Introduction
Heterochromatin protein 1 (HP1) is a histone code
reader which specifically recognizes and binds to methylated H3
lysine 9 (H3K9) (1). Through its
activities in DNA and its interacting proteins, the mammalian HP1
family plays a critical role in a variety of cellular processes
including centromere stability, telomere stability, regulation of
gene expression, DNA repair, cellular senescence and cancer
progression (2–4). Three human HP1 homologs (HP1α, HP1β
and HP1γ) contain two conserved domains, which are separated by a
flexible hinge region: a chromodomain (CD) interacting with
methylated H3K9 and a chromoshadow domain (CSD) interacting with
the PxVxL motif of its partner (5). HP1 homologs are known to exhibit
different subnuclear localizations in interphase: HP1α and HP1β are
centromeric while HP1γ is located in both euchromatic and
heterochromatic regions (3,6,7).
As might be expected given their name and localization, HP1 in mice
and human represses transcription and suppresses tumor through
formation of multimolecular complex with transcriptional
corepressor TIF1β, histone methyltransferase SETDB1 and
NuRD-histone deacetylase complex (8). However, it has been recently reported
that HP1 may work at euchromatic regions and as a positive
regulator in gene expression (5,9,10).
Several reports show that expression of HP1 is
changed in various tumor tissues compared with normal tissues. In
papillary thyroid carcinomas and embryonal brain tumor, the mRNA
level of HP1α was decreased (11,12),
which makes HP1α a predictor in these cancers. HP1α expression is
also decreased in invasive/metastatic breast cancer tissue and HP1α
knockdown (KD) reduces the invasion rate of metastatic breast
cancer cells (13,14), suggesting HP1α is a metastatic
suppressor. In addition to HP1α, reduction of HP1β expression is
correlated with invasive activity in human melanoma cells (15). Different from these beneficial
effects, the three homologs of HP1 were highly expressed in
patients with acute myeloid leukemia and chronic myeloid leukemia
(16). However, the molecular
mechanism whereby the expression of HP1 proteins is regulated in
cancer is not well understood.
Matrix metallopeptidases (MMPs) are a family of
zinc-dependent extracellular matrix (ECM) remodeling endopeptidases
capable of degrading almost all components of ECM (17). MMPs are important not only in
normal, physiological and biological processes such as
embryogenesis, normal tissue remodeling, wound healing and
angiogenesis but also in diseases such as arthritis and cancer
(18). Expression and activation
of MMP are increased in almost all human cancers compared to normal
tissue. Indeed, MMPs play important roles in carcinogenesis as well
as invasion and metastasis (19).
MMPs can regulate cell migration by removing sites of adhesion,
exposing new sites, cleaving cell-cell or cell-matrix receptors and
releasing chemo attractants from ECM (20). MMP2/14 degrades laminin 5 and
reveals a cryptic site that triggers motility (19). In addition to migration, MMP2/9 are
localized in invadopodia and promote invasion by degrading type IV
collagen of basement membrane (21,22).
To understand this issue, we have identified the
expression patterns of HP1 in various cancer cells. We show that
HP1β inhibits de novo expression and activation of MMP2
through the control of mRNA level of MMP2 and membrane type 1
metallopeptidase (MT1-MMP). Collectively, this study is the first
to elucidate the functional relationship of HP1β and MMP2 in cancer
metastasis.
Materials and methods
Cell culture and transfection
Human cancer cell lines were cultured according to
the instructions from ATCC and were maintained under a fully
humidified atmosphere of 95% air and 5% CO2 at 37°C.
Cancer cells were grown to 70% confluence in plates, the cells were
transfected with siRNA or GFP-tagged vectors using Lipofectamine
2000 reagent, according to manufacturer’s protocol (Invitrogen Life
Technologies, Carlsbad, CA, USA). After incubation for 48 h, mRNAs
and proteins were extracted from the cells. The sequences of siRNAs
targeting HP1 genes were: HP1α, 5′-GUUCCAGUCCUCUCUCAAAGC-3′ and
5′-GCUUUG AGAGAGGACUGGAAC-3′; HP1β, 5′-GACUCCAGUGGA GAGCUCAUG-3′
and 5′-CAUGAGCUCUCCACUGGA GUC-3′; HP1γ, 5′-AUUCUUCAGGCUCUGCCUC-3′
and 5′-GAGGCAGAGCCUGAAGAAU-3′ (HP1α HP1β, and HP1γ primers were
described previously) (23).
Western blotting
Immunoblotting was performed as previously described
(24). Cell lysates were prepared
using lysis buffer supplemented with protease and phosphatase
inhibitors (Thermo Scientific Pierce, Rockford, IL, USA). Protein
concentrations were quantified according to the BCA Protein Assay
kit (Thermo Scientific Pierce, Rockford, IL, USA).
RT-PCR and quantitative real-time PCR
(qRT-PCR)
Total RNA was extracted using easy-Blue reagent
(Intron Biotechnology, Seoul, Korea) and 1 μg of RNA with oligo dT
primers was subjected to reverse transcription using the ImProm-II™
Reverse Transcription system (Promega Corporation, Madison, WI,
USA). cDNA was amplified using Super Premix Sapphire PCR master mix
(Mbiotech, Inc., Seoul, Korea). qRT-PCR was performed with the
KAPA™ SYBR® FAST qPCR (Kapa Biosystems, Inc.,
Wilmington, MA, USA) using CFX96™ or Chromo4™ real-time PCR
Detector (Bio-Rad, Hercules, CA, USA). Relative levels of mRNA were
normalized to the values of GAPDH mRNA for each reaction. Primer
sequences used for PCR were: MMP2, 5′-ACCAGCTGGCCTAGTGATGATG-3′ and
5′-GGCTT CCGCATGGTCTCGATG-3′; MT1-MMP, 5′-GGAATAAC
CAAGTGATGGATGG-3′ and 5′-TTGTTTCCACGGAAGA AGTAGG-3′; tissue
inhibitor of metallopeptidase (TIMP)2, 5′-GCGGTCAGTGAGAAGGAAGTGG-3′
and 5′-CTTGCA CTCGCAGCCCATCTG-3′; GAPDH, 5′-GAGTCAACGGAT
TTGGTCGT-3′ and 5′-TTGATTTTGGAGGGATCTCG-3′.
Migration assay
Human cancer cells grown in 6-well plates were
scratched with a yellow pipette tip to form a thin wound.
Microscope images were observed immediately and 48 h after the
scratch.
Results
Analysis of HP1 expression in human
cancer cell lines
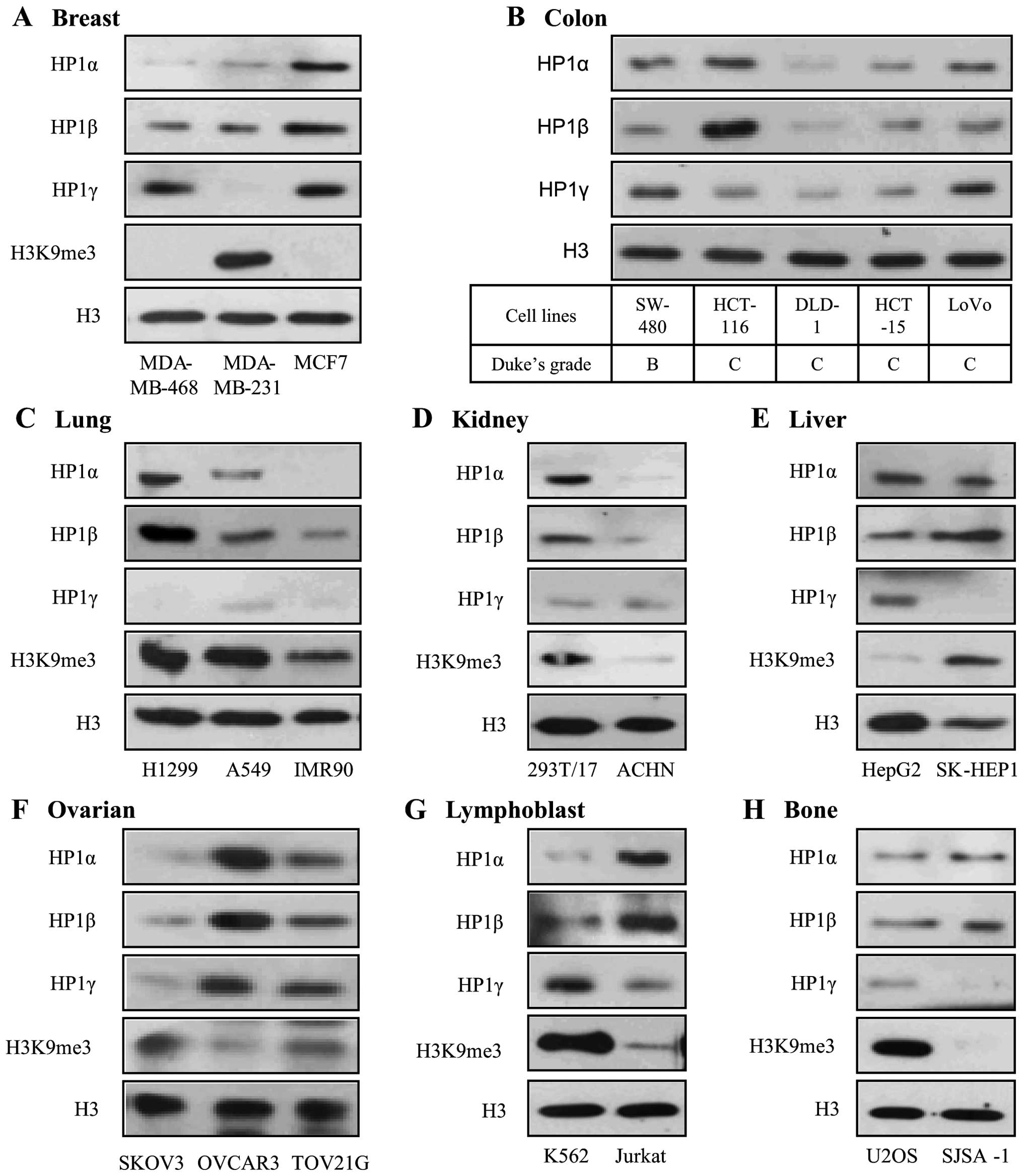
To investigate the potential role of HP1 in human
cancer, we first examined the expression levels of the three HP1
proteins in diverse human cancer cell lines, which originated from
eight different organs. The cell lines we used are indicated in
Table I. HP1α and HP1β were more
highly expressed in MCF7, a non-invasive breast cancer cell line,
than the two invasive breast cancer cell lines (Fig. 1A). HP1γ was uniquely repressed and
histone H3 lysine 9 methylation (H3K9me3) was dramatically
increased in only MDA-MB-231, invasive breast cancer cells. Colon
cancer cells are sorted according to the Dukes grade. Among the
five colon cancer cell lines, only SW-480 is classified as Dukes
grade B, which has invasion through the bowel wall, but does not
involve lymph nodes. The expression levels of HP1α, β and γ were
all high in SW-480 (Fig. 1B). The
other colon cancer cell lines, which are classified as Dukes grade
C, involving invasion of the lymph nodes, expressed high levels of
all three HP1 proteins. In IMR90, a normal lung cell line, the
three HP1 proteins and H3K9me3 were all suppressed compared to the
two lung cancer cell lines (Fig.
1C). On the other hand, the HP1 proteins and H3K9me3 were more
highly expressed in 293T/17, normal kidney cell line, than ACHN,
renal cell adenocarcinoma (Fig.
1D). These results suggest that HP1 proteins are related to
tumor progression in lung, but tumor suppression in kidney. In the
liver cancer cell lines, HP1α and HP1γ were more highly expressed
in HepG2 while HP1β and H3K9me3 were more highly expressed in
SK-HEP1 (Fig. 1E). In the ovarian
cancer cell lines, the three HP1 proteins showed similar expression
patterns, but H3K9me3 showed an opposite pattern (Fig. 1F). In leukemia and osteosarcoma
cell lines, HP1γ and H3K9me3 were expressed in the same pattern
(Fig. 1G and H). Taken together,
these results show that the expression patterns of the HP1 proteins
vary in different cancer types and degree of invasiveness (Table I).
 | Table IThe human cancer cell lines
investigated. |
Table I
The human cancer cell lines
investigated.
| Organ | Cell line | Tumor type/stage | HP1α | HP1β | HP1γ |
|---|
| Breast | MDA-MB-468 | Malignant
adenocarcinoma | −− | − | + |
| MDA-MB-231 | Malignant
adenocarcinoma | −− | − | −− |
| MCF7 | Adenocarcinoma | + | + | + |
| Colon | SW-480 | Colorectal
adenocarcinoma/Duke’s grade B | + | + | + |
| HCT-116 | Colorectal
carcinoma/Duke’s grade C | + | ++ | − |
| DLD-1 | Colorectal
adenocarcinoma/Duke’s grade C | −− | −− | −− |
| HCT-15 | Colorectal
adenocarcinoma/Duke’s grade C | − | − | − |
| LoVo | Colorectal
adenocarcinoma/Duke’s grade C | − | − | + |
| Lung | H1299 | Carcinoma | + | ++ | −− |
| A549 | Carcinoma | − | + | − |
| IMR90 | Normal
fibroblast | −− | − | −− |
| Kidney | 293T/17 | Normal epithelial
cell | + | + | − |
| ACHN | Renal cell
adenocarcinoma | −− | −− | − |
| Liver | HepG2 | Hepatocellular
carcinoma | + | − | + |
| SK-HEP1 | Adenocarcinoma | − | + | − |
| Ovarian | SKOV3 | Adenocarcinoma | − | − | − |
| OVCAR3 | Adenocarcinoma | ++ | ++ | ++ |
| TOV21G | Malignant
adenocarcinoma/grade 3, Stage III | + | + | + |
| Lymphoblast | K562 | Chronic myelogenous
leukemia | −− | − | + |
| Jurkat | Acute T cell
leukemia | + | ++ | − |
| Bone | U2OS | Osteosarcoma | − | − | − |
| SJSA-1 | Multipotential
osteosarcoma | + | + | −− |
Inverse correlation of MMP2 and HP1β in
colon cancer cells
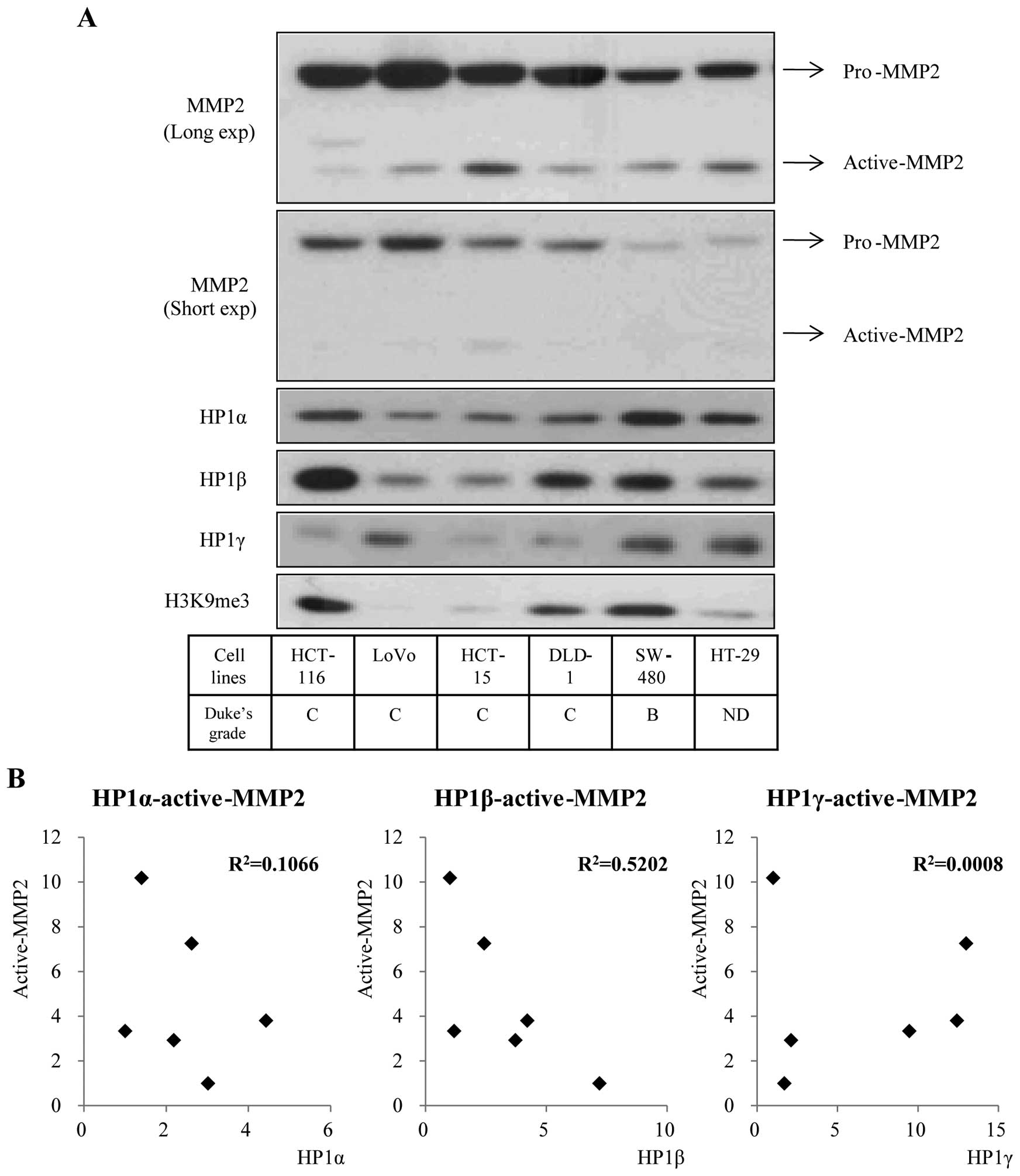
Although to different extents, the three HP1
proteins were highly expressed in colon cancer cell lines. To
determine whether HP1 and MMP2 are involved in colorectal cancer,
we first analyzed the expression pattern of MMP2 and HP1 proteins
in six colon cancer cell lines. MMP2, which degrades the basic
component of cellular membrane, is regarded as a leading molecule
in the metastatic process of cancer development (17,19).
Inactive pro-MMP2 is 70 kDa and active-MMP2, derived from cleavage
of pro-MMP2 is smaller (35–50 kDa). Among the members of HP1
family, HP1β showed a reverse expression pattern with active-MMP2
(Fig. 2A). Using ImageJ program,
we quantified the expression level of active-MMP2 and HP1s
displayed in Fig. 2A and assessed
the correlation between the values. We confirmed a strong
correlation between active-MMP2 and HP1β through the coefficient of
determination (R2=0.5202) (Fig. 2B, middle). HP1α showed a weak
correlation with active-MMP2, as R2 is 0.1066 (Fig. 2B, left) and HP1γ had no correlation
with active-MMP2 (Fig. 2B, right).
These data suggest the possibility of a biological relationship
between HP1β and MMP2.
HP1β negatively regulates the expression
and activation of MMP2 protein
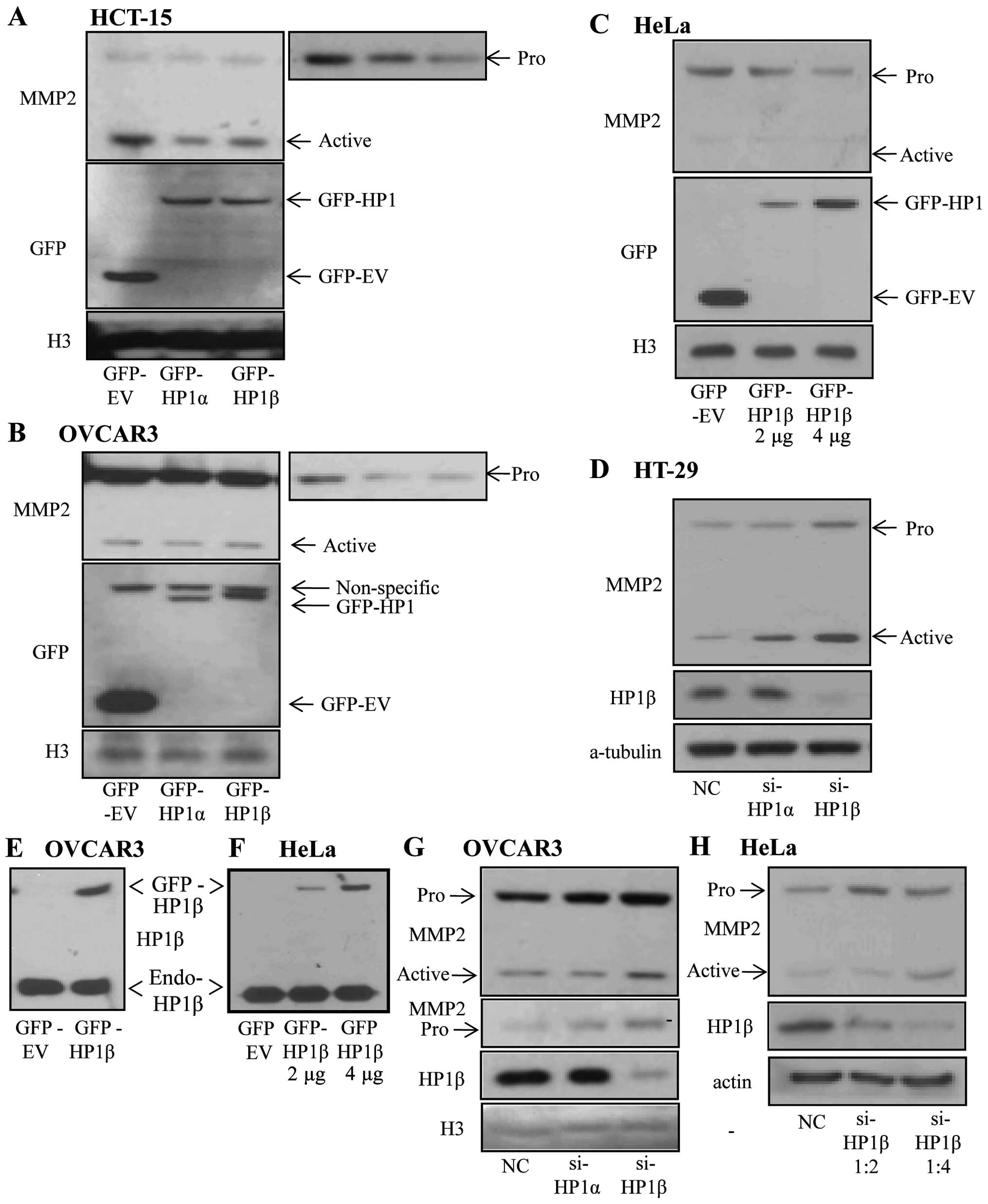
To investigate whether HP1β has influence on the
MMP2 protein level, we transfected GFP-empty vector or GFP-tagged
HP1 vectors into colon cancer cell line, HCT-15, which showed a
weak HP1β signal. Overexpression of HP1β in HCT-15 cells resulted
in reduction of pro-MMP2 as well as active-MMP2 (Fig. 3A). In OVCAR3, an ovarian cancer
cell line, overexpressing HP1β caused a reduction of pro-MMP2, but
not active-MMP2 (Fig. 3B).
Similarly, only the pro-MMP2 level in HeLa cells was decreased
depending on the amounts of ectopically expressed HP1β (Fig. 3C). We cannot exclude the
possibility that, because the high basal expressions of HP1β is
enough to saturate the active-MMP2 level, exogenously expressed
HP1β results in no significant change in the active-MMP2 level in
OVCAR3 and HeLa cells (Fig. 3E and
F). Therefore, we next examined the MMP2 level in HP1β-depleted
cells. In HT-29, a colon cancer cell line, KD of HP1β promoted both
pro- and active-MMP2 (Fig. 3D).
Increase of pro-MMP2 and active-MMP2 levels following HP1β KD, was
observed in OVCAR3 and HeLa cells (Fig. 3G and H). These effects were also
observed in HP1α but to a lesser extent (Fig. 3A–D and G). Thus, these results
suggest that HP1β is a major regulator of MMP2 expression and
activation and HP1α is a minor regulator.
HP1β regulates the mRNA expression of
MMP2 and MT1-MMP
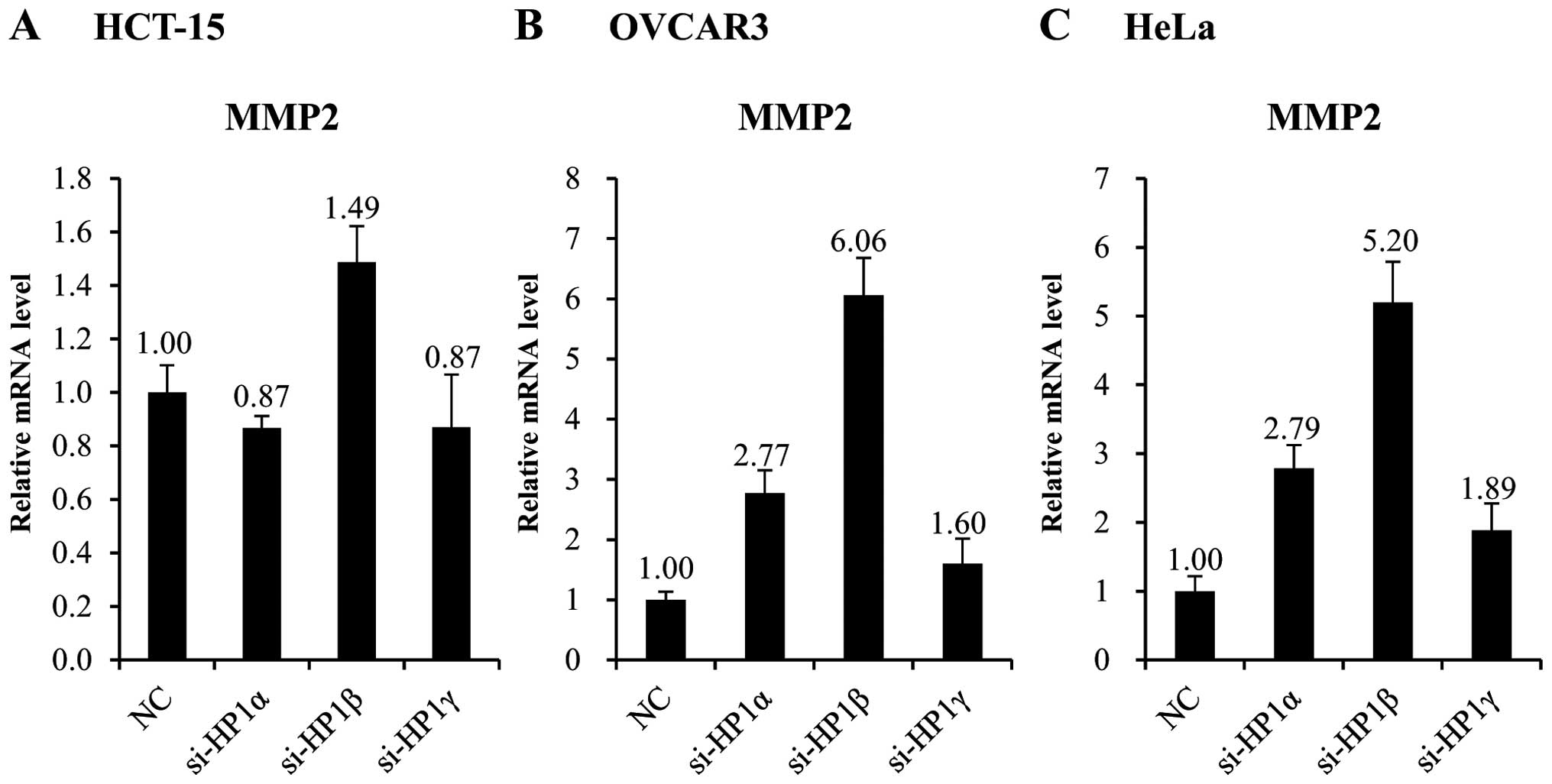
To examine whether HP1β regulates MMP2 expression at
the transcription level, we measured the mRNA level of MMP2 in
HP1β-deleted cells. The mRNA level of the MMP2 gene increased
1.5-fold, following KD of HP1β in HCT-15 cells (Fig. 4A). However, considering that HCT-15
cells express very low basal level of HP1β, this is regarded as a
significant change. In OVCAR3 and HeLa cells, which express a high
basal level of HP1β, the mRNA level of MMP2 increased >5-fold
following KD of HP1β (Fig. 4B and
C).
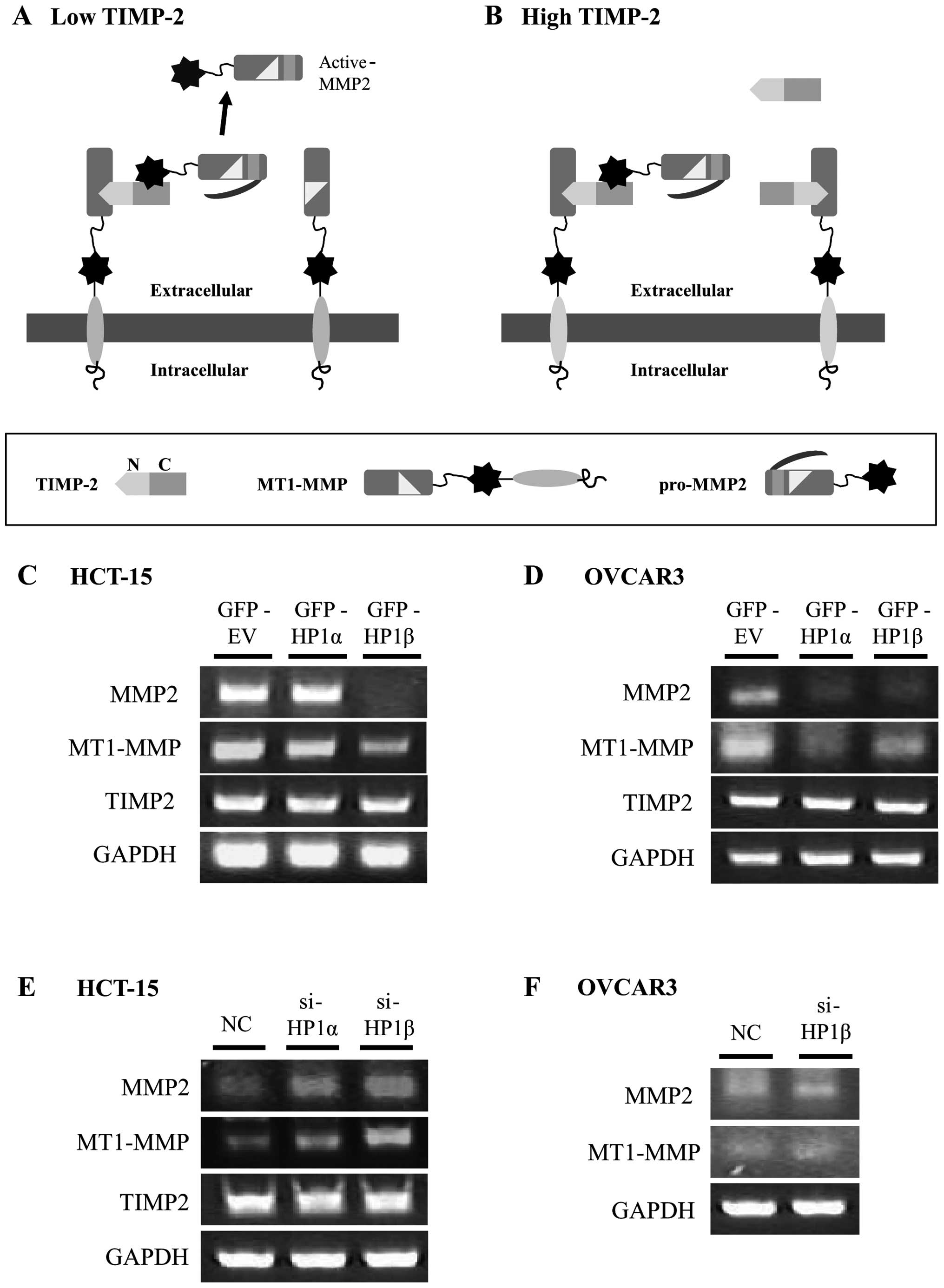
The activation of MMP2 is triggered by the cleavage
of pro-MMP2 by MT1-MMP, which is influenced by the level of TIMP.
TIMP2 binds to the active site of MT1-MMP and this complex acts as
a receptor for pro-MMP2. Then, the free MT1-MMP cleaves the
propeptide of pro-MMP2, leading to the intermediate stage. Through
further autocatalytic proteolysis, fully active MMP2 is generated
(Fig. 5A) (25). However, when the TIMP2 level is
high enough to saturate all MT1-MMP, cleavage of pro-MMP2 is
impossible preventing MMP2 activation (Fig. 5B). We found that active-MMP2 as
well as pro-MMP2 was changed by HP1β (Figs. 3 and 4). Thus, we examined whether the mRNA
levels of MT1-MMP and TIMP2 are regulated by HP1β. The mRNA levels
of MMP2 and MT1-MMP decreased following overexpression of HP1β in
HCT-15 and OVCAR3 cells, while the level of TIMP2 did not
significantly change (Fig. 5C and
D). Consistent with this result, KD of HP1β promotes the mRNA
level of MMP2 and MT1-MMP in both cell lines (Fig. 5E and F).
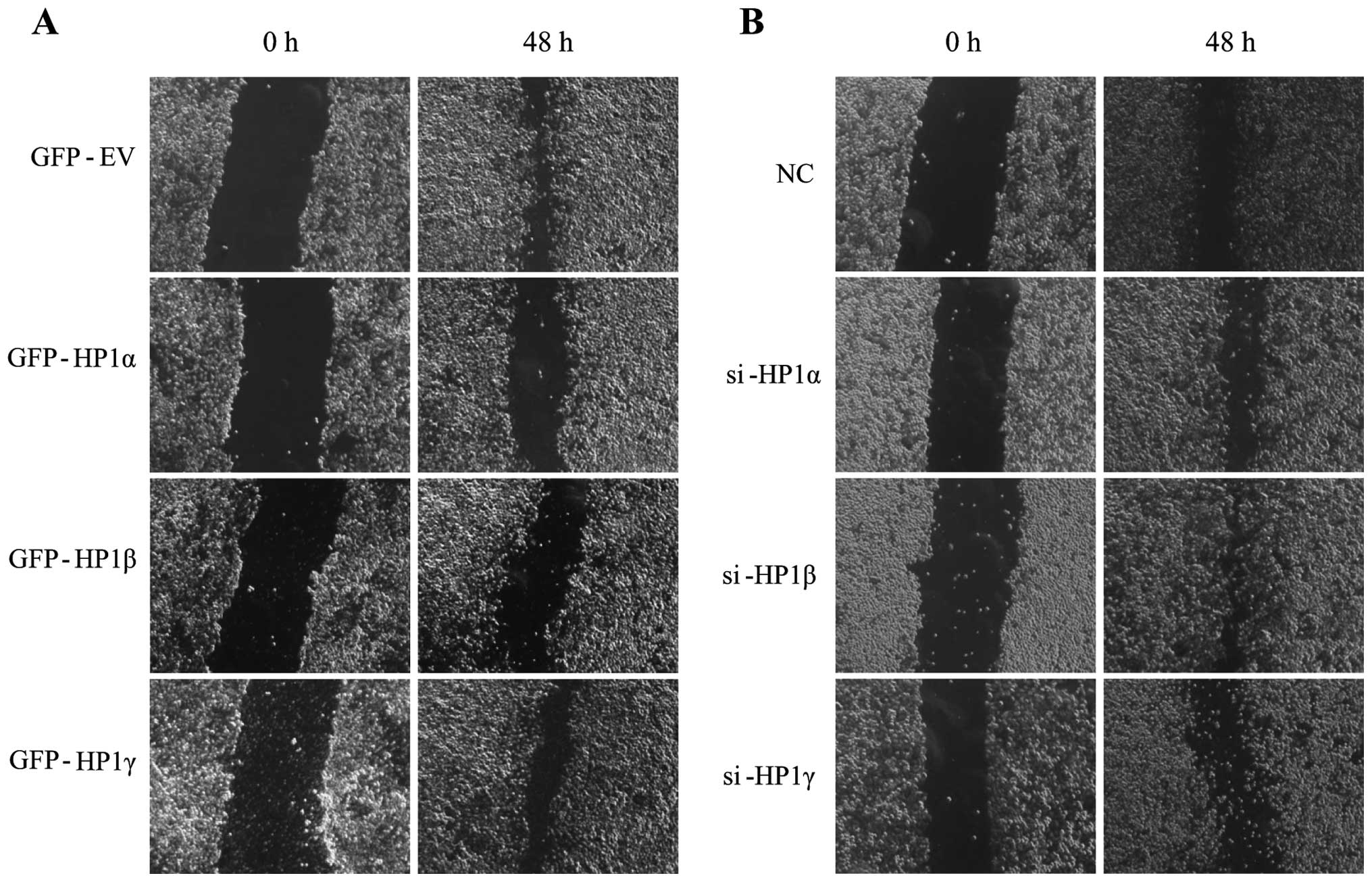
HP1β inhibits migration of human cancer
cells
Recent studies of breast cancer cells revealed an
inverse correlation between HP1α expression and tumor cell
invasiveness (13,14). We therefore investigated whether
tumor cell motility is regulated by all three HP1 protein or in an
HP1 isoform-specific manner. To do this, we assessed the migratory
ability of HeLa cells transfected with GFP-tagged vectors or siRNA
following scratch with a yellow tip. Overexpression of HP1β
markedly decreased the migration rate of HeLa cells (Fig. 6A), while KD of HP1β promoted
migration of HeLa cells (Fig. 6B).
Interestingly, overexpression or KD of HP1α resulted in the
similar, but lesser effects, on the migration rate compared to
HP1β. In contrast, modulating the expression of HP1γ did not affect
the migration. Thus, it suggests that HP1β and HP1α, but not HP1γ,
are responsible for cancer metastasis.
Discussion
The HP1 family of proteins was identified >25
years ago (26), but the common or
divergent functions of the three homologs remain unclear. There are
several indications that the three mammalian HP1 homologs, HP1α, β
and γ, may not fulfil identical functions. First, they show
differences in cellular distribution. HP1α marks strongly in the
pericentric heterochromatin, whereas HP1γ shows less specificity
for these regions (3,6,7).
Second, despite their high similarity in structure and function,
the three HP1 homologs are not always present together and can
interact with different binding partners (3). Finally, distinct post-translational
modifications on an individual HP1 homolog (6,27)
may further diversify their functions. Here, we show that in human
cells, HP1β has unique properties in cancer development, which are
not shared by HP1α and γ. First, we find opposite expression
patterns of HP1β and MMP2, which promotes metastasis in cancer.
Second, we reveal that overexpression of HP1β reduces protein
levels of both pro-MMP2 and active-MMP2; this is mediated by
suppressed mRNA level of MMP2 and MT1-MMP, which are required for
the activation of MMP2. Consistently, KD of HP1β increases protein
levels of pro-MMP2 and active-MMP2. mRNA level of MMP2 and MT1-MMP
are also increased by HP1β KD. Furthermore, we demonstrate that
overexpression of HP1β represses the migration of human cancer cell
and KD of HP1β promotes cell migration.
The links between HP1 proteins and tumorigenesis
have emerged. In vitro studies of HP1 expression in cancer
have suggested that these proteins protect against tumor cell
aggressiveness and invasiveness. HP1α is overexpressed in
carcinomas and the expression level correlates with clinical data
and disease outcome (28). This
has been most convincingly shown in breast cancer cell lines
(13,14). Downregulation of the HP1α protein
is linked to a higher invasive potential of cancer cells (3,11,13,29).
HP1α downregulation has been observed in most highly invasive and
metastatic breast cancer cells versus non-metastatic cells
(13,14,29).
Decreasing the HP1α expression in non-invasive MCF-7 cells enhanced
the invasive potential; increasing the HP1α expression in
metastatic MDA-MB-231 cells decreased the invasive potential
(30). In this study, we show that
HP1β overexpression causes impaired migratory ability, whereas HP1β
KD results in increased migration. Moreover, our findings reveal
the molecular mechanism by which HP1β regulates cancer migration
and metastasis. HP1β negatively regulates MMP2 expression at the
transcriptional level and prevents MMP2 activation by reducing the
expression of MT1-MMP. In line with this finding, decreased HP1β
expression is associated with melanoma oncogenesis and high
invasiveness in human melanoma cells (15). Consequently, because HP1α and HP1β
have been shown to attenuate metastasis in cancer cells, it may be,
given the data presented here, that HP1 is a suppressor of cell
migration and metastasis. Interestingly, the reverse pattern of
euchromatic HP1γ and H3K9me3 was observed in different cancer cells
including breast, ovarian, lung, and liver cancer cells (Fig. 1). This result supports the
hypothesis that HP1γ might regulate certain cancer-associated genes
via a different epigenetic mechanism, not shared by HP1α and β.
Taken together, our results elucidate the role of
HP1β as a key regulator of cancer metastasis by reducing both
expression and activation of MMP2, which is mediated by altered
mRNA levels of MMP2 and MT1-MMP. These findings suggest the
epigenetic regulation of MMP2 by HP1β and provide the mechanistic
rationale for the targeting of HP1β to relieve cancer
metastasis.
Acknowledgements
This research was supported by Medical Research
Center programs (2012-0009849) and the Basic Science Research
Program through the National Research Foundation of Korea (NRF)
funded by the Ministry of Education, Science and Technology
(2012013998).
Abbreviations:
|
ECM
|
extracellular matrix
|
|
H3K9me
|
histone H3 lysine 9 methylation
|
|
HP1
|
heterochromatin protein 1
|
|
KD
|
knockdown
|
|
MMP
|
matrix metallopeptidase
|
|
MT1-MMP
|
membrane type 1 matrix
metallopeptidase
|
|
TIMP
|
tissue inhibitor of
metallopeptidase
|
References
|
1
|
Nielsen PR, Nietlispach D, Mott HR, et al:
Structure of the HP1 chromodomain bound to histone H3 methylated at
lysine 9. Nature. 416:103–107. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ayoub N, Jeyasekharan AD and Venkitaraman
AR: Mobilization and recruitment of HP1: a bimodal response to DNA
breakage. Cell Cycle. 8:2945–2950. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Dialynas GK, Vitalini MW and Wallrath LL:
Linking Heterochromatin Protein 1 (HP1) to cancer progression.
Mutat Res. 647:13–20. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zhang R and Adams PD: Heterochromatin and
its relationship to cell senescence and cancer therapy. Cell Cycle.
6:784–789. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kwon SH and Workman JL: The changing faces
of HP1: from heterochromatin formation and gene silencing to
euchromatic gene expression: HP1 acts as a positive regulator of
transcription. Bioessays. 33:280–289. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Minc E, Allory Y, Worman HJ, Courvalin JC
and Buendia B: Localization and phosphorylation of HP1 proteins
during the cell cycle in mammalian cells. Chromosoma. 108:220–234.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Nielsen AL, Oulad-Abdelghani M, Ortiz JA,
Remboutsika E, Chambon P and Losson R: Heterochromatin formation in
mammalian cells: interaction between histones and HP1 proteins. Mol
Cell. 7:729–739. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Nielsen AL, Ortiz JA, You J, et al:
Interaction with members of the heterochromatin protein 1 (HP1)
family and histone deacetylation are differentially involved in
transcriptional silencing by members of the TIF1 family. EMBO J.
18:6385–6395. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hediger F and Gasser SM: Heterochromatin
protein 1: don’t judge the book by its cover! Curr Opin Genet Dev.
16:143–150. 2006.PubMed/NCBI
|
|
10
|
Piacentini L, Fanti L, Berloco M, Perrini
B and Pimpinelli S: Heterochromatin protein 1 (HP1) is associated
with induced gene expression in Drosophila euchromatin. J
Cell Biol. 161:707–714. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Pomeroy SL, Tamayo P, Gaasenbeek M, et al:
Prediction of central nervous system embryonal tumour outcome based
on gene expression. Nature. 415:436–442. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wasenius VM, Hemmer S, Kettunen E,
Knuutila S, Franssila K and Joensuu H: Hepatocyte growth factor
receptor, matrix metalloproteinase-11, tissue inhibitor of
metalloproteinase-1, and fibronectin are up-regulated in papillary
thyroid carcinoma: a cDNA and tissue microarray study. Clin Cancer
Res. 9:68–75. 2003.
|
|
13
|
Kirschmann DA, Lininger RA, Gardner LM, et
al: Down-regulation of HP1Hsalpha expression is associated with the
metastatic phenotype in breast cancer. Cancer Res. 60:3359–3363.
2000.PubMed/NCBI
|
|
14
|
Thomsen R, Christensen DB, Rosborg S,
Linnet TE, Blechingberg J and Nielsen AL: Analysis of HP1α
regulation in human breast cancer cells. Mole Carcinog. 50:601–613.
2011.
|
|
15
|
Nishimura K, Hirokawa YS, Mizutani H and
Shiraishi T: Reduced heterochromatin protein 1-beta (HP1beta)
expression is correlated with increased invasive activity in human
melanoma cells. Anticancer Res. 26:4349–4356. 2006.PubMed/NCBI
|
|
16
|
Lukásová E, Koristek Z, Falk M, et al:
Methylation of histones in myeloid leukemias as a potential marker
of granulocyte abnormalities. J Leukoc Biol. 77:100–111.
2005.PubMed/NCBI
|
|
17
|
Vihinen P and Kähäri VM: Matrix
metalloproteinases in cancer: prognostic markers and therapeutic
targets. Int J Cancer. 99:157–166. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Gupta A, Kaur CD, Jangdey M and Saraf S:
Matrix metalloproteinase enzymes and their naturally derived
inhibitors: novel targets in photocarcinoma therapy. Ageing Res
Rev. 13:65–74. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Egeblad M and Werb Z: New functions for
the matrix metalloproteinases in cancer progression. Nat Rev
Cancer. 2:161–174. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
McCawley LJ and Matrisian LM: Matrix
metalloproteinases: they’re not just for matrix anymore! Curr Opin
Cell Biol. 13:534–540. 2001.
|
|
21
|
Ikebe T, Shinohara M, Takeuchi H, et al:
Gelatinolytic activity of matrix metalloproteinase in tumor tissues
correlates with the invasiveness of oral cancer. Clin Exp
Metastasis. 17:315–323. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Thomas GT, Lewis MP and Speight PM: Matrix
metalloproteinases and oral cancer. Oral Oncol. 35:227–233. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
du Chéné I, Basyuk E, Lin YL, et al:
Suv39H1 and HP1gamma are responsible for chromatin-mediated HIV-1
transcriptional silencing and post-integration latency. EMBO J.
26:424–435. 2007.PubMed/NCBI
|
|
24
|
Kwon S, Zhang Y and Matthias P: The
deacetylase HDAC6 is a novel critical component of stress granules
involved in the stress response. Genes Dev. 21:3381–3394. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lafleur MA, Handsley MM and Edwards DR:
Metalloproteinases and their inhibitors in angiogenesis. Expert Rew
Mol Med. 5:1–39. 2003.PubMed/NCBI
|
|
26
|
James TC and Elgin SC: Identification of a
nonhistone chromosomal protein associated with heterochromatin in
Drosophila melanogaster and its gene. Mol Cell Biol.
6:3862–3872. 1986.PubMed/NCBI
|
|
27
|
Lomberk G, Bensi D, Fernandez-Zapico ME
and Urrutia R: Evidence for the existence of an HP1-mediated
subcode within the histone code. Nat Cell Biol. 8:407–415. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
De Koning L, Savignoni A, Boumendil C, et
al: Heterochromatin protein 1alpha: a hallmark of cell
proliferation relevant to clinical oncology. EMBO Mol Med.
1:178–191. 2009.PubMed/NCBI
|
|
29
|
De Lange R, Burtscher H, Jarsch M and
Weidle UH: Identification of metastasis-associated genes by
transcriptional profiling of metastatic versus non-metastatic colon
cancer cell lines. Anticancer Res. 21:2329–2339. 2001.PubMed/NCBI
|
|
30
|
Norwood LE, Moss TJ, Margaryan NV, et al:
A requirement for dimerization of HP1Hsalpha in suppression of
breast cancer invasion. J Biol Chem. 281:18668–18676. 2006.
View Article : Google Scholar : PubMed/NCBI
|