Introduction
The high mobility group A1 (HMGA1) belongs to
super-family of nonhistone chromatin binding proteins and has two
isoforms, HMGA1a and HMGA1b. HMGA1 is an architectural
transcription factor and participates in multiple cellular biology
processes, including transcriptional regulation, embryogenesis,
transformation, cell cycle regulation, differentiation, viral
integration, and DNA repair (1–3).
Recently, HMGA1 was found to be associated with the occurrence and
development of many malignant tumors, including breast (4), pancreas (5), lung (6), ovary (7), colon (8) and thyroid carcinomas (9). HMGA1 is also reported to be
associated with high invasion and metastasis of the tumors. It may
be a molecular prognostic marker of tumors (10).
Transforming growth factor-β (TGF-β) is reported to
exert an essential role on cell proliferation, differentiation,
apoptosis, invasion, and cellular microenvironment (11–14).
The role of TGF-β in cancer occurrence and development has been
extensively studied, and previous studies have shown that TGF-β1 is
a tumor suppressor in the early phase of tumor and it becomes a
tumor promoting factor during the late stages of cancer (15–17).
The TGF-β receptor is composed of type I TGF-β receptors (TβRI) and
type II TGF-β receptors (TβRII), and both of them participate in
classic TGF-β1 signaling. With the presence of ligand binding, the
type II receptors activate the type I receptors and recruit Smad2
and Smad3. Activated Smad2 and Smad3 bind to Smad4, and transfer
into the nucleus to regulate gene expression (18). In addition to the classic
TGF-β1/Smad pathway, TGF-β1 can also be activated by activating
non-Smad pathways, such as the phosphatidylinositol-3 kinase
(PI3K), extracellular signal-regulated kinase [ERK, mitogen
activated protein kinase (MAPK)], c-Jun NH2 terminal kinase (JNK)
and p38 MAPK pathways and Rho GTPases (19). TGF-β1 is generally considered to be
a necessary promoter for epithelial-mesenchymal transition (EMT)
and EMT is considered to be an important initiator for tumor
invasive behavior during cancer progression (20 23).
We aimed to determine the effects of TGF-β1 on the
expression of HMGA1 in thyroid cancer cells. To our knowledge, the
present study provides the first link between TGF-β1 and HMGA1 in
thyroid cancer cells, and revealed that PI3K and ERK signaling are
involved in the TGF-β1-induced HMGA1 expression. In addition,
matrix metalloproteinase-2 (MMP-2) was also found to be correlated
with the invasion induced by HMGA1 in thyroid cancer cells. This
study also provided further evidence for the pivotal role of HMGA1
in the progress of thyroid cancer.
Materials and methods
Cell culture
Human thyroid cancer cell line TPC1 and SW579 were
purchased from American Type Culture Collection (USA). TPC1 cells
were cultured in DMEM medium with 10% fetal bovine serum at 37°C in
a humidified atmosphere containing 5% CO2. SW579 cells
were cultured in L15 medium supplemented with 10% fetal bovine
serum at 37°C in a humidified atmosphere without
CO2.
Ethics approval and consent to
participate
The ethics approval and consent for the use of human
tissue was confirmed by the ethics committee of the First
Affiliated Hospital of University of South China.
Reagents
The ERK inhibitor PD98059 (20 µM) and U0126
(25 µM), PI3K inhibitors wortmannin (100 nM) were purchased
from Calbiochem Corp. (San Diego, CA, USA). while wortmannin,
PD98059 and U0126 were dissolved in 0.1% (vol/vol) dimethyl
sulfoxide (DMSO).
Transient transfection and luciferase
activity assay
Transient gene delivery was carried out as described
previously (24). A luciferase
assay kit (Promega) was used to measure the reporter activity
according to the manufacturer's instructions. SW579 cells were
cultured in serum free medium for 12 h in a 12 well plate. The
cells were transfected with the HMGA1 promoter vector containing
luciferase or with the control vector, pGL4.1 and after 6 h of
transfection, the cells were treated with or without TGF-β1 at the
indicated concentration for 12 h. Luciferase activity was
normalized by using a Renilla luciferase internal
control.
Immunofluorescence
SW579 cells were seeded on a round glass cover
placed into a 6-well microtiter plate. The cells treated with
TGF-β1 at the indicated concentration were fixed with 4%
paraformaldehyde-PBS for 15 min at room temperature, washed with
PBS twice and then permeabilized by incubation with 0.1% Triton
X-100 for 5 min. The cells were washed twice with PBS again and
stained with anti-HMGA1 (1:100; ab4078, Abcam) antibody for 2 h at
room temperature. After washing with PBS, each sample was incubated
with FITC-conjugated secondary antibodies (Boster, Wuhan, China)
for 1 h. The samples were washed with PBS twice and incubated with
4′,6-diamidino-2-phenylindole (DAPI) 15 min. The samples were
analyzed under a fluorescence microscope (Olympus, Tokyo,
Japan).
RNA isolation and real-time RT-PCR
The SW579 were treated with TGF β1 (0, 1, 2, 5 and
10 ng/ml) for 12 h or wortmannin (100 nM), PD98059 (20 µM)
and U0126 (25 µM), and maintained in culture medium for 2 h.
Total RNA of SW579 cells was extracted using TRIzol reagent
(Invitrogen) and was reverse transcribed into cDNA using the
first-strand synthesis kit (Gibco-BRL, Carlsbad, CA, USA). The
mRNAs of HMGA1 and GAPDH were amplified with a denaturation step
(95°C for 1 min), followed by 35 cycles of denaturation (95°C for
10 sec), annealing and extension (60°C for 20 sec). Results from 3
separate experiments were analyzed, and GAPDH as a reference.
Construction and screening of lentiviral
vectors harboring HMGA1-specific siRNA
The siRNA sequences targeting to human HMGA1 gene
(GenBank accession no. NM_145901) were selected: Target1:
ACTCCAGGAAGGAAACCAA; Target2: AGCGAAGTGCCAACACCTA; and Target3:
GCTACCAGCGCCAAATGTT. Three pairs of complementary oligonucleotides
were designed, and cloned into a lentivirus-based vector
(pGCSIL-GFP, Genechem, Shanghai, China). Lentiviral particles were
prepared as previously described (25).
Three lentiviral constructs carrying siRNAs were
used to infect SW579 cells at a multiplicity of infection (MOI) of
20 (low MOI) and 40 (high MOI). Three days after infection, GFP
expression was measured to calculate the infection efficiency. Five
days after infection, cells were harvested for test HMGA1 knockdown
efficiency with real-time RT-PCR, and the siRNA with the highest
knockdown efficiency was used for subsequent experiments.
Cell proliferation and colony formation
assays
Cells were seeded in 96-well plates (2,000
cells/well) and counted using an automated cell counter (Nexcelom
Bioscience, Lawrence, MA, USA). For colony formation assay, cells
were seeded in 12-well plates (400 cells/well) and cultured for 8
days. Each experiment was repeated in triplicate and performed at
least twice.
Cell cycle analysis by flow
cytometry
The transfected cells were seeded in 6 well plates
at 2×105/well. After the indicated treatments, the cells
were harvested by trypsinization and washed with PBS and fixed
overnight in ice-cold 75% ethanol at −20°C. The immobilized cells
were washed, and dissolved in RNAse, followed by incubation at 37°C
for 30 min. Next, cells were stained with propidium iodide (PI) for
30 min. The DNA content of the cells was measured using a BD Accuri
C6 flow cytometer (BD Biosciences).
Cell invasion assays
The transfected cells (10,000 cells/well) were
resuspended in serum free medium and seeded in the upper chamber of
a 24-well Matrigel™ Invasion Chamber (BD Biosciences, San Diego,
CA, USA) coated with Matrigel. Cell invasion was calculated as the
percentage of total cells that had invaded the bottom chamber
containing complete medium with serum.
Scratch-wound assays
Cells were transiently transfected and grown to
confluence. The central linear wound area was carefully generated
by scratching the cell monolayer with a sterile 200-µl
pipette tip, and images were taken after 24 h. Bars represent as
the average percentage of wound closure relative to the initial
wound area.
Western blot analysis
The lysates of cell or tissue were lysed for 30 min
on ice. Soluble proteins (30 µg) were probed with anti-HMGA1
(1:1,000; ab4078, Abcam), anti-E-cadherin antibodies (1:500; #3195,
CST) and MMP-2 (1:500; ab37150, Abcam). Loading variations were
normalized with GAPDH, and was identified with anti-GAPDH
monoclonal Ab.
Tissue microarray and immunohistochemical
analysis
IHC staining of the tissue microarray (TH8010a, US
Biomax) was performed as detailed in our previous studies (26). The rabbit polyclonal HMGA1 antibody
(1:150; ab4078, Abcam) and MMP-2 antibody (1:100; ab37150, Abcam)
were used.
Statistical analysis
All experiments were performed in triplicate and the
results were expressed as mean ± SD. Statistical analysis was
performed using SPSS, version 13.0 (SPSS, Inc., Chicago, IL, USA).
Non-parametrically two-tailed Mann-Whitney U test was used to test
statistical association between clinicopathological and molecular
parameters. P-values <0.05 were considered significant.
Spearman's rank correlation coefficients were used to assess the
correlation of HMGA1 and MMP-2 expression with clinicopathological
parameters.
Results
HMGA1 expression is increased by TGF-β1
in thyroid cancer SW579 cells
It has been shown that TGF-β1 could stimulate the
invasion and metastasis of cancer cells. TGF-β1 has high expression
in most malignant tumors, and is a necessary factor for cancer
initiation and development (27).
In this study, we investigated the effects of TGF-β1 on the
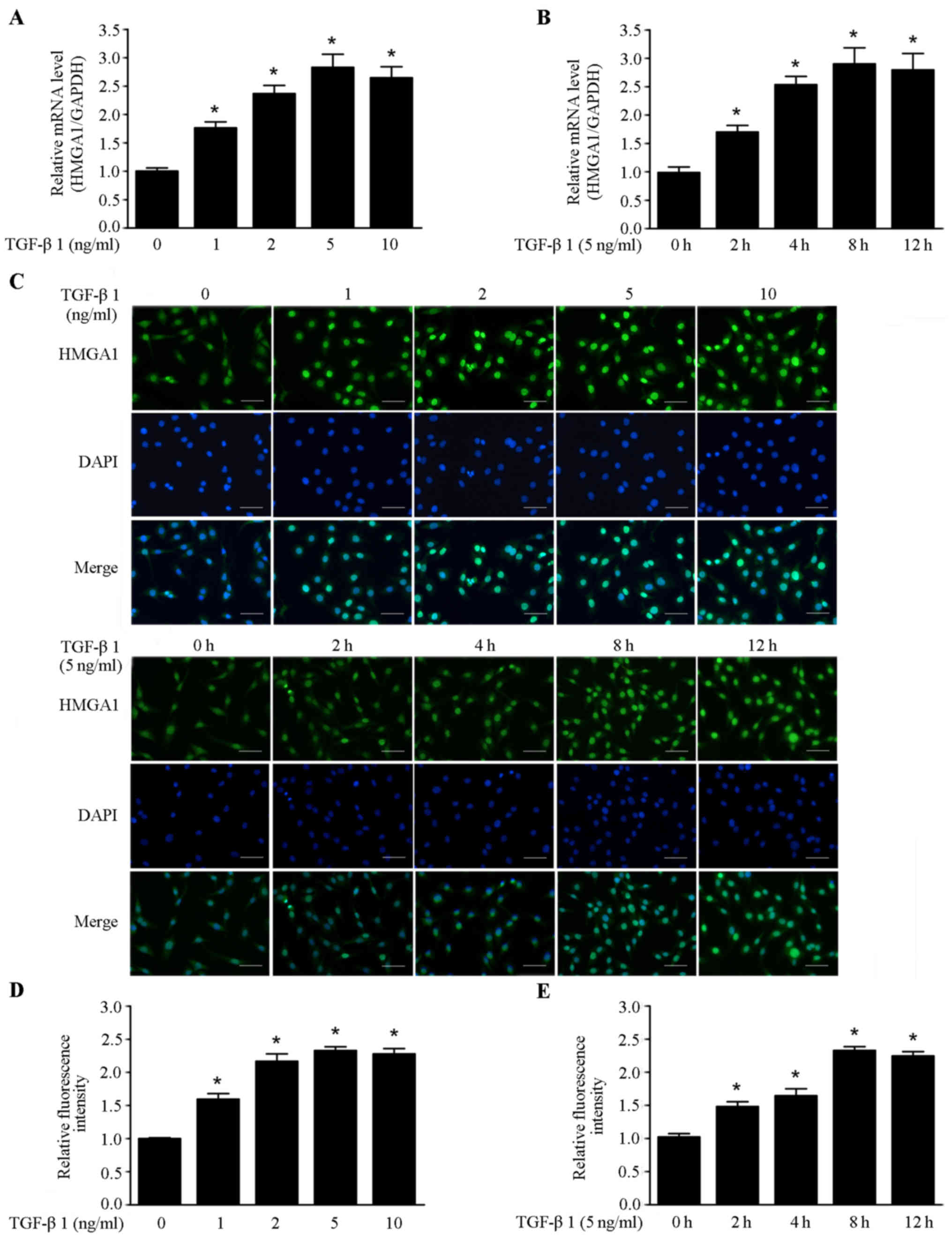
expression of HMGA1 in thyroid cancer SW579 cell line. As shown in
Fig. 1A and B, TGF-β1 elevated the
HMGA1 mRNA level in a dose and time-dependent manner in SW579
cells. Immunofluorescence assay revealed that the HMGA1 expression
was also enhanced in a dose- and time-dependent manner by TGF-β1 in
SW579 cells (Fig. 1C–E). These
data indicate that TGF-β1 is a positive regulator of HMGA1
expression in thyroid cancer cells.
TGF-β1 upregulates HMGA1 expression
through the PI3K/Akt and ERK pathway
Previous studies have shown that PI3K/Akt and MAPK
were involved in the cellular and molecular events of TGF-β1
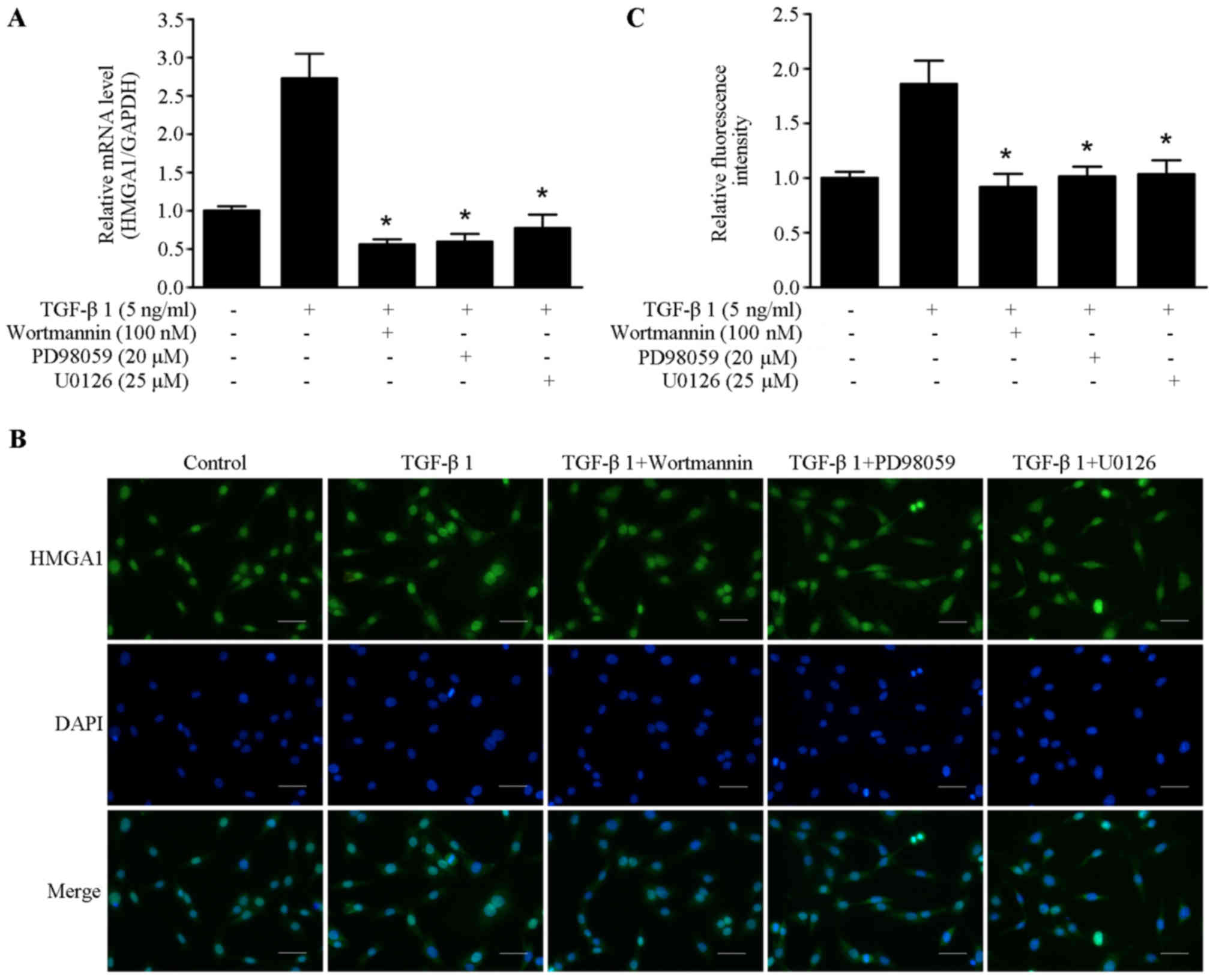
signaling (28,29). The inhibitors of PI3K/Akt and ERK
pathway wortmannin, PD98059 and U0126 were utilized to elucidate
the underlying molecular mechanism of TGF-β1-induced HMGA1
expression. As shown in Fig. 2A,
TGF-β1-induced HMGA1 mRNA transcription was abrogated by treatment
with wortmannin, PD98059 and U0126 in SW579 cells.
Immunofluorescence staining showed that wortmannin, PD98059 and
U0126 could block the TGF-β1-induced HMGA1 expression in SW579
cells (Fig. 2B and C). These
results suggest that PI3K/Akt and ERK pathway are involved in
TGF-β1-induced HMGA1 expression in thyroid cancer cells.
TGF-β1 upregulates HMGA1 expression by
enhancing the promoter activity of HMGA1 in thyroid cancer
cells
To further elucidate the molecular mechanism of
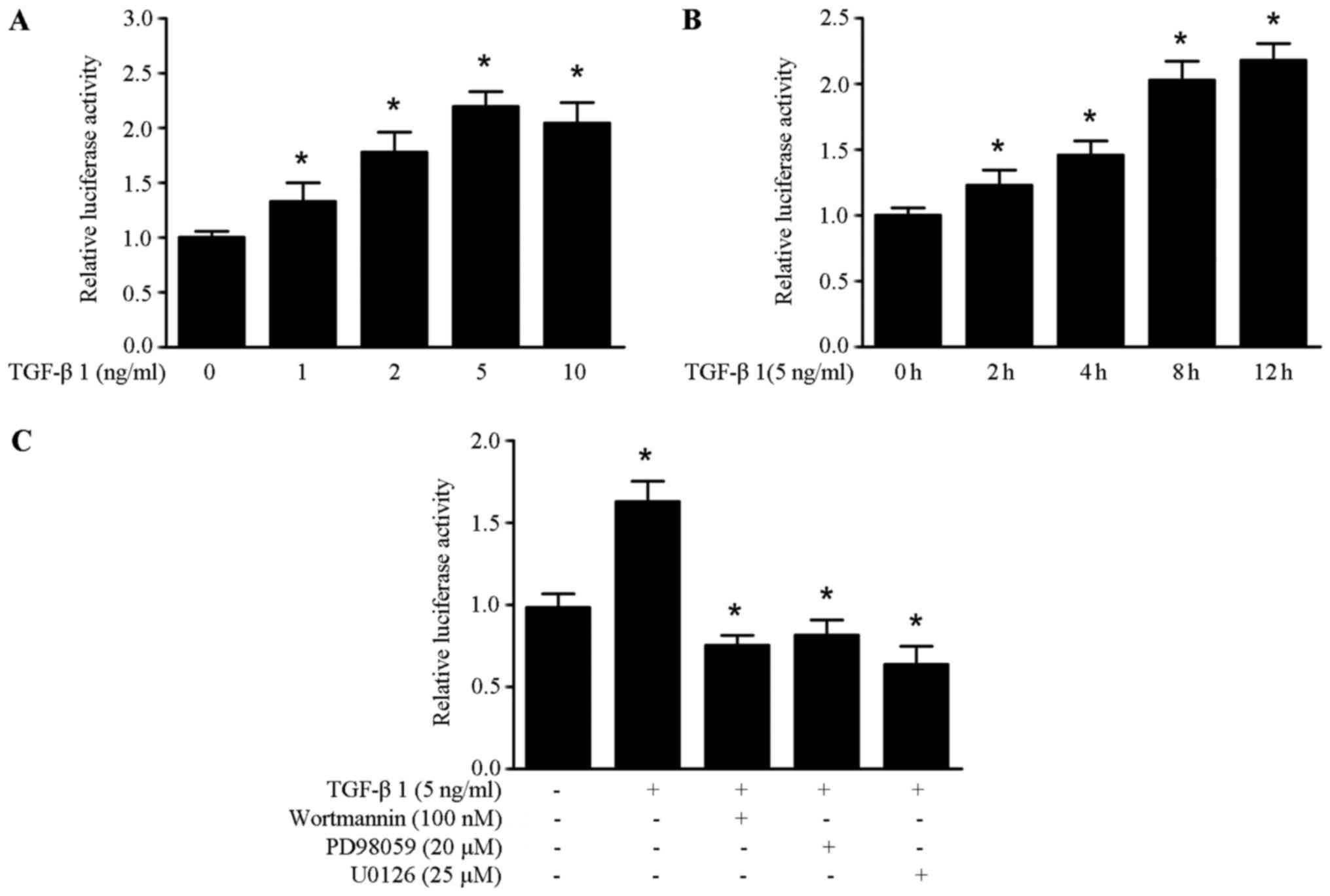
TGF-β1 induced HMGA1 expression, we obtained the HMGA1
promoter sequence from the SW579 cells. It was found that TGF-β1
could enhance the promoter activity of HMGA1 in a dose and
time-manner in the SW579 cells (Fig.
3A and B). As shown in Fig.
3C, the increased promoter activity of HMGA1 induced by
TGF-β1 was abrogated by treatment with wortmannin, PD98059 and
U0126 in SW579 cells. These data suggest that TGF-β1 upregulates
HMGA1 expression by enhancing the promoter activity of HMGA1
in thyroid cancer cells.
Lentivirus-mediated HMGA1 knockdown
inhibits cell growth in the (least)non-invasive cell line in
vitro
We have reported that siRNA mediated HMGA1 silencing
in thyroid cancer cells could inhibit the cellular oncogenic
characteristics (30). To
determine whether lentivirus-mediated HMGA1 knockdown have effect
on the cellular oncogenic properties of thyroid cancer, three
lentivirus-mediated shRNA targeting HMGA1 gene constructs with
different shRNAs (KD1, KD2 and KD3) were used to infect thyroid
cancer TPC1 and SW579 cells. The infection efficiencies of these
lentiviral vectors were >90% (Fig.
4A and B). Real time RT PCR assay showed that all three
constructs, used at high or low MOI, significantly downregulated
HMGA1 expression in TPC1 cells (Fig.
4C). The highest knockdown efficiency was obtained by using KD3
(low MOI, 81% relative to the NC group; high MOI, 82%), which was
named as HMGA1/GV248RNAi-LV-3.
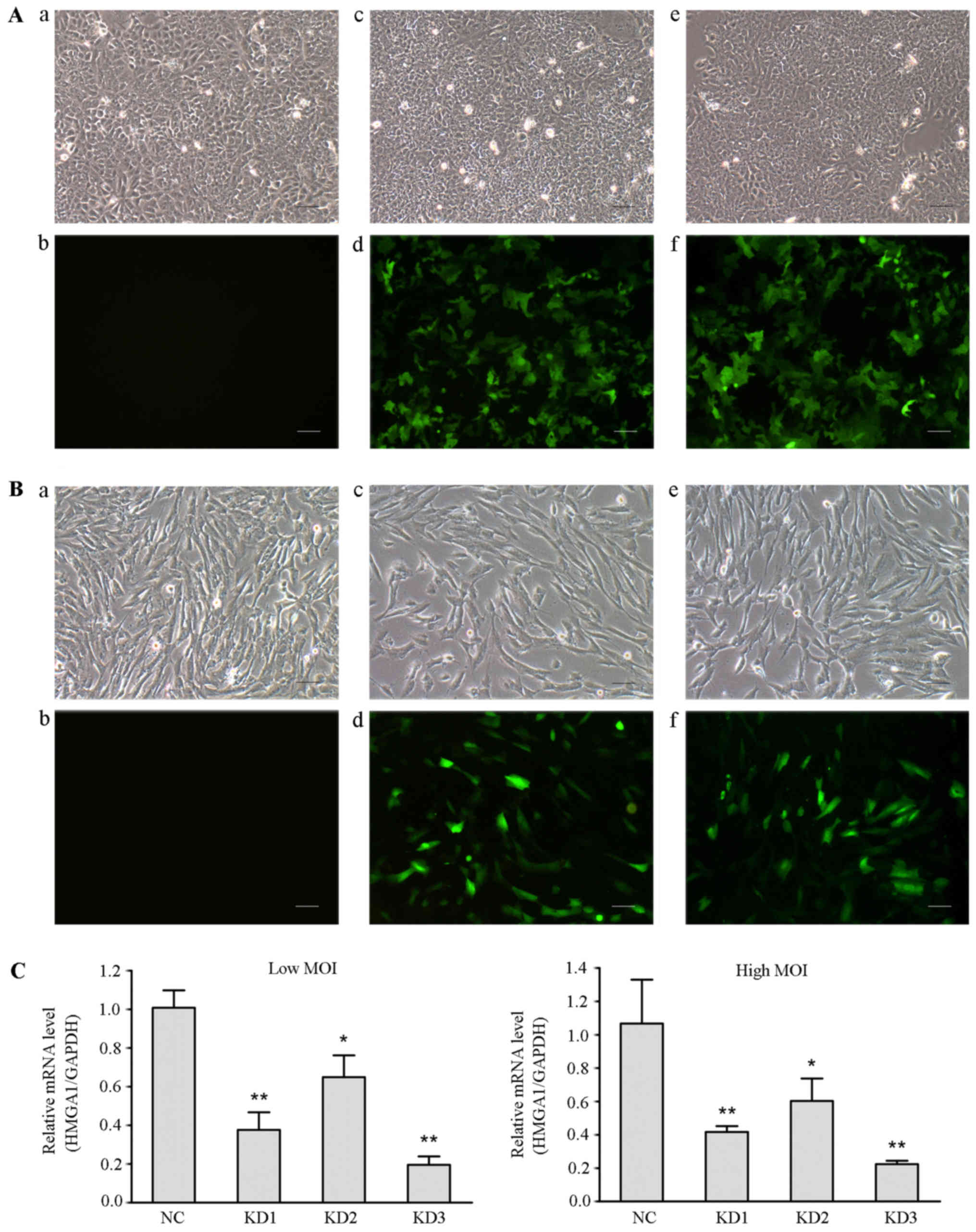 | Figure 4Identification of the lentiviral
vector with the highest knockdown efficiency. (A) Fluorescence
microscopy was used to observe the infection efficiency of
different lentiviral vectors in TPC1 cells (magnification, x100).
a, TPC1 cells without lentiviral infection in the optical
microscope (Con group); b, TPC1 cells of Con group in the
fluorescence microscope; c, TPC1 cells were infected with negative
lentivirus NC/GV248 RNAi-LV (NC group) in the optical microscope;
d, TPC1 cells of NC group in the fluorescence microscope; e, TPC1
cells were infected with HMGA1/GV248RNAi-LV#3 RNAi (KD group) at a
high MOI in the optical microscope; f, TPC1 cells of KD group at a
high MOI in the fluorescence microscope. Scale bar, 50 µm.
(B) Fluorescence microscopy examination of the infection
efficiencies of different lentiviral vectors in SW579 cells
(magnification, x100). The panels are described as similar with
that in (A). Scale bar, 50 µm. (C) Relative levels of HMGA1
in SW579 cells infected with different groups of lentiviral
particles. Either low or high MOI, HMGA1 expression significantly
decreased in SW579 cells infected with different groups of
lentiviral particles. The highest knockdown efficiency was obtained
using KD3 lentiviral particles. *P<0.05,
**P<0.01. |
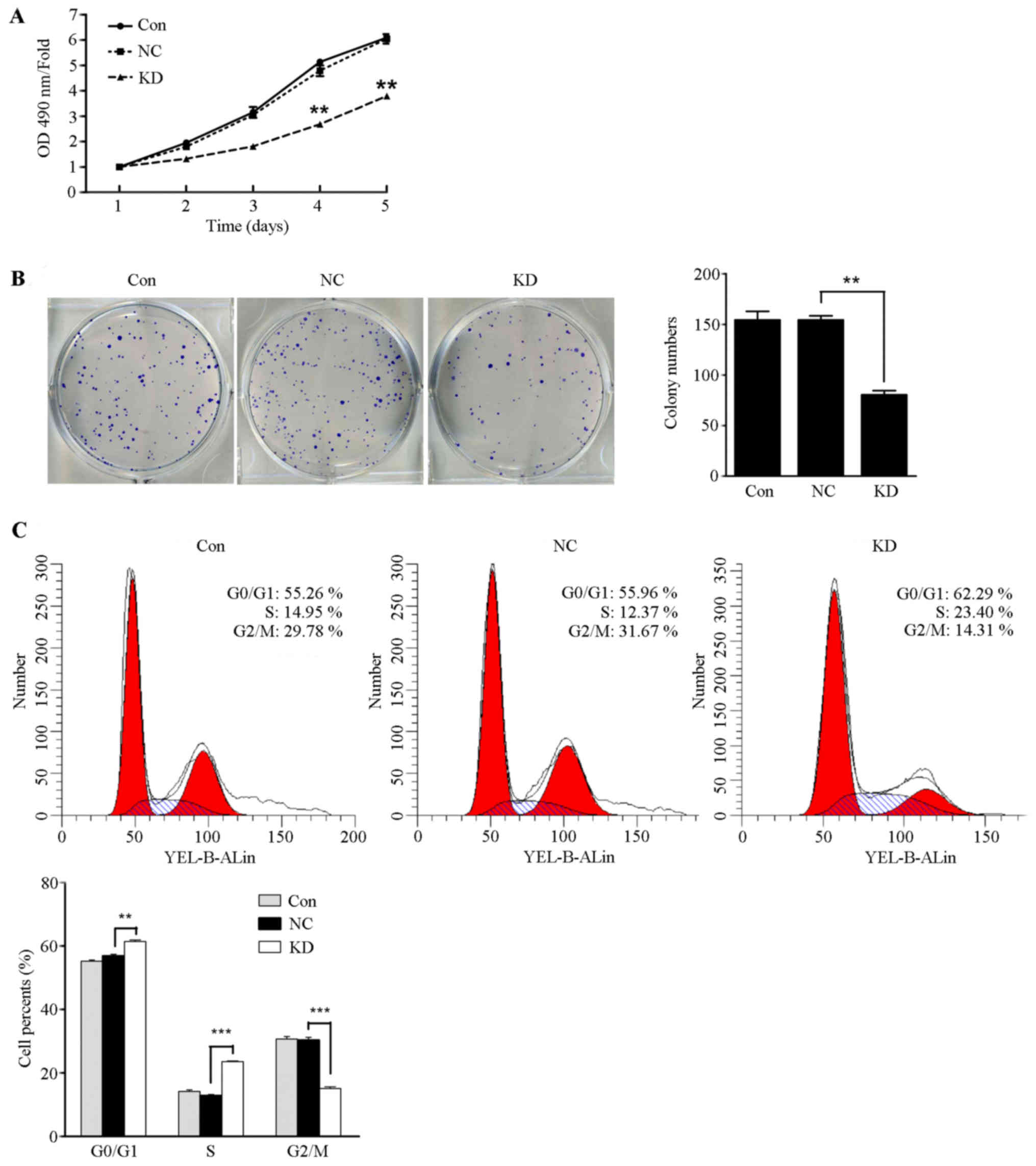
HMGA1/GV248RNAi-LV-3 was transfected into thyroid
cancer TPC1 cells and SW579 cells. As shown in Fig. 5A, TPC1 cell proliferation was
significantly inhibited in the HMGA1 knockdown group compared with
the control group and the NC group at day four (P<0.05), whereas
the proliferate capacity of SW579 cells showed no significant
difference in the three groups (data not shown). It was found that
the number of formed colonies by lentivirus-mediated knockdown of
HMGA1 was significantly decreased compared to those in control and
NC group (Fig. 5B, P<0.01). The
number of formed colonies of SW579 cells have no significant
different in the three groups (data not shown). In additions, cell
cycle assay analysis showed that the cell populations in the
G0–G1 and S phases of TPC1 cells with HMGA1
knockdown markedly increased (Fig.
5C, P<0.01 and P<0.001) and the population of
G2-M phases significantly decreased (Fig. 5C, P<0.001). Those results
indicated that HMGA1 knockdown inhibits the proliferation capacity
in the (least)non-invasive thyroid cancer TPC1 cells, but has no
obvious effect on invasive thyroid cancer SW579 cells.
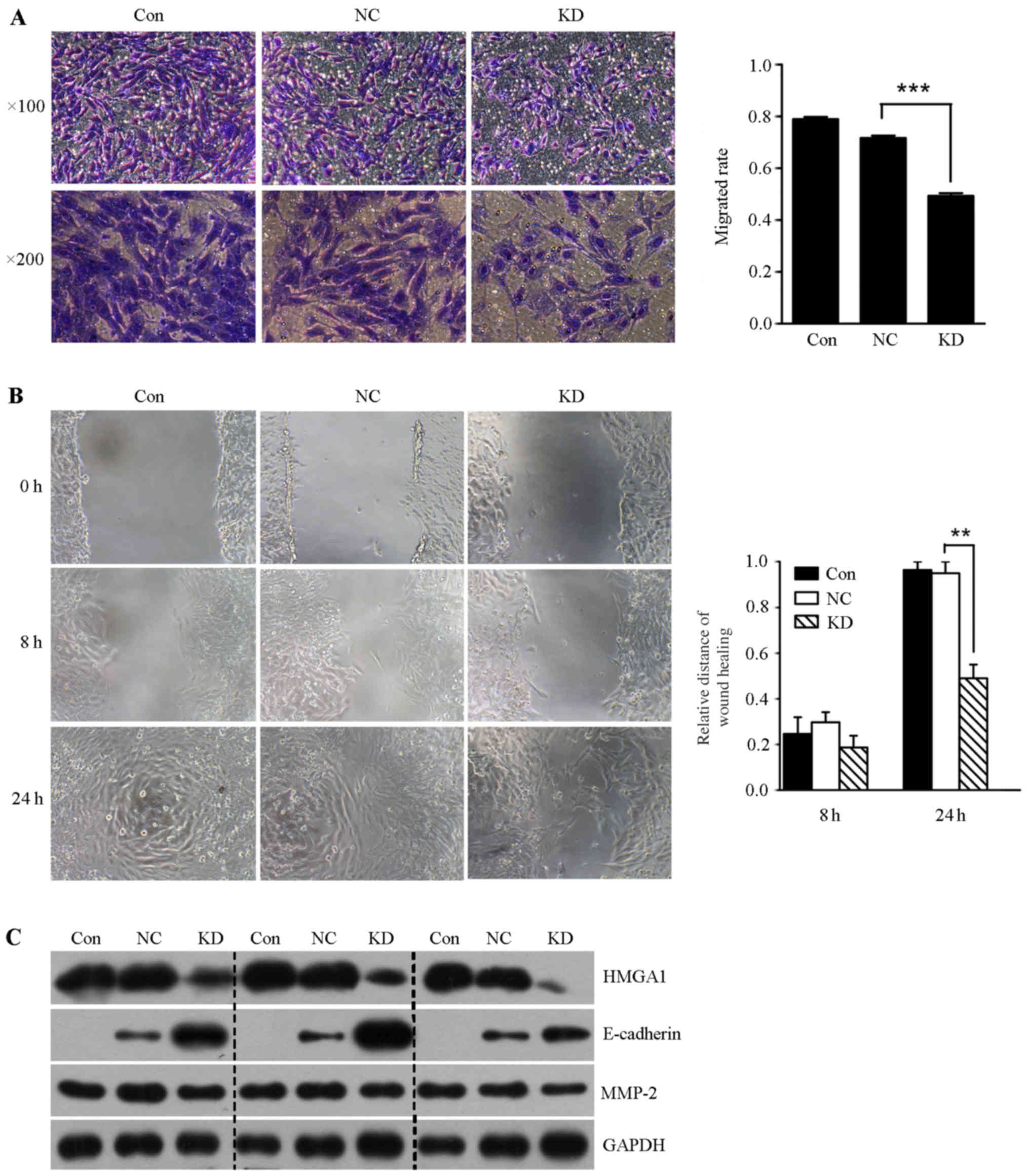
Lentivirus-mediated HMGA1 knockdown
inhibits the invasion and migration in SW579 cells
In order to determine whether lentivirus mediated
HMGA1 knockdown could affect the invasion of thyroid cancer SW579
cells, we performed the transwell invasion assay and scratch-wound
assay. It was shown that the invasion rate and the migration
ability of SW579 cells in HMGA1 knockdown group was significantly
lower than that in control and NC groups (Fig. 6A and B). In addition, we found that
lentivirus mediated knockdown of HMGA1 resulted in downregulation
of MMP-2, and upregulation of E-cadherin in SW579 cells (Fig. 6C).
HMGA1 correlates with MMP-2 expression in
thyroid carcinoma
To further indentify the relationship between HMGA1
and MMP2 in thyroid cancer, we examined the expression of HMGA1 and
MMP2 using a tissue microarray (TH8010a, US Biomax), consisting of
70 thyroid cancer cases and 10 normal cases. The
clinicopathological data are available in Table I. Statistical analysis showed that
the expression of HMGA1 in thyroid carcinoma was not significantly
correlated with the clinicopathological parameters of thyroid
carcinoma (Table I, P>0.05).
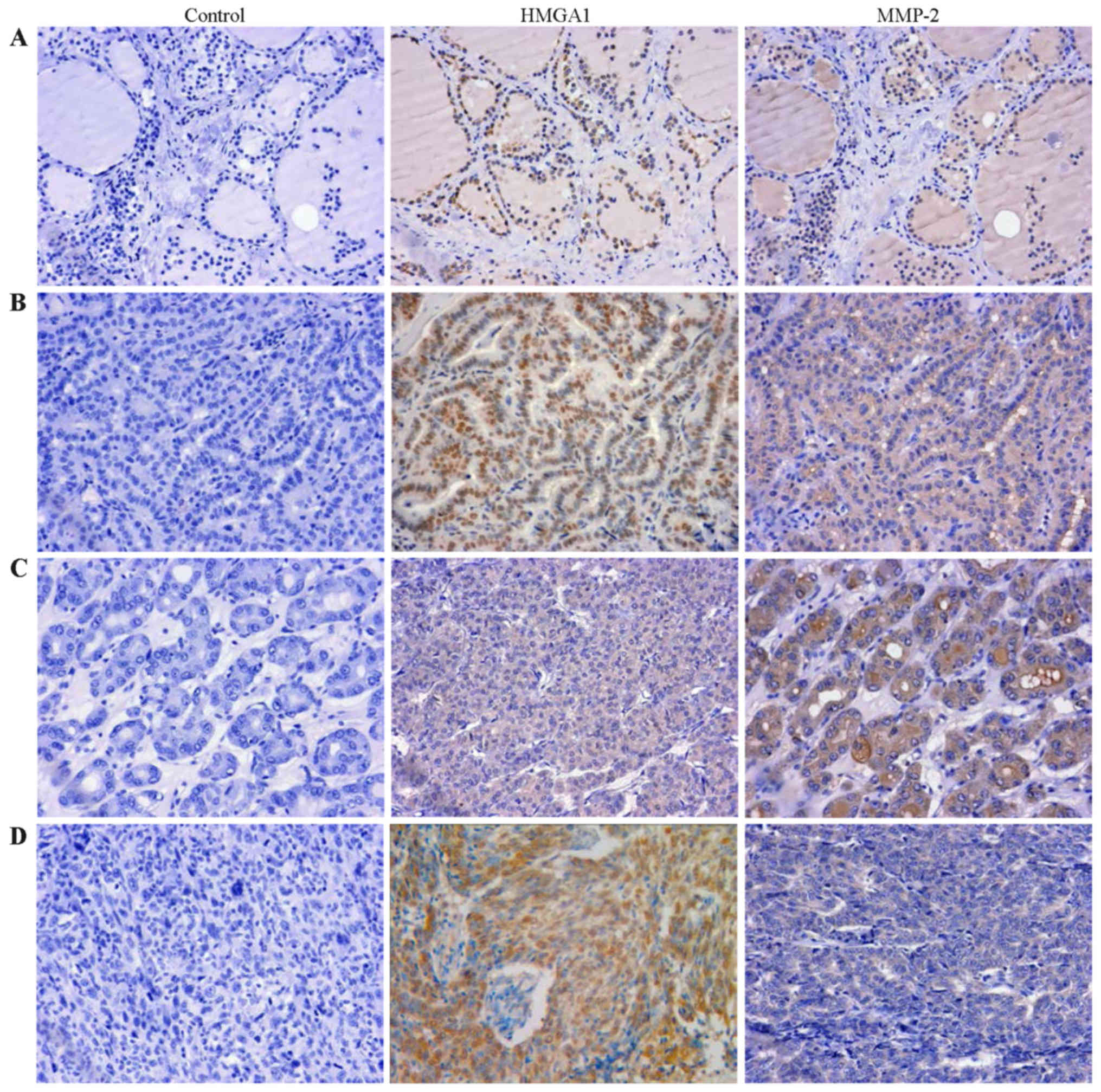
HMGA1 and MMP-2 immunostaining of thyroid carcinoma and normal
tissue of representative cases are shown in Fig. 7. The immunohistochemistry analysis
revealed that HMGA1 was stained in 40.0% in normal thyroid tissue
and 98.6% in thyroid tumors (P<0.001, Table II). MMP-2 expressed in 58.6% of
thyroid tumors, and completely loss in normal thyroid tissue
(P<0.001, Table II).
Furthermore, the expression of MMP-2 in papillary carcinoma was
also significantly higher than that in follicular carcinoma and
undifferentiated carcinoma (Table
I, P<0.001). In addition, expression of HMGA1 and MMP-2 was
found to be positively correlated in thyroid tumors (Table III, r=0.284, P=0.017).
 | Table ICorrelation of HMGA1 and MMP-2
expression with clinicopathological parameters in thyroid
cancer. |
Table I
Correlation of HMGA1 and MMP-2
expression with clinicopathological parameters in thyroid
cancer.
| n | HMGA1
| P-value | MMP-2
| P-value |
|---|
| − | + | ++ | − | + | ++ | +++ |
|---|
| Age (years) | | | | | 0.469 | | | | | 0.312 |
| >50 | 32 | 0 | 11 | 21 | | 12 | 5 | 10 | 5 | |
| ≤50 | 38 | 1 | 15 | 22 | | 17 | 7 | 12 | 2 | |
| Gender | | | | | 0.777 | | | | | 0.113 |
| Male | 14 | 0 | 5 | 9 | | 9 | 1 | 3 | 1 | |
| Female | 56 | 1 | 21 | 34 | | 20 | 11 | 19 | 6 | |
| Node
metastasis | | | | | 0.094 | | | | | 0.725 |
| Positive | 15 | 0 | 3 | 12 | | 6 | 4 | 4 | 1 | |
| Negative | 55 | 1 | 23 | 31 | | 23 | 8 | 18 | 6 | |
| Tumor size
(cm) | | | | | 0.940 | | | | | 0.117 |
| T2 | 31 | 0 | 11 | 20 | | 11 | 4 | 11 | 5 | |
| T3 | 23 | 1 | 10 | 12 | | 10 | 5 | 6 | 2 | |
| T4 | 16 | 0 | 5 | 11 | | 8 | 3 | 5 | 0 | |
| Tumor types | | | | | 0.574 | | | | | <0.001 |
| Papillary | 44 | 1 | 14 | 29 | | 11 | 8 | 19 | 6 | |
| Follicular | 20 | 0 | 11 | 9 | | 14 | 3 | 2 | 1 | |
|
Undifferentiated | 6 | 1 | 5 | 0 | | 4 | 1 | 1 | 0 | |
| Stage | | | | | 0.589 | | | | | 0.977 |
| I | 27 | 1 | 10 | 16 | | 13 | 6 | 8 | 0 | |
| II | 17 | 0 | 6 | 11 | | 2 | 2 | 9 | 4 | |
| III | 19 | 0 | 9 | 10 | | 10 | 2 | 4 | 3 | |
| IV | 7 | 0 | 1 | 6 | | 4 | 2 | 1 | 0 | |
 | Table IIExpressions of HMGA1 and MMP-2 in
thyroid normal tissue and cancer. |
Table II
Expressions of HMGA1 and MMP-2 in
thyroid normal tissue and cancer.
| n | HMGA1
| P-value | MMP-2
| P-value |
|---|
| Positive | Negative | Positive | Negative |
|---|
| Tissue types | | | | <0.001 | | | <0.001 |
| Normal | 10 | 4 | 6 | | 0 | 10 | |
| Tumor | 70 | 69 | 1 | | 41 | 29 | |
 | Table IIICorrelation of HMGA1 and MMP-2
expression in thyroid cancer. |
Table III
Correlation of HMGA1 and MMP-2
expression in thyroid cancer.
| n | HMGA1
| P-value | Spearman (r) |
|---|
| − | + | ++ |
|---|
| MMP 2 | | | | | 0.017 | 0.284 |
| − | 29 | 1 | 15 | 13 | | |
| + | 12 | 0 | 4 | 8 | | |
| ++ | 22 | 0 | 5 | 17 | | |
| +++ | 7 | 0 | 2 | 5 | | |
Discussion
HMGA1 plays a carcinogenic role in the initiation
and progression of different types of tumors. HMGA1 overexpression
promoted pancreatic adenocarcinoma cells Akt activation (31), and its knockdown inhibited breast
cancer cell proliferation and migration of immunodeficient mice
(32). HMGA1 was able to regulate
basal-like breast cancer EMT, and to promote breast cancer
metastasis (33). HMGA1 knockdown
increased p53 levels, restore normal stem cell properties of colon
cancer stem cells (34). HMGA1 is
associated with poor clinical outcome in patients with uveal
melanomas (35).
TGF-β1 is a ubiquitous cytokine exerting a necessary
role in many aspects of cellular behavior, including cell
proliferation, differentiation and apoptosis. TGF-β1 is a tumor
suppressor in the early phase of tumor and becomes a tumor
promoting factor during the late stages of cancer and embryonic
development (15–17). This study offers evidence of
association between TGF-β1 and HMGA1 in the development of thyroid
cancer, and TGF-β1 increased the level of HMGA1 mRNA and protein in
thyroid cancer SW579 cells. Since PI3K signaling and ERK signaling
have previously been described as two kinds of non-Smad signaling
pathways regulating the TGF-β1 signaling pathway (19), we aimed to assess their
involvements in the TGF-β1 induced HMGA1 expression. We found that
both of them participated in this process, whether PI3K signaling
and ERK signaling work in parallel or direct coordination with Smad
proteins in TGF-β1 induced HMGA1 expression remains to be further
clarified.
With lentivirus-mediated HMGA1 silencing, this study
revealed that HMGA1 knockdown could inhibit cellular oncogenic
properties of thyroid cancer cells in vitro. Furthermore,
the lentivirus-mediated HMGA1 downregulation decreased expression
of MMP-2, and increased E-cadherin expression in SW579 cells,
indicating the involvement of MMP-2 and E-cadherin in HMGA1 induced
cellular invasion. Our previous study also confirmed that the siRNA
mediated HMGA1 knockdown can decrease Snail expression, and
increase E-cadherin expression, resulting in the suppression of
thyroid cancer cell growth and invasion (30). It was reported that HMGA1
overexpression could promote the DNA-damage response, and stimulate
Akt activation in colon and thyroid anaplastic carcinoma (36). HMGA1 downregulation by siRNA
enhanced transcriptional activity of p53, and increased thyroid
cancer apoptosis (37). It was
also reported that HMGA1 proteins can inhibit Hand1 promoter
activity, and HMGA1 overexpression exerts an important role on
HAND1 silencing in differentiated thyroid carcinomas (38). In addition, HMGA1 silencing
decreased the expression of vimentin and Snail, increased the
expression of E-cadherin in triple-negative breast cancer
MDA-MB-231 cells (4). Moreover,
our previous study also found that TGF-β1 could upregulate HMGA1
expression through the PI3K/Akt pathway, and HMGA1 enhanced the
proliferation and migration ability in breast cancer cells
(39), consistent with the results
of this study. Those results suggest that HMGA1 may play a
necessary role in the progress of thyroid cancer and breast cancer,
and it could contribute to the understanding of the molecular basis
of solid cancer progression and invasion, opening new perspectives
in cancer management and precision therapy.
To further reveal the significance of HMGA1 in
clinical prognosis, we used the tissue microarray to detect the
expression of HMGA1 in thyroid carcinoma and normal thyroid
tissues. We observed that HMGA1 was expressed in thyroid carcinoma
more than that in normal thyroid tissues. However, we did not
obtain data for the association of HMGA1 expression with node
metastasis in this study. The expression level of MMP-2 in thyroid
carcinoma was significantly higher than that in normal thyroid
tissue. Moreover, MMP-2 expression in thyroid carcinoma is
associated with the tumor types, which was not reported previously
in other cancers. HMGA1 and MMP-2 are reported to be positively
correlated in a subgroups of carcinosarcomas (40), and HMGA1 drives transformation
through upregulation of MMP-2 in undifferentiated, large cell
carcinoma (41). HMGA1 also
upregulates expression of MMP-2 in prostate cancer (42). In addition, HMGA1 promotes cellular
invasiveness through PI3K/Akt dependent regulation of MMP-9
activity in pancreatic cancer (43), and induces MMP-13 expression in
breast cancer (44), indicating
that HMGA1-MMP axis plays an important role in a variety of human
cancers. The tissue microarray data revealed a positive correlation
between MMP-2 and HMGA1 expression (P=0.017), which was consistent
with cellular model results, suggesting that HMGA1 plays an
important role in regulating MMP-2 expression in human thyroid
cancer. Further clarification of underlying molecular mechanisms
will help to understand the role of HMGA1 in TGF-β1 signaling in
the progress of thyroid cancer.
In conclusion, the present study established the
first link between HMGA1 and TGF-β1 in the regulation of thyroid
cancer proliferation and invasion, and provides evidence for the
pivotal role of HMGA1 in the progression of thyroid cancer, rending
HMGA1 to be potential biological marker for the diagnosis of
thyroid cancer.
Abbreviations:
|
TGF-β1
|
transforming growth factor-β1
|
|
EMT
|
epithelial-mesenchymal transition
|
|
HMGA1
|
high mobility group A
|
|
PI3K
|
phosphoinositide 3-kinase
|
|
ERK
|
extracellular signal related
kinase
|
|
MMP-2
|
matrix metalloproteinase-2
|
|
DMSO
|
dimethyl sulfoxide
|
|
GFP
|
green fluorescent protein
|
Acknowledgments
This study was supported by projects from the
National Natural Science Foundation of China (grant nos. 31200573,
81172542 and 81472608), the Key project of Education Department of
Hunan Province (16A189), Natural Science Foundation of Hunan
Province (2016JJ4077) and Young Talents Program of the University
of South China.
References
|
1
|
Fusco A and Fedele M: Roles of HMGA
proteins in cancer. Nat Rev Cancer. 7:899–910. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Reeves R: Nuclear functions of the HMG
proteins. Biochim Biophys Acta. 1799:3–14. 2010. View Article : Google Scholar
|
|
3
|
Resar LM: The high mobility group A1 gene:
Transforming inflammatory signals into cancer? Cancer Res.
70:436–439. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Shah SN, Cope L, Poh W, Belton A, Roy S,
Talbot CC Jr, Sukumar S, Huso DL and Resar LM: HMGA1: A master
regulator of tumor progression in triple-negative breast cancer
cells. PLoS One. 8:e634192013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Abe N, Watanabe T, Masaki T, Mori T,
Sugiyama M, Uchimura H, Fujioka Y, Chiappetta G, Fusco A and Atomi
Y: Pancreatic duct cell carcinomas express high levels of high
mobility group I(Y) proteins. Cancer Res. 60:3117–3122.
2000.PubMed/NCBI
|
|
6
|
Meyer B, Loeschke S, Schultze A, Weigel T,
Sandkamp M, Goldmann T, Vollmer E and Bullerdiek J: HMGA2
overexpression in non-small cell lung cancer. Mol Carcinog.
46:503–511. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Masciullo V, Baldassarre G, Pentimalli F,
Berlingieri MT, Boccia A, Chiappetta G, Palazzo J, Manfioletti G,
Giancotti V, Viglietto G, et al: HMGA1 protein over-expression is a
frequent feature of epithelial ovarian carcinomas. Carcinogenesis.
24:1191–1198. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Belton A, Gabrovsky A, Bae YK, Reeves R,
Iacobuzio Donahue C, Huso DL and Resar LM: HMGA1 induces intestinal
polyposis in transgenic mice and drives tumor progression and stem
cell properties in colon cancer cells. PLoS One. 7:e300342012.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chiappetta G, Tallini G, De Biasio MC,
Manfioletti G, Martinez-Tello FJ, Pentimalli F, de Nigris F, Mastro
A, Botti G, Fedele M, et al: Detection of high mobility group I
HMGI(Y) protein in the diagnosis of thyroid tumors: HMGI(Y)
expression represents a potential diagnostic indicator of
carcinoma. Cancer Res. 58:4193–4198. 1998.PubMed/NCBI
|
|
10
|
Huang R, Huang D, Dai W and Yang F:
Overexpression of HMGA1 correlates with the malignant status and
prognosis of breast cancer. Mol Cell Biochem. 404:251–257. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Heldin CH, Landström M and Moustakas A:
Mechanism of TGF beta signaling to growth arrest, apoptosis, and
epithelial-mesenchymal transition. Curr Opin Cell Biol. 21:166–176.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ikushima H and Miyazono K: TGFbeta
signalling: A complex web in cancer progression. Nat Rev Cancer.
10:415–424. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Massagué J: TGFbeta in cancer. Cell.
134:215–230. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yang L, Pang Y and Moses HL: TGF beta and
immune cells: An important regulatory axis in the tumor
microenvironment and progression. Trends Immunol. 31:220–227. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Inman GJ: Switching TGFβ from a tumor
suppressor to a tumor promoter. Curr Opin Genet Dev. 21:93–99.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Joshi A and Cao D: TGF beta signaling,
tumor microenvironment and tumor progression: The butterfly effect.
Front Biosci (Landmark Ed). 15:180–194. 2010. View Article : Google Scholar
|
|
17
|
Meulmeester E and Ten Dijke P: The dynamic
roles of TGF-β in cancer. J Pathol. 223:205–218. 2011. View Article : Google Scholar
|
|
18
|
Smith AL, Robin TP and Ford HL: Molecular
pathways: Targeting the TGF-β pathway for cancer therapy. Clin
Cancer Res. 18:4514–4521. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Derynck R, Akhurst RJ and Balmain A:
TGF-beta signaling in tumor suppression and cancer progression. Nat
Genet. 29:117–129. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lenferink AE, Cantin C, Nantel A, Wang E,
Durocher Y, Banville M, Paul Roc B, Marcil A, Wilson MR and
O'Connor-McCourt MD: Transcriptome profiling of a TGF-beta-induced
epithelial-to-mesenchymal transition reveals extracellular
clusterin as a target for therapeutic antibodies. Oncogene.
29:831–844. 2010. View Article : Google Scholar
|
|
21
|
Vincent T, Neve EP, Johnson JR, Kukalev A,
Rojo F, Albanell J, Pietras K, Virtanen I, Philipson L, Leopold PL,
et al: A SNAIL1-SMAD3/4 transcriptional repressor complex promotes
TGF-beta mediated epithelial-mesenchymal transition. Nat Cell Biol.
11:943–950. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
22
|
Voulgari A and Pintzas A:
Epithelial-mesenchymal transition in cancer metastasis: Mechanisms,
markers and strategies to overcome drug resistance in the clinic.
Biochim Biophys Acta. 1796:75–90. 2009.PubMed/NCBI
|
|
23
|
Wendt MK, Allington TM and Schiemann WP:
Mechanisms of the epithelial-mesenchymal transition by TGF-beta.
Future Oncol. 5:1145–1168. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhong J, Cao RX, Zu XY, Hong T, Yang J,
Liu L, Xiao XH, Ding WJ, Zhao Q, Liu JH, et al: Identification and
characterization of novel spliced variants of PRMT2 in breast
carcinoma. FEBS J. 279:316–335. 2012. View Article : Google Scholar
|
|
25
|
Lois C, Hong EJ, Pease S, Brown EJ and
Baltimore D: Germline transmission and tissue-specific expression
of transgenes delivered by lentiviral vectors. Science.
295:868–872. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhong J, Cao RX, Liu JH, Liu YB, Wang J,
Liu LP, Chen YJ, Yang J, Zhang QH, Wu Y, et al: Nuclear loss of
protein arginine N-methyltransferase 2 in breast carcinoma is
associated with tumor grade and overexpression of cyclin D1
protein. Oncogene. 33:5546–5558. 2014. View Article : Google Scholar
|
|
27
|
Welch DR, Fabra A and Nakajima M:
Transforming growth factor beta stimulates mammary adenocarcinoma
cell invasion and metastatic potential. Proc Natl Acad Sci USA.
87:7678–7682. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Derynck R and Zhang YE: Smad-dependent and
Smad-independent pathways in TGF-beta family signalling. Nature.
425:577–584. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Moustakas A and Heldin CH: Non-Smad
TGF-beta signals. J Cell Sci. 118:3573–3584. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhong J, Liu C, Chen YJ, Zhang QH, Yang J,
Kang X, Chen SR, Wen GB, Zu XY and Cao RX: The association between
S100A13 and HMGA1 in the modulation of thyroid cancer proliferation
and invasion. J Transl Med. 14:802016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Liau SS, Jazag A, Ito K and Whang EE:
Overexpression of HMGA1 promotes anoikis resistance and
constitutive Akt activation in pancreatic adenocarcinoma cells. Br
J Cancer. 96:993–1000. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Di Cello F, Shin J, Harbom K and Brayton
C: Knockdown of HMGA1 inhibits human breast cancer cell growth and
metastasis in immunodeficient mice. Biochem Biophys Res Commun.
434:70–74. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Pegoraro S, Ros G, Piazza S, Sommaggio R,
Ciani Y, Rosato A, Sgarra R, Del Sal G and Manfioletti G: HMGA1
promotes metastatic processes in basal-like breast cancer
regulating EMT and stemness. Oncotarget. 4:1293–1308. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Puca F, Colamaio M, Federico A, Gemei M,
Tosti N, Bastos AU, Del Vecchio L, Pece S, Battista S and Fusco A:
HMGA1 silencing restores normal stem cell characteristics in colon
cancer stem cells by increasing p53 levels. Oncotarget.
5:3234–3245. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Qu Y, Wang Y, Ma J, Zhang Y, Meng N, Li H,
Wang Y and Wei W: Overexpression of high mobility group A1 protein
in human uveal melanomas: Implication for prognosis. PLoS One.
8:e687242013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
D'Angelo D, Mussnich P, Rosa R, Bianco R,
Tortora G and Fusco A: High mobility group A1 protein expression
reduces the sensitivity of colon and thyroid cancer cells to
antineoplastic drugs. BMC Cancer. 14:8512014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Frasca F, Rustighi A, Malaguarnera R,
Altamura S, Vigneri P, Del Sal G, Giancotti V, Pezzino V, Vigneri R
and Manfioletti G: HMGA1 inhibits the function of P53 family
members in thyroid cancer cells. Cancer Res. 66:2980–2989. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Martinez Hoyos J, Ferraro A, Sacchetti S,
Keller S, De Martino I, Borbone E, Pallante P, Fedele M, Montanaro
D, Esposito F, et al: HAND1 gene expression is negatively regulated
by the High Mobility Group A1 proteins and is drastically reduced
in human thyroid carcinomas. Oncogene. 28:876–885. 2009. View Article : Google Scholar
|
|
39
|
Zu X, Zhong J, Tan J, Tan L, Yang D, Zhang
Q, Ding W, Liu W, Wen G, Liu J, et al: TGF-β1 induces HMGA1
expression in human breast cancer cells: Implications of the
involvement of HMGA1 in TGF-β signaling. Int J Mol Med. 35:693–701.
2015.PubMed/NCBI
|
|
40
|
Hillion J, Roy S, Heydarian M, Cope L,
Xian L, Koo M, Luo LZ, Kellyn K, Ronnett BM, Huso T, et al: The
high mobility group A1 (HMGA1) gene is highly overexpressed in
human uterine serous carcinomas and carcinosarcomas and drives
matrix metalloproteinase-2 (MMP-2) in a subset of tumors. Gynecol
Oncol. 141:580–587. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Hillion J, Wood LJ, Mukherjee M,
Bhattacharya R, Di Cello F, Kowalski J, Elbahloul O, Segal J,
Poirier J, Rudin CM, et al: Upregulation of MMP-2 by HMGA1 promotes
transformation in undifferentiated, large cell lung cancer. Mol
Cancer Res. 7:1803–1812. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Takaha N, Resar LM, Vindivich D and Coffey
DS: High mobility group protein HMGI(Y) enhances tumor cell growth,
invasion, and matrix metalloproteinase-2 expression in prostate
cancer cells. Prostate. 60:160–167. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Liau SS, Jazag A and Whang EE: HMGA1 is a
determinant of cellular invasiveness and in vivo metastatic
potential in pancreatic adenocarcinoma. Cancer Res. 66:11613–11622.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Reeves R, Edberg DD and Li Y:
Architectural transcription factor HMGI(Y) promotes tumor
progression and mesenchymal transition of human epithelial cells.
Mol Cell Biol. 21:575–594. 2001. View Article : Google Scholar : PubMed/NCBI
|