Introduction
Cancer of colon and rectum (colorectal cancer, CRC)
is a malignant neoplasm arising from the lining of the large
intestine. Patients with inflammatory bowel disease (IBD),
including both ulcerative colitis (UC) and Crohn’s disease (CD),
are at increased risk of developing CRC (1). CRC is a worldwide health-care problem
with a continually increasing incidence. In Asia, IBD was
considered rare until two decades ago (2), but recent population-based and
referral center cohorts have shown a rising incidence and
prevalence of IBD in Asia (3).
Importantly, the incidence and prevalence of CD and UC in Korea are
still lower than those in Western countries, but are rapidly
increasing (4). IBD can occur in
combination of defected immune response, luminal, environment and
genetic factors including tumor necrosis factor (TNF)-α. The
evaluated level of TNF-α was especially found in the blood,
intestinal mucosa and stools of patients with IBD. In addition to
TNF-α, the increases of other pro-inflammatory mediators have been
observed in stools and rectal dialysates from patients with IBD as
well (5). The existing data
revealed that TNF-α inhibitors are increasingly being used in
IBD-related studies and patients. The most commonly used biologics
in IBD are TNF-α antibodies, such as infliximab, a chimeric IgG1
monoclonal antibody; adalimumab, a human monoclonal IgG1 antibody;
and certolizumab pegol, a pegylated Fab fragment of a humanized
IgG4 isotype monoclonal antibody (6). Even though these TNF inhibitors may
increase the risk of tuberculosis, varicella and other
opportunistic infections, there is little evidence suggesting that
anti-TNF agents specifically raise the overall risk of serious
infections. Similarly, there is little evidence that TNF
antagonists raise the risk of developing malignancy over and above
the risks from concomitant therapies and the underlying disease
process (7). Therefore, more
studies are needed on the use of TNF inhibitors in patients with
IBD.
Betaine is an essential biochemical molecule of the
methionine/homeocysteine cycle and is synthesized by conversion of
choline. It was first discovered in the juice of sugar beets
(Beta vulgaris) in the 19th century (8), and since then has been found in
various microorganisms, plants and animals (9,10).
It plays central roles in choline-mediated one-carbon metabolism,
structural integrity and signaling functions of cell membranes, and
neurotransmitter synthesis (11).
Previous studies showed dietary choline and betaine intakes and
associations with inflammatory markers in healthy free-eating
adults enrolled in the ATTICA study. Moreover, betaine and choline
may be involved in reducing inflammation, including their important
role as a source of one-carbon units for the metabolism of
homocysteine. In highest tertile for dietary intake of choline and
betaine had significantly lower plasma C-reactive protein,
interleukin (IL)-6, and TNF-α concentration than did persons in the
lowest tertile of intake (12).
Previous studies have shown that betaine has
anti-inflammatory activity through inhibition of reactive species
(RS) and modulation of reduced glutathione (GSH)/oxidized
glutathione (GSSG) ratio in aging process both in vitro and
in vivo studies (13–15).
However, the protective role of betaine on the expression and
regulation of inflammatory mediators associated with colon cancer
has not explored yet. Hence, in the present study we aimed to
evaluate the anti-inflammatory effects of betaine on
AOM/DSS-induced colitis-associated colon cancer in mice. We
demonstrate that betaine is a potent anti-inflammatory agent that
may act through the inactivation of inflammatory cytokines.
Materials and methods
Chemicals
Betaine was purchased from Sigma-Aldrich Co. (St.
Louis, MO, USA). Betaine was freshly prepared before each
experiment and was solubilized with phosphate buffered saline
(PBS).
Animal study
The animal protocol used in this study has been
reviewed by the Pusan National University-Institutional Animal Care
and Use Committee (PNU-IACUC, Busan, Korea) on their ethical
procedures and scientific care, and it has been approved
(PNU-2013-0318) as previously described (16). Five-week-old male ICR mice were
purchased from Samtako Co., Ltd. (Osan, Korea). All animals were
housed in plastic cages (4 mice/cage) and had free access to
drinking water and a basal diet (Formula M07; Feed Lab, Guri,
Korea) ad libitum, under controlled conditions of humidity
(50±10%), light (12/12 h light/dark cycle), and temperature
(23±1°C). After arrival, the animals were quarantined for 7 days,
and then randomized by body weights into experimental and control
groups. A colonic carcinogen AOM was purchased from Sigma-Aldrich.
DSS with a molecular weight of 36,000–50,000 (cat. no. 160110) was
purchased from MP Biomedicals, LLC (Aurora, OH, USA). The
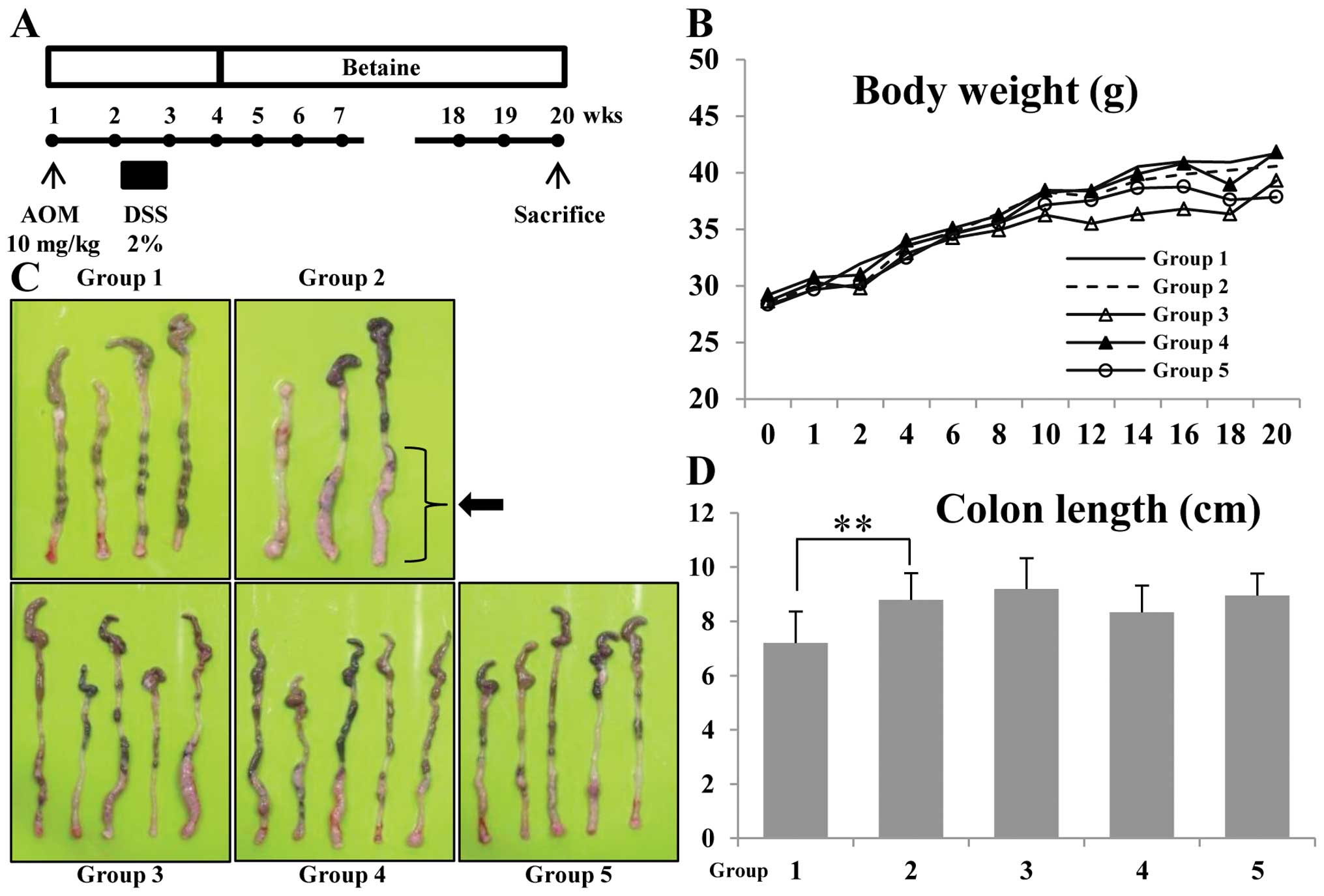
experimental protocol is shown in Fig.
1A. Animals were divided into four experimental groups and
control group (n=15 per group). Group 1 was the control. Animals in
group 2 through 5 were given a single intraperitoneal injection of
AOM (10 mg/kg body weight). Seven days after the AOM injection,
animals received 2% DSS (w/v) in the drinking water for 7 days.
Seven days after the DSS treatment, betaine-containing diets were
started. Subsequently, groups 3 through 5 received the diets
containing 1, 5 and 10 mg/kg betaine for 16 weeks, respectively.
All animals were sacrificed at week 16 after administration of
betaine. At sacrifice, a complete necropsy was performed on all
mice. Histopathological examination was performed on
paraffin-embedded sections after hematoxylin and eosin (H&E)
staining.
GSH assay
To analyze the total glutathione level, using
OxiSelect™ Total Glutathione (GSSG/GSH) Assay kit (Cell Biolabs,
Inc., San Diego, CA, USA) according to company protocol. GSH
reductase solution (25 μl), was added to each well in a 96-well
plate and 25 μl of the NADPH was added onto it; 100 μl of the
prepared GSH standards or samples was added to each well and mixed
thoroughly. Then 50 μl of the chromogen was added and the
absorbance was read at 405 nm by using a multi-well reader (Thermo
Fisher Scientific, Vantaa, Finland).
Assessment of reactive species (RS)
generation
RS generation was measured as previously described
using a fluorescence probe (17).
Briefly, 2′,7′-dichlorofluorescin diacetate (DCF-DA; final
concentration 2.5 μM) was added to homogenates and the changes in
fluorescence intensity were measured every 5 min for 30 min using a
fluorescence plate reader (GENios, Tecan Instruments, Salzburg,
Austria) at excitation and emission wavelengths of 485 and 530 nm,
respectively.
Total RNA extraction and quantitative
real-time PCR
Total RNA was extracted from colonic mucosa using
the TRIzol (Qiagen, Tokyo, Japan) according to the manufacturer’s
protocol. The cDNA was then synthesized from total RNA using
TOPscript™ RT DryMix (Enzynomics, Daejeon, Korea). A quantitative
real-time PCR (qPCR) analysis of individual cDNA was performed with
Takara TP800 instrument (Takara Bio Inc., Shiga, Japan) using
SYBR-Green gene expression assay (Enzynomics). The primers used for
each reverse transcription-polymerase chain reaction reactions are
as follows: GAPDH, 5′-aactttggcattgtggaagg-3′ and
5′-acacattgggggtaggaaca-3′; TNF-α, 5′-cgtcagccgatttgctatct-3′ and
5′-cggactccgcaaagtctaag-3′; inducible nitric oxide synthase (iNOS),
5′-ctcactgggacagcacagaa-3′ and 5′-gcttgtctctgggtcct ctg-3′;
cyclooxygenase (COX)-2; 5′-gctgtacaagcagtggcaaa-3′ and
5′-ccccaaagatagcatctgga-3′; IL-6, 5′-agttgccttcttgggactga-3′ and
5′-ttctgcaagtgcatcatcgt-3′ (forward and reverse, respectively). The
PCR cycling conditions were 95°C for 15 min, followed by 40 cycles
of 95°C for 15 sec and 54°C for 10 sec and 72°C for 20 sec. The
expression level of each gene was normalized to the GAPDH
expression level. Each assay was performed in triplicate and the
average was calculated.
Cell culture and cell viability
assay
The murine macrophage RAW 264.7 cells were obtained
from American Type Culture Collection (Manassas, VA, USA) and
cultured in DMEM (HyClone, Logan, UT, USA) supplemented with 10%
fetal bovine serum (FBS, HyClone), 100 U/ml penicillin and 100
μg/ml streptomycin (HyClone) at 37°C in a humidified 5% CO. Cell
viability was determined by MTT assay. For the MTT assay, RAW 264.7
cells were seeded in a 24-well culture plate at a density of
4×104 cells/well, cultured for 24 h in the growth media
and then treated with or without betaine for the indicated
concentrations. The cells were incubated with 0.5 mg/ml MTT
[3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide]
(Sigma-Aldrich) at 37°C for 2 h. The formazan granules generated by
the live cells were dissolved in DMSO and the absorbance at 540 nm
was monitored by using a multi-well reader (Thermo Fisher
Scientific).
Western blot analysis
The cells were treated under the appropriate
conditions, harvested and washed with cold PBS and were lysed in
lysis buffer [40 mM Tris (pH 8.0), 120 mM NaCl, 0.5% NP-40, 0.1 mM
sodium orthovanadate, 2 μg/ml aprotinin, 2 μg/ml leupeptin and 100
μg/ml phenymethylsulfonyl fluoride (PMSF)]. The supernatant was
collected and protein concentrations were measured (Bio-Rad,
Hercules, CA, USA). Protein extracts were denatured by boiling at
100°C for 5 min in sample buffer [0.5 M Tris-HCl (pH 6.8), 4% SDS,
20% glycerol, 0.1% bromophenol blue, 10% β-mercaptoethanol]. Equal
amount of the total proteins were subjected to 6–15% SDS-PAGE and
transferred to PVDF. The membranes were blocked with 5% non-fat dry
milk in Tris-buffered saline with Tween-20 buffer (TBS-T) (20 mM
Tris, 100 mM NaCl, pH 7.5 and 0.1% Tween-20) for 1 h at room
temperature. Then, the membranes were incubated overnight at 4°C
with the primary antibodies. The membranes were washed 4 times for
10 min with TBS-T buffer and then incubated for 1 h with
horseradish peroxidase-conjugated anti-rabbit or anti-mouse
immunoglobin (Santa Cruz Biotechnology Inc., Santa Cruz, CA, USA).
The membranes were washed again for 10 min with TBS-T buffer.
Antigen-antibody complexes were detected by the enhanced
chemiluminescence (ECL) detection system (GE Healthcare
Biosciences, Pittsburgh, PA, USA).
Statistical analysis
Measurements of cell viability of RAW 264.7 cells
and multiplicity of colonic lesions were analyzed using Student’s
t-test. The statistical analysis of mRNA expression was performed
by the Kruskal-Wallis test. P-value of <0.05 was considered to
be statistically significant.
Results
Betaine inhibits AOM/DSS-induced
colitis-associated tumorigenesis
The AOM/DSS model is a widely used
inflammation-associated colon cancer model in rodents (18–22).
In the present study, antitumor effect of dietary administration of
betaine was evaluated in AOM/DSS-induced tumorigenesis model. The
study protocol is summarized in Fig.
1A. During the experiments, feeding the mice with the different
doses of betaine did not produce any observable clinical toxicity
or significant changes in body weight compared to control (Fig. 1B). There were no relative changes
in colon length between AOM/DSS-induced tumorigenesis groups and
betaine-containing diets groups (Fig.
1C and D). Although slight changes were found in colon length,
other positive effects such as tumor incidence and inflammatory
cytokines were observed on betaine-treated groups with
AOM/DSS-induced tumorigenesis.
Macroscopically, the AOM/DSS model resulted in 100%
incidence of colonic tumors, which were most frequently observed in
the middle and distal colon (Fig.
1C, group 2, arrow). The colonic tumors developed in the mice
of groups 2 through 5 with different incidence rate and
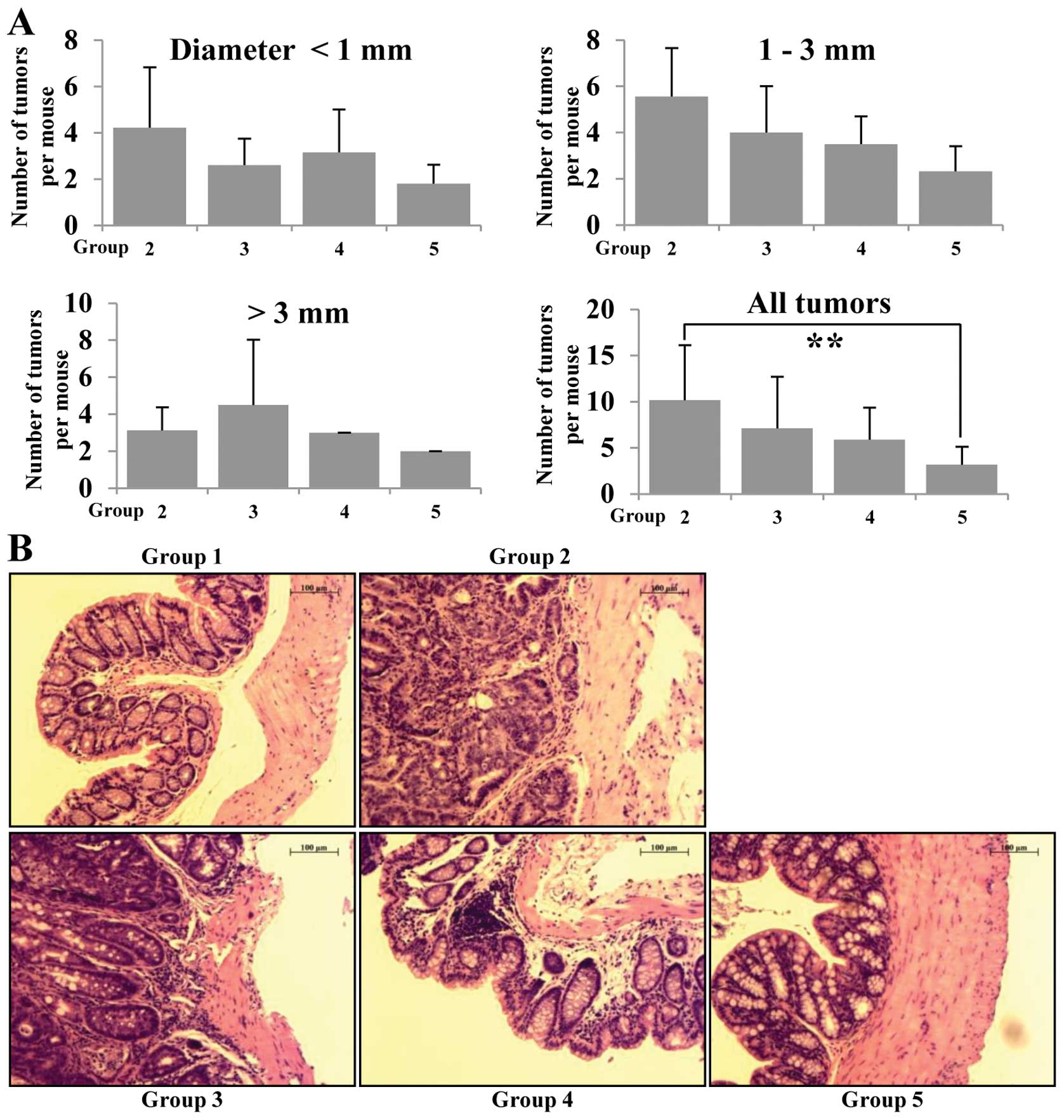
multiplicity (Fig. 2A). AOM/DSS
group (group 2) had mainly adenocarcinoma (ADC) with a multiplicity
of 9.67±5.03. The incidence of ADC in betaine fed (groups 3–5) was
less than that of group 2 and the multiplicity of ADC in groups 3,
4 and 5 is 7.03±2.69, 5.25±2.24 and 3.48±1.41, respectively. The
multiplicity of colonic ADC in groups 3, 4 and 5 was significantly
smaller than group 2 (p<0.01) (Fig.
2A). In H&E staining, group 2 (AOM/DSS group) animals
showed increased high grade dysplasia, colonic adenoma and tissue
inflammation, but administration of betaine resulted in reduction
of all of these phenomena in groups 3–5 compared to group 2
dose-dependently (Fig. 2B).
Betaine suppresses inflammatory
mediators
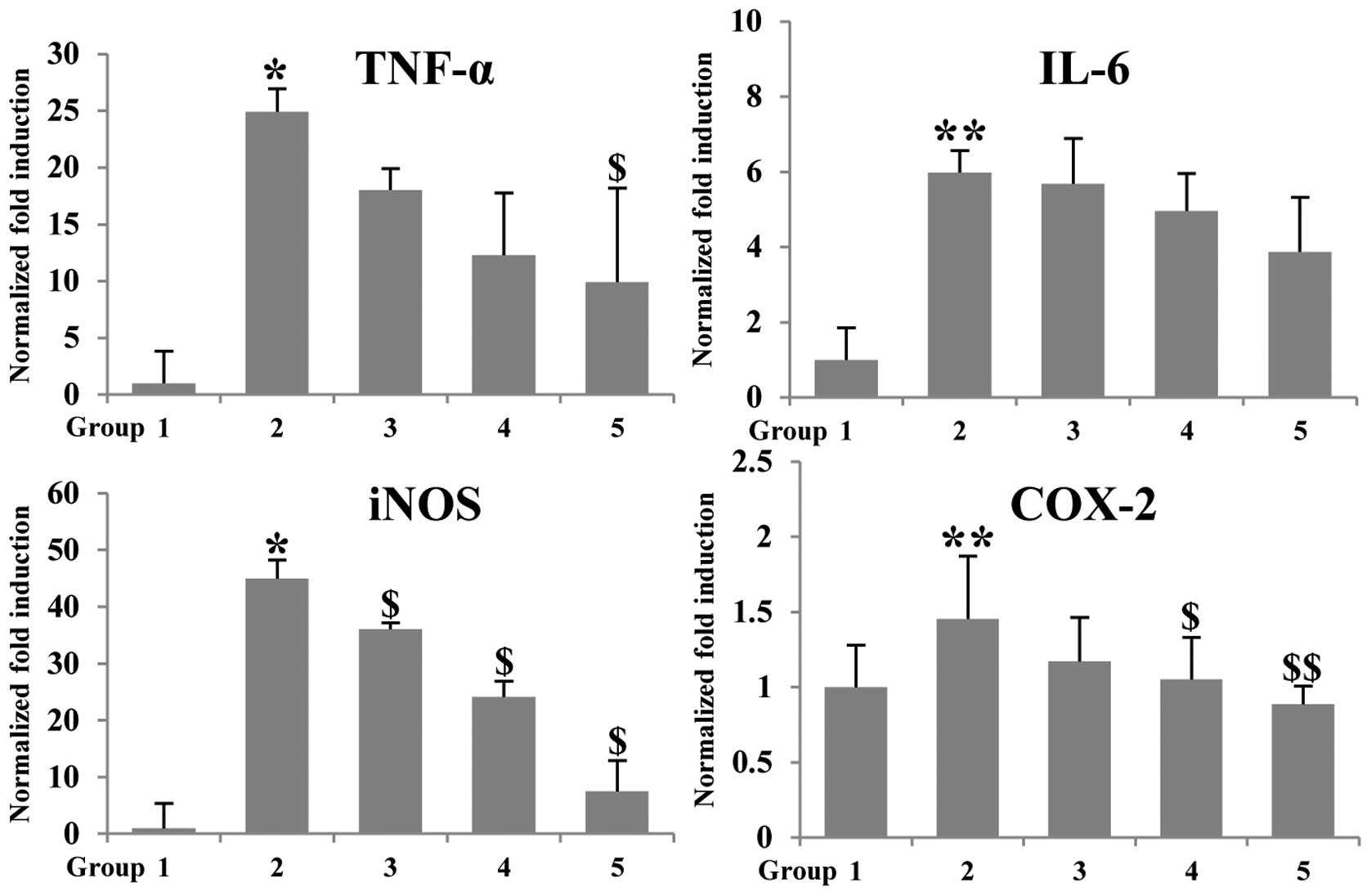
Expression of inflammation-associated genes was
further confirmed in colonic mucosa by using real-time PCR. AOM/DSS
upregulated the mRNA expression of pro-inflammatory genes such as
TNF-α, IL-6, iNOS and COX-2, compared with group 1 (control group).
In contrast, when betaine was given to mice, mRNA expression of
TNF-α, IL-6, iNOS and COX-2 was decreased when compared with the
group 2 (AOM/DSS group) (Fig. 3).
The expression level of TNF-α and iNOS was decreased 2.5- and
5.25-fold, respectively, compared to group 2. These results suggest
that administration of betaine could reduce inflammation, which may
contribute, at least in part, the anticancer effect in
AOM/DSS-induced colon tumorigenesis.
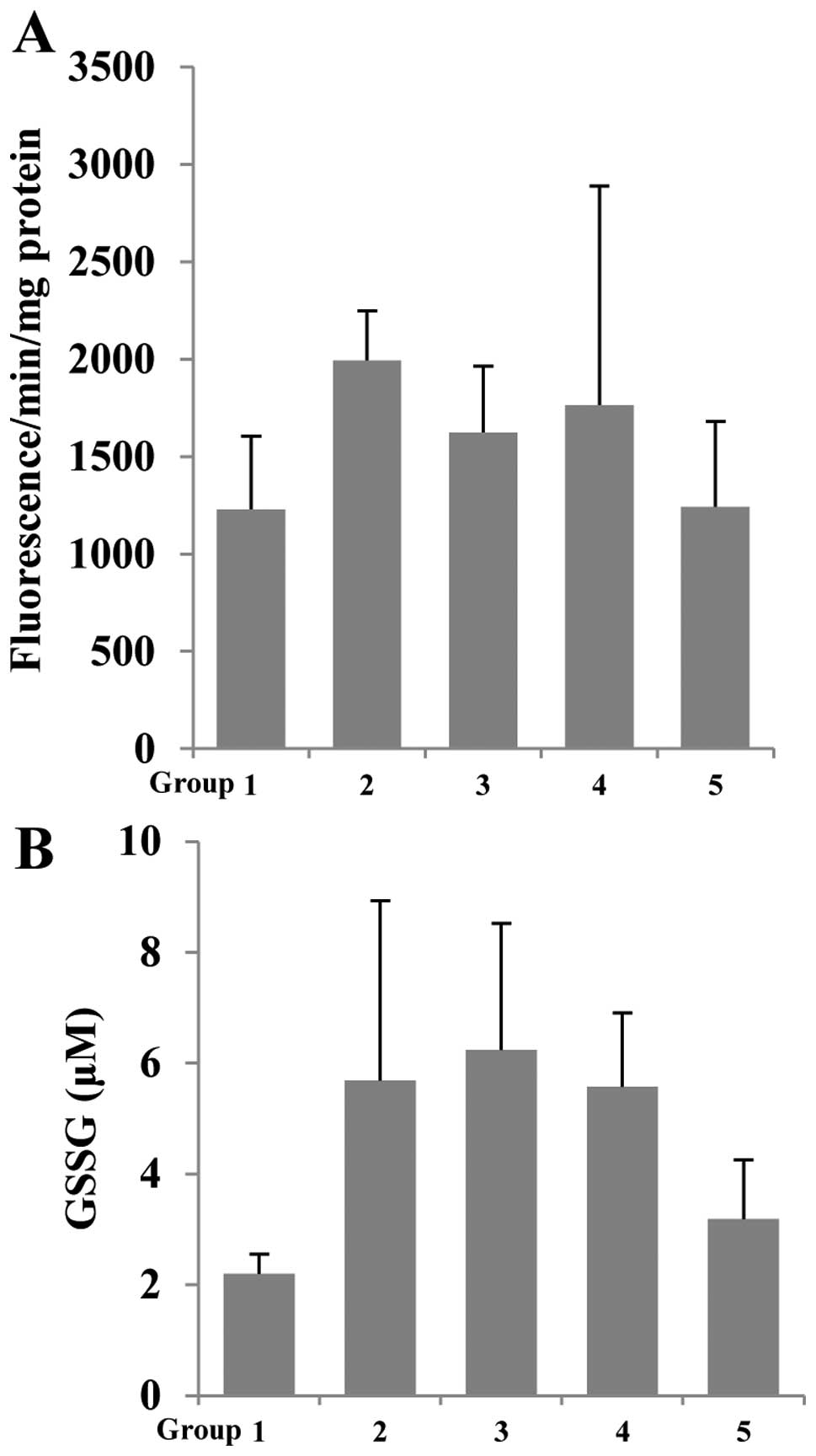
Betaine inhibits RS generation and GSSG
concentration
Previous studies have shown that betaine suppressed
aging-related inflammatory status through inhibition of RS
generation and modulation of glutathione (13–15).
Thus, we determined the effect of betaine on AOM/DSS-induced RS
generation in colonic mucosa using DCF-DA, which is oxidized by RS
to fluorescence DCF. As shown in Fig.
4A, the RS formation increased due to AOM/DSS group (group 2).
In contrast, betaine-treated groups (groups 3–5) showed decreased
RS generation in colonic mucosa. Glutathione is a key intracellular
thiol composed of glutamic acid, cysteine, and glycine. Glutathione
protects cells from free radical damage by acting as an
antioxidant. Within cells, glutathione exists in reduced (GSH) and
oxidized (GSSG) states. In healthy cell and tissue, more than 90%
of the total glutathione pool is in the reduced form (GSH) while
less than 10% exists in the disulfide form (GSSG). Thus, GSH
depletion can cause redox imbalance through increased oxidative
stress (23). To confirm whether
betaine can modulate the glutathione levels, we measured GSSG
concentration in the homogenate from colonic mucosa. AOM/DSS (group
2) was increased in GSSG concentration compared to group 1 (control
group). However, betaine-treated groups (groups 3–5) showed
decreased GSSG concentration compared to group 2 (Fig. 4B). Taken together, in part, betaine
can overcome oxidative stress including AOM/DSS-induced
tumorigenesis through modulation of RS and glutathione levels.
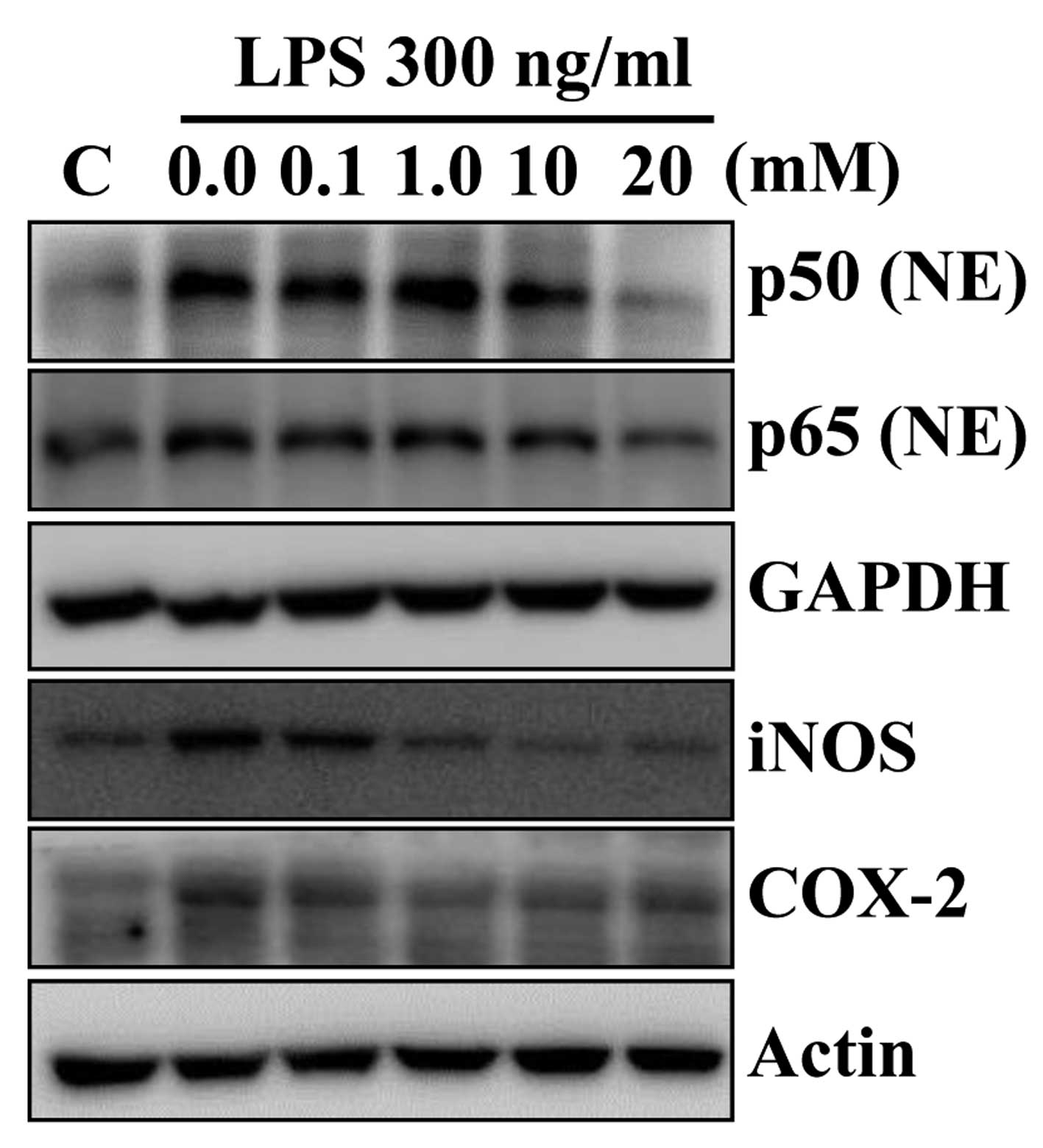
Betaine inhibits LPS-induced
pro-inflammatory gene expression in RAW 264.7 cells
After evaluation of anti-inflammatory activity of
betaine in the AOM/DSS-induced mouse colon cancer model, we
examined its effect on expression of pro-inflammatory genes in
macrophages upon stimulation with lipopolysaccharide (LPS), which
is one of the most potent pro-inflammatory agonists for monocytes
and macrophages. Betaine pretreatment down-regulated LPS-induced
expression of p50, p65, iNOS and COX-2 protein levels in murine
macrophage RAW 264.7 cells (Fig.
5). No cytotoxicity was observed under the experimental
condition (data not shown). These data suggested that betaine may
regulate the macrophage functions in AOM/DSS-induced mouse colon
cancer, thereby decreasing pro-inflammatory cytokine
productions.
Discussion
Inflammation is an important tumor promoter, and
several cytokines including TNF-α, IL-6, induced by inflammation
can promote tumor growth (18,24).
Many proto-oncogenes and carcinogens cause activation of NF-κB,
whereas chemicals with known chemopreventive properties can
suppress NF-κB activation (25).
Previous studies showed treatment with betaine inhibited NF-κB
activation through modulation of ROS and thiol homeostasis during
aging process in vitro and in vivo (13–15).
In this study, we demonstrated that three doses of betaine
administration (1, 5, and 10 mg/kg in diet) inhibited
colitis-associated colon tumorigenesis in ICR male mice.
Choline and its oxidation product betaine are
nutrients involved in one-carbon metabolism (26). Betaine is a nutrient abundant in
animal foods, especially seafoods, and plant foods including wheat
bran and spinach (27). Betaine
can donate a methyl group to homocysteine to methionine. It serves
as an osmolyte that regulates cell volume and protect cells and
protein from environmental stresses including ionic stress
(28). Methylation of homocyteine
by betaine is confined to the liver and the kidney, but the pathway
involving folate exists in all body cells (29). Betaine has been reported as a
nutrient preventing inflammatory processes by blocking the
expression of pro-inflammatory genes as a consequence of
suppressing the NF-κB activation in aging process (13). It also suppresses the production of
RS and modulation of GSH levels (14). So far, there are limited studies on
intake of choline and betaine and cancer risk in humans, because
food composition data were not available until recently (30). Several epidemiologic studies have
examined the association between dietary intake of choline and
betaine and cancer risk. Especially, higher betaine intake, may be
protective against lung cancer through mitigating the adverse
effect of smoking (31). In the
current study, we found that administration of betaine inhibited
tumor incidence with inflammation in AOM/DSS-induced colon
tumorigenesis in ICR male mice (Fig.
2A). During the experiments, betaine-containing diets did not
show any cytotoxicity regarding body weight or food intake.
Unfortunately, we did not observe difference of colon length
compared to AOM/DSS group (group 2) and betaine-treated groups
(groups 3–5). However, other factors including gene expression of
pro-inflammatory mediators, oxidative stress status (e.g., RS
generation and GSSG concentration), and H&E staining results,
were affected by betaine treatment. The qPCR data showed that
AOM/DSS-induced inflammatory cytokines including TNF-α, IL-6, COX-2
and iNOS were inhibited by betaine treatment in colonic mucosa
(Fig. 3). Furthermore, H&E
staining data showed administration of betaine (groups 3–5)
decreased AOM/DSS-induced inflammatory-related damage in colonic
mucosa compared to group 2 (Fig.
2B).
Redox homeostasis plays a critical role in the
protection of cells from both internal and external oxidative and
other forms of stress, and it maintains the regulatory role of
redox-sensitive transcription factors including NF-κB (32,33).
A previous study showed ROS to play an important role in cancer
development, both in the initiation and promotion stages of
carcinogenesis (34). In the
multi-step process of colon carcinogenesis, ROS were also found to
enhance colon carcinogenesis at all stages (35). It has been reported that
carcinogenic metals, such as As(III) and/or Cr(VI), in drinking
water promoted tumorigenesis in murine AOM/DSS colitis-associated
colorectal cancer model through modulation of the redox status.
Importantly, ROS-mediated β-catenin activation by carcinogenic
metals, As(III) and/or Cr(VI), may play an important role in this
promotion effect (36). Betaine
has been reported to prevent lysophosphatidylcholine-triggered RS
generation and NF-κB activation in endothelial cells (15). In addition, a previous study
reported that dietary betaine supplementation was capable of
restoring the redox balance by maintaining thiol homeostasis,
thereby suppressing pro-inflammatory NF-κB activation during aging
(14). Betaine-treated groups
showed inhibition of AOM/DSS-induced RS generation and GSSG levels
in colonic mucosa (Fig. 4).
Therefore, in part, betaine can reduce oxidative stress by
modulation of GSSG levels in various types of stress.
LPS is an endotoxin released by gram-negative
bacteria that can be transferred to cluster of differentiation 14
by LPS-binding protein and recognized by Toll-like receptor 4 on
the cellular surface of macrophages (37). LPS triggers the translocation of
NF-κB lead to the expression of NF-κB-regulated genes including
TNF-α, IL-6, COX-2 and iNOS in murine macrophage RAW 264.7 cells
(38). Our results demonstrated
that betaine treatment inhibited the LPS-induced TNF-α, IL-6, COX-2
and iNOS in RAW 264.7 cells (Fig.
5).
In conclusion, administration with betaine
effectively suppressed AOM/DSS-induced mouse colon tumor incidence
with inflammation by suppressing the expression of cytokines, such
as TNF-α, IL-6, COX-2 and iNOS. In addition, treatment with betaine
decreased RS generation and modulation of total glutathione
concentration. Collectively, betaine is a candidate cancer
chemopreventive agent against cancer development in
inflammation-associated colon tumorigenesis.
Acknowledgements
This study was supported by the National Research
Foundation of Korea (NRF) grant funded by the Korea government
(MSIP) (no. 2009-0083538) and the R&D Program of MKE/KEIT
(10040391, Development of Functional Food Materials and Device for
Prevention of Aging-associated Muscle Function Decrease). We thank
Aging Tissue Bank for providing research information.
References
|
1
|
Itzkowitz SH and Yio X: Inflammation and
cancer IV. Colorectal cancer in inflammatory bowel disease: the
role of inflammation. Am J Physiol Gastrointest Liver Physiol.
287:G7–G17. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Loftus EV Jr: Clinical epidemiology of
inflammatory bowel disease: Incidence, prevalence, and
environmental influences. Gastroenterology. 126:1504–1517. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hou JK, El-Serag H and Thirumurthi S:
Distribution and manifestations of inflammatory bowel disease in
Asians, Hispanics, and African Americans: a systematic review. Am J
Gastroenterol. 104:2100–2109. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yang SK, Yun S, Kim JH, et al:
Epidemiology of inflammatory bowel disease in the Songpa-Kangdong
district, Seoul, Korea, 1986–2005: a KASID study. Inflamm Bowel
Dis. 14:542–549. 2008.
|
|
5
|
Pedersen J, Coskun M, Soendergaard C,
Salem M and Nielsen OH: Inflammatory pathways of importance for
management of inflammatory bowel disease. World J Gastroenterol.
20:64–77. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Nielsen OH, Seidelin JB, Munck LK and
Rogler G: Use of biological molecules in the treatment of
inflammatory bowel disease. J Intern Med. 270:15–28. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Targownik LE and Bernstein CN: Infectious
and malignant complications of TNF inhibitor therapy in IBD. Am J
Gastroenterol. 108:1835–1842; quiz 1843. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Craig SA: Betaine in human nutrition. Am J
Clin Nutr. 80:539–549. 2004.PubMed/NCBI
|
|
9
|
Steenge GR, Verhoef P and Katan MB:
Betaine supplementation lowers plasma homocysteine in healthy men
and women. J Nutr. 133:1291–1295. 2003.PubMed/NCBI
|
|
10
|
Kim SK and Kim YC: Attenuation of
bacterial lipopolysaccharide-induced hepatotoxicity by betaine or
taurine in rats. Food Chem Toxicol. 40:545–549. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zeisel SH and Blusztajn JK: Choline and
human nutrition. Annu Rev Nutr. 14:269–296. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Detopoulou P, Panagiotakos DB,
Antonopoulou S, Pitsavos C and Stefanadis C: Dietary choline and
betaine intakes in relation to concentrations of inflammatory
markers in healthy adults: the ATTICA study. Am J Clin Nutr.
87:424–430. 2008.PubMed/NCBI
|
|
13
|
Go EK, Jung KJ, Kim JY, Yu BP and Chung
HY: Betaine suppresses proinflammatory signaling during aging: the
involvement of nuclear factor-kappaB via nuclear factor-inducing
kinase/IkappaB kinase and mitogen-activated protein kinases. J
Gerontol A Biol Sci Med Sci. 60:1252–1264. 2005. View Article : Google Scholar
|
|
14
|
Go EK, Jung KJ, Kim JM, et al: Betaine
modulates age-related NF-kappaB by thiol-enhancing action. Biol
Pharm Bull. 30:2244–2249. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lee EK, Jang EJ, Jung KJ, Kim DH, Yu BP
and Chung HY: Betaine attenuates lysophosphatidylcholine-mediated
adhesion molecules in aged rat aorta: modulation of the nuclear
factor-kappaB pathway. Exp Gerontol. 48:517–524. 2013. View Article : Google Scholar
|
|
16
|
Kim DH, Hossain MA, Kang YJ, et al:
Baicalein, an active component of Scutellaria baicalensis
Georgi, induces apoptosis in human colon cancer cells and prevents
AOM/DSS-induced colon cancer in mice. Int J Oncol. 43:1652–1658.
2013.PubMed/NCBI
|
|
17
|
LeBel CP, Ischiropoulos H and Bondy SC:
Evaluation of the probe 2′,7′-dichlorofluorescin as an indicator of
reactive oxygen species formation and oxidative stress. Chem Res
Toxicol. 5:227–231. 1992.
|
|
18
|
Tanaka T, Kohno H, Suzuki R, Yamada Y,
Sugie S and Mori H: A novel inflammation-related mouse colon
carcinogenesis model induced by azoxymethane and dextran sodium
sulfate. Cancer Sci. 94:965–973. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kim M, Miyamoto S, Sugie S, et al: A
tobacco-specific carcinogen, NNK, enhances AOM/DSS-induced colon
carcinogenesis in male A/J mice. In Vivo. 22:557–563.
2008.PubMed/NCBI
|
|
20
|
Tanaka T, Yasui Y, Tanaka M, Oyama T and
Rahman KM: Melatonin suppresses AOM/DSS-induced large bowel
oncogenesis in rats. Chem Biol Interact. 177:128–136. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Miyoshi N, Nagasawa T, Mabuchi R, et al:
Chemoprevention of azoxymethane/dextran sodium sulfate-induced
mouse colon carcinogenesis by freeze-dried yam sanyaku and its
constituent diosgenin. Cancer Prev Res (Phila). 4:924–934. 2011.
View Article : Google Scholar
|
|
22
|
Yasui Y, Hosokawa M, Mikami N, Miyashita K
and Tanaka T: Dietary astaxanthin inhibits colitis and
colitis-associated colon carcinogenesis in mice via modulation of
the inflammatory cytokines. Chem Biol Interact. 193:79–87. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Bizzozero OA, Ziegler JL, De Jesus G and
Bolognani F: Acute depletion of reduced glutathione causes
extensive carbonylation of rat brain proteins. J Neurosci Res.
83:656–667. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Tanaka T: Colorectal carcinogenesis:
Review of human and experimental animal studies. J Carcinog.
8:52009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Bharti AC and Aggarwal BB: Chemopreventive
agents induce suppression of nuclear factor-kappaB leading to
chemosensitization. Ann NY Acad Sci. 973:392–395. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lee JE, Giovannucci E, Fuchs CS, Willett
WC, Zeisel SH and Cho E: Choline and betaine intake and the risk of
colorectal cancer in men. Cancer Epidemiol Biomarkers Prev.
19:884–887. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Konstantinova SV, Tell GS, Vollset SE,
Nygard O, Bleie O and Ueland PM: Divergent associations of plasma
choline and betaine with components of metabolic syndrome in middle
age and elderly men and women. J Nutr. 138:914–920. 2008.PubMed/NCBI
|
|
28
|
Teixido N, Canamas TP, Usall J, Torres R,
Magan N and Vinas I: Accumulation of the compatible solutes,
glycine-betaine and ectoine, in osmotic stress adaptation and heat
shock cross-protection in the biocontrol agent Pantoea agglomerans
CPA-2. Lett Appl Microbiol. 41:248–252. 2005. View Article : Google Scholar
|
|
29
|
Olthof MR and Verhoef P: Effects of
betaine intake on plasma homocysteine concentrations and
consequences for health. Curr Drug Metab. 6:15–22. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ueland PM: Choline and betaine in health
and disease. J Inherit Metab Dis. 34:3–15. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ying J, Rahbar MH, Hallman DM, et al:
Associations between dietary intake of choline and betaine and lung
cancer risk. PLoS One. 8:e545612013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Liu H, Colavitti R, Rovira II and Finkel
T: Redox-dependent transcriptional regulation. Circ Res.
97:967–974. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Palozza P, Serini S, Torsello A, et al:
Beta-carotene regulates NF-kappaB DNA-binding activity by a redox
mechanism in human leukemia and colon adenocarcinoma cells. J Nutr.
133:381–388. 2003.PubMed/NCBI
|
|
34
|
Pelicano H, Carney D and Huang P: ROS
stress in cancer cells and therapeutic implications. Drug Resist
Updat. 7:97–110. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Erdelyi I, Levenkova N, Lin EY, et al:
Western-style diets induce oxidative stress and dysregulate immune
responses in the colon in a mouse model of sporadic colon cancer. J
Nutr. 139:2072–2078. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Wang X, Mandal AK, Saito H, et al: Arsenic
and chromium in drinking water promote tumorigenesis in a mouse
colitis-associated colorectal cancer model and the potential
mechanism is ROS-mediated Wnt/beta-catenin signaling pathway.
Toxicol Appl Pharmacol. 262:11–21. 2012. View Article : Google Scholar
|
|
37
|
Takeda K and Akira S: Toll-like receptors
in innate immunity. Int Immunol. 17:1–14. 2005. View Article : Google Scholar
|
|
38
|
Ci X, Ren R, Xu K, et al: Schisantherin A
exhibits anti-inflammatory properties by down-regulating NF-kappaB
and MAPK signaling pathways in lipopolysaccharide-treated RAW 264.7
cells. Inflammation. 33:126–136. 2010. View Article : Google Scholar : PubMed/NCBI
|