Introduction
Numerous studies have confirmed that oncogenes
and/or tumor suppressors act as critical regulators for various
cellular processes (1,2), including the cell proliferation,
apoptosis, differentiation and movements. Moreover, emerging
studies have demonstrated that dysregulated oncogenes and/or tumor
suppressors could modulate numerous oncogenic and tumor suppressive
pathways in cancer cells and were actively implicated in the
initiation of human cancers including glioma (3–5). In
addition, oncogenes and/or tumor suppressors have been regarded as
attractive therapeutic targets and promising biomarkers for human
malignancies (6–8).
B-cell CLL/lymphoma 3 (BCL-3) is an atypical member
of the IκB family (9) and can bind
NF-κB homodimeric complexes of p50 or p52, which switches the
transcriptional properties of the homodimers from a repressive to
an activating state (10). The
expression of mRNA and protein of BCL-3 has been reported to be
overexpressed in breast cancer (10,11),
nasopharyngeal carcinoma (12),
endometrial cancer (13),
hepatocellular carcinoma (14) and
colorectal cancer (15).
Functionally, BCL-3 was found to regulate the colony formation and
cell cycle progression by regulating ubiquitination-mediated
degradation of c-Myc in colorectal cancer (16). Recently, Tu et al (14) reported that BCL3 promotes the tumor
growth of hepatocellular carcinoma by regulating cell proliferation
and the cell cycle through cyclin D1. A previous study demonstrated
that STAT3, an important oncogene in human cancer, was a bona fide
target of BCL3 in cervical cancer cell line (17). Recently, Mansour et al
(18) reported that the decoy
receptor DcR1 was induced in a p50/Bcl3-dependent manner and
attenuated the efficacy of temozolomide in glioblastoma cells.
However, the status of BCL3 and its exact roles in glioma have not
been investigated.
In the present study, BCL3 was found to be
overexpressed in glioma specimens and cells. The positive
expression of BCL3 conferred adverse clinical parameters and
reduced overall survival of the glioma patients. Moreover, BCL3
promoted the glioma cell proliferation and cell cycle progression
and inhibited apoptosis. Interestingly, STAT3 pathway was
recognized as a direct functional mediator of BCL3 in glioma.
Materials and methods
Human specimens and cell culture
A total of 86 human glioma specimens and 20 normal
brain tissues were collected from the People’s Hospital of Gaozhou.
Diagnoses were confirmed by pathologist. None of the patients
received preoperative immunotherapy, chemotherapy or radiotherapy.
These clinical samples were immediately fixed with formalin and
embedded with paraffin after surgical resection. The written
informed consents were obtained from every patient included in this
study. The clinical stage was based on World Health Orgnization
(WHO) grade (19). The demographic
and clinical information of patients are shown in Table I. The protocols involving clinical
tissues in the present study were approved by the Ethics Review
Committee of the People’s Hospital of Gaozhou.
 | Table ICorrelation between the
clinicopathological characteristics and BCL3 expression in
gliomas. |
Table I
Correlation between the
clinicopathological characteristics and BCL3 expression in
gliomas.
| Characteristics | Total no. of patients
(n=86) | No. of patients | P-value |
|---|
|
|---|
| BCL3 positive | BCL3 negative |
|---|
| Age (years) |
| <50 | 42 | 20 | 22 | 0.286 |
| ≥50 | 44 | 26 | 18 | |
| Gender |
| Male | 49 | 25 | 24 | 0.597 |
| Female | 37 | 21 | 16 | |
| Tumor size
(cm) |
| <5 | 31 | 11 | 20 | 0.012a |
| ≥5 | 55 | 35 | 20 | |
| KPS score |
| <80 | 51 | 22 | 29 | 0.020a |
| ≥80 | 35 | 24 | 11 | |
| WHO grade |
| I+II | 28 | 17 | 21 | 0.029a |
| III+IV | 58 | 39 | 19 | |
The normal human astrocyte (NHA) cell line was
obtained from the American Type Culture Collection (ATCC; Manassas,
VA, USA). The human glioma cell lines (U87, T98, A172 and U251)
were purchased from the Chinese Academy of Sciences (Shanghai,
China). All cells were cultured in Dulbecco’s modified Eagle’s
medium (DMEM; HyClone Laboratories, Logan, UT, USA) along with
fetal bovine serum (10%) (FBS; HyClone Laboratories), penicillin
(100 U/ml) and streptomycin (100 μg/ml). Cell cultures were kept in
an incubator containing 5% CO2 and humidified atmosphere
at 37°C.
Immunohistochemistry (IHC)
Before IHC staining, glioma specimens and normal
brain tissues were fixed with formalin and embedded with paraffin.
Then, the embedded tissues were cut into 4 μm thick sections. IHC
staining followed standard protocol to evaluate the expression
level of BCL3 (sc-185; Santa Cruz Biotechnology, Santa Cruz, CA,
USA) and STAT3 (#9139; Cell Signaling Technology, Boston, MA, USA)
in human tissues. The percentage of positive tumor cells was graded
as per the following criteria: 0, <10%; 1, 10–30%; 2, 31–50%;
and 3, >50%.
Cell transfection
BCL3 shRNA (sc-29789-SH) and corresponding
non-target (NT) shRNA (sc-108060) were obtained from Santa Cruz
Biotechnology. shRNAs transfection was performed using plasmid
transfection reagent (sc-108061; Santa Cruz Biotechnology).
Retroviral vector pMMP-BCL3 and pMMP-STAT3 were generated by
inserting the cDNA into pMMP. Retrovirus packaging and transduction
were previously described (20). A
STAT3 specific siRNA and scrambled siRNA was obtained from OriGene
Technologies, Inc. (Shanghai, China). The siRNAs were transduced
into glioma cells with Lipofectamine 2000 (Invitrogen, Carlsbad,
CA, USA) following the manufacturer’s protocol.
CCK-8 and BrdU incorporation assay
Glioma cells that were treated with corresponding
vectors were seeded into 96-well plates (1.5×103
cells/well); 24, 48, 72 and 96 h after transfection, the cell
proliferation assay was performed by addition of 10 μl Cell
Counting kit-8 (CCK-8) solution (Beyotime Institute of
Biotechnology, Shanghai, China) to each well, followed by
incubation at 37°C for 2 h. Absorbance was measured at a wavelength
of 490 nm using a microplate reader (FlexStation III ROMV2.1.28;
Molecular Devices, Sunnyvale, CA, USA). For 5-bromodeoxyuridine
(BrdU) assays, 48 h after transfection, glioma cells grown on
coverslips were incubated with BrdU at room temperature for 60 min
and subsequently were incubated with the antibody of BrdU
(Sigma-Aldrich, St. Louis, MO, USA) following the manufacturer’s
protocol.
Cell cycle assay
Forty-eight hours after transfection, glioma cells
were collected with trypsin and fixed overnight at 4°C using 80%
ethanol. Glioma cells were then incubated with propidium iodide
(PI; Sigma-Aldrich) for 20 min. Then, flow cytometry assays for
glioma cells were performed with a FACSCalibur (BD Biosciences,
Bedford, MA, USA).
Cell apoptosis assay
Apoptosis of cells was evaluated with the
Annexin-V-FLUOS staining kit (Roche Diagnostics, Indianapolis, IN,
USA) following standard protocol provided by the manufacturer. Then
flow cytometry assay was performed with a FACSCalibur (BD
Biosciences).
Western blot analysis
Total proteins were collected with RIPA lysis
buffer, and 40 μg protein were subjected to 4–20% SDS gel
electrophoresis and were then transferred to PVDF membranes. Then,
5% milk blocked membranes were incubated with BCL3 (Santa Cruz
Biotechnology), STAT3 (Cell Signaling Technology), p-STAT3 (Cell
Signaling Technology), BCL2 (Cell Signaling Technology), MCL-1
(Cell Signaling Technology) or cyclin D1 (Cell Signaling
Technology) antibodies, respectively, and subsequently incubated
with matched secondary antibodies (Cell Signaling Technology).
Then, signals for each protein expression were detected with the
Bio-Rad Gel imaging system. GAPDH (Cell Signaling Technology) was
used as a loading control.
In vivo experiments
The subcutaneous tumor formation experiments were
performed on nude mice. U251 cells (5×106) with BCL3
knockdown or cells of control group were suspended in PBS and were
implanted into the back of mice via subcutaneous injection. Tumor
volume was measured with calipers every 3 days. The xenograft tumor
tissues were subjected to immunoblotting for Ki-67 (Cell Signaling
Technology) and caspase-3 (Cell Signaling Technology). The
protocols of in vivo experiments were approved by the
Institutional Animal Care and Use Committee of the People’s
Hospital of Gaozhou.
Statistical analysis
All data were collected and showed as mean ±
standard error (SE). GraphPad Prism 5 software (GraphPad Software,
Inc., San Diego, CA, USA) was used in the present study to perform
statistical analysis. P<0.05 was considered to be statistically
different.
Results
Overexpression of BCL3 in glioma tissues
and cells confers disease progression
IHC was initially performed to detect BCL3
expression in glioma specimens and normal brain tissues. IHC
sections were scored according to the percentage of positive cells
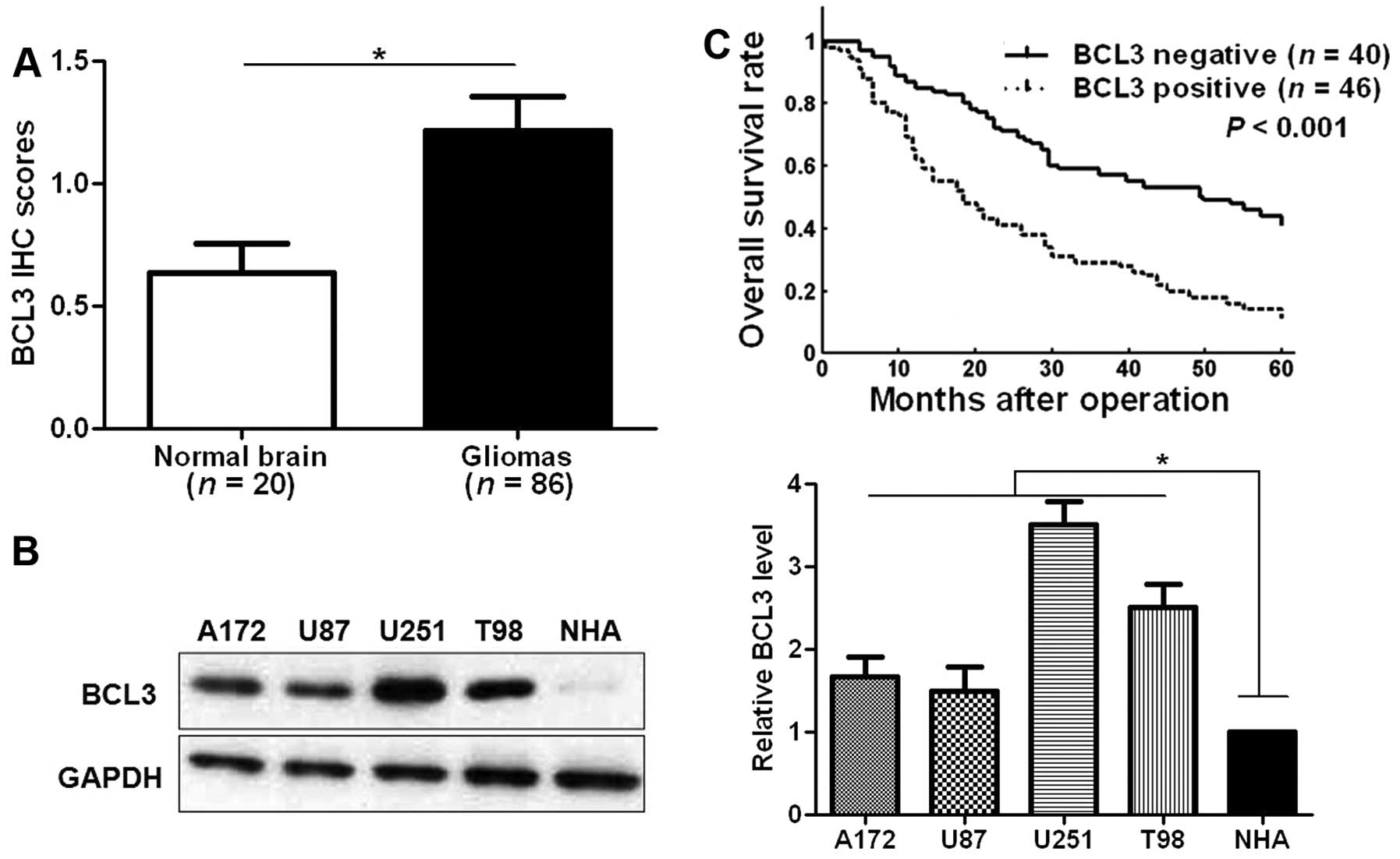
(Fig. 1). The level of BCL3 was
significantly upregulated in glioma tissues as compared with normal
brain tissues (P<0.05; Fig.
2A). Furthermore, our data revealed that BCL3 expression in
four glioma cell lines (A172, U87, U251 and T98 cells) was
evidently increased compared to an NHA cell line (P<0.05,
respectively; Fig. 2B). BCL3
staining was considered as positive expression in 46 cases of
glioma according to IHC scores (1–3). As
shown in Table I, positive
expression of BCL3 in glioma patients was positively correlated
with large tumor size (P=0.012), high Karnofsky performance status
(KPS) score (P=0.020) and advanced WHO grade (P=0.029). Then, we
examined the prediction value of BCL3 for the prognosis of glioma
patients. Compared with those with negative expression of BCL3,
patients that showed positive expression of BCL3 had significant
reduced 5-year overall survival (P<0.001; Fig. 2C). The above indicate that BCL3 can
potentially act as biomarker and prognostic indicator for
glioma.
BCL3 regulates biological function in
glioma cell lines
Next, we disclosed the exact roles of BCL3 in glioma
cells. Since increased proliferation, aberrant cell cycle
progression and reduced apoptosis are well-recognized hallmarks of
human cancer (21), we examined
whether BCL3 regulated these biological processes of glioma cells.
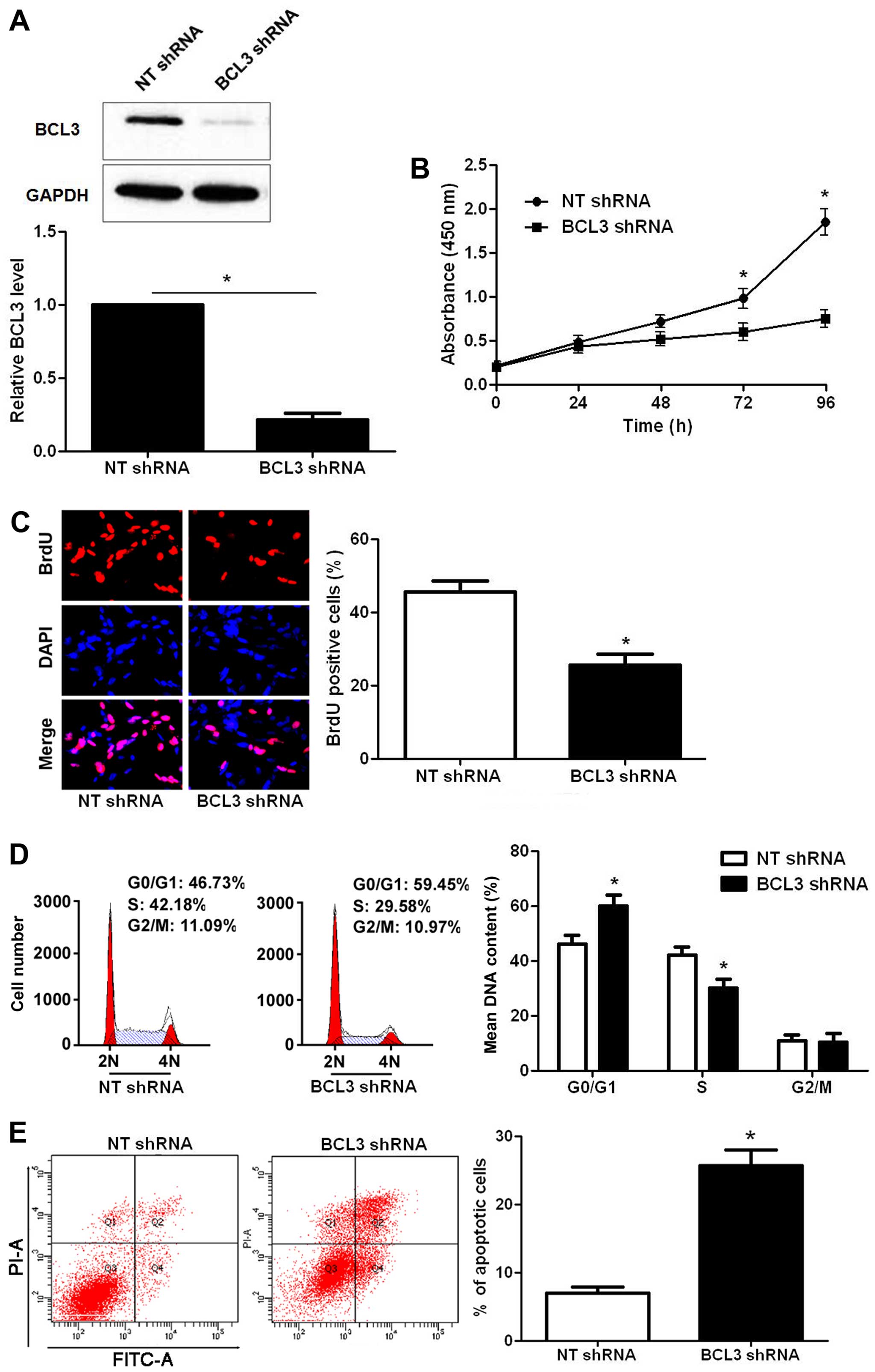
BCL3 knockdown was confirmed by immunoblotting after BCL3 shRNA
transfection (P<0.05; Fig. 3A).
CCK-8 and BrdU assays indicated that BCL3 knockdown significantly
reduced the proliferative ability of U251 cells (P<0.05,
respectively; Fig. 3B and C).
Moreover, BCL3 knockdown led to a prominent cell cycle arrest at
G1-phase (P<0.05; Fig. 3D). The
portion of apoptotic cells were evidently increased after BCL3
silencing (P<0.05; Fig. 3E). On
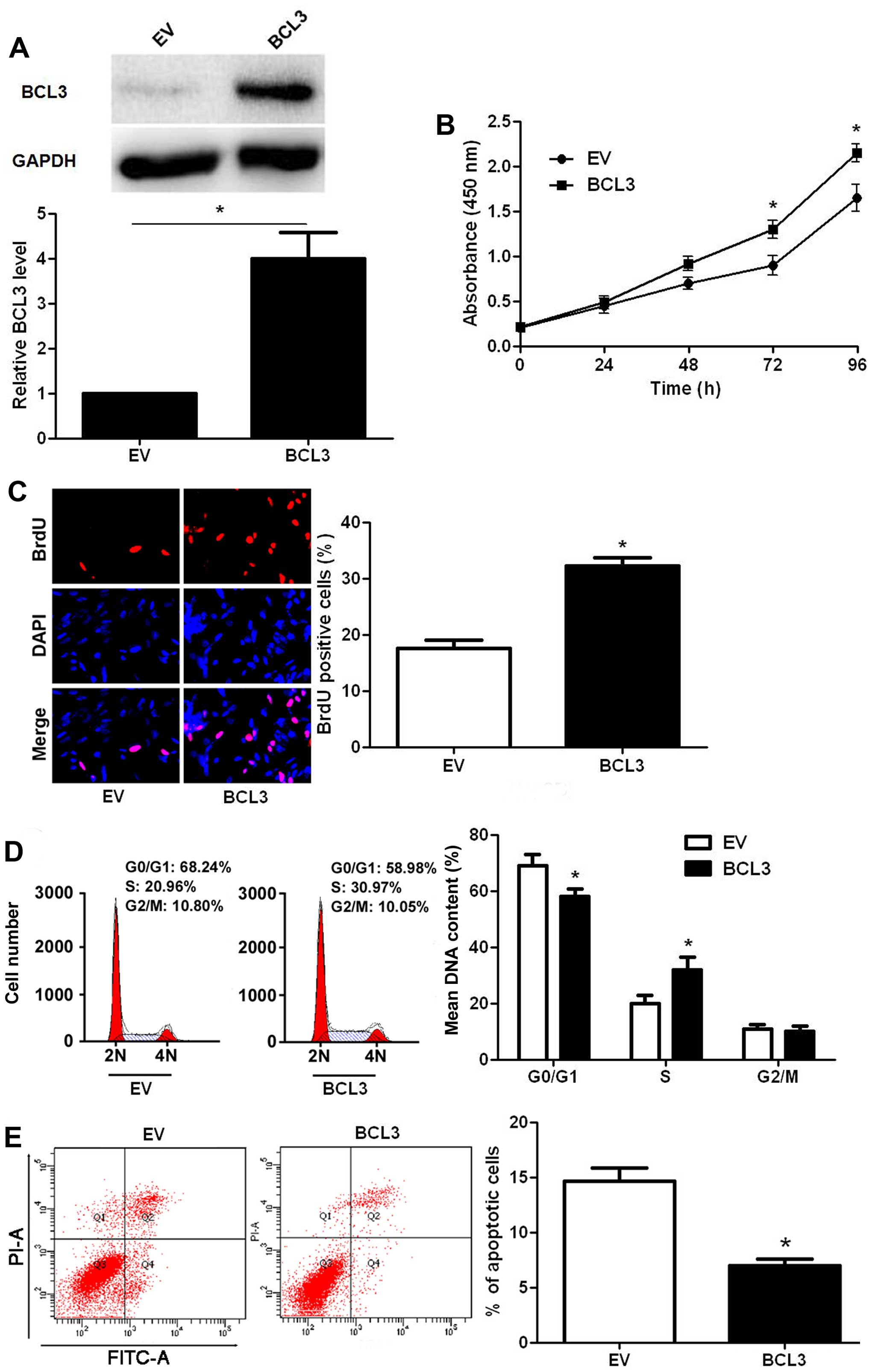
the contrary, infection of BCL3 retroviruses led to a significantly
increased expression of BCL3 in U87 cells (P<0.05; Fig. 4A). Functionally, BCL3
overexpression facilitated cell proliferation (P<0.05,
respectively; Fig. 4B and C) and
cell cycle progression (P<0.05; Fig. 4D), while suppressed apoptosis in
U87 cells (P<0.05; Fig. 4E).
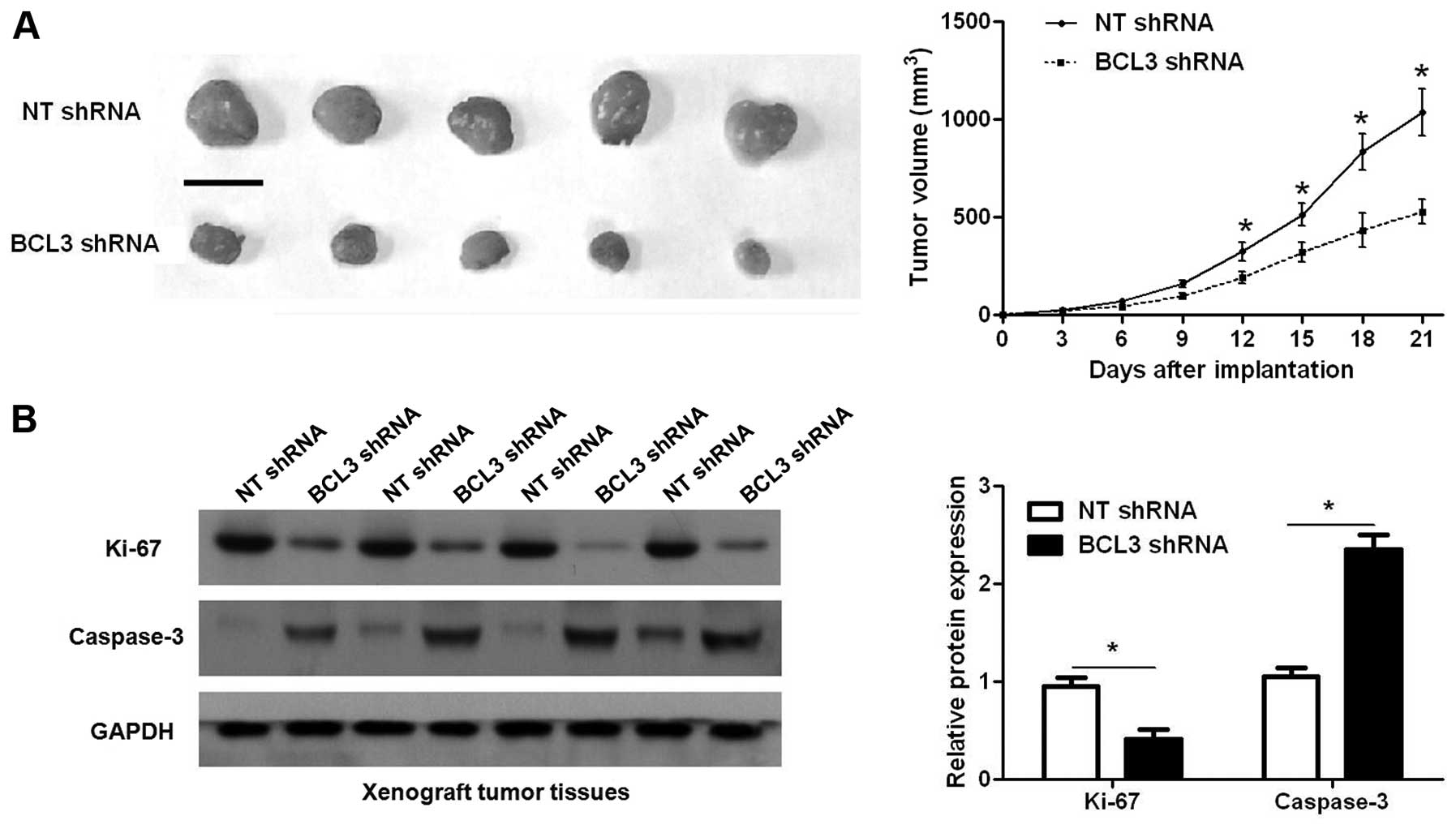
Furthermore, we used a subcutaneous tumor formation model to
investigate whether BCL3 could affect the growth of glioma cells in
a mouse xenograft model. As shown in Fig. 5A, BCL3 knockdown significantly
reduced the tumor growth of U251 cells in mice (P<0.05).
Furthermore, immunoblotting of Ki-67 and caspase-3 indicated BCL3
knockdown inhibited proliferation and induced apoptosis of glioma
cells in vivo (P<0.05, respectively; Fig. 5B). Thus, BCL3 functions as an
oncogene by promoting proliferation and the process of cell cycle,
and inhibiting apoptosis in glioma.
BCL3 positively regulates STAT3 abundance
in glioma
A previous study demonstrated that STAT3, an
important oncogene in human cancer, was a bona fide target of BCL3
in cervical cancer cell line (17). U251 cells that were transduced with
NT shRNA and BCL3 shRNA, respectively, were subjected to
immunoblotting for BCL3, STAT3 and p-STAT3 expression. As expected,
BCL3 knockdown significantly reduced STAT3 and p-STAT3 expression
in vitro (Fig. 6). Since
BCL2, MCL-1 and cyclin D1 are downstream molecules involved in
STAT3 signaling pathway (22).
Notably, BCL3 knockdown coordinately reduced the levels of BCL2,
MCL-1 and cyclin D1 in U251 cells (Fig. 6). In contrast, BCL3 overexpression
resulted in an obvious increase of STAT3, p-STAT3, BCL2, MCL-1 and
cyclin D1 protein expression (Fig.
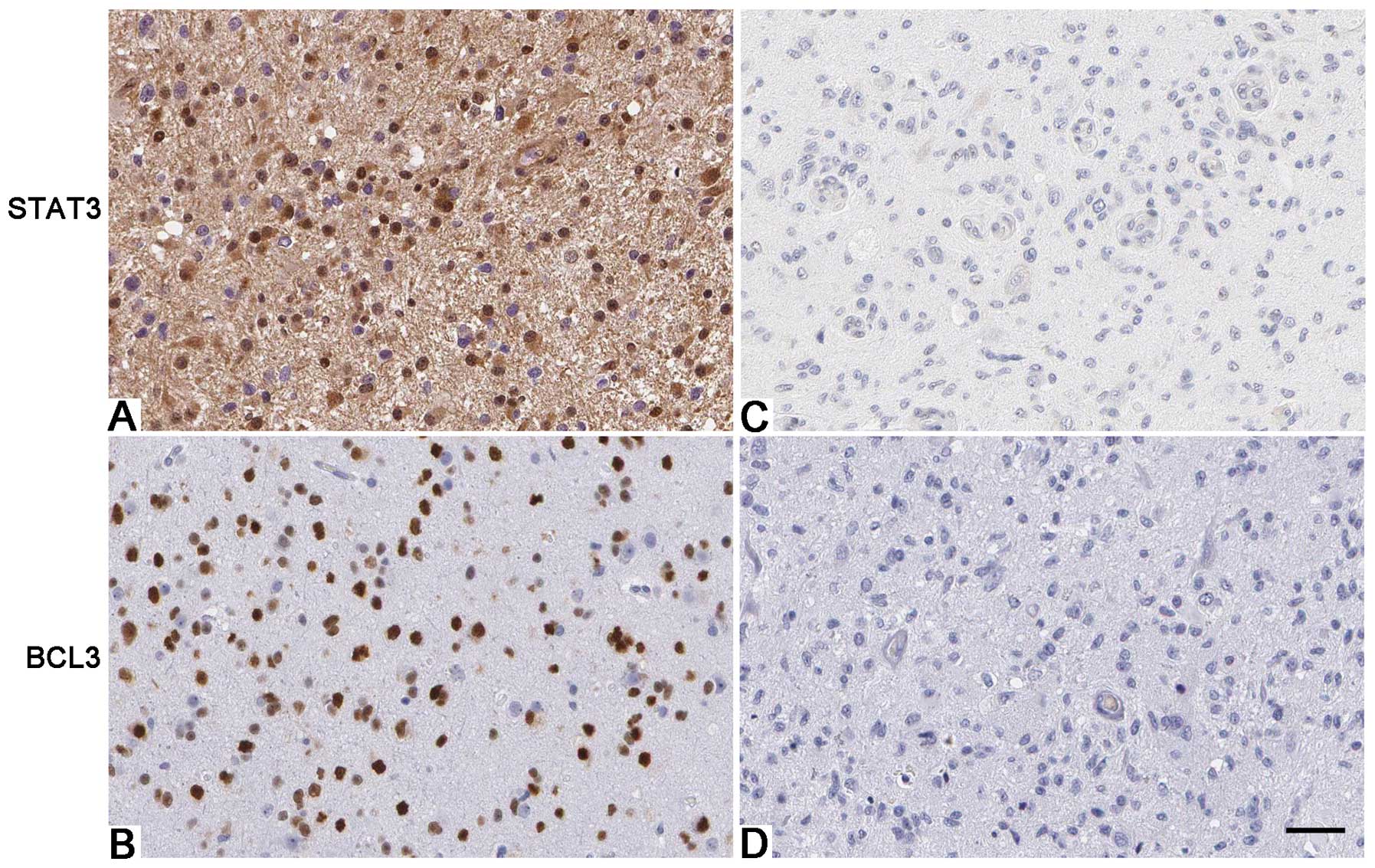
6) in U87 cells. Furthermore, IHC was performed to detect the
expression of STAT3 in glioma specimens. Interestingly, Spearman’s
correlation analysis indicated that BCL3 was positively correlated
with STAT3 expression in glioma specimens (r=0.673, P<0.001;
Fig. 7).
STAT3 knockdown reverses the oncogenic
role of BCL3 in vitro
To determine whether STAT3 silencing could attenuate
the oncogene function of BCL3 in glioma, a series of rescue
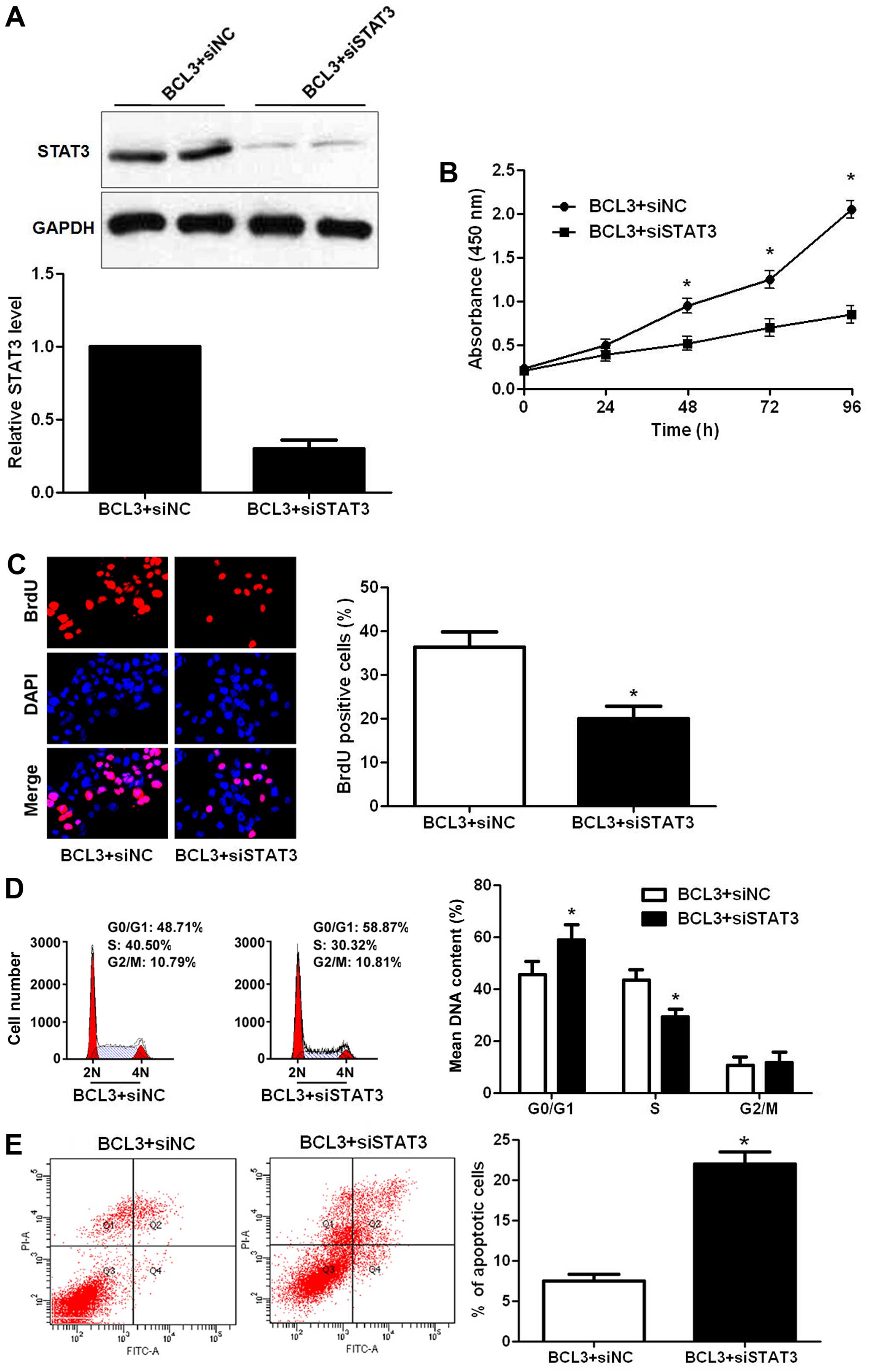
experiments were performed. STAT3 siRNA were employed for STAT3
knockdown in BCL3 overexpressing U87 cells (P<0.05; Fig. 8A). Notably, STAT3 knockdown
abolished the oncogenic effects of BCL3 overexpression on U87 cells
with decreased cell proliferation (P<0.05, respectively;
Fig. 8B and C), cell cycle arrest
(P<0.05; Fig. 8D) and increased
apoptosis (P<0.05; Fig. 8E).
Our data indicate that STAT3 mediates the functions of BCL3 in
glioma cells.
Discussion
Increasing evidence confirms that oncogenes play
critical roles in the progression of human malignancies including
glioma (23). Functionally,
oncogenes were found to modulate the proliferation, apoptosis and
metastasis of glioma cells (24).
Moreover, oncogenes were proposed as novel biomarkers and treatment
targets for glioma (25).
Therefore, elucidating the expression level, clinical significance
and biological function of specific oncogene in glioma will greatly
contribute to the diagnosis and treatment of glioma. Here,
overexpression of BCL3 was observed in glioma specimens and cells.
In addition, positive expression of BCL3 in glioma was directly
correlated with the adverse clinical features. Importantly, we
suggest that positive expression of BCL3 was associated with the
reduced 5-year overall survival of glioma patients. Therefore,
these data in the present study demonstrate that BCL3 can act as a
promising biomarker and prognostic indicator for glioma.
BCL3, a well-known proto-oncogene, is deregulated in
solid malignancies and exerts an oncogenic role by regulating
proliferation and cell death (17). Study of breast cancer confirmed
that BCL3-dependent stabilization of CtBP1 was crucial for the
inhibition of apoptosis and tumor progression (11). Moreover, BCL3 promoted the cell
proliferation of ovarian cancer cells (26). In this study, in vitro
experiments confirmed that BCL3 promoted the proliferative ability
and cell cycle progression of glioma cells while induced apoptosis.
Moreover, in vivo experiments showed that BCL3 knockdown
could inhibit the tumor growth of glioma in nude mice. Therefore,
this study confirms that BCL3 exerts an oncogenic role by
modulating proliferation, cell cycle progression and apoptosis in
glioma.
STAT3, an important oncogene, was found to be
over-expressed and persistently active in ~70% of human cancers
including glioma (27–30). STAT3 activation is implicated in
cell proliferation, apoptosis, migration, invasion and angiogenesis
in glioma (30). Phosphorylation
of tyrosine residue (Y705) is necessary for STAT3 activation and
positively regulates its downstream targets including BCL2, MCL-1
and cyclin D1. Cyclin D1 knockdown confers cell cycle arrest at
G1-phase (14). Moreover, p-STAT3
knockdown facilitates apoptosis via downregulation of MCL-1 and
BCL2 (31). Here, we presented
that BCL3 positively regulated the levels of STAT3, p-STAT3 and its
downstream molecules including BCL2, MCL-1 and cyclin D1.
Furthermore, IHC staining indicated that BCL3 protein levels
positively correlated with STAT3 protein expression in human glioma
specimens. These data further demonstrate that STAT3 is a
downstream target of BCL3 in glioma. Notably, STAT3 knockdown could
abrogate the influence of BCL3 overexpression on proliferation, the
process of cell cycle and apoptosis in glioma cells. Therefore,
these results indicate that STAT3 serves as not only a downstream
target of BCL3 but also a functional mediator of BCL3 in
glioma.
Collectively, we present that BCL3 is overexpressed
in glioma and its positive expression is correlated with poor
clinical parameters and survival. BCL3 promotes the tumor growth of
glioma through regulating proliferative ability, process of cell
cycle and apoptosis. Mechanistically, this study finds that BCL3
functions as an oncogenic factor by activating STAT3 pathway. Taken
together, BCL3 may serve as a clinical indicator and a drug target
for glioma patients.
Acknowledgements
The authors thank all the patients who participated
in the present study.
References
|
1
|
Viale A, Pettazzoni P, Lyssiotis CA, Ying
H, Sánchez N, Marchesini M, Carugo A, Green T, Seth S, Giuliani V,
et al: Oncogene ablation-resistant pancreatic cancer cells depend
on mitochondrial function. Nature. 514:628–632. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Antal CE, Hudson AM, Kang E, Zanca C,
Wirth C, Stephenson NL, Trotter EW, Gallegos LL, Miller CJ, Furnari
FB, et al: Cancer-associated protein kinase C mutations reveal
kinase’s role as tumor suppressor. Cell. 160:489–502. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chistiakov DA and Chekhonin VP:
Extracellular vesicles shed by glioma cells: Pathogenic role and
clinical value. Tumour Biol. 35:8425–8438. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zhang XQ and Leung GK: Long non-coding
RNAs in glioma: Functional roles and clinical perspectives.
Neurochem Int. 77:78–85. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chautard E, Ouédraogo ZG, Biau J and
Verrelle P: Role of Akt in human malignant glioma: From oncogenesis
to tumor aggressiveness. J Neurooncol. 117:205–215. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Katsetos CD, Reginato MJ, Baas PW,
D’Agostino L, Legido A, Tuszyn Ski JA, Dráberová E and Dráber P:
Emerging microtubule targets in glioma therapy. Semin Pediatr
Neurol. 22:49–72. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Awad AJ, Burns TC, Zhang Y and Abounader
R: Targeting MET for glioma therapy. Neurosurg Focus. 37:E102014.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Huse JT and Aldape KD: The evolving role
of molecular markers in the diagnosis and management of diffuse
glioma. Clin Cancer Res. 20:5601–5611. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bours V, Franzoso G, Azarenko V, Park S,
Kanno T, Brown K and Siebenlist U: The oncoprotein Bcl-3 directly
transactivates through kappa B motifs via association with
DNA-binding p50B homodimers. Cell. 72:729–739. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Cogswell PC, Guttridge DC, Funkhouser WK
and Baldwin AS Jr: Selective activation of NF-kappa B subunits in
human breast cancer: Potential roles for NF-kappa B2/p52 and for
Bcl-3. Oncogene. 19:1123–1131. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Choi HJ, Lee JM, Kim H, Nam HJ, Shin HJ,
Kim D, Ko E, Noh DY, Kim KI, Kim JH, et al: Bcl3-dependent
stabilization of CtBP1 is crucial for the inhibition of apoptosis
and tumor progression in breast cancer. Biochem Biophys Res Commun.
400:396–402. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Thornburg NJ, Pathmanathan R and
Raab-Traub N: Activation of nuclear factor-kappaB p50
homodimer/Bcl-3 complexes in nasopharyngeal carcinoma. Cancer Res.
63:8293–8301. 2003.PubMed/NCBI
|
|
13
|
Pallares J, Martínez-Guitarte JL, Dolcet
X, Llobet D, Rue M, Palacios J, Prat J and Matias-Guiu X:
Abnormalities in the NF-kappaB family and related proteins in
endometrial carcinoma. J Pathol. 204:569–577. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tu K, Liu Z, Yao B, Xue Y, Xu M, Dou C,
Yin G and Wang J: BCL-3 promotes the tumor growth of hepatocellular
carcinoma by regulating cell proliferation and the cell cycle
through cyclin D1. Oncol Rep. 35:2382–2390. 2016.PubMed/NCBI
|
|
15
|
Puvvada SD, Funkhouser WK, Greene K, Deal
A, Chu H, Baldwin AS, Tepper JE and O’Neil BH: NF-κB and Bcl-3
activation are prognostic in metastatic colorectal cancer.
Oncology. 78:181–188. 2010. View Article : Google Scholar :
|
|
16
|
Liu Z, Jiang Y, Hou Y, Hu Y, Cao X, Tao Y,
Xu C, Liu S, Wang S, Wang L, et al: The IκB family member Bcl-3
stabilizes c-Myc in colorectal cancer. J Mol Cell Biol. 5:280–282.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Maldonado V, Espinosa M, Pruefer F, Patiño
N, Ceballos-Canciono G, Urzua U, Juretic N and Melendez-Zajgla J:
Gene regulation by BCL3 in a cervical cancer cell line. Folia Biol
(Praha). 56:183–193. 2010.
|
|
18
|
Mansour NM, Bernal GM, Wu L, Crawley CD,
Cahill KE, Voce DJ, Balyasnikova IV, Zhang W, Spretz R, Nunez L, et
al: Decoy receptor DcR1 is induced in a p50/Bcl3-dependent manner
and attenuates the efficacy of temozolomide. Cancer Res.
75:2039–2048. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Pessôa IA, Sagica FE, Anselmo NP, Brito JR
and de Oliveira EH: IDH1 and IDH2 mutations in different histologic
subtypes and WHO grading gliomas in a sample from Northern Brazil.
Genet Mol Res. 14:6533–6542. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tu K, Li J, Verma VK, Liu C, Billadeau DD,
Lamprecht G, Xiang X, Guo L, Dhanasekaran R, Roberts LR, et al:
Vasodilator-stimulated phosphoprotein promotes activation of
hepatic stellate cells by regulating Rab11-dependent plasma
membrane targeting of transforming growth factor beta receptors.
Hepatology. 61:361–374. 2015. View Article : Google Scholar
|
|
21
|
Cipriano R, Miskimen KL, Bryson BL, Foy
CR, Bartel CA and Jackson MW: Conserved oncogenic behavior of the
FAM83 family regulates MAPK signaling in human cancer. Mol Cancer
Res. 12:1156–1165. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Li H, Liu YW, Wang H, Zhou Q, Li JJ, Annie
H, Qi ST and Lu YT: MiR-519a functions as a tumor suppressor in
glioma by targeting the oncogenic STAT3 pathway. J Neurooncol.
128:35–45. 2016. View Article : Google Scholar
|
|
23
|
Hartel I, Ronellenfitsch M, Wanka C,
Wolking S, Steinbach JP and Rieger J: Activation of AMP-activated
kinase modulates sensitivity of glioma cells against epidermal
growth factor receptor inhibition. Int J Oncol. 49:173–180.
2016.PubMed/NCBI
|
|
24
|
Foster KA, Jane EP, Premkumar DR, Morales
A and Pollack IF: NVP-BKM120 potentiates apoptosis in tumor
necrosis factor-related apoptosis-inducing ligand-resistant glioma
cell lines via upregulation of Noxa and death receptor 5. Int J
Oncol. 47:506–516. 2015.PubMed/NCBI
|
|
25
|
Wang H, Wang Y, Bao Z, Zhang C, Liu Y, Cai
J and Jiang C: Hypomethylated Rab27b is a progression-associated
prognostic biomarker of glioma regulating MMP-9 to promote
invasion. Oncol Rep. 34:1503–1509. 2015.PubMed/NCBI
|
|
26
|
Guan Y, Yao H, Zheng Z, Qiu G and Sun K:
MiR-125b targets BCL3 and suppresses ovarian cancer proliferation.
Int J Cancer. 128:2274–2283. 2011. View Article : Google Scholar
|
|
27
|
Wu ZL, Song YQ, Shi YF and Zhu J: High
nuclear expression of STAT3 is associated with unfavorable
prognosis in diffuse large B-cell lymphoma. J Hematol Oncol.
4:312011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chen Y, Wang J, Wang X, Liu X, Li H, Lv Q,
Zhu J, Wei B and Tang Y: STAT3, a poor survival predicator, is
associated with lymph node metastasis from breast cancer. J Breast
Cancer. 16:40–49. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Takemoto S, Ushijima K, Kawano K,
Yamaguchi T, Terada A, Fujiyoshi N, Nishio S, Tsuda N, Ijichi M,
Kakuma T, et al: Expression of activated signal transducer and
activator of transcription-3 predicts poor prognosis in cervical
squamous-cell carcinoma. Br J Cancer. 101:967–972. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Brantley EC and Benveniste EN: Signal
transducer and activator of transcription-3: A molecular hub for
signaling pathways in gliomas. Mol Cancer Res. 6:675–684. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Iwamaru A, Szymanski S, Iwado E, Aoki H,
Yokoyama T, Fokt I, Hess K, Conrad C, Madden T, Sawaya R, et al: A
novel inhibitor of the STAT3 pathway induces apoptosis in malignant
glioma cells both in vitro and in vivo. Oncogene. 26:2435–2444.
2007. View Article : Google Scholar
|