Introduction
Osteosarocoma (OS) is well established as the most
common primary malignant bone tumor. OS is usually observed in
children, adolescents and young adults. OS treatment requires a
multidisciplinary strategy of surgery and chemotherapy including
radiotherapy (1). The
identification of effective chemotherapy for the treatment of OS
has led to significant improvement in patient outcome; the 5-year
survival rate for patients with a localized tumor has reached ~70%
(2). However, the 5-year
event-free survival of metastatic OS is only ~20% (3–8). The
combination of chemotherapeutic agents, such as doxorubicin,
cisplatin, methotrexate, and ifosfamide, is widely accepted to have
efficacy against OS (9–13). However, these agents for OS have
been used for over ten years now and there is a continued need for
new therapeutic approaches for further improvement of OS patient
prognosis. Recently, the use of molecular-targeted cancer therapy
has been receiving attention for various tumors, because of several
potential advantages in features such as drug metabolism and
accumulation, optimum doses, and side effects, over conventional
anticancer agents (14).
Molecular-targeted therapy is currently favored as a replacement
for conventional OS therapies.
The development of clinical agents remains a costly
and time-consuming process. Identification of new uses for existing
drugs has been recognized as being a more efficient approach for
drug discovery than the development of novel drugs. The aim of this
study was to identify existing compounds that are capable of
killing OS cells. First, we screened the anti-proliferative effects
of 324 anticancer drugs using five OS cell lines and selected
candidate agents for new OS treatment. Second, we investigated the
intracellular mechanism of the anti-proliferative activity of the
candidate agent and examined its inhibitory effect on tumor growth
using an in vivo model.
Materials and methods
Osteosarcoma cell culture
Five human OS cell lines (143B, HOS, MG63, SAOS-2,
and HUO9) were used in this study. 143B, HOS, MG63 were cultured in
minimum essential media (MEM) (Gibco, Carlsbad, CA, USA) containing
10% fetal bovine serum (FBS), 100 U/ml penicillin and 100 mg/ml
streptomycin (Invitrogen, Carlsbad, CA, USA). SAOS-2 was cultured
in McCoy's 5A (modified) medium (Gibco) containing 15% FBS, 100
U/ml penicillin and 100 mg/ml streptomycin. HUO9 was cultured in
RPMI-1640 medium (Gibco) containing 10% FBS, 100 U/ml penicillin
and 100 mg/ml streptomycin. Cells were maintained as attached
monolayers and were incubated in a humidified atmosphere with 5%
CO2 at 37°C.
Chemical compounds
The Screening Committee of Anticancer Drugs (SCADS)
compound library, containing 324 compounds in four 96-well
microplates (http://gantoku-shien.jfcr.or.jp/), was kindly provided
by Grant-in-Aid for Scientific Research on the Priority Area
‘Cancer’ from the Ministry of Education, Culture, Sports, Science
and Technology of Japan. The compounds, mainly composed of
antitumor drugs and kinase inhibitors, were provided at a
concentration of 10 mM in dimethyl sulfoxide (DMSO) solution.
Cucurbitacin I (Sigma-Aldrich, St. Louis, MO, USA)
was initially dissolved in DMSO and stored at −20°C. For the
experiments, cucurbitacin I was diluted with culture media to the
final concentration used.
Measurement of cell viability
For measurement of cell proliferation, the five
human OS cell lines were placed in monolayer culture at a density
of 3.0×104 cells/well (100 μl) and were treated with
either diluent control (DMSO) or 10 mM of each compound in 96-well
plates. Cell viability was measured using the Cell-Titer
96® AQueous One Solution Cell Proliferation Assay kit
(Promega, Madison, WI, USA). After compound screening, candidate
compounds were examined for their anti-proliferative effect in a 2D
monolayer culture (as above) and a 3D collagen gel culture
(cellmatrix type 1A; Nitta Gelatin Inc., Japan). After 24 h of
incubation, the compounds, dissolved in DMSO, were added to the
culture at the indicated final concentrations. The cells were then
cultured for 24 h. Cell viability in 2D monolayers was measured
using cell proliferation assay kit as above. Cell viability in 3D
collagen gels was measured using the Cell-Titer-Glo™ Luminescent
Cell Viability assay (Promega). For dose-response tests, cells were
exposed to media with various concentrations (10 nM, 100 nM, 1.0 μM
and 10 μM) of cucurbitacin I or DMSO (negative control) for 24 h.
For time-response tests, cells were exposed to media with 10 mM
cucurbitacin I or DMSO for 12, 24 or 48 h.
Flow cytometry
Cell cycle progression and apoptosis were analysed
by flow cytometry. For apoptosis analysis, cells were incubated
with cucurbitacin I (10 μM) for 24 h followed by Annexin V-FITC and
propidium isodide (PI) double staining performed according to the
manufacturer's instructions (Beckman Coulter, Miami, FL, USA).
Western blot analysis
After treatment with or without cucurbitacin I (10
μM) for 12 or 24 h, cells were lysed with radioimmunoprecipitation
(RIPA) buffer (Millipore-Upstate, Temecula, CA, USA) supplemented
with a protease inhibitor cocktail, 0.5 mM PMSF, and 0.2 mM
Na3VO4. Proteins were separated by SDS-PAGE,
and samples were adjusted to the same protein concentration before
loading. Proteins were transferred to a nitrocellulose membrane,
and blotted. Antibodies were obtained from the following sources
and used at the dilutions recommended by the manufacturer: STAT3
and phospho-STAT3 antibodies 1:2,000 dilution (Cell Signaling
Technology Beverly, MA, USA) and cleaved caspase-3, phospho-cyclin
D1, c-Myc, Mcl-1, survivin and cleaved PARP antibodies 1:1,000
dilution (Cell Signaling Technology). The β-actin protein was
assayed as a loading control.
Enzyme linked immunosorbent assay
(ELISA)
The effect of cucurbitacin I on apoptosis was
evaluated by measurement of caspase-3 activation. OS cells were
cultured with or without cucurbitacin I (10 μM) for 24 h. Caspase-3
levels were determined using the Caspase-3 (Active) Human ELISA kit
(Invitrogen) according to the manufacturer's instructions.
Growth of 143B xenografts in athymic nude
mice with in vivo cucurbitacin I treatment
All animal experiments strictly followed the
guidelines of Cedars-Sinai Medical Center and the National
Institute of Health (NIH). 143B (6.0×106) human OS cells
were inoculated subcutaneously into the back of female nude mice.
Forty mice were randomly assigned to each of the following
experimental groups: i) saline with DMSO (diluent-specific
control); ii) 0.25 mg/kg cucurbitacin I; iii) 0.5 mg/kg
cucurbitacin I; iv) 1.0 mg/kg cucurbitacin I. Saline or
cucurbitacin I was administered three times a week
intraperitoneally. Body weight and tumor size were measured every
week, and the tumor volume was calculated using the following
formula: V = lw2/2, where (l) is the length, (w) the
width, and (V) is the volume as described previously (15). The treatment was stopped at 28
days. The total observation period from the start of treatment of
the xenografts was 60 days.
Histologic examination and terminal
deoxynucleotide trans-ferase-mediated deoxyuridine triphosphate
nick-end labeling (TUNEL) assay
For histologic examination, tumor samples were fixed
in 4% paraformaldehyde phosphate buffer solution, embedded in
paraffin and cut into 5-mm-thick sections. The sections were
stained with hematoxylin-eosin and examined under a light
microscope. Immunohistochemistry was carried out using antibodies
obtained from the following sources and used at the dilutions
recommended by the manufacturer: phospho-STAT3 antibodies 1:400
dilution (Cell Signaling Technology) and cleaved caspase-3
antibodies 1:300 dilution, phospho-cyclin D1 antibodies 1:200
dilution, c-Myc antibodies 1:200 dilution, surviving antibodies
1:400 dilution and cleaved PARP antibodies 1:50 dilution (Cell
Signaling Technology). TUNEL staining was carried out on
paraformaldehyde-fixed, paraffin-embedded sections using the
ApopTag® Peroxidase In Situ Apoptosis Detection kit
(Intergen, NY, USA) according to the manufacturer's
instructions.
Statistical analysis
All in vitro experiments were repeated at
least three times to ensure reproducibility. Data are expressed as
the means ± SD. A non-parametric analysis of variance test
(Mann-Whitney) was used to compare differences between two groups.
Kaplan-Meier analysis was used to estimate survival time of treated
mice. A repeated ANOVA or one-way ANOVA was used to compare tumor
volume and weight. P-values are indicated in the figures. A p-value
of <0.05 was considered statistically significant.
Results
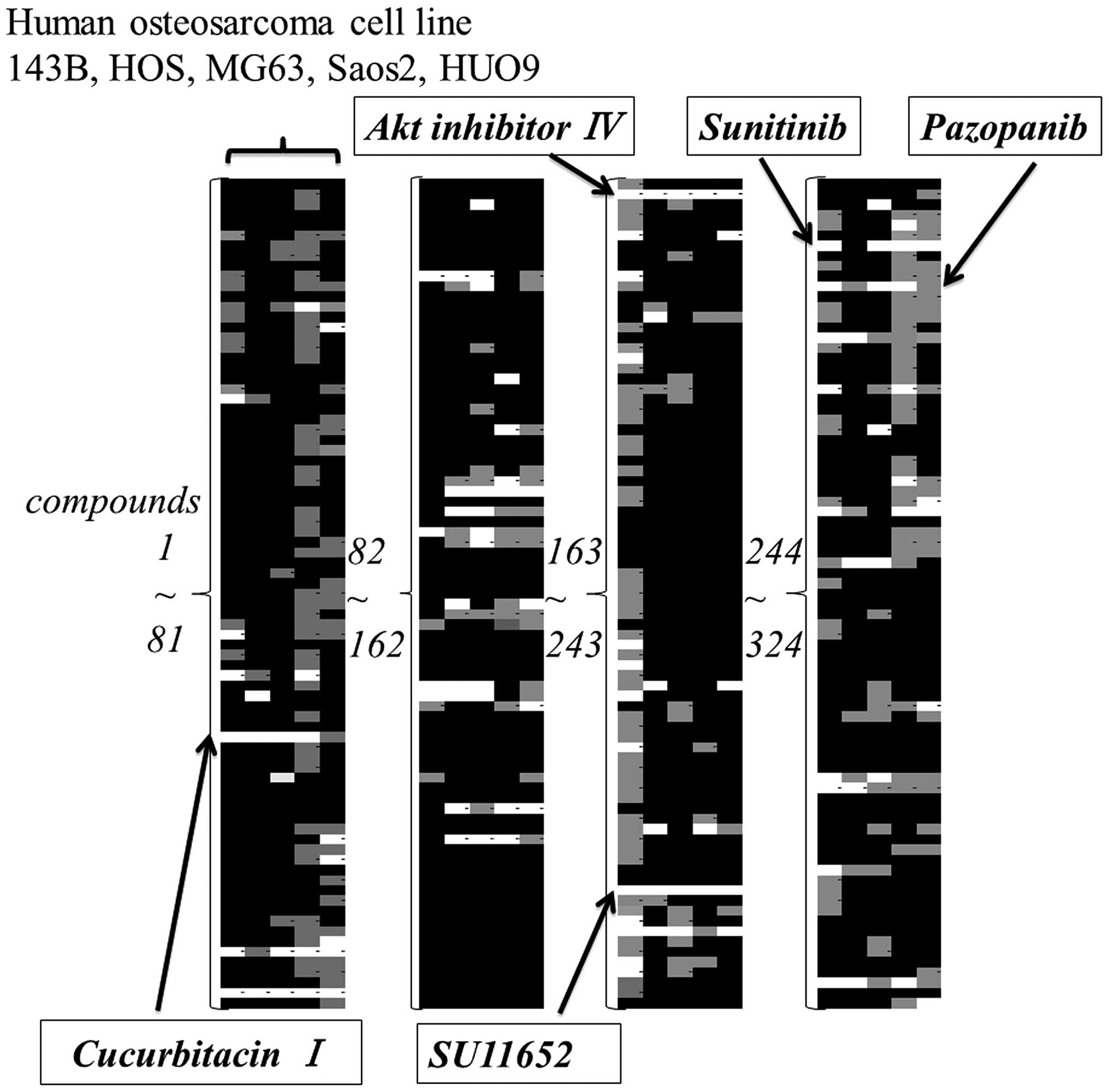
Compound screening
The anti-proliferative effect of 324 compounds
against five OS cell lines was screened. The results were
color-coded according to the percentage decrease in cell viability
compared to control. White color indicated >50% reduction and a
gray indicated from 20 to 50% reduction. Based on this color
analysis, compounds that had a broad effect on 5 cell lines were
narrowed down to pazopanib, sunitinib, cucurbitacin I,
Akt-inhibitor IV, and SU11652 (Fig.
1). Cytotoxic compounds and compounds unsuitable for in
vivo administration were excluded from this screening.
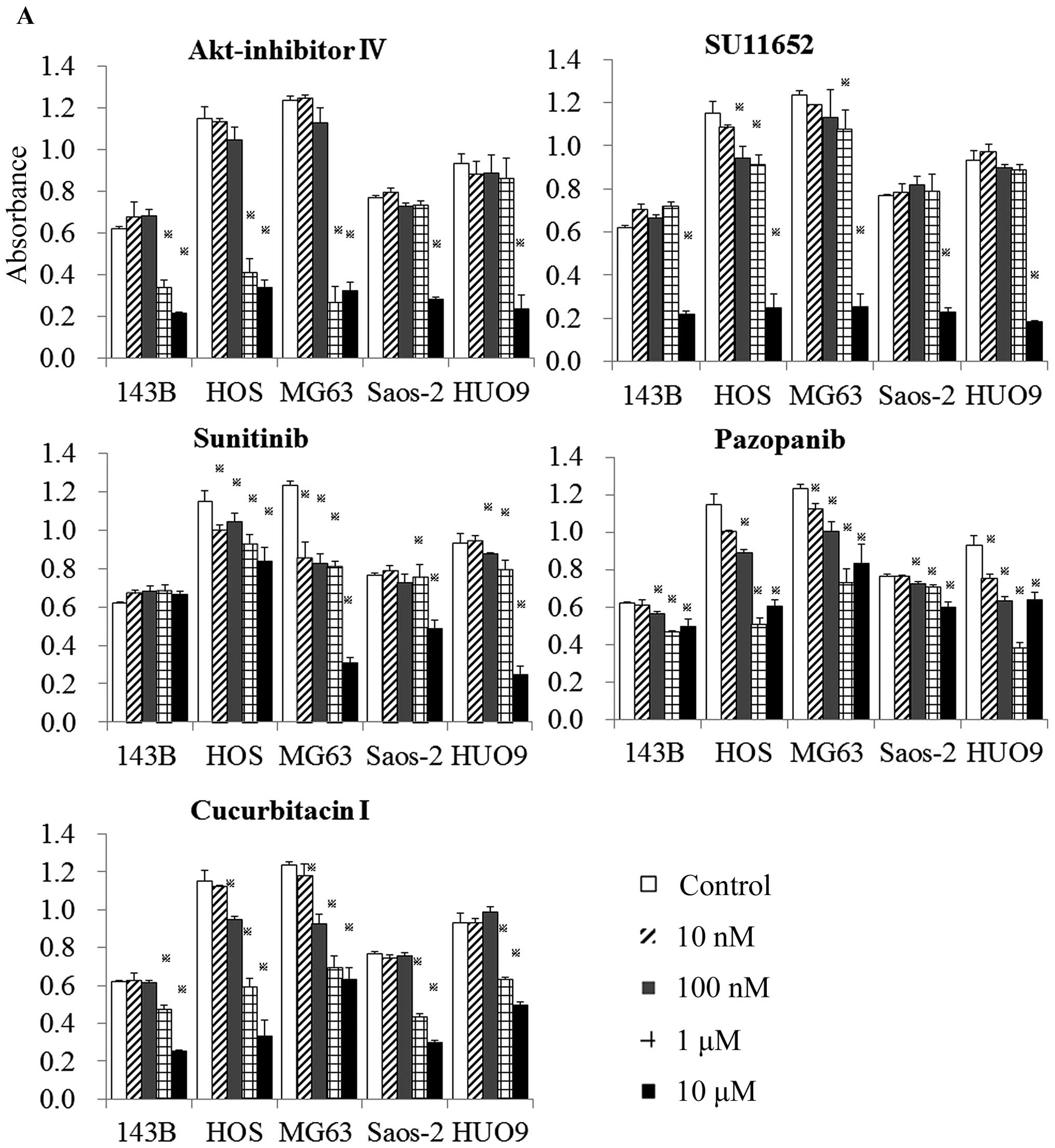
Effect of identified compounds on cell
viability
The 5 compounds were then examined more specifically
for their effects on cell viability in 2D monolayer (Fig. 2A) and 3D collagen cultures
(Fig. 2B).
Cucurbitacin I and Akt inhibitor IV demonstrated a
dose-dependent and significant reduction in cell viability of all
five OS cell lines in both monolayer and 3D cultures. Pazopanib
displayed a weak reduction in the viability of 143B, Saos-2 and
HUO9 cells in monolayer culture and of 143B, HOS and Saos-2 cells
in 3D culture. SU11652, at a concentration of 10 μM, showed a broad
inhibition of cell viability in monolayer and 3D cultures.
Sunitinib had a weak effect on the viability of 143B cells in
monolayer and of 143B and HOS cells in 3D culture (Fig. 2A and B). Sunitinib and SU11652 are
established inhibitors of receptor tyrosine kinases that are
essential for angiogenesis, tumor cell proliferation, and tumor
cell survival. These inhibitors have been developed by Sugen
(Redwood City, CA, USA). Sunitinib (SU11248) was made by
replacement of chlorine in SU11562 with fluorine. Only sunitinib
has been applied in clinical situations (16). Based on their above effects on cell
viability, not only pazopanib but also sunitinib and SU11652 were
excluded from further experiments.
Derivatives of cucurbitacin I (cucurbitacin B) and
of Akt-inhibitor IV (three oral Akt-inhibitors) were then assayed
for their effect on cell viability. The oral Akt-inhibitors,
AZD5363, GDC0068 and GSK690693, had only a small inhibitory effect
on cell viability and Akt-inhibitor IV exhibited toxicity in
vivo (data not shown). Akt-inhibitors were therefore excluded
from this study. The family Cucurbitaceae (cucurbitacin I
and cucurbitacin B) demonstrated broad and significant inhibition
of the viability of five OS cell lines in a dose-dependent manner.
Compared to cucurbitacin B, viability of the OS cell lines Saos2
and MG63 was significantly decreased to a greater extent by
cucurbitacin I when the cells were exposed to 10 μM of each agent
for 48 h (data not shown). Therefore, subsequent experiments were
performed using cucurbitacin I. Cucurbitacin I displayed similar
results in the five OS cell lines in a time-dependent manner
(Fig. 2C).
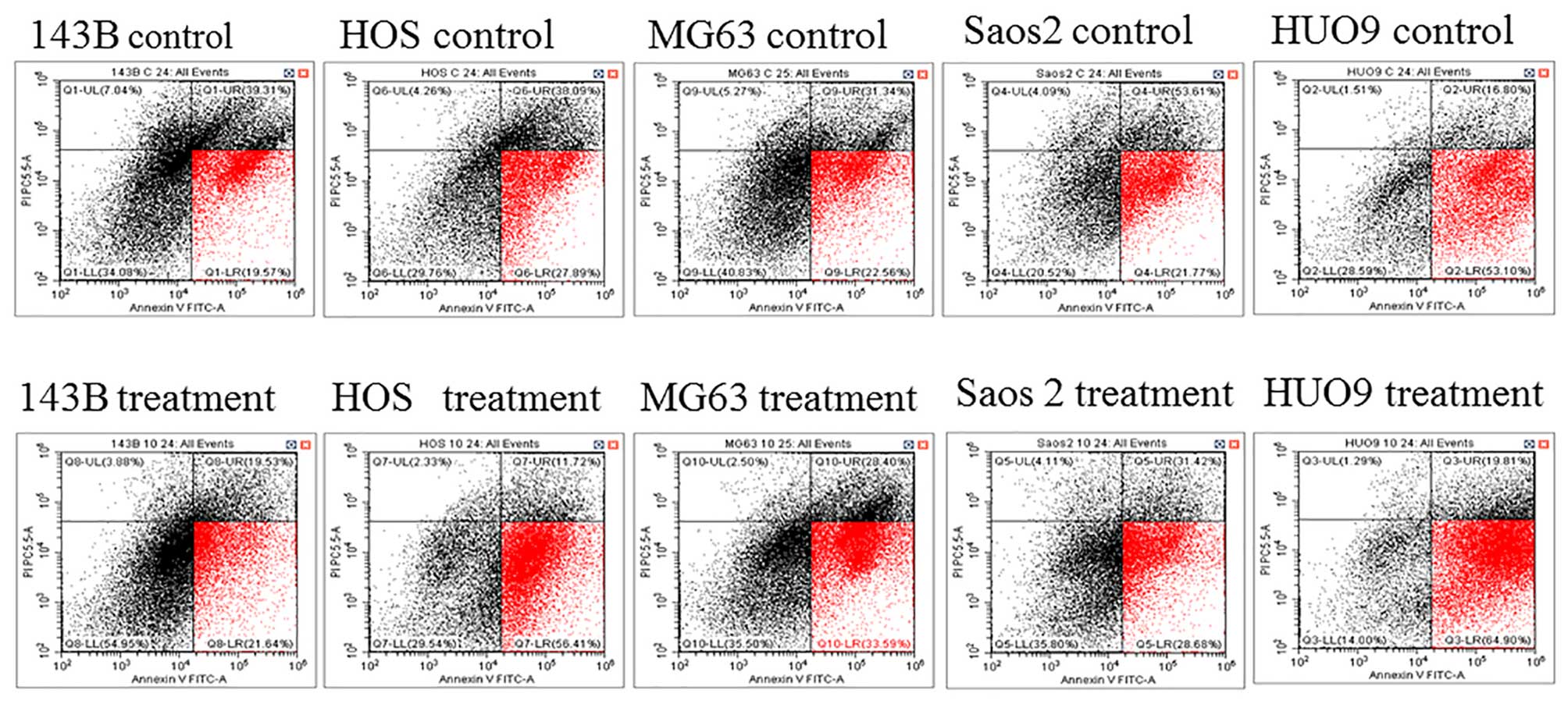
Cucurbitacin I induces apoptosis in
osteosarcoma cells
Annexin V (x-axis) is a protein that binds to the
phospholipid phosphatidylserine, but cannot enter the cell.
Phosphatidylserine is located at the inner side of the membrane.
However, upon apoptosis induction, its normal distribution in the
cell is perturbed and it becomes exposed at the outer membrane.
This feature is used in Annexin V staining for the detection of
early apoptotic cells. The membrane-impermeable dye PI (y-axis)
binds directly to the DNA, which is only possible upon membrane
damage, occurring at late apoptotic or necroptotic events. The
early apoptosis rate (red zone) increased in the cucurbitacin I
treatment group compared to the control group in all five OS cell
lines (143B 19.57 to 21.64%, HOS 27.89 to 56.41%, MG63 22.56 to
33.59%, HUO9 53.1 to 64.9%, Saos2 21.77 to 28.68%) (Fig. 3). These results confirmed that
cucurbitacinI causes osteosarcoma cell death through apoptosis.
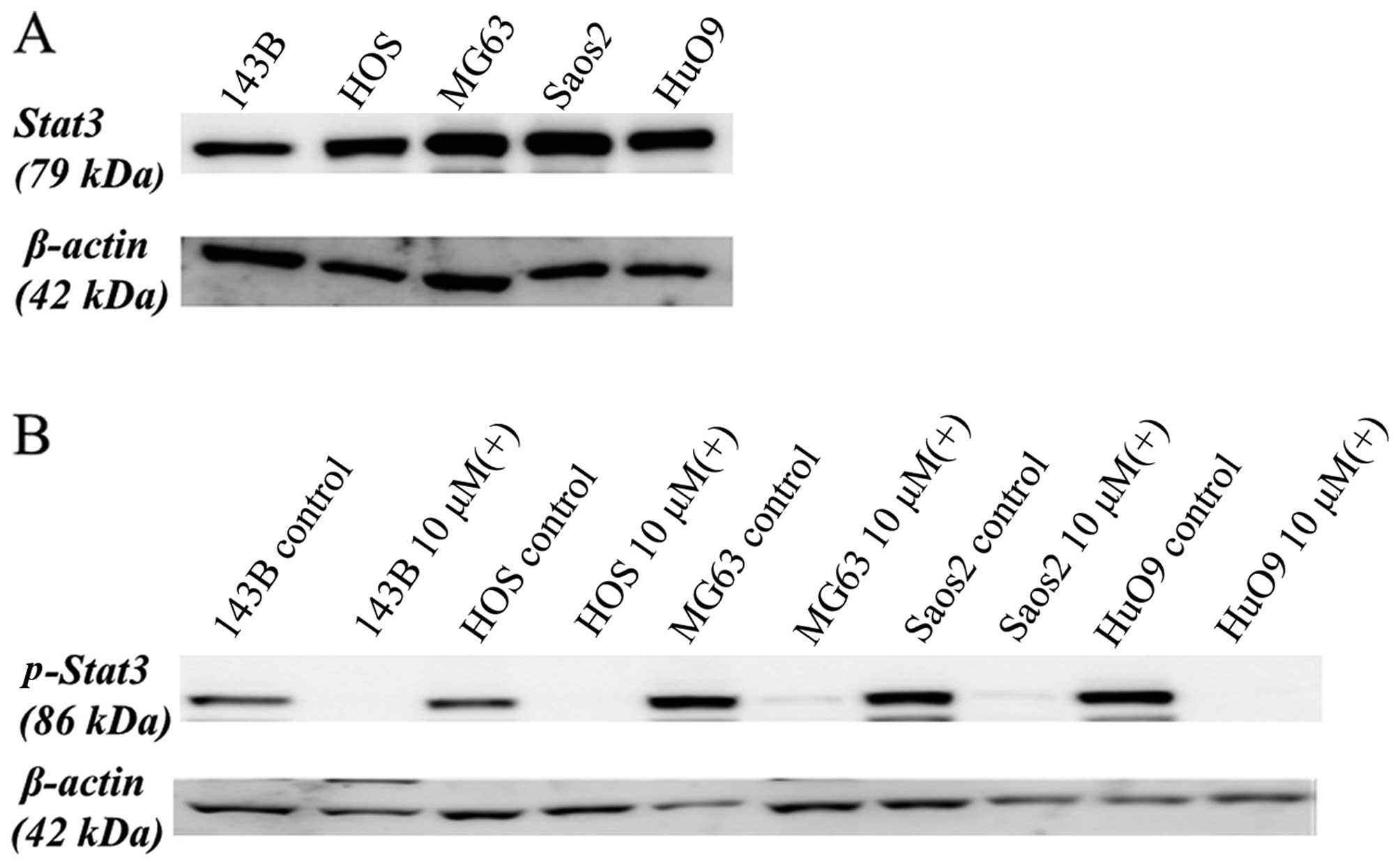
Cell signaling mechanism of cucurbitacin
I
STAT3 is tyrosine phosphorylated and constitutively
activated in many human cancer types (17–19).
We first assayed STAT3 expression of the five human OS cell lines
(143B, HOS, MG63, SAOS-2, and HUO9) by western blotting of the cell
lysates with antibodies specific for STAT3. All five cell lines
expressed high levels of STAT3 (Fig.
4A).
These five human OS cell lines were then treated
with or without 10 μM of cucurbitacin I for 12 h, following which,
the cell lysates were analyzed by western blotting with antibodies
specific for phospho-STAT3. As shown in Fig. 4B, cucurbitacin I suppressed the
levels of phosphorylated STAT3 in all five cell lines.
STAT3-mediated signals are involved in regulation of
the apoptotic pathway. Caspase-3, a member of the caspase family
that plays a central role in apoptosis, is primarily responsible
for the cleavage of PARP during cell death and receives inhibitory
signals from Mcl-1. The effect of cucurbitacin I on PARP cleavage,
cleaved caspase-3 and Mcl-1 was analyzed by western blotting, and
its effect on activated caspase-3 was measured using ELISA.
Cucurbitacin I clearly downregulated Mcl-1 protein levels and
upregulated cleavage of the PARP protein in all OS cell lines
treated for 24 h (Fig. 4C). As
shown in Fig. 4D, activated
caspase-3 was observed in all OS cell lines, except for HUO9 cells,
that were treated with cucurbitacin I for 24 h. Thus, cucurbitacin
I induced apoptosis in human OS cells.
The effect of cucurbitacin I on cell cycle regulator
proteins, such as phospho-cyclin D1, c-Myc and survivin, was also
investigated by western blotting with each specific antibody.
Phospho-cyclin D1 plays a role as a cell cycle regulator. c-Myc is
a key regulator of cellular proliferation and growth factor
stimulation. Survivin is a member of the inhibitors of apoptosis
protein family and inhibits cell death through interference with
both caspase-dependent and -independent cell apoptosis. As shown in
Fig. 4C, cucurbitacin I clearly
downregulated phospho-cyclin D1, c-Myc and survivin levels
following treatment for 24 h.
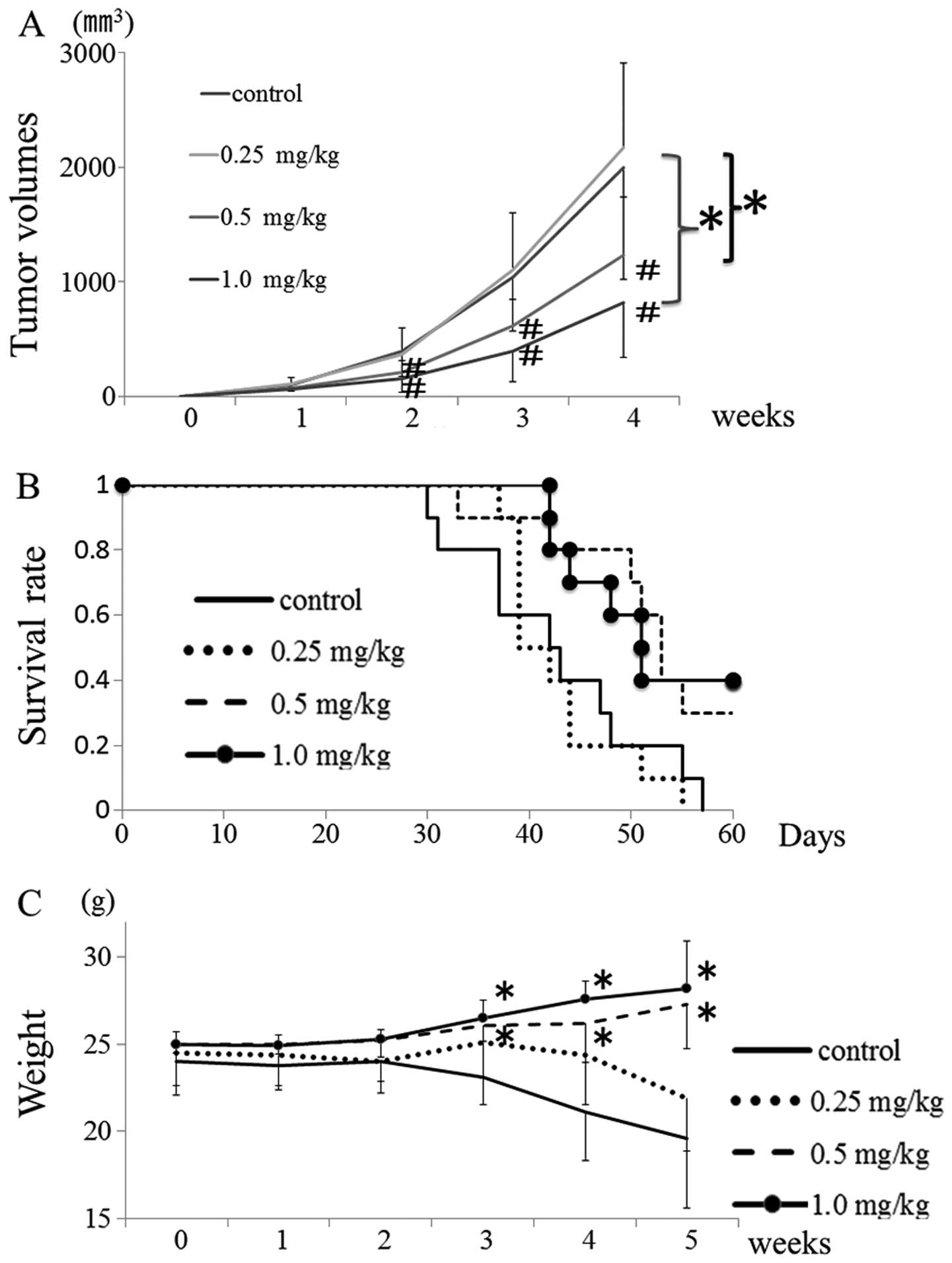
Cucurbitacin I inhibits growth and
induces apoptosis in mice with tumors
To determine the effect of cucurbitacin I on tumor
growth inhibition, we evaluated time-dependent changes in 143B
xenografts in vivo following cucurbitacin I treatment.
Fig. 5A shows that, in the absence
of cucurbitacin I, the growth of the 143B tumor was highly
aggressive, but that treatment with 0.5 or 1.0 mg/kg of
cucurbitacin I significantly and dose-dependently inhibited the
tumor growth. Furthermore, treatment with 0.5 and 1.0 mg/kg of
cucurbitacin I improved overall survival rate, and body weight
significantly increased over time (Fig. 5B and C). In addition, the tumor was
histopathologically evaluated after 14 days of treatment with 1.0
mg/kg cucurbitacin I. Cucurbitacin I suppressed phospho-STAT3
phospho-cyclin D1, survivin and c-Myc expression in the 143B tumor.
Moreover, the phospho-STAT3-negative area was positive by TUNEL,
cleaved caspase-3 and cleaved PARP in immunohistochemical analysis.
Thus, suppression of tumor cell growth signal by cucurbitacin I
administration led to tumor cell apotosis (Fig. 6).
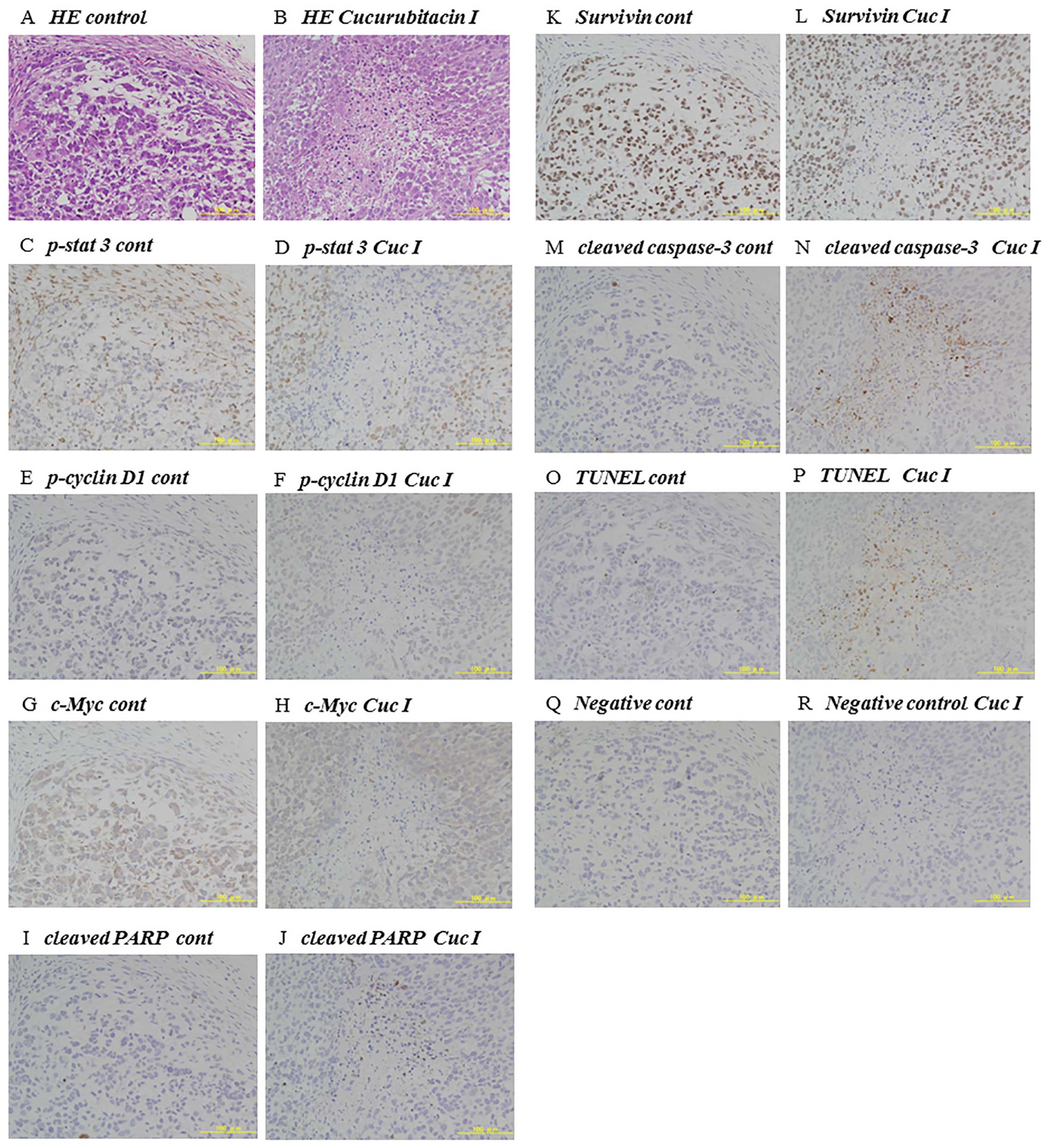 | Figure 6Immunohistochemical analysis of
cucurbitacin I-treated tumors. Representative 143B tumors of
athymic nude mice were immunohistochemically analyzed on day 14
after administration of control (A, C, E, G, I, K, M, O and Q) or
1.0 mg/kg cucurbitacin I (B, D, F, H, J, L, N, P and R). (A and B)
Hematoxylin-eosin (H&E) staining. (C and D) Immunohistochemical
staining of phospho-STAT3. (E and F) p-cyclin D1. (G and H) c-Myc.
(I and J) Cleaved PARP. (K and L) Suvivin. (M and N) Cleaved
caspase-3. (O and P) TUNEL assay. (Q and R) Negative control. |
Discussion
Progress in the development of therapeutic agents
for primary musculoskeletal malignant tumors including OS has been
much slower than that for molecular targeted agents for other
cancers. This is because, firstly, the disease prevalence rate is
extremely low, and, secondly, the tumor tissues are formed
heterogeneously by various cells. Although screening of a compound
library to detect agents for tumor treatment is not difficult, few
such screenings have been performed for rare malignant tumors such
as OS (20). The rarity of these
tumors may result in an economic disadvantage for pharmaceutical
companies and difficulty in performing clinical trials. In this
study, we attempted to identify new target molecules for OS through
cell-based screening using the SCADS inhibitor kit and five OS cell
lines. We selected cucurbitacin I among many candidates based on
its ability to inhibit the viability of the five OS cell lines.
The cucurbitacins have been used for centuries as
unpurified molecules in folk medicine for their anti-inflammatory
analgesic effects. However, little was known about the biological
activities of the cucurbitacins for a long time. Recently, the
derivatives of cucurbitacin: B, D, E and Q, have been widely
recognized for their anti-proliferative activity in in vitro
studies using endothelial cells, leukemias, and a variety of solid
cancer cell lines (18,21–24).
Cucurbitacin I has been found to have anti-proliferative properties
against adenocarcinoma cells (25,26),
nasopharyngeal carcinoma cells (27), anaplastic large cell lymphoma
(28), and non-small cell
carcinoma (29). In this study, we
first demonstrated that cucurbitacin I inhibited viability of human
OS cell lines in monolayer and collagen 3D cultures. A similar
result was previously shown for cucurbitacin B in combination with
methotrexate, which showed promising anti-proliferative activity
against human OS (30). However,
in this study, comparison of the effect of cucurbitacin I and
cucurbitacin B alone in vitro indicated that cucurbitacin I
showed greater inhibition of tumor cell viability than cucurbitacin
B.
Initial studies suggested that cucurbitacin I was a
selective inhibitor of janus kinase (JAK)/STAT3 activation
(22) and that it reduced the
levels of activated STAT3 in human cancer cell lines including
pancreatic, lung and breast carcinomas (25). STAT3 is a critical mediator of
oncogenic signaling, and is activated in many human cancers
(19,31–33),
including in 82% of prostate cancers (34), 70% of breast cancers (35), >90% of head and neck cancers
(36), and >50% of lung cancers
(37). In OS cell lines and
tissues, STAT3 and phospho-STAT3 are overexpressed (38). Furthermore, activation of STAT3
signaling induces the expression of specific genes such as
phosphocyclin D1, c-Myc, Mcl-1, and survivin, which may stimulate
cell proliferation and anti-apoptosis, and promote tumor growth
(29). In the tumors investigated,
aberrant STAT3 activation has been demonstrated to be required for
tumor cell growth and survival (19,39,40).
In this study, STAT3 was highly expressed in the OS
cell lines and cucurbitacin I suppressed phospho-STAT3 expression.
Our results indicated that cucurbitacin I treatment suppressed the
expression of anti-apoptotic factors, such as Mcl-1, and enhanced
apoptotic factors such as caspase-3 and cleaved PARP. Additionally,
cucurbitacin I also inhibited the expression of the cell
proliferation factors, survivin, c-Myc and phospho-cyclin D1
(Fig. 4). These changes in
expression of the downstream targets of STAT3 signaling led to an
increase in apoptosis and a decrease in the proliferation of OS
cells. Moreover, the phospho-STAT3 negative area of tumors in
vivo was positive by TUNEL assay in immunohistochemical
analysis indicating apoptosis (Fig.
6). We successfully demonstrated cucurbitacin I induced tumor
cell death by demonstrating an increase in apoptosis and a decrease
in proliferative signaling via inactivation of STAT3. These
findings may explain why administration of purified cucurbitacin I
to athymic nude mice with human OS xenografts resulted in
significant inhibition of tumor growth and improvement in the
overall survival rate. It has also been shown that inhibition of
STAT3-dependent signaling pathways can reverse tumor growth in
experimental systems with few effects on normal cells (18,19).
In this study, the administration of 0.5 or 1.0 mg/kg of
cucurbitacin I showed inhibition of tumor growth without body
weight decrease. It was therefore considered that cucurbitacin I
did not have a severe toxic effect.
In conclusion, we demonstrated significant
inhibition of tumor growth and improvement in the overall survival
rate in vivo by cucurbitacin I treatment. These effects
resulted from inactivation of STAT3, leading to the induction of
apoptosis and the suppression of proliferative signaling mediated
by signaling molecules such as survivin, c-Myc and phospho-cyclin
D1. These results shed light on a therapeutic strategy by which
cucurbitacin I might be used as a clinical agent and suggest that
STAT3 might be used as a therapeutic target for OS treatment.
Acknowledgements
We would like to express our thanks to the SCADS
Inhibitor kit, Screening Committee of Anticancer Drugs that was
supported by a Grant-in-Aid for Scientific Research on Innovative
Areas, Scientific Support Programs for Cancer Research, from The
Ministry of Education, Culture, Sports, Science and Technology,
Japan.
References
|
1
|
Picci P: Osteosarcoma (osteogenic
sarcoma). Orphanet J Rare Dis. Jan 27–2007.(Epub ahead of print).
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Meyers PA, Heller G, Healey J, Huvos A,
Lane J, Marcove R, Applewhite A, Vlamis V and Rosen G: Chemotherapy
for nonmetastatic osteogenic sarcoma: The Memorial Sloan-Kettering
experience. J Clin Oncol. 10:5–15. 1992.PubMed/NCBI
|
|
3
|
Harris MB, Gieser P, Goorin AM, Ayala A,
Shochat SJ, Ferguson WS, Holbrook T and Link MP: Treatment of
metastatic osteosarcoma at diagnosis: A Pediatric Oncology Group
Study. J Clin Oncol. 16:3641–3648. 1998.PubMed/NCBI
|
|
4
|
Bacci G, Ferrari S, Longhi A, Forni C,
Zavatta M, Versari M and Smith K: High-grade osteosarcoma of the
extremity: Differences between localized and metastatic tumors at
presentation. J Pediatr Hematol Oncol. 24:27–30. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Meyers PA, Heller G, Healey JH, Huvos A,
Applewhite A, Sun M and LaQuaglia M: Osteogenic sarcoma with
clinically detectable metastasis at initial presentation. J Clin
Oncol. 11:449–453. 1993.PubMed/NCBI
|
|
6
|
Pacquement H, Kahfa C, Fagnou C, Demaille
MC, Brunat-Mentigny M, Sariban E, Perel Y and Zucker JM: Metastatic
osteogenic sarcoma (OS) at diagnosis. Study of 73 cases from the
French Society of Pediatric Oncology (SFOP) between 1980 and 1990.
Eur J Cancer. 33:S1241997. View Article : Google Scholar
|
|
7
|
Marina NM, Pratt CB, Rao BN, Shema SJ and
Meyer WH: Improved prognosis of children with osteosarcoma
metastatic to the lung(s) at the time of diagnosis. Cancer.
70:2722–2727. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kaste SC, Pratt CB, Cain AM, Jones-Wallace
DJ and Rao BN: Metastases detected at the time of diagnosis of
primary pediatric extremity osteosarcoma at diagnosis: Imaging
features. Cancer. 86:1602–1608. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Baum ES, Gaynon P, Greenberg L, Krivit W
and Hammond D: Phase II study of cis-dichlorodiammineplatinum (II)
in childhood osteosarcoma: Children's Cancer Study Group Report.
Cancer Treat Rep. 63:1621–1627. 1979.PubMed/NCBI
|
|
10
|
Cores EP, Holland JF, Wang JJ and Sinks
LF: Doxorubicin in disseminated osteosarcoma. JAMA. 221:1132–1138.
1972. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Jaffe N, Paed D, Farber S, Traggis D,
Geiser C, Kim BS, Das L, Frauenberger G, Djerassi I and Cassady JR:
Favorable response of metastatic osteogenic sarcoma to pulse
high-dose methotrexate with citrovorum rescue and radiation
therapy. Cancer. 31:1367–1373. 1973. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Marti C, Kroner T, Remagen W, Berchtold W,
Cserhati M and Varini M: High-dose ifosfamide in advanced
osteosarcoma. Cancer Treat Rep. 69:115–117. 1985.PubMed/NCBI
|
|
13
|
Nitschke R, Starling KA, Vats T and Bryan
H: Cis-diamminedichloroplatinum (NSC-119875) in childhood
malignancies: A Southwest Oncology Group study. Med Pediatr Oncol.
4:127–132. 1978. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hamakawa H, Nakashiro K, Sumida T,
Shintani S, Myers JN, Takes RP, Rinaldo A and Ferlito A: Basic
evidence of molecular targeted therapy for oral cancer and salivary
gland cancer. Head Neck. 30:800–809. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sun J, Blaskovich MA, Knowles D, Qian Y,
Ohkanda J, Bailey RD, Hamilton AD and Sebti SM: Antitumor efficacy
of a novel class of non-thiol-containing peptidomimetic inhibitors
of farnesyltransferase and geranylgeranyltransferase I: Combination
therapy with the cytotoxic agents cisplatin, Taxol, and
gemcitabine. Cancer Res. 59:4919–4926. 1999.PubMed/NCBI
|
|
16
|
Sun L, Liang C, Shirazian S, Zhou Y,
Miller T, Cui J, Fukuda JY, Chu JY, Nematalla A, Wang X, et al:
Discovery of
5-[5-fluoro-2-oxo-1,2-dihydroindol-(3Z)-ylidenemethyl]-2,4-dimethyl-1H-pyrrole-3-carboxylic
acid (2-diethylaminoethyl) amide, a novel tyrosine kinase inhibitor
targeting vascular endothelial and platelet-derived growth factor
receptor tyrosine kinase. J Med Chem. 46:1116–1119. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Bowman T, Yu H, Sebti S, Dalton W and Jove
R: Signal transducers and activators of transcription: Novel
targets for anticancer therapeutics. Cancer Control. 6:427–435.
1999.
|
|
18
|
Turkson J and Jove R: STAT proteins: Novel
molecular targets for cancer drug discovery. Oncogene.
19:6613–6626. 2000. View Article : Google Scholar
|
|
19
|
Bowman T, Garcia R, Turkson J and Jove R:
STATs in oncogenesis. Oncogene. 19:2474–2488. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Shoemaker RH: The NCI60 human tumour cell
line anticancer drug screen. Nat Rev Cancer. 6:813–823. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Jayaprakasam B, Seeram NP and Nair MG:
Anticancer and antiinflammatory activities of cucurbitacins from
Cucurbita andreana. Cancer Lett. 189:11–16. 2003. View Article : Google Scholar
|
|
22
|
Duncan KLK, Duncan MD, Alley MC and
Sausville EA: Cucurbitacin E-induced disruption of the actin and
vimentin cytoskeleton in prostate carcinoma cells. Biochem
Pharmacol. 52:1553–1560. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sun J, Blaskovich MA, Jove R, Livingston
SK, Coppola D and Sebti SM: Cucurbitacin Q: A selective STAT3
activation inhibitor with potent antitumor activity. Oncogene.
24:3236–3245. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Rivat C, Rodrigues S, Bruyneel E, Piétu G,
Robert A, Redeuilh G, Bracke M, Gespach C and Attoub S: Implication
of STAT3 signaling in human colonic cancer cells during intestinal
trefoil factor 3 (TFF3) - and vascular endothelial growth
factor-mediated cellular invasion and tumor growth. Cancer Res.
65:195–202. 2005.PubMed/NCBI
|
|
25
|
Blaskovich MA, Sun J, Cantor A, Turkson J,
Jove R and Sebti SM: Discovery of JSI-124 (cucurbitacin I), a
selective Janus kinase/signal transducer and activator of
transcription 3 signaling pathway inhibitor with potent antitumor
activity against human and murine cancer cells in mice. Cancer Res.
63:1270–1279. 2003.PubMed/NCBI
|
|
26
|
Iwanski GB, Lee DH, En-Gal S, Doan NB,
Castor B, Vogt M, Toh M, Bokemeyer C, Said JW, Thoennissen NH, et
al: Cucurbitacin B, a novel in vivo potentiator of gemcitabine with
low toxicity in the treatment of pancreatic cancer. Br J Pharmacol.
160:998–1007. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lui VWY, Yau DMS, Wong EYL, Ng YK, Lau
CPY, Ho Y, Chan JP, Hong B, Ho K, Cheung CS, et al: Cucurbitacin I
elicits anoikis sensitization, inhibits cellular invasion and in
vivo tumor formation ability of nasopharyngeal carcinoma cells.
Carcinogenesis. 30:2085–2094. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Shi X, Franko B, Frantz C, Amin HM and Lai
R: JSI-124 (cucur-bitacin I) inhibits Janus kinase-3/signal
transducer and activator of transcription-3 signalling,
downregulates nucleophosmin-anaplastic lymphoma kinase (ALK), and
induces apoptosis in ALK-positive anaplastic large cell lymphoma
cells. Br J Haematol. 135:26–32. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Jing N and Tweardy DJ: Targeting Stat3 in
cancer therapy. Anticancer Drugs. 16:601–607. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lee DH, Thoennissen NH, Goff C, Iwanski
GB, Forscher C, Doan NB, Said JW and Koeffler HP: Synergistic
effect of low-dose cucurbitacin B and low-dose methotrexate for
treatment of human osteosarcoma. Cancer Lett. 306:161–170. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Bromberg JF, Wrzeszczynska MH, Devgan G,
Zhao Y, Pestell RG, Albanese C and Darnell JE Jr: Stat3 as an
oncogene. Cell. 98:295–303. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yu H and Jove R: The STATs of cancer - new
molecular targets come of age. Nat Rev Cancer. 4:97–105. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Darnell JE: Validating Stat3 in cancer
therapy. Nat Med. 11:595–596. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Mora LB, Buettner R, Seigne J, Diaz J,
Ahmad N, Garcia R, Bowman T, Falcone R, Fairclough R, Cantor A, et
al: Constitutive activation of Stat3 in human prostate tumors and
cell lines: Direct inhibition of Stat3 signaling induces apoptosis
of prostate cancer cells. Cancer Res. 62:6659–6666. 2002.PubMed/NCBI
|
|
35
|
Dolled-Filhart M, Camp RL, Kowalski DP,
Smith BL and Rimm DL: Tissue microarray analysis of signal
transducers and activators of transcription 3 (Stat3) and
phospho-Stat3 (Tyr705) in node-negative breast cancer shows nuclear
localization is associated with a better prognosis. Clin Cancer
Res. 9:594–600. 2003.PubMed/NCBI
|
|
36
|
Leeman RJ, Lui VW and Grandis JR: STAT3 as
a therapeutic target in head and neck cancer. Expert Opin Biol
Ther. 6:231–241. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Song L, Turkson J, Karras JG, Jove R and
Haura EB: Activation of Stat3 by receptor tyrosine kinases and
cytokines regulates survival in human non-small cell carcinoma
cells. Oncogene. 22:4150–4165. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Ryu K, Choy E, Yang C, Susa M, Hornicek
FJ, Mankin H and Duan Z: Activation of signal transducer and
activator of transcription 3 (Stat3) pathway in osteosarcoma cells
and overexpression of phosphorylated-Stat3 correlates with poor
prognosis. J Orthop Res. 28:971–978. 2010.PubMed/NCBI
|
|
39
|
Niu G, Heller R, Catlett-Falcone R,
Coppola D, Jaroszeski M, Dalton W, Jove R and Yu H: Gene therapy
with dominant-negative Stat3 suppresses growth of the murine
melanoma B16 tumor in vivo. Cancer Res. 59:5059–5063.
1999.PubMed/NCBI
|
|
40
|
Duncan MD and Duncan KL: Cucurbitacin E
targets proliferating endothelia. J Surg Res. 69:55–60. 1997.
View Article : Google Scholar : PubMed/NCBI
|