Introduction
Ameloblastoma is a common benign odontogenic tumor
worldwide and is characterized by slow but steady invasion into the
maxillary and mandibular bones. A histopathological classification
of ameloblastoma by the World Health Organization in 2005 defined
four types: solid/multicystic, extraosseous/peripheral,
desmoplastic and unicystic. Solid/multicystic ameloblastoma is
further divided into follicular and plexiform types, including the
basal cell type (1,2). Although ameloblastoma is not a
malignant lesion, treatment of any type of ameloblastoma is limited
to surgical treatments such as enucleation and resection, although
recurrence with significant morbidity is common after enucleation,
particularly in young people (1–4).
Accordingly, resection remains the best way to remove
ameloblastoma, although this method is not without its drawbacks.
Therefore, a better understanding of the pathophysiology of
ameloblastoma is necessary, because there is a high demand for
drugs capable of acting as selective inhibitors of
ameloblastoma.
Expansion of solid/multicystic ameloblastoma in bone
is thought to occur as a result of accelerated bone resorption
activities by peritumoral osteoclasts. The activation of
osteoclasts is triggered by the binding of receptor activator of
nuclear factor kappa-B ligand (RANKL), which is released from
vicinal ameloblastoma cells, to bind receptor activator of nuclear
factor kappa-B (RANK) on the plasma membrane of osteoclasts, in a
manner similar to that seen in bone-invasive cancers, particularly
oral squamous cell carcinoma (SCC) (5–8).
Furthermore, several matrix metalloproteinases (MMP; MMP-1, MMP-2
and MMP-9) released from ameloblastoma cells are also involved in
progression of invasive lesions similar to that seen in oral
invasive SCC (9–15). However, ameloblastoma exhibits
clinical features that differ from those of oral SCC, including its
bone invasion patterns, rate of spread and clinical symptoms. For
example, solid/multicystic ameloblastoma and bone invasion of SCC
show clear differences on X-ray transmission images (1,16,17).
The border of a solid/multicystic ameloblastoma of the jaw bone is
well defined, smooth and scalloped, and the stroma exhibits a
characteristic soap bubble or honeycomb appearance, often
accompanied by knife-edge-like dental root resorption. In contrast,
the borders of other bone invasive cancers are less well defined,
often showing marked bone resorption similar to that seen in severe
periodontitis, and are characterized by floating teeth without root
resorption (1,16,17).
These differences are likely caused by the extremely slow spread of
ameloblastoma relative to that of bone-invading cancer cells
derived from oral tissues, breast, lung and other organs, which
tend to spread more rapidly (6).
We hypothesized that the expansion mechanism(s) of
ameloblastoma in jaw bone differs from those of invasive cancer
cells. In the present study, we compared the expression levels and
release of RANKL in ameloblastoma and invasive oral SCC cell lines,
to determine its effect on osteoclast differentiation. We also
examined the possibility that ameloblastoma could directly resorb
bone or dentine minerals. We found that ameloblastoma cells
expressed lower amounts of RANKL than oral SCC cells but resorbed
bone mineral materials by activation of vacuolar-type
H+-ATPase (V-ATPase) and H+/Cl−
exchange transporter 7 (CLC-7) on their plasma membranes.
Materials and methods
Cell culture
The human ameloblastoma cell line AM-1 was
established from a plexiform-type ameloblastoma representing
typical features of native cells (18,19).
Cells were grown in defined keratinocyte serum-free medium (D-KSFM;
Invitrogen, San Diego, CA, USA). Human normal skin keratinocytes
(HaCaT), human tongue squamous carcinoma (HSC-3), human lip
fibroblasts (KD; purchased from JCRB Cell bank, Osaka, Japan), and
RAW264.7 mouse macrophage cells (purchased from DS Pharma
Biomedical, Osaka, Japan) were grown in Dulbecco's modified Eagle's
medium (DMEM; Invitrogen), supplemented with 10% fetal bovine serum
(FBS; PAA Laboratories, Pasching, Austria). Human osteoclast (hOC)
precursor cells derived from bone marrow (purchased from Lonza,
Basel, Switzerland) were grown in an original culture solution
based on modified Eagle's medium (MEM) containing 100 ng/ml human
synthetic RANKL (Wako Pure Chemical Industries, Ltd., Osaka, Japan)
and 50 ng/ml human macrophage colony stimulating factor (M-CSF;
PeproTech, Rocky Hill, NJ, USA). Cells were reseeded for the next
passage after trypsin (Invitrogen) dispersion when they reached
~80% confluency. HaCaT and HSC-3 were gifts from M. Furue (Kyushu
University, Fukuoka, Japan) and H. Takeuchi (Kyushu Dental
University, Kitakyushu, Japan), respectively.
Western blot analysis
Western blots were performed as previously described
(20). Briefly, cells (AM-1,
HaCaT, HSC-3 KD and hOCs) were homogenized in 1 ml ice-cold lysis
buffer and centrifuged at 50,000 × g for 30 min at 4°C. The
supernatants (20 μg) were then separated on 10 or 12% sodium
dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) gels
and transferred to polyvinyldifluoride membranes (Millipore,
Darmstadt, Germany). Immunoblot analyses were performed using mouse
anti-human RANKL monoclonal antibody (1:500; Sigma-Aldrich, St.
Louis, MO, USA), mouse antihuman V-ATPase isoform α3
monoclonal antibody (V-ATPase α3 or TCIRG1; 1:500; Abcam,
Cambridge, MA, USA), rabbit anti-human CLC-7 (1:500; Abgent, Inc.,
San Diego, CA, USA), rabbit anti-human chloride transporter 3
(CLC-3 polyclonal antibody, 1:500; Abcam), or mouse anti-human
cathepsin K monoclonal antibody (1:1,000; Sigma-Aldrich). Rabbit
antihuman β-actin monoclonal antibody (1:1,000; Cell Signaling
Technology, Danvers, MA, USA) was used as an internal standard.
Blots were developed with horseradish peroxidase (HRP)-linked
secondary antibodies (1:3,000; Cell Signaling Technology) and
visualized using the enhanced chemiluminescence (ECL) system,
LAS-4000 (GE Healthcare, Cleveland, OH, USA). Immobilon western
chemiluminescent HRP substrate (Millipore) was used for detection.
For the biotinylation assay, cells (AM-1 and HaCaT; 80% confluent
in a 6-cm dish) were lysed in lysis buffer following incubation in
cold biotin reagent (1 mg/ml sulpho-NHS-SS-biotin; Thermo Fisher
Scientific, Waltham, MA, USA) for 30 min at 4°C, and centrifuged at
50,000 × g for 30 min at 4°C. The supernatant was then incubated
with 300 μl cold avidin beads (Thermo Fisher Scientific) at 4°C for
2 h, followed by centrifugation at 3,500 × g for 30 min at 4°C.
Supernatants were then aspirated, and the beads were washed three
times with 1 ml cold lysis buffer, once with cold 500 mM NaCl and
Tris-HCl (pH 7.5), and once with cold 10 mM Tris-HCl (pH 7.5). The
beads were boiled with 50 μl Laemmli sample buffer, and a 20-μl
aliquot was analyzed by SDS-PAGE. Antibodies used for the
immunoblot analysis included anti-V-ATPase α3, anti-CLC-7
and anti-E-cadherin (rabbit anti-human monoclonal; 1:1,000; Cell
Signaling Technology).
Coculture and osteoclastogenesis
experiments
AM-1 and KD cells were cocultured at a ratio of 1:1
in a 6-cm dish in a 1:1 mixture of D-KSFM and 10% FBS-containing
α-MEM, as previously described (5,21).
Bone marrow cells were collected from C57BL/6J mice at 6 weeks of
age. Cells (1.5×105)/well in 24-well plates were
cultured in 10% FBS-containing α-MEM with 20 ng/ml M-CSF. After 2
days, adherent cells were used as bone marrow-derived
monocyte/macrophage precursor cells (BMM). AM-1 or HSC3 cells were
cocultured with RAW264.7 cells or BMM in 24-well plastic plates at
a ratio of 1:5 in 5% FBS-containing α-MEM medium (α-MEM), or a
mixture of D-KSFM and 10% FBS-containing α-MEM (mixed medium). All
media were supplemented with 50 ng/ml M-CSF. As a positive control,
100 ng/ml RANKL was added to the media. After 5–7 days, cells were
fixed in 4% paraformaldehyde (PFA), and stained for
tartrate-resistant acid phosphatase (TRAP) using a TRAP kit
(Sigma-Aldrich). TRAP-positive multinuclear cells containing more
than three nuclei were considered to be osteoclasts (22). For assessment of osteoclast
differentiation, RAW264.7 cells (5×104 cells/well) were
cultured in 24-well plates in α-MEM. PP2, PP3 (Abcam), and
(3Z)-3-[(1-Methylindol-3-yl)methylidene]-2-oxo-1H-indole-5-sulfonamide
[spleen tyrosine kinase (Syk) inhibitor (Syk inh); Abcam] were
applied 1 h prior to changing the medium to mixed medium.
Recombinant mouse semaphorin 3A (R&D Systems, Minneapolis, MN,
USA) was applied 12 h prior to changing the medium to the mixed
medium. The culture medium was changed every second day. After 7
days, cells were fixed in 4% PFA and stained for TRAP. The method
for counting TRAP-positive cells was as described above.
Cell observation and pit assay
Calcium phosphate- and collagen I-coated coverslips,
and 24-well calcium phosphate-coated plates (BD BioCoat Osteologic;
BD Biosciences, San Jose, CA, USA; and Osteo Assay surface; Corning
Incorporated, Corning, NY, USA, respectively) were used for the pit
assay (23). AM-1 and HaCaT cells
were cultured on Osteologic coverslips for 2–10 days. AM-1 and hOCs
were cultured on osteo assay surface plates for 10 days. Cells and
pits were observed at magnifications of 4× and 10× with an inverted
microscope (IX71; Olympus, Tokyo, Japan); images were captured
using cellSens imaging software (ver. 1.7.1; Olympus). The pits in
three samples were counted in random regions with pit areas
quantified using ImageJ software (NIH, Bethesda, MD, USA).
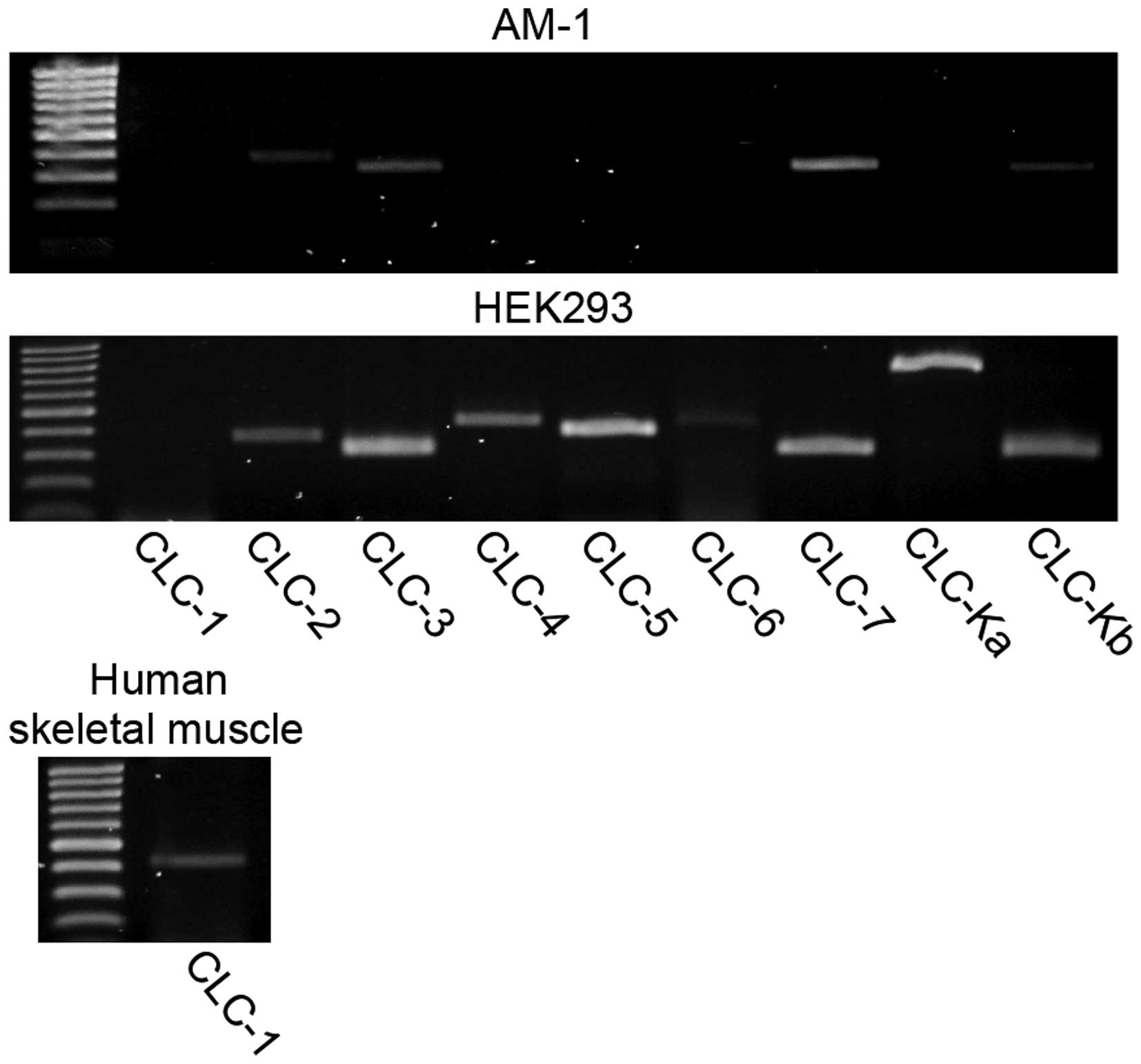
Reverse transcription-polymerase chain
reaction (RT-PCR) analysis
Total RNA was extracted from AM-1 using TRIzol
reagent (Thermo Fisher Scientific). Isolated total RNA (4 μg) was
subjected to RT-PCR analysis using PCR Super Mix High Fidelity
(Thermo Fisher Scientific) and the CLC-1 primers
5′-ctgagccagcctgtctgtttt-3′ (forward) and 5′-ctccaactcgccctc
tacctt-3′ (reverse); CLC-2 primers 5′-tagccctgaggcttctgtctg-3′
(forward) and 5′-ggagcaggatcaattttgcag-3′ (reverse); CLC-3 primers
5′-tagggcaaatattgcctggtg-3′ (forward) and
5′-gatggaaccttgatgccaaaa-3′ (reverse); CLC-4 primers
5′-ctcctcccatacaaagggacac-3′ (forward) and
5′-taatgctgtcctcctgtgctgt-3′ (reverse); CLC-5 primers
5′-gcatatagcacagatggcgaac-3′ (forward) and
5′-acggttggaatttctcttgcat-3′ (reverse); CLC-6 primers
5′-ctggaatgggagacagaggtg-3′ (forward) and
′5-cctccatggtccagtcttcac-3′ (reverse); CLC-7 primers
5′-gactcgtagcaccagggtttg-3′ (forward) and
5′-catgtgctaggggaagacctg-3′ (reverse); CLC-Ka primers
5′-gaggaggtggtcaaggttgtg-3′ (forward) and
5′-ttctcaggagcctctcactgg-3′ (reverse); and CLC-Kb primers
5′-gaggaggtggtcaaggttgtg-3′ (forward) and
5′-tttcttcatctccacccagga-3′ (reverse). Total RNA extracted from
HEK293 (kindly provided by Dr H. Takeuchi) or human skeletal muscle
(Agilent Technologies, Santa Clara, CA, USA) was used as a positive
control.
Fluorescent immunohistochemistry
Clinical sample collection was performed at the
Department of Oral and Maxillofacial Surgery, Kyushu University
Hospital (Fukuoka, Japan). Specimens were removed surgically from
three patients with primary ameloblastoma (plexiform, follicular
and basal cell types); all patients provided informed consent
before enrollment. Immunohistochemistry was performed as previously
described (20). Briefly,
following the initial biopsy, all specimens were fixed in 4% PFA in
phosphate-buffered saline (pH 7.4) overnight, embedded in paraffin
wax and sectioned at 5 μm. After deparaffinization and blocking
procedures, specimens were stained with primary antibodies (mouse
anti-human V-ATPase α3 antibody, 1:200; rabbit anti-human
CLC-7 antibody, 1:200) and secondary antibodies (Alexa Fluor 594
conjugated anti-mouse IgG, HRP-linked antibody, 1:1,000; Alexa
Fluor 488 conjugate anti-rabbit IgG, HRP-linked antibody, 1:1,000;
Invitrogen). Sections were then mounted using PermaFluor mountant
(Lab Vision Products, Thermo Fisher Scientific) and visualized at
the appropriate wavelength using a fluorescence microscope (BioRevo
BZ-9000; Keyence). For cell staining, AM-1 cells and hOCs were
fixed in 4% PFA. After permeabilization with digitonin (100 μg/ml;
Wako Pure Chemical) and blocking with 2.5% bovine serum albumin
(Sigma-Aldrich), cells were stained with the aforementioned primary
antibodies (mouse anti-human E-cadherin antibody, 1:200; BD
Biosciences; rabbit anti-human CLC-3 antibody, 1:200; Abcam; mouse
anti-human V-ATPase α3 antibody, 1:200; rabbit anti-human
CLC-7 antibody, 1:200). This was followed by incubation with
secondary antibodies, and then mounting with PermaFluor mountant
for visualization. Single-cell samples were visualized with a
confocal microscope (LSM700; Carl Zeiss, Oberkochen, Germany; or
A1; Nikon, Tokyo, Japan). Images were processed using Adobe
Photoshop CS3 (Adobe Systems, San Jose, CA, USA).
Drugs
Bafilomycin A1 was obtained from Merck. All other
chemicals were purchased from Sigma-Aldrich.
Statistical analyses
All data are expressed as the mean ± standard error
of the mean (SEM). Student's t-test and one-way analysis of
variance (ANOVA) were used for statistical evaluations. Statistical
significance was set as P<0.05.
Results
Assessment of bone demineralization and
RANKL expression by AM-1 cells
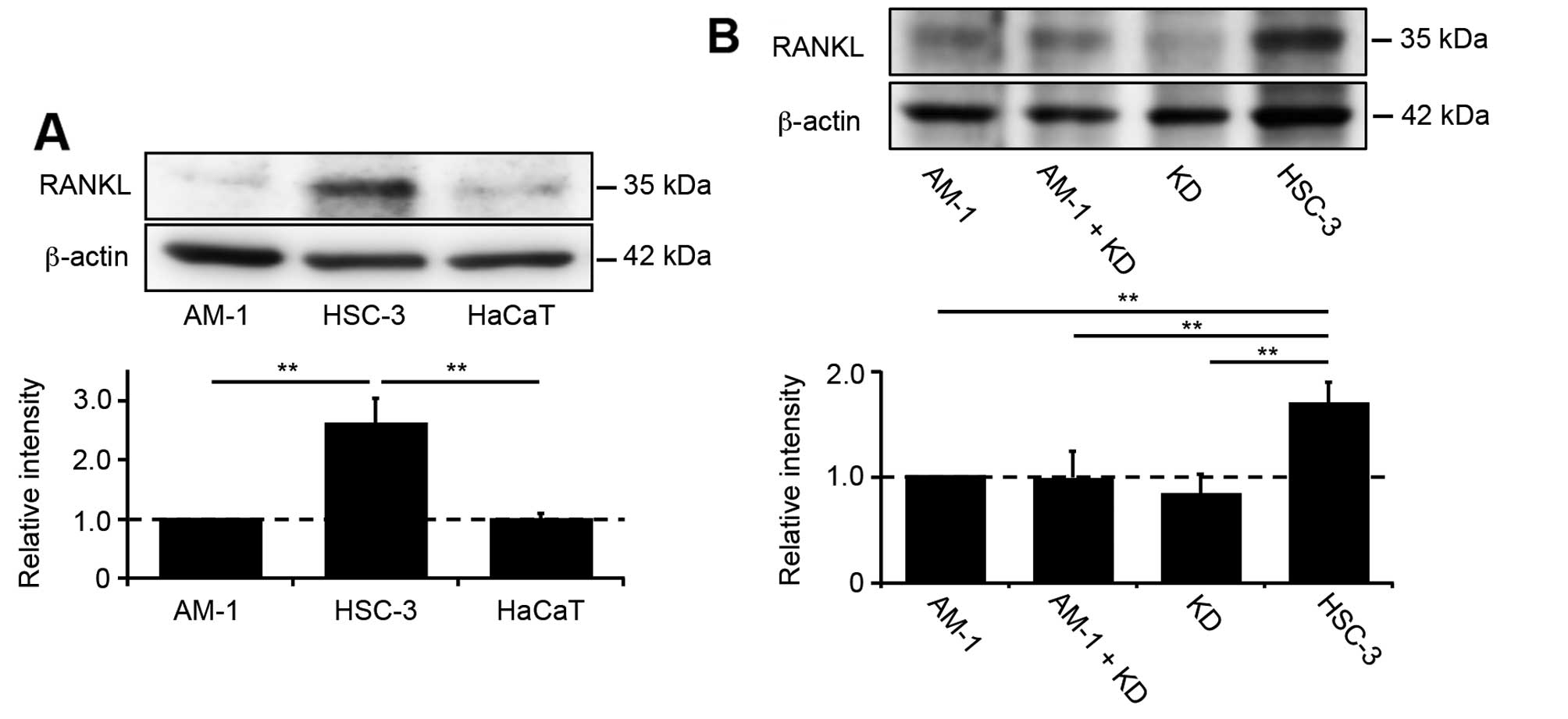
Western blot analysis using anti-RANKL antibody was
performed to detect expression of RANKL in AM-1, HSC-3 and HaCaT
cells. HSC-3, bone invasive cancer cells and HaCaT, normal skin
keratinocyte, were used as a positive and negative control,
respectively. RANKL expression was high in HSC-3 cells, whereas in
AM-1 cells, its expression was similar to that in the negative
control (Fig. 1A). To evaluate the
effect of tumor-stromal interactions on RANKL expression by
ameloblastoma cells, AM-1 and KD cells (human lip fibroblasts) were
cocultured in mixed medium, but this did not increase RANKL
expression (Fig. 1B). Next, we
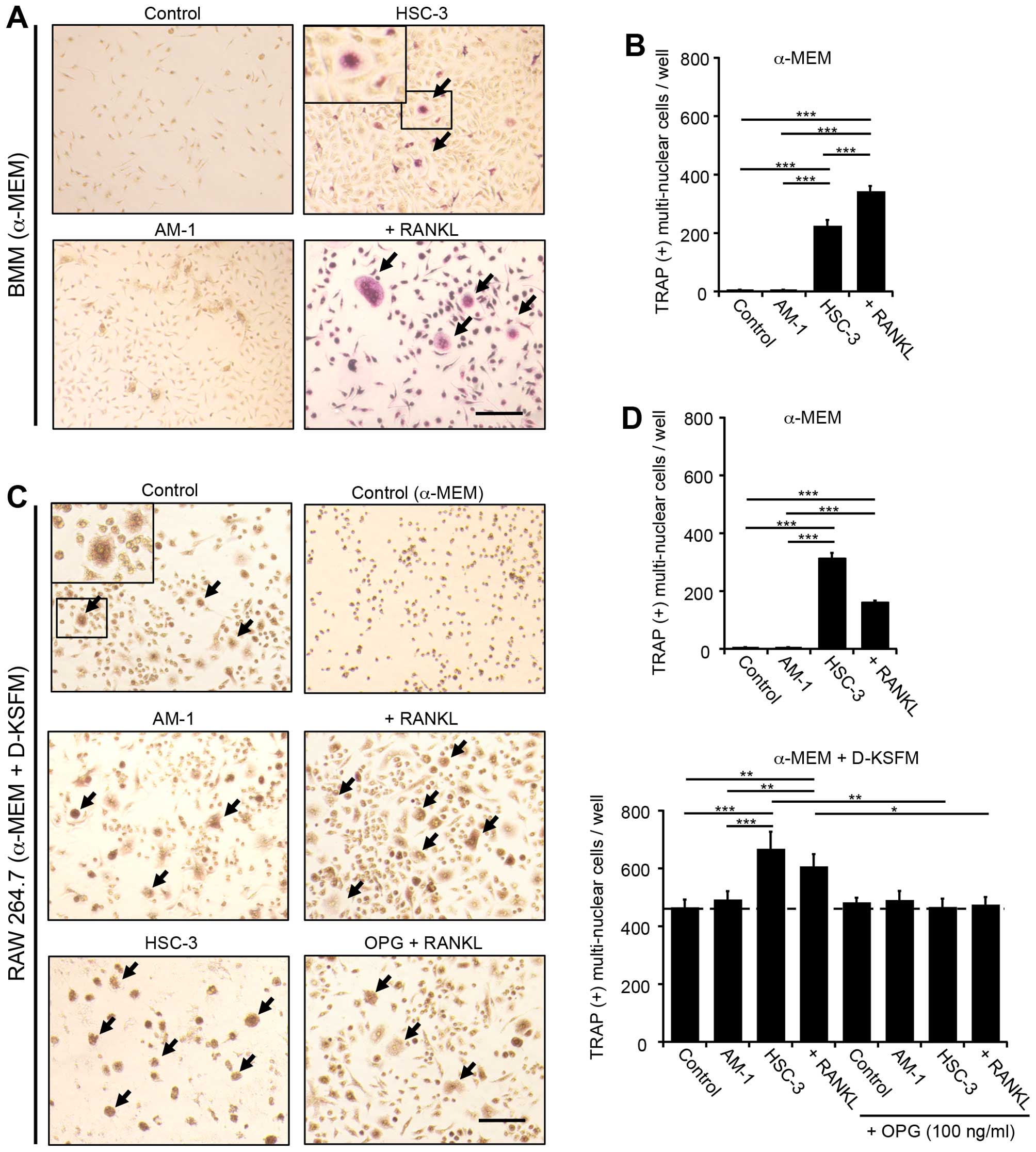
examined the osteoclastic differentiation of BMM and RAW264.7 cells
cocultured with AM-1 or HSC-3 cells grown in a medium of α-MEM
alone or α-MEM plus D-KSFM (mixed medium); as a positive control,
100 ng/ml RANKL was added to the medium in the absence of coculture
cells. TRAP-positive multinuclear cells were detected in the
cocultures with HSC-3 cells, but not in those with AM-1 grown in
α-MEM medium (Fig. 2). On the
other hand, cocultures grown in mixed medium produced more
TRAP-positive cells, which were inhibited to the control level by
the addition of osteoprotegerin (OPG; 100 ng/ml) (Fig. 2C and D), indicating the presence of
RANKL-independent osteoclastogenesis in the mixed medium. To
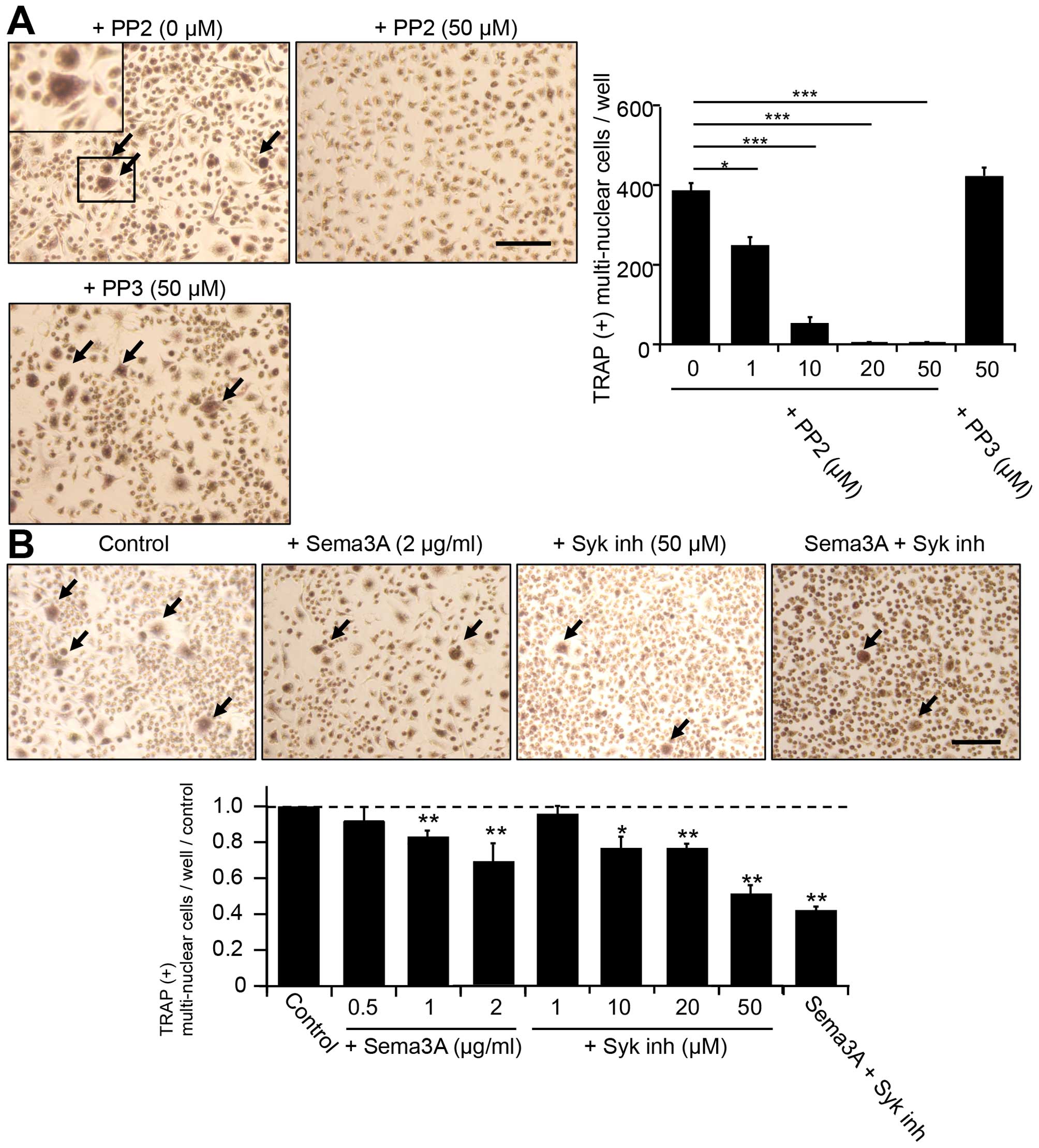
investigate the RANK-independent osteoclastogenesis of RAW264.7 in
mixed medium, we tested several inhibitors of non-receptor tyrosine
kinases, which are related to immunoreceptor tyrosine-based
activation motif (ITAM)-bearing receptor pathways. PP2, a specific
Src kinase family inhibitor, inhibited the formation of
TRAP-positive multinuclear cells in a dose-dependent manner
(Fig. 3A). In contrast, PP3 (50
μM), a negative control for PP2, did not detectably inhibit
TRAP-positive multinuclear cell formation. However, semaphorin 3A
(sema3A; >1 μg/ml), a negative regulator of the formation of
plexin-A1-triggering receptor expressed in myeloid cells-2
(TREM-2), which is an immunoreceptor, DNAX activating protein of
12-kDa (DAP12) complex, and Syk inh (50 μM) partially inhibited the
RANKL-independent TRAP-positive cell formation (Fig. 3). Interestingly, further inhibition
was observed by application of a mixture of sema3A (2 μg/ml) and
Syk inh (50 μM) (Fig. 3B).
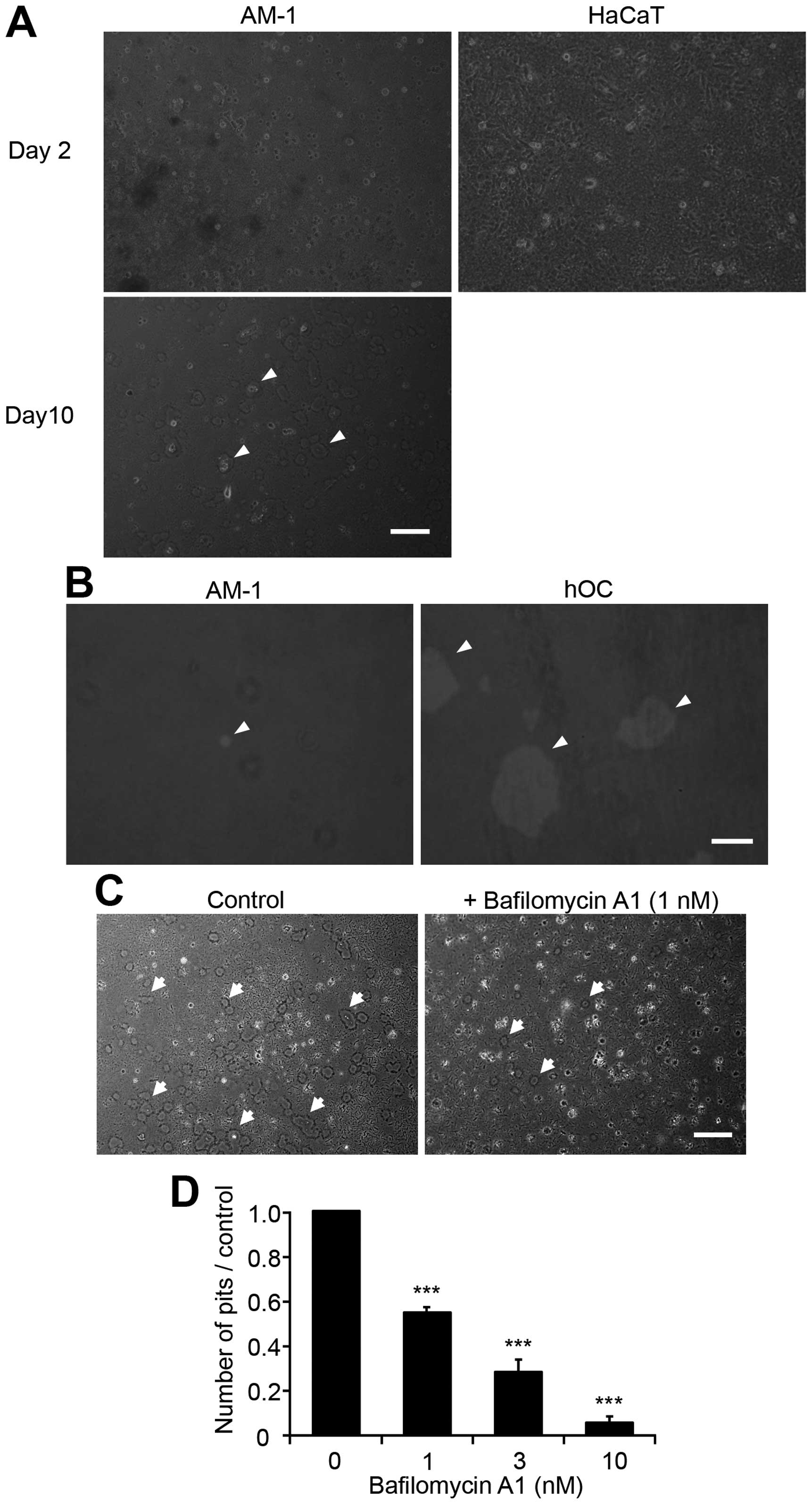
AM-1 cells dissolve the mineral
substrate
Based on the above results, we hypothesized that
ameloblastoma cells might directly demineralize bone. Their
demineralization ability was assessed using calcium phosphate- and
collagen I-coated coverslips (Osteologic), which mimic bone mineral
substrate. AM-1 cells grew very slowly and more than 10 days were
needed to reach ~80% confluency after an initial seeding of
6.0×105 cells in a non-coated 6-cm dish. After reaching
confluency, cells (2.0×105 cells) were reseeded on
Osteologic coverslips in 24-well culture plates and cultured for an
additional 10 days. Small round pits with a diameter of 30–40 μm
were observed on the coverslips at a density of 47±3
pits/mm2 (n=3; Fig. 4A,
left panel). In contrast, HaCaT cells grew quickly, similarly to
HSC-3 cells, reaching ~80% confluency within 2 days of culture in
DMEM containing 10% FBS. HaCaT cells produced no pits on Osteologic
coverslips, even after reaching full confluency (Fig. 4A). Addition of bafilomycin A1, an
V-ATPase inhibitor, to the culture medium inhibited pit formation
in a dose-dependent manner at concentrations ranging from 1 to 10
nM (Fig. 4C and D). Addition of 10
nM concanamycin, another V-ATPase inhibitor, also completely
inhibited pit formation by AM-1 cells (data not shown). Next, we
determined the demineralization ability of AM-1 cells compared with
hOCs, which were differentiated from bone marrow-derived osteoclast
precursor cells using RANKL. AM-1 and hOCs were initially seeded at
a density of 1×104 cells on Osteologic coverslips. Cells
were then cultured for 10 days, and the absorbed pits were analyzed
as described above. The mean pit area produced by AM-1 cells was
1.7% of that produced by hOCs (0.1±0.03% for AM-1 vs. 5.9±2.5% for
hOCs; n=3 for each treatment; Fig.
4B). The addition of bisphosphonates, either alendronate (10
μM) or pamidronate (10 μM), which are inhibitors of osteoclasts,
had no inhibitory effect on the viability of AM-1 cells, although
the RAW264.7 cells were susceptible (data not shown).
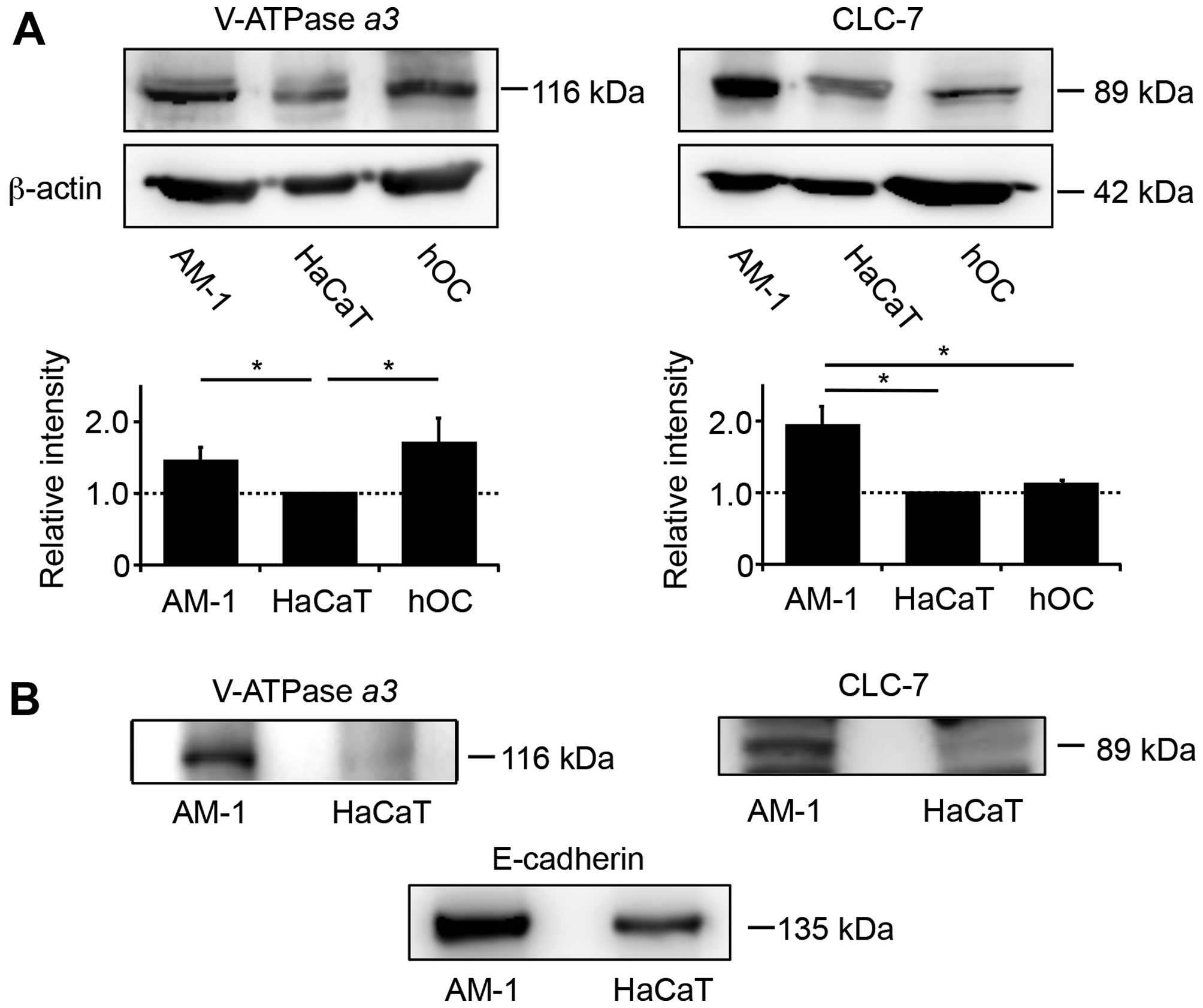
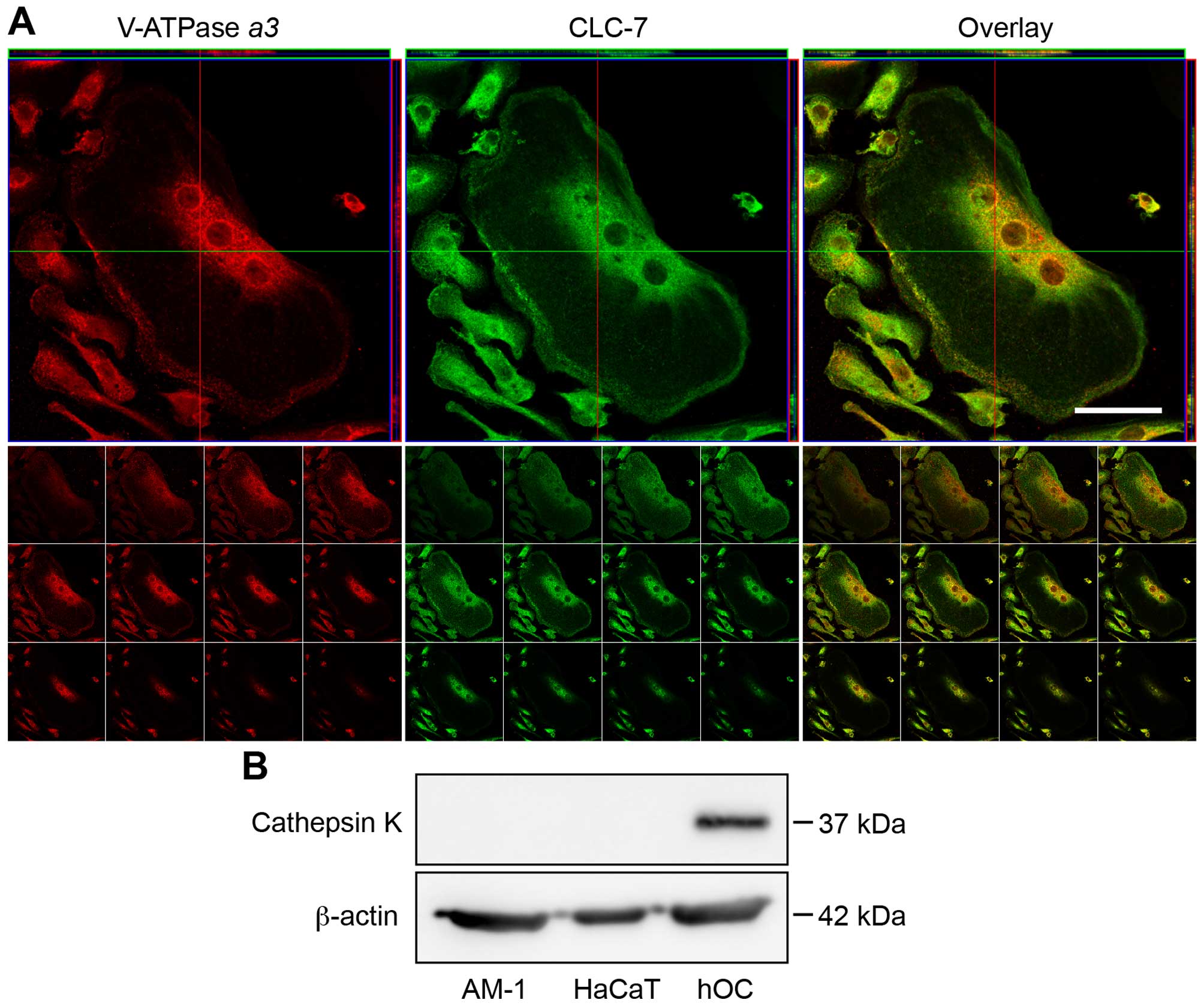
V-ATPase and CLC-7 are expressed on the
plasma membrane of AM-1 cells
Based on the inhibitory effects of bafilomycin A1
and concanamycin, we assumed that demineralization of calcium
phosphate by AM-1 cells could be caused by V-ATPase, a proton pump
expressed on the surface of the plasma membrane. V-ATPase is
coexpressed with the chloride transporter CLC-7 on the surface of
organelles, such as lysosomes, in most eukaryotic cells, and on the
plasma membrane of osteoclasts. Western blot analysis of AM-1 cell
lysates showed the presence of V-ATPase α3 at levels similar
to that of hOCs, while CLC-7 was present at levels greater than
those in hOCs and HaCaT cells. The relative expression of V-ATPase
and CLC-7 was hOCs ≈ AM-1 >HaCaT and AM-1 >hOCs ≈ HaCaT,
respectively (Fig. 5A). To examine
whether these proteins were present on the cell-surface, we labeled
intact AM-1 and HaCaT cells with a biotinylation reagent, followed
by lysis and detection with streptavidin. Biotinylated V-ATPase
α3 and CLC-7 were observed in the cell-surface extracts of
only AM-1 cells but were absent in HaCaT cells (Fig. 5B). As a positive control,
E-cadherin, a transmembrane protein in the plasma membrane, was
detected in the cell-surface extracts of both AM-1 and HaCaT cells
(Fig. 5B). In addition, the
expression profile of the CLC chloride channel family in AM-1 cells
was assessed by RT-PCR analysis; the results showed that several
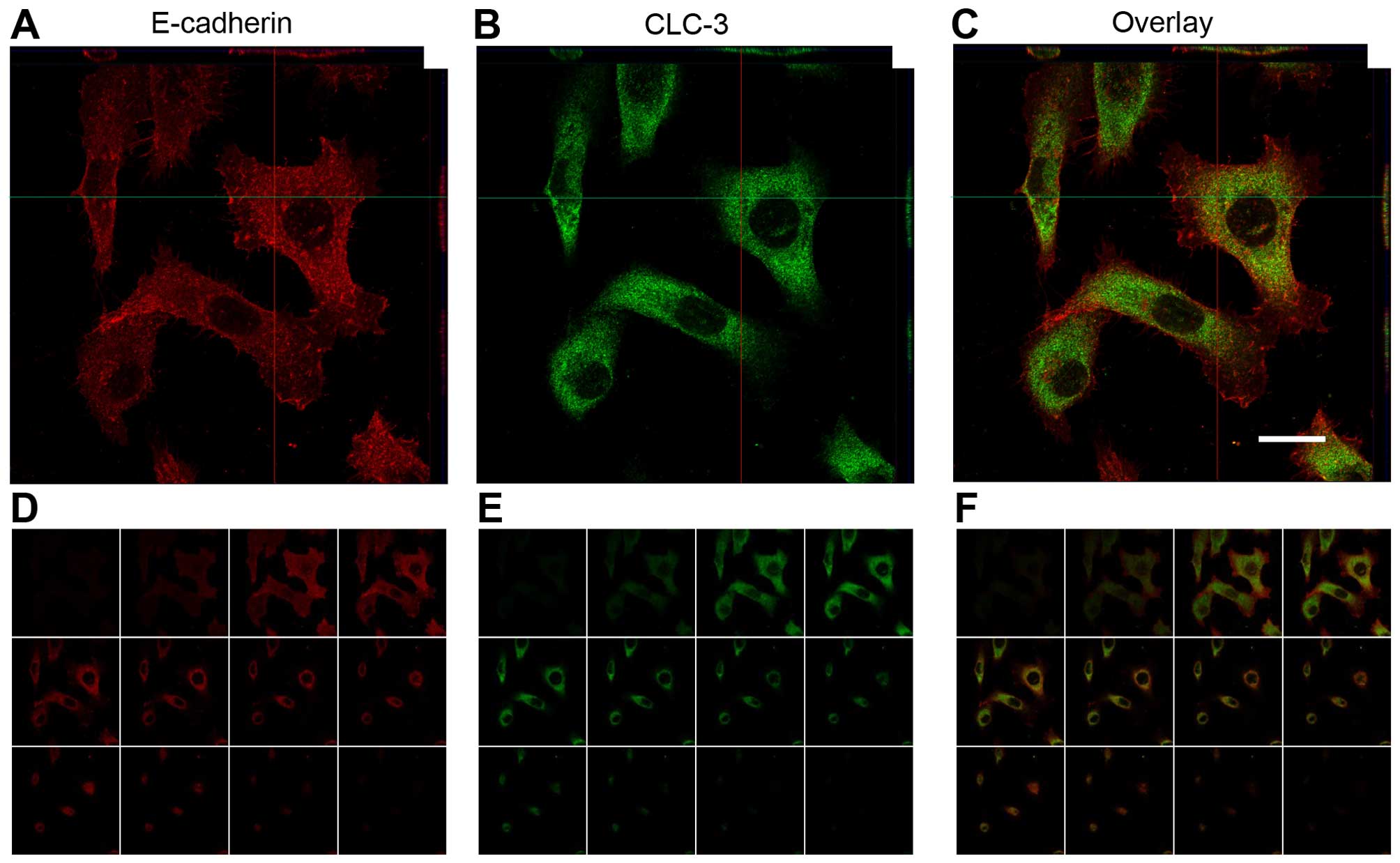
mRNA for CLC-2, CLC-3, CLC-7 and CLC-Kb were detected (Fig. 6). Immunofluorescence analysis of
CLC-3 demonstrated that the positive signals were localized almost
entirely in the cytosol of AM-1 cells (Fig. 7).
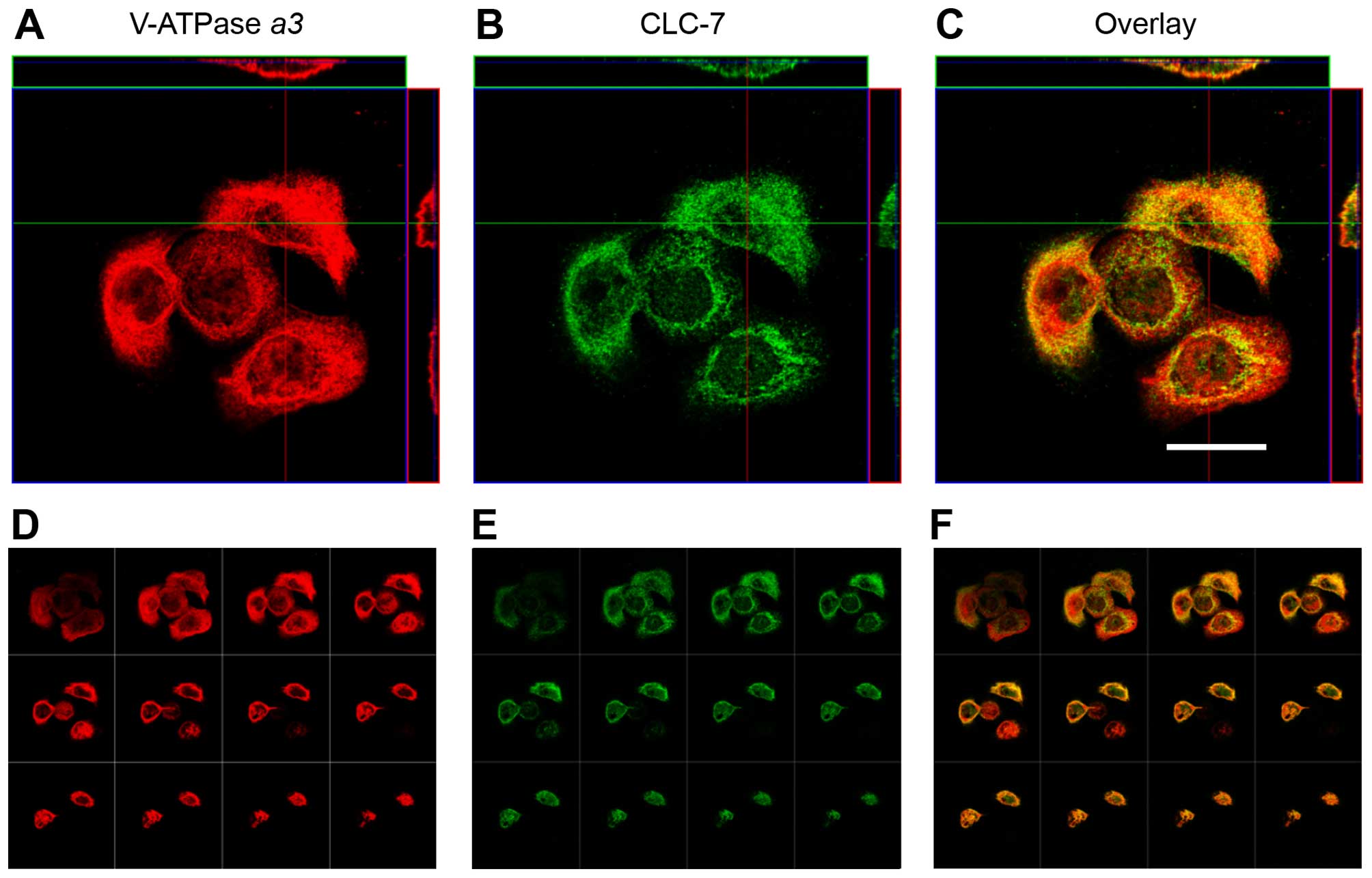
Comparison of osteoclastogenic features
of AM-1 cells and osteoclasts
Immunofluorescence analysis was performed to examine
the membrane localization of both V-ATPase α3 and CLC-7
(Fig. 8, Z-axis and tiled images).
Merged images of V-ATPase α3 and CLC-7 are shown in Fig. 8C and F. As a positive control, an
identical pattern of membrane localization of V-ATPase α3
and CLC-7 was evident in hOC (Fig.
9A). Nevertheless, the cytosol of both AM-1 cells and hOCs was
also stained by antibodies for V-ATPase α3 and CLC-7,
because these factors are usually expressed in the lysosomes of all
eukaryotic cells (Figs. 8 and
9A). Next, we performed TRAP
staining of AM-1 cells. AM-1 cells were negative for TRAP, in clear
contrast to the findings for osteoclasts (data not shown).
Furthermore, neither AM-1 nor HaCaT cells expressed cathepsin K, a
cysteine proteinase released from osteoclasts to digest the organic
materials of bone (Fig. 9B).
Distribution of V-ATPase and CLC-7 in
clinical specimens of ameloblastoma lesions
Finally, we performed immunofluorescence staining of
clinically dissected specimens from patients bearing three types of
ameloblastoma: plexiform, follicular and basal cell types. All
types of solid/multicystic ameloblastoma specimens were clearly
stained with V-ATPase α3 and CLC-7 antibodies in the
cytosol, particularly on the plasma membrane of the epithelium
rather than the stroma (Fig.
10).
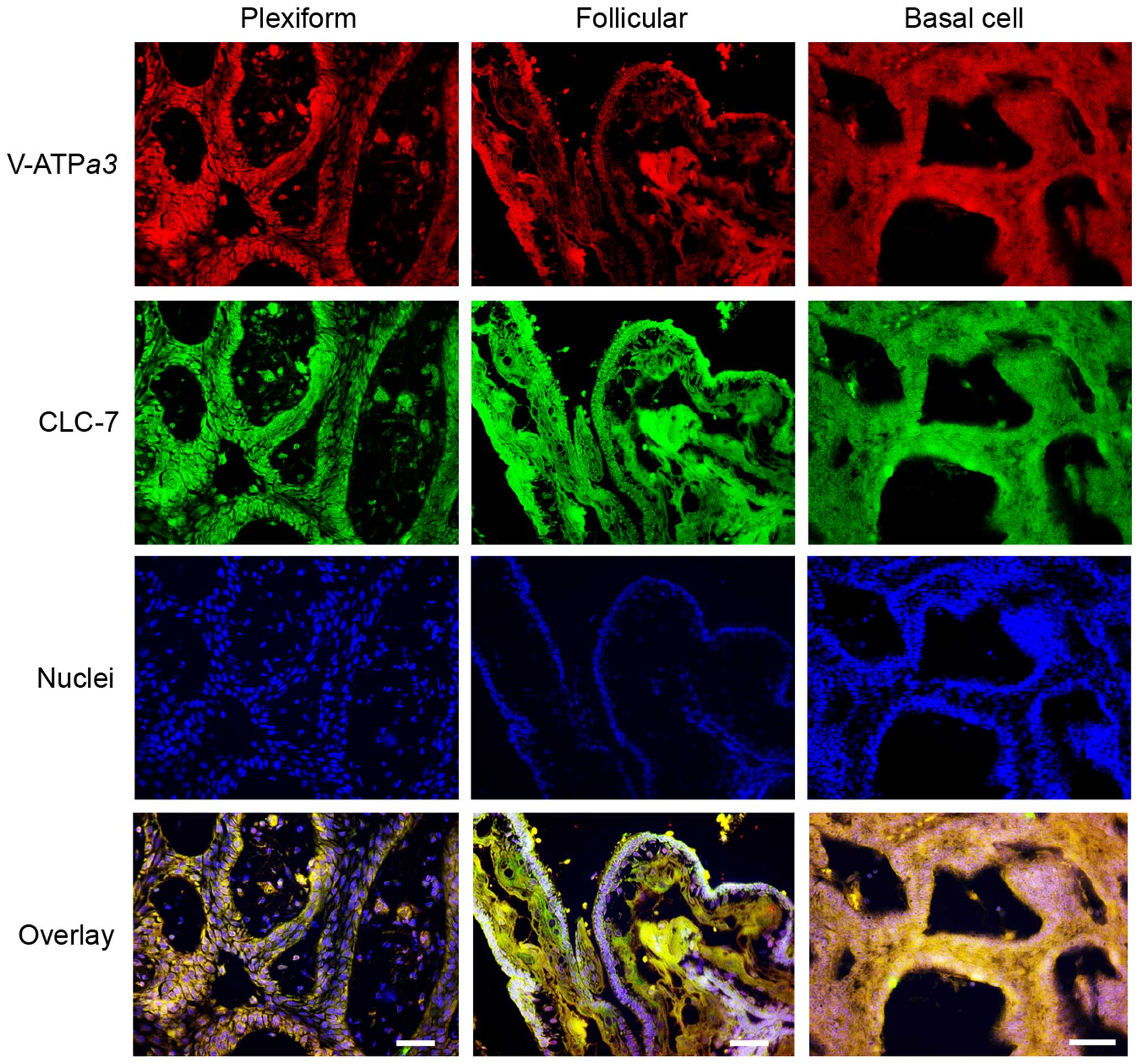 | Figure 10Distribution of V-ATPase and CLC-7 in
several types of ameloblastoma. Immunohistochemical analyses were
performed using mouse anti-human V-ATPase α3 (1:200; top,
red), rabbit anti-human CLC-7 (1:200; second, green) antibodies,
and Hoechst 33342 (1:500; third, blue) in human plexiform,
follicular, and basal cell-type ameloblastomas. Merged images
(bottom) show colocalization of V-ATPase, CLC-7 and Hoechst 33342.
An Alexa Fluor 594-conjugated anti-mouse IgG HRP-linked antibody
(1:1,000) and 488-conjugated anti-rabbit IgG HRP-linked antibody
(1:1,000) were used as secondary antibodies. Scale bar, 200 μm. |
Discussion
Disruptive bone resorption as a result of invasion
by cancer cells is caused by maturation and functional activation
of osteoclasts by RANKL released from cancer cells (7,24,25).
Ameloblastoma cells are thought to behave in a similar manner,
expressing RANKL on the plasma membrane, thereby activating
peripheral osteoclasts (5),
suggesting that the mechanisms for ameloblastoma expansion in jaw
bone are similar to those of bone-invasive cancers. However,
despite these apparent similarities, the clinical features of bone
cancer and ameloblastoma are quite different (3,6),
which led us to examine the osteoclastogenic responses induced by
ameloblastoma cells. We found that the expression and release of
RANKL by AM-1 cells, an ameloblastoma cell line, appeared to be too
low to activate osteoclasts, even in the presence of other cell
types such as fibroblasts. Many reports have highlighted the
importance of the tumor-stroma interaction in tumor cell invasion,
and the role of osteoclastogenesis in odontogenic tumors; however,
no increases in RAW264.7 differentiation or RANKL expression in
AM-1 were observed in cocultures with AM-1 or KD cells,
respectively (26–30). In fact, Kumamoto and Ooya (31) reported little expression of RANKL
in either plexiform or follicular ameloblastoma specimens. In
addition, RANKL-positive cells were shown by qualitative and
quantitative analysis of immunoreactivity to be distributed more
commonly throughout the stroma rather than in the epithelium
surrounding the ameloblastoma (32). RANKL and OPG positive cells were
also more commonly found in the stromal cells of ameloblastoma,
implying that RANKL expression in ameloblastoma may play a key role
in the proliferation and tumor progression of associated stromal
cells, similar to that seen in breast cancer (33,34).
However, Sandra et al (5) and Kibe et al (21) demonstrated osteoclastic
differentiation in cocultures of RAW264.7 macrophages with AM-3
cells, a follicular-type ameloblastoma cell line, and with AM-1
cells, results that differ from those of the present study.
Differences in the culture medium used in the present study may be
a possible explanation for this difference: untreated RAW264.7
cells cultured in a mixed medium (α-MEM plus D-KSFM) alone, which
was used in the studies by both Sandra et al (5) and Kibe et al (21), differentiated into TRAP-positive
multinuclear cells. In contrast, when cultured in α-MEM alone, BMM
and RAW246.7 cells did not differentiate into TRAP-positive cells
without the support of HSC-3 cells or added RANKL. Recently, it has
been identified that osteoclastic differentiation comprises two
different types of receptor-mediated signaling pathways, including
both RANK and immunoreceptor tyrosine-based activation motif
(ITAM)-bearing receptors (35).
ITAM-bearing receptors are classified into two types: Fc receptor
common γ subunit (FcRγ) receptors [osteoclast-associated receptor
(OSCAR) and paired immunoglobulin-like receptor-A (PIR-A)] and
DAP12 receptors [signal-regulatory protein b1 (SIRPβ1) and TREM-2].
ITAM-bearing receptors commonly activate phosphorylation of Syk and
Src kinases, which are members of the non-receptor tyrosine kinase
family (36). The present study
showed that osteoclast formation by RAW264.7 in a mixed medium was
completely inhibited by PP2 (>20 mM). Src kinases are related
not only to the activation of ITAM-bearing receptors, but also to
activation of M-CSF receptors (37,38).
M-CSF and its receptors are involved in an initial step of
differentiation (38).
Consequently, an Src kinase inhibitor fully suppressed osteoclastic
differentiation, while sema3A (>1 μg/ml), a negative regulator
of plexin-1A-TREM-2-DAP12 complex formation, and Syk inh (50 μM),
an inhibitor of FcR, partially inhibited RAW264.7 differentiation
(39,40). Together, these results indicate
that sema3A suppresses DAP12 receptor signaling, while Syk inh
suppresses FcRγ signaling. Therefore, we conclude that the D-KSFM
used in the previous studies (5,21)
must contain some ligands for M-CSF receptors and ITAM-bearing
receptors, thus promoting osteoclastogenesis.
From these observations, we hypothesized that
ameloblastoma might itself be able to resorb bone, given the fact
that their radiographic images show well-defined tumor margins and
knife-edge-like dental root resorption, in clear contrast to the
images of bone-invasive cancers (1). We speculate that there is little
space for osteoclasts to exist between the peritumor wall of
ameloblastoma and the dental roots, a feature that is far more
typical of ameloblastoma than that of other bone-related tumors. We
demonstrated that AM-1 cells formed resorption pits even in the
absence of osteoclastic cells, and at a magnitude much greater than
that of osteoclasts alone, indicating the probable activation of
V-ATPase and CLC-7 expressed on their plasma membranes. Thus, AM-1
exhibited bone demineralization activities similar to osteoclasts.
However, AM-1 showed differing features from osteoclasts with
regard to the absence of TRAP staining, the insensitivity to
bisphosphonates and lack of cathepsin K expression. These results
support direct bone demineralization of AM-1, but not experimental
contamination of osteoclasts.
In osteoclasts, the α3 subunit of V-ATPase is
thought to be essential for bone resorption (41–44),
because its absence results in autosomal recessive osteopetrosis
(45,46). We thus examined the α3
subunit as a marker of V-ATPase on the plasma membrane of AM-1
cells and clinical specimens. V-ATPase inhibitors bafilomycin A1
and concanamycin effectively blocked pit formation, indicating that
AM-1 cells have the ability to resorb calcium phosphate with
V-ATPase, a mechanism similar to that of osteoclasts. However,
CLC-7 is also a key regulator of bone demineralization, releasing
Cl− in cooperation with H+ release by
V-ATPase in osteoclasts (44). We
also detected CLC-7 expression on the plasma membrane of both AM-1
cells and ameloblastoma clinical specimens. Thus, we showed that
ameloblastoma cells demineralized bone matrix by means of V-ATPase
and CLC-7 expressed on their plasma membranes. Among CLC chloride
channel family members, CLC-1, 2, Ka and Kb are classified into
voltage-activated chloride channels, while CLC-3, 4, 5, 6 and 7 as
chloride transporters. An isotype of these chloride transporter
proteins, CLC-3, acts as a main chloride transporter in the
endosomes and lysosomes of ameloblastoma cells and other cell types
(47).
Taken together, these data show that ameloblastoma
cells appear to resorb bone in a manner similar to that of
osteoclasts, but at only 1.7% of the activity. Furthermore, AM-1
cells grew considerably slower than other cell lines, such as HaCaT
and HSC-3, a phenotype likely related to their slow expansion in
the jaw bone. The organic bone matrix consists of collagen, bone
Gla protein, bone sialoprotein and other proteins. This complex is
degraded by several MMP (MMP-1, MMP-2 and MMP-9), which are
released from ameloblastoma, but the cathepsin K released from
osteoclasts was not found in ameloblastoma (9,10,12–14,46).
Remarkably, both MMP-2 and MMP-9 are abundantly expressed in AM-1
and AM-3 cells relative to their expression in other oral cancer
cells (21,48). These findings appear to suggest
that ameloblastoma can resorb bone and dental roots, including the
periapical tissue, in concert with their activity in resorption of
inorganic hard tissues.
In conclusion, we demonstrated direct bone
demineralization, possibly by activation of V-ATPase plus CLC-7
present on the plasma membrane of ameloblastoma rather than by
RANKL release by ameloblastoma cells, and therefore we propose a
new concept for the slow pathophysiological progression of several
typical types of ameloblastomas.
Acknowledgements
The present study was supported by a Grant-in-Aid
for Scientific Research from the Japan Society for the Promotion of
Science (KAKENHI, #24592991 to H.M., #26861732 to Y.I. and
#24229009 to M.H.). We would like to thank Professors K. Okabe and
H. Takeuchi for critical comments and Professors T. Furue and H.
Takeuchi for providing us with HaCaT and HSC-3 cells, respectively.
We also thank Drs M. Matsuda, H. Umebayashi, T. Kawakubo-Yasukochi,
A. Mizokami, H. Kondo and S. Kanda for the helpful comments and
technical assistance. We would also like to thank the Research
Support Center, Graduate School of Medical Sciences, Kyushu
University for technical support in using the Carl Zeiss LSM700 and
Nikon A1 laser scanning confocal microscopes.
References
|
1
|
Thompson L: World Health Organization
classification of tumours: Pathology and genetics of head and neck
tumours. Ear Nose Throat J. 85:742006.PubMed/NCBI
|
|
2
|
Hertog D, Bloemena E, Aartman IHA and
van-der-Waal I: Histopathology of ameloblastoma of the jaws; some
critical observations based on a 40 years single institution
experience. Med Oral Patol Oral Cir Bucal. 17:e76–e82. 2012.
View Article : Google Scholar :
|
|
3
|
Reichart PA, Philipsen HP and Sonner S:
Ameloblastoma: Biological profile of 3677 cases. Eur J Cancer B
Oral Oncol. 31B:86–99. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hong J, Yun PY, Chung IH, Myoung H, Suh
JD, Seo BM, Lee JH and Choung PH: Long-term follow up on recurrence
of 305 ameloblastoma cases. Int J Oral Maxillofac Surg. 36:283–288.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sandra F, Hendarmin L, Kukita T, Nakao Y,
Nakamura N and Nakamura S: Ameloblastoma induces
osteoclastogenesis: A possible role of ameloblastoma in expanding
in the bone. Oral Oncol. 41:637–644. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hirshberg A, Shnaiderman-Shapiro A, Kaplan
I and Berger R: Metastatic tumours to the oral cavity -
pathogenesis and analysis of 673 cases. Oral Oncol. 44:743–752.
2008. View Article : Google Scholar
|
|
7
|
Jimi E, Shin M, Furuta H, Tada Y and
Kusukawa J: The RANKL/RANK system as a therapeutic target for bone
invasion by oral squamous cell carcinoma (Review). Int J Oncol.
42:803–809. 2013.PubMed/NCBI
|
|
8
|
Casimiro S, Mohammad KS, Pires R,
Tato-Costa J, Alho I, Teixeira R, Carvalho A, Ribeiro S, Lipton A,
Guise TA, et al: RANKL/RANK/MMP-1 molecular triad contributes to
the metastatic phenotype of breast and prostate cancer cells in
vitro. PLoS One. 8:e631532013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Souza Freitas V, Ferreira de Araújo CR,
Alves PM, de Souza LB, Galvão HC and de Almeida Freitas R:
Immunohistochemical expression of matrilysins (MMP-7 and MMP-26) in
ameloblastomas and adenomatoid odontogenic tumors. Oral Surg Oral
Med Oral Pathol Oral Radiol Endod. 108:417–424. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ribeiro BF, Iglesias DPP, Nascimento GJF,
Galvão HC, Medeiros AM and Freitas RA: Immunoexpression of MMPs-1,
-2, and -9 in ameloblastoma and odontogenic adenomatoid tumor. Oral
Dis. 15:472–477. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhang B, Zhang J, Xu ZY and Xie HL:
Expression of RECK and matrix metalloproteinase-2 in ameloblastoma.
BMC Cancer. 9:4272009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Qian Y and Huang HZ: The role of RANKL and
MMP-9 in the bone resorption caused by ameloblastoma. J Oral Pathol
Med. 39:592–598. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Shen LC, Chen YK, Hsue SS and Shaw SY:
Expression of osteonectin/secreted protein acidic and rich in
cysteine and matrix metalloproteinases in ameloblastoma. J Oral
Pathol Med. 39:242–249. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Henriques ÁCG, Vasconcelos MG, Galvão HC,
de Souza LB and de Almeida Freitas R: Comparative analysis of the
immunohistochemical expression of collagen IV, MMP-9, and TIMP-2 in
odontogenic cysts and tumors. Oral Surg Oral Med Oral Pathol Oral
Radiol Endod. 112:468–475. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sharma M, Sah P, Sharma SS and
Radhakrishnan R: Molecular changes in invasive front of oral
cancer. J Oral Maxillofac Pathol. 17:240–247. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Whitehouse GH: Radiological bone changes
produced by intraoral squamous carcinomata involving the lower
alveolus. Clin Otolaryngol Allied Sci. 1:45–52. 1976. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Murrin RJA and Mahendra P: ‘Floating’
teeth at presentation in sporadic Burkitt's lymphoma. Br J
Haematol. 127:12004. View Article : Google Scholar
|
|
18
|
Harada H, Mitsuyasu T, Nakamura N, Higuchi
Y, Toyoshima K, Taniguchi A and Yasumoto S: Establishment of
ameloblastoma cell line, AM-1. J Oral Pathol Med. 27:207–212. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sweeney RT, McClary AC, Myers BR, Biscocho
J, Neahring L, Kwei KA, Qu K, Gong X, Ng T, Jones CD, et al:
Identification of recurrent SMO and BRAF mutations in
ameloblastomas. Nat Genet. 46:722–725. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sugihara M, Morita H, Matsuda M,
Umebayashi H, Kajioka S, Ito S, Nishida M, Inoue R, Futatsuki T,
Yamazaki J, et al: Dual signaling pathways of arterial constriction
by extracellular uridine 5′-triphosphate in the rat. J Pharmacol
Sci. 115:293–308. 2011. View Article : Google Scholar
|
|
21
|
Kibe T, Fuchigami T, Kishida M, Iijima M,
Ishihata K, Hijioka H, Miyawaki A, Semba I, Nakamura N, Kiyono T,
et al: A novel ameloblastoma cell line (AM-3) secretes MMP-9 in
response to Wnt-3a and induces osteoclastogenesis. Oral Surg Oral
Med Oral Pathol Oral Radiol. 115:780–788. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Tsutsumi K, Matsuda M, Kotani M, Mizokami
A, Murakami A, Takahashi I, Terada Y, Kanematsu T, Fukami K,
Takenawa T, et al: Involvement of PRIP, phospholipase C-related,
but catalytically inactive protein, in bone formation. J Biol Chem.
286:31032–31042. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Loomer PM, Ellen RP and Tenenbaum HC:
Osteogenic and osteoclastic cell interaction: Development of a
co-culture system. Cell Tissue Res. 294:99–108. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Thomas RJ, Guise TA, Yin JJ, Elliott J,
Horwood NJ, Martin TJ and Gillespie MT: Breast cancer cells
interact with osteoblasts to support osteoclast formation.
Endocrinology. 140:4451–4458. 1999.PubMed/NCBI
|
|
25
|
Michigami T, Ihara-Watanabe M, Yamazaki M
and Ozono K: Receptor activator of nuclear factor kappaB ligand
(RANKL) is a key molecule of osteoclast formation for bone
metastasis in a newly developed model of human neuroblastoma.
Cancer Res. 61:1637–1644. 2001.PubMed/NCBI
|
|
26
|
Blankenstein T: The role of tumor stroma
in the interaction between tumor and immune system. Curr Opin
Immunol. 17:180–186. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Bhowmick NA and Moses HL: Tumor-stroma
interactions. Curr Opin Genet Dev. 15:97–101. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Guzmán-Medrano R, Arreola-Rosales RL,
Shibayama M, Silva-Olivares DA, Bologna-Molina R and Rodríguez MA:
Tumor-associated macrophages and angiogenesis: A statistical
correlation that could reflect a critical relationship in
ameloblastoma. Pathol Res Pract. 208:672–676. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Fuchigami T, Kibe T, Koyama H, Kishida S,
Iijima M, Nishizawa Y, Hijioka H, Fujii T, Ueda M, Nakamura N, et
al: Regulation of IL-6 and IL-8 production by reciprocal
cell-to-cell interactions between tumor cells and stromal
fibroblasts through IL-1α in ameloblastoma. Biochem Biophys Res
Commun. 451:491–496. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wang HC, Jiang WP, Sima ZH and Li TJ:
Fibroblasts isolated from a keratocystic odontogenic tumor promote
osteoclastogenesis in vitro via interaction with epithelial cells.
Oral Dis. 21:170–177. 2015. View Article : Google Scholar
|
|
31
|
Kumamoto H and Ooya K: Expression of
parathyroid hormone-related protein (PTHrP), osteoclast
differentiation factor (ODF)/receptor activator of nuclear
factor-kappaB ligand (RANKL) and osteoclastogenesis inhibitory
factor (OCIF)/osteoprotegerin (OPG) in ameloblastomas. J Oral
Pathol Med. 33:46–52. 2004. View Article : Google Scholar
|
|
32
|
da Silva TA, Batista AC, Mendonça EF,
Leles CR, Fukada S and Cunha FQ: Comparative expression of RANK,
RANKL, and OPG in keratocystic odontogenic tumors, ameloblastomas,
and dentigerous cysts. Oral Surg Oral Med Oral Pathol Oral Radiol
Endod. 105:333–341. 2008. View Article : Google Scholar
|
|
33
|
Schramek D, Leibbrandt A, Sigl V, Kenner
L, Pospisilik JA, Lee HJ, Hanada R, Joshi PA, Aliprantis A,
Glimcher L, et al: Osteoclast differentiation factor RANKL controls
development of progestin-driven mammary cancer. Nature. 468:98–102.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Gonzalez-Suarez E, Jacob AP, Jones J,
Miller R, Roudier-Meyer MP, Erwert R, Pinkas J, Branstetter D and
Dougall WC: RANK ligand mediates progestin-induced mammary
epithelial proliferation and carcinogenesis. Nature. 468:103–107.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Koga T, Inui M, Inoue K, Kim S, Suematsu
A, Kobayashi E, Iwata T, Ohnishi H, Matozaki T, Kodama T, et al:
Costimulatory signals mediated by the ITAM motif cooperate with
RANKL for bone homeostasis. Nature. 428:758–763. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Bradshaw JM: The Src, Syk, and Tec family
kinases: Distinct types of molecular switches. Cell Signal.
22:1175–1184. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Saltel F, Chabadel A, Zhao Y,
Lafage-Proust MH, Clézardin P, Jurdic P and Bonnelye E:
Transmigration: A new property of mature multinucleated
osteoclasts. J Bone Miner Res. 21:1913–1923. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Redlich K and Smolen JS: Inflammatory bone
loss: Pathogenesis and therapeutic intervention. Nat Rev Drug
Discov. 11:234–250. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Hayashi M, Nakashima T, Taniguchi M,
Kodama T, Kumanogoh A and Takayanagi H: Osteoprotection by
semaphorin 3A. Nature. 485:69–74. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Lai JY, Cox PJ, Patel R, Sadiq S, Aldous
DJ, Thurairatnam S, Smith K, Wheeler D, Jagpal S, Parveen S, et al:
Potent small molecule inhibitors of spleen tyrosine kinase (Syk).
Bioorg Med Chem Lett. 13:3111–3114. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Toyomura T, Oka T, Yamaguchi C, Wada Y and
Futai M: Three subunit a isoforms of mouse vacuolar
H+-ATPase. Preferential expression of the α3 isoform
during osteoclast differentiation. J Biol Chem. 275:8760–8765.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Toyomura T, Murata Y, Yamamoto A, Oka T,
Sun-Wada GH, Wada Y and Futai M: From lysosomes to the plasma
membrane: Localization of vacuolar-type H+-ATPase with
the α3 isoform during osteoclast differentiation. J Biol Chem.
278:22023–22030. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Forgac M: Vacuolar ATPases: Rotary proton
pumps in physiology and pathophysiology. Nat Rev Mol Cell Biol.
8:917–929. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Matsumoto N, Daido S, Sun-Wada GH, Wada Y,
Futai M and Nakanishi-Matsui M: Diversity of proton pumps in
osteoclasts: V-ATPase with a3 and d2 isoforms is a major form in
osteoclasts. Biochim Biophys Acta. 1837:744–749. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Frattini A, Orchard PJ, Sobacchi C,
Giliani S, Abinun M, Mattsson JP, Keeling DJ, Andersson AK,
Wallbrandt P, Zecca L, et al: Defects in TCIRG1 subunit of the
vacuolar proton pump are responsible for a subset of human
autosomal recessive osteopetrosis. Nat Genet. 25:343–346. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Sobacchi C, Schulz A, Coxon FP, Villa A
and Helfrich MH: Osteopetrosis: Genetics, treatment and new
insights into osteoclast function. Nat Rev Endocrinol. 9:522–536.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Jentsch TJ: Chloride and the
endosomal-lysosomal pathway: Emerging roles of CLC chloride
transporters. J Physiol. 578:633–640. 2007. View Article : Google Scholar
|
|
48
|
Zhang B, Zhang J, Huang HZ, Xu ZY and Xie
HL: Expression and role of metalloproteinase-2 and endogenous
tissue regulator in ameloblastoma. J Oral Pathol Med. 39:219–222.
2010. View Article : Google Scholar
|