Introduction
The cancer stem cells (CSCs) are a rare population
of tumor cells and enable them to simultaneously self-perpetuate
with a consistently maintained CSC subpopulation and to generate
differentiated progeny via asymmetrical cell division, giving rise
to heterogenic tumors (1–3). CSCs are relatively quiescent and have
the properties necessary for tumor initiation, resistance to
therapy, and progression (4–7). The
CSC population expands during periods of stress, such as radiation
(8,9), chemotherapy (5), and castration (10), which are likely the initiating
cells of chemoresistant cancer relapses and metastases. Based on
CSC markers, a number of CSC populations have been identified. For
instance, the side population (SP) cells, which express high levels
of ABCG2 and ABCB1 multi-drug efflux pumps that may contribute to
chemoresistance, possess the stem cell-like properties (2,3,11).
The aldehyde dehydrogenases (ALDH) are cytosolic isoenzymes
responsible for oxidizing intracellular aldehydes. The ALDH1-high
cell subpopulations show distinct stem-like characteristics and are
highly resistant to chemotherapeutic agents commonly used as
first-line therapy, such as cisplatin and docetaxel (2,3). The
current anticancer therapies fail to destroy, but tend to favor the
selection and expansion of resistant CSCs in tumors, resulting in
poor responses and outcomes. The elimination of CSCs is of utmost
importance at the time of therapeutic intervention in order to
prevent CSC expansion and subsequent tumor recurrence, relapse, and
metastasis.
Secretory phospholipase A2 group IIa (sPLA2-IIa) is
distributed in trace amounts in a variety of normal mammalian
tissues, but found at high levels in various inflamed tissues and
some cancers. sPLA2-IIa, a NF-κB target gene (12,13),
has traditionally been associated with their enzymatic activity and
participates in biosynthesis of potent biologically active lipid
mediators, particularly arachidonic acid-derived eicosanoids, which
promote inflammation, angiogenesis, and tumorigenesis (14,15).
Independent of its enzymatic activity, sPLA2-IIa can also serve as
a ligand for cell membrane receptors and stimulate integrin
activation, COX-2 expression, and secretion of cytokines (16–18).
sPLA2-IIa binds to integrin avβ3 at KD of 0.2 mM, and
induces cell proliferation (16).
sPLA2-IIa also weakly interacts with M-type receptor in human cells
and induces pro-inflammatory signaling (15,19,20).
sPLA2-IIa is associated with the pathology of
several types of malignancies, including cancers of the colon,
breast, stomach, esophagus, ovary, lung, and prostate (21,22).
sPLA2-IIa is overexpressed in almost all human prostate cancer
tissues and elevated levels are associated with advanced tumor
grades (13,23–26).
sPLA2-IIa remains elevated in androgen-independent prostate cancers
(27) and is significantly
increased in metastatic lesions (28). We are the first to demonstrate that
cancer cells overexpress and secrete sPLA2-IIa into the
interstitial fluids, i.e., tumor microenvironment and blood, in
patients with both prostate and lung cancers (13,29,30).
High levels of plasma sPLA2-IIa are associated with poor prognosis,
advanced cancer stage, and short cancer survival. We confirmed that
tumors secret sPLA2-IIa into the circulation to a detectable level
in the mouse model of human cancer (29). The most recent report by others
supports our finding in that high levels of plasma sPLA2-IIa are
associated with poor prognosis of cancers (31). More importantly, we revealed that
elevated HER/ERBB-PI3K-Akt-NF-κB signaling induces sPLA2-IIa
overexpression and secretion in both lung and prostate cancer
cells; in turn, sPLA2-IIa stimulates its overexpression via
HER/ERBB-elicited signaling in the positive feedback regulation
manner (26,32). sPLA2-IIa induces phosphorylation of
HER2 and HER3 in a dose-dependent manner in NSCSC A549 and H1975
cells (32). However, the
underlying molecular mechanisms of sPLA2-IIa at an aberrant high
level in the tumor microenvironment in stimulating cancer
progression and metastasis remains to be elucidated.
The HER/ERBB-elicited signaling is essential for
cell growth and survival. Recent studies further demonstrate that
this signaling pathway is of critical importance in supporting CSC
properties (33–35). We determined a role of sPLA2-IIa in
CSCs and revealed that sPLA2-IIa is overexpressed in both side
population (SP) CSCs from NSCSC cells and ALDH1-high CSCs from CRPC
cells. Furthermore, sPLA2-IIa directly interacts with and activates
EGFR family receptors, suggesting that sPLA2-IIa is a ligand for
both EGFR and HER3. These findings, together with our previous data
(13,29,30,32),
support the notion that high levels of sPLA2-IIa in the tumor
micro-environment support CSC phenotype via HER/ERBB-elicited
signaling, and sPLA2-IIa is a novel therapeutic target against
cancer and CSCs.
Materials and methods
Reagents
RPMI-1640 medium was purchased from Invitrogen
(Gaithersburg, MD, USA). Fetal bovine serum (FBS) and
charcoal/dextran-treated FBS were purchased from Hyclone
Laboratories (Logan, UT, USA). sPLA2-IIa antibodies were obtained
from Life Span BioSciences (Seattle, WA, USA) and Cayman Chemical
(Ann Arbor, MI, USA). Recombinant human sPLA2-IIa was obtained from
BioVendor (Candler, NC, USA). EGFR, HER2, and HER3 antibodies were
from Cell Signaling Technology (Danvers, MA, USA) and Santa Cruz
Biotechnology (Santa Cruz, CA, USA). RT-PCR primers were customly
synthesized by Genscript (Piscataway, NJ, USA). Plasmid
sPLA2-IIa(−800)-Luc was constructed as we described previously
(13).
Cell culture
The human prostate adenocarcinoma cell lines LNCaP
and 22Rv1, and lung cancer adenocarcinoma H1975 were obtained from
ATCC (Rockville, MD, USA) and maintained in RPMI-1640 medium
supplemented with 10% FBS (complete medium) at 37°C in 5%
CO2. LNCaP-AI cells were generated by us previously
(36) and maintained in RPMI-1640
medium supplemented with 10% charcoal/dextran-treated FBS (stripped
medium). Lung cancer adenocarcinoma A549 cells were obtained from
ATCC and maintained in MEM medium supplemented with 5% FBS
(complete medium) at 37°C in 5% CO2.
3D cell culture
A 96-well plate was coated with 100 µl/well
of medium containing 0.9% agarose. SP CSCs and non-SP control cells
in medium with 10% FBS on ice were mixed with a same volume of cold
(4°C) medium containing 10% FBS and 10% growth factor-reduced
Matrigel matrix (BD Biosciences, Bedford, MA, USA) and seeded into
the agarose plate at 100 µl/well. The cells were cultured at
37°C for 14 days.
CSC sorting
The ALDH1-high CSCs from 22Rv1 cells was isolated
using the Aldefluor assay kit (StemCell Technologies, Vancouver,
Canada) and BD FACSAria II cell sorters. ALDH1 cleaves
boron-dipyrromethene-aminoacetaldehyde (BAAA) to release
fluorescent dye, which can be blocked by diethylaminobenzaldehyde
(DEAB). After gating with DEAB control, the fluorescent cells were
sorted out as ALDH1-high CSCs.
SP CSCs were isolated as previously described
(37). Briefly, A549 and H1975
cells were resuspended at 1×106/ml in prewarmed DMEM
with 2% FCS and 10 mmol/l HEPES buffer. Hoechst 33342 dye was added
at a final concentration of 5 µg/ml in the absence or
presence of reserpine (50 µmol/l; Sigma) and the cells were
incubated at 37°C for 90 min with intermittent shaking. At the end
of the incubation, the cells were washed with ice-cold HBSS with 2%
FCS and 10 mmol/l HEPES, centrifuged down at 4°C, and resuspended
in ice-cold HBSS containing 2% FCS and 10 mmol/l HEPES. Propidium
iodide at a final concentration of 2 µg/ml was added to gate
viable cells. Gated by control cells treated with reserpine that
blocks Hoechst 33342 transporter, SP CSCs were sorted using BD
FACSAria II cell sorters. SP CSCs were defined as the missing
region in the presence of reserpine.
Tumorigenesis in mice
One thousand of CSCs or control cells in 50
µl PBS were mixed with 50 µl of cold Matrigel (Thermo
Fisher Scientific) and inoculated subcutaneously into nude mice.
Tumor incidence and sizes were measured using calipers and recorded
twice a week. The mice were maintained in a facility approved by
the American Association for Accreditation of Laboratory Animal
Care (AAALAC) and in accordance with current regulations and
standards of the U.S. Department of Agriculture, U.S. Department of
Health and Human Services, and NIH. The animal studies were
approved by the Institutional Animal Care and Use Committee (IACUC)
and executed according to IACUC guidelines.
RT-PCR
RNAs from both CSCs and control cells were isolated
using RNeasy Plus Universal Mini kit (Qiagen, Germany). RNA samples
were then treated with DNase using DNA-free™ kit (Thermo Fisher
Scientific) and subjected to reverse transcription reactions using
High Capacity cDNA Reverse Transcription kits (Applied Biosystems,
Thermo Fisher Scientific). Real-time RT-PCR was performed using the
Fast SYBR® Green Master Mix and 7300 Real-Time PCR
system (Applied Biosystems, Thermo Fisher Scientific).
Co-immunoprecipitation assay
Cell extracts from LNCaP-AI cells were prepared.
Co-immunoprecipitation was performed in a modified RIPA buffer
(PBS, 0.1% NP-40, 0.1% sodium deoxycholate, 150 mM NaCl, 1 mM EDTA,
1 mM dithiothreitol, 1 mM phenylmethylsulfonyl fluoride, and 1X
protease inhibitor cocktail). Antibody was first incubated with 1
mg of cell extract at 4°C on Rocker platform for 1–2 h of Protein G
plus/protein A agarose beads (50 µl) (Calbiochem, Thermo
Fisher Scientific) was then added, and the samples were incubated
at 4°C on Rocker platform overnight. The beads were washed with the
modified RIPA buffer four times and boiled in SDS loading buffer.
The protein samples were subjected to SDS-PAGE and western blot
analysis.
Western blot analysis
Western blot analysis was performed as previously
described (13). Briefly, aliquots
of samples with the same amount of protein, determined using the DC
Protein assay kit (Bio-Rad, Hercules, CA, USA), were mixed with
loading buffer (final concentrations of 62.5 mM Tris-HCl, pH 6.8,
2.3% SDS, 100 mM dithiothreitol, and 0.005% bromophenol blue),
boiled, fractionated in an SDS-PAGE, and transferred onto a 0.45-mm
nitrocellulose membrane (Bio-Rad). The membranes were blocked with
2% fat-free milk in PBS, and probed with first antibody in PBS
containing 0.01% Tween-20 (PBST) and 1% fat-free milk. The
membranes were then washed four times in PBS and incubated with
IRDye 800CW secondary antibody (LI-COR Biosciences, Lincoln, NE,
USA) in PBST containing 1% fat-free milk for 30 min. After washing
four times in PBS, the membranes were visualized using Odyssey
imaging system (LI-COR).
Reporter assay
Cells (105/well) were seeded in 12-well
tissue culture plates. The next day, Lipofectamine 3000 reagent was
used for the transient transfection assay according to the protocol
provided by Invitrogen/Life Technologies, Inc. The cells were then
treated for 24 h. Subsequently, the cell extracts were prepared and
luciferase activity was assessed in a Berthold Detection system
(Titertek-Berthold, Pforzheim, Germany) using a Luciferase assay
kit (Promega, Madison, WI, USA) according to the manufacturer's
instructions. For each assay, cell extract (20 µl) was used
and the reaction was started by injection of 50 µl of
luciferase substrate. Each reaction was measured for 10 sec in the
luminometer. Luciferase activity was defined as light units/mg
protein.
Results
sPLA2-IIa stimulates HER/ERBB-elicited
signaling in cancer cells
We reported previously that treatment of NSCLC A549
and H1975 cells with recombinant human sPLA2-IIa induces
phosphorylation of HER2 and HER3 within 2 h in a dose-dependent
manner (32). The reporter assay
revealed that sPLA2-IIa enhances the promoter activities of NF-κB
and sPLA2-IIa genes in a dose-dependent manner in lung cancer
cells. For validation, we determined the effects of sPLA2-IIa in
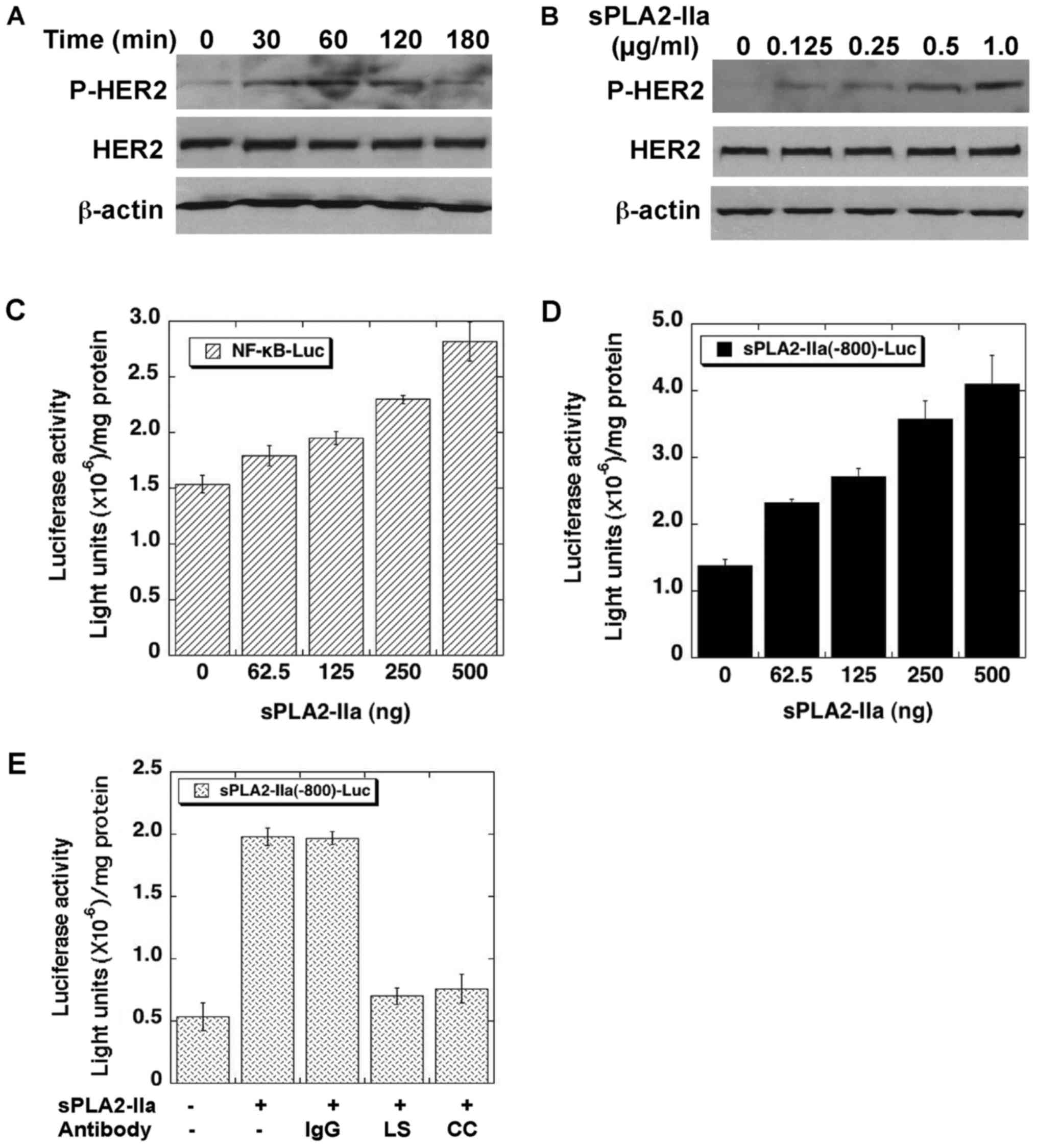
prostate cancer cells. As shown in Fig. 1A and B, recombinant human sPLA2-IIa
induces phosphorylation of HER2 within 2 h in a dose- and
time-dependent manner in CRPC LNCaP-AI cells (36). sPLA2-IIa also enhances the promoter
activities of NF-κB (Fig. 1C) and
sPLA2-IIa (Fig. 1D) genes in a
dose-dependent manner in the cells. The stimulatory effects of
sPLA2-IIa on sPLA2-IIa promoter activity were abolished by
antibodies against sPLA2-IIa (Fig.
1E). These data implicated that sPLA2-IIa enhances
HER/ERBB-elicited signaling in prostate cancer cells.
sPLA2-IIa functions as a ligand for EGFR
family receptors
A structural analysis we performed previously
suggests that the sPLA2-IIa β hairpin shares significant similarity
with the EGF hairpin and could be displaced to provide additional
contacts with EGFR, and protein docking computation shows that
sPLA2-IIa directly interacts with the extracellular domain (ECD) of
EGFR in such a way as to stabilize EGFR in its active conformation
(32). For validation of the
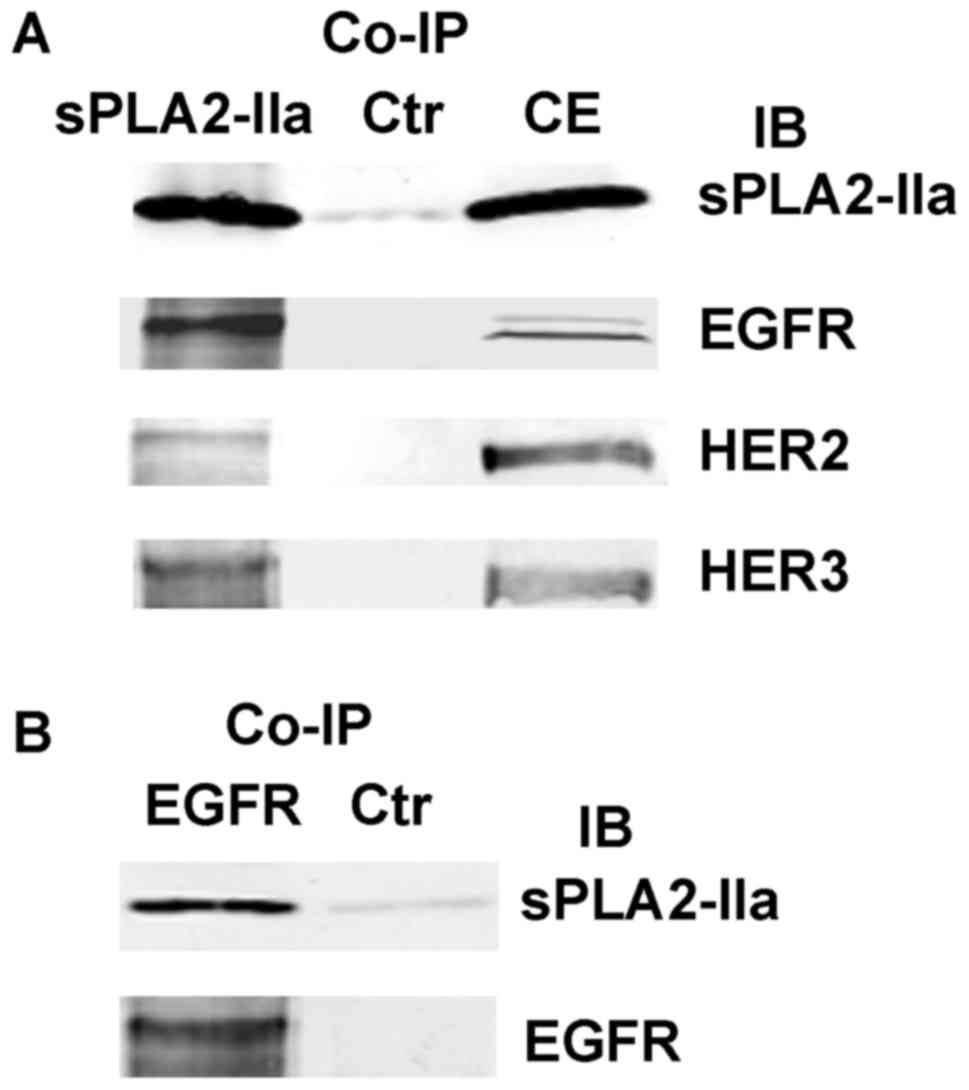
interactions of sPLA2-IIa with EGFR family receptors,
co-immunoprecipitation (co-IP) was performed using cell extracts
from LNCaP-AI cells. As shown in Fig.
2A, EGFR, HER2, and HER3 were detected in the co-IP complex
pulled down by an antibody to sPLA2-IIa. Reciprocally, sPLA2-IIa
was detected in the co-IP complexes pulled down by an antibody to
EGFR (Fig. 2B). These data reveal
that sPLA2-IIa activates EGFR family receptors and complexes with
EGFR, HER2, and HER3, strongly suggesting that sPLA2-IIa is a novel
ligand for EGFR and HER3 (32).
Overexpression of sPLA2-IIa in CSCs
SP CSCs express high levels of ABCG2 and/or ABCB1
multidrug efflux pump proteins and possess stem cell-like
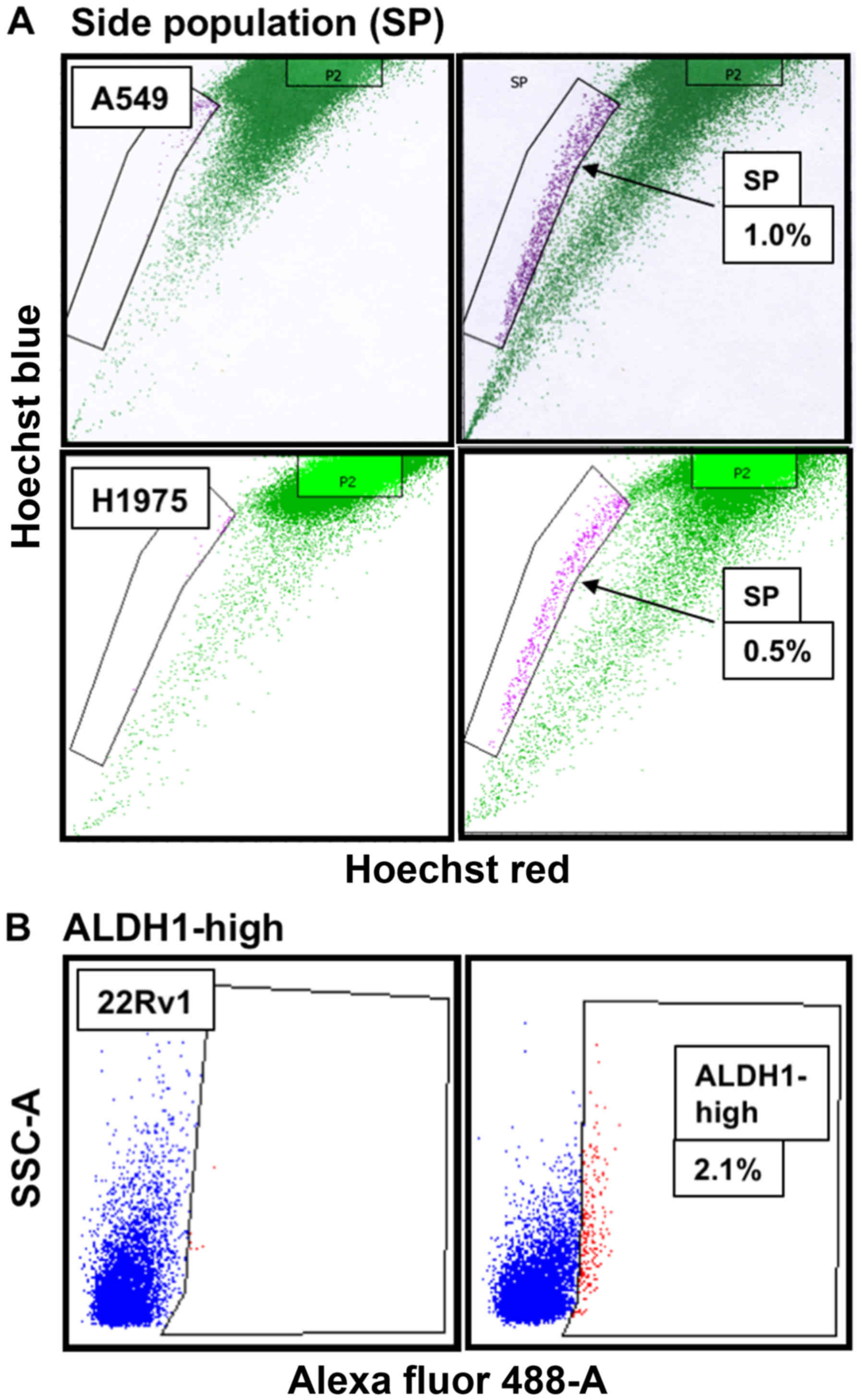
properties (37). We isolated SP
CSCs from NSCLC cells using flow cytometry and Hoechst 33342 dye
efflux assay (37) and found ~1%
of SP CSCs in A549 cells and 0.5% of SP CSCs in H1975 cells
(Fig. 3A). ALDH1 is a universal
functional marker for CSCs (38–44)
and is involved in cellular responses to oxidative stress (42) and drug resistance (41). Overexpression of ALDH1 correlates
with poor prognosis in prostate cancer (38). ALDH1-based approach has been used
to successfully isolate CSCs from many cell lines of diverse cancer
types (45,46), including CRPC 22Rv1 and PC-3 cells
(39,44). Consistent with data reported in the
literature (44), we found ~2.1%
of ALDH1-high CSCs in 22Rv1 cells (Fig. 3B).
The real-time RT-PCR was performed to determine
expression of CSC marker genes in the CSCs. As shown in (Tables I and II), the CSC markers, including ABCG2,
ALDH1, CD133, CD44, CD56, Focx2, MDR1, Nanog, NKX3.1, NSE, Slug,
Snail, Sox2, and Twist (2,3,47),
are heterogeneously overexpressed in SP CSCs relative to non-SP
control cells or in ALDH1-high CSCs relative to ALDH1-low control
cells. In addition, HER2, HER3, or AR gene is also moderately
increased in these CSCs. Interestingly, sPLA2-IIa levels in SP and
ALDH1-high CSCs are elevated 41-fold (H1975), 4.3-fold (A549), and
6-fold (22Rv1), respectively, suggesting that sPLA2-IIa is a novel
CSC marker and supports CSC properties (Table II).
 | Table IPrimers used for RT-PCR. |
Table I
Primers used for RT-PCR.
| ABCG2 | Upper primer |
5′-AAACCTGGTCTCAACGCCATCC-3′ |
| Lower primer |
5′-TGCCCATCACAACATCATCTTG-3′ |
| ALDH1 | Upper primer |
5′-CTTACCTGTCCTACTCACCGATTTG-3′ |
| Lower primer |
5′-CCTTGTCAACATCCTCCTTATCTCC-3′ |
| AR | Upper primer |
5′-GTCTTCGGAAATGTTATGAAGCA-3′ |
| Lower primer |
5′-ACGATCGAGTTCCTTGATGTAG-3′ |
| CD133 | Upper primer |
5′-TGAGACCCAAGACTCCCATAAAGC-3′ |
| Lower primer |
5′-GGACACAGCATAGAATAATCCCTGC-3′ |
| CD44 | Upper primer |
5′-CGTGGAGAAAAATGGTCGCTAC-3′ |
| Lower primer |
5′-TACTGGGAGGTGTTGGATGTGAGG-3′ |
| CD56 | Upper primer |
5′-CGGCATTTACAAGTGTGTGG-3′ |
| Lower primer |
5′-GACATCTCGGCCTTTGTGTT-3′ |
| Focx2 | Upper primer |
5′-AGAATTACTACCGGGCTGCG-3′ |
| Lower primer |
5′-TGAGCGCGATGTAGCTGTAG-3′ |
| HER2 | Upper primer |
5′-AATGGAGACCCGCTGAACAATAC-3′ |
| Lower primer |
5′-CACAAAATCGTGTCCTGGTAGCAG-3′ |
| HER3 | Upper primer |
5′-TACGAGAGGTGTGAGGTGGTGATG-3′ |
| Lower primer |
5′-GGAGGTTGGGCAATGGTAGAGTAG-3′ |
| MDR1 | Upper primer |
5′-TGGGAAGAGCACAACAGTCCAG-3 |
| Lower primer |
5′-CGTGGTGGCAAACAATACAGGTTC-3′ |
| Nanog | Upper primer |
5′-CCAGTCCCAAAGGCAAACAAC-3′ |
| Lower primer |
5′-TGGAGGCTGAGGTATTTCTGTCTC-3′ |
| NKX3.1 | Upper primer |
5′-AAAGGCACTTGGGGTCTTATCTG-3′ |
| Lower primer |
5′-CTTCTGATGGCTGAACTTCCTCTC-3′ |
| NSE | Upper primer |
5′-GTCCCACGTGTCTTCCACTT-3′ |
| Lower primer |
5′-CCCAAGTCAGGCCAGTTTTA-3′ |
| Slug | Upper primer |
5′-GAGCATTTGCAGACAGGTCA-3′ |
| Lower primer |
5′-GCTTCGGAGTGAAGAAATGC-3′ |
| Snail | Upper primer |
5′-ACCCCACATCCTTCTCACTG-3′ |
| Lower primer |
5′-TACAAAAACCCACGCAGACA-3′ |
| SOX2 | Upper primer |
5′-GCACCGCTACGACGTGA-3′ |
| Lower primer |
5′-TGCGAGTAGGACATGCTGTAGG-3′ |
| sPLA2-IIa | Upper primer |
5′-TTGACGACAGGAAAGGAAGCCG-3′ |
| Lower primer |
5′-TCTGCTCCCCGAGTTGCTAAAC-3′ |
| Twist | Upper primer |
5′-GGAGTCCGCAGTCTTACGAG-3′ |
| Lower primer |
5′-CCAGCTTGAGGGTCTGAATC-3′ |
| Vimentin | Upper primer |
5′-AATCCAAGTTTGCTGACCTCTCTG-3′ |
| Lower primer |
5′-CTCTTCCATTTCACGCATCTGG-3′ |
 | Table IICSCs express a high level of
sPLA2-IIa. |
Table II
CSCs express a high level of
sPLA2-IIa.
| H1975 cells | None-SP
control | SP CSCs | Fold increase |
|---|
| ABCG2 | 2.26E-04 | 3.05E-03 | 13.53 |
| ALDH1 | 2.01E-06 | 4.00E-05 | 19.92 |
| CD133 | 1.01E-06 | 4.53E-02 | 45033.82 |
| Focx2 | 4.95E-05 | 2.46E-04 | 4.97 |
| HER3 | 1.20E-04 | 5.07E-04 | 4.21 |
| MDR1 | 3.43E-07 | 7.19–05 | 209.32 |
| Nanog | 4.59E-05 | 7.80E-05 | 1.7 |
| NKX3.1 | 4.31E-03 | 2.83E-02 | 6.57 |
| Slug | 1.26E-02 | 6.91E-02 | 5.5 |
| Snail | 5.60E-02 | 1.83E-01 | 3.27 |
|
sPLA2-IIa | 7.66E-08 | 3.15E-06 | 41.1 |
| Twist | 7.66E-08 | 3.33E-05 | 435.24 |
| A549 cells | | | |
| SOX2 | 5.05E-04 | 2.22E-03 | 4.39 |
|
sPLA2-IIa | 1.83E-04 | 7.91E-04 | 4.31 |
| Twist | 3.64E-04 | 1.15E-03 | 3.16 |
|
| 22RV1 cells | ALDH1-low
control | ALDH1-high
CSCs | Fold increase |
|
| ALDH1 | 2.80E-04 | 1.81E-02 | 64.63 |
| AR | 3.72E-05 | 9.44E-05 | 2.54 |
| CD133 | 1.26E-07 | 3.56E-05 | 283.24 |
| CD44 | 3.40E-03 | 3.11E-02 | 9.16 |
| CD56 | 5.99E-06 | 2.33E-05 | 3.89 |
| Focx2 | 5.17E-05 | 1.79E-04 | 3.47 |
| HER2 | 1.01E-02 | 1.87E-02 | 1.85 |
| HER3 | 1.08E-02 | 2.95E-02 | 1.56 |
| Nanog | 4.77E-04 | 7.85E-04 | 1.6 |
| NSE | 1.24E-02 | 8.63E-02 | 6.44 |
| Slug | 3.31E-06 | 1.09E-05 | 2.25 |
| Snail | 2.19E-04 | 2.23E-03 | 10.2 |
| SOX2 | 1.93E-05 | 6.20E-05 | 3.21 |
|
sPLA2-IIa | 3.93E-06 | 2.34E-05 | 5.95 |
Characterization of CSCs in 3D cell
culture and in mice
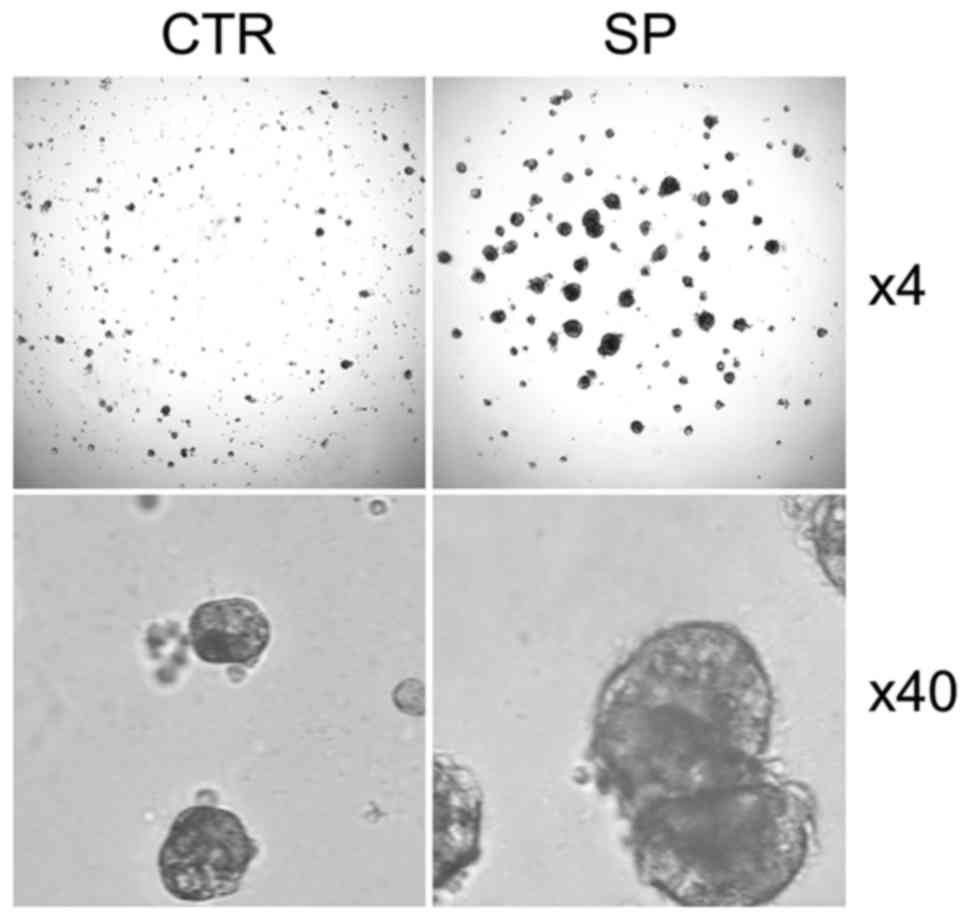
We performed 3D cell culture to validate CSCs
properties and found that SP CSCs from A549 cells generate more and
much larger spheroids than those by non-SP control cells (Fig. 4). Similar observation was also made
in ALDH1-high CSCs from 22RV1 cells and SP CSCs from H1975 cells
(data not shown).
To further characterize the tumor initiating
properties of CSCs in mice, a subcutaneous inoculation of 1,000
cells/mouse of SP CSCs, but not non-SP control cells, into nude
mice produced visible tumors on day 20. The tumors reached ~100
mm3 on day 30. A subcutaneous inoculation of 1,000
cells/mouse of ALDH1-high CSCs, but not ALDH1-low control cells,
into nude mice produced tumors on day 25. The tumors reached ~100
mm3 on day 35.
Overexpression of CSC markers in CRPC
LNCaP-AI cells and taxane-resistant 22Rv1-R cells
CRPC LNCaP-AI cells were established by culturing
androgen-dependent LNCaP cells in the stripped medium as we
described previously (36). CRPC
22Rv1 cells express constitutively active androgen receptor
(48,49). We cultured 22Rv1 cells in the
presence of increasing concentrations of paclitaxel to derive
22Rv1-R cells. As shown in Table
III, 22Rv1-R cells are resistant to all taxenes.
 | Table IIIEstablishment of taxane-resistant
22Rv1-R cell line. |
Table III
Establishment of taxane-resistant
22Rv1-R cell line.
| Gene | pTx | dTx | cTx |
|---|
| 22Rv1 | 2 | 1 | 1 |
| 22Rv1-R | 327 | 305 | 15 |
| R index | 164 | 305 | 15 |
We found that CSC markers, including ABCG2, ALDH1,
CD44, CD56, Focx2, MDR1, Nanog, NSE, Snail, Sox2, and Vimentin,
were heterogeneously overexpressed in LNCaP-AI cells relative to
parental LNCaP cells (Table IV)
and in taxane-resistant 22Rv1-R cells relative to parental 22Rv1
cells (Table V). Strikingly, MDR1
level is increased by 1,380-fold in 22Rv1-R cells and 66-fold in
LNCaP-AI cells. The expression of sPLA2-IIa in LNCaP-AI cells
relative to LNCaP cells is increased 60-fold (Table IV), confirming the finding
reported in our previous study (13). sPLA2-IIa is not overexpressed in
22Rv1-R cells relative to 22Rv1 cells, but is overexpressed in CSCs
from 22Rv1 cells (Table II),
indicating the heterogeneity of gene expression in cancer cells.
These findings suggest that CSC population is elevated in LNCaP-AI
and 22Rv1-R cells, supporting the notion that the current
anticancer therapies favor the selection and expansion of CSCs.
 | Table IVOverexpression of CSC markers in CRPC
LNCaP-AI cells. |
Table IV
Overexpression of CSC markers in CRPC
LNCaP-AI cells.
| Gene | LNCaP cells | LNCaP-AI cells | Fold increase |
|---|
| ALDH1 | 5.60E-05 | 2.77E-04 | 4.94 |
| CD44 | 8.96E-05 | 1.19E-04 | 2.44 |
| CD56 | 1.20E-06 | 8.10E-05 | 67.74 |
| MDR1 | 4.49E-06 | 2.97E-04 | 66.2 |
| Nanog | 2.95E-04 | 5.54E-04 | 1.88 |
| NSE | 2.64E-03 | 8.11E-03 | 3.07 |
| SOX2 | 2.40E-07 | 1.26E-06 | 8 |
|
sPLA2-IIa | 9.19E-03 | 5.50E-01 | 59.9 |
| Vimentin | 2.35E-03 | 8.55E-02 | 36.4 |
 | Table VOverexpression of CSC markers in
taxane-resistant CRPC 22RV1-R cells. |
Table V
Overexpression of CSC markers in
taxane-resistant CRPC 22RV1-R cells.
| Gene | 22RV1 cells | 22RV1-R cells | Fold increase |
|---|
| ABCG2 | 1.98E-03 | 1.17E-02 | 5.89 |
| ALDH1 | 1.35E-03 | 5.63E-03 | 4.18 |
| CD44 | 2.30E-03 | 6.56E-03 | 2.85 |
| Focx2 | 6.70E-06 | 3.29E-05 | 4.91 |
| MDR1 | 1.56E-04 | 2.15E-01 | 1,380.42 |
| Nanog | 3.02E-04 | 1.03E-03 | 3.4 |
| NSE | 3.19E-02 | 9.42E-02 | 3 |
| Snail | 5.56E-04 | 3.80E-03 | 9.9 |
| SOX2 | 1.04E-04 | 2.52E-04 | 2.57 |
Discussion
We demonstrated that cancer cells overexpress and
secrete sPLA2-IIa into the interstitial fluid, i.e., tumor
microenvironment and blood (13,29,30).
Plasma sPLA2-IIa continuously increased with prostate cancer
progression and reached as high as 18 ng/ml at the late stage in
metastatic prostate cancer (29).
High levels of plasma sPLA2-IIa, based on the optimum cutoff value
of 2.0 ng/ml, significantly predicted advanced stage and high
Gleason score in prostate cancer (13,29).
We further showed that sPLA2-IIa is overexpressed in almost all
lung cancers and is significantly elevated in the blood of lung
cancer patients (30). High levels
of plasma sPLA2-IIa, at the optimum cutoff value of 2.4 ng/ml, are
significantly associated with advanced lung cancer stage and
decreased overall cancer survival (30). The most recent report by others
supports our finding in that high levels of plasma sPLA2-IIa are
associated with poor prognosis of cancers (31).
The ultimate cause of cancer treatment failure is
that tumor cells evolve and develop multiple mechanisms to escape
the cytotoxic effects of anticancer drugs, including enhancement of
cell survival pathway, impaired apoptotic machinery, increased DNA
repair mechanisms, and multidrug resistance phenotype by
overexpression of drug-efflux pump proteins P-glycoprotein (MDR1)
and ABCG-2 (50–52). These mechanisms may also drive CSC
properties and tumor progression. ATP-binding cassette (ABC)
transporters, such as P-glycoprotein (MDR1), multidrug resistant
associated protein (MRP1), and ATP-binding cassette membrane
transporter G2 [ABCG2, also known as breast cancer resistance
protein 1 (BCRP1)], are membrane transporters that can pump
cytotoxic chemotherapeutic drugs out of the cell. CSCs express high
levels of ABC transporters leading to low intracellular drug
concentrations and conferring multidrug resistance to many
anticancer drugs. The efflux capacity of SP CSCs, determined by
high ABCG2 activity, is associated with tumor growth, progression,
and metastasis. The aldehyde dehydrogenase (ALDH) family members
are cytosolic isoenzymes responsible for oxidizing intracellular
aldehydes. ALDH1-high CSCs are highly resistant to chemotherapeutic
agents commonly used as first-line therapy in the clinical setting,
such as cisplatin, gemcitabine, doxorubicin, vinorelbine and
docetaxel, whereas ALDH1-low cells are sensitive to the cytotoxic
activity of these drugs. In the present study, we found that both
SP CSCs from NSCLC cells and ALDH1-high CSCs from CRPC cells
overexpress a number of CSC markers, supporting the notion that
multiple mechanisms contribute to CSC phenotype (Table II). More importantly, we found
that both SP CSCs and ALDH1-high CSCs overexpress sPLA2-IIa. It was
reported that ALDH1-high CSCs from lung cancer cells also
overexpress sPLA2-IIa (53). These
findings strongly suggest that sPLA2-IIa is a marker for CSCs and
may support CSC properties.
Gene amplification, overexpression, and mutations in
EGFR family receptors have been well described in various cancers,
including breast, head and neck, prostate, and NSCLC, and
contribute to resistance to therapy and cancer progression
(54–59). EGF is a preferable ligand for
EGFR/EGFR homodimer or EGFR/HER2 heterodimer, while heregulin-a is
a preferable ligand for HER2/HER3 heterodimer (34,60,61).
HER2 has no ligand and HER3 has no tyrosine kinase activity, and
they function by forming heterodimer with other HER receptor.
Because HER3 has six tyrosine containing binding sites for p85, the
regulatory subunit of PI3K, HER2/HER3 complex is much more
effective than EGFR/HER2 in activation of the PI3K/Akt pathway.
HER3, which signaling function cannot be inhibited by tyrosine
kinase inhibitor (TKI), provides a focal point in resistance to TKI
therapy (62). Increasing body of
evidence highlight the role of HER3 in lung cancer, which has not
been successfully addressed in the targeted therapy to date. By
coupling to numerous signaling pathways, such as the RAS-ERK and
PI3K-Akt pathways, and multiple feedback regulatory loops,
HER/ERBB-elicited signaling propels the clonal expansion of CSCs
(33–35). One such positive feedback loop is
stimulation of sPLA2-IIa overexpression via HER/ERBB-PI3K-Akt-NF-κB
signaling (13,29,30).
We uncovered that elevated HER/ERBB-PI3K-Akt-NF-κB signaling
induces sPLA2-IIa overexpression and secretion in both lung and
prostate cancer cells, and in turn, sPLA2-IIa activates EGFR family
receptors and HER/ERBB-elicited signaling and stimulates sPLA2-IIa
overexpression in a positive feedback manner (13,29,30).
We further investigated the molecular action of
sPLA2-IIa. Given the potential ligand activity of sPLA2-IIa, we
hypothesized that sPLA2-IIa functions as a ligand for EGFR family
receptors, leading to an enhanced HER/ERBB-elicited signaling.
Indeed, we found that sPLA2-IIa enhances HER/ERBB-PI3K-Akt-NF-κB
signaling in both prostate cancer cells (Figs. 1 and 2) and lung cancer cells (32). Our protein docking analysis and
co-immunoprecipitation experiments revealed that sPLA2-IIa
interacts with EGFR family receptors (Fig. 2) (32). sPLA2-IIa directly or indirectly
interacts with EGFR, HER2, and HER3, suggesting that it may be a
ligand for both EGFR and HER3. Given that both SP and ALDH1-high
CSCs overexpress sPLA2-IIa (Table
II), sPLA2-IIa in the tumor microenvironment may function as a
ligand for EGFR family receptors, stimulates HER/ERBB- elicited
signaling, and promotes the clonal expansion of CSCs and cancer
progression (33–35).
sPLA2-IIa stimulates growth of prostate cancer cells
(13,27,63),
colon cancer cells (64), and
brain tumor cells (65,66), which may be via the EGFR-, MAPK-,
PI3K/Akt-, NF-κB-mediated cell growth and survival signaling
pathways (67–70). sPLA2-IIa abrogates TNF-α-induced
apoptosis and compromises immune surveillance function (71). In the transgenic adenocarcinoma of
the mouse prostate (TRAMP) model, sPLA2-IIa contributes to
aggressive phenotypes, androgen-independent growth, and metastasis
(72). sPLA2-IIa binds to
integrins and induces proliferation of monocytic cells in an
integrin-dependent manner (16).
We showed that sPLA2-IIa is overexpressed in CSCs, which may
support CSC properties. On the other hand, knocking down expression
of sPLA2-IIa reduces lung cancer growth (73). Our previous and current studies
revealed the underlying mechanisms of sPLA2-IIa action, in which
sPLA2-IIa functions as a ligand for EGFR family receptors, leading
to sPLA2-IIa overexpression via HER/ERBB-PI3K-Akt-NF-κB signaling
in a positive feedback manner. Aberrant high levels of sPLA2-IIa in
the tumor microenvironment support CSC properties and contribute to
cancer progression and metastasis. sPLA2-IIa is a novel therapeutic
target against cancer.
It has been shown that the treatment with taxol and
cisplatin may not affect growth, but can even stimulate growth in
CSCs (74,75). Similarly, castration has been shown
to induce epithelial-mesenchymal transition, promote growth of CSCs
in prostate cancer, and lead to castration-resistance and
metastasis (10). Consistent with
these observations, we found that CRPC LNCaP-AI cells, selected in
the stripped medium (36), and
multidrug resistant 22Rv1-R cells, derived from CRPC 22Rv1 cells
selected in the medium containing paclitaxel, overexpress several
CSC markers and drug efflux pump proteins (Tables IV and V). Therefore, LNCaP-AI and 22Rv1-R cells
provide valuable tools for studying CSCs and determine the roles of
sPLA2-IIa in tumor progression.
Acknowledgments
This study was supported in part by the Millennium
Scholar funds of University of Cincinnati Cancer Center and a pilot
grant from the Department of Internal Medicine, University of
Cincinnati College of Medicine.
References
|
1
|
O'Flaherty JD, Barr M, Fennell D, Richard
D, Reynolds J, O'Leary J and O'Byrne K: The cancer stem-cell
hypothesis: Its emerging role in lung cancer biology and its
relevance for future therapy. J Thorac Oncol. 7:1880–1890. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Leon G, MacDonagh L, Finn SP, Cuffe S and
Barr MP: Cancer stem cells in drug resistant lung cancer: Targeting
cell surface markers and signaling pathways. Pharmacol Ther.
158:71–90. 2016. View Article : Google Scholar
|
|
3
|
MacDonagh L, Gray SG, Breen E, Cuffe S,
Finn SP, O'Byrne KJ and Barr MP: Lung cancer stem cells: The root
of resistance. Cancer Lett. 372:147–156. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Liu T, Xu F, Du X, Lai D, Liu T, Zhao Y,
Huang Q, Jiang L, Huang W, Cheng W, et al: Establishment and
characterization of multidrug resistant, prostate
carcinoma-initiating stem-like cells from human prostate cancer
cell lines 22RV1. Mol Cell Biochem. 340:265–273. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Fang DD, Cao J, Jani JP, Tsaparikos K,
Blasina A, Kornmann J, Lira ME, Wang J, Jirout Z, Bingham J, et al:
Combined gemcitabine and CHK1 inhibitor treatment induces apoptosis
resistance in cancer stem cell-like cells enriched with tumor
spheroids from a non-small cell lung cancer cell line. Front Med.
7:462–476. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Vlashi E and Pajonk F: The metabolic state
of cancer stem cells - a valid target for cancer therapy? Free
Radic Biol Med. 79:264–268. 2015. View Article : Google Scholar
|
|
7
|
Pfeiffer MJ and Schalken JA: Stem cell
characteristics in prostate cancer cell lines. Eur Urol.
57:246–254. 2010. View Article : Google Scholar
|
|
8
|
Lagadec C, Vlashi E, Della Donna L,
Dekmezian C and Pajonk F: Radiation-induced reprogramming of breast
cancer cells. Stem Cells. 30:833–844. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ghisolfi L, Keates AC, Hu X, Lee DK and Li
CJ: Ionizing radiation induces stemness in cancer cells. PLoS One.
7:e436282012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Li P, Yang R and Gao WQ: Contributions of
epithelial-mesen-chymal transition and cancer stem cells to the
development of castration resistance of prostate cancer. Mol
Cancer. 13:552014. View Article : Google Scholar
|
|
11
|
Brown MD, Gilmore PE, Hart CA, Samuel JD,
Ramani VA, George NJ and Clarke NW: Characterization of benign and
malignant prostate epithelial Hoechst 33342 side populations.
Prostate. 67:1384–1396. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Antonio V, Brouillet A, Janvier B, Monne
C, Bereziat G, Andreani M and Raymondjean M: Transcriptional
regulation of the rat type IIA phospholipase A2 gene by cAMP and
interleukin-1beta in vascular smooth muscle cells: Interplay of the
CCAAT/enhancer binding protein (C/EBP), nuclear factor-kappaB and
Ets transcription factors. Biochem J. 368:415–424. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Dong Z, Liu Y, Scott KF, Levin L, Gaitonde
K, Bracken RB, Burke B, Zhai QJ, Wang J, Oleksowicz L, et al:
Secretory phospholipase A2-IIa is involved in prostate cancer
progression and may potentially serve as a biomarker for prostate
cancer. Carcinogenesis. 31:1948–1955. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Cummings BS: Phospholipase A2 as targets
for anti-cancer drugs. Biochem Pharmacol. 74:949–959. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Triggiani M, Granata F, Giannattasio G and
Marone G: Secretory phospholipases A2 in inflammatory and allergic
diseases: Not just enzymes. J Allergy Clin Immunol. 116:1000–1006.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Saegusa J, Akakura N, Wu CY, Hoogland C,
Ma Z, Lam KS, Liu FT, Takada YK and Takada Y: Pro-inflammatory
secretory phospholipase A2 type IIA binds to integrins alphavbeta3
and alpha4beta1 and induces proliferation of monocytic cells in an
integrin-dependent manner. J Biol Chem. 283:26107–26115. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Triggiani M, Granata F, Balestrieri B,
Petraroli A, Scalia G, Del Vecchio L and Marone G: Secretory
phospholipases A2 activate selective functions in human
eosinophils. J Immunol. 170:3279–3288. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Tada K, Murakami M, Kambe T and Kudo I:
Induction of cyclooxygenase-2 by secretory phospholipases A2 in
nerve growth factor-stimulated rat serosal mast cells is
facilitated by interaction with fibroblasts and mediated by a
mechanism independent of their enzymatic functions. J Immunol.
161:5008–5015. 1998.PubMed/NCBI
|
|
19
|
Cupillard L, Mulherkar R, Gomez N, Kadam
S, Valentin E, Lazdunski M and Lambeau G: Both group IB and group
IIA secreted phospholipases A2 are natural ligands of the mouse
180-kDa M-type receptor. J Biol Chem. 274:7043–7051. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Nicolas JP, Lambeau G and Lazdunski M:
Identification of the binding domain for secretory phospholipases
A2 on their M-type 180-kDa membrane receptor. J Biol Chem.
270:28869–28873. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Scott KF, Sajinovic M, Hein J, Nixdorf S,
Galettis P, Liauw W, de Souza P, Dong Q, Graham GG and Russell PJ:
Emerging roles for phospholipase A2 enzymes in cancer. Biochimie.
92:601–610. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Meyer AM, Dwyer-Nield LD, Hurteau GJ,
Keith RL, O'Leary E, You M, Bonventre JV, Nemenoff RA and Malkinson
AM: Decreased lung tumorigenesis in mice genetically deficient in
cytosolic phospholipase A2. Carcinogenesis. 25:1517–1524. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kallajoki M, Alanen KA, Nevalainen M and
Nevalainen TJ: Group II phospholipase A2 in human male reproductive
organs and genital tumors. Prostate. 35:263–272. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Jiang J, Neubauer BL, Graff JR, Chedid M,
Thomas JE, Roehm NW, Zhang S, Eckert GJ, Koch MO, Eble JN, et al:
Expression of group IIA secretory phospholipase A2 is elevated in
prostatic intraepithelial neoplasia and adenocarcinoma. Am J
Pathol. 160:667–671. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Graff JR, Konicek BW, Deddens JA, Chedid
M, Hurst BM, Colligan B, Neubauer BL, Carter HW and Carter JH:
Expression of group IIa secretory phospholipase A2 increases with
prostate tumor grade. Clin Cancer Res. 7:3857–3861. 2001.PubMed/NCBI
|
|
26
|
Dong Z, Liu Y, Levin L, Oleksowicz L, Wang
J and Lu S: Vav3 oncogene is involved in regulation of secretory
phospholipase A2-IIa expression in prostate cancer. Oncol Rep.
25:1511–1516. 2011.PubMed/NCBI
|
|
27
|
Sved P, Scott KF, McLeod D, King NJ, Singh
J, Tsatralis T, Nikolov B, Boulas J, Nallan L, Gelb MH, et al:
Oncogenic action of secreted phospholipase A2 in prostate cancer.
Cancer Res. 64:6934–6940. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Mirtti T, Laine VJ, Hiekkanen H, Hurme S,
Rowe O, Nevalainen TJ, Kallajoki M and Alanen K: Group IIA
phospholipase A as a prognostic marker in prostate cancer:
Relevance to clinicopathological variables and disease-specific
mortality. APMIS. 117:151–161. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Oleksowicz L, Liu Y, Bracken RB, Gaitonde
K, Burke B, Succop P, Levin L, Dong Z and Lu S: Secretory
phospholipase A2-IIa is a target gene of the HER/HER2-elicited
pathway and a potential plasma biomarker for poor prognosis of
prostate cancer. Prostate. 72:1140–1149. 2012. View Article : Google Scholar :
|
|
30
|
Kupert E, Anderson M, Liu Y, Succop P,
Levin L, Wang J, Wikenheiser-brokamp K, Chen P, Pinney SM,
Macdonald T, et al: Plasma secretory phospholipase A2-IIa as a
potential biomarker for lung cancer in patients with solitary
pulmonary nodules. BMC Cancer. 11:5132011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Menschikowski M, Hagelgans A, Schuler U,
Froeschke S, Rosner A and Siegert G: Plasma levels of phospholipase
A2-IIA in patients with different types of malignancies: Prognosis
and association with inflammatory and coagulation biomarkers.
Pathol Oncol Res. 19:839–846. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Dong Z, Meller J, Succop P, Wang J,
Wikenheiser-Brokamp K, Starnes S and Lu S: Secretory phospholipase
A2-IIa upregulates HER/HER2-elicited signaling in lung cancer
cells. Int J Oncol. 45:978–984. 2014.PubMed/NCBI
|
|
33
|
Mimeault M, Hauke R, Mehta PP and Batra
SK: Recent advances in cancer stem/progenitor cell research:
Therapeutic implications for overcoming resistance to the most
aggressive cancers. J Cell Mol Med. 11:981–1011. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Schneider MR and Yarden Y: The EGFR-HER2
module: A stem cell approach to understanding a prime target and
driver of solid tumors. Oncogene. 35:2949–2960. 2016. View Article : Google Scholar :
|
|
35
|
Singh S, Trevino J, Bora-Singhal N,
Coppola D, Haura E, Altiok S and Chellappan SP: EGFR/Src/Akt
signaling modulates Sox2 expression and self-renewal of stem-like
side-population cells in non-small cell lung cancer. Mol Cancer.
11:732012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Lu S, Tsai SY and Tsai MJ: Molecular
mechanisms of androgen- independent growth of human prostate cancer
LNCaP-AI cells. Endocrinology. 140:5054–5059. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Ho MM, Ng AV, Lam S and Hung JY: Side
population in human lung cancer cell lines and tumors is enriched
with stem-like cancer cells. Cancer Res. 67:4827–4833. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Li T, Su Y, Mei Y, Leng Q, Leng B, Liu Z,
Stass SA and Jiang F: ALDH1A1 is a marker for malignant prostate
stem cells and predictor of prostate cancer patients' outcome. Lab
Invest. 90:234–244. 2010. View Article : Google Scholar
|
|
39
|
Doherty RE, Haywood-Small SL, Sisley K and
Cross NA: Aldehyde dehydrogenase activity selects for the holoclone
phenotype in prostate cancer cells. Biochem Biophys Res Commun.
414:801–807. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Marcato P, Dean CA, Giacomantonio CA and
Lee PW: Aldehyde dehydrogenase: Its role as a cancer stem cell
marker comes down to the specific isoform. Cell Cycle.
10:1378–1384. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Januchowski R, Wojtowicz K and Zabel M:
The role of aldehyde dehydrogenase (ALDH) in cancer drug
resistance. Biomed Pharmacother. 67:669–680. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Singh S, Brocker C, Koppaka V, Chen Y,
Jackson BC, Matsumoto A, Thompson DC and Vasiliou V: Aldehyde
dehydrogenases in cellular responses to oxidative/electrophilic
stress. Free Radic Biol Med. 56:89–101. 2013. View Article : Google Scholar :
|
|
43
|
Wu A, Luo W, Zhang Q, Yang Z, Zhang G, Li
S and Yao K: Aldehyde dehydrogenase 1, a functional marker for
identifying cancer stem cells in human nasopharyngeal carcinoma.
Cancer Lett. 330:181–189. 2013. View Article : Google Scholar
|
|
44
|
Nishida S, Hirohashi Y, Torigoe T,
Kitamura H, Takahashi A, Masumori N, Tsukamoto T and Sato N: Gene
expression profiles of prostate cancer stem cells isolated by
aldehyde dehydrogenase activity assay. J Urol. 188:294–299. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Nishida S, Hirohashi Y, Torigoe T, Inoue
R, Kitamura H, Tanaka T, Takahashi A, Asanuma H, Masumori N,
Tsukamoto T, et al: Prostate cancer stem-like
cells/cancer-initiating cells have an autocrine system of
hepatocyte growth factor. Cancer Sci. 104:431–436. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Jiang F, Qiu Q, Khanna A, Todd NW, Deepak
J, Xing L, Wang H, Liu Z, Su Y, Stass SA, et al: Aldehyde
dehydrogenase 1 is a tumor stem cell-associated marker in lung
cancer. Mol Cancer Res. 7:330–338. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Mimeault M and Batra SK: Recent progress
on tissue-resident adult stem cell biology and their therapeutic
implications. Stem Cell Rev. 4:27–49. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Hu R, Dunn TA, Wei S, Isharwal S, Veltri
RW, Humphreys E, Han M, Partin AW, Vessella RL, Isaacs WB, et al:
Ligand-independent androgen receptor variants derived from splicing
of cryptic exons signify hormone-refractory prostate cancer. Cancer
Res. 69:16–22. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Dehm SM, Schmidt LJ, Heemers HV, Vessella
RL and Tindall DJ: Splicing of a novel androgen receptor exon
generates a constitutively active androgen receptor that mediates
prostate cancer therapy resistance. Cancer Res. 68:5469–5477. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Munoz M, Henderson M, Haber M and Norris
M: Role of the MRP1/ABCC1 multidrug transporter protein in cancer.
IUBMB Life. 59:752–757. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Modok S, Mellor HR and Callaghan R:
Modulation of multidrug resistance efflux pump activity to overcome
chemoresistance in cancer. Curr Opin Pharmacol. 6:350–354. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Signore M, Ricci-Vitiani L and De Maria R:
Targeting apoptosis pathways in cancer stem cells. Cancer Lett.
332:374–382. 2013. View Article : Google Scholar
|
|
53
|
Bennett DT, Deng XS, Yu JA, Bell MT,
Mauchley DC, Meng X, Reece TB, Fullerton DA and Weyant MJ: Cancer
stem cell phenotype is supported by secretory phospholipase A2 in
human lung cancer cells. Ann Thorac Surg. 98:439–445; discussion
445–436. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Di Lorenzo G, Tortora G, D'Armiento FP, De
Rosa G, Staibano S, Autorino R, D'Armiento M, De Laurentiis M, De
Placido S, Catalano G, et al: Expression of epidermal growth factor
receptor correlates with disease relapse and progression to
androgen-independence in human prostate cancer. Clin Cancer Res.
8:3438–3444. 2002.PubMed/NCBI
|
|
55
|
Shi Y, Brands FH, Chatterjee S, Feng AC,
Groshen S, Schewe J, Lieskovsky G and Cote RJ: Her-2/neu expression
in prostate cancer: High level of expression associated with
exposure to hormone therapy and androgen independent disease. J
Urol. 166:1514–1519. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Osman I, Scher HI, Drobnjak M, Verbel D,
Morris M, Agus D, Ross JS and Cordon-Cardo C: HER-2/neu (p185neu)
protein expression in the natural or treated history of prostate
cancer. Clin Cancer Res. 7:2643–2647. 2001.PubMed/NCBI
|
|
57
|
Signoretti S, Montironi R, Manola J,
Altimari A, Tam C, Bubley G, Balk S, Thomas G, Kaplan I, Hlatky L,
et al: Her-2-neu expression and progression toward androgen
independence in human prostate cancer. J Natl Cancer Inst.
92:1918–1925. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Yeh S, Lin HK, Kang HY, Thin TH, Lin MF
and Chang C: From HER2/Neu signal cascade to androgen receptor and
its coacti-vators: A novel pathway by induction of androgen target
genes through MAP kinase in prostate cancer cells. Proc Natl Acad
Sci USA. 96:5458–5463. 1999. View Article : Google Scholar
|
|
59
|
Craft N, Shostak Y, Carey M and Sawyers
CL: A mechanism for hormone-independent prostate cancer through
modulation of androgen receptor signaling by the HER-2/neu tyrosine
kinase. Nat Med. 5:280–285. 1999. View
Article : Google Scholar : PubMed/NCBI
|
|
60
|
Schulze WX, Deng L and Mann M:
Phosphotyrosine interactome of the ErbB-receptor kinase family. Mol
Syst Biol. 1:2005 00082005. View Article : Google Scholar
|
|
61
|
Hsieh AC and Moasser MM: Targeting HER
proteins in cancer therapy and the role of the non-target HER3. Br
J Cancer. 97:453–457. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Tsao MS, Sakurada A, Cutz JC, Zhu CQ,
Kamel-Reid S, Squire J, Lorimer I, Zhang T, Liu N, Daneshmand M, et
al: Erlotinib in lung cancer - molecular and clinical predictors of
outcome. N Engl J Med. 353:133–144. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Patel MI, Singh J, Niknami M, Kurek C, Yao
M, Lu S, Maclean F, King NJ, Gelb MH, Scott KF, et al: Cytosolic
phospholipase A2-alpha: A potential therapeutic target for prostate
cancer. Clin Cancer Res. 14:8070–8079. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Belinsky GS, Rajan TV, Saria EA, Giardina
C and Rosenberg DW: Expression of secretory phospholipase A2 in
colon tumor cells potentiates tumor growth. Mol Carcinog.
46:106–116. 2007. View Article : Google Scholar
|
|
65
|
Hernández M, Martín R, García-Cubillas MD,
Maeso- Hernández P and Nieto ML: Secreted PLA2 induces
proliferation in astrocytoma through the EGF receptor: Another
inflammation-cancer link. Neuro-oncol. 12:1014–1023. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Martín R, Hernández M, Ibeas E, Fuentes L,
Salicio V, Arnés M and Nieto ML: Secreted phospholipase A2-IIA
modulates key regulators of proliferation on astrocytoma cells. J
Neurochem. 111:988–999. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Valentin E and Lambeau G: Increasing
molecular diversity of secreted phospholipases A(2) and their
receptors and binding proteins. Biochim Biophys Acta. 1488:59–70.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Lambeau G and Lazdunski M: Receptors for a
growing family of secreted phospholipases A2. Trends Pharmacol Sci.
20:162–170. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Hernández M, Burillo SL, Crespo MS and
Nieto ML: Secretory phospholipase A2 activates the cascade of
mitogen-activated protein kinases and cytosolic phospholipase A2 in
the human astrocytoma cell line 1321N1. J Biol Chem. 273:606–612.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Park DW, Kim JR, Kim SY, Sonn JK, Bang OS,
Kang SS, Kim JH and Baek SH: Akt as a mediator of secretory
phospholipase A2 receptor-involved inducible nitric oxide synthase
expression. J Immunol. 170:2093–2099. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Ibeas E, Fuentes L, Martín R, Hernández M
and Nieto ML: Inflammatory protein sPLA(2)-IIA abrogates
TNFalpha-induced apoptosis in human astroglioma cells: Crucial role
of ERK. Biochim Biophys Acta. 1793:1837–1847. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Morgenbesser SD, McLaren RP, Richards B,
Zhang M, Akmaev VR, Winter SF, Mineva ND, Kaplan-Lefko PJ, Foster
BA, Cook BP, et al: Identification of genes potentially involved in
the acquisition of androgen-independent and metastatic tumor growth
in an autochthonous genetically engineered mouse prostate cancer
model. Prostate. 67:83–106. 2007. View Article : Google Scholar
|
|
73
|
Yu JA, Mauchley D, Li H, Meng X, Nemenoff
RA, Fullerton DA and Weyant MJ: Knockdown of secretory
phospholipase A2 IIa reduces lung cancer growth in vitro and in
vivo. J Thorac Cardiovasc Surg. 144:1185–1191. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Larzabal L, El-Nikhely N, Redrado M,
Seeger W, Savai R and Calvo A: Differential effects of drugs
targeting cancer stem cell (CSC) and non-CSC populations on lung
primary tumors and metastasis. PLoS One. 8:e797982013. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Lopez-Ayllon BD, Moncho-Amor V, Abarrategi
A, Ibañez de Cáceres I, Castro-Carpeño J, Belda-Iniesta C, Perona R
and Sastre L: Cancer stem cells and cisplatin-resistant cells
isolated from non-small-lung cancer cell lines constitute related
cell populations. Cancer Med. 3:1099–1111. 2014. View Article : Google Scholar : PubMed/NCBI
|