Introduction
Breast cancer (BC) is the most common malignancy in
women and the mortality rate has been continuously increasing over
the past 30 years. About one million women worldwide are diagnosed
with BC every year (1,2). So it is very important to prevent
tumorigenesis and treat cancer. Establishment of an animal model
for the development of preventative measures and effective mammary
cancer treatment is needed. Mouse models are excellent tools to
understand the natural biology of breast cancer. Since human breast
cancers are clustered into several phenotypes (subtypes) based on
grade and molecular markers, a good animal model for a subtype is
one which mimics most subtype characteristics morphology, molecular
markers, metastatic pattern, grade, hormone-dependency,
parity/pregnancy status and so on (3–5).
Kunming mice are closed colony mice and the largest
number in production capacity in China. They are widely used in
biology, pharmacology, toxicology and other areas of scientific
research in China (6–11). Chinese scientist detected Kunming
mice in one of the heterozygous breeding females (12–16).
In our study, we found that Kunming female species after breeding
can develop spontaneous breast cancer in 10–12 months (average 11.5
months). The pathological diagnosis was primarily invasive ductal
carcinoma (17,18).
But BC is a biologically heterogeneous disease and
patients with the same diagnostic and clinical prognostic profiles
can have markedly different clinical outcomes. This difference is
possibly caused by the limitation of our current taxonomy of breast
cancers, which groups molecularly distinct diseases into clinical
classes based mainly on morphology. Molecular profiling has
provided biological evidence for heterogeneity of breast cancer
through the identification of intrinsic subtypes. Analysis of gene
expression data suggest that breast cancers can be divided into
molecular subtypes which have distinct clinical features, with
markedly differing prognosis and clinical outcomes. Therefore,
immunohistochemical (IHC) markers have been used as surrogates in
subtyping breast cancer. The updated IHC subtype definition was
given as luminal A [ER+ and/or progesterone receptor
(PR+), HER2−], luminal B (ER+
and/or PR+, HER2+),
HER2+/ER− (ER−, PR−,
HER2+), basal-like (ER−, PR−,
HER2−, CK5/6+), and unclassified (negative
for all five markers) (19,20).
In the present study, in order to understand the
histopathological and molecular characteristics of the model we
elucidated the pathogenesis of breast cancer to identify specific
therapeutic targets. We have subclassified the breast cancers in
the spontaneous breast cancer model of Kunming mice (21,22).
We used IHC staining to determine the expression of ER-α, PR,
HER-2/neu and to identify intrinsic subtypes using formalin-fixed,
paraffin-embedded tumor blocks, western blot analysis of c-Myc,
cyclin D1 and VEGF gene expression. We also determined how relevant
the model is for human breast cancer associations between tumor
subtypes and tumor characteristics.
Materials and methods
Mouse model
Female Kunming species mice (female), 6–8
generation, average 12 month-old) were purchased from Shanghai
Experimental Animal Center. The animal studies were in compliance
with the university rules of conduct and adhered to the principles
of Institutional Animal Care and Use Committee Guidebook
(http://en.wikipedia.org). The use, management and
welfare of the study animals met the Chinese Animal Care
Regulations for Captive and Laboratory Animals as outlined in the
1988 National Regulation on Laboratory Animal Research, issued by
the Ministry of Science and Technology. The animals were housed in
accordance with Guidelines for the Care and Use of Laboratory
Animals in scientific research (Chinese National Science Academy)
in registered animal facility. The animals were maintained in Cabin
type isolators at standard environmental condition (temperature
22–25°C, humidity 40–70%) with 12:12 dark/ light period. The mice
were palpated on the breast every 3 days, trained technicians
palpated all mammary glands of all animals and noted the location
and size of all nodules, using standard technique (13–15).
The mice were split to breed after cancer being found. Tumor weight
was estimated by palpation. No precise quantitative guidelines such
as the acceptable upper limit of tumor burden was available, since
the adverse effects on the host depend on the biology of the tumor,
the site and mode of growth.
In total 398, mice aged 11 to 12 months, were used
in this study. Of the 89 cancer-bearing mice, spontaneous breast
cancer was found with an average of 307 days after birth (306 to
448 days). After euthanasia, mammary glands and spontaneous breast
cancer tissues were collected from each cancer-bearing animal at
difference stages. The control mammary glands were collected from
18 month-old mice in two abdominal mammary glands. The final volume
of cancer tissue was measured by the method of water immersion
(23).
Histopathological analysis
Histopathological evaluations were done. After
euthanasia, mammary tumors and all organs were collected in 10%
buffered formalin (liver, lungs, kidneys, heart, spleen, brain,
pancreas, bone, adrenals, small and large intestine, uterus, ovary,
cervix and urinary bladder). Formalin-fixed and paraffin-embedded
tissues were cut at 5 μm thickness, stained with haematoxylin and
eosin following standard procedure and examined under a light
microscope.
Immunohistochemistry
Sections from formalin-fixed, paraffin embedded
tumors were cut and mounted on slides. After deparaffinization in
xylene, slides were rehydrated through graded series of alcohol and
placed in Tris buffer. Endogenous peroxidase activity was blocked
with 3% hydrogen peroxidase and methanol. Commercially available
antibodies to ER, PR, and Her2/neu, were used in the study. After
tissue pretreatment including steam antigen retrieval and protein
block, slides were incubated with antibody followed by incubation
with horseradish peroxidase conjugated HRP. 3,3′-Diaminobenzidine
tetrahydrochloride (DAB) chromogen was used for visualization of
the antibody/ enzyme complex. Appropriate positive and negative
controls were included with each IHC run. All cases were studied
for ER, PR and Her2 antibodies. Staining results were assessed by
two of the authors on a double headed microscope. A case was
considered positive for a given marker only when both observers
agreed upon its specificity and distribution. Nuclear or membrane
immunostaining for ER, PR and Her2 was evaluated counting a total
of 1,000 cells in 10 representative fields at high magnification
(×200). The number of immunopositive cells was expressed as a
percentage (mean, median, minimum and maximum values).
The intensity of ER, PR and Her2 immunoreactivity
was graded on a scale of 0 to 3, in which 0, no reactivity; 1,
weak; 2, moderate; and 3, strong reactivity.
Western immunoblot analysis
Protein was extracted from fresh-frozen biopsy
specimens from Kunming mice mammary carcinomas and normal mammary
glands. Each sample was placed in 2-ml Eppendorf safe-lock tubes
and immersed in Laemmli buffer for lysis. After incubation on ice
for 20 min, tissue lysates were clarified for 10 min at 12,000 × g
at 4°C, denatured at 95°C for 5 min, and stored at −80°C until
needed. Protein concentrations were normalized using BCA reagent
according to the manufacturer’s protocol (Pierce, Rockford, IL).
For electrophoresis, protein extracts from fresh-frozen mammary
cancer were subjected to SDS-PAGE in 8% polyacrylamide gels
according to Laemmli et al (24). Electrophoresis was stopped when the
tracker dye reached the end of gels.
For western immunoblots, electrophoresed proteins
were transferred to nitrocellulose membranes and blocked in
phosphate-buffered saline, 0.05% Tween-20 (PBS-T), plus 5% skim
milk for 1 h or overnight. The membrane was then incubated with the
cyclin D1, VEGF, c-Myc HER-2/ neu polyclonal rabbit anti-human
antibody at 1:1,000 dilution (DakoCytomation) in PBS-T plus 2% skim
milk for 2 h, washed five times with PBS-T, and incubated for 1 h
with peroxidase-conjugated goat anti-rabbit secondary antibodies
(Sigma-Aldrich) in PBS-T plus 2% milk. Membranes were visualized
with ECL+ after incubation with anti-mouse or rabbit
secondary antibody (1:5,000) (GE Healthcare).
Statistical analysis
Statistical analysis was performed by Statistical
Package for Social Sciences (SPSS) Version 17.0. All of the
statistical tests were two-tailed and P-value less than 0.05 was
considered as statistically significant.
Results
Animals
Kunming mammary tumors were observed in female
breeding mice, mostly after delivering the 6–8th litter (about
average 11.5 months), after stopping breeding they continued to
rear 1–8 weeks. Among the 398 females bred, 89 mice developed
mammary carcinoma (incidence rate 25%). The age of tumor occurrence
was on average about 13.5 months (range 12–16 months), the life
span of mammary tumor mice was about 8 weeks (6–10 weeks). The
females that were not mated did not develop tumors during their
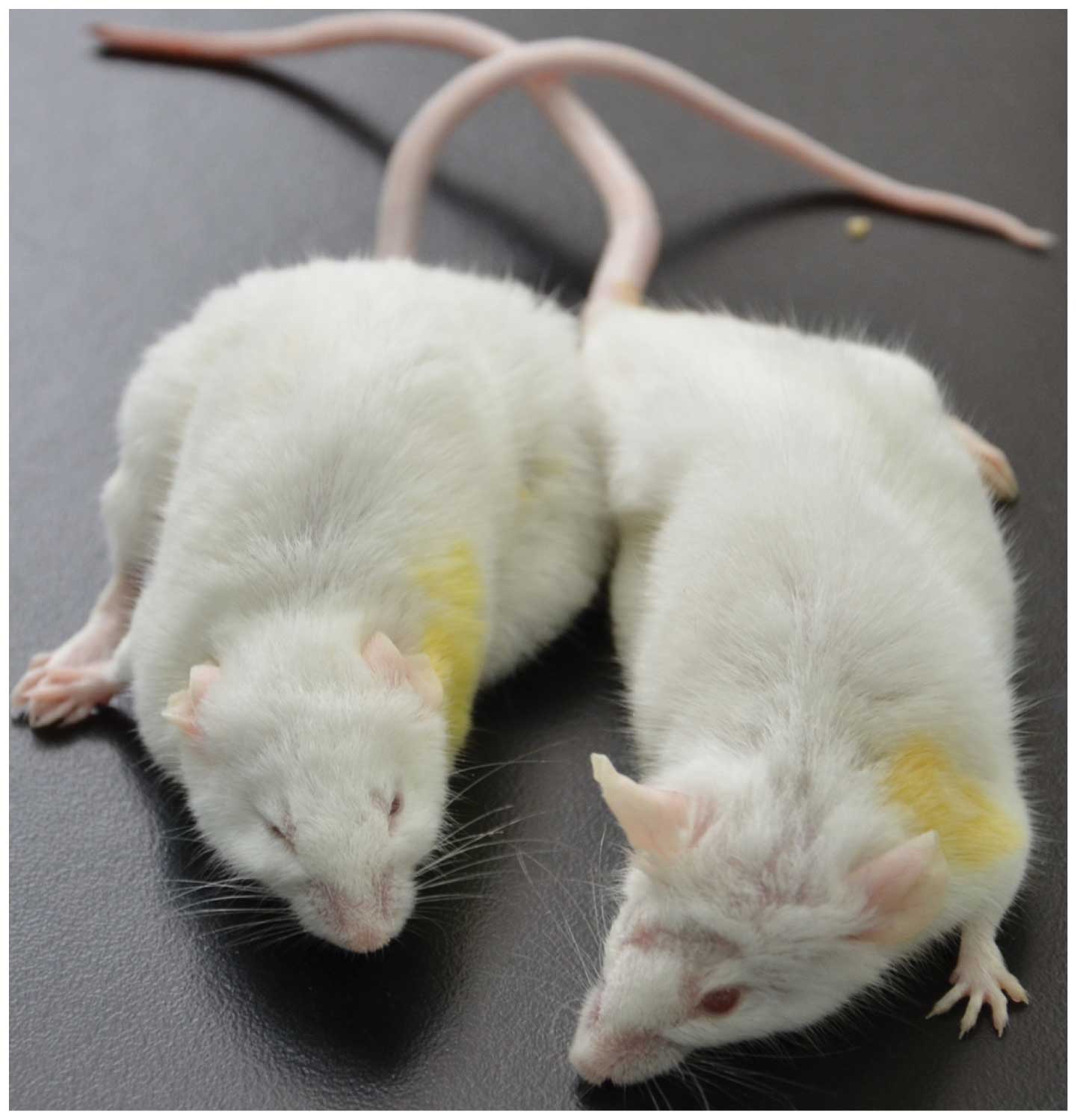
life span (data not shown). The tumors were unilateral i.e.,
developed either in right or left side mammary glands and were
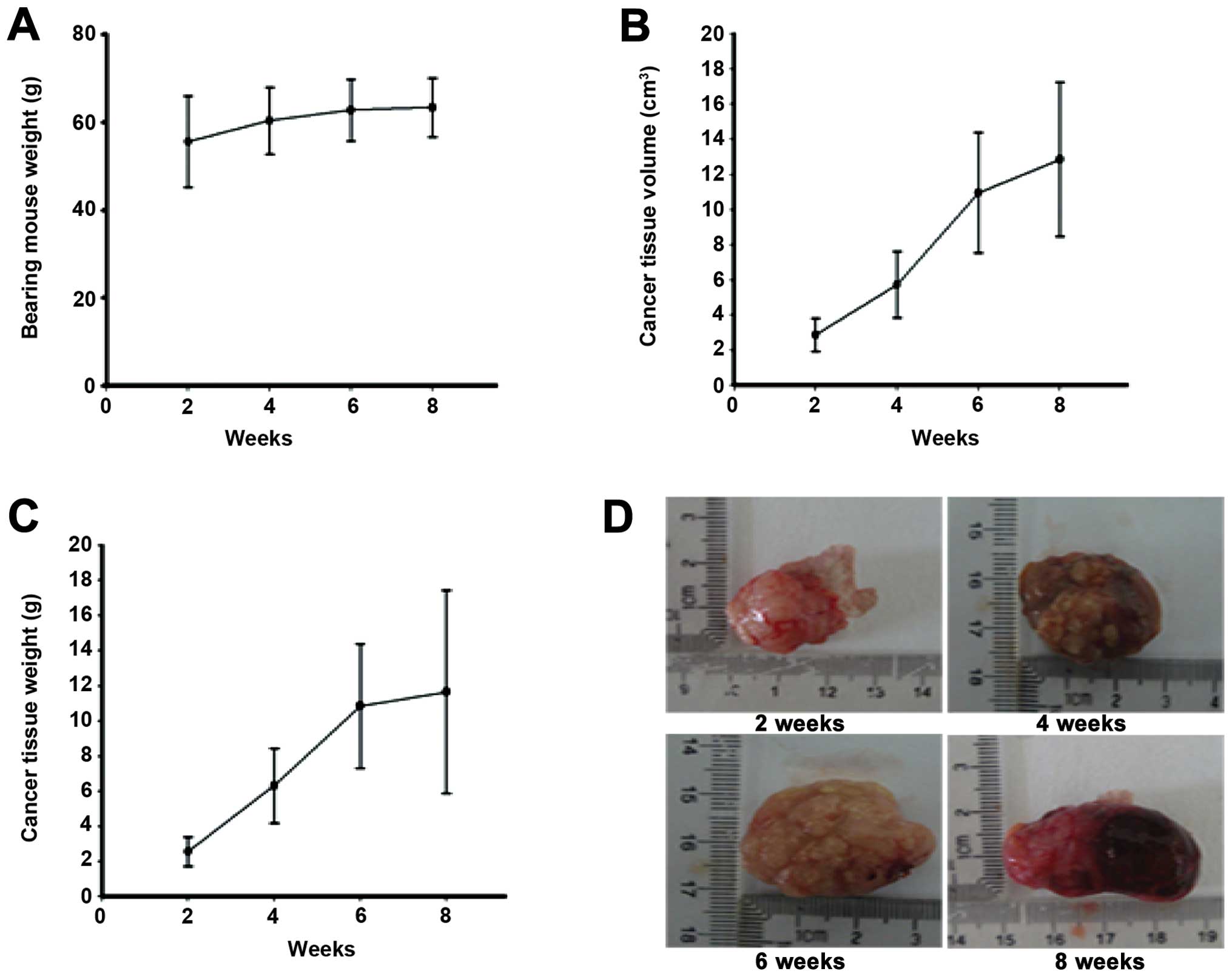
observed only in the 2nd or 5th or both mammary glands (Fig. 1). The tumor grew rapidly in the
initial stage and reached 10% body weight in about 2 weeks. At the
time, the body weight of the bearing mice was increased. During 4–6
weeks, the mice got thin gradually, and the body weight did not
increase obviously (Fig. 2A). At
the stage that they were euthanasized for ethical reasons the
average body weight was about 62 g, cancer weight was about 12 g
and the tumor-size was about 13 cm3 (Fig. 2B and C). The life span was about 62
days.
Foci develop in mammary cancer of Kunming
mice
About 4 weeks after cancer development, some of
neoplastic mass appeared as multi-lobes with ulceration on the skin
surface. On palpation, fluid thrills were observed in each lobe.
Exteriorized tumor mass revealed multi-lobes at 6, 8 weeks, each
lobe had multiple solid nodules enclosing a central necrosed tissue
with foul smelling inflammatory exudates and clotted blood
(Fig. 2D).
Histologically, the expansile tumor mass pushed
through the overlying dermis and was found infiltrating the muscle
layer underlying the mammary gland and had indistinct borders at 6
weeks. The tumor mass was similar to tubular epithelial-like
morphology with differentiated lobes (Fig. 2D).
Histopathological analysis
Resected specimens were sectioned at 5 μm
continuously and microscopically examined after hematoxylin and
eosin staining. The morphologically normal ducts are surrounded by
a well-developed basement membrane with a terminal duct entering a
cluster of lobules (Fig. 3A1 and
A2). Such a lobular-like structure, however, is similar to the
lobular development found in the mouse mammary glands of early to
mid-pregnant mice and also appear similar in structure to the
terminal duct lobular unit in a normal adult on white adipose cells
(25–27). One marked difference between the
mouse mammary gland and human breast tissue is that the mouse tumor
and ducts are surrounded by a thin fibroblastic stroma adjacent to
the adipocytes that form the majority of the gland and which are
often very close to the epithelial structures (Fig. 3B). In contrast, in humans, the
lobules have extensive extracellular connective tissue stroma,
therefore the adipocytes are less proximal to the ductal epithelium
human breast (28,29).
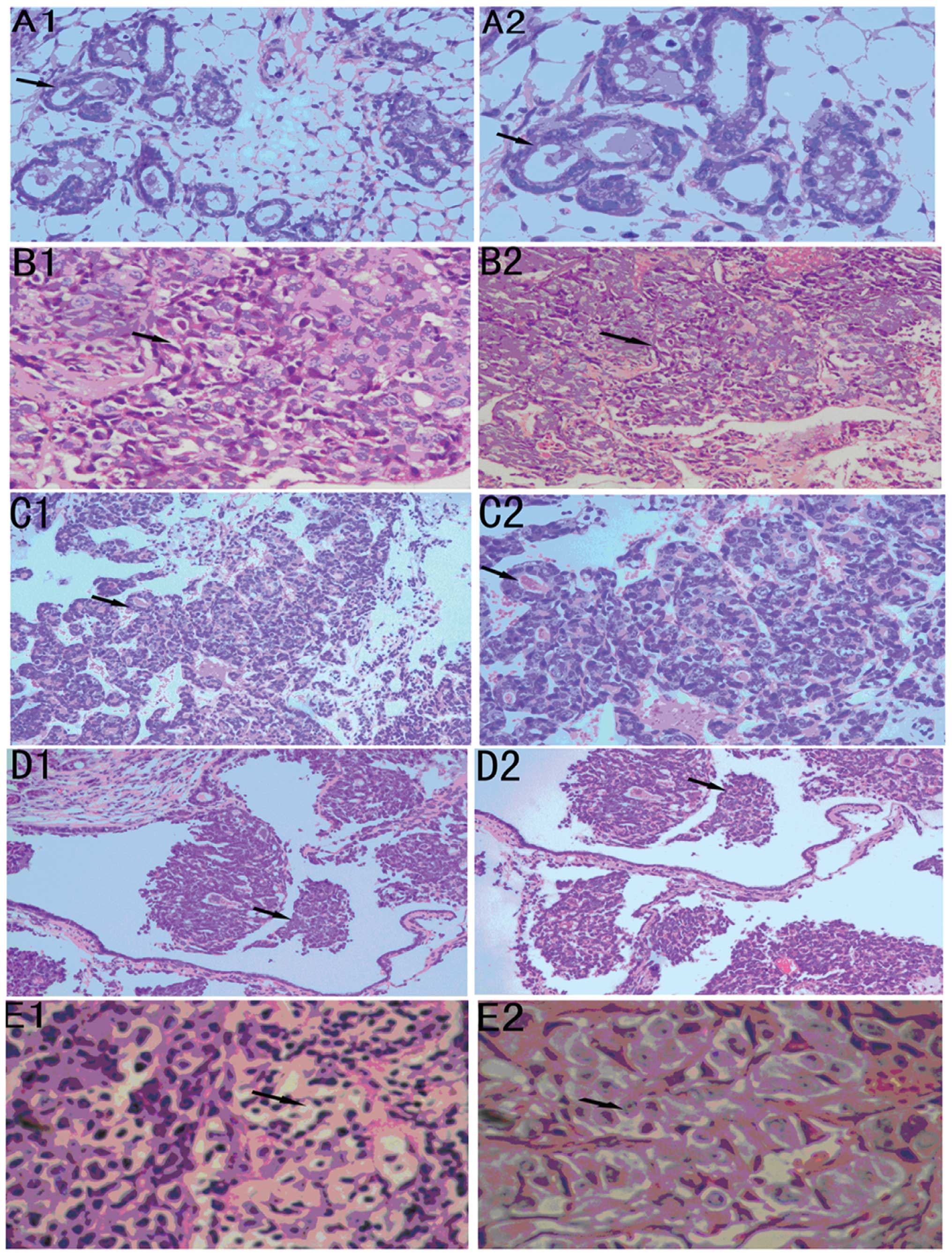 | Figure 3Mouse and human breast cancer
pathology at different stages. (A) Histopathology of normal mouse
mammary gland, the epithelial cells are vacuolated with cytoplasmic
accumulation of fat droplets (A1, ×200; A2, ×400). (B) At two weeks
cancer tissue cells are lined with hyperchromatic nuclei and rarely
prominent nucleoli. Few myoepithelial cells are admixed in the
hyperplastic epithelium (B1, ×100; B2, ×200). (C) At four weeks
cancer tissue was high-grade comedo ductal carcinoma in situ
and there were few mammary gland ducts. Highly pleomorphic cuboidal
to oval cells with abundant eosinophilic granular cytoplasm, round
nuclei and prominent nucleoli (C1, ×100; C2, ×200). (D) At eight
weeks cancer tissue was in a distended duct with central necrosis.
Lymphocytes and plasma cells infiltrate the periductal stroma (D1,
×200; D2, ×400). (E) Intermediate-grade ductal carcinoma in
situ in woman with proliferation of pleomorphic cuboidal cells
with moderate eosinophilic cytoplasm, oval to elongate nuclei, and
single prominent nucleoli (E1, ×200; E2, ×400). |
The cytological atypia is a distinct morphological
change typically found in the center of the tumor at 2–4 weeks in
cancer tissue, the tumor cells now appear pleomorphic, showing a
moderate variation in size and shape (Fig. 3B1 and B2) and nuclear pleomorphism.
The majority of cells are mononuclear with macrophage morphology.
An increased vessel density is found in the vicinity of the tumor
adjacent to the areas with dense leukocytic infiltration (Fig. 3C1 and B2). High density of
leukocytic infiltration is often observed in the human tumors
(28,29). The majority of the ducts in the
mammary glands that carry these early carcinomas are still
morphologically normal, except for focal areas in which there is
mild ductal epithelial hyperplasia with a small increase in the
number of cell layers (data not shown).
By 6 to 8 weeks the primary tumors progressed to the
advanced carcinoma stage, termed late carcinoma. At this stage the
tumor is composed of solid sheets of epithelial cells with little
or no remaining acinar structures visible (Fig. 3D1 and D2). The malignant cells in
the tumor have marked variation in cellular and nuclear size and
shape with vesicular nuclei and prominent nucleoli.
The patterns in the human tissue are heterogeneous,
some solid sheets of cells filled the terminal duct lobular unit
surrounded by a well-formed basement membrane and connective
tissue. At this late carcinoma stage, multiple tumor nodules as
well as ductal hyperplasia were found throughout the mammary gland
(data not shown). Kunming mouse tumors at this stage have many
characteristics that are similar to those of a human breast cancer
classified as poorly differentiated invasive ductal carcinoma. The
tubular structure disappeared. Multiple area of necrosis with
infiltration of phlegmonosis material appeared in between and
within mammary glands duct at 8 weeks. These infiltrates are
composed of cells with the morphology of macrophages, fibroblasts
and neutrophils. There was evidence of necrosis at higher
magnification (Fig. 3E1 and E2).
An increased vascularity was also observed at these sites.
Neoplastic tubular epithelial cells metastasized
into the liver and lungs (Fig. 4A and
B, long arrows). No metastatic foci in bone, heart, adrenal,
kidney, brain, intestines and pancreas were detected (Fig. 4C–T). Nodular-type tumors were found
in the liver and lungs. Single foci of tumor emboli consisting of
pleomorphic neoplastic cells spreading through lung blood vessels
were present in the lungs. Large sized pleomorphic neoplastic cells
were found in live follicles. Nodular-type tumors consisting of
pleomorphic tubular epithelial cells were found attached to inner
wall of the lung aorta (data not shown).
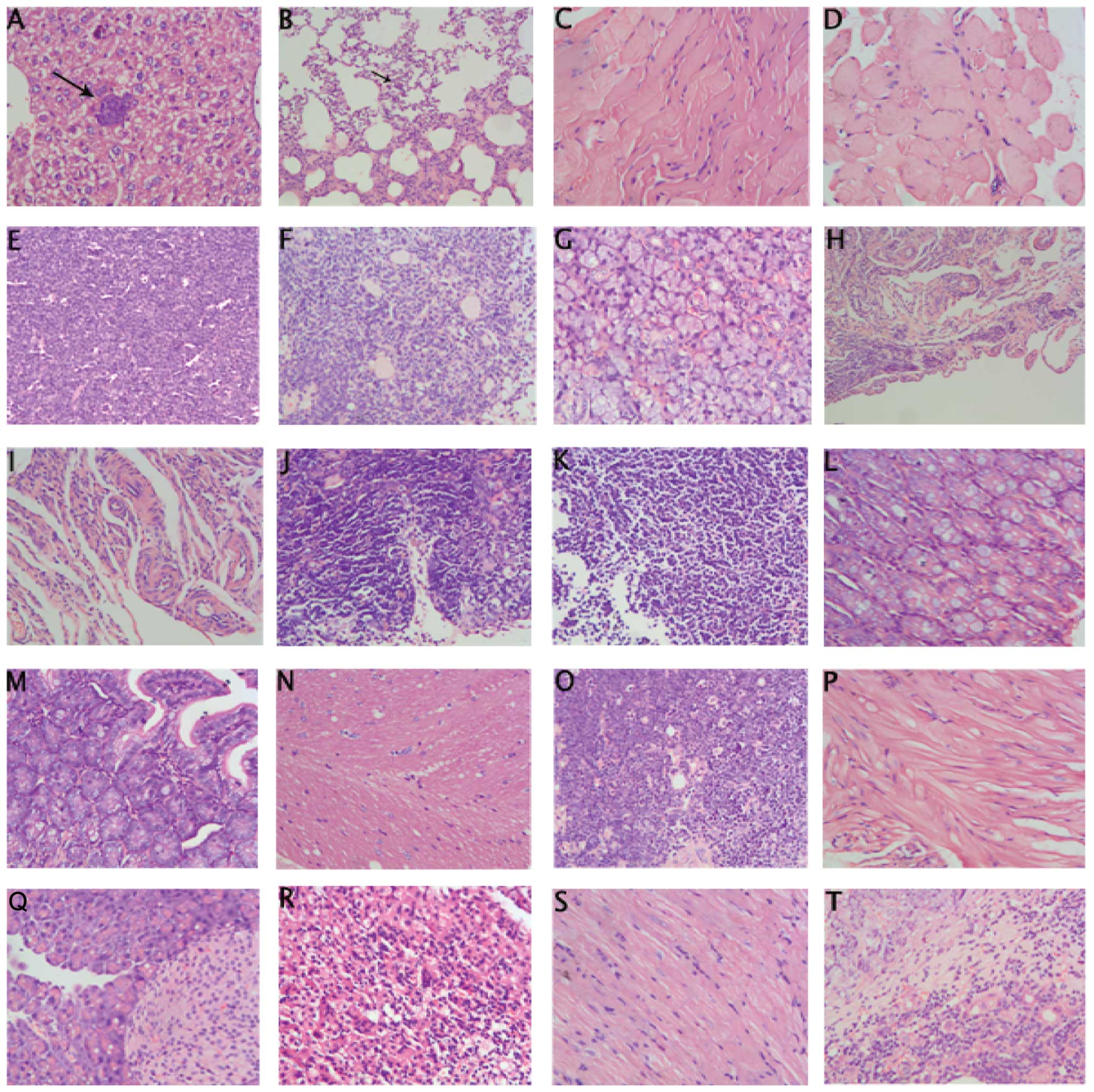 | Figure 4Tumor metastasis and non-tumor
metastasis tissue. i) Metastatic foci in liver showing luminal
epithelial-like morphology as in the primary (A, arrow, ×200). ii)
Metastatic foci in lung by single or multiple layers of pleomorphic
epithelial cells (B, arrow, ×200). iii) Non-tumor metastasis tissue
(C, sternum; D, pectoralis major; E, lymphaden; F, ovary; G,
submandibular gland; H, uterus; I, cervix; J, thymus; K, spleen; L,
large intestine; M, small intestine; N, cerebrum; O, omentum majus;
P, bladder; Q, pancreas; R, adrenal glands; S, heart; T, liver;
magnification, ×200). |
Immunochemistry of ER/PR and HER2
expression
Breast cancer is a heterogeneous disease with a
variety of pathological entities and varied clinical behavior and
different molecular alterations driving its growth, survival and
response to treatment. Estrogen-α and progesterone-receptor (ER and
PR) expression is routinely used to determine the prognosis of
human breast cancers, expression loss being associated with poor
prognosis (20). To further
examine the relevance of the Kunming mouse model for studies on
human breast cancer, the expression of ER-α and PR in the tumors of
Kunming mice at difference time was examined with IHC.
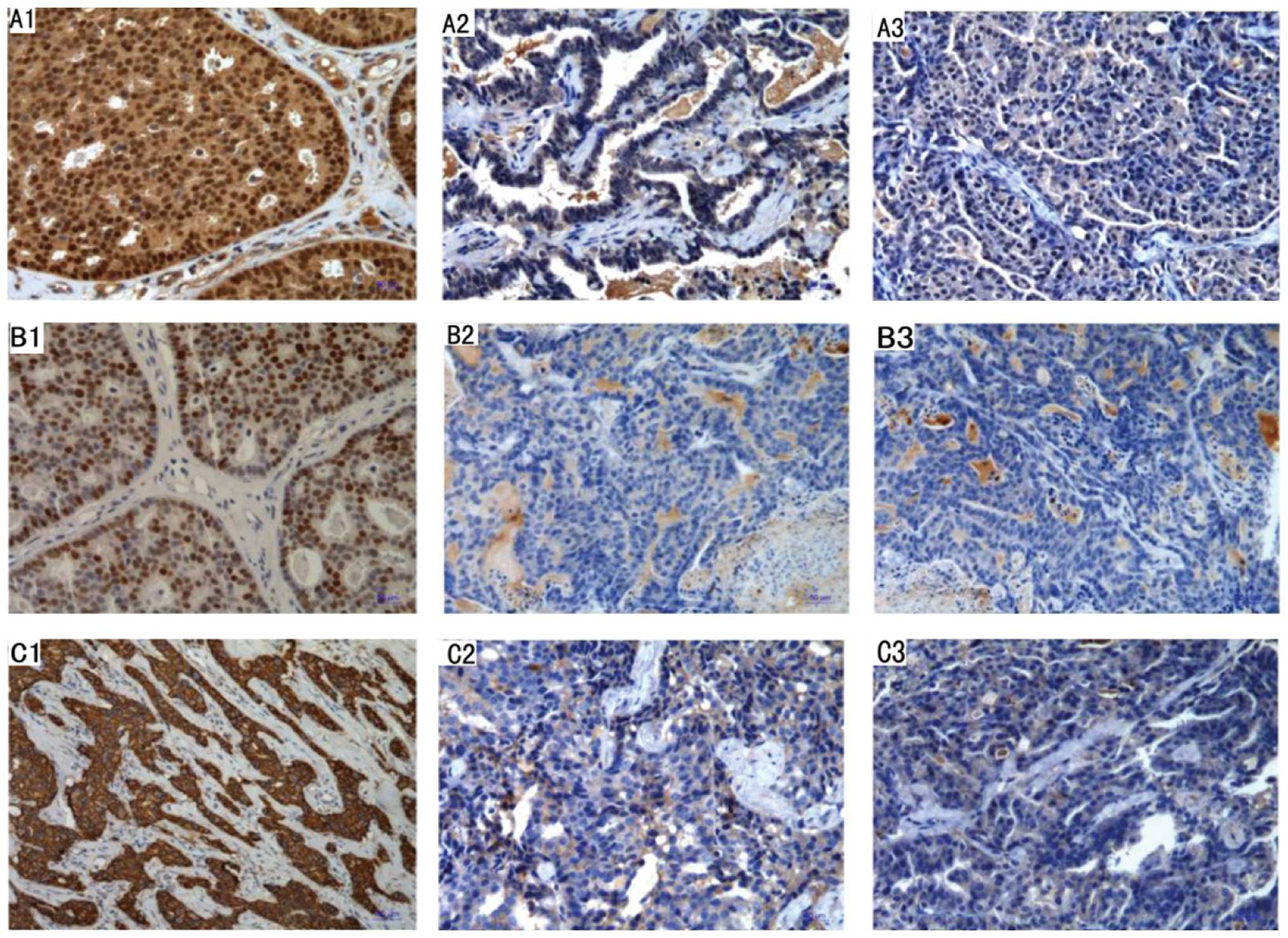
The IHC result indicated that both nuclear ER-α and
PR immunoreactivity and nuclear colocalization were not present in
the difference stages of breast cancer cells (Fig. 5A2, A3, B2 and B3). There was
virtually no ER-α, PR-positive cells in the tumor, except in the
residual ductal structures inside or adjacent to the tumor, but
HER2 was weakly positive (Fig. 5C1 and
C2).
All tumors were ER−, PR−, but
HER2 was weakly positive [20% cells with nuclear staining (Fig. 5C1 and C2). Tumors with
ER− and/or PR− are known to have higher risk
of mortality after diagnosis compared to women with ER+
and/or PR+ disease. Several clinical studies have
demonstrated women with HR− tumors have poor survival
advantage by treatment with adjuvant hormonal and/or
chemotherapeutic regimens (30–33).
Marker expression detected by immunoblot
analysis
To investigate maker gene expression in normal
mammary tissue and cancer tissue, 8 normal mouse mammary glands and
8 malignant mouse mammary tumors were subjected to western
immunoblot analysis. The antibodies were also used to probe western
blots of protein lysates of age-matched mammary glands and tumors
at different stages of progression that had been carefully
dissected away from the surrounding tissues.
A 62-kD band, corresponding to c-Myc (Abcom), was
observed in tumor samples at different stages (Fig. 4A). Differences in band intensity
were observed at comparable total protein loads. In particular,
blotting of neoplastic tissues at four different stages produced
four different types of bands, the strongest intensity was at 2
weeks (Fig. 4, lane 2), and
intermediate intensity at 4 to 8 weeks (Fig. 4, lanes 3–5). Normal mammary tissues
had a clearly detectable band of weak intensity (Fig. 4, lane 1).
The expression of cyclin D1 (Santa Cruz
Biotechnology, Santa Cruz, CA) is found in the developing tumor and
the mammary gland of Kunming mice. There is an increase in the
tumor cell expression while at the same time the expression in
normal ducts decreases. Anti-cyclin D1 antibodies recognizes a
43-kD protein in mammary tumors at various ages (Fig. 4C). The blotting at 2 to 4 weeks
were the brightest at comparable total protein intensity (Fig. 4C, lanes 2 and 3), but weaker at 6
to 8 weeks. Normal mammary tissue was the weakest (Fig. 4C, lanes 4 and 5).
The expression of VEGF gene was not different
(Fig. 4B). The band intensity in 6
to 8 weeks was the strongest in all the bands (Fig. 4B, lanes 4 and 5), but weaker at 2
to 4 weeks (Fig. 4B, lanes 2 and
3). The normal bands were the weakest (Fig. 4B, lane 1).
Discussion
Breast cancer is the most frequent cancer in women
(23% of all cancers), and it ranks second overall when both sexes
are considered together (34). So
it is very important to investigate breast carcinogenesis and
cancer progression. Animal models are powerful tools to analyze the
mechanism of the induction of human breast cancer. Most tumors are
ductal infiltrating carcinomas expressing estrogen and progesterone
receptors. The majority of the genetically modified mouse breast
cancer models as well as most spontaneous, chemically or mouse
mammary tumor virus (MMTV)-induced mammary tumors in mice do not
express ER and PR, or if they do (some MMTV models), they are
pregnancy-dependent (35).
Although the application of transgenic technology in mice to study
the progression of mammary cancer has proven extremely powerful to
understand important principles of tumorigenesis and evaluating
response to therapy, few of these models reflect the complexity of
human breast cancers, especially their progression to metastasis as
these models lack many aspects of human cancers. A lack of
understanding about the natural history of the disease is a major
contributory factor to this limitation (2–6). So
a successful animal model that develops spontaneous mammary tumors
that resemble human breast cancer in many aspects is needed.
Kunming mouse (KM mouse) is a genetically
heterogeneous mouse. So the mice bear genetically heterogeneous
spontaneous mammary tumors, similarly to randomly selected groups
of cancer patients. Therefore, the model is an alternative to other
rodents. To our knowledge, this is the first report to use
closed-population Kunming mouse to make spontaneous breast
cancer.
KM mice are a unique closed population of laboratory
mice in China. They are widely used in pharmacology, toxicology and
other experiments (6–11). Some scholars in China have made
all-round studies and thought that KM mice is different from NIH
and other international renowned out-bred, in that it is a out-bred
mouse with unique genetic traits. The ancestor of Kunming mice was
traceable from Western Europe Mus Musculus domesticus and
was introduced to China’s Yunnan province. The ancestor of KM mice
was polluted by Mus Musculus domesticus paternal genetics
and gradually evolved into a different one from other out-bred
populations with unique genetic characteristics. Experimental
animals in a closed group require more than 5 years of closed
breeding, random mating breeding method, production and
reproduction (6–16). The species average age was 11.5
months at cessation of births. Those continuing to feed for 2–3
weeks suffered spontaneous breast cancer. On pathological
diagnosis, the model is invasive ductal carcinoma (17,18).
Ductal carcinoma is the most common histological
category of malignant breast tumors, lobular carcinoma is the
second major type while medullary carcinoma is a relatively rare
entity. On clinical diagnosis, the various presentations are
classified on the basis of morphological and molecular examination.
Prognosis is defined according to a number of parameters, tumor
size and grade, the presence/absence of estrogen and/or
progesterone receptors, HER2/neu (HER2, c-erbB2) protein, vascular
or perineural tumor invasion (33,34).
Our HIC data show that all the different stages of
cancer cells were ER−, PR− and HER2 weak
positive. So the sub-classification of the spontaneous breast
cancer was luminal B. Most mice (80%) tend to have ER−,
highly aggressive mammary tumors; thus, the spontaneous breast
cancer model may be particularly suitable as an animal model of
human hormone-resistance breast cancer.
HER-2/neu is a cell-membrane receptor tyrosine
kinase, normally involved in the signal transduction pathways
leading to cell growth and differentiation (2). Approximately 15–20% of breast cancers
have amplification of the HER-2/neu gene or overexpression of its
protein product (33). So the weak
expression of Her2 resulted in tumor cell morphological differences
among different stages of Kunming tumor-bearing mice in cancer
tissue and partial necrosis in 6–8 weeks of tumor cells.
Most of the model displayed metastases to the liver
and lungs. The tumors predominantly had luminal/tubular
epithelial-like morphology (Fig. 4A
and B). But other organs were free of metastasis. The spleens
were enlarged at 6 to 8 weeks. The result showed hematogenous
spread almost exclusively to the lungs, in contrast to human
tumors, which show regional lymph node involvement with
preferential spread to the liver.
In recent years, understanding of the underlying
biological mechanisms of carcinogenesis and the altered molecular
events has led to the identification of novel molecular targets and
development of targeted therapies. Targeting the pathways that
promote or sustain growth and invasion of carcinoma cells is
critical to effective treatment of breast cancer. A better
understanding of the biology of ER, PR-negative breast cancer is
therefore needed. In this study, we investigated the common
chromosomal amplifications found in human breast cancer, such as
c-Myc and cyclin D1 (37–39).
The blotting result showed that c-Myc expression was
significantly elevated in all the tumors tissues. Prior studies
have examined Myc expression in breast cancers and the basal breast
cancer subtype exhibits enrichment for a c-Myc transcriptional gene
signature (36–39); so the hight expression of c-Myc in
Kunming mice leads to proliferation of spontaneous breast cancer
cells, and the cancer volume is greatly enlarged (Fig. 2D). Following tumor growth, the
cancer needs additional nutrition, for new blood vessels formation.
Thus, the expression of VEGF was increased significantly (Fig. 2D and 6B).
The differences in c-Myc expression allowed
reciprocal studies on induction or repression of the metastatic
phenotype by manipulation of c-Myc expression and its downstream
targets. Clearly human breast cancers that overexpress c-Myc may
still metastasize if other factors override its function (38,39).
Our in vivo assays demonstrated that expression of c-Myc
will support increased growth of those few metastatic cells that
escape the inhibitory function of Myc by means of other mutations
or by changes in gene expression (Fig.
6A). This phenomenon can help us explain why some Kunming mouse
breast cancer cells metastasize to the lungs and the liver.
Several converging studies have suggested that c-Myc
can be involved in the activation of cyclins (D1, D2, E1 and A2),
cyclin-dependent kinases (CDK4), and in the downregulation of cell
cycle inhibitors. The expression of Myc and CCND1 constitutes an
early and transient event. The regulation of human CCND1 by
progestins may be more complicated, while it has been suggested
that PR regulates CCND1 expression by non-genomic mechanisms
(36). The high expression of
cyclin D1 may be related with this mechanism.
Loss of estrogen and progesterone receptor gene
expression has been found in 30% of human breast cancers, and this
condition is associated with less differentiated tumors and poor
clinical outcome. Similarly, overexpression of ErbB2/neu and cyclin
D1 has been found in ~20% of cases and this also correlates with
poor prognosis (40,41). Remarkably, these phenomena seem to
be recapitulated in the model with loss of ER and PR and weak
expression of Her2/neu and overexpression of cyclin D1, suggesting
a common pathway to malignancy between mammary cancers in mouse and
human.
In the present study we performed a detailed
histological and molecular marker analysis that showed many
similarities to the histology of human tumors. We also analyzed a
series of biomarkers associated with poor prognosis in human breast
cancer. Remarkably in the Kunming mouse model, loss of estrogen and
progesterone receptors and low expression of Her2/neu and
overexpression of c-Myc, cyclin D1 and VEGF were observed, which is
recapitulated in a manner similar to that observed in human breast
cancer with poor prognosis. The animal spontaneous tumors are
suitable models for human cancer, primarily because both animal
population and the tumors are genetically heterogeneous. It
provides a new model for future study on prognosis, drug trials and
clinical management of breast cancer in women.
Acknowledgements
This study was supported by Chinese National Natural
Science Fundation (no. 81160531), Jiangxi Natural Science Fundation
(no. 20114BAB205051), Jiangxi Department of Education (no.
GJJ10528).
References
|
1
|
Silber JH, Rosenbaum PR, Clark AS, et al:
Characteristics associated with differences in survival among black
and white women with breast cancer. JAMA. 310:389–397. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Mahesh Kumar MJ, Ponvijay KS and Nandhini
R: A mouse model for luminal epithelial-like ER positive subtype of
human breast cancer. BMC Cancer. 7:180–189. 2007.PubMed/NCBI
|
|
3
|
Rostoker R, Bitton-Worms K, Caspi A, et
al: Investigating new therapeutic strategies targeting
hyperinsulinemia’s mitogenic effects in a female mouse breast
cancer model. Endocrinology. 154:1701–1710. 2013.
|
|
4
|
Pierpaoli E, Viola V, Barucca A, et al:
Effect of annatto-tocotrienols supplementation on the development
of mammary tumors in HER-2/neu transgenic mice. Carcinogenesis.
34:1352–1360. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Futakuchi M and Singh RK: Animal model for
mammary tumor growth in the bone microenvironment. Breast Cancer.
20:195–203. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lin EY, Jones JG, Li P, et al: Progression
to malignancy in the polyoma middle T oncoprotein mouse breast
cancer model provides a reliable model for human diseases. Am J
Pathol. 163:2113–2126. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Dai Y, Cui J, Cun Y, et al:
Tetrahydrobiopterin ameliorates hepatic ischemia-reperfusion injury
by coupling with eNOS in mice. J Surg Res. 176:e65–e71. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lin Y, Wu D, Zeng WX, et al: Effect of
threonine on immunity and reproductive performance of male mice
infected with pseudorabies virus. Anima. 6:1821–1829. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wang W, Zheng H, Yang S, et al: Origin and
evolution of new exons in rodents. Genome Res. 15:1258–1264. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yu N, Yang J and Yin T: Extracts from a
traditional Chinese herbal remedy (Zhuyun recipe) improve
endometrial receptivity in mice with embryonic implantation
dysfunction and ovulatin stimulation. Ethnopharmacology.
137:389–395. 2011. View Article : Google Scholar
|
|
11
|
Ma P, Wu Y, Zeng Q, et al: Oxidative
damage induced by chlorpyrifos in the hepatic and renal tissue of
Kunming mice and the antioxidant role of vitamin E. Food Chem
Toxicol. 58:177–183. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Peng Q, Li RY, Jia B, et al: Sex control
by Zfy siRNA in the mouse. Theriogenology. 76:507–511. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Fan Y, Huang ZY, Cao CC, et al: Genome of
the Chinese tree shrew. Nat Commun. 4:1426–1436. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Shi P, Zhang J, Yang H, et al: Adaptive
diversification of bitter taste receptor genes in mammalian
evolution. Mol Biol Evol. 20:805–814. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wu X, Wang C, Katoh H, et al: Genetic
profile of LIBP/1 inbred strain derived from the Kunming outbred
stock of the mouse. Jikken Dobutsu. 41:541–543. 1992.(In
Japanese).
|
|
16
|
Zhang WV, Gong CM, Wei YL, et al: A new
way for inbred strain mice genetic monitoring and the discovery of
sex-linkaging RAPD markers. Shi Yan Sheng Wu Xue Bao. 29:59–69.
1996.(In Chinese).
|
|
17
|
Zheng LX, Liu HN, Qiao YD, et al:
Expression of vascular endothelial growth factor and Cyclin D3 gene
on Liu Wei Di Huang Pill in spontaneous breast cancer tissue. Chin
J Exp Trad Med Form. 11:117–119. 2010.
|
|
18
|
Zheng LX, Lin DM, Liu HN, et al: Mechanism
of nourishing Yin prescription inhibiting spontaneous breast cancer
grown by TGF-β signal pathway. Chin Trad Pat Med. 10:1793–1795.
2011.
|
|
19
|
Bouchalova K, Cizkova M, Cwiertka K, et
al: Triple negative breast cancer - current status and prospective
targeted treatment based on Her1 (EGFR), Top2A and C-MYC gene
assessment. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub.
153:13–17. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ortiz AP, Frías O, Pérez J, et al: Breast
cancer molecular subtypes and survival in a hospital-based sample
in Puerto Rico. Cancer Med. 2:343–350. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sørlie T, Perou CM, Tibshirani R, et al:
Gene expression patterns of breast carcinomas distinguish tumor
subclasses with clinical implications. Proc Natl Acad Sci USA.
98:10869–10874. 2001.PubMed/NCBI
|
|
22
|
Sørlie T, Tibshirani R, Parker J, et al:
Repeated observation of breast tumor subtypes in independent gene
expression data sets. Proc Natl Acad Sci USA. 100:8418–8423.
2003.PubMed/NCBI
|
|
23
|
Cornish BH, Thomas BJ, Ward LC, et al: A
new technique for the quantification of peripheral edema with
application in both unilateral and bilateral cases. Angiology.
53:41–47. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Laemmli C, Werlen C and van der Meer JR:
Mutation analysis of the different tfd genes for degradation of
chloroaromatic compounds in Ralstonia eutropha JMP134. Arch
Microbiol. 181:112–121. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hermes GL and McClintock MK: Isolation and
the timing of mammary gland development, gonadarche, and ovarian
senescence: Implications for mammary tumor burden. Dev Psychobiol.
50:353–360. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Russo J and Russo IH: Atlas and histologic
classification of tumors of the rat mammary gland. J Mammary Gland
Biol Neoplasia. 5:187–200. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hermes GL, Delgado B, Tretiakova M, et al:
Social isolation dysregulates endocrine and behavioral stress while
increasing malignant burden of spontaneous mammary tumors. Proc
Natl Acad Sci USA. 29:22393–22398. 2009. View Article : Google Scholar
|
|
28
|
Mallon E, Osin P, Nasiri N, Blain I, et
al: The basic pathology of human breast cancer. J Mammary Gland
Biol Neoplasia. 5:139–163. 2000. View Article : Google Scholar
|
|
29
|
Howard BA and Gusterson BA: Human breast
development. J Mammary Gland Biol Neoplasia. 5:119–137. 2000.
View Article : Google Scholar
|
|
30
|
Burrai GP, Mohammed SI, Miller MA, Marras
V, et al: Spontaneous feline mammary intraepithelial lesions as a
model for human estrogen receptor- and progesterone
receptor-negative breast lesions. BMC Cancer. 10:156–167. 2010.
View Article : Google Scholar
|
|
31
|
Munjal K, Ambaye A, Evans MF, et al:
Immunohistochemical analysis of ER, PR, Her2 and CK5/6 in
infiltrative breast carcinomas in Indian patients. Asian Pac J
Cancer Prev. 10:773–778. 2009.PubMed/NCBI
|
|
32
|
Adamczyk A, Niemiec J, Ambicka A, et al:
Expression of ER/PR/HER2, basal markers and adhesion molecules in
primary breast cancer and in lymph nodes metastases: a comparative
immunohistochemical analysis. Pol J Pathol. 63:228–234. 2012.
View Article : Google Scholar
|
|
33
|
Sutton LM, Han JS and Molberg KH:
Intratumoral expression level of epidermal growth factor receptor
and cytokeratin 5/6 is significantly associated with nodal and
distant metastases in patients with basal-like triple-negative
breast carcinoma. Am J Clin Pathol. 134:782–787. 2010. View Article : Google Scholar
|
|
34
|
Parkin DM, Bray F, Ferlay J, et al: Global
cancer statistics 2002. CA Cancer J Clin. 55:74–108. 2005.
View Article : Google Scholar
|
|
35
|
Kordon EC: MTV-induced pregnancy-dependent
mammary tumors: early history and new perspectives. J Mammary Gland
Biol Neoplasia. 13:289–297. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Giulianelli S, Vaqué JP, Soldati R, et al:
Estrogen receptor alpha mediates progestin-induced mammary tumor
growth by interacting with progesterone receptors at the cyclin
D1/Myc promoter. Cancer Res. 72:2416–2427. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Alles MC, Gardiner-Garden M, Nott DJ, et
al: Meta-analysis and gene set enrichment relative to er status
reveal elevated activity of MYC and E2F in the ‘basal’ breast
cancer subgroup. PLoS One. 4:e47102009.PubMed/NCBI
|
|
38
|
Chandriani S, Frengen E, Cowling VH, et
al: A core MYC gene expression signature is prominent in basal-like
breast cancer but only partially overlaps the core serum response.
PLoS One. 4:e66932009. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Horiuchi D, Kusdra L, Huskey NE,
Chandriani S, et al: MYC pathway activation in triple-negative
breast cancer is synthetic lethal with CDK inhibition. J Exp Med.
209:679–696. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Li Y, Chen H, Hardy TM and Tollefsbol TO:
Epigenetic regulation of multiple tumor-related genes leads to
suppression of breast tumorigenesis by dietary genistein. PLoS One.
8:e543692013. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Duncan JS, Whittle MC, Nakamura K, Abell
AN, Midland AA, Zawistowski JS, Johnson NL, et al: Dynamic
reprogramming of the kinome in response to targeted MEK inhibition
in triple-negative breast cancer. Cell. 149:307–321. 2012.
View Article : Google Scholar : PubMed/NCBI
|