillance for HCC recurrence and
adequate therapy for recurrence will be more important than
nutritional therapy in these patients for prolonging OS.
This study included several limitations. First, this
is a retrospective observational study although propensity score
matching analyses were performed for reducing selection biases in
this study. Second, our patient cohorts included heterogeneous
patient populations with various stages of HCC and various causes
of underlying liver diseases. Third, BCAA treatment adherence in
each individual, antiviral therapies such as interferon therapy for
patients with HCV or nucleoside analogue therapy for those with
hepatitis B virus during observation period were not included in
the present analyses, leading to bias (38–40).
Hence, further studies with well selected patient population will
be needed. However, our study results demonstrated that early
interventional therapy using BCAA granules may be effective in some
selected patients.
We concluded that in HCC patients with pretreatment
serum albumin level ≥3.6g/dl, early BCAA supplementation can be a
treatment option for improving clinical outcome.
Acknowledgements
We would like to thank Haruko Takada for the data
collection.
References
|
1
|
El-Serag HB: Epidemiology of viral
hepatitis and hepatocellular carcinoma. Gastroenterology.
142:1264–1273. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lencioni: Loco-regional treatment of
hepatocellular carcinoma. Hepatology. 52:762–773. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Nishikawa H and Osaki Y: Non-B, non-C
hepatocellular carcinoma (Review). Int J Oncol. 43:1333–1342.
2013.PubMed/NCBI
|
|
4
|
Llovet JM: Update treatment approach to
hepatocellular carcinoma. J Gastoenterol. 40:225–235. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Llovet JM, Ricci S, Mazzaferro V, Hilgard
P, Gane E, Blanc JF, de Oliveira AC, Santoro A, Raoul JL, Forner A,
Schwartz M, Porta C, Zeuzem S, Bolondi L, Greten TF, Galle PR,
Seitz JF, Borbath I, Häussinger D, Giannaris T, Shan M, Moscovici
M, Voliotis D and Bruix J: SHARP Investigators Study Group:
Sorafenib in advanced hepatocellular carcinoma. N Engl J Med.
359:378–390. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
El-Serag HB: Hepatocellular carcinoma. N
Engl J Med. 365:1118–1127. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wo JY, Dawson LA, Zhu AX and Hong TS: An
emerging role for radiation therapy in the treatment of
hepatocellular carcinoma and intrahepatic cholangiocarcinoma. Surg
Oncol Clin North Am. 23:353–368. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kawaguchi T, Izumi N, Charlton MR and Sata
M: Branched-chain amino acids as pharmacological nutrients in
chronic liver disease. Hepatology. 54:1063–1070. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
No authors listed. Nutritional status in
cirrhosis. Italian Multicentre Cooperative Project on Nutrition in
Liver Cirrhosis. J Hepatol. 21:317–335. 1994.PubMed/NCBI
|
|
10
|
Kuroda H, Ushio A, Miyamoto Y, Sawara K,
Oikawa K, Kasai K, Endo R, Takikawa Y, Kato A and Suzuki K: Effects
of branched-chain amino acid-enriched nutrient for patients with
hepatocellular carcinoma following radiofrequency ablation: a
one-year prospective trial. J Gastroenterol Hepatol. 25:1550–1555.
2010.
|
|
11
|
Kasugai H, Osaki Y, Oka H, Kudo M and Seki
T: Osaka Liver Cancer Study Group: Severe complications of
radiofrequency ablation therapy for hepatocellular carcinoma: an
analysis of 3,891 ablations in 2,614 patients. Oncology. 72(Suppl
1): 72–75. 2007. View Article : Google Scholar
|
|
12
|
Lautz HU, Selberg O, Körber J, Bürger M
and Müller MJ: Protein-calorie malnutrition in liver cirrhosis.
Clin Investig. 70:478–486. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kato M, Miwa Y, Tajika M, Hiraoka T, Muto
Y and Moriwaki H: Preferential use of branched-chain amino acids as
an energy substrate in patients with liver cirrhosis. Intern Med.
37:429–434. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kajiwara K, Okuno M, Kobayashi T, Honma N,
Maki T, Kato M, Ohnishi H, Muto Y and Moriwaki H: Oral
supplementation with branched chain amino acids improves survival
rate of rats with carbon tetrachloride-induced liver cirrhosis. Dig
Dis Sci. 43:1572–1579. 1998. View Article : Google Scholar
|
|
15
|
Yosida T, Muto Y, Moriwaki H and Yamato M:
Effect of long-term oral supplementation with branched chain amino
acid granules on the prognosis of liver cirrhosis. J Gastroenterol.
24:692–698. 1989.PubMed/NCBI
|
|
16
|
Marchesini G, Dioguardi FS, Bianchi GP,
Zoli M, Bellati G, Roffi L, Martines D and Abbiati R: Long-term
oral branched-chain amino acid treatment in chronic hepatic
encephalopathy: a randomized double blind casein-controlled trial.
J Hepatol. 11:92–101. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Marchesini G, Bianchi G, Merli M, Amodio
P, Panella C, Loguercio C, Rossi Fanelli F and Abbiati R: Italian
BCAA Study Group: Nutritional supplementation with branched chain
amino acids in advanced cirrhosis: a double-blind, randomized
trial. Gastroenterology. 124:1792–1801. 2003. View Article : Google Scholar
|
|
18
|
Keshavarzian A, Meek J, Sutton C, Emery
VM, Hughes EA and Hodgson HJ: Dietary protein supplementation from
vegetable sources in the management of chronic portal systemic
encephalopathy. Am J Gastroenterol. 79:945–949. 1984.
|
|
19
|
Kumada H, Okanoue T, Onji M, Moriwaki H,
Izumi N, Tanaka E, Chayama K, Sakisaka S, Takehara T, Oketani M,
Suzuki F, Toyota J, Nomura H, Yoshioka K, Seike M, Yotsuyanagi H
and Ueno Y: Study Group for the Standardization of Treatment of
Viral Hepatitis Including Cirrhosis, Ministry of Health, Labour and
Welfare of Japan: Guidelines for the treatment of chronic hepatitis
and cirrhosis due to hepatitis C virus infection for the fiscal
year 2008 in Japan. Hepatol Res. 40:8–13. 2010.
|
|
20
|
Saito Y, Saito H, Nakamura M, Wakabayashi
K, Takagi T, Ebinuma H and Ishii H: Effect of the molar ratio of
branched-chain to aromatic amino acids on growth and albumin mRNA
expression of human liver cancer cell lines in a serum-free medium.
Nutr Cancer. 39:126–131. 2001. View Article : Google Scholar
|
|
21
|
Muto Y, Sato S, Watanabe A, Moriwaki H,
Suzuki K, Kato A, Kato M, Nakamura T, Higuchi K, Nishiguchi S,
Kumada H and Ohashi Y: Long-Term Survival Study (LOTUS) Group:
Overweight and obesity increase the risk for liver cancer in
patients with liver cirrhosis and long-term oral supplementation
with branched-chain amino acid granules inhibits liver
carcinogenesis in heavier patients with liver cirrhosis. Hepatol
Res. 35:204–214. 2006.
|
|
22
|
Yoshiji H, Noguchi R, Kitade M, Kaji K,
Ikenaka Y, Namisaki T, Yoshii J, Yanase K, Yamazaki M, Tsujimoto T,
Akahane T, Kawaratani H, Uemura M and Fukui H: Branched-chain amino
acids suppress insulin-resistance-based hepatocarcinogenesis in
obese diabetic rats. J Gastroenterol. 44:483–491. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sugiyama K, Yu L and Nagasue N: Direct
effect of branched-chain amino acids on the growth and metabolism
of cultured human hepatocellular carcinoma cells. Nutr Cancer.
31:62–68. 1998. View Article : Google Scholar
|
|
24
|
Nishikawa H, Osaki Y, Iguchi E, Koshikawa
Y, Ako S, Inuzuka T, Takeda H, Nakajima J, Matsuda F, Sakamoto A,
Henmi S, Hatamaru K, Ishikawa T, Saito S, Nasu A, Kita R and Kimura
T: The effect of long-term supplementation with branched-chain
amino acid granules in patients with hepatitis C virus-related
hepatocellular carcinoma after radiofrequency thermal ablation. J
Clin Gastroenterol. 47:359–366. 2013. View Article : Google Scholar
|
|
25
|
Nishiguchi S and Habu D: Effect of oral
supplementation with branched-chain amino acid granules in the
early stage of cirrhosis. Hepatol Res. 30S:36–41. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Habu D, Nishiguchi S, Nakatani S, Kawamura
E, Lee C, Enomoto M, Tamori A, Takeda T, Tanaka T and Shiomi S:
Effect of oral supplementation with branched-chain amino acid
granules on serum albumin level in the early stage of cirrhosis: a
randomized pilot trial. Hepatol Res. 25:312–318. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Nishikawa H, Osaki Y, Inuzuka T, Takeda H,
Nakajima J, Matsuda F, Henmi S, Sakamoto A, Ishikawa T, Saito S,
Kita R and Kimura T: Branched-chain amino acid treatment before
transcatheter arterial chemoembolization for hepatocellular
carcinoma. World J Gastroenterol. 18:1379–1384. 2012. View Article : Google Scholar
|
|
28
|
Bruix J and Sherman M: Practice Guidelines
Committee, American Association for the Study of Liver Diseases:
Management of hepatocellular carcinoma. Hepatology. 42:1208–1236.
2005. View Article : Google Scholar
|
|
29
|
Yamasaki T, Kurokawa F, Shirahashi H,
Kusano N, Hironaka K and Okita K: Percutaneous radiofrequency
ablation therapy with combined angiography and computed tomography
assistance for patients with hepatocellular carcinoma. Cancer.
91:1342–1348. 2001. View Article : Google Scholar
|
|
30
|
Park JW, Amarapurkar D, Chao Y, Chen PJ,
Geschwind JF, Goh KL, Han KH, Kudo M, Lee HC, Lee RC, Lesmana LA,
Lim HY, Paik SW, Poon RT, Tan CK, Tanwandee T, Teng G and Cheng AL:
Consensus recommendations and review by an International Expert
Panel on Interventions in Hepatocellular Carcinoma (EPOIHCC). Liver
Int. 33:327–337. 2013. View Article : Google Scholar
|
|
31
|
Kudo M, Izumi N, Kokudo N, Matsui O,
Sakamoto M, Nakashima O, Kojiro M and Makuuchi M: HCC Expert Panel
of Japan Society of Hepatology. Management of hepatocellular
carcinoma in Japan: Consensus-Based Clinical Practice Guidelines
proposed by the Japan Society of Hepatology (JSH) 2010 updated
version. Dig Dis. 29:339–364. 2011. View Article : Google Scholar
|
|
32
|
Austin PC: A comparison of 12 algorithms
for matching on the propensity score. Stat Med. 33:1057–1069. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
D’Agostino RB Jr: Propensity score methods
for bias reduction in the comparison of a treatment to a
non-randomized control group. Stat Med. 17:2265–2281. 1998.
|
|
34
|
Kawaguchi T and Sata M: Importance of
hepatitis C virus-associated insulin resistance: therapeutic
strategies for insulin sensitization. World J Gastroenterol.
16:1943–1952. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kawaguchi T, Yamagishi S and Sata M:
Branched-chain amino acids and pigment epithelium-derived factor:
novel therapeutic agents for hepatis c virus-associated insulin
resistance. Curr Med Chem. 16:4843–4857. 2009. View Article : Google Scholar
|
|
36
|
Choudry HA, Pan M, Karinch AM and Souba
WW: Branched-chain amino acid-enriched nutritional support in
surgical and cancer patients. J Nutr. 136(Suppl 1): S314–S318.
2006.PubMed/NCBI
|
|
37
|
Ichikawa K, Okabayashi T, Maeda H,
Namikawa T, Iiyama T, Sugimoto T, Kobayashi M, Mimura T and
Hanazaki K: Oral supplementation of branched-chain amino acids
reduces early recurrence after hepatic resection in patients with
hepatocellular carcinoma: a prospective study. Surg Today.
43:720–726. 2013. View Article : Google Scholar
|
|
38
|
Takaguchi K, Moriwaki H, Doyama H, Iida M,
Yagura M, Shimada N, Kang M, Yamada H and Kumada H: Effects of
branched-chain amino acid granules on serum albumin level and
prognosis are dependent on treatment adherence in patients with
liver cirrhosis. Hepatol Res. 43:459–466. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Mazzaferro V, Romito R, Schiavo M, Mariani
L, Camerini T, Bhoori S, Capussotti L, Calise F, Pellicci R, Belli
G, Tagger A, Colombo M, Bonino F, Majno P and Llovet JM: HCC
Italian Task Force: Prevention of hepatocellular carcinoma
recurrence with alpha-interferon after liver resection in HCV
cirrhosis. Hepatology. 44:1543–1554. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Nishikawa H, Nishijima N, Arimoto A,
Inuzuka T, Kita R, Kimura T and Osaki Y: Effect of nucleoside
analog use in patients with hepatitis B virus-related
hepatocellular carcinoma. Hepatol Res. May 24–2013.(Epub ahead of
print). View Article : Google Scholar
|