Introduction
Lung cancer is the most frequent cause of
cancer-related deaths worldwide, with mortality estimated to exceed
1 million deaths each year (1). An
estimated 224,210 new cases of lung and bronchial cancers were
diagnosed in 2014, and 159,260 deaths are estimated to occur from
the disease (2). Non-small cell
lung cancer (NSCLC) accounts for almost 85% of all cases of lung
cancer and comprises mainly adenocarcinoma, squamous cell
carcinoma, and large-cell carcinoma (3). The most important risk factor for
NSCLC is cigarette smoking, followed by occupational and
environmental exposures (4).
Although the predominant treatment for NSCLC still involves a
combination of surgery, radiation therapy, and chemotherapy, some
patients have conditions that make them ineligible for surgical
treatment (5). Thus, the discovery
and development of an effective chemotherapeutic agent might
improve survival rates for patients with NSCLC.
Coffee beans contain more than a thousand compounds,
one of which is kahweol (6).
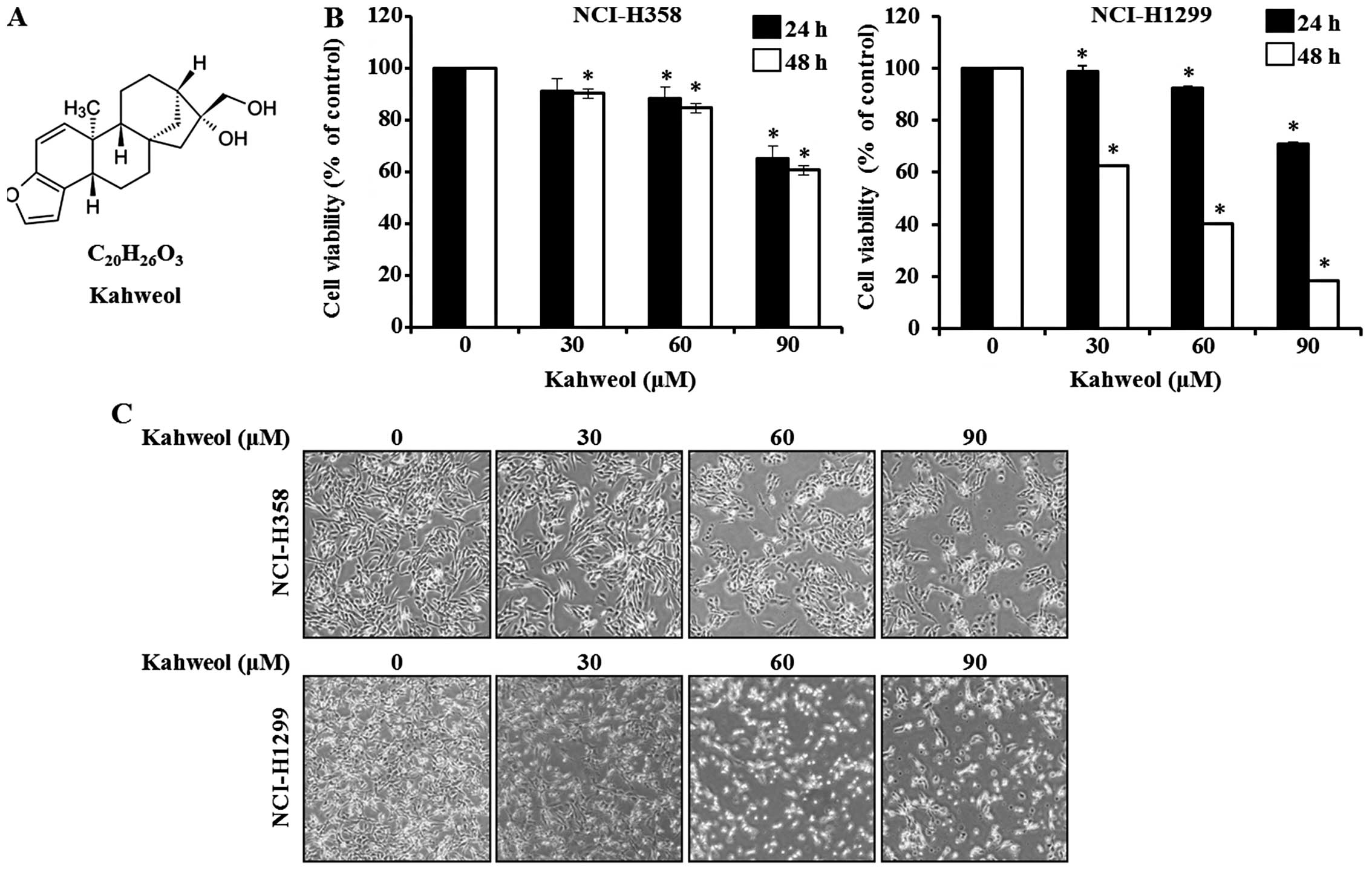
Kahweol, a diterpene molecule (Fig.
1A), is present in oil derived from Arabica coffee beans
(7) and has been shown to have a
wide variety of biological activities, including anti-angiogenic
and anti-inflammatory effect in HUVEC, antitumor effect on human
breast cancer, antiproliferative properties in oral squamous
cancer, suppression of iNOS and cyclooxygenase in RAW 264.7 cell
and chemoprotective and antitumorigenic effect in organs of rat
(8–14). However, in NSCLC, the
anti-apoptotic mechanisms and molecular targets of kahweol are
poorly understood.
Basic transcription factor 3 (BTF3), a general RNA
polymerase II transcription factor, acts as a modulator of
apoptosis and is differentially expressed in several types of
cancer (15). The biological
important role of BTF3 was shown in mouse embryos, homozygous for
loss of function mutation in the BTF3 gene, that died at the early
stage of development (16) and
changes in BTF3 expression have been shown to be related to
apoptosis in the BL60-2 Burkitt lymphoma cell line (17). In several cancer cell lines, BTF3
is overexpressed (18–20) but as an apoptosis-related protein,
its pattern of expression in NSCLC is still unknown.
The use of chemotherapy in patients with advanced
NSCLC requires further investigation. Therefore, we investigated
the potential regulatory effect of kahweol on viability and
apoptosis of the NSCLC cell lines NCI-H358 and NCI-H1299, and its
anti-apoptotic mechanism in relation to BTF3.
Materials and methods
Reagent and antibodies
Kahweol was purchased from Santa Cruz Biotechnology
(Dallas, TX, USA). The MEK1 inhibitor PD98059 was purchased from
Sigma-Aldrich (St. Louis, MO, USA). Antibodies, including BTF3,
PARP, caspase-3, Bcl-2, Bcl-xl, Bax, p27, p21, cyclin D1, survivin,
and β-actin, were obtained from Santa Cruz Biotechnology (Paso
Robles, CA, USA). Anti-ERK and anti-phospho-ERK antibodies were
purchased from Cell Signaling Technology (Danvers, MA, USA).
Cell lines and culture conditions
NCI-H358 (ATCC CRL-5807) and NCI-H1299 (ATCC
CRL-5803) were obtained from the American Type Culture Collection
(ATCC). NCI-H358 was derived from tumor tissue obtained from a
patient prior to initiation of chemotherapy and NCI-H1299 was
established from a lymph node metastasis of the lung from a patient
who had received prior radiation therapy (Manassas, VA, USA). These
NSCLC lines were grown routinely in RPMI-1640 medium (Welgene,
Deagu, Korea) with 10% fetal bovine serum (FBS) and 100 U each of
penicillin and streptomycin (Gibco, Grand Island, NY, USA) at 37°C
with CO2 in a humidified atmosphere.
Cell viability assay
We performed the MTS assay
(3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium)
to assess cell viability. NCI-H358 cells (2.5×103) and
NCI-H1299 cells (2.5×103) were seeded in 96-well plates
and were incubated overnight. The cells were treated with different
concentrations of kahweol [0 (control), 30, 60 and 90 μM] and then
incubated for 24 and 48 h. Absorbance was measured at 490 nm using
a BioTek Microplate Reader (BioTek, Winooski, VT, USA). The
percentages of viable kahweol-treated cells were normalized to
those of untreated cells.
DAPI staining
Apoptosis of kahweol-treated cells, nuclear
condensation, and fragmentation were detected by means of
4′-6-diamidino-2-phenylindole (DAPI) staining. NCI-H358 and
NCI-H1299 cells were treated with different concentrations of
kahweol for 48 h. The cells were harvested by trypsinization and
were then fixed in 100% methanol at room temperature for 30 min and
washed with phosphate-buffered saline (PBS). The washed cells were
stained with DAPI solution (Sigma-Aldrich) (2 μg/ml) for 20 min in
the dark, and the stained cells were imaged by confocal microscopy
using a Nikon C2 Plus System (Nikon Corp., Tokyo, Japan).
Immunocytochemical testing
Glass coverslips were sterilized on 6-well tissue
culture plates, and the NCI-H358 and NCI-H1299 cells were seeded
for 24 h. After being treated with different concentrations of
kahweol for 48 h, the cells were fixed and permeabilized with
Cytofix/Cytoperm solution (BD Biosciences, San Jose, CA, USA) for
30 min at 4°C. The cells were incubated with monoclonal BTF3
antibody containing 1% bovine serum albumin (BSA) at 4°C overnight
in the dark and then washed with PBS. The BTF3 antibody was reacted
with Alexa Fluor 546-conjugated anti-mouse IgG at room temperature
for 1 h in the dark. The reacted cells were washed with PBS-T and
were stained with DAPI solution (Sigma-Aldrich) (2 μg/ml) for 20
min in the dark. The stained cells were observed under the FluoView
confocal laser scanning microscope.
Annexin V assay and PI staining
Apoptosis can be evaluated by means of simultaneous
staining with Annexin V-FITC and propidium iodide (PI). Annexin
V-FITC staining reveals the early stage of apoptosis, and PI
staining shows the late stage. The NCI-H358 and NCI-H1299 cells
were incubated with various concentrations of kahweol for 48 h,
after which the cells were harvested using a scraper. The harvested
cells were stained with Annexin V-FITC and PI and then assessed by
means of fluorescence-activated cell sorting (FACS) (BD
Biosciences).
Western blot analysis
The kahweol-treated NCI-H358 and NCI-H1299 cells
were cultured for 48 h, washed with cold PBS, and then lysed using
M-PER Mammalian Protein Extraction Reagent (Thermo Scientific,
Rockford, IL, USA) that contained a protease inhibitor cocktail
(Roche, Basel, Switzerland). The protein concentration was measured
using the BCA Protein Assay kit (Thermo Scientific). Samples were
separated on SDS-polyacrylamide gels and transferred to
polyvinylidene difluoride membranes (Millipore, Billerica, MA,
USA). The membranes were blocked with 5% skim milk in Tris-buffered
saline (TBS) with 0.1% Tween-20 for 20–30 min at room temperature
and were incubated with the primary antibodies at 4°C overnight.
Subsequently, the membranes were washed five times in TBS buffer
with 0.1% Tween-20 for 10 min and rotated for 1 h at room
temperature in a solution of horseradish peroxidase-conjugated
anti-mouse, anti-rabbit, or anti-goat IgG antibodies. The membranes
were developed using a chemiluminescent ECL Detection kit (Thermo
Scientific) and detected using ImageQuant LAS 4000 Mini software
(GE Healthcare Life Sciences, Buckinghamshire, UK) according to the
manufacturer’s instructions.
Statistical analysis
Data are presented as means ± SD from three
independent experiments. Data analysis for statistical significance
were obtained with use of Student’s t-test. As compared with the
vehicle control, p-values <0.05 indicated statistical
significance.
Results
Growth inhibitory effect of kahweol on
NSCLC cells
We investigated whether kahweol could effectively
suppress the cell proliferative capability of the two NSCLC cell
lines NCI-H358 and NCI-H1299. To determine cell viability, we
treated the cells with different concentrations of kahweol (30, 60
and 90 μM) at different time-points (24 or 48 h). Cell viability
was calculated 48 h after treatment using the MTS assay (Fig. 1B). The values for NCI-H358 were
90.1±0.02, 84.6±0.02 and 60.6±0.05% at kahweol concentrations of
30, 60 and 90 μM, respectively; the corresponding values for
NCI-H1299 were 62.5±0.01, 40.4±0.01 and 18.4±0.01%, respectively,
as compared with the untreated control cells. On microscopy, we
observed morphological changes in the cells in the
kahweol-containing medium that reflected apoptosis. As shown in
Fig. 1C, the apoptotic phenotype
at 48 h included cell rounding, cytoplasmic blebbing, and
irregularities in shape. In light of inhibition of cell viability
by treating kahweol in a dose- and time- dependent manner, kahweol
treatment affected the cell viability decrease.
Kahweol induces apoptosis in NSCLC
cells
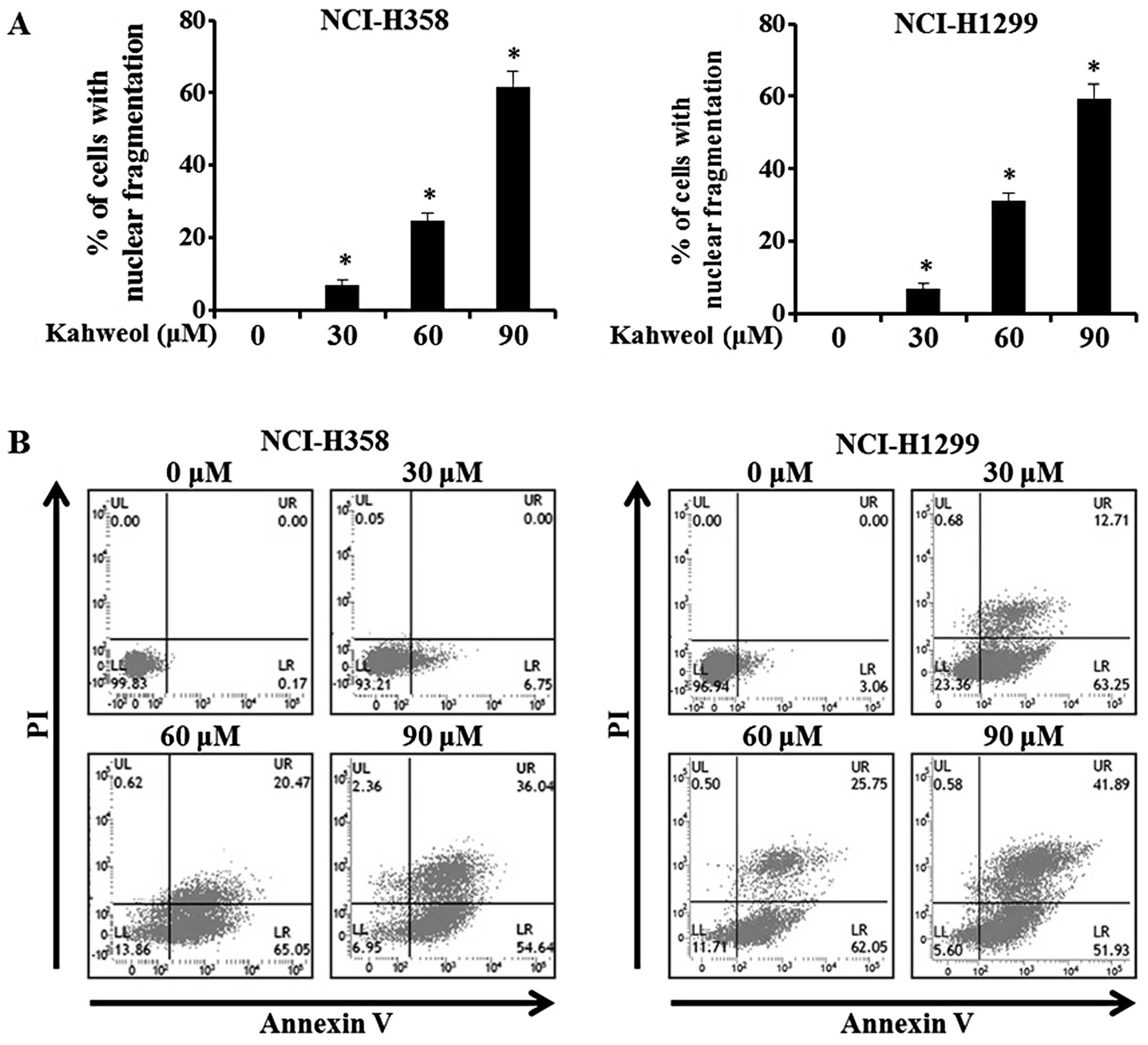
Cytoplasmic blebbing and morphological
irregularities are critical hallmarks of apoptosis (21). We examined the nuclear integrity of
the NSCLC cells treated with kahweol to determine whether this
compound would induce apoptosis. To detect any apoptotic changes in
the kahweol-treated NCI-H358 and NCI-H1299 cells, we used DAPI
staining and then viewed the cells using a confocal laser scanning
microscope. The percentage of cells with nuclear condensation in
the kahweol-treated group versus the DMSO-treated group is shown in
Fig. 2A. Using an Annexin V assay,
we verified the presence of kahweol-mediated apoptosis and found
that the ratio of early-to-late apoptotic cells was significantly
increased in the kahweol-treated NCI-H358 and NCI-H1299 cells
relative to the untreated control cells (Fig. 2B). These results indicate that
apoptosis of NSCLS cell lines result from increase of apoptotic
changes and nuclear condensation by treating kahweol.
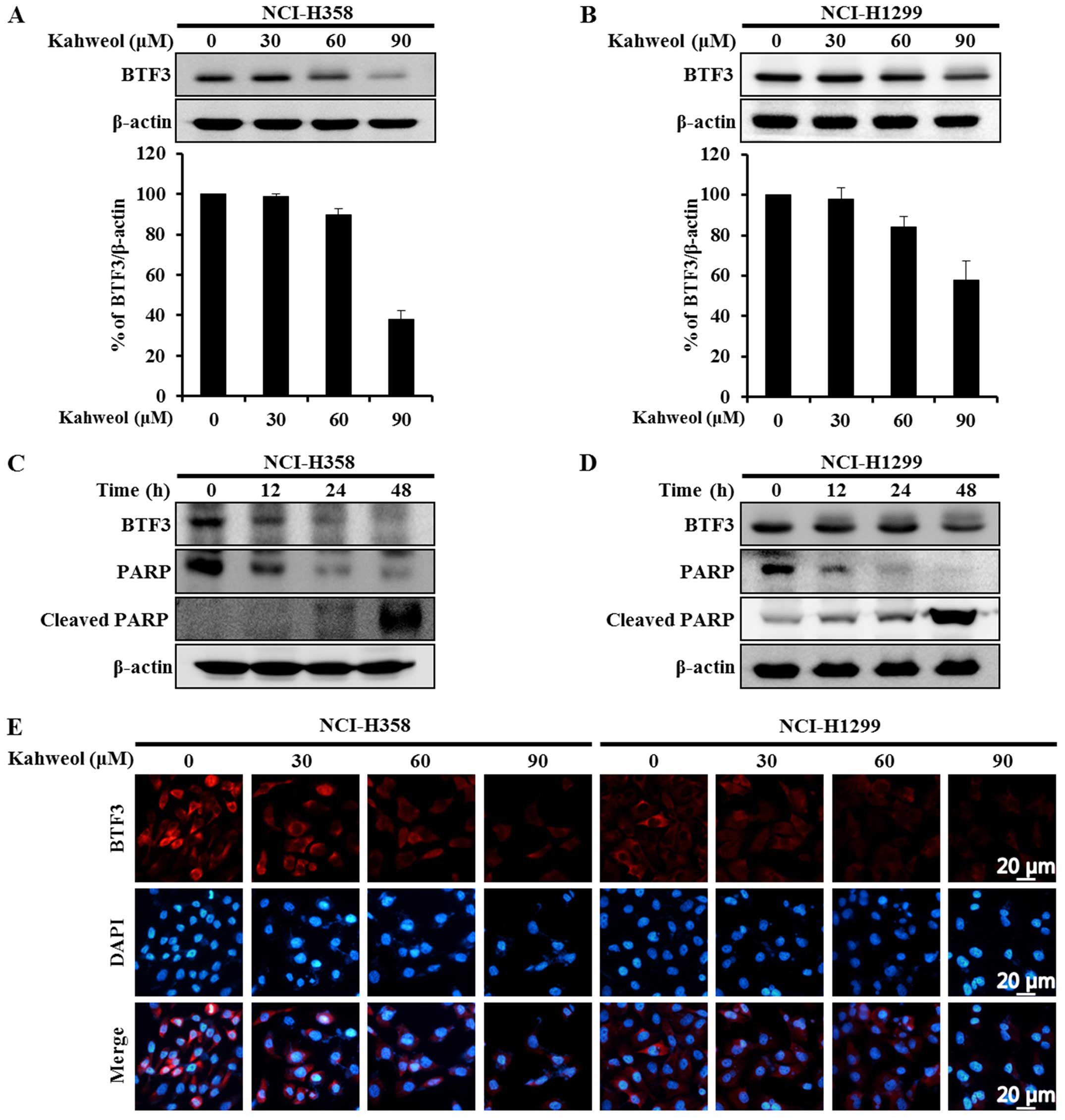
Kahweol suppresses BTF3 expression levels
in NSCLC cell lines
The transcription factor BTF3 has been shown to
cause significant proliferation of several cancer cell lines
(18–20) indicating that it critically
influences cell cycle arrest and apoptosis (17,22,23).
If the level of BTF3 expression could be effectively modulated by a
chemotherapeutic agent, that agent might be useful in anticancer
therapy. To determine whether BTF3-mediated apoptosis of NSCLC
cells might be induced by treatment with kahweol, we used western
blot analysis to examine NSCLC cells treated for 48 h with
different concentrations of kahweol (30, 60 and 90 μM). Indeed,
this treatment induced a marked decrease in the expression levels
of BTF3 in the NCI-H358 and NCI-H1299 cells in a dose-dependent
manner (Fig. 3A and B). To further
investigate the apoptotic effects of BTF3 downregulation, we
examined the cells at 0, 12, 24, and 48 h. BTF3 expression levels
decreased significantly as time progressed. Kahweol also induced
the cleavage of poly-(ADP-ribose) polymerase (PARP), resulting in
apoptosis (Fig. 3C and D).
Immunocytochemical testing was used to confirm these results. The
levels of BTF3 expression were reduced in the kahweol-treated NSCLC
cells in a dose-dependent manner (Fig.
3E). Collectively, these data indicate that in light of BTF3
expression decrease by treatment with kahweol, downregulation of
BTF3 by kahweol leads to apoptotic cell death.
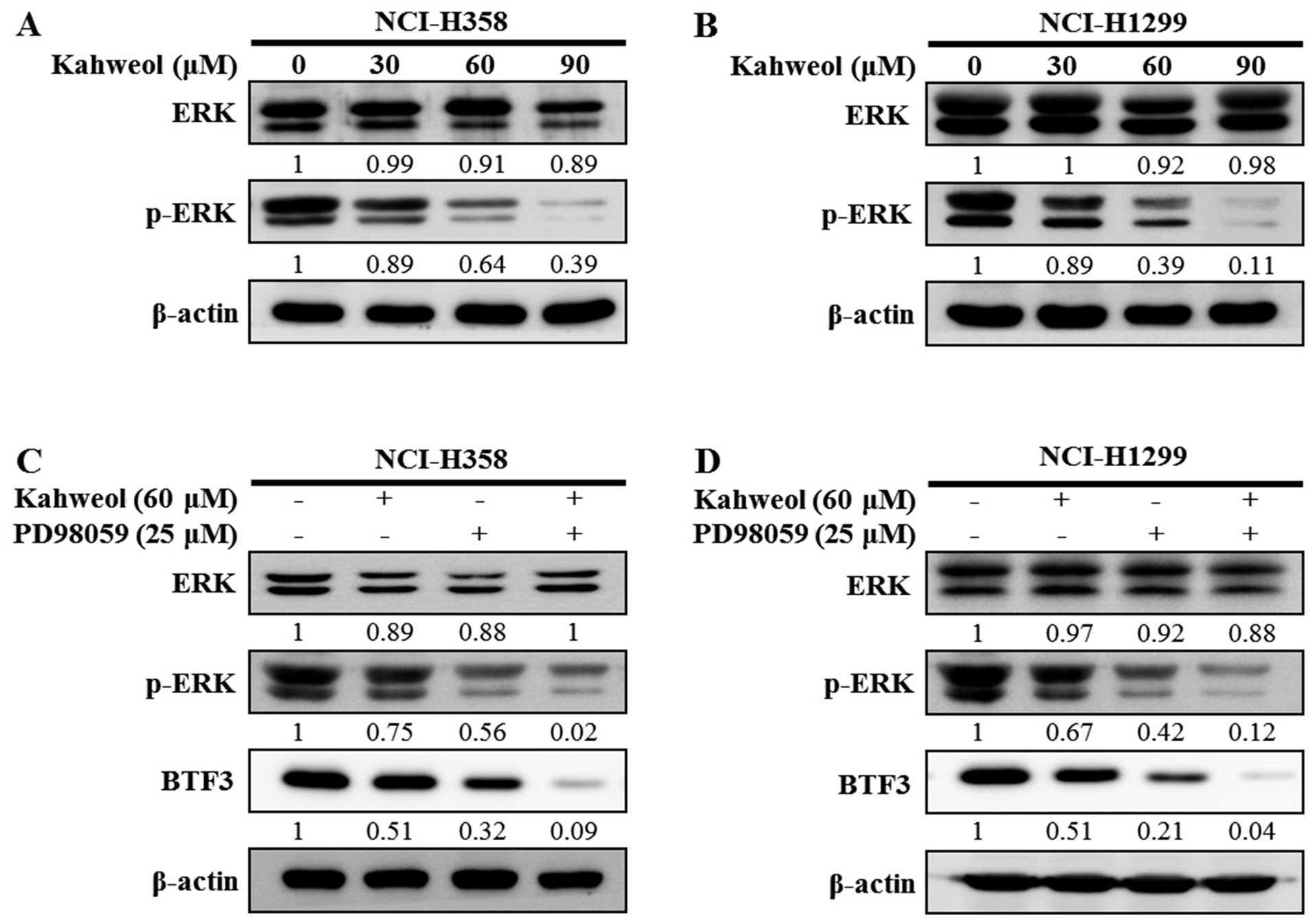
Kahweol induces inactivation of the ERK
signaling pathway in NSCLC cells
The ERK pathway plays an important role in cell
proliferation (24) and can be
activated by various stimuli, including chemotherapeutic agents
(25). We performed western blot
analysis to investigate the role of ERK pathways in the
kahweol-induced reduction of BTF3 expression levels. Although the
expression levels of extracellular signal-regulated kinase 1 and 2
(ERK1/2) were not changed, the level of phospho-ERK1/2 expression
was decreased by various concentration of kahweol (Fig. 4A and B). Furthermore, in order to
investigate whether BTF3 is a target of the ERK signaling pathway,
we applied PD98059, an ERK-specific inhibitor. When the NSCLC cells
were exposed to kahweol or PD98059, the phosphorylation of ERK was
significantly suppressed (Fig. 4C and
D). In addition, when the cells were treated with kahweol plus
PD98059, definite suppressive effect was observed. These results
indicate that BTF3 was regulated by inhibiting the ERK signaling
pathway through the effect of kahweol similar to PD98059 in NSCLC
cells.
Kahweol regulates the arrest of cell
cycle proteins and apoptosis-regulating proteins in NSCLC
cells
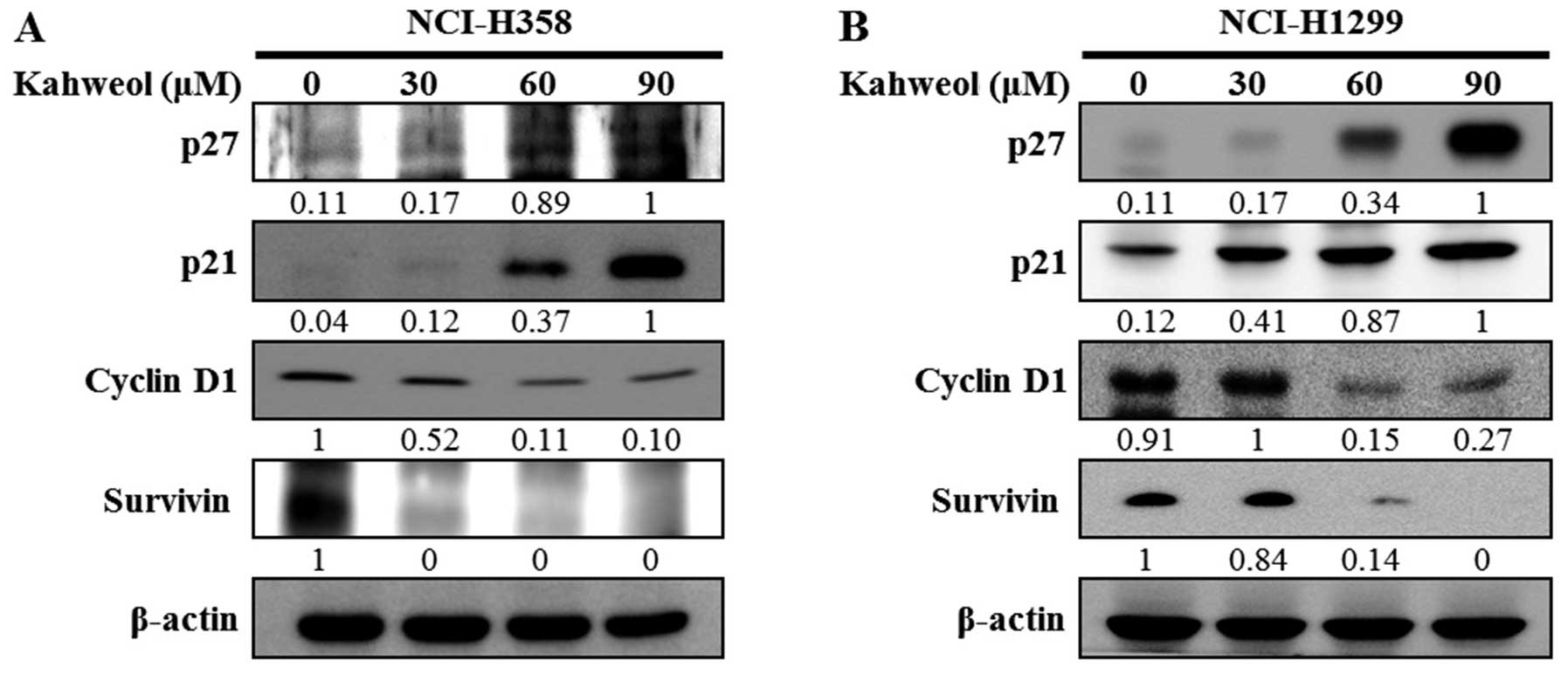
Next, we examined whether kahweol treatment
regulated the expression levels of various proteins related to cell
cycle arrest and apoptosis. The levels of cell cycle arrest-related
proteins, including p27 and p21, were increased, whereas the levels
of proteins related to cell proliferation and survival, including
cyclin D1 and survivin, were decreased (Fig. 5A and B). Moreover, when we tested
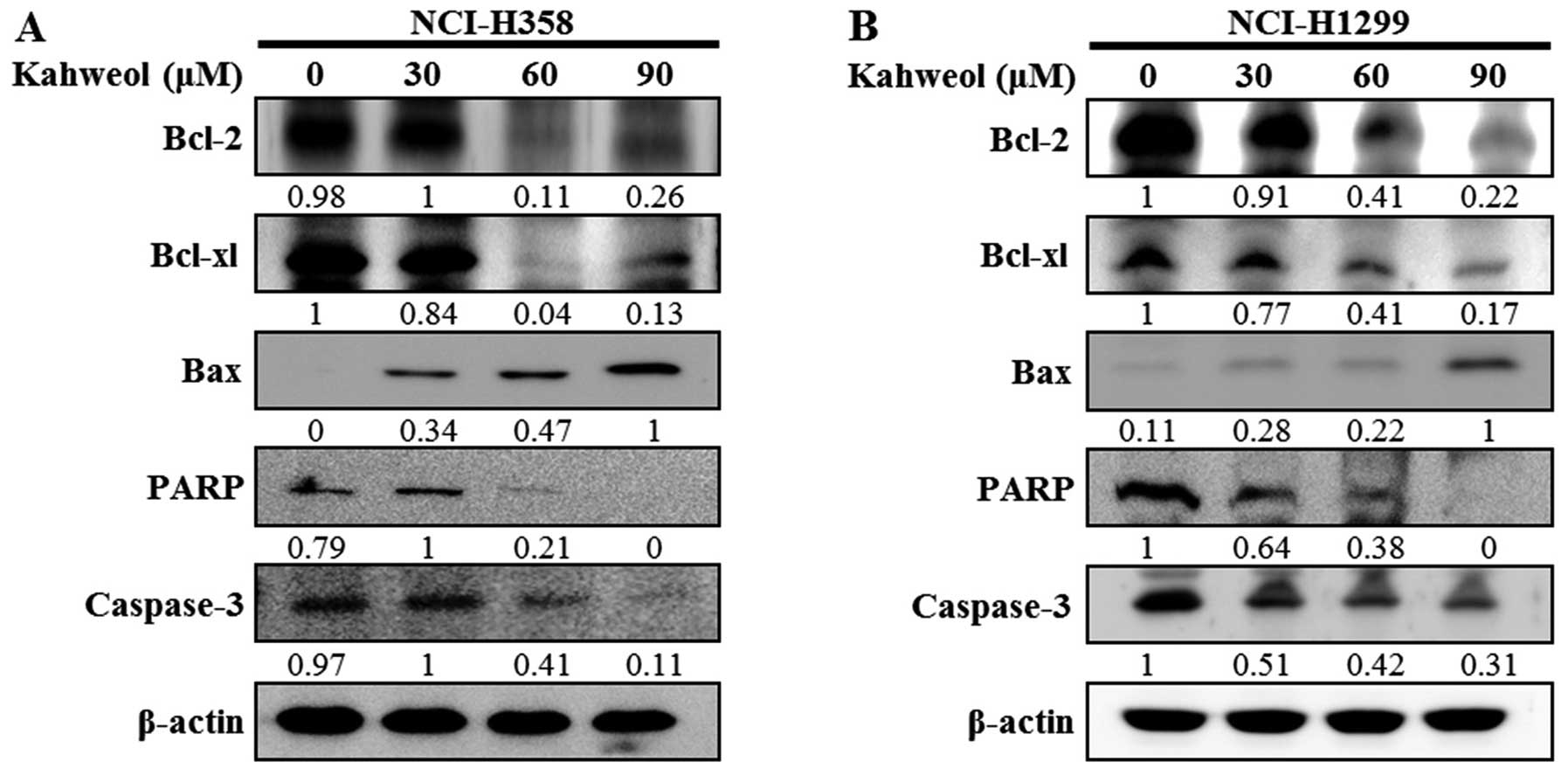
pro-apoptotic and anti-apoptotic protein levels at different doses
of kahweol, caspase-3 and PARP were activated in a dose-dependent
manner. In addition, the downregulation of Bcl-2 and Bcl-xl and the
upregulation of Bax appeared to be involved in apoptotic cell death
induced by kahweol (Fig. 6A and
B). These results suggest that kahweol-induced cytotoxicity
regulates BTF3 through inhibition of the ERK signaling pathway and
leads to both cell cycle arrest and apoptotic cell death.
Discussion
Coffee contains numerous antioxidants and phenolic
compounds, some of which have antitumor effects in various cancers
(26). One of the bioactive
compounds associated with the antitumor effects of coffee is
kahweol (7,27,28).
Kahweol has been shown to have anti-inflammatory, anticarcinogenic,
and antitumor effects (8,9,12–14).
Moreover, several animal studies showed that kahweol increased
blood cholesterol and was compatible with a chemoprotective
activity against various toxicants and procarcinogens (29–33).
Although studies of kahweol-induced apoptosis have been carried out
involving many cancer cell lines, its antiproliferative mechanisms
in human NSCLC cells remained to be investigated. We therefore
treated NSCLC cells with different concentrations of kahweol and
conducted various analyses to determine the potential effects of
this compound on tumor cell proliferation and survival. Our results
showed that apoptosis induced by kahweol was associated with
characteristic morphological changes, such as membrane blebbing and
chromatin condensation.
In our study, BTF3 was downregulated in
kahweol-treated NSCLC cells. BTF3 activates the transcription of
RNA polymerase II through physiologic binding to promoter regions
such as the TATA and CAAT boxes (34,35).
BTF is also known to regulate cell cycle arrest and apoptosis
(17,22,23).
Recent research has shown that BTF3 is associated with the
apoptotic pathway related to DNA damage (36). DNA damage leads to apoptosis
through the activation of ATM in mitochondria and MAPK
(mitogen-activated protein kinase) through phosphorylation
(37).
Moreover, we found that kahweol-induced apoptosis
occurred via inactivation of the ERK pathway. The ERK1/2 pathway is
known to be associated with various cellular processes, including
differentiation, transformation, proliferation, and apoptosis
(38–40). In previous studies, several
antiproliferative agents such as fucoidan, cucurbitacin B and
apicidin, is able to inactivate the ERK1/2 pathway for apoptosis
(41–43). In our study, we found that
inactivation of ERK1/2 pathway is involved in kahweol-mediated
apoptosis, as suggested by the use of PD98059, an inhibitor of the
activation of MEK as a result of ERK pathway inhibition by kawheol
(44).
To further characterize the effects of kahweol on
BTF3, we also analyzed its effects on p27 p21, cyclin D1, and
survivin. The cell cycle plays an important role in cell survival,
growth, and proliferation (45)
and involves several regulatory proteins between DNA synthesis and
mitosis (45) that are closely
associated with complexes containing cyclins and cyclin-dependent
kinases (46). The G1 stage of the
cell cycle, which precedes DNA synthesis, involves the p21 family
(e.g., p21 and p27) which inhibits proliferation and DNA repair
(45,47,48).
Cyclin D1 regulates the G1 restriction point (49). Survivin inhibits apoptosis (in the
cytosol) and controls cell division (in the nucleus) (50). In addition, kahweol both induced
Bax and reduced Bcl-2 and Bcl-xL expression while also activating
caspase-3 and PARP, which suggests that kahweol suppressed BTF3
expression and ultimately led to apoptotic cell death. To the best
of our knowledge, ours is the first report to demonstrate a
potential role for kahweol in cancer chemoprevention, as shown in
NSCLC cells. These results indicate that kahweol may inhibit cell
proliferation and induce apoptosis due to BTF3 via the ERK
signaling pathway. Thus, kahweol might be a promising
chemotherapeutic agent in the treatment of patients with NSCLC.
Acknowledgements
This study was supported by a grant from the
Next-Generation BioGreen 21 Program (project no. PJ01116401), Rural
Development Administration, Republic of Korea.
References
|
1
|
Jemal A, Siegel R, Ward E, Murray T, Xu J
and Thun MJ: Cancer statistics, 2007. CA Cancer J Clin. 57:43–66.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Siegel R, Ma J, Zou Z and Jemal A: Cancer
statistics, 2014. CA Cancer J Clin. 64:9–29. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wang L, Xiong Y, Sun Y, Fang Z, Li L, Ji H
and Shi T: HLungDB: An integrated database of human lung cancer
research. Nucleic Acids Res. 38(Database): D665–D669. 2010.
View Article : Google Scholar :
|
|
4
|
Hecht SS: Tobacco smoke carcinogens and
lung cancer. J Natl Cancer Inst. 91:1194–1210. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Saintigny P and Burger JA: Recent advances
in non-small cell lung cancer biology and clinical management.
Discov Med. 13:287–297. 2012.PubMed/NCBI
|
|
6
|
Spiller MA: The chemical components of
coffee. Prog Clin Biol Res. 158:91–147. 1984.PubMed/NCBI
|
|
7
|
Lee KA, Chae JI and Shim JH: Natural
diterpenes from coffee, cafestol and kahweol induce apoptosis
through regulation of specificity protein 1 expression in human
malignant pleural mesothelioma. J Biomed Sci. 19:602012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Cárdenas C, Quesada AR and Medina MA:
Insights on the antitumor effects of kahweol on human breast
cancer: Decreased survival and increased production of reactive
oxygen species and cytotoxicity. Biochem Biophys Res Commun.
447:452–458. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chae JI, Jeon YJ and Shim JH:
Anti-proliferative properties of kahweol in oral squamous cancer
through the regulation specificity protein 1. Phytother Res.
28:1879–1886. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Cárdenas C, Quesada AR and Medina MA:
Anti-angiogenic and anti-inflammatory properties of kahweol, a
coffee diterpene. PLoS One. 6:e234072011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Huber WW, Prustomersky S, Delbanco E, Uhl
M, Scharf G, Turesky RJ, Thier R and Schulte-Hermann R: Enhancement
of the chemoprotective enzymes glucuronosyl transferase and
glutathione transferase in specific organs of the rat by the coffee
components kahweol and cafestol. Arch Toxicol. 76:209–217. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kim JY, Jung KS and Jeong HG: Suppressive
effects of the kahweol and cafestol on cyclooxygenase-2 expression
in macrophages. FEBS Lett. 569:321–326. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kim JY, Jung KS, Lee KJ, Na HK, Chun HK,
Kho YH and Jeong HG: The coffee diterpene kahweol suppress the
inducible nitric oxide synthase expression in macrophages. Cancer
Lett. 213:147–154. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tao KS, Wang W, Wang L, Cao DY, Li YQ, Wu
SX and Dou KF: The multifaceted mechanisms for coffee’s
anti-tumorigenic effect on liver. Med Hypotheses. 71:730–736. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kusumawidjaja G, Kayed H, Giese N, Bauer
A, Erkan M, Giese T, Hoheise JD, Friess H and Kleeff J: Basic
transcription factor 3 (BTF3) regulates transcription of
tumor-associated genes in pancreatic cancer cells. Cancer Biol
Ther. 6:367–376. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Deng JM and Behringer RR: An insertional
mutation in the BTF3 transcription factor gene leads to an early
postimplantation lethality in mice. Transgenic Res. 4:264–269.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Brockstedt E, Otto A, Rickers A, Bommert K
and Wittmann-Liebold B: Preparative high-resolution two-dimensional
electrophoresis enables the identification of RNA polymerase B
transcription factor 3 as an apoptosis-associated protein in the
human BL60-2 Burkitt lymphoma cell line. J Protein Chem.
18:225–231. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Dunican DS, McWilliam P, Tighe O,
Parle-McDermott A and Croke DT: Gene expression differences between
the microsatellite instability (MIN) and chromosomal instability
(CIN) phenotypes in colorectal cancer revealed by high-density cDNA
array hybridization. Oncogene. 21:3253–3257. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Odreman F, Vindigni M, Gonzales ML,
Niccolini B, Candiano G, Zanotti B, Skrap M, Pizzolitto S, Stanta G
and Vindigni A: Proteomic studies on low- and high-grade human
brain astrocytomas. J Proteome Res. 4:698–708. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Roy L, Laboissière S, Abdou E, Thibault G,
Hamel N, Taheri M, Boismenu D, Lanoix J, Kearney RE and Paiement J:
Proteomic analysis of the transitional endoplasmic reticulum in
hepatocellular carcinoma: An organelle perspective on cancer.
Biochim Biophys Acta. 1804:1869–1881. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Saraste A and Pulkki K: Morphologic and
biochemical hallmarks of apoptosis. Cardiovasc Res. 45:528–537.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Bloss TA, Witze ES and Rothman JH:
Suppression of CED-3-independent apoptosis by mitochondrial betaNAC
in Caenorhabditis elegans. Nature. 424:1066–1071. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Thiede B, Dimmler C, Siejak F and Rudel T:
Predominant identification of RNA-binding proteins in Fas-induced
apoptosis by proteome analysis. J Biol Chem. 276:26044–26050. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Pearson G, Robinson F, Beers Gibson T, Xu
BE, Karandikar M, Berman K and Cobb MH: Mitogen-activated protein
(MAP) kinase pathways: Regulation and physiological functions.
Endocr Rev. 22:153–183. 2001.PubMed/NCBI
|
|
25
|
Persons DL, Yazlovitskaya EM and Pelling
JC: Effect of extracellular signal-regulated kinase on p53
accumulation in response to cisplatin. J Biol Chem.
275:35778–35785. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kris-Etherton PM, Hecker KD, Bonanome A,
Coval SM, Binkoski AE, Hilpert KF, Griel AE and Etherton TD:
Bioactive compounds in foods: Their role in the prevention of
cardiovascular disease and cancer. Am J Med. 113(Suppl 9B):
S71–S88. 2002. View Article : Google Scholar
|
|
27
|
Kim HG, Hwang YP and Jeong HG: Kahweol
blocks STAT3 phosphorylation and induces apoptosis in human lung
adenocarcinoma A549 cells. Toxicol Lett. 187:28–34. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Oh JH, Lee JT, Yang ES, Chang JS, Lee DS,
Kim SH, Choi YH, Park JW and Kwon TK: The coffee diterpene kahweol
induces apoptosis in human leukemia U937 cells through
down-regulation of Akt phosphorylation and activation of JNK.
Apoptosis. 14:1378–1386. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Weusten-Van der Wouw MP, Katan MB, Viani
R, Huggett AC, Liardon R, Liardon R, Lund-Larsen PG, Thelle DS,
Ahola I, Aro A, et al: Identity of the cholesterol-raising factor
from boiled coffee and its effects on liver function enzymes. J
Lipid Res. 35:721–733. 1994.PubMed/NCBI
|
|
30
|
Ratnayake WM, Pelletier G, Hollywood R,
Malcolm S and Stavric B: Investigation of the effect of coffee
lipids on serum cholesterol in hamsters. Food Chem Toxicol.
33:195–201. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Schilter B, Perrin I, Cavin C and Huggett
AC: Placental glutathione S-transferase (GST-P) induction as a
potential mechanism for the anti-carcinogenic effect of the
coffeespecific components cafestol and kahweol. Carcinogenesis.
17:2377–2384. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Hammons GJ, Fletcher JV, Stepps KR, Smith
EA, Balentine DA, Harbowy ME and Kadlubar FF: Effects of
chemoprotective agents on the metabolic activation of the
carcinogenic arylamines PhIP and 4-aminobiphenyl in human and rat
liver microsomes. Nutr Cancer. 33:46–52. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Miller EG, Formby WA, Rivera-Hidalgo F and
Wright JM: Inhibition of hamster buccal pouch carcinogenesis by
green coffee beans. Oral Surg Oral Med Oral Pathol. 65:745–749.
1988. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Cavallini B, Huet J, Plassat JL, Sentenac
A, Egly JM and Chambon P: A yeast activity can substitute for the
HeLa cell TATA box factor. Nature. 334:77–80. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zheng XM, Black D, Chambon P and Egly JM:
Sequencing and expression of complementary DNA for the general
transcription factor BTF3. Nature. 344:556–559. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Kastan MB and Lim DS: The many substrates
and functions of ATM. Nat Rev Mol Cell Biol. 1:179–186. 2000.
View Article : Google Scholar
|
|
37
|
Powers SK, Kavazis AN and McClung JM:
Oxidative stress and disuse muscle atrophy. J Appl Physiol.
102:2389–2397. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Wada T and Penninger JM: Mitogen-activated
protein kinases in apoptosis regulation. Oncogene. 23:2838–2849.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Tibbles LA and Woodgett JR: The
stress-activated protein kinase pathways. Cell Mol Life Sci.
55:1230–1254. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Zhang W and Liu HT: MAPK signal pathways
in the regulation of cell proliferation in mammalian cells. Cell
Res. 12:9–18. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Ahn MY, Ahn JW, Kim HS, Lee J and Yoon JH:
Apicidin inhibits cell growth by downregulating IGF-1R in salivary
mucoepidermoid carcinoma cells. Oncol Rep. 33:1899–1907.
2015.PubMed/NCBI
|
|
42
|
Hyun JH, Kim SC, Kang JI, Kim MK, Boo HJ,
Kwon JM, Koh YS, Hyun JW, Park DB, Yoo ES, et al: Apoptosis
inducing activity of fucoidan in HCT-15 colon carcinoma cells. Biol
Pharm Bull. 32:1760–1764. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Zheng Q, Liu Y, Liu W, Ma F, Zhou Y, Chen
M, Chang J, Wang Y, Yang G and He G: Cucurbitacin B inhibits growth
and induces apoptosis through the JAK2/STAT3 and MAPK pathways in
SH-SY5Y human neuroblastoma cells. Mol Med Rep. 10:89–94.
2014.PubMed/NCBI
|
|
44
|
Sheng Z, Knowlton K, Chen J, Hoshijima M,
Brown JH and Chien KR: Cardiotrophin 1 (CT-1) inhibition of cardiac
myocyte apoptosis via a mitogen-activated protein kinase-dependent
pathway. Divergence from downstream CT-1 signals for myocardial
cell hypertrophy. J Biol Chem. 272:5783–5791. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Schafer KA: The cell cycle: A review. Vet
Pathol. 35:461–478. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Pines J: Cyclins and cyclin-dependent
kinases: Theme and variations. Adv Cancer Res. 66:181–212. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Deng C, Zhang P, Harper JW, Elledge SJ and
Leder P: Mice lacking p21CIP1/WAF1 undergo normal development, but
are defective in G1 checkpoint control. Cell. 82:675–684. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Pan ZQ, Reardon JT, Li L, Flores-Rozas H,
Legerski R, Sancar A and Hurwitz J: Inhibition of nucleotide
excision repair by the cyclin-dependent kinase inhibitor p21. J
Biol Chem. 270:22008–22016. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Yasutis KM and Kozminski KG: Cell cycle
checkpoint regulators reach a zillion. Cell Cycle. 12:1501–1509.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Khan S, Ferguson Bennit H, Asuncion
Valenzuela MM, Turay D, Diaz Osterman CJ, Moyron RB, Esebanmen GE,
Ashok A and Wall NR: Localization and upregulation of survivin in
cancer health disparities: A clinical perspective. Biologics.
9:57–67. 2015.PubMed/NCBI
|