Introduction
Although substantial efforts have been made by
medical scientists over several decades, pancreatic ductal
adenocarcinoma (PDAC), which is currently the fourth most
life-threatening type of cancer, remains a problem requiring a
solution. Without effective early detection and desirable
therapies, prognostic improvement for PDAC has not been achieved up
until now. The relative 5-year survival rate remains <8%, even
in developed countries (1,2). It is critical to further our
understanding of the molecular mechanisms underlying pancreatic
cancer in order to pinpoint promising targets for successful
therapy. Gremlin 1, an antagonist of bone morphogenetic protein
(BMP)2/4/7 (3), is expressed at
high levels in pancreatic tumor niches and may be a potential
therapeutic target.
Gremlin 1, in either its soluble or cell-associated
form, is a highly conserved 184-amino acid protein. This protein is
also known as cell proliferation-inducing gene 2 protein (PIG-2),
cysteine knot superfamily 1 BMP antagonist 1, DAN domain family
member (DAN)-2, downregulated in Mos-transformed cells protein, and
increased in high glucose protein 2 (IHG-2) (4,5).
Together with DAN, Cerberus and Mucin 2, Gremlin 1 belongs to the
Dan family, whose members share a conserved cysteine structure that
includes a cysteine knot motif (6,7). Of
note, this feature is also present in transforming growth factor
(TGF)-β and vascular endothelial growth factor (VEGF), indicating
that Gremlin 1 is a member of the TGF-β superfamily. The human
Gremlin 1 gene maps to chromosome 15q13-q15 (8). This gene was primarily segregated
using a differential screen involving a transformation-resistant
revertant of a v-mos-transformed rat fibroblast cell line, and its
expression in adult rats appears to be associated with the final
differentiation of cells in several organs (8,9). The
mRNA expression of human Gremlin 1 is widely observed in normal
organs, including the small intestine, brain, colon, pancreas,
ovary, prostate and skeletal muscle, and it appears to be expressed
at high levels in specific types of cells, including neurons,
astrocytes and fibroblasts (8,10).
Aberrant expression of Gremlin 1 is also found in malignancies. It
has been demonstrated that the expression of Gremlin 1 is
significantly higher in specimens of various types of cancer,
specifically in the stroma in pancreatic, esophageal, colon,
pulmonary, breast, and bladder cancer (11). However, the precise mechanism
accounting for this widely confirmed character remains to be fully
elucidated.
Sonic hedgehog (SHH) was originally identified as a
factor that does not contribute to the normal development of the
pancreas. The aberrant expression of SHH is associated with
malignant diseases of the pancreas (12-15).
SHH potently binds Patched (Ptch), a 12-pass transmembrane protein,
which overrides the inhibitory effect of Ptch on Smoothened (SMO),
another transmembrane protein. In a complex signaling cascade, GLI
family zinc finger (Gli)1/2/3, transcription factors of SHH, are
activated and induce the activation of SHH target genes. SHH
exhibits a multifunctional role in a paracrine manner in the tumor
microenvironment (16). Several
studies involving limb-bud development have revealed that
fibroblast growth factor (FGF)4/8 triggers a reciprocal interaction
network, which includes FGF4/8, SHH, BMP4 and Gremlin 1 (17-21).
Regarding the correlation between Gremlin 1 and SHH, there is merit
in investigating their association in the pancreatic cancer
niche.
Based on the findings of our previous studies on the
SHH-associated tumor-stroma interaction in the pancreas (14,22,23),
the present study aimed to examine the association between SHH and
Gremlin 1, and the contribution of the latter factor in the
progression of pancreatic cancer. It was found that the
overexpression of Gremlin 1 in malignant tissue was correlated with
pT status and tumor-node-metastasis (TNM) stage. The SHH signal
from tumor cells elevated the stromal expression of Gremlin 1,
which contributed to the proliferation and migration of pancreatic
stellate cells (PSCs). In addition, the proliferation, invasion,
and epithelial to mesenchymal transition (EMT) of pancreatic cancer
cells were promoted by Gremlin 1, which was promoted by SHH
signaling in a ligand-independent manner. Taken together, the data
suggested that Gremlin 1 was overexpressed in PDAC by SHH signaling
to induce tumor progression.
Materials and methods
Patients and tissue samples and
immunohistochemistry (IHC)
Pancreatic cancer (n=66) and normal (n=7) tissue
samples were obtained from the surgical pathology bank at the
Department of Pathology, Shaanxi Provincial People's Hospital
(Shaanxi, China), which comprised 39 cases (including four normal
patients) and from the First Affiliated Hospital of Xi'an Jiaotong
University (Xi'an, China), which comprised 27 cases (including
three normal patients). The study was approved by the ethics
committees of both organizations. Based on the 7th edition of the
TNM classification of the American Joint Commission on Cancer
(2010) (24), the pathologic TNM
status of these specimens were evaluated. The tumor tissues were
from 66 cases of Whipple resection for PDAC. The seven normal
control specimens were derived from patients who had undergone
partial pancreatectomy with benign diseases. Each patient signed an
informed consent form. The clinico-pathologic data are summarized
in Table I. Follow-up data of all
the cases were available, and the deadline was October 31, 2017.
IHC analyses were performed with Gremlin 1 antibody (rabbit
monoclonal antibody, 1:200 dilution; cat. no. ab22138; Abcam,
Cambridge, MA, USA), according to the manufacturer's instructions
using a SABC kit (Maxim, Fuzhou, China). The samples were incubated
with the primary antibody at 4°C overnight and with secondary
antibodies (SP-9001; Beijing Zhongshan Golden Bridge Biotechnology
Co., Ltd., Beijing, China). Following immunohistochemical
procedures, the slides were stained with the 3,3′-diaminobenzidine
(DAB) liquid chromogen substrate kit (ZLI-9017; Beijing Zhongshan
Golden Bridge Biotechnology Co., Ltd.) and counterstained with
hematoxylin. Finally, the results of IHC were observed under a
microscope (SCN 400; Leica Microsystems GmbH, Mannheim, Germany).
The protein expression was evaluated in four grades as follows: 0
(negative), 1 (weak), 2 (medium), and 3 (strong). Based on the
percentage of positive staining area relative to the total tumor
area, the extent of staining was classified into four grades as
follows: 0 (0%), 1 (1-10%), 2 (11-50%), 3 (51-80%) and 4 (>81%).
The overall expression score was equal to the sum of the expression
grade and the extent grade.
 | Table IStatistical association between the
expression of Gremlin 1 and clinicopathological features in 66
cases of pancreatic ductal adenocarcinoma. |
Table I
Statistical association between the
expression of Gremlin 1 and clinicopathological features in 66
cases of pancreatic ductal adenocarcinoma.
| Feature | Cases, (n) | Gremlin 1
| P-valuea |
|---|
| Normal expression,
n (%) | Overexpression, n
(%) |
|---|
| Sex | | | | 0.603 |
| Male | 41 | 14 (34.1) | 27 (65.9) | |
| Female | 25 | 7 (28.0) | 18 (72.0) | |
| Mean age
(years) | | | | 0.336 |
| ≤58.88 | 32 | 12 (37.5) | 20 (62.5) | |
| >58.88 | 34 | 9 (26.5) | 25 (73.5) | |
| Histological
grade | | | | 0.155 |
| 1 | 35 | 14 (40.0) | 21 (60.0) | |
| 2 | 20 | 6 (30.0) | 14 (70.0) | |
| 3 | 11 | 1 (9.1) | 10 (90.9) | |
| pT status | | | | 0.009b |
| 1 | 8 | 6 (75.0) | 2 (25.0) | |
| 2 | 19 | 8 (42.1) | 11 (57.9) | |
| 3 | 31 | 5 (16.1) | 26 (83.9) | |
| 4 | 8 | 2 (25.0) | 6 (75.0) | |
| pN status | | | | 0.107 |
| 0 | 41 | 16 (39.0) | 25 (61.0) | |
| 1 | 25 | 5 (20.0) | 20 (80.0) | |
| pM status | | | | 0.151 |
| 0 | 57 | 20 (35.1) | 37 (64.9) | |
| 1 | 9 | 1 (11.1) | 8 (88.9) | |
| TNM stage | | | | 0.029b |
| I | 24 | 13 (54.2) | 11 (45.8) | |
| II | 28 | 6 (21.4) | 22 (78.6) | |
| III | 5 | 1 (20.0) | 4 (80.0) | |
| IV | 9 | 1 (11.1) | 8 (88.9) | |
Cancer cell culture
In the present study, the origin of the human
pancreatic cancer cell lines (AsPC-1 and BxPC-3) was the American
Type Culture Collection (Manassas, VA, USA), as described
previously (14), wich were
purchased from the Shanghai Institutes for Biological Sciences,
Chinese Academy of Sciences (Shanghai, China). The general culture
conditions for all tumor cell lines was 37°C with a 5%
CO2 atmosphere in DMEM containing 10% FBS (both from
HyClone; GE Healthcare Life Sciences, Logan, UT, USA) and 1%
penicillin and streptomycin. As described by Li et al, the
cells were exposed to SHH and/or in different conditions (14). The cells
(1×105/μl) were cultured under standard
conditions in a 5% CO2 atmosphere at 37°C for 72 h and
exposed to SHH, cyclopamine, Gli-1 small interfering RNA (siRNA)
and Gremlin 1 siRNA (to avoid confusion, details of different
conditions are shown in the Results section, separately).
Quantification of SHH in conditioned
medium (CM)
According to the manufacturer's protocol of the
enzyme-linked immunosorbent assay (ELISA) kit (R&D Systems,
Inc., Minneapolis, MN, USA), the SHH concentrations in the CM of
the pancreatic cancer cells and PSCs were quantified.
Isolation and culture of human PSCs
According to the methods described by Vonlaufen
et al (25) and our
previous study (14,26), human PSCs were isolated from the
normal pancreatic tissue samples that were obtained from patients
who underwent partial pancreatectomy with benign disease at Shaanxi
Provincial People's Hospital and the First Affiliated Hospital of
Xi'an Jiaotong University. The cell culture conditions were 37°C
with 5% CO2 in DMEM/F12 media supplemented with 10%
heat-inactivated FBS (both from HyClone; GE Healthcare Life
Sciences), together with 1% penicillin and streptomycin. Several
methods, including Oil Red O staining of the fat droplets in the
cytoplasm and immunofluorescence of α-smooth muscle actin (α-SMA).
Oil Red O staining was applied to visualize intracellular lipid
content in PSCs. Briefly, PSCs on the slides were washed with
phosphate-buffered saline (PBS) and fixed in 4% paraformaldehyde
for 1 h at room temperature. After washing the PSCs with
isopropanol, pre-warmed 0.25% Oil Red O working solution was used
to stain intracellular lipid content for 15 min in a 60°C oven.
After being washed with PBS twice, the cells were re-stained with
hematoxylin for 15 sec and sealed with glycerin on glass slides.
Finally, a light microscope (Nikon Eclipse Ti-S; Nikon, Tokyo,
Japan) at a magnification of ×200 was used to photograph the cells
stained with Oil Red O. After the designated treatment, PSCs were
fixed with 4% paraformaldehyde for 10 min at room temperature,
permeabilized in 0.5% Triton X-100 for 10 min, and blocked in 1%
BSA for 1 h. Fixed cells were then incubated with α-SMA antibodies
at 1:100 dilution at 4°C overnight. Cells were washed and incubated
with Goat anti-rabbit FITC (green) IgG antibody (ZSGB-BIO Inc.,
Beijing, China) at 1:100 dilution for 60 min. Nuclei were stained
with DAPI for 5 min. The cells were visualized by a fluorescent
microscope (Nikon) using appropriate excitation and emission
spectra at a ×400 magnification) were used to confirm the PSCs.
Cell proliferation assay
The cancer cells and PSCs were seeded into 96-well
culture plates at a density of 2,000-5,000 cells per well. First,
the cells were starved for 24 h, and they were then cultured in
specific media [according to given concentrations of cyclopamine
and SHh, the drugs (or solvent only) were administered in medium
containing 1% FBS] separately. At 24, 48, 72, or 96 h following
removal of the media, the optical densities at 492 nm were
monitored with 3-(4,5-dimethylthiazolyl-2)-2,5-diphenyltetrazolium
bromide (MTT) reagent using a multifunction microplate reader
(POLARstar OPTIMA; BMG Labtech, Offenburg, Germany).
Indirect co-culture of pancreatic cancer
cells and PSCs
Prior to the media being replaced with DMEM
supplemented with 2% FBS and 1% penicillin and streptomycin, 10%
FBS was added to the cultured pancreatic cancer cells until they
reached 50% confluence. After 48 h, the CM were collected and
incubated with the PSCs for 72 h. The cells (1×106/ml)
were cultured under standard conditions with a 5% CO2
atmosphere at 37°C.
Cell migration and invasion assays
For the assessment of cell migration and invasion,
wound-healing and Transwell migration assays were performed based
on the protocol described in our previous study (14).
RT-qPCR assay
According to the methods previously described
(14), an RT-qPCR assay was
performed. The extraction of the total ribonucleic acid (RNA) was
achieved using the Fastgen1000 RNA isolation system (Fastgen,
Shanghai, China) according to the manufacturer's instructions. A
Prime Script RT reagent kit (Takara, Dalian, China) was used to
reverse-transcribe the total RNA into cDNA. Quantitative
(real-time) PCR was performed as previously described (27). The PCR primer sequences used were
as follows: H-β-actin forward, 5′-AGC TACGAGCTGCCTGACG-3′ and
reverse, 5′-GCATTTGCG GTGGACGAT-3′; H-Gremlin 1 forward,
5′-AACTACAGCCAC CTACCAAG-3′ and reverse, 5′-CACGAACTACGCACAAG
CAG-3′; R-Gremlin 1 forward, 5′-ACTGTGCTTCAGATGGT CGG-3′ and
reverse, 5′-AATGCGGCCCTCAGAGTTAC-3′; H-SHH forward,
5′-GCGACTTCCTCACTTTCCTG-3′ and reverse, 5′-CCGGTTGATGAGAATGGTG-3′;
R-SHH forward, 5′-TATGAGGGTCGAGCAGTGGA-3′ and reverse,
5′-CGGGACGTAAGTCCTTCACC-3′. The expression level of each target
gene was determined using β-actin as the normalization control. The
relative gene expression was calculated using the 2−ΔΔCq
method (28).
Western blot analysis
As previously described (29), primary antibodies were obtained
from different sources as follows: α-SMA (1:1,000 dilution; goat
polyclonal antibody; cat. no. ab21027) antibody was provided by
Abcam, and N-cadherin, E-cadherin, Snail and Vimentin antibodies
were provided by Cell Signaling Technology, Inc., Danvers, MA, USA
(1:1,000 dilution; cat. no. 9782). The secondary antibodies
(1:10,000 dilution; goat anti-rabbit IgG; cat. no. ab6721) and
anti-mouse IgG (1:10,000 dilution; rabbit anti-mouse HRP; cat. no.
ab6728) were provided by Abcam. Whole-cell lysates of the AsPc-1
and BxPC-3 cells were prepared by using the RIPA buffer (Beyotime,
Guangzhou, China) according to the manufacturer's instructions.
Then, the concentration was determined via a BCA protein assay kit
(Pierce, Rockford, IL, USA). The protein lysates were subsequently
resolved on a 10% polyacrylamide gel with a 5% stacking gel. Next,
the proteins were blotted on polyvinylidene difluoride membranes.
Before incubating with the primary antibodies overnight at 4°C, the
membranes were blocked for 2 h in TBS containing 0.1% (vol/vol)
Tween-20 and 10% (wt/vol) non-fat dry milk powder. Following the
incubation of the secondary HRP-coupled antibodies for 2 h at room
temperature, the membranes were washed via 0.1% TBS/Tween-20, and
the signal was detected using the enhanced chemiluminescence kit
and a Molecular Imager ChemiDoc XRS System (Bio-Rad Laboratories,
Hercules, CA, USA).
RNA interference
The siRNA for Gremlin 1 (GREM1-Homo-482, forward,
5′-GCAACAGUCGCACCAUCAUTT-3′ and reverse,
5′-AUGAUGGUGCGACUGUUGCTT-3′), siRNA for GLI1 (GLI1-Homo-2758,
forward, 5′-GGCUCAGCUUG UGUGUAAUTT-3′ and reverse,
5′-AUUACACACAAGCUG AGCCTT-3′), and negative control siRNA (NC,
forward, 5′-GUA UGACAACAGCCUCAAGTT-3′ and reverse, 5′-CUUGAG
GCUGUUGUCAUACTT-3′) were provided by GenePharm (Shanghai, China).
Using Lipofectamine RNAi MAX reagent (Invitrogen; Thermo Fisher
Scientific, Inc.), the PSCs or pancreatic cancer cells
(2×105/well in 6-well plates) were transfected with 100
nM siRNA, based on the previously reported protocol (30). After 48 h, the transfected cells
were collected for further analysis.
Statistical analysis
Data are expressed as the mean ± standard deviation.
Statistical analysis of the human tissue data was performed using
Pearson's χ2 test. The in vitro and in
vivo data were assessed using one-way analysis of variance for
multiple comparisons between the groups with the S-N-K method as a
post hoc test. The SPSS statistical software package (version 19.0;
IBM SPSS, Armonk, NY, USA) was used to perform the statistical
tests. P<0.05 was considered to indicate a statistically
significant difference.
Results
Gremlin 1 is overexpressed in pancreatic
cancer tissue and correlates with poor clinical prognosis
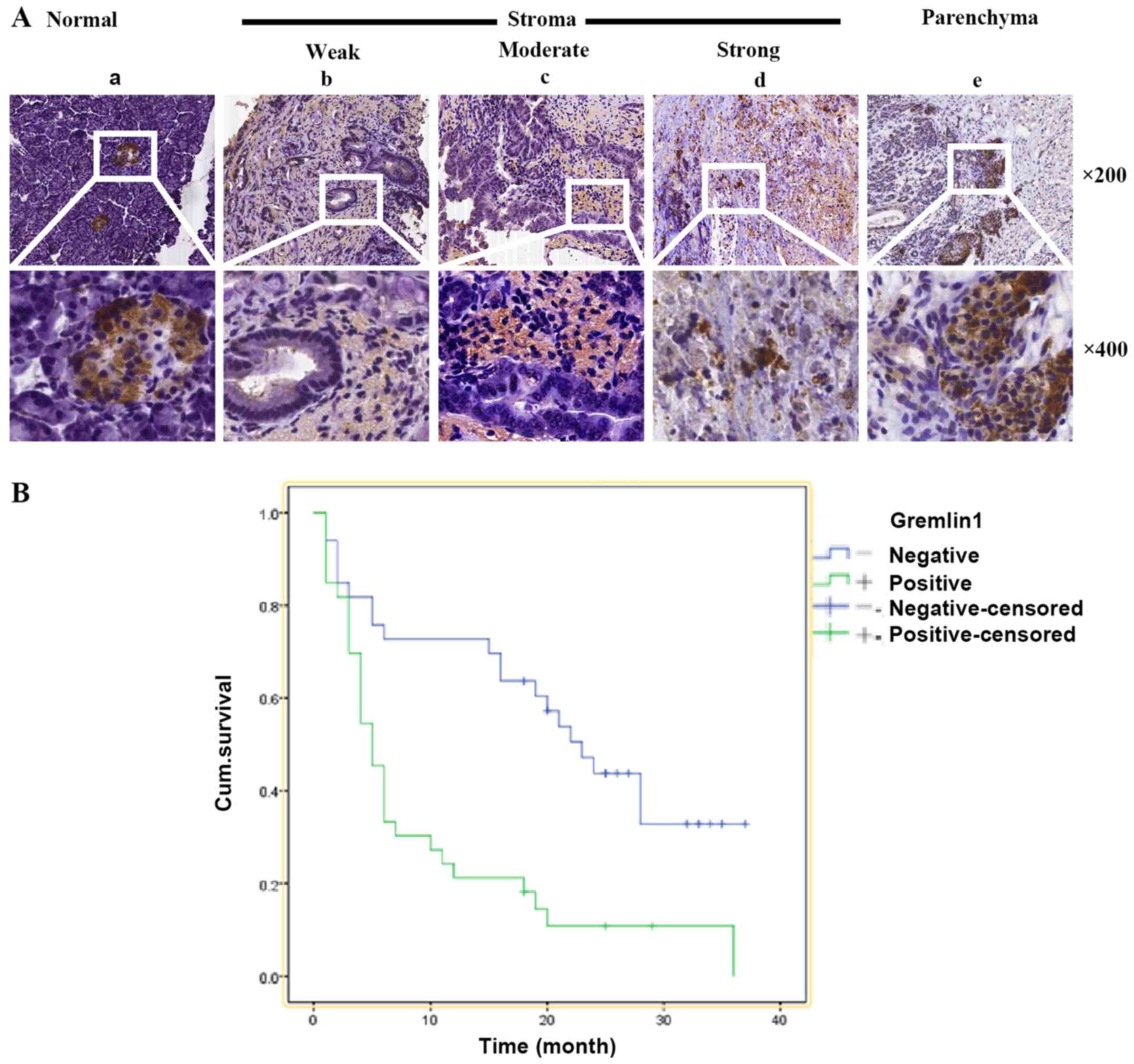
To elucidate the patterns of Gremlin 1 expression in
normal and malignant pancreas tissue, specimens from 73 patients,
including seven normal cases and 66 malignant cases, were analyzed.
According to the methods described above, the expression of Gremlin
1 was categorized into four staining levels as follows: Negative,
weak, moderate and strong. The normal pancreatic tissues were
mainly negative for the expression of Gremlin 1. Correspondingly,
its expression was positive in 45/66 cancer cases, including weak
in 18 cases, moderate in 15 cases and strong in 12 cases.
In 38/45 cases, the IHC staining confirmed extensive
staining of Gremlin 1 in the cancer stroma. However, in the
remaining seven cases, Gremlin 1 was predominantly expressed in the
cancer parenchyma (Fig. 1A). These
data indicated that Gremlin 1 may be functional in cancer cells and
stroma cells. In addition, the associations among the
clinicopathological features and expression of Gremlin 1 were
analyzed in the PDAC specimens, which are summarized in Table I. Notably, the correlation analysis
showed that the total Gremlin 1 expression was positively
associated with the pT status. Specifically, the expression of
Gremlin 1 was detected in 2/8 pT1 cases (25%), 11/19 pT2 cases
(57.9%), 26/31 pT3 cases (83.9%), and 6/8 pT4 cases (75.0%). The
associations between sex, age, and histological grade and the
staining level of Gremlin 1 were also analyzed, however, there were
no statistically significant associations.
Kaplan-Meier survival analysis was performed, and it
was found that the median survival rate of the Gremlin 1-positive
and Gremlin 1-negative groups were 9.5 and 21.7 months,
respectively (Fig. 1B). These
findings indicated that Gremlin 1 may be involved and be a valuable
biomarker in PDAC.
Paracrine SHH signaling by pancreatic
cancer cells regulates the expression of Gremlin 1 in PSCs
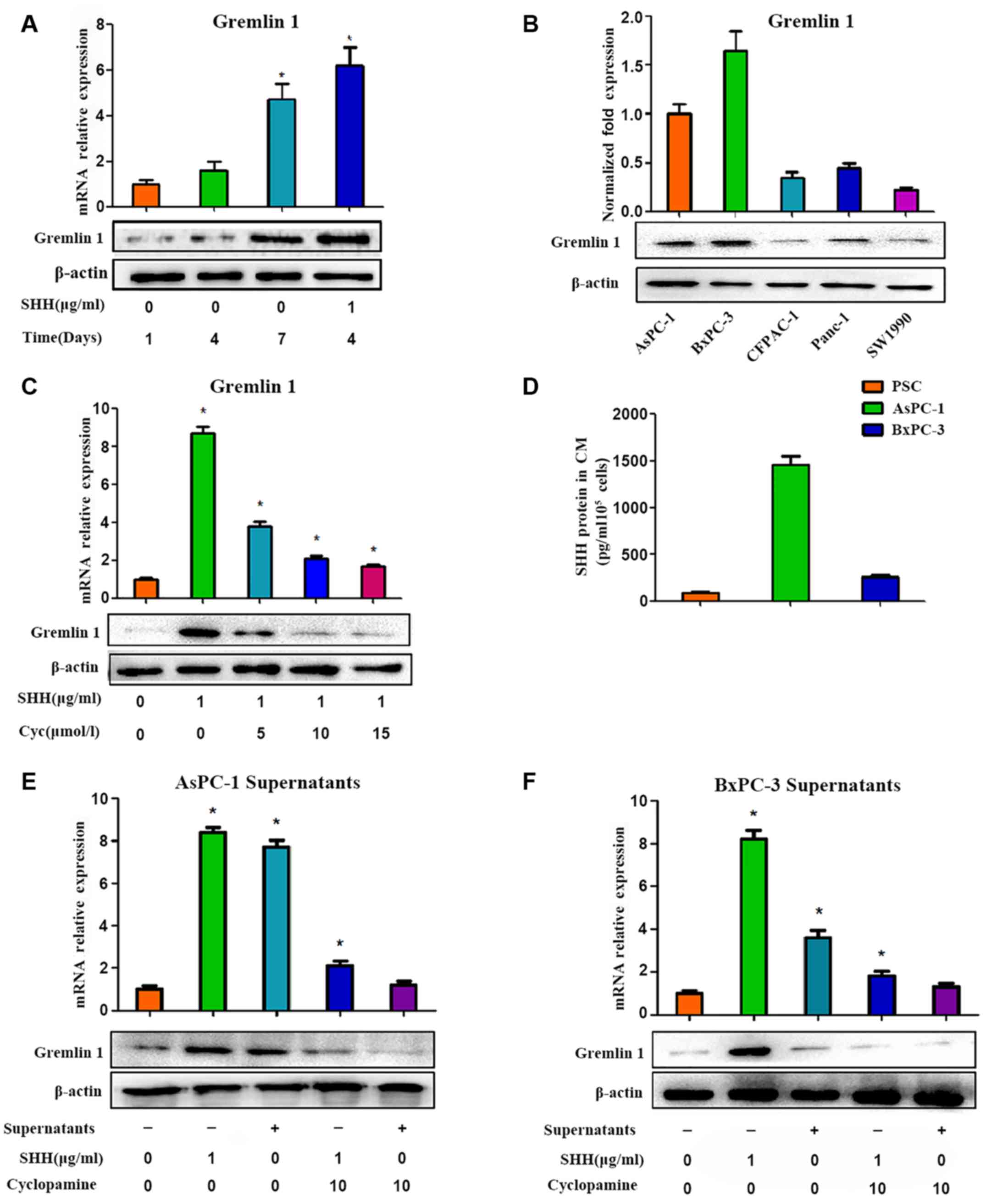
To detect the role of Gremlin 1 in the pancreatic
cancer microenvironment, the expression of Gremlin 1 in PSCs was
first investigated. Gremlin 1 was undetected in quiescent PSCs.
However, in activated PSCs, the expression of Gremlin 1 increased
with culture duration and this was marked. At 1 week following
isolation of the PSCs, a high level of Gremlin 1 was observed in
the activated PSCs (Fig. 2A). It
was also found that two pancreatic cancer cell lines (AsPc-1 and
BxPc-3) overexpressed Gremlin 1, whereas the other three pancreatic
cancer cell lines (CFPAC, Panc-1 and SW1990) exhibited low
expression levels of Gremlin 1 (Fig.
2B).
As reported in our previous study (14), SHH is important in promoting the
activation of PSCs. The present study further examined the variance
in the expression of Gremlin 1 in PSCs under the influence of SHH.
The effect of recombinant human (rh)SHH on quiescent PSCs was
investigated, and it was observed that Gremlin 1 was upregulated
following 24 h of treatment (Fig.
2C). This elevation was more marked than in the self-activated
PSCs (Fig. 2A). However, such
promotion was shut down by cyclopamine, a special inhibitor of SHH
signaling, via the direct inhibition of SMO in a dose-dependent
manner (Fig. 2C).
According to a previous study (14), high levels of SHH are found in the
AsPC-1 culture medium, whereas low levels are found in BxPC-3
culture medium. This finding was confirmed by an ELISA assay in the
present study (Fig. 2D).
Therefore, the culture supernatants of the AsPC-1 cells were
collected following 48 h of culture and were used in an indirect
co-culture system of PSCs and pancreatic cancer cells. As a result,
the supernatants of the AsPC-1 cells promoted the expression of
Gremlin 1 in the PSCs, and cyclopamine exerted a negative effect in
a dose-dependent manner (Fig. 2E).
However, such marked variances were not observed in the BxPC-3
groups (Fig. 2F). These results
indicated that SHH signaling was crucial for the modulation of
Gremlin 1 in PSCs.
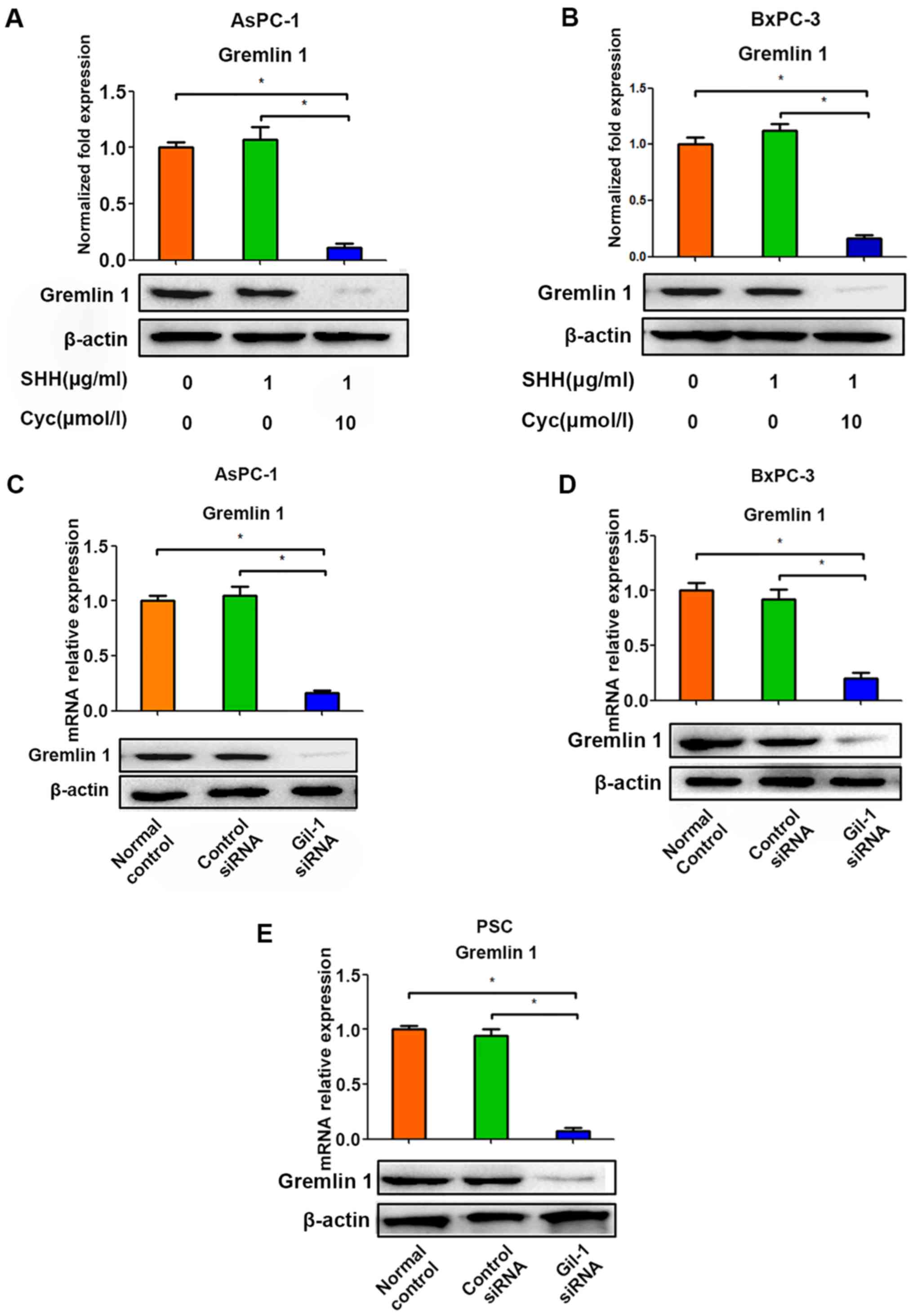
Silencing of Gli-1 impedes the expression
of Gremlin 1 in pancreatic cancer cells and PSCs
Based on the above findings, the effects of rh-SHH
and cyclopamine were examined in AsPC-1 and BxPC-3 cells (Fig. 3A and B). However, no significant
change in the expression of Gremlin 1 was observed under the
influence of rh-SHH in either of the two tumor cell lines, whereas
cyclopamine exerted a negative effect on the expression of Gremlin
1 in these cancer cells (Fig. 3A and
B). In addition, the expression of Gremlin 1 was suppressed by
Gli-1 siRNA (Fig. 3C–E). According
to a previous study, in certain situations, cyclopamine and Gli-1,
the critical downstream factors of SHH, are involved in SHH
signaling in pancreatic cancer cells in a ligand-independent manner
(29). Therefore, the present
study investigated whether Gli-1 siRNA modulated the expression of
Gremlin 1 in cancer cells. It was observed that the silencing of
Gli-1 restricted the expression of Gremlin 1 in the AsPC-1 and
BxPC-3 cells (Fig. 3C and D). The
above data indicated that the expression of Gremlin 1 in pancreatic
tumor cells may be modulated by SHH indirectly, which depends on
the activation of Gli-1 rather than the SHH ligand.
Variability in the expression of Gremlin
1 induced by SHH signaling modulates the proliferation of
pancreatic cancer cells and PSCs
To further uncover the functional relevance of
Gremlin 1 in PSCs, MTT assays were performed, which demonstrated
that Gremlin 1 siRNA significantly decreased the proliferation
ability of PSCs. Cyclopamine also resulted in a negative effect.
Furthermore, although SHH increased the growth of the PSCs, it was
found that either Gremlin 1 siRNA or cyclopamine attenuated this
positive effect. However, no statistically significant difference
was observed among the remaining groups (Fig. 4A and B). The cell proliferation
assay revealed that the growth of the AsPC-1 and BxPC-3 cells was
inhibited by silencing Gremlin 1. However, this inhibition was not
significantly different from that of cyclopamine or Gli-1 siRNA
(Fig. 4C and D).
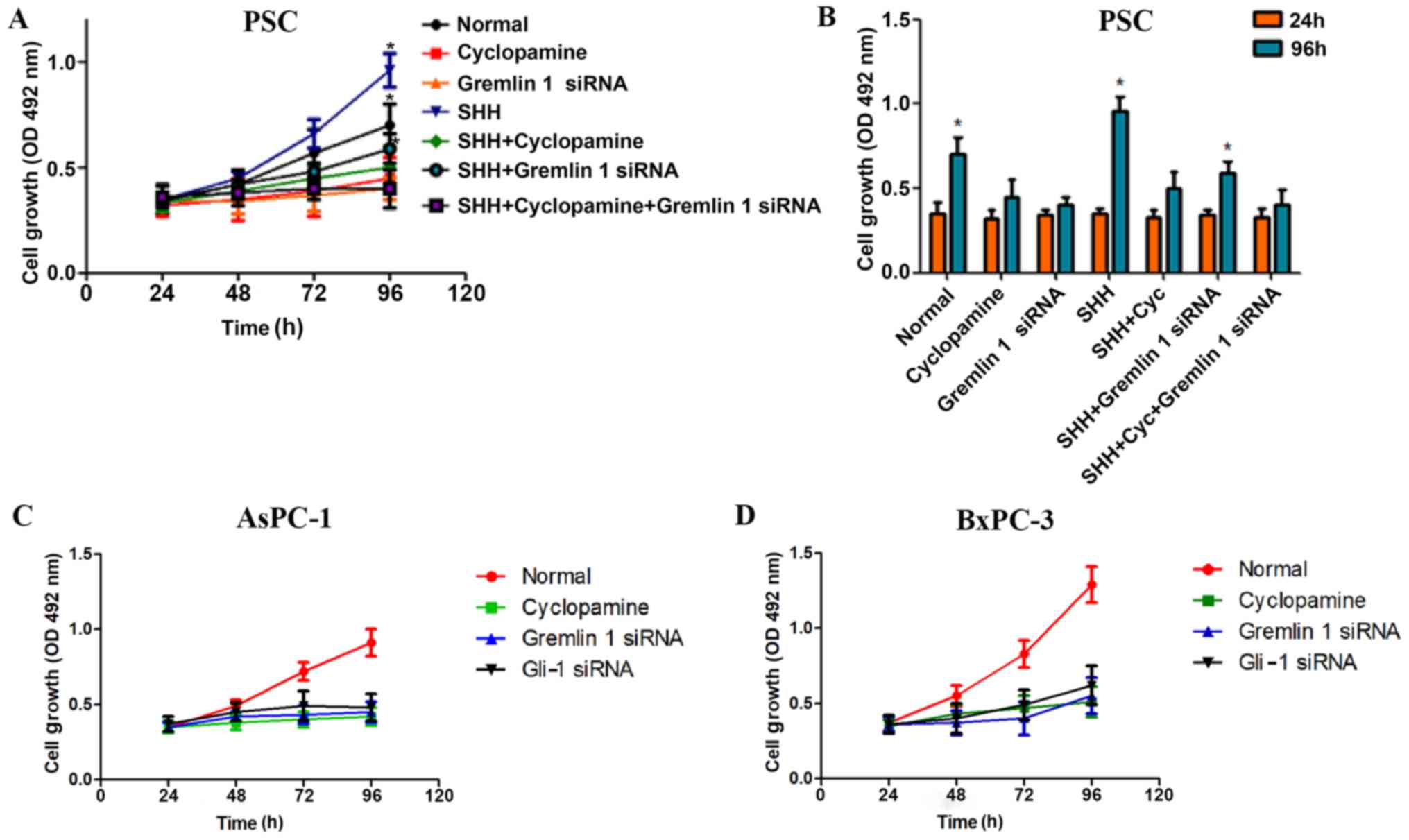 | Figure 4Variability of the expression of
Gremlin 1 induced by SHH signaling modulates the proliferation of
pancreatic cancer cells and PSCs. Effects of Gremlin 1 siRNA, SHH,
cyclopamine, and Gli-1 siRNA on the proliferation of PSCs (A) over
time and (B) compared between and 24 and 96 h. Effects of Gremlin 1
siRNA, cyclopamine, and Gli-1 on the proliferation of (C) AsPC-1
and (D) BxPC-3 cells. *P<0.05, compared with control
group. SHH, sonic hedgehog; PSCs, pancreatic stellate cells; Gli-1,
GLI family zinc finger-1; siRNA, small interfering RNA; OD, optical
density. |
Overexpression of Gremlin 1 induced by
SHH signaling promotes the invasion and migration ability of
pancreatic cancer cells in vitro
The findings from the above experiments revealed
that Gremlin 1 may have an active role in cancer cells. Therefore,
wound-healing migration assays and Transwell invasion assays were
performed using AsPC-1 and BxPC-3 cells in different
conditions.
In the Transwell invasion assays, it was found that
Gremlin 1 siRNA and cyclopamine inhibited the aggression of the
cancer cells. In accordance with the negative effects on the AsPC-1
cells, no significant difference was observed in the Gremlin 1
siRNA + cyclopamine group, compared with the siRNA group, and
cyclopamine group. For the BxPC-3 cells, the downregulation of
Gremlin 1 not only significantly decreased the invasion of the
siRNA group but also inhibited that of the cyclopamine group
(Fig. 5A and B). To quantify the
migration ability of the PSCs, a wound-induced migration assay was
performed. As shown in Fig. 5C and
D, a positive effect was observed in the SHH group, which was
suppressed by cyclopamine, whereas the siRNA groups had a negative
effect. However, no statistically significant difference was
observed between the Gremlin 1 siRNA and cyclopamine groups
(Fig. 5C and D). These data
indicated that Gremlin 1 siRNA and cyclopamine may share a similar
mechanism in their inhibition of PSC proliferation and
migration.
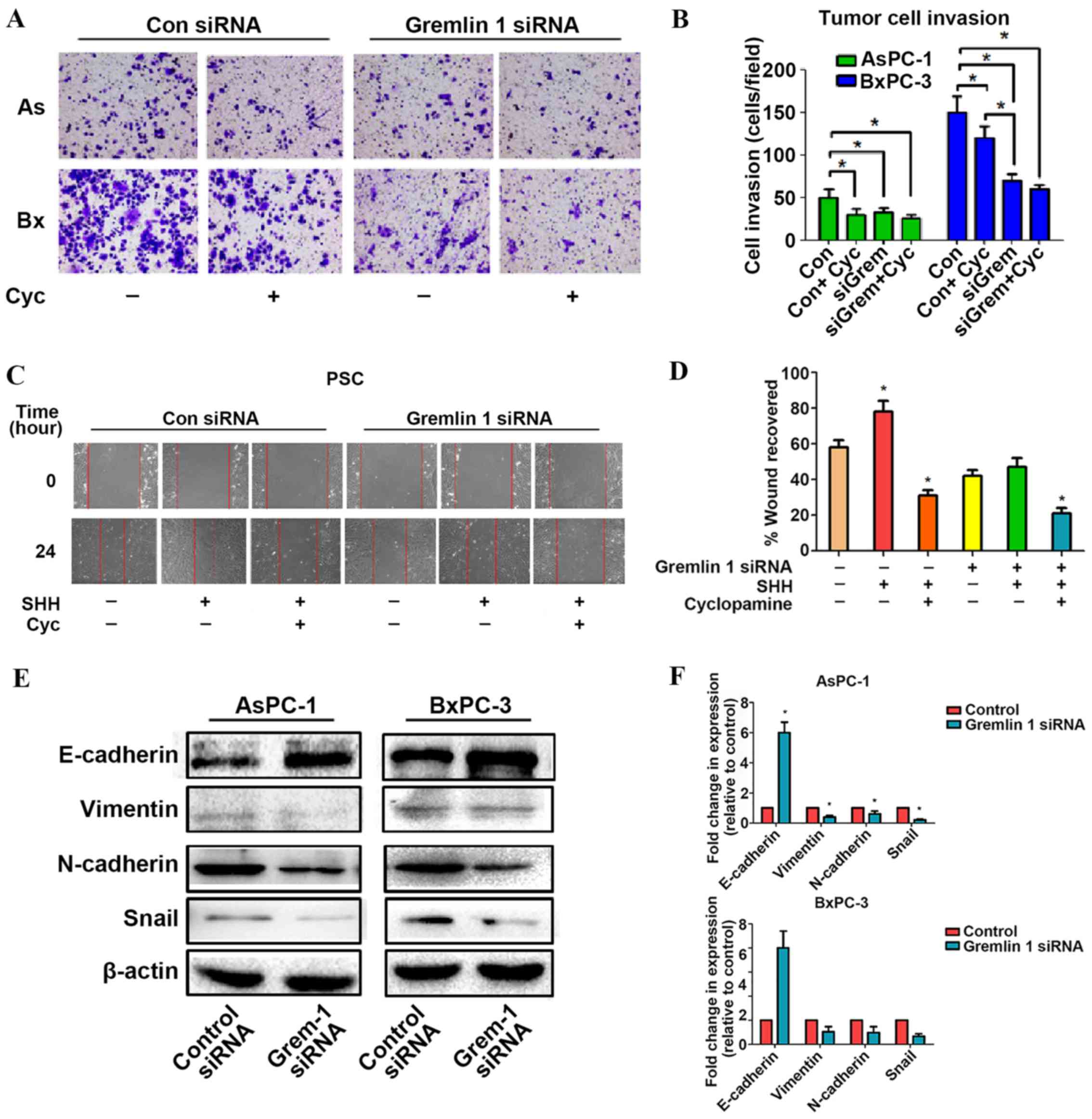 | Figure 5Overexpression of Gremlin 1 induced
by SHH signaling promotes the invasion and migration ability of
pancreatic cancer cells in vitro. (A) Images (magnification,
×200) and (B) quantification of the effect of Gremlin 1 on the
invasion of AsPC-1 and BxPC-3 cells. (C) Images (magnification,
×200) and (D) quantification of the effect of Gremlin 1 on the
migration of PSCs. (E) Blots and (F) quantification showed that
Gremlin 1 promoted epithelial to mesenchymal transition in
pancreatic cancer cells. SHH, sonic hedgehog; PSCs, pancreatic
stellate cells; siRNA, small interfering RNA; Con, control; AS,
AsPC-1 cells; Bx, BxPC-3 cells; Cyc, cyclopamine; Grem, Gremlin
1. |
Subsequently, markers of EMT, namely, E-cadherin,
vimentin, N-cadherin, and Snail, were examined the silencing of
Gremlin 1 resulted in suppression of the expression of E-cadherin
and elevation in the expression of the other three proteins
(Fig. 5E and F). The above data
suggested that Gremlin 1 is a promoter of pancreatic cancer cell
invasion, migration and EMT.
Discussion
Accumulating evidence supports the tumor promoting
role of Gremlin 1. The available microarray data in the Oncomine
database further confirms that the expression of Gremlin 1 is
substantially upregulated in tumor specimens compared with that in
normal samples (31-33). According to studies by Sneddon
et al and Namkoong et al (11,34),
the overexpression of Gremlin 1 is observed in diverse human tumors
and typically has an oncogenic role in malignancies of the
pancreas, cervix, ovary, kidney, lung, sarcoma, colon and breast.
Notably, in a study of 165 pancreas specimens, the RNA levels of
Gremlin 1 increased consistently from 5% in normal tissue to
>70% in malignant tissue (11).
This finding was further confirmed by experiments in a study by
Segara et al (31). As its
other name, PIG-2, indicates, Gremlin 1 is overexpressed in various
malignancies (tumor and stromal cells) and promotes tumor cell
proliferation (35). As BMP4
inhibits the growth of tumor cells and BMPs may act as inhibitors
of carcinogenesis and recurrence (36), Gremlin 1 may effectively reverse
their effects. Another mechanism may involve the downregulation of
cell cycle inhibitor p27kip1, the hyperphosphorylation of
Retinoblastoma protein and the activation of E2F (37). Gremlin 1 is important in tumor
angiogenesis, involving VEGF receptor (VEGFR)2 signaling. Kim et
al also revealed a possible BMP-independent and
VEGFR2-independent mechanism of Gremlin 1 during pathogenesis
(38). The present study provided
evidence that Gremlin 1, which is induced by SHH signaling, acts as
an enhancer for tumor progression in PDAC.
In the present study, weak, but not completely
negative, expression of Gremlin 1 was found in the normal pancreas,
mainly in islet cells. Gremlin 1 is also known as IHG-2. The name
IHG-2 is derived from the finding that the expression of Gremlin 1
in mesangial cells, originating from the glomerular mesangium of
the kidney, is promoted by high ambient glucose (5). The positive contribution of BMP
signaling to the modulation of insulin secretion indicates that the
expression of Gremlin 1 may be a subtle response to the impairment
of pancreas islet cells by high glucose during the pathogenesis of
diabetes mellitus (39,40). In normal C57 mice, following 8 or
12 weeks of a high-fat diet, which is necessary to impair glucose
homeostasis and induce diabetes mellitus, the expression of Gremlin
1 exhibited an increasing trend (41). These findings further support a
close association between IHG-2 (Gremlin 1), and high glucose,
revealing a novel understanding of the inner mechanism of pancreas
islet cells in diabetes mellitus. In previous years, studies have
provided support for the connection between diabetes mellitus and
pancreatic cancer (42-44). The hypothesis that aberrant insulin
regulation is a growth promoter for pancreatic cancer has been
substantiated by in vivo and in vitro experiments
(45,46). The implication that Gremlin 1 is
positively expressed in the islet cells of the normal pancreas, and
the role of Gremlin 1 during carcinogenesis from acinar-to-ductal
metaplasia to cancer require further investigation.
The histopathological analysis performed in the
present study on clinical specimens and orthotopic transplant model
tissues revealed that Gremlin 1 was predominantly expressed in the
stroma, although weak staining in certain cases was found in the
parenchyma; and patients with negative Gremlin 1 staining tended to
have a higher survival rate. In the pancreatic cancer stroma,
desmoplasia is a common pathological feature and is regarded as a
promoter of malignant progression (47). This fibrotic response in the tumor
stroma regularly involves the activation of PSCs. In vitro,
a high expression level of Gremlin 1 was found in activated PSCs.
According to previous articles, such a finding is not rare. In the
liver, Gremlin 1 is recognized as a marker of hepatic fibrosis as
this secretory protein is expressed at a high level in activated
hepatic stellate cells and is involved in EMT (49). In the kidney, the expression of
Gremlin 1 is considerably higher and is predominantly observed in
regions of tubulointerstitial fibrosis and glomeru-losclerosis
(48-51). In the intestine, stromal Gremlin 1
is regarded as a potential biomarker and a promising drug target of
cancer (52,53). In the brain, the knockdown of
Gremlin 1 inhibits glioma carcinogenesis (54). In the skin, Gremlin 1 is considered
a marker of activated myofibroblasts and the tumor-stromal
interface (55). PSCs are a type
of myofibroblast-like cell, which are involved in fibrosis and are
the main source of the extracellular matrix, which is essential for
excessive fibrous tissue (56).
Although the traditional view is that PSCs are likely of local
origin, an emerging concept is that the bone marrow (BM) is also a
source. BM-derived cancer-associated PSCs, with Gremlin 1 as a
novel marker (57), are increased
when the pancreas regenerates or undergoes carcinogenesis (58-60).
These reports support the clinical findings of the present
study.
According to the statistical analysis of the
clinicopathological features, the present study found that Gremlin
1 was positively correlated with the pT status but not with the pM
status. It was observed that Gremlin 1 promoted the migration of
PSCs and the invasion and EMT of cancer cells. This result appears
paradoxical. Of note, although invasion, migration, and EMT are
crucial steps of metastasis, every phrase of metastasis is subject
to a multitude of complicated and subtle controls. Certain subtle
mechanisms may modulate Gremlin 1-induced metastasis. Despite the
pT status referring to the size and direct extent of the primary
tumor, this concept contains several vital components, including
the infiltration, invasion and destruction of the surrounding
tissue. For example, pT3 (7th edition of the TNM staging system)
refers to cases where the tumor extends beyond the pancreas,
without involvement of the celiac axis or superior mesenteric
artery. Therefore, a positive correlation between Gremlin 1
staining and pT status is reasonable with the findings from the
in vitro experiments.
Based on our previous investigations on the SHH
pathway, it was hypothesized that there is an association between
Gremlin 1 and SHH in the cancer-stroma interaction. In the context
of vertebrate limb formation, a network involving SHH and Gremlin 1
has been identified, in which Gremlin 1 is a downstream factor of
the cellular reaction to the SHH signal depending on Gli-1
(19). Although the FGF-SHH signal
is reduced by BMP4, Gremlin 1 maintains the signal by antagonizing
BMP4 (19). Therefore, in this
positive feedback loop, SHH maintains the expression of Gremlin 1
to reverse the suppressive effects of BMP4 on the expression of
FGF4, and the latter factor increasingly upregulates SHH (21). Accordingly, in SHH−/−
mice, Gremlin 1 activation is considerably suppressed in
vivo, leading to limb deformation. Similarly, limb deformation
is rescued by grafting Gremlin-expressing cells, as Gremlin 1 is a
potent promoter of the expression of FGF4 and restores the FGF-SHH
feedback loop (17). In addition,
Gremlin 1 deletion in genetically modified animals induces
irregular BMP4 signaling, which actively suppresses FGF-SHH
signaling. However, as limb-bud outgrowth is essentially controlled
by signals in this FGF-SHH positive feedback loop, high levels of
FGF signaling also trigger the FGF-Gremlin 1 negative feedback loop
and, subsequently, inhibiting of the expression of Gremlin 1
(6,17). Therefore, a subtle balance between
the promotion and termination of growth is achieved in organ
formation and regeneration, and Gremlin 1 is a critical regulator
in this delicate network, although several unidentified molecules
may make substantial contributions to this process. In the in
vitro experiments in the present study, Gremlin 1 acted as a
downstream factor of the cellular reaction to the SHH signal, which
correlated with Gli-1. It was confirmed that the two pancreatic
cancer cells and activated PSCs expressed Gremlin 1. In addition,
extrinsic SHH was a potent trigger causing an increase of Gremlin 1
in the PSCs, whereas this effect was negligible in cancer cells.
However, in the present study, the level of Gremlin 1 succumbed to
RNA interference-mediated Gli-1 interference on both sides. It has
been confirmed that tumor cells secrete SHH, which cannot activate
SHH pathways in tumor cells but instead trigger signaling in
stromal cells, including PSCs (14). Therefore, the present study
suggested that SHH signaling was crucial for the expression of
Gremlin 1 in pancreatic cancer.
In the present study, it was found that Gremlin 1
was not only a promoter of PSC proliferation and migration, but
also an enhancer of the proliferation, invasion and EMT of
pancreatic cancer cells. Gremlin 1 is involved in EMT in
hepatocellular cancer, and additional clues regarding the function
of Gremlin 1 have arisen from studies involving the BMP pathway.
Initially, Gremlin 1 was recognized as a gene that produces
dorsalizing activity via its antagonism of BMP signaling from a
screen of a Xenopus cDNA library (9). As with the majority of members of the
BMP antagonist family, Gremlin 1 has eight conserved cysteine
residues, also known as the eight-membered ring. This structure
enables Gremlin 1 to bind BMP molecules tightly and to obstruct the
binding site for BMP receptors (3). Active BMP dimers bind certain BMP
receptors on the cell membrane, generating their signal via the
phosphorylation of R-Smads and several non-canonical intracellular
effectors. The function of BMPs in development and in various
diseases is versatile. In the intestine and colon, BMPs inhibit
stem cell self-renewal via the inhibition of Wnt signaling
(61). A number of these functions
are shared among diverse family members, whereas other functions
are specific to certain proteins. For example, BMP2/4/7 exert
specific effects in a wide variety of manners, including the
induction of EMT in several human pancreatic cancer cell lines via
the phosphorylation of Smad1 and the upregulation of MMP2 (62). The concentration of active BMPs is
critical in controlling their influence (63,64).
It has been observed that BMP4 potently reduces the growth of and
increases the migration and invasion of pancreatic cancer cells,
including Panc-1 and MiaPaCa-2 cells, when its concentration is 250
ng/ml (65). Further
investigations have shown that the EMT and migration of Panc-1
cells are triggered when the BMP4 concentration is ~50 ng/ml
(66,67). Such a delicate
concentration-dependent mechanism indicates that Gremlin 1, a
specific inhibitor that fine-tunes BMP2/4/7 signaling, is effective
at exerting a unique effect on the self-renewal, invasion,
migration and EMT of pancreatic cancer cells.
Despite the fact that the results of the present
study support the hypothesis that Gremlin 1 acts as a downstream
factor of SHH signaling and contributes to the progression of
pancreatic cancer, details require precise investigation to locate
the cellular signaling factors. In addition, the reciprocal
interaction network, including FGF4/8, SHH, BMP4 and Gremlin 1, has
not been fully examined, and several other factors in the
pathogenesis cannot be ruled out. Therefore, SHH-Gremlin
1-associated oncogenesis requires further investigation.
In conclusion, the present study showed that the
aberrant expression of Gremlin 1 in the pancreatic cancer
microenvironment was induced by SHH signaling. The data from the
in vitro experiments suggest that Gremlin 1 promoted the
proliferation and migration of PSCs, and the proliferation,
invasion and EMT of pancreatic cancer cells. Collectively, Gremlin
1 may be a therapeutic target and emerging marker of pancreatic
cancer.
Acknowledgments
Not applicable.
Funding
This study was supported by grants from the National
Natural Scientific Foundation of China (grant nos. 81472248,
81502074, 81672434 and 81702916).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YY, LC, QM and JM designed the experiments; YY and
LC carried out the majority of the experiments; BY, CZ and WQ
analyzed the data; YX and TQ organized the figures and contributed
to the data analysis and interpretation, and the writing and
revision of the manuscript; YC and LH wrote the manuscript and help
performed the immunohistochemistry experiments; QM and JM reviewed
it.
Ethics approval and consent to
participate
The study was approved by the Ethical Committees of
Shaanxi Provincial People's Hospital and the First Affiliated
Hospital of Xi'an Jiaotong University. Each patient signed an
informed consent form.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
Statistics, 2017. CA Cancer J Clin. 67:7–30. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chen W, Zheng R, Baade PD, Zhang S, Zeng
H, Bray F, Jemal A, Yu XQ and He J: Cancer statistics in China,
2015. CA Cancer J Clin. 66:115–132. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Nolan K, Kattamuri C, Luedeke DM, Deng X,
Jagpal A, Zhang F, Linhardt RJ, Kenny AP, Zorn AM and Thompson TB:
Structure of protein related to Dan and Cerberus: Insights into the
mechanism of bone morphogenetic protein antagonism. Structure.
21:1417–1429. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chen B, Blair DG, Plisov S, Vasiliev G,
Perantoni AO, Chen Q, Athanasiou M, Wu JY, Oppenheim JJ and Yang D:
Cutting edge: Bone morphogenetic protein antagonists Drm/Gremlin
and Dan interact with Slits and act as negative regulators of
monocyte chemotaxis. J Immunol. 173:5914–5917. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
McMahon R, Murphy M, Clarkson M, Taal M,
Mackenzie HS, Godson C, Martin F and Brady HR: IHG-2, a mesangial
cell gene induced by high glucose, is human gremlin. Regulation by
extracellular glucose concentration, cyclic mechanical strain, and
transforming growth factor-beta1. J Biol Chem. 275:9901–9904. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Walsh DW, Godson C, Brazil DP and Martin
F: Extracellular BMP-antagonist regulation in development and
disease: Tied up in knots. Trends Cell Biol. 20:244–256. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Li Q, Huo Y, Guo Y, Zheng X, Sun W and Hao
Z: Generation and applications of a DNA aptamer against Gremlin-1.
Molecules. 22:222017.
|
|
8
|
Topol LZ, Modi WS, Koochekpour S and Blair
DG: DRM/GREMLIN (CKTSF1B1) maps to human chromosome 15 and is
highly expressed in adult and fetal brain. Cytogenet Cell Genet.
89:79–84. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hsu DR, Economides AN, Wang X, Eimon PM
and Harland RM: The Xenopus dorsalizing factor Gremlin identifies a
novel family of secreted proteins that antagonize BMP activities.
Mol Cell. 1:673–683. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Topol LZ, Bardot B, Zhang Q, Resau J,
Huillard E, Marx M, Calothy G and Blair DG: Biosynthesis,
post-translation modification, and functional characterization of
Drm/Gremlin. J Biol Chem. 275:8785–8793. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sneddon JB, Zhen HH, Montgomery K, van de
Rijn M, Tward AD, West R, Gladstone H, Chang HY, Morganroth GS, Oro
AE, et al: Bone morphogenetic protein antagonist Gremlin 1 is
widely expressed by cancer-associated stromal cells and can promote
tumor cell proliferation. Proc Natl Acad Sci USA. 103:14842–14847.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Thayer SP, di Magliano MP, Heiser PW,
Nielsen CM, Roberts DJ, Lauwers GY, Qi YP, Gysin S, Fernández-del
Castillo C, Yajnik V, et al: Hedgehog is an early and late mediator
of pancreatic cancer tumorigenesis. Nature. 425:851–856. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Feldmann G, Dhara S, Fendrich V, Bedja D,
Beaty R, Mullendore M, Karikari C, Alvarez H, Iacobuzio-Donahue C,
Jimeno A, et al: Blockade of hedgehog signaling inhibits pancreatic
cancer invasion and metastases: A new paradigm for combination
therapy in solid cancers. Cancer Res. 67:2187–2196. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Li X, Wang Z, Ma Q, Xu Q, Liu H, Duan W,
Lei J, Ma J, Wang X, Lv S, et al: Sonic hedgehog paracrine
signaling activates stromal cells to promote perineural invasion in
pancreatic cancer. Clin Cancer Res. 20:4326–4338. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Islam SS, Mokhtari RB, Noman AS, Uddin M,
Rahman MZ, Azadi MA, Zlotta A, van der Kwast T, Yeger H and Farhat
WA: Sonic hedgehog (Shh) signaling promotes tumorigenicity and
stemness via activation of epithelial-to-mesenchymal transition
(EMT) in bladder cancer. Mol Carcinog. 55:537–551. 2016. View Article : Google Scholar
|
|
16
|
Yauch RL, Gould SE, Scales SJ, Tang T,
Tian H, Ahn CP, Marshall D, Fu L, Januario T, Kallop D, et al: A
paracrine requirement for hedgehog signalling in cancer. Nature.
455:406–410. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Verheyden JM and Sun X: An Fgf/Gremlin
inhibitory feedback loop triggers termination of limb bud
outgrowth. Nature. 454:638–641. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Panman L, Galli A, Lagarde N, Michos O,
Soete G, Zuniga A and Zeller R: Differential regulation of gene
expression in the digit forming area of the mouse limb bud by SHH
and Gremlin 1/FGF-mediated epithelial-mesenchymal signalling.
Development. 133:3419–3428. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zúñiga A, Haramis AP, McMahon AP and
Zeller R: Signal relay by BMP antagonism controls the SHH/FGF4
feedback loop in vertebrate limb buds. Nature. 401:598–602. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Niswander L, Jeffrey S, Martin GR and
Tickle C: A positive feedback loop coordinates growth and
patterning in the vertebrate limb. Nature. 371:609–612. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Bénazet J-D, Bischofberger M, Tiecke E,
Gonçalves A, Martin JF, Zuniga A, Naef F and Zeller R: A
self-regulatory system of interlinked signaling feedback loops
controls mouse limb patterning. Science. 323:1050–1053. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Cao L, Xiao X, Lei J, Duan W, Ma Q and Li
W: Curcumin inhibits hypoxia-induced epithelial mesenchymal
transition in pancreatic cancer cells via suppression of the
hedgehog signaling pathway. Oncol Rep. 35:3728–3734. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Li W, Cao L, Chen X, Lei J and Ma Q:
Resveratrol inhibits hypoxia-driven ROS-induced invasive and
migratory ability of pancreatic cancer cells via suppression of the
Hedgehog signaling pathway. Oncol Rep. 35:1718–1726. 2016.
View Article : Google Scholar
|
|
24
|
Edge SB and Compton CC: The American Joint
Committee on Cancer: The 7th edition of the AJCC cancer staging
manual and the future of TNM. Ann Surg Oncol. 17:1471–1474. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Vonlaufen A, Phillips PA, Yang L, Xu Z,
Fiala-Beer E, Zhang X, Pirola RC, Wilson JS and Apte MV: Isolation
of quiescent human pancreatic stellate cells: A promising in vitro
tool for studies of human pancreatic stellate cell biology.
Pancreatology. 10:434–443. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Han L, Ma J, Duan W, Zhang L, Yu S, Xu Q,
Lei J, Li X, Wang Z, Wu Z, et al: Pancreatic stellate cells
contribute pancreatic cancer pain via activation of sHH signaling
pathway. Oncotarget. 7:18146–18158. 2016.PubMed/NCBI
|
|
27
|
Jiang Z, Chen X, Chen K, Sun L, Gao L,
Zhou C, Lei M, Duan W, Wang Z, Ma Q, et al: YAP Inhibition by
Resveratrol via Activation of AMPK Enhances the Sensitivity of
Pancreatic Cancer Cells to Gemcitabine. Nutrients. 8:82016.
View Article : Google Scholar
|
|
28
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-DeltaDeltaC(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
29
|
Lei J, Ma J, Ma Q, Li X, Liu H, Xu Q, Duan
W, Sun Q, Xu J, Wu Z, et al: Hedgehog signaling regulates hypoxia
induced epithelial to mesenchymal transition and invasion in
pancreatic cancer cells via a ligand-independent manner. Mol
Cancer. 12:66. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Li X, Ma Q, Xu Q, Liu H, Lei J, Duan W,
Bhat K, Wang F, Wu E and Wang Z: SDF-1/CXCR4 signaling induces
pancreatic cancer cell invasion and epithelial-mesenchymal
transition in vitro through non-canonical activation of Hedgehog
pathway. Cancer Lett. 322:169–176. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Segara D, Biankin AV, Kench JG, Langusch
CC, Dawson AC, Skalicky DA, Gotley DC, Coleman MJ, Sutherland RL
and Henshall SM: Expression of HOXB2, a retinoic acid signaling
target in pancreatic cancer and pancreatic intraepithelial
neoplasia. Clin Cancer Res. 11:3587–3596. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Iacobuzio-Donahue CA, Maitra A, Olsen M,
Lowe AW, van Heek NT, Rosty C, Walter K, Sato N, Parker A, Ashfaq
R, et al: Exploration of global gene expression patterns in
pancreatic adenocarcinoma using cDNA microarrays. Am J Pathol.
162:1151–1162. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Koli K, Sutinen E, Rönty M, Rantakari P,
Fortino V, Pulkkinen V, Greco D, Sipilä P and Myllärniemi M:
Gremlin-1 overex-pression in mouse lung reduces silica-induced
lymphocyte recruitment - A link to idiopathic pulmonary fibrosis
through negative correlation with CXCL10 chemokine. PLoS One.
11:e01590102016. View Article : Google Scholar
|
|
34
|
Namkoong H, Shin SM, Kim HK, Ha SA, Cho
GW, Hur SY, Kim TE and Kim JW: The bone morphogenetic protein
antagonist Gremlin 1 is overexpressed in human cancers and
interacts with YWHAH protein. BMC Cancer. 6:74. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Karagiannis GS, Musrap N, Saraon P, Treacy
A, Schaeffer DF, Kirsch R, Riddell RH and Diamandis EP: Bone
morphogenetic protein antagonist gremlin-1 regulates colon cancer
progression. Biol Chem. 396:163–183. 2015. View Article : Google Scholar
|
|
36
|
Piccirillo SG, Reynolds BA, Zanetti N,
Lamorte G, Binda E, Broggi G, Brem H, Olivi A, Dimeco F and Vescovi
AL: Bone morphogenetic proteins inhibit the tumorigenic potential
of human brain tumour-initiating cells. Nature. 444:761–765. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Maciel TT, Melo RS, Schor N and Campos AH:
Gremlin promotes vascular smooth muscle cell proliferation and
migration. J Mol Cell Cardiol. 44:370–379. 2008. View Article : Google Scholar
|
|
38
|
Kim M, Yoon S, Lee S, Ha SA, Kim HK, Kim
JW and Chung J: Gremlin-1 induces BMP-independent tumor cell
proliferation, migration, and invasion. PLoS One. 7:e351002012.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Goulley J, Dahl U, Baeza N, Mishina Y and
Edlund H: BMP4-BMPR1A signaling in β cells is required for and
augments glucose-stimulated insulin secretion. Cell Metab.
5:207–219. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Scott GJ, Ray MK, Ward T, McCann K,
Peddada S, Jiang FX and Mishina Y: Abnormal glucose metabolism in
heterozygous mutant mice for a type I receptor required for BMP
signaling. Genesis. 47:385–391. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Henley KD, Gooding KA, Economides AN and
Gannon M: Inactivation of the dual Bmp/Wnt inhibitor Sostdc1
enhances pancreatic islet function. Am J Physiol Endocrinol Metab.
303:E752–E761. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Eibl G, Cruz-Monserrate Z, Korc M, Petrov
MS, Goodarzi MO, Fisher WE, Habtezion A, Lugea A, Pandol SJ, Hart
PA, et al: Diabetes mellitus and obesity as risk factors for
pancreatic cancer. J Acad Nutr Diet. 118:555–567. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Kleeff J, Costello E, Jackson R, Halloran
C, Greenhalf W, Ghaneh P, Lamb RF, Lerch MM, Mayerle J, Palmer D,
et al: The impact of diabetes mellitus on survival following
resection and adjuvant chemotherapy for pancreatic cancer. Br J
Cancer. 115:887–894. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Hart PA, Law RJ, Frank RD, Bamlet WR,
Burch PA, Petersen GM, Rabe KG and Chari ST: Impact of diabetes
mellitus on clinical outcomes in patients undergoing surgical
resection for pancreatic cancer: A retrospective, cohort study. Am
J Gastroenterol. 109:1484–1492. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Chen K, Qian W, Jiang Z, Cheng L, Li J,
Sun L, Zhou C, Gao L, Lei M, Yan B, et al: Metformin suppresses
cancer initiation and progression in genetic mouse models of
pancreatic cancer. Mol Cancer. 16:1312017. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Duan W, Chen K, Jiang Z, Chen X, Sun L, Li
J, Lei J, Xu Q, Ma J, Li X, et al: Desmoplasia suppression by
metformin-mediated AMPK activation inhibits pancreatic cancer
progression. Cancer Lett. 385:225–233. 2017. View Article : Google Scholar
|
|
47
|
Liu J, Zhong Y, Liu G, Zhang X, Xiao B,
Huang S, Liu H and He L: Role of Stat3 Signaling in Control of EMT
of Tubular Epithelial Cells During Renal Fibrosis. Cell Physiol
Biochem. 42:2552–2558. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Roxburgh SA, Murphy M, Pollock CA and
Brazil DP: Recapitulation of embryological programmes in renal
fibrosis–the importance of epithelial cell plasticity and
developmental genes. Nephron, Physiol. 103:139–148. 2006.
View Article : Google Scholar
|
|
49
|
Walsh DW, Roxburgh SA, McGettigan P,
Berthier CC, Higgins DG, Kretzler M, Cohen CD, Mezzano S, Brazil DP
and Martin F: Co-regulation of Gremlin and Notch signalling in
diabetic nephropathy. Biochim Biophys Acta. 1782:10–21. 2008.
View Article : Google Scholar
|
|
50
|
Dolan V, Murphy M, Sadlier D, Lappin D,
Doran P, Godson C, Martin F, O'Meara Y, Schmid H, Henger A, et al:
Expression of gremlin, a bone morphogenetic protein antagonist, in
human diabetic nephropathy. Am J Kidney Dis. 45:1034–1039. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
McKnight AJ, Patterson CC, Pettigrew KA,
Savage DA, Kilner J, Murphy M, Sadlier D and Maxwell AP; Warren
3/U.K. Genetics of Kidneys in Diabetes (GoKinD) Study Group: A
GREM1 gene variant associates with diabetic nephropathy. J Am Soc
Nephrol. 21:773–781. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Jang BG, Kim HS, Chang WY, Bae JM, Oh HJ,
Wen X, Jeong S, Cho NY, Kim WH and Kang GH: Prognostic significance
of stromal GREM1 expression in colorectal cancer. Hum Pathol.
62:56–65. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Pelli A, Väyrynen JP, Klintrup K, Mäkelä
J, Mäkinen MJ, Tuomisto A and Karttunen TJ: Gremlin1 expression
associates with serrated pathway and favourable prognosis in
colorectal cancer. Histopathology. 69:831–838. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Guan Y, Cheng W, Zou C, Wang T, Cao Z and
Wu A: Gremlin1 promotes carcinogenesis of glioma in vitro. Clin Exp
Pharmacol Physiol. 44:244–256. 2017. View Article : Google Scholar
|
|
55
|
Kim HS, Shin MS, Cheon MS, Kim JW, Lee C,
Kim WH, Kim YS and Jang BG: GREM1 is expressed in the
cancer-associated myofibroblasts of basal cell carcinomas. PLoS
One. 12:e01745652017. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Koikawa K, Ohuchida K, Takesue S, Ando Y,
Kibe S, Nakayama H, Endo S, Abe T, Okumura T, Horioka K, et al:
Pancreatic stellate cells reorganize matrix components and lead
pancreatic cancer invasion via the function of Endo180. Cancer
Lett. 412:143–154. 2018. View Article : Google Scholar
|
|
57
|
Quante M, Tu SP, Tomita H, Gonda T, Wang
SS, Takashi S, Baik GH, Shibata W, Diprete B, Betz KS, et al: Bone
marrow-derived myofibroblasts contribute to the mesenchymal stem
cell niche and promote tumor growth. Cancer Cell. 19:257–272. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Ishii G, Sangai T, Oda T, Aoyagi Y, Hasebe
T, Kanomata N, Endoh Y, Okumura C, Okuhara Y, Magae J, et al:
Bone-marrow-derived myofibroblasts contribute to the cancer-induced
stromal reaction. Biochem Biophys Res Commun. 309:232–240. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Marrache F, Pendyala S, Bhagat G, Betz KS,
Song Z and Wang TC: Role of bone marrow-derived cells in
experimental chronic pancreatitis. Gut. 57:1113–1120. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Scarlett CJ: Contribution of bone marrow
derived cells to the pancreatic tumor microenvironment. Front
Physiol. 4:56. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Kosinski C, Li VS, Chan ASY, Zhang J, Ho
C, Tsui WY, Chan TL, Mifflin RC, Powell DW, Yuen ST, et al: Gene
expression patterns of human colon tops and basal crypts and BMP
antagonists as intestinal stem cell niche factors. Proc Natl Acad
Sci USA. 104:15418–15423. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Gordon KJ, Kirkbride KC, How T and Blobe
GC: Bone morpho-genetic proteins induce pancreatic cancer cell
invasiveness through a Smad1-dependent mechanism that involves
matrix metalloproteinase-2. Carcinogenesis. 30:238–248. 2009.
View Article : Google Scholar
|
|
63
|
Yin Y, Yang Y, Yang L, Yang Y, Li C, Liu X
and Qu Y: Overexpression of Gremlin promotes non-small cell lung
cancer progression. Tumour Biol. 37:2597–2602. 2016. View Article : Google Scholar
|
|
64
|
Wellbrock J, Sheikhzadeh S,
Oliveira-Ferrer L, Stamm H, Hillebrand M, Keyser B, Klokow M,
Vohwinkel G, Bonk V, Otto B, et al: Overexpression of Gremlin-1 in
patients with Loeys-Dietz syndrome: Implications on pathophysiology
and early disease detection. PLoS One. 9:e1047422014. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Virtanen S, Alarmo EL, Sandström S, Ampuja
M and Kallioniemi A: Bone morphogenetic protein -4 and -5 in
pancreatic cancer--novel bidirectional players. Exp Cell Res.
317:2136–2146. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Hamada S, Satoh K, Hirota M, Kimura K,
Kanno A, Masamune A and Shimosegawa T: Bone morphogenetic protein 4
induces epithelial-mesenchymal transition through MSX2 induction on
pancreatic cancer cell line. J Cell Physiol. 213:768–774. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Kallioniemi A: Bone morphogenetic protein
4-a fascinating regulator of cancer cell behavior. Cancer Genet.
205:267–277. 2012. View Article : Google Scholar : PubMed/NCBI
|