Introduction
Tumor cells and stromal fibroblasts mutually
interact. Particularly, a process of degradation and reconstruction
of the neighboring stroma is essential for cancer invasion. Matrix
metalloproteinases (MMPs) play a central role in this process.
Emmprin regulates the production of MMP-2 by mediating this
interaction between the tumor cells and the fibroblasts. Emmprin is
a membrane glycoprotein with two extracellular immunoglobulin-like
domains and has molecular weight of 44–66 kDa depending on the
degree of glycosylation (1,2).
Emmprin is expressed more abundantly in tumor cells than in
fibroblasts. A number of studies have reported that emmprin is
expressed at a significantly higher level in malignant tumors
compared to benign or normal tissues, and that the high emmprin
expression is correlated with malignancy and/or poor prognosis
(3,4). In humans, 24 MMPs that degrade stroma
have been discovered. They are broadly classified into secretory
and membrane types. MMP-2 represents the secretory MMPs in tumors.
MMP-2 is expressed in both tumor cells and fibroblasts, with
predominant expression observed in fibroblasts in direct contact
with tumor cells. However, not only the fibroblasts neighboring the
tumor cells but also those located at sites distant from tumor
cells express MMP-2.
Co-culture is one of the methods of observing
interactions between the tumor cells and the fibroblasts.
Co-culture of fibroblasts and tumor cells significantly enhances
the MMP-2 expression by fibroblasts via emmprin. This phenomenon is
also observed when an epithelioid sarcoma cell line is used
(5). Our laboratory previously
demonstrated that conditioned medium of epithelioid sarcoma cells
contains full-length emmprin, and that the addition of this
conditioned medium to fibroblasts led to enhanced MMP-2 expression
by the fibroblasts, which was suppressed by anti-emmprin
neutralizing antibody (5). This
result indicated that emmprin is released from the sarcoma cells
and acts on fibroblasts located at sites distant from the tumor
cells.
Emmprin is likely secreted in two forms: released by
proteolytic cleavage, or released with the membrane in the form of
microvesicles. Egawa et al have reported the proteo-lytic
cleavage of emmprin by MT1-MMP based on their experiments using
human epidermoid carcinoma cell line and human fibrosarcoma cell
line (6). In our prior experiments
using sarcoma cells, emmprin in the conditioned medium was detected
as a full length protein. However, the precise mechanism, by which
full length emmprin is released, have not been determined.
In the present study, we have used microvesicles to
directly examine our phenomenon-based hypothesis that emmprin
exists in the form of microvesicles in the conditioned medium, and
that emmprin packaged in the microvesicles facilitates MMP-2
production by acting on fibroblasts located at distant sites from
the tumor cells.
Materials and methods
Cell culture
Epithelioid sarcoma cell line FU-EPS-1 was
established in our laboratory from a patient with epithelioid
sarcoma who had not received any chemotherapy before surgical
resection (7). The cell line was
maintained in growth medium, D-MEM/Ham's F-12 (Wako, Japan),
supplemented with 10% fetal calf serum (FCS). The human dermal
fibroblast ST353 was obtained from non-lesional dermis around
nodular fascitis. ST353i was immortalized by transduction of human
telomerase reverse transcriptase (hTERT).
Preparation of microvesicles
Microvesicles from cell-cultured medium were
prepared as previously described (8–10).
FU-EPS-1 cells were cultured to sub-confluency in the growth
medium. The cells were washed with serum-free medium and cultured
further in DMEM/Ham's-F12 without serum for 48 h. Conditioned
medium was centrifuged at 1500 g 15 min to remove cells and large
debris. Supernatant was centrifuged at 50,000 g for 1 h at 4°C.
Pelleted microvesicles were re-suspended in serum-free medium
containing 0.2% lactalbumin enzymatic hydrolysate. Vesicles were
quantified based on tumor cell counts.
Electron microscopy
Microvesicles were applied on 400 mesh nickel
parlodion-coated grids and allowed to settle. Samples were blocked
in 5% normal goat serum for 30 min, rinsed in PBS and incubated
with anti-emmprin antibody at 4°C overnight. Grids were washed with
PBS, incubated with gold nanoparticle conjugated anti-mouse
secondary antibody (Abcam, Cambridge, UK) for 1 h and then rinsed
with PBS again. Grids were fixed in 2% glutaraldehyde in PBS,
rinsed, and then negatively stained with 1% phosphotungstic acid
(pH 6.0), before being analyzed under an electron microscope.
Specificity of immunolabeling was determined by comparing results
obtained with gold-labeled secondary antibody only.
Immunoblotting
SDS-PAGE and immunoblotting of microvesicles and
cell lysates were performed using 4–15% Mini-PROTEAN TGX gel
(Bio-Rad, Hercules, CA, USA), immobilon membrane (Millipore,
Bedford, MA, USA), antibodies against emmprin (monoclonal antibody,
clone 109403, R&D System, Flanders, NJ, USA; goat polyclonal
antibody against N- and C-terminal region of emmprin, Santa Cruz
Biotechnology, Santa Cruz, CA, USA), and MMP-2 monoclonal antibody
(Daiichi Fine Chemical, Toyama, Japan) as described previously
(5).
Glycosidase digestions
Extracted membrane protein from microvesicles were
reacted with incubation buffer (100 mM
NaH2PO4 (pH 7.0), 10 mM EDTA, 1% NP-40, 0.1%
SDS, 1% 2-mercaptoethanol) containing 10 U/ml N-glycosidase F
(Roche, Basel, Switzerland) for 24 h at 37°C. The trichloroacetic
acid was added to a final concentration of 3.3%, and the samples
were incubated at 4°C overnight. The samples were centrifuged at
10,000 rpm for 10 min to remove supernatant. The pellet was lysed
in 4X concentrated Laemmli sample buffer and analyzed by SDS/PAGE
and immunoblotting.
Precipitation with biotinylated Sophora
japonica agglutinin
Microvesicles were lysed in precipitation buffer (1%
CHAPS, 25 mM HEPES, pH 7.5, 150 mM NaCl, 5 mM MgCI2,
protease inhibitor cocktail) at 4°C overnight. After centrifugation
(42,000 rpm, 1 h), the supernatant was incubated at 4°C overnight
with biotinylated Sophora japonica agglutinin (PHA-L, Vector
laboratories, Burlingame, CA, USA), followed by incubation with
avidin agarose beads (Thermo Fisher Scientific, Waltham, MA, USA)
at 4°C for 1 h. After washing with precipitation buffer and
centrifugation (4,000 rpm, 1 min), the pellet was lysed in 4X
concentrated Laemmli sample buffer and analyzed by SDS/PAGE and
immunoblotting.
Transient knockdown of emmprin by RNA
interference
Small interference RNAs (siRNAs) sequences were used
for transient knockdown of emmprin mRNA. siRNAs targeting the
emmprin were designed and synthesized (Invitrogen Corp., Carlsbad,
CA, USA), and transfection was carried out using Oligofectamine
transfection reagent in Opti-MEM (Thermo Fisher Scientific) in the
absence of serum and antibiotics according to the manufacturer's
instructions. Knockdown of emmprin expression in FU-EPS-1 cells was
analyzed by immunoblotting.
Stable knockdown of emmprin by RNA
interference
To establish the cells stably expressing shRNA
against emmprin, we used the BLOCK-iT Lentiviral RNAi Expression
system (Invitrogen Corp.), as described previously (11,12).
Gene-specific insert
(5′-CACAGTCTTCACTACCGTAGCGAACTACGGTAGTGAAGACTGTGC-3′) was cloned
into plenti6 according to the manufacturer's instructions. The
lentivirus was produced in HEK 293FT cells using the ViraPower
lentiviral expression system (Invitrogen), and the virus-containing
media were harvested for infection. Lentivirus expressing LacZ
shRNA was used as a control. Stable shRNA expressing cells were
propagated and maintained in the presence of blasticidin (5
µg/ml; Invitrogen).
Enzyme-linked immunosorbent assay
(ELISA)
The protein concentrations of MMP-2 were measured
using human MMP-2 Quantikine ELISA kit (R&D System,
Minneapolis, MN, USA) as per the manufacturer's instructions.
Results
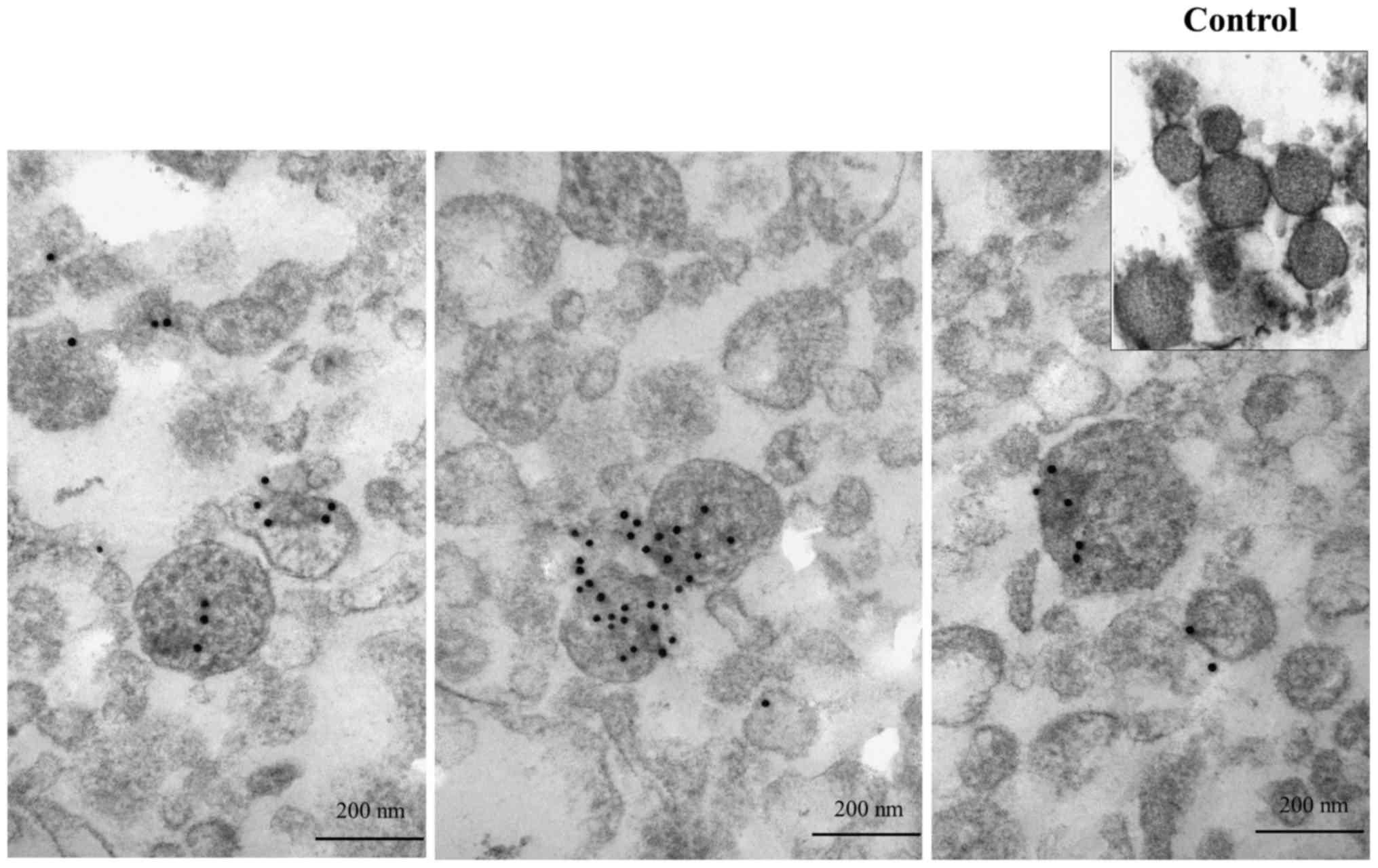
Determination of emmprin localization in
microvesicles using immunoelectron micrographs
We first examined whether emmprin protein exists in
microvesicles using immunoelectron microscopy. Microvesicles
purified by ultracentrifugation obtained from the conditioned
medium of the FU-EPS-1 cells were subjected to immunoelectron
micrography using postembedding labeling methods. Microvesicles
showed closed circle structures of 100–300 nm in diameter, similar
to those of prior reports (10,13).
Black spots represent gold particles conjugated with anti-emmprin
monoclonal antibody and indicate emmprin localization in
microvesicles (Fig. 1). No gold
particles were found in the control electron micrographs. Our data
indicates the presence of emmprin in the microvesicles collected
from FU-EPS-1 cells.
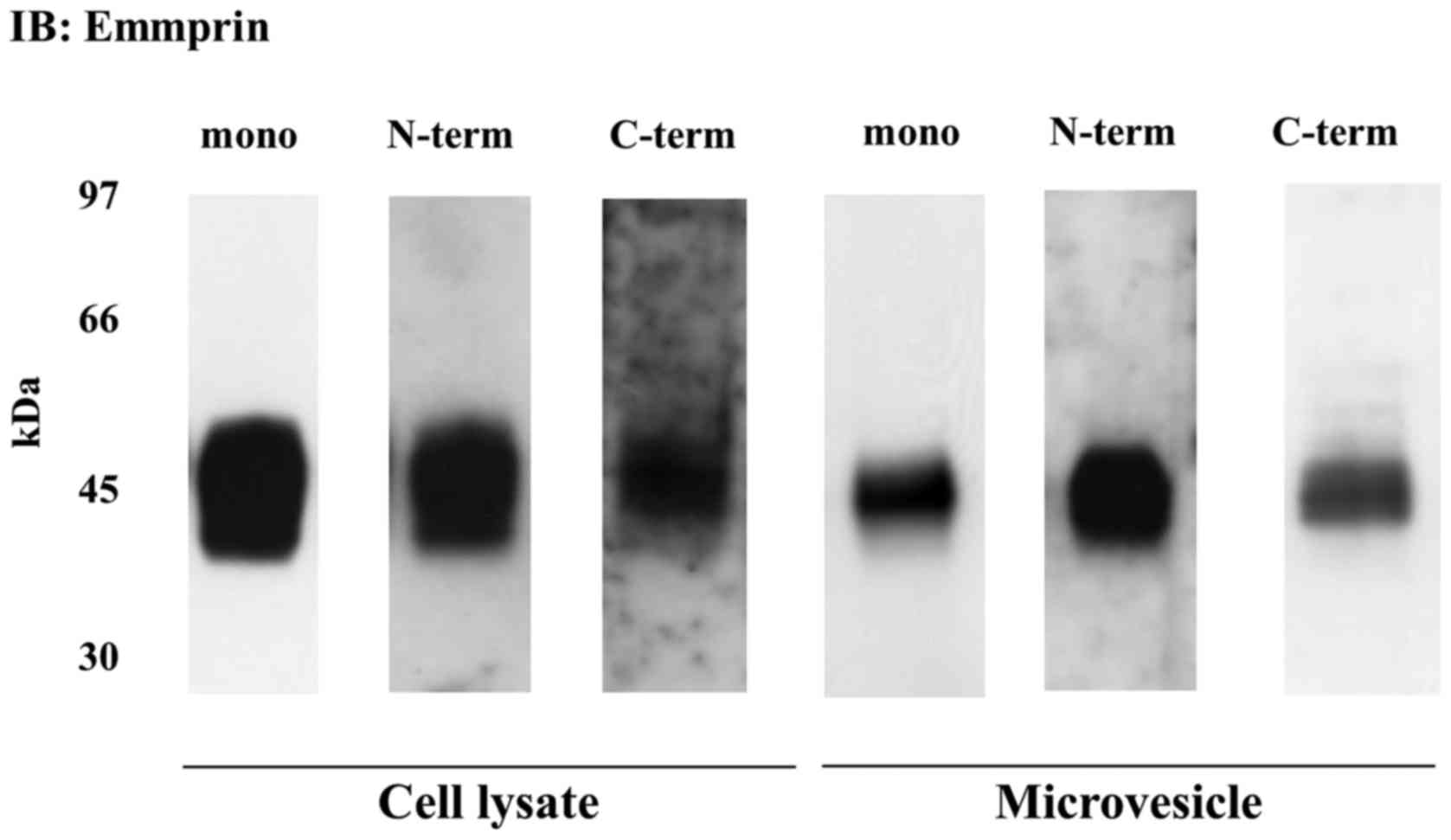
Full length emmprin expression in
microvesicles released from epithelioid sarcoma cells
We next examined whether emmprin contained in the
microvesicles was the full-length protein identical to the form
expressed on the tumor cell surface. Membrane proteins were
extracted from total cell lysates and microvesicles, both obtained
from the FU-EPS-1 cells. Immunoblotting with anti-emmprin
monoclonal antibody detected ~ 45 kDa emmprin band, which was also
observed with antibodies raised against the N- and C-terminal
domain of emmprin, indicative of the release of full-length
emmprin. Almost identical emmprin immunoreactivity was observed in
total cell lysate and microvesicles (Fig. 2). These findings indicate that
microvesicles also contain the full-length emmprin.
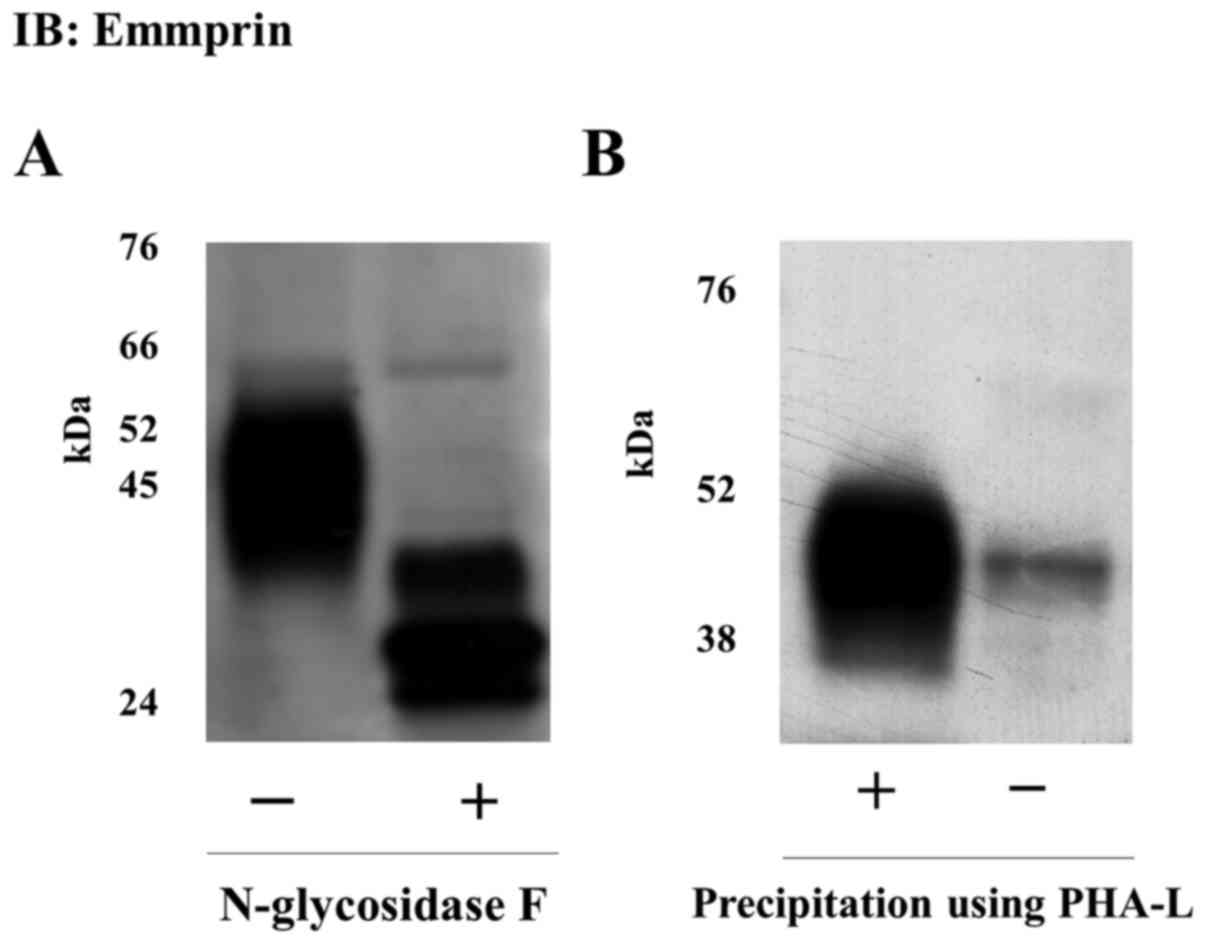
Presence of N-glycosylation with
polylactosamine in emmprin released as microvesicles
We assessed the glycosylation of the emmprin
released in the microvesicles. It is reported that emmprin-induced
stimulation of MMP production in fibroblasts is dependent on
N-glycosylation of its extracellular domains (14–16).
Treatment of microvesicles with N-glycosidase F, which removes all
glycosylation, increased the electrophoretic mobility of the
observed emmprin protein band. A 27-kDa band was observed upon
treatment with N-glycosidase F, which is consistent with the size
of the core emmprin protein (Fig.
3A). Further, emmprin released as microvesicles was
precipitated by PHA-L lectin, indicative of the presence of β1,6
(beta 1,6) branching and polylactosamines (Fig. 3b) (17,18).
Overall, the data suggest that the full-length 45-kDa emmprin
protein, identified in the microvesicles released from FU-EPS-1
cells, was characterized by polylactosamine glycosylation.
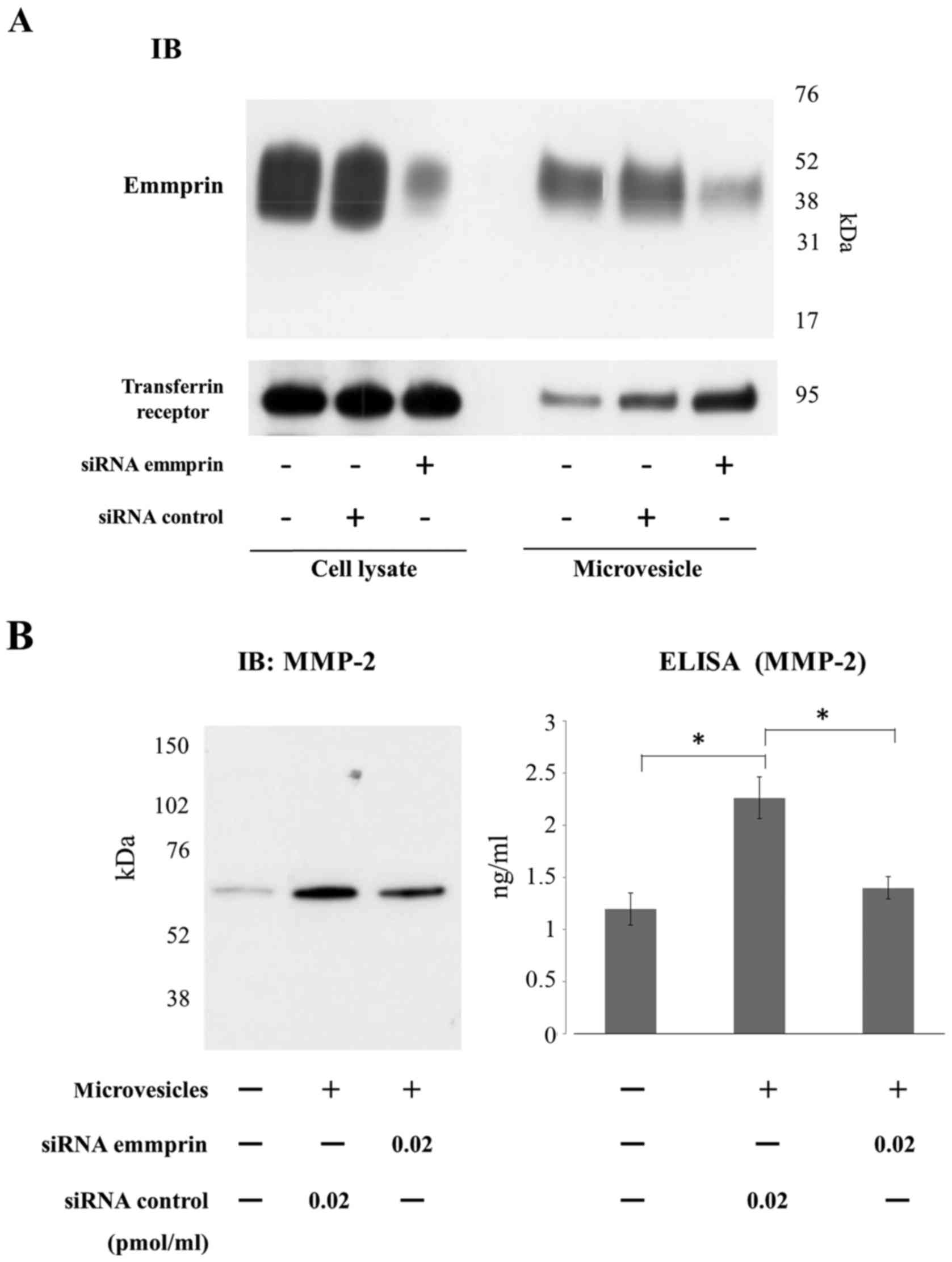
Emmprin derived from microvesicles
regulates MMP-2 production by fibroblasts
Finally, we examined the function of emmprin in
microvesicles. In particular, we were interested in elucidating
whether emmprin in microvesicles also regulates the production of
MMP-2 from fibroblasts. siRNA or shRNA inhibition of emmprin
expression in tumor cells was used to assess the role of
microvesicles associated emmprin in regulation of MMP-2 production
from fibroblasts (Figs. 4A and
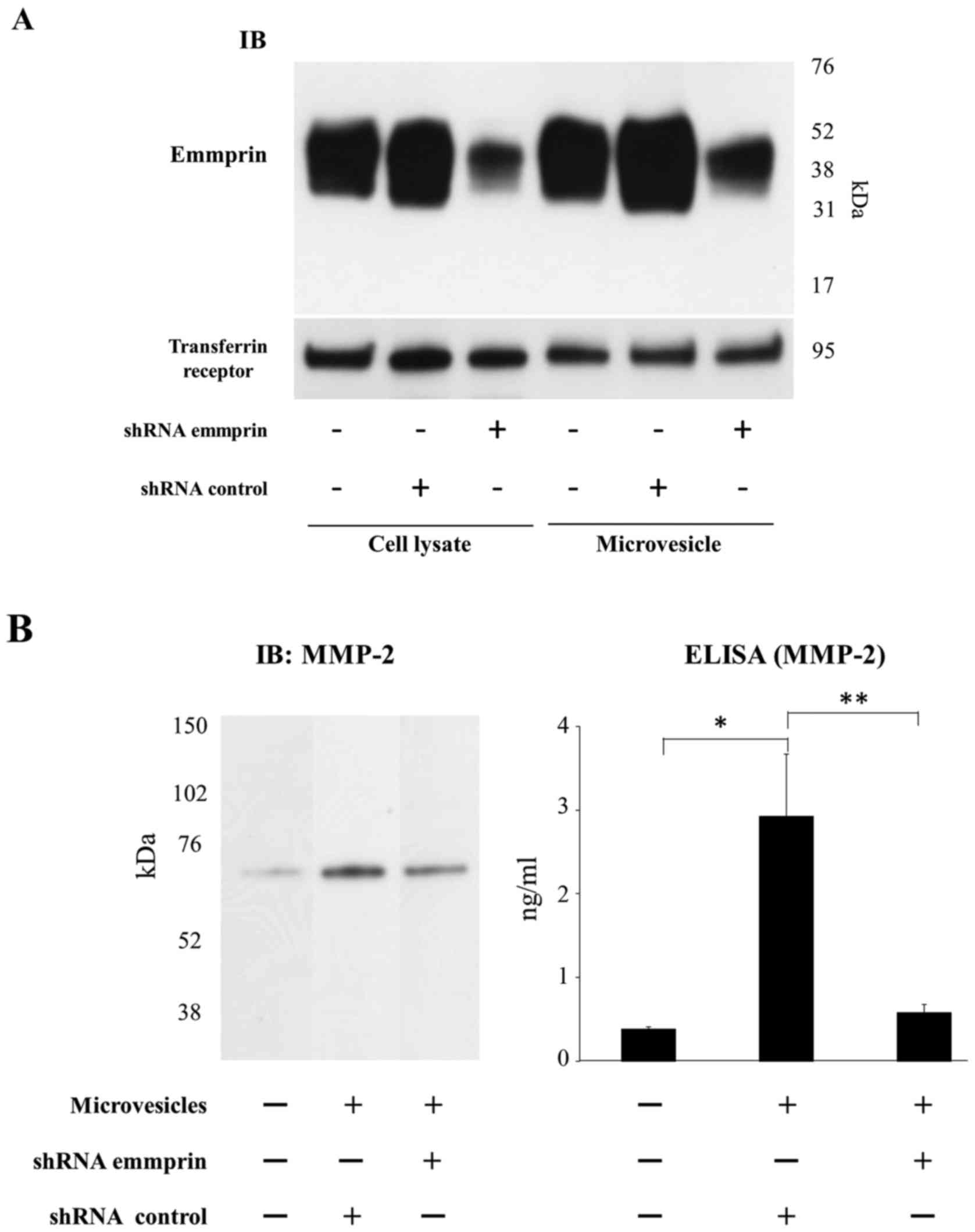
5A). Immunoblots showed that
emmprin expression was markedly reduced in both cell lysates and
microvesicles derived from FU-EPS cells transfected with emmprin
siRNA (Fig. 4A) or transduced with
emmprin shRNA (Fig. 5A). MMP-2
production by ST353i fibroblasts was determined using ELISA upon
treatment with microvesicles isolated from control siRNA
transfected tumor cells or those transfected with either emmprin
siRNA or shRNA. MMP-2 production from fibroblasts was increased
when tumor cell microvesicles were added to the fibroblast cultures
(Figs. 4b and 5b). Microvesicles collected from FU-EPS-1
transfected with emmprin-specific siRNA and transduced with
emmprin-specific shRNA displayed significantly reduced MMP-2
production by fibroblasts compared with that of control cells
(Figs. 4b and 5b). These findings indicate that emmprin
contained in microvesicles stimulates MMP-2 production from
fibroblasts.
Discussion
This is the first study to demonstrate the presence
of full-length emmprin with polylactosamine in microvesicles
derived from sarcoma cells and to provide evidence for the
involvement of such released emmprin in the regulation of MMP-2
expression by fibroblasts.
Microvesicles, including the tumor-derived ones, are
microparticles that are produced from the cell surface of plasma
membrane. A recent study revealed that microvesicles shed from
tumor cell lines are rather heterogeneous in size, ranging from 100
nm to 200 nm, and more heterogeneous in shape than exosomes
(19,20). Various substances such as proteins,
RNA, mRNA, and miRNA are present inside or on the surface of
microvesicles. The constant secretion of microvesicles from plasma
membrane results in the transport of a variety of molecules to
distant sites to influence cellular processes (10,19,21,22).
In the present study, we established the presence of
glycosylated full-length emmprin in microvesicles derived from
epithelioid sarcoma cells. Briefly, using multiple antibodies
against emmprin (monoclonal as well as N- and C-terminal
polyclonal), we demonstrated that emmprin contained in
microvesicles is the full-length protein identical to the form
present on the plasma membrane (Fig.
1). Furthermore, the N-glycosylation modification with the
enrichment of polylactos-amine was demonstrated in emmprin protein
structure (Fig. 2). Previously we
showed that the synthesized first immunoglobulin-like domain can
mimic emmprin activity when substituted with chitobiose, the
disaccharide with which N-glycosylation starts, indicating an
essential role of N-glycosylation for the emmprin activity
(16). These results support our
hypothesis that biologically active emmprin is released by
epithelioid sarcoma cells predominantly by microvesicle shedding,
which is based on our previous report that emmprin exists in the
conditioned medium as a full length protein (5).
The presence of emmprin in microvesicles was
previously reported using immunoelectron microscopy in a lung
carcinoma cell line (8).
Expression of protein and mRNA of emmprin in microvesicles were
also demonstrated in ovarian cancer and pancreatic adenocarcinoma
(10,13). However, there have been very few
reports regarding microvesicles in sarcomas compared to those in
epithelial malignant tumors. Almost 30 years ago, the presence of
microvesicles was reported in a human osteogenic sarcoma cell line
(23). Recently, it was reported
that EWS/Fli-1 fusion mRNA of Ewing sarcoma is secreted by
microvesicles (24).
Emmprin is also cleaved by MT1-MMP/MMP-14 from the
cell surface and released into the culture medium (6,25).
Predominance between the emmprin cleaved and secreted and the
emmprin shed as a vesicle is plausibly dependent upon the
malignancy of the cells, and/or the difference in cell types
including their aggressiveness. The variability in the abundance of
microvesicles as a function of the cell type was highlighted
recently. Embryonal carcinoma cells, a more aggressive testicular
germ cell tumor, had a higher amount of microvesicles and a
significantly higher emmprin expression level compared to seminoma
cells which manifest a lesser degree of malignancy (26). In addition, it was previously
reported that electron microscopy of dermatofibrosarcoma
protuberans (DFSP) and dermatofibroma revealed that DFSP, the more
aggressive of the two tumors, contained multivesicular buds whereas
dermatofibroma lacked them (27).
It was previously reported using co-culture
experiments of laryngeal cancer cells and fibroblasts that MMP-2
expression by fibroblasts requires direct cell-cell contact
(28). The expression of MMP-2 by
direct cell-cell contact of tumor cells and fibroblasts is
undeniably established as the co-culture of tumor cells and
fibroblasts clearly enhances MMP-2 expression (5). However, the upregulation of MMP-2
expression in fibroblasts in the vicinity of a tumor expressing
emmprin but lacking direct contact with tumor cells, is supported
by certain pathological observations (29,30).
Emmprin likely exists in three cellular and
extracellular forms: i) on the cell surface, ii) released after
cleavage by MT1-MMP/MMP-14, and iii) secreted in the form of
microvesicle shedding (31).
Predominance among these forms of emmprin is likely dependent on
the cell type differences including malignancy. Limitations of this
study include using only one type of cells, epithelioid sarcoma,
for the experiments. It remains to be tested that the physiological
activity of emmprin in microvesicles is preserved in
vivo.
In conclusion, we provided evidence that emmprin
contained in microvesicles derived from sarcoma cells is involved
in the regulation of MMP-2 production by fibroblasts located at
sites distant from the tumor cells. We have successfully clarified
a mechanism of action of emmprin, and provided insights into its
important role in the invasion of sarcoma cells.
Acknowledgments
We thank M. Onitsuka, and H. Fukagawa for their
excellent technical assistance.
Abbreviations:
|
emmprin
|
extracellular matrix metalloproteinase
inducer, CD147
|
|
MMP
|
matrix metalloproteinase
|
|
MV
|
microvesicle
|
|
siRNA
|
small interference RNA
|
|
shRNA
|
short hairpin RNA
|
|
FCS
|
fetal calf serum
|
References
|
1
|
Biswas C, Zhang Y, DeCastro R, Guo H,
Nakamura T, Kataoka H and Nabeshima K: The human tumor cell-derived
collagenase stimulatory factor (renamed EMMPRIN) is a member of the
immunoglobulin superfamily. Cancer Res. 55:434–439. 1995.PubMed/NCBI
|
|
2
|
Grass GD and Toole BP: How, with whom and
when: An overview of CD147-mediated regulatory networks influencing
matrix metalloproteinase activity. Biosci Rep. 36:e002832015.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Nakamura K, Kodama J, Hongo A and
Hiramatsu Y: Role of emmprin in endometrial cancer. BMC Cancer.
12:1912012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sameshima T, Nabeshima K, Toole BP,
Yokogami K, Okada Y, Goya T, Koono M and Wakisaka S: Glioma cell
extracellular matrix metalloproteinase inducer (EMMPRIN) (CD147)
stimulates production of membrane-type matrix metalloproteinases
and activated gelatinase A in co-cultures with brain-derived
fibroblasts. Cancer Lett. 157:177–184. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Koga K, Nabeshima K, Aoki M, Kawakami T,
Hamasaki M, Toole BP, Nakayama J and Iwasaki H: Emmprin in
epithelioid sarcoma: Expression in tumor cell membrane and
stimulation of MMP-2 production in tumor-associated fibroblasts.
Int J Cancer. 120:761–768. 2007. View Article : Google Scholar
|
|
6
|
Egawa N, Koshikawa N, Tomari T, Nabeshima
K, Isobe T and Seiki M: Membrane type 1 matrix metalloproteinase
(MT1-MMP/MMP-14) cleaves and releases a 22-kDa extracellular matrix
metalloproteinase inducer (EMMPRIN) fragment from tumor cells. J
Biol Chem. 281:37576–37585. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Nishio J, Iwasaki H, Nabeshima K, Ishiguro
M, Naumann S, Isayama T, Naito M, Kaneko Y, Kikuchi M and Bridge
JA: Establishment of a new human epithelioid sarcoma cell line,
FU-EPS-1: Molecular cytogenetic characterization by use of spectral
karyotyping and comparative genomic hybridization. Int J Oncol.
27:361–369. 2005.PubMed/NCBI
|
|
8
|
Sidhu SS, Mengistab AT, Tauscher AN,
LaVail J and Basbaum C: The microvesicle as a vehicle for EMMPRIN
in tumor-stromal interactions. Oncogene. 23:956–963. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Murayama T, Kataoka H, Koita H, Nabeshima
K and Koono M: Glycocalyceal Bodies in a human rectal carcinoma
cell line and their interstitial collagenolytic activities.
Virchows Arch B Cell Pathol Incl Mol Pathol. 60:263–270. 1991.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Baj-Krzyworzeka M, Szatanek R, Weglarczyk
K, Baran J, Urbanowicz B, Brański P, Ratajczak MZ and Zembala M:
Tumour-derived microvesicles carry several surface determinants and
mRNA of tumour cells and transfer some of these determinants to
monocytes. Cancer Immunol Immunother. 55:808–818. 2006. View Article : Google Scholar
|
|
11
|
Niiya D, Egawa N, Sakamoto T, Kikkawa Y,
Shinkawa T, Isobe T, Koshikawa N and Seiki M: Identification and
characterization of Lutheran blood group glycoprotein as a new
substrate of membrane-type 1 matrix metalloproteinase 1 (MT1-MMP):
A systemic whole cell analysis of MT1-MMP-associating proteins in
A431 cells. J Biol Chem. 284:27360–27369. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Koga K, Aoki M, Sameshima T, Hamasaki M,
Egawa N, Seiki M, Toole BP, Suzumiya J and Nabeshima K: Synthetic
emmprin peptides inhibit tumor cell-fibroblast
interaction-stimulated upregulation of MMP-2 and tumor cell
invasion. Int J Oncol. 39:657–664. 2011.PubMed/NCBI
|
|
13
|
Millimaggi D, Mari M, D'Ascenzo S, Carosa
E, Jannini EA, Zucker S, Carta G, Pavan A and Dolo V: Tumor
vesicle-associated CD147 modulates the angiogenic capability of
endothelial cells. Neoplasia. 9:349–357. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Guo H, Zucker S, Gordon MK, Toole BP and
Biswas C: Stimulation of matrix metalloproteinase production by
recombinant extracellular matrix metalloproteinase inducer from
transfected Chinese hamster ovary cells. J Biol Chem. 272:24–27.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sun J and Hemler ME: Regulation of MMP-1
and MMP-2 production through CD147/extracellular matrix
metalloproteinase inducer interactions. Cancer Res. 61:2276–2281.
2001.PubMed/NCBI
|
|
16
|
Kawakami T, Sameshima T, Hojo H, Koga K,
Nakahara Y, Toole BP, Suzumiya J, Okada Y, Iwasaki A and Nabeshima
K: Synthetic emmprin peptides with chitobiose substitution
stimulate MMP-2 production by fibroblasts. BMC Cancer. 11:3002011.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Cummings RD and Kornfeld S:
Characterization of the structural determinants required for the
high affinity interaction of asparagine-linked oligosaccharides
with immobilized Phaseolus vulgaris leukoagglutinating and
erythroagglutinating lectins. J Biol Chem. 257:11230–11234.
1982.PubMed/NCBI
|
|
18
|
Tang W, Chang SB and Hemler ME: Links
between CD147 function, glycosylation, and caveolin-1. Mol Biol
Cell. 15:4043–4050. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Cocucci E, Racchetti G and Meldolesi J:
Shedding microvesicles: Artefacts no more. Trends Cell Biol.
19:43–51. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Muralidharan-Chari V, Clancy JW, Sedgwick
A and D'Souza-Schorey C: Microvesicles: Mediators of extracellular
communication during cancer progression. J Cell Sci. 123:1603–1611.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Khalyfa A, Khalyfa AA, Akbarpour M, Connes
P, Romana M, Lapping-Carr G, Zhang C, Andrade J and Gozal D:
Extracellular microvesicle microRNAs in children with sickle cell
anaemia with divergent clinical phenotypes. Br J Haematol.
174:786–798. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhang H, Bai M, Deng T, Liu R, Wang X, Qu
Y, Duan J, Zhang L, Ning T, Ge S, et al: Cell-derived microvesicles
mediate the delivery of miR-29a/c to suppress angiogenesis in
gastric carcinoma. Cancer Lett. 375:331–339. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Grignani G and Jamieson GA: Tissue
factor-dependent activation of platelets by cells and microvesicles
of SK-OS-10 human osteogenic sarcoma cell line. Invasion
Metastasis. 7:172–182. 1987.PubMed/NCBI
|
|
24
|
Tsugita M, Yamada N, Noguchi S, Yamada K,
Moritake H, Shimizu K, Akao Y and Ohno T: Ewing sarcoma cells
secrete EWS/Fli-1 fusion mRNA via microvesicles. PloS One.
8:e774162013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Huang W, Luo WJ, Zhu P, Tang J, Yu XL, Cui
HY, Wang B, Zhang Y, Jiang JL and Chen ZN: Modulation of
CD147-induced matrix metalloproteinase activity: Role of CD147
N-glycosylation. Biochem J. 449:437–448. 2013. View Article : Google Scholar
|
|
26
|
Milia-Argeiti E, Mourah S, Vallée B, Huet
E, Karamanos NK, Theocharis AD and Menashi S:
EMMPRIN/CD147-encriched membrane vesicles released from malignant
human testicular germ cells increase MMP production through
tumor-stroma interaction. Biochim Biophys Acta. 1840:2581–2588.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Dominguez-Malagon H, Valdez-Carrillo MC
and Cano-Valdez AM: Dermatofibroma and dermatofibrosarcoma
protuberans: A comparative ultrastructural study. Ultrastruct
Pathol. 30:283–291. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Suzuki S, Sato M, Senoo H and Ishikawa K:
Direct cell-cell interaction enhances pro-MMP-2 production and
activation in co-culture of laryngeal cancer cells and fibroblasts:
Involvement of EMMPRIN and MT1-MMP. Exp Cell Res. 293:259–266.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kanekura T, Chen X and Kanzaki T: Basigin
(CD147) is expressed on melanoma cells and induces tumor cell
invasion by stimulating production of matrix metalloproteinases by
fibroblasts. Int J Cancer. 99:520–528. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Nabeshima K, Suzumiya J, Nagano M, Ohshima
K, Toole BP, Tamura K, Iwasaki H and Kikuchi M: Emmprin, a cell
surface inducer of matrix metalloproteinases (MMPs), is expressed
in T-cell lymphomas. J Pathol. 202:341–351. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Nabeshima K, Iwasaki H, Koga K, Hojo H,
Suzumiya J and Kikuchi M: Emmprin (basigin/CD147): Matrix
metalloproteinase modulator and multifunctional cell recognition
molecule that plays a critical role in cancer progression. Pathol
Int. 56:359–367. 2006. View Article : Google Scholar : PubMed/NCBI
|