Introduction
Human epidermal growth factor receptor
(HER2)-overexpressing breast cancer accounts for 20–30% of the
total number of breast cancer (1),
and the HER2 expression level is as much as 100 times higher when
compared to non-cancerous breast tissue (2). HER2-overexpressing breast cancer has
a higher degree of malignancy and is more prone to drug resistance
than luminal A and luminal B, thus resulting in poorer prognosis
and shorter survival time (3).
Trastuzumab (Herceptin) is the first targeted anti-cancer drug in
breast cancer, and it brings a remarkable breakthrough in the
prognosis of HER2-overexpressing breast cancer (4). After binding to the HER2 receptor,
trastuzumab inhibits the formation of HER2 heterodimers and the
activation of downstream signaling pathways, which inhibits the
proliferation and metastasis of breast cancer cells. Studies
reported that patients with relapsed metastatic breast cancer have
a median overall survival of up to 37.4 months and a median
progression-free survival of up to 13.6 months after receiving
trastuzumab combined with chemotherapy (5,6).
Although trastuzumab has brought significant
clinical benefits for breast cancer patients, primary and secondary
resistance to trastuzumab has caused great confusion for clinicians
and patients. The molecular mechanisms of trastuzumab resistance
are poorly understood. It may be related to many factors. One
factor is the absence of an effective binding site for HER2 or the
destruction of molecular structure of HER2. Other possible factors
include the reactivation of HER2-related or unrelated signaling
pathways (7,8), changes in receptor function induced
by the rearrangement of HER dimer axis (9–11),
the abnormal activation of the phosphatidylinositol 3
kinase/protein kinase B (PI3K/Akt) signaling pathway, or the loss
of phosphatase and tensin homolog deleted on chromosome ten
(12–14).
HER3 is one of the members of HER family. The HER3
kinase domain cannot catalytically activate other kinases, due to
lack of crucial residues, was once considered as a signaling
substrate for other HER members. Therefore, the clinical value and
research of HER3 were greatly ignored in the past. Currently,
researchers found that its kinase domain possesses a specific
allosteric activation function and acts as an activator kinase to
activate receptor kinases (HER1, HER2, HER4) by forming
heterodimers (15). Another study
revealed HER3 could automatically phosphorylate since it has the
capability to bind ATP by using its intracellular segments
(16). HER3 has been found to be
involved in the tumorigenesis and progression in many kinds of
cancer. A study by Lee-Hoeflich et al demonstrated that HER3
formed powerful carcinogenic dimers with HER2, and that HER3 was as
important as HER2 in promoting proliferation of HER2-overexpressing
breast cancer cells (17). The
cooperation between HER2 and HER3 is unique, but the underlying
molecular mechanism still needs further study.
NRG1 acts as a ligand of HER3 and promotes the
interaction of HER2/HER3 (18).
NRG1/HER3 has been confirmed to promote cell proliferation through
autocrine or paracrine modes in ovarian and colon cancer cells
(19,20). There is less research on the
effects of NRG1/HER3 of breast cancer cells. It has been found that
NRG1 upregulated the expression of matrix
metalloproteinase-1/matrix metalloproteinase-9 and promoted
invasion of HER2-overexpressing breast cancer cells. One study
showed that HER3 gene silencing downregulated NRG1 level and
inhibited tumor cell invasion, and in turn, NRG1 gene silencing
inhibited HER3 activity and cell proliferation (21). This study indicated that HER3
promoted the proliferation of HER2-overexpressing breast cancer
cells in an NRG1-dependent manner. Moreover, a study by Göstring
et al demonstrated that the activation loop of NRG1/HER3,
independent of HER2, played a vital role in the proliferation
process of HER2-overexpressing breast cancer cells (22).
In light of the promoted role NRG1/HER3 has in the
proliferation of HER2-overexpressing breast cancer cells, the
inactivation of NRG1-dependent HER3 signal path must play an
important role in the inhibition of tumor cell growth when
trastuzumab downregulates HER2. Otherwise, primary resistance to
trastuzumab would occur. The study was performed to determine the
role of NRG1-dependent HER3 activation in primary resistance to
trastuzumab, and to clarify the inhibitory effect of HER3
monoclonal antibody on HER2-overexpressing breast cancer cells.
HER2 and HER3 were both highly expressed in MDA-MB-453 and BT474
cells. One reference showed that MDA-MB-453 belongs to
HER2-enriched subtype based on the PAM50 gene signature (23), and it was also shown as intrinsic
trastuzumab-resistant, HER2-positive breast cancer cell line
(24,25). Thus, we chose BT474 and MDA-MB-453
cell lines as our research objective.
Materials and methods
Cell culture, reagents and
antibodies
BT474 and MDA-MB-453 cell lines were purchased from
the Institute of Basic Medical Sciences, Chinese Academy of Medical
Sciences. BT474 cells (26) were
cultured in DMEM/high-glucose medium. MDA-MB-453 cells (11) were cultured first in L-15 Leibovitz
medium and then in modified RPMI medium. 1% penicillin-streptomycin
and 20% FBS were added to all media. The cells were cultured in a
petri dish at 37°C and in a humidified atmosphere containing 5%
CO2. After incubation without FBS for 4 h, the cells
were treated with anti-human HER2 antibody trastuzumab (Herceptin)
(54 µg/ml) for 0.5 h, anti-HER3 monoclonal therapeutic
antibody (clone 3D4) (5 µg/ml) for 2.5 h, and recombinant
NRG1 (100 ng/ml) for 10 min.
Trastuzumab (Herceptin) was purchased from Shanghai
Roche Pharmaceuticals Co., Ltd. The anti-human HER3 monoclonal
antibody (clone 3D4) was generously provided by Beijing Cotimes
Biotech Co., Ltd. Recombinant NRG1 was purchased from Cell
Signaling Technology. Primary antibodies to p-HER2/ErbB2 (Tyr1196)
(1:1500), HER2/ErbB2 (1:1500), p-HER3/ErbB3 (Tyr1289) (1:1500),
HER3/ErbB3 (1:1500), p-Akt (Ser473) (1:1500), Akt (pan) (1:1500),
p-MAPK (Thr202/Tyr204) (1:1500), MAPK (1:1500) were purchased from
Cell Signaling Technology. β-actin rabbit polyclonal antibody
(1:1500) was purchased from Beijing Guan Xing Yu Sci-Tech Co., Ltd.
Peroxidase-conjugated AffiniPure goat anti-rabbit IgG (H+L)
(1:5000) was purchased from ZSGB-Bio.
SDS-PAGE and western blotting
After BCA quantitative operation, cell proteins were
boiled at 99°C for 5 min in 5X buffer with protein phosphatase
inhibitor (1:100) and then electrophoresed on a 10% SDS-PAGE gel.
Target protein was transferred to a polyvinylidene fluoride
membranes, and the membrane was blocked with skim milk (5%) for 1
h. After 2 washes with phosphate buffer solution (PBS), membranes
were incubated with primary antibodies at 4°C overnight. After 3
washes with PBS, membranes were incubated with
peroxidase-conjugated second antibodies for 1 h. After 3 washes
with PBS, membranes were colored using the Immobilon Western
Chemiluminescent HRP Substrate (Millipore, Billerica, MA, USA).
RNA interference transfection in
MDA-MB-453 cells
A small interfering RNA (siRNA) targeting human HER3
was purchased from Gene Pharma Co., Ltd. (Shanghai, China). siRNA
transfection was performed in 6-well plates using Lipofectamine™
3000 Reagent (Thermo Fisher Scientific, Waltham, MA, USA) according
to the manufacturer's protocols. Approximately 4000–5000 cells were
seeded on each well on 6-well culture plate, and allowed to adhere
overnight in supplemented medium. Lipofectamine 3000 Reagent (7.5
µl) was diluted in Opti-MEM™ Medium (125 µl), and
mixed well. Dilute DNA in Opti-MEM and mix well to prepared DNA
master mix. Mix diluted DNA and diluted Lipofectamine 3000 Reagent
(1:1 ratio) in one tube and set to incubate for 10–15 min at room
temperature. DNA-lipid complex was then added to cells, which were
set to incubate cells for 48 h at 37°C.
Apoptotic assay
Approximately 70% of the cell density per well were
seeded on 6-well plates. The two types of cells, including the
positive and negative controls, were incubated with different
reagents for an appropriate time to induce apoptosis, followed by 2
washes with PBS, and then separated the cells from the well with
450 µl of 0.25% trypsin-EDTA solution and re-suspended with
200 µl of their respective culture medium. After that, 100
µl of cells in suspension and 100 µl of Muse™ Annexin
V and Dead Cell Reagent was added to each tube and incubated for 20
min at room temperature. Finally, the percentage of apoptotic cells
was detected using the Muse Cell Analyzer (Muse 1.4 Analysis,
Millipore).
Cell viability assay
Approximately 10000 cells per well were plated onto
96-well plates, and adhered at least 24 h in culture medium. The
treatments were divided into four groups (control group, HER3
antibody alone group, trastuzumab alone group, and trastuzumab
combined with HER3 antibody group) in a range of drug
concentrations (0.1, 1, 10, 100 and 1000 nM), and then incubated
for 48 h in the presence or absence of NRG1 (5 nM). The final live
cell number was determined by thiazolyl blue tetrazolium bromide
(MTT; Amresco LLC, Solon, OH, USA).
Statistical analysis
GraphPad Prism 5.01 (GraphPad Software Inc., USA)
were used to perform the statistical analysis. Data obtained from
western blotting were analyzed by AlphaView SA 3.4.0 (Protein
Simple, San Jose, CA, USA). The comparison between any two groups
was determined by unpaired t-test or one-way ANOVA. A P-value of
<0.05 was considered to indicate a statistically significant
difference. Each experiment was repeated at least three times.
Results
Activation of NRG1-dependent HER3
abolished inhibitory effects of trastuzumab in BT474 cells
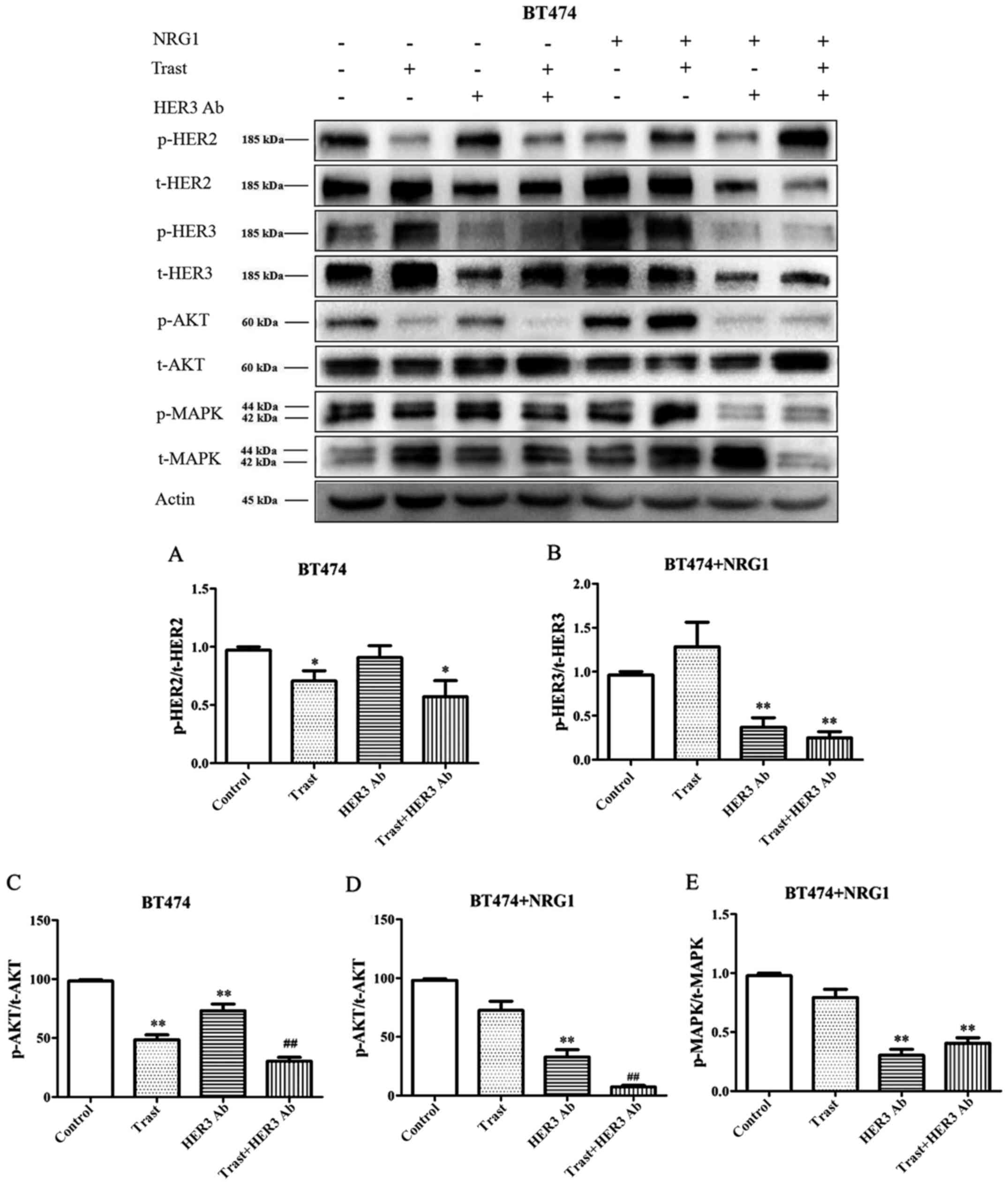
Without NRG1 stimulation, the expression of p-HER2
was significantly downregulated by trastuzumab (P<0.05),
whereas, the inhibitory effect of trastuzumab disappeared after
NRG1 stimulation (Fig. 1). The
HER3 antibody could not downregulate p-HER2 expression before NRG1
stimulation, but it showed a slight downregulation effect after
NRG1 stimulation. The expression of p-HER3 was significantly
increased after NRG1 stimulation (Fig.
1). The HER3 antibody had an inhibitory effect on p-HER3 before
NRG1 stimulation, however, the downregulation role was
significantly enhanced after NRG1 stimulation (P<0.01). The HER3
antibody combined with trastuzumab showed synergistic inhibitory
effect on p-HER3 (Fig. 1).
After BT474 cells were stimulated with NRG1, the
expression of p-Akt was upregulated. In the absence of NRG1
stimulation, the expression of p-Akt was significantly
down-regulated by trastuzumab and HER3 antibody (P<0.01), but
the inhibitory effect of trastuzumab was superior to that of the
HER3 antibody. Whereas, with NRG1 stimulation, the inhibitory
effect of trastuzumab decreased and the inhibitory effect of the
HER3 antibody became more obvious (P<0.01). Combined application
of the two antibodies showed synergistic effect (Fig. 1C and D). Without stimulation of
NRG1, the expression of phospho mitogen-activated protein kinase
(p-MAPK) was inhibited by trastuzumab in BT474 cells. The
inhibitory effect of trastuzumab disappeared after NRG1
stimulation, however, the HER3 antibody showed significant
downregulation effect (P<0.01) (Fig. 1E).
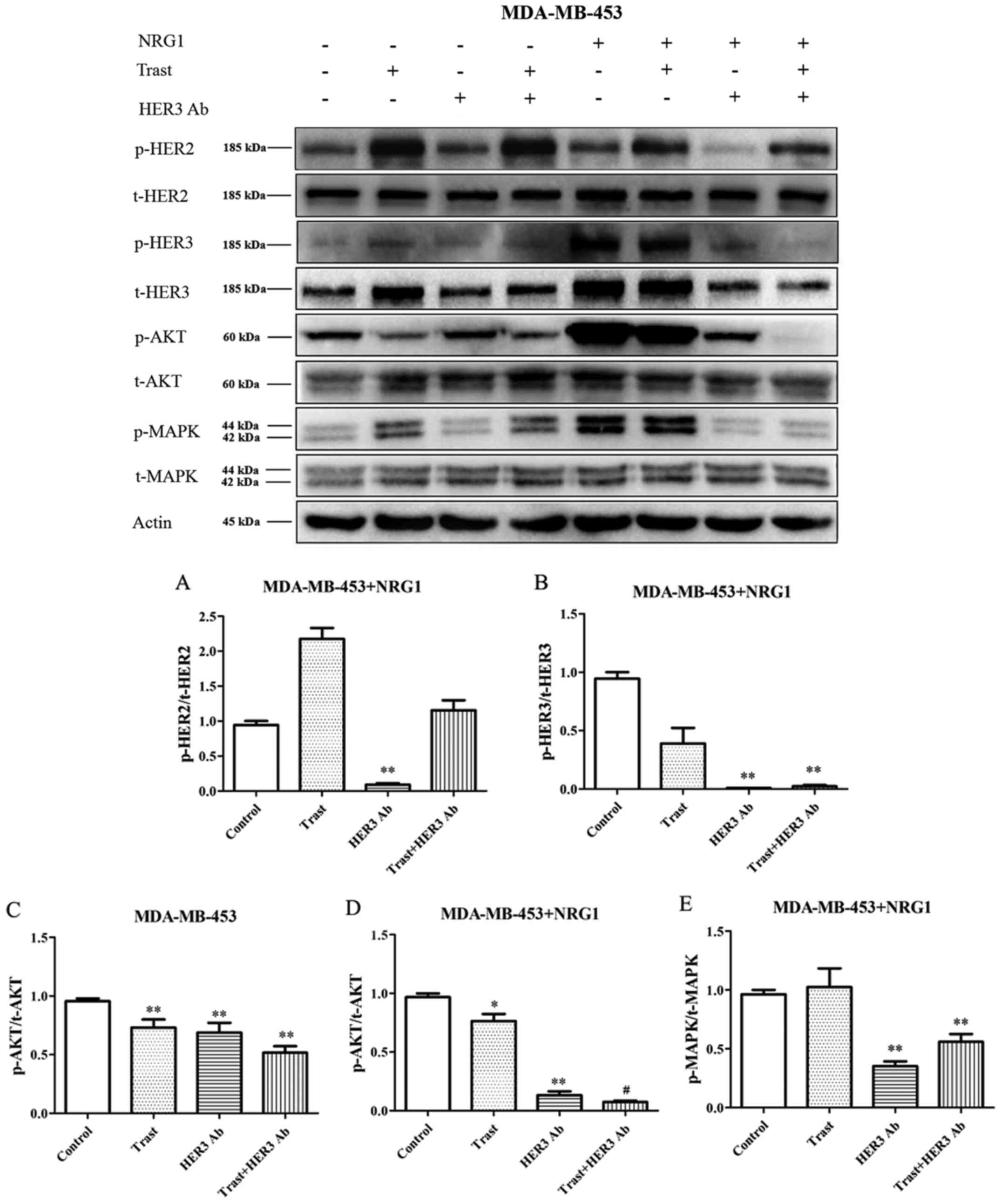
NRG1-depedent HER3 antibody inhibited
p-HER2/p-HER3/p-Akt(p-MAPK) pathways in MDA-MB-453 cells
The expression of p-HER3 and HER3 was significantly
increased after NRG1 stimulation. The expression of p-HER2 and
p-HER3 was not downregulated by trastuzumab without or with NRG1
stimulation (Fig. 2). After NRG1
stimulation, the expression of p-HER2 and p-HER3 were significantly
down-regulated by the HER3 antibody (P<0.01) (Fig. 2A and B).
In the absence of NRG1 stimulation, trastuzumab and
HER3 antibody significantly downregulated the expression of p-Akt
(P<0.01), and trastuzumab combined with the HER3 antibody seemed
to have a synergistic trend (Fig.
2C). By adding NRG1 stimulation, the expression of p-Akt was
significant increased, and the HER3 antibody significantly
downregulated the raised p-Akt expression, moreover, the HER3
antibody combined with trastuzumab showed significant synergistic
effect (P<0.05) (Fig. 2D).
After NRG1 stimulation, trastuzumab did not downregulate p-MAPK
expression, however, the HER3 antibody still significantly
downregulated p-MAPK expression (P<0.01) (Fig. 2E).
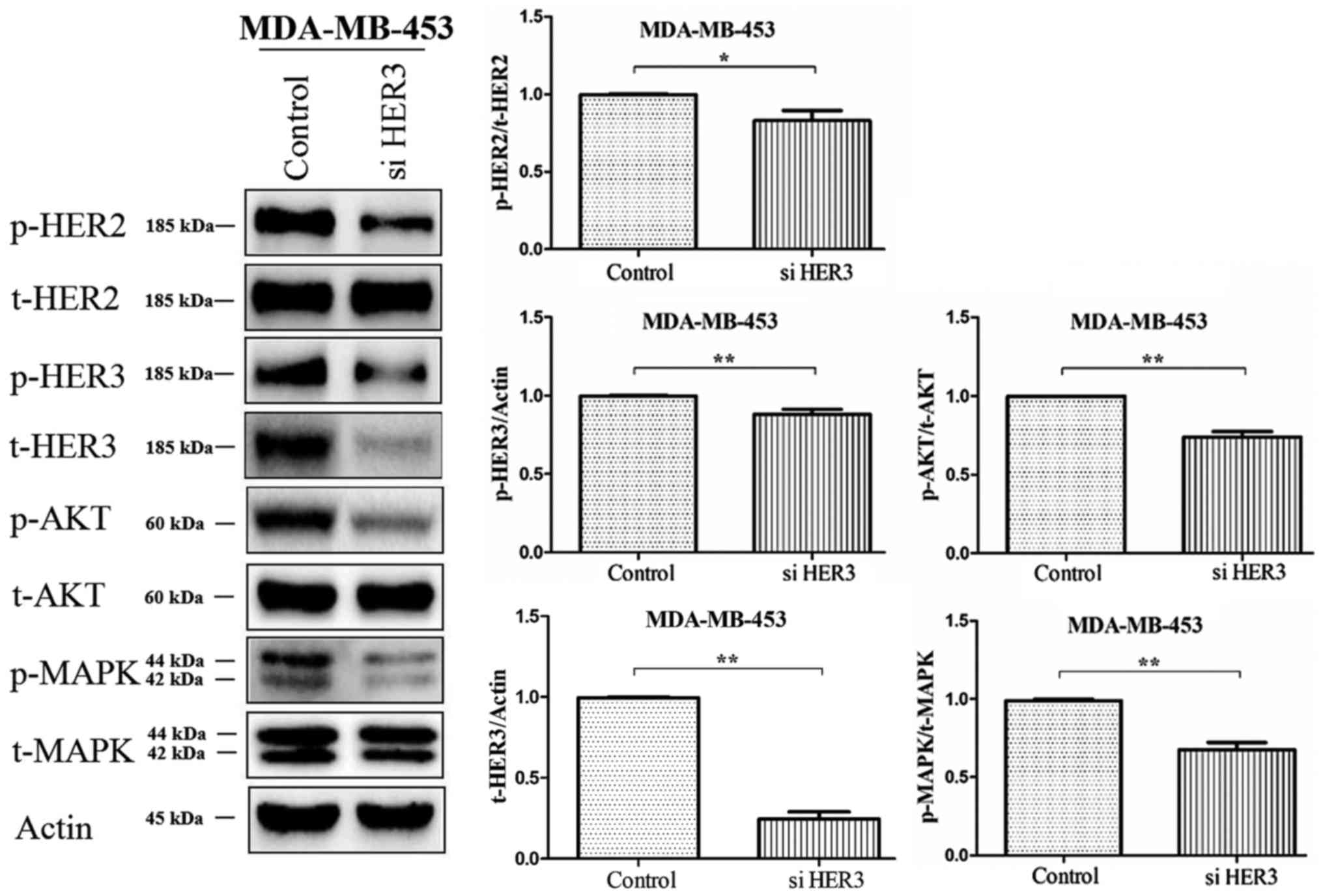
The silencing role of siHER3 in
MDA-MB-453 cells
The expression of HER2, HER3, Akt and MAPK was
detected by western blotting after silencing HER3 gene in
MDA-MB-453 cells. The results showed that the expression of p-HER3
was significantly downregulated after HER3 gene silencing. The
expression of p-HER2, p-Akt and p-MAPK was also downregulated
compared to the control (Fig.
3).
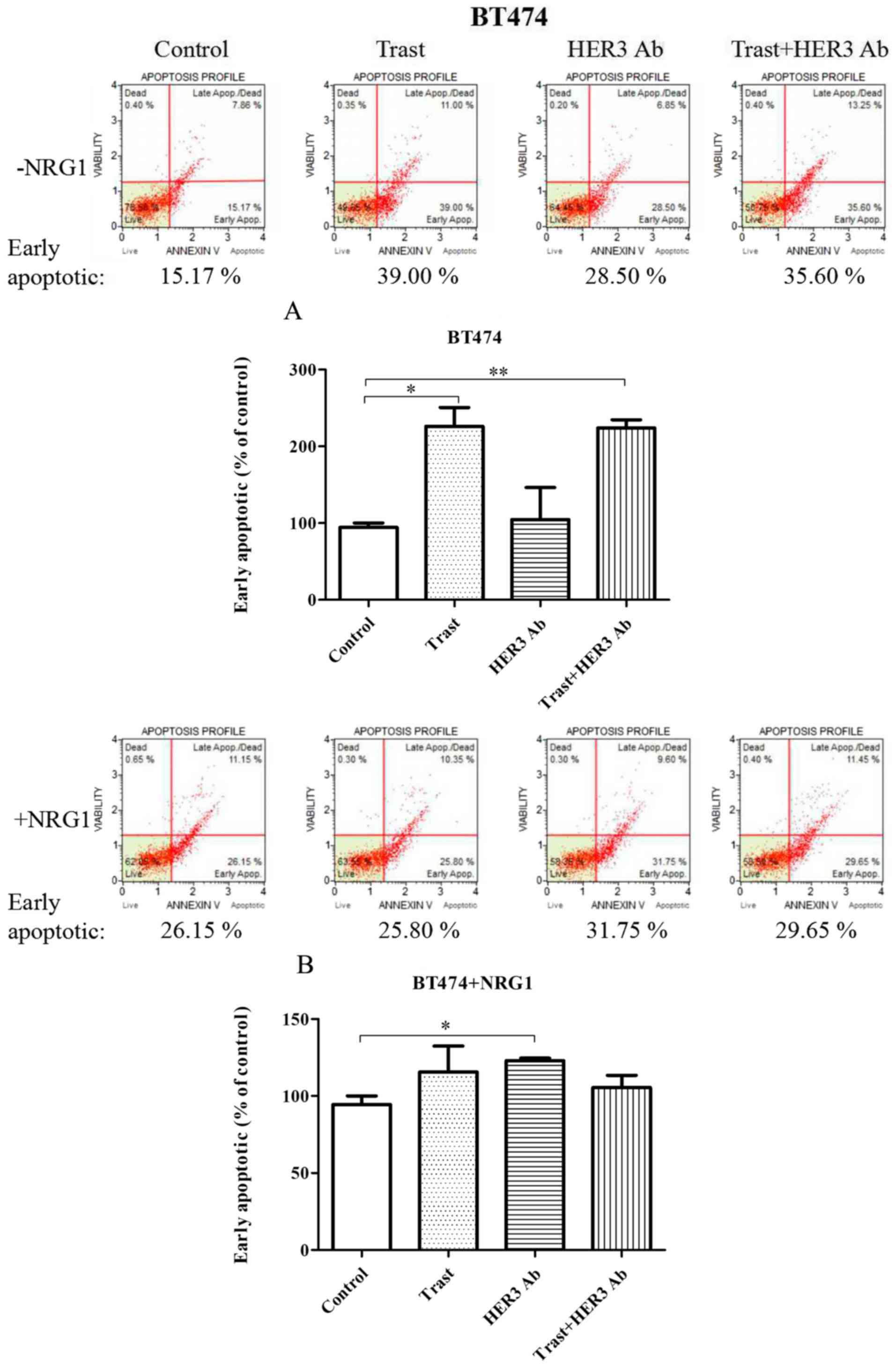
NRG1-depedent HER3 antibody promotes
early apoptosis
Trastuzumab significantly induced an increase in the
proportion of early apoptosis in BT474 cells without NRG1
stimulation (P<0.05). After NRG1 stimulation, the role of
trastuzumab significantly decreased. However, HER3 antibody
significantly promoted early apoptosis of BT474 cells compared to
the control (P<0.05). The combination therapy did not show
synergistic effect (Fig. 4).
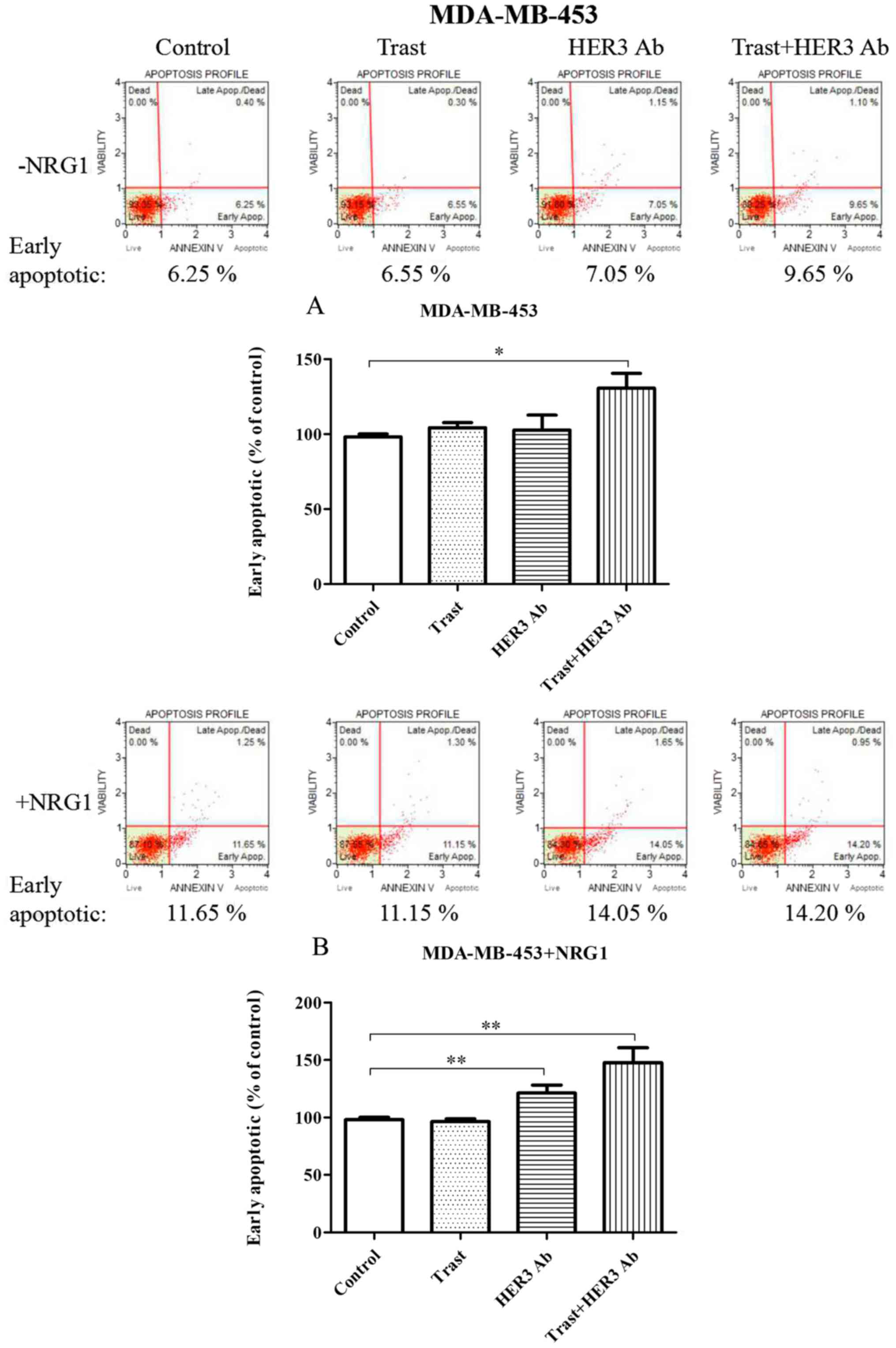
In the absence of NRG1 stimulation, trastuzumab and
HER3 antibody seemed to mildly induce an increase of early
apoptosis in MDA-MB-453 cells. However, combined therapy showed
significantly synergistic effect (P<0.05). After NRG1
stimulation, early apoptosis of MDA-MB-453 cells was not affected
by trastuzumab, but it was significantly promoted by the HER3
antibody when compared to the control (P<0.01). The combined
therapy seemed to have a synergistic trend (Fig. 5).
HER3 antibody combined with trastuzumab
synergistically inhibits cell viability
In BT474 cells, with the increase of drug
concentration, the inhibitory effect of trastuzumab on cell
viability was gradually enhanced. While the effect of trastuzumab
on MDA-MB-453 cells was weak and stable, even the drug
concentration increased to 1000 nM. The inhibitory effects of
trastuzumab on cell viability are different between the two cell
lines. After NRG1 combined with trastuzumab, the cell viability was
significantly increased in BT474 cells (Fig. 6A), however, this significant
increase was not observed in MDA-MB-453 cells (Fig. 6D). With NRG1 stimulation, the
combined therapy showed significantly inhibitory effect compared to
trastuzumab treatment when the dosages were higher than 1 nM in
BT474 and MDA-MB-453 cells (Fig. 6C
and F). Synergistic inhibitory effect of combined therapy was
observed at the concentration of 10 nM in BT474 cells after NRG1
stimulation and at the concentration of 1 nM and 10 nM in
MDA-MB-453 cells before NRG1 stimulation (Fig. 6C and E).
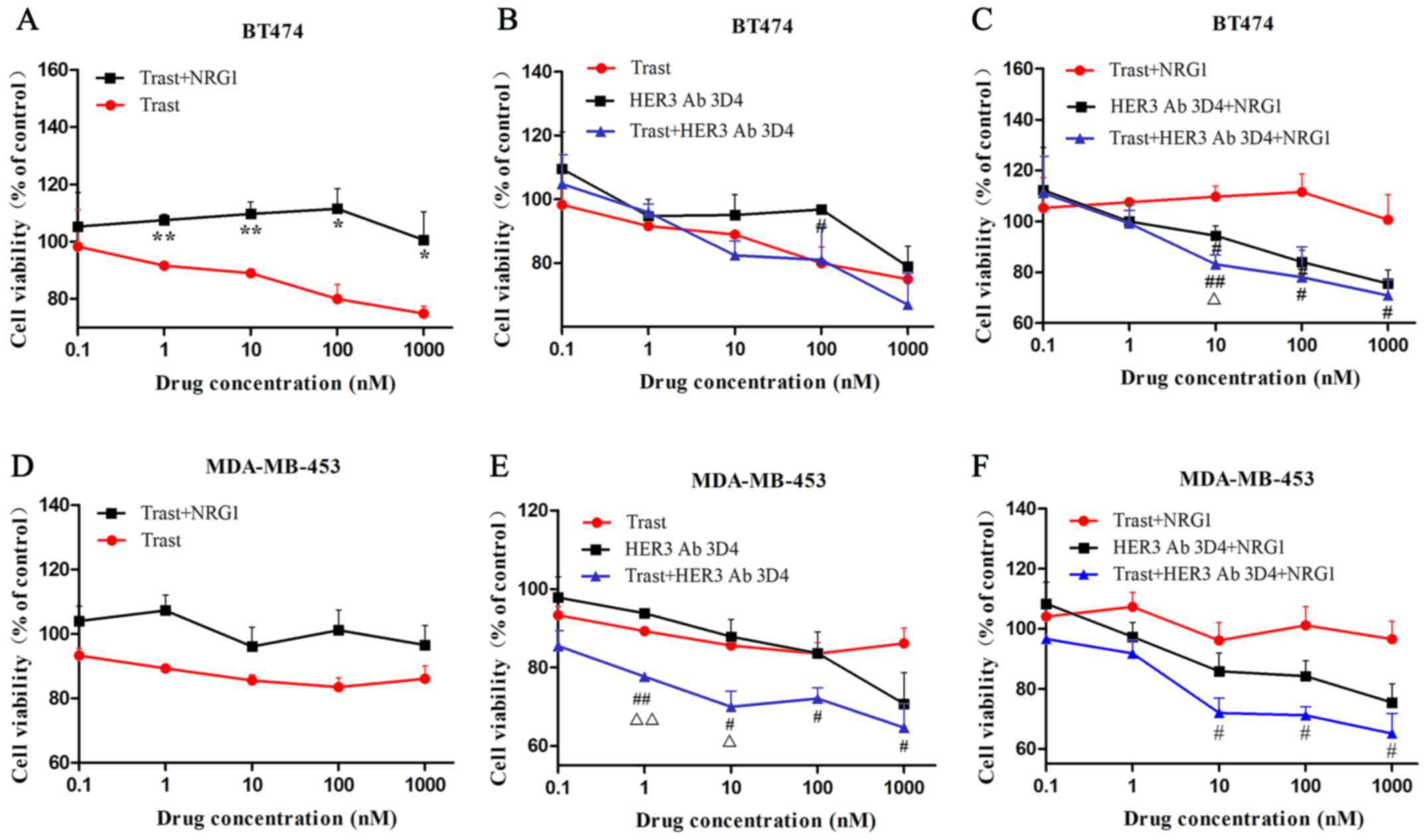 | Figure 6HER3 antibody combined with
trastuzumab synergistically inhibits cell viability. (A and D) In
BT474 cells, the inhibitory effect of trastuzumab on cell viability
was gradually enhanced with the increase of drug concentration.
However, the effect on MDA-MB-453 cells was weak and stable. After
NRG1 combined with trastuzumab, the cell viability was
significantly increased in BT474 cells, however, this significant
increase was not observed in MDA-MB-453 cells. (C and F) With NRG1
stimulation, the combined therapy showed significantly inhibitory
effect compared to trastuzumab treatment when the dosages were
higher than 1 nM in BT474 and MDA-MB-453 cells. (C and E)
Synergistic inhibitory effect of combined therapy was observed at
the concentration of 10 nM in BT474 cells after NRG1 stimulation
and at the concentration of 1 and 10 nM in MDA-MB-453 cells before
NRG1 stimulation. (A) *P<0.05, **P<0.01
compared to trastuzumab. (B, C, E and F) #P<0.05,
##P<0.01 compared to trastuzumab or trastuzumab+NRG1;
△P<0.05, △△P<0.01 compared to HER3
antibody or HER3 antibody+NRG1. |
Discussion
HER3 was initially thought to be functionally
passive and clinically insignificant. Currently, research has
confirmed that HER3, as a functional activator, has the ability to
activate recipient kinase (15).
Moreover, HER3 is proved to be involved in tumorigenesis and
progression, it may serve as a new therapeutic target (27). The research significance and
clinical value of HER3 are becoming more and more important. A
study by Lee-Hoeflich et al demonstrated that simultaneous
downregulation of HER3 and HER2 was crucial in inhibiting
proliferation of HER2-overexpressing breast cancer cells (17). Resistance to trastuzumab, which
functions to block HER2, is a serious problem faced by clinicians.
Therefore, the goal of our study was to analyze the role
NRG1-depedent HER3 activation has on inducing trastuzumab
resistance and the inhibitory effect of HER3 monoclonal antibody on
HER2-overexpressing breast cancer cells.
In absence of NRG1 stimulation, trastuzumab
significantly downregulated the expression of p-HER2 and induced an
increase in early apoptosis in BT474 cells. After NRG1 stimulation,
the inhibitory effects of trastuzumab disappeared. However, we
observed that the expression of p-HER3 was increased after NRG1
stimulation. The addition of HER3 antibody significantly
downregulated the expression of HER3 and induced an increase in
early apoptosis. The data suggest that the p-HER3 upregulation,
stimulated by NRG1, may counteract the p-HER2 downregulation effect
caused by trastuzumab, potentially leading to trastuzumab
resistance. A study by Garrett et al showed that trastuzumab
and lapatinib double blockade of HER2 caused a transcriptional and
post-translational upregulation of HER3, partially offsetting the
targeted inhibition efficacy of HER2-directed therapies (28). Our result coincides with that of
Göstring et al, who concluded that the activation loop of
NRG1/HER3 played a vital role in the proliferation process of
HER2-overexpressing breast cancer cells, independently of HER2
(22). Trastuzumab did not
downregulate p-HER2 or p-HER3 and had no effect on apoptosis before
or after the addition of NRG1 to MDA-MB-453 cells, confirming that
MDA-MB-453 cells were indeed resistant to trastuzumab. After NRG1
stimulation, the HER3 antibody significantly downregulated p-HER2
and p-HER3 and significantly promoted apoptosis in MDA-MB-453
cells, indicating that the inhibition of NRG1/HER2/HER3 led to the
apoptosis of MDA-MB-453 cells. Therefore, the activation of
NRG1/HER3 may be one of the key factors causing primary resistance
to trastuzumab in MDA-MB-453 cells. A study by Wu et al
showed that antisense oligonucleotide (EZN-3920) could sustain an
anti-proliferation effect in trastuzumab-resistant cells by
effectively downregulating the expression of HER3 (29). A study by Ebbing et al
showed that the metallo-proteinase ADAM10 activated HER3 and
downstream signaling by releasing NRG1 from the cell membrane and
induced resistance to trastuzumab (30). These studies suggest that NRG1/HER3
was closely related to trastuzumab resistance, and the inhibition
of HER3 might reverse trastuzumab resistance. Moreover, after the
silencing of HER3 gene, the expression of p-HER2, p-HER3 and the
downstream proteins p-Akt and p-MAPK were significantly
downregulated, consistent with the inhibitory effect of HER3
antibody.
For HER3 antibody (3D4), apoptotic-promoting effect
was not present before NRG1 stimulation, but it was observed after
NRG1 stimulation in MDA-MB-453 cells, indicating that HER3 antibody
might reverse NRG1 initiated primary resistance to trastuzumab. A
study by Leung et al showed that lapatinib, a HER1/HER2
small molecule tyrosine kinase inhibitor, could not continuously
inhibit the signaling pathway of HER dimers. The elevation of NRG1
was observed in resistance process, whereas the addition of the
HER3 antibody SPG1 effectively reversed the resistance to lapatinib
(31), which was almost in line
with our findings. A study by Xia et al showed that
NRG1-positive expression was an independent negative predictive
factor for HER2-overexpressing breast cancer, indirectly hinting
that NRG1-dependent HER3 activation might promote the progression
of breast cancer (32).
The expression of p-Akt and p-MAPK was downregulated
by the HER3 antibody in BT474 and MDA-MB-453 cells after NRG1
stimulation. These results suggest that NRG1-dependent HER3
inducing the primary resistance to trastuzumab was mediated by Akt
and MAPK-related signaling pathways. A study by Dey et al
showed that simultaneous inhibition of the HER2 and PI3K/Akt/mTOR
signaling pathways was more effective than the single inhibition of
HER2 (33). A combination of the
new Akt inhibitor AZD5363 and the EGFR/HER2/HER3 inhibitor AZD8931
synergistically inhibited the proliferation of HER2-overexpressing
breast cancer cells (34,35), and S100P induced trastuzumab
resistance by activating the RAS/MEK/MAPK signaling pathway
(36). These findings were
consistent with our results that the HER3 antibody promoted
apoptosis in MDA-MB-453 cells by downregulating Akt and MAPK
phosphorylation. Therefore, HER3 antibody reversed NRG1-induced
trastuzumab primary resistance by interfering with the
phosphorylation of HER3, blocking the formation of heterodimers,
and downregulating p-Akt and p-MAPK in the downstream signaling
pathway.
HER3 targeting therapy is rapidly developing.
Sensitivity of colorectal cancer DiFi cells to cetuximab was
restored by Patritumab, a HER3 monoclonal antibody, which functions
by inhibiting the activity of NRG1/HER3 (37). HER3 monoclonal antibody LMAb3 fully
inhibited proliferation of trastuzumab-resistant SKOV3-T cells by
downregulating the activity of HER3 and its related signaling
proteins (38). Wang et al
reported that HER3 monoclonal antibody and trastuzumab have
synergistically inhibitory effect in HER2-positive gastric cancer
cells (39). A study by Canonici
et al showed that the irreversible panHER inhibitor
Neratinib reversed trastuzumab-resistance (40). In our study, we found synergistic
effects between trastuzumab and HER3 monoclonal antibody (3D4) in
the inhibition of cell viability in both BT474 and MDA-MB-453 cells
after NRG1 stimulation.
In conclusion, NRG1/HER3 activation is one of the
key factors inducing primary resistance to trastuzumab in
HER2-overexpressing breast cancer cells. The HER3 antibody may
reverse trastuzumab primary resistance by significantly inhibiting
the activation of NRG1-dependent HER3. Because of the diversity and
complexity of the HER family and the signal transduction pathways,
the combined targeted drugs or multi-targeted drugs treatment may
become the trend in the future. Trastuzumab combined with HER3
monoclonal antibody may be a treatment choice for patients with
primary resistance to trastuzumab.
Acknowledgments
We thank Professor Wang Ping and Professor Zhang
Dong for experimental guidance. Springer Nature Author Services
helped to prepare our manuscript. This study was funded by the
National Natural Science Foundation of China (no. 81301912, to
Q.L.), the Beijing Municipal Health System High-level Health Person
Foundation Project (no. 2014-3-005, to Q.L.), and the Beijing
Municipal Science and Technology Commission (Capital Features,
Z161100000516083, awarded to Q.L.).
Glossary
Abbreviations
Abbreviations:
|
HER
|
human epidermal growth factor
receptor
|
|
p-HER2
|
phospho human epidermal growth factor
receptor 2
|
|
p-HER3
|
phospho human epidermal growth factor
receptor 3
|
|
p-Akt
|
phospho protein kinase B
|
|
p-MAPK
|
phospho mitogen-activated protein
kinase
|
|
HER1
|
human epidermal growth factor receptor
1
|
|
HER2
|
human epidermal growth factor receptor
2
|
|
HER3
|
human epidermal growth factor receptor
3
|
|
HER4
|
human epidermal growth factor receptor
4
|
|
Akt
|
protein kinase B
|
|
MAPK
|
mitogen-activated protein kinase
|
|
NRG1
|
neuregulin 1
|
|
PI3K
|
phosphatidylinositol 3 kinase
|
|
siRNA
|
small interfering RNA
|
|
FBS
|
fetal bovine serum
|
References
|
1
|
Slamon DJ, Godolphin W, Jones LA, Holt JA,
Wong SG, Keith DE, Levin WJ, Stuart SG, Udove J, Ullrich A, et al:
Studies of the HER-2/neu proto-oncogene in human breast and ovarian
cancer. Science. 244:707–712. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Schulz R, Streller F, Scheel AH, Rüschoff
J, Reinert MC, Dobbelstein M, Marchenko ND and Moll UM: HER2/ErbB2
activates HSF1 and thereby controls HSP90 clients including MIF in
HER2-overexpressing breast cancer. Cell Death Dis. 5:e9802014.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Slamon DJ, Clark GM, Wong SG, Levin WJ,
Ullrich A and McGuire WL: Human breast cancer: Correlation of
relapse and survival with amplification of the HER-2/neu oncogene.
Science. 235:177–182. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Nahta R: Molecular mechanisms of
trastuzumab-based treatment in HER2-overexpressing breast cancer.
ISRN Oncol. 2012:4280622012.PubMed/NCBI
|
|
5
|
Gelmon KA, Boyle FM, Kaufman B, Huntsman
DG, Manikhas A, Di Leo A, Martin M, Schwartzberg LS, Lemieux J,
Aparicio S, et al: Lapatinib or trastuzumab plus taxane therapy for
human epidermal growth factor receptor 2-positive advanced breast
cancer: Final results of NCIC CTG MA.31. J Clin Oncol.
33:1574–1583. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Valero V, Forbes J, Pegram MD, Pienkowski
T, Eiermann W, von Minckwitz G, Roche H, Martin M, Crown J, Mackey
JR, et al: Multicenter phase III randomized trial comparing
docetaxel and trastuzumab with docetaxel, carboplatin, and
trastuzumab as first-line chemotherapy for patients with
HER2-gene-amplified metastatic breast cancer (BCIRG 007 study): Two
highly active therapeutic regimens. J Clin Oncol. 29:149–156. 2011.
View Article : Google Scholar
|
|
7
|
Ritter CA, Perez-Torres M, Rinehart C,
Guix M, Dugger T, Engelman JA and Arteaga CL: Human breast cancer
cells selected for resistance to trastuzumab in vivo overexpress
epidermal growth factor receptor and ErbB ligands and remain
dependent on the ErbB receptor network. Clin Cancer Res.
13:4909–4919. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Madrid-Paredes A, Cañadas-Garre M,
Sánchez-Pozo A and Calleja-Hernández MA: Non-HER2 signaling
pathways activated in resistance to anti-HER2 therapy in breast
cancer. Breast Cancer Res Treat. 153:493–505. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Scaltriti M, Rojo F, Ocaña A, Anido J,
Guzman M, Cortes J, D Cosimo S, Matias-Guiu X, Ramon y Cajal S,
Arribas J, et al: Expression of p95HER2, a truncated form of the
HER2 receptor, and response to anti-HER2 therapies in breast
cancer. J Natl Cancer Inst. 99:628–638. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ozkavruk Eliyatkin N, Aktas S, Ozgur H,
Ercetin P and Kupelioglu A: The role of p95HER2 in trastuzumab
resistance in breast cancer. J BUON. 21:382–389. 2016.PubMed/NCBI
|
|
11
|
Narayan M, Wilken JA, Harris LN, Baron AT,
Kimbler KD and Maihle NJ: Trastuzumab-induced HER reprogramming in
'resistant' breast carcinoma cells. Cancer Res. 69:2191–2194. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Deguchi Y, Okabe H, Oshima N, Hisamori S,
Minamiguchi S, Muto M and Sakai Y: PTEN loss is associated with a
poor response to trastuzumab in HER2-overexpressing
gastroesophageal adenocarcinoma. Gastric Cancer. 20:416–427. 2017.
View Article : Google Scholar
|
|
13
|
Luque-Cabal M, García-Teijido P,
Fernández-Pérez Y, Sánchez-Lorenzo L and Palacio-Vázquez I:
Mechanisms behind the resistance to Trastuzumab in HER2-amplified
breast cancer and strategies to overcome it. Clin Med Insights
Oncol. 10(Suppl 1): 21–30. 2016.PubMed/NCBI
|
|
14
|
Liu J, Pan C, Guo L, Wu M, Guo J, Peng S,
Wu Q and Zuo Q: A new mechanism of trastuzumab resistance in
gastric cancer: MACC1 promotes the Warburg effect via activation of
the PI3K/AKT signaling pathway. J Hematol Oncol. 9:762016.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Jura N, Shan Y, Cao X, Shaw DE and Kuriyan
J: Structural analysis of the catalytically inactive kinase domain
of the human EGF receptor 3. Proc Natl Acad Sci USA.
106:21608–21613. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Shi F, Telesco SE, Liu Y, Radhakrishnan R
and Lemmon MA: ErbB3/HER3 intracellular domain is competent to bind
ATP and catalyze autophosphorylation. Proc Natl Acad Sci USA.
107:7692–7697. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lee-Hoeflich ST, Crocker L, Yao E, Pham T,
Munroe X, Hoeflich KP, Sliwkowski MX and Stern HM: A central role
for HER3 in HER2-amplified breast cancer: Implications for targeted
therapy. Cancer Res. 68:5878–5887. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Jeong H, Kim J, Lee Y, Seo JH, Hong SR and
Kim A: Neuregulin-1 induces cancer stem cell characteristics in
breast cancer cell lines. Oncol Rep. 32:1218–1224. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sheng Q, Liu X, Fleming E, Yuan K, Piao H,
Chen J, Moustafa Z, Thomas RK, Greulich H, Schinzel A, et al: An
activated ErbB3/NRG1 autocrine loop supports in vivo proliferation
in ovarian cancer cells. Cancer Cell. 17:298–310. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
De Boeck A, Pauwels P, Hensen K, Rummens
JL, Westbroek W, Hendrix A, Maynard D, Denys H, Lambein K, Braems
G, et al: Bone marrow-derived mesenchymal stem cells promote
colorectal cancer progression through paracrine neuregulin 1/HER3
signalling. Gut. 62:550–560. 2013. View Article : Google Scholar
|
|
21
|
Kim S, Han J, Shin I, Kil WH, Lee JE and
Nam SJ: A functional comparison between the HER2(high)/HER3 and the
HER2(low)/HER3 dimers on heregulin-β1-induced MMP-1 and MMP-9
expression in breast cancer cells. Exp Mol Med. 44:473–482. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Göstring L, Malm M, Höidén-Guthenberg I,
Frejd FY, Ståhl S, Löfblom J and Gedda L: Cellular effects of
HER3-specific affibody molecules. PLoS One. 7:e400232012.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ni M, Chen Y, Lim E, Wimberly H, Bailey
ST, Imai Y, Rimm DL, Liu XS and Brown M: Targeting androgen
receptor in estrogen receptor-negative breast cancer. Cancer Cell.
20:119–131. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Shim JS, Rao R, Beebe K, Neckers L, Han I,
Nahta R and Liu JO: Selective inhibition of HER2-positive breast
cancer cells by the HIV protease inhibitor nelfinavir. J Natl
Cancer Inst. 104:1576–1590. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Barok M, Tanner M, Köninki K and Isola J:
Trastuzumab-DM1 causes tumour growth inhibition by mitotic
catastrophe in trastuzumab-resistant breast cancer cells in vivo.
Breast Cancer Res. 13:R462011. View
Article : Google Scholar : PubMed/NCBI
|
|
26
|
Walsh AJ, Cook RS, Sanders ME, Aurisicchio
L, Ciliberto G, Arteaga CL and Skala MC: Quantitative optical
imaging of primary tumor organoid metabolism predicts drug response
in breast cancer. Cancer Res. 74:5184–5194. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Li Q, Yuan Z and Cao B: The function of
human epidermal growth factor receptor-3 and its role in tumors
(Review). Oncol Rep. 30:2563–2570. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Garrett JT, Sutton CR, Kuba MG, Cook RS
and Arteaga CL: Dual blockade of HER2 in HER2-overexpressing tumor
cells does not completely eliminate HER3 function. Clin Cancer Res.
19:610–619. 2013. View Article : Google Scholar :
|
|
29
|
Wu Y, Zhang Y, Wang M, Li Q, Qu Z, Shi V,
Kraft P, Kim S, Gao Y, Pak J, et al: Downregulation of HER3 by a
novel antisense oligonucleotide, EZN-3920, improves the antitumor
activity of EGFR and HER2 tyrosine kinase inhibitors in animal
models. Mol Cancer Ther. 12:427–437. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ebbing EA, Medema JP, Damhofer H, Meijer
SL, Krishnadath KK, van Berge Henegouwen MI, Bijlsma MF and van
Laarhoven HW: ADAM10-mediated release of heregulin confers
resistance to trastuzumab by activating HER3. Oncotarget.
7:10243–10254. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Leung WY, Roxanis I, Sheldon H, Buffa FM,
Li JL, Harris AL and Kong A: Combining lapatinib and pertuzumab to
overcome lapatinib resistance due to NRG1-mediated signalling in
HER2-amplified breast cancer. Oncotarget. 6:5678–5694. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Xia W, Petricoin EF III, Zhao S, Liu L,
Osada T, Cheng Q, Wulfkuhle JD, Gwin WR, Yang X, Gallagher RI, et
al: An heregulin-EGFR-HER3 autocrine signaling axis can mediate
acquired lapatinib resistance in HER2+ breast cancer
models. Breast Cancer Res. 15:R852013. View Article : Google Scholar
|
|
33
|
Dey N, Sun Y, Carlson JH, Wu H, Lin X,
Leyland-Jones B and De P: Anti-tumor efficacy of BEZ235 is
complemented by its anti-angiogenic effects via downregulation of
PI3K-mTOR-HIF1alpha signaling in HER2-defined breast cancers. Am J
Cancer Res. 6:714–746. 2016.PubMed/NCBI
|
|
34
|
Crafter C, Vincent JP, Tang E, Dudley P,
James NH, Klinowska T and Davies BR: Combining AZD8931, a novel
EGFR/HER2/HER3 signalling inhibitor, with AZD5363 limits AKT
inhibitor induced feedback and enhances antitumour efficacy in
HER2-amplified breast cancer models. Int J Oncol. 47:446–454. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
O'Brien NA, McDonald K, Tong L, von Euw E,
Kalous O, Conklin D, Hurvitz SA, di Tomaso E, Schnell C, Linnartz
R, et al: Targeting PI3K/mTOR overcomes resistance to HER2-targeted
therapy independent of feedback activation of AKT. Clin Cancer Res.
20:3507–3520. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Merry CR, McMahon S, Forrest ME, Bartels
CF, Saiakhova A, Bartel CA, Scacheri PC, Thompson CL, Jackson MW,
Harris LN, et al: Transcriptome-wide identification of mRNAs and
lincRNAs associated with trastuzumab-resistance in HER2-positive
breast cancer. Oncotarget. 7:53230–53244. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kawakami H, Okamoto I, Yonesaka K, Okamoto
K, Shibata K, Shinkai Y, Sakamoto H, Kitano M, Tamura T, Nishio K,
et al: The anti-HER3 antibody patritumab abrogates cetuximab
resistance mediated by heregulin in colorectal cancer cells.
Oncotarget. 5:11847–11856. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Li X, Duan Y, Qiao C, Zhou T, Yu M, Geng
J, Feng J, Shen B, Lv M and Li Y: Anti-HER3 monoclonal antibody
inhibits acquired trastuzumab-resistant gynecologic cancers.
Technol Cancer Res Treat. 15:573–582. 2016. View Article : Google Scholar
|
|
39
|
Wang Q, Zhang X, Shen E, Gao J, Cao F,
Wang X, Li Y, Tian T, Wang J, Chen Z, et al: The anti-HER3 antibody
in combination with trastuzumab exerts synergistic antitumor
activity in HER2-positive gastric cancer. Cancer Lett. 380:20–30.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Canonici A, Gijsen M, Mullooly M, Bennett
R, Bouguern N, Pedersen K, O'Brien NA, Roxanis I, Li JL, Bridge E,
et al: Neratinib overcomes trastuzumab resistance in HER2 amplified
breast cancer. Oncotarget. 4:1592–1605. 2013. View Article : Google Scholar : PubMed/NCBI
|