Introduction
Epithelial ovarian carcinomas (EOCs) account for 90%
of total cases of ovarian cancer. Unfortunately, almost 70% of
women with ovarian cancer are not diagnosed until the disease is
advanced in stage leading to the highest mortality of any of the
gynecologic cancers (1).
Paclitaxel has been used as a first-line chemotherapeutic agent
against ovarian cancer (2).
However, the development of chemoresistance causes the primary
failure in the treatment of ovarian cancer (3). Although improvement in survival rate
has been observed, the majority of patients experience recurrent
disease due to resistance to chemotherapy (4). The identification of suitable
biomarkers for chemosensitivity diagnosis to paclitaxel and the
development of new therapeutic agent for overcoming chemoresistance
may be important research fields to improve the therapeutic outcome
of patient with ovarian cancer.
The fibroblast growth factor receptors (FGFRs)
consist of an extracellular ligand domain with three
immunoglobulin-like domains (I–III), a transmembrane domain, and an
intracellular tyrosine kinase domain that transmit the signal to
the intracellular binding proteins (5). There are four FGFRs (FGFR1/2/3/4) as
membrane-bound receptors with seven isoforms due to the alternative
splicing in Ig-III-like domain with different ligand-binding
affinity (5). While each FGFR can
be activated by several FGFs, the FGF/FGFR signaling is further
controlled with different tissue distribution (5,6). For
example, high level expression of FGFR1 was observed in the skin,
cornea, lung, heart, placenta, kidney and uterus. In contrast, high
expression of FGFR2 was detected in prostate and stomach. The
expressions of FGFR3 and FGFR4 were more restricted than FGFR1 and
FGFR2. FGFR3 expression is found in appendix, colon, liver,
sublingual gland, placenta, and cervix with restricted and lower
patterns. FGFR4 expression is observed in the liver, sublingual
gland duct, kidney and ureter (6).
Differential tissue distribution of FGFR expressions suggests that
FGFR families play important functional roles in normal tissue
homeostasis (7). Recent studies
have indicated that FGFR signaling is also implicated in tumor
development (8–10). Cumulative evidence revealed that
FGFR aberrations are common in a wide variety of cancers, with the
majority being gene amplification or activating mutations (11). Helsten et al (12) reported that FGFR aberrations were
found in 7.1% of cancers using next-generation sequencing.
Moreover, ovarian cancer is the fifth mosy commonly altered (~9%)
in FGFR activity among the cancer malignancies implicating that
FGFR inhibition could be an important therapeutic approach against
ovarian carcinoma (12).
AKT/PKB is an intracellular serine/threonine kinase.
It has been extensively studied that AKT regulates a variety of
cellular processes by mediating extracellular and intracellular
signals (13–16). Activation of AKT mediates its
pleckstrin homology (PH) domain binding to products of
phosphatidylinositol 3-kinase (PI3K). This activation is feedback
regulated by a dual phosphatase PTEN (17). Activation of AKT through
platelet-derived growth factor (PDGF), insulin, epidermal growth
factor (EGF), basic fibroblast growth factor (bFGF) has been
identified (18–21). These events regulate cell growth,
survival, differentiation, angiogenesis, migration and metabolism
(22). Moreover, studies have
shown that AKT signaling is frequently impaired in many
malignancies (23) and the
overexpression of AKT induces chemoresistance (24). To date, many studies have
demonstrated that AKT signaling is the major target for anticancer
drug development and overcoming chemoresistance.
Three-dimensional sphere culture model, a strategy
with cell anchorage-independent growth potential, have been
reported to establish reliable methodologies and techniques for the
high-throughput drug development against various cancers (25–27).
Although the sphere culture model is a better recapitulating system
of primary tumors than conventional monolayer culture model, the
sphere culture model approaches have not yet been used widely in
cancer research and drug development fields because of higher costs
and technical problems than conventional monolayer culture model
(28). The wide application of
sphere culture model in cancer cell research and the accumulation
of available data through the sphere culture model may facilitate
the understanding of tumor growth and metastasis in specific tumor
microenvironment (29,30). Especially, epithelial ovarian
cancer frequently spreads by direct metastasis from the primary
site (31). Unlike primary tumor
in ovary, premetastatic ovarian cancer cells undergo
epithelial-to-mesenchymal (EMT) transition, which loosens the
intercellular attachment between cancer cell-to-cell and cancer
cell-to-extracellular matrix through the remodeling of cadherins
(31). Detached ovarian cancer
cells from the primary site disseminate within the abdominal cavity
and often associated with ascitic fluid, particularly in high-grade
serous carcinoma. In addition, the disseminated ovarian cancer is
faced with a harsh growth condition with the specific tumor
microenvironment involving hypoxia and nutrient-deprived conditions
(31). Nonetheless, to date the
conventional monolayer ovarian cancer culture model has been used
for ovarian cancer research preferentially. Here, we report the
molecular characterization of ovarian carcinoma SKOV3ip1 cell line
in conventional monolayer culture model and sphere cultured model.
We evaluated the antitumor activity of the selective FGFR inhibitor
BGJ398 against SKOV3ip1 in sphere culture model. These data suggest
that BGJ398 is a potent anticancer drug candidate against
epithelial ovarian carcinoma. The present data also showed that the
inhibition of FGFR/AKT signaling pathway represents a therapeutic
target for overcoming metastatic ovarian carcinoma.
Materials and methods
Antibodies and reagents
Antibodies against STAT3 (H-190), phosphorylated
STAT3 at Tyr 705 (B-7) and Actin (C-2) were purchased from Santa
Cruz Biotechnology (Santa Cruz, CA, USA). Rabbit monoclonal
antibodies against AKT, phosphorylated AKT at Ser473 (D9E),
phosphorylated FGFR1 at Tyr 653/654, p42/44 MAPK, phosphorylated
p42/44 MAPK, p38 MAPK and phosphorylated p38 MAPK at Tyr 180/182
antibodies were from Cell Signaling Technology (Danvers, MA, USA).
Goat anti-mouse and rabbit secondary antibodies conjugated to
horseradish peroxidase (HRP) were purchased from Jackson
Laboratories (Bar Harbor, ME, USA). BGJ398 (against FGFR1/2/3),
TAE684 (against ALK) and imatinib (against Abl) were purchased from
Selleck Chemicals LLC (Houston, TX, USA). AG490 (against JAK) was
from Tocris Bioscience (Bristol, UK). All reagents were solubilized
with dimethyl sulfoxide (DMSO) at the following concentration,
paclitaxel at 100 µM, BGJ398 at 5 mM, TAE684 at 3 and
imatinib at 5 mM.
Cell lines and sphere culture
SKOV3ip1 ovarian carcinoma cell line (32) was a kind gift from Professor A.K.
Sood (University of Texas MD Anderson Cancer Center, Houston, TX,
USA). Cells were maintained as a 2D monolayer in RPMI-1640 medium
(Corning Incorporated, Corning, NY, USA) supplemented with 10%
fetal bovine serum (FBS; Gibco, Grand Island, NY, USA) and 10 U/ml
penicillin/streptomycin (Gibco) at 37°C in a 5% CO2
humidified incubator. To culture as 3D sphere system, SKOV3ip1
cells (1×106) were seeded in ultra-low attachment 6-well
plate (Corning Incorporated) and were cultured in RPMI-1640 medium
supplemented with 10% FBS (Gibco) and 10 U/ml
penicillin/streptomycin (Gibco) at 5% CO2 incubator.
Immunoblotting
The expression of protein and their phosphorylation
status in SKOV3ip1 cells was detected by immunoblotting.
Monolayer-cultured or sphere-cultured SKOV3ip1 cells were harvested
and washed once with ice-cold 1X phosphate-buffered saline (PBS),
and then lysed by 100 µl ice-cold RIPA buffer (20 mM
Tris-Cl, pH 8.0, 125 mM NaCl, 100 mM phenylmethylsulfonyl fluoride,
1 mM ethylene diamine tetraacetic acid, 1% Triton X-100, 0.5%
sodium deoxycholate, 0.1% sodium dodecyl sulfate, 1X Complete
Protease Inhibitor Cocktail) (Roche, Mannheim, Germany) on ice.
Protein concentrations were determined using Bradford protein assay
kit (Bio-Rad Laboratories, Hercules, CA, USA). Equal amount of
proteins (30 µg) were separated by SDS-PAGE and transferred
to nitrocellulose membranes, followed by immunoblotting with the
specific antibodies and horseradish peroxidase-conjugated secondary
antibodies. Immunoreactive bands were detected by SuperSignal West
Pico Chemiluminescent Substrate (Thermo Fisher Scientific,
Rockford, IL, USA) using Fusion Solo chemiluminescence analyzer
(Vilber Lourmat, Marne la Vallee, France).
Cell viability assay
The crystal violet staining assay was examined to
determine the cell viability in monolayer culture and sphere
culture model. To examined the crystal violet staining assay in
monolayer culture model, SKOV3ip1 cells (5×104) were
seeded in conventional 24-well plate and incubated overnight. The
drugs were then treated with the following concentration to each
well; vehicle control (DMSO); paclitaxel at 3.125, 6.25, 12.5, 25,
50 and 100 nM, BGJ398 at 0.3125, 0.625, 1.25, 2.5 and 5 µM,
TAE684 at 0.1875, 0.375, 0.75, 1.5 and 3 µM, and imatinib at
0.3125, 0.625, 1.25, 2.5 and 5 µM. Cells were further
incubated for 72 h. Three hundred microliter 0.2% crystal violet
solution was added to each well and further incubated for 20 min
with gently shaking. Stained cells were washed with distilled water
until a clear background was visible. For colorimetric analysis,
crystal violet dye in each well was extracted using 1% SDS/PBS
solution and the the absorbance was determined using EMax PLUS
microplate reader (Molecular Devices, Sunnyvale, CA, USA) at 570 nm
wavelength. For determination of cell viability in sphere-cultured
cells, SKOV3ip1 cells (5×104) were seeded in ultra-low
attachment 24-well plate and incubated overnight. Then drugs were
added and the cells were further incubated for 72 h. Cells in each
well were harvested and transferred to conventional 24-well plate
and further incubated for 12 h. Attached viable cells were stained
by 0.2% crystal violet solution for 20 min with gently shaking.
Stained cells were washed with distilled water. For colorimetric
analysis, crystal violet dye was extracted using 1% SDS/PBS then
the absorbance was determined at 570 nm using EMax PLUS microplate
reader (Molecular Devices).
MTS assay
The cell viability was analyzed by MTS assay in
monolayer culture and sphere culture model. For the MTS assay,
EZ-Cytox (Daeillab Service, Seoul, Korea) was used following the
manufacturer's instructions. SKOV3ip1 cells were seeded into
conventional 96-well plate or ultra-low attachment 96-well plate at
a density of 1×104/well and incubated overnight. The
drugs were then treated with the following concentration to each
well; vehicle control (DMSO); paclitaxel at 3.125, 6.25, 12.5, 25,
50 and 100 nM, BGJ398 at 0.3125, 0.625, 1.25, 2.5 and 5 µM,
TAE684 at 0.1875, 0.375, 0.75, 1.5 and 3 µM, and imatinib at
0.3125, 0.625, 1.25, 2.5 and 5 µM. Cells were further
incubated for 72 h. Sphere-cultured cells in ultra-low attachment
96-well plate were harvested and transferred to conventional
96-well plate and further incubated for 12 h. Wells with culture
media were used as negative control. The absorbance was determined
at 450 nm using EMax PLUS microplate reader (Molecular
Devices).
Statistical analysis
All statistical analyses of data were performed from
three independent experiments. All experiments were repeated three
times. Significant differences by concentration were calculated
using one-way ANOVA test and P≤0.05 analyzed by concentration were
considered significant. All graphs showed the mean with the
standard deviation. Cell viability data were analyzed with GraphPad
Prism 6 statistical software and presented as the mean value of
viable cells ± standard deviation (SD).
Results
Differential expressions of survival
signaling molecules in SKOV3ip1 ovarian cancer cell cultured in
conventional 2D monolayer culture model and 3D sphere culture
model
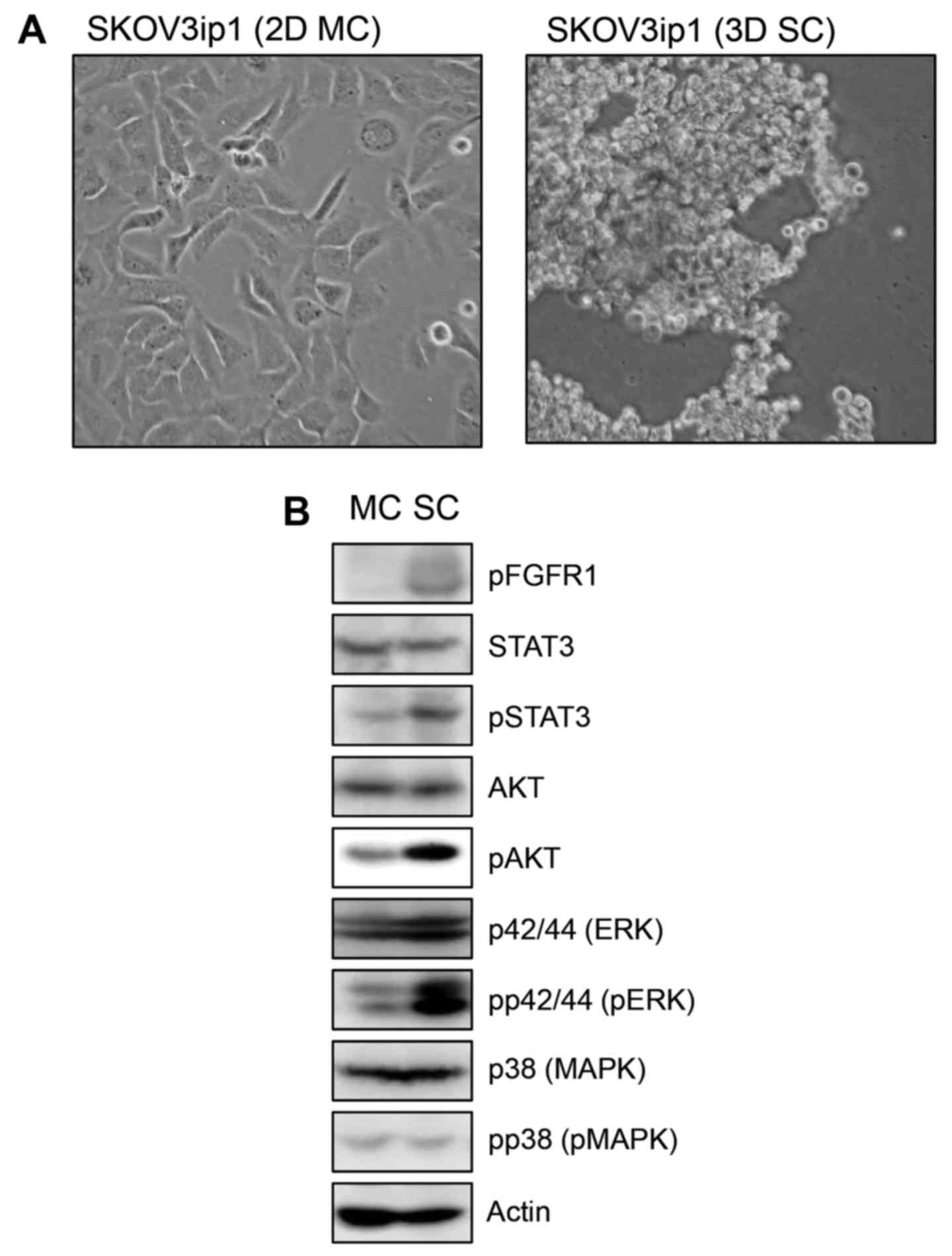
We examined the morphological analysis of SKOV3ip1
ovarian cancer cells in conventional 2D monolayer culture and 3D
sphere culture model. At 72 h after sphere cultivation, SKOV3ip1
cells formed loose sheet-like aggregates and did not accumulate as
compact spheroids (Fig. 1A). We
examined the expression of cellular signaling molecules which have
a role in cell proliferation including AKT, STAT3, p42/44 ERK and
p38 MAPK and their activation status in monolayer-cultured SKOV3ip1
cells and sphere-cultured SKOV3ip1 cells. Immunoblotting showed
that the expression level of molecules was not different in
monolayer-cultured SKOV3ip1 cells and sphere-cultured SKOV3ip1
cells. Notably, p42/44 ERK, AKT and STAT3 were activated in
sphere-cultured SKOV3ip1 cells than in 2D monolayer-cultured
SKOV3ip1 cells (Fig. 1B).
Additionally, phosphorylated FGFR1 was upregulated in
sphere-cultured SKOV3ip1 cells (Fig.
1B). These results showed that the sphere culture condition
upregulated the prosurvival signaling pathway in SKOV3ip1
cells.
Selective pan-FGFR inhibitor BGJ398
inhibits cell viability of SKOV3ip1 cells in sphere culture
model
FGFR inhibitor has been reported as a novel agent
with potential anti-angiogenic and anticancer effect (33). It has been reported that FGFR
signaling could regulate PI3K/AKT and STAT3 pathway in mammalian
cells (34). Thus, we examined the
effect of a highly selective pan-FGFR inhibitor BGJ398 on SKOV3ip1
cell viability. Tyrosine kinase inhibitor TAE684 (against ALK) and
imatinib also known as Gleevec (against ABL), and standard
anti-ovarian cancer chemotherapeutic agent paclitaxel were used and
compared in antitumor activities of SKOV3ip1 cells.
Monolayer-cultured SKOV3ip1 cells were treated with various
concentrations [vehicle control (DMSO)]; paclitaxel at 3.125, 6.25,
12.5, 25, 50 and 100 nM, BGJ398 at 0.3125, 0.625, 1.25, 2.5 and 5
µM, TAE684 at 0.1875, 0.375, 0.75, 1.5 and 3 µM, and
imatinib at 0.3125, 0.625, 1.25, 2.5 and 5 µM) of tyrosine
kinase inhibitors and paclitaxel for 72 h, and the cell viability
was determined using crystal violet colorimetric analysis (Fig. 2A) and MTS assay (Fig. 2B). Visualization of crystal violet
stained cells confirmed the result of colorimetric analysis
(Fig. 2C). As shown in Fig. 2A and B, monolayer-cultured SKOV3ip1
cell viability was effectively decreased by paclitaxel and TAE648
in a dose-dependent manner (P>0.05). But the cell viability of
monolayer-cultured SKOV3ip1 was not affected by BGJ398 and imatinib
(Fig. 2A). Next, the cell
viability of sphere-cultured SKOV3ip1 by treatment of paclitaxel or
tyrosine kinase inhibitors was compared. Cell viability analysis
showed that sphere-cultured SKOV3ip1 cells were clearly more
resistant to paclitaxel than SKOV3ip1 in monolayer (Fig. 2A). TAE648-resistance was not
observed in sphere-cultured SKOV3ip1 cells (Fig. 2A). Also imatinib did not influence
the cell viability in sphere-cultured SKOV3ip1 cells (Fig. 2A). Notably, BGJ398 differentially
affected the cell viability in monolayer-cultured and
sphere-cultured SKOV3ip1 (Fig. 2A and
B). BGJ398 reduced the cell viability of sphere-cultured
SKOV3ip1 cells in a dose-dependent manner (P>0.05; Fig. 2A). MTS assay was used to confirm
viability of cells treated with inhibitors. The inhibition of cell
viability by BGJ398 was also observed in sphere cultured SKOV3ip1
cells (P>0.05; Fig. 2B).
BGJ398-treated sphere-cultured SKOV3ip1 cells did not maintain the
healthy aggregate formation (Fig.
2D). Dissociation of spheroids were not observed at
TAE684-treated and imatinib-treated sphere-cultured SKOV3ip1
(Fig. 2D). Next, we examined the
cell viability assay with BGJ398 at 24–72 h. The result showed that
the cell viability was decreased in a dose-dependent manner both in
monolayer cultured model and sphere cultured model at 24–72 h
(Fig. 2E). Interestingly,
decreased cell viability was not significant in monolayer-cultured
SKOV3ip1 cell at 48- and 72-h treatments (P>0.05) (Fig. 2E), but in sphere-cultured SKOV3ip1
cells, the cell viability was gradually decreased in a
time-dependent manner (Fig. 2E).
These data revealed that BGJ398 is a potent chemotherapeutic agent
against SKOV3p1 cells depending on sphere growth conditions.
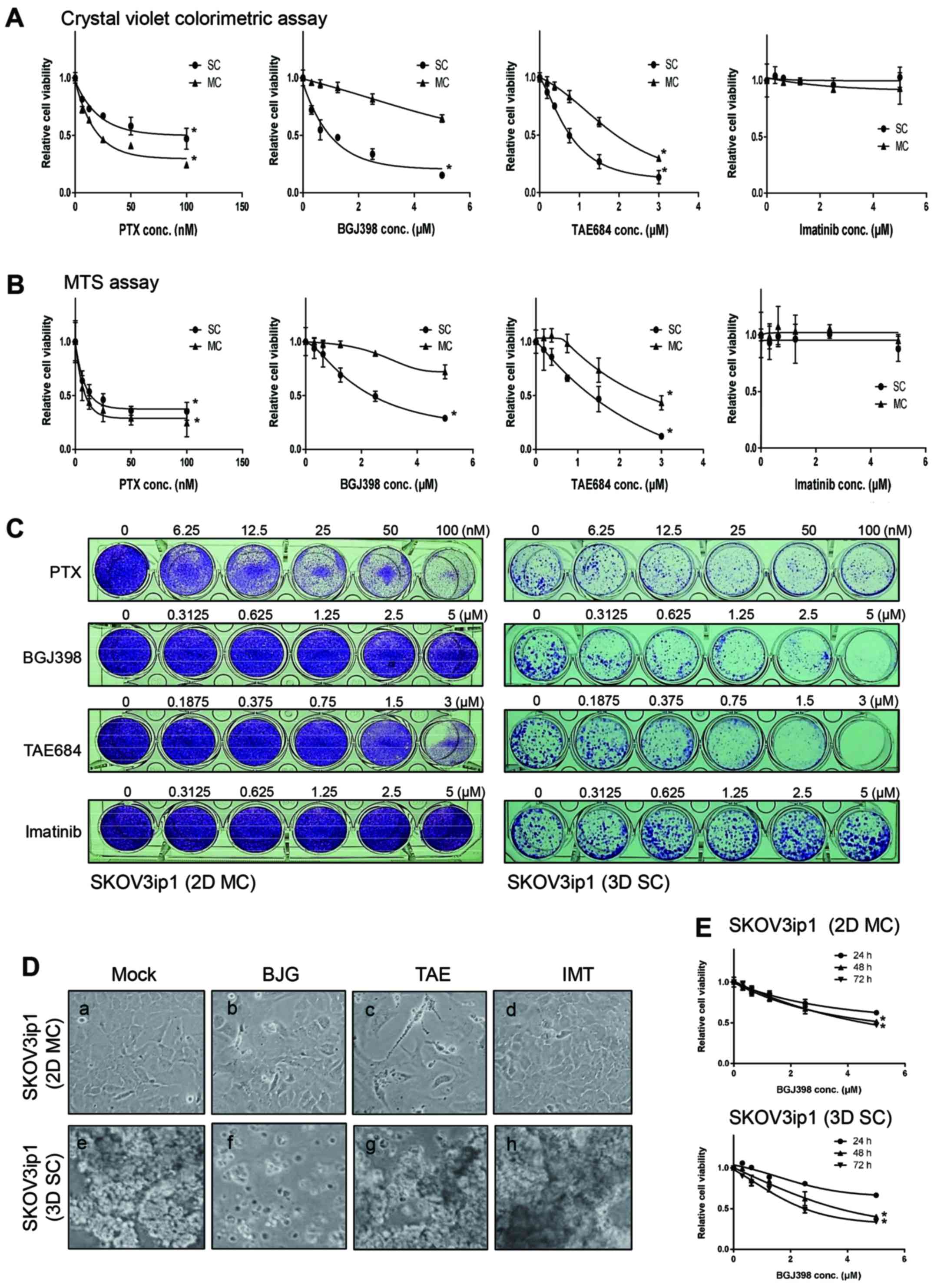 | Figure 2Selective FGFR inhibitor BGJ398
reduces the cell viability in sphere-cultured SKOV3ip1. Cells
(5×104) were seeded in conventional 24-well plate and
incubated overnight. Then drugs were added with the following
concentration to each well; vehicle control DMSO (mock); paclitaxel
at 3.125, 6.25, 12.5, 25, 50 and 100 nM, BGJ398 at 0.3125, 0.625,
1.25, 2.5 and 5 µM, TAE684 at 0.1875, 0.375, 0.75, 1.5 and 3
µM, and imatinib at 0.3125, 0.625, 1.25, 2.5 and 5
µM. Cells were further cultured for 72 h and the cell
viability was determined by crystal violet colorimetric analysis
(A) and MTS assay (B). (C) Viable cells at 72 h after treatment
paclitaxel or inhibitors as indicated concentration were visualized
by crystal violet staining. (D) Monolayer (2D MC) or spheroid
formation (3D SC) of SKOV3ip1 at 72 h after treatment of vehicle
solution DMSO (Mock), 5 µM BGJ398 (BGJ), 3 µM TAE648
(TAE) and 5 µM imatinib (IMT). (E) SKOV3ip1 cells
(5×104) were seeded in conventional 24-well plate and
incubated overnight. Then DMSO (mock) or BGJ398 (0.3125, 0.625,
1.25, 2.5 and 5 µM) were added to each well. The cell
viability was determined by crystal violet colorimetric analysis at
24, 48 and 72 h. All experiments were repeated three times.
Significant differences by concentration were calculated using
one-way ANOVA test and *P≤0.05 analyzed by concentration
were considered significant. |
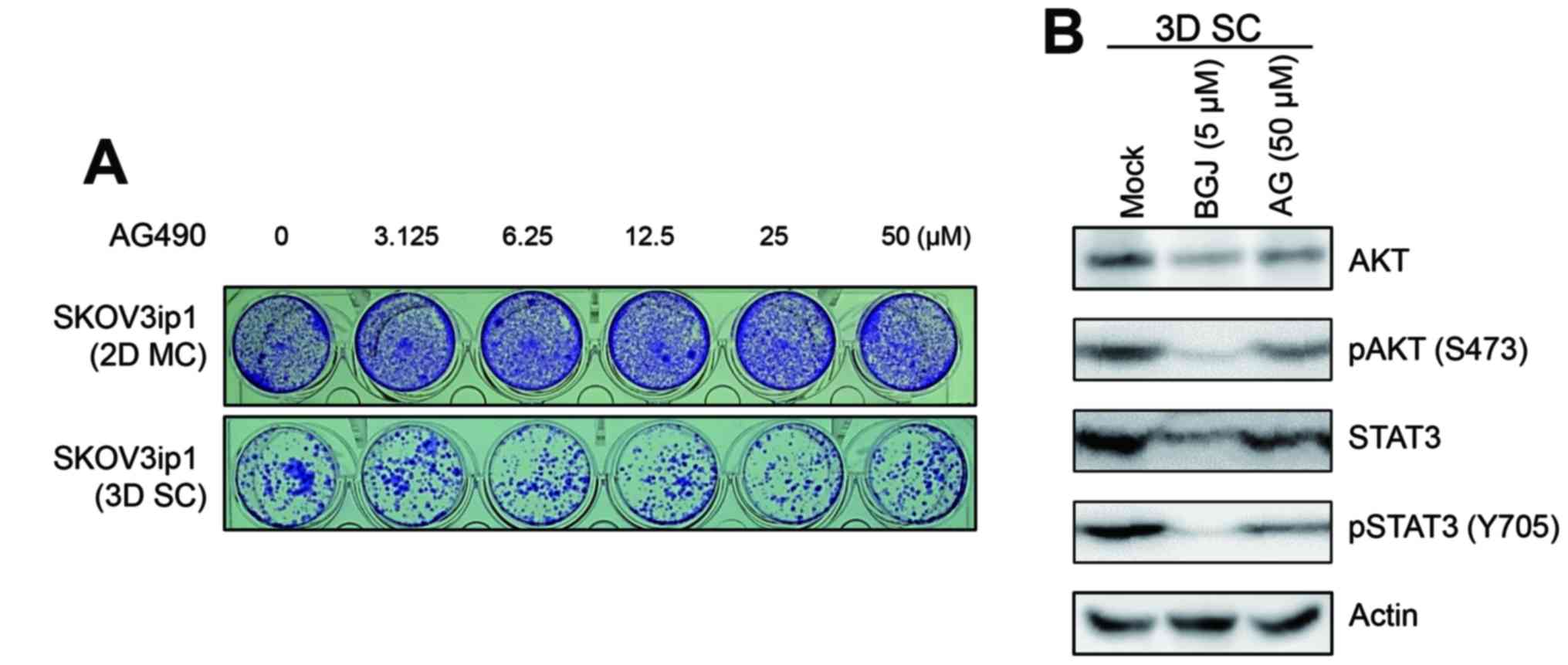
BGJ398 inhibits the activated AKT and
STAT3 in sphere-cultured SKOV3ip1 cells
Since major survival signaling molecules, AKT and
STAT3 were activated in sphere-cultured SKOV3ip1 cells, we examined
whether BGJ398 was able to affect the activation status of AKT and
STAT3 in sphere-cultured SKOV3ip1 cells. Immunoblotting analysis
indicated that BGJ398 suppressed the phosphorylated AKT at Ser473
residue and STAT3 at Tyr 705 residue in sphere-cultured SKOV3ip1
cells (Fig. 3A). Also BGJ398
decreased the phosphorylation of AKT at Ser473 residue and STAT3 at
Tyr 705 residue in a dose-dependent manner (Fig. 3B). In contrast, TAE684 and imatinib
did not affect the status of phosphorylation of AKT at Ser473
residue and STAT3 at Tyr 705 residue in sphere-cultured SKOV3ip1
cells (Fig. 3C). These results
demonstrated that BGJ398 suppressed the activated AKT and STAT3
signaling pathway in sphere-cultured SKOV3ip1 cells, not in
monolayer-cultured SKOV3ip1 cells. Several published studies have
demonstrated that JAK/STAT signaling regulates PI3K/AKT pathway in
mammalian cells (35,36). Inhibition of the cell viability of
sphere-cultured SKOV3ip1 cells by BGJ398 resulted in inhibition of
AKT and STAT3 activation leading us to further examine whether
JAK/STAT3 signaling mediates sphere culture-induced AKT and STAT3
activation in SKOV3ip1 cells. Thus, we examined whether the
selective JAK tyrosine kinase inhibitor AG490 affected the
activation of AKT and STAT3 status in sphere-cultured SKOV3ip1
cells. As shown in Fig. 4A, the
treatment of AG490 is not able to inhibit cell growth of
sphere-cultured SKOV3ip1 cells. Also, the phosphorylation of AKT
and STAT3 was not inhibited by treatment of AG490 in
sphere-cultured SKOV3ip1 cells (Fig.
4B). Collectively, these results indicated that BGJ398
effectively suppressed cell growth of sphere-cultured SKOV3ip1
through the inhibition of major survival signaling molecules, AKT
and STAT3 activation irrelevant to JAK/STAT3 signaling pathway.
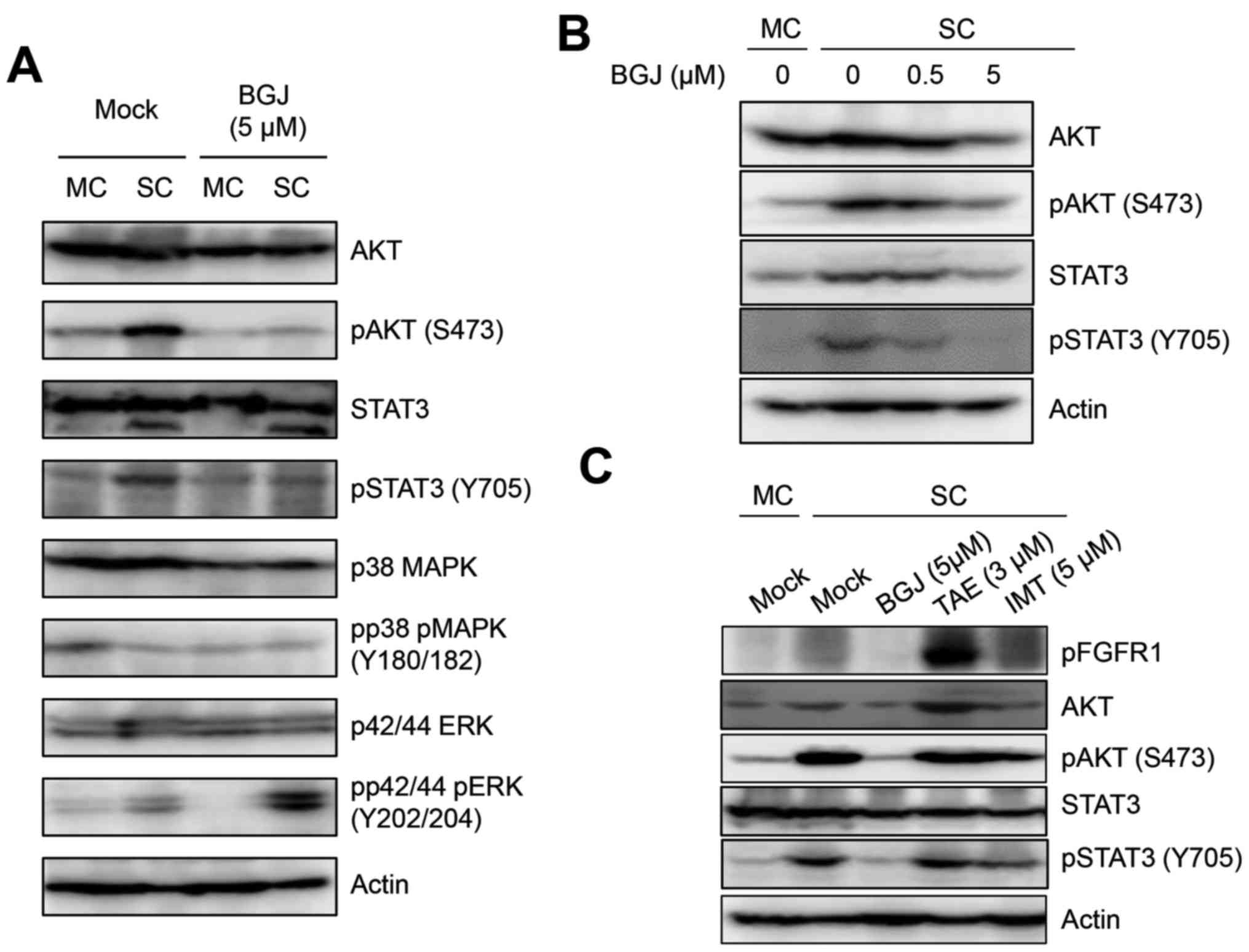 | Figure 3BGJ398 suppresses the phosphoryaltion
of AKT and STAT3 in sphere-cultured SKOV3ip1. (A)
Monolayer-cultured (MC) and sphere-cultured (SC) SKOV3ip1 cells
were treated with DMSO (Mock) and 5 µM BGJ398, respectively.
At 72 h after treatment, cells were harvested and were analyzed the
activation status of AKT, STAT3, p38 MAPK and p42/44 ERK by
immunoblotting. (B) SKOV3ip1 cells were treated with DMSO (Mock)
and 0.5 and 5 µM BGJ398, respectively. At 72 h after
treatment, immunoblotting was performed to analyze the cellular
expression and activation of AKT and STAT3 with indicated
antibodies. (C) Cell lysates at 72 h after treatment of DMSO
(Mock), 5 µM BGJ398 (BGJ), 3 µM TAE648 (TAE), and 5
µM imatinib (IMT) in sphere-cultured SKOV3ip1 were subjected
to immunoblotting and the cellular expression of AKT, STAT3 and its
activation status were analyzed with indicated antibodies. The
level of phosphorylated FGFR1 was shown as a positive control of
BGJ treatment. Actin was used as a loading control. |
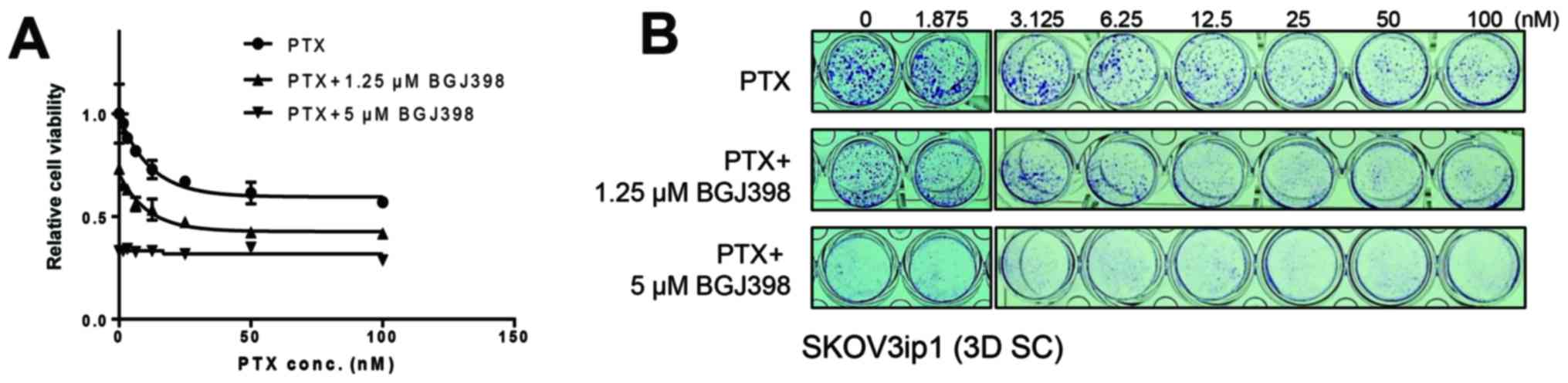
BGJ398 synergistically inhibits the cell
viability of sphere-cultured SKOV3ip1 with the combinational
treatment of paclitaxel
Previous results that the metastatic
microenvironment mimetic sphere culture of SKOV3ip1 enhances
paclitaxel resistance led us test whether BGJ398 sensitizes
paclitaxel-resistance in sphere-cultured SKOV3ip1 cells. To
determine whether the combination of BGJ398 and paclitaxel can
synergistically inhibit cell viability in sphere-cultured SKOV3ip1,
serial diluted paclitaxel with dose-different combinations (0, 1.25
and 5 µM) of BGJ398 were treated in sphere-cultured SKOV3ip1
and crystal violet colorimetric analysis was examined at 72 h after
treatment. As shown in Fig. 5,
treatment of sphere-cultured SKOV3ip1 with BGJ398 in combination
with paclitaxel led to a synergistic inhibition of cell viability
compared to treatment of paclitaxel alone.
Discussion
Ovarian cancer is the most lethal among
gynecological cancers (1). Despite
the active pharmaceutical research against ovarian cancer to date,
paclitaxel is still a first-line therapeutic anticancer drug for
ovarian cancer (2). Moreover, the
recurrent ovarian cancer in patients due to the development of
chemoresistant phenotype is the main obstacle to managing advanced
ovarian cancer (37). Together
with understanding of the chemo-resistance molecular mechanism of
ovarian cancer, developing new pharmaceutical agents will be
important to overcoming of ovarian cancer. We describe here a novel
antitumor activity of pan-FGFR inhibitor BGJ398 against ovarian
cancer dependent on sphere culture model. Also, BGJ398 induced
dissociation of the spheroid formation of ovarian cancer cells.
Although many anticancer drugs showed promising
effects in vitro cell line model, most failed to develop
into an in vivo model system. Application of the modified
in vitro culture model considering in vivo tumor
microenvironment will be important at the developmental stage of
anticancer drug. It has been reported sphere culture of epithelial
ovarian carcinoma cells represented the feature of in vivo
histological differentiation rather than the cells which were
cultured in conventional monolayer model (38). Moreover, the transition from
monolayer culture model to sphere culture model induced changes in
the expression of molecular biomarkers relevant to malignancy
including cadherins, vimentin and β-catenin (38). In this study, we suggested that the
activated AKT and STAT3, the key regulators in cell survival, also
changed by the transition of the culture model (Fig. 1) result in sensitizing the
antitumoral activity of BGJ398 against SKOV3ip1. Many studies have
reported the characterization of cancer cell spheroid as a model of
cancer stem cells. Ovarian cancer cell spheroids with stem
cell-like phenotype have been also reported to upregulate stem cell
markers, metastatic capability and acquiring chemotherapy
resistance (39–41). In the present study, we did not
incubate SKOV3ip1 cells with stem cell enrichment media, nor
separated the side population by FACS sorting for the enrichment of
ovarian cancer stem cells. AKT and STAT3 activation was
demonstrated by SKOV3ip1 incubation with anchorage-independent
growth. Moon and colleagues (42)
have reported that PI3K/Akt and Stat3 signaling are important roles
in maintenance of cancer stem cells of glioblastoma cell model.
Moreover, it has been reported that STAT3 signaling pathway has a
role in drug-resistance, migration, and invasion and the blockade
of STAT3 potentiates chemotherapy in ovarian cancer stem cell model
(43–46). Although FGFR signaling function in
maintenance of cancer stem cells has been reported (47), further examinations how ovarian
cancer sphere culture system upregulates cellular AKT and STAT3
activation and what the molecular mechanism of FGFR inhibitor
BGJ398 in regulation of AKT and STAT3 in sphere cultured-SKOV3ip1
were needed.
BGJ398 is a selective pan-FGFR inhibitor with
IC50 of 0.9, 1.4, 1.0 and 60 nM for FGFR1, 2, 3 and 4 in
cell-free assay, respectively (48) and with potential antiangiogenic and
anti-neoplastic activities (11).
Cheng et al (49) have
reported that BGJ398 inhibited the growth of E-cadherin-positive
epithelial type bladder cell lines at drug concentrations of 1
µM or lower. They also have shown that BGJ-398 did not
inhibit mesenchymal type bladder cancer cells and primary tumor
growth but block tumor metastasis in a mouse model in vivo
(49). In this study, we did not
observe changes of signaling molecules on EMT in SKOV3ip1 cells
following sphere cultivation. Although it has been reported that
SKOV3ip1 is highly malignant and has metastasis potential by
overexpressing ERBB2 (50),
whether FGFR may be responsible for mediating EMT signaling in
ovarian cancer spheres will be an important research area. TAE684
is a selective ALK kinase inhibitor (51). TAE684 treatment induces inhibition
of phosphorylation of NPM-ALK and its downstream effectors
including STAT3 and STAT5 and blocked the growth of anaplastic
large-cell lymphoma (ALCL)-drived and ALK-dependent cell lines
(51). Imatinib is a multi-target
kinase inhibitor against Abl (52), PDGF (53), and c-Kit (54) used in the treatment of various
cancers, mostly BCR/ABL-mutated chronic myelogenous leukemia. It
has been reported that imatinib reverses doxorubicin-resistance by
repression of the NF-κB signaling and preventing activation of
STAT3/HSP27/p38/Akt survival pathway in highly active c-Abl cancer
cells (55). Despite TAE684 having
cytotoxic activity in monolayer-cultured SKOV3ip1 cells as well as
in sphere-cultured SKOV3ip1 cells, our data showed that TAE684 and
imatinib do not suppress AKT and STAT3 pathways (Fig. 3A).
The present study showing the inhibition of the
SKOV3ip1 ovarian cancer growth in sphere culture system by BGJ398
treatment implies that FGFR signaling potentiated the specific
antitumor target against ovarian cancer spheroids. It has been
reported that RNAi-induced FGFR1/2 reduction inhibits proliferation
of SKOV3 ovarian cancer cell lines in vitro and increases
cisplatin sensitivity (56).
However, we demonstrated that significant cell toxicity was not
observed in BGJ398-treated monolayer-cultured SKOV3ip1 cells.
Specifically, the cell viability was decreased by BGJ398 in
sphere-cultured SKOV3ip1 cells (Fig.
2). Despite the absence of the expression profile of FGFR
isotypes in sphere-cultured SKOV3ip cells, we revealed the
downregulation of AKT and STAT3 activation via a pan-FGFR
inhibition in sphere-cultured SKOV3ip1 cells (Fig. 3A). Furthermore, we observed
cytotoxicity and dissociation of spheroids of SKOV3ip1 by treatment
of PI3K/AKT inhibitor wortmannin in SKOV3ip1 cells implying that
AKT activation is critical for the cell survival in sphere-cultured
SKOV3ip1 cells (data not shown). Potent therapeutic targeting of
the JAK/STAT3 pathway for ovarian cancer growth inhibition has been
demonstrated (57,58). Several reports have demonstrated
that JAK signaling regulates PI3K/AKT pathway in cells (35,36).
Recently, Wen et al (59)
reported that JAK/STAT3 inhibition using small molecule inhibitor
suppressed tumor progression and metastasis in peritoneal mouse
model of ovarian cancer. Moreover, it has been reported that the
inhibition of Jak2 suppresses the progression of ovarian cancer
(60). In the present study, we
did not investigate the defined signaling mechanism of AKT and
STAT3 activation in sphere-cultured SKOV3ip1 cells. The underlying
molecular mechanisms remain to be elucidated.
As shown in Fig. 2,
evidence have been reported that cells formed 3D spheroids have
resistance to antitumor chemotherapeutics including paclitaxel and
cisplatin. This has been largely considered due to several
mechanisms including a decreased penetration rate of drugs into
spheroid structure and alteration of prosurvival signaling pathway
(61,62). Cumulative evidence have showed that
β-integrin signaling is important in the maintenance of ovarian
cancer spheroid. Casey et al (63) reported that ovarian cancer spheroid
formation and the adhesion of spheroids to extracellular matrix
components is a β-integrin-dependent event. Sodek et al
(64) demonstrated that the
overexpression of β1-integrin induces compact spheroid formation
and invasive behavior in ovarian cancer cells. Yoshida et al
(62) reported that
laminin-1-derived synthetic peptide AG73 enhances the expression of
β1-integrin and induced the activation of downstream effector genes
including MAPK, ERK and AKT. Therefore, we cannot rule out the
possibility that the interference of the β-integrin pathway by
BGJ398 may exist and contribute to BGJ398-induced cytotoxicity in
sphere-cultured SKOV3ip1 cells.
Solid tumors on primary tissue were organized upon
its specific tumor microenvironment with cell-to-cell or
cell-to-extracellular matrix association. Unlike other cancers,
ovarian cancer metastasis is distinctly developed by dissemination
into peritoneal cavity and associated with the ascitic fluid to
form metastatic microenvironment (65). Within ascitic fluid, ovarian cancer
cells exist as individual cells or multicellular aggregates with
absence of anchorage-dependent signaling. In ovarian
cancer-specific microenvironment, metastases aggregates have an
enhanced resistance to anticancer drugs, including paclitaxel
(66). In the present study,
BGJ398 sensitized the cell cytotoxic activity with a combination
treatment of paclitaxel in sphere-cultured SKOV3ip1 cells implying
a possibility of new therapeutic approaches for targeting against
ovarian cancers spheroid regarding ovarian tumor microenvironment
(Fig. 5).
In conclusion, the present study suggests that
pan-FGFR inhibitor BGJ398 is a potent chemotherapeutic agent for
ovarian cancer spheroid. Our data also indicated that FGFR may have
a unique prosurvival role in the spheroid maintenance of ovarian
cancer. This study gives us a more comprehensive insight into 3D
sphere cell culture model during drug development considering
ovarian cancer specific microenvironment and the antitumor activity
of FGFR inhibitor against metastatic ovarian carcinoma.
Acknowledgments
The present study was supported by the Basic Science
Research Program through the National Research Foundation of Korea
(NRF) funded by the Ministry of Education
(2015R1D1A1A01060688).
References
|
1
|
Jelovac D and Armstrong DK: Recent
progress in the diagnosis and treatment of ovarian cancer. CA
Cancer J Clin. 61:183–203. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kampan NC, Madondo MT, McNally OM, Quinn M
and Plebanski M: Paclitaxel and its evolving role in the management
of ovarian cancer. BioMed Res Int. 2015:4130762015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Vaughan S, Coward JI, Bast RC Jr, Berchuck
A, Berek JS, Brenton JD, Coukos G, Crum CC, Drapkin R,
Etemadmoghadam D, et al: Rethinking ovarian cancer: Recommendations
for improving outcomes. Nat Rev Cancer. 11:719–725. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Brouwer-Visser J, Lee J, McCullagh K,
Cossio MJ, Wang Y and Huang GS: Insulin-like growth factor 2
silencing restores taxol sensitivity in drug resistant ovarian
cancer. PLoS One. 9:e1001652014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kelleher FC, O'Sullivan H, Smyth E,
McDermott R and Viterbo A: Fibroblast growth factor receptors,
developmental corruption and malignant disease. Carcinogenesis.
34:2198–2205. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hughes SE: Differential expression of the
fibroblast growth factor receptor (FGFR) multigene family in normal
human adult tissues. J Histochem Cytochem. 45:1005–1019. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Turner N and Grose R: Fibroblast growth
factor signalling: From development to cancer. Nat Rev Cancer.
10:116–129. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Touat M, Ileana E, Postel-Vinay S, André F
and Soria JC: Targeting FGFR signaling in cancer. Clin Cancer Res.
21:2684–2694. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wesche J, Haglund K and Haugsten EM:
Fibroblast growth factors and their receptors in cancer. Biochem J.
437:199–213. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hallinan N, Finn S, Cuffe S, Rafee S,
O'Byrne K and Gately K: Targeting the fibroblast growth factor
receptor family in cancer. Cancer Treat Rev. 46:51–62. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Katoh M: FGFR inhibitors: Effects on
cancer cells, tumor micro-environment and whole-body homeostasis
(Review). Int J Mol Med. 38:3–15. 2016.PubMed/NCBI
|
|
12
|
Helsten T, Elkin S, Arthur E, Tomson BN,
Carter J and Kurzrock R: The FGFR landscape in cancer: Analysis of
4,853 tumors by next-generation sequencing. Clin Cancer Res.
22:259–267. 2016. View Article : Google Scholar
|
|
13
|
Xue M, Cao X, Zhong Y, Kuang D, Liu X,
Zhao Z and Li H: Insulin-like growth factor-1 receptor (IGF-1R)
kinase inhibitors in cancer therapy: Advances and perspectives.
Curr Pharm Des. 18:2901–2913. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Asati V, Mahapatra DK and Bharti SK:
PI3K/Akt/mTOR and Ras/Raf/MEK/ERK signaling pathways inhibitors as
anticancer agents: Structural and pharmacological perspectives. Eur
J Med Chem. 109:314–341. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chen J, Elfiky A, Han M, Chen C and Saif
MW: The role of Src in colon cancer and its therapeutic
implications. Clin Colorectal Cancer. 13:5–13. 2014. View Article : Google Scholar
|
|
16
|
Schultze SM, Hemmings BA, Niessen M and
Tschopp O: PI3K/AKT, MAPK and AMPK signalling: Protein kinases in
glucose homeostasis. Expert Rev Mol Med. 14:e12012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Dillon LM and Miller TW: Therapeutic
targeting of cancers with loss of PTEN function. Curr Drug Targets.
15:65–79. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Franke TF, Yang SI, Chan TO, Datta K,
Kazlauskas A, Morrison DK, Kaplan DR and Tsichlis PN: The protein
kinase encoded by the Akt proto-oncogene is a target of the
PDGF-activated phosphatidylinositol 3-kinase. Cell. 81:727–736.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wu J, Feng X, Zhang B, Li J, Xu X, Liu J,
Wang X, Wang J and Tong X: Blocking the bFGF/STAT3 interaction
through specific signaling pathways induces apoptosis in
glioblastoma cells. J Neurooncol. 120:33–41. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kohn AD, Kovacina KS and Roth RA: Insulin
stimulates the kinase activity of RAC-PK, a pleckstrin homology
domain containing ser/thr kinase. EMBO J. 14:4288–4295.
1995.PubMed/NCBI
|
|
21
|
Henson ES and Gibson SB: Surviving cell
death through epidermal growth factor (EGF) signal transduction
pathways: Implications for cancer therapy. Cell Signal.
18:2089–2097. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hemmings BA and Restuccia DF: PI3K-PKB/Akt
pathway. Cold Spring Harb Perspect Biol. 4:a0111892012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Altomare DA and Testa JR: Perturbations of
the AKT signaling pathway in human cancer. Oncogene. 24:7455–7464.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kim D, Dan HC, Park S, Yang L, Liu Q,
Kaneko S, Ning J, He L, Yang H, Sun M, et al: AKT/PKB signaling
mechanisms in cancer and chemoresistance. Front Biosci. 10:975–987.
2005. View Article : Google Scholar
|
|
25
|
Mehta G, Hsiao AY, Ingram M, Luker GD and
Takayama S: Opportunities and challenges for use of tumor spheroids
as models to test drug delivery and efficacy. J Control Release.
164:192–204. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hickman JA, Graeser R, de Hoogt R, Vidic
S, Brito C, Gutekunst M and van der Kuip H; IMI PREDECT Consortium:
Three-dimensional models of cancer for pharmacology and cancer cell
biology: Capturing tumor complexity in vitro/ex vivo. Biotechnol J.
9:1115–1128. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hirschhaeuser F, Menne H, Dittfeld C, West
J, Mueller-Klieser W and Kunz-Schughart LA: Multicellular tumor
spheroids: An underestimated tool is catching up again. J
Biotechnol. 148:3–15. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ke N, Albers A, Claassen G, Yu DH,
Chatterton JE, Hu X, Meyhack B, Wong-Staal F and Li QX: One-week
96-well soft agar growth assay for cancer target validation.
Biotechniques. 36:826–828. 830832–833. 2004.PubMed/NCBI
|
|
29
|
Cao D, Kishida S, Huang P, Mu P, Tsubota
S, Mizuno M and Kadomatsu K: A new tumorsphere culture condition
restores potentials of self-renewal and metastasis of primary
neuroblastoma in a mouse neuroblastoma model. PLoS One.
9:e868132014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Weiswald LB, Bellet D and Dangles-Marie V:
Spherical cancer models in tumor biology. Neoplasia. 17:1–15. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ahmed N and Stenvers KL: Getting to know
ovarian cancer ascites: Opportunities for targeted therapy-based
translational research. Front Oncol. 3:2562013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yu D, Wolf JK, Scanlon M, Price JE and
Hung MC: Enhanced c-erbB-2/neu expression in human ovarian cancer
cells correlates with more severe malignancy that can be suppressed
by E1A. Cancer Res. 53:891–898. 1993.PubMed/NCBI
|
|
33
|
Dieci MV, Arnedos M, Andre F and Soria JC:
Fibroblast growth factor receptor inhibitors as a cancer treatment:
From a biologic rationale to medical perspectives. Cancer Discov.
3:264–279. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ornitz DM and Itoh N: The fibroblast
growth factor signaling pathway. Wiley Interdiscip Rev Dev Biol.
4:215–266. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Yamada O, Ozaki K, Akiyama M and Kawauchi
K: JAK-STAT and JAK-PI3K-mTORC1 pathways regulate telomerase
transcriptionally and posttranslationally in ATL cells. Mol Cancer
Ther. 11:1112–1121. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Gu YJ, Sun WY, Zhang S, Li XR and Wei W:
Targeted blockade of JAK/STAT3 signaling inhibits proliferation,
migration and collagen production as well as inducing the apoptosis
of hepatic stellate cells. Int J Mol Med. 38:903–911.
2016.PubMed/NCBI
|
|
37
|
Jayson GC, Kohn EC, Kitchener HC and
Ledermann JA: Ovarian cancer. Lancet. 384:1376–1388. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Lee JM, Mhawech-Fauceglia P, Lee N,
Parsanian LC, Lin YG, Gayther SA and Lawrenson K: A
three-dimensional microen-vironment alters protein expression and
chemosensitivity of epithelial ovarian cancer cells in vitro. Lab
Invest. 93:528–542. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Liao J, Qian F, Tchabo N,
Mhawech-Fauceglia P, Beck A, Qian Z, Wang X, Huss WJ, Lele SB,
Morrison CD, et al: Ovarian cancer spheroid cells with stem
cell-like properties contribute to tumor generation, metastasis and
chemotherapy resistance through hypoxia-resistant metabolism. PLoS
One. 9:e849412014. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
He QZ, Luo XZ, Wang K, Zhou Q, Ao H, Yang
Y, Li SX, Li Y, Zhu HT and Duan T: Isolation and characterization
of cancer stem cells from high-grade serous ovarian carcinomas.
Cell Physiol Biochem. 33:173–184. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Wang L, Mezencev R, Bowen NJ, Matyunina LV
and McDonald JF: Isolation and characterization of stem-like cells
from a human ovarian cancer cell line. Mol Cell Biochem.
363:257–268. 2012. View Article : Google Scholar
|
|
42
|
Moon SH, Kim DK, Cha Y, Jeon I, Song J and
Park KS: PI3K/Akt and Stat3 signaling regulated by PTEN control of
the cancer stem cell population, proliferation and senescence in a
glioblastoma cell line. Int J Oncol. 42:921–928. 2013.PubMed/NCBI
|
|
43
|
Han Z, Feng J, Hong Z, Chen L, Li W, Liao
S, Wang X, Ji T, Wang S, Ma D, et al: Silencing of the STAT3
signaling pathway reverses the inherent and induced chemoresistance
of human ovarian cancer cells. Biochem Biophys Res Commun.
435:188–194. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Han Z, Hong Z, Gao Q, Chen C, Hao Z, Ji T,
Hu W, Yan Y, Feng J, Liao S, et al: A potent oncolytic adenovirus
selectively blocks the STAT3 signaling pathway and potentiates
cisplatin antitumor activity in ovarian cancer. Hum Gene Ther.
23:32–45. 2012. View Article : Google Scholar
|
|
45
|
Zhang X, Liu P, Zhang B, Wang A and Yang
M: Role of STAT3 decoy oligodeoxynucleotides on cell invasion and
chemosensitivity in human epithelial ovarian cancer cells. Cancer
Genet Cytogenet. 197:46–53. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Fujiwara Y, Takaishi K, Nakao J, Ikeda T,
Katabuchi H, Takeya M and Komohara Y: Corosolic acid enhances the
antitumor effects of chemotherapy on epithelial ovarian cancer by
inhibiting signal transducer and activator of transcription 3
signaling. Oncol Lett. 6:1619–1623. 2013.PubMed/NCBI
|
|
47
|
Chang JY, Wang C, Liu J, Huang Y, Jin C,
Yang C, Hai B, Liu F, D'Souza RN, McKeehan WL, et al: Fibroblast
growth factor signaling is essential for self-renewal of dental
epithelial stem cells. J Biol Chem. 288:28952–28961. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Guagnano V, Kauffmann A, Wöhrle S, Stamm
C, Ito M, Barys L, Pornon A, Yao Y, Li F, Zhang Y, et al: FGFR
genetic alterations predict for sensitivity to NVP-BGJ398, a
selective pan-FGFR inhibitor. Cancer Discov. 2:1118–1133. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Cheng T, Roth B, Choi W, Black PC, Dinney
C and McConkey DJ: Fibroblast growth factor receptors-1 and -3 play
distinct roles in the regulation of bladder cancer growth and
metastasis: Implications for therapeutic targeting. PLoS One.
8:e572842013. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Ueno NT, Bartholomeusz C, Herrmann JL,
Estrov Z, Shao R, Andreeff M, Price J, Paul RW, Anklesaria P, Yu D,
et al: E1A-mediated paclitaxel sensitization in
HER-2/neu-overexpressing ovarian cancer SKOV3.ip1 through apoptosis
involving the caspase-3 pathway. Clin Cancer Res. 6:250–259.
2000.PubMed/NCBI
|
|
51
|
Galkin AV, Melnick JS, Kim S, Hood TL, Li
N, Li L, Xia G, Steensma R, Chopiuk G, Jiang J, et al:
Identification of NVP-TAE684, a potent, selective, and efficacious
inhibitor of NPM-ALK. Proc Natl Acad Sci USA. 104:270–275. 2007.
View Article : Google Scholar :
|
|
52
|
Buchdunger E, Zimmermann J, Mett H, Meyer
T, Müller M, Druker BJ and Lydon NB: Inhibition of the Abl
protein-tyrosine kinase in vitro and in vivo by a
2-phenylaminopyrimidine derivative. Cancer Res. 56:100–104.
1996.PubMed/NCBI
|
|
53
|
Ranza E, Mazzini G, Facoetti A and Nano R:
In-vitro effects of the tyrosine kinase inhibitor imatinib on
glioblastoma cell proliferation. J Neurooncol. 96:349–357. 2010.
View Article : Google Scholar
|
|
54
|
Heinrich MC, Griffith DJ, Druker BJ, Wait
CL, Ott KA and Zigler AJ: Inhibition of c-kit receptor tyrosine
kinase activity by STI 571, a selective tyrosine kinase inhibitor.
Blood. 96:925–932. 2000.PubMed/NCBI
|
|
55
|
Sims JT, Ganguly SS, Bennett H, Friend JW,
Tepe J and Plattner R: Imatinib reverses doxorubicin resistance by
affecting activation of STAT3-dependent NF-κB and HSP27/p38/AKT
pathways and by inhibiting ABCB1. PLoS One. 8:e555092013.
View Article : Google Scholar
|
|
56
|
Cole C, Lau S, Backen A, Clamp A, Rushton
G, Dive C, Hodgkinson C, McVey R, Kitchener H and Jayson GC:
Inhibition of FGFR2 and FGFR1 increases cisplatin sensitivity in
ovarian cancer. Cancer Biol Ther. 10:495–504. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Gritsina G, Xiao F, O'Brien SW, Gabbasov
R, Maglaty MA, Xu RH, Thapa RJ, Zhou Y, Nicolas E, Litwin S, et al:
Targeted blockade of JAK/STAT3 signaling inhibits ovarian carcinoma
growth. Mol Cancer Ther. 14:1035–1047. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
McCann GA, Naidu S, Rath KS, Bid HK,
Tierney BJ, Suarez A, Varadharaj S, Zhang J, Hideg K, Houghton P,
et al: Targeting constitutively-activated STAT3 in hypoxic ovarian
cancer, using a novel STAT3 inhibitor. Oncoscience. 1:216–228.
2014. View Article : Google Scholar
|
|
59
|
Wen W, Liang W, Wu J, Kowolik CM, Buettner
R, Scuto A, Hsieh MY, Hong H, Brown CE, Forman SJ, et al: Targeting
JAK1/STAT3 signaling suppresses tumor progression and metastasis in
a peritoneal model of human ovarian cancer. Mol Cancer Ther.
13:3037–3048. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Kobayashi A, Tanizaki Y, Kimura A, Ishida
Y, Nosaka M, Toujima S, Kuninaka Y, Minami S, Ino K and Kondo T:
AG490, a Jak2 inhibitor, suppressed the progression of murine
ovarian cancer. Eur J Pharmacol. 766:63–75. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Desoize B and Jardillier J: Multicellular
resistance: A paradigm for clinical resistance? Crit Rev Oncol
Hematol. 36:193–207. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Yoshida Y, Kurokawa T, Nishikawa Y, Orisa
M, Kleinman HK and Kotsuji F: Laminin-1-derived scrambled peptide
AG73T disaggregates laminin-1-induced ovarian cancer cell spheroids
and improves the efficacy of cisplatin. Int J Oncol. 32:673–681.
2008.PubMed/NCBI
|
|
63
|
Casey RC, Burleson KM, Skubitz KM,
Pambuccian SE, Oegema TR Jr, Ruff LE and Skubitz AP: Beta
1-integrins regulate the formation and adhesion of ovarian
carcinoma multicellular spheroids. Am J Pathol. 159:2071–2080.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Sodek KL, Ringuette MJ and Brown TJ:
Compact spheroid formation by ovarian cancer cells is associated
with contractile behavior and an invasive phenotype. Int J Cancer.
124:2060–2070. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Lengyel E: Ovarian cancer development and
metastasis. Am J Pathol. 177:1053–1064. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Thibault B, Castells M, Delord JP and
Couderc B: Ovarian cancer microenvironment: Implications for cancer
dissemination and chemoresistance acquisition. Cancer Metastasis
Rev. 33:17–39. 2014. View Article : Google Scholar
|