Introduction
Thyroid cancer is the leading cause of increased
morbidity and mortality for endocrine malignancies, and papillary
thyroid carcinoma (PTC) accounts for 80% of thyroid cancer cases
(1). Reports have indicated that
PTC have a homogeneous molecular signature during tumorigenesis
compared with other human cancers, with wide variability in
clinical behaviors (2). Due to the
refractory nature of PTC to conventional radiation and drug
treatment, a subset of PTC is clinically aggressive and fatal
(3). However, the current
prognostic factors are not fully able to provide the molecular
information that is potentially useful for the prognostic
evaluation and treatment of PTC (4).
The mammalian genome contains several hundred
microRNAs (miRNAs), which are non-coding RNAs, 18–25 nucleotides
(nt) in length, that regulate the expression of 30% of human genes
(5). miRNAs can suppress the
expression of oncogenes, thus serving as tumor suppressors. Also,
they can promote the progression of neoplasms by reducing the
expression of some tumor suppressors (6). The deregulation of miRNAs has been
evidenced in a broad spectrum of diseases, including all major
cancers (7). However, only a few
human miRNAs have been shown to be dysregulated in PTC, including
miR-449 (8), miR-129-5p (9) and miR-146-5p (10). Accumulated reports indicate that
miR-449 serves as a tumor suppressor by inducing senescence and
apoptosis (11). However, to our
knowledge, no functional evidence of miR-449 in PTC has been
documented.
The RET proto-oncogene (RET) is a transmembrane
receptor-type tyrosine kinase. Under normal cellular conditions,
RET is activated by binding of both glial cell line-derived
neurotrophic factor (GDNF) ligands and cell surface-bound
co-receptors of the GDNF family receptor a (GFRa) proteins
(12). The RET receptor tyrosine
kinase has essential roles in cell survival, differentiation and
proliferation (13). Oncogenic
activation of RET causes the cancer syndrome multiple endocrine
neoplasia type 2 (MEN2) and is a frequent event in sporadic thyroid
carcinomas (14). RET activation
has been reported to stimulate RAS-ERK, c-Jun-NH2-kinase,
phosphoinositide 3-kinase, p38 mitogen-activated protein kinase
(MAPK) and signal transducer and activator of transcription (STAT)
(15). However, the identity of
the critical secondary oncogenic signals involved in the
progression of RET-mediated PTC is still largely unknown.
β-catenin is a key downstream transcriptional
activator of the Wnt signaling pathway. Under normal conditions,
cytoplasmic β-catenin is constantly degraded by a destruction
complex. However, the activation of Wnt signaling disrupts this
complex, enables β-catenin stabilization and cytoplasmic
accumulation, and finally translocation to the nucleus (16). Once inside the nucleus, β-catenin
interacts with the transcription factor lymphoid enhancer-binding
factor 1 (LEF)/T cell-specific transcription factor (TCF) to
upregulate the expression of genes involved in cell migration,
growth, differentiation and survival (17). Loss of membrane-associated
β-catenin, often with an accompanying relative increase in
cytosolic or nuclear expression, has been noted in anaplastic and
poorly differentiated thyroid carcinomas and in thyroid papillary
microcarcinoma (18). Reports have
indicated that the β-catenin-RET kinase pathway is a critical
contributor to the development and metastasis of human thyroid
carcinoma (19). Thus, inhibiting
the RET-involved β-catenin pathway may hinder PTC progression.
The present study investigated the potential
involvement of miR-449 in PTC. We hypothesized that miR-449 could
inhibit tumor growth of PTC by targeting RET expression and
inactivating β-catenin signaling. Our results suggest that miR-449
may provide a better understanding of the process of PTC.
Materials and methods
TPC sample
Twenty-five pairs of human PTC and adjacent normal
tissues were obtained from the Second Affiliated Hospital of Xi’an
Jiaotong University (Shaanxi, China). The tissues were frozen in
liquid nitrogen and stored at −80°C until use. Written informed
consent for tissue donation (for research purposes) was obtained
from the patients, and the protocol was approved by the
Institutional Review Board of the Second Affiliated Hospital of
Xi’an Jiaotong University.
Cell lines
Four human PTC cell lines, including TPC-1, K1,
IHH-4 and CGTH-W3, were obtained from the American Type Culture
Collection (ATCC; Manassas, VA, USA). The human thyroid epithelial
cell lines Nthy-ori3-1 and HTori-3 were purchased from Shanghai
Cell Collection (SCC; Shanghai, China). These cell lines were
cultured in Dulbecco’s modified Eagle’s medium (DMEM) plus 10%
fetal bovine serum (FBS; Life Technologies, Inc., Grand Island, NY,
USA) at 37°C in a humidified atmosphere containing 5%
CO2.
Quantitative real-time polymerase chain
reaction (qRT-PCR)
The total RNA was extracted from TRIzol reagent
(Invitrogen, Carlsbad, CA, USA) following the manufacturer’s
instructions. The RT-PCR primers for miR-449 and U6 were purchased
from GeneCopoeia (San Diego, CA, USA). The PCR primers for RET
were: 5′-AATTTGGAAAAGTGGTCAAGGC-3′ (sense) and
5′-CTGCAGGCCCCATACAAT-3′ (antisense) (186 bp). To analyze the gene
expression, the qRT-PCR mixture system containing the cDNA
templates, primers and SYBR-Green qPCR Master Mix was subjected to
qRT-PCR quantification according to the standard methods. β-actin
and U6 snRNA were used as the internal control of the mRNA or
miRNA, respectively. Relative gene expression was quantified by the
2−ΔΔCt method.
Northern blot analysis
miR-449 expression levels in PTC samples, adjacent
normal tissues, PTC cell lines, and human thyroid epithelial cell
lines were further determined by Northern blot assay. Northern blot
analysis was performed according to previously described procedures
(20).
Cell cycle analysis and cell
proliferation assay
Cells (5×103) were plated into each well
of 96-well plates. Six hours later, the cells were treated with
miR-control or miR-449 mimic. The procedures of cell cycle analysis
were previously described (21).
At 24, 48, 72, 96 and 120 h, cell numbers were determined by
Scepter™ 2.0 handheld automated cell counter.
Western blotting
Whole cells were lysed in lysis buffer (Beyotime
Institute of Biotechnology, Haimen, China) supplemented with 1 mM
phenylmethanesulfonyl fluoride (PMSF). The protein concentration
was determined using the BCA protein assay (Tiangen Biotech, Co.,
Ltd., Beijing, China). Twenty micrograms of protein in each sample
was separated by 12% SDS-PAGE and electrotransferred to
polyvinylidene dluoride (PVDF) membranes (Millipore, Billerica MA,
USA) for immunoblotting. The following primary antibodies were
used: anti-RET (1:1,000, ab134100; Abcam, Shanghai, China),
anti-Ki-67 (1:1000, ab15580; Abcam), anti-proliferating cell
nuclear antigen (PCNA) (1:500, ab18197; Abcam), anti-β-catenin
(1:4000, ab16051; Abcam) and anti-GAPDH (1:2500, ab9485; Abcam),
which was used as the internal reference. After incubation with the
appropriate horseradish peroxidase (HRP)-conjugated secondary
antibody, proteins were detected using a ChemiDoc XRS imaging
system and Quantity One analysis software (Bio-Rad Laboratories,
San Francisco, CA, USA).
Luciferase reporter assay
The cDNA of RET was amplified by PCR, and general
method of recombinant DNA was used to clone the wild-type (WT)
3′-UTR and mutant (MUT) 3′-UTR of RET. The WT and MUT sequences of
RET were then cloned into a pMIR-REPORT luciferase reporter vector
(Ambion, Austin, TX, USA) to generate constructs of Luc-RET and
Luc-RET-mut, followed by DNA sequencing verification. The
pMIR-REPORT control vector (Luc-control) was also cloned. All three
vectors were transfected into TPC-1cells/IHH-4 cells in 6-well
plates with a Lipofectamine 2000 reagent kit (Sigma-Aldrich, St.
Louis, MO, USA). After 1 day, the luciferase activity was measured
using a luciferase reporter assay system (Promega, Madison, WI,
USA). All relative luciferase activities were normalized to the
condition of Luc-control with β-galactosidase transfection.
siRNA transfection
The small interfering RNA (siRNA), to genetically
downregulate RET (RET-siRNA), as well as its non-specific scramble
siRNA (NC-siRNA), were purchased from Guangzhou RiboBio Co., Ltd.
(Guangzhou, China). Transfection of siRNAs into TPC-1 cells was
conducted using Lipofectamine 2000 reagent kit. In TPC-1 cells, 100
nM siRNAs (RET-siRNA and NC-siRNA) were used. The efficiency of
siRNA knockdown was confirmed by western blot analysis 48 h after
transfection.
RET and β-catenin overexpression
RET and β-catenin over-expression was achieved by
PCR amplification using their cDNA as templates, separately, and
the RET and β-catenin expressing vectors were constructed by
inserting their cDNA into pcDNA 3.1 vector. The recombinant
plasmids and other agents were co-transfected into 3×106
TPC-1 cells using a nucleofector instrument. Forty-eight hours
later, subsequent experiments were performed on the cells. The
experiment was replicated thrice for data calculations.
Immunofluorescence staining
Fluorescent cells were cultured on 8-well chamber
CultureSlides (Becton Dickinson, Bedford, MA, USA). After 8 h,
cells were fixed in 3% paraformaldehyde in PBS at room temperature
for 8 min, then permeabilized with 0.2% Triton X-100 for 15 min at
room temperature. After washing in PBS, the cells were incubated
with primary mouse anti-β-catenin monoclonal antibody (1 mg/ml;
Transduction Laboratories, Lexington, KY, USA) at 4°C overnight.
After washing, cells were incubated with biotinylated goat
anti-mouse IgG (Pierce, Rockford, IL, USA) at room temperature for
1 h. The immunoreactivity was revealed using Alexa568-conjugated
streptavidin (Molecular Probes, Eugene, OR, USA), and cells were
counterstained with 10 mg/ml DAPI. The cells were examined under a
Nikon fluorescence microscope (Image Systems, Columbia, MD,
USA).
TOP-FLASH/FOP-FLASH luciferase reporter
assay
The assay was conducted according to a previous
report (22), where each well was
incubated with a mixture containing 20 μl of serum-free DMEM, 0.6
μl of FuGENE, 0.15 μg of the firefly luciferase reporter plasmid,
0.15 μg of the β-catenin expression vector, 0.15 μg of the TCF-4
expression vector, and 0.8 ng of the Renilla luciferase
vector phRG-TK. Then, 24 h after transfection, the cells were lysed
in 50 μl of passive lysis buffer, and the luciferase activity was
determined with a luminometer using the Dual-Luciferase assay
aystem (Promega) on 20 μl of lysate. Results were expressed as fold
induction. Fold induction was determined by normalizing each
firefly luciferase value to the Renilla luciferase internal
control value and by dividing these normalized values with the mean
normalized value of the corresponding reporter construct
transfected with the empty expression vectors.
PTC xenografts
Male athymic nude mice were housed and manipulated
according to the protocols approved by the Experimental Animal
Center of the Second Affiliated Hospital of Xi’an Jiaotong
University. For each mouse, 5×106 miR-449-overexpressing
TPC-1 cells were injected subcutaneously into the right scapula in
100 serum-free medium. After the development of a palpable tumor,
the tumor volume was monitored every 5 days and assessed by
measuring the 2 perpendicular dimensions using a caliper and the
formula (a × b2)/2, where a is the larger and b is the
smaller dimension of the tumor. At 25 days after the inoculation,
the mice were sacrificed and tumor weights were assessed. A portion
of each tumor was selected for western blotting for RET and key
components of the Wnt/β-catenin pathway and qRT-PCR analysis for
miR-449.
Statistical analysis
All results were presented as mean ± SD from a
minimum of 3 replicates. Differences between groups were evaluated
by the SPSS version 15.0 statistical software with Student’s t-test
when comparing only 2 groups or assessed by one-way ANOVA when
>2 groups were compared. For the comparison of paired tissues, a
paired Student’s t-test was used to determine statistical
significance. The relationship between RET and miR-449 expression
was explored by Spearman’s correlation. Differences were considered
statistically significant at P<0.05.
Results
The levels of miR-449 expression are
downregulated in PTC tissues and cell lines
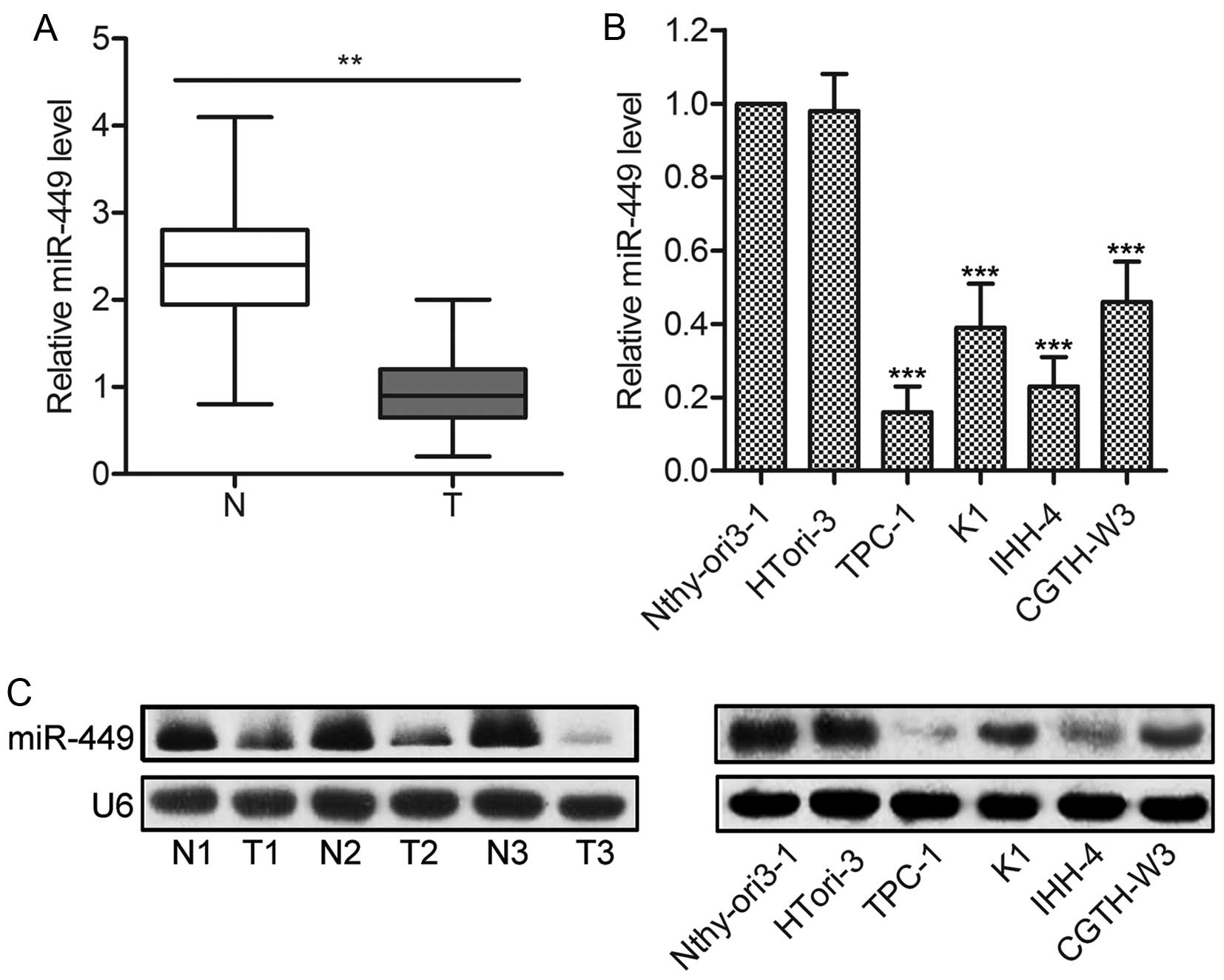
Firstly, the expression of miR-449 was measured in
20 fresh-frozen PTC specimens and adjacent normal tissues. The
results of qPCR showed that the average expression levels of
miR-449 were significantly lower in PTC tissues than in adjacent
normal tissues (P<0.01; Fig.
1A). Consistently, miR-449 expression was also decreased in PTC
cell lines, such as TPC-1, CGTH-W3, K1 and IHH-4, compared with the
non-cancerous human TECs (P<0.001; Fig. 1B). Northern blot analysis was used
to further confirm the decreased expression of miR-449 in PTC
specimens and cell lines (Fig.
1C). Taken together, these results provide novel evidence for
miR-449 downregulation in human PTC tissues and cell lines.
miR-449 overexpression suppresses the
proliferation of PTC cells
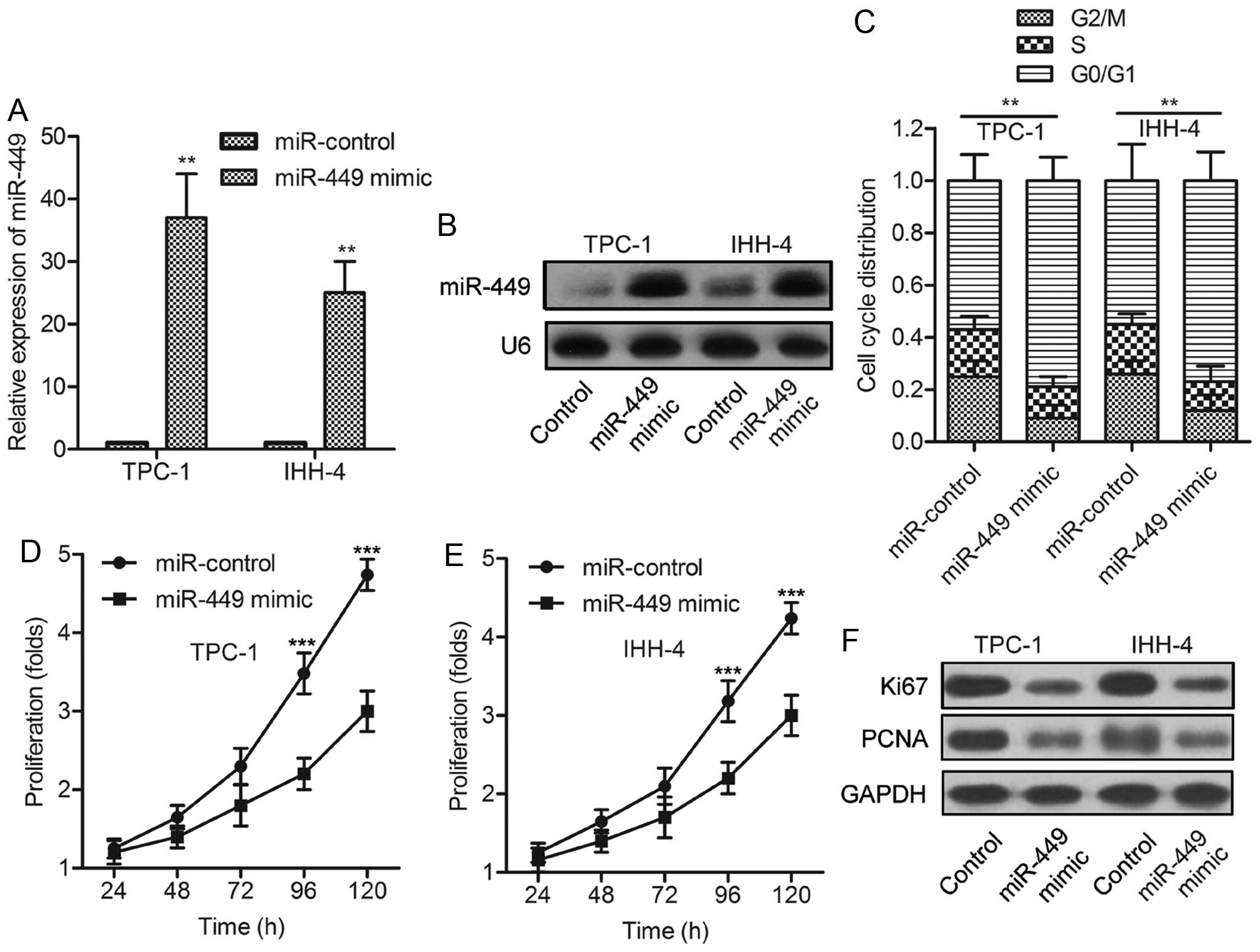
To determine whether miR-449 regulates PTC cell
growth, a synthetic activator specific for miR-449 was employed to
suppress the expression of endogenous miR-449. The efficiency of
this miR-449 activator was confirmed by qPCR assay and Northern
blot analysis (Fig. 2A and B).
FACS analysis indicated that miR-449 overexpression induced an
accumulation in G0/G1 phase (Fig.
2C), implying the cell cycle arrest of PTC cells. The treatment
of miR-449 mimic decreased the proliferation rates of TPC-1 and
IHH-4 cells (Fig. 2D and E).
Moreover, Ki-67, a mitosis-associated nuclear antigen in
proliferating cells, and PCNA were consistently found to be
underexpressed in TPC-1 and IHH-4 cells treated with miR-449 mimic
(Fig. 2F). The above data
indicated that miR-449 overexpression inhibits the proliferation of
PTC cells in vitro.
RET is a direct target of miR-449
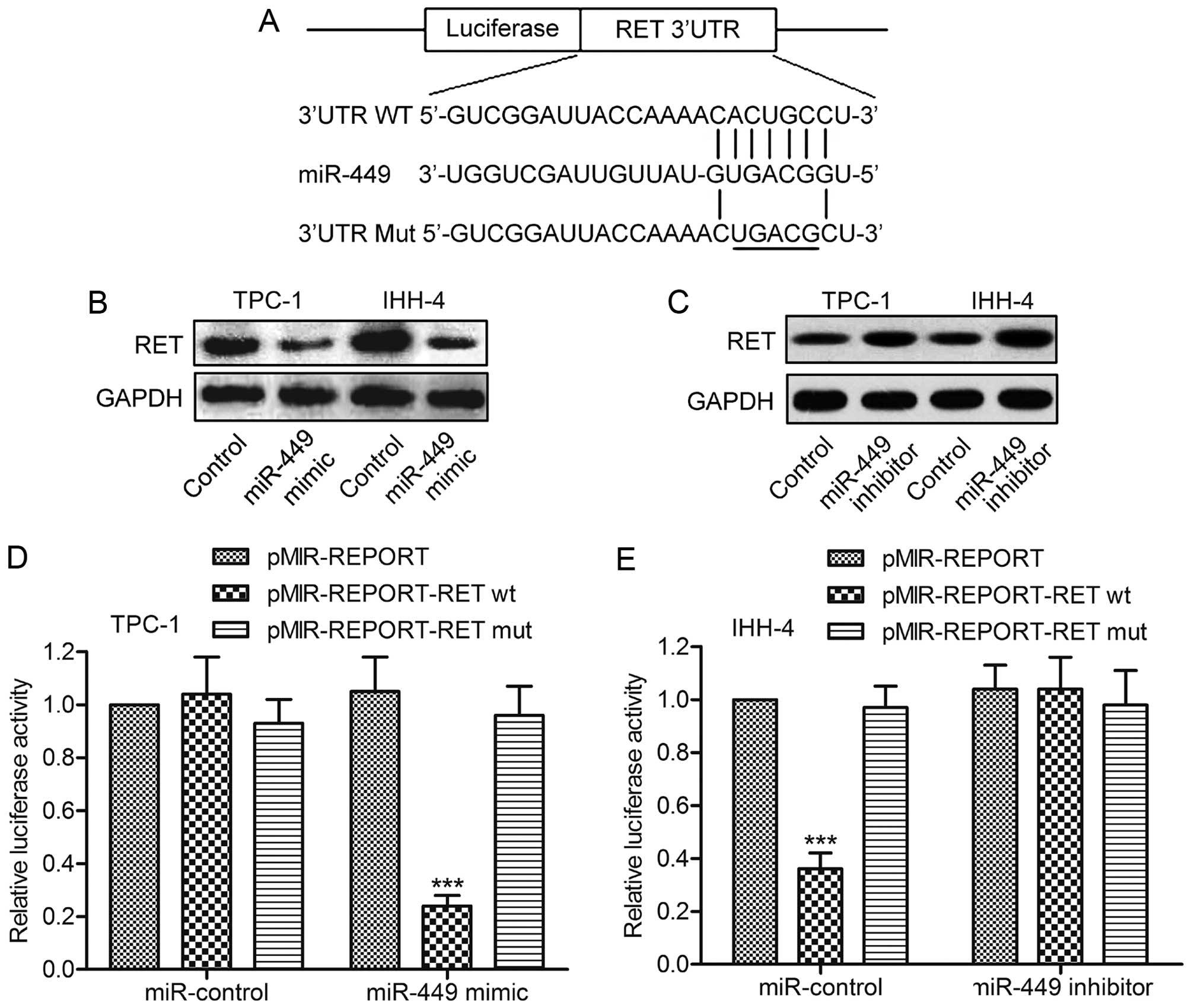
To investigate the molecular mechanisms of miR-449
in PTC cell growth, putative miR-449 targets were predicted using
TargetScan. The results revealed that RET was a potential target of
miR-449. The 3′-UTR of RET mRNA contains a complementary site for
the seed region of miR-449 (Fig.
3A). To experimentally confirm RET as an authentic target of
miR-449, the expression level of miR-449 was changed in TPC-1 and
IHH-4 cells, followed by immunoblot analysis of RET levels. The
data indicated that miR-449 overexpression caused the decline of
RET expression, whereas miR-449 inhibition induced the restoration
of RET (Fig. 3B and C). To further
verify the regulatory effect of miR-449 on RET, the plasmid
pMIR-REPORT-RETwt or pMIR-REPORT-RETmut was transfected into TPC-1
cells together with miR-449 mimics. Luciferase expression levels by
pMIR-REPORT-RETwt, but not pMIR-REPORT-RETmut, were found to be
suppressed by synthetic miR-449 mimics (Fig. 3D). Consistently, miR-449 inhibitor
rescued the reduction in the expression level of luciferase with
wild-type RET 3′-UTR in U-87 MG glioma cells (Fig. 3E). Taken together, these results
support the bioinformatics predictions indicating the RET 3′-UTR to
be a direct target of miR-449.
RET is involved in miR-449-induced PTC
cell growth promotion
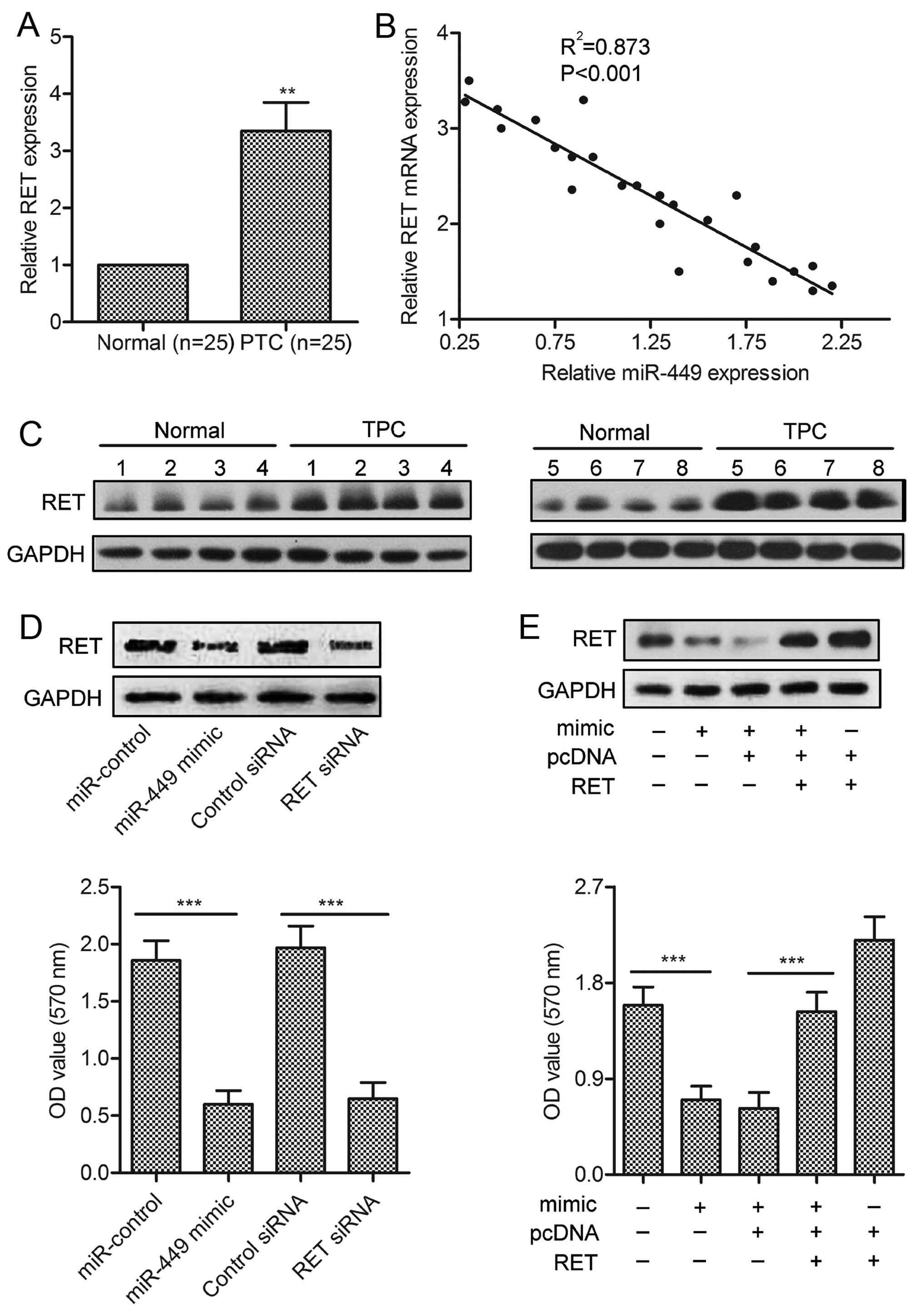
To investigate whether RET is a functional target of
miR-449, firstly, we measured the expression of RET in 25 pairs of
clinical PTC samples using western blotting and qRT-PCR. Compared
with paired normal tissues, PTC showed significantly higher RET
expression (P<0.01; Fig. 4A and
B). We also analyzed the correlation between RET level and
miR-449 expression in the same patients. As shown in Fig. 4C, a significant inverse correlation
was observed (P<0.01, R=0.873). We further performed
loss-of-function and gain-of-function studies by transfecting siRNA
or RET plasmids in TPC-1 cells. As shown in Fig. 4D, siRNA against RET significantly
inhibited cell growth, which is similar to those induced by
miR-449. Besides, overexpression of RET could abolish the
inhibitive effect of miR-449 (Fig.
4E).
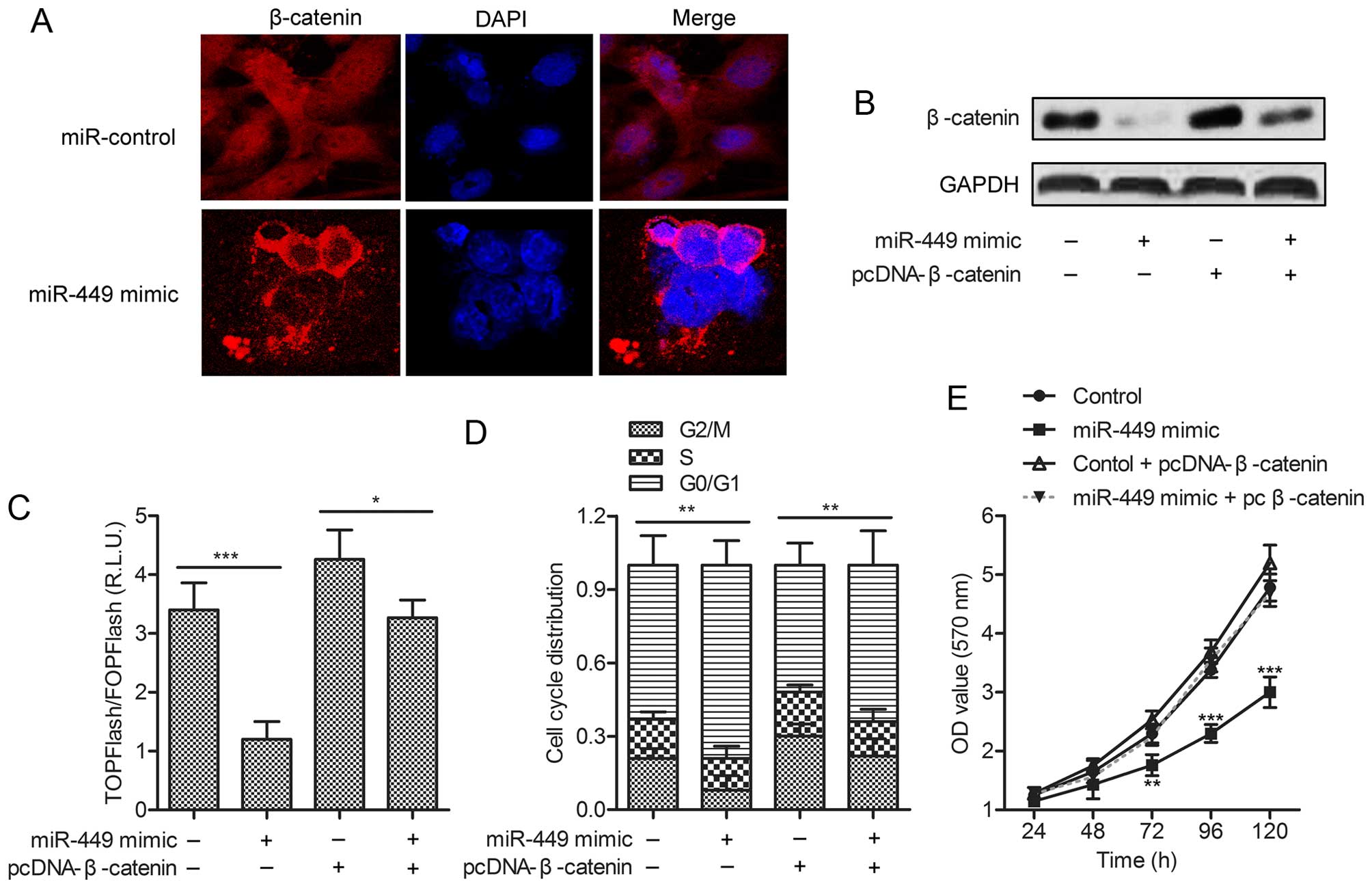
Overexpression of miR-449 suppresses
β-catenin nuclear translocation
To further explore the mechanism of G-17 on the
growth of TPC-1 cells, the subcellular localization of β-catenin
was measured. Cells in miR-control group displayed cytoplasmic and
nuclear staining of β-catenin, whereas PGL group showed membrane
β-catenin staining with minimal cytoplasmic or nuclear staining
(Fig. 5A). To determine whether
miR-449 could regulate Wnt/β-catenin signaling, gain of-function
analyses were performed by overexpressing β-catenin in TPC-1 cells
transfected with miR-449 mimic (Fig.
5B). TPC-1 cells were transiently transfected with the Wnt
signaling reporter TOPFlash or the negative control FOPFlash, along
with miR-449 mimic or miR-control. The results indicated that
TCF/LEF transcriptional activity was significantly decreased in
miR-449-overexpressing cells, whereas this effect could be promoted
by pcDNA-β-catenin (Fig. 5C). Flow
cytometric analysis demonstrated that G0/G1 arrest was prevented in
miR-449 overexpressing cells by pcDNA-β-catenin (Fig. 5D). Shown by MTT assays,
over-expressing RET level could restore the proliferation rates of
TPC-1 cells treated with miR-449 mimic (Fig. 5E). These data clearly suggested
that miR-449 inhibited the nuclear translocation of β-catenin to
inactivate the β-catenin pathway in TPC-1 cells.
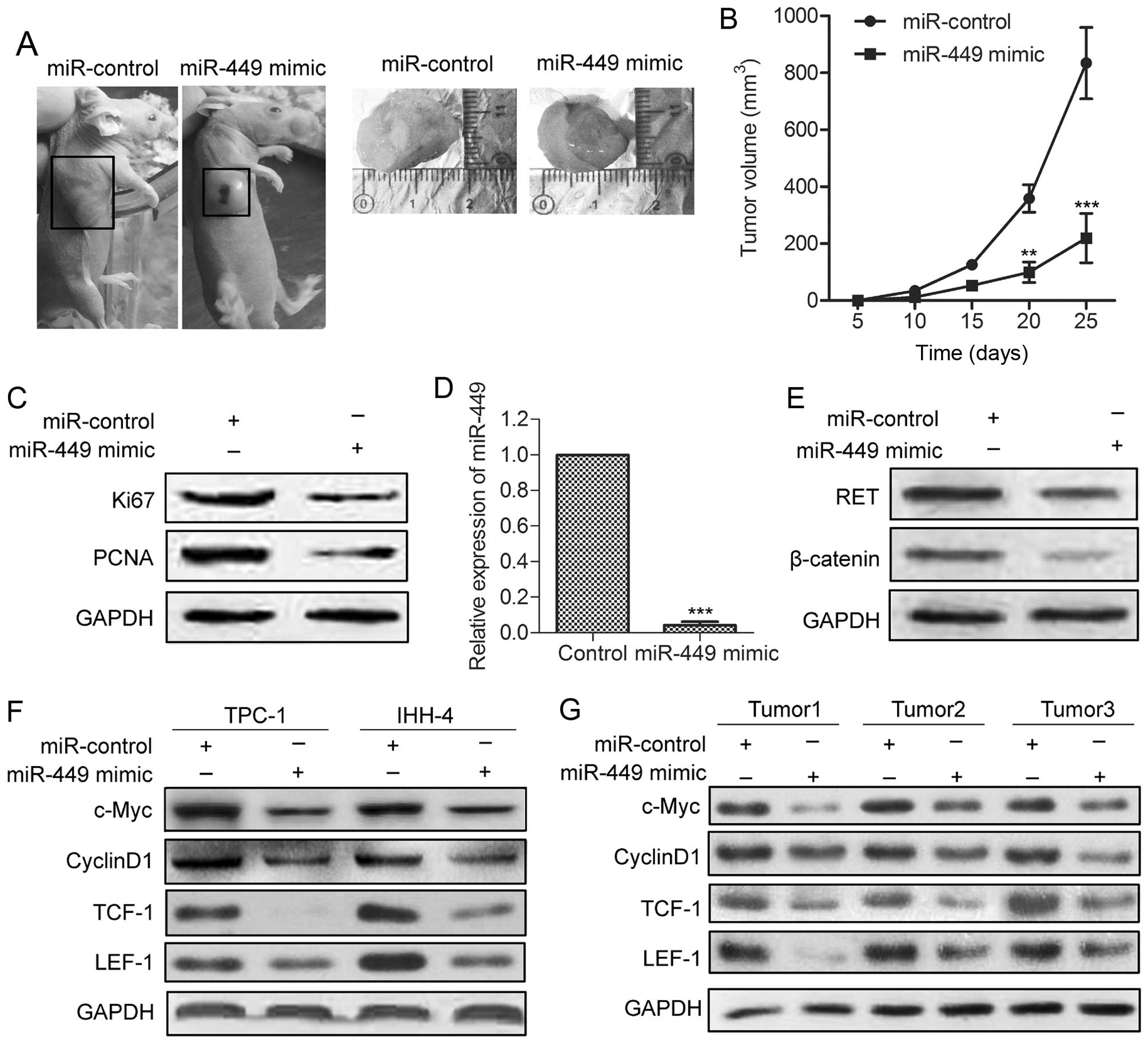
miR-449 reduces tumor growth and the
expression of key downstream genes of β-catenin in xenograft models
of PTC
To further investigate the inhibitive effect of
miR-449 on tumor growth in vivo, miR-449-overexpressing and
control TPC-1 cells were injected subcutaneously into the right
scapula of each mouse (n=5). The tumor growth of the
miR-449-over-expressing tumors was significantly suppressed
(Fig. 6A). The average tumor
volume of miR-449-overexpressing tumors was lower compared with
control tumors (Fig. 6B). The
expression of Ki-67 and PCNA was significantly decreased compared
with control group (Fig. 6C).
qRT-PCR and western blot analyses revealed elevated miR-449 with
decreased RET and β-catenin levels in miR-449-overexpressing tumors
(Fig. 6D and E). Finally, we
examined the effect of miR-449 mimic on the expression of c-Myc,
cyclin D1, TCF-1 and LEF-1, which are key downstream genes of
Wnt/β-catenin signaling in tumor growth. As shown in Fig. 6F and 6G, the levels of these
molecules were all decreased in TPC-1 and IHH-4 cells stably
overexpressing miR-449 in vitro, and in
miR-449-over-expressing tumors. These findings indicate that
miR-449 inhibits tumor growth and the expression of Wnt/β-catenin
downstream genes in human PTC cells.
Discussion
The downregulation of miR-449 has been observed
frequently in a variety of human malignancies, including lung
(23), gastric cancers (11) and colon cancer stem cells (24). In accordance, the decreased
expression of miR-449 was observed in human PTC tissues and cell
lines in the present study. However, to date, the relationship
between miR-449 and PTC is not fully understood. In this study, we
demonstrated for the first time that miR-449 inhibits tumor growth
of PTC by targeting a novel target, RET, which leads to the
inactivation of β-catenin signaling.
Researchers have been convinced that aberrant miRNA
expression is closely related to the changes of cell function. Bou
Kheir et al (11) suggested
that the deregulation of miR-449 not only leads to deregulated
control of cell cycle proteins, but also of growth factors and
their receptors. For instance, MCF-7 cells treated with miR-449
mimics were completely arrested at G1 phase. In gastric cancer
cells, miR-449 induces apoptosis by inhibiting histone deacetylase,
leading to p53 pathway activation and thus, the induction of
apoptosis markers (11). In
agreement with these reports, miR-449 overexpression in this study
induced an accumulation in G0/G1 phase and decreased the
proliferation of TPC-1 and IHH-4 cells. Thus, our results suggest
that miR-449 plays an inhibitive role in human PTC cell growth.
To date, several targets of miR-449 have been
identified, such as AREG, CDK6 and HDAC1 (25,26).
On the basis of bioinformatics analysis, we further predicted
another miR-449 target, RET. Reports have indicated that mutations
in RET induce papillary carcinoma and familial medullary thyroid
carcinoma in lung (27) and
thyroid cancer (28). RET has been
evidenced as an oncogene in a number of cancers, including head and
neck squamous cell carcinoma (29), thyroid (30) and lung cancer (31). From the aspect mentioned above,
targeting hNUDC with miR-449 may offer a novel strategy to prevent
the oncogenic effect of RET in PTC. Our present experimental
results confirmed that RET is a functional target of miR-449 in PTC
cells. There are several pieces of evidence to support this.
Firstly, confirmed by luciferase-reporter gene assays, miR-449
overexpression significantly downregulated RET by directly
targeting the 3′-UTR of RET mRNA. This effect was largely
eliminated when sites in the RET 3′-UTR targeted by miR-449 were
mutated. Also, the expression of RET was significantly decreased in
miR-449-overexpressing cells. Moreover, a significant inverse
correlation was observed between RET and miR-449 expression in
patients. We further found that the knockdown of RET inhibited cell
growth similar to the phenotypes induced by miR-449 mimics, whereas
RET overexpression could abolish the growth inhibitive effect of
miR-449. These results strongly suggest that miR-449-reduced cell
growth is partly mediated by the repression of RET expression.
Reports indicate that abnormal expression or
localization of β-catenin has been found in different tumor types
(32,33), and loss of cell-cell adhesion
mediated by β-catenin has been implicated in anchorage-independent
cell growth (19). The nuclear
localization of β-catenin may be an important marker of tumor
progression or advanced disease in human medullary thyroid
carcinoma. According to the report by Gujral et al (19), through RET-mediated tyrosine
phosphorylation, β-catenin escapes cytosolic downregulation by the
adenomatous polyposis coli/Axin/glycogen synthase kinase-3 complex
and accumulates in the nucleus, where it can stimulate
β-catenin-specific transcriptional programs in a RET-dependent
fashion. Their research evidenced that RET-induced activation of
the β-catenin pathway may contribute to cell proliferation and
tumorigenesis in thyroid carcinoma. Consistent with that report,
our results demonstrate that miR-449-mediated downregulation of RET
leads to inactivation of Wnt/β-catenin signaling in PTC cells.
First, miR-449 overexpression inhibited β-catenin accumulation and
nuclear translocation. Second, miR-449 overexpression increased
TCF/LEF transcriptional activity, and this effect was supported by
the observation that overexpression of β-catenin prevented
miR-449–reduced PTC viability. Moreover, miR-449 overexpression was
found to enhance the expression of several β-catenin downstream
genes in human PTC patients and cells. These results indicated that
miR-449 inhibited the nuclear translocation of β-catenin to
inactivate the Wnt pathway in TPC-1 cells.
Upregulation of β-catenin occurs in various cancers,
namely hepatocellular carcinoma (34), prostate cancer (35) and cervical carcinoma (36). Increased free β-catenin pools have
been observed in thyroid carcinomas secondary to reduced E-cadherin
expression (19). Elevated levels
of β-catenin were also reported in human PTC cells, and it has been
suggested as a key molecule implicated in tumorigenesis in PTC
(8). In medullary thyroid
carcinoma cells, RET induces β-catenin-mediated transcription, cell
proliferation and transformation in vitro and β-catenin
nuclear localization and the resultant RET-mediated β-catenin
signaling appears to be a key secondary event in tumor growth and
spreading in vivo. Our findings in this study demonstrate a
key role of miR-449 for β-catenin activation in human PTC cells.
Thus, miR-449 over-expression is a new mechanism that inactivates
β-catenin in human PTC via targeting RET.
In conclusion, this study demonstrated that miR-449
was generally downregulated in both primary carcinoma and PTC cell
lines. Overexpression of miR-218 reduced proliferation of PTC cells
and reduced tumor growth of PTC cells in vivo. We also
showed that RET was directly involved in the regulation of miR-218
during PTC development, possibly through β-catenin signaling
pathway. Thus, the present study suggests that miR-449 may be a
potential target for future prevention and treatment of human
PTC.
Acknowledgements
The present study was funded by the Science and
Technology Plan Project of Social Development Plans for Public
Relations of Shaanxi Province, China (nos. 2012SF2-13 and
2015SF068).
Abbreviations:
|
PTC
|
papillary thyroid carcinoma
|
|
miR
|
microRNA
|
|
RET
|
RET proto-oncogene
|
|
PCNA
|
proliferating cell nuclear antigen
|
|
TCF
|
T cell-specific transcription
factor
|
|
LEF-1
|
lymphoid enhancer-binding factor 1
|
References
|
1
|
Powell DJ Jr, Russell J, Nibu K, Li G,
Rhee E, Liao M, Goldstein M, Keane WM, Santoro M, Fusco A, et al:
The RET/PTC3 oncogene: Metastatic solid-type papillary carcinomas
in murine thyroids. Cancer Res. 58:5523–5528. 1998.PubMed/NCBI
|
|
2
|
Nikiforova MN, Kimura ET, Gandhi M,
Biddinger PW, Knauf JA, Basolo F, Zhu Z, Giannini R, Salvatore G,
Fusco A, et al: BRAF mutations in thyroid tumors are restricted to
papillary carcinomas and anaplastic or poorly differentiated
carcinomas arising from papillary carcinomas. J Clin Endocrinol
Metab. 88:5399–5404. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hong DS, Cabanillas ME, Wheler J, Naing A,
Tsimberidou AM, Ye L, Busaidy NL, Waguespack SG, Hernandez M, El
Naggar AK, et al: Inhibition of the Ras/Raf/MEK/ERK and RET kinase
pathways with the combination of the multikinase inhibitor
sorafenib and the farnesyltransferase inhibitor tipifarnib in
medullary and differentiated thyroid malignancies. J Clin
Endocrinol Metab. 96:997–1005. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Nikiforov YE and Nikiforova MN: Molecular
genetics and diagnosis of thyroid cancer. Nat Rev Endocrinol.
7:569–580. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yu H, Lu Y, Li Z and Wang Q: microRNA-133:
Expression, function and therapeutic potential in muscle diseases
and cancer. Curr Drug Targets. 15:817–828. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhi F, Wang Q, Deng D, Shao N, Wang R, Xue
L, Wang S, Xia X and Yang Y: MiR-181b-5p downregulates NOVA1 to
suppress proliferation, migration and invasion and promote
apoptosis in astrocytoma. PLoS One. 9:e1091242014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Croce CM: Causes and consequences of
microRNA dysregulation in cancer. Nat Rev Genet. 10:704–714. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhang X, Li M, Zuo K, Li D, Ye M, Ding L,
Cai H, Fu D, Fan Y and Lv Z: Upregulated miR-155 in papillary
thyroid carcinoma promotes tumor growth by targeting APC and
activating Wnt/β-catenin signaling. J Clin Endocrinol Metab.
98:E1305–E1313. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Brest P, Lassalle S, Hofman V, Bordone O,
Gavric Tanga V, Bonnetaud C, Moreilhon C, Rios G, Santini J, Barbry
P, et al: MiR-129-5p is required for histone deacetylase
inhibitor-induced cell death in thyroid cancer cells. Endocr Relat
Cancer. 18:711–719. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Geraldo MV, Fuziwara CS, Friguglieti CU,
Costa RB, Kulcsar MA, Yamashita AS and Kimura ET: MicroRNAs
miR-146-5p and let-7f as prognostic tools for aggressive papillary
thyroid carcinoma: A case report. Arq Bras Endocrinol Metabol.
56:552–557. 2012. View Article : Google Scholar
|
|
11
|
Bou Kheir T, Futoma-Kazmierczak E,
Jacobsen A, Krogh A, Bardram L, Hother C, Grønbæk K, Federspiel B,
Lund AH and Friis-Hansen L: miR-449 inhibits cell proliferation and
is down-regulated in gastric cancer. Mol Cancer. 10:12011.
View Article : Google Scholar
|
|
12
|
Trupp M, Arenas E, Fainzilber M, Nilsson
AS, Sieber BA, Grigoriou M, Kilkenny C, Salazar-Grueso E, Pachnis
V, Arumäe U, et al: Functional receptor for GDNF encoded by the
c-ret proto-oncogene. Nature. 381:785–789. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gujral TS, van Veelen W, Richardson DS,
Myers SM, Meens JA, Acton DS, Duñach M, Elliott BE, Höppener JW and
Mulligan LM: A novel RET kinase-beta-catenin signaling pathway
contributes to tumorigenesis in thyroid carcinoma. Cancer Res.
68:1338–1346. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Marx SJ: Molecular genetics of multiple
endocrine neoplasia types 1 and 2. Nat Rev Cancer. 5:367–375. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Arighi E, Borrello MG and Sariola H: RET
tyrosine kinase signaling in development and cancer. Cytokine
Growth Factor Rev. 16:441–467. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hoppler S and Kavanagh CL: Wnt signalling:
Variety at the core. J Cell Sci. 120:385–393. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Moon RT, Kohn AD, De Ferrari GV and Kaykas
A: WNT and beta-catenin signalling: Diseases and therapies. Nat Rev
Genet. 5:691–701. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
18
|
Garcia-Rostan G, Camp RL, Herrero A,
Carcangiu ML, Rimm DL and Tallini G: Beta-catenin dysregulation in
thyroid neoplasms: Down-regulation, aberrant nuclear expression,
and CTNNB1 exon 3 mutations are markers for aggressive tumor
phenotypes and poor prognosis. Am J Pathol. 158:987–996. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Gujral TS, van Veelen W, Richardson DS,
Myers SM, Meens JA, Acton DS, Duñach M, Elliott BE, Höppener JW and
Mulligan LM: A novel RET kinase-β-catenin signaling pathway
contributes to tumorigenesis in thyroid carcinoma. Cancer Res.
68:1338–1346. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Liu J, Ma L, Li C, Zhang Z, Yang G and
Zhang W: Tumor-targeting TRAIL expression mediated by miRNA
response elements suppressed growth of uveal melanoma cells. Mol
Oncol. 7:1043–1055. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ma L, Liu J, Liu L, Duan G, Wang Q, Xu Y,
Xia F, Shan J, Shen J, Yang Z, et al: Overexpression of the
transcription factor MEF2D in hepatocellular carcinoma sustains
malignant character by suppressing G2-M transition genes. Cancer
Res. 74:1452–1462. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Gilles C, Polette M, Mestdagt M,
Nawrocki-Raby B, Ruggeri P, Birembaut P and Foidart JM:
Transactivation of vimentin by β-catenin in human breast cancer
cells. Cancer Res. 63:2658–2664. 2003.PubMed/NCBI
|
|
23
|
Luo W, Huang B, Li Z, Li H, Sun L, Zhang
Q, Qiu X and Wang E: MicroRNA-449a is downregulated in non-small
cell lung cancer and inhibits migration and invasion by targeting
c-Met. PLoS One. 8:e647592013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Fang Y, Gu X, Li Z, Xiang J and Chen Z:
miR-449b inhibits the proliferation of SW1116 colon cancer stem
cells through downregulation of CCND1 and E2F3 expression. Oncol
Rep. 30:399–406. 2013.PubMed/NCBI
|
|
25
|
Yang X, Feng M, Jiang X, Wu Z, Li Z, Aau M
and Yu Q: miR-449a and miR-449b are direct transcriptional targets
of E2F1 and negatively regulate pRb-E2F1 activity through a
feedback loop by targeting CDK6 and CDC25A. Genes Dev.
23:2388–2393. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Buurman R, Gürlevik E, Schäffer V, Eilers
M, Sandbothe M, Kreipe H, Wilkens L, Schlegelberger B, Kühnel F and
Skawran B: Histone deacetylases activate hepatocyte growth factor
signaling by repressing microRNA-449 in hepatocellular carcinoma
cells. Gastroenterology. 143:811–820.e15. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Takeuchi K, Soda M, Togashi Y, Suzuki R,
Sakata S, Hatano S, Asaka R, Hamanaka W, Ninomiya H, Uehara H, et
al: RET, ROS1 and ALK fusions in lung cancer. Nat Med. 18:378–381.
2012. View
Article : Google Scholar : PubMed/NCBI
|
|
28
|
Melillo RM, Castellone MD, Guarino V, De
Falco V, Cirafici AM, Salvatore G, Caiazzo F, Basolo F, Giannini R,
Kruhoffer M, et al: The RET/PTC-RAS-BRAF linear signaling cascade
mediates the motile and mitogenic phenotype of thyroid cancer
cells. J Clin Invest. 126:16032016. View
Article : Google Scholar :
|
|
29
|
Lin C, Lu W, Ren Z, Tang Y, Zhang C, Yang
R, Chen Y, Cao W, Wang L, Wang X, et al: Elevated RET expression
enhances EGFR activation and mediates EGFR inhibitor resistance in
head and neck squamous cell carcinoma. Cancer Lett. 377:1–10. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Bano G and Hodgson S: Diagnosis and
management of hereditary thyroid cancer. Recent Results Cancer Res.
205:29–44. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Drilon A, Bergagnini I, Delasos L, Sabari
J, Woo KM, Plodkowski A, Wang L, Hellmann MD, Joubert P, Sima CS,
et al: Clinical outcomes with pemetrexed-based systemic therapies
in RET-rearranged lung cancers. Ann Oncol. 27:1286–1291. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Bauman TM, Vezina CM, Ricke EA, Halberg
RB, Huang W, Peterson RE and Ricke WA: Expression and
colocalization of β-catenin and lymphoid enhancing factor-1 in
prostate cancer progression. Hum Pathol. 51:124–133. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Lan L, Deng W, Chen H, Huo L, Deng L,
Zhang G and Luo Y: Nuclear translocation of β-catenin represses
membrane localization of NIS in human thyroid cancer cells.
Zhonghua Yi Xue Za Zhi. 96:891–896. 2016.(In Chinese). PubMed/NCBI
|
|
34
|
Li T, Zhong J, Dong X, Xiu P, Wang F, Wei
H, Wang X, Xu Z, Liu F, Sun X, et al: Meloxicam suppresses
hepatocellular carcinoma cell proliferation and migration by
targeting COX-2/PGE2-regulated activation of the β-catenin
signaling pathway. Oncol Rep. 35:3614–3622. 2016.PubMed/NCBI
|
|
35
|
Lombardi AP, Pisolato R, Vicente CM,
Lazari MF, Lucas TF and Porto CS: Estrogen receptor beta (ERβ)
mediates expression of β-catenin and proliferation in prostate
cancer cell line PC-3. Mol Cell Endocrinol. 430:12–24. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Chen Q, Zheng PS and Yang WT:
EZH2-mediated repression of GSK-3β and TP53 promotes Wnt/β-catenin
signaling-dependent cell expansion in cervical carcinoma.
Oncotarget. Apr 15–2016.(Epub ahead of print). View Article : Google Scholar
|