Introduction
Liver cancer is the third most common cause of
cancer death worldwide and an estimated 696,000 deaths from liver
cancer occurred in 2008 (1).
Surgery is currently the most effective treatment for patients with
hepatocellular carcinoma (HCC) (2). However, the indication of surgery is
restricted by limited criteria (2,3). In
addition, although the survival rate has improved during the past
decade, the rate of recurrence after surgery still remains high in
patients with HCC (4). Therefore,
systemic chemotherapy is required for patients with advanced stages
of HCC in order to prolong their survival.
Cisplatin [cis-diamminedichloroplatinum (II)]
is a commonly used anticancer drug, the biological activity of
which was first reported in 1965 (5). Cisplatin exerts cytotoxic effects
primarily by an interaction with cellular DNA; its binding alters
the structure of DNA and affects its ability to act as a template
during transcription, which ultimately triggers apoptotic cell
death (6,7). Despite a stable rate of initial
responses, cisplatin treatment often results in therapeutic failure
due to the development of chemoresistance (6). Several mechanisms related to the
cisplatin-induced antitumor effects have been reported in the past
several decades. Apoptosis is one of the major components of
cisplatin-induced cytotoxicity (8). With respect to the relationship
between the cytotoxic effects of cisplatin and the aberrant
expression of microRNAs (miRNAs), several studies have identified
the presence of dysregulated miRNAs in various cancers, such as
breast, gastric, lung, esophageal, ovarian, and tongue cancers
(9). However, the mechanism
involved in the relationship between cisplatin and HCC remains
elusive.
miRNAs are essentially 18–22 nucleotide-long
endogenous noncoding RNAs (3,10).
The effect of miRNAs on the regulation of the expression of various
genes is so broad that one miRNA controls more than 200 genes
(11). Among various human
cancers, it has been reported that aberrant expression of miRNAs is
a common feature and is related to patient survival (12–15).
In addition, with regard to the relationship between miRNAs and
HCC, several studies have shown aberrant expression of specific
miRNAs in HCC tissues compared with normal tissues (16–19).
These previous studies indicate that the modulation of non-coding
RNAs, especially miRNAs, might be a valuable target for HCC
formation.
Therefore, in our present study, we intended to
elucidate the profiles of miRNAs that are associated with the
cisplatin-induced antitumor effects observed in HCC cell lines, as
well as the role of cell cycle regulatory molecules and
apoptosis-related caspase proteins. An analysis of the miRNA
profiles after treatment with cisplatin may be a novel approach for
the treatment of patients with cisplatin-resistant HCC.
Materials and methods
Chemicals
Cisplatin was purchased from Nippon Kayaku (Tokyo,
Japan). A Cell Counting kit (CCK-8) was purchased from Dojindo
Laboratories (Kumamoto, Japan), and all other chemicals were
obtained from Sigma Chemical (Tokyo, Japan).
Antibodies
In this study, the following antibodies were used:
anti-β-actin monoclonal antibody (Sigma-Aldrich, St. Louis, MO,
USA; A5441, used at 1:2,000), cyclin D1 (Thermo Fisher Scientific,
Waltham, MA, USA; RB-9041, used at 1:1,000), Cdk6 (Santa Cruz
Biotechnology, Santa Cruz, CA, USA; sc-177, used at 1:500), Cdk4
(Cell Signaling Technology, Danvers, MA, USA; #2906, used at
1:1,000), horseradish peroxidase (HRP)-linked anti-mouse and
anti-rabbit IgG secondary antibodies (GE Healthcare UK,
Buckinghamshire, UK; used at 1:2,000).
Cell lines and culture
The human hepatocellular carcinoma cell lines HLE,
HLF, HuH7, Li-7, Hep3B and HepG2 were obtained from the Japanese
Cancer Research Resources Bank and were passaged in our laboratory.
The cell lines were authenticated by the cell bank using short
tandem repeat PCR. Cells were grown in Minimum Essential Medium
(MEM) (Gibco Invitrogen, Carlsbad, CA, USA) supplemented with 10%
FBS (533–69545; Wako) and penicillin-streptomycin (100 mg/l;
Invitrogen) in a humidified atmosphere of 5% CO2 at
37°C.
Cell proliferation assay
Cell proliferation assays were conducted using the
CCK-8 according to the manufacturer’s instructions. Cells from each
cell line (0.5×104) were seeded into a 96-well plate and
cultured in 100 μl of MEM supplemented with 10% FBS. After 24 h,
the seeded cells were treated with 0, 1, 3 or 10 μg/ml of cisplatin
added to the culture medium. At the indicated time points, the
medium was changed to 100 μl of MEM with CCK-8 reagent (10 μl of
CCK-8 and 90 μl of MEM). Absorbance was measured for each well at a
wavelength of 450 nm using an auto-microplate reader.
Cell lysates
The lysates were collected according to the methods
described in our previous studies (20). All steps were performed at 4°C.
Protein concentrations were measured using a dye-binding protein
assay based on the Bradford method (21).
Gel electrophoresis and western
blotting
Samples were electrophoresed using 7.5 to 10%
SDS-PAGE (22) after which the
proteins were transferred to nitrocellulose membranes. The
membranes were incubated with primary antibodies after blocking and
were then incubated with HRP-conjugated secondary antibodies
(23). Immunoreactive proteins
were visualized with an enhanced chemiluminescence detection system
(Perkin Elmer Co.) on X-ray film.
Apoptosis
The detection of caspase-cleaved cytokeratin 18
(CK18) was performed using an M30 Apoptosense ELISA kit which was
purchased from Peviva AB (Bromma, Sweden). HuH7 cells
(0.5×104) were seeded into a 96-well plate and cultured
in 100 μl of MEM supplemented with 10% FBS. After 24 h, the seeded
cells were washed once with PBS and treated with 1 μg/ml of
cisplatin added to the culture medium. At the indicated time
points, 10 μl of PRO-PREP™ protein extraction solution was added to
each well. The plates were shaken on a rotary shaker for 5 min at
room temperature in order to lyse the cells. Next, 25 μl of M30
Standard (A–G), M30 Control Low, M30 Control High or samples was
pipetted into the appropriate wells. A total of 75 μl of the
diluted M30 HRP Conjugate solution was also added to each well. The
cells were incubated at room temperature for 4 h. The incubation
solution was discarded, and the plate was washed five times with
250 μl of diluted washing solution. Next, 200 μl of TMB substrate
was added to each well. The cells were incubated in the dark at
room temperature for 20 min. Then, 50 μl of stop solution was added
to each well. After 5 min, the absorbance was measured at a
wavelength of 450 nm using an auto-microplate reader.
Antibody arrays of phosphorylated
receptor tyrosine kinase
The RayBio Human Phospho Array kit (catalog no. ARY
001) was purchased from RayBiotech, Inc (Norcross, GA, USA). An
array to detect phosphorylated receptor tyrosine kinase (p-RTK) was
conducted according to the manufacturer’s instructions. Briefly,
p-RTK array membranes were blocked with 5% bovine serum albumin
(BSA)/TBS (0.01 mol/l Tris-HCl, pH 7.6) for 1 h. The membranes were
then incubated with 1.5 ml of lysate prepared from cell lines after
normalization with equal amounts of protein. After extensive
washing with TBS containing 0.1% v/v Tween-20 (3 washes for 10 min
each), the membranes were then incubated with an
anti-phospho-tyrosine-HRP detection antibody for 2 h at room
temperature. The unbound HRP antibody was washed away with TBS with
0.1% Tween-20. Finally, each array membrane was exposed to X-ray
film using a chemiluminescence detection system (Perkin Elmer Co.).
The density of the immunoreactive band obtained on the p-RTK array
was analyzed by densitometric scanning (Tlc scanner, Shimizu Co.,
Ltd.).
Angiogenic profile analysis using an
antibody array
The RayBio Human Angiogenesis Antibody Array 1 kit
(catalog no. AAH-ANG-1) was purchased from RayBiotech, Inc. The
assay for the antibody array was performed according to the
manufacturer’s instructions. Briefly, the angiogenesis antibody
membranes were blocked with blocking buffer for 30 min. The
membranes were then incubated with 1 ml of lysate prepared from the
cell lines after normalization with equal amounts of protein. After
extensive washing with TBS with 0.1% v/v Tween-20 (3 washes for 5
min each) and with TBS alone (2 washes for 5 min each) to remove
unbound lysate, the membranes were then incubated with
biotin-conjugated antibodies for 1 h at room temperature. After
washing the membranes with TBS with 0.1% Tween-20 and with TBS
alone, the membranes were incubated with HRP-conjugated
streptavidin for 2 h at room temperature. After washing these
membranes, finally, each array membrane was exposed to X-ray film
using a chemiluminescence detection system (Perkin Elmer Co.). The
density of the immunoreactive band obtained on this array was
analyzed by densitometric scanning (Tlc scanner, Shimizu Co.,
Ltd.).
Analysis of the microRNA array
Total RNA was extracted from the samples derived
from the cancer cell lines using a miRNeasy Mini kit (Qiagen,
Hilden, Germany) according to the manufacturer’s instructions. RNA
samples typically showed A260/280 ratios between 1.9 and
2.1, using an Agilent 2100 Bioanalyzer (Agilent Technologies, Santa
Clara, CA, USA).
After measurement of the RNA with an RNA 6000 Nano
kit (Agilent Technologies), the samples were labeled using a
miRCURY Hy3/Hy5 power labeling kit and were hybridized onto a human
miRNA Oligo chip (version 19.0; Toray Industries, Tokyo, Japan).
Scanning was conducted with the 3D-Gene Scanner 3000 (Toray
Industries), and 3D-Gene extraction version 1.2 software (Toray
Industries) was used to read the raw intensity of the image. To
determine the change in miRNA expression between the
cisplatin-treated samples and the control samples, the raw data
were analyzed via GeneSpringGX version 10.0 (Agilent Technologies).
Samples were first normalized relative to the 28S RNA, and then the
baseline was corrected to the median of all samples.
Replicate data were consolidated into two groups:
those from the cisplatin-treated cells and those from the control
cells were organized by the hierarchical clustering and ANOVA
functions in GeneSpring software. Hierarchical clustering was
performed with the clustering function (condition tree) and
Euclidean correlation as a distance metric. Two-way ANOVA analysis
and asymptotic p-value computation without any error correction of
the samples were conducted to determine the miRNAs that varied most
prominently across the different groups. The p-value cutoff was set
to 0.05. Only changes >50% in at least one of the time points
for each sample were considered significant. All of the analyzed
data were scaled by global normalization. The statistical
significance of the differentially expressed miRNAs was analyzed by
Student’s t-test.
Statistical analysis
All analyses were conducted using the
computer-assisted JMP8.0 (SAS Institute, Cary, NC, USA). A paired
analysis between the groups was conducted using Student’s t-test. A
p-value of 0.05 indicated a significant difference between the
groups.
Results
Cisplatin inhibits the proliferation of
human HCC cells
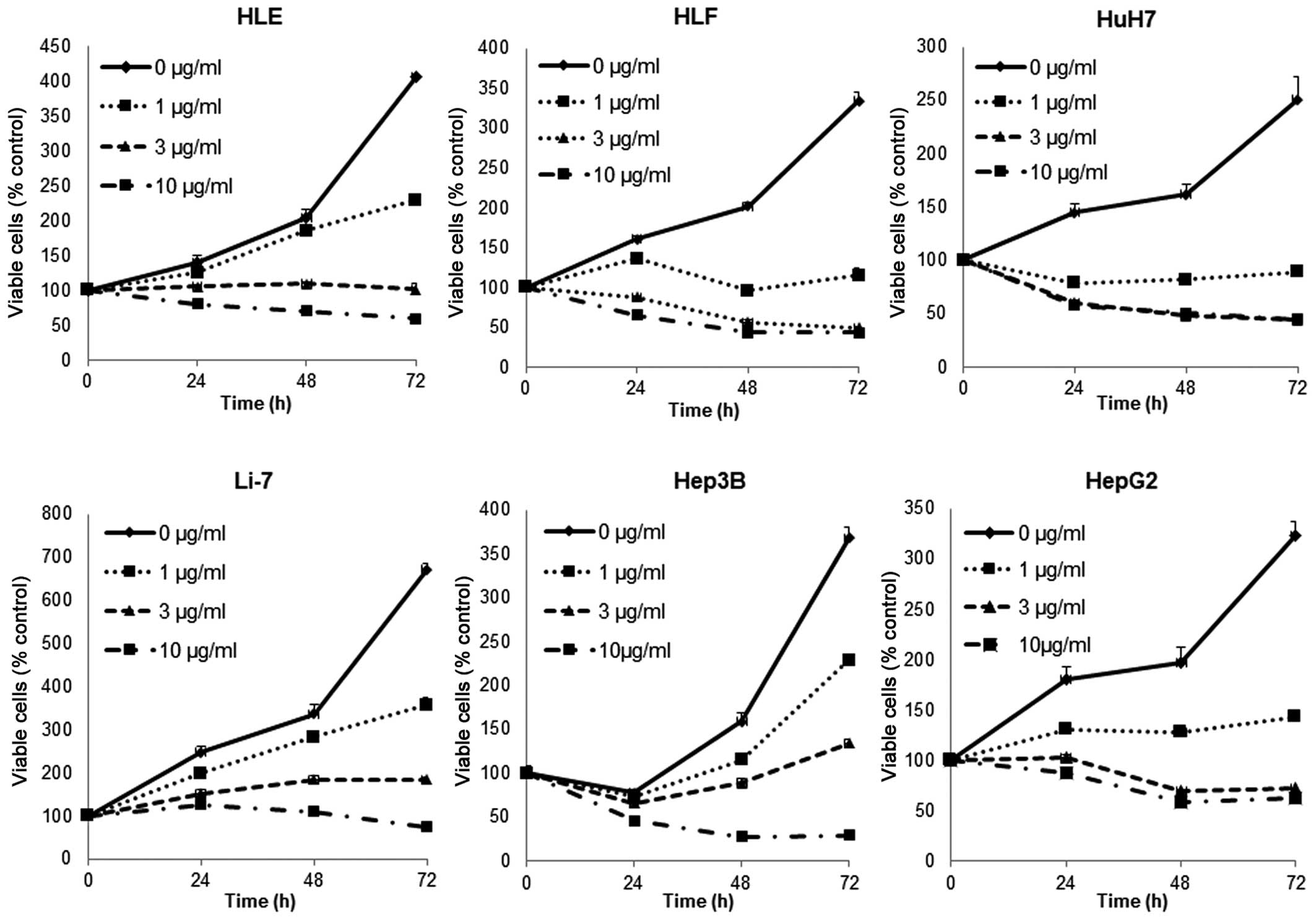
To evaluate the effect on the growth activity of
human HCC cell lines by cisplatin in vitro, we examined the
effect of cisplatin on the proliferation of six human HCC cell
lines: HLE, HLF, HuH7, Li-7, Hep3B and HepG2. To understand the
direct relationship between the decrease in cell viability and the
inhibition of cell proliferation, we followed the course of
proliferation over three days after the addition of cisplatin.
Cells were grown in culture medium and treated with 0, 1, 3 or 10
μg/ml of cisplatin. As shown in Fig.
1, cisplatin led to a strong dose- and time-dependent
inhibition of cell proliferation in the human HCC cell lines HLE,
HLF, HuH7, Li-7, Hep3B and HepG2. These results show that cisplatin
inhibits the proliferation of human HCC cells.
Differences in phosphorylated receptor
tyrosine kinases (p-RTKs) in HuH7 cells with or without cisplatin
treatment in vitro
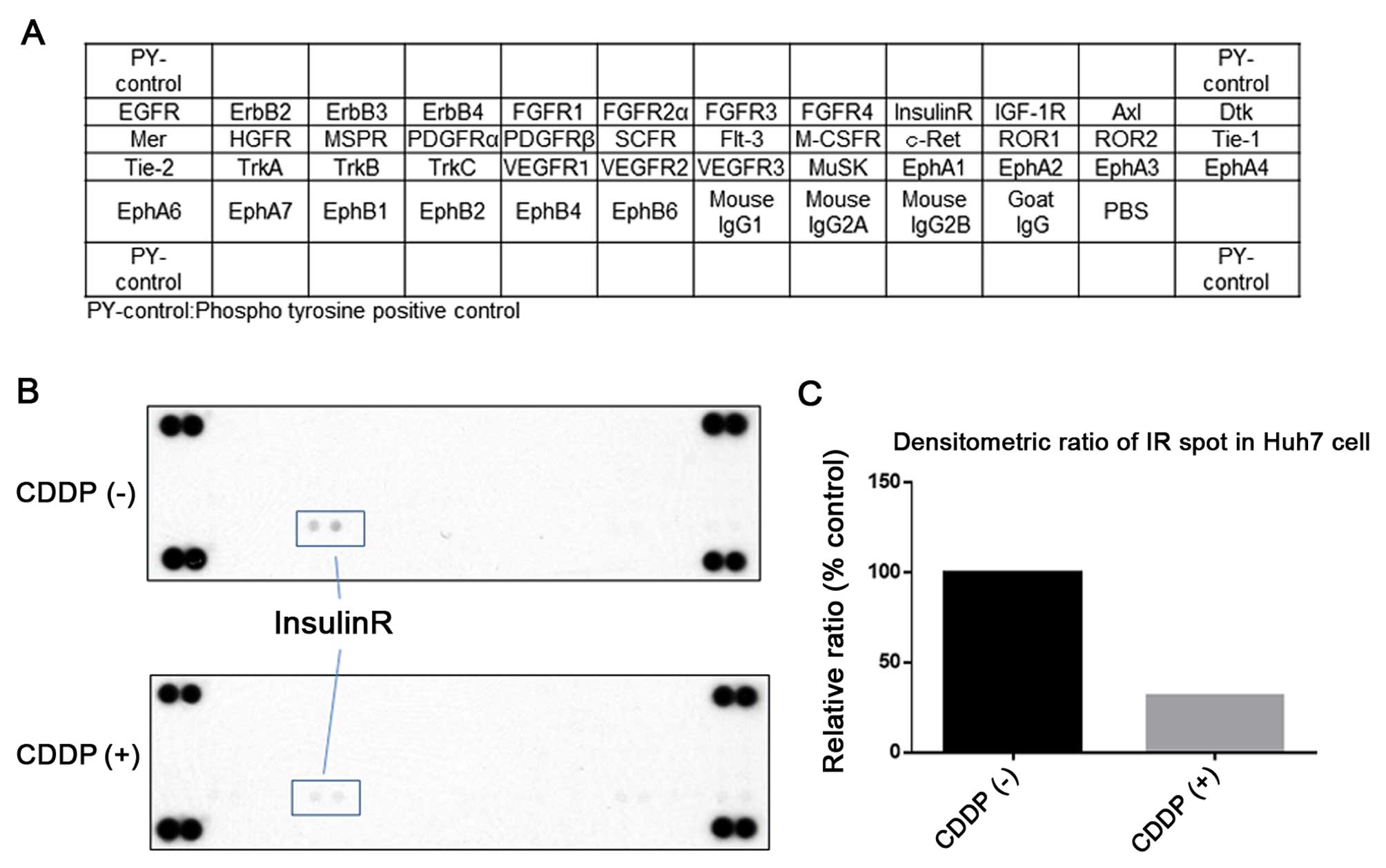
After the antitumor effects of cisplatin in human
HCC cell lines were established, we next used a phosphorylated-RTK
array system to identify the ‘key’ RTKs that are responsible for
these antitumor effects. With an antibody array (Fig. 2A), we simultaneously screened the
expression of 42 different RTKs in HuH7 cells with or without
cisplatin treatment. The results showed that the expression of
insulin receptor (Insulin R) (Fig.
2B) was reduced by the treatment with cisplatin.
The density of the Insulin R obtained from the
membrane array was analyzed by a Kodak Image Station (Eastman
Kodak, Rochester, NY, USA). The densitometric ratio of the Insulin
R spots of the cisplatin-treated cell lines to the Insulin R spots
of the untreated cell lines was reduced to 31.2% (Fig. 2C).
Differences in the expression of
angiogenesis-related protein in HuH7 cells with or without
cisplatin treatment in vitro
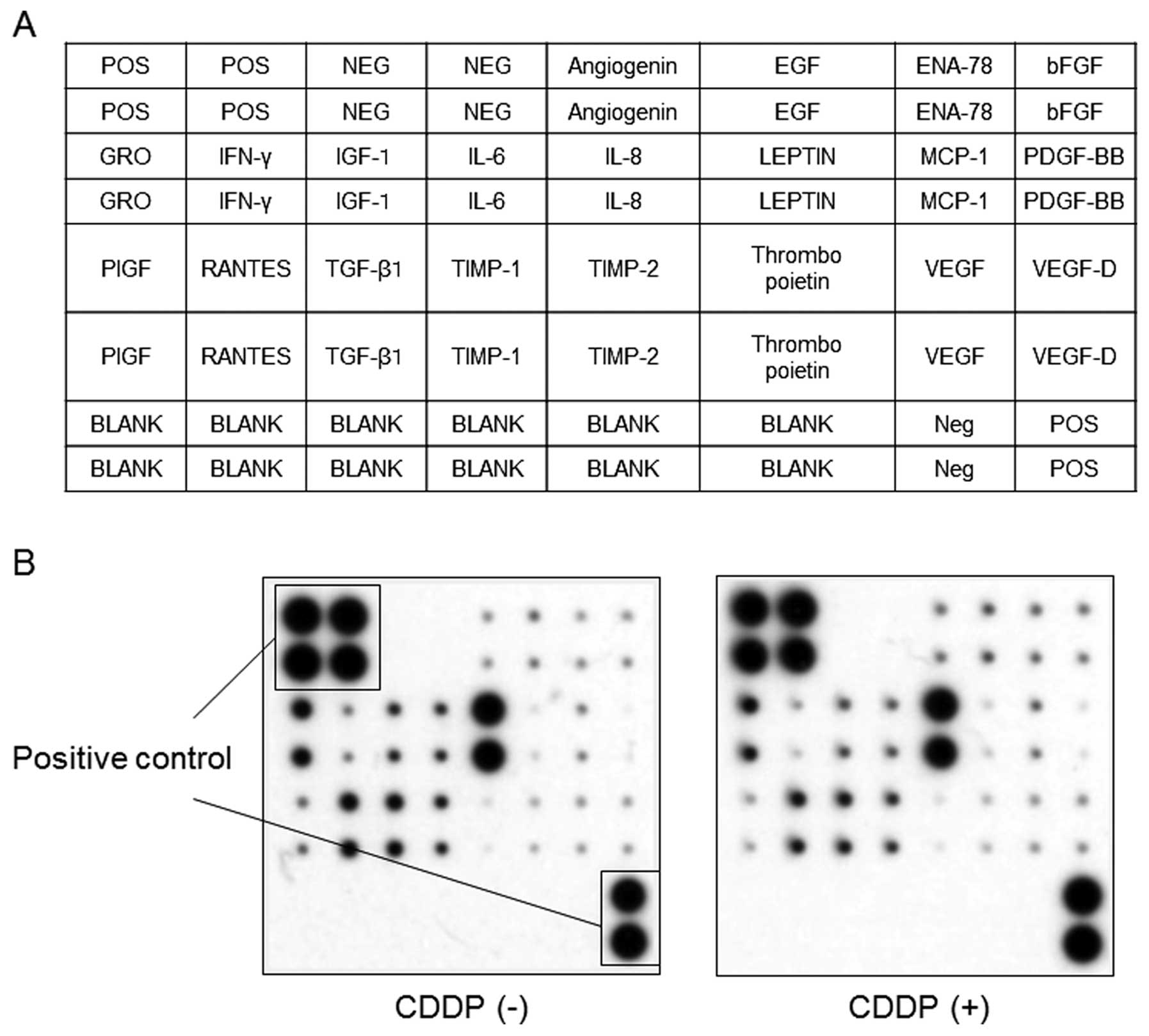
We used an angiogenesis antibody array system to
identify the ‘key’ angiogenesis-related proteins responsible for
the antitumor effects of cisplatin. By using this antibody array
(Fig. 3A), we simultaneously
screened the expression of 20 different angiogenesis markers in the
human HCC cell line HuH7 with or without cisplatin treatment. None
of the markers of angiogenesis were changed by treatment with
cisplatin as detected by the protein array (Fig. 3B).
Effects of cisplatin on cell cycle
regulatory proteins in HuH7 cells
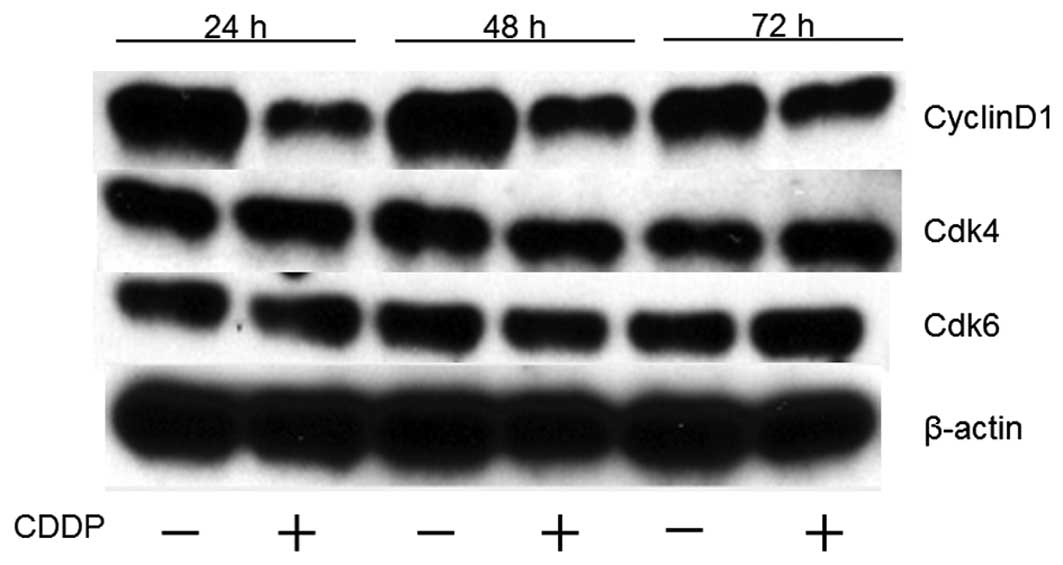
To determine whether cisplatin affects the cell
cycle in HuH7 cells, western blot analysis was used to examine the
expression of various cell cycle-related molecules in HuH7 cells
with and without cisplatin treatment. Cells were treated with 1
μg/ml of cisplatin or were left untreated for 24–72 h. The most
remarkable change was the loss of cyclin D1, a key protein that has
been implicated in the G0/G1 transition (Fig. 4). We then studied the expression of
other cell cycle-related proteins (Cdk4 and Cdk6) that have also
been implicated in the G0/G1 transition. Cdk4 and Cdk6, the
catalytic subunits of cyclin D1, were not changed after the
addition of cisplatin to the culture medium. The amount of β-actin
(an internal control for protein loading) was almost the same in
each lane in sodium dodecyl sulfate polyacrylamide gel
electrophoresis (Fig. 4).
Cisplatin induces apoptosis of HuH7
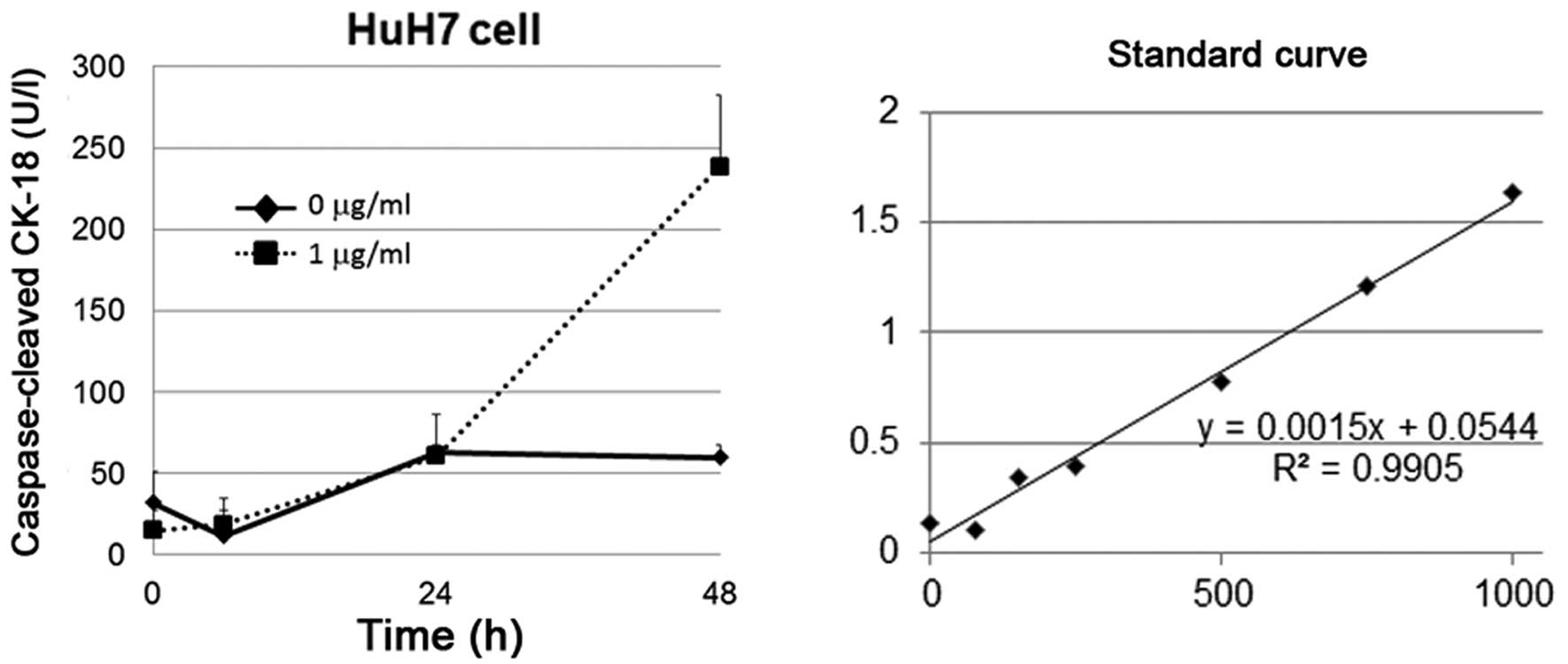
cells
In order to establish that cisplatin induces
apoptosis in HuH7 cells, we used the M30 Apoptosense method which
specifically measures caspase-cleaved cytokeratin 18 in apoptotic
cells. The activity in this assay is inhibited by a pan-caspase
inhibitor. The M30 Apoptosense method is a useful screening tool as
it measures the accumulation of the apoptotic product in cell
cultures, which allows for an integrative determination of
apoptosis until the cells are harvested. Cisplatin induced strong
expression of caspase-cleaved cytokeratin-18 in HuH7 cells after 48
h of treatment (Fig. 5).
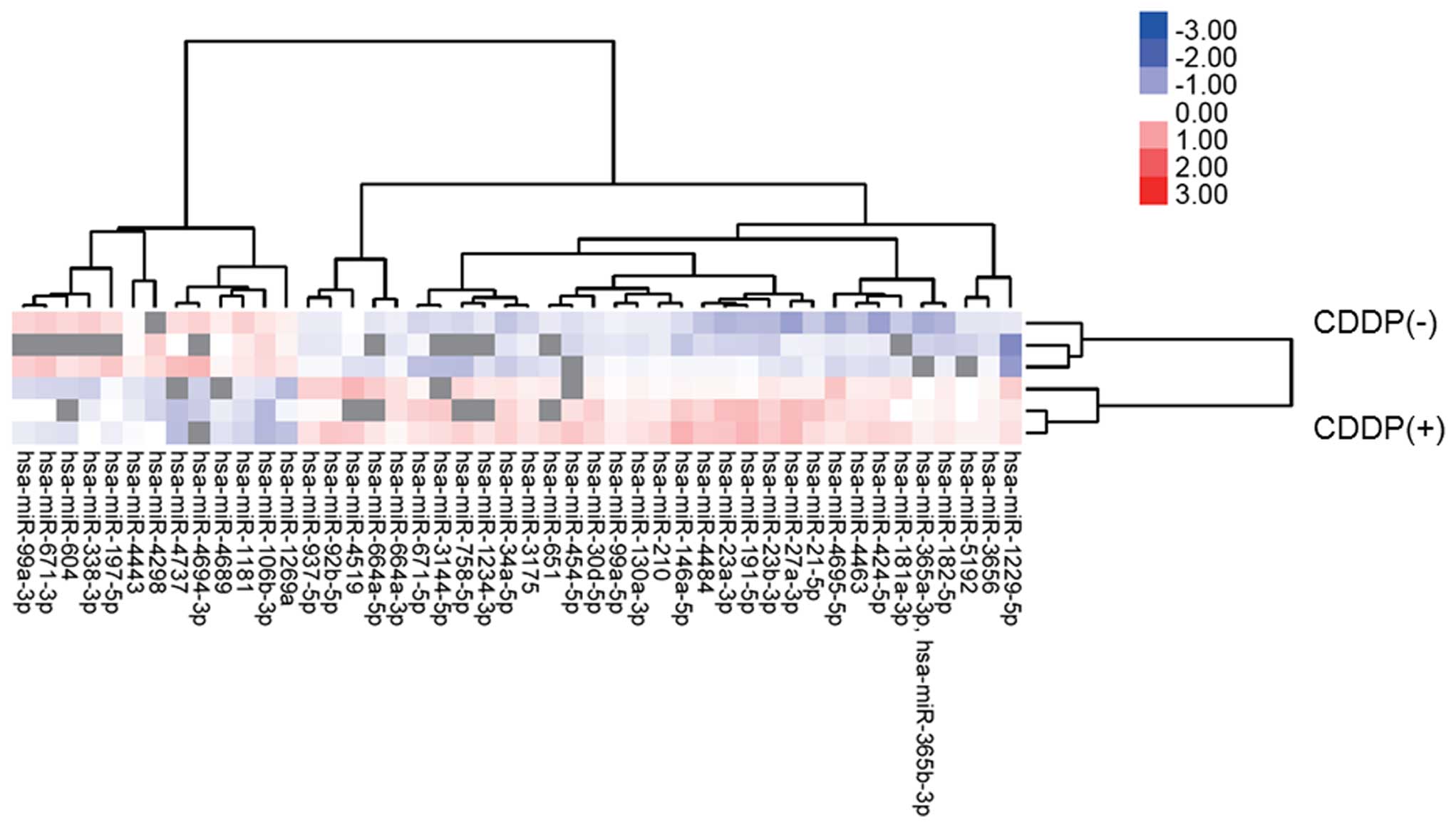
Differences in miRNA expression in HuH7
cells with or without cisplatin treatment in vitro
Using a custom micro-array platform, we analyzed the
expression levels of 2019 human miRNAs in HuH7 cells with or
without cisplatin treatment in vitro. As shown in Fig. 6, Tables I and II, when the expression of miRNAs was
examined in HuH7 cells treated with 1 μg/ml of cisplatin in
vitro and in those not treated with cisplatin, 36 miRNAs were
significantly upregulated (Table
I) in HuH7 cells after 24 h of cisplatin treatment, while 10
miRNAs were downregulated (Table
II) out of the 2019 total miRNAs. Unsupervised hierarchical
clustering analysis, with Pearson’s correlation, showed that HuH7
cells treated with cisplatin clustered together and separately from
the untreated cells (Fig. 6).
 | Table IStatistical results and chromosomal
locations of the miRNAs that were upregulated in HuH7 cells that
were treated with cisplatin. |
Table I
Statistical results and chromosomal
locations of the miRNAs that were upregulated in HuH7 cells that
were treated with cisplatin.
| Upregulated
miRNA | Fold (treated/
non-treated) mean ± SD | p-value | Chromosomal
localization |
|---|
|
hsa-miR-1229-5p | 2.08±0.624 | 0.028559 | 5 |
|
hsa-miR-1234-3p | 1.35 | 0.00523 | 8 |
|
hsa-miR-130a-3p | 1.25±0.077 | 0.007693 | 11q12.1 |
|
hsa-miR-146a-5p | 1.69±0.377 | 0.037223 | 5q34 |
|
hsa-miR-181a-3p | 2.18 | 0.049811 | 1q32.1 |
| hsa-miR-182-5p | 1.46±0.278 | 0.030763 | 7q32.2 |
| hsa-miR-191-5p | 1.77±0.254 | 0.047376 | 3p21.31 |
| hsa-miR-197-5p | 1.09 | 0.017823 | 1p13.3 |
| hsa-miR-210 | 1.24±0.086 | 0.041995 | 11p15.5 |
| hsa-miR-21-5p | 1.47±0.132 | 0.033956 | 17q23.1 |
| hsa-miR-23a-3p | 1.73±0.236 | 0.036484 | 19p13.13 |
| hsa-miR-23b-3p | 1.72±0.127 | 0.008715 | 9q22.32 |
| hsa-miR-27a-3p | 1.85±0.222 | 0.04843 | 19p13.13 |
| hsa-miR-30d-5p | 1.39±0.160 | 0.007856 | 8q24.22 |
|
hsa-miR-3144-5p | 1.78 | 0.047758 | 6 |
| hsa-miR-3175 | 1.36±0.112 | 0.004832 | 15 |
| hsa-miR-338-3p | 1.00 | 0.039909 | 17q25.3 |
| hsa-miR-34a-5p | 1.67±0.151 | 0.002676 | 1p36.22 |
| hsa-miR-3656 | 1.20±0.089 | 0.01424 | 11 |
| hsa-miR-365a-3p,
hsa-miR-365b-3p | 2.35 | 0.02913 |
16p13.12,17q11.2 |
| hsa-miR-424-5p | 1.63±0.452 | 0.04736 | Xq26.3 |
| hsa-miR-4463 | 1.45±0.173 | 0.015803 | 6 |
| hsa-miR-4484 | 1.52±0.040 | 0.037087 | 10 |
| hsa-miR-4519 | 1.08±0.951 | 0.014617 | 16 |
| hsa-miR-454-5p | 1.62 | 0.038449 | 17q22 |
|
hsa-miR-4695-5p | 1.73±0.665 | 0.021036 | 1 |
| hsa-miR-5192 | 1.88 | 0.028807 | 2 |
| hsa-miR-651 | 1.37 | 0.044509 | Xp22.31 |
|
hsa-miR-664a-3p | 1.24±0.151 | 0.017514 | 1 |
|
hsa-miR-664a-5p | 1.49 | 0.042349 | 1 |
| hsa-miR-671-5p | 1.48±0.213 | 0.033916 | 7q36.1 |
| hsa-miR-758-5p | 1.83 | 0.024661 | 14q32.31 |
| hsa-miR-92b-5p | 1.38±0.180 | 0.040637 | 1q22 |
| hsa-miR-937-5p | 1.30±0.166 | 0.033649 | 8q24.3 |
| hsa-miR-99a-3p | 1.03 | 0.015059 | 21q21.1 |
| hsa-miR-99a-5p | 1.14±0.059 | 0.028724 | 21q21.1 |
 | Table IIStatistical results and chromosomal
locations of the miRNAs that were downregulated in HuH7 cells that
were treated with cisplatin. |
Table II
Statistical results and chromosomal
locations of the miRNAs that were downregulated in HuH7 cells that
were treated with cisplatin.
| Downregulated
miRNA | Fold (treated/
non-treated) mean ± SD | p-value | Chromosomal
localization |
|---|
|
hsa-miR-106b-3p | 0.50±0.135 | 0.00563 | 7q22.1 |
| hsa-miR-1181 | 0.70±0.042 | 0.006913 | 19 |
| hsa-miR-1269a | 0.69±0.170 | 0.033337 | 4 |
| hsa-miR-4298 | 1.00 | 0.030744 | 11 |
| hsa-miR-4443 | 0.88±0.032 | 0.041175 | 3 |
| hsa-miR-4689 | 0.56±0.500 | 0.024162 | 1 |
|
hsa-miR-4694-3p | 0.50 | 0.023221 | 11 |
| hsa-miR-4737 | 0.35±0.337 | 0.029994 | 17 |
| hsa-miR-604 | 0.67 | 0.022309 | 10p11.23 |
| hsa-miR-671-3p | 0.93 | 0.014697 | 7q36.1 |
Discussion
Herein we present evidence for the reduction of a
phosphorylated RTK (p-RTK), the IR, the downregulation of cyclin D1
among cell cycle regulatory molecules, and the miRNA profiles in
HCC cells after treatment with cisplatin.
First of all, the activation of IR induces the
expression of IR substrates 1 and 2 (IRS1/2), which in turn mediate
mitogenic and anti-apoptotic signaling (24). In addition, the overexpression of
IRS1 prevents transforming growth factor β1-induced apoptosis
(25). In our present study, the
phosphorylation of IR was inhibited by cisplatin according to the
phosphorylated RTK array (Fig. 2B and
C). Cisplatin also induced apoptosis (Fig. 5). These results suggest that IR
signaling may be one of the important pathways that mediate
cisplatin-induced apoptosis. Noteworthy, the phosphatase and tensin
homolog (PTEN), which is one of the most important suppressors of
the IR signaling pathway, was negatively regulated by microRNA-21
(16). Furthermore, microRNA-21
confers cisplatin resistance via the regulation of PTEN (26). This suggests that microRNA-21 may
be induced in response to cisplatin during initial therapy, but
that continuous treatment with cisplatin may induce the acquisition
of resistance through the overexpression of microRNA-21. As shown
in Table I, our data demonstrated
the upregulation of microRNA-21 after the treatment of HCC cells
with cisplatin. Therefore, microRNAs may modulate the IR signaling
pathway during cisplatin-induced apoptosis.
In addition, cyclin D1 is regarded as one of the key
molecules in the transition from G1 to S phase. On the one hand,
the upregulation of cyclin D1 results in the rapid progression of
HCC, on the other hand, cyclin D1 is downregulated by microRNA-338p
(27,28) in HCC cells. These microRNAs then
induce G1 arrest (29) and promote
cell apoptosis (30). In our
study, cellular proliferation was significantly inhibited after
cisplatin treatment in a dose-dependent manner (Fig. 1), and cyclin D1 expression was
reduced at the protein level by cisplatin treatment (Fig. 4). Moreover, cisplatin induced
apoptosis in these cells. Of note, microRNA-338 was significantly
upregulated in cisplatin-treated HCC cells compared to non-treated
HCC cells (Table I). This
indicates that cisplatin inhibits the expression of cyclin D1 via
the upregulation of microRNA-338-3p. Furthermore, the expression of
microRNA-34a and microRNA-99a was also upregulated in HCC cells
after treatment with cisplatin (Table
I). Guo et al reported that microRNA-34a inhibits the
potential for lymphatic metastasis by inducing the down-regulation
of cyclin D1 and Cdk6 (31). It
was demonstrated that microRNA-99a suppresses the growth of
hepatocellular carcinoma (HCC) via the induction of G1-phase cell
cycle arrest and also correlates with patient survival (32). These data suggest that cisplatin
inhibits cellular proliferation by modulating cell cycle regulatory
molecules through microRNA-34a and microRNA-99a. MicroRNA-338-3p,
microRNA-34a and microRNA-99a may be novel cell cycle regulators,
and therefore, miRNA profiling may also be a powerful tool to
discover targetable molecules in HCC.
In conclusion, microRNAs were strongly associated
with the mechanisms of cisplatin-induced cell proliferation and
apoptosis in HCC cells. Therefore, the analysis of microRNA
profiles may be a powerful tool to elucidate new mechanisms of
action of cisplatin and to discover new targetable molecules for
the treatment of HCC.
References
|
1
|
Ferlay J, Shin HR, Bray F, Forman D,
Mathers C and Parkin DM: Estimates of worldwide burden of cancer in
2008: GLOBOCAN 2008. Int J Cancer. 127:2893–2917. 2010. View Article : Google Scholar
|
|
2
|
Małkowski P, Pacholczyk M, Łagiewska B,
Adadyński L, Wasiak D, Kwiatkowski A, Chmura A and Czerwiński J:
Hepatocellular carcinoma - epidemiology and treatment. Przegl
Epidemiol. 60:731–740. 2006.(In Polish).
|
|
3
|
Belghiti J and Kianmanesh R: Surgical
treatment of hepatocellular carcinoma. HPB Oxf. 7:42–49. 2005.
View Article : Google Scholar
|
|
4
|
Lee PH, Lin WJ, Tsang YM, Hu RH, Sheu JC,
Lai MY, Hsu HC, May W and Lee CS: Clinical management of recurrent
hepatocellular carcinoma. Ann Surg. 222:670–676. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Rosenberg B, Vancamp L and Krigas T:
Inhibition of cell division in Escherichia coli by electrolysis
products from a platinum electrode. Nature. 205:698–699. 1965.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Galluzzi L, Senovilla L, Vitale I, Michels
J, Martins I, Kepp O, Castedo M and Kroemer G: Molecular mechanisms
of cisplatin resistance. Oncogene. 31:1869–1883. 2012. View Article : Google Scholar
|
|
7
|
Fuertes MA, Castilla J, Alonso C and Pérez
JM: Cisplatin biochemical mechanism of action: From cytotoxicity to
induction of cell death through interconnections between apoptotic
and necrotic pathways. Curr Med Chem. 10:257–266. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Asechi H, Hatano E, Nitta T, Tada M,
Iwaisako K, Tamaki N, Nagata H, Narita M, Yanagida A, Ikai I, et
al: Resistance to cisplatin-induced apoptosis via PI3K-dependent
survivin expression in a rat hepatoma cell line. Int J Oncol.
37:89–96. 2010.PubMed/NCBI
|
|
9
|
Drayton RM: The role of microRNA in the
response to cisplatin treatment. Biochem Soc Trans. 40:821–825.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Masaki T: MicroRNA and hepatocellular
carcinoma. Hepatol Res. 39:751–752. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Krek A, Grün D, Poy MN, Wolf R, Rosenberg
L, Epstein EJ, MacMenamin P, da Piedade I, Gunsalus KC, Stoffel M,
et al: Combinatorial microRNA target predictions. Nat Genet.
37:495–500. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Calin GA, Dumitru CD, Shimizu M, Bichi R,
Zupo S, Noch E, Aldler H, Rattan S, Keating M, Rai K, et al:
Frequent deletions and down-regulation of micro-RNA genes miR15 and
miR16 at 13q14 in chronic lymphocytic leukemia. Proc Natl Acad Sci
USA. 99:15524–15529. 2002. View Article : Google Scholar
|
|
13
|
Michael MZ, O’Connor SM, van Holst
Pellekaan NG, Young GP and James RJ: Reduced accumulation of
specific microRNAs in colorectal neoplasia. Mol Cancer Res.
1:882–891. 2003.PubMed/NCBI
|
|
14
|
Lee EJ, Gusev Y, Jiang J, Nuovo GJ, Lerner
MR, Frankel WL, Morgan DL, Postier RG, Brackett DJ and Schmittgen
TD: Expression profiling identifies microRNA signature in
pancreatic cancer. Int J Cancer. 120:1046–1054. 2007. View Article : Google Scholar
|
|
15
|
Takamizawa J, Konishi H, Yanagisawa K,
Tomida S, Osada H, Endoh H, Harano T, Yatabe Y, Nagino M, Nimura Y,
et al: Reduced expression of the let-7 microRNAs in human lung
cancers in association with shortened postoperative survival.
Cancer Res. 64:3753–3756. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Meng F, Henson R, Wehbe-Janek H, Ghoshal
K, Jacob ST and Patel T: MicroRNA-21 regulates expression of the
PTEN tumor suppressor gene in human hepatocellular cancer.
Gastroenterology. 133:647–658. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Gramantieri L, Ferracin M, Fornari F,
Veronese A, Sabbioni S, Liu CG, Calin GA, Giovannini C, Ferrazzi E,
Grazi GL, et al: Cyclin G1 is a target of miR-122a, a microRNA
frequently down-regulated in human hepatocellular carcinoma. Cancer
Res. 67:6092–6099. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wong QW, Lung RW, Law PT, Lai PB, Chan KY,
To KF and Wong N: MicroRNA-223 is commonly repressed in
hepatocellular carcinoma and potentiates expression of Stathmin1.
Gastroenterology. 135:257–269. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Varnholt H, Drebber U, Schulze F,
Wedemeyer I, Schirmacher P, Dienes HP and Odenthal M: MicroRNA gene
expression profile of hepatitis C virus-associated hepatocellular
carcinoma. Hepatology. 47:1223–1232. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Masaki T, Tokuda M, Yoshida S, Nakai S,
Morishita A, Uchida N, Funaki T, Kita Y, Funakoshi F, Nonomura T,
et al: Comparison study of the expressions of myristoylated
alanine-rich C kinase substrate in hepatocellular carcinoma, liver
cirrhosis, chronic hepatitis, and normal liver. Int J Oncol.
26:661–671. 2005.PubMed/NCBI
|
|
21
|
Bradford MM: A rapid and sensitive method
for the quantitation of microgram quantities of protein utilizing
the principle of protein-dye binding. Anal Biochem. 72:248–254.
1976. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Laemmli UK: Cleavage of structural
proteins during the assembly of the head of bacteriophage T4.
Nature. 227:680–685. 1970. View
Article : Google Scholar : PubMed/NCBI
|
|
23
|
Towbin H, Staehelin T and Gordon J:
Electrophoretic transfer of proteins from polyacrylamide gels to
nitrocellulose sheets: Procedure and some applications. Proc Natl
Acad Sci USA. 76:4350–4354. 1979. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Reuveni H, Flashner-Abramson E, Steiner L,
Makedonski K, Song R, Shir A, Herlyn M, Bar-Eli M and Levitzki A:
Therapeutic destruction of insulin receptor substrates for cancer
treatment. Cancer Res. 73:4383–4394. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Tanaka S and Wands JR: Insulin receptor
substrate 1 overexpression in human hepatocellular carcinoma cells
prevents transforming growth factor beta1-induced apoptosis. Cancer
Res. 56:3391–3394. 1996.PubMed/NCBI
|
|
26
|
Yang SM, Huang C, Li XF, Yu MZ, He Y and
Li J: miR-21 confers cisplatin resistance in gastric cancer cells
by regulating PTEN. Toxicology. 306:162–168. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Fu X, Tan D, Hou Z, Hu Z and Liu G:
miR-338-3p is down-regulated by hepatitis B virus X and inhibits
cell proliferation by targeting the 3′-UTR region of cyclinD1. Int
J Mol Sci. 13:8514–8539. 2012. View Article : Google Scholar
|
|
28
|
Fu X, Tan D, Hou Z, Hu Z, Liu G, Ouyang Y
and Liu F: The effect of miR-338-3p on HBx deletion-mutant
(HBx-d382) mediated liver-cell proliferation through CyclinD1
regulation. PLoS One. 7:e432042012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chen L, Zheng J, Zhang Y, Yang L, Wang J,
Ni J, Cui D, Yu C and Cai Z: Tumor-specific expression of
microRNA-26a suppresses human hepatocellular carcinoma growth via
cyclin-dependent and -independent pathways. Mol Ther. 19:1521–1528.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yang X, Liang L, Zhang XF, Jia HL, Qin Y,
Zhu XC, Gao XM, Qiao P, Zheng Y, Sheng YY, et al: MicroRNA-26a
suppresses tumor growth and metastasis of human hepatocellular
carcinoma by targeting interleukin-6-Stat3 pathway. Hepatology.
58:158–170. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Guo Y, Li S, Qu J, Wang S, Dang Y, Fan J,
Yu S and Zhang J: MiR-34a inhibits lymphatic metastasis potential
of mouse hepatoma cells. Mol Cell Biochem. 354:275–282. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Li D, Liu X, Lin L, Hou J, Li N, Wang C,
Wang P, Zhang Q, Zhang P, Zhou W, et al: MicroRNA-99a inhibits
hepatocellular carcinoma growth and correlates with prognosis of
patients with hepatocellular carcinoma. J Biol Chem.
286:36677–36685. 2011. View Article : Google Scholar : PubMed/NCBI
|