Introduction
Ovarian cancer, the most lethal gynecologic
malignancy, exists predominantly in the form of epithelial ovarian
cancer (EOC) (1,2). In the United States, EOC is the
leading cause of death among gynecologic malignancies with ~22,280
cases and 15,500 deaths every year (3). Despite the use of aggressive
treatment, most EOC patients develop recurrent cancer, and cancer
metastasis is one of the leading causes of death.
Ginsenosides are the pharmacologically active
components of Panax ginseng (4)
that has long been utilized as a traditional Chinese medicine for
officinal or recuperative purposes (5,6). To
date, >100 ginsenoside compounds have been identified (7), among which the stereoisomer Rg3 is
one of the bioactive extracts with antitumor effect (8,9). It
is reported that Rg3 could play multiple roles similarly to other
ginsenosides, including anti-inflammation properties (10,11),
inhibiting scar hyperplasia of skin (12) and angiogenesis (13). Furthermore, accumulating evidence
supports that Rg3 could become a new medicine for cancer
preventionin, because it can inhibit cancer growth, metastasis and
development (8,9), but the mechanisms are still unclear.
Ginsenoside Rg3 has two optically active chiral molecules 20(R)-Rg3
and 20(S)-Rg3, which differ in the orientation of the hydroxyl (OH)
group on carbon-20 (5). Although
both were reported to inhibit tumor metastasis (8), stereospecifity of Rg3 appears to be
linked to their bioactivities (6).
In our preliminary studies (unpublished), 20(S)-Rg3 robustly
blocked EMT process in ovarian cancer. In this study, we discovered
for the first time that 20(S)-Rg3 effectively inhibits the Warburg
effect of human ovarian cancer cells, promising a novel natural
agent for anti-ovarian cancer therapy.
The Warburg effect, which was first described by
Warburg in the 1930s, is a metabolic reprogramme used by cancer
cells to support their high energy requirements and high rates of
macromolecular synthesis (14,15).
In cancer cells, the main hallmark of the Warburg effect is aerobic
glycolysis, in which glucose consumption and lactate production are
both increased even in the presence of oxygen (16). The Warburg effect not only allows
cancer cells to meet their high energy demands and supply the
anabolic precursors for nucleotide and lipid synthesis, but it also
minimizes reactive oxygen species production in mitochondria,
thereby providing a growth advantage for tumors. Since Warburg
effect plays a crucial role in proliferation and progression of
cancer cells, it provides a novel target for anticancer
therapy.
The mechanism of Warburg effect is also complicated,
because Warburg effect could be impacted by many factors. There is
evidence that the signal transducer and activator of transcription
3 (STAT3) is involved in Warburg effect. STAT3 has emerged as a
potential anti-cancer target as it is crucial in the regulation of
genes involved in cell proliferation and survival, and is
constitutively activated in common human cancers (17,18).
STAT3 can be phosphorylated by tyrosine kinase which is activated
by cytokines, growth factors, or hormones (19). In ovarian cancer, STAT3 siRNA
downregulates cyclin D1, survivin, and vascular endothelial growth
factor expression in HOC cells; inhibits STAT3 and its related
genes; inhibits HOC cell growth; and induces apoptosis in
vitro (20). Recent studies
have found that STAT3 was also involved in regulating glycolysis.
In breast cancer cells, the activation of STAT3 can combine to
hexokinase 2 (HK2) promoter, and then promote HK2 transcription
activation, regulate aerobic glycolysis of breast cancer cells
(21). Sustained activation of
STAT3 can promote glycolysis and inhibit mitochondrial function in
two ways (22). As a nuclear
transcription factor c-myc can participate in regulating the
glycolytic pathway by regulating a variety of enzymes (23), and c-myc is also involved in STAT3
signaling network (24).
Interleukin-6 (IL-6)-STAT3 signaling pathway can affect glycolysis
through regulating HK2 and PFKFB3 (25). Therefore, STAT3 plays an important
role in regulating the Warburg effect.
We discovered that 20(S)-Rg3 significantly inhibits
the Warburg effect in ovarian cancer cells and confirmed that this
process depends on the activation of STAT3. This will add to our
understanding of human ovarian cancer and provide future clinical
approaches to treat this disease.
Materials and methods
Reagents and antibodies
Ginsenoside standard 20(S)-Rg3 was purchased from
Ambo Institute (Seoul, South Korea) and dissolved at a
concentration of 50 mg/ml in DMSO as a stock solution (stored at
−20°C). It was then further diluted in cell culture medium to
create working concentrations. The maximum final concentration of
DMSO was <0.1% for each treatment, and was also used as a
control. Other antibodies such as pyruvate kinase M2 (PKM2), HK2,
phospho-STAT3 (Tyr705), and STAT3 were from Cell Signaling
Technology, Inc. (Beverly, MA, USA).
Cell culture and 20(S)-Rg3 treatment
Human ovarian cancer cell lines SKOV3 (obtained from
ATCC, Manassas, VA, USA) and 3AO (purchased from the Chinese
Academy of Sciences Type Culture Collection, Shanghai, China) were
maintained in RPMI-1640 medium (Gibco-BRL, Gaithersburg, MD, USA)
supplemented with 10% (v/v) fetal bovine serum, 1% penicillin
antibiotics-antimycotics at 37°C under a humidified 5%
CO2 atmosphere. Cells were incubated with 160 μg/ml
(SKOV3) and 80 μg/ml (3AO) concentrations of 20(S)-Rg3 for 24 or 48
h.
Plasmid and transient transfection
The human STAT3 expression vector pcDNA3 hId1
(POSE230074807) was purchased from GeneChem (Shanghai, China),
ovarian cancer cells were seeded into 6-well plates until 80%
confluent and transiently transfected with pcDNA3 hId1 or empty
vector (pcDNA3) as a control using X-tremeGENE HP DNA Transfection
Reagent (Roche Diagnostics, Indianapolis, IN, USA) following the
manufacturer’s instructions. After 48 h of transfection, the cells
were harvested for further study.
Quantitative real-time PCR
Total RNA was isolated using TRIzol reagent
(Invitrogen Life Technologies, Carlsbad, CA, USA) according to the
manufacturer’s instructions. For mRNA detection, first-strand cDNA
was synthesized using a PrimeScript RT Reagent kit (Perfect Real
Time; Takara Bio, Inc., Liaoning, China). Quantitative real-time
PCR was performed using a SYBR Premix Ex Taq™ II kit (Takara Bio,
Inc.) on a CFX96 real-time PCR system (Bio-Rad, Hercules, CA, USA).
β-actin was used as an internal control to normalize the results.
Gene expression was normalized to internal controls, and fold
changes were calculated using relative quantification
(2−ΔΔCt).
Western blot analysis
Cell lysates were collected using Mammalian Protein
Extraction Reagent (Pierce Biotechnology, Inc., Rockford, IL, USA)
containing protease inhibitors (Roche Diagnostics). The protein
concentrations in each sample were determined using the BCA-200
Protein Assay kit (Pierce Biotechnology, Inc.). The proteins were
resolved on 12 or 10% (for other protein detection)
SDS-polyacrylamide gels and transferred onto nitrocellulose
membranes. The membranes were then blocked using blocking buffer
(5% non-fat milk in TBST) and incubated overnight at 4°C with
rabbit anti-human PKM2 (no. D78A4), HK2 (no. C64G5), phospho-STAT3
(Tyr705) (no. 9139), and STAT3 (no. 9145), and mouse anti-human
β-actin (no. 3700S) (all from Cell Signaling Technology, Inc.) at
dilutions of 1:2,000, 1:500, 1:500, 1:500, and 1:1,000,
respectively. After washing with TBST, the blots were visualized
using peroxidase (HRP)-conjugated anti-rabbit or anti-mouse IgG and
ECL reagents (Pierce Biotechnology, Inc.).
Immunohistochemical staining
Paraffin-embedded mouse ovarian cancer tissue
sections on Poly-L-Lysine-coated slides were deparaffinized and
rinsed with 10 mM Tris-HCl (pH 7.4) and 150 mM sodium chloride.
Paroxidase was quenched with methanol and 3% hydrogen peroxide.
Slides were then placed in 10 mM citrate buffer (pH 6.0) at 100°C
for 20 min in a pressurized heating chamber. After incubation with
1:50 dilution of rabbit monoclonal antibody to HK2 (no. C64G5), or
with 1:800 dilution of rabbit monoclonal antibody to PKM2 (no.
D78A4) (both from Cell Signaling Technology, Inc.) for 1 h at room
temperature, slides were thoroughly washed three times with
phosphate-buffered saline. Bound antibodies were detected using the
EnVision Detection Systems Peroxidase/DAB, Rabbit/Mouse kit (Dako,
Glostrup, Denmark). The slides were then counterstained with
hematoxylin.
Glucose consumption and lactate
production assay
To determine the levels of glucose and lactate, the
supernatants of cell culture media were collected and detected
using a glucose and lactate assay kit (BioVision, Inc., Milpitas,
CA, USA) according to the manufacturer’s instructions. The values
at different time periods were analyzed by the optical density
values. Glucose consumption and lactate production were calculated
based on the standard curve, and normalized to the cell number.
Statistical analysis
All experiments were performed at least in
triplicate, and each experiment was independently performed at
least three times. Data are presented as the means ± standard
deviation (SD) and were analyzed using SPSS 19.0 and GraphPad Prism
5 software. Statistical significance was assessed using the
two-tailed unpaired Student’s t-test. Differences were considered
statistically significant when the P-value was <0.05.
Results
20(S)-Rg3, but not 20(R)-Rg3 induces a
metabolic shift in SKOV3 and 3AO ovarian cancer cells
In cancer cells, glucose is preferentially
metabolized by aerobic glycolysis, which differs from mitochondrial
oxidative phosphorylation in normal, non-tumourigenic cells. This
phenomenon, termed the Warburg effect, is characterized by
increased glycolysis and lactate production regardless of oxygen
availability. In our study, we first examined whether 20(S)-Rg3 or
20(R)-Rg3 was capable of inducing a metabolic shift in two human
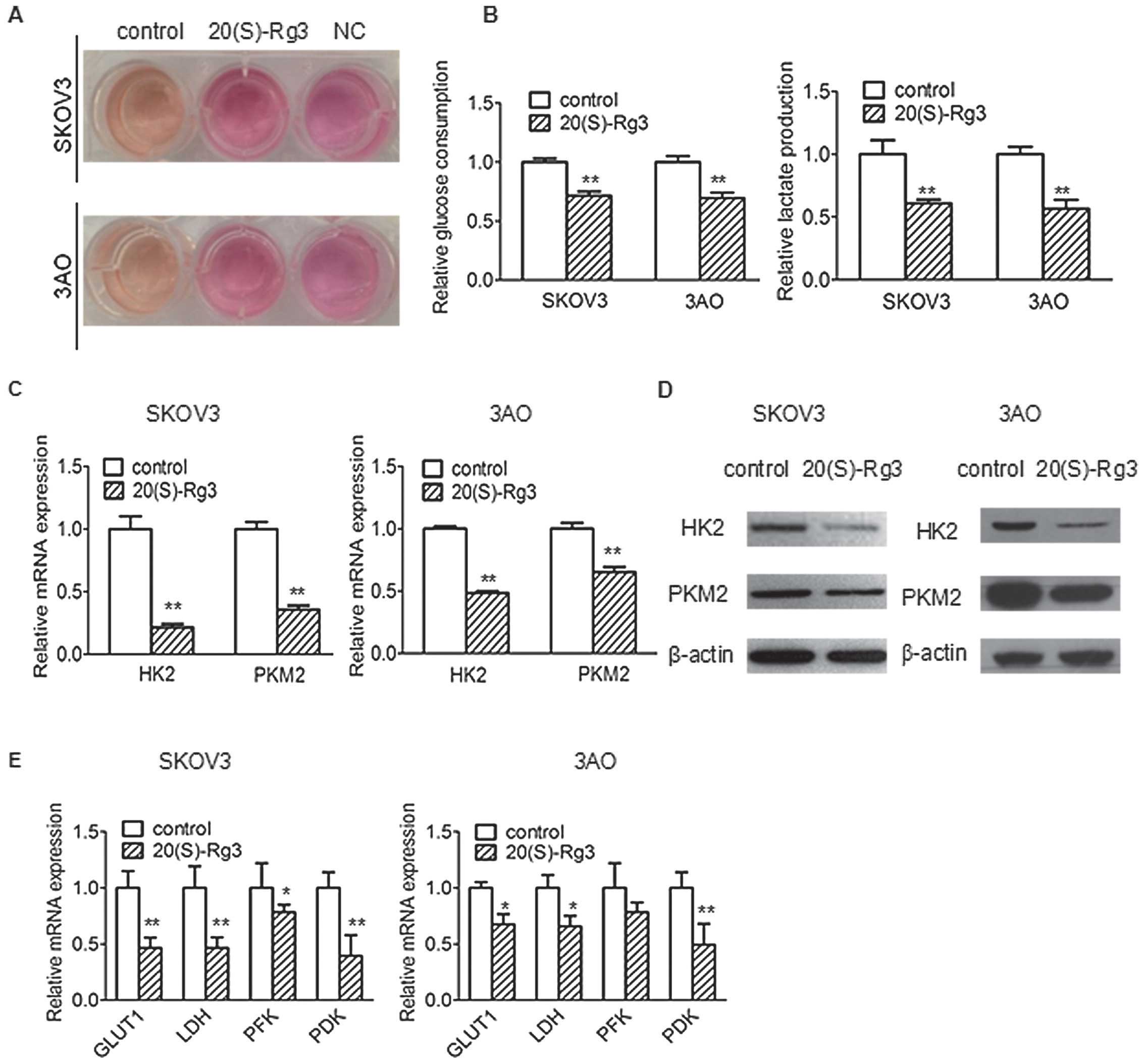
ovarian cancer cell lines SKOV3 and 3AO. We found that 20(S)-Rg3
inhibited glucose uptake and lactate secretion >30% (Fig. 1B) and reduced the acidity of cell
culture media (Fig. 1A), but
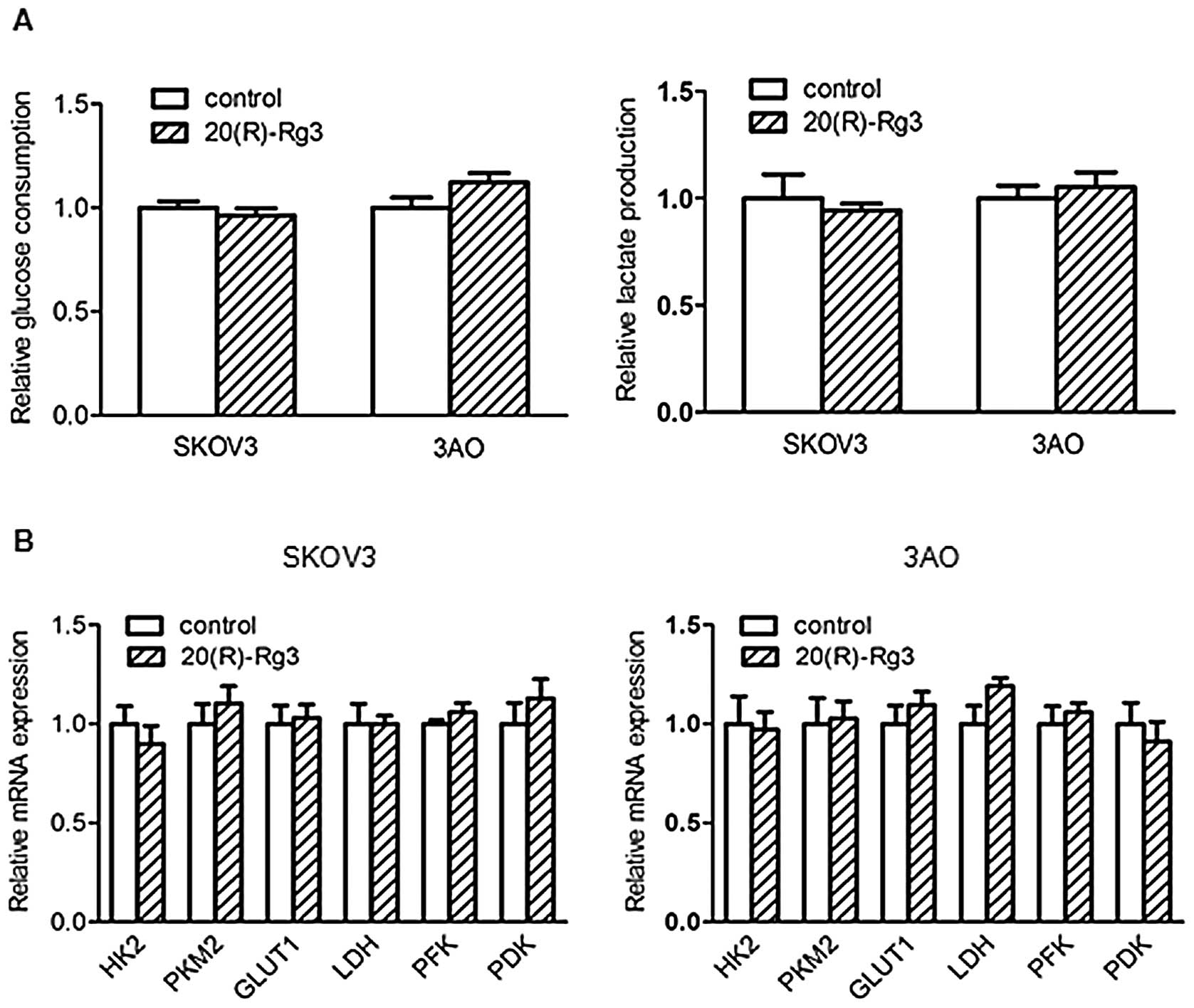
20(R)-Rg3 had no effect on glucose uptake, lactate secretion and
metabolic enzyme genes (Fig. 2).
HK2 and PKM2 are key cell glycolysis genes. The
RT-qPCR analysis and western blot analysis showed that 20(S)-Rg3
inhibited the expression of HK2 and PKM2 (Fig. 1C and D). 20(S)-Rg3 can also inhibit
other metabolic enzyme genes including GLUT1, LDH,
PDK and PFK in Warburg effect (Fig. 1E).
Downregulation of p-STAT3 contributes to
the inhibition of Warburg effect by 20(S)-Rg3 in SKOV3 and 3AO
cells
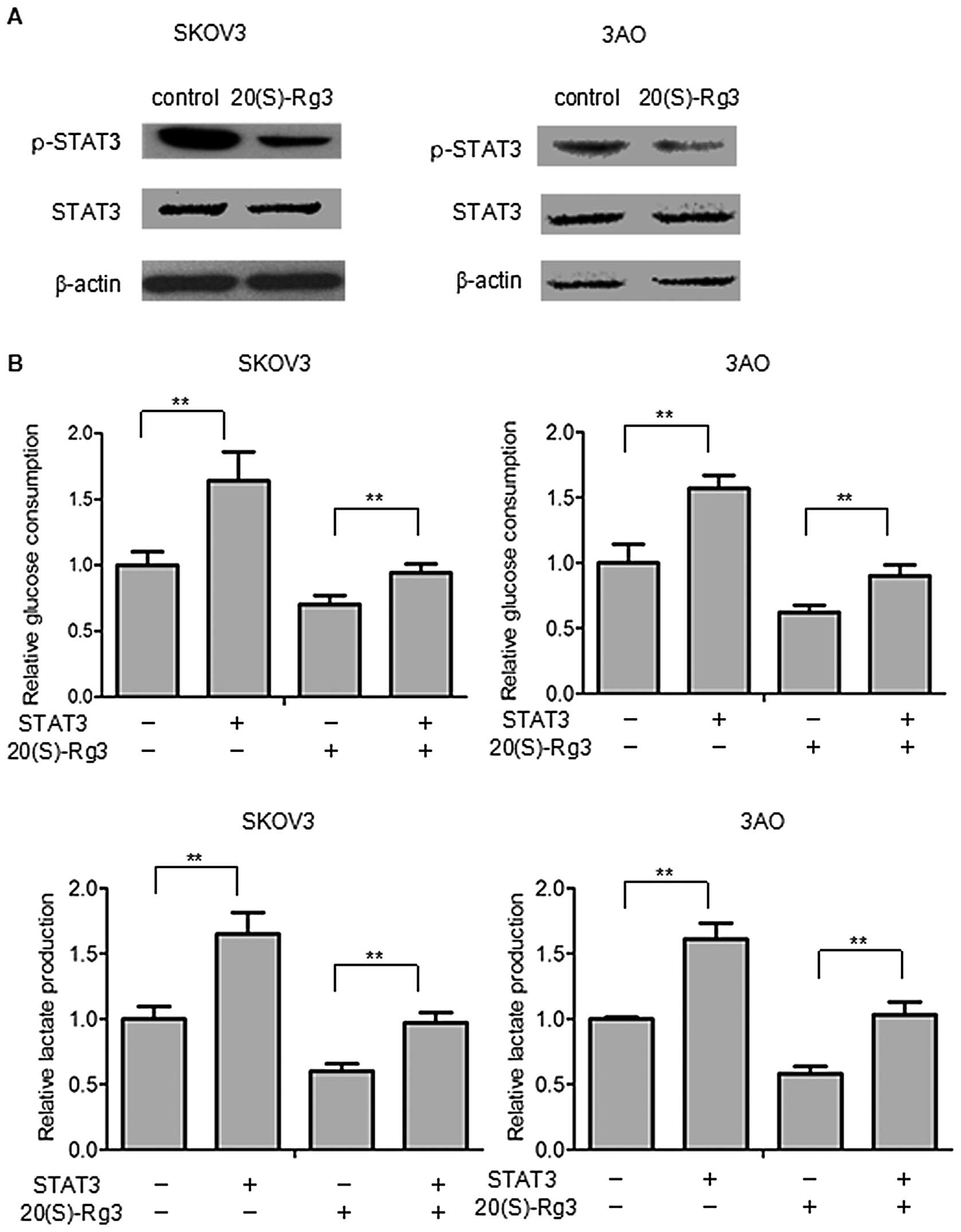
Previously (Fig.
3B) we found that STAT3 was also involved in regulating
glycolysis. Thus, we further confirmed that STAT3 played an
important role in Warburg effect of SKOV3 and 3AO cells. We found
that 20(S)-Rg3 downregulated p-STAT3 (Y705) without STAT3 in SKOV3
and 3AO cells (Fig. 3A). To
demonstrate the role of STAT3 in 20(S)-Rg3-induced metabolic shift
in ovarian cancer cells, STAT3 was ectopically transfected into
SKOV3 and 3AO cells. Overexpression of STAT3 significantly promoted
glucose uptake and lactate secretion and reduced the effects of
20(S)-Rg3 on glucose uptake and lactate secretion in SKOV3 and 3AO
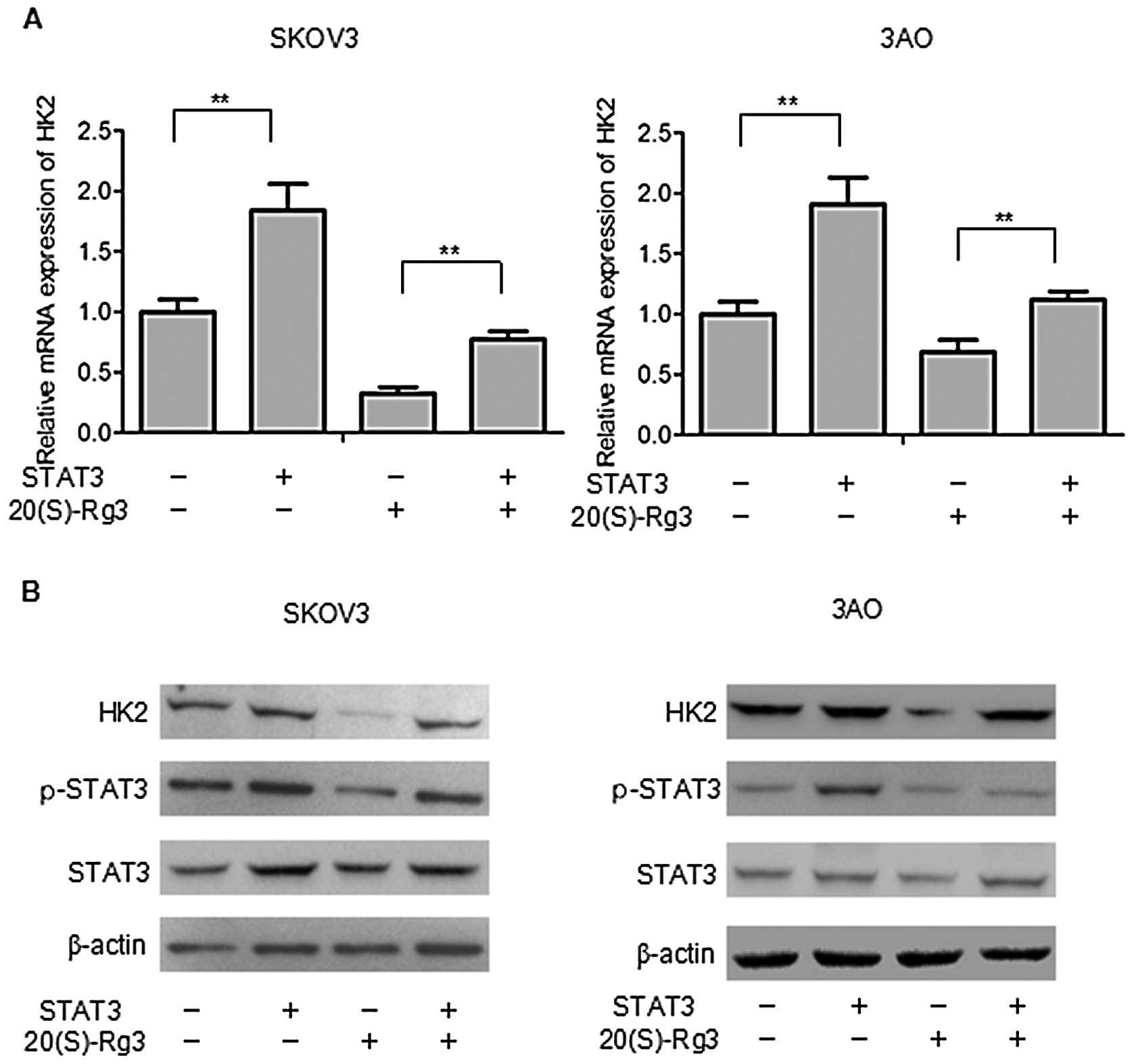
cells (Fig. 3B). In a previous
study (Fig. 1C and E), the
expression of HK2 had the greatest reduction after 20(S)-Rg3
treatment, so we chose HK2 for further study. The RT-qPCR and
western blot analysis showed that overexpression of STAT3
significantly promoted HK2 and reduced the effects of 20(S)-Rg3 on
expression of HK2 (Fig. 4). These
results indicated that STAT3 plays an important role in the process
of aerobic glycolysis regulated by 20(S)-Rg3 in SKOV3 and 3AO
cells.
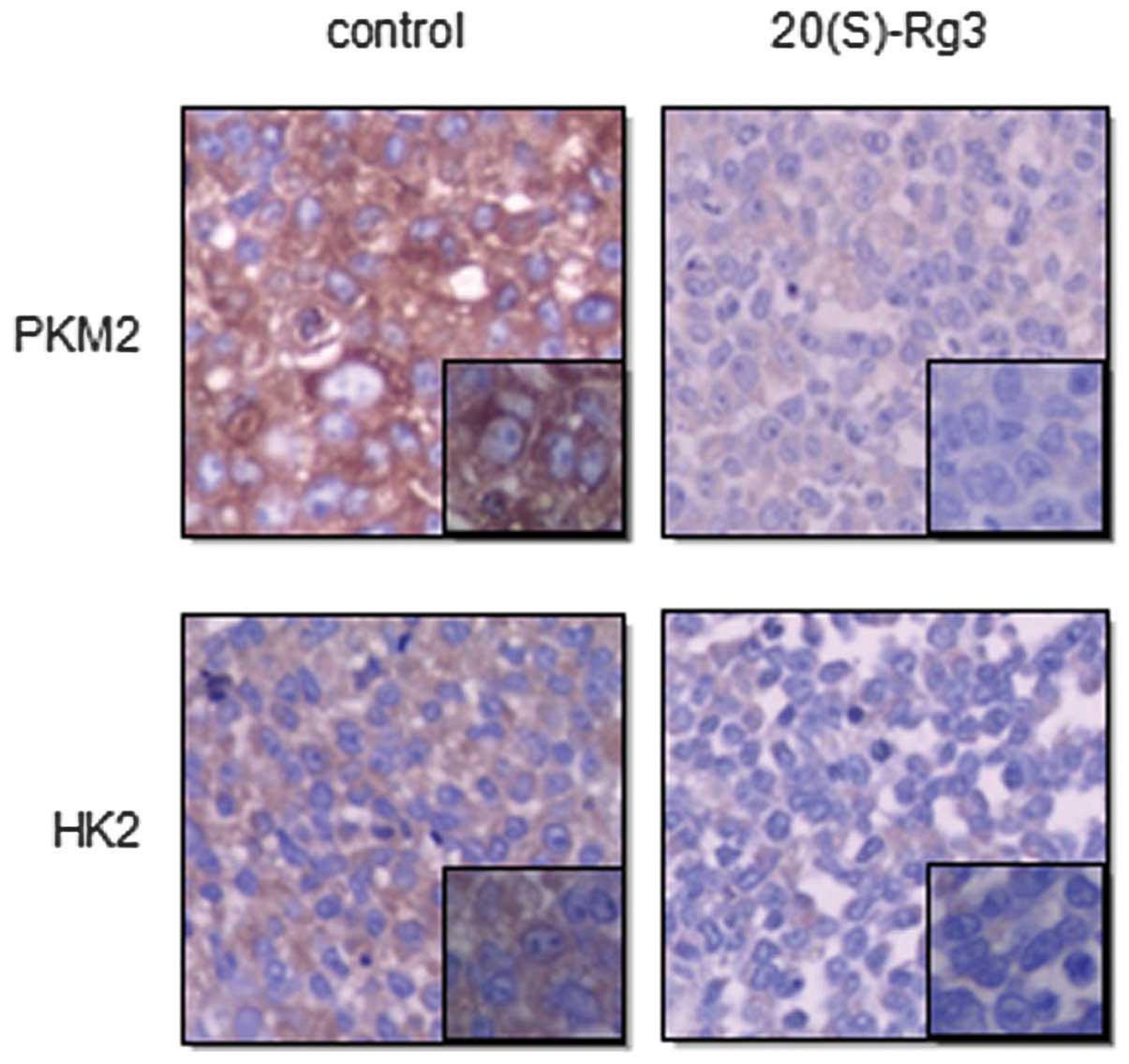
Expression of HK2 and PKM2 in ovarian
cancer tissue subcutaneous tumors of nude mice
In preliminary studies (unpublished), we found that
20(S)-Rg3 inhibited growth of xenografts of ovarian cancer in nude
mice. Here, to further investigate whether 20(S)-Rg3 could alter
metabolism of SKOV3 cells in vivo, the subcutaneous tumors
were fixated for confirmation by H&E staining and
immunohistochemistry analysis of HK2 and PKM2. As shown in Fig. 5, subcutaneous tumors in control
mice exhibited a significantly high level of HK2 and PKM2.
Contrarily, tumors in 20(S)-Rg3-treated mice displayed a decrease
of HK2 and PKM2. These in vivo findings coincided with the
in vitro changes observed in the cell models, demonstrating
that 20(S)-Rg3 robustly inhibited the Warburg effect in ovarian
cancer.
Discussion
In this study, we demonstrated that 20(S)-Rg3, the
pharmacologically active components of Panax ginseng, inhibited
Warburg effect in ovarian cancer cells. The Warburg effect
inhibited by 20(S)-Rg3 was accompanied by a decrease in levels of
glucose uptake and lactate secretion as well as some metabolic
enzymes in glycolysis including PKM2, HK2, GLUT1, and LDH. The
expression of HK2 had the greatest reduction (Fig. 1). 20(S)-Rg3 downregulated p-STAT3
(Y705) without STAT3 in SKOV3 and 3AO cells, and overexpression of
STAT3 can rescue the inhibitor effect induced by 20(S)-Rg3
(Figs. 3 and 4). We also found that 20(S)-Rg3 weakened
HK2 and PKM2 in vivo through immunohistochemical staining of
HK2, PKM2 in subcutaneous tumor samples (Fig. 5). Collectively, our data revealed
that STAT3, has important roles in mediating 20(S)-Rg3-induced
inhibition of aerobic glycolysis in ovarian cancer cells.
The Warburg effect not only allows cancer cells to
meet their high energy demands and supply the anabolic precursors
for nucleotide and lipid synthesis. The importance of Warburg
effect in survival and proliferation of cancer cells in the tumour
microenvironment is well documented. In this study, we have
demonstrated for the first time that the ginsenoside 20(S)-Rg3,
isolated from the traditional Chinese herb Panax ginseng,
effectively inhibits aerobic glycolysis by inducing p-STAT3
inactivation in ovarian cancer cells.
Substantial research has been performed on the
antitumor effect of 20(S)-Rg3, but the specific mechanism is still
unclear. 20(S)-Rg3 has a broad spectrum of antitumor activities,
ranging from the prevention of tumor growth and the inhibition of
tumor progression to the enhancement of chemotherapeutic response.
20(S)-Rg3 has been shown to restrain HT29 colorectal cancer cell
proliferation by inhibiting mitosis and inducing apoptosis
(8). Kim et al demonstrated
that 20(S)-Rg3 sensitized prostate cancer cells to docetaxel and
other chemotherapeutics by inhibiting cell growth and augmenting
apoptosis via suppression of constitutively activated and TNF
α-induced NF-κB (26). 20(S)-Rg3
promoted TRAIL-induced apoptosis in hepatocellular carcinoma cells
via C/EBP homology protein-mediated DR5 upregulation (27). Collectively, these findings,
together with our in vitro and in vivo data, shed new
light on the metabolic mechanism of ginsenosides in general, and
validate potential clinical use of 20(S)-Rg3 in particular as an
adjuvant chemotherapeutic for patients with advanced ovarian
cancer.
STAT3 is associated with cell proliferation,
survival, and carcinogenesis. Numerous studies have reported that
STAT3 is constitutively phosphorylated in a wide variety of cancers
through the increased activities of positive regulators, such as
the IL-6 cytokines, receptor tyrosine kinases EGFR and VEGFR
(28). It has been reported that
IL-6-STAT3 signaling pathway can affect glycolysis through
regulating HK2 and PFKFB3 (25).
It has also been reported that in breast cancer cells, the
activation of STAT3 can combine to HK2 promoter, and then promote
HK2 transcription activation, regulate aerobic glycolysis of breast
cancer cells (21). HK2 is
overexpressed in tumors and contributes to aerobic glycolysis.
Thus, it is characterized as a pivotal player in the Warburg effect
and an emerging target for cancer metabolism therapeutics (29,30).
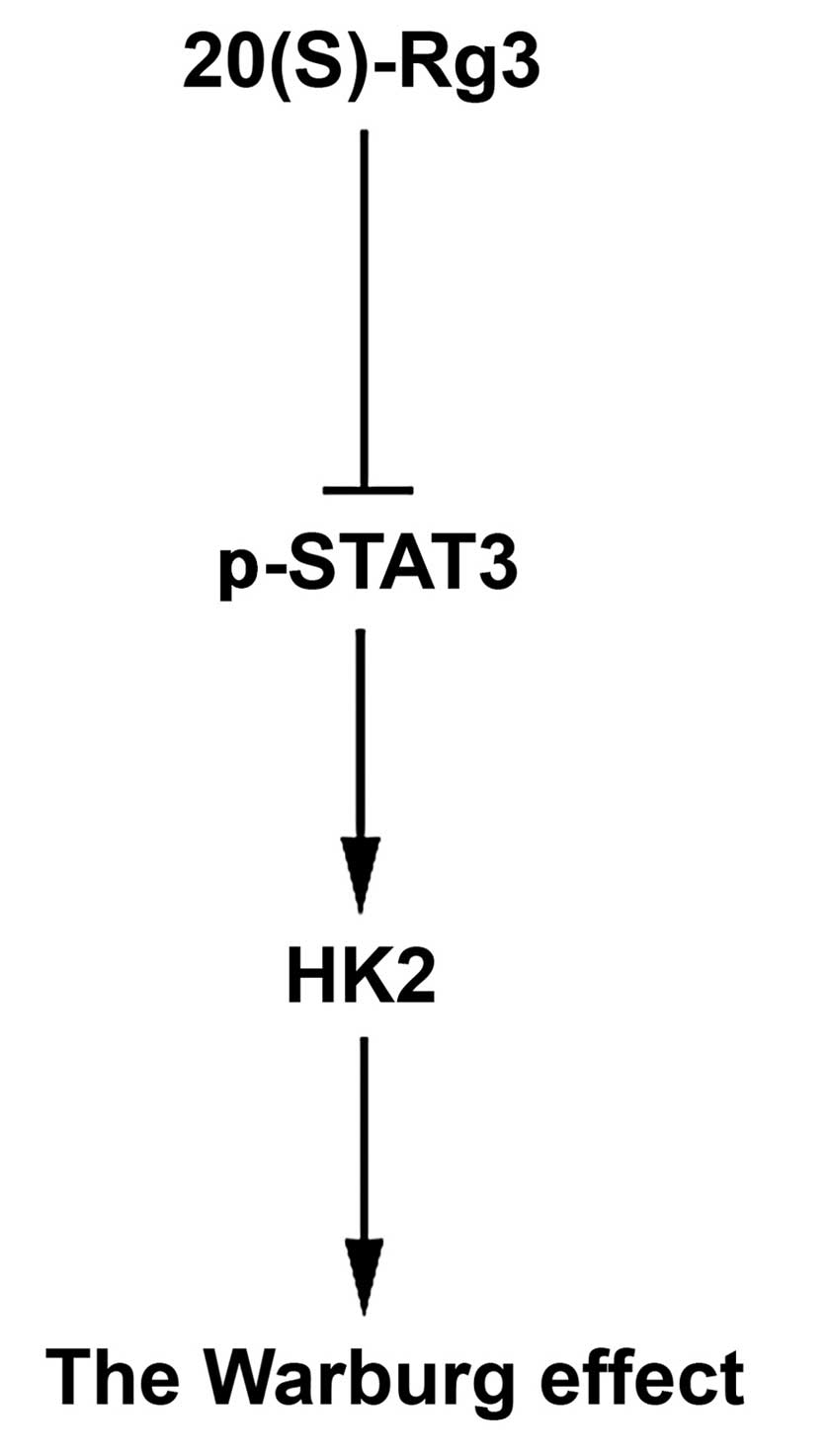
Our results showed that Warburg effect was inhibited by 20(S)-Rg3
though STAT3/HK2 pathways (Fig.
6). The experimental results confirmed our hypothesis,
providing the first experimental demonstration that Warburg effect
is inhibited by 20(S)-Rg3 through downregulating p-STAT3.
Futhermore, we also found that 20(S)-Rg3 decreased metabolic
enzymes in glycolysis including PKM2, HK2, GLUT1, and LDH, but the
mechanism still needed further study. Our data lay the foundation
for necessary additional studies of this compound to warrant its
clinical use as a new anti-cancer drug for the treatment of various
diseases including ovarian cancer.
Acknowledgements
We thank Ms. Yan Zhang, Ms. Jing Li, and Dr Mei Xue
for technical assistance. This study was supported by the National
Natural Science Foundation of China (Beijing, China) (no.
30973429).
References
|
1
|
Cho KR and Shih IeM: Ovarian cancer. Annu
Rev Pathol. 4:287–313. 2009. View Article : Google Scholar :
|
|
2
|
Swisher EM, Taniguchi T and Karlan BY:
Molecular scores to predict ovarian cancer outcomes: a worthy goal,
but not ready for prime time. J Natl Cancer Inst. 104:642–645.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2013. CA Cancer J Clin. 63:11–30. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Gillis CN: Panax ginseng pharmacology: a
nitric oxide link? Biochem Pharmacol. 54:1–8. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Liao B, Newmark H and Zhou R:
Neuroprotective effects of ginseng total saponin and ginsenosides
Rb1 and Rg1 on spinal cord neurons in vitro. Exp Neurol.
173:224–234. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Nag SA, Qin JJ, Wang W, Wang MH, Wang H
and Zhang R: Ginsenosides as anticancer agents: in vitro and in
vivo activities, structure-activity relationships, and molecular
mechanisms of action. Front Pharmacol. 3:252012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lü JM, Yao Q and Chen C: Ginseng
compounds: an update on their molecular mechanisms and medical
applications. Curr Vasc Pharmacol. 7:293–302. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lee SY, Kim GT, Roh SH, et al: Proteomic
analysis of the anti-cancer effect of 20S-ginsenoside Rg3 in human
colon cancer cell lines. Biosci Biotechnol Biochem. 73:811–816.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Mochizuki M, Yoo YC, Matsuzawa K, et al:
Inhibitory effect of tumor metastasis in mice by saponins,
ginsenoside-Rb2, 20(R)- and 20(S)-ginsenoside-Rg3, of red ginseng.
Biol Pharm Bull. 18:1197–1202. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bae EA, Kim EJ, Park JS, Kim HS, Ryu JH
and Kim DH: Ginsenosides Rg3 and Rh2 inhibit the activation of AP-1
and protein kinase A pathway in
lipopolysaccharide/interferon-gamma-stimulated BV-2 microglial
cells. Planta Med. 72:627–633. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Keum YS, Han SS, Chun KS, et al:
Inhibitory effects of the ginsenoside Rg3 on phorbol ester-induced
cyclooxygenase-2 expression, NF-kappaB activation and tumor
promotion. Mutat Res. 523–524:75–85. 2003. View Article : Google Scholar
|
|
12
|
Cui W, Cheng L, Hu C, Li H, Zhang Y and
Chang J: Electrospun poly(L-lactide) fiber with ginsenoside rg3 for
inhibiting scar hyperplasia of skin. PLoS One. 8:e687712013.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yue PY, Wong DY, Wu PK, et al: The
angiosuppressive effects of 20(R)-ginsenoside Rg3. Biochem
Pharmacol. 72:437–445. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Warburg O: On the origin of cancer cells.
Science. 123:309–314. 1956. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kim JW and Dang CV: Cancer’s molecular
sweet tooth and the Warburg effect. Cancer Res. 66:8927–8930. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Vander Heiden MG, Cantley LC and Thompson
CB: Understanding the Warburg effect: the metabolic requirements of
cell proliferation. Science. 324:1029–1033. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Diaz N, Minton S, Cox C, et al: Activation
of stat3 in primary tumors from high-risk breast cancer patients is
associated with elevated levels of activated SRC and survivin
expression. Clin Cancer Res. 12:20–28. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Germain D and Frank DA: Targeting the
cytoplasmic and nuclear functions of signal transducers and
activators of transcription 3 for cancer therapy. Clin Cancer Res.
13:5665–5669. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang SW and Sun YM: The IL-6/JA K/STAT3
pathway: Potential therapeutic strategies in treating colorectal
cancer (Review). Int J Oncol. 44:1032–1040. 2014.PubMed/NCBI
|
|
20
|
Zhu Q, Hu J, Meng H, Shen Y, Zhou J and
Zhu Z: S-phase cell cycle arrest, apoptosis, and molecular
mechanisms of aplasia ras homolog member I-induced human ovarian
cancer SKOV3 cell lines. Int J Gynecol Cancer. 24:629–634. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Jiang S, Zhang LF, Zhang HW, et al: A
novel miR-155/miR-143 cascade controls glycolysis by regulating
hexokinase 2 in breast cancer cells. EMBO J. 31:1985–1998. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Demaria M, Giorgi C, Lebiedzinska M, et
al: A STAT3-mediated metabolic switch is involved in tumour
transformation and STAT3 addiction. Aging (Albany NY). 2:823–842.
2010.
|
|
23
|
Sawayama H, Ishimoto T, Sugihara H, et al:
Clinical impact of the Warburg effect in gastrointestinal cancer
(Review). Int J Oncol. 45:1345–1354. 2014.PubMed/NCBI
|
|
24
|
Kiuchi N, Nakajima K, Ichiba M, et al:
STAT3 is required for the gp130-mediated full activation of the
c-myc gene. J Exp Med. 189:63–73. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ando M, Uehara I, Kogure K, et al:
Interleukin 6 enhances glycolysis through expression of the
glycolytic enzymes hexokinase 2 and
6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase-3. J Nippon
Med Sch. 77:97–105. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kim SM, Lee SY, Cho JS, et al: Combination
of ginsenoside Rg3 with docetaxel enhances the susceptibility of
prostate cancer cells via inhibition of NF-kappaB. Eur J Pharmacol.
631:1–9. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lee JY, Jung KH, Morgan MJ, et al:
Sensitization of TRAIL-induced cell death by 20(S)-ginsenoside Rg3
via CHOP-mediated DR5 upregulation in human hepatocellular
carcinoma cells. Mol Cancer Ther. 12:274–285. 2013. View Article : Google Scholar
|
|
28
|
Liu CY, Tseng LM, Su JC, et al: Novel
sorafenib analogues induce apoptosis through SHP-1 dependent STAT3
inactivation in human breast cancer cells. Breast Cancer Res.
15:R632013. View
Article : Google Scholar : PubMed/NCBI
|
|
29
|
Mathupala SP, Ko YH and Pedersen PL:
Hexokinase-2 bound to mitochondria: cancer’s stygian link to the
‘Warburg Effect’ and a pivotal target for effective therapy. Semin
Cancer Biol. 19:17–24. 2009. View Article : Google Scholar :
|
|
30
|
Vander Heiden MG, Lunt SY, Dayton TL, et
al: Metabolic pathway alterations that support cell proliferation.
Cold Spring Harb Symp Quant Biol. 76:325–334. 2011. View Article : Google Scholar
|