Introduction
Glioma is the most common brain tumor, accounting
for 1/3–2/3 of adult intracranial tumors. Its pathogenesis is
complicated and no radical cure is available. Current treatment is
mainly surgery, supplemented by chemotherapy and radiation.
However, due to glioma features such as invasive growth and
metastasis, this comprehensive treatment cannot achieve good
efficacy and also have negatively influences on the quality of life
(1). Therefore, it is essential to
discover novel therapeutic agents for improving the treatment
outcome in patients diagnosed with glioma.
CXCR4 is a well-known G-protein coupled receptor
(GPCR) for the small chemokine stromal-derived factor SDF-1α, which
is also known as CXCL12. It has been reported that CXCR4 is
overexpressed in many tumor cells, including glioma, colorectal,
breast, lung, prostate and cervical cancer (2–4).
Consistently, high expression of CXCR4 was observed in highly
invasive glioma stem cells (5).
SDF-1α promotes tumor growth in a paracrine fashion by directly
stimulating tumor cell proliferation and survival via CXCR4.
Moreover, interruption of CXCR4 and SDF-1α axis has demonstrated
antitumor growth activities in a variety of preclinical tumor
models (6–8). Therefore, it is necessary and of
great significance to develop a CXCR4 antagonist targeting CXCR4
for providing more effective strategy for the treatment of
glioma.
The CXCR4 antagonist Plerixafor (AMD3100) is the
most studied and clinically advanced compound that inhibits
SDF-1α/CXCR4 signaling (9,10). It has been reported that long-term
use of AMD3100 could result in cardiotoxicity and other adverse
events (11). Thus, it is
important to search for new safer and selective CXCR4 inhibitors
suitable for glioma.
Virus macrophage inflammatory protein-II (vMIP-II)
is a low molecular weight protein encoded by the HHV-8 K4 gene,
which is an analogue of human chemotactic factor (12). The viral macrophage inflammatory
protein-II (vMIP-II) shows a broad spectrum interaction with both
CC and CXC chemokine receptors including CCR5 and CXCR4 (13). Its N-terminal has been reported to
be the site with high affinity binding to CXCR4 (14). In our previous study, we developed
a synthetic version of the 21-residue N-terminal of vMIP-II
(NT21MP), and demonstrated that NT21MP is a potent antagonist of
SDF-1α and CXCR4, in that it inhibited SDF-1α-induced migration,
inhibited cellular proliferation, promoted apoptosis by
downregulating CXCR4 expression, and inhibited breast cancer
progression and metastasis in vitro and in vivo
(15,16). In the present study, we explored
whether NT21MP inhibits cell growth and invasion, as well as
induces apoptosis in U251 and SHG-44 cells. Moreover, we determined
whether NT21MP exhibits its antitumor function through regulation
of SDF-1α/CXCR4 in glioma cells.
Material and methods
Reagents and antibodies
Human glioma cell lines SHG-44 and U251 were
purchased from Cell Bank of the Chinese Academy of Sciences
(Shanghai, China). NT21MP was designed by our laboratory and
synthesized by GL Biochem Ltd. (Shanghai, China). The amino acid
sequence information of the NT21MP is
H-D-leu-D-Gly-D-Ala-D-Ser-D-Trp-D-His-D-Arg-D-Pro-D-Asp-D-Lys-Cys-Cys-Leu-Gly-Tyr-Gln-Lys-Arg-Pro-Leu-Pro-OH.
Human-SDF-1α was purchased from PeproTech (Rocky Hill, NJ, USA).
AMD3100 and 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium
bromide (MTT) were obtained from Sigma-Aldrich (St. Louis, MO,
USA). Primary antibodies against Bcl-2, Bax, caspase-3, cyclin D1
and β-actin were obtained from Santa Cruz Biotechnology (Santa
Cruz, CA, USA). A mouse anti-human CXCR4 mAb was purchased from
Abcam (clone: 44716.111). Secondary antibodies conjugated to
horseradish peroxidase (HRP) were purchased from ZSGB-Bio, Co.,
Ltd. (Beijing, China). Apoptosis kit was obtained from BD
Biosciences (San Jose, CA, USA). Hoechst 33258 was purchased from
Sigma-Aldrich. Reverse transcription kit was obtained from Thermo
Fisher Scientific (Waltham, MA, USA) and the SYBR Premix Dimer
Eraser™ reagent kit from Takara, Co., Ltd. (Shiga, Japan).
Cell culture and treatment
The human glioma cell lines SHG-44 and U251 were
cultured in Dulbecco's modified Eagle's medium (DMEM)/high glucose
medium containing 10% fetal bovine serum (FBS) at 37°C, in a humid
atmosphere with 5% CO2 and passaged every 3 days. Cells
were stimulated or not with 0.1 µg/ml SDF-1α and cultured in
absence or in presence of 1 µg/ml NT21MP or AMD3100. Cells
in logarithmic phase were used in all experiments.
Quantitative RT-PCR
Total RNA extraction and cDNA synthesis were
conducted with the above kits according to the manufacturer's
instructions. All real-time PCR experiments were performed by using
an ABI 7500 real-time PCR system (Applied Biosystems, Inc., Foster
City, CA, USA) and the SYBR Premix Dimer Eraser™ reagent kit
(Takara Bio). All reactions were performed in triplicate. GAPDH was
used as internal reference to normalize the variability in
expression levels and the fold-change of target gene in cells
relative to their respective control was reported as
2−ΔΔCt. Primers used for real-time PCR are shown in
Table I.
 | Table IPrimers used for real-time PCR. |
Table I
Primers used for real-time PCR.
| Name | Forward primer | Reverse primer |
|---|
| Bak1 |
CCCAGGACACAGAGGAGGTTT |
GCCTCCTGTTCCTGCTGATG |
| CDK4 |
GGTGACAAGTGGTGGAACAG |
GCCCAATCAGGTCAAAGATT |
| CDK6 |
CCCACTGAAACCATAAAGGA |
ACCACAGCGTGACGACCA |
| Cyclin D1 |
AGGAGAACAAACAGAATCA |
TAGGACAGGAAGTTGTTG |
| Bcl-2 |
ATGTGTGTGGAGAGCGTCAA |
ACAGTTCCACAAAGGCATCC |
| Bax |
GGGGACGAACTGGACAGTAA |
CAGTTGAAGTTGCCGTCAGA |
| SDF-1α |
CCGCGCTCTGCCTCAGCGACGGGAAG |
CTTGTTTAAAGCTTTCTCCAGGTACT |
| GAPDH |
CAGCCTCAAGATCATCAGCA |
TGTGGTCATGAGTCCTTCCA |
Immunofluorescence analysis
Cells were plated into the 6-well plate at the
density of 2×105 cells/ml, treated with SDF-1α, NT21MP
or with AMD3100 as described in 'Cell culture and treatment' and
incubated at 37°C with 5% CO2 overnight; after washing
with pre-cooled PBS twice, cells were fixed for 20 min with
fixative, treated with 0.2% Triton X-100 for 5 min at room
temperature, washed with phosphate-buffered saline (PBS) 3 times
and blocked with 4% BSA for 30 min. Then, anti-CXCR4 primary
antibody (1:200) and goat anti-mouse secondary antibody labeled
with FITC (1:200) were sequentially added. Counterstaining was
carried out with Hoechst 33258 at room temperature. After washing,
cells were stained in 10 mg/ml Hoechst solution. Image acquisition
and processing were conducted by using Leica Confocal Software
(Leica Microsystems). All reactions were performed in
triplicate.
Cell proliferation analysis
Cells were seeded in 96-well plates at the density
of 5×104 cells in 100 µl/well in triplicate and
were treated with SDF-1α, NT21MP or with AMD3100 as described in
'Cell culture and treatment' for 24, 48 and 72 h at 37°C and 5%
CO2. Afterwards, 20 µl MTT solution (5 mg/ml) was
added in each well and further incubated for 4 h. After removing
the cell culture medium, dimethyl sulfoxide (DMSO) (150 µl)
was added in each well and OD values were obtained on a microplate
reader at 490 nm to assess growth inhibition rate of human glioma
as follows: Growth inhibition rate (%) = 1- (average OD in
experiment group/average OD in control group) × 100%. Each
experimental group was performed in five replicates for each
time-point and all the experiments were repeated three times
independently.
Wound healing analysis
Cells were seeded in 96-well plates at the density
of 1×105 cells/ml, treated with SDF-1α, NT21MP or with
AMD3100 as described in 'Cell culture and treatment' and incubated
at 37°C overnight. At 80–90% confluency, a wound was generated by
scraping with a 10-µl sterile pipette tip. Then cells were
washed twice with PBS and were further cultured at 37°C with 5%
CO2. After 20 h, the cells in the wounded monolayer were
photographed and cell migration was assessed by measuring gap sizes
at multiple fields. At least three independent experiments were
performed in each cell line.
Transwell analysis
Cell migration assay was performed with Transwell
chambers (Corning Costar Corp., Cambridge, MA, USA). Cells were
seeded in serum-free medium (3×105 cells/well) in the
Transwell chambers either with or without NT21MP or AMD3100. Then,
the chamber were placed into wells of the 24-well plate which was
added with the medium containing 10% FBS with or without SDF-1α.
Cells were cultured at 37°C with 5% CO2 for 18–24 h and
those did not pass through the membrane were removed.
Paraformaldehyde (4%) was used to fix the remaining ones for 20 min
followed by Giemsa staining. The migrated cells on the lower side
of the filters were defined as invasive cells and counted at ×200
magnification in 10 different fields of each filter. All reactions
were performed in triplicate.
Cell cycle analysis
Cells were plated into a 6-well plate at the density
of 1×105 cells/ml, treated with SDF-1α, NT21MP or with
AMD3100 as described in 'Cell culture and treatment' and incubated
at 37°C with 5% CO2 for 48 h; then, cells were fixed
with 70% alcohol at 4°C overnight. Afterwards, cells were washed
with PBS. In order to remove RNA, RNase A (100 µg/ml) was
used to digest the fixed cells for 30 min. The staining was carried
out with 50 mg/ml propidium iodide (PI) in PBS Triton X-100 in the
dark for another 30 min at room temperature. Finally, results were
acquired by the Muse™ cell analyzer. At least three independent
experiments were performed in each cell line.
Apoptosis analysis
Cells were plated into the 6-well plate at the
density of 5×105 cells/ml, treated with SDF-1α, NT21MP
or with AMD3100 as described in 'Cell culture and treatment' and
incubated at 37°C with 5% CO2. After 24 h, cells were
collected and washed twice with PBS. Then, cell density was
adjusted to 5×105 cells/ml with DMEM/high glucose medium
containing 1% FBS; 100 µl cell suspension were mixed with
100 µl apoptosis reagent and incubated for 30 min in the
dark at room temperature. Finally, results were acquired by the
Muse™ cell analyzer. At least three independent experiments were
performed in each cell line.
Transfection
Transient transfections were performed using
Lipofectamine or Lipofectamine 2000 reagents (Thermo Fisher
Scientific) according to the manufacturer's instructions. CXCR4
siRNA and the negative control siRNA products were purchased from
Suzhou GenePharma, Co., Ltd. (Suzhou, China). Transfection were
performed as previously reported. CXCR4 siRNA fragments are
summarized in Table II.
 | Table IICXCR4 siRNA fragments. |
Table II
CXCR4 siRNA fragments.
| Name | Sense | Antisense |
|---|
| CXCR4-homo-1 |
GAAGCAUGACGGACAAGUA |
UACUUGUCCGUCAUGCUUC |
| CXCR4-homo-2 |
GGAAGCUGUUGGCUGAAAA |
UUUUCAGCCAACAGCUUCC |
| CXCR4-homo-3 |
CUGUCCUGCUAUUGCAUUA |
UAAUGCAAUAGCAGGACAG |
Western blot analysis
Cells were seeded at the density of
2.5×105 cells/ml, treated with SDF-1α, NT21MP or with
AMD3100 as described above and lysed in RIPA buffer (150 mM NaCl,
50 mM Tris-HCl, pH 7.5, 1% Nonidet P-40, 0.5% sodium deoxycholate
and 0.1% SDS), supplemented with protease and phosphatase inhibitor
cocktails (Hoffman-La Roche Ltd., Basel, Switzerland). Protein
concentrations were determined by BCA protein assay kit (Beyotime
Institute of Biotechnology, Haimen, China) and proteins (20
µg/lane) were isolated by SDS-PAGE (10% polyacrylamide)
electrophoresis and then transferred to NC membranes. Then, the
membranes were blocked for 2 h and incubated with primary
antibodies overnight at 4°C. After washing with TBST buffer, the
membrane was incubated with secondary antibodies at 37°C for 2 h.
The membrane was washed with TBST buffer and detected the protein
expression levels with a Bio-Rad Gel Imaging System. The
anti-β-actin antibody was used as loading control.
Statistical analysis
Each experiment consisted of three replications.
Statistical comparisons between different groups were evaluated
using GraphPad Prism 4.0 (Graphpad Software, Inc., La Jolla, CA,
USA). Statistical analyses were performed with mean ± standard
deviation (SD) values. P<0.05 was considered statistically
significant.
Results
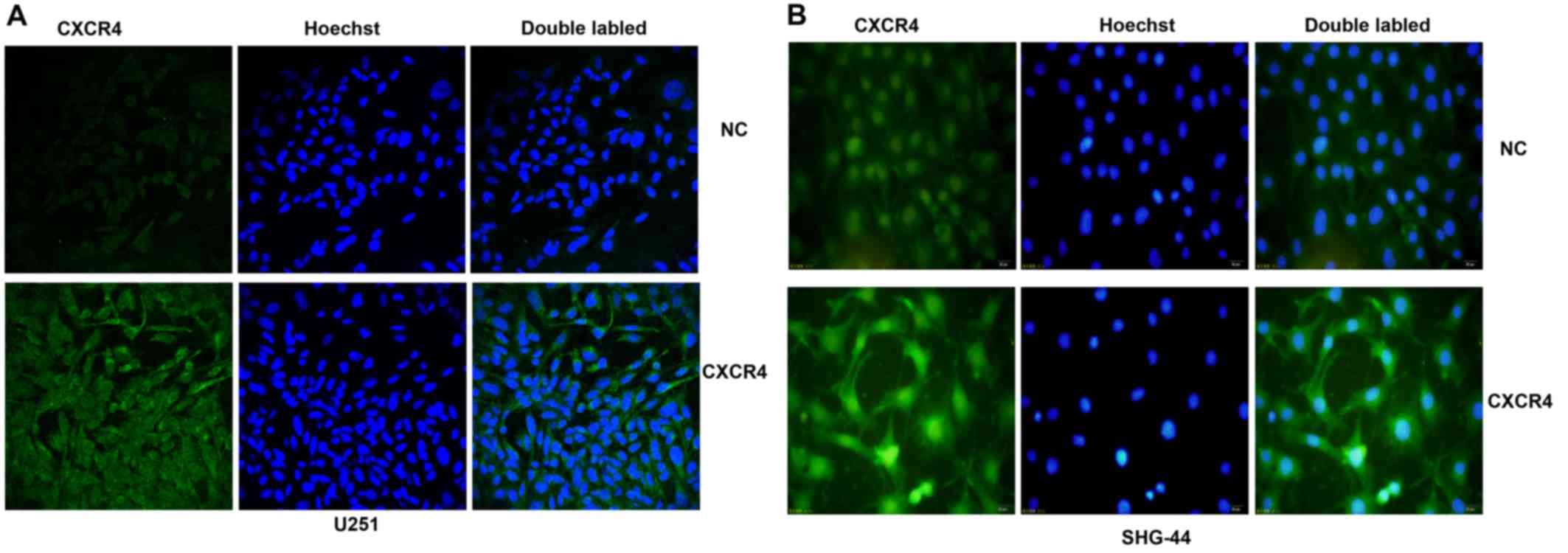
CXCR4 is expressed at different levels in
human glioma U251 and SHG-44 cells
CXCR4 expression in glioma cells was detected by
immunofluorescence. As shown in Fig.
1, the results indicated that both U251 and SHG-44 expressed
the CXCR4 protein. Compared with U251 cells, the SHG-44 cell line
showed higher expression levels.
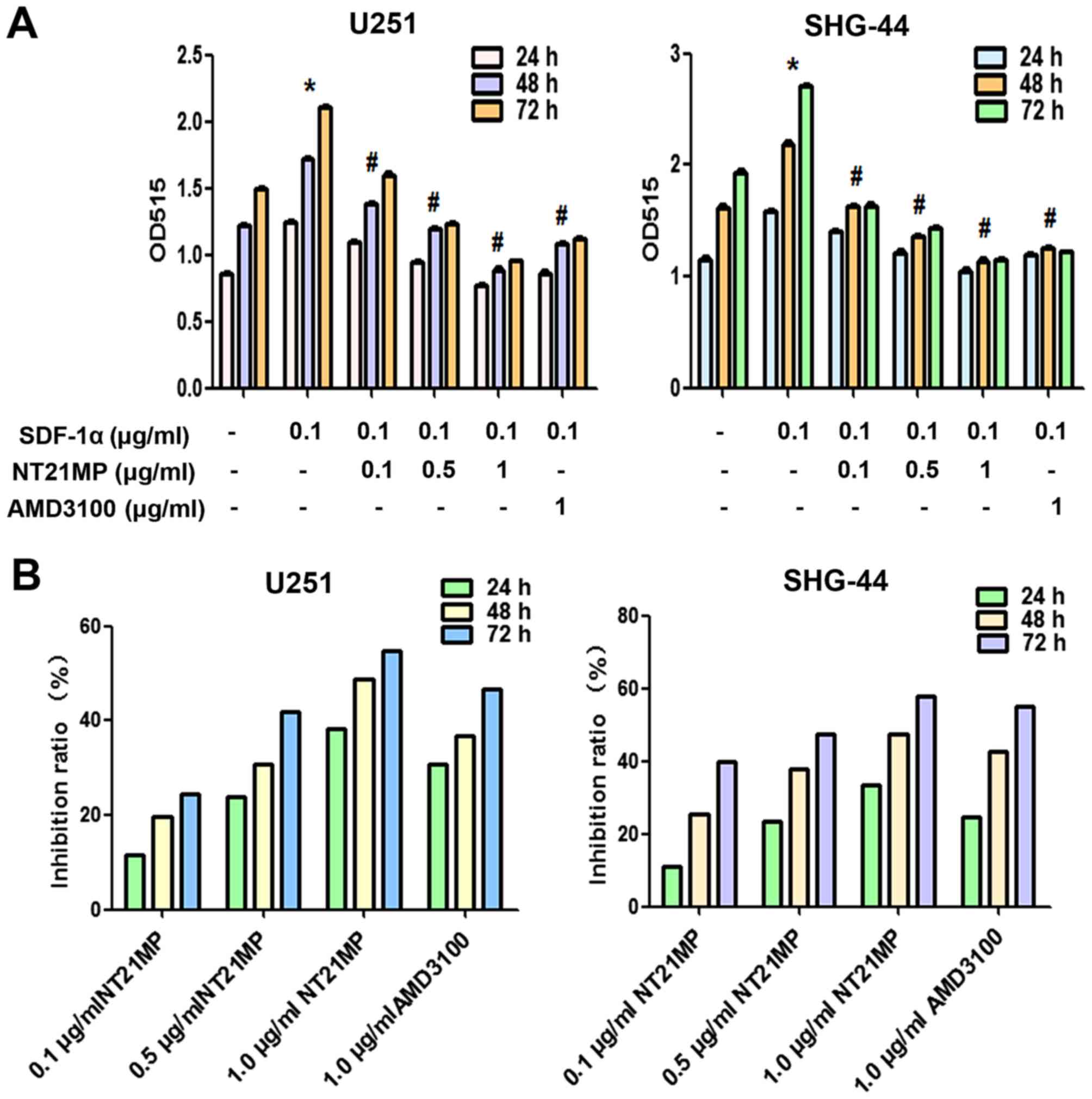
NT21MP inhibits SDF-1α-induced human
glioma cell proliferation
In order to investigate the possible functional
effects of NT21MP on SDF-1α-induced glioma cells, we assessed cell
proliferation ability after exposure of U251 and SHG-44 cells to
various doses of NT21MP using the MTT assay at 24, 48 and 72 h. As
shown in Fig. 2A, treatment with
SDF-1α significantly increased cell viability, NT21MP inhibited
cell growth in a dose- and time-dependent manner in U251 and SHG-44
cells (Fig. 2B). AMD3100, as a
specific inhibitor of CXCR4, inhibited the SDF-1α induced
proliferation (P<0.05; Fig. 2A)
in a time-dependent manner (P<0.05; Fig. 2B).
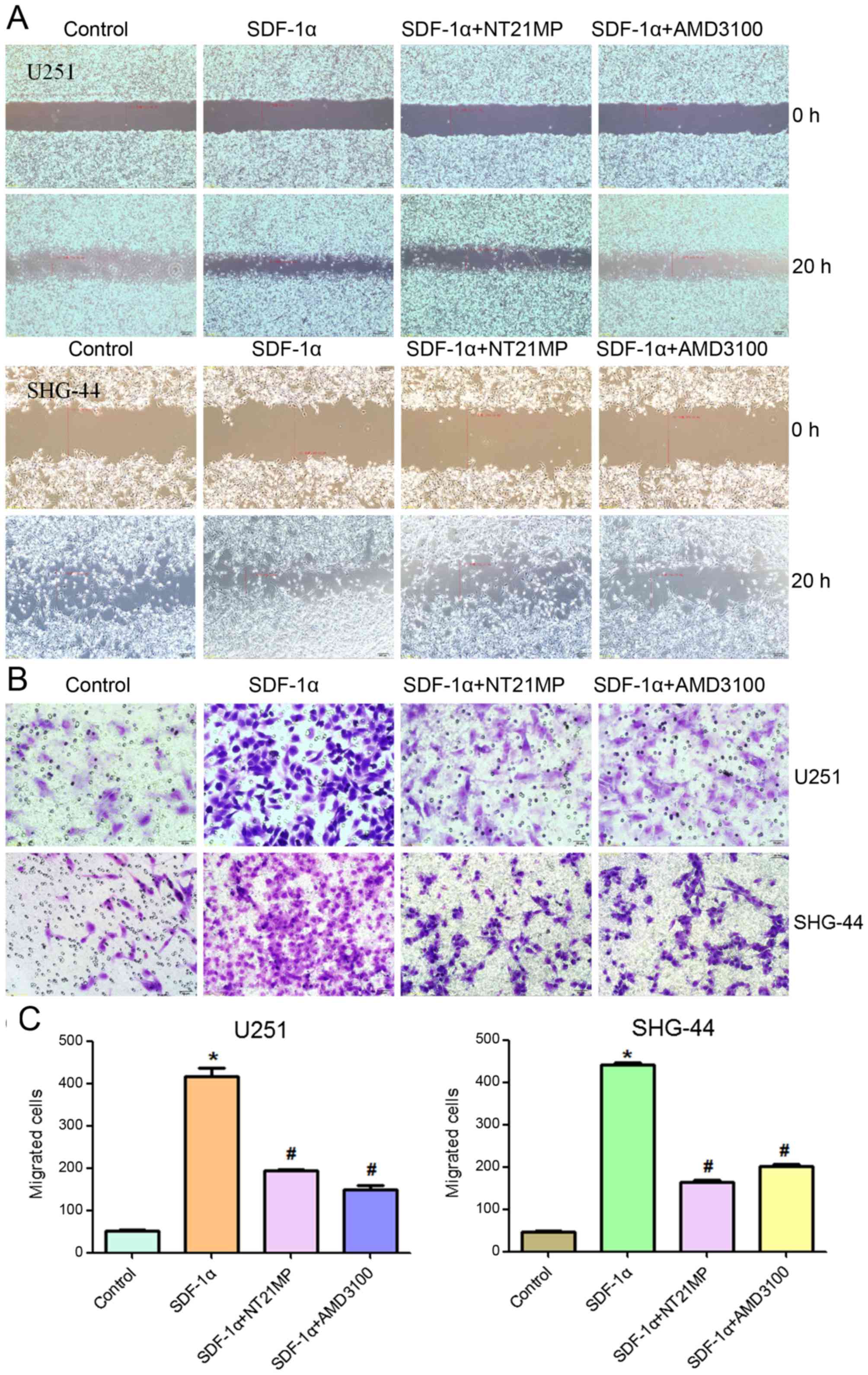
NT21MP inhibits SDF-1α-induced migration
and invasion in human glioma cell
One of the important functions of SDF-1α/CXCR4
interaction is to regulate cell migration. Therefore, the effects
of NT21MP on cell migration were evaluated using a wound healing
and Transwell invasion assay, and the results were compared to
those cells treated with AMD3100. As shown in Fig. 3, as expected, SDF-1α promoted cell
migration and invasion in U251 and SHG-44 cells. AMD3100 did not
induce significant changes, while NT21MP significantly reduced the
percentage of area occupied by migrating cells, further confirming
that NT21MP acted as an antagonist.
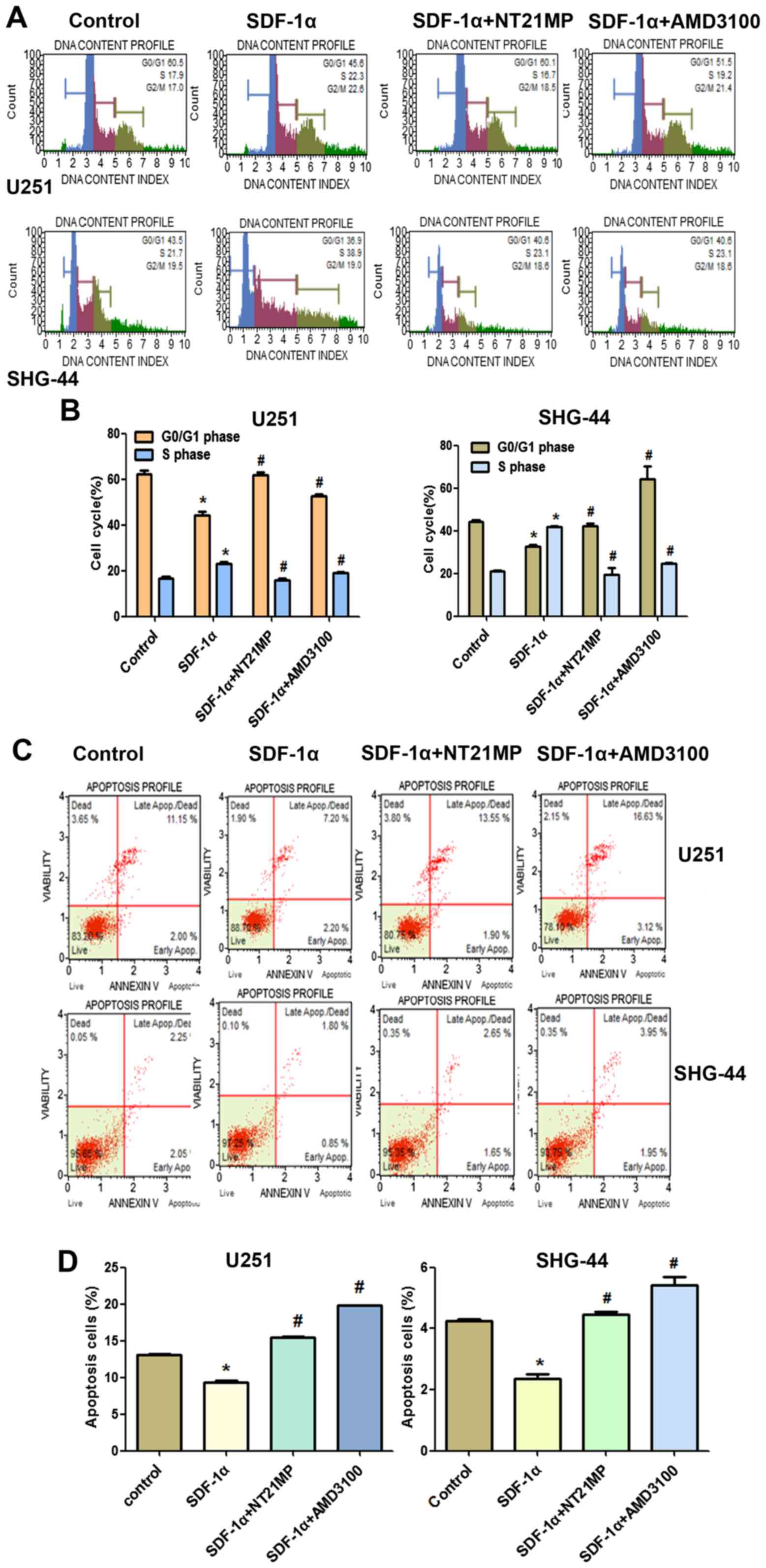
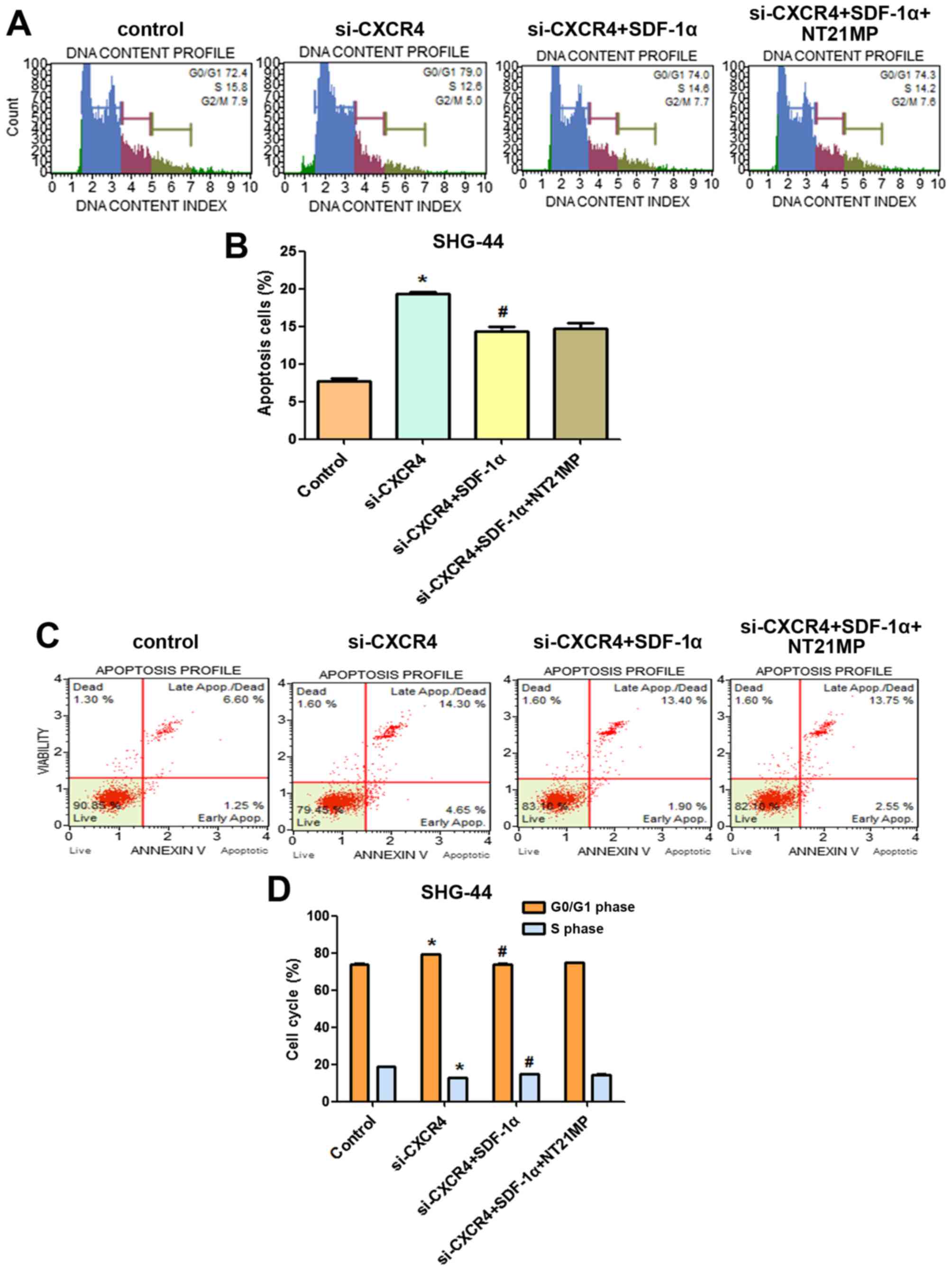
NT21MP arrests glioma cells in G0/G1
phase and promotes apoptosis
The role of cell cycle arrest has been implicated in
tumor cells proliferation, we performed cell cycle analysis using
flow cytometry to determine whether the inhibition of NT21MP on
U251 and SHG-44 cell proliferation involved cell cycle changes.
As shown in Fig. 4A and
B, when glioma cells were treated with SDF-1α, the percentage
of cells in the G0/G1 phase decreased (from 60.5 to 45.6% for U251
and from 43.5 to 36.9% for SHG-44) and the percentage of cells in
the S phase increased (from 17.1 to 22.3% for U251, from 21 to
38.9% for SHG-44, respectively). These results indicated that
SDF-1α induced the cell transition from G0/G1 to S phase. Compared
with the SDF-1α treatment group, the rate of cell growth in the S
phase is significantly reduced in glioma cells when treated with
NT21MP (for U251 cells, the percentage was from 22.3 to 16.7%, and
for SHG-44 cells, from 38.9 to 23.1%), while the number of cells in
G0/G1 phase was increased, indicating that NT21MP could block the
cell cycle of glioma cells at the G0/G1 phase.
The results of Annexin V/PI double-staining
demonstrated that the number of apoptotic cells was significantly
decreased by SDF-1α treatment compared with the untreated cells. As
shown in Fig. 4C and D, compared
with the SDF-1α treatment group, NT21MP significantly attenuated
the anti-apoptotic effects of SDF-1α-induced in both cell lines,
indicating that NT21MP could induce apoptosis in glioma cells. In
addition, CXCR4-specific antagonist, AMD3100, prevented
SDF-1α-induced anti-apoptosis in glioma cells.
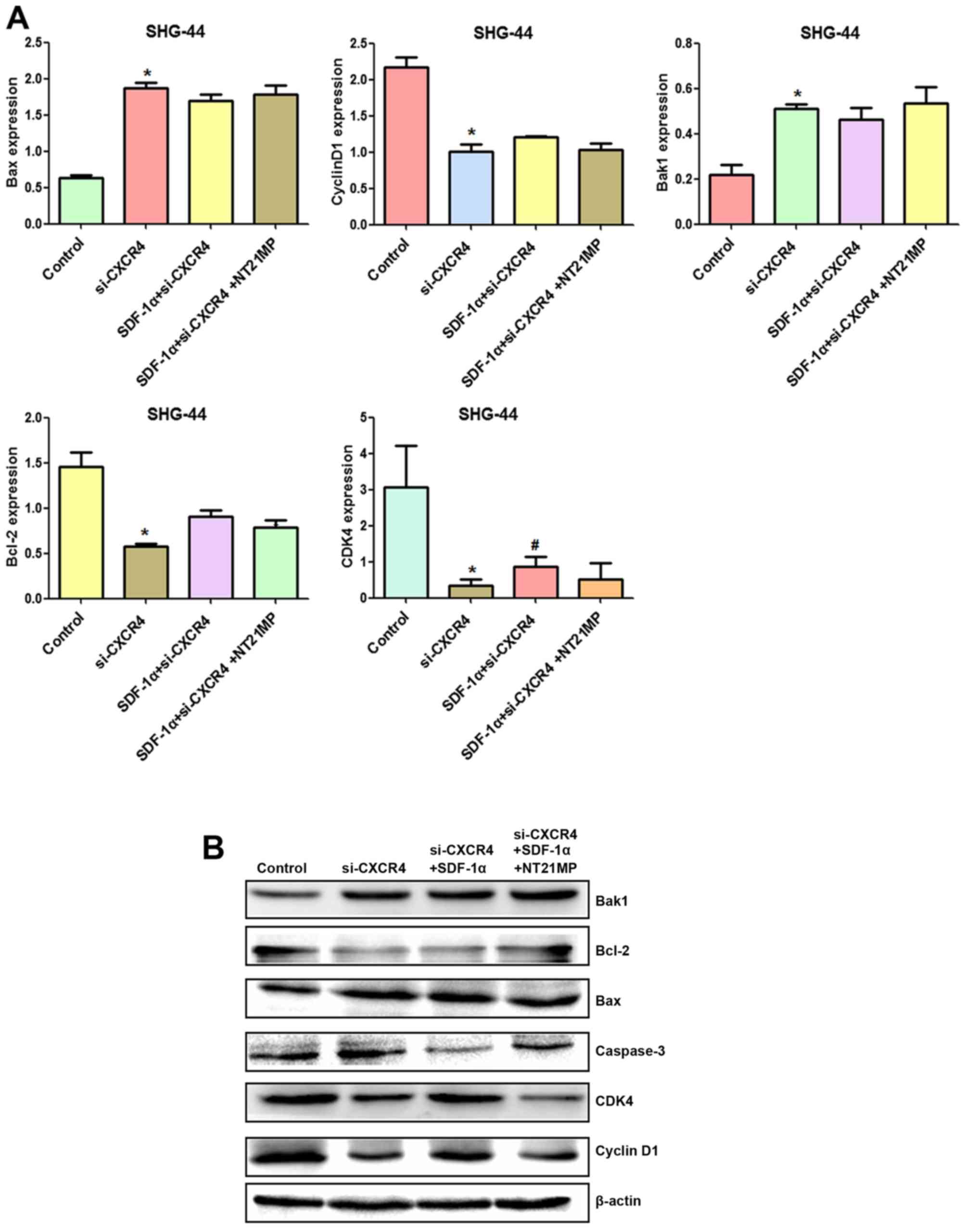
Effects of NT21MP on the expression of
cell cycle and apoptosis-related genes
To further explore the molecular mechanisms by which
NT21MP arrest glioma cell in G0/G1 phase, qRT-PCR and western
blotting assay were applied to analyze the changes of mRNA and
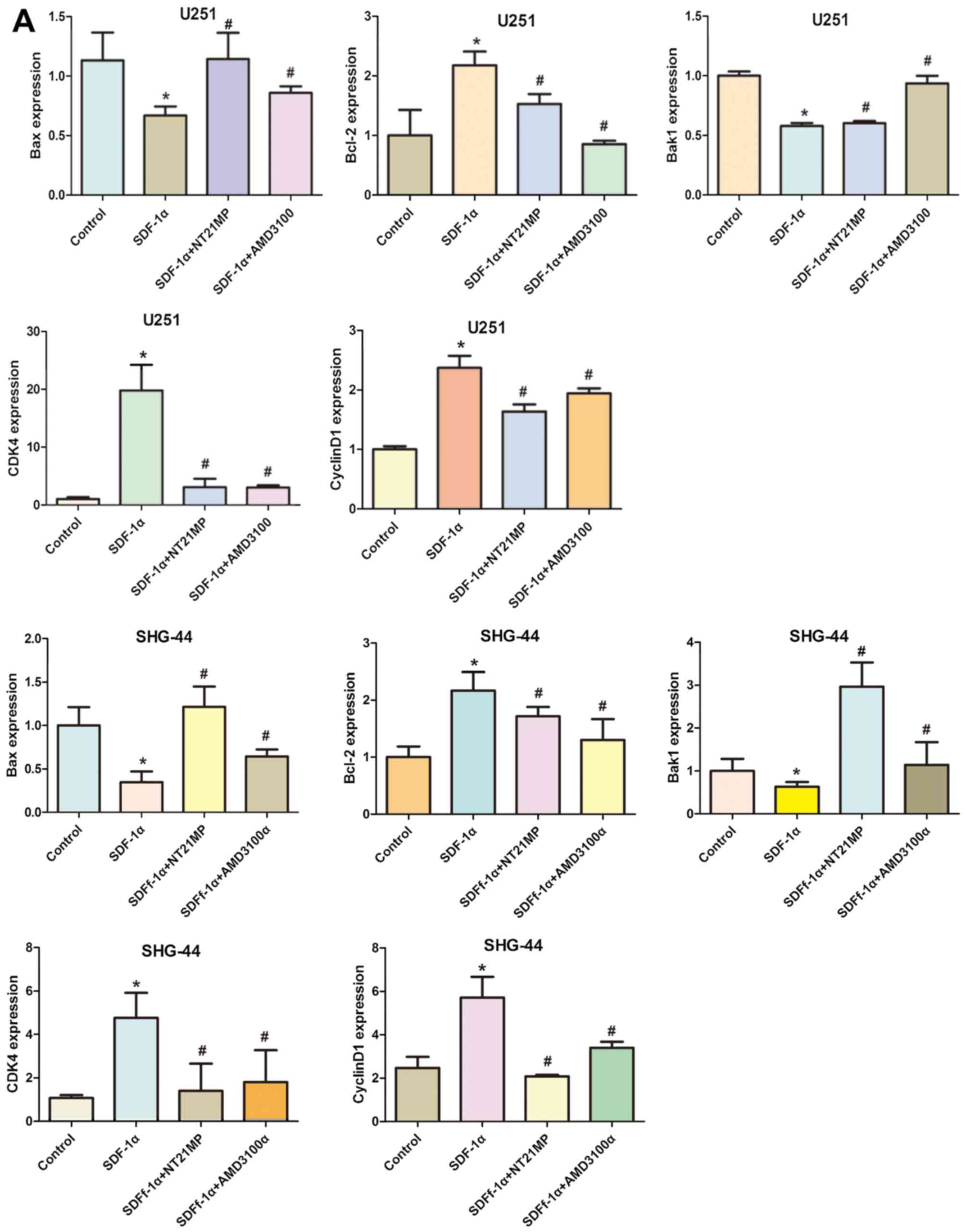
protein levels of cell cycle related factors.
Cyclin D1 expression could be used as a biomarker in
cell cycle progression. Besides, CDK4 is also recommended as a
master regulatory protein in the cell cycle. As shown in Fig. 5, the mRNA and protein levels of
cyclin D1 and CDK4 in the SDF-1α group in the two cell lines were
clearly upregulated compared with the control group. A reduction in
CDK4 and cyclin D1 mRNA and protein levels was observed in NT21MP
treatment group. Quantification of western blots confirmed that the
CDK4 and cyclin D1 protein level decrease in NT21MP treatment
group.
To evaluate potential mechanisms of SDF-1α-induced
activation of the intrinsic pathway, we assessed the effect of
SDF-1α on the mRNA levels of Bcl-2 as well as Bak1, Bax in U251 and
SHG-44 cells. As shown in Fig. 5A,
Bcl-2 mRNA levels in U251 and SHG-44 cells were substantially
altered under SDF-1α treatment (P<0.05), but the Bax and Bak1
levels in glioma cells were decreased by SDF-1α treatment.
Consistent with these results, western blotting showed that SDF-1α
also upregulate Bcl-2 and downregulate Bax and Bak1 at the protein
level in the two cell lines (Fig.
5B). The expression level of Bcl-2 was significantly
downregulated by NT21MP whereas the expression levels of Bax and
Bak1 were upregulated. Together these results suggested that NT21MP
significantly attenuated the anti-apoptotic effects of SDF-1α,
indicating that NT21MP could induce apoptosis in glioma cells.
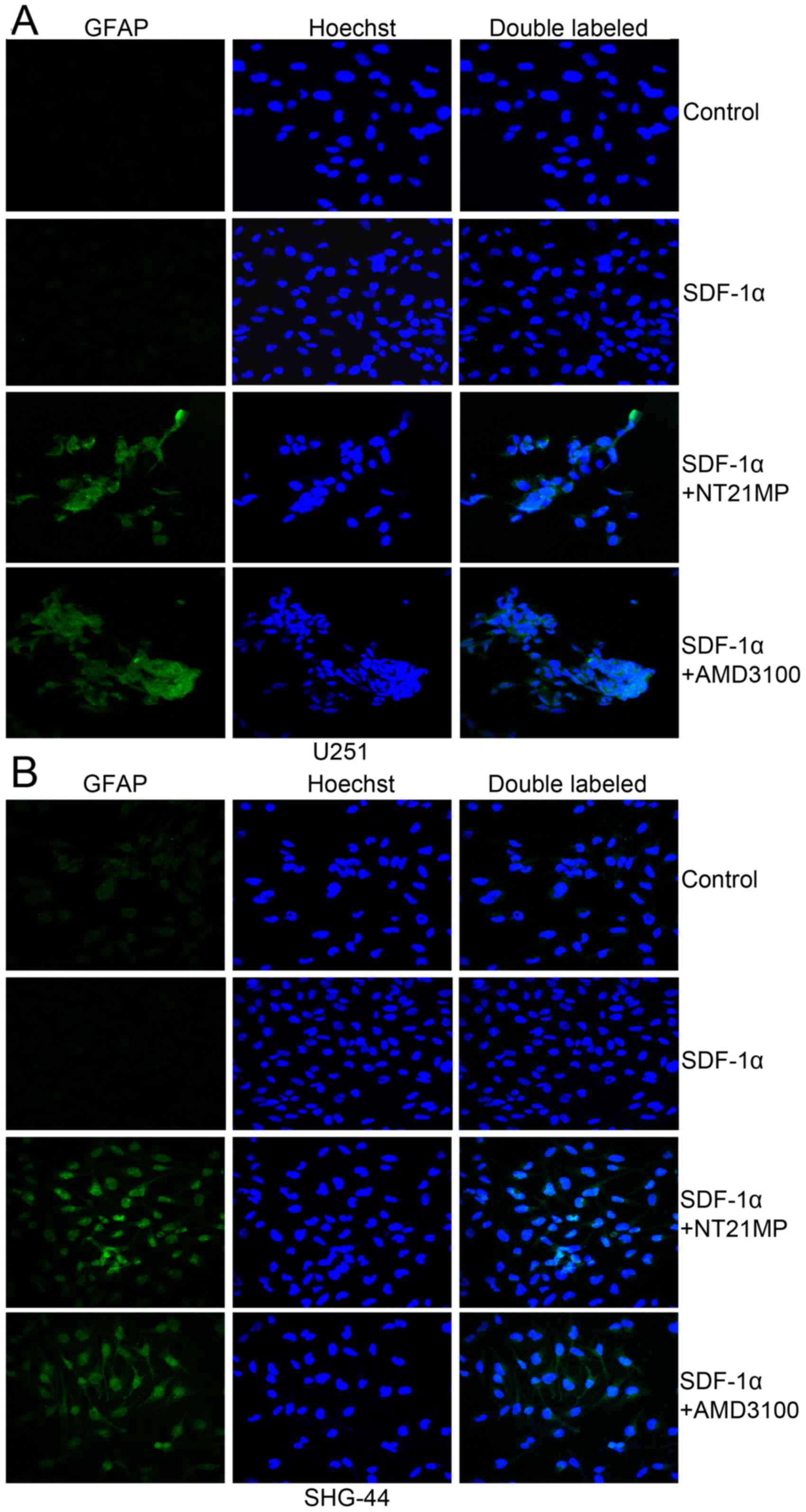
NT21MP induces the expression of
differentiation-related markers (GFAP) in glioma cells
Previous studies suggested that plasma glial
fibrillary acidic protein (GFAP) levels are sensitive and specific
for glioma. Therefore, we performed immunofluorescence analysis to
investigate the expression levels of differentiation-related
markers (GFAP) in glioma cells. As shown in Fig. 6, the results revealed very low
expression of GFAP in the two glioma cells. Pre-treatment with
NT21MP, markedly increased the expression of GFAP in U251 and
SHG-44 cells. These results suggested that NT21MP increased the
expression of differentiation-related markers in glioblastoma cells
in vitro.
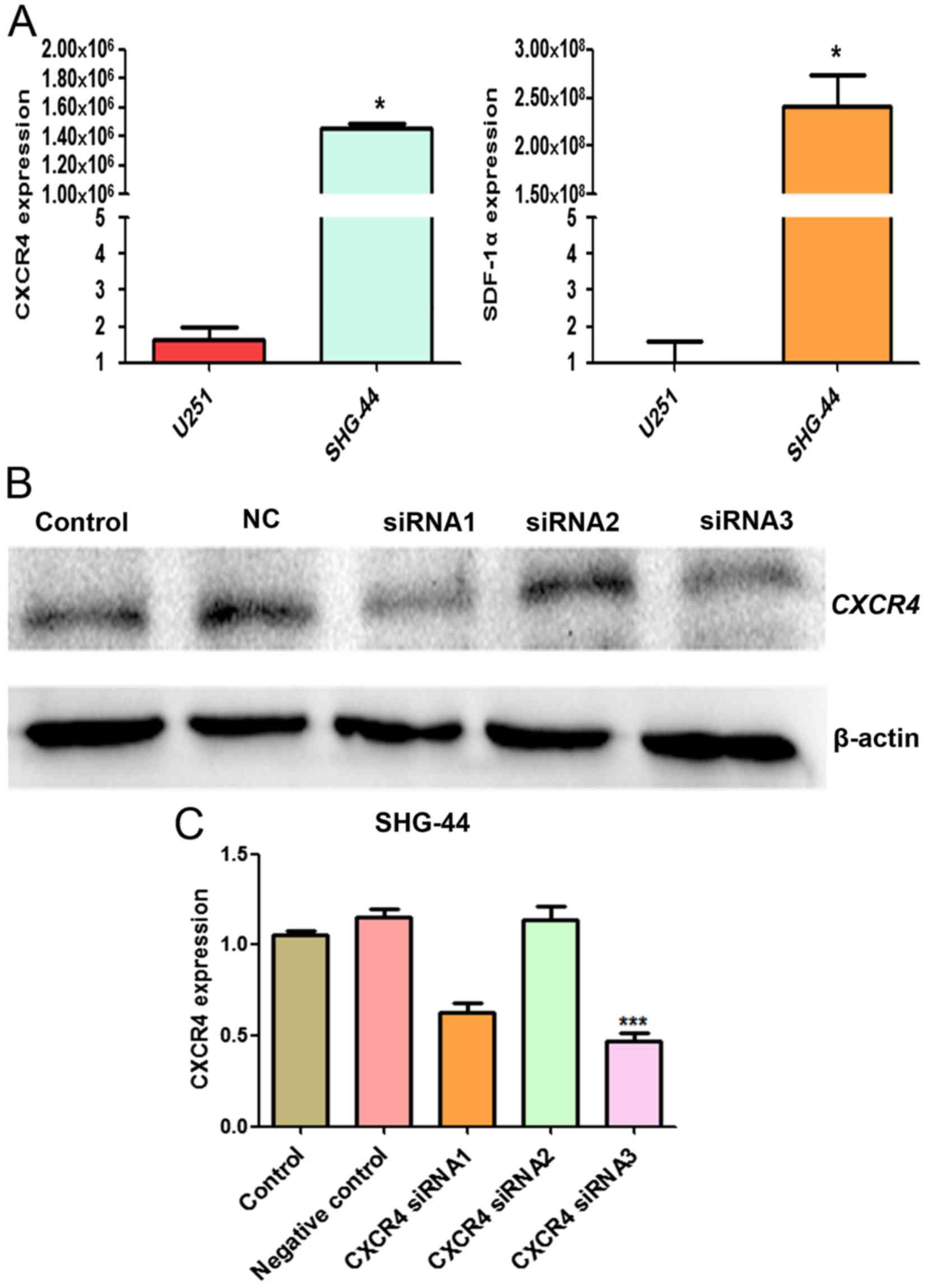
Assessment of the effect of CXCR4 siRNA
in SHG-44 cells
We evaluated the mRNA expression level of SDF-1α and
CXCR4 in U251 and SHG-44 cells by RT-qPCR. SDF-1α and CXCR4 mRNA
expression in SHG-44 was significantly higher than U251 cells
(P<0.05) (Fig. 7A). To detect
the effect of siRNA targeting CXCR4 expression in SHG-44 cells,
western blot analysis was performed to evaluate the expression of
CXCR4 protein. As shown in Fig.
7B, transient transfection of CXCR4-specific siRNA resulted in
the suppression of CXCR4 protein expression, thus, siRNA3 was more
effective and selected for the subsequent experiments.
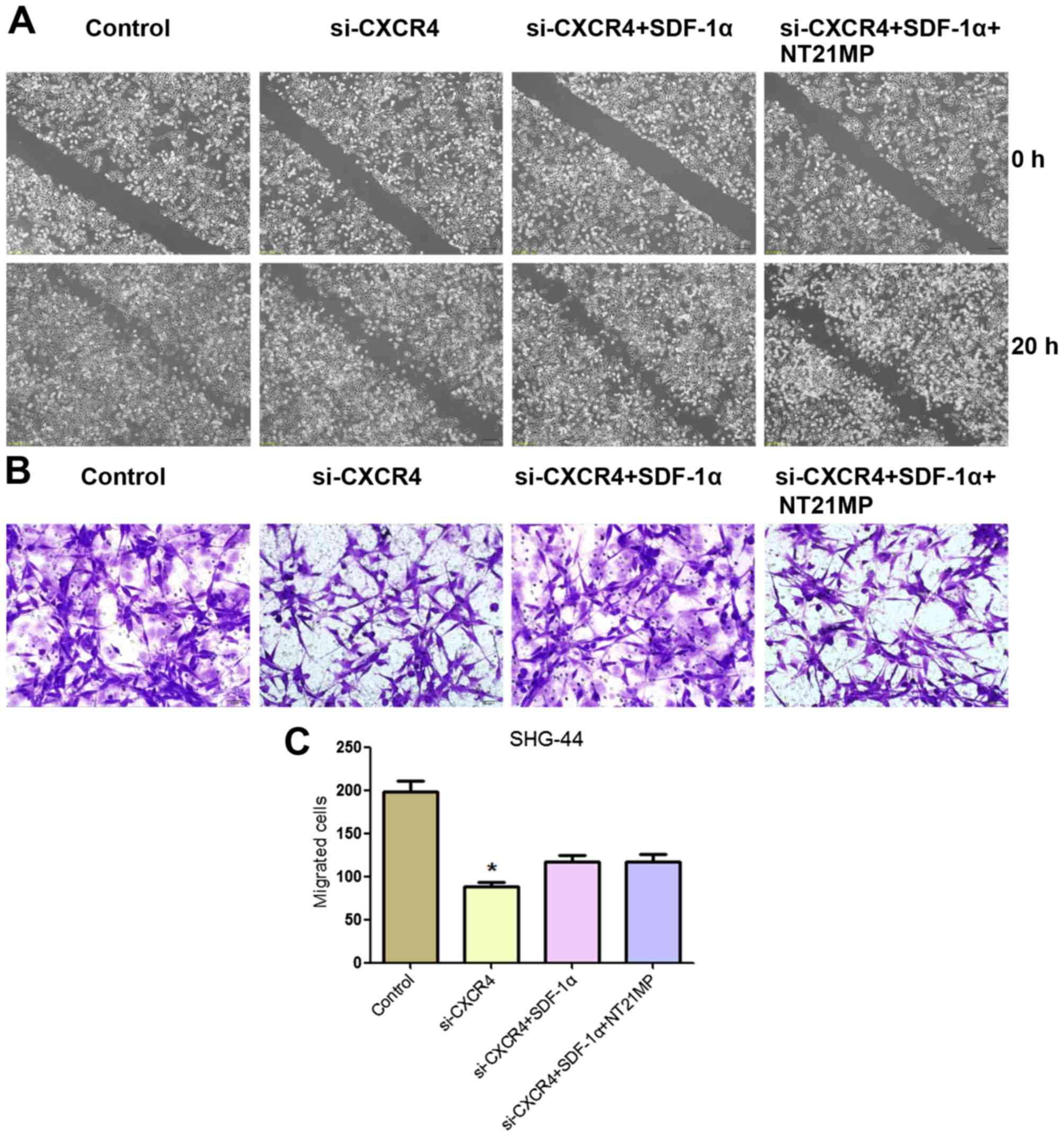
Effects of NT21MP on SHG-44 cell
migration and invasion after CXCR4 depletion
In order to detect the effects of NT21MP on glioma
cell, we measured the effect of CXCR4 silencing on the migration
and invasion ability of cells in vitro by wound healing and
Transwell assay. As shown in Fig.
8, a slower migration was observed and the number of migrated
cells was significantly reduced in SHG-44 cells treated with
si-CXCR4 group compared with the control group. These results
indicated that the invasion and migration ability were affected by
the depletion of CXCR4 in SHG-44 cells.
Cells treated with si-CXCR4-SDF-1α group exhibited
an increase in the number of migrated cells compared with the
si-CXCR4 group. Notably, in comparison with si-CXCR4-SDF-1α group,
the ability of migration and invasion ability of SHG-44 cells with
NT21MP-si-CXCR4-SDF-1α treatment was significantly decreased. These
results indicated that NT21MP inhibited SHG-44 cell migration and
invasion by blocking the SDF-1α/CXCR4 biological axis.
Effects of NT21MP on cell cycle and
apoptosis in SHG-44 cells after CXCR4 silencing
In order to further define the role of NT21MP in
SHG-44 cells, we next analyzed cell cycle following siRNA depletion
of CXCR4 by flow cytometry. As shown in Fig. 9A and B, SHG-44 cells treated with
si-CXCR4 for the same period of time showed a significant increase
in the proportion of cells in G0/G1 phase and a significant
decrease in the proportion of cells in S phase compared with that
of the control group. Treatment with si-CXCR4-SDF-1α resulted in a
significant decrease in the proportion of cells in G0/G1 phase and
a significant increase in the proportion of cells in S phase. We
also investigated the impact of CXCR4 siRNA and NT21MP combination
therapy on SDF-1α-induced SHG-44 cells. In comparison with
si-CXCR4-SDF-1α treatment, NT21MP-si-CXCR4-SDF-1α decreased the
percentage of S phase in SHG-44 cells.
Furthermore, we analyzed cell apoptosis in different
treatment groups. Compared with the control group, cell apoptosis
increased after CXCR4 depletion (Fig.
9C and D), demonstrating that high expression of CXCR4 was
responsible for the anti-apoptotic effect. However, the percentage
of apoptotic cells in si-CXCR4-SDF-1α group was lower than that of
si-CXCR4 group, but NT21MP-si-CXCR4-SDF-1α increased the percentage
of apoptotic cells compared with the si-CXCR4-SDF-1α group.
Effects of NT21MP on the expression of
cell cycle and apoptosis-related genes in SHG-44 cells after CXCR4
silencing
To further explore the molecular mechanism by which
NT21MP causes glioma cell arrest in G0/G1 phase and promotes
apoptosis, RT-qPCR and western blotting were applied to assess the
changes of mRNA and protein levels of cell cycle and
apoptosis-related factors after CXCR4 silencing.
Compared with the control group, cyclin D1, CDK4 and
Bcl-2/Bax expression levels were decreased after CXCR4 depletion,
while Bak1 and caspase-3 amounts were increased (Fig. 10A and B). Combining with the
results in Fig. 5, SDF-1α
upregulated expression of cyclin D1, CDK4 and Bcl-2/Bax and
downregulated expression of Bak1 and caspase-3 slightly after CXCR4
silencing, suggesting that weakened SDF-1α induced G0/G1 to S phase
transition and anti-apoptosis.
After CXCR4 silencing, NT21MP slightly decreased
cyclin D1, CDK4 and Bcl-2/Bax levels and increased Bak1 and
caspase-3 amounts slightly, suggesting that regulation by NT21MP of
the above genes was significantly decreased after CXCR4 silencing.
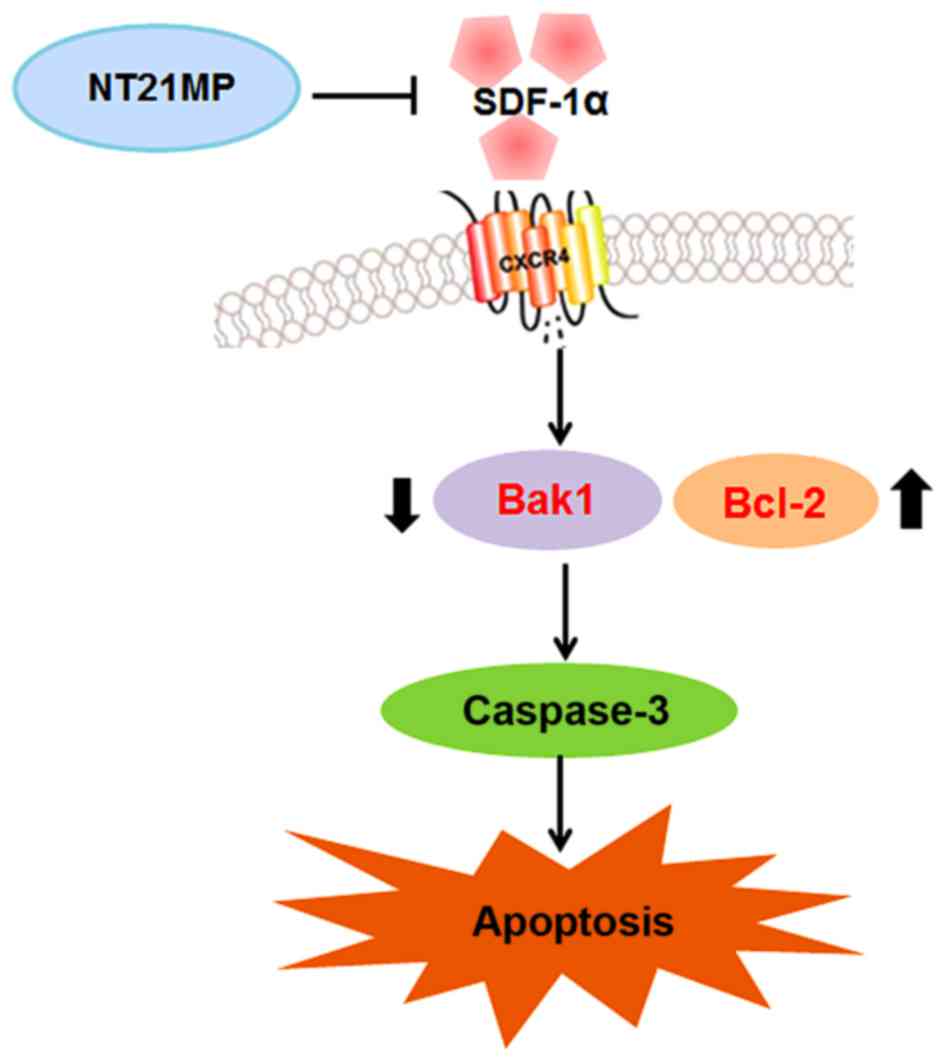
Together, these findings indicated that NT21MP caused cell cycle
arrest in G0/G1 phase and promoted apoptosis by inhibiting the
SDF-1α/CXCR4 biological axis (Fig.
11).
Discussion
Accumulating evidence has revealed that SDF-1α/CXCR4
plays a significant role in tumor genesis (17). It is known that SDF-1α binds to
CXCR4 and subsequently activates their multiple target genes
including extracellular regulated kinase 2 (ERK2), protein kinase B
(PKB), phosphatidylinositol 3-kinase (PI3K), steroid receptor
coactivator (SRC) and mitogen activated protein kinase (MAPK),
through these pathways CXCR4 regulates many different cellular
functions including survival, growth and chemotaxis as well as
having diverse effects on gene expression (18,19).
The activation of CXCR4 has been reported to promote
tumor growth, motility, invasion and metastasis in a variety of
human cancers including glioma (20). AMD3100 is also employed in the
treatment of glioma through multiple mechanisms. However, AMD3100
lacks CXCR4 specificity because it also binds the other
high-affinity receptors for CXCL12, CXCR7 (10). Therefore, development of an
antagonists targeting CXCR4 could provide more effective strategy
for human cancers (21). To
achieve better treatment of glioma, we recently developed NT21MP,
which is capable of antagonising the function of CXCR4 pathway. In
the present study, we found that NT21MP caused cell proliferation
inhibition, cell cycle arrest and induced apoptosis in U251 and
SHG-44 cells. The expression of GFAP was also markedly increased.
To gain more insight into the effect of NT21MP on glioma cells, we
selectively knocked down CXCR4 using siRNA and observed the
subsequent effects on SHG-44 cells. Silencing of CXCR4 could
inhibit cell proliferation. Moreover, in comparison with
si-CXCR4-SDF-1α treatment, NT21MP-si-CXCR4-SDF-1α treatment
decreased the ability of migration and invasion and the percentage
of S phase; however, the percentage of apoptotic cells was
increased. These results identified that NT21MP could be a
promising agent by targeting CXCR4 for treatment of glioma.
It has been reported that SDF-1α/CXCR4 could enhance
cell proliferation in various human cancers (22,23).
For example, Barbero et al (24) reported that exogenous SDF-1α
promotes proliferation of glioma cells in a dose-dependent manner.
In this study, we found that SDF-1α promoted glioma cell growth,
whereas NT21MP was capable of inducing growth inhibition in U251
and SHG-44 cells.
High ability of migration is a hallmark of malignant
gliomas and is the main reason for therapeutic failure and
recurrence of tumors (25). It is
known that SDF-1α/CXCR4 plays a pivotal role in cell migration and
invasion in glioma (26). Thus, to
further explore the anti-metastasis activity of NT21MP, we detected
cell invasion in glioma cells after NT21MP treatment. We observed a
marked decrease in cell invasion ability in NT21MP treated
group.
Cyclin D1 is a positive cell cycle regulator during
the G1/S transition (27). In
addition, CDK4 is also recommended as a master regulatory protein
in the cell cycle (28). We showed
that SDF-1α increased the active level of cyclin D1 and CDK4.
Conversely, this level was decreased by NT21MP. Moreover, the
combination of SDF-1α and CXCR4 activates Akt to promote changes of
Bcl-2 expression. The activity and ratio of Bcl-2, Bak1 and Bax can
regulate mitochondrial function and cytochrome c release,
playing a role in the mitochondrial pathway of apoptosis. Chen
et al (29) found that
Bcl-2 downregulation and Bax upregulation could change the
permeability of the mitochondrial membrane, activate caspase-9 and
caspase-3, and induce apoptosis in glioma cells. Our results showed
that NT21MP upregulated Bak1 and downregulated Bcl-2/Bax, further
promoting caspase-3 expression and apoptosis in glioma cells.
GFAP is an intermediate filament protein, and a
specific marker for astrocytes and related tumors, oligodendroglia
cells and ependymocytes (30). It
is expressed in normal brain tissue and glioma cells, and with the
increasing glioma tumor grade the expression of GFAP was decreased
(31), making GFAP a good
indicator for differentiation degree and prognosis of glioma.
NT21MP induced GFAP expression, suggesting a possible glioma cell
transformation into normal cells.
The importance of CXCR4 in glioma biology has been
inferred by others. Sehgal et al (32) found that overexpression of CXCR4 in
glioma lines enhanced their soft agar colony-forming capability,
whereas the treatment with antibodies to CXCR4 and SDF-1α can cause
inhibition of cell proliferation. Consistently, we found that
silencing of CXCR4 suppressed cell migration and invasion but
induced cell cycle arrest and apoptosis in SHG-44 cells.
Importantly, we observed a synergistic effect between si-CXCR4 and
NT21MP on cell apoptosis, migration and invasion.
In summary, this study demonstrated that through the
SDF-1α/CXCR4 biological axis, NT21MP exerts anti-glioma effects,
blocking cell cycle, inhibiting proliferation and inducing
apoptosis. These findings suggest that targeting CXCR4 may
represent a potential approach to enhance the efficacy of NT21MP to
treat glioma.
Acknowledgments
The present study was supported by the Major Program
of Anhui Educational Committee (nos. KJ2015ZD29 and KJ2016SD37),
the Natural Science Foundation of Anhui (no. 1508085MH159), the Key
Program of college discipline (major) top-notch talent academic
subsidize of Anhui (no. gxbjZD2016069), the Program for science
research of Bengbu Medical College (nos. BYKY1419ZD and BYKY1420ZD)
and the Program for graduates research of Bengbu Medical College
(no. Byycx1524).
References
|
1
|
Omuro A and DeAngelis LM: Glioblastoma and
other malignant gliomas: A clinical review. JAMA. 310:1842–1850.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Fishman M and Seigne J: Immunotherapy of
metastatic renal cell cancer. Cancer Control. 9:293–304.
2002.PubMed/NCBI
|
|
3
|
Vicari AP and Caux C: Chemokines in
cancer. Cytokine Growth Factor Rev. 13:143–154. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Li W and Graeber MB: The molecular profile
of microglia under the influence of glioma. Neuro Oncol.
14:958–978. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Flüh C, Hattermann K, Mehdorn HM, Synowitz
M and Held-Feindt J: Differential expression of CXCR4 and CXCR7
with various stem cell markers in paired human primary and
recurrent glioblastomas. Int J Oncol. 48:1408–1416. 2016.PubMed/NCBI
|
|
6
|
Cho KS, Yoon SJ, Lee JY, Cho NH, Choi YD,
Song YS and Hong SJ: Inhibition of tumor growth and
histopathological changes following treatment with a chemokine
receptor CXCR4 antagonist in a prostate cancer xenograft model.
Oncol Lett. 6:933–938. 2013.PubMed/NCBI
|
|
7
|
Maddirela DR, Kesanakurti D, Gujrati M and
Rao JS: MMP-2 suppression abrogates irradiation-induced microtubule
formation in endothelial cells by inhibiting αvβ3-mediated
SDF-1/CXCR4 signaling. Int J Oncol. 42:1279–1288. 2013.PubMed/NCBI
|
|
8
|
Sekuła M, Miekus K and Majka M:
Downregulation of the CXCR4 receptor inhibits cervical carcinoma
metastatic behavior in vitro and in vivo. Int J Oncol.
44:1853–1860. 2014.
|
|
9
|
Broxmeyer HE, Orschell CM, Clapp DW,
Hangoc G, Cooper S, Plett PA, Liles WC, Li X, Graham-Evans B,
Campbell TB, et al: Rapid mobilization of murine and human
hematopoietic stem and progenitor cells with AMD3100, a CXCR4
antagonist. J Exp Med. 201:1307–1318. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Labrosse B, Brelot A, Heveker N, Sol N,
Schols D, De Clercq E and Alizon M: Determinants for sensitivity of
human immunodeficiency virus coreceptor CXCR4 to the bicyclam
AMD3100. J Virol. 72:6381–6388. 1998.PubMed/NCBI
|
|
11
|
Kalatskaya I, Berchiche YA, Gravel S,
Limberg BJ, Rosenbaum JS and Heveker N: AMD3100 is a CXCR7 ligand
with allosteric agonist properties. Mol Pharmacol. 75:1240–1247.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Khan A, Greenman J and Archibald SJ: Small
molecule CXCR4 chemokine receptor antagonists: Developing drug
candidates. Curr Med Chem. 14:2257–2277. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kledal TN, Rosenkilde MM, Coulin F,
Simmons G, Johnsen AH, Alouani S, Power CA, Lüttichau HR, Gerstoft
J, Clapham PR, et al: A broad-spectrum chemokine antagonist encoded
by Kaposi's sarcoma-associated herpesvirus. Science. 277:1656–1659.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yang QL, Ding YQ, Chen CJ, Tang J, Zhang J
and Yang ZF: Suppression of murine breast cancer metastasis by
selective inhibition of CXCR4 by synthetic polypeptide derived from
viral macrophage inflammatory protein II. Chin Sci Bull.
55:2152–2159. 2010. View Article : Google Scholar
|
|
15
|
Yang Q, Chen C, Yang Z, Gao Y and Tang J:
Suppression of breast cancer proliferation and induction of
apoptosis via AKT and ERK1/2 signal transduction pathways by
synthetic polypeptide derived from viral macrophage inflammatory
protein II. J Huazhong Univ Sci Technolog Med Sci. 31:497–503.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yang QL, Ding YX, Chen CJ, Yang ZF and Gao
YJ: The mechanism of polypeptide derived from viral macrophage
inflammatory protein II modulates SDF-1α/CXCR4-induced migration.
Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi. 28:137–140. 2012.In Chinese.
PubMed/NCBI
|
|
17
|
Domanska UM, Kruizinga RC, Nagengast WB,
Timmer-Bosscha H, Huls G, de Vries EG and Walenkamp AM: A review on
CXCR4/CXCL12 axis in oncology: No place to hide. Eur J Cancer.
49:219–230. 2013. View Article : Google Scholar
|
|
18
|
Epstein RJ: The CXCL12-CXCR4 chemotactic
pathway as a target of adjuvant breast cancer therapies. Nat Rev
Cancer. 4:901–909. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
19
|
Portella L, Vitale R, De Luca S, D'Alterio
C, Ieranò C, Napolitano M, Riccio A, Polimeno MN, Monfregola L,
Barbieri A, et al: Preclinical development of a novel class of
CXCR4 antagonist impairing solid tumors growth and metastases. PLoS
One. 8:e745482013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Berghuis D, Schilham MW, Santos SJ, Savola
S, Knowles HJ, Dirksen U, Schaefer KL, Vakkila J, Hogendoorn PC and
Lankester AC: The CXCR4-CXCL12 axis in Ewing sarcoma: Promotion of
tumor growth rather than metastatic disease. Clin Sarcoma Res.
2:24–32. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Peled A, Wald O and Burger J: Development
of novel CXCR4-based therapeutics. Expert Opin Investig Drugs.
21:341–353. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Skommer J, Wlodkowic D and Pelkonen J:
CXCR4 expression during tumour cell death. Leuk Res. 31:1155–1156.
2007. View Article : Google Scholar
|
|
23
|
Teicher BA and Fricker SP: CXCL12
(SDF-1)/CXCR 4 pathway in cancer. Clin Cancer Res. 16:2927–2931.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Barbero S, Bonavia R, Bajetto A, Porcile
C, Pirani P, Ravetti JL, Zona GL, Spaziante R, Florio T and
Schettini G: Stromal cell-derived factor 1alpha stimulates human
glioblastoma cell growth through the activation of both
extracellular signal-regulated kinases 1/2 and Akt. Cancer Res.
63:1969–1974. 2003.PubMed/NCBI
|
|
25
|
Tu H, Zhou Z, Liang Q, Li Z, Li D, Qing J,
Wang H and Zhang L: CXCR4 and SDF-1 production are stimulated by
hepatocyte growth factor and promote glioma cell invasion.
Onkologie. 32:331–336. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ehtesham M, Winston JA, Kabos P and
Thompson RC: CXCR4 expression mediates glioma cell invasiveness.
Oncogene. 25:2801–2806. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Koepp DM, Schaefer LK, Ye X, Keyomarsi K,
Chu C, Harper JW and Elledge SJ: Phosphorylation-dependent
ubiquitination of cyclin E by the SCFFbw7 ubiquitin ligase.
Science. 294:173–177. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Tamamori-Adachi M, Ito H,
Sumrejkanchanakij P, Adachi S, Hiroe M, Shimizu M, Kawauchi J,
Sunamori M, Marumo F, Kitajima S, et al: Critical role of cyclin D1
nuclear import in cardiomyocyte proliferation. Circ Res. 92:9–12.
2003. View Article : Google Scholar
|
|
29
|
Chen S, Zhu L, Huang J, Cai Y, Lu X, Yang
Q, Wu Q, Chen C and Wang Z: Arsenic trioxide targets miR-125b in
glioma cells. Curr Pharm Des. 20:5354–5361. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Middeldorp J and Hol EM: GFAP in health
and disease. Prog Neurobiol. 93:421–443. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhou J, Liu Q, Wang J, Guo X and Song L:
Expressions of peroxiredoxin 1, peroxiredoxin 6 and GFAP in human
brain astrocytoma and their clinical significance. Nan Fang Yi Ke
Da Xue Xue Bao. 32:1255–1259. 2012.In Chinese. PubMed/NCBI
|
|
32
|
Sehgal A, Keener C, Boynton AL, Warrick J
and Murphy GP: CXCR-4, a chemokine receptor, is overexpressed in
and required for proliferation of glioblastoma tumor cells. J Surg
Oncol. 69:99–104. 1998. View Article : Google Scholar : PubMed/NCBI
|