Introduction
Photodynamic therapy (PDT), a clinically-approved
cancer therapy, utilizes a photosensitizer, a light at a wavelength
corresponding to the photosensitizer’s absorbance, and molecular
oxygen to produce reactive oxygen species in malignant cellular
targets, and ultimately causing their destruction (1). Apoptosis is an important mechanism
for eliminating tumors (2–6), and the effectiveness of PDT regimens
correlates with tumor cell apoptosis (7). Because PDT itself is not always
effective as a tumor treatment (8,9), PDT
is combined with other anticancer agents for improved therapeutic
benefit. 4HPR [N-(4-hydroxyphenyl) retinamide; fenretinide], a
proapoptotic, clinically-relevant anticancer synthetic retinoid
(10), is a potential candidate
for combined treatment.
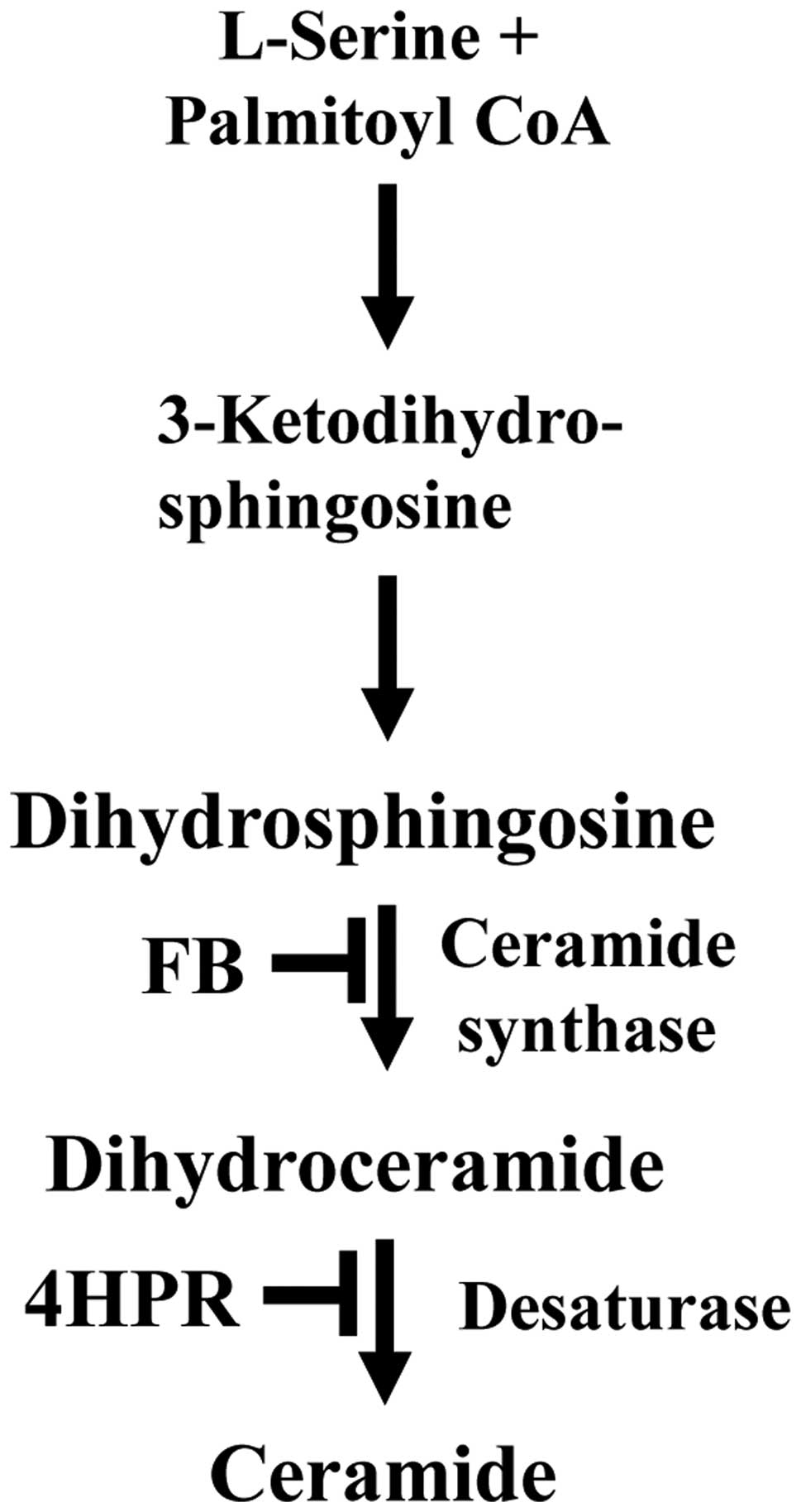
As others and we have shown, sphingolipids (SLs),
e.g., ceramide, generated via the de novo SL biosynthetic
pathway (Fig. 1), have been
implicated in apoptotic cell death after PDT and 4HPR in various
malignant cell lines (11–14). Ceramide synthase catalyzes a
reaction in the de novo SL biosynthesis pathway, in which a
fatty acyl group is added to dihydrosphingosine to form
dihydroceramide. Ceramide is formed in the subsequent
desaturase-dependent reaction, which can be inhibited by 4HPR
(15). The ceramide synthase
inhibitor fumonisin B1 (FB) renders cells resistant to apoptosis
after PDT and 4HPR (14,16).
There have been no reports on combining PDT with
4HPR for improving the efficacy of PDT. The objective of the
present study was to test the hypothesis that combining PDT with
4HPR enhances cell killing via apoptosis and the de novo SL
biosynthesis pathway. We used SCC17B cells, a human head and neck
squamous cell carcinoma cell line, representing a model that is
potentially PDT-treatable in the clinic (17).
Materials and methods
Materials
The phthalocyanine photosensitizer Pc4,
HOSiPcOSi(CH3)2(CH2)3N(CH3)2,
was kindly provided by Dr Malcolm E. Kenney (Department of
Chemistry, Case Western Reserve University, Cleveland, OH, USA).
4HPR [N-(4-hydroxyphenyl) retinamide], fetal bovine serum and goat
serum were purchased from Sigma-Aldrich (St. Louis, MO, USA).
Cellgro DMEM/F-12 medium was obtained from Thermo Fisher Scientific
(Waltham, MA, USA). Inhibitors were from the sources indicated in
brackets: zVAD-fmk (MBL International Corp., Woburn, MA, USA),
fumonisin B1 (Cayman Chemicals, Ann Arbor, MI, USA) and ABT-199
(Selleck Chemicals, Houston, TX, USA).
Cell culture and treatments
SCC17B cells were obtained from Dr Thomas Carey
(University of Michigan, Ann Arbor, MI, USA). Cells were grown in
DMEM/F-12 medium containing 10% fetal bovine serum, 100 U/ml
penicillin and 100 μg/ml streptomycin (Life Technologies, Carlsbad,
CA, USA) in a humidified incubator at 37°C and 5% CO2.
For all experiments, unless indicated otherwise, incubation of
cells was carried out in a humidified incubator at 37°C and 5%
CO2. All treatments, as well as staining with
Mitotracker Red CMXRos (see below) were added to cells in growth
medium. After overnight incubation with Pc4 (20 nM), 4HPR (2.5 μM)
was added immediately prior to irradiation. Cells were irradiated
at room temperature with red light (2 mW/cm2;
λmax ~670 nm) using a light-emitting diode array light
source (EFOS, Mississauga, ON, Canada) at the fluence of 200
mJ/cm2 and incubated for 10 h. Phosphate-buffered saline
(PBS) without calcium and magnesium was used for confocal
microscopy. PBS containing calcium and magnesium was used for mass
spectrometry (MS). Both types of PBS were purchased from Life
Technologies.
Clonogenic assay
Cell survival was assessed using clonogenic assay
according to the modified pre-plating protocol, as we have
described (18). Cells were
resuspended in growth medium containing Pc4 (20 nM) and seeded (250
cells/well) in a 6-well plate (Thermo Fisher Scientific). After
overnight incubation, the cells were irradiated. 4HPR was added
immediately prior to irradiation. The inhibitors FB, zVAD-fmk
(zVAD) and ABT-199 (ABT) were added 1 h prior to PDT±4HPR. After 14
days of incubation, the medium was aspirated, the plates were
stained with crystal violet (0.1% in 20% ethanol; Sigma-Aldrich)
for 30 sec, rinsed with water and air-dried. Colonies (≥50 cells)
were counted using eCount Colony Counter (VWR International,
Radnor, PA, USA). Plating efficiency was 36% (n=16).
Quantitative confocal microscopy
Cells were grown on coverslips (Thermo Fisher
Scientific) in 6-well plates (Thermo Fisher Scientific). To
visualize mitochondria, treated cells were incubated with
Mitotracker Red CMXRos (250 nM; Life Technologies) in growth medium
for 30 min. After treatments, the coverslips were washed with cold
PBS, and fixed by incubation for 15 min in 4% formaldehyde (Thermo
Fisher Scientific) in PBS. After washing with PBS, cells were
permeabilized with ice-cold acetone/methanol (1:1) for 10 min.
After blocking with 3% goat serum and 3% fetal bovine serum in PBS
for 1 h at 4°C, cells were incubated at room temperature for 45 min
with mouse monoclonal anti-dihydroceramide/ceramide antibodies
(1:30; ALX-804-196; Enzo Life Sciences, Ann Arbor, MI, USA). After
washing with cold PBS, cells were incubated at room temperature for
45 min with Alexa 488-conjugated goat anti-mouse monoclonal IgM
antibodies (1:200; 115-546-075; Jackson ImmunoResearch, West Grove,
PA, USA) and washed again with cold PBS. To visualize the nuclei,
cells were stained with 4′6-diamidino-2-phenylindole (DAPI; Life
Technologies; 1 μg/ml in cold PBS) for 10 min at room temperature
and washed with cold PBS. The coverslips were mounted on slides
using the ProLong Antifade kit (P7481; Life Technologies). Zeiss
LSM780 confocal microscope equipped with a 100×1.4 NA OIL DIC D
objective (Carl Zeiss, Thornwood, NY, USA) was used for acquiring
the images.
For ER and dihydroceramide/ceramide detection, mouse
monoclonal anti-KDEL antibody (1:50; ab12223; Abcam, Cambridge, MA,
USA) and anti-dihydroceramide/ceramide antibodies were combined
with Alexa 594-conjugated goat anti-mouse IgG (115-585-071) and
Alexa 488-conjugated goat anti-mouse IgM antibodies (both 1:200;
monoclonal, from Jackson ImmunoResearch), respectively. For
visualization of Bax, mouse monoclonal anti-Bax antibodies (1:50;
ab5714; Abcam) were combined with Alexa 488-conjugated monoclonal
goat anti-mouse IgG antibodies (1:200; 115-545-071; Jackson
ImmunoResearch). For visualization of cytochrome c (cyt
c) redistribution, mouse monoclonal anti-cyt c
antibodies (1:50; 556432; BD Biosciences, San Jose, CA, USA) were
combined with Alexa 594-conjugated goat anti-mouse IgG (1:200;
Jackson ImmunoResearch). As a criterion to score the cells positive
for the redistribution of cyt c the margin of cyt c
staining around the nuclei greater than 10 μm was used. At least
100 cells were assessed for every condition in each experiment.
Confocal microscopy imaging was performed at the Microscopy,
Imaging and Cytometry Resources Core at Wayne State University,
School of Medicine.
Quantifications were carried out as follows:
dihydroceramide/ceramide fluorescence associated with the ER and
the mitochondria, as well as Bax fluorescence associated with the
mitochondria, were all quantified with MetaXpress software (version
5 5.00.20; Molecular Devices, LLC; Sunnyvale, CA, USA) (19). Mitotracker or anti-KDEL antibodies
were employed to demarcate the mitochondria or the ER.
Dihydroceramide/ceramide or Bax-pixel intensities associated with
these subcellular compartments were measured with the
multiwavelength cell scoring module. The correct compartment
identification was verified by visual inspection. All images were
corrected for background contribution before quantification. For
Bax associated with mitochondria a minimum of 488 regions were
measured for each data point. For dihydroceramide/ceramide
associated with the ER and mitochondria a minimum of 680 or 518
regions were measured for each data point, respectively.
Significant differences (p<0.05) were determined using
comparison of multiple samples by one-way ANOVA.
Electrospray ionization/double mass
spectrometry (MS) analysis
After treatments, cells were collected on ice,
washed twice with cold PBS, resuspended in a mixture of ethyl
acetate/methanol (1:1, v/v; EMD Millipore, Billerica, MA, USA),
dried under nitrogen in the N-EVAP analytical evaporator
(Organomation; Berlin, MA, USA), and shipped overnight on dry ice
to the Lipidomics Shared Resource Facility (Medical University of
South Carolina, Charleston, SC, USA) for further processing. After
extraction, SLs were separated by high performance liquid
chromatography, introduced to the electrospray ionization source
and then analyzed by double MS using TSQ 7000 triple quadrupole
mass spectrometer (Thermo-Fisher Scientific) as described
previously (20). The pmoles of
SLs were normalized per mg protein. Protein content was determined
by a modified Bradford assay per manufacturer’s instructions
(Bio-Rad, Hercules, CA, USA).
Statistical analysis
Significant differences (p<0.05) were determined
using Student’s t-test or one-way ANOVA.
Results
Enhanced cell killing after PDT+4HPR is
FB-, zVAD-fmk- and ABT-199-sensitive
Clonogenic assay was used to test whether combining
PDT with 4HPR sensitizes SCC17B cells to PDT. As shown in Table I, when PDT and 4HPR were used at
LD20 each, i.e., the dose reducing survival by 20%, 63% of
PDT+4HPR-treated cells were unable to form colonies. To determine
whether apoptosis and ceramide synthase are necessary for enhanced
cell killing after PDT+4HPR, we used the pancaspase inhibitor
zVAD-fmk (zVAD), the Bcl2 inhibitor ABT-199 (ABT), and the ceramide
synthase inhibitor FB (21–23).
We have previously shown that FB and zVAD rendered cells resistant
to PDT (16). In contrast, ABT
sensitized cells to PDT (16).
Apoptosis is a major pathway involved in the anti-cancer action of
4HPR (24) and the de novo
SL biosynthesis pathway plays a role in 4HPR-induced apoptosis
(14,25). As shown in Table I, all inhibitors were non-toxic
(LD<5). FB and zVAD rendered the cells resistant not only to PDT
and 4HPR alone, but also to PDT+4HPR. In contrast, ABT sensitized
SCC17B cells to PDT±4HPR.
 | Table IPDT+4HPR-induced augmented cell
killing is FB-, zVAD- and ABT-sensitive in SCC17B cells. |
Table I
PDT+4HPR-induced augmented cell
killing is FB-, zVAD- and ABT-sensitive in SCC17B cells.
| Treatment | % Survival |
|---|
| FB | 96±0.9 |
| zVAD | 96±0.8 |
| ABT | 97±1.3 |
| 4HPR | 80±0.6a |
| 4HPR+FB | 91±0.9a,b |
| 4HPR+zVAD | 95±1.0b |
| 4HPR+ABT | 73±0.7a,b |
| PDT | 79±0.6a |
| PDT+FB | 93±0.7b |
| PDT+zVAD | 91±1.3a,b |
| PDT+ABT | 67±2.2a,b |
| PDT+4HPR | 37±0.7a,c |
| PDT+4HPR+FB | 73±1.7a,b |
| PDT+4HPR+zVAD | 91±1.6b |
| PDT+4HPR+ABT | 32±0.9a,b |
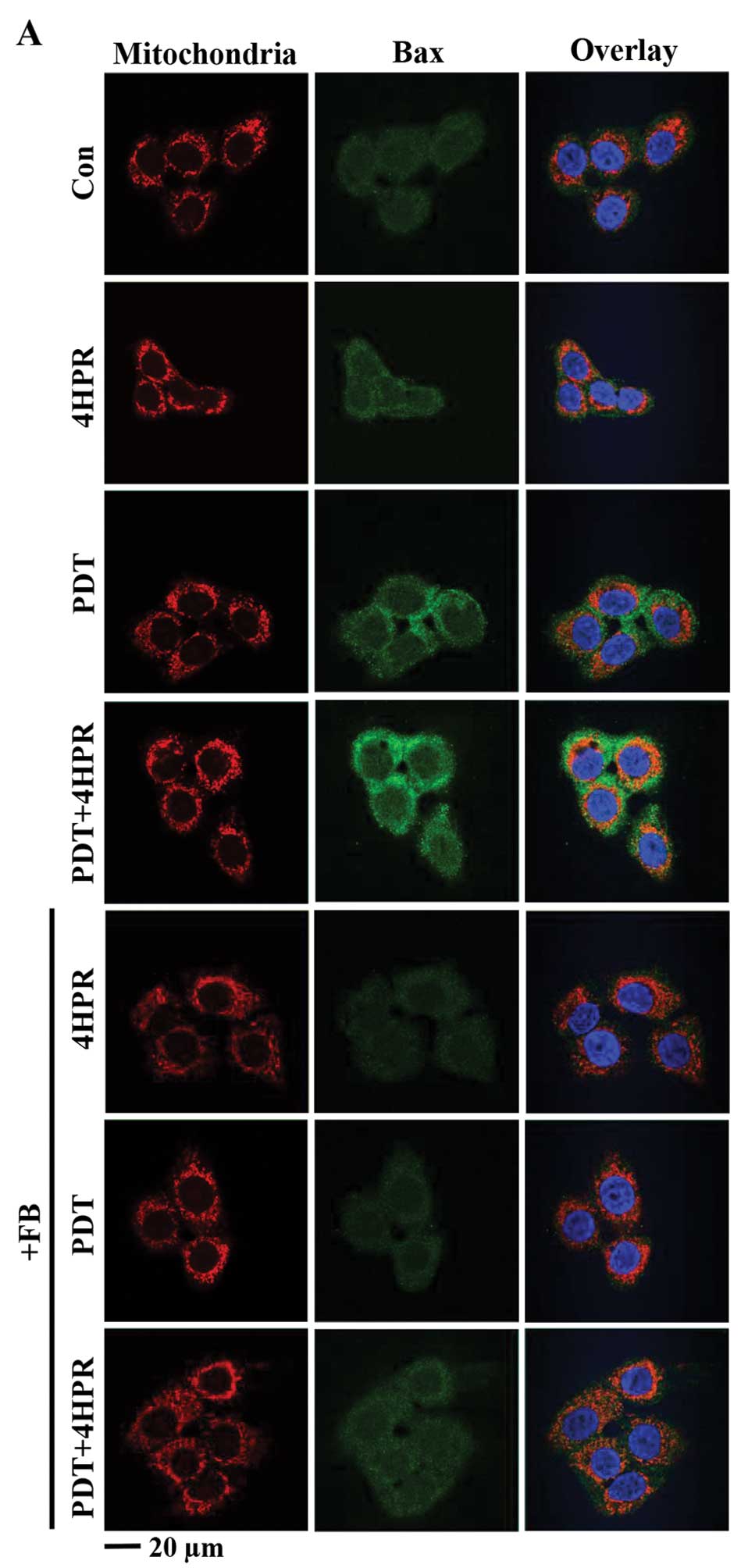
PDT+4HPR-enhanced Bax associated with
mitochondria and cyt c redistribution is inhibited by FB
The mitochondrial apoptosis pathway, including Bax
translocation to mitochondria and cyt c
redistribution/release, is induced by PDT and 4HPR (16,26–28).
Both of these processes are inhibited by FB after PDT (16). The question is whether the
mitochondrial apoptosis pathway is affected by combining PDT with
4HPR, and whether the process is ceramide synthase-dependent. Using
quantitative confocal microscopy we found that Bax associated with
mitochondria and cyt c redistribution were induced after
each individual treatment (Fig.
2). PDT+4HPR enhanced Bax associated with mitochondria and cyt
c redistribution, and FB inhibited both processes.
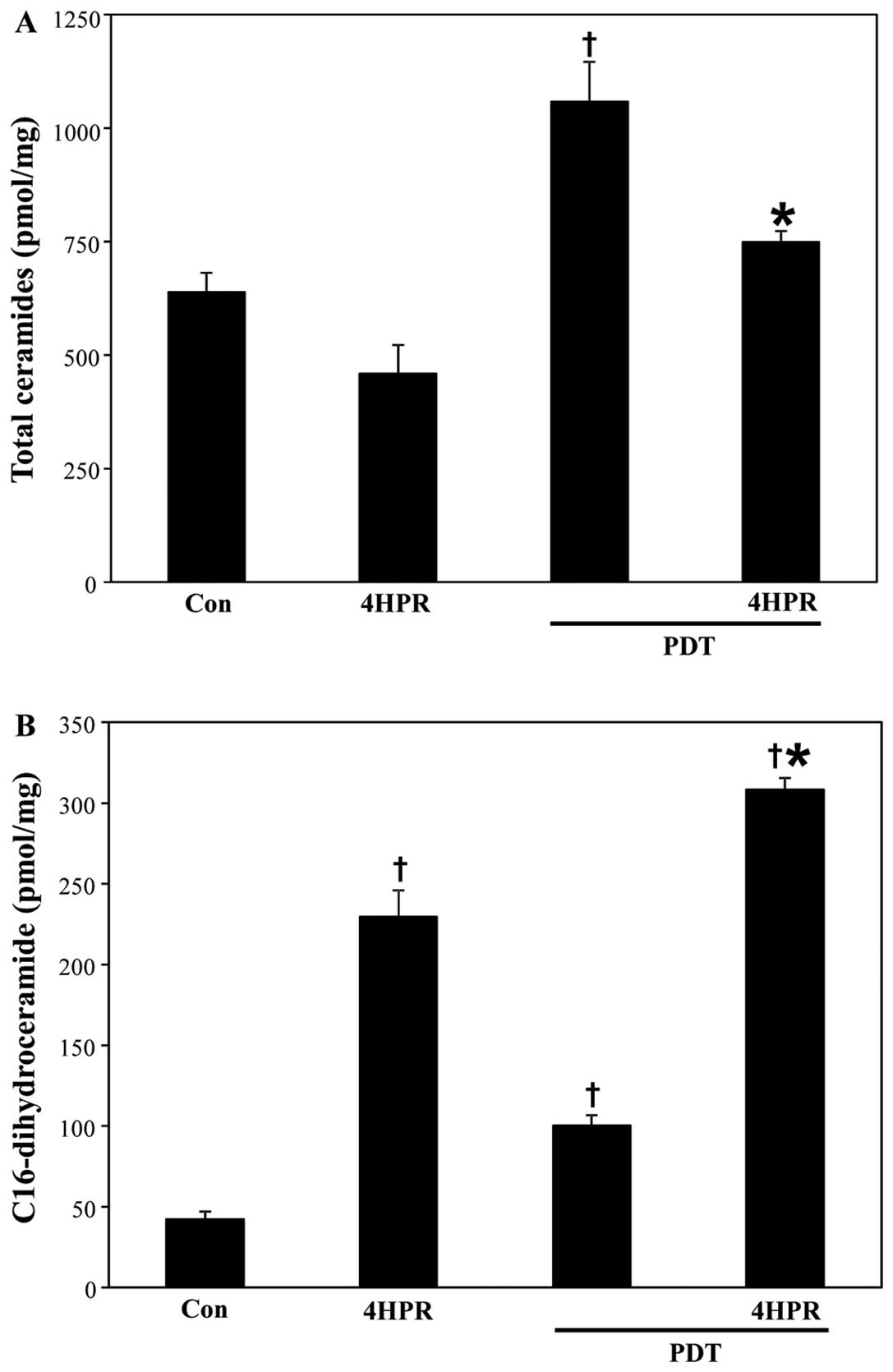
PDT+4HPR enhances C16-dihydroceramide,
not ceramide, accumulation
Both PDT and 4HPR regulate the de novo SL
biosynthesis pathway (12–16,29).
The question is what the effects of combining PDT with 4HPR on the
cellular SL profile are. We used MS to address the question. As
depicted in Fig. 3A, in contrast
to 4HPR, PDT increased total ceramide accumulation. Combining PDT
with 4HPR attenuated PDT-induced increase in total ceramide levels.
The accumulation of individual ceramides, by and large, followed
the same pattern (Table II). 4HPR
did not significantly raise the levels of any individual ceramide.
In contrast, 4HPR increased accumulation of C16-dihydroceramide, a
de novo SL biosynthesis pathway metabolite by 445% above
basal levels (Fig. 3B). PDT also
increased the levels of C16-dihydroceramide by 138% beyond resting
levels. Combining PDT with 4HPR enhanced accumulation of
C16-dihydroceramide by 632%.
 | Table IIEffect of PDT±4HPR on individual
ceramides in SCC17B cells. |
Table II
Effect of PDT±4HPR on individual
ceramides in SCC17B cells.
| Ceramide | Con | 4HPR | PDT | PDT+4HPR |
|---|
| C14-ceramide | 16.1±0.9 | 16.7±1.8 | 29.4±1.7a | 22.6±0.8a,d |
| C16-ceramide | 91.0±11.9 | 51.7±6.4a | 129.6±8.5a | 93.5±3.6d |
| C18-ceramide | 17.8±2.1 | 21.5±3.0 | 61.6±4.2a | 48.3±0.8a,d |
| C18:1-ceramide | 7.6±0.3 | 10.8±1.1 | 25.5±2.6a | 22.4±0.8a,b |
| C20-ceramide | 5.2±0.7 | 9.1±0.5 | 21.4±1.8a | 17.6±1.2a,b |
| C20:1-ceramide | 1.5±0.2 | 2.3±0.2 | 5.3±0.6a | 3.9±0.3a |
| C22-ceramide | 51.6±2.7 | 37.5±3.2 | 132.7±10.6a | 100.3±3.0a,d |
| C22:1-ceramide | 18.7±1.7 | 16.1±1.9 | 49.6±4.5a | 34.6±1.5a,d |
| C24-ceramide | 161.9±9.1 | 97.7±5.1 | 252.6±22.4a | 175.2±8.9d |
| C24:1-ceramide | 199.3±9.4 | 110.0±8.6a | 305.4±28.6a | 196.7±9.0d |
| C26-ceramide | 15.8±3.6 | 10.6±0.8 | 13.9±1.3 | 11.0±1.0 |
| C26:1-ceramide | 33.4±3.2 | 20.1±1.8a | 33.5±2.3 | 23.0±2.3a,c |
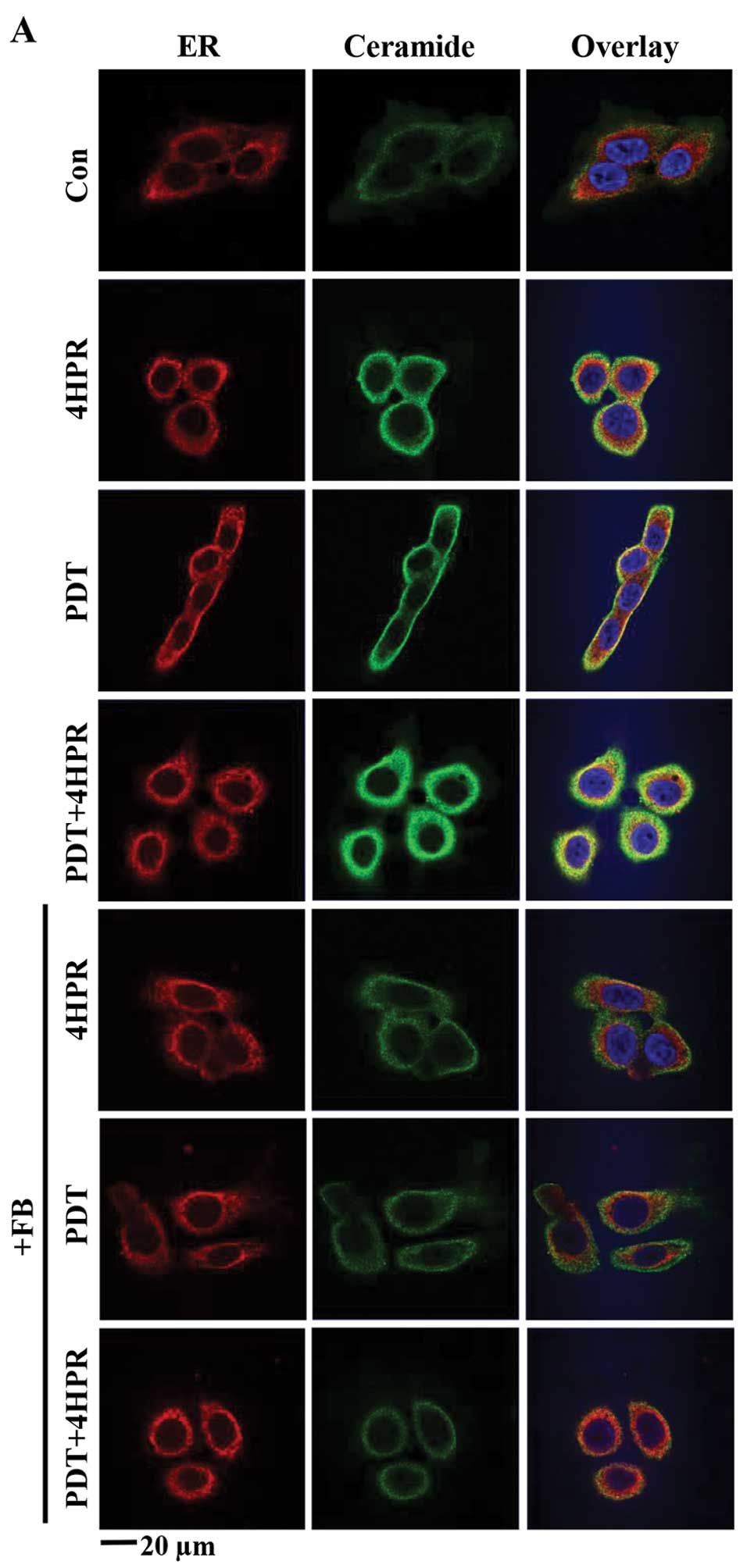
PDT+4HPR-induced enhanced ceramide
accumulation in the ER is inhibited by FB
Because the de novo SL biosynthesis pathway
is localized to the ER, we tested the effect of combining PDT with
4HPR on dihydroceramide/ceramide accumulation in the ER. Using an
antibody that recognizes dihydroceramide and ceramide (30) and the ER marker anti-KDEL for
quantitative confocal microscopy, we found that PDT+4HPR enhanced
dihydroceramide/ceramide accumulation in the ER (Fig. 4A and B). FB inhibited ER-associated
ceramide accumulation after PDT±4HPR.
We have shown that PDT-induced mitochondrial
dihydroceramide/ceramide accumulation is FB-sensitive (16). However, it is unknown what impact
on mitochondrial dihydroceramide/ceramide accumulation combining
PDT with 4HPR might have. Using the same
anti-dihydroceramide/ceramide antibody and the mitochondrial marker
Mitotracker for quantitative confocal microscopy, we found that PDT
and 4HPR alone did induce mitochondrial dihydroceramide/ceramide
accumulation (Fig. 4C). However,
the effect was not enhanced after PDT+4HPR. FB inhibited
mitochondrial ceramide accumulation after all the treatments.
Discussion
The present study is novel because, to our
knowledge, no report exists on combining PDT with 4HPR. The results
suggest that PDT+4HPR-induced enhanced killing of SCC17B cells
depends on ceramide synthase, caspase activation and inhibition of
Bcl2, and is associated with ceramide synthase-dependent
mitochondrial apoptosis pathway. We have reported similar findings
after PDT alone (16). In
contrast, in breast cancer cells FB did not affect cell death after
4HPR (14). The discrepancy
between the findings could be due to the use of different assays,
i.e. clonogenic vs. short-term viability assay, respectively
(31), or cell type-specificity of
FB-induced resistance.
Our observation that 4HPR increased the levels of
C16-dihydroceramide, the substrate of desaturase in the de
novo SL biosynthesis pathway (Fig.
1), is consistent with the report showing 4HPR-induced
inhibition of the enzyme (15).
Our present findings that PDT-induced increased ceramides and
C16-dihydroceramide in cells undergoing apoptosis are similar to
our previous results in SCCVII mouse squamous carcinoma cells
(18,32). Here we also demonstrate that
combining PDT with 4HPR enhances C16-dihydroceramide levels,
apoptosis and loss of clonogenicity. In addition, total ceramide
levels, as well as most of the individual ceramides remained above
baseline levels after PDT+4HPR. These data support the notion that
dihydroceramide, together with ceramide, has a
pro-apoptotic/pro-death role.
We demonstrate in the present report that
PDT+4HPR-induced enhanced killing of SCC17B cells is associated
with ER-localized and FB-sensitive de novo SL biosynthesis.
This is in accordance with previous findings for PDT and 4HPR
itself (15,16,29).
Although each treatment alone increased mitochondrial
dihydroceramide/ceramide accumulation in a FB-sensitive manner,
combining PDT with 4HPR did not affect the process. The ER and
mitochondria are connected by the mitochondria-associated membrane.
Ceramide synthase activation has been shown in
mitochondria-associated membrane after radiation (33). Reportedly, 4HPR can activate
ceramide synthase (29). It
remains to be established whether and at what subcellular site
ceramide synthase is activated after PDT+4HPR.
We believe that our findings have important clinical
implications. Combining PDT with another anticancer agent is
advantageous because lower doses of individual treatments (LD20;
Table I) are required for improved
therapeutic benefit. This study also shows that combining PDT with
4HPR enhances cell killing via the de novo SL biosynthesis
and mitochondrial apoptotic pathway. Thus, targeting these pathways
could augment the therapeutic value of PDT.
Acknowledgements
This study was supported by U.S. Public Health
Service Grants: to D.S., R01 CA77475 from the National Cancer
Institute (NCI), National Institutes of Health (NIH) and Wayne
State University (WSU) Bridge Funding; to the MS-related work at
the Lipidomics Shared Resource Facility (Medical University of
South Carolina), NCI Grants IPO1CA097132 and P30 CA 138313,
NIH/NCRR SC COBRE Grant P20 RR017677, C06 RR018823 from the
Extramural Research Facilities Program of the National Center for
Research Resources. The Microscopy, Imaging and Cytometry Resources
Core was supported, in part, by the NIH Grant P30 CA022453 to the
Karmanos Cancer Institute/WSU, and the Perinatology Research Branch
of the National Institutes of Child Health and Development/WSU.
References
|
1
|
Agostinis P, Berg K, Cengel KA, Foster TH,
Girotti AW, Gollnick SO, Hahn SM, Hamblin MR, Juzeniene A, Kessel
D, Korbelik M, Moan J, Mroz P, Nowis D, Piette J, Wilson BC and
Golab J: Photodynamic therapy of cancer: an update. CA Cancer J
Clin. 61:250–281. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Boisteau O, Gautier F, Cordel S, Henry F,
Harb J, Douillard JY, Vallette FM, Meflah K and Gregoire M:
Apoptosis induced by sodium butyrate treatment increases
immunogenicity of a rat colon tumor cell line. Apoptosis.
2:403–412. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sato M, Harada K, Yura Y, Azuma M,
Kawamata H, Iga H, Tsujimoto H, Yoshida H and Adachi M: The
treatment with differentiation- and apoptosis-inducing agent,
vesnarinone, of a patient with oral squamous cell carcinoma.
Apoptosis. 2:313–318. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Schiffer IB, Gebhard S, Heimerdinger CK,
Heling A, Hast J, Wollscheid U, Seliger B, Tanner B, Gilbert S,
Beckers T, Baasner S, Brenner W, Spangenberg C, Prawitt D, Trost T,
Schreiber WG, Zabel B, Thelen M, Lehr HA, Oesch F and Hengstler JG:
Switching off HER-2/neu in a tetracycline-controlled mouse tumor
model leads to apoptosis and tumor-size-dependent remission. Cancer
Res. 63:7221–7231. 2003.PubMed/NCBI
|
|
5
|
Stahnke K, Eckhoff S, Mohr A, Meyer LH and
Debatin KM: Apoptosis induction in peripheral leukemia cells by
remission induction treatment in vivo: selective depletion and
apoptosis in a CD34+ subpopulation of leukemia cells.
Leukemia. 17:2130–2139. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Urosevic M, Maier T, Benninghoff B, Slade
H, Burg G and Dummer R: Mechanisms underlying imiquimod-induced
regression of basal cell carcinoma in vivo. Arch Dermatol.
139:1325–1332. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Henderson BW, Gollnick SO, Snyder JW,
Busch TM, Kousis PC, Cheney RT and Morgan J: Choice of
oxygen-conserving treatment regimen determines the inflammatory
response and outcome of photodynamic therapy of tumors. Cancer Res.
64:2120–2126. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wilson JJ, Jones H, Burock M, Smith D,
Fraker DL, Metz J, Glatstein E and Hahn SM: Patterns of recurrence
in patients treated with photodynamic therapy for intraperitoneal
carcinomatosis and sarcomatosis. Int J Oncol. 24:711–717.
2004.PubMed/NCBI
|
|
9
|
Rhodes LE, de Rie M, Enstrom Y, Groves R,
Morken T, Goulden V, Wong GA, Grob JJ, Varma S and Wolf P:
Photodynamic therapy using topical methyl aminolevulinate vs
surgery for nodular basal cell carcinoma: results of a multicenter
randomized prospective trial. Arch Dermatol. 140:17–23. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Mariani L, Formelli F, DePalo G, Manzari
A, Camerini T, Campa T, Di Mauro MG, Crippa A, Delle Grottaglie M,
Del Vecchio M, Marubini E, Costa A and Veronesi U: Chemoprevention
of breast cancer with fenretinide (4-HPR): study of long-term
visual and ophthalmologic tolerability. Tumori. 82:444–449.
1996.PubMed/NCBI
|
|
11
|
Dolgachev V, Nagy B, Taffe B, Hanada K and
Separovic D: Reactive oxygen species generation is independent of
de novo sphingolipids in apoptotic photosensitized cells. Exp Cell
Res. 288:425–436. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Dolgachev V, Farooqui MS, Kulaeva OI,
Tainsky MA, Nagy B, Hanada K and Separovic D: De novo ceramide
accumulation due to inhibition of its conversion to complex
sphingolipids in apoptotic photosensitized cells. J Biol Chem.
279:23238–23249. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wispriyono B, Schmelz E, Pelayo H, Hanada
K and Separovic D: A role for the de novo sphingolipids in
apoptosis of photosensitized cells. Exp Cell Res. 279:153–165.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Rehman F, Shanmugasundaram P and Schrey
MP: Fenretinide stimulates redox-sensitive ceramide production in
breast cancer cells: potential role in drug-induced cytotoxicity.
Br J Cancer. 91:1821–1828. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kraveka JM, Li L, Szulc ZM, Bielawski J,
Ogretmen B, Hannun YA, Obeid LM and Bielawska A: Involvement of
dihydroceramide desaturase in cell cycle progression in human
neuroblastoma cells. J Biol Chem. 282:16718–16728. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Boppana NB, Kodiha M, Stochaj U, Lin HS,
Haimovitz-Friedman A, Bielawska A, Bielawski J, Divine GW, Boyd JA,
Korbelik M and Separovic D: Ceramide synthase inhibitor fumonisin
B1 inhibits apoptotic cell death in SCC17B human head and neck
squamous carcinoma cells after Pc4 photosensitization. Photochem
Photobiol Sci. 13:1621–1627. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Biel MA: Photodynamic therapy of head and
neck cancers. Methods Mol Biol. 635:281–293. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Separovic D, Saad ZH, Edwin EA, Bielawski
J, Pierce JS, Van Buren E and Bielawska A: C16-ceramide analog
combined with Pc 4 photodynamic therapy evokes enhanced total
ceramide accumulation, promotion of DEVDase activation in the
absence of apoptosis, and augmented overall cell killing. J Lipids.
2011.1–9. 2011. View Article : Google Scholar
|
|
19
|
Kodiha M, Brown CM and Stochaj U: Analysis
of signaling events by combining high-throughput screening
technology with computer-based image analysis. Sci Signal.
1:122008.
|
|
20
|
Separovic D, Semaan L, Tarca AL, Awad
Maitah MY, Hanada K, Bielawski J, Villani M and Luberto C:
Suppression of sphingomyelin synthase 1 by small interference RNA
is associated with enhanced ceramide production and apoptosis after
photodamage. Exp Cell Res. 314:1860–1868. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wang E, Norred WP, Bacon CW, Riley RT and
Merrill AH Jr: Inhibition of sphingolipid biosynthesis by
fumonisins. Implications for diseases associated with Fusarium
moniliforme. J Biol Chem. 266:14486–14490. 1991.PubMed/NCBI
|
|
22
|
Garcia-Calvo M, Peterson EP, Leiting B,
Ruel R, Nicholson DW and Thornberry NA: Inhibition of human
caspases by peptide-based and macromolecular inhibitors. J Biol
Chem. 273:32608–32613. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Souers AJ, Leverson JD, Boghaert ER,
Ackler SL, Catron ND, Chen J, Dayton BD, Ding H, Enschede SH,
Fairbrother WJ, Huang DC, Hymowitz SG, Jin S, Khaw SL, Kovar PJ,
Lam LT, Lee J, Maecker HL, Marsh KC, Mason KD, Mitten MJ, Nimmer
PM, Oleksijew A, Park CH, Park CM, Phillips DC, Roberts AW, Sampath
D, Seymour JF, Smith ML, Sullivan GM, Tahir SK, Tse C, Wendt MD,
Xiao Y, Xue JC, Zhang H, Humerickhouse RA, Rosenberg SH and Elmore
SW: ABT-199, a potent and selective BCL-2 inhibitor, achieves
antitumor activity while sparing platelets. Nat Med. 19:202–208.
2013. View
Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hail N Jr, Kim HJ and Lotan R: Mechanisms
of fenretinide-induced apoptosis. Apoptosis. 11:1677–1694. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Erdreich-Epstein A, Tran LB, Bowman NN,
Wang H, Cabot MC, Durden DL, Vlckova J, Reynolds CP, Stins MF,
Groshen S and Millard M: Ceramide signaling in fenretinide-induced
endothelial cell apoptosis. J Biol Chem. 277:49531–49537. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chiu SM, Xue LY, Usuda J, Azizuddin K and
Oleinick NL: Bax is essential for mitochondrion-mediated apoptosis
but not for cell death caused by photodynamic therapy. Br J Cancer.
89:1590–1597. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Tiwari M, Kumar A, Sinha RA, Shrivastava
A, Balapure AK, Sharma R, Bajpai VK, Mitra K, Babu S and Godbole
MM: Mechanism of 4-HPR-induced apoptosis in glioma cells: evidences
suggesting role of mitochondrial-mediated pathway and endoplasmic
reticulum stress. Carcinogenesis. 27:2047–2058. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ulukaya E, Pirianov G, Kurt MA, Wood EJ
and Mehmet H: Fenretinide induces cytochrome c release, caspase 9
activation and apoptosis in the absence of mitochondrial membrane
depolarisation. Cell Death Differ. 10:856–859. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wang H, Maurer BJ, Reynolds CP and Cabot
MC: N-(4-hydroxyphenyl)retinamide elevates ceramide in
neuroblastoma cell lines by coordinate activation of serine
palmitoyltransferase and ceramide synthase. Cancer Res.
61:5102–5105. 2001.PubMed/NCBI
|
|
30
|
Cowart LA, Szulc Z, Bielawska A and Hannun
YA: Structural determinants of sphingolipid recognition by
commercially available anti-ceramide antibodies. J Lipid Res.
43:2042–2048. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Oplustilova L, Wolanin K, Mistrik M,
Korinkova G, Simkova D, Bouchal J, Lenobel R, Bartkova J, Lau A,
O’Connor MJ, Lukas J and Bartek J: Evaluation of candidate
biomarkers to predict cancer cell sensitivity or resistance to
PARP-1 inhibitor treatment. Cell Cycle. 11:3837–3850. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Separovic D, Joseph N, Breen P, Bielawski
J, Pierce JS, van Buren E, Bhatti G, Saad ZH, Bai A and Bielawska
A: Combining anticancer agents photodynamic therapy and LCL85 leads
to distinct changes in the sphingolipid profile, autophagy,
caspase-3 activation in the absence of cell death, and long-term
sensitization. Biochem Biophys Res Commun. 409:372–377. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Lee H, Rotolo JA, Mesicek J, Penate-Medina
T, Rimner A, Liao WC, Yin X, Ragupathi G, Ehleiter D, Gulbins E,
Zhai D, Reed JC, Haimovitz-Friedman A, Fuks Z and Kolesnick R:
Mitochondrial ceramide-rich macrodomains functionalize Bax upon
irradiation. PLoS One. 6:e197832011. View Article : Google Scholar : PubMed/NCBI
|