Introduction
Ovarian cancer is the seventh most common cancer in
women under the age of 65 years. Epithelial ovarian cancer (EOC)
constitutes the majority of ovarian neoplasms (1). It is often not detected until the
advanced stages and is the most frequent cause of death among
gynecologic cancers (2). Over the
last several years, the treatment of ovarian cancer has not
appreciably changed. Cytoreductive surgery followed by adjuvant
chemotherapy is generally recommended as the primary treatment for
advanced EOC. Over 70% of patients respond to chemotherapy
initially, but ~50% of advanced cases will relapse (3). Many drugs have been developed so far
to treat EOC; however, the improvement in the prognosis of EOC
patients is insufficient. Therefore, new clinically useful
biomarkers and new targets for EOC treatment need to be
identified.
Clofibric acid (CA), a peroxisome
proliferator-activated receptor α (PPARα) ligand, which is commonly
used for the treatment of hyperlipidemia, may be one of the
possible EOC treatments. Our previous in vivo and in
vitro studies have demonstrated that CA has an antitumor effect
against human ovarian cancer, which is comparable to that of
cisplatin (4). We have previously
shown that CA treatment induces the expression of carbonyl
reductase 1 (CR1), which in turn decreased prostaglandin (PG)
E2 levels; the treatment also causes significant
induction of apoptosis and profound repression of angiogenesis
(4). CR1 is a NADPH-dependent
oxidoreductase with a broad specificity for carbonyl compounds,
which reduces aldehydes and ketones (5). CR1 is present in a variety of organs,
such as the liver, kidney, breast, ovary, and vascular endothelial
cells, and its primary function is considered to control fatty acid
metabolism (6). Earlier reports
have shown that there is a negative relationship between CR1
expression levels and malignant tumor growth (7–10).
In order to elucidate antitumor effect of CR1, we transfected mouse
ovarian cancer cells with a CR1 cDNA expression vector and
investigated the effect of overexpressed CR1 on tumor growth in
vivo, and found that tumor growth was significantly inhibited
in mice from the CR1 induction group compared to tumor development
in animals injected with intact, unmodified cell lines (11). Furthermore, the milk fat globule
EGF factor 8 (MFG-E8), an ‘eat-me signal’ for phagocytes such as
macrophages, was expressed extensively in the cytoplasm of tumor
cells and interstitial cells of mice from the CR1 induction group.
MFG-E8 is released by apoptotic endothelial cells and induces
engulfment of apoptotic cells by macrophage (12). Activated macrophages are the major
source of tumor necrosis factor α (TNFα) and TNFα is a key cytokine
involved in inflammation, cellular homeostasis, tumor progression
and carcinogenesis (13).
Therefore, we focused on TNFα, a potent cytokine,
which is produced by many types of cells, including macrophages.
TNFα elicits a particularly broad spectrum of whole body and
cellular responses, including activation and migration of immune
cells, fever, acute phase response, cell proliferation,
differentiation, and apoptosis (14). TNFα exerts its effects through two
distinct receptors, TNF receptor 1 (TNFR1) and TNFR2 (15). Binding of the inherently trimeric
TNFα to TNFR1 and TNFR2 induces receptor trimerization and
recruitment of several signaling proteins to the cytoplasmic
domains of the receptor (15).
TNFR1 transduces apoptotic and anti-inflammatory signals through
the recruitment of the Fas-associated death domain protein (FADD)
and subsequent recruitment of caspase-8 (16,17).
Thereafter, activated caspase-8 initiates a proteolytic cascade
that involves other caspases (caspase-3, -6 and -7) and ultimately
induces apoptosis (16,17). TNFR1 also mediates anti-apoptotic
and inflammatory responses such as the induction of necrosis factor
(NF)- κB through the recruitment of TNF-receptor-associated factor
2 (TRAF2) and receptor-interacting protein 1 (RIP1) (18,19).
On the other hand, TNFR2 recruits TRAF2 and TRAF1 to transmit
anti-apoptotic and inflammatory signals inducing the expression of
NF-κB and c-Jun (20).
In this study, we investigated the mechanism of
antitumor effects of CR1 mediated by TNFR1 and TNFR2 signaling.
Materials and methods
Cell lines and cell culture
OVCAR-3 and TOV-21G cell lines were obtained from
the American Type Culture Collection (Rockville, MD, USA). Both
OVCAR-3 and TOV-21G cells are derived from human ovarian cancer
tissues and are commonly used to produce xenografted solid tumors
(10,21–23).
The cells were cultured in the RPMI-1640 medium supplemented with
10% fetal calf serum (FCS), 100 U/ml penicillin, and 100 mg/ml
streptomycin at 37°C in a humidified air containing 5%
CO2.
Animal experiments
Animal experiments were conducted in accordance with
the Guidelines for Animal Experimentation of Hirosaki University.
Eight-week-old female BALB/c nu/nu mice were used in this study.
All mice were group housed in plastic cages with stainless-steel
grid tops in an air-conditioned room at the Institute for Animal
Experiments of Hirosaki University. Mice were kept on a 12/12-h
light/dark cycle and given ad libitum access to food and water.
Plasmid DNA preparation
To achieve highly efficient transfection, we used
the pCMV6-AC-GFP vector (OriGene Technologies, Rockville, MD, USA)
that encodes human CR1, the green fluorescent protein (GFP), and
the ampicillin-resistant gene. For amplification, pCMV6-AC-GFP was
transformed into E. coli DH5α competent cells by heat shock
transformation according to standard laboratory protocols. The
transformed bacteria were amplified in LB-ampicillin medium. The
plasmids were purified from cultured-transformed bacteria using
Maxiprep PureLink HiPure Plasmid Filter DNA Purification kits
(Invitrogen, Carlsbad, CA, USA) according to the manufacturer's
protocol. Plasmid DNA (pDNA) was diluted in sterile water to a
concentration of 2 μg/μl.
Transfection
OVCAR-3 and TOV-21G cells were plated into 10-cm
well plates and cultured to 70–80% confluence in the RPMI-1640
medium supplemented with 10% fetal calf serum (FCS). Then, 24 μg of
the coding plasmid was transfected into OVCAR-3 and TOV-21G cells
using Lipofectamine (Life Technologies, Rockville, MD, USA)
according to the manufacturer's protocol. The vector without CR1
pDNA was used as control. Transfected cells were cultured in the
RPMI-1640 medium supplemented with 10% fetal calf serum (FCS) for
48 h. We confirmed the expression of the CR construct using
fluorescence microscopy.
Xenograft mouse model
The mice were divided into two groups (n=5 for each
group) for each of the two types of cancer cells used. Normal
OVCAR-3 cells or OVCAR-3 overexpressing the CR1-DNA
(5.0×106 cells) were injected subcutaneously in 0.2 ml
of RPMI-1640 medium into the back region of nude mice. All mice
were numbered, housed separately, and tumor development was
examined by measuring 2 diameters twice a week using a caliper.
Tumor dimensions were measured twice a week using a caliper. Tumor
volume was calculated using the following equation: V
(mm3) = A x B2/2, where A is the largest diameter and B
is the smallest diameter (10). On
the third or fourth week of the experiment, animals were sacrificed
and tumors were isolated for pathological and biochemical
examinations.
Immunohistochemistry
Six-micrometer-thick sections of formalin-fixed and
paraffin-embedded tissue specimens were stained by an established
method, as described previously (4). Sections were incubated with
antibodies specific for TNFR1, TNFR2, caspase-8, caspase-3, NF-κB,
and c-Jun (Santa Cruz Biotechnology, Santa Cruz, CA, USA) overnight
at 4°C. Slides were incubated with appropriate biotinylated
species-specific secondary antibodies for 1 h and then exposed to
avidin-biotin-peroxidase complex. Sections were treated with 0.02%
diaminobenzidine as a chromogen and counterstained with
hematoxylin.
Western blot analysis
Cell lysates (25 μg protein) were prepared from
tumor tissues, electrophoresed using a 12% precast polyacrylamide
gel onto nitrocellulose membranes (Bio-Rad Laboratories). The
protein concentration was determined using the Bradford assay. The
blots were probed for 2 h with the diluted antibodies against the
following proteins: CR1 (Santa Cruz Biotechnology) at 1:500, human
caspase-8 (Santa Cruz Biotechnology) at 1:200, caspase-3 (Santa
Cruz Biotechnology) at 1:200, NF-κB (Santa Cruz Biotechnology) at
1:500, c-jun (Santa Cruz Biotechnology) at 1:500, and β-actin
(Sigma-Aldrich, St. Louis, MO, USA) at 1:2,000. The membranes were
then incubated for 1 h with the appropriate biotinylated secondary
antibodies, and protein bands were visualized using enhanced
chemiluminescence (Amersham Pharmacia Biotech, Piscataway, NJ, USA)
according to the manufacturer's procedure.
Statistical analysis
Differences in the number of apoptotic cells between
the CR1-overexpressing group and control were evaluated using
Student's t-test. Differences in tumor volume between
CR1-overexpressing group and control were also evaluated by
Student's t-test. A result was deemed significant at a P-value
<0.05.
Results
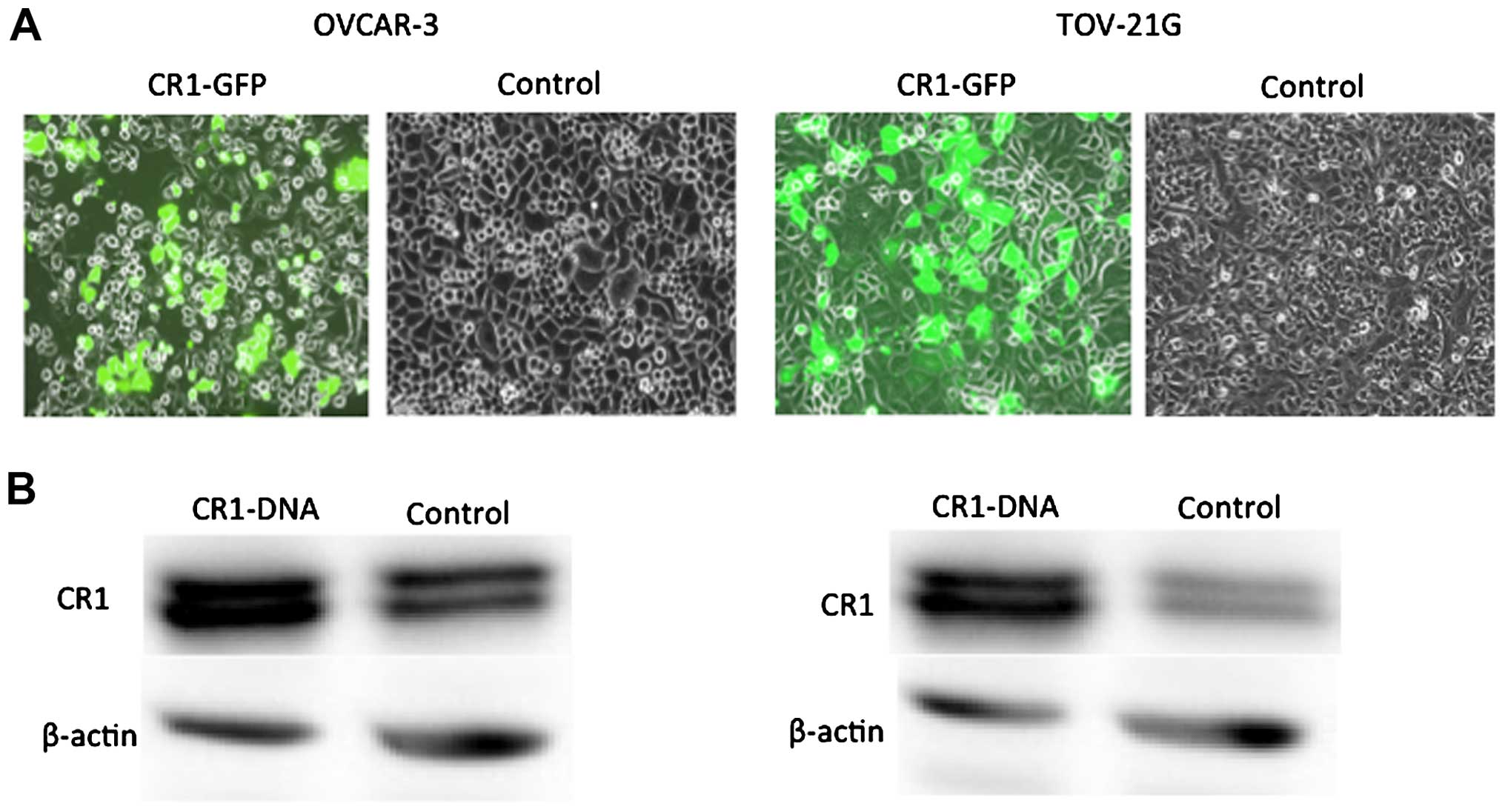
CR1 expression levels in normal and
CR1-transfected OVAR-3 and TOV21-G cells
CR1 expression levels were compared in normal OVAR-3
and TOV21-G cells and cells transfected with CR1 cDNA. CR1-GFP
protein was clearly detected in CR1-DNA transfected cells (Fig. 1A). Western blot analysis clearly
showed high expression of CR1 protein in the CR1-DNA transfected
cells compared to control (Fig.
1B).
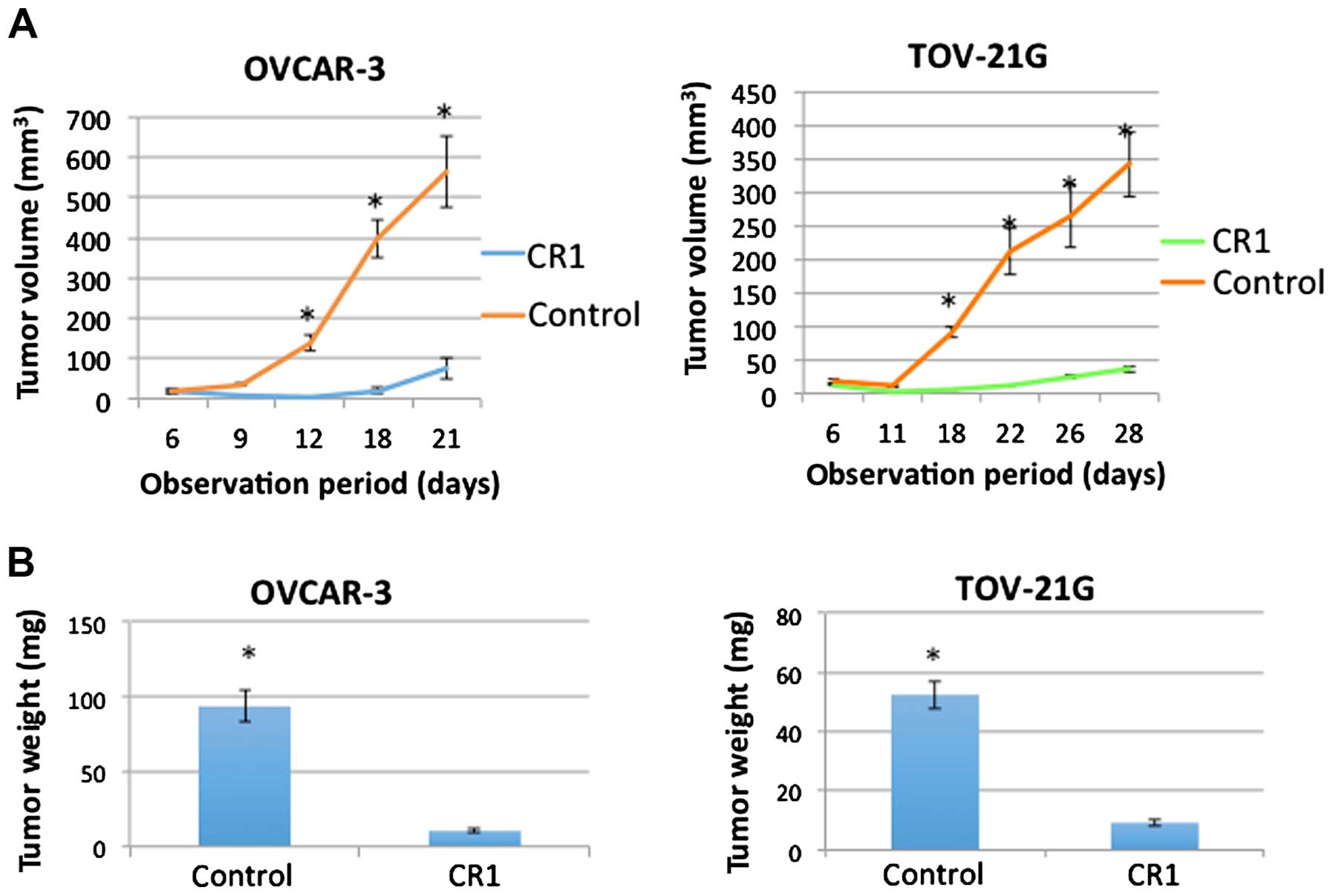
Effects of CR1-transfection on tumor
growth in vivo
As shown in Fig.
2A, OVCAR-3 and TOV-21G cells injected in mice formed tumors
and showed significant increase in tumor volume by 12 or 18 days
after injection (P<0.001), respectively. However, OVCAR-3 and
TOV-21G cells transfected with CR1 showed no increase until 12 or
18 days after the injection. Although they showed a slight increase
in the volume by the end of the experiment, both size and weight
(Fig. 2, P<0.001) were
significantly lower that those of normal OVCAR-3 and TOV-21G cells.
These results suggested that transfection of CR1 caused an
inhibitory effect on tumor growth.
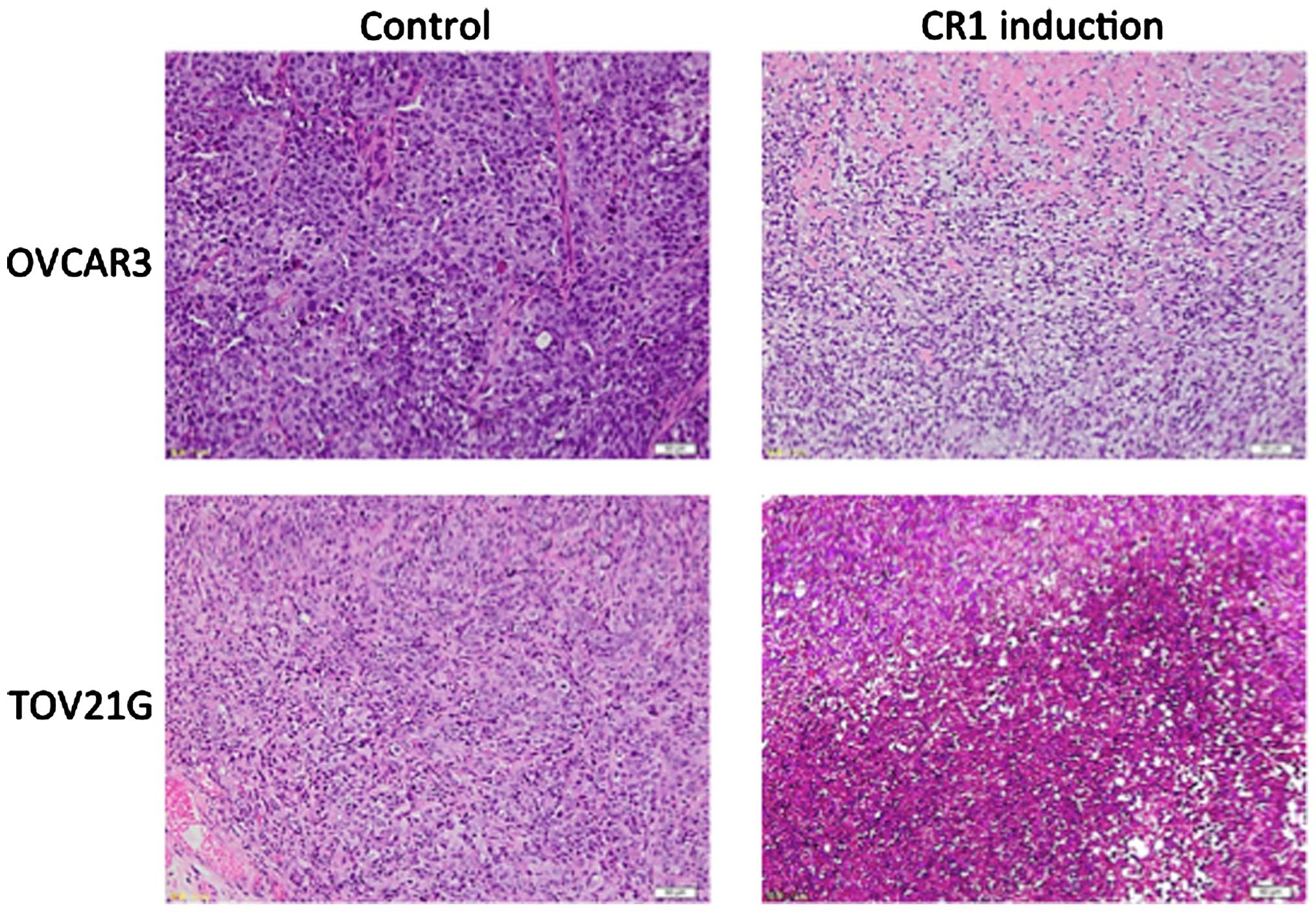
Histological examination on the isolated tumor
showed that while malignant cells were densely packed in tumor
tissues of control groups (normal OVCAR-3 and TOV-21G cells),
malignant cells were sparsely distributed in tumors derived from
CR1-overexpressing cell lines (Fig.
3). Necrosis with inflammatory cells was observed in large
areas of tumors in CR1 induction groups (Fig. 3).
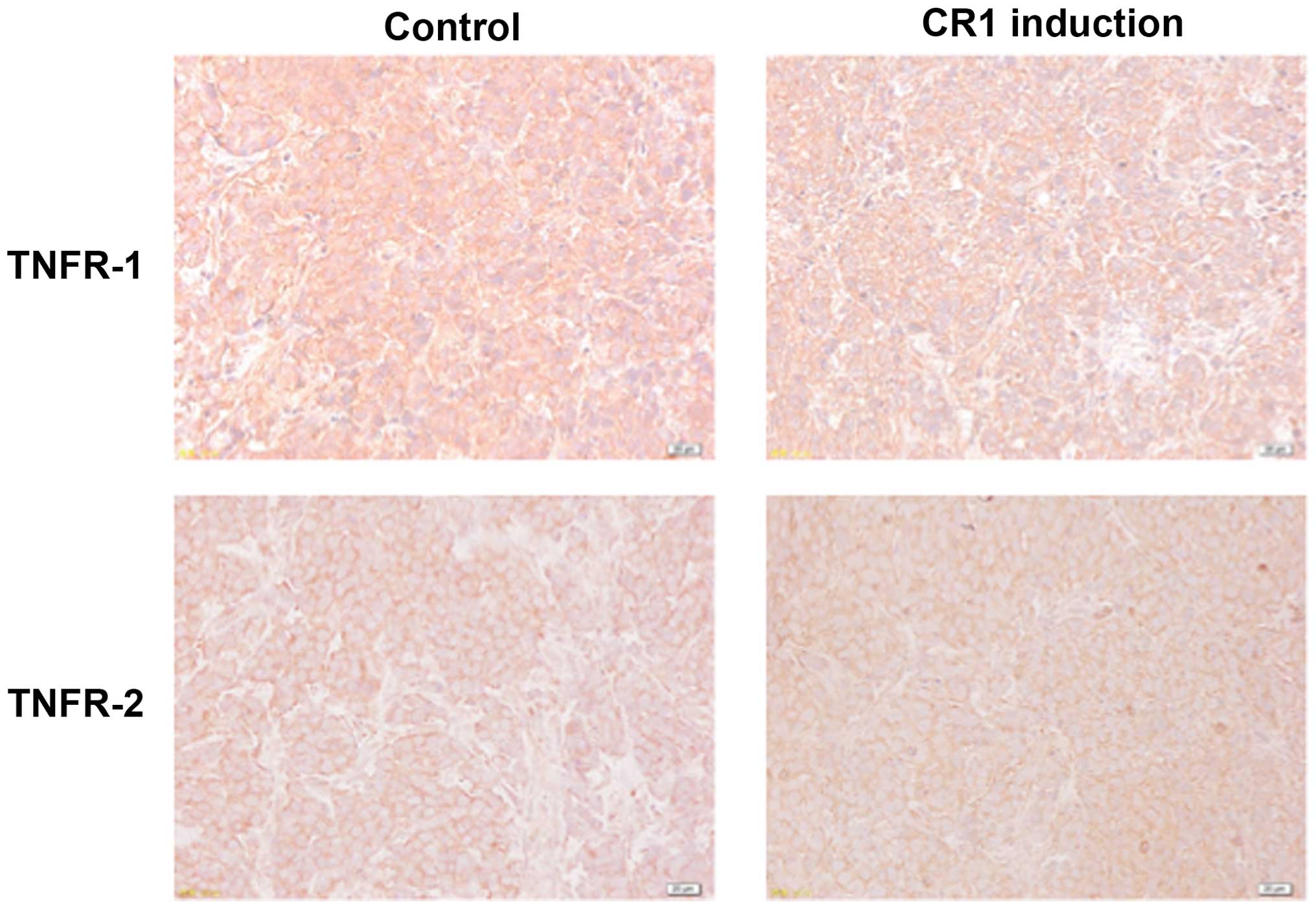
Effects of CR1-transfection on TNFR1 and
TNFR2 expression
Immunohistochemical analysis showed that TNFR1 was
expressed on cell membranes of tumors from all treatment groups and
there were no significant differences in its expression levels
between CR1 induction and control groups (Fig. 4). TNFR2 expression levels were
comparatively weak in both groups (Fig. 4).
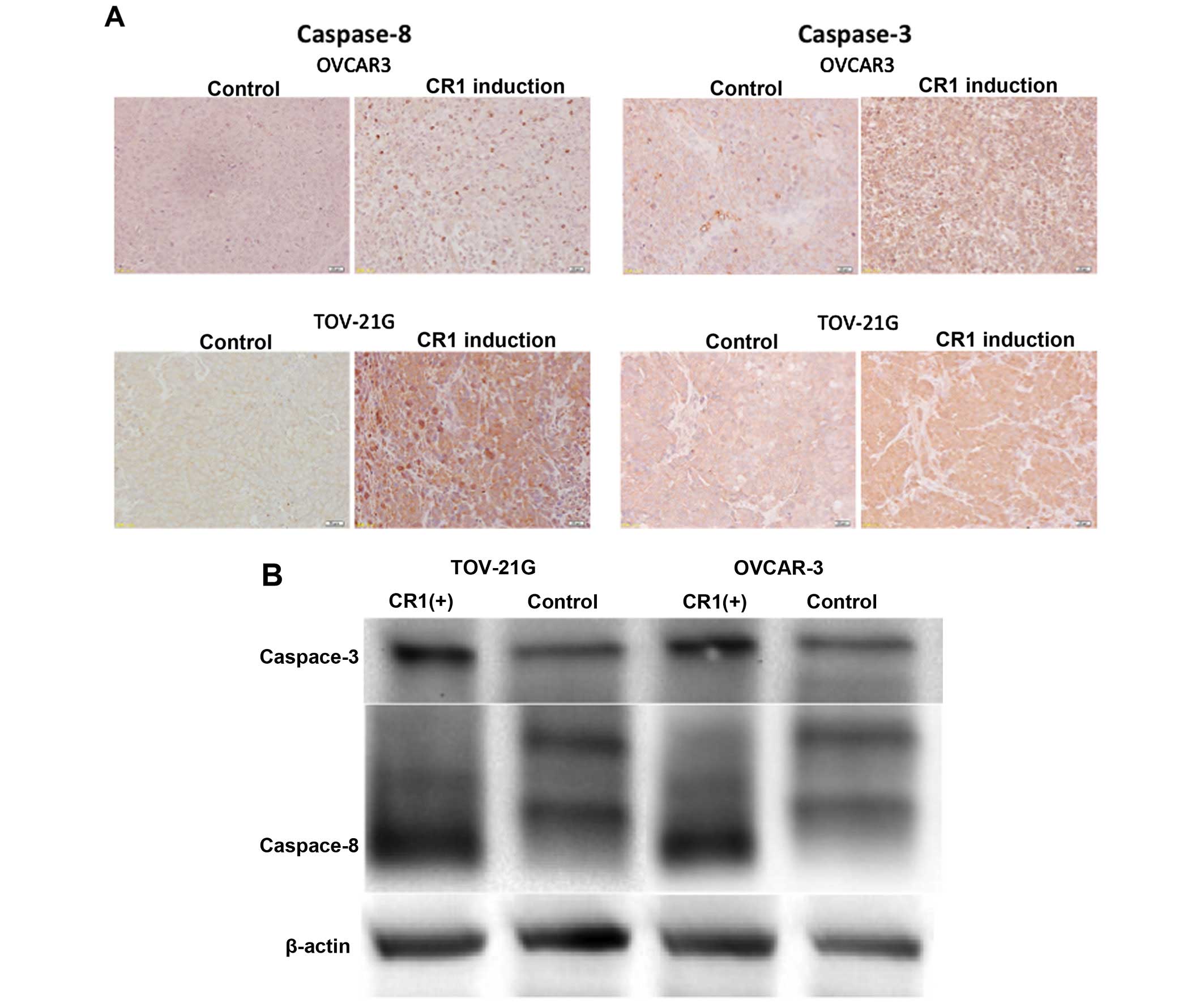
Effect of CR1-transfection on caspase-8
and -3 expression
Immunohistochemical and western blot analysis showed
a high expression of caspase-8 and -3 in tumors formed after
injections of CR1-overexpressing OVCAR-3 and TOV-21G cells,
respectively. In addition, very weak expression was observed in
tumors caused by injections of intact cell lines (Fig. 5).
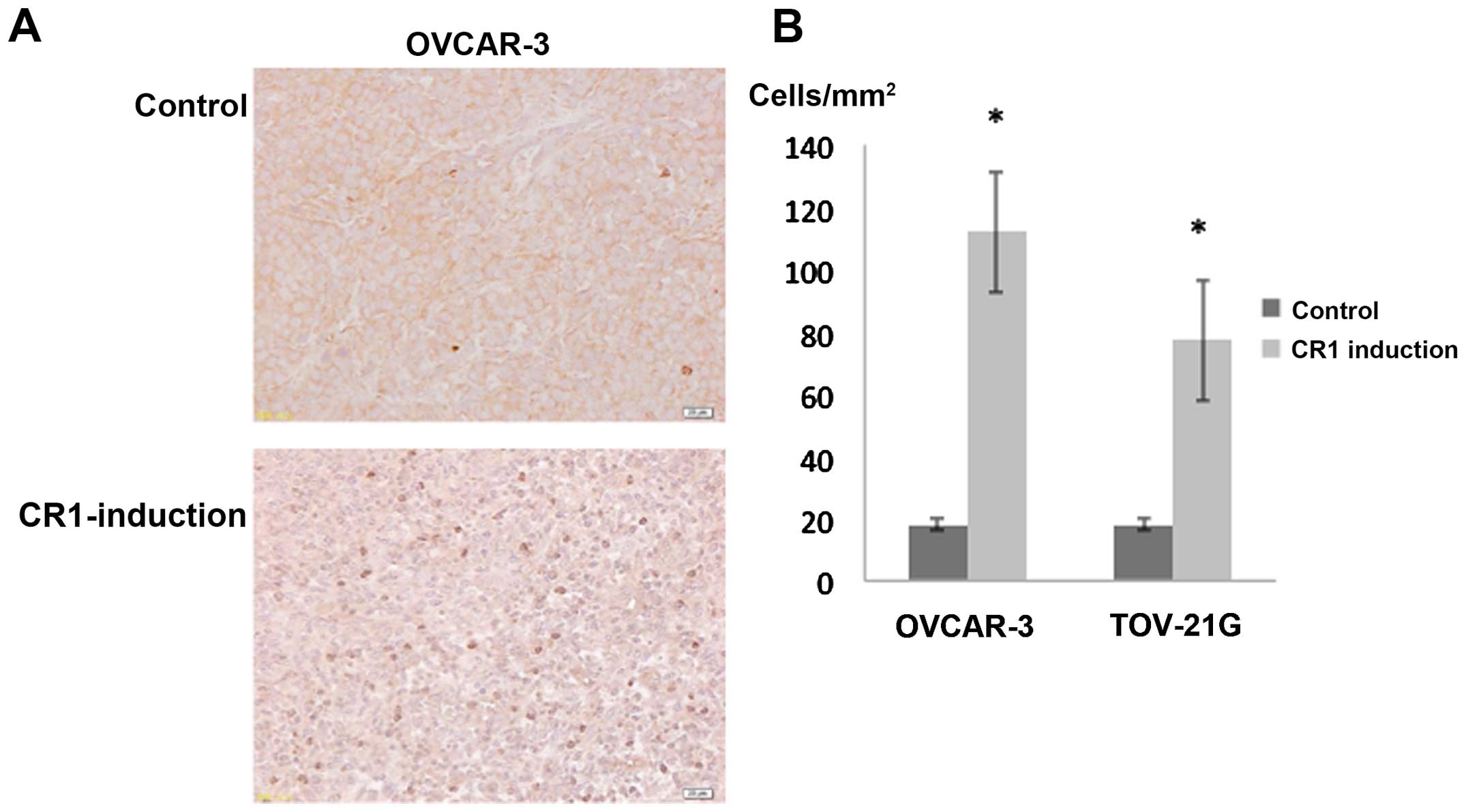
Effects of CR1-transfection on apoptotic
cell number
The number of apoptotic cells per mm2
identified with an anti-caspase-8 antibody (24) was 112.0±19.5 and 77.2±12.8 in
tumors caused by CR1-overexpressing OVCAR-3 and TOV-21G cells,
respectively. Tumors induced by the injection of control OVCAR-3
and TOV-21G cells exhibited fewer apoptotic cells per
mm2, that is, 18.0±7.7 and 17.6±1.8, respectively. The
differences between control and CR1 induction groups were
statistically significant (Fig. 6,
P<0.001 each).
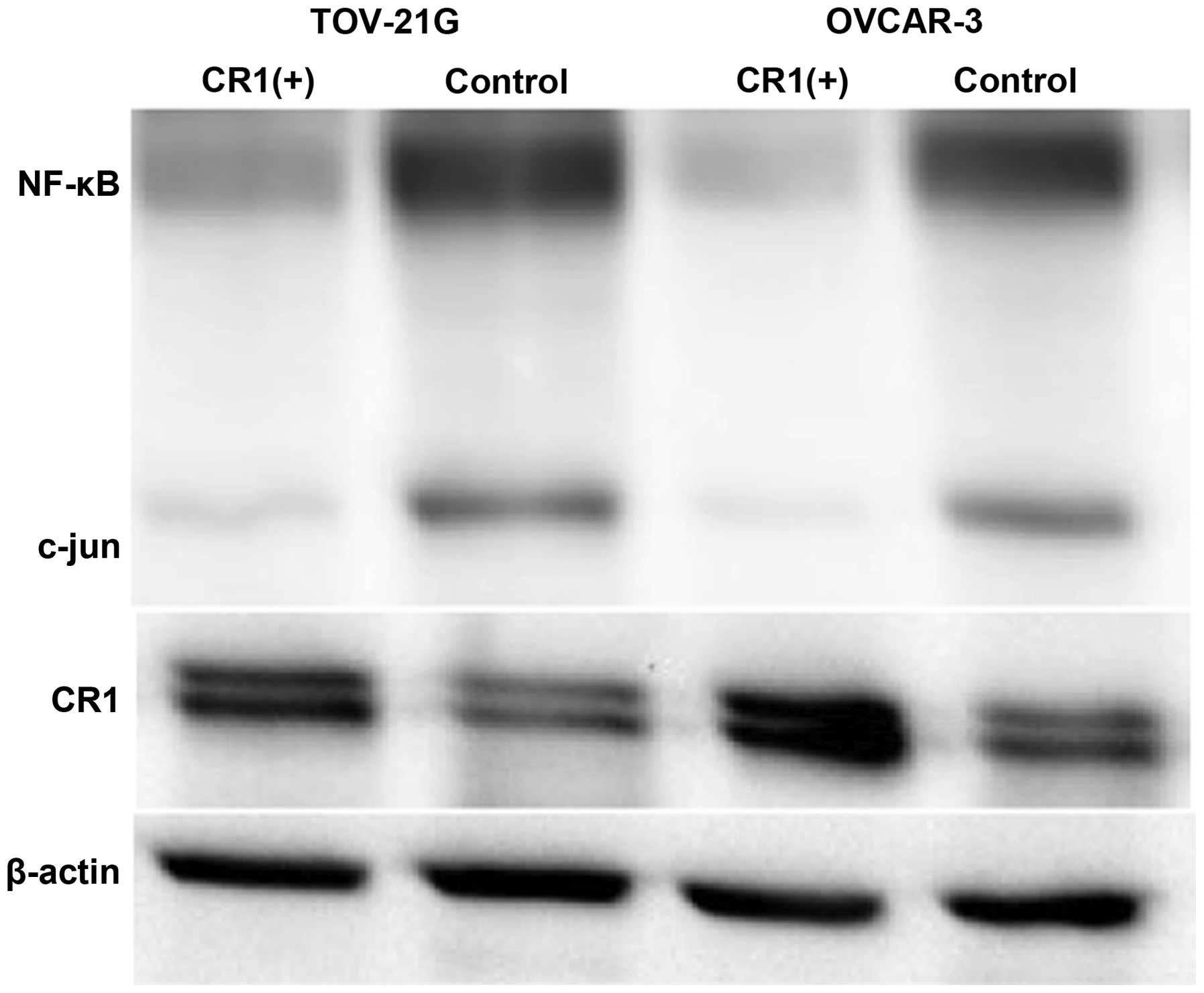
Effect of CR1-transfection on NF-κB and
c-Jun expression
Western blot analysis showed that both NF-κB and
c-Jun expression levels were lower in tumors caused by
CR1-overexpressing OVCAR-3 and TOV-21G cells compared to their
expression in tumors induced by control cell lines (Fig. 7).
Discussion
The results of this study show that subcutaneous
injections of CR1-overexpressing OVCAR-3 and TOV-21G cells into the
back region of nude mice formed smaller tumors in volume than
injection with normal OVCAR-3 and TOV-21G cell lines and were
compatible with our previous studies that showed spontaneous
regression of malignant ovarian tumors with high expression of CR1
(11) and growth promotion of
ovarian cancer cell lines by CR1 suppression (10). Because an increased expression of
MFG-E8 in macrophages was also observed in our previous study
(11), we speculated apoptosis as
a putative mechanism of CR1 function. As shown in Fig. 5, we found that expression levels of
caspase-8 and -3 were higher in tumors with CR1 overexpressed cell
lines, confirming our hypothesis. In addition, expression levels of
NF-κB and c-Jun were negatively affected in tumors with CR1
overexpression. NF-κB and c-Jun are known to cooperate to prevent
apoptosis induced by TNFα, therefore we were interested to study
TNFRs for TNFα with CR1.
TNFR1 is ubiquitously expressed in most tissues,
whereas TNFR2 is mainly expressed in immune cells (13). Although both receptors bind TNFα,
cellular effects of TNFα in most cell types are predominantly
mediated by TNFR1 (25). TNFR1 is
an important member of the death receptor family and is capable of
inducing apoptotic cell death (26). TNFR2 can also mediate cell death
signals, which may be indirectly communicated through TNFR1
(26). As shown in Fig. 4, TNFR1 was expressed to equal
levels regardless of CR1 expression, while TNFR2 was expressed
weaker in both groups, suggesting that TNFR1 signaling has a more
important role in tumor development. In order that TNFR1 elicits
physiological function, it trimerizes and releases the silencer of
death domain protein, which recruits adaptor proteins such as RIP,
TRAF-2, and FADD (15). FADD binds
to pro-caspase-8 and activated caspase-8 subsequently initiates a
proteolytic cascade that involves other caspases (caspase-3, -6 and
-7) and ultimately induces apoptosis (16,27).
As shown in Fig. 5, because
expression levels of caspase-8 and -3 were higher in CR1
overexpressing tumors, it is presumed that CR1 induced apoptosis
through the activation of caspase pathway. In addition, expression
of NF-κB and c-Jun was lower in CR1-overexpressing tumors. NF-κB
and c-Jun have been shown to induce transcription of genes related
to proliferation and anti-apoptosis (13). Therefore, apoptosis induced by CR-1
overexpression can be accounted for by reduced expression of NF-κB
and c-Jun. These results were compatible with earlier reports that
showed an inhibitory effect of NF-κB on ovarian cancer growth
(28,29).
In conclusion, the results of this study show that
CR1 has anticancer effects by inducing apoptosis through the TNFα
system. Of interest, CR1 induced apoptosis of TOV-21G cell lines
which are derived from chemo-resistant ovarian clear cell
adenocarcinoma (30). Clear cell
carcinoma is one of the most frequent of ovarian cancers and has
poor prognosis (30), then the
results of present study suggests that CR1 might become a new
candidate for treatment of clear cell carcinoma. Further studies
are required for clinical application of a PPARα ligand or CR1 gene
therapy.
Acknowledgements
This study was supported by a Grant-in Aid for
Cancer Research from the Ministry of Education, Science and Culture
of Japan (no. 20591935 to Y. Yokoyama).
References
|
1
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2013. CA Cancer J Clin. 63:11–30. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Heintz AP, Odicino F, Maisonneuve P, Quinn
MA, Benedet JL, Creasman WT, Ngan HY, Pecorelli S and Beller U:
Carcinoma of the ovary = FIGO 26th Annual Report on the Results of
Treatment in Gynecological Cancer. Int J Gynaecol Obstet. 95(Suppl
1): S161–S192. 2006. View Article : Google Scholar
|
|
3
|
McGuire WP, Hoskins WJ, Brady MF, Kucera
PR, Partridge EE, Look KY, Clarke-Pearson DL and Davidson M:
Cyclophosphamide and cisplatin compared with paclitaxel and
cisplatin in patients with stage III and stage IV ovarian cancer. N
Engl J Med. 334:1–6. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yokoyama Y, Xin B, Shigeto T, Umemoto M,
Kasai-Sakamoto A, Futagami M, Tsuchida S, Al-Mulla F and Mizunuma
H: Clofibric acid, a peroxisome proliferator-activated receptor α
ligand, inhibits growth of human ovarian cancer. Mol Cancer Ther.
6:1379–1386. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gonzalez-Covarrubias V, Ghosh D, Lakhman
SS, Pendyala L and Blanco JG: A functional genetic polymorphism on
human carbonyl reductase 1 (CBR1 V88I) impacts on catalytic
activity and NADPH binding affinity. Drug Metab Dispos. 35:973–980.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wermuth B, Bohren KM, Heinemann G, von
Wartburg JP and Gabbay KH: Human carbonyl reductase. Nucleotide
sequence analysis of a cDNA and amino acid sequence of the encoded
protein. J Biol Chem. 263:16185–16188. 1988.PubMed/NCBI
|
|
7
|
Umemoto M, Yokoyama Y, Sato S, Tsuchida S,
Al-Mulla F and Saito Y: Carbonyl reductase as a significant
predictor of survival and lymph node metastasis in epithelial
ovarian cancer. Br J Cancer. 85:1032–1036. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Murakami A, Fukushima C, Yoshidomi K,
Sueoka K, Nawata S, Yokoyama Y, Tsuchida S, Ismail E, Al-Mulla F
and Sugino N: Suppression of carbonyl reductase expression enhances
malignant behaviour in uterine cervical squamous cell carcinoma:
Carbonyl reductase predicts prognosis and lymph node metastasis.
Cancer Lett. 311:77–84. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Murakami A, Yakabe K, Yoshidomi K, Sueoka
K, Nawata S, Yokoyama Y, Tsuchida S, Al-Mulla F and Sugino N:
Decreased carbonyl reductase 1 expression promotes malignant
behaviours by induction of epithelial mesenchymal transition and
its clinical significance. Cancer Lett. 323:69–76. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Osawa Y, Yokoyama Y, Shigeto T, Futagami M
and Mizunuma H: Decreased expression of carbonyl reductase 1
promotes ovarian cancer growth and proliferation. Int J Oncol.
46:1252–1258. 2015.PubMed/NCBI
|
|
11
|
Wang H, Yokoyama Y, Tsuchida S and
Mizunuma H: Malignant ovarian tumors with induced expression of
carbonyl reductase show spontaneous regression. Clin Med Insights
Oncol. 6:107–115. 2012.PubMed/NCBI
|
|
12
|
Hanayama R, Tanaka M, Miwa K, Shinohara A,
Iwamatsu A and Nagata S: Identification of a factor that links
apoptotic cells to phagocytes. Nature. 417:182–187. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
van Horssen R, Ten Hagen TL and Eggermont
AM: TNF-alpha in cancer treatment: Molecular insights, antitumor
effects, and clinical utility. Oncologist. 11:397–408. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tracey KJ and Cerami A: Tumor necrosis
factor, other cytokines and disease. Annu Rev Cell Biol. 9:317–343.
1993. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Vandenabeele P, Declercq W, Beyaert R and
Fiers W: Two tumour necrosis factor receptors: Structure and
function. Trends Cell Biol. 5:392–399. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Rath PC and Aggarwal BB: TNF-induced
signaling in apoptosis. J Clin Immunol. 19:350–364. 1999.
View Article : Google Scholar
|
|
17
|
Degterev A, Boyce M and Yuan J: A decade
of caspases. Oncogene. 22:8543–8567. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Barnes PJ and Karin M: Nuclear
factor-kappaB: A pivotal transcription factor in chronic
inflammatory diseases. N Engl J Med. 336:1066–1071. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Karin M, Liu Z and Zandi E: AP-1 function
and regulation. Curr Opin Cell Biol. 9:240–246. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Rothe M, Pan M-G, Henzel WJ, Ayres TM and
Goeddel DV: The TNFR2-TRAF signaling complex contains two novel
proteins related to baculoviral inhibitor of apoptosis proteins.
Cell. 83:1243–1252. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Shigeto T, Yokoyama Y, Xin B and Mizunuma
H: Peroxisome proliferator-activated receptor α and γ ligands
inhibit the growth of human ovarian cancer. Oncol Rep. 18:833–840.
2007.PubMed/NCBI
|
|
22
|
Wakui M, Yokoyama Y, Wang H, Shigeto T,
Futagami M and Mizunuma H: Efficacy of a methyl ester of
5-aminolevulinic acid in photodynamic therapy for ovarian cancers.
J Cancer Res Clin Oncol. 136:1143–1150. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Cebulla J, Huuse EM, Pettersen K, van der
Veen A, Kim E, Andersen S, Prestvik WS, Bofin AM, Pathak AP,
Bjørkøy G, et al: MRI reveals the in vivo cellular and vascular
response to BEZ235 in ovarian cancer xenografts with different
PI3-kinase pathway activity. Br J Cancer. 112:504–513. 2015.
View Article : Google Scholar
|
|
24
|
Hirakawa H, Yokoyama Y, Yoshida H and
Mizunuma H: Inhibitory effects of aromatase inhibitor on estrogen
receptor-alpha positive ovarian cancer in mice. J Ovarian Res.
7:42014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
McFarlane SM, Pashmi G, Connell MC,
Littlejohn AF, Tucker SJ, Vandenabeele P and MacEwan DJ:
Differential activation of nuclear factor-kappaB by tumour necrosis
factor receptor subtypes. TNFR1 predominates whereas TNFR2
activates transcription poorly. FEBS Lett. 515:119–126. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wajant H, Pfizenmaier K and Scheurich P:
Tumor necrosis factor signaling. Cell Death Differ. 10:45–65. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Naudé PJ, den Boer JA, Luiten PG and Eisel
UL: Tumor necrosis factor receptor cross-talk. FEBS J. 278:888–898.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zerbini LF, Tamura RE, Correa RG, Czibere
A, Cordeiro J, Bhasin M, Simabuco FM, Wang Y, Gu X, Li L, et al:
Combinatorial effect of non-steroidal anti-inflammatory drugs and
NF-κB inhibitors in ovarian cancer therapy. PLoS One. 6:e242852011.
View Article : Google Scholar
|
|
29
|
Nishio H, Yaguchi T, Sugiyama J, Sumimoto
H, Umezawa K, Iwata T, Susumu N, Fujii T, Kawamura N, Kobayashi A,
et al: Immunosuppression through constitutively activated NF-κB
signalling in human ovarian cancer and its reversal by an NF-κB
inhibitor. Br J Cancer. 110:2965–2974. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Takano M, Sugiyama T, Yaegashi N, Sagae S,
Kuzuya K, Udagawa Y, Tsuda H, Suzuki M, Kigawa J, Goto T, et al:
Less impact of adjuvant chemotherapy for stage I clear cell
carcinoma of the ovary: A retrospective Japan Clear Cell Carcinoma
Study. Int J Gynecol Cancer. 20:1506–1510. 2010.PubMed/NCBI
|