Introduction
Colorectal cancer is the leading, and second leading
cause of death in developed and developing countries, respectively.
The survival rate of colorectal cancer patients has increased due
to the early diagnosis and treatment strategies (1). However, the 5-year survival rate
still remains at less than 60% (2). For colorectal cancer, surgery is the
primary treatment method. While during the later phases, for
example, the node-positive stage III, adjuvant chemotherapy is
necessary (1). Vincristine (VCR)
is widely used in tumor treatment. However, in colorectal cancer
chemotherapy treatment, the development of acquired
multidrug-resistance (MDR) to conventional chemotherapeutics has
been the main restriction (3,4). MDR
is associated with decrease of drug accumulation in cell due to
active energy-dependent efflux of drugs or metabolites (5–8).
Given this premise, novel treatment strategies which could help
overcome MDR, as well as increase tumor cell response to
chemotherapy drugs are greatly needed.
RLIP76 is a 76-kDa splice variant, which was encoded
by the human gene RALBP1 (18p11.22). It is a Ral-interacting
protein of 76 kDa, also known as RalBP1. It was identified as a Ral
GTPase effector protein that connects the Ral with Rho pathways
originally (9–11). This protein participates in the ATP
hydrolysis-dependent movement of substances, including glutathione
conjugates (GS-E) and chemotherapy drugs, out of cells (12–14).
GS-Es are toxic to the cells and need to be transported out of
cells in order to keep cells from death. As a result, RLIP76
mediates resistance to its substrates, which range from weakly
cationic compounds, such as doxorubicin (DOX), vinblastine (VBL),
vincristine (VCR), vinorelbine (VRL) (15–17),
colchicine, sunitinib and sorafenib (11,18),
to anionic metabolites, including glutathione conjugates of
electrophiles (19). Knockout of
RLIP76 with targeting antibodies or antisense molecules can be able
to increase the sensitivity to radiation and chemotherapy of tumors
and cause solid tumors regression in non-small cell lung cancer,
colorectal carcinomas (20),
prostate cancer (21), and B16
melanomas (22) in mice and
pancreatic cancer (23),
glioblastoma (24) in human. One
of the mechanisms is that knockout of RLIP76 increased cellular
accumulation of chemotherapy drugs.
Most early studies concentrated on research of the
transport functions of RLIP76, whereas growing evidence has shown
that RLIP76 is necessary in a variety of cellular functions, such
as mitosis, proliferation, differentiation, apoptosis and
endocytosis (25–27). It takes part in the formation of
multi-functional protein complexes, like the mitotic spindle and
the receptor signaling complexes of EGF, TGF-β, insulin and
clathrin-dependent endocytosis (28–30)
and determine the rate of receptor-ligand signaling. RLIP76 exists
in many human tissues, including liver, heart and ovary, but
overexpressed in various types of cancer cells, including lung and
ovarian carcinomas and melanomas (16,31,32).
Blocking RLIP76 with targeting antibodies or knockout RLIP76 with
antisense results in apoptosis in many types of cancer cells in
vitro (11,18,33–37),
and sensitivity to apoptosis on RLIP76 depletion in malignant cells
is greater than in non-malignant cells (22). RLIP76 belongs to Ras family and
transmits signals from Ral to the downstream protein, cdc42.
Activation of the Rho family G-protein cdc42, has been shown to
induce apoptosis (38). RLIP76 is
also involved in various cellular signaling pathways, such as
PI3K/Akt and Erk signaling pathway which regulates resistance to
chemo-radiotherapy and basal survival in a variety of cancers
(25–27). Phosphorylation of Erk and PI3K is
markedly and consistently decreased in all human kidney cancer cell
lines due to RLIP76 deletion (13). These data show that RLIP76 is a
potential target for tumor treatment, but the roles of RLIP76 in
colorectal cancer, especially in multidrug resistance (MDR) of
colorectal cancer are still unknown.
In the present study, we verified RLIP76 level in
MDR cancer cells and cancer cells without drug resistance. Then,
the function of RLIP76 on chemotherapy, migration, invasion,
apoptosis and signaling pathway was detected after knockdown of
RLIP76. Our findings provide important insights into VCR resistance
and highlight RLIP76 as a novel molecular target that can be
specifically inhibited to sensitize colorectal cancer cells to
VCR.
Materials and methods
Cell culture and reagents
The human colorectal cancer HCT-8 cell line and the
MDR HCT-8/V cell line were obtained from Nanjing KeyGen Biotech.
Co., Ltd (Nanjing, China). Cells were grown in RPMI-1640 medium
supplemented with 10% heat-inactivated fetal bovine serum (FBS), 2
mM glutamine, 100 μ/ml penicillin, and 100 ng/ml streptomycin
(Invitrogen, Carlsbad, CA, USA) at 37°C in a 5% CO2
humidified atmosphere. HCT-8/V cells were routinely maintained in a
medium containing 1000 ng/l VCR (vincristine sulfate; Dalian Meilun
Biotech Co., Ltd., Dalian, China) and incubated in a drug-free
medium for at least a week before use.
Drug sensitivity was determined by Cell
Counting kit-8 (CCK-8)
Cells were counted and plated into 96-well plates at
a density of 3×103 (1.2 μl) cells/well. The VCR used was
dissolved with RPMI-1640 medium and then diluted at different
concentrations including 5, 10, 50, 100, 200, 500 and 1000 μg/ml
with RPMI-1640 medium supplemented with 10% FBS, 2 mM glutamine,
100 μ/ml penicillin and 100 ng/ml streptomycin. Cells were cultured
overnight and then were cultured in the medium at various
concentrations of VCR for 48 h, then 20 μl of CCK-8 (Beyotime
Institute of Biotechnology, Haimen, China) was added to each well,
following incubation for 4 h at 37°C. Each solution was subjected
to spectrophotometry at 450 nm in a Multiskan Ascent microplate
reader (Thermo Fisher Scientific, Vantaa, Finland). The drug
sensitivity is expressed as the half maximal inhibitory
concentration (IC50) for each of the cell lines, which
represents the concentration of the drug that caused a 50%
reduction in the absorbance at 450 nm relative to the untreated
cells (control). GraphPad Prism 5 was used to calculate the
IC50. In cell proliferation assay, 5×103
cells were plated into 96-well plates. Each well contained medium
supplemented with 10% FBS. The cultures were stained using a Cell
Counting kit-8 at various time-points.
Transwell assay
Cell migration and invasion were detected by a
Transwell assay. Cells were starved overnight in serum-free medium,
trypsinized, and washed three times in RPMI-1640 medium without
FBS. For migration, 8.76 μl cells (1×105) were seeded
into the upper chambers in 200 μl serum-free media without Matrigel
membrane. In addition, the lower chambers were loaded with 600 μl
RPMI-1640 supplemented with 10% FBS. After 24 h, the cells in the
upper chambers that had not migrated were removed with a cotton
swab. For the invasion assay, colorectal cancer cells
(2×105) were seeded into the upper chambers with a
Matrigel (8-μm pore size; BD Biosciences, San Jose, CA, USA)
membrane and after 48 h, the cells in the upper chambers that had
not migrated were removed with a cotton swab. The cells on the
lower surface of the membrane were fixed in formaldehyde and
stained with hematoxylin staining solution. Then, the cells were
counted and photographed.
Western blot analysis
The total protein was extracted from the cancer
cells, and the proteins were separated by sodium dodecyl
sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). Crude
fraction containing 40 mg of proteins were subjected to SDS-PAGE
and proteins were transferred onto PVDF membrane. The detection of
β-actin (1:10,000; Santa Cruz Biotechnology, Santa Cruz, CA, USA)
on the same membrane was used as the internal control. Specific
antibodies for RLIP76 (ab133549; monoclonal, 1/10,000–1/50,000;
Abcam, Cambridge, MA, USA), caspase-3 (#9662; 1:1,000, polyclonal;
Cell Signaling Technology, Danvers, MA, USA), caspase-8 (#9746;
1:1,000; monoclonal; Cell Signaling Technology), caspase-9 (#9508;
1:1,000; monoclonal; Cell Signaling Technology), PARP (#9542;
1:1,000; monoclonal; Cell Signaling Technology), phosphorylated Erk
(#4370; 1:2,000, monoclonal; Cell Signaling Technology) and Erk
(#4695; 1:1,000, monoclonal; Cell Signaling Technology) were used
for the immunodetection of the corresponding proteins.
Subsequently, HRP-conjugated secondary antibodies (1:10,000;
Beijing Zhongshan Golden Bridge Biotechnology Co., Ltd., Beijing,
China), followed by enhanced chemiluminescence (Millipore Corp.,
Billerica, MA, USA), were used. The same amount of protein was used
each time.
Lentiviral infection and stable cell line
selection
The lenti-virus that encoded RLIP76-specific shRNA
and the scrambled shRNA lentivirus were generated by GenomeDitech
Co., Ltd., Shanghai, China. MDR colorectal cancer cells (HCT-8/V)
were infected with recombinant shRNA that was specific for RLIP76
lentiviral stocks or scrambled shRNA lentiviral stocks. qRT-PCR and
western blot analysis were used to select stable RLIP76 knockdown
cell lines (KD) and the control cell lines (NC). The lentiviral
vectors expressed the green fluorescent protein, which allowed for
the measurement of infection efficiency in the transfected
cells.
Quantitative RT-PCR (qRT-PCR)
analysis
The total cellular RNA was extracted with the TRIzol
(Takara Bio, Dalian, China) reagent and reverse transcribed to cDNA
according to the manufacturer’s protocols. The qPCR products were
detected with SYBR-Green (Takara) in a LightCycler® 480
Real-Time PCR System (Roche Diagnostics). The β-actin gene
was amplified as an internal control. The primers for RLIP76:
5′-ggCATgAAgTgTgAAggCATCTAC-3′ and 5′-CT CgCAAATACTgCTTCAgCAAAC-3′
were used for qPCR.
Statistical analysis
The data were evaluated with a two-tailed unpaired
Student’s t-test or with a two-tailed paired Student’s t-test.
Values with P<0.05 were considered to be statistically
significant.
Results
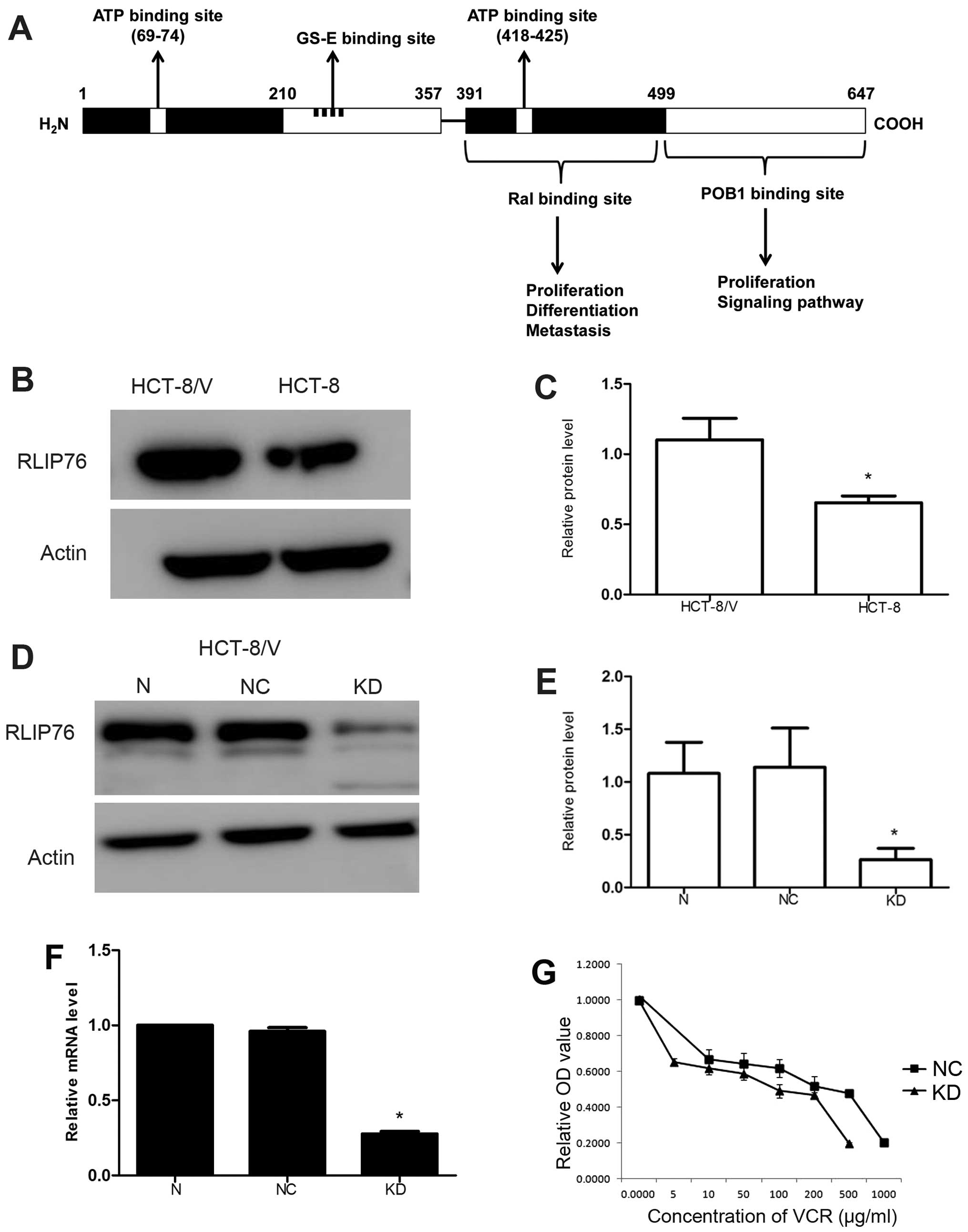
RLIP76 is overexpressed in HCT-8/V
RLIP76 contains ATP binding domain, GS-E binding
site, Ral binding domain and POB1 binding domain, which indicates
it may play a role in proliferation, metastasis, ATP-dependent
transport and apoptosis (Fig. 1A).
Vincristine (VCR) is one of the substrates of RLIP76. The level of
RLIP76 was detected by western blot analysis, and the HCT-8/V cell
line exhibited higher expression compared with that of the HCT-8
cell line (Fig. 1B and C;
P<0.05).
RLIP76-specific shRNA decreases RLIP76
expression in HCT-8/V
Lentiviral vector-mediated RNA interference
technology was used to infect the HCT-8/V cells with the negative
control (NC) and RLIP76-specific shRNA lentivirus (KD) to generate
stable cell lines. Transfection of HCT-8/V cells with
RLIP76-specific shRNA lentivirus (KD) markedly downregulated the
RLIP76 protein levels compared with NC cells to 0.264±0.106 as
determined by western blot analysis (Fig. 1D and E; P<0.05). No significant
difference of relative RLIP76 mRNA level was found by qRT-PCR
between cells without transfection and the NC cells. However,
relative RLIP76 mRNA level decreased to 0.277±0.016 (P<0.05) in
KD cells (Fig. 1F).
RLIP76 knockdown decreases the
IC50 of VCR in HCT-8/V
MDR cells often have decreased intracellular drug
accumulation because RLIP76 could transport VCR; thus, we verified
the capacity of the RLIP76-specific shRNA lentivirus to enhance the
sensitivity to VCR of HCT-8/V. The IC50 of VCR in
HCT-8/V cells decreased from 164.4±1.734 to 13.95±2.008 (μg/ml)
(P<0.05) in KD cells (Fig. 1G
and Table I).
 | Table IEffect of RLIP76 knockdown on VCR
cytotoxicity in HCT-8/V cells. |
Table I
Effect of RLIP76 knockdown on VCR
cytotoxicity in HCT-8/V cells.
| Cell line | IC50 of
VCR (μg/ml) |
|---|
| HCT-8/V | NC | 164.4±1.734 |
| KD | 13.95±2.008 |
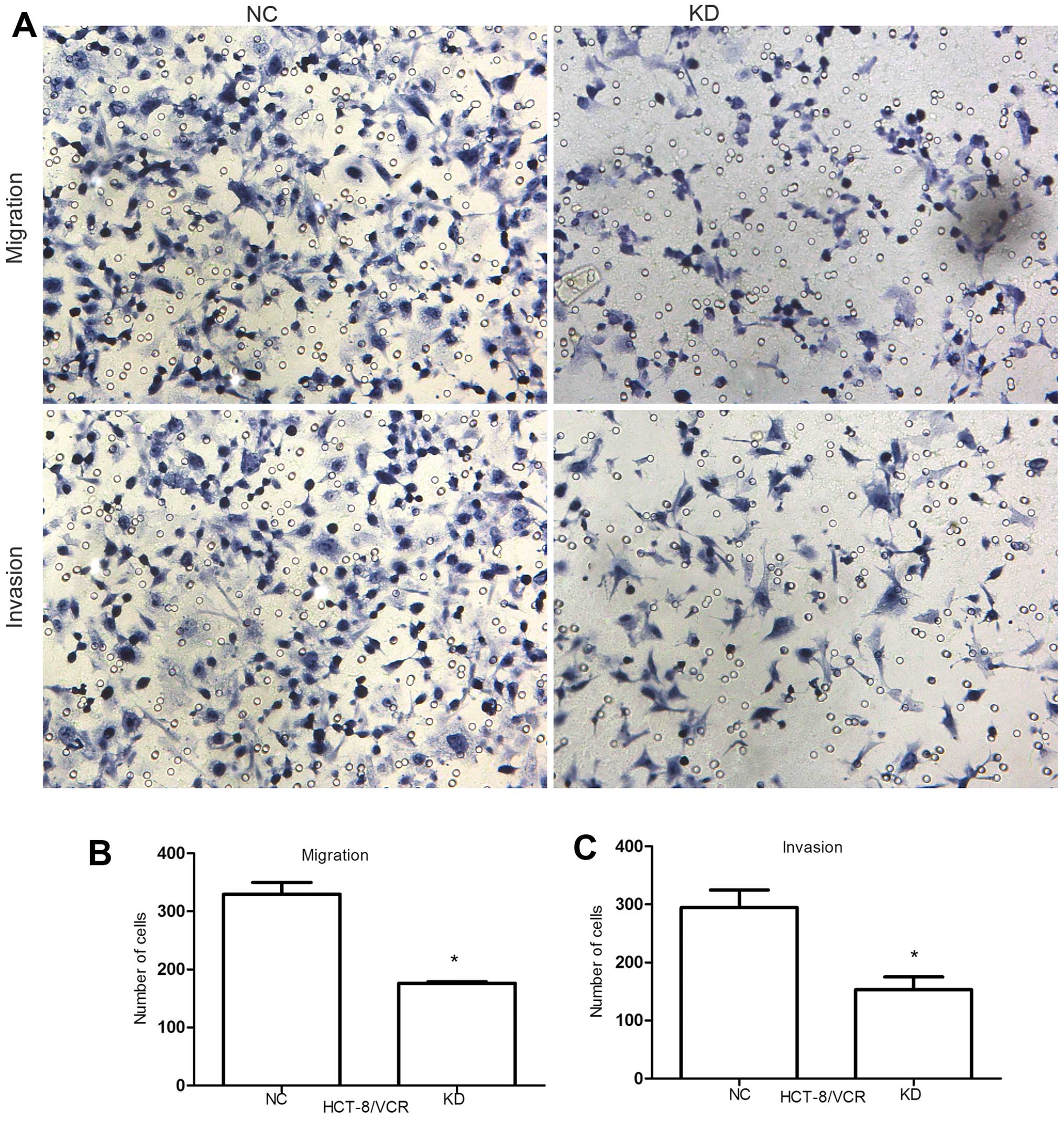
Knockdown of RLIP76 decreases the
migration and invasion of HCT-8/V cells
To assess the effect of RLIP76 knockdown on HCT-8/V
cell migration and invasion, we performed the in vitro
migration and invasion assays of KD HCT-8/V cells and the NC. Cells
that migrated across the membrane were quantified after incubation
for 24 h and 48 h respectively. Cell migration decreased from
329.67±20.23 to 176.33±2.52 (P<0.05) and cell invasion reduced
from 294.67±30.07 to 153±22.11 (P<0.05), suggesting that RLIP76
knockdown significantly suppressed the migration and invasion of
colorectal cancer cells (Fig.
2A–C). Before this, we performed another Transwell assay
compared with HCT-8 in migration and invasion. The migration and
invasion level did not decrease similarly to HCT-8 even though the
RLIP76 was knocked down (Fig.
2D).
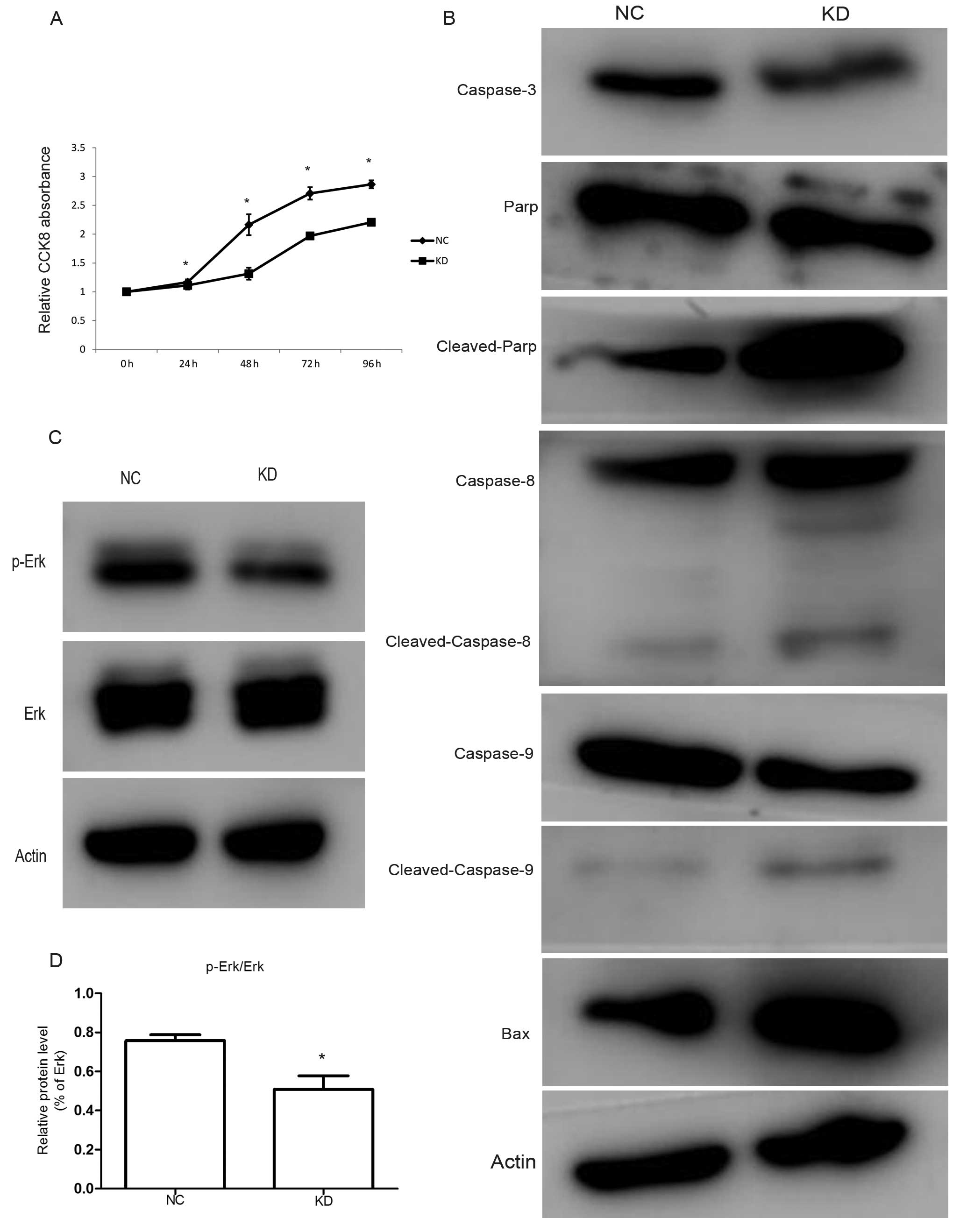
RLIP76 knockdown decreases growth and
increases apoptosis of HCT-8/V cells through downregulating Erk
phosphorylation
We performed a CCK-8 assay to investigate the
biological function of RLIP76 on cell proliferation. Knockdown of
RLIP76 decreased the growth of cancer cells (Fig. 3A; P<0.05). Apoptosis is a
process of programmed cell death that occurs in multi-cellular
organisms. In the present study, we analyzed the expression levels
of Bax, caspase-3, PARP, caspase-8 and caspase-9 by western blot
analysis. We found that the protein levels of caspase-3 decreased,
whereas the Bax, cleaved-PARP, cleaved-caspase-8 and
cleaved-caspase-9 increased in KD cells compared with the control
(Fig. 3B).
The MAPK signaling pathways are well-known as
important signaling pathways for cancer cell growth. To identify
the potential molecular mechanisms of RLIP76 knockdown in HCT-8/V
cell proliferation and apoptosis, we analyzed the expression levels
of signaling proteins by western blot analysis and found that
RLIP76 knockdown markedly reduced the phosphorylated-Erk from
75.8±3.02 to 50.8±7.02% (P<0.05), however, Erk protein level
remained constant (Fig. 3C and
D).
Discussion
Colorectal cancer is one of the most common causes
of cancer-related deaths in developed countries (39). For colorectal cancer, chemotherapy
is a vital prevention and treatment method. Even though significant
advances have been achieved in recent years, resistance to
chemotherapy is still a major problem (40). The main reasons for chemotherapy
failure are insufficient intratumoral drug concentration, intrinsic
overexpression of drug efflux transporters in tumor cells and tumor
microenvironment-related factors (9,41,42).
The present study used wild-type, HCT-8 and MDR colorectal cancer
cells, HCT-8/VCR, to investigate the molecular mechanisms and
cellular behavior involved in VCR resistance. A significant finding
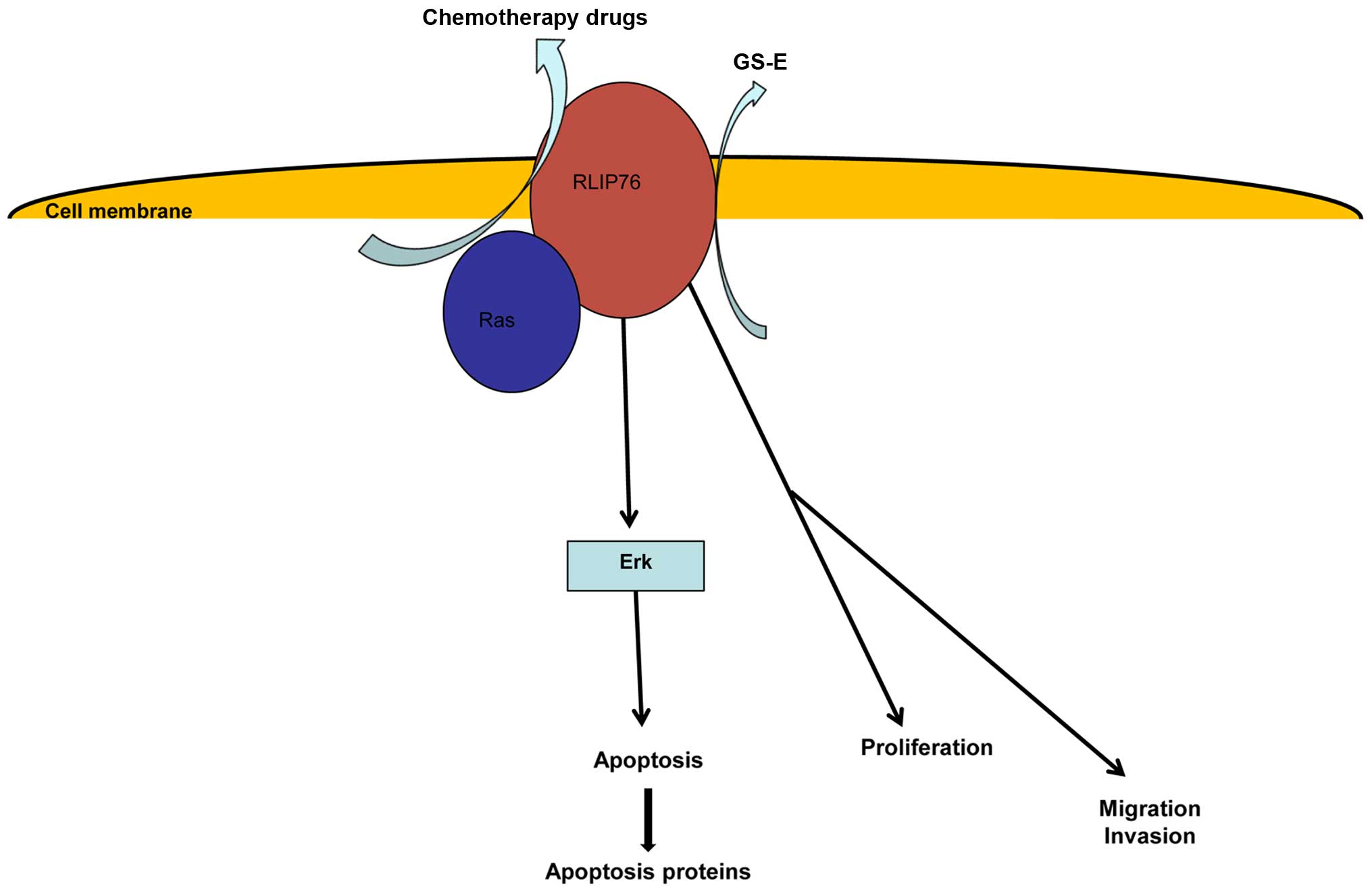
of the present study was that RLIP76 knockdown could reduce
IC50 of HCT-8/V to VCR. We developed a model to describe
the functions of RLIP76, which shows that RLIP76 may play a role in
chemotherapy drug transportation, cancer cell apoptosis,
proliferation and metastasis (Fig.
4).
Sui et al (43) used VCR to prove that JNK or COX-2
inhibition increased intracellular VCR accumulation and the
sensitivity to VCR in HCT-8/V cells. In addition, in the present
study, we showed that RLIP76 is overexpressed in MDR cancer cells
compared to the wild-type cancer cells on protein level and
knockdown of RLIP76 significantly also reduced IC50 of
HCT-8/V to VCR. The higher resistance to VCR in HCT-8/V as compared
with the HCT-8 cells is associated with a higher RLIP76-mediated
efflux of VCR in HCT-8/V. Knockdown of RLIP76 with shRNA sensitizes
HCT-8/V to VCR. On the contrary, MDR is a phenotype exhibited by
many cancers to develop resistance to the cytotoxic effects of many
structurally divergent cytotoxic agents. Accumulation defective MDR
is mediated by various transporter proteins such as MRP and Pgp
(44–46). However, RLIP76, a
stress-responsive, stress-protective ATP-dependent transporter,
also plays an important role in chemotherapy agents and glutathione
conjugate (GS-E) transport. Knockout of the mouse homolog of RLIP76
leads to 80% loss of transport capacity for GS-E, and markedly
increased sensitivity to stress, xenobiotics, as well as ionizing
radiation (11).
Apoptosis can be initiated via one of two pathways.
In the intrinsic pathway, the cell undergoes cellular stress,
whereas in the extrinsic pathway, apoptosis is caused by signals
from other cells. Both pathways activate initiator caspases to
activate executioner caspases, which consequently induce cell death
by indiscriminately degrading proteins. Several studies have
reported that RLIP76 depletion can increase the apoptosis induced
by chemotherapy drugs by suppressing cellular transport (21). In the present study, we also found
that RLIP76 knockdown in HCT-8/V cells without chemotherapy
significantly increased apoptosis as compared with controls. We
carried out western blot analysis to detect apoptosis proteins,
including caspase-3, caspase-8, caspase-9, Parp and Bax, finding
that knockdown of RLIP76 decreased caspase-3, increased
cleaved-caspase-8, cleaved-caspase-9, cleaved-Parp and Bax, which
implies a functional interaction between RLIP76 and the caspase
pathways in colorectal cancer. This observation is consistent with
previous findings that RLIP76 deletion or inhibition in animal
models causes rapid, complete, and sustained regression of
malignancy in human xenografts (20). The apoptotic effect of RLIP76 maybe
related to Ras as it is also a member of the Ras family. As Ras is
a upstream protein of Erk, a relationship between RLIP76 and Erk
may exist. In addition, Erk signaling represents a primary axis of
a signal relay pathway that determines the basal survival and
resistance to apoptotic effects. Therefore, in the present study,
we investigated the interaction of Erk and RLIP76. Knockdown of
RLIP76 reduced the phosphorylation level of Erk and as a result, it
can enhance the effects of some chemotherapy drugs which target Erk
pathways, such as sunitinib, sorafenib and temsirolimus.
Invasion and migration are features that result in
poor prognosis in colorectal cancer (47). In the present study, we found that
RLIP76 knockdown significantly suppressed the invasiveness and
migration of KD HCT-8/V colorectal cancer cells compared with the
control cells.
Our findings show that RLIP76 is overexpressed in
MDR colorectal cancer cells, and RLIP76 is a very important
anticancer target that functions as an anti-apoptosis protein
necessary for the survival of cancer cells. It also regulates the
important signaling pathways of cancer cells, such as
down-regulating phosphorylation level of Erk. Though further study
is still needed, RLIP76 is an important target to improve drug
resistance and tumor treatment.
Acknowledgements
The present study was supported in part by grants
from the National Natural Science Foundation of China (81472685),
the Science and Technology Development Project of Shandong Province
(2013GSF11852), the Major Science and Technology Projects of
Shandong Province (2015ZDXX0802A01), the Postdoctoral Innovation
Project Special Foundation of Shandong Province (201302031) and the
Promotive research fund for excellent young and middle-aged
scientists of Shandong Province (BS2014YY037).
References
|
1
|
Cunningham D, Atkin W, Lenz HJ, Lynch HT,
Minsky B, Nordlinger B and Starling N: Colorectal cancer. Lancet.
375:1030–1047. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Verdecchia A, Francisci S, Brenner H,
Gatta G, Micheli A, Mangone L and Kunkler I; EUROCARE-4 Working
Group. Recent cancer survival in Europe: A 2000–02 period analysis
of EUROCARE-4 data. Lancet Oncol. 8:784–796. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kong Y, Bai PS, Sun H, Nan KJ, Chen NZ and
Qi XG: The deoxycholic acid targets miRNA-dependent CAC1 gene
expression in multidrug resistance of human colorectal cancer. Int
J Biochem Cell Biol. 44:2321–2332. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yasunaga M and Matsumura Y: Role of SLC6A6
in promoting the survival and multidrug resistance of colorectal
cancer. Sci Rep. 4:48522014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Nooter K and Sonneveld P: Clinical
relevance of P-glycoprotein expression in haematological
malignancies. Leuk Res. 18:233–243. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Leith C: Multidrug resistance in leukemia.
Curr Opin Hematol. 5:287–291. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sharma R, Awasthi YC, Yang Y, Sharma A,
Singhal SS and Awasthi S: Energy dependent transport of xenobiotics
and its relevance to multidrug resistance. Curr Cancer Drug
Targets. 3:89–107. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Takara K, Sakaeda T and Okumura K: An
update on overcoming MDR1-mediated multidrug resistance in cancer
chemotherapy. Curr Pharm Des. 12:273–286. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Jullien-Flores V, Dorseuil O, Romero F,
Letourneur F, Saragosti S, Berger R, Tavitian A, Gacon G and
Camonis JH: Bridging Ral GTPase to Rho pathways. RLIP76, a Ral
effector with CDC42/Rac GTPase-activating protein activity. J Biol
Chem. 270:22473–22477. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Park SH and Weinberg RA: A putative
effector of Ral has homology to Rho/Rac GTPase activating proteins.
Oncogene. 11:2349–2355. 1995.PubMed/NCBI
|
|
11
|
Awasthi S, Cheng J, Singhal SS, Saini MK,
Pandya U, Pikula S, Bandorowicz-Pikula J, Singh SV, Zimniak P and
Awasthi YC: Novel function of human RLIP76: ATP-dependent transport
of glutathione conjugates and doxorubicin. Biochemistry.
39:9327–9334. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Singhal SS, Singhal J, Nair MP, Lacko AG,
Awasthi YC and Awasthi S: Doxorubicin transport by RALBP1 and ABCG2
in lung and breast cancer. Int J Oncol. 30:717–725. 2007.PubMed/NCBI
|
|
13
|
Singhal SS, Sehrawat A, Sahu M, Singhal P,
Vatsyayan R, Rao Lelsani PC, Yadav S and Awasthi S: Rlip76
transports sunitinib and sorafenib and mediates drug resistance in
kidney cancer. Int J Cancer. 126:1327–1338. 2010.
|
|
14
|
Singhal SS, Sehrawat A, Mehta A, Sahu M
and Awasthi S: Functional reconstitution of RLIP76 catalyzing
ATP-dependent transport of glutathione-conjugates. Int J Oncol.
34:191–199. 2009.
|
|
15
|
Drake KJ, Singhal J, Yadav S, Nadkar A,
Pungaliya C, Singhal SS and Awasthi S: RALBP1/RLIP76 mediates
multidrug resistance. Int J Oncol. 30:139–144. 2007.
|
|
16
|
Awasthi S, Singhal SS, Srivastava SK,
Zimniak P, Bajpai KK, Saxena M, Sharma R, Ziller SA III, Frenkel EP
and Singh SV: Adenosine triphosphate-dependent transport of
doxorubicin, daunomycin, and vinblastine in human tissues by a
mechanism distinct from the P-glycoprotein. J Clin Invest.
93:958–965. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Awasthi S, Singhal SS, Pandya U, Gopal S,
Zimniak P, Singh SV and Awasthi YC: ATP-Dependent colchicine
transport by human erythrocyte glutathione conjugate transporter.
Toxicol Appl Pharmacol. 155:215–226. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Awasthi S, Singhal SS, Singhal J, Yang Y,
Zimniak P and Awasthi YC: Role of RLIP76 in lung cancer doxorubicin
resistance: III. Anti-RLIP76 antibodies trigger apoptosis in lung
cancer cells and synergistically increase doxorubicin cytotoxicity.
Int J Oncol. 22:721–732. 2003.PubMed/NCBI
|
|
19
|
Yadav S, Zajac E, Singhal SS, Singhal J,
Drake K, Awasthi YC and Awasthi S: POB1 over-expression inhibits
RLIP76-mediated transport of glutathione-conjugates, drugs and
promotes apoptosis. Biochem Biophys Res Commun. 328:1003–1009.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Singhal SS, Singhal J, Yadav S, Dwivedi S,
Boor PJ, Awasthi YC and Awasthi S: Regression of lung and colon
cancer xenografts by depleting or inhibiting RLIP76 (Ral-binding
protein 1). Cancer Res. 67:4382–4389. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Singhal SS, Roth C, Leake K, Singhal J,
Yadav S and Awasthi S: Regression of prostate cancer xenografts by
RLIP76 depletion. Biochem Pharmacol. 77:1074–1083. 2009. View Article : Google Scholar :
|
|
22
|
Singhal SS, Awasthi YC and Awasthi S:
Regression of melanoma in a murine model by RLIP76 depletion.
Cancer Res. 66:2354–2360. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Leake K, Singhal J, Nagaprashantha LD,
Awasthi S and Singhal SS: RLIP76 regulates PI3K/Akt signaling and
chemo-radiotherapy resistance in pancreatic cancer. PLoS One.
7:e345822012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wang Q, Qian J, Wang J, Luo C, Chen J, Hu
G and Lu Y: Knockdown of RLIP76 expression by RNA interference
inhibits invasion, induces cell cycle arrest, and increases
chemosensitivity to the anticancer drug temozolomide in glioma
cells. J Neurooncol. 112:73–82. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Singhal SS, Yadav S, Vatsyayan R,
Chaudhary P, Borvak J, Singhal J and Awasthi S: Increased
expression of cdc2 inhibits transport function of RLIP76 and
promotes apoptosis. Cancer Lett. 283:152–158. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hu Y and Mivechi NF: HSF-1 interacts with
Ral-binding protein 1 in a stress-responsive, multiprotein complex
with HSP90 in vivo. J Biol Chem. 278:17299–17306. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wang Q, Wang JY, Zhang XP, Lv ZW, Fu D, Lu
YC, Hu GH, Luo C and Chen JX: RLIP76 is overexpressed in human
glioblastomas and is required for proliferation, tumorigenesis and
suppression of apoptosis. Carcinogenesis. 34:916–926. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Jullien-Flores V, Mahé Y, Mirey G,
Leprince C, Meunier-Bisceuil B, Sorkin A and Camonis JH: RLIP76, an
effector of the GTPase Ral, interacts with the AP2 complex:
Involvement of the Ral pathway in receptor endocytosis. J Cell Sci.
113:2837–2844. 2000.PubMed/NCBI
|
|
29
|
Quaroni A and Paul EC: Cytocentrin is a
Ral-binding protein involved in the assembly and function of the
mitotic apparatus. J Cell Sci. 112:707–718. 1999.PubMed/NCBI
|
|
30
|
Awasthi S, Singhal SS, Sharma R, Zimniak P
and Awasthi YC: Transport of glutathione conjugates and
chemotherapeutic drugs by RLIP76 (RALBP1): A novel link between
G-protein and tyrosine kinase signaling and drug resistance. Int J
Cancer. 106:635–646. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Awasthi S, Singhal SS, Awasthi YC, Martin
B, Woo JH, Cunningham CC and Frankel AE: RLIP76 and Cancer. Clin
Cancer Res. 14:4372–4377. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Awasthi YC, Singhal SS, Gupta S, Ahmad H,
Zimniak P, Radominska A, Lester R and Sharma R: Purification and
characterization of an ATPase from human liver which catalyzes ATP
hydrolysis in the presence of the conjugates of bilirubin bile
acids and glutathione. Biochem Biophys Res Commun. 175:1090–1096.
1991. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Awasthi S, Singhal SS, Singhal J, Cheng J,
Zimniak P and Awasthi YC: Role of RLIP76 in lung cancer doxorubicin
resistance: II. Doxorubicin transport in lung cancer by RLIP76. Int
J Oncol. 22:713–720. 2003.PubMed/NCBI
|
|
34
|
Yadav S, Singhal SS, Singhal J,
Wickramarachchi D, Knutson E, Albrecht TB, Awasthi YC and Awasthi
S: Identification of membrane-anchoring domains of RLIP76 using
deletion mutant analyses. Biochemistry. 43:16243–16253. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Stuckler D, Singhal J, Singhal SS, Yadav
S, Awasthi YC and Awasthi S: RLIP76 transports vinorelbine and
mediates drug resistance in non-small cell lung cancer. Cancer Res.
65:991–998. 2005.PubMed/NCBI
|
|
36
|
Singhal SS, Yadav S, Singhal J, Zajac E,
Awasthi YC and Awasthi S: Depletion of RLIP76 sensitizes lung
cancer cells to doxorubicin. Biochem Pharmacol. 70:481–488. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Awasthi S, Cheng JZ, Singhal SS, Pandya U,
Sharma R, Singh SV, Zimniak P and Awasthi YC: Functional reassembly
of ATP-dependent xenobiotic transport by the N- and C-terminal
domains of RLIP76 and identification of ATP binding sequences.
Biochemistry. 40:4159–4168. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Su JL, Lin MT, Hong CC, Chang CC, Shiah
SG, Wu CW, Chen ST, Chau YP and Kuo ML: Resveratrol induces
FasL-related apoptosis through Cdc42 activation of
ASK1/JNK-dependent signaling pathway in human leukemia HL-60 cells.
Carcinogenesis. 26:1–10. 2005. View Article : Google Scholar
|
|
39
|
Rohwer N and Cramer T: Hypoxia-mediated
drug resistance: Novel insights on the functional interaction of
HIFs and cell death pathways. Drug Resist Updat. 14:191–201. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Wang H, Zhao L, Zhu LT, Wang Y, Pan D, Yao
J, You QD and Guo QL: Wogonin reverses hypoxia resistance of human
colon cancer HCT116 cells via downregulation of HIF-1α and
glycolysis, by inhibiting PI3K/Akt signaling pathway. Mol Carcinog.
53(Suppl 1): E107–E118. 2014. View
Article : Google Scholar
|
|
41
|
Cho K, Shin HW, Kim YI, Cho CH, Chun YS,
Kim TY and Park JW: Mad1 mediates hypoxia-induced doxorubicin
resistance in colon cancer cells by inhibiting mitochondrial
function. Free Radic Biol Med. 60:201–210. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Murono K, Tsuno NH, Kawai K, Sasaki K,
Hongo K, Kaneko M, Hiyoshi M, Tada N, Nirei T, Sunami E, et al:
SN-38 overcomes chemoresistance of colorectal cancer cells induced
by hypoxia, through HIF1alpha. Anticancer Res. 32:865–872.
2012.PubMed/NCBI
|
|
43
|
Sui H, Zhou S, Wang Y, Liu X, Zhou L, Yin
P, Fan Z and Li Q: COX-2 contributes to P-glycoprotein-mediated
multidrug resistance via phosphorylation of c-Jun at Ser63/73 in
colorectal cancer. Carcinogenesis. 32:667–675. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Choudhuri S and Klaassen CD: Structure,
function, expression, genomic organization, and single nucleotide
polymorphisms of human ABCB1 (MDR1), ABCC (MRP), and ABCG2 (BCRP)
efflux transporters. Int J Toxicol. 25:231–259. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Higgins CF: Multiple molecular mechanisms
for multidrug resistance transporters. Nature. 446:749–757. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Sharom FJ: ABC multidrug transporters:
Structure, function and role in chemoresistance. Pharmacogenomics.
9:105–127. 2008. View Article : Google Scholar
|
|
47
|
Poon RT, Fan ST, Ng IO, Lo CM, Liu CL and
Wong J: Different risk factors and prognosis for early and late
intrahepatic recurrence after resection of hepatocellular
carcinoma. Cancer. 89:500–507. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Singhal SS, Singhal J, Figarola J, Horne D
and Awasthi S: RLIP76 targeted therapy for kidney cancer. Pharm
Res. 32:3123–3136. 2015. View Article : Google Scholar : PubMed/NCBI
|