Introduction
Colorectal cancer (CRC) is the second most common
cause of cancer death in the Western world (1), and the incidence of colorectal cancer
in the Asia-Pacific region is increasing (2). Surgical resection of primary tumors
and adjuvant chemotherapy improved patient survival, but nearly
half of the patients eventually died of local recurrence and
metastasis (3). At present,
targeted therapy has become an important treatment for various
malignant tumors (including CRC). Bevacizumab (targeted to VEGF)
and cetuximab (targeted to EGFR) are two drugs approved for use in
the treatment of progressive and/or metastatic colorectal cancer.
Molecular targeted therapy also has the problem of multi-target
combination therapy.
Now, exploring new therapeutic targets in colorectal
cancer has become an essential goal. The fibroblast growth factor
receptor (FGFRs) is a polygene family, an immunoglobulin gene
superfamily member, which is distributed in a variety of cells on
the membrane protein. FGFR4 is a class of transmembrane tyrosine
kinase receptors with autophosphorylation activity that plays a
very important role in embryonic development, tissue repair and
angiogenesis (4). Hagel et
al discovered Blu9931, which is a novel irreversible kinase
inhibitor that specifically targets FGFR4 and is also the first
FGFR4-selective molecule for the treatment of patients with
hepatocellular carcinoma (5).
Our previous studies have shown that the expression
of FGFR4 mRNA is significantly increased in gastric cancer tissue
when compared with that of the corresponding normal tissue
(6). The knockdown of FGFR4
expression resulted in a decrease in the proliferation of MKN45 and
SGC7901 GC cell lines and an increase in apoptosis. Western blot
analysis showed that the expression of caspase-3 was increased and
the expression of Bcl-xL in MKN45 and SGC7901 cells was decreased
after FGFR4-siRNA transfection (7). The apoptosis rates were obviously
increased in GC cells treated with the single and combination of
5-FU and PD173074 (an inhibitor of FGFR) (8). Nonetheless we have not yet studied
the role of FGFR4 in colorectal cancer.
In order to investigate the clinical value of FGFR4
expression in CRC and explore new targeted drugs, Blu9931 as a
specific inhibitor of FGFR4 was introduced in this study. We
selected two colorectal cancer cell lines, HCT116 and SW620, that
overexpress FGFR4. A series of functional tests were then performed
to study the effects of single agent treatment and combinations of
5-FU and Blu9931 on the biological behavior of the above two cell
lines, including proliferation assay, apoptosis detection, cell
cycle distribution assessment and the expression of related
molecules by western blot analysis. Epithelial-mesenchymal
transition (EMT) is considered a key event in metastasis and plays
a critical role in the progression of colorectal cancer. Ectopic
FGF19 expression promotes EMT and invasion in epithelial-like HCC
cells by inhibiting of E-cadherin expression (9). FGF19 interacts with FGFR4 and
promotes FGFR4 expression. We found that FGFR4 and E-cadherin
expression are negatively correlated in colorectal cancer cells.
Through these functional tests, our aim is to elucidate the
mechanism of Blu9931 and 5-FU on colorectal cancer cells. We
believe that FGFR4 is a potential therapeutic target for colorectal
cancer and that Blu9931 may be used in the treatment of colorectal
cancer patients.
Materials and methods
Cell lines and cell culture
Human colorectal cancer cell lines LS47 and SW620
were purchased from the American Type Culture collection. DLD1,
SW116 and HCT116 cell lines were purchased from the Chinese Academy
of Sciences, the Science Cell Bank of the Type Culture Collection
(CBTCCCAS, Shanghai, China). Cell lines were cultivated in
Dulbecco's modified Eagle's medium (DMEM, Sweden) supplemented with
10% fetal bovine serum (FBS; Gibco, USA), 100 U/ml of penicillin
and 100 μg/ml of streptomycin (Caisson labs, UT, USA) at
37°C in a humidified atmosphere containing 5% CO2.
Antibodies and reagents
Rabbit monoclonal anti-FGFR4 antibody,
anti-E-cadherin, anti-vimentin, anti-cleaved caspase-3, anti-STAT3,
anti-cyclin D1, anti-p27kip1 and anti-β-actin antibody
as well as mouse monoclonal anti-PCNA antibody were all purchased
from Cell Signaling Technology (Beverly, MA, USA). Secondary
horseradish peroxidase-conjugated antibodies were goat anti-mouse
and goat anti-rabbit from Sigma-Aldrich Corp. (St. Louis, MO, USA).
Blu9931 (HY-12823) was purchased from Shanghai Haoyuan Chemexpress
(Shanghai, China). 5-FU was from the clinical trial group in our
research center.
Reverse transcription PCR and
quantitative real-time PCR
According to the protocol, TRIzol reagents are used
to collect total RNA from colorectal cancer cell lines. The first
strand cDNA synthesis kit (MBI, Fermentas, Canada) was reverse
transcribed into cDNA for each RNA sample. The primers were:
FGFR-4, 5′-AGATGCTCAAAGACAACGCCT-3′ and 5′-CGCACTCCACGATCACGTA-3′;
β-actin, 5′-CACGATGGAGGGGCCGGACTCATC-3′ and
5′-TAAAGACCTCTATGCCAACACAGT -3 ′. The 2X Taq PCR MasterMix (Tiangen
Biotech, China) was used for PCR. The FGFR4 annealing temperature
was 57°C. The PCR product was subjected to 2% agarose gel
electrophoresis and stained with ethidium bromide. Gene-specific
primers of FGFR4 and GAPDH were the same as the reverse
transcriptase PCR in our study. Five colorectal cancer cell lines
were examined by real-time PCR according to the specification by
Takara (Japan). The experiment was carried out in duplicate.
Relative differences (-fold) were calculated according to the
comparative Ct method.
Protein extraction and western
blotting
Whole-cell lysates were prepared using the mammalian
Protein Extraction reagent (Merck, Darmstadt, Germany) in
accordance with the manufacturer's instructions. Protein
concentrations of the samples were determined by the bicinchoninic
acid (BCA) protein assay (Pierce, Rockford, IL, USA). Protein
samples (30 μg of each protein) boiled for 5 min were
separated on 10% SDS-polyacrylamide gels and transferred onto PVDF
membranes. The membranes were blocked for 1 h at room temperature
with phosphate-buffered saline (PBS) containing 0.05% Tween-20 and
5% non-fat dried milk, and incubated overnight at 4°C with the
primary antibodies following the manufacturer's instructions.
Immunoblots were washed three times with PBS containing 0.05%
Tween-20 and 1% non-fat milk. Then PVDF membranes incubated with
secondary antibodies conjugated with horseradish peroxidase against
mouse IgG or rabbit IgG for 1 h at room temperature. Immunoreactive
proteins were using the ECl detection system (ImageQuant LAS 3000;
General Electric Co., Fairfeld, CT, USA). Three independent western
blot assays were performed for all samples.
Cell viability assay
HCT116 cells and SW620 cells in logarithmic growth
phase were harvested, and cell density was adjusted to
3×105/ml. Then, these cells were seeded into 96-well
plates (100 μl/well) and there were 4 wells in each group.
After 24-h incubation, the cells were treated with single and
combination of Blu9931 and 5-FU at different concentrations.
According to Blu9931 (0, 1.875, 3.75, 7.5,15 and 30 μM) and
5-FU (0, 3.125, 6.25, 12.5, 25 and 50 μM) were studied.
Cells were incubated at 37°C in an environment of 5% CO2
for 72 h. After addition of 5 mg/ml MTT (10 μl/well), cells
were incubated for 4 h and the supernatant was removed. DMSO (100
μl/well) was added to each well and cells were further
incubated for 10 min with constant shaking to resolve purple
crystals. The absorbance was measured at 490 nm using a microplate
reader. The cell viability and IC50 were calculated and
analyzed.
Proliferation assay
HCT116 cells and SW620 cells in logarithmic growth
phase were harvested, and cell density was adjusted to
2×105/ml. Then, these cells were seeded into 96-well
plates (100 μl/well) and there were 4 wells in each group.
After 24-h incubation, the cells were treated with single and
combination of Blu9931 and 5-FU at IC50. After culturing
for 1, 2, 3, 4 and 5 days, cells were incubated for 4 h with 5
mg/ml MTT (10 μl/well) and the supernatant was removed. DMSO
(100 μl/well) was added to each well and cells were further
incubated for 10 min with constant shaking to resolve purple
crystals. The absorbance was measured at 490 nm using a microplate
reader.
Apoptosis rate detection
HCT116 cells and SW620 cells were treated with
single and combination of Blu9931 and 5-FU at each proper
concentration for 72 h. Then the cells were harvested. Annexin V
and APC were used to detect apoptosis by flow cytometry. Cells were
suspended in 500 μl of Annexin V binding buffer and the
cells were incubated with 5 μl of Annexin V for 15 min in
the dark and at room temperature. Then 5 μl of propidium
iodide was added. Thereafter, all samples were analyzed by a
FACSCalibur (BD Bioscience) flow cytometer with CellQuest
software.
Cell cycle analysis
HCT116 cells and SW620 cells were treated with
single and combination of Blu9931 and 5-FU at each proper
concentration for 72 h. The cells were then collected and
centrifuged at 1,000 rpm for 5 min. The supernatant was discarded
and the pre-cooled PBS was added to wash the cells twice. Then 70%
pre-cooled ethanol was added, overnight at 4°C. The
ethanol-immobilized cells were centrifuged, the supernatant was
discarded and cells washed three times with precooled PBS. The
cells were resuspended using 1 ml of PI/Triton X-100 staining
solution (20 μg PI/0.1% Triton X-100) containing 0.2 mg
RNase A and stained at 37°C for 15 min. Samples were placed on ice
and immediately analysed on Beckman Coulter to separate G0/G1, S,
G2/M and hypodiploid nuclei. All assays were carried out in
triplicate.
Statistical analysis
Statistical analysis was carried out with SPSS 13.0
software (USA). Data were showed by means ± SD and three individual
experiments were performed. Student's t-test was used to compare
data between two groups. One-way ANOVA and Tukey's test were
applied to compare data between three or more groups. P<0.05 was
considered statistically significant.
Results
FGFR4 expression is different in various
colorectal cell lines
FGFR4 was expressed in colorectal cancer cell lines
at mRNA and protein levels using reverse transcription PCR,
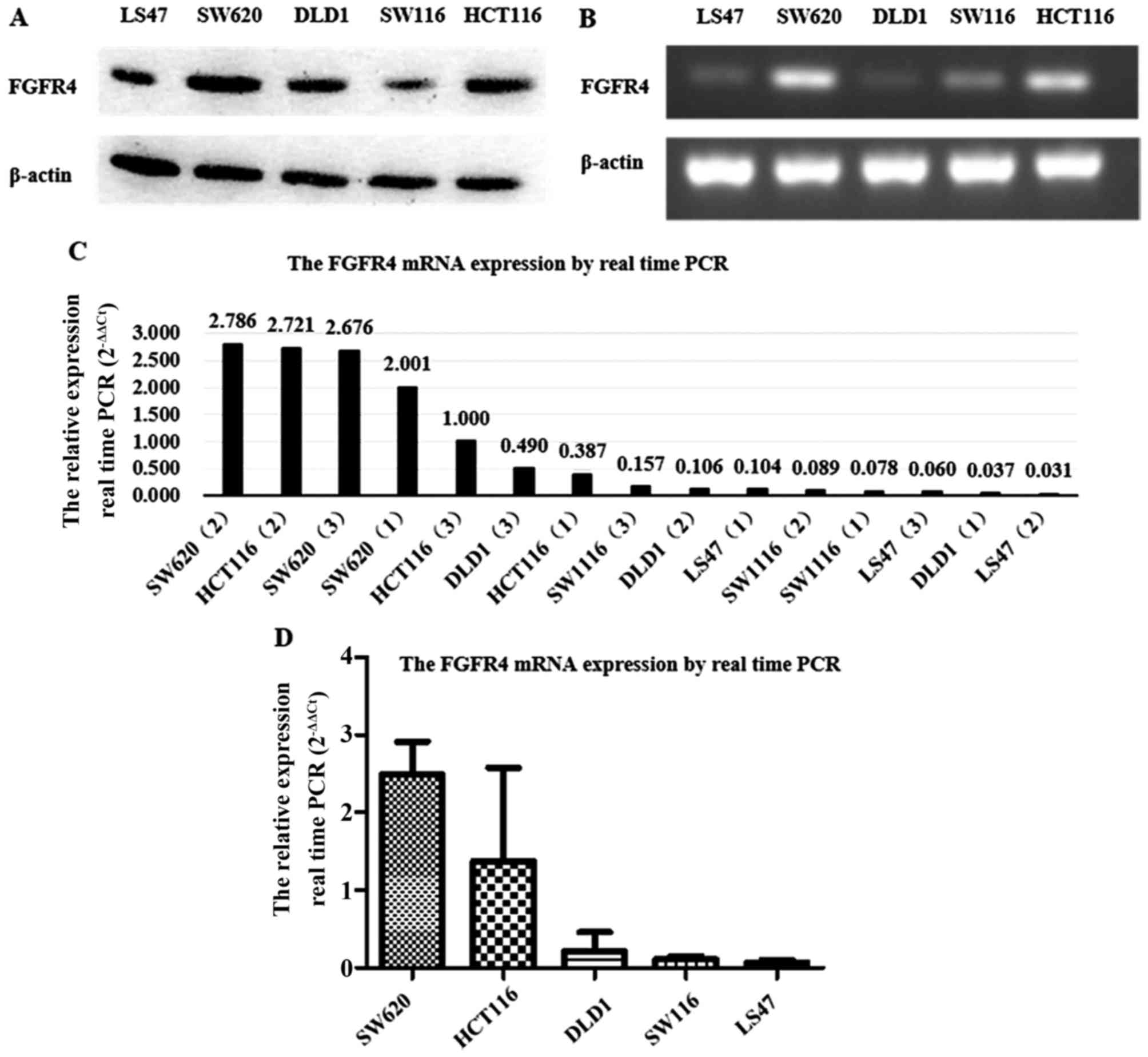
real-time PCR and western blot analysis. As Fig. 1A illustrates, FGFR4 protein
expression was stronger in HCT116 and SW620, while weaker in LS47,
DLD1 and SW116. Reverse transcription PCR results showed that FGFR4
mRNA expression was significantly higher in HCT116 and SW620 than
in the other three colorectal cancer cell lines (Fig. 1B), which was verified by
quantitative real-time PCR (2−ΔΔct) through at least
three independent detectors (Fig.
1C). As shown in Fig. 1D, the
quantitative analysis results by real-time PCR confirmed that FGFR4
mRNA expression in HCT116 and SW620 were much higher than the other
colorectal cancer lines. Therefore, the HCT116 and SW620 cell lines
were chosen to undergo subsequent assays.
5-FU and Blu9931 have an effect on the
cell viability of HCT116 and SW620 cells
In order to evaluate the growth effect of different
reagents on HCT116 and SW620 cells, MTT assays were used to detect
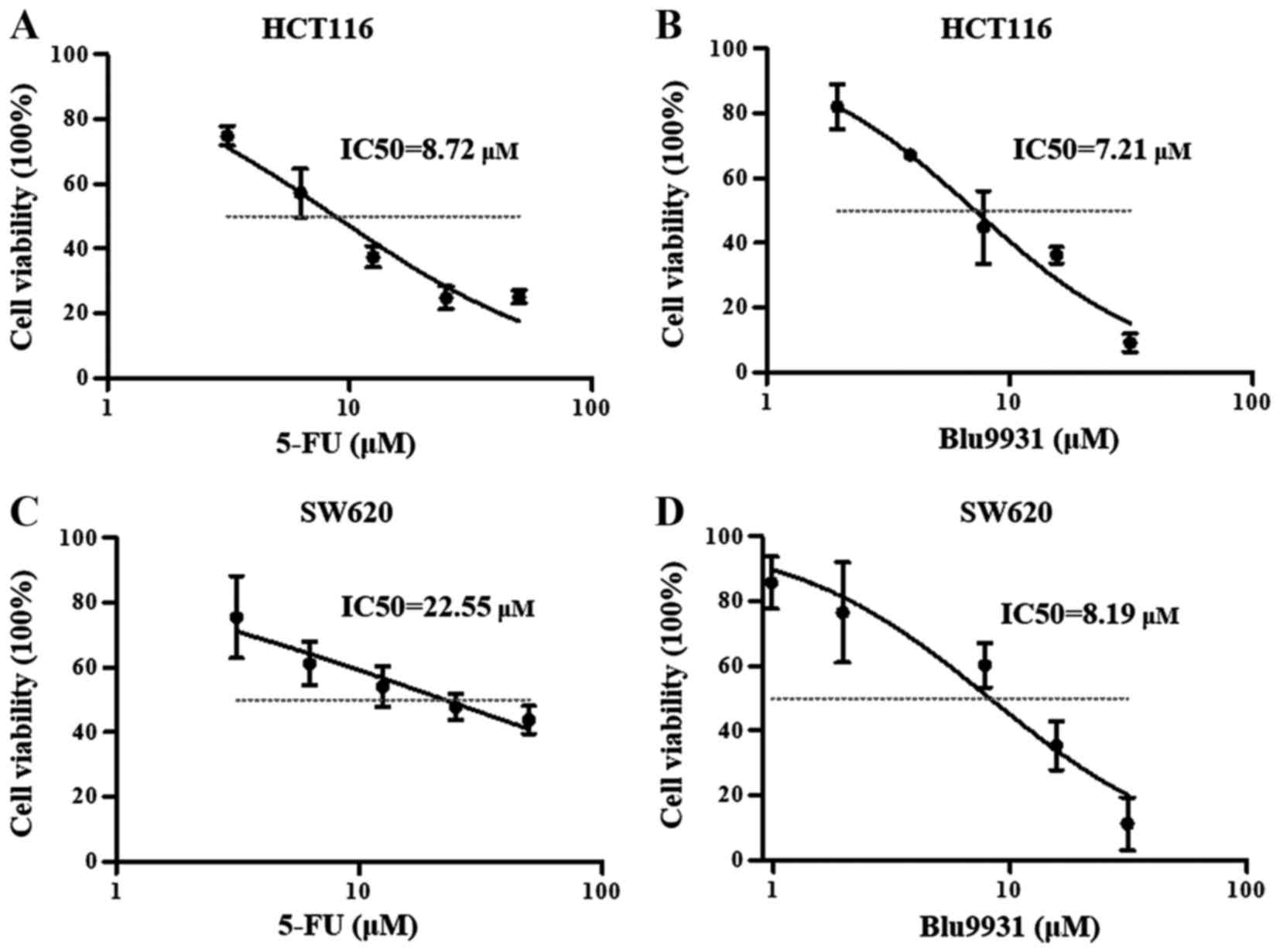
the cell viability using a microplate reader. Fig. 2A and B showed that the
concentration of 5-FU and Blu9931 increased, the cell viability of
HCT116 cells progressively decreased, the IC50 of 5-FU
and Blu9931 were 8.72 and 7.21 μM, respectively. When 5-FU
and Blu9931 acted on SW620 cells, the cell viability also decreased
with increasing concentration, and IC50 of 5-FU and
Blu9931 were 22.55 and 8.19 μM, respectively (Fig. 2C and D). For subsequent analysis,
the appropriate concentration of each agent is selected in the
linear region. According to the above results, in HCT116 cells, the
appropriate concentration of 5-FU and Blu9931 is 10 μM. In
SW620 cells, the appropriate concentrations of 5-FU and Blu9931 are
20 μM and 10 μM, respectively.
Single agent treatments and the
combination of 5-FU and Blu9931 affect the proliferation of HCT116
and SW620 cells
In order to explore whether FGFR4 is a therapeutic
target for colorectal cancer, we have introduced FGFR4-specific
inhibitor Blu9931 and 5-FU to intervene in HCT116 and SW620 cells.
MTT assay was used to detected cell absorbance to evaluate cell
proliferation.
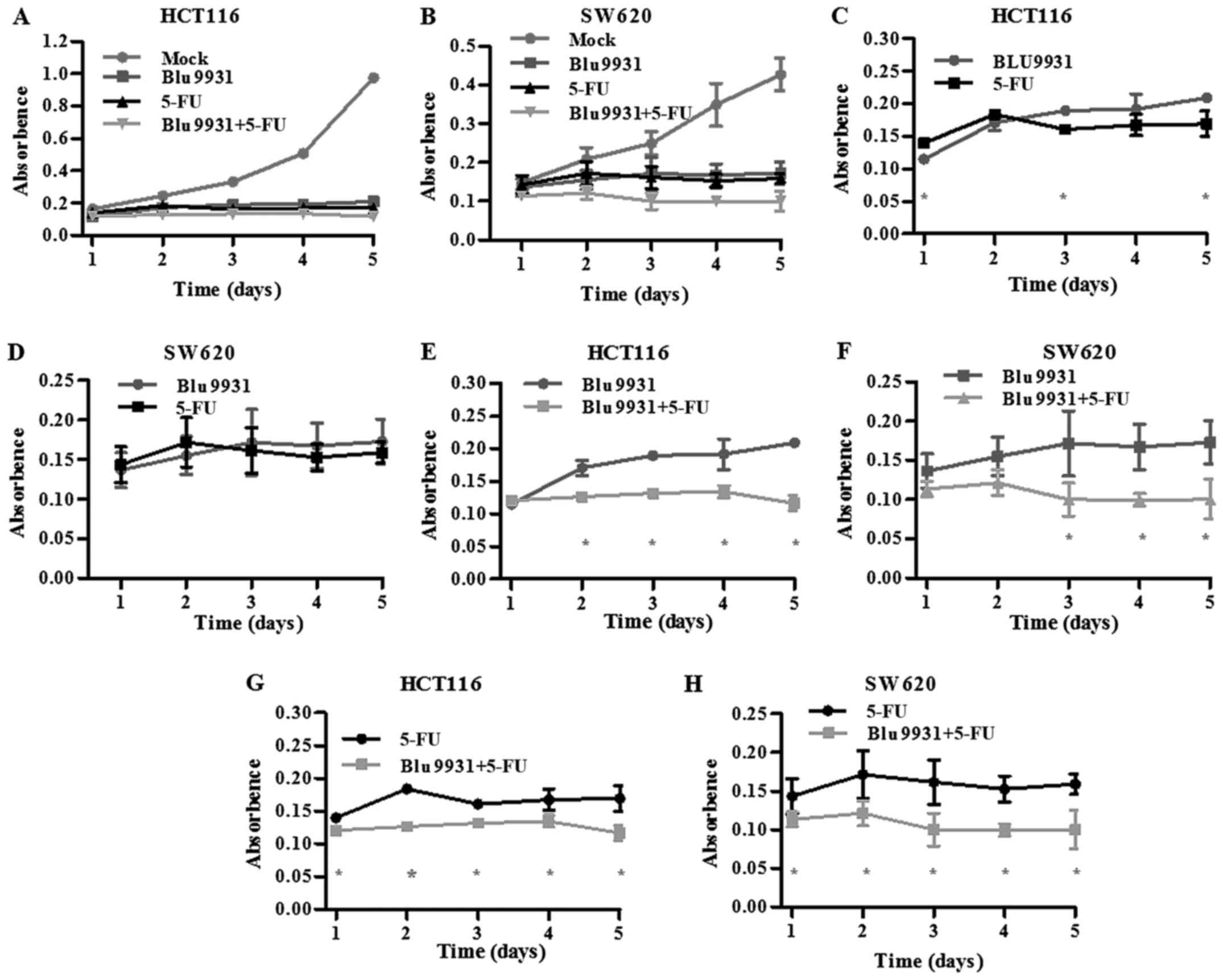
The single and combination of 5-FU and Blu9931
obviously weakened the proliferative ability of HCT116 and SW620
cells when compared with its corresponding Mock group (Fig. 3A and B; one-way ANOVA; P<0.05).
As shown in Fig. 3C, the
proliferative ability of the Blu9931 group was slightly stronger
than that of the 5-FU group on days 1, 3 and 5 in HCT116 cells
(Student's t-test; P<0.05). However, the proliferation ability
had no significant difference between the 5-FU group and Blu9931
group SW620 cells (Fig. 3D;
Student's t-test; P<0.05). Compared with Blu9931 group, the
proliferation ability in HCT116 cells treated with the combination
of 5-FU and Blu9931 significantly decreased from day 2 to day 5
while it significantly decreased in SW620 cells from day 3 to day 5
after MTT assay (Fig. 3E and F;
Student's t-test; P<0.05). Compared with 5-FU group, the
proliferation ability significantly decreased in HCT116 and SW620
cells treated with the combination of 5-FU and Blu9931 from day 1
to day 5 after MTT assay (Fig. 3G and
H; Student's t-test; P<0.05).
Single agent treatment and the
combination of 5-FU and Blu9931 affect the apoptosis rate of HCT116
and SW620 cells
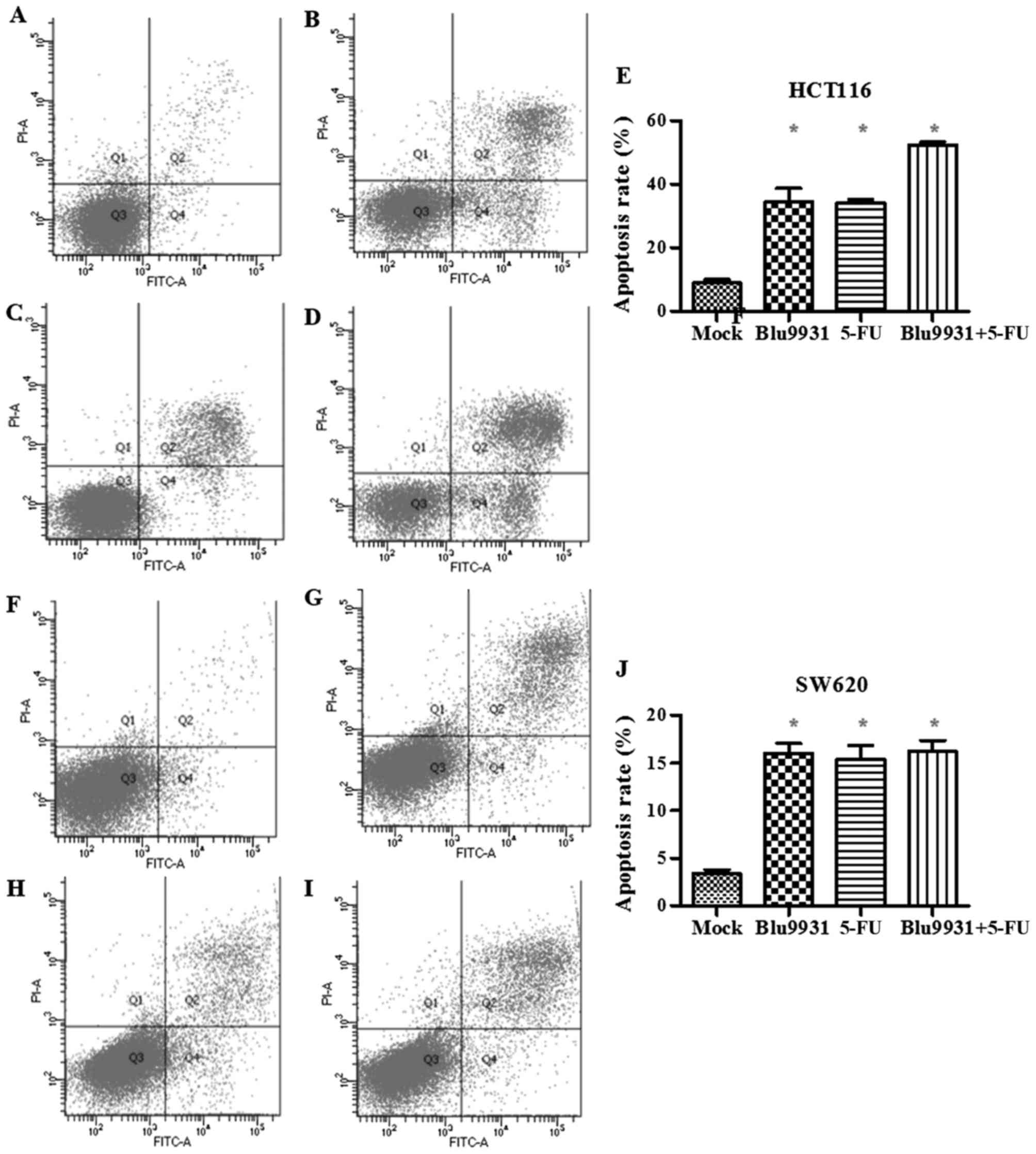
To study whether the different reagents affect
apoptosis of the colorectal cancer cells, the apoptotic rate of
HCT116 and SW620 cells was detected by flow cytometry and treated
with the single agent treatment and the combination of 5-FU and
Blu9931 for 72 h. In both HCT116 cells and SW620 cells, the
apoptotic rate of each treatment group increased, the difference
was statistically significant (Fig.
4; one-way ANOVA; P<0.05). However, in HCT116 cells, the
apoptosis rate of 5-FU plus Blu9931 was higher than that in the
single of 5-FU and Blu9931 (one-way ANOVA; P<0.05). However,
there was no statistically significant difference among 5-FU,
Blu9931 and 5-FU plus Blu9931 in SW620 cells (one-way ANOVA;
P<0.05).
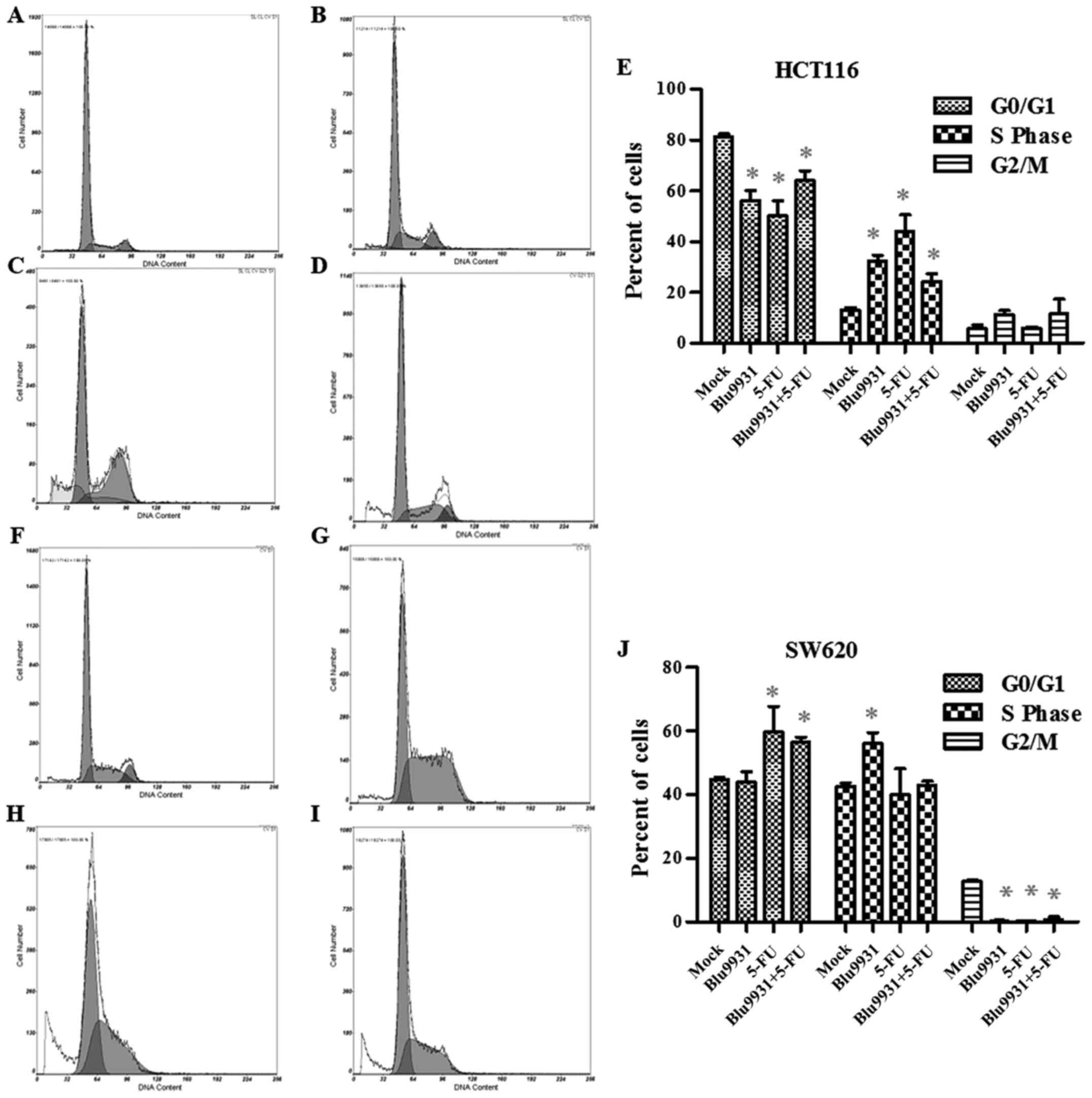
Single agent treatment and the
combination of 5-FU and Blu9931 affect cell cycle distribution of
HCT116 and SW620 cells
The distribution of cell cycle was evaluated by flow
cytometry in HCT116 and SW620 cells with the single and combined
treatment of Blu9931 and 5-FU. As Fig.
5A–E shows, the HCT116 cells treated with the single and
combined Blu9931 and 5-FU in S stage evidently increased while
those in G0/G1 stage remarkably decreased compared to the mock
group (one-way ANOVA; P<0.05). However, in SW620 cells, the G2/m
stage cells in the three treatment groups were much less than those
in the mock group. The SW620 cells treated with the single Blu9931
in S stage significantly increased while 5-FU group and 5-FU plus
Blu9931 group remarkably increased in G0/G1 stage (Fig. 5F–J; one-way ANOVA; P<0.05).
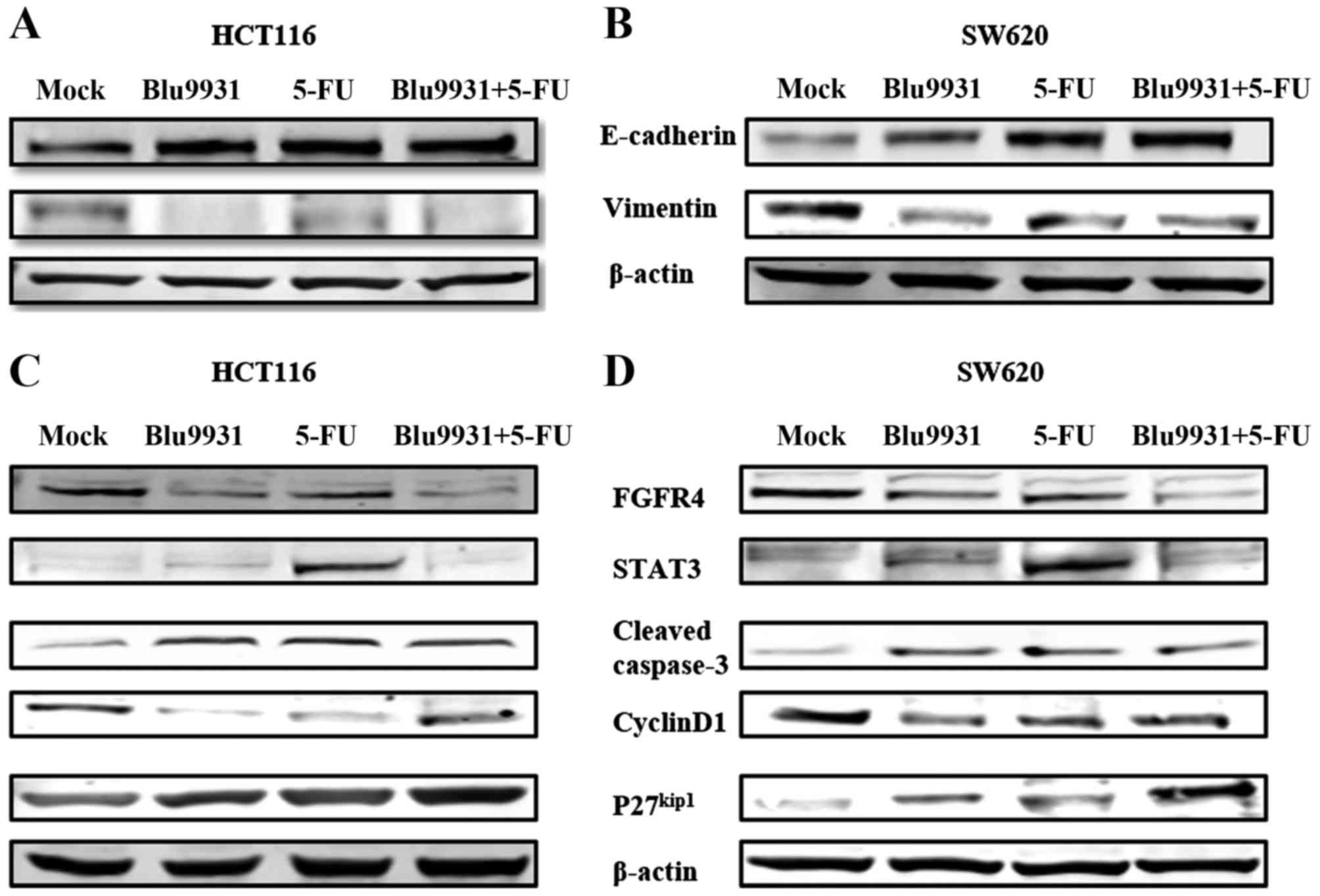
Single agent treatment and the
combination of 5-FU and Blu9931 suppress EMT in HCT116 and SW620
cells
The epithelial-mesenchymal transition (EMT) plays an
important role in tumor invasion and metastasis. In the EMT
process, the cell phenotype transforms from epithelial into
mesenchymal, and cell markers also change (10). The expression of E-cadherin was
decreased and the expression of vimentin was increased. To
investigate whether the different reagents affect EMT in the
colorectal cancer cells, we detected the expression of E-cadherin
and vimentin by western blotting. In HCT116 cells, expression of
E-cadherin was increased in the single and combination of 5-FU and
Blu9931 compared with control group while vimentin expression was
weakened. The same result was seen in SW620 cells. The above
results show that Blu9931 and 5-FU can suppress EMT (Fig. 6A and B).
Single agent treatment and the
combination of 5-FU and Blu9931 affect the expression of related
molecules in HCT116 and SW620 cells
Western blotting was used to detect the expression
of related molecules including signal pathway (STAT3), apoptosis
(cleaved caspase-3), and cell cycle (cyclin D1 and
P27kip1) in HCT116 and SW620 cells when treated by the
single and combination of 5-FU and Blu9931. As shown in Fig. 6C and D, expression of FGFR4 in
HCT116 and SW620 cells was decreased in the single Blu9931 group
and the combination of 5-FU and Blu9931 group while no significant
difference was noted in the single 5-FU group when compared with
the control group. When compared with the control group, expression
of STAT3 was markedly increased in the single agent 5-FU group.
Compared to the control group, cleaved caspase-3 expression in
HCT116 and SW620 cells was obviously increased in the single and
combination of 5-FU and Blu9931. In other words, the single and
combination of 5-FU and Blu9931 had an effect on promoting
apoptosis, but 5-FU plus Blu9931 group was no significant
difference in the single agent 5-FU and single Blu9931 treatment
groups. When compared to the control group, P27kip1
expression was obviously increased in the single and combination of
5-FU and Blu9931 while cyclin D1 expression was prominently
weakened.
Discussion
With the development of tumor molecular biology, the
study of tumor molecular targeted therapy has become the current
hot spot. Molecular targeted drugs have also made some progress in
the application of colorectal cancer. A number of phase II clinical
studies have shown that cetuximab (anti-EGFR) has a high efficacy
(11,12) in the treatment of advanced
colorectal cancer. However, the curative effect was not
satisfactory. So exploring new therapeutic targets in CRC has
become an essential goal. Katoh (13) suggested that the FGFR family may be
important in clinical cancer diagnostics and therapeutics. FGFR4
plays a very important role in embryonic development, tissue repair
and angiogenesis and has become a popular research molecule in a
variety of tumors. However, the effect of FGFR4 in CRC has rarely
been studied.
There are only two reports associated with Blu9931,
both of which are not associated with colorectal cancer.
Furthermore, 5-FU is a cell cycle-specific chemotherapeutic agent
that is primarily resistant to cells in the S phase. In this study,
we reported that the treatment of Blu9931 significantly reduced
cell viability and increased cell apoptosis rates when compared
with the negative control group. The effects of the combination
treatment of 5-FU and Blu9931 in proliferation and arresting cell
cycle were superior to that of the single agent treatments.
As shown in Fig. 1,
in five common colorectal cancer cell lines, the expression of
FGFR4 at mRNA and protein levels in HCT116 and SW620 cells was
significantly higher than the other three cells. Thus, HCT116 and
SW620 cells were chosen for the subsequent assays. With the
concentration of 5-FU and Blu9931 increasing, the cell viability of
HCT116 and SW620 cells gradually decreased after administration as
single agents. This finding shows that both Blu9931 and 5-FU can
significantly reduce the viability of colorectal cancer cells.
Hagel et al showed Blu9931 markedly inhibited the viability
of hepatocellular cancer cells (EC50<1 μM)
(5).
The single and combination of 5-FU and Blu9931
obviously weakened the proliferative ability of HCT116 and SW620
cells when compared with its corresponding mock group (Fig. 3). Moreover, proliferation ability
of the combination treatment of 5-FU and Blu9931 was significantly
less than that in the single agent treatments. It suggests that the
combination of 5-FU and Blu9931 had synergistic effects on the
inhibition of proliferation rates in CRC cells. As shown in
Fig. 6, expression of FGFR4 in the
HCT116 and SW620 cells was decreased in the single Blu9931 group
and the combination of 5-FU and Blu9931 group while no significant
difference was noted in the single 5-FU group when compared with
the control group. Therefore, Blu9931 mainly reduced the
proliferation of CRC cells by inhibiting the activity of FGFR4,
whereas 5-FU was not. Bai et al (14) indicated that FGFR4 inhibitor
PD173074 obviously suppressed colon cancer cell proliferation and
neovascularization. Also, in embryonal rhabdomyosarcoma, inhibition
of FGFR4 expression reduces cell proliferation in vitro and
xenograft formation in vivo (15).
In both HCT116 and SW620 cells, the apoptosis rate
of single agent treatment and combinations of 5-FU and Blu9931
increased when compared with the control group. Only in HCT116
cells, the apoptosis rate of 5-FU plus Blu9931 was higher than that
in the single of 5-FU and Blu9931. Also, western blot analysis
showed that the single and combination of 5-FU and Blu9931
obviously increased cleaved caspase-3 expression, and 5-FU plus
Blu9931 group had no significant difference in the single agent
5-FU and single Blu9931 treatment groups in HCT116 and SW620 cells.
In other words, the single agent treatment 5-FU and Blu9931 can
induce colorectal cancer cell apoptosis, but Blu9931 plus 5-FU does
not seem to make the apoptosis rate higher. Blu9931-induced
apoptosis may be mediated by decreasing FGFR4 expression. Our
previous studies have shown that silencing FGFR4 can increase tumor
cell apoptosis rates (7). Zaid
et al (16) indicated that
the downregulation of FGFR4 significantly prevented the WNT
pathway, suggesting that WNT may be one of the signaling pathways
that affect apoptosis. Only in the single agent treatment 5-FU,
stat3 expression was significantly elevated relative to the
negative control group and the other two treatment groups. This
result is very interesting, because usually high expression of
stat3 promotes cell growth and reduces the rate of apoptosis. We
hypothesized that 5-FU may induce STAT3 expression, whereas STAT3
is essential for lysosomal pathways that induce cell death
(17).
Our study also investigated the effects of different
treatments on cell cycle distribution in HCT116 and SW620 cells by
flow cytometry. HCT116 cells treated with the single and combined
of Blu9931 and 5-FU in S stage evidently increased while those in
G0/G1 stage remarkably decreased compared to mock group, which
indicated that cell cycle was blocked in the S phase. SW620 cells
treated with the single Blu9931 arrested in the S stage while 5-FU
group and 5-FU plus Blu9931 group arrested in the G0/G1 stage. The
antitumor effect of 5-FU is mainly mediated by inhibition of
thymidylate synthase (TS) and its incorporation metabolites into
RNA and DNA (18).
Twenty-four-hour exposure to 5-FU produced S-phase accumulation in
MCF-7 breast cancer cells (19).
However, Chong et al showed that 5-FU-induced cell cycle
arrested at the G0/G1 phases in SW480 and HT29 cells (20). Now, we are not particularly aware
of the mechanism of 5-FU on the cell cycle. However, we can see
that 5-FU has different effects on human tumor cell cycle. The
western blot results also demonstrated that the cell cycle was
inhibited. When the P27 protein expression increased, the most
critical cell cycle protein E-CDK2 complex activity was inhibited,
and cell cycle stagnated in G1 phase (21,22).
Cyclin D1 plays an important role in the development of tumor as an
important positive regulator of the cell cycle (23-25).
In this study, P27kip1 expression remarkably
strengthened in the three treatment groups compared to the negative
control while cyclin D1 observably weakened in the HCT116 and SW620
cells. Moreover, the P27kip1 expression following the
combination treatment of 5-FU and Blu9931 was stronger than that in
the single agent treatments which suggesting that the combination
of 5-FU and Blu9931 had synergistic effects on the inhibition of
the cell cycle.
EMT plays a key role in the development,
progression, invasion and metastasis of malignant tumors. There are
EMT phenomena in the process of malignancy and invasion of
peripheral tissues (26). Brabletz
et al (27) found that
inhibition of E-cadherin expression, induced β-catenin nuclear
translocation can enhance the ability of colorectal cancer cell
invasion, and low expression of E-cadherin is associated with
distant metastasis of colorectal cancer. Zhao et al
(28) showed that FGFR4
overexpression inhibited E-cadherin expression by activation of
GSK3β/β-catenin pathway and knocked it down to produce an opposite
effect in hepatocellular carcinoma cells. Our findings suggest that
Blu9931 as a specific inhibitor of FGFR4 can inhibit EMT in
colorectal cancer cells. Inhibition of FGFR4 expression can promote
the expression of E-cadherin and reduce the expression of
vimentin.
In conclusion, this study explored the effects of
single agent treatments and combination of Blu9931 and 5-FU on the
biological characteristics of colorectal cancer cells and its
mechanism. Inhibiting the activity of FGFR4 may be one of the
principal mechanisms of Blu9931 to inhibit the proliferation of
colorectal cancer cells, increase the apoptosis rate, prevent the
cell cycle and inhibit EMT. The combination of 5-FU and Blu9931 has
a synergistic effect in reducing colorectal cancer cell
proliferation and preventing the cell cycle. The results of this
study further demonstrate that the first specific inhibitor of
FGFR4, Blu9931, can be a novel target drug for the treatment of
colorectal cancer. Obviously, our results should be validated by
further studies in vitro and in vivo.
Abbreviations:
|
CRC
|
colorectal cancer
|
|
5-FU
|
5-fluorouracil
|
|
FGFR
|
fibroblast growth factor receptor
|
|
FGFR4
|
fibroblast growth factor receptor
4
|
Acknowledgments
This study was supported by Department of
Gastrointestinal Surgery and institute of Clinical medicine, The
First Affiliated Hospital, Zhengzhou University and National
Natural Science Foundation of China, grant no. 81201955.
Reference
|
1
|
Longley DB and Johnston PG: Molecular
mechanisms of drug resistance. J Pathol. 205:275–292. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kimman M, Norman R, Jan S, Kingston D and
Woodward M: The burden of cancer in member countries of the
Association of Southeast Asian Nations (ASEAN). Asian Pac J Cancer
Prev. 13:411–420. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Punt CJ and Tol J: More is less -
combining targeted therapies in metastatic colorectal cancer. Nat
Rev Clin Oncol. 6:731–733. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Eswarakumar VP, Lax I and Schlessinger J:
Cellular signaling by fibroblast growth factor receptors. Cytokine
Growth Factor Rev. 16:139–149. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hagel M, Miduturu C, Sheets M, Rubin N,
Weng W, Stransky N, Bifulco N, Kim JL, Hodous B, Brooijmans N, et
al: First selective small molecule inhibitor of FGFR4 for the
treatment of hepatocellular carcinomas with an activated FGFR4
signaling pathway. Cancer Discov. 5:424–437. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Packer LM and Pollock PM: Paralog-specific
kinase inhibition of FGFR4: Adding to the arsenal of anti-FGFR
Agents. Cancer Discov. 5:355–357. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ye YW, Zhou Y, Yuan L, Wang CM, Du CY,
Zhou XY, Zheng BQ, Cao X, Sun MH, Fu H, et al: Fibroblast growth
factor receptor 4 regulates proliferation and antiapoptosis during
gastric cancer progression. Cancer. 117:5304–5313. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Li J, Ye Y, Wang M, Lu L, Han C, Zhou Y,
Zhang J, Yu Z, Zhang X, Zhao C, et al: The over-expression of FGFR4
could influence the features of gastric cancer cells and inhibit
the efficacy of PD173074 and 5-fluorouracil towards gastric cancer.
Tumour Biol. 37:6881–6891. 2016. View Article : Google Scholar
|
|
9
|
Sawey ET, Chanrion M, Cai C, Wu G, Zhang
J, Zender L, Zhao A, Busuttil RW, Yee H, Stein L, et al:
identification of a therapeutic strategy targeting amplified FGF19
in liver cancer by Oncogenomic screening. Cancer Cell. 19:347–358.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Jang MJ, Baek SH and Kim JH: UCH-L1
promotes cancer metastasis in prostate cancer cells through EMT
induction. Cancer Lett. 302:128–135. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Dfaz Rubio E, Tabernero J, van Cutsem E,
Cavantes A, Andre T, Hu-inblet Y, Soulie P, Corretge S, Kisker O
and de Gramont A: Cetuximab in combination with
oxaliplatin/5-fluorouracil(5-FU)/folinic acid(FA)(FoLFoX-4) in the
first-1ine treatment of patients with epidermal growth factor
receptor EGFR)-expressing metastatic colorectal cancer: An
international Phase ii study. J Clin Oncol. 23:S162005.
|
|
12
|
Folprecht G, Lutz MP, Schöffski P,
Seufferlein T, Nolting A, Pollert P and Köhne CH: Cetuximab and
irinotecan/5-fluorouracil/folinic acid is a safe combination for
the first-line treatment of patients with epidermal growth factor
receptor expressing metastatic colorectal carcinoma. Ann Oncol.
17:450–456. 2006. View Article : Google Scholar
|
|
13
|
Katoh M: Genetic alterations of FGF
receptors: An emerging field in clinical cancer diagnostics and
therapeutics. Expert Rev Anticancer Ther. 10:1375–1379. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bai YP, Shang K, Chen H, Ding F, Wang Z,
Liang C, Xu Y, Sun MH and Li YY: FGF-1/-3/FGFR4 signaling in
cancer-associated fibroblasts promotes tumor progression in colon
cancer through Erk and MMP-7. Cancer Sci. 106:1278–1287. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Crose LE, Etheridge KT, Chen C, Belyea B,
Talbot LJ, Bentley RC and Linardic CM: FGFR4 blockade exerts
distinct antitumorigenic effects in human embryonal versus alveolar
rhabdomyosarcoma. Clin Cancer Res. 18:3780–3790. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zaid TM, Yeung TL, Thompson MS, Leung CS,
Harding T, Co NN, Schmandt RS, Kwan SY, Rodriguez-Aguay C,
Lopez-Berestein G, et al: Identification of FGFR4 as a potential
therapeutic target for advanced-stage, high-grade serous ovarian
cancer. Clin Cancer Res. 19:809–820. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wake MS and Watson CJ: STAT3 the oncogene
- still eluding therapy? FEBS J. 282:2600–2611. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Longley DB, Harkin DP and Johnston PG:
5-fluorouracil: mechanisms of action and clinical strategies. Nat
Rev Cancer. 3:330–338. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
19
|
Grem JL, Nguyen D, Monahan BP, Kao V and
Geoffroy FJ: Sequence-dependent antagonism between fluorouracil and
paclitaxel in human breast cancer cells. Biochem Pharmacol.
58:477–486. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chong D, Ma L, Liu F, Zhang Z, Zhao S, Huo
Q, Zhang P, Zheng H and Liu H: Synergistic antitumor effect of
3-bromopyruvate and 5-fluorouracil against human colorectal cancer
through cell cycle arrest and induction of apoptosis. Anticancer
Drugs. 28:831–840. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Nourse J, Firpo E, Flanagan WM, Coats S,
Polyak K, Lee MH, Massague J, Crabtree GR and Roberts JM:
Interleukin-2-mediated elimination of the p27Kip1
cyclin-dependent kinase inhibitor prevented by rapamycin. Nature.
372:570–573. 1994. View
Article : Google Scholar : PubMed/NCBI
|
|
22
|
Peng D, Fan Z, Lu Y, DeBlasio T, Scher H
and Mendelsohn J: Anti-epidermal growth factor receptor monoclonal
antibody 225 up-regulates p27KiP1 and induces G1 arrest
in prostatic cancer cell line DU145. Cancer Res. 56:3666–3669.
1996.PubMed/NCBI
|
|
23
|
Zhou JX, Niehans GA, Shar A, Rubins JB,
Frizelle SP and Kratzke RA: mechanisms of G1 checkpoint loss in
resected early stage non-small cell lung cancer. Lung Cancer.
32:27–38. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Moghaddam SJ, Haghighi EN, Samiee S,
Shahid N, Keramati AR, Dadgar S and Zali MR: Immunohistochemical
analysis of p53, cyclin D1, RB1, c-fos and N-ras gene expression in
hepatocellular carcinoma in Iran. World J Gastroenterol.
13:588–593. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Rose SL and Buller RE: The role of p53
mutation in BRCA1-associated ovarian cancer. Minerva Ginecol.
54:201–209. 2002.PubMed/NCBI
|
|
26
|
Li Y, Wang W, Wang W, Yang R, Wang T, Su
T, Weng D, Tao T, Li W, Ma D, et al: Correlation of TWIST2
up-regulation and epithelial-mesenchymal transition during
tumorigenesis and progression of cervical carcinoma. Gynecol Oncol.
124:112–118. 2012. View Article : Google Scholar
|
|
27
|
Braumbtz T, Jung A, Reu S, Porzner M,
Hlubek F, Leoni A, Kunz S, Knuechel R and Kirchner T: Variable
beta-catenin expression in colorectal cancers indicate tumor
progression driven by the tumor environment. Proc Natl Acad Sci
USA. 98:10356–10361. 2001. View Article : Google Scholar
|
|
28
|
Zhao H, Lv F, Liang G, Huang X, Wu G,
Zhang W, Yu L, Shi L and Teng Y: FGF19 promotes
epithelial-mesenchymal transition in hepatocellular carcinoma cells
by modulating the GSK3β/β-catenin signaling cascade via FGFR4
activation. Oncotarget. 7:13575–13586. 2016. View Article : Google Scholar
|