Introduction
Urinary bladder cancer (UBC), which is mainly
accompanied by symptoms of intermittent hematuria and vesical
irritability, is the ninth most common type of cancer worldwide
(1) and the second most common
cancer of the genitourinary tract (2), with an estimated 76,960 newly
diagnosed cases and 16,390 deaths in the United States in 2016
(3). Cigarette smoking is the most
important risk factor for this heterogeneous cancer; other risk
factors include schistosoma haematobium, occupational exposure to
benzidine, β-naphthylamine, dyes or leather and physical trauma to
the uroepithelium from infection, instrumentation or the presence
of calculi. With respect to histopathology, transitional cell
carcinomas account for >90% of all bladder cancers, whereas
adenocarcinomas, squamous cell carcinomas (SCC) and
undifferentiated bladder carcinomas constitute the remaining 10% of
bladder cancers (4).
UBC displays the characteristic of frequent
progression both at the time of diagnosis and after initial
treatment. Such progression is the outcome of a tumorous natural
history that includes the two closely related processes of invasion
and metastasis. Tumor progression involves the migration of tumor
cells and the invasion of other tissues and remains the most common
cause of cancer-related deaths (5). Although non-muscle-invasive bladder
cancer (NMIBC) accounts for ~70% of UBCs at initial presentation
(6) and carries a 5-year survival
rate of 90% (7), NMIBC tumors
recur at a rate of 50–70% and progress at a rate of 15% in
recurrent UBC patients (8). When
NMIBC progresses to muscle-invasive bladder cancer (MIBC), which
may display the characteristics of metastatic malignant tumors and
subsequently lead to death, the 5-year survival rate falls to 60%
(7). Even worse, ~50% of MIBC
patients succumb to this disease despite optimal therapy (9), and ~80% of UBC patients with positive
lymph node metastases die within the first 5 years after initial
confirmed diagnosis (10,11). Tumor metastasis, which includes
hematogenous and lymphatic metastasis, is one of the most critical
aspects of tumor progression and contributes to ~90% of
cancer-associated deaths (12).
Despite advances in surgical techniques and in adjuvant
chemotherapies and immunotherapies resulting from recent extensive
studies of treatment options for advanced UBC with or without
metastatic disease, the 5-year survival rate for patients with
metastatic UBC was 5% (13), and
that of patients with lymph node metastasis was <30% (14). Although conventional
clinicopathological characteristics such as tumor grade and stage
provide important prognostic information in UBC, they are of
limited use in the prediction of tumor recurrence, progression,
treatment response, and survival (15), partially due to the shortcomings of
staging and grading subjectivity that can lead to high
interobserver variability (16).
Therefore, the investigation of novel molecular markers that are
positively associated with tumor progression, metastasis and
survival and determination of the molecular mechanisms of these
markers in tumor progression are crucial for improving poor
survival in UBC and developing a more exact prognosis-predictive
and therapeutic strategy.
Various chemokines and their specific receptors have
been shown to be involved in tumor progression, and some receptors
have been particularly proposed as biomarkers for predicting
survival, such as the CXCL12/CXCR7 axis in pancreatic (17), the CXCL12/CXCR4 axis in breast
(18,19) and oral SCC (20), the CCL5/CCR1 axis in prostate
(21) and the CCL21/CCR7 axis in
gastric cancer (22). Among these,
the most important chemokine/receptor axes are CXCL12/CXCR4 and
CCL21/CCR7, mainly because of their determinant role in regulating
the directional migration and organ-selective metastasis of tumor
cells. An early breast cancer study showed significantly elevated
CXCR4 and CCR7 expression in breast cancer cell lines and in tissue
samples from patients and from mice in an established breast cancer
model compared with normal controls (18). Given the preferential expression of
CXCL12 in lung, liver and bone marrow and of CCL21 in lymph nodes
found in the present study, the authors concluded that the
CXCL12/CXCR4 axis may be responsible for metastasis to lung, liver
and bone marrow and that CCL21 may be responsible for metastasis to
lymph nodes (18). Notably, these
hypotheses were confirmed by the results of subsequent experiments
in vivo.
In the present study, we were most interested in the
expression profile of CCR7 and the significance of the CCL21/CCR7
axis in progression, metastasis and survival in human UBC.
Therefore, in the present study, we have initially evaluated the
expression of CCR7 by immunohistochemical staining and determined
its correlation with clinicopathological characteristics in 62
closely screened UBC patients. In addition, we investigated the
microlymphatic vessel density (MLVD) and the microvessel density
(MVD) in this cohort and determined their associations with CCR7
expression and clinicopathological parameters. These data provide
preliminary evidence for a significant role of CCR7 in tumor
progression, angiogenesis, lymphangiogenesis, survival and
prognosis in UBC. In addition, the invasion and migration abilities
of UBC cell lines with or without the intervention of CCL21
treatment or CCR7 gene silencing were tested in vitro. To
further elucidate the mechanisms through which the CCL21/CCR7 axis
regulates tumor behavior, the activities of the AKT and ERK-1/2
signaling pathways were also analyzed.
Materials and methods
Cell lines and reagents
The human bladder cancer cell lines T24, 5637,
UM-UC-3 and RT4, as well as the SV-40 immortalized human
uroepithelial cell line SV-HUC-1, which was used as a normal
control, were purchased from the Cell Bank of the Chinese Academy
of Sciences (Shanghai, China). The cells were cultured in
Dulbecco's modified Eagle's medium (DMEM; Gibco, Grand Island, NY,
USA) (UM-UC-3), RPMI-1640 medium (T24, 5637 and RT4) and F12K
medium (SV-HUC-1) supplemented with 10% fetal bovine serum (FBS;
Gibco), 100 units/ml penicillin, 100 μg/ml streptomycin and
2 mmol/l glutamine at 37°C in a humidified chamber supplemented
with 5% CO2. When the cells reached 80–90% confluency,
they were treated with various concentrations of recombinant human
CCL21 purchased from PeproTech (Rocky Hill, NJ, USA) or with
specific concentrations of CCL21 for 48 h to analyze the effect of
activation of the CCL21/CCR7 axis on the invasion and migration
capacity of UBC cells and the expression of possible related
signaling pathway biomarkers.
Streptavidin-peroxidase (SP) ready-to-use kits,
diaminobenzidine (DAB) tetrahydrochloride chromogenic reagent and
mouse monoclonal antibody against CD34 were purchased from Beijing
Zhongshan Golden Bridge Biotechnology (Beijing, China). Rabbit
monoclonal antibody to CCR7, rabbit polyclonal antibody to
total-ERK1/2 and rabbit monoclonal antibody to phospho-ERK1/2 were
obtained from Abcam (Cambridge, MA, USA). Rabbit polyclonal
antibody to AKT and rabbit polyclonal antibody to phospho-AKT were
from ImmunoWay Biotechnology Inc., (Newark, DE, USA). Mouse
monoclonal antibody to β-actin and horseradish peroxidase
(HRP)-conjugated secondary antibodies were from Santa Cruz
Biotechnology (Santa Cruz, CA, USA). Mouse monoclonal antibody to
D2-40 was from Dako (Carpinteria, CA, USA). RIPA lysis buffer and
the BCA protein assay kit were from Beyotime Institute of
Biotechnology (Haimen, China). Sodium dodecyl sulfate
polyacrylamide gel electrophoresis (SDS-PAGE) loading buffer was
from Auragene Bioscience (Changsha, China). Lipofectamine 2000 was
from Invitrogen (Carlsbad, CA, USA). Small interfering RNA (siRNA)
oligonucleotides targeting the CCR7 gene and a control scrambled
sequence siRNA were designed and synthesized by Shanghai GenePharma
Co., Ltd. (Shanghai, China). PD98059 was obtained from
Sigma-Aldrich (St. Louis, MO, USA).
Patient population and tissue specimen
collection
After obtaining approval from the Xiangya Hospital
Institutional Review Board, the clinical and pathological data of
consecutive UBC patients who underwent radical cystectomy with
bilateral pelvic and iliac lymphadenectomy at the authors'
institution between June 2006 and June 2011 were reviewed. We
included only patients for whom sufficient tissue and valid
follow-up data were available and from whom written informed
consent was obtained before they were enrolled in the study. We
also excluded patients who received neoadjuvant chemotherapy or
radiotherapy and those who exhibited synchronous or metachronous
cancer in other organs, which finally left 62 patients for the
analysis.
Tissue specimens were immediately formalin-fixed
after surgery and then embedded in paraffin for immunohistochemical
analysis. Sections from each case were stained with hematoxylin and
eosin and investigated by light microscopy to confirm
histopathology. The pathological tumor stage and grade were
assigned according to the 2009 UICC-TNM classification system and
the 2004 WHO system, respectively. Eight additional samples of
normal bladder tissues were also obtained from sites apparently
distant from the tumors. All procedures were performed in
accordance with the ethical principles set forth in the Declaration
of Helsinki. We had no access to information that could be used to
identify individual participants during or after data
collection.
Immunohistochemical staining and
evaluation
UBC tissue samples were fixed in 10% formalin,
embedded in paraffin, and cut into 4-μm slices.
Immunohistochemical staining was performed on a single
representative block from each case using the
streptavidin-peroxidase method with an SP ready-to-use kit
according to the manufacturer's instructions. Tissue blocks with at
least 1 mm of peripheral tissue surrounding the tumor mass were
selected. The tissue blocks were deparaffinized by incubating them
twice in xylenol for 10 min at room temperature, dehydrated in a
graded ethanol series (50, 75 and 100%), incubated in 3% hydrogen
peroxide for 30 min to block endogenous peroxidases, subjected to
antigen retrieval in 10 mmol/l citric acid buffer (pH 6.0) in a
microwave oven for 10 min and allowed to cool at room temperature.
Following three 5-min washes in phosphate-buffered saline (PBS),
the sections were treated with normal goat serum for 10 min at room
temperature to block non-specific antibody reactions. The sections
were further incubated overnight at 4°C with the following diluted
primary antibodies according to the manufacturer's instructions:
rabbit monoclonal anti-CCR7 (1:200), mouse monoclonal anti-CD34
(1:200) and mouse monoclonal anti-D2-40 (1:50). The sections were
then sequentially incubated with anti-goat polyvalent antibody for
10 min and with streptavidin-peroxidase for 10 min and subsequently
visualized by incubation in 0.03% DAB tetrahydrochloride
chromogenic reagent followed by counterstaining with hematoxylin.
For negative controls, PBS was used as a substitute for the primary
antibody. Human spleen tissues and normal lymph nodes were used as
positive controls for CCR7 and D2-40 staining, respectively, as
suggested by the manufacturer.
For each tissue section, immunohistochemical
detection of CCR7 expression was evaluated independently by two
pathologists who were blinded to the patients' clinicopathological
data. Expression was evaluated using a semi-quantitative scoring
system that depended on the percentage of positively stained tumor
cells and the staining intensity. The percentage of positively
stained cells was graded on a semi-quantitative 5-point scale as
follows: 0 (none); 1 (1–10%); 2 (11–50%); 3 (51–75%); and 4
(76–100%). The immunostaining intensity was graded on a
semi-quantitative 4-point scale as follows: 0 (none, equivalent to
the negative control); 1 (weak, slightly darker than the negative
control); 2 (moderate, equivalent to an intensity between that of
scores 1 and 3); and 3 (intense, equivalent to or darker than the
positive control). Then, the percentage of positively stained cells
and the staining intensity score were multiplied to yield a final
score ranging from 0 to 12. Borderline cases were re-evaluated by
joint review and discussion or by consultation with a third
investigator familiar with immunohistochemical evaluation of
pathology.
Evaluation of MLVD and MVD
For evaluation of MLVD and MVD, tissue sections were
stained with D2-40 (a monoclonal antibody containing 166 amino
acids that recognizes a sialoglycoprotein expressed in lymphatic
endothelial cells with high specificity and affinity and thus is
widely used for labeling microlymphatic vessels) and CD34 (a highly
glycosylated transmembrane glycoprotein that is expressed in
vascular endothelial cells and thus is widely used for labeling
microvessels) antibodies, respectively. MLVD and MVD were assessed
by two independent pathologists who had no prior knowledge of the
patient information according to the following steps: Firstly,
areas with the most intense vascularization (hot spots) were
selected under low-power magnification (×40 or ×100); secondly,
average MLVD/MVD was measured at high-power magnification (×200) in
3 randomized fields within the hot spots following agreement of the
two pathologists. For each section, MLVD/MVD was defined as the
average number of positively stained vessels per high-power field
(HPF) in three HPFs within the selected hot spots. Hot spots within
both the intratumoral area, which was defined as the area within
the tumor mass, and peritumoral area, which was defined as the area
within 1 mm of the tumor border, were analyzed. In addition to
distinct stained vessels, single immunoreactive endothelial cells
or clusters of immunoreactive endothelial cells (containing
brown-colored particles due to the positive expression of CD34 or
D2-40) that were clearly separated from the adjacent clusters or
vessels, regardless of the presence of lumen, were also counted as
one microvessel or as one microlymphatic vessel.
CCR7 gene silencing by siRNA
The target sequences of three siRNAs of CCR7 and one
siRNA with a scrambled sequence, which served as a non-silencing
control due to its random sequence that does not target any gene
product, were as follows: CCR7 siRNA1 sense:
5′-GCGUCCUUCUCAUCAGCAAdTdT-3′ and antisense:
5′-UUGCUGAUGAGAAGGACGCdTdT-3′; CCR7 siRNA2 sense:
5′-GCUGGUCGUGUUGACCUAUdTdT-3′ and antisense:
5′-AUAGGUCAACACGACCAGCdTdT-3′; CCR7 siRNA3 sense:
5′-GAUGAGGUCACGGACGAUUdTdT-3′ and antisense:
5′-AAUCGUCCGUGACCUCAUCdTdT-3′; scrambled siRNA sense:
5′-AGUUCAACGACCAGUAGUCdTdT-3′ and antisense:
5′-GACUACUGGUCGUUGAACUdTdT-3′. Cells in 6-well culture plates were
transfected with siRNA using Lipofectamine 2000 transfection
reagent according to the manufacturer's instruction, and the
transfection efficiency was assessed by western blot assay. Cells
that showed effective depletion of CCR7 were selected for use in
cell migration assays, cell invasion assays and western blot
analysis.
Protein extraction and western blot
analysis
Cells were lysed in RIPA lysis buffer for 30 min at
4°C and centrifuged at 130,00 rpm for 20 min at 4°C; the protein
concentration of the lysate was measured using the BCA protein
assay kit. Protein samples were mixed with an equal amount of
SDS-PAGE loading buffer and separated using 10% SDS-PAGE. After
electrophoresis, proteins were transferred to polyvinylidene
fluoride membranes by semi-dry electrophoretic transfer. The
membranes were blocked in 5% skim milk and incubated with primary
antibodies to CCR7 (1:5,000), phospho-ERK (1:500), total-ERK
(1:2,000), phospho-AKT (1:2,000), and total-AKT (1:2,000) overnight
at 4°C. Monoclonal β-actin antibody (1:500) was used to provide a
loading control. The membranes were washed five times in TBST and
incubated with HRP-conjugated secondary antibodies. Immunoreactive
bands were imaged with an EC3 300 Imaging System (UVP LLC, Upland,
CA, USA), and the OD values corresponding to the image intensity
were measured using Image-Pro Plus version 6.0 (IPP6.0; Media
Cybernetics, Inc., Rockville, MD, USA) software.
Wound-healing assay
To assess the migration ability of human UBC cells,
a wound-healing assay was performed. Cells were plated in 6-well
culture dishes and incubated with DMEM containing 10% FBS with or
without CCL21 treatment for 24 h at 37°C under 5% CO2.
When the cells had formed a confluent monolayer, a scratch was made
with a fine pipette tip, forming a linear wound in the central area
of the cell monolayer, and the detached cells were carefully
removed using PBS. The wound area was photographed at the beginning
of the experiment and at 24 and 48 h after wounding the cell
monolayer. Cell migration was assessed by measuring the size of the
wound gap in at least six fields. The wound-healing assay was
performed in triplicate.
Matrigel invasion assay
To assess the invasion ability of human UBC cells, a
Matrigel invasion assay was performed using a polycarbonate
membrane Transwell chamber containing a filter 6.5 mm in diameter
with 8-μm pores (Corning Inc., Corning, NY, USA). The
Transwell filter was precoated with basement membrane Matrigel (BD
Biosciences, San Jose, CA, USA). Briefly, cells pretreated with or
without CCL21 for 48 h were resuspended in serum-free DMEM medium,
and 300 μl of the cell suspension (5×104 cells)
was added to the upper chamber of the device. DMEM containing 10%
FBS was added to the lower chamber as a chemoattractant. After
incubation for 24 h at 37°C under 5% CO2, non-invasive
cells on the upper surface of the filter were removed completely
and carefully using cotton swabs, and the lower surface of the
filter was fixed in 4% formaldehyde and stained with 0.5% crystal
violet for 20 min. After washing the filter twice with PBS, the
cells on the lower side of the filter were photographed using an
inverted microscope (×100 magnification), and absorbance was
measured at 570 nm. The Matrigel invasion assay was performed in
triplicate.
Statistical analysis
Data are presented as the mean ± standard deviation
(SD) of the values obtained in three independent experiments or as
the median (minimum-maximum) for non-normally distributed data.
Qualitative variables such as sex, histological grade, tumor stage
and lymph node involvement are presented as frequencies or
percentages. Qualitative variables were analyzed using the
Chi-square test or Fisher's exact test as appropriate. Quantitative
variables were compared using the independent t-test or one-way
analysis of variance (ANOVA) to compare differences in 2 or more
groups, and the least significant difference (LSD) t-test, the
Dunnett's t-test or the Student-Newman-Keuls (SNK) q-test, as
appropriate, were used for post hoc subgroup analysis. Survival
curves were calculated using the Kaplan-Meier method, and
differences in the survival curves were examined using the log-rank
test. Cox's proportional hazard regression model was used for
multivariate analysis to assess the effect of tumor variables on
the prognosis of UBC. Statistical analysis was performed using SPSS
statistical software, version 19.0 (IBM, Armonk, NY, USA). The
significance of differences was accepted at P<0.05
(2-tailed).
Results
Correlation between CCR7 expression and
clinicopathological parameters and prognosis in 62 UBC
patients
The expression and distribution of CCR7 protein in
UBC tissues of different grades and stages, including papillary
urothelial neoplasm of low malignant potential (PUNLMP), low-grade
urothelial carcinoma (LGPUC) and high-grade urothelial carcinoma
(HGPUC), as well as stage T1 (tumor invades the subepithelial
connective tissue), stage T2 (tumor invades the muscularis propria
bladder wall), stage T3 (tumor invades the perivesical tissue), and
stage T4 (tumor invades any of the following: prostate, uterus,
vagina, pelvic wall, or abdominal wall) cancerous tissues, were
measured by immunohistochemical analysis in clinical samples
obtained from a total of 62 patients (49 men and 13 women) with
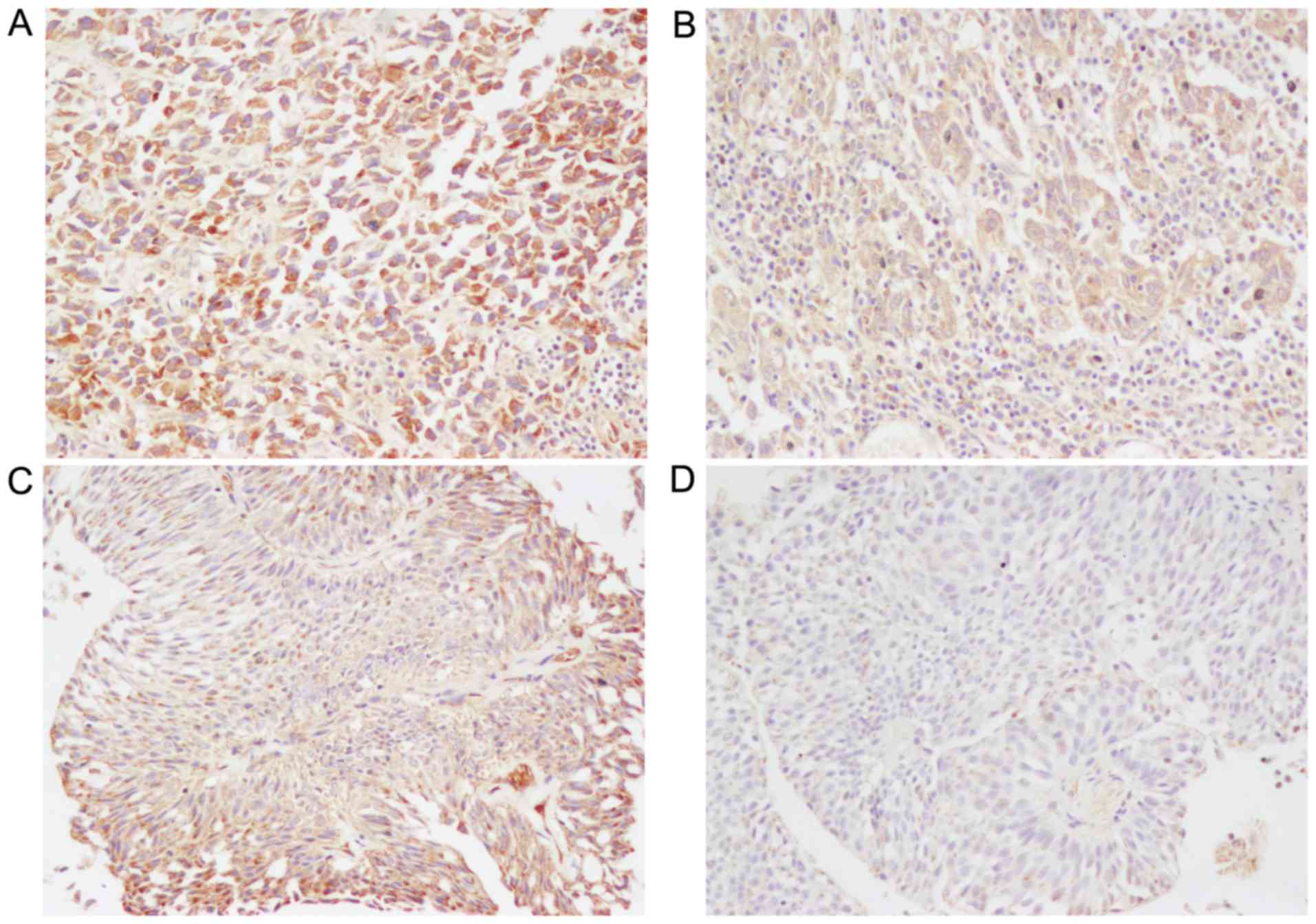
UBC. Fig. 1 shows representative
immunostaining of CCR7 protein, which was positively stained only
in the cytoplasm and cell membranes of UBC cells. The majority of
cases showed a heterogeneous staining pattern that was
characterized by variable staining intensity within the same
section or field (Fig. 1C). The
average CCR7 staining score for all 62 tumors was 5.80±0.04.
The clinicopathological data of all cases are
summarized in Table I. The
follow-up time was defined as the interval between surgery and
death or between surgery and the last observation for surviving
patients. The median (range) follow-up time after operation was
43.5 months (6–74 months). Thirty-nine patients were alive at the
last follow-up examination; the others died of metastasis, local
tumor invasion, severely compromised immunity and other causes. The
median age of the study subjects at the time of treatment was 60.5
years (range, 34–77 years).
 | Table ICorrelations between
clinicopathological characteristics and CCR7, MLVD and MVD in 62
urinary bladder cancer patients. |
Table I
Correlations between
clinicopathological characteristics and CCR7, MLVD and MVD in 62
urinary bladder cancer patients.
|
Characteristics | No. of cases | CCR7 expression
| MLVD
| MVD
|
|---|
| High (n=40) | Low (n=22) | P-value | MLVD/HPF (mean ±
SD) | P-value | MVD/HPF (mean ±
SD) | P-value |
|---|
| Age (years) | | | | 0.618a | | 0.872c | | 0.632c |
| <60 | 28 | 19 | 9 | | 6.40±2.06 | | 34.52±6.33 | |
| ≥60 | 34 | 21 | 13 | | 6.59±2.43 | | 33.74±7.26 | |
| Sex | | | | 0.756b | | 0.463c | | 0.164c |
| Male | 49 | 31 | 18 | | 6.37±2.53 | | 34.83±6.27 | |
| Female | 13 | 9 | 4 | | 7.02±2.09 | | 31.32±7.56 | |
| Primary tumor
(pT)e | | | | 0.015a | | 0.027c | | 0.016c |
| pT1-T2 | 24 | 11 | 13 | | 3.87±2.37 | | 30.81±8.36 | |
| pT3-T4 | 38 | 29 | 9 | | 8.17±3.16 | | 36.17±7.24 | |
| Regional lymph
nodes(pN)e | | | | 0.008a | | 0.006c | | 0.385c |
| pN0 | 40 | 21 | 19 | | 4.03±2.03 | | 33.43±7.82 | |
| pN1-N3 | 22 | 19 | 3 | | 11.00±2.64 | | 35.30±6.59 | |
| Classification of
2004 | | | | 0.010a | | 0.246d | | 0.447d |
| WHO grading
system | | | | | | | | |
| PUNLMP | 5 | 2 | 3 | | 5.23±2.28 | | 34.16±8.93 | |
| LGPUC | 24 | 11 | 13 | | 5.45±2.74 | | 33.78±7.02 | |
| HGPUC | 33 | 27 | 6 | | 7.46±2.93 | | 34.31±7.40 | |
For statistical analyses, the mean CCR7 staining
score was used as a cut-off to separate tissue sections with high
CCR7 expression (final scores ranging from 6 to 12) from those with
low CCR7 expression (final scores ranging from 0 to 4). The
correlations between CCR7 immunoreactivity and the
clinicopathological characteristics of the subjects are summarized
in Table I. High expression of
CCR7 protein was identified in 64.52% (40/62) of the tumors, and
low expression was identified in 35.48% (22/62) of the tumors. CCR7
expression was significantly higher in patients with lymph node
status of pN1-N3 than in patients with lymph node status of pN0
(P=0.008, Chi-square test) and was significantly associated with
primary tumor stage (P=0.015; Chi-square test) and tumor grade
(P=0.010; Chi-square test), whereas there was no significant
correlation between CCR7 expression and patient age (P>0.05;
Chi-square test) or sex (P>0.05; Fisher's exact test). Patients
with high CCR7 expression exhibited a significantly worse overall
survival rate than those with low CCR7 expression by the log-rank
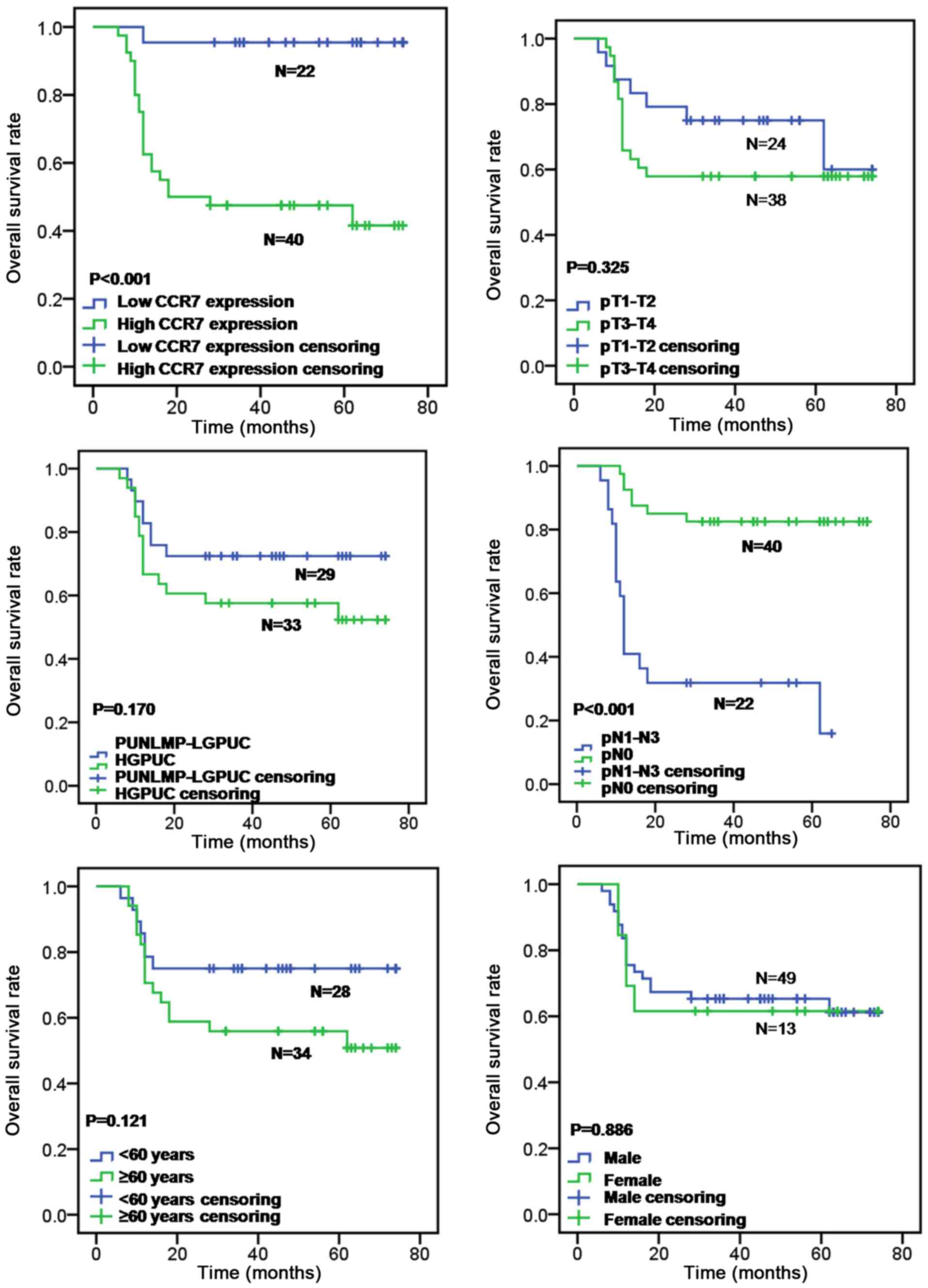
test (P<0.001; Fig. 2).
Furthermore, lymph node metastasis was also correlated with overall
survival rate (log-rank test, P<0.001; Fig. 2), whereas patient's age (log-rank
test, P=0.121; Fig. 2), sex
(log-rank test, P=0.886; Fig. 2),
primary tumor stage (log-rank test, P=0.325; Fig. 2) and tumor grade (log-rank test,
P=0.170; Fig. 2) had no prognostic
significance for overall survival. Prognostic factors of UBC and
their variable assignment as determined by the multivariate
analysis are shown in Table II.
By multivariate analysis based on the Cox's proportional hazard
regression model, CCR7 protein expression level and pN were
independent prognostic factors for overall survival in UBC patients
(HR, 10.413, 95% CI, 1.242–87.313, P=0.031 and HR, 0.211, 95% CI,
0.083–0.534, P=0.001, respectively), whereas, the patient's sex,
age, tumor grade and primary tumor stage were not independent
prognostic factors (P=0.318, P=0.792, P=0.816 and P=0.351,
respectively) (Table III). These
results indicate that high levels of CCR7 expression may be
associated with lymph node metastasis and poor overall survival in
patients with UBC.
 | Table IIPrognostic factors of urinary bladder
cancer and their variable categorization. |
Table II
Prognostic factors of urinary bladder
cancer and their variable categorization.
| Prognostic
factors | Variable names | Variable
values |
|---|
| Age | X1 | 0=<60 years,
1=≥60 years |
| Sex | X2 | 0=male,
1=female |
| pT | X3 | 0=pT1-T2,
1=pT3-T4 |
| pN | X4 | 0=pN1-N3,
1=pN0 |
| Pathological
grade | X5 | 0=PUNLMP-LGPUC,
1=HGPUC |
| CCR7
expression | X6 | 0=low, 1=high |
| Survival time | t | (months) |
| Survival
status | Y | 0=censoring,
1=death |
 | Table IIICox multivariate analysis of the
factors associated with overall survival. |
Table III
Cox multivariate analysis of the
factors associated with overall survival.
| Variables | HR | 95% CI | P-value |
|---|
| Age (<60 vs. ≥60
years) | 0.854 | 0.264–2.761 | 0.792 |
| Sex (male vs.
female) | 1.706 | 0.598–4.869 | 0.318 |
| pT (pT1-T2 vs.
pT3-T4) | 1.583 | 0.603–4.157 | 0.351 |
| pN (pN1-N3 vs.
pN0) | 0.211 | 0.083–0.534 | 0.001 |
| Pathological grade
(PUNLMP-LGPUC vs. HGPUC) | 1.141 | 0.374–3.488 | 0.816 |
| CCR7 (low vs.
high) | 10.413 | 1.242–87.313 | 0.031 |
Identification of D2-40-positive
lymphatic vessels and CD34-positive blood vessels and measurement
of MVD/MLVD
D2-40 positive staining was seen in thin-walled
vessels devoid of erythrocytes, but no D2-40 staining was observed
in tumor cells or blood vessels, indicating that D2-40 is specific
for the lymphatic vasculature (Fig. 3A
and B). D2-40-positive lymphatic vessels and CD34-positive
blood vessels were detected in all tumor samples, and
D2-40-positive lymphatic vessels were detected within peritumoral
areas in 62 (100%) cases and within intratumoral areas in 21
(33.87%) cases. The morphological characteristics of D2-40-positive
lymphatic vessels within intratumoral and peritumoral areas are
shown in Fig. 3A and B.
Peritumoral lymphatic vessels had more dilated lumina, were denser
and more numerous, and showed a greater number of hotspots than
intratumoral lymphatic vessels, which appeared collapsed and
elongated. The anatomic locational correlation of peritumoral
lymphatics with blood vessels was higher than that of intratumoral
lymphatics (Fig. 3A and B). With
regard to the eight normal bladder tissues obtained from obviously
non-tumorous regions of bladder far from the tumor boundaries in
patients who underwent radical cystectomy, both D2-40-positive
lymphatic vessels and CD34-positive blood vessels were present in
all normal control tissues, but were found only in the lamina
propria and submucosa, and no epithelial expression was found
(Fig. 3C). The average MLVD of
normal bladder tissues was (2.56±1.08)/HPF, which was significantly
lower than the average peritumoral MLVD [(6.50±2.01)/HPF;
P<0.001, SNK q-test; Fig. 3H]
and higher than the average intratumoral MLVD [(2.08±0.92)/HPF;
P>0.05, SNK q-test; Fig. 3H],
but the MLVD did not differ significantly between normal control
tissues and intratumoral areas. Unlike the distribution of
D2-40-positive vessels in serial tumor sections, CD34-positive
blood vessels were homogeneously present in the peritumoral and
intratumoral areas in the great majority of cases (Fig. 3D). The average MVDs of normal
bladder tissues, peritumoral areas and intra-tumoral areas were
(22.40±4.59)/HPF, (15.33±10.23)/HPF and (34.09±7.26)/HPF,
respectively, and the differences between any two groups were
significant (P<0.05, SNK q-test; Fig. 3G). Inflammatory infiltration by
lymphocytes was frequently observed in the peritumoral area
(Fig. 3D).
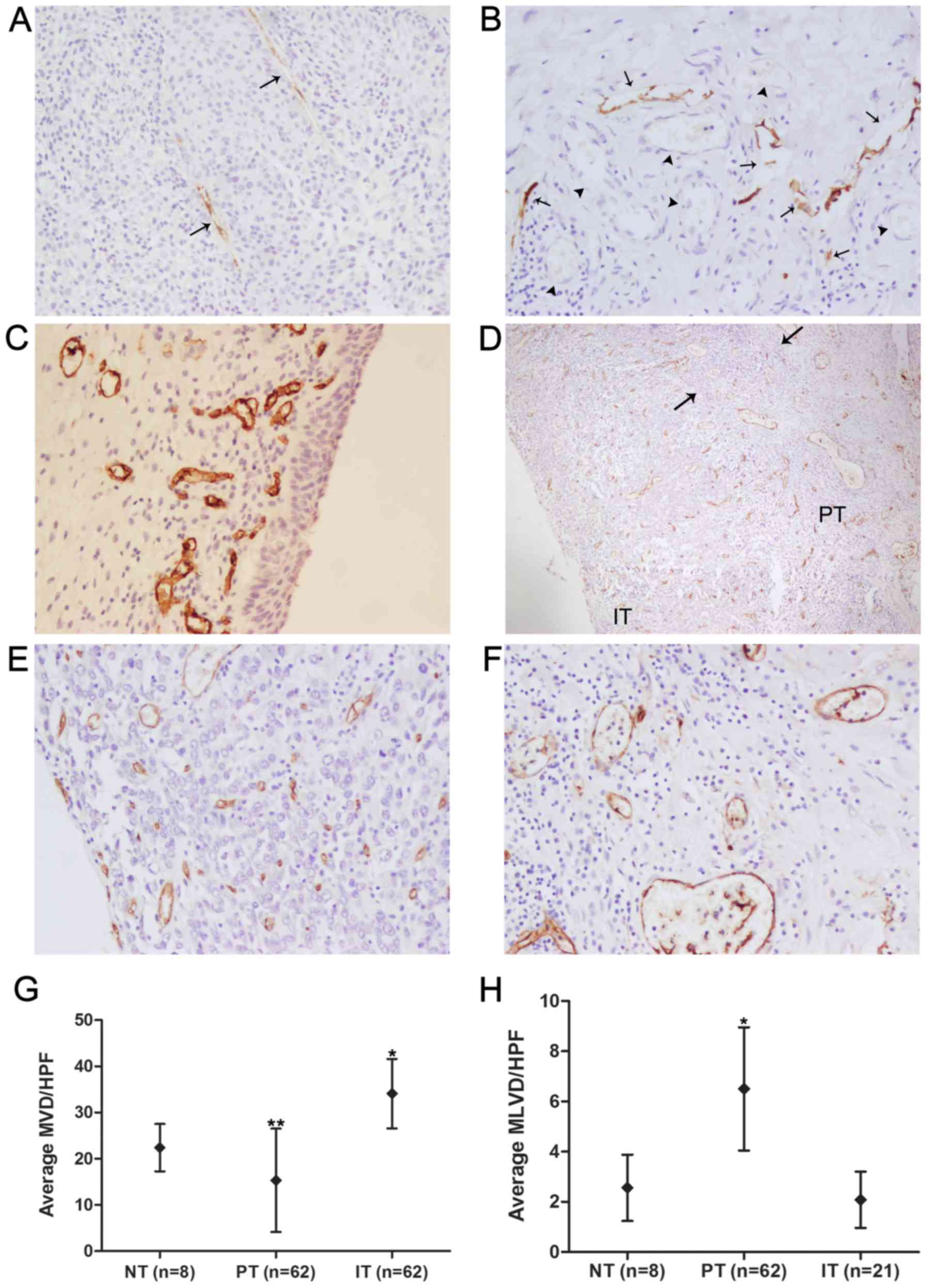 | Figure 3Immunohistochemical detection of
D2-40 and CD34 proteins in patient with urinary bladder cancer. (A)
Intratumoral lymphatic vessels (arrows) detected with D2-40
antibody in urinary bladder cancer samples presented collapsed and
elongated size. (B) Peritumoral lymphatic vessels (arrows) detected
with D2-40 antibody in urinary bladder cancer samples were found to
be more dilated in the lumen, more numerous in vessel density and
closer to anatomic locational correlation with blood vessels
(arrowheads), which were identified by the characteristic of
intraluminal erythrocytes compared with intratumoral lymphatics.
(C) CD34-positive vessels representing blood vessels in normal
control tissues were detected in the lamina propria and submucosa,
while the epithelium expression was not found. (D) CD34-positive
blood vessels were homogeneously present in the peritumoral (PT)
and intratumoral areas (IT) of urinary bladder cancer cases, and
inflammatory infiltration (the area between the two arrows) by
lymphocytes was observed in the peritumoral area. Representative
intratumoral and peritumoral blood vessels detected with CD34
antibody are shown, respectively (E and F). (G) Intratumoral MVD
(n=62) was the highest, followed by the MVD of normal bladder
tissues (NT, n=8) and peritumoral MVD (n=62). *P<0.05
compared to the PT group and the NT group, **P<0.05
compared to the IT group and the NT group (one-way ANOVA followed
by the SNK q-test). (H) Peritumoral MLVD (n=62) was the highest,
followed by the MLVD of normal bladder tissues (n=8) and
intratumoral MLVD (n=21). *P<0.001 compared to the IT
group and the NT group (one-way ANOVA followed by the SNK q-test).
Original magnification, ×200 in A, B, C, E and F; ×40 in D. |
Correlation of MVD/MLVD with
clinicopathological parameters and CCR7 expression in 62 UBC
patients
Microlymphatic vessel density per high-power field
(MLVD/HPF) and microvessel density per high-power field (MVD/HPF)
were assessed by immunohistochemical staining of 62 UBC tissues
with antibodies against CD34 and D2-40. Due to the low detection
rate and potentially non-functional characteristics of collapsed
intratumoral lymphatic vessels, we only studied the relationship of
peritumoral MLVD with clinical pathological parameters. As shown in
Table I, a significantly higher
MLVD/HPF was observed in patients with primary tumor stage of
pT3-T4 (P=0.027) and lymph node status of pN1-N3 (P=0.006) than in
patients with primary tumor stage of pT1-T2 and lymph node status
of pN0. However, no obvious relationship was found between MLVD/HPF
and patient age, sex or tumor grade (all P>0.05). In addition,
the data also showed that higher MVD/HPF was significantly related
to a primary tumor stage of pT3-T4 (P=0.016) rather than pT1-T2.
MVD/HPF, however, was not significantly associated with patient
age, sex, lymph node metastasis or tumor grade (all P>0.05).
For further analysis of the possible correlation
between CCR7 expression and MLVD/MVD, the 62 UBC patients were
grouped into a low-CCR7-expression group and a high-CCR7-expression
group according to their CCR7 expression levels. Table IV shows that increased CCR7
expression was significantly correlated with higher MLVD/HPF
(P=0.014) and higher MVD/HPF (P=0.002). These results imply that
CCR7 is a promoting factor that induces both lymphangiogenesis and
angiogenesis but that it may be correlated with lymph node
metastasis by virtue of its lymphangiogenic role rather than its
angiogenic role.
 | Table IVCorrelations between CCR7 expression
and MVD/MLVD in 62 urinary bladder cancer patients. |
Table IV
Correlations between CCR7 expression
and MVD/MLVD in 62 urinary bladder cancer patients.
| CCR7
expression | No. of cases | MLVD
| MVD
|
|---|
| MLVD/HPF (mean ±
SD) | P-value | MVD/HPF (mean ±
SD) | P-value |
|---|
| High | 40 | 8.11±2.39 | 0.014a | 37.03±8.03 | 0.002a |
| Low | 22 | 3.57±1.84 | | 28.76±7.19 | |
Influence of the CCL21/CCR7 axis on the
migration and invasion capacity of UBC cells
Prior to the investigation of the effect of the
CCL21/CCR7 axis on the migration and invasion ability of UBC cells,
we screened the constitutive expression of CCR7 in T24, 5637,
UM-UC-3, RT4 UBC cells and SV-HUC-1 normal uroepithelial cells by
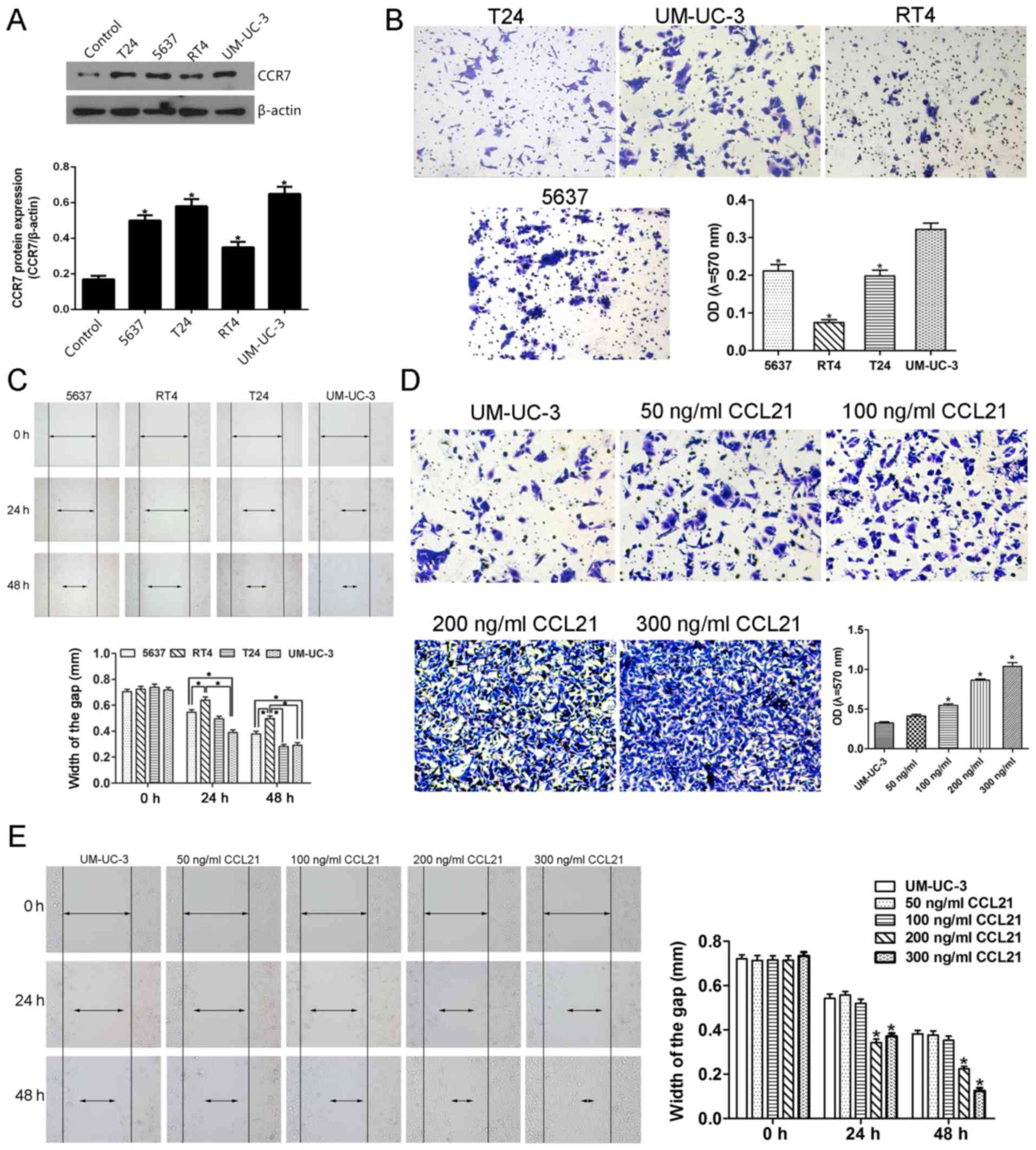
western blot analysis. A significantly higher CCR7 expression level
was observed in T24, 5637, UM-UC-3 and RT4 UBC cell lines than in
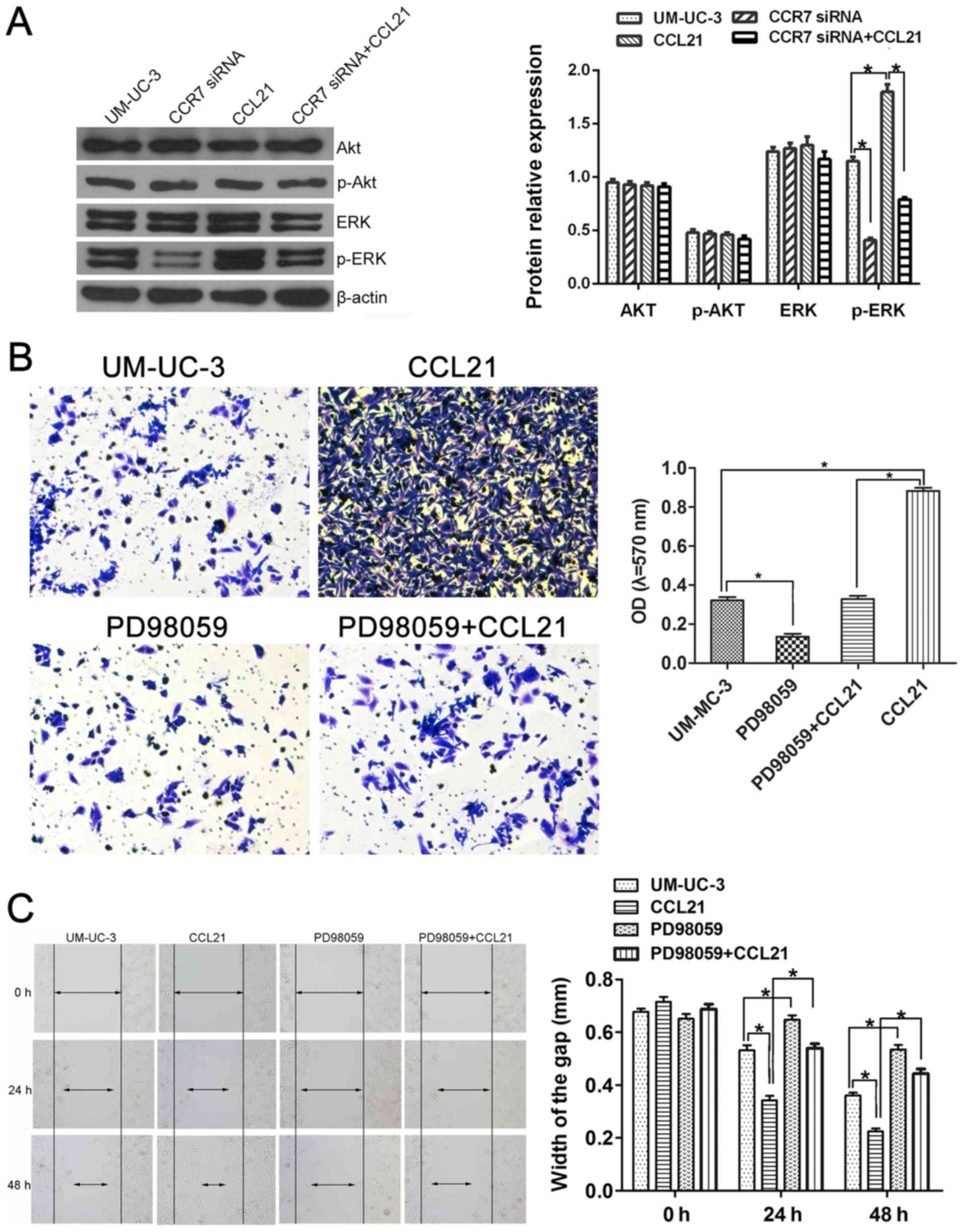
SV-HUC-1 cell line which was used as the control cells (Fig. 4A). Furthermore, after treatment
with 200 ng/ml CCL21, the migration ability of the UM-UC-3 cells
assessed by the mean of wound-healing assay was significantly
enhanced at 24 h compared with other cells, and that of UM-UC-3 and
T24 cells was significantly enhanced at 48 h compared with other
cells (Fig. 4C). Similarly, the
invasive ability of UM-UC-3 cells determined by the mean of
Matrigel invasion assay using a polycarbonate membrane Transwell
chamber was significantly higher than that of other UBC cells
(Fig. 4B). Therefore, based on the
results that the migration and invasion abilities of UM-UC-3 UBC
cells were significantly higher than that of other UBC cells, the
UM-UC-3 UBC cell line was selected for the following in
vitro study even though the T24, 5637 and UM-UC-3 cells show
similar CCR7 protein expression level (Fig. 4A).
The effect of CCL21 at various concentrations on the
invasion and migration capacity of UM-UC-3 cells is shown in
Fig. 4D and E. To determine
whether CCL21 was able to modulate invasion ability in UM-UC-3
cells, the Matrigel invasion assay was used to evaluate the cell's
invasion ability. As presented in Fig.
4D, the OD of the cell suspensions of CCL21-treated cells
increased gradually and significantly as the concentration of CCL21
was increased from 100 to 300 ng/ml, indicating that CCL21
treatment significantly enhanced the invasion ability of UM-UC-3
cells in a dose-dependent manner (P<0.05). When the UM-UC-3
cells were treated with CCL21 at 50 and 100 ng/ml, the migration
ability of the cells did not change significantly (Fig. 4E). However, as the treatment time
increased and as the concentration of CCL21 protein was increased
to 100 ng/ml and higher, an obvious effect of increased cell
migration occurred. These results show that treatment of UM-UC-3
cells with CCL21 enhances their migration ability in a dose- and
time-dependent manner.
To confirm the influence of the CCL21/CCR7 axis on
the migration and invasion capacity of UM-UC-3 cells, small
interfering RNAs (siRNAs) targeting the CCR7 gene were used for
CCR7 knockdown, and exogenous CCL21 was used for CCR7 activation.
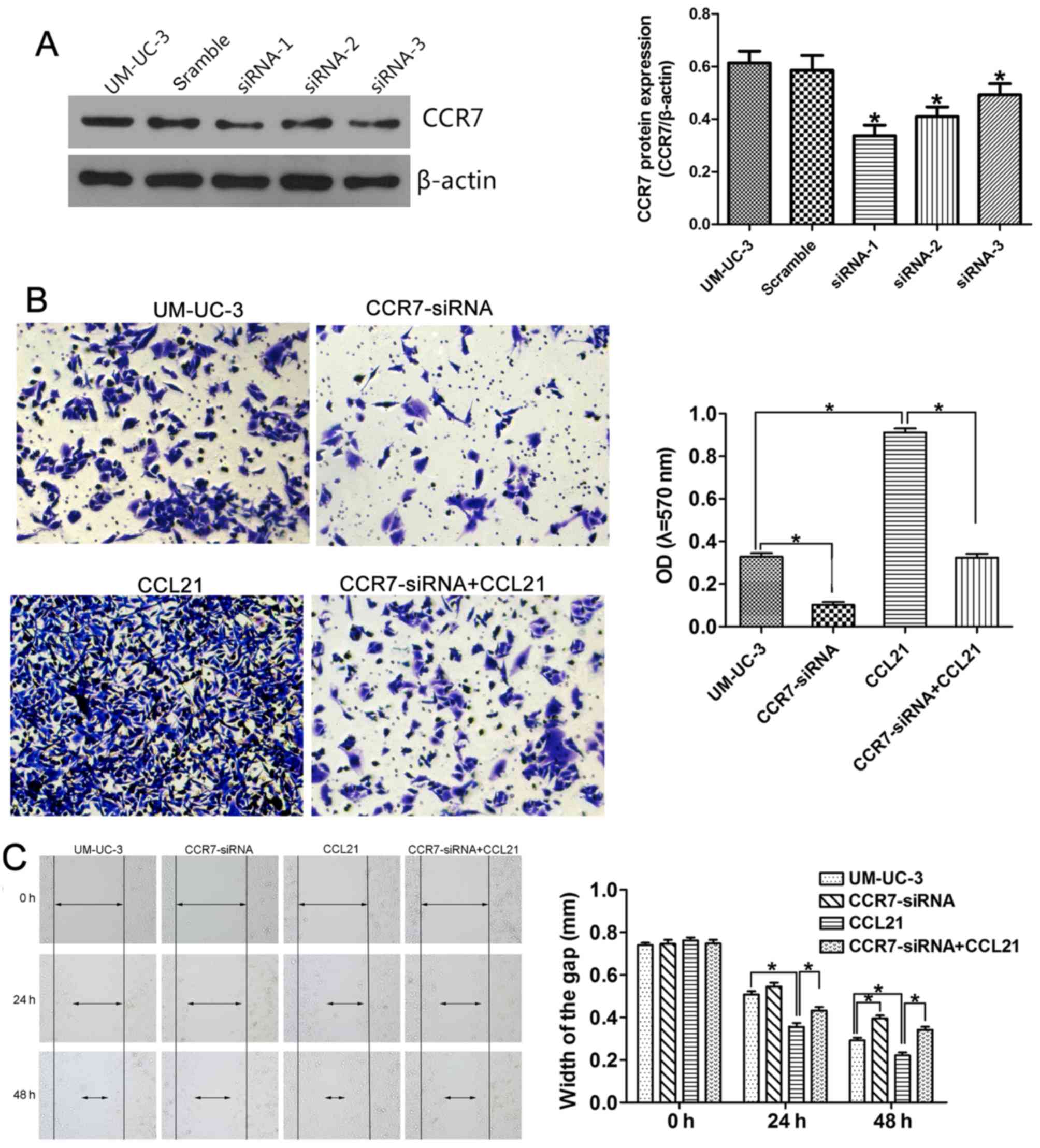
Fig. 5A shows that all three CCR7
siRNA sequences (siRNA-1, siRNA-2 and siRNA-3) significantly
depleted CCR7 expression in the UM-UC-3 cell line, as determined by
western blotting, compared with cells transfected with negative
control siRNA. UM-UC-3 cells transfected with CCR7 siRNA-1 were
selected for use in the following in vitro study. The
effects of CCR7 knockdown on the invasion behavior of the cells, as
represented by the OD values, are shown in Fig. 5B. The OD values were 0.328±0.028 in
the control group, 0.102±0.024 in UM-UC-3 cells transfected with
CCR7 siRNA-1 (CCR7-siRNA group; P<0.05 compared with the control
group), 0.912±0.033 in UM-UC-3 cells pretreated with 200 ng/ml
CCL21 for 48 h (CCL21 group; P<0.05 compared with the control
group), and 0.324±0.032 in UM-UC-3 cells treated with 200 ng/ml
CCL21 after transfection with CCR7 siRNA-1 (CCR7-siRNA+CCL21 group;
P<0.05 compared with the CCL21 group). These results indicate
that CCR7 knockdown attenuates the enhancement by CCL21 of the
invasive behavior of UM-UC-3 cells. CCL21 treatment significantly
enhanced cell migration ability, and CCR7 knockdown by siRNA
significantly abrogated the enhanced effect of CCL21 on the
migration of UM-UC-3 cells (Fig.
5C). After transfection with CCR7 siRNA-1 for 24 h, the
difference in the migration ability of the CCR7-siRNA group and the
control group was not statistically significant. However, when the
UM-UC-3 cells were transfected for 48 h, the difference was
significant.
The ERK/AKT signaling pathway in the
CCL21/CCR7 axis induces enhanced migration and invasion capacity of
UBC cells
To investigate whether CCL21/CCR7 interaction
enhanced the invasion and migration ability of UM-UC-3 cells via
the MEK/ERK1/2 pathway or the PI3K/AKT pathway, the expression
levels of total-ERK1/2, phospho-ERK1/2, total-AKT and phospho-AKT
were first assessed using western blotting (Fig. 6A). The expression levels of
total-ERK1/2, total-AKT and phospho-AKT in UM-UC-3 cells did not
significantly change either after CCR7 gene knockdown or CCL21
treatment (P>0.05). However, the phospho-ERK1/2 protein level
increased when the cells were treated with CCL21 and decreased when
the CCR7 gene was silenced (P<0.05). These results indicate that
CCL21/CCR7 interaction may modulate the action of the MEK/ERK
signaling pathway but not that of the PI3K/AKT pathway in UM-UC-3
cells.
To determine how CCL21/CCR7 interaction promoted the
invasion and migration functions of UM-UC-3 cells via the
MEK/ERK1/2 pathway, PD98059 was used to inhibit the activation of
MEK. PD98059 significantly suppressed the invasion ability of
UM-UC-3 cells and abrogated the enhancement of the invasion ability
of UM-UC-3 cells by CCL21/CCR7 (P<0.05; Fig. 6B). Fig. 6C shows that inhibition of MEK (the
upstream regulatory protein of ERK1/2) by PD98059 in UM-UC-3 cells
led to significantly delayed wound healing at all times examined
compared with the control group and significantly interfered with
the rapid wound healing induced by CCL21 (P<0.05).
Discussion
The CC-chemokine receptor 7 (CCR7), which belongs to
the Class A subfamily of G protein-coupled receptors with seven
transmembrane domains, is widely expressed in various types of
immune cells including naive, regulatory and central memory T
cells, naive B cells, double-negative and single-positive
thymocytes, and (semi-) mature dendritic cells (DCs) and is
functionally involved in the homing of various subpopulations of T
cells and antigen-presenting DCs to the T cell areas of lymphoid
tissues upon activation by the ligand of CCL21 (23,24).
Furthermore, aberrantly increased CCR7 expression has been reported
in breast (18,25), gastric (22,26,27),
colorectal (28–30), non-small cell lung (31,32),
cervical (33), SCC of the head
and neck (34), prostate (35,36),
melanoma (37) and esophageal,
oral and oropharyngeal SCC cancer (38–41)
and is generally associated with lymph node metastasis and worse
prognosis. In addition, a previous study conducted at our
institution in which the CCR7 expression levels in 115 patients
with UBC and 10 patients with benign prostatic hyperplasia (BPH)
were compared found a significantly higher frequency of detectable
CCR7 expression in the UBC group than in the BPH group and the
clinical feasibility of CCR7 expression level as an independent
predictive biomarker in the diagnosis of lymph node metastasis
(42). However, little is known
about the association of CCR7 expression with survival, prognosis,
demographic factors or other clinicopathological features of UBC,
such as tumor TNM staging and tumor differentiation, and the
underlying mechanisms through which CCR7 affects the lymphatic
metastasis of tumor cells remain obscure.
Our finding that high CCR7 expression was
significantly correlated with lymph node metastasis and poor
survival is consistent with the results of almost all related
previous studies (22,27,30,33,39,40).
However, whether the CCR7 expression level can be used as an
independent predictive factor in tumor prognosis remained
controversial. Ding et al (39) reported that the CCR7 expression
level was not identified as an independent prognostic factor in
esophageal SCC by multivariate analysis. In contrast, some
investigators reported that CCR7 was an independent prognostic
factor for overall survival in gastric cancer, colorectal
carcinoma, and cervical cancer (27,28,33).
It is unclear why these studies resulted in two completely
different conclusions, and no explanations for this contradictory
phenomenon are evident. We hypothesize that discrepancies in the
weight ratio of CCR7 to other pro-metastatic factors, including
MMP-9, IL-8, VEGF and EGFR, within different tumor environments as
well as heterogeneity in tumor type, study population and study
method may partially account for the above phenomena. Our
multivariate analysis showed that high CCR7 expression and lymph
node metastasis were independent prognostic factors for overall
survival in UBC, consistent with the findings reported by Ma et
al (27), Gunther et al
(28) and Kodama et al
(33). We suggest that UBC
patients who have negative lymph node metastasis but detectable
high CCR7 expression should still be closely monitored, even though
emphasis has formerly been placed on patients with positive lymph
node involvement.
MVD, as assessed by immunohistochemistry using
antibodies against CD34, CD31 or factor VIII-related antigen
markers to identify vasculature, has been widely used as a
surrogate marker for angiogenesis, which is characterized by
neovascularization and is required for tumors to grow beyond a
certain size and to metastasize to new sites (43). Of all these specific endothelial
markers used for quantitative angiogenic studies, CD34 is widely
accepted as the optimal marker especially in the invasive tumors
due to its robustness and ease of use (44,45),
and thus, the antibody against CD34 was selected in this study. It
is known that MVD is associated with tumor progression and survival
in UBC (46–49). In addition, several studies have
reported that altered MVD is also associated with lymph node
metastasis and decreased survival rate in UBC (50–52).
In contrast to the extensive studies on tumor-induced angiogenesis,
little is known about MLVD and tumor-induced lymphangiogenesis,
which has been evaluated using similiar methods as for MVD except
that the antibodies used were LYVE-1-, D2-40/podoplanin- or
VEGFR-3-specific. In our literature review, we found that the use
of VEGFR-3 as a marker for lymphatic vessels is controversial. A
considerable amount of evidence shows that VEGFR-3 expression in
tumors was detectable in the endothelium of lymphatic vessels, and
VEGFR-3-positive vessels were counted when measuring MLVD (53–55).
Additionally, however, it was reported that VEGFR-3 lacks
specificity for lymphatic vessels in human tumors; tumoral VEGFR-3
expression was also present in the endothelium of blood vessels,
and VEGFR-3 stained vessels were therefore used for MVD
determination (56–58). The high specificity and sensitivity
of D2-40 as a lymphatic endothelial marker to quantitatively assess
lymphangiogenesis were verified by means of the immunohistochemical
single staining using a single antibody to D2-40 (59), the immunohistochemical double
staining using antibodies to D2-40 and CD34 (60) and the ultrastructure study using
electron microscopy (61).
Therefore, the antibody against D2-40 was selected as the specific
lymphatic marker in this study. MLVD has been widely used as a
marker for lymphangiogenesis characterized by the de novo
formation of lymphatic vessels. With regard to the clinical
significance and prognostic value of MLVD, a considerable number of
studies reported that increased MLVD was statistically
significantly correlated with lymph node metastasis and survival in
head and neck SCC (62–64), breast (65), gastric cancer (66), prostate adenocarcinoma (67), cutaneous malignant melanoma
(68,69) and UBC (53,70).
There is compelling evidence that pro-angiogenic and
pro-lymphangiogenic factors (such as agents in the vascular growth
factor family) trigger angiogenesis and lymphangiogenesis,
respectively, and are related to adverse tumor outcomes. However,
the role of CCR7 as a pro-angiogenic or pro-lymphangiogenic factor
was unclear. Based on the notably significant relationship of lymph
node metastasis to MLVD/MVD and CCR7 expression in human cancers,
as discussed above, we speculate that CCR7 may play a pivotal role
in lymph node metastasis and poor prognosis of UBC by acting as a
pro-angiogenic or pro-lymphangiogenic factor to induce angiogenesis
or lymphangiogenesis.
Our finding that MLVD was significantly higher in
peritumoral areas than in normal control tissue suggested that at
least some of the peritumoral lymphatics were indeed derived from
the newly formed lymphatic vessels by lymphangiogenesis rather than
being pre-existing vessels. The lower MLVD in intratumoral areas
compared with the normal and peritumoral compartments is consistent
with observations reported in other studies (62,71–73),
as are the collapsed and elongated morphological characteristics of
intratumoral lymphatics. These observations suggest a possible
mechanism for the formation of intratumoral lymphatics in which the
early-stage rapidly growing tumor grows into pre-existing lymphatic
vessels to form partitions that ultimately surround the whole
lymphatic vessel; the increased intratumoral pressure resulting
from the tumor merisis then further compresses the separated
lymphatics into a collapsed and elongated shape. Although this
hypothetical non-lymphangiogenic pattern for the formation of
intratumoral lymphatics requires further functional studies in
vitro and in vivo to verify its validity, we speculate
that intratumoral non-lymphangiogenic lymphatic vessels are
probably non-functional for tumor progression. Reports by others
(74–76) that no lymphatic vessels were
detected within the intratumoral areas of malignant tumors of the
female genital system further support our hypothesis. In view of
the poor function of intratumoral lymphatics in tumor metastasis,
the clinical significance of peritumoral lymphangiogenesis and
peritumoral MLVD was analyzed. Our results showed that regional
lymph node invasion was significantly associated with increased
levels of peritumoral MLVD rather than MVD, consistent with the
findings of other studies (53,62,65–69)
and suggesting that peritumoral lymphangiogenesis rather than
angiogenesis is involved in the process of regional lymph node
metastasis. On the other hand, in view of the significant
correlation between MVD and tumor stage that was observed in this
study (Table I), we presumed that
tumor-associated angiogenesis is mainly involved in tumor growth
and progression by acting as a pipeline for the transpor nutrients
required for tumor cell growth. This presumption is consistent with
our finding that MVD was highest within the intratumoral areas,
followed by normal controls and peritumoral areas (Fig. 3G), indicating a greater demand for
blood supply and nutrients within the tumor. Further analysis of
correlations between CCR7 and MVD/MLVD revealed that high CCR7
expression level was associated with both increased MVD and MLVD,
suggesting a promoting role of CCR7 in angiogenesis and
lymphangiogenesis. Based on these findings, we concluded that, as a
pro-angiogenic and pro-lymphangiogenic factor, CCR7 expression
correlates with lymph node metastasis due to its lymphangiogenic
role rather than its angiogenic role. A recent study reported the
interesting finding that the CCL21/CCR7 axis is indirectly involved
in breast cancer-induced lymphangiogenesis through regulation of
the expression of VEGF-C (77).
Whether the CCL21/CCR7 axis induces tumor lymphangiogenesis through
a direct role as a pro-lymphangiogenic factor like the VEGF/VEGFR
family or through indirect regulation of VEGF-C or VEGF-D remains
an interesting question for future scientific research.
Several studies have shown that CCR7 activity is
associated with migration and invasion by certain cancer cell lines
(22,27,78–81).
However, whether the upregulation and downregulation of CCR7
expression modulates the biological behavior of UBC cell lines
in vitro is poorly defined, despite the fact that a higher
CCR7 level was observed in UBC tissues than in normal controls
(42). Our results demonstrate
that CCL21/CCR7 interaction significantly enhances the migration
and invasion capacity of human UM-UC-3 cells in a dose- and
time-dependent manner. Moreover, knock down of the CCR7 gene by
small interfering RNAs targeting CCR7 sequences was shown in the
present study to significantly suppress the enhancement of
migration and invasion by human UM-UC-3 cells that was induced by
treatment of the cells with CCL21. These results further confirmed
that the activation of CCR7 is responsible for the increased
migration and invasion abilities of human UBC cells. Some previous
studies reported that the CCL21/CCR7 axis enhances the migration
and invasion of cancer cells via the ERK1/2 pathway (78), whereas others found that the
CCL21/CCR7-induced enhanced behavior of cancer cells were mediated
by the PI3K/AKT pathway (81). The
present study shows that CCR7 activation by CCL21 treatment result
in a significant increase in ERK1/2 phosphorylation but not AKT
phosphorylation in UBC cells, and CCR7 knockdown by efficacious
siRNA transfection significantly decreases ERK1/2 phosphorylation
only. Furthermore, PD98059, a specific inhibitor of MEK, the
upstream activator protein of ERK1/2, remarkably suppressed the
enhanced invasion and migration abilities of UBC cells induced by
CCL21. Based on these results, we came to a conclusion that the
PI3K/AKT and ERK pathways play a critical role in the
carcinogenesis and progression of UBC (78,81–83),
but the ERK pathway may play a more important role in the
CCL21/CCR7-induced progression of UBC than the AKT pathway.
This study is limited by its retrospective design
and by the semi-quantitative immunohistochemical methodology used
in the analysis of clinical tumor samples. Although
immunohistochemistry has been widely used in the study of protein
expression, it has inherent defects, including high variability in
technical procedures and staining scoring standards in different
studies. Another limitation of the present study is the isolation
of the tumor cells from their microenvironment in our in
vitro experiments. Animal models, especially the orthotopic
xenograft model, which most closely mimics human primary tumor
carcinogenesis, remain a crucial connection between cell-based
experiments and the translation of novel agents into cancer
therapeutics, and our results lack testing with in vivo
experiments or appropriate animal models that provide a realistic
microenvironment for tumor survival. A statistical methological
defect of our research is the small sample size of 62 subjects that
may result in the reduced test power (1-β) and the increased type
II error (β).
In conclusion, this study provides novel insights
into the multifaceted role played by the CCL21/CCR7 chemoaxis in
the complex mechanics of lymph node metastasis in patients with
UBC. On the one hand, CCR7 is a promoting factor that induces both
lymphangiogenesis and angiogenesis; however, it may correlate with
lymph node metastasis due to its lymphangiogenic role rather than
due to its angiogenic role. On the other hand, the CCL21/CCR7
chemoaxis promotes the migration and invasion by UBC cells via the
MEK/ERK1/2 signaling pathway rather than the PI3K/AKT pathway.
These insights into the mechanisms of CCL21/CCR7-mediated
migration, invasion and lymph node metastasis in UBC enable us to
inhibit the molecular processes involved in these events and
thereby deliver therapeutic benefit to patients with advanced
disease.
Glossary
Abbreviations
Abbreviations:
|
MLVD
|
microlymphatic vessel density
|
|
MVD
|
microvessel density
|
|
CCL21
|
chemokine (C-C motif) ligand 21
|
|
CCR7
|
C-C chemokine receptor 7
|
|
ERK
|
extracellular signal-regulated
kinase
|
|
siRNA
|
small interfering RNA
|
References
|
1
|
Ploeg M, Aben KK and Kiemeney LA: The
present and future burden of urinary bladder cancer in the world.
World J Urol. 27:289–293. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Johansson SL and Cohen SM: Epidemiology
and etiology of bladder cancer. Semin Surg Oncol. 13:291–298. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2016. CA Cancer J Clin. 66:7–30. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Metts MC, Metts JC, Milito SJ and Thomas
CR Jr: Bladder cancer: A review of diagnosis and management. J Natl
Med Assoc. 92:285–294. 2000.PubMed/NCBI
|
|
5
|
Xu X, Chen H, Lin Y, Hu Z, Mao Y, Wu J, Xu
X, Zhu Y, Li S and Zheng X: MicroRNA-409-3p inhibits migration and
invasion of bladder cancer cells via targeting c-Met. Mol Cells.
36:62–68. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Babjuk M, Burger M, Zigeuner R, Shariat
SF, van Rhijn BW, Compérat E, Sylvester RJ, Kaasinen E, Böhle A,
Palou Redorta J, et al European Association of Urology: EAU
guidelines on non-muscle-invasive urothelial carcinoma of the
bladder: Update 2013. Eur Urol. 64:639–653. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Luke C, Tracey E, Stapleton A and Roder D:
Exploring contrary trends in bladder cancer incidence, mortality
and survival: Implications for research and cancer control. Intern
Med J. 40:357–362. 2010. View Article : Google Scholar
|
|
8
|
Zuiverloon TC, Nieuweboer AJ, Vékony H,
Kirkels WJ, Bangma CH and Zwarthoff EC: Markers predicting response
to bacillus Calmette-Guérin immunotherapy in high-risk bladder
cancer patients: A systematic review. Eur Urol. 61:128–145. 2012.
View Article : Google Scholar
|
|
9
|
Abdollah F, Gandaglia G, Thuret R,
Schmitges J, Tian Z, Jeldres C, Passoni NM, Briganti A, Shariat SF,
Perrotte P, et al: Incidence, survival and mortality rates of
stage-specific bladder cancer in United States: A trend analysis.
Cancer Epidemiol. 37:219–225. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Meeks JJ, Bellmunt J, Bochner BH, Clarke
NW, Daneshmand S, Galsky MD, Hahn NM, Lerner SP, Mason M, Powles T,
et al: A systematic review of neoadjuvant and adjuvant chemotherapy
for muscle-invasive bladder cancer. Eur Urol. 62:523–533. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Itesako T, Seki N, Yoshino H, Chiyomaru T,
Yamasaki T, Hidaka H, Yonezawa T, Nohata N, Kinoshita T, Nakagawa
M, et al: The microRNA expression signature of bladder cancer by
deep sequencing: The functional significance of the miR-195/497
cluster. PLoS One. 9:e843112014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Gupta GP and Massagué J: Cancer
metastasis: Building a framework. Cell. 127:679–695. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Siegel R, Ma J, Zou Z and Jemal A: Cancer
statistics, 2014. CA Cancer J Clin. 64:9–29. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Shariat SF, Karakiewicz PI, Palapattu GS,
Amiel GE, Lotan Y, Rogers CG, Vazina A, Bastian PJ, Gupta A,
Sagalowsky A, et al: Nomograms provide improved accuracy for
predicting survival after radical cystectomy. Clin Cancer Res.
12:6663–6676. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Habuchi T, Marberger M, Droller MJ,
Hemstreet GP III, Grossman HB, Schalken JA, Schmitz-Dräger BJ,
Murphy WM, Bono AV, Goebell P, et al: Prognostic markers for
bladder cancer: International Consensus Panel on bladder tumor
markers. Urology. 66(Suppl 1): 64–74. 2005. View Article : Google Scholar
|
|
16
|
Bol MG, Baak JP, Buhr-Wildhagen S, Kruse
AJ, Kjellevold KH, Janssen EA, Mestad O and Øgreid P:
Reproducibility and prognostic variability of grade and lamina
propria invasion in stages Ta, T1 urothelial carcinoma of the
bladder. J Urol. 169:1291–1294. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Guo JC, Li J, Zhou L, Yang JY, Zhang ZG,
Liang ZY, Zhou WX, You L, Zhang TP and Zhao YP: CXCL12-CXCR7 axis
contributes to the invasive phenotype of pancreatic cancer.
Oncotarget. 7:62006–62018. 2016.PubMed/NCBI
|
|
18
|
Müller A, Homey B, Soto H, Ge N, Catron D,
Buchanan ME, McClanahan T, Murphy E, Yuan W, Wagner SN, et al:
Involvement of chemokine receptors in breast cancer metastasis.
Nature. 410:50–56. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yasuoka H, Tsujimoto M, Yoshidome K,
Nakahara M, Kodama R, Sanke T and Nakamura Y: Cytoplasmic CXCR4
expression in breast cancer: Induction by nitric oxide and
correlation with lymph node metastasis and poor prognosis. BMC
Cancer. 8:3402008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yu T, Wu Y, Helman JI, Wen Y, Wang C and
Li L: CXCR4 promotes oral squamous cell carcinoma migration and
invasion through inducing expression of MMP-9 and MMP-13 via the
ERK signaling pathway. Mol Cancer Res. 9:161–172. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kato T, Fujita Y, Nakane K, Mizutani K,
Terazawa R, Ehara H, Kanimoto Y, Kojima T, Nozawa Y, Deguchi T, et
al: CCR1/CCL5 interaction promotes invasion of taxane-resistant PC3
prostate cancer cells by increasing secretion of MMPs 2/9 and by
activating ERK and Rac signaling. Cytokine. 64:251–257. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Mashino K, Sadanaga N, Yamaguchi H, Tanaka
F, Ohta M, Shibuta K, Inoue H and Mori M: Expression of chemokine
receptor CCR7 is associated with lymph node metastasis of gastric
carcinoma. Cancer Res. 62:2937–2941. 2002.PubMed/NCBI
|
|
23
|
Comerford I, Harata-Lee Y, Bunting MD,
Gregor C, Kara EE and McColl SR: A myriad of functions and complex
regulation of the CCR7/CCL19/CCL21 chemokine axis in the adaptive
immune system. Cytokine Growth Factor Rev. 24:269–283. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Förster R, Davalos-Misslitz AC and Rot A:
CCR7 and its ligands: Balancing immunity and tolerance. Nat Rev
Immunol. 8:362–371. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Cabioglu N, Yazici MS, Arun B, Broglio KR,
Hortobagyi GN, Price JE and Sahin A: CCR7 and CXCR4 as novel
biomarkers predicting axillary lymph node metastasis in T1 breast
cancer. Clin Cancer Res. 11:5686–5693. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ishigami S, Natsugoe S, Nakajo A, Tokuda
K, Uenosono Y, Arigami T, Matsumoto M, Okumura H, Hokita S and
Aikou T: Prognostic value of CCR7 expression in gastric cancer.
Hepatogastroenterology. 54:1025–1028. 2007.PubMed/NCBI
|
|
27
|
Ma H, Gao L, Li S, Qin J, Chen L, Liu X,
Xu P, Wang F, Xiao H, Zhou S, et al: CCR7 enhances TGF-β1-induced
epithelial-mesenchymal transition and is associated with lymph node
metastasis and poor overall survival in gastric cancer. Oncotarget.
6:24348–24360. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Günther K, Leier J, Henning G, Dimmler A,
Weissbach R, Hohenberger W and Förster R: Prediction of lymph node
metastasis in colorectal carcinoma by expressionof chemokine
receptor CCR7. Int J Cancer. 116:726–733. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yu S, Duan J, Zhou Z, Pang Q, Wuyang J,
Liu T, He X, Xinfa L and Chen Y: A critical role of CCR7 in
invasiveness and metastasis of SW620 colon cancer cell in vitro and
in vivo. Cancer Biol Ther. 7:1037–1043. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Malietzis G, Lee GH, Bernardo D, Blakemore
AI, Knight SC, Moorghen M, Al-Hassi HO and Jenkins JT: The
prognostic significance and relationship with body composition of
CCR7-positive cells in colorectal cancer. J Surg Oncol. 112:86–92.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Koizumi K, Kozawa Y, Ohashi Y, Nakamura
ES, Aozuka Y, Sakurai H, Ichiki K, Doki Y, Misaki T and Saiki I:
CCL21 promotes the migration and adhesion of highly lymph node
metastatic human non-small cell lung cancer Lu-99 in vitro. Oncol
Rep. 17:1511–1516. 2007.PubMed/NCBI
|
|
32
|
Takanami I: Overexpression of CCR7 mRNA in
nonsmall cell lung cancer: Correlation with lymph node metastasis.
Int J Cancer. 105:186–189. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kodama J, Hasengaowa, Kusumoto T, Seki N,
Matsuo T, Ojima Y, Nakamura K, Hongo A and Hiramatsu Y: Association
of CXCR4 and CCR7 chemokine receptor expression and lymph node
metastasis in human cervical cancer. Ann Oncol. 18:70–76. 2007.
View Article : Google Scholar
|
|
34
|
Wang J, Xi L, Hunt JL, Gooding W,
Whiteside TL, Chen Z, Godfrey TE and Ferris RL: Expression pattern
of chemokine receptor 6 (CCR6) and CCR7 in squamous cell carcinoma
of the head and neck identifies a novel metastatic phenotype.
Cancer Res. 64:1861–1866. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Yousefieh N, Hahto SM, Stephens AL and
Ciavarra RP: Regulated expression of CCL21 in the prostate tumor
microenvironment inhibits tumor growth and metastasis in an
orthotopic model of prostate cancer. Cancer Microenviron. 2:59–67.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Heresi GA, Wang J, Taichman R, Chirinos
JA, Regalado JJ, Lichtstein DM and Rosenblatt JD: Expression of the
chemokine receptor CCR7 in prostate cancer presenting with
generalized lymphadenopathy: Report of a case, review of the
literature, and analysis of chemokine receptor expression. Urol
Oncol. 23:261–267. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Mori T, Kim J, Yamano T, Takeuchi H, Huang
S, Umetani N, Koyanagi K and Hoon DS: Epigenetic up-regulation of
C-C chemokine receptor 7 and C-X-C chemokine receptor 4 expression
in melanoma cells. Cancer Res. 65:1800–1807. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Ishida K, Iwahashi M, Nakamori M, Nakamura
M, Yokoyama S, Iida T, Naka T, Nakamura Y and Yamaue H: High CCR7
mRNA expression of cancer cells is associated with lymph node
involvement in patients with esophageal squamous cell carcinoma.
Int J Oncol. 34:915–922. 2009.PubMed/NCBI
|
|
39
|
Ding Y, Shimada Y, Maeda M, Kawabe A,
Kaganoi J, Komoto I, Hashimoto Y, Miyake M, Hashida H and Imamura
M: Association of CC chemokine receptor 7 with lymph node
metastasis of esophageal squamous cell carcinoma. Clin Cancer Res.
9:3406–3412. 2003.PubMed/NCBI
|
|
40
|
Shi M, Chen D, Yang D and Liu XY:
CCL21-CCR7 promotes the lymph node metastasis of esophageal
squamous cell carcinoma by up-regulating MUC1. J Exp Clin Cancer
Res. 34:1492015. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Shang ZJ, Liu K and Shao Z: Expression of
chemokine receptor CCR7 is associated with cervical lymph node
metastasis of oral squamous cell carcinoma. Oral Oncol. 45:480–485.
2009. View Article : Google Scholar
|
|
42
|
Chen J, Cui YU, Liu L, Li C, Tang Y, Zhou
XU, Qi L and Zu X: CCR7 as a predictive biomarker associated with
computed tomography for the diagnosis of lymph node metastasis in
bladder carcinoma. Oncol Lett. 11:735–740. 2016.PubMed/NCBI
|
|
43
|
Elfiky AA and Rosenberg JE: Targeting
angiogenesis in bladder cancer. Curr Oncol Rep. 11:244–249. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Vermeulen PB, Gasparini G, Fox SB, Toi M,
Martin L, McCulloch P, Pezzella F, Viale G, Weidner N, Harris AL,
et al: Quantification of angiogenesis in solid human tumours: An
international consensus on the methodology and criteria of
evaluation. Eur J Cancer. 32A:2474–2484. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Vermeulen PB, Gasparini G, Fox SB,
Colpaert C, Marson LP, Gion M, Beliën JA, de Waal RM, Van Marck E,
Magnani E, et al: Second international consensus on the methodology
and criteria of evaluation of angiogenesis quantification in solid
human tumours. Eur J Cancer. 38:1564–1579. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Dickinson AJ, Fox SB, Persad RA, Hollyer
J, Sibley GN and Harris AL: Quantification of angiogenesis as an
independent predictor of prognosis in invasive bladder carcinomas.
Br J Urol. 74:762–766. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Bochner BH, Cote RJ, Weidner N, Groshen S,
Chen SC, Skinner DG and Nichols PW: Angiogenesis in bladder cancer:
Relationship between microvessel density and tumor prognosis. J
Natl Cancer Inst. 87:1603–1612. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Goddard JC, Sutton CD, Furness PN, O'Byrne
KJ and Kockelbergh RC: Microvessel density at presentation predicts
subsequent muscle invasion in superficial bladder cancer. Clin
Cancer Res. 9:2583–2586. 2003.PubMed/NCBI
|
|
49
|
Canoğlu A, Göğüş C, Bedük Y, Orhan D,
Tulunay O and Baltaci S: Microvessel density as a prognostic marker
in bladder carcinoma: Correlation with tumor grade, stage and
prognosis. Int Urol Nephrol. 36:401–405. 2004. View Article : Google Scholar
|
|
50
|
Chaudhary R, Bromley M, Clarke NW, Betts
CD, Barnard RJ, Ryder WD and Kumar S: Prognostic relevance of
micro-vessel density in cancer of the urinary bladder. Anticancer
Res. 19:3479–3484. 1999.
|
|
51
|
Jaeger TM, Weidner N, Chew K, Moore DH,
Kerschmann RL, Waldman FM and Carroll PR: Tumor angiogenesis
correlates with lymph node metastases in invasive bladder cancer. J
Urol. 154:69–71. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Shariat SF, Youssef RF, Gupta A, Chade DC,
Karakiewicz PI, Isbarn H, Jeldres C, Sagalowsky AI, Ashfaq R and
Lotan Y: Association of angiogenesis related markers with bladder
cancer outcomes and other molecular markers. J Urol. 183:1744–1750.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Zhou M, He L, Zu X, Zhang H, Zeng H and Qi
L: Lymphatic vessel density as a predictor of lymph node metastasis
and its relationship with prognosis in urothelial carcinoma of the
bladder. BJU Int. 107:1930–1935. 2011. View Article : Google Scholar
|
|
54
|
Jacquemier J, Mathoulin-Portier MP,
Valtola R, Charafe-Jauffret E, Geneix J, Houvenaeghel G, Puig B,
Bardou VJ, Hassoun J, Viens P, et al: Prognosis of breast-carcinoma
lymphagenesis evaluated by immunohistochemical investigation of
vascular-endothelial-growth-factor receptor 3. Int J Cancer.
89:69–73. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Zeng Y, Opeskin K, Baldwin ME, Horvath LG,
Achen MG, Stacker SA, Sutherland RL and Williams ED: Expression of
vascular endothelial growth factor receptor-3 by lymphatic
endothelial cells is associated with lymph node metastasis in
prostate cancer. Clin Cancer Res. 10:5137–5144. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Longatto Filho A, Martins A, Costa SM and
Schmitt FC: VEGFR-3 expression in breast cancer tissue is not
restricted to lymphatic vessels. Pathol Res Pract. 201:93–99. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Valtola R, Salven P, Heikkilä P, Taipale
J, Joensuu H, Rehn M, Pihlajaniemi T, Weich H, deWaal R and Alitalo
K: VEGFR-3 and its ligand VEGF-C are associated with angiogenesis
in breast cancer. Am J Pathol. 154:1381–1390. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Partanen TA, Alitalo K and Miettinen M:
Lack of lymphatic vascular specificity of vascular endothelial
growth factor receptor 3 in 185 vascular tumors. Cancer.
86:2406–2412. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Kahn HJ, Bailey D and Marks A: Monoclonal
antibody D2-40, a new marker of lymphatic endothelium, reacts with
Kaposi's sarcoma and a subset of angiosarcomas. Mod Pathol.
15:434–440. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Afonso J, Santos LL, Amaro T, Lobo F and
Longatto-Filho A: The aggressiveness of urothelial carcinoma
depends to a large extent on lymphovascular invasion - the
prognostic contribution of related molecular markers.
Histopathology. 55:514–524. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Braun M, Flucke U, Debald M,
Walgenbach-Bruenagel G, Walgenbach KJ, Höller T, Pölcher M,
Wolfgarten M, Sauerwald A, Keyver-Paik M, et al: Detection of
lymphovascular invasion in early breast cancer by D2-40
(podoplanin): A clinically useful predictor for axillary lymph node
metastases. Breast Cancer Res Treat. 112:503–511. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Franchi A, Gallo O, Massi D, Baroni G and
Santucci M: Tumor lymphangiogenesis in head and neck squamous cell
carcinoma: A morphometric study with clinical correlations. Cancer.
101:973–978. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Beasley NJ, Prevo R, Banerji S, Leek RD,
Moore J, van Trappen P, Cox G, Harris AL and Jackson DG:
Intratumoral lymphangiogenesis and lymph node metastasis in head
and neck cancer. Cancer Res. 62:1315–1320. 2002.PubMed/NCBI
|
|
64
|
Maula SM, Luukkaa M, Grénman R, Jackson D,
Jalkanen S and Ristamäki R: Intratumoral lymphatics are essential
for the metastatic spread and prognosis in squamous cell carcinomas
of the head and neck region. Cancer Res. 63:1920–1926.
2003.PubMed/NCBI
|
|
65
|
Nakamura Y, Yasuoka H, Tsujimoto M, Imabun
S, Nakahara M, Nakao K, Nakamura M, Mori I and Kakudo K: Lymph
vessel density correlates with nodal status, VEGF-C expression, and
prognosis in breast cancer. Breast Cancer Res Treat. 91:125–132.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Wang XL, Fang JP, Tang RY and Chen XM:
Different significance between intratumoral and peritumoral
lymphatic vessel density in gastric cancer: A retrospective study
of 123 cases. BMC Cancer. 10:2992010. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Roma AA, Magi-Galluzzi C, Kral MA, Jin TT,
Klein EA and Zhou M: Peritumoral lymphatic invasion is associated
with regional lymph node metastases in prostate adenocarcinoma. Mod
Pathol. 19:392–398. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Dadras SS, Paul T, Bertoncini J, Brown LF,
Muzikansky A, Jackson DG, Ellwanger U, Garbe C, Mihm MC and Detmar
M: Tumor lymphangiogenesis: A novel prognostic indicator for
cutaneous melanoma metastasis and survival. Am J Pathol.
162:1951–1960. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Dadras SS, Lange-Asschenfeldt B, Velasco
P, Nguyen L, Vora A, Muzikansky A, Jahnke K, Hauschild A, Hirakawa
S, Mihm MC, et al: Tumor lymphangiogenesis predicts melanoma
metastasis to sentinel lymph nodes. Mod Pathol. 18:1232–1242. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Ma Y, Hou Y, Liu B, Li X, Yang S and Ma J:
Intratumoral lymphatics and lymphatic vessel invasion detected by
D2-40 are essential for lymph node metastasis in bladder
transitional cell carcinoma. Anat Rec (Hoboken). 293:1847–1854.
2010. View Article : Google Scholar
|
|
71
|
Fernández MI, Bolenz C, Trojan L, Steidler
A, Weiss C, Alken P, Grobholz R and Michel MS: Prognostic
implications of lymphangiogenesis in muscle-invasive transitional
cell carcinoma of the bladder. Eur Urol. 53:571–578. 2008.
View Article : Google Scholar
|
|
72
|
Miyata Y, Kanda S, Ohba K, Nomata K,
Hayashida Y, Eguchi J, Hayashi T and Kanetake H: Lymphangiogenesis
and angiogenesis in bladder cancer: prognostic implications and
regulation by vascular endothelial growth factors-A, -C, and -D.
Clin Cancer Res. 12:800–806. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Liang P, Hong JW, Ubukata H, Liu HR,
Watanabe Y, Katano M, Motohashi G, Kasuga T, Nakada I and Tabuchi
T: Increased density and diameter of lymphatic microvessels
correlate with lymph node metastasis in early stage invasive
colorectal carcinoma. Virchows Arch. 448:570–575. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Williams CS, Leek RD, Robson AM, Banerji
S, Prevo R, Harris AL and Jackson DG: Absence of lymphangiogenesis
and intratumoural lymph vessels in human metastatic breast cancer.
J Pathol. 200:195–206. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Birner P, Schindl M, Obermair A, Plank C,
Breitenecker G, Kowalski H and Oberhuber G: Lymphatic microvessel
density in epithelial ovarian cancer: Its impact on prognosis.
Anticancer Res. 20:2981–2985. 2000.PubMed/NCBI
|
|
76
|
Schoppmann SF, Birner P, Stöckl J, Kalt R,
Ullrich R, Caucig C, Kriehuber E, Nagy K, Alitalo K and Kerjaschki
D: Tumor-associated macrophages express lymphatic endothelial
growth factors and are related to peritumoral lymphangiogenesis. Am
J Pathol. 161:947–956. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Tutunea-Fatan E, Majumder M, Xin X and
Lala PK: The role of CCL21/CCR7 chemokine axis in breast
cancer-induced lymphangiogenesis. Mol Cancer. 14:352015. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Redondo-Muñoz J, José Terol M,
García-Marco JA and García-Pardo A: Matrix metalloproteinase-9 is
up-regulated by CCL21/CCR7 interaction via extracellular
signal-regulated kinase-1/2 signaling and is involved in
CCL21-driven B-cell chronic lymphocytic leukemia cell invasion and
migration. Blood. 111:383–386. 2008. View Article : Google Scholar
|
|
79
|
Li F, Zou Z, Suo N, Zhang Z, Wan F, Zhong
G, Qu Y, Ntaka KS and Tian H: CCL21/CCR7 axis activating chemotaxis
accompanied with epithelial-mesenchymal transition in human breast
carcinoma. Med Oncol. 31:1802014. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Xia X, Liu K, Zhang H and Shang Z:
Correlation between CCR7 expression and lymph node metastatic
potential of human tongue carcinoma. Oral Dis. 21:123–131. 2015.
View Article : Google Scholar
|
|
81
|
Yang J, Wang S, Zhao G and Sun B: Effect
of chemokine receptors CCR7 on disseminated behavior of human T
cell lymphoma: clinical and experimental study. J Exp Clin Cancer
Res. 30:512011. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Calderaro J, Rebouissou S, de Koning L,
Masmoudi A, Hérault A, Dubois T, Maille P, Soyeux P, Sibony M, de
la Taille A, et al: PI3K/AKT pathway activation in bladder
carcinogenesis. Int J Cancer. 134:1776–1784. 2014. View Article : Google Scholar
|
|
83
|
Islam SS, Mokhtari RB, Akbari P, Hatina J,
Yeger H and Farhat WA: Simultaneous targeting of bladder tumor
growth, Survival, and epithelial-to-mesenchymal transition with a
novel therapeutic combination of acetazolamide (AZ) and
sulforaphane (SFN). Target Oncol. 11:209–227. 2016. View Article : Google Scholar
|