Introduction
Tumor invasion and metastasis are the main
biological characteristics of malignant cancers. Mortality in
cancer patients principally results from the metastatic spread of
cancer cells to distant organs. Tumor metastasis is a highly
complex and multistep process, which includes changes in cell-cell
adhesion properties. A number of molecules, including matrix
metalloproteinases (MMPs) (1),
integrins (2), and Rac GTPases
(3), have been found to be
responsible for cancer cell motility.
Alterations in integrin-mediated signaling pathways
and integrin trafficking are involved in nearly all steps of
carcinogenesis including adhesion and migration, which include
changes in the utilization of αβ heterodimers, aberrant expression
of integrins, and constitutive activation of downstream molecules
of integrin signaling pathways (4). Integrins play important roles in
pathological angiogenesis and tumor metastasis, making them
attractive targets for cancer therapy strategies (5). Integrins α5β1, α6β4, αvβ3, and αvβ6
have been extensively studied in cancer and their expressions are
closely associated with cancer progression in various tumor types
(6). Upregulation of these
integrins renders cancer cells more motile, invasive, and resistant
to anticancer drugs (7). Integrins
transmit signals across the plasma membrane via the tyrosine
kinases Src and focal adhesion kinase (FAK) and the CRK-associated
tyrosine kinase substrate p130Cas, and thereby regulate cell
adhesion, migration, invasion, proliferation, and differentiation
(8). In addition, numerous studies
have indicated that many signaling molecules, including
AMP-activated protein kinase (AMPK) (9,10),
protein kinase C (PKC) (11), and
mitogen-activated protein kinases (MAPK) (12), are associated with
integrin-mediated regulation of metastasis in cancer cells.
Integrin trafficking regulates cell adhesion to
extracellular matrix (ECM), establishes and maintains cell
polarity, redefines signaling pathways, and controls migration
(13). It is regulated by members
of the Ras-associated binding (Rab) family of small GTPases, which
function as molecular switches regulating vesicular transport in
eukaryotic cells. Rab11 mediates slow integrin recycling through
recycling endosomes, whereas Rab4 mediates fast integrin recycling
directly from early endosomes (14). The deregulation of Rab GTPases is
closely related to cancer development and progression (15). Because of the involvement of Rab4
in the recycling of αvβ3 integrin, inhibition of Rab4 effector
protein (Rab IP4) blocks integrin recycling, leading to inhibition
of cell adhesion and cell spreading (16). Integrin αvβ6 is internalized by a
clathrin-dependent mechanism by interaction with HS1-associated
protein X1 (HAX1). HAX1 is found in clathrin-coated vesicles. When
the cytodomain of β6 integrin interacts with HAX1 and is
endocytosed, carcinoma migration and invasion is increased
(17).
Heavy-particle radiotherapy, including the use of
protons and carbon ions, has been producing noteworthy clinical
results worldwide (18). However,
the detailed regulatory mechanisms underlying their functions are
not yet well understood. In our previous studies (19,20),
we demonstrated that proton beam irradiation suppresses metastatic
capabilities such as migration, invasion, and MMP-2 and −9
expression, as well as increasing chemopreventive enzymes such as
quinone reductase (QR) and glutathione S-transferase (GST) in human
colorectal adenocarcinoma HT-29 cells. In the present study, to
elucidate the regulatory mechanisms underlying the inhibitory
effect of proton beam irradiation on metastatic potential, we
investigated the effects of proton beam on the expression of
members of the integrin family and trafficking regulators, and
integrin signaling pathways related to tumor progression.
Materials and methods
Materials
The following items were purchased from the stated
commercial sources: 12-O-tetradecanoyl phorbol-13-acetate
(TPA) from Sigma-Aldrich Co. (St. Louis, MO, USA); mouse anti-human
FAK (pY397), rabbit anti-phospho Src (Tyr416), rabbit anti-phospho
p130Cas (Tyr410), rabbit anti-phospho AMPKα (Thr172), and rabbit
anti-phospho PKC (pan) (ζ Thr410) from Cell Signaling Technology
(Danvers, MA, USA); mouse anti-human phospho MAPK (Tyr204), mouse
anti-β-actin, horseradish peroxidase (HRP)-conjugated anti-mouse
IgG, and anti-rabbit IgG-HRP antibodies from Santa Cruz
Biotechnology Inc. (Santa Cruz, CA, USA); ECL Plus Western Blotting
Substrate from Pierce Biotechnology (Rockford, IL, USA); TRIzol
from Invitrogen Life Technologies (Carlsbad, CA, USA); PrimeScript™
1st strand cDNA Synthesis kit from Takara Bio Inc. (Shiga, Japan);
FastStart Universal SYBR Green Master from Roche Applied Science
(Mannheim, Germany); phosphatase inhibitor cocktail and protease
inhibitor cocktail solutions from GenDEPOT (Barker, TX, USA).
Cell culture
The human colon adenocarcinoma cell line, HT-29, was
obtained from the Korean Cell Line Bank (KCLB no. 30038, Seoul,
Korea). Cells were grown in 5% CO2 at 37°C in RPMI-1640
medium supplemented with 10% fetal bovine serum, penicillin, and
streptomycin. To induce metastatic potential, cells were incubated
with 150 nM TPA for 1 h before proton beam irradiation.
Proton beam irradiation
Cell irradiation with a 35-MeV proton beam using the
MC-50 cyclotron (Scanditronix, Uppsala, Sweden) was carried out at
the Korean Institute of Radiological and Medical Sciences (Seoul,
Korea) according to a previous study (21). Cells anchored in a
12.5-cm2 flask filled with medium were placed on a beam
stage and then irradiated. Cells were irradiated (0.5, 2, 8 and 16
Gy) at the center of Bragg peaks modulated to 6-cm width. Flasks
were oriented such that the growth surface was orthogonal to the
horizontal beam entering from the top of the flask. A
mono-energetic proton beam cannot be applied for cancer cells
because the Bragg peak is too narrow to give a uniform dose to a
tumor of any significant depth. Thus, a region of high dose
uniformity in the percent depth-dose, known as spread-out
Bragg-peak (SOBP) dose distribution was created by traversing a
rotating range modulator designed to obtain a uniform dose
distribution to an indicated depth in cells plated and the media.
It was assumed that the thickness of the cell monolayer was between
3–6 μm and that of media was 1 cm. Thus dose distribution by SOBP
was enough to target live cells. The average dose rate was 2.31
Gy/sec. Radiochromic film (GAF-MD55) was used as an in situ
measuring tool of the dose at each beam irradiation.
Western blot analysis
After irradiation, cells were incubated for 1 and 3
days, washed with ice-cold PBS, and lysed in RIPA buffer (50 mM
NaCl, 10 mM Tris, 0.1% SDS, 1% Triton X-100, 0.1% sodium
deoxycholate, 5 mM EDTA, and 1 mM Na3VO4, pH
7.4). Proteins (40 μg) were separated by SDS-polyacrylamide gel
electrophoresis and transferred to nitrocellulose membranes
(Whatman, Dassel, Germany). The membranes were blocked with 5%
skimmed milk for 1 h and incubated with primary antibody (diluted
1:1,000) overnight at 4°C. After washing with Tris-buffered saline
containing 0.1% Tween-20, the membranes were incubated with
HRP-conjugated secondary antibodies (diluted 1:3,000) for 1 h at
room temperature. Antibodies binding on the nitrocellulose
membranes were detected with an enhanced chemiluminescence solution
(Amersham Bioscience, Buckinghamshire, UK) and radiography. The
images were obtained with a Lumino image analyzer (LAS-4000 Mini,
Fujifilm, Tokyo, Japan) and analyzed with image analysis software
(Multi Gauge ver. 3.0, Fujifilm).
Quantitative RT-PCR (qRT-PCR)
analysis
Total RNA was isolated from HT-29 using TRIzol
(Invitrogen Life Technologies), and cDNA was synthesized using
PrimeScript™ 1st strand cDNA synthesis kit (Takara Bio Inc.),
according to the instructions of the manufacturer. qRT-PCR was
performed in triplicate using a FastStart SYBR Green Master Mix
(Roche Diagnostics, Mannheim, Germany) in ABI PRISM 7300 Sequence
Detection System (Applied Biosystems, Foster City, CA, USA). The
expression levels of target genes relative to that of the
endogenous reference gene, actin, were calculated using the delta
cycle threshold method (22)
(Table I).
 | Table IPrimers for quantitative RT-PCR
analysis. |
Table I
Primers for quantitative RT-PCR
analysis.
| Forward | Reverse |
|---|
| ITGA5 |
GGCAGCTATGGCGTCCCACTGTGG |
GGCATCAGAGGTGGCTGGAGGCTT |
| ITGA6 |
GGAGCCCCACAGTATTTTGA |
TTCCATTTGCAGATCCATGA |
| ITGAV |
ACTCAAGCAAAAGGGAGCAA |
TGCAAGCCTGTTGTATCAGC |
| ITGB1 |
AATGAAGGGCGTGTTGGT |
CTGCCAGTGTAGTTGGGGTT |
| ITGB3 |
CGTCCAGGTCACCTTTGATT |
GTGGCAGACACATTGACCAC |
| ITGB4 |
ATGAGGCCTGAGAAGCTGAA |
GCTGACTCGGTGGAGAAGAC |
| ITGB6 |
TGCGACCATCAGTGAAGAAG |
GTAGGACAACCCCGATGAGA |
| FAK |
TGGTGAAAGCTGTCATCGAG |
CTGGGCCAGTTTCATCTTGT |
| CDH1 |
TGCCCAGAAAATGAAAAAGG |
GGATGACACAGCGTGAGAGA |
| RAB 4 |
CACTCGAGCAATGTCCGAAACCTACG |
GTGAATTCCTAACAACCACACTCCTGAGC |
| RAB 11 |
CACTCGAGCAATGGGCACCCGCGACGAC |
GTGAATTCCTTAGATGTTCTGACAGCAC |
| HAX1 |
ATGGACCCCCATCCTAGAAC |
CTGCTATCTGCTTCGTGTCG |
| Rab IP4 |
CCTTTGGAACTGGTGGAGAA |
ACCAGCAGCCCAACAATTAC |
Statistical analysis
All data are presented as the mean ± SEM. The data
were evaluated by one-way analysis of variance (ANOVA). Differences
between the mean values were assessed using Dunnett’s multiple
comparisons test. Statistical significance was defined as
P<0.05.
Results
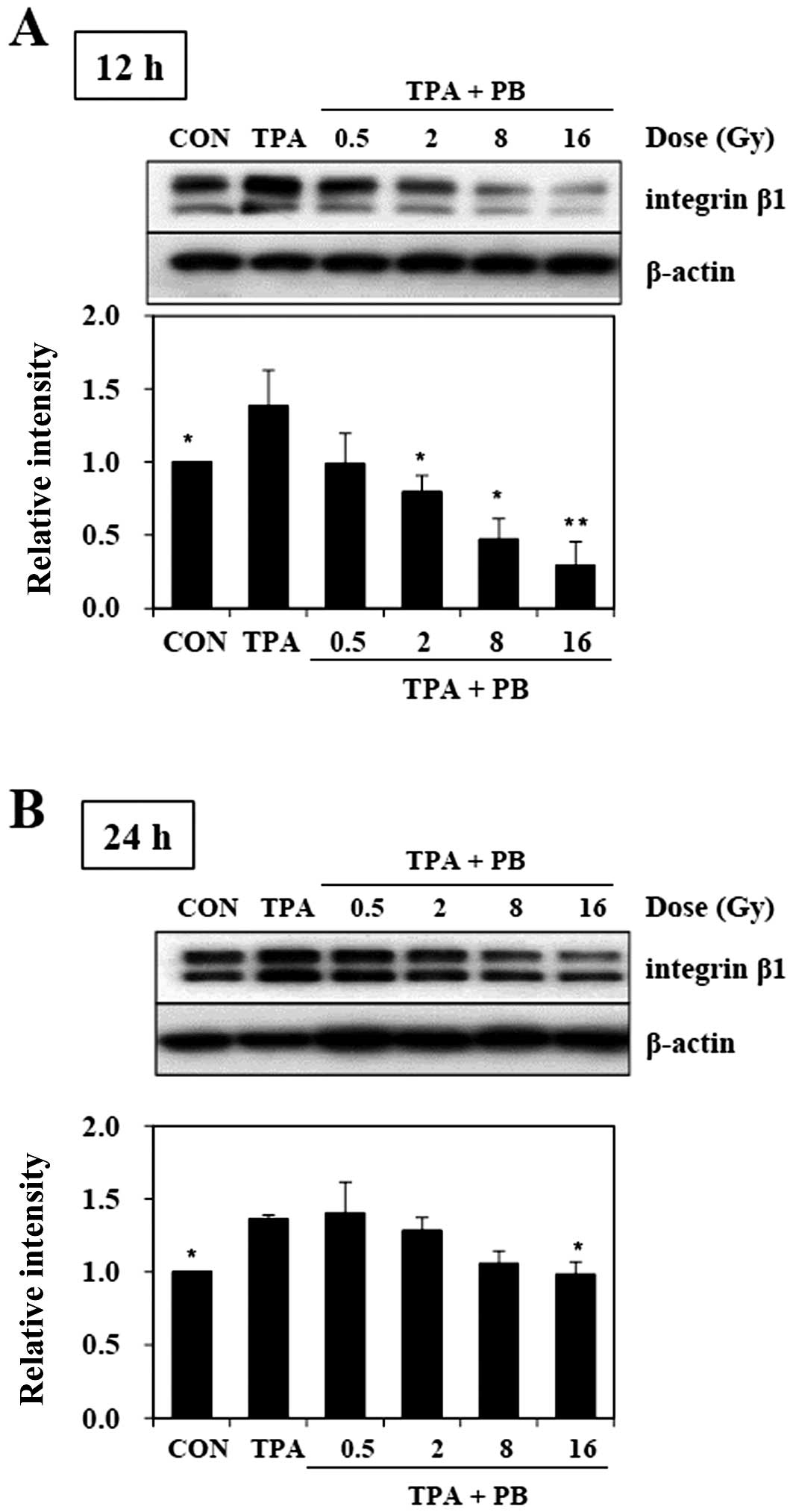
The inhibitory effect of proton beam
irradiation on the TPA-induced integrin β1 expression
To determine whether proton beam irradiation
regulates the expression of integrin β1, we investigated the
expression of integrin β1 in TPA-induced aggressive HT-29 human
colorectal adenocarcinoma cells after the cells were irradiated by
proton beam at 0.5, 2, 8 and 16 Gy. The treatment of TPA for 1 h
resulted in higher expression of integrin β1 than that of
non-treated control, while proton beam irradiation severely
inhibited TPA-induced integrin β1 expression in a dose-dependent
manner 12 h (Fig. 1A) and 24 h
(Fig. 1B) after irradiation.
Twelve hours after proton beam irradiation, the dose of 16 Gy
showed the strongest inhibitory effect on the expression of
integrin β1. Therefore, these findings suggest that proton beam can
inhibit metastatic potential, including migration and invasion, in
TPA-induced HT-29 human colorectal adenocarcinoma cells.
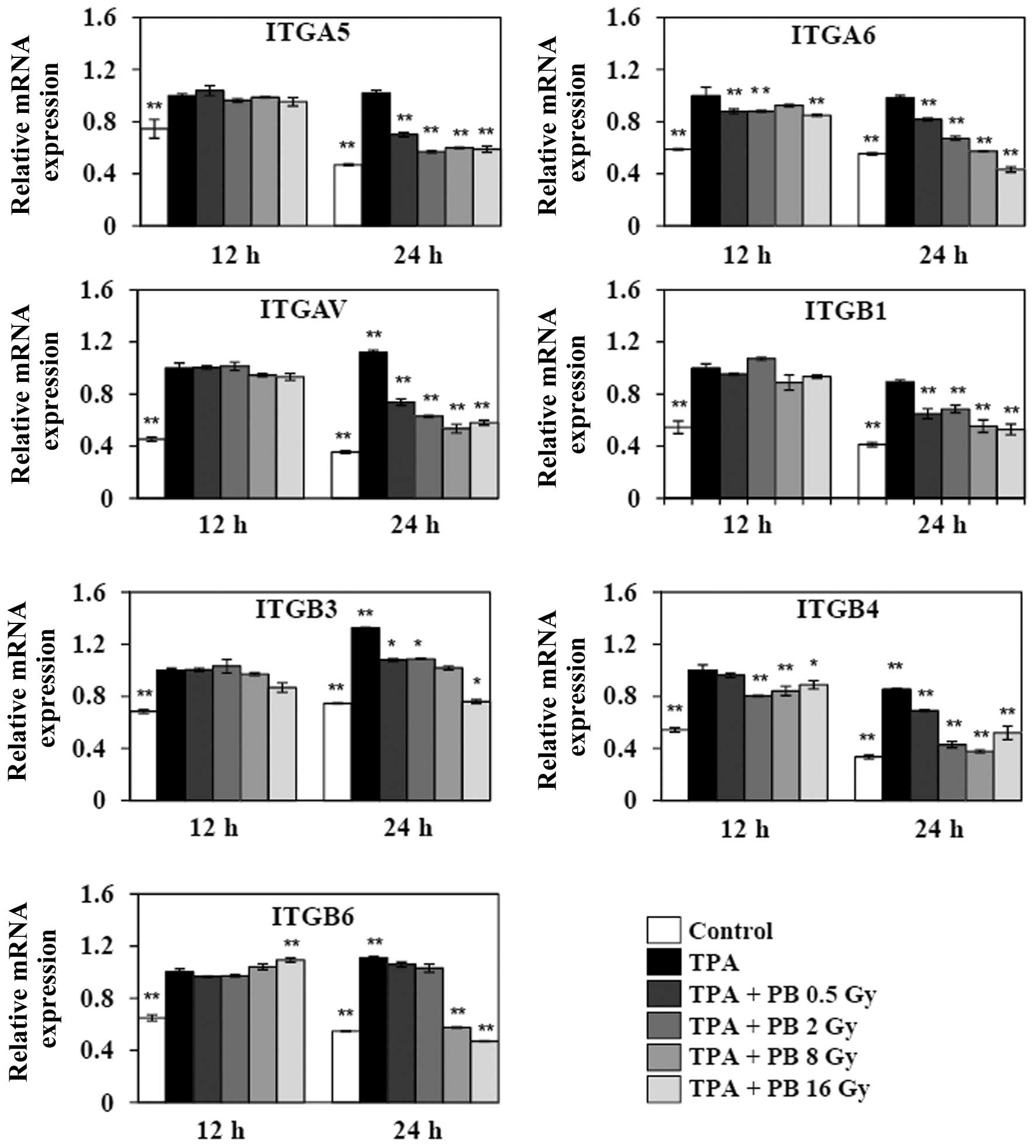
Proton beam irradiation inhibits
TPA-induced gene expressions of integrins involved in migration and
invasion
To further explore the effect of proton beam
irradiation on integrin expression, we next investigated the gene
expressions of integrin subunits, such as ITGA5 and ITGB1 (α5β1)
that form a fibronectin receptor, ITGA6 and ITGB4 (α6β4) that form
a laminin receptor, ITGAV and ITGB3 (αvβ3) that form fibronectin
and vitronectin receptors, and ITGAV and ITGB3 (αvβ6). The
expressions of these subunits have been extensively shown to be
correlated with cancer progression in various tumor types. The
treatment with TPA for 1 h increased the expression of ITGAV and
ITGB3 in a time-dependent manner. Although the TPA-induced gene
expressions did not significantly changed 12 h after irradiation,
proton beam irradiation remarkably decreased the gene expression of
all subunits after 24 h (Fig. 2).
Therefore, these findings suggest that proton beam can inhibit
migration and invasion through the inhibition of the gene
expression of members of the integrin family in TPA-induced HT-29
human colorectal adenocarcinoma cells.
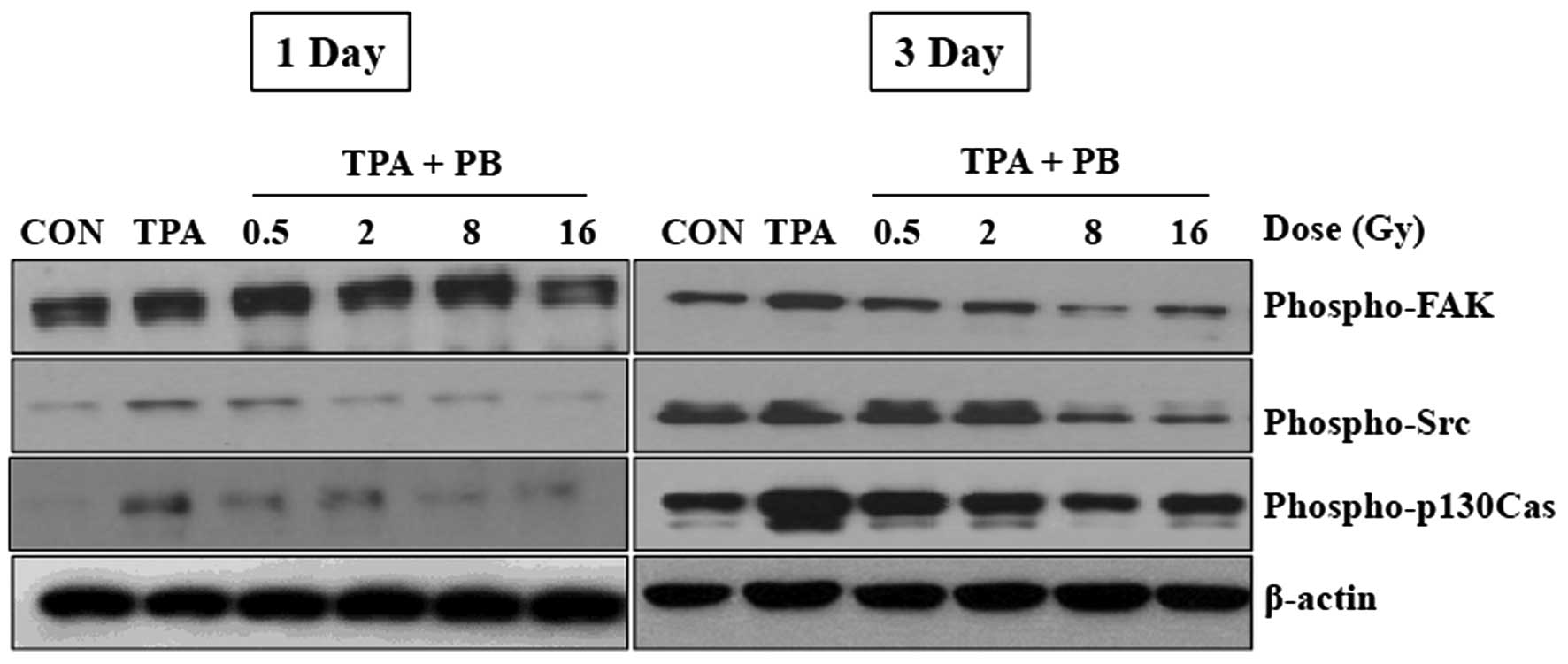
Proton beam irradiation inhibits the
phosphorylation of key molecules involved in integrin
signaling
To investigate whether proton beam irradiation
regulates integrin signaling pathways, we assessed the effects of
proton beam irradiation on the phosphorylation status of key
molecules involved in integrin signaling pathways 1 and 3 days
after irradiation. Three days after irradiation, the
phosphorylation of Src, a non-receptor tyrosine kinase, as well as
p130Cas, an adaptor protein were consistently increased. The
phosphorylation of FAK by TPA was increased 1 day after
irradiation, but was decreased at 3 days after irradiation. Proton
beam irradiation at 16 Gy completely suppressed the phosphorylation
of the proteins (Fig. 3).
Moreover, the phosphorylation of Src and p130Cas, but not FAK, was
increased in the control group. These results suggest that proton
beam irradiation may regulate cell adhesion and migration through
the inhibition of the activities of downstream integrin signaling
molecules.
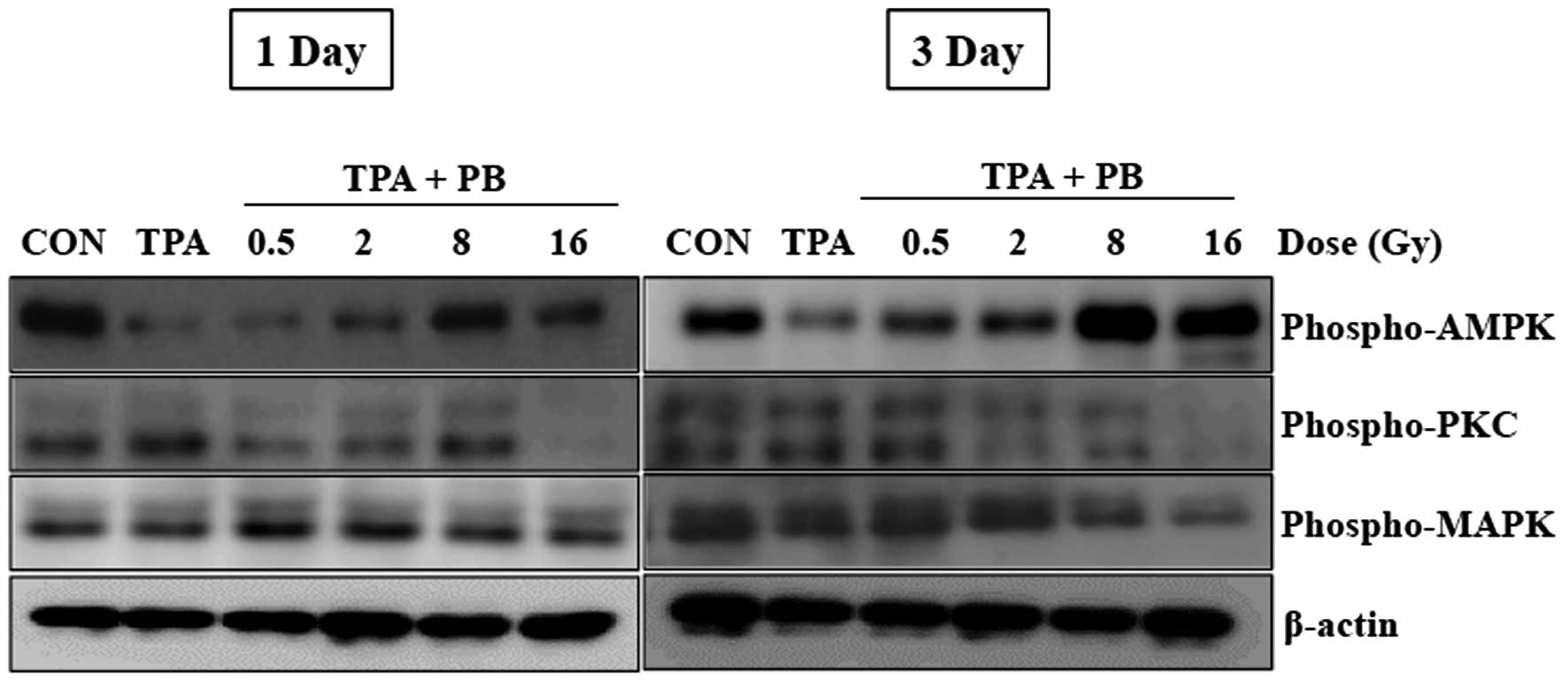
Proton beam irradiation regulates the
phosphorylation of molecules downstream of integrin signaling
To further study the regulatory mechanism underlying
proton beam irradiation, we next investigated the phosphorylation
of AMPK, PKC, and MAPK, which are molecules downstream of integrin
signaling and well known for their roles in the regulation of
various integrin-dependent cellular functions. As shown in Fig. 4, the treatment with TPA for 1 h
consistently decreased the phosphorylation of AMPK as compared with
that in the control group. However, proton beam irradiation at 8
and 16 Gy resulted in notable increases in the phosphorylation of
AMPK. On the contrary, an inhibitory effect on the phosphorylation
of PKC and MAPK was observed 3 days after irradiation at 16 Gy.
These results suggest that proton beam irradiation may regulate
integrin-mediated functions by upregulating the activity of
AMPK.
Proton beam irradiation inhibits the
expressions of genes related to integrin-mediated cell adhesion and
integrin trafficking
To study how proton beam irradiation affects
integrin-mediated functions, we investigated the expression of
genes that regulate integrin-mediated adhesion to ECM, as well as
integrin trafficking. As shown in Fig.
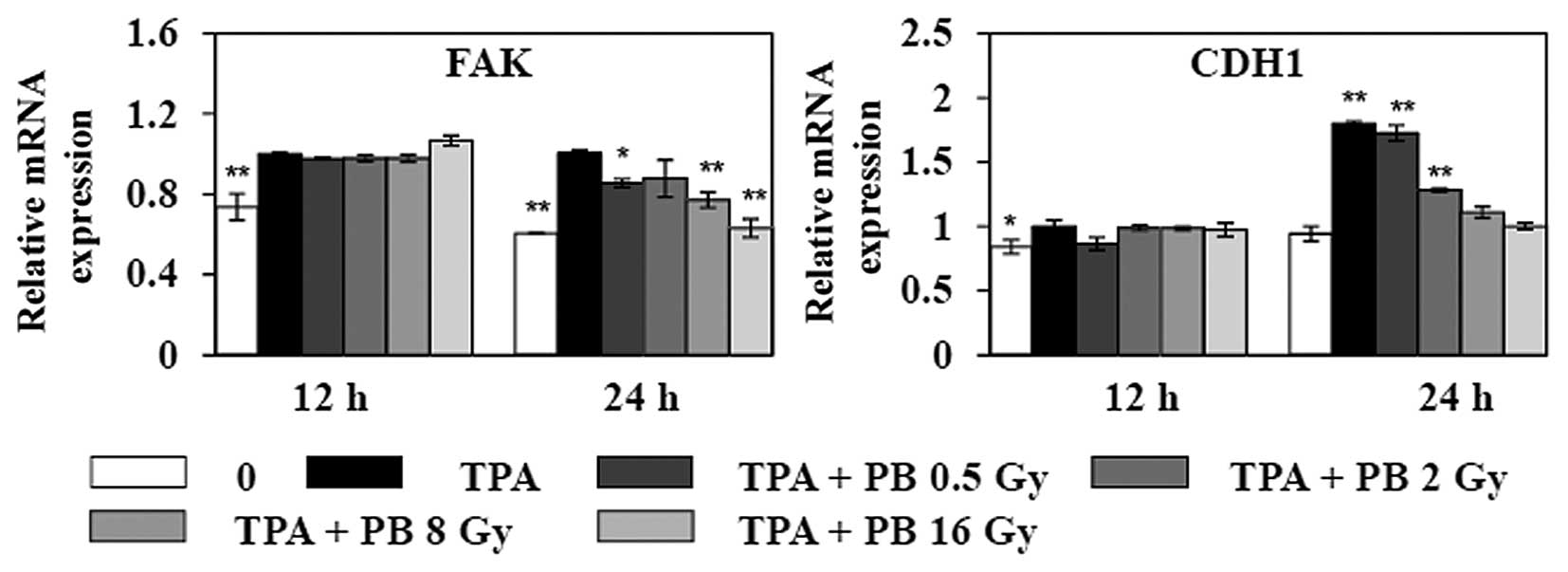
5, the treatment with TPA increased the expressions of CDH1 and
FAK 12 h after irradiation. Proton beam irradiation
dose-dependently decreased the expressions of FAK and CDH1 24 h
after irradiation. To further study the effects of proton beam
irradiation on integrin functions, we assessed the effect of proton
beam irradiation on the expressions of genes related to integrin
trafficking (Fig. 6). Proton beam
irradiation dose-dependently decreased the gene expression of Rab
family of small GTPases, such as RAB4, which mediates fast integrin
recycling from the early endosome RAB11, which mediates slow
integrin recycling through recycling endosome; and HAX1, which
mediates clathrin-dependent endocytosis of integrin, 24 h after
irradiation. In contrast, TPA decreased the gene expression of Rab
IP4, which blocks integrin recycling, leading to inhibition of cell
adhesion and cell spreading. However, the expression of Rab IP4 was
increased by proton beam irradiation at 8 Gy. Therefore, these
results suggest that proton beam irradiation can modulate cell
adhesion and migration, as well as integrin trafficking necessary
for tumor progression.
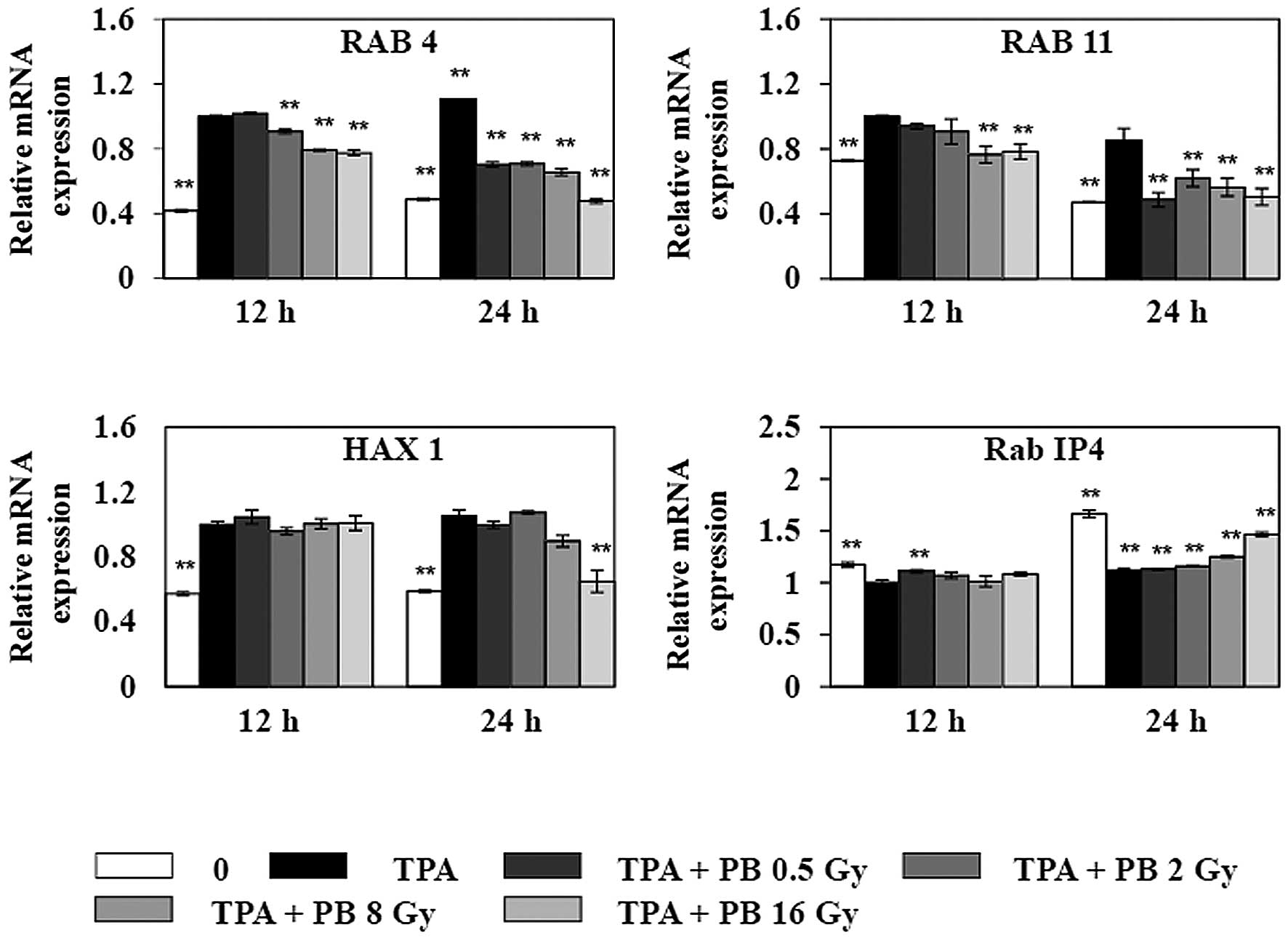 | Figure 6Effects of proton beam irradiation on
the gene expressions of RAB4, RAB11, HAX1, and Rab IP4, which are
related to integrin trafficking, in TPA-induced HT-29 human
colorectal adenocarcinoma cells. Before irradiation, cells were
treated with 150 nM TPA for 1 h. After cells were irradiated with
proton beam at different doses, expressions of RAB4, RAB11, HAX1,
and Rab IP4 were determined by qRT-PCR. Each value represents the
mean ± SEM of three independent experiments. *P<0.05,
**P<0.01 vs. the TPA group. |
Discussion
In the present study, we demonstrated that proton
beam irradiation suppresses protein expression of integrin β1, gene
expressions of members of the integrin family, integrin-mediated
signaling pathways involving FAK, Src, and p130Cas, the
phosphorylation of kinases such as PKC and MAPK, and gene
expression of regulators involved in integrin trafficking such as
RAB4, RAB11, and HAX1, as well as increasing the phosphorylation of
AMPK and Rab IP4, which are well known as negative regulators on
tumor progression and metastasis.
Proton beam therapy (PBT) has recently attracted
attention for its use as an alternative to gamma or X-ray
irradiation therapy and been producing promising clinical results
worldwide (23). PBT has been used
for decades, mainly to treat hepatocellular carcinoma (24), non-small cell lung cancer (25), prostate cancer (26), and head and neck tumors (27). In addition, many in vitro
studies have shown that PBT suppresses metastatic capability,
including adhesion and migration, in diverse human cancer cell
lines (28–30). However, a more detailed
investigation on the inhibitory effect of proton beam on metastatic
potential of cancer cells is still needed.
Integrin β1 is the most widely expressed integrin in
cells, and it has been implicated in the clinical course and
prognosis of several types of cancer (31). Integrin β1 plays a significant role
in tumor metastasis. It binds to ECM and initiates adhesion by
recruiting cytoplasmic proteins, such as Src, FAK, and p130Cas
(32). The multiple parallel
signaling pathways downstream of integrin engagement that promote
tumor growth include FAK, Src, PKC, MAP kinase, Akt, and Ras
pathways. These pathways are upregulated as the level of integrin
β1 increases (33). Indeed,
several studies have demonstrated the correlation of the expression
of integrin β1 with malignant features, including metastasis
(34,35). Accordingly, integrin β1 signaling
in tumor cells has been shown to promote resistance to multiple
treatment modalities, including cytotoxic drugs, radiotherapy
(36), and targeted therapies such
as trastuzumab (37) and lapatinib
(38). Studies using conditional
genetic models point to critical roles of integrin β1 in
initiation, growth, or progression of a variety of cancers
(4). In a prostate adenocarcinoma
model, deletion of integrin β1 led to more dramatic expansion of
the tumor cell population, enhanced the rate of prostate tumor
progression, and decreased overall animal survival (39).
The metastatic potential of tumors depends on
integrin complexes, which function as intracellular signaling
mediators. Integrins promote migration of cells on the surrounding
ECM, and the signals initiated by integrin binding to ECM proteins
are necessary for the maintenance of cell survival. Focal adhesion
sites contain integrins and complexes of signaling elements, such
as Src, FAK, p130Cas, MAP kinases, small GTPases, and
phosphoinositide 3-kinase (40).
Although it is not clear which regulatory mechanism is employed by
proton beam irradiation to modulate the expression of integrin β1,
we demonstrated that proton beam irradiation suppressed the protein
expression of integrin β1, leading to an inhibition of the
phosphorylated forms of FAK, Src, and p130Cas, which are molecules
downstream of the integrin β1 signaling pathway. The decrease in
the expression of integrin β1 protein was accompanied by changes in
mRNA levels of integrin β1 as well as other members of the integrin
family, suggesting a post-translational mechanism. Therefore, our
findings demonstrate that downregulation of the protein level of
integrin β1 and activities of downstream signaling molecules is a
novel mechanism underlying the suppressive effect of proton beam
irradiation on the migration of cancer cells.
On the contrary, recent studies have shown not only
integrin signaling, but also integrin trafficking contribute to
cancer growth and progression (4,6).
Abundant evidence suggests that integrin trafficking regulates cell
adhesion to ECM, establishes and maintains cell polarity, redefines
signaling pathways, and controls migration (41). Therefore, transcriptional changes,
mutational alterations, and deregulated cellular signaling changing
endocytosis and recycling of integrins confer invasive and
metastatic properties to tumor cells. Although at least 24 αβ
integrin heterodimers are known, α5β1, α6β4, αvβ3, and αvβ6
integrins have been extensively studied in cancer and the
expression is correlated with cancer progression in various tumor
types (7). Upregulation of these
integrins renders cancer cells more motile, invasive, and resistant
to anticancer drugs (42). Unlike
these integrins, expression levels of certain integrins, such as
α2β1 (43) and α1β1 (44), decrease in tumor cells, which
potentially increase tumor cell dissemination. In addition to
changes in expression, changes in the functions of these integrins
also play a critical role in cancer progression. Therefore, our
findings suggest proton beam irradiation as a general and strong
inhibitor on the expression of members of the integrin family. AMPK
is a metabolic sensor that maintains cellular energy homeostasis.
AMPK regulates lipid, cholesterol, and glucose metabolism in
specialized metabolic tissues, such as liver, muscle, and adipose
tissues. This function has made AMPK a key therapeutic target in
patients with obesity and diabetes (45). The connection of AMPK with several
tumor suppressors suggests that therapeutic manipulation of this
pathway using established diabetes drugs warrants further
investigation in patients with cancer (46). Although previous studies showed
that LKB1-deficient (47) or
AMPK-deficient cells (48) are
resistant to oncogenic transformation and tumorigenesis, the role
of AMPK in tumorigenesis and tumor metabolism is unknown. Recent
studies have indicated that AMPK controls metastasis of cancer
cells (49,50). Interestingly, our findings
indirectly show that an increase in AMPK phosphorylation by proton
beam irradiation may suppress metastatic potentials of TPA-induced
HT-29 human colorectal adenocarcinoma cells. However, a more
detailed investigation on the regulatory mechanism underlying
proton beam irradiation-induced AMPK phosphorylation is still
needed.
In conclusion, our findings suggest that proton beam
irradiation can inhibit metastatic potential including cell
adhesion and migration by modulating gene expression of integrins,
genes involved in integrin trafficking, and activities of molecules
involved in integrin sigmaling necessary for tumor progression.
Acknowledgements
This study was supported by the National Research
Foundation of Korea (NRF) grant funded by the Korea government
(MSIP) (NRF-2014M2B2A4030340).
Abbreviations:
|
TPA
|
12-O-tetradecanoylphorbol-13-acetate
|
|
FAK
|
focal adhesion kinase
|
|
AMPK
|
AMP-activated protein kinase
|
|
PKC
|
protein kinase C
|
|
MAPK
|
mitogen-activated protein kinases
|
|
ECM
|
extracellular matrix
|
|
Rab GTPase
|
Ras-associated binding small
GTPase
|
|
Rab IP4
|
Rab4 effector protein
|
|
HAX1
|
HS1-associated protein X1
|
|
PBT
|
proton beam therapy
|
References
|
1
|
Chaudhary AK, Pandya S, Ghosh K and
Nadkarni A: Matrix metalloproteinase and its drug targets therapy
in solid and hematological malignancies: An overview. Mutat Res.
753:7–23. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ganguly KK, Pal S, Moulik S and Chatterjee
A: Integrins and metastasis. Cell Adhes Migr. 7:251–261. 2013.
View Article : Google Scholar
|
|
3
|
Cook DR, Rossman KL and Der CJ: Rho
guanine nucleotide exchange factors: Regulators of Rho GTPase
activity in development and disease. Oncogene. 33:4021–4035. 2014.
View Article : Google Scholar
|
|
4
|
Xiong J, Balcioglu HE and Danen EH:
Integrin signaling in control of tumor growth and progression. Int
J Biochem Cell Biol. 45:1012–1015. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kapp TG, Rechenmacher F, Sobahi TR and
Kessler H: Integrin modulators: a patent review. Expert opinion on
therapeutic patents. 23:1273–1295. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Shin S, Wolgamott L and Yoon SO: Integrin
trafficking and tumor progression. Int J Cell Biol.
2012:5167892012. View Article : Google Scholar
|
|
7
|
Desgrosellier JS and Cheresh DA: Integrins
in cancer: Biological implications and therapeutic opportunities.
Nat Rev Cancer. 10:9–22. 2010. View
Article : Google Scholar
|
|
8
|
Mitra SK and Schlaepfer DD:
Integrin-regulated FAK-Src signaling in normal and cancer cells.
Curr Opin Cell Biol. 18:516–523. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hahn SS, Tang Q, Zheng F, Zhao S, Wu J and
Chen J: Repression of integrin-linked kinase by antidiabetes drugs
through crosstalk of PPARγ- and AMPKα-dependent signaling: Role of
AP-2α and Sp1. Cell Signal. 26:639–647. 2014. View Article : Google Scholar
|
|
10
|
Caino MC, Chae YC, Vaira V, Ferrero S,
Nosotti M, Martin NM, Weeraratna A, O’Connell M, Jernigan D,
Fatatis A, et al: Metabolic stress regulates cytoskeletal dynamics
and metastasis of cancer cells. J Clin Invest. 123:2907–2920. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang J, Wu J, Hong J, Chen R, Xu K, Niu W,
Peng C, Liu E, Wang J, Liu S, et al: PKC promotes the migration of
colon cancer cells by regulating the internalization and recycling
of integrin αvβ6. Cancer Lett. 311:38–47. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chen J, Elfiky A, Han M, Chen C and Saif
MW: The role of Src in colon cancer and its therapeutic
implications. Clin Colorectal Cancer. 13:5–13. 2014. View Article : Google Scholar
|
|
13
|
Caswell PT and Norman JC: Integrin
trafficking and the control of cell migration. Traffic. 7:14–21.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hutagalung AH and Novick PJ: Role of Rab
GTPases in membrane traffic and cell physiology. Physiol Rev.
91:119–149. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Subramani D and Alahari SK:
Integrin-mediated function of Rab GTPases in cancer progression.
Mol Cancer. 9:3122010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Vukmirica J, Monzo P, Le Marchand-Brustel
Y and Cormont M: The Rab4A effector protein Rabip4 is involved in
migration of NIH 3T3 fibroblasts. J Biol Chem. 281:36360–36368.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ramsay AG, Keppler MD, Jazayeri M, Thomas
GJ, Parsons M, Violette S, Weinreb P, Hart IR and Marshall JF:
HS1-associated protein X-1 regulates carcinoma cell migration and
invasion via clathrin-mediated endocytosis of integrin alphavbeta6.
Cancer Res. 67:5275–5284. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Mitin T and Zietman AL: Promise and
pitfalls of heavy-particle therapy. J Clin Oncol. 32:2855–2863.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Nam KS, Kim MK and Shon YH: Cancer
chemopreventive enzymes of human colorectal adenocarcinoma cells
irradiated with proton beams. J Korean Phys Soc. 52:945–948. 2008.
View Article : Google Scholar
|
|
20
|
Nam KS and Shon YH: Suppression of
metastatic potential in human colorectal adenocarcinoma cells
irradiated with proton beams. J Korean Phys Soc. 59:709–712. 2011.
View Article : Google Scholar
|
|
21
|
Lee KB, Kim KR, Huh TL and Lee YM: Proton
induces apoptosis of hypoxic tumor cells by the p53-dependent and
p38/JNK MAPK signaling pathways. Int J Oncol. 33:1247–1256.
2008.PubMed/NCBI
|
|
22
|
Sikorsky JA, Primerano DA, Fenger TW and
Denvir J: Effect of DNA damage on PCR amplification efficiency with
the relative threshold cycle method. Biochem Biophys Res Commun.
323:823–830. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Combs SE, Djosanjh M, Pötter R, Orrechia
R, Haberer T, Durante M, Fossati P, Parodi K, Balosso J, Amaldi U,
et al: Towards clinical evidence in particle therapy: ENLIGHT,
PARTNER, ULICE and beyond. J Radiat Res (Tokyo). 54(Suppl 1):
i6–i12. 2013. View Article : Google Scholar
|
|
24
|
Lee SU, Park JW, Kim TH, Kim YJ, Woo SM,
Koh YH, Lee WJ, Park SJ, Kim DY and Kim CM: Effectiveness and
safety of proton beam therapy for advanced hepatocellular carcinoma
with portal vein tumor thrombosis. Strahlenther Onkol. 190:806–814.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chang JY, Komaki R, Lu C, Wen HY, Allen
PK, Tsao A, Gillin M, Mohan R and Cox JD: Phase 2 study of
high-dose proton therapy with concurrent chemotherapy for
unresectable stage III nonsmall cell lung cancer. Cancer.
117:4707–4713. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hoppe B, Henderson R, Mendenhall WM,
Nichols RC, Li Z and Mendenhall NP: Proton therapy for prostate
cancer. Oncology. 25:644–650. 6522011.PubMed/NCBI
|
|
27
|
Frank SJ and Selek U: Proton beam
radiation therapy for head and neck malignancies. Curr Oncol Rep.
12:202–207. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Gridley DS, Pecaut MJ, Mao XW, Wroe AJ and
Luo-Owen X: Biological effects of passive versus active scanning
proton beams on human lung epithelial cells. Technol Cancer Res
Treat. 14:81–98. 2015.
|
|
29
|
Wéra AC, Heuskin AC, Riquier H, Michiels C
and Lucas S: Low-LET proton irradiation of A549 non-small cell lung
adenocarcinoma cells: Dose response and RBE determination. Radiat
Res. 179:273–281. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zaboronok A, Isobe T, Yamamoto T, Sato E,
Takada K, Sakae T, Tsurushima H and Matsumura A: Proton beam
irradiation stimulates migration and invasion of human U87
malignant glioma cells. J Radiat Res (Tokyo). 55:283–287. 2014.
View Article : Google Scholar
|
|
31
|
Brakebusch C and Fässler R: beta 1
integrin function in vivo: Adhesion, migration and more. Cancer
Metastasis Rev. 24:403–411. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Canel M, Serrels A, Frame MC and Brunton
VG: E-cadherin-integrin crosstalk in cancer invasion and
metastasis. J Cell Sci. 126:393–401. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Jahangiri A, Aghi MK and Carbonell WS: β1
integrin: Critical path to antiangiogenic therapy resistance and
beyond. Cancer Res. 74:3–7. 2014. View Article : Google Scholar :
|
|
34
|
Parvani JG, Galliher-Beckley AJ, Schiemann
BJ and Schiemann WP: Targeted inactivation of β1 integrin induces
β3 integrin switching, which drives breast cancer metastasis by
TGF-β. Mol Biol Cell. 24:3449–3459. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Howe GA and Addison CL: β1 integrin: An
emerging player in the modulation of tumorigenesis and response to
therapy. Cell Adhes Migr. 6:71–77. 2012. View Article : Google Scholar
|
|
36
|
Nam JM, Chung Y, Hsu HC and Park CC: beta1
integrin targeting to enhance radiation therapy. Int J Radiat Biol.
85:923–928. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Lesniak D, Sabri S, Xu Y, Graham K,
Bhatnagar P, Suresh M and Abdulkarim B: Spontaneous
epithelial-mesenchymal transition and resistance to HER-2-targeted
therapies in HER-2-positive luminal breast cancer. PLoS One.
8:e719872013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Huang C, Park CC, Hilsenbeck SG, Ward R,
Rimawi MF, Wang YC, Shou J, Bissell MJ, Osborne CK and Schiff R: β1
integrin mediates an alternative survival pathway in breast cancer
cells resistant to lapatinib. Breast Cancer Res. 13:R842011.
View Article : Google Scholar
|
|
39
|
Moran-Jones K, Ledger A and Naylor MJ: β1
integrin deletion enhances progression of prostate cancer in the
TRAMP mouse model. Sci Rep. 2:5262012. View Article : Google Scholar
|
|
40
|
Harburger DS and Calderwood DA: Integrin
signalling at a glance. J Cell Sci. 122:159–163. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Caswell PT, Vadrevu S and Norman JC:
Integrins: Masters and slaves of endocytic transport. Nat Rev Mol
Cell Biol. 10:843–853. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
42
|
Makrilia N, Kollias A, Manolopoulos L and
Syrigos K: Cell adhesion molecules: Role and clinical significance
in cancer. Cancer Invest. 27:1023–1037. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Ramirez NE, Zhang Z, Madamanchi A, Boyd
KL, O’Rear LD, Nashabi A, Li Z, Dupont WD, Zijlstra A and Zutter
MM: The α(2)β(1) integrin is a metastasis suppressor in mouse
models and human cancer. J Clin Invest. 121:226–237. 2011.
View Article : Google Scholar :
|
|
44
|
Mattila E, Pellinen T, Nevo J, Vuoriluoto
K, Arjonen A and Ivaska J: Negative regulation of EGFR signalling
through integrin-alpha1beta1-mediated activation of protein
tyrosine phosphatase TCPTP. Nat Cell Biol. 7:78–85. 2005.
View Article : Google Scholar
|
|
45
|
Grahame Hardie D: AMP-activated protein
kinase: A key regulator of energy balance with many roles in human
disease. J Intern Med. 276:543–559. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Shackelford DB and Shaw RJ: The LKB1-AMPK
pathway: Metabolism and growth control in tumour suppression. Nat
Rev Cancer. 9:563–575. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Bardeesy N, Sinha M, Hezel AF, Signoretti
S, Hathaway NA, Sharpless NE, Loda M, Carrasco DR and DePinho RA:
Loss of the Lkb1 tumour suppressor provokes intestinal polyposis
but resistance to transformation. Nature. 419:162–167. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Kato K, Ogura T, Kishimoto A, Minegishi Y,
Nakajima N, Miyazaki M and Esumi H: Critical roles of AMP-activated
protein kinase in constitutive tolerance of cancer cells to
nutrient deprivation and tumor formation. Oncogene. 21:6082–6090.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Chan KT, Asokan SB, King SJ, Bo T, Dubose
ES, Liu W, Berginski ME, Simon JM, Davis IJ, Gomez SM, et al: LKB1
loss in melanoma disrupts directional migration toward
extracellular matrix cues. J Cell Biol. 207:299–315. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Goodwin JM, Svensson RU, Lou HJ, Winslow
MM, Turk BE and Shaw RJ: An AMPK-independent signaling pathway
downstream of the LKB1 tumor suppressor controls Snail1 and
metastatic potential. Mol Cell. 55:436–450. 2014. View Article : Google Scholar : PubMed/NCBI
|