Introduction
Hepatocellular carcinoma (HCC) is a major cause of
cancer-related death (1). It is
highly resistant to available chemotherapeutic agents, leaving HCC
patients with no effective therapeutic option and a poor prognosis.
The ability to avoid cell death is a hallmark of cancer cells, and
their survival in an adverse environment under cellular stresses
(e.g., hypoxia, nutrient deficiency and treatment stress)
contributes to therapeutic failure and tumor progression (2,3).
Although cell death has been attributed to unrestrained autophagy,
increasing data indicate that autophagy may also function as an
important tumor-protective mechanism in HCC (4,5).
Chaperone-mediated autophagy (CMA) refers to
selective autophagy of a subset of cytosolic proteins containing
amino acid sequence motif KFERQ in lysosomes. Around 30%
oxidatively modified proteins targeted to lysosomes by this
pathway, and directly transport through the lysosome membrane for
degradation (6). Modulation of CMA
is known to underlie the pathogenesis of some systemic diseases
such as Parkinson’s disease (7),
Danon disease (8) and
mucolipidosis type IV (9).
Therefore, understanding of CMA activities under different
pathological and physiological conditions is increasing day by day,
especially in cancer (10).
Although in most cells a basal level of CMA activity may exist, the
pathway is further activated by nutritional stress (11). Activation during prolonged
starvation is associated with increased levels of
lysosome-associated membrane protein type 2a (Lamp2a) at the
lysosomal membrane, which represents a limiting step in this
pathway (10,12). Lamp2a in the lysosomal membrane
acts as a receptor of substrate proteins for CMA, and Lamp2a in the
lysosomal membrane have been shown to correlate directly with CMA
activity under pathological and physiological conditions (13–15).
We previously demonstrated that macroautophagy
defects at an early stage of oncogenesis may contribute to the
malignant differentiation and invasive phenotype in HCC (16). Furthermore, once a tumor is formed,
macroautophagy can protect HCC cells against apoptosis induced by
antineoplastic agents through its degradative process (17–19).
We further investigated CMA status and function in HCC by focusing
on the regulatory role of Lamp2a during cancer cell starvation.
Materials and methods
Cell lines and animals
The human HCC cell lines HepG2, Hep3B, Huh7,
MHCC97L, MHCC97H and HCCLM3, and the human normal hepatic cell line
L-02, were routinely maintained in high-glucose Dulbecco’s modified
Eagle’s media (DMEM) supplemented with 10% heat-inactivated fetal
bovine serum (FBS), 100 U/ml penicillin and 100 mg/ml streptomycin.
All cell lines were cultured at 37°C in a humidified incubator in
an atmosphere of 5% CO2. Male athymic BALB/c nude mice
(4-weeks old; Shanghai Institute of Material Medicine, Chinese
Academy of Science, Shanghai, China) were raised under
pathogen-free conditions. All animal care and experimental
protocols were carried out in accordance with the guidelines
established by the Shanghai Medical Experimental Animal Care
Commission.
Patient samples
Patient samples were collected after obtaining
informed consent according to an established protocol approved by
the Ethics Committee of Fudan University. The data did not contain
any information that could lead to the identification of the
individual patients.
Samples for real-time polymerase chain reaction
(PCR) studies were randomly collected from patients undergoing
curative resection for HCC at the Liver Cancer Institute, Zhongshan
Hospital, Fudan University, in March 2006. Samples were collected
immediately after resection, transported in liquid nitrogen, and
stored at −80°C. Sixteen frozen tissue samples were also obtained
from the above patients for western blot studies.
Tumor specimens for tissue microarray (TMA) studies
were obtained from 228 consecutive HCC patients who underwent
curative resection without preoperative treatment at the Liver
Cancer Institute, Zhongshan Hospital, Fudan University, between
2005 and 2006. Complete follow-up data were available for each
patient, and the diagnosis of HCC was confirmed by pathological
examination.
Real-time PCR
Total RNA was extracted from cell lines and frozen
tumor specimens using TRIzol reagent (Invitrogen, Carlsbad, CA,
USA). Total RNA (2 μg) was reverse transcribed using a RevertAid
First-Strand cDNA Synthesis kit (Fermentas, Burlington, ON,
Canada). Reverse transcription-PCR was performed before
quantitative real-time PCR. Lamp2a mRNA expression was determined
by real-time PCR using SYBR Premix Ex Taq (Takara Bio, Dalian,
China). PCR amplification cycles were carried out for 10 sec at
95°C, followed by 40 cycles of 95°C for 5 sec and 60°C for 30 sec.
Data were collected after each annealing step. β-actin was used as
an endogenous control to normalize for differences in the amounts
of total RNA in each sample. Relative gene expression levels were
calculated and were expressed as 2−ΔCt, as previously
described (16). The following
primers were used: β-actin 5′-CAACTGGGACGACATGGAGAAAAT-3′ and
5′-CCAGAGGCGTACAGGGATAGCAC-3′; Lamp2a 5′-AGACTGCAGTGCAGATGACGAC-3′
and 5′-GACCAATAAAATAAGCCAGCAAC-3′.
Western blot analysis
Western blot analysis was performed as previously
described (16). Briefly, proteins
from total cell lysates were separated by standard sodium dodecyl
sulfate-polyacrylamide gel electrophoresis and then transferred to
polyvinylidene difluoride membranes. The membranes were washed,
blocked and incubated with specific primary anti-human antibodies
against β-actin (1:1,000; ab8226; Abcam, Cambridge, MA, USA) or
Lamp2a (1:1,000; ab18528; Abcam), followed by incubation with
horseradish peroxidase-conjugated secondary antibodies. The
reactions were detected by enhanced chemiluminescence assay.
Transfection and clone selection
Lentiviral-mediated pGC-GFP-Lamp2a and
pGCSIL-GFP-Lamp2a short hairpin RNAs (shRNAs) were constructed
(Shanghai Genechem, Co., Ltd., Shanghai, China). The shRNA
targeting sequence (5′-AAGCACCATCATGCTGGATAT-3′) for Lamp2a was
used. Huh7 and HCCLM3 cells were transfected with lentivirus
particles and the cell populations expressing GFP-Lamp2a or
GFP-Lamp2a shRNA were isolated by flow cytometry (BD Biosciences,
San Jose, CA, USA). Stable transfectant clones were further
validated by real-time PCR, and immunoblotting was used to
determine the expression levels of Lamp2a protein.
Cell viability, migration and invasion
assays
Cell viability was assessed using an MTT
(3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2-H-tetrazolium bromide)
kit (Trevigen, Gaithersburg, MD, USA), according to the
manufacturer’s protocol. Cells (5×103) were seeded in
96-well plates, incubated for 24 h at 37°C, and treated with the
specified agents at defined time-points.
Cell migration was evaluated using the scratch-wound
assay. Cells were cultured for 2 days to form a tight cell
monolayer and then serum-starved for 6 h. After serum starvation,
the cell monolayer was wounded using a 20-μl plastic pipette tip.
The remaining cells were washed twice with culture medium to remove
cell debris and incubated at 37°C with normal serum-containing
culture medium. Migrating cells at the wound front were
photographed at the indicated times, using an inverted microscope
(Leica Microsystems, Wetzlar, Germany). The percentage of the
cleared area at each time-point compared with time zero was
measured using Image-Pro Plus software v6.2.
Cell invasion assays were performed using
Transwell filters
Filters coated with Matrigel (BD Biosciences) in the
upper compartment were loaded with 100 μl medium containing
1×105 cells and the lower compartment was filled with
conditioned culture medium mixed with DMEM and supplemented with
10% FBS, NIH3T3 and HCC cell super supplements. After 36 h,
migrated cells on the bottom surface were fixed with 4%
paraformaldehyde and counted after Giemsa staining.
Autophagy analysis
Autophagy was assessed using GFP-LC3 redistribution.
Redistribution of GFP-LC3 was detected using an inverted
fluorescence microscope. The number of GFP-LC3-positive dots per
cell was determined in three independent experiments. Eight
randomly selected fields representing 200 cells were counted.
In vivo tumorigenicity
HCCLM3-control, HCCLM3-Lamp2a, Huh7-control and
Huh7-Lamp2a shRNA cells (5×106) were suspended in 100 μl
serum-free DMEM and Matrigel (BD Biosciences) (1:1), and then
inoculated into the liver parenchyma or the right upper flank
subcutaneous region of nude mice, as previously described (20). The mice were sacrificed 5 weeks
after tumor implantation. At necropsy, the volumes of the largest
(a) and smallest (b) tumors were measured and the tumor volume was
calculated as: V = a × b2 × π/6. The tumor sections were
prepared for immunohistochemical staining. Immunoreactivity was
analyzed using Ki-67 (1:1,000; ab15580; Abcam) and P62 (1:1,000;
ab56416; Abcam) in tumor tissues. Terminal deoxynucleotidyl
transferase-mediated deoxyuridine triphosphate nick-end labeling
(TUNEL) staining was performed using an In Situ Apoptosis Detection
kit (VB-4005; GeneCopoeia, Rockville, MD, USA), according to the
manufacturer’s instructions.
TMA and immunohistochemistry
TMA was constructed as previously described
(21). Briefly, all the HCC
tissues were reviewed by two histopathologists, and representative
areas free from necrotic and hemorrhagic material were premarked in
the paraffin blocks. Two core biopsies (1 mm in diameter) were
taken from the donor blocks and transferred to the recipient
paraffin block at defined array positions. Three different TMA
blocks were constructed, each containing 200 cylinders. Consecutive
sections (4 μm in thickness) were placed on
3-aminopropyltriethoxysilane-coated slides (Shanghai Biochip, Co.,
Ltd., Shanghai, China).
Immunohistochemistry was performed with monoclonal
rabbit antibodies against human Lamp2a (1:100; ab18528; Abcam),
using a two-step protocol (Novolink Polymer Detection system;
Novocastra/Leica Biosystems, Richmond, IL, USA), as previously
described (21). Briefly, after
microwave antigen retrieval, tissues were incubated with primary
antibodies for 60 min at room temperature, followed by incubation
for 30 min with the secondary antibody (RE7112; Novolink Polymer;
Leica Microsystems GmbH, Wetzlar, Germany). The sections were
developed in 3,3′-diaminobenzidine solution under microscopic
observation and counterstained with hematoxylin. Negative control
slides, in which the primary antibodies were omitted, were included
in all assays.
Evaluation of immunohistochemical
variables
Immunohistochemical staining was evaluated by 3
independent pathologists with no knowledge of the patient
characteristics. Scores were assigned for the intensity and
percentage of positive staining in the cytoplasm in the whole
cylinder. Discrepancies were resolved by consensus among the three
pathologists, using a multihead microscope. The criteria for
Lamp2a-positivity included moderate or strong immunoreactivity in
>20% of the cells. In the event of a difference between
duplicate tissue cores, the higher score was considered to be the
final score.
Statistical analyses
Means were compared between two groups using
unpaired, two-tailed Student’s t-test, and among multiple groups
using one-way analysis of variance. Categorical data were analyzed
using χ2 or Fisher’s exact tests. The Kaplan-Meier
method was used to determine survival probability and differences
were assessed by the log-rank test. Statistical significance was
set at P<0.05. All analyses were performed using SPSS software
(v.16.0).
Results
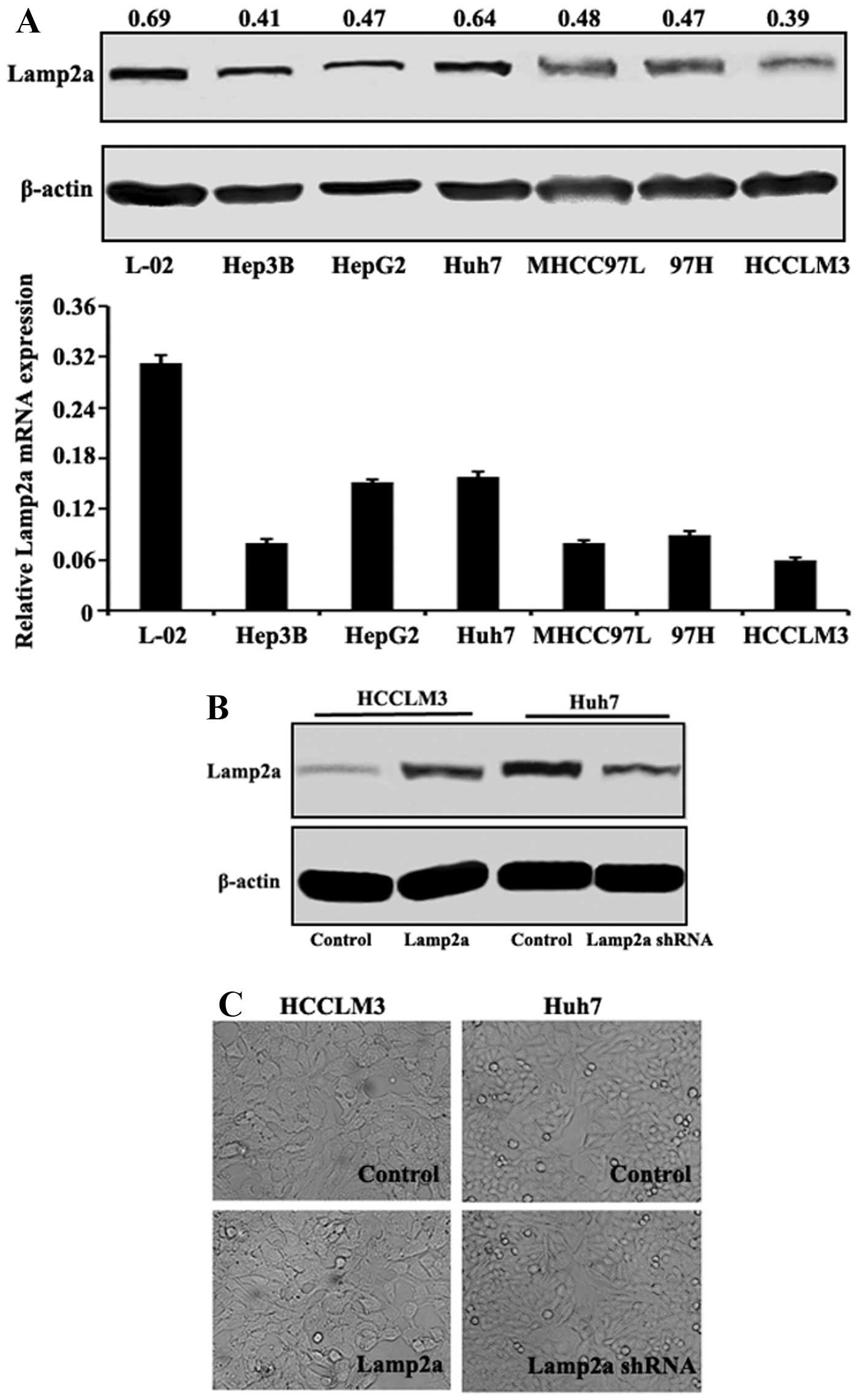
Lamp2a expression in HCC cell lines
In several HCC cell lines with different malignant
phenotypes and invasive potentials as previously described
(16), Lamp2a mRNA and protein
expression levels were evaluated. Lamp2a mRNA expression levels
were lower in HCC cells compared with normal hepatic L-02 cells, as
confirmed at the protein level by western blotting (Fig. 1A). Among the HCC cell lines, Lamp2a
expression levels were high in Huh7, intermediate in MHCC97L,
MHCC97H and HepG2, and low in Hep3B and HCCLM3 cells, which did not
correlate with their malignancy or invasiveness. We further
evaluated the effect of Lamp2a expression in HCC cells in paired
isogenic HCC cell lines (termed HCCLM3-Control/HCCLM3-Lamp2a and
Huh7-Control/Huh7-Lamp2a shRNA) in which Lamp2a expression was
modified by RNA interference or cDNA transfection (Fig. 1B). Light microscopy showed no
significant difference in cell morphology in relation to Lamp2a
expression (Fig. 1C).
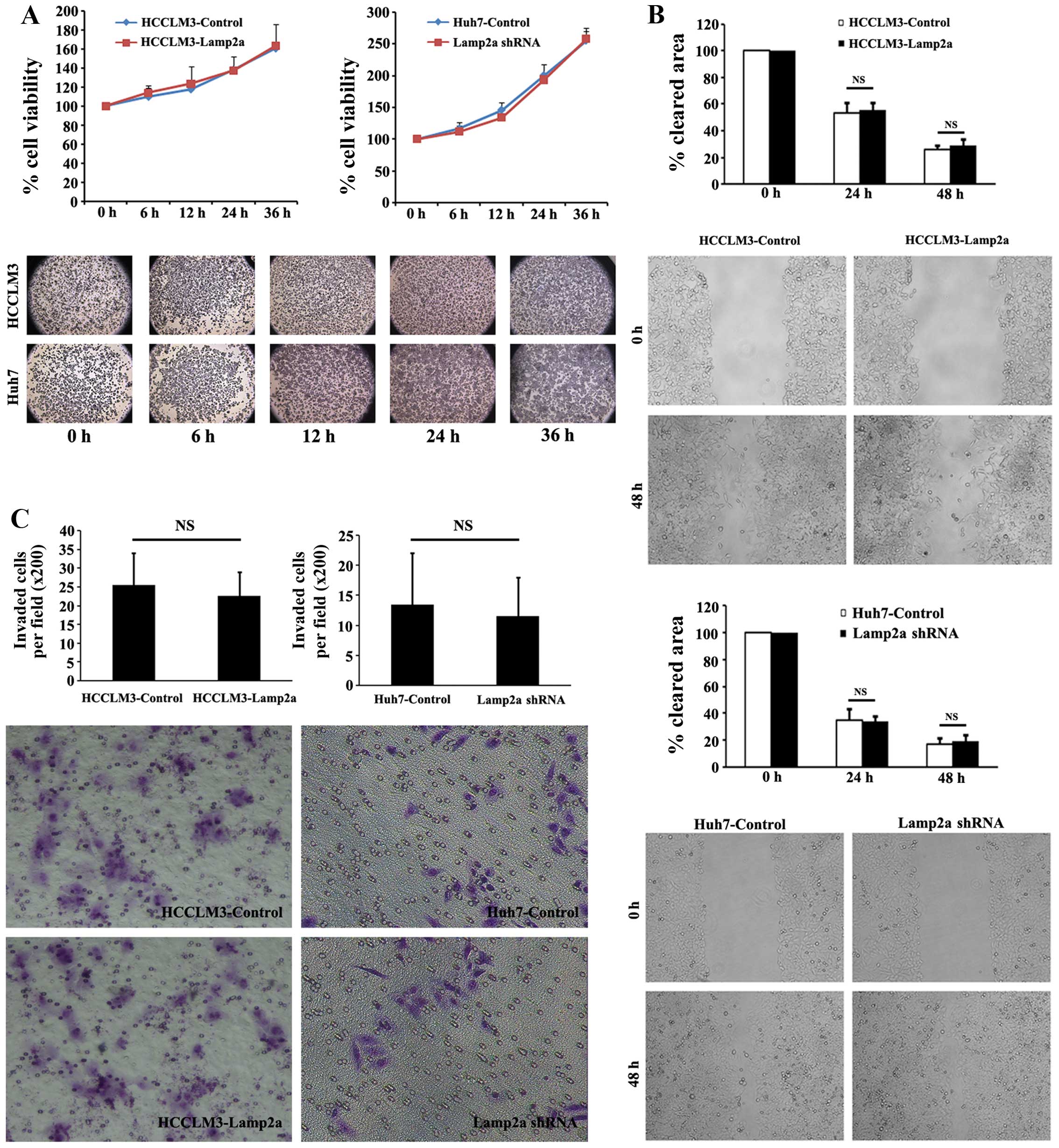
We also assessed cellular viability, migration
ability and invasion ability in paired HCC cell lines. Two groups
of HCC cells were incubated under normal conditions for 6–36 h and
cellular viability was measured using MTT assays. There was no
significant difference in survival in cells with upregulated or
downregulated Lamp2a expression compared with control cells
(Fig. 2A). Cell migration as the
first step of tumor metastasis is a characteristic of invasive
tumors. Wound-healing migration assays were adopted to assess the
effect of Lamp2a expression on HCC cell migration. No significant
difference was revealed in wound-closure rate of HCCLM3-Lamp2a and
Huh7-Lamp2a shRNA cells compared with the control cells under
microscopic examination after 24 and 48 h (Fig. 2B). Invasive capacity of the HCC
cells was assessed by Matrigel invasion assays. As shown in
Fig. 2C, Lamp2a overexpression in
HCCLM3 and Lamp2a silencing in Huh7 cells had no effect on cellular
invasion compared with control cells.
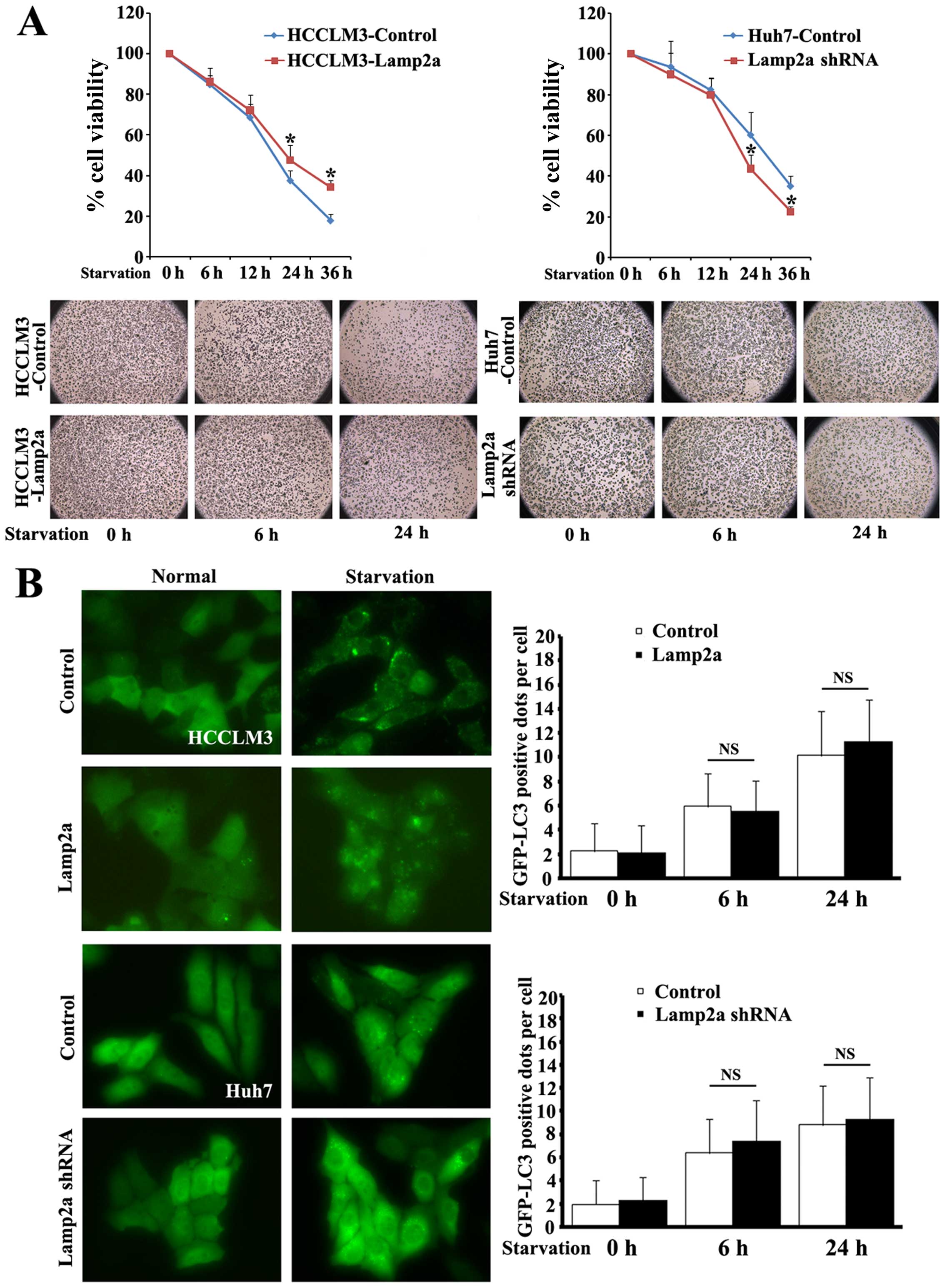
Lamp2a protected HCC cells from prolonged
starvation
CMA is activated maximally in response to stressors
and changes in cellular nutritional status (22). We therefore explored the role of
Lamp2a as the critical receptor of CMA in HCC during cell
starvation. Isogenic paired HCC cell lines
(HCCLM3-Control/HCCLM3-Lamp2a and Huh7-Control/Huh7-Lamp2a shRNA)
were incubated in the absence of serum for 6–32 h and cellular
viability was measured using MTT assays (Fig. 3A). There were no differences in
viability between the cell line pairs after 6 and 12 h of
starvation, but upregulation of Lamp2a in HCCLM3 cells markedly
increased cell survival after prolonged starvation for 24 and 36 h
by 10 and 16.5%, respectively, compared with control cells.
Similarly, silencing Lamp2a expression in Huh7-Lamp2a shRNA cells
abolished the protective effect of Lamp2a and reduced survival to
16.6 and 12.5%, respectively, compared with control cells.
Macroautophagy is also activated by starvation and
is involved in the protective mechanism against environmental and
cellular stress (23). We further
quantified the level of autophagy by transfecting cells with
GFP-LC3 fusion protein (Fig. 3B).
Following starvation GFP-LC3 protein changed from a diffuse
cytoplasmic distribution to punctate GFP-LC3 dots, indicating a
formation of autophagy vacuoles. Morphometric analysis revealed
similar numbers of GFP-LC3-positive dots per cell in
Lamp2a-modified cells after short and prolonged starvation,
indicating that the protection offered by Lamp2a was independent of
macroautophagy.
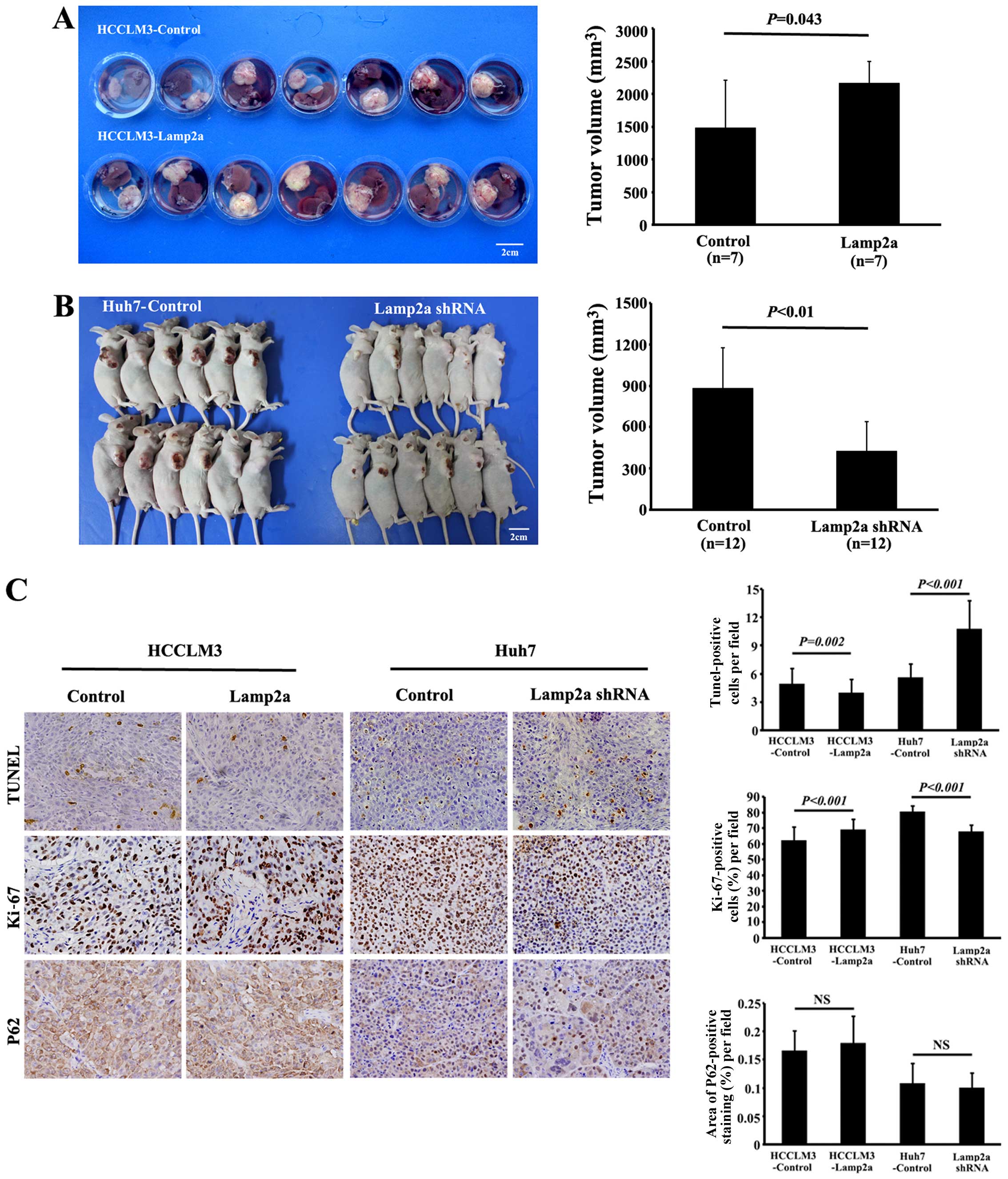
Lamp2a promotes growth of HCC xenograft
models
Ortho-topic injection of HCCLM3-Control and
HCCLM3-Lamp2a, or subcutaneous injection of Huh7-Control and
Huh7-Lamp2a shRNA cells in nude mice led to tumor formation in all
the groups. However, tumors from HCCLM3-Lamp2a-derived xenografts
were significantly larger (2,164.6±332.0 mm3) than those
from HCCLM3-Control xenografts (1,477.6±732.4 mm3;
P<0.05) (Fig. 4A). Moreover,
Huh7-Lamp2a shRNA-derived xenografts were significantly smaller
(426.5±214.4 mm3) than Huh7-Control-derived tumors
(880.3±293.3 mm3; P<0.01) (Fig. 4B).
Lamp2a overexpression caused a moderate decrease of
TUNEL-positive tumor cells compared with HCCLM3-Control-derived
tumors (4.0±1.5 vs. 4.9±1.7; P=0.002). Significantly more
TUNEL-positive tumor cells were also observed in Huh7-shRNA-derived
xenografts compared with control tumors (10.7±3.0 vs. 5.6±1.4;
P<0.001) (Fig. 4C). Similarly,
Ki-67 staining indicated a significant increase in proliferative
activity in the Lamp2a-overexpressing HCCLM3 orthotopic model and
control Huh7 subcutaneous model compared with the control HCCLM3
and Huh7-shRNA groups, respectively (69.0±6.6 vs. 62.0±8.9%;
P<0.001, and 80.4±4.1 vs. 67.7±4.5%; P<0.001) (Fig. 4C). We examined the effect of Lamp2a
expression on macroautophagy in tumor xenografts in vivo by
investigating the expression of P62, which accumulates during
defective autophagy. Immunohistochemical analysis revealed no
significant difference of P62 expression between the two groups,
suggesting that macroautophagy activity was independent of Lamp2a
expression (Fig. 4C).
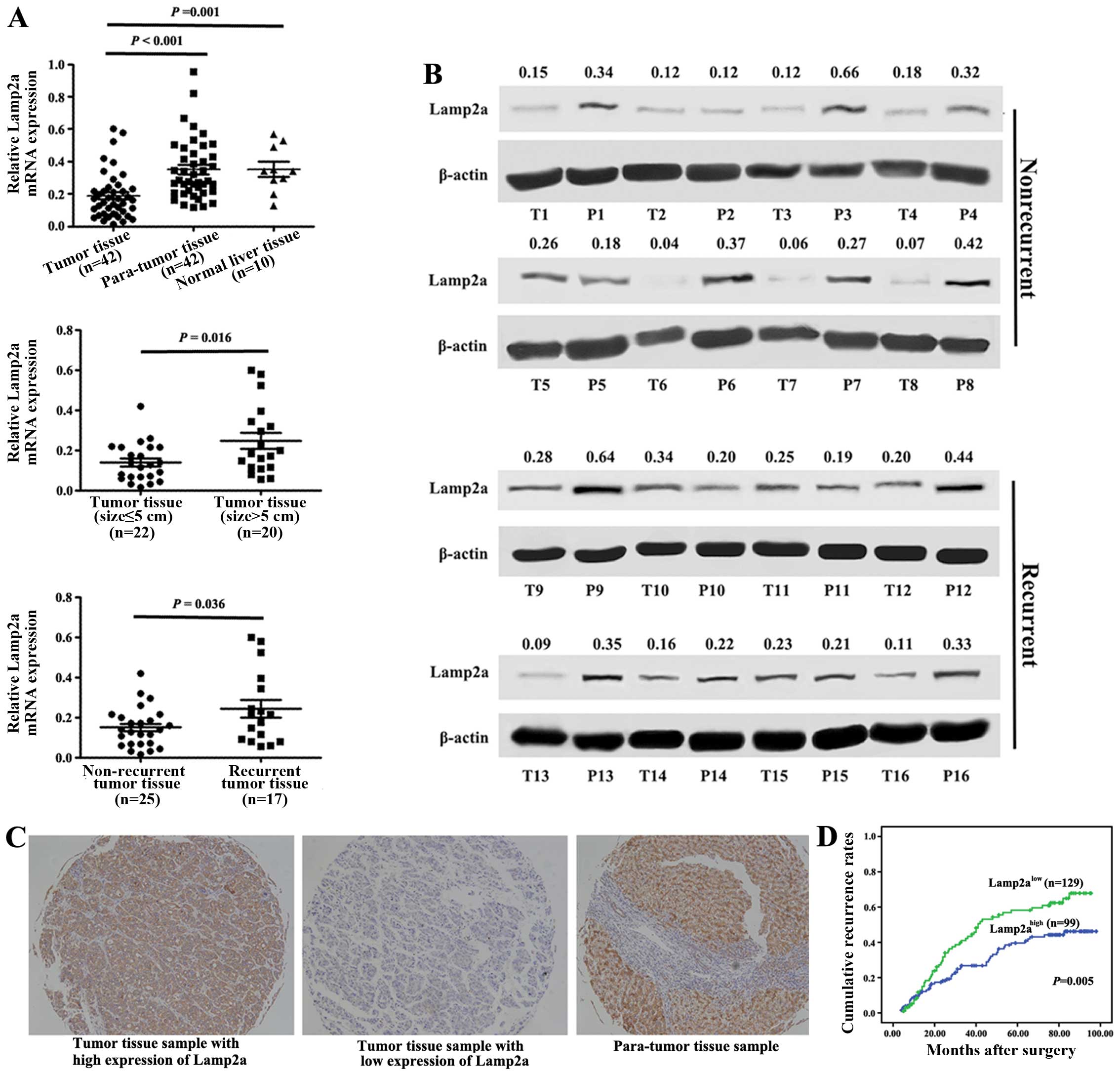
Lamp2a expression in HCC tissues
We compared Lamp2a mRNA expression in 42 HCC tissue
samples and para-tumor tissues and 10 normal livers. Compared with
para-tumor tissue samples and normal liver samples, Lamp2a
expression was significantly decreased in tumor tissue samples
(P<0.001 and P=0.001, respectively) (Fig. 5A). Lamp2a expression was lower in
83.3% (35/42) of tumor tissues compared with matched para-tumor
tissues. Levels were decreased >3-fold in 10 samples, and 2- to
3-fold in 11 samples among the above tumor samples. These findings
were further confirmed in 16 of the 42 HCC cases by western blot
analysis (Fig. 5B). Lamp2a protein
levels were decreased in 11 of the 16 samples compared with the
para-tumor tissues, especially in cases of non-recurrent HCC.
Higher Lamp2a mRNA expression was associated with HCC recurrence
(P=0.036) and larger HCC size (P=0.016). Together with the results
of the in vivo studies, these findings suggest that Lamp2a
expression in HCC tissues contributes to tumor growth and may also
influence the clinical prognosis.
We further validated our hypothesis using TMA to
evaluate the correlation between Lamp2a and prognosis in 228 HCC
patients who underwent curative resection. Strong Lamp2a staining
in hepatocytes and tumor cells was observed in most para-tumor
tissue samples (176/228) and some tumor tissue samples (47/228)
(Fig. 5C). High Lamp2a expression,
indicated by moderate or strong immunohistochemical staining in
>20% of the cells, was present in 43% (99/228) of HCC patients.
However, there was no correlation between Lamp2a expression and
clinicopathological characteristics such as gender, liver
cirrhosis, tumor number, tumor capsule, tumor differentiation or
vascular invasion. Older patients and patients with high
α-fetoprotein levels had lower Lamp2a expression. Moreover, high
expression of Lamp2a was significantly correlated with large tumor
size (P=0.001) (Table I). The
overall 3-, 5- and 7-year cumulative recurrence rates in these HCC
patients were 34.1, 47.9 and 54.5%, respectively, though patients
with high Lamp2a expression had a significantly higher recurrence
rate than patients with low Lamp2a expression (P=0.005) (Fig. 5D). The 3-, 5- and 7-year cumulative
recurrence rates for Lamp2ahigh and Lamp2alow
groups were 43.4 vs. 26.8, 58.2 vs. 39.6 and 62.2 vs. 46.2%,
respectively.
 | Table ICorrelation between Lamp2a expression
and clinicopathological characteristics. |
Table I
Correlation between Lamp2a expression
and clinicopathological characteristics.
| Lamp2a
expression | |
|---|
|
| |
|---|
| Variables | Low (n=129) | High (n=99) | P-value |
|---|
| Gender |
| Female | 17 | 10 | |
| Male | 112 | 89 | 0.476 |
| Age (years) |
| ≤50 | 54 | 62 | |
| >50 | 75 | 37 | 0.002 |
| Preoperative AFP
(ng/ml) |
| ≤20 | 33 | 45 | |
| >20 | 96 | 54 | 0.002 |
| Liver
cirrhosis |
| No | 34 | 18 | |
| Yes | 95 | 81 | 0.145 |
| Tumor size
(cm) |
| ≤5 | 85 | 44 | |
| >5 | 44 | 55 | 0.001 |
| Tumor number |
| Single | 112 | 77 | |
| Multiple | 17 | 22 | 0.072 |
| Vascular
invasion |
| No | 110 | 91 | |
| Yes | 19 | 8 | 0.124 |
| Capsule |
| No | 81 | 64 | |
| Yes | 48 | 35 | 0.773 |
| Tumor
differentiation |
| I–II | 73 | 61 | |
| III–IV | 56 | 38 | 0.445 |
Discussion
In contrast to macroautophagy and microautophagy,
CMA is a pathway for the selective degradation of individual
proteins, mediated by binding to heat shock cognate 70/90-kDa
protein (HSC70/90) (24), followed
by unfolding and translocation of the proteins through the
lysosomal membrane by Lamp2a (25). Although both microautophagy and CMA
share the HSC70 as a targeting molecule, only CMA depends on Lamp2a
(22). The interaction between
Lamp2a and substrate proteins is a critical step in the CMA
pathway, and changes in the Lamp2a levels of lysosomal membrane
therefore modulate the activity (26).
Lamp2a-related CMA activity was shown to decline in
the liver of aged mice, and this failure in cellular clearance was
proposed to lead to cellular proteotoxicity and accumulation of
intracellular damage, ultimately contributing to functional failure
in aged organisms (15,27,28).
These key observations contribute to a complex but growing
convergence between our understandings of the biology of ageing and
the mechanisms that underlie cancer (29).
The results of the present study demonstrated that
Lamp2a expression was significantly lower in HCC cells compared
with the normal hepatic cells. We also compared its mRNA expression
levels in HCC, para-tumor and normal liver tissues and, consistent
with aged livers in mice, found a significant decrease of Lamp2a
expression in HCC tissues compared with para-tumor tissues and
normal livers. Furthermore, immunohistochemical analysis of
high-throughput TMA showed stronger staining for Lamp2a protein in
hepatocytes of para-tumor tissues compared with HCC cells of tumor
tissues. These findings suggest that Lamp2a-related CMA may be
suppressed in HCC.
Previous studies of CMA activities in breast
(14), lung (30) and gastric cancers (31) demonstrated an inconsistent increase
in basal CMA activity. Moreover, CMA activation in these cells and
tumors was mostly associated with an increase in Lamp2a levels.
Knock-down of Lamp2a in these cells established that CMA was
essential for cancer cell proliferation, tumor growth and
metastasis. Lamp2a-related CMA may thus be a tumor-promoter
mechanism, and its increased activity may promote human cancer
progression.
The results of the present study suggested that
Lamp2a expression was downregulated in HCC tissues, but the
function of the remaining Lamp2a protein in HCC is still unclear.
Gain and loss of Lamp2a function were explored in HCCLM3 and Huh7
HCC cell lines, respectively. HCC cell lines with stable
upregulation or downregulation of Lamp2a expression were confirmed
by western blotting, but no differences in cell morphology or
function were observed between the two groups under normal culture
conditions. However, autophagy induces survival mechanisms that
prevent against tumor cell death during hypoxia, starvation and
oxidative/metabolic stress (32).
We therefore observed cellular viability during starvation for 6–36
h, and found no effect of Lamp2a expression on cell viability
during short-term starvation, but Lamp2a blockage significantly
inhibited HCC cell survival under prolonged starvation. These
results suggest that Lamp2a-mediated CMA activation may promote
long-term cancer cell survival. Moreover, Lamp2a overexpression
induced HCC xenograft growth in vivo. Downregulation of
Lamp2a resulted in a marked increase in TUNEL-positive tumor cells
and a decrease in Ki-67 staining, suggesting that Lamp2a aids tumor
growth by helping cells to avoid apoptosis and promoting cell
proliferation.
The present study raises the question of why
impaired Lamp2a expression increased HCC tumor survival under
stress. We speculate that, similar to macroautophagy (33,34),
Lamp2a-related CMA may have an opposing effect, via the degradation
of modified and oxidatively damaged proteins (35). Reduced CMA activity in aged
organisms contributes to the proteins aggregation and abnormal
intracellular homeostasis (15,27,28).
Lamp2a defects at an early stage of oncogenesis may thus accelerate
HCC tumorigenesis as a result of reduced CMA activity and abnormal
protein degradation. However, once the tumor is formed,
Lamp2a-related CMA acts as part of a stress-response mechanism to
protect the cancer cells against oxidative insults or low nutrient
supply.
As a result of high recurrence rate after surgery
and development of tumors in intrahepatic metastases or cirrhotic
liver, the prognosis of HCC is poor (36). As a critical receptor for CMA,
Lamp2a may play a role in tumor growth, ultimately contributing to
the poor prognosis. These real-time PCR and immunohistochemical
analyses of HCC tissue samples supported a strong correlation
between Lamp2a expression and tumor size, while 3-, 5- and 7-year
cumulative recurrence rates were also significantly lower in
patients with higher Lamp2a expression levels. To the best of our
knowledge, this study is the first to report Lamp2a-mediated tumor
survival and recurrence in human HCC.
In conclusion, impaired Lamp2a expression in HCC is
required for tumor growth by enabling cells to avoid apoptosis and
by promoting cell proliferation. Lamp2a may serve as a predictive
marker for HCC viability and recurrence, indicating the need for
more aggressive treatment. Targeting chaperone-mediated autophagy
through Lamp2a may also represent a potentially novel therapeutic
strategy for HCC.
Acknowledgements
The present study was supported by the funds from
the National Natural Science Fund of China (nos. 81472219 and
81602037).
Abbreviations:
|
HCC
|
hepatocellular carcinoma
|
|
Lamp2a
|
lysosome-associated membrane protein
type 2a
|
|
DMEM
|
Dulbecco’s modified Eagle’s media
|
|
HSC70/90
|
heat shock cognate 70/90-kDa
protein
|
|
MTT
|
3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2-H-tetrazolium
bromide
|
|
PCR
|
real-time polymerase chain
reaction
|
|
TUNEL
|
terminal deoxynucleotidyl
transferase-mediated deoxyuridine triphosphate nick-end
labeling
|
|
TMA
|
tissue microarray
|
References
|
1
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Amaravadi RK and Thompson CB: The roles of
therapy-induced autophagy and necrosis in cancer treatment. Clin
Cancer Res. 13:7271–7279. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kondo Y, Kanzawa T, Sawaya R and Kondo S:
The role of autophagy in cancer development and response to
therapy. Nat Rev Cancer. 5:726–734. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lee YJ and Jang BK: The role of autophagy
in hepatocellular carcinoma. Int J Mol Sci. 16:26629–26643. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Amaravadi RK, Lippincott-Schwartz J, Yin
XM, Weiss WA, Takebe N, Timmer W, DiPaola RS, Lotze MT and White E:
Principles and current strategies for targeting autophagy for
cancer treatment. Clin Cancer Res. 17:654–666. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chiang HL and Dice JF: Peptide sequences
that target proteins for enhanced degradation during serum
withdrawal. J Biol Chem. 263:6797–6805. 1988.PubMed/NCBI
|
|
7
|
Xilouri M, Brekk OR, Kirik D and Stefanis
L: LAMP2A as a therapeutic target in Parkinson disease. Autophagy.
9:2166–2168. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Fidziańska A, Walczak E and Walski M:
Abnormal chaperone-mediated autophagy (CMA) in cardiomyocytes of a
boy with Danon disease. Folia Neuropathol. 45:133–139. 2007.
|
|
9
|
Venugopal B, Mesires NT, Kennedy JC,
Curcio-Morelli C, Laplante JM, Dice JF and Slaugenhaupt SA:
Chaperone-mediated autophagy is defective in mucolipidosis type IV.
J Cell Physiol. 219:344–353. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Cuervo AM and Dice JF: Regulation of
lamp2a levels in the lysosomal membrane. Traffic. 1:570–583. 2000.
View Article : Google Scholar
|
|
11
|
Cuervo AM, Knecht E, Terlecky SR and Dice
JF: Activation of a selective pathway of lysosomal proteolysis in
rat liver by prolonged starvation. Am J Physiol. 269:C1200–C1208.
1995.PubMed/NCBI
|
|
12
|
Cuervo AM and Dice JF: Unique properties
of lamp2a compared to other lamp2 isoforms. J Cell Sci.
113:4441–4450. 2000.PubMed/NCBI
|
|
13
|
Park Y, Liu C, Luo T, Dietrich WD,
Bramlett H and Hu B: Chaperone-mediated autophagy after traumatic
brain injury. J Neurotrauma. 32:1449–1457. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Saha T: LAMP2A overexpression in breast
tumors promotes cancer cell survival via chaperone-mediated
autophagy. Autophagy. 8:1643–1656. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhang C and Cuervo AM: Restoration of
chaperone-mediated autophagy in aging liver improves cellular
maintenance and hepatic function. Nat Med. 14:959–965. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ding ZB, Shi YH, Zhou J, Qiu SJ, Xu Y, Dai
Z, Shi GM, Wang XY, Ke AW, Wu B, et al: Association of autophagy
defect with a malignant phenotype and poor prognosis of
hepatocellular carcinoma. Cancer Res. 68:9167–9175. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hui B, Shi YH, Ding ZB, Zhou J, Gu CY,
Peng YF, Yang H, Liu WR, Shi GM and Fan J: Proteasome inhibitor
interacts synergistically with autophagy inhibitor to suppress
proliferation and induce apoptosis in hepatocellular carcinoma.
Cancer. 118:5560–5571. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ding ZB, Hui B, Shi YH, Zhou J, Peng YF,
Gu CY, Yang H, Shi GM, Ke AW, Wang XY, et al: Autophagy activation
in hepatocellular carcinoma contributes to the tolerance of
oxaliplatin via reactive oxygen species modulation. Clin Cancer
Res. 17:6229–6238. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Shi YH, Ding ZB, Zhou J, Hui B, Shi GM, Ke
AW, Wang XY, Dai Z, Peng YF, Gu CY, et al: Targeting autophagy
enhances sorafenib lethality for hepatocellular carcinoma via ER
stress-related apoptosis. Autophagy. 7:1159–1172. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Peng YF, Shi YH, Ding ZB, Ke AW, Gu CY,
Hui B, Zhou J, Qiu SJ, Dai Z and Fan J: Autophagy inhibition
suppresses pulmonary metastasis of HCC in mice via impairing
anoikis resistance and colonization of HCC cells. Autophagy.
9:2056–2068. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Gao Q, Qiu SJ, Fan J, Zhou J, Wang XY,
Xiao YS, Xu Y, Li YW and Tang ZY: Intratumoral balance of
regulatory and cytotoxic T cells is associated with prognosis of
hepatocellular carcinoma after resection. J Clin Oncol.
25:2586–2593. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kaushik S, Bandyopadhyay U, Sridhar S,
Kiffin R, Martinez-Vicente M, Kon M, Orenstein SJ, Wong E and
Cuervo AM: Chaperone-mediated autophagy at a glance. J Cell Sci.
124:495–499. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lebovitz CB, Bortnik SB and Gorski SM:
Here, there be dragons: Charting autophagy-related alterations in
human tumors. Clin Cancer Res. 18:1214–1226. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chiang HL, Terlecky SR, Plant CP and Dice
JF: A role for a 70-kilodalton heat shock protein in lysosomal
degradation of intracellular proteins. Science. 246:382–385. 1989.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Cuervo AM and Dice JF: A receptor for the
selective uptake and degradation of proteins by lysosomes. Science.
273:501–503. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Massey AC, Kaushik S, Sovak G, Kiffin R
and Cuervo AM: Consequences of the selective blockage of
chaperone-mediated autophagy. Proc Natl Acad Sci USA.
103:5805–5810. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Schneider JL, Villarroya J, Diaz-Carretero
A, Patel B, Urbanska AM, Thi MM, Villarroya F, Santambrogio L and
Cuervo AM: Loss of hepatic chaperone-mediated autophagy accelerates
proteostasis failure in aging. Aging Cell. 14:249–264. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Schneider JL, Suh Y and Cuervo AM:
Deficient chaperone-mediated autophagy in liver leads to metabolic
dysregulation. Cell Metab. 20:417–432. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Finkel T, Serrano M and Blasco MA: The
common biology of cancer and ageing. Nature. 448:767–774. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kon M, Kiffin R, Koga H, Chapochnick J,
Macian F, Varticovski L and Cuervo AM: Chaperone-mediated autophagy
is required for tumor growth. Sci Transl Med. 3:109ra1172011.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhou J, Yang J, Fan X, Hu S, Zhou F, Dong
J, Zhang S, Shang Y, Jiang X, Guo H, et al: Chaperone-mediated
autophagy regulates proliferation by targeting RND3 in gastric
cancer. Autophagy. 12:515–528. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Hait WN, Jin S and Yang JM: A matter of
life or death (or both): Understanding autophagy in cancer. Clin
Cancer Res. 12:1961–1965. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
White E and DiPaola RS: The double-edged
sword of autophagy modulation in cancer. Clin Cancer Res.
15:5308–5316. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Cicchini M, Karantza V and Xia B:
Molecular pathways: Autophagy in cancer - a matter of timing and
context. Clin Cancer Res. 21:498–504. 2015. View Article : Google Scholar
|
|
35
|
Hubbi ME, Hu H, Kshitiz, Ahmed I,
Levchenko A and Semenza GL: Chaperone-mediated autophagy targets
hypoxia-inducible factor-1α (HIF-1α) for lysosomal degradation. J
Biol Chem. 288:10703–10714. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Sherman M: Recurrence of hepatocellular
carcinoma. N Engl J Med. 359:2045–2047. 2008. View Article : Google Scholar : PubMed/NCBI
|