Introduction
Breast cancer is the most common type of malignancy
worldwide and the second leading cause of cancer-related mortality
in women (1,2). Recent advances in breast cancer
detection and treatment have decreased the mortality rate
attributable to breast cancer. However, the side effects of
radiotherapy and chemotherapy, such as immunosuppression,
recurrence and metastasis, remain challenging and adversely affect
the quality of life of breast cancer patients (3,4). A
number of traditional Chinese herbal medicines have been suggested
as potential therapeutic options, due to their relative lack of
side effects.
Constitutive activation of the transcription factor
nuclear factor (NF)-κB stimulates proliferation and metastasis and
inhibits apoptosis of breast cancer cells. NF-κB
activity-stimulating signals have been shown to cause dissociation
of IκB, allowing NF-κB dimers to translocate to the nucleus and
alter gene expression, in order to activate anti-apoptotic genes
and promote the transcription of genes associated with cancer
growth, invasion and metastasis. This makes NF-κB a key factor in
enabling precancerous and malignant cells to escape apoptosis
(5-7). Inhibition of NF-κB activity results
in the partial release of cells from the G2/M arrest following
curcumin treatment of human breast cancer MCF-7 cells (8,9).
Therefore, NF-κB may represent an effective target for controlling
tumor invasion and metastasis.
Baicalin is one of the predominant flavonoids
isolated from the dry root of Scutellaria baicalensis Georgi
(Huang-Qin, a medicinal plant), an important traditional Chinese
medicinal herb used to treat diseases of the central nervous system
(CNS), hepatic disorders and inflammatory conditions, among others
(10,11). Furthermore, baicalin has multiple
biological functions, including anti-inflammatory, antioxidant,
anti-apoptotic and immune regulation properties (12,13).
Baicalin reduces the acute hepatic injury induced by
CCl4 and promotes early recovery of liver function
(14). Baicalin inhibits HepG2
hepatoblastoma cells through oxidative/nitrative stress (15). Evidence suggests that the potential
antioxidant and anti-inflammatory properties of this compound are
largely due to its ability to enhance an antioxidant status and to
suppress the expression of several inflammatory cytokines by
attenuating the activity of NF-κB (12,16).
Dinda et al reported that baicalin inhibited reactive oxygen
species (ROS) production in arteriosclerotic vascular disease by
suppressing the activation of the NF-κB signaling pathway (17). Wang et al reported that
baicalin exerts an inhibitory effect on inflammation-related
collagen-induced arthritis via inhibition of the activation of the
NF-κB signaling pathway (18).
Given its ability to inhibit the activation of NF-κB signaling, it
was hypothesized that baicalin may suppress breast cancer growth.
To the best of our knowledge, no previous studies have investigated
this possible effect of baicalin.
In the present study, the breast cancer cell lines
MCF-7 and MDA-MB-231 and a xenograft mouse model were treated with
baicalin, with the aim of determining the therapeutic potential of
this compound in breast cancer and elucidating the role of NF-κB in
the effects of baicalin.
Materials and methods
Cell treatment
The breast cancer cell lines MCF-7, MDA-MB-231 and
MCF-10 were purchased from Cell Applications, Inc. (San Diego, CA,
USA). The cells were cultured in cell growth medium supplemented
with 100 IU/ml penicillin and 100 μg/ml streptomycin at 37°C
in a humidified incubator with 5% carbon dioxide (CO2)
and 95% air.
The cell growth medium was replaced with serum-free
medium for an additional 24-h culture prior to further treatments.
Cells were suspended at a density of 2×106 cells/ml for
experiments. In the mechanism experiments, cells treated with
serum-free medium alone served as the control group. The cells were
treated with 20 or 30 μM baicalin in Dulbecco’s modified
Eagle’s medium for 48 h. The concentrations of baicalin were
selected as previously described (10-13,18).
In vivo tumorigenicity and baicalin
treatment
The present study was approved by the Ethics
Committee for Animal Experimentation of the School of Life Science
and Technology, Harbin Institute of Technology. All animal
experiments were performed according to the Guidelines for the care
and use of experimental animals approved by the Heilongjiang
Province People’s Congress (http://www.nicpbp.org.cn/sydw/ CL0249/2730.html).
Female BALB/c nude mice, 5 weeks old and weighing ~15 g, were
purchased from the Harbin Veterinary Research Institute (Harbin,
China). The animals were housed in a clean and ventilated
environment under standard laboratory conditions (temperature
22±1°C, relative humidity 50-70%, and a 12-h light/dark cycle).
Standard food and water were provided ad libitum throughout
the experiments. The animals were allowed to acclimatize to their
surroundings over 7 days to eliminate the effect of stress prior to
the initiation of the experiments, and then randomly divided into
three groups (control, 100 and 200 mg/kg groups; n=6 per group) for
the following tumorigenicity and baicalin treatment
experiments.
MDA-MB-231 cells (1×105/injection) were
suspended in 100 μl phosphate-buffered saline (PBS)
containing 50% Matrigel (BD Biosciences, Franklin Lakes, NJ, USA)
and injected into the mammary fat pad of 4-5-week-old female nude
mice. One week later, the control mice received PBS (1 ml/kg, once
daily i.p.), while 100 and 200 mg/kg group mice received daily i.p.
injections of baicalin (>95% purity; Sigma-Aldrich; Merck KGaA,
St. Louis, MO, USA) at doses of 100 and 200 mg/kg, respectively.
The concentrations of baicalin were selected as previously
described (10-13,18).
Tumor size was measured every 5 days for 4 weeks, starting 5 days
after tumor cell implantation. The tumor volume was calculated
according to the following formula: V = L x W2/2, where
V, volume (mm3); L, length (mm); and W, width (mm).
Cell viability
Cells were cultured using the abovementioned method,
diluted to 2×106 cells/ml, seeded into 96-well plates
(100 μl/well) and incubated for 24 h, followed by the
addition of 20 or 30 μM baicalin and further incubation for
72 h. According to the manufacturer’s recommendations, 20 μl
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-2H-tetrazoliumbro-mide
solution (5 mg/ml in PBS; Sigma-Aldrich; Merck KGaA) was added to
each well and incubated with cells under standard conditions for 4
h. Subsequently, the formazan crystals in each well were dissolved
in dimethyl sulfoxide, after removal of the medium. Finally, the
optical density at 490 nm was measured using an enzyme-linked
immunosorbent microplate reader (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA). The results are expressed as the mean of
triplicate experiments.
Cell cycle analysis
Untreated cells and those treated with baicalin were
dissociated and washed with PBS for cell cycle analysis. The cells
were incubated in PBS containing 500 U/ml RNase (Sigma-Aldrich;
Merck KGaA) at 37°C for 15 min, incubated with 50 μg/ml
propidium iodide (PI) at 4°C in the dark for 2 h, and analyzed to
determine the percentage of 10,000 cells in each of three cell
cycle compartments (G0/G1, S and G2/M) using a
fluorescence-activated cell analyzer (Becton-Dickinson, Mountain
View, CA, USA). Modifit LT 2.0 software was used to perform data
analysis, and CELLQuest software (both from Becton-Dickinson) was
used to quantify the percentage of cells in each compartment.
Transwell assay
To measure cell invasion, 8-μm pore 24-well
Matrigel invasion chambers (Corning Inc., Corning, NY, USA) were
used according to the manufacturer’s instructions. A total of
2×104 cells were seeded into each well. DMEM with 0.1%
FBS was added to the upper chamber, while DMEM supplemented with
10% FBS was added to the lower chamber well to promote cell
invasion. After 24 h of incubation at 37°C with 5% CO2,
non-invading cells were removed from the top well, while migrated
cells were quantified by capturing photographs of 6 independent
visual fields under an Olympus BX51 microscope (Olympus Co., Tokyo,
Japan), and was stained with 0.1% crystal violet solution.
Independent experiments were repeated at least three times.
Scratch wound healing assay
A scratch wound healing assay was performed to
evaluate the migration of untreated cells and cells treated with
baicalin. In brief, MCF-7 and MDA-MB-231 cells were seeded in
6-well plates and cultured with DMEM supplemented with 10% FBS.
After reaching confluence, each well was scratched with a
200-μl pipette tip. After 12 and 24 h of incubation, 6 wound
healing areas were imaged and the distance between the two edges
was analyzed by ImageJ software, version 1.48 (National Institutes
of Health, Bethesda, MD, USA).
Dual-luciferase reporter assay
The pRL-TK and recombinant NF-ĸB luciferase reporter
plasmids (Thermo Fisher Scientific, Inc., Waltham, MA, USA) were
co-transfected into MCF-7 and MDA-MB-231 cells for measurement of
NF-ĸB reporter activity. Transfected cells were harvested 48 h
after transfection, and luciferase activity was determined by
Fluoroskan Ascent FL (Thermo Fisher Scientific, Inc.) configured
for dual assays. The relative luciferase activity was normalized to
Renilla luciferase activity. All experiments were performed
in triplicate wells.
Enzyme-linked immunosorbent assay (ELISA)
measurement of TNF-α and IL-1β levels in mouse serum and cell
culture supernatants
At day 30 of baicalin treatment, the mice were
anesthetized with intraperitoneal injection of 10% chloral hydrate
(400 mg/kg body weight). Blood samples were collected from the
abdominal aorta using syringes, and centrifuged for 30 min (3,000 ×
g at 4°C). Serum samples were stored at -80°C until use. The mice
were then euthanized by cervical dislocation. To determine cytokine
levels in vitro, MCF-7 and MDA-MB-231 cells were treated
with or without baicalin using the method described above. The
supernatants of culture plates were harvested from each well. TNF-α
and IL-1β levels in the serum and culture supernatants were
measured using mouse TNF-α and IL-1β ELISA kits (R&D Systems,
Minneapolis, MN, USA), according to the manufacturer’s
instructions.
Western blotting
MCF-7 and MDA-MB-231 cells and 0.3-g tumor samples
from mice were treated with RIPA lysis buffer supplemented with
protease inhibitor cocktail and protein phosphatase inhibitor. The
protein concentration was quantified using a DC protein kit
(Bio-Rad Laboratories). Samples were then separated, transferred to
PVDF membranes, and incubated with 1:1,000 dilution primary
antibodies overnight at 4°C. The primary antibodies used were
anti-phospho-NF-ĸB-p65 (Ser536) (rabbit mAb, catalog no. 3033),
anti-phospho-IKKα/β (Ser176/180) (rabbit mAb, catalog no. 2697) and
anti-IĸBα (rabbit mAb, catalog no. 4812). The anti-total NF-ĸB-p65
(rabbit mAb, catalog no. 8242), anti-total-IKKβ (rabbit mAb,
catalog no. 2370) and anti-β-actin antibodies (rabbit mAb, catalog
no. 4970) were used as loading controls. On the following day, the
membranes were incubated with secondary antibody (anti-rabbit IgG,
catalog no. 4414) for 2 h at room temperature. All antibodies were
purchased from Cell Signaling Technology (Danvers, MA, USA). The
bands were visualized using an ECL Plus western blotting detection
system (GE Healthcare, Milwaukee, WI, USA).
Reverse transcription-polymerase chain
reaction (RT-PCR) and quantitative PCR (RT-qPCR)
Total RNA was extracted from MCF-7 and MDA-MB-231
cells and xenograft tumor tissues using TRIzol reagent (Invitrogen;
Thermo Fisher Scientific, Grand Island, NY, USA) according to the
manufacturer’s instructions. Equal amounts (1 μg) of total
RNA were used to perform reverse transcription using PrimeScript™
RT reagent kit (Takara, Tokyo, Japan). For the determination of the
expression of various genes in MCF-7 and MDA-MB-231 cells and
xenograft tumor tissues, RT-qPCR was performed using the following
primers: CCND1, forward, 5′-AGAAGCTGTGCATCTACACCGACA-3′ and
reverse, 5′-TGATCTGTTTGTTCTCCTCCGCCT-3′; BCL2, forward,
5′-TGAGCAGAGTCTTCAGAGACAGCC-3′ and reverse,
5′-ATGTGTGTGGAGAGCGTCAACC-3′; BIRC2, forward,
5′-CCCAAAGACTTTTCCCAGGTCCC-3′ and reverse,
5′-ACTGAGCTTCCCACCACAGGCA-3′; BIRC3, forward,
5′-GAATACTCCCTGTGATTAATGCTGCCGTGG-3′ and reverse,
5′-TCTCTTGCTTGTAAAGACGTCTGTGTCTTC-3′. The ACTB (β-actin)
control primers were 5′-CGTTGCTATCCAGGCTGTGCTA-3′ and
5′-CCAGGTCCAGACGCAGGATGGC-3′. Quantification of gene expression was
determined by comparative quantity, using ACTB gene
expression as internal control. The PCR conditions were
denaturation at 94°C for 45 sec, annealing at 57°C for 30 sec, and
extension at 72°C for 1 min, for 40 cycles.
Statistical analysis
Data are expressed as mean ± standard error of the
mean and were analyzed using GraphPad Prism version 5.0 (GraphPad
Software, La Jolla, CA, USA). All calculated significant
differences were based on one-way analysis of variance and the post
hoc Tukey’s test. For direct comparisons between two groups, the
Student’s t-test was used. P<0.05 was considered to indicate
statistically significant differences.
Results
Baicalin suppresses proliferation and
induces G1/S arrest in breast cancer cells
To evaluate the effect of baicalin on breast cancer,
cell proliferation was assessed. The cells were manually counted in
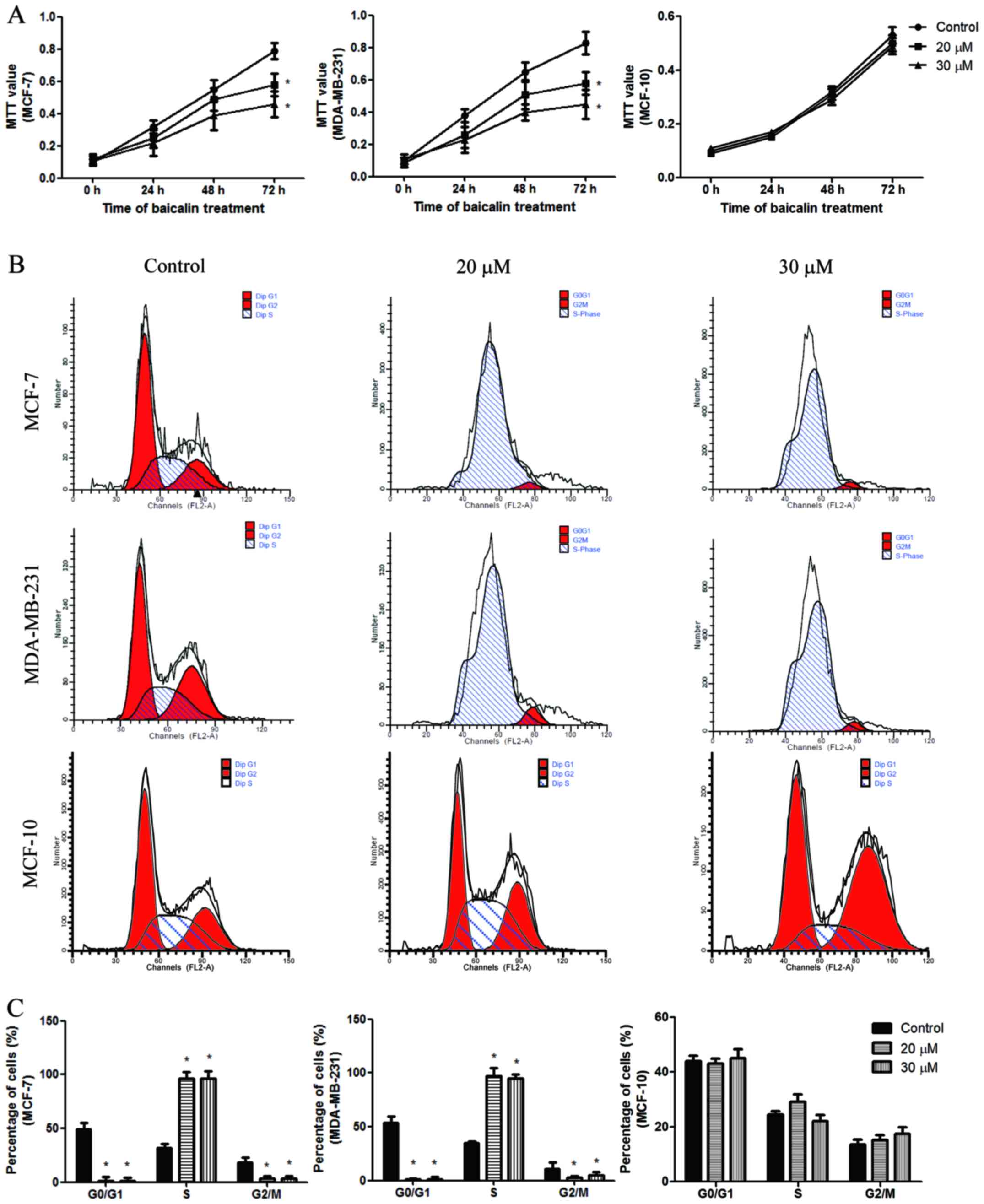
6 wells per group. The results demonstrated that baicalin reduced
cell proliferation from 48 to 72 h in MCF-7 and MDA-MB-231 cells,
indicating that this compound can inhibit breast cancer cell
proliferation (Fig. 1A). In
addition, the effect of baicalin on non-tumorigenic normal breast
epithelial cells was assessed by comparing the proliferation of
MCF-7 and MCF-10 cells. Baicalin did not affect the proliferation
of MCF-10 cells, indicating that this compound mediated growth
arrest of breast cancer cell lines without affecting the
non-tumorigenic normal breast epithelial cells (Fig. 1A). To further elucidate the
mechanism of action of baicalin in breast cancer development, after
48 h of treatment, cell cycle analysis was performed by flow
cytometry. In MCF-7 cells, the cell phase distributions before and
after treatment with 20 and 30 μM baicalin were as follows:
G0/1 phase, 49.53±0.62 vs. 55.34±1.74 and 63.52±3.64%; S phase,
31.75±1.24 vs. 25.73±2.79 and 25.51±3.61%; and G2/M phase,
18.71±0.45 vs. 17.93±1.87 and 17.66±2.2, respectively. In
MDA-MB-231 cells, the cell phase distributions before and after
treatment with 20 and 30 μM baicalin were as follows: G0/1
phase, 42.96±2.01 vs. 53.1±1.74 and 64.96±3.33%; S phase,
29.53±1.12 vs. 24.17±2.71 and 24±2.29%; and G2/M phase, 17.51±1.79
vs. 18.17±1.74 and 13.41±2.48%, respectively (Fig. 1B and C). These results indicate
that the proportion of cells in the S phase was significantly
reduced following treatment with baicalin, whereas the proportion
of cells in the G0/1 phase was significantly increased, suggesting
that baicalin induces G1/S arrest. Compared with MCF-7 cells,
baicalin did not affect the cell cycle distribution of MCF-10 cells
(Fig. 1B and C).
Baicalin suppresses the invasion and
migration of breast cancer cells
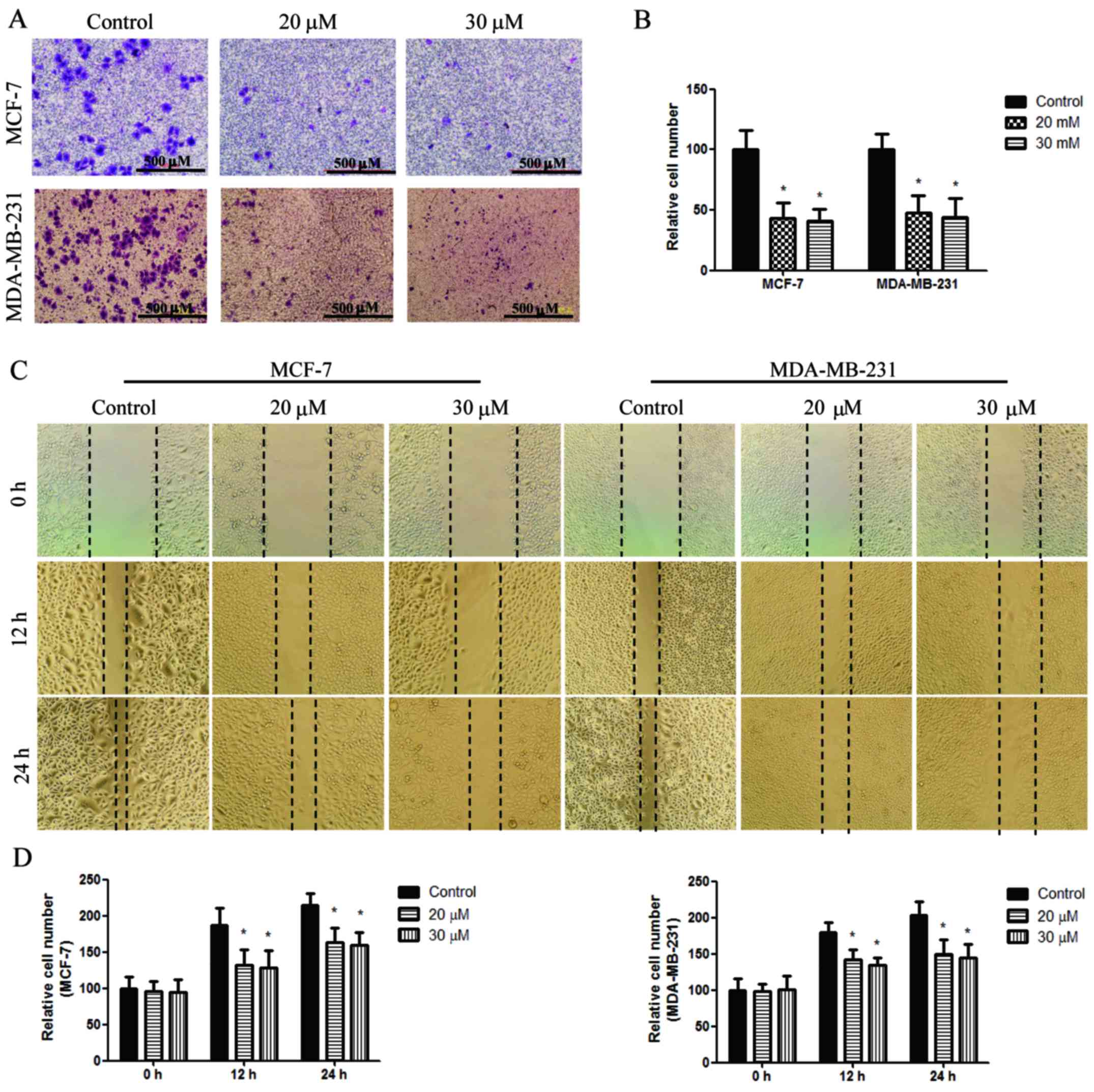
The Transwell assay was employed to evaluate the
effect of baicalin on the invasion of MCF-7 and MDA-MB-231 cells.
The cells were manually counted in 6 wells per group. The mean
number of invasive cells, as determined by microscopy, was
100±19.5, 43±13.7 and 41±13.3 in untreated MCF-7 cells, and those
treated with 20 and 30 μM baicalin, respectively. The mean
number of invading cells, as determined by microscopy, was
100±13.6, 54±15.3 and 44±16.3 in untreated MBA-MB-231 cells, and
those treated with 20 and 30 μM baicalin, respectively.
Therefore, the number of invading cells in the treatment groups was
significantly reduced compared with that in the untreated control
group (Fig. 2A and B).
Next, a wound healing assay was performed to explore
the effects of baicalin on the migration of MCF-7 and MDA-MB-231
cells. The cells were manually counted in 6 wells per group. After
24 h, the number of migrating MCF-7 cells was 115±16.3, 67±7.7 and
64±10.1 in untreated cells, and those treated with and 20 and 30
μM baicalin, respectively. The number of migrating MDA-MB
cells was 103±12.6, 52±10.6 and 44±7.3 in untreated cells, and
those treated with 20 and 30 μM baicalin, respectively.
Therefore, the number of migrating cells in the treatment groups
was significantly reduced compared with that in the untreated
control group (Fig. 2C and D).
Baicalin suppresses breast cancer tumor
growth in xenograft mice
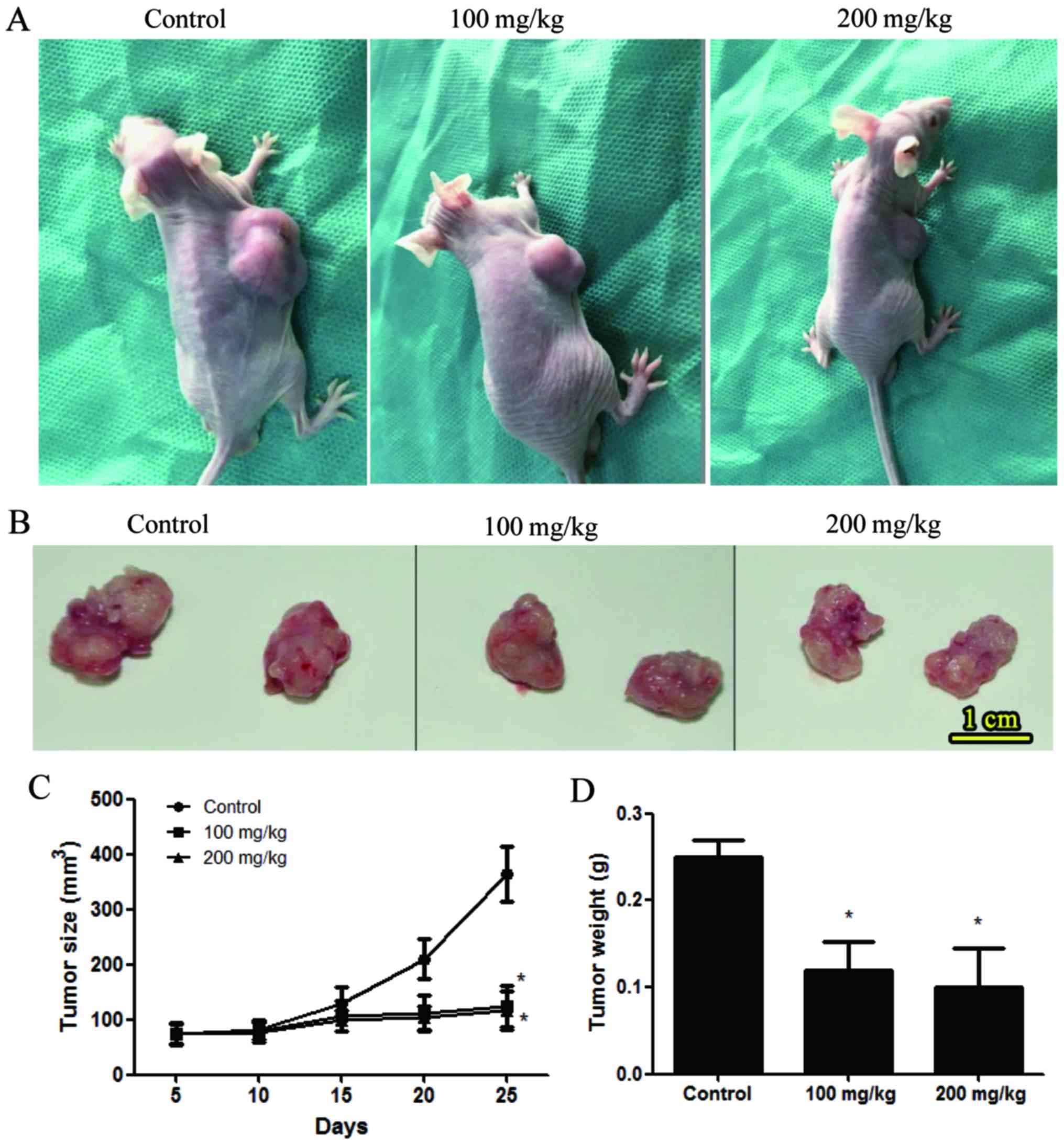
To further explore the effects of baicalin on breast
cancer growth, an MBA-MB-231 xenograft mouse model was employed,
using 6 mice per group. After 25 days, the tumor volumes were
smaller in the MBA-MB-231 cell xenograft mice treated with 100 and
200 mg/kg baicalin compared with the untreated group (124.77±38.33
and 116.22±35.64 vs. 363.63±47.63 mm3, respectively;
Fig. 3A-C). In the control group,
the longest diameter of the largest subcutaneous tumor was 15.62
mm. The tumor weights were also reduced in the 100 and 200 mg/kg
baicalin treatment groups compared with the untreated group
(0.12±0.03 and 0.10±0.05 vs. 0.25±0.02 g, respectively; Fig. 3B and D). No multiple subcutaneous
tumors or peritonitis were observed in mice after being injected
with MDA-MB-231 cells or baicalin.
Baicalin inhibits the secretion of TNF-α
and IL-1β into cell media and mouse serum
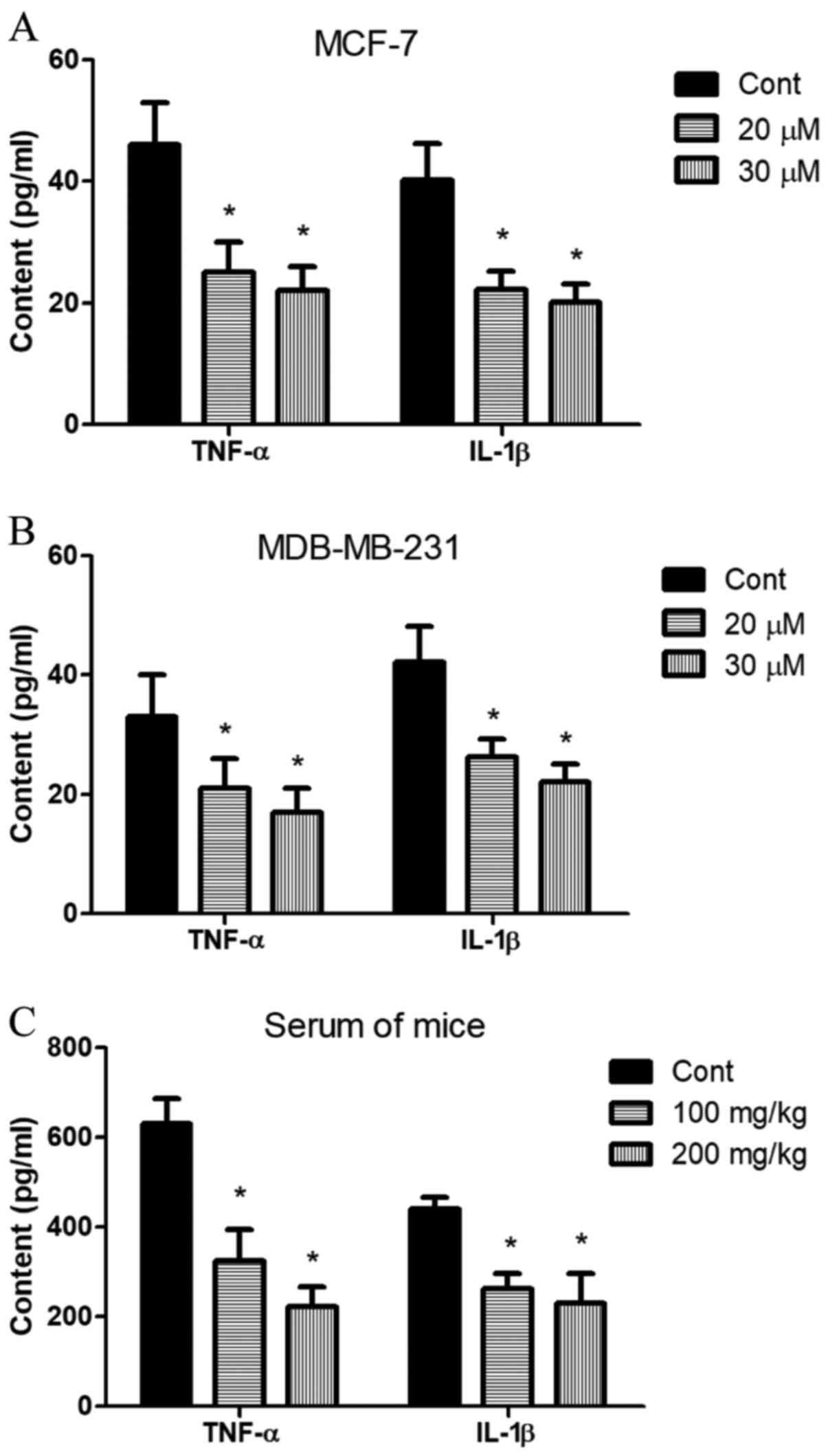
To investigate whether baicalin modulates the
inflammatory process by regulating the secretion of cytokines, the
serum levels of TNF-α and IL-1β were measured in vitro and
in vivo by ELISA. The cells were manually counted in 6 wells
per group; the number of mice per group was n=6. As shown in
Fig. 4A and B, high levels of the
pro-inflammatory cytokines TNF-α and IL-1β were detected in the
medium of MCF-7 and MBA-MB-231 cells in the control group. By
contrast, the levels of these cytokines were significantly lower in
the baicalin-treated groups (20 and 30 μM) compared with the
untreated group. However, there was no significant difference in
the cytokine levels between mice treated with 20 and those treated
with 30 μM baicalin. Similar results were observed in serum
samples obtained from mice on day30 following treatment with 100 or
200 mg/kg baicalin (Fig. 4C).
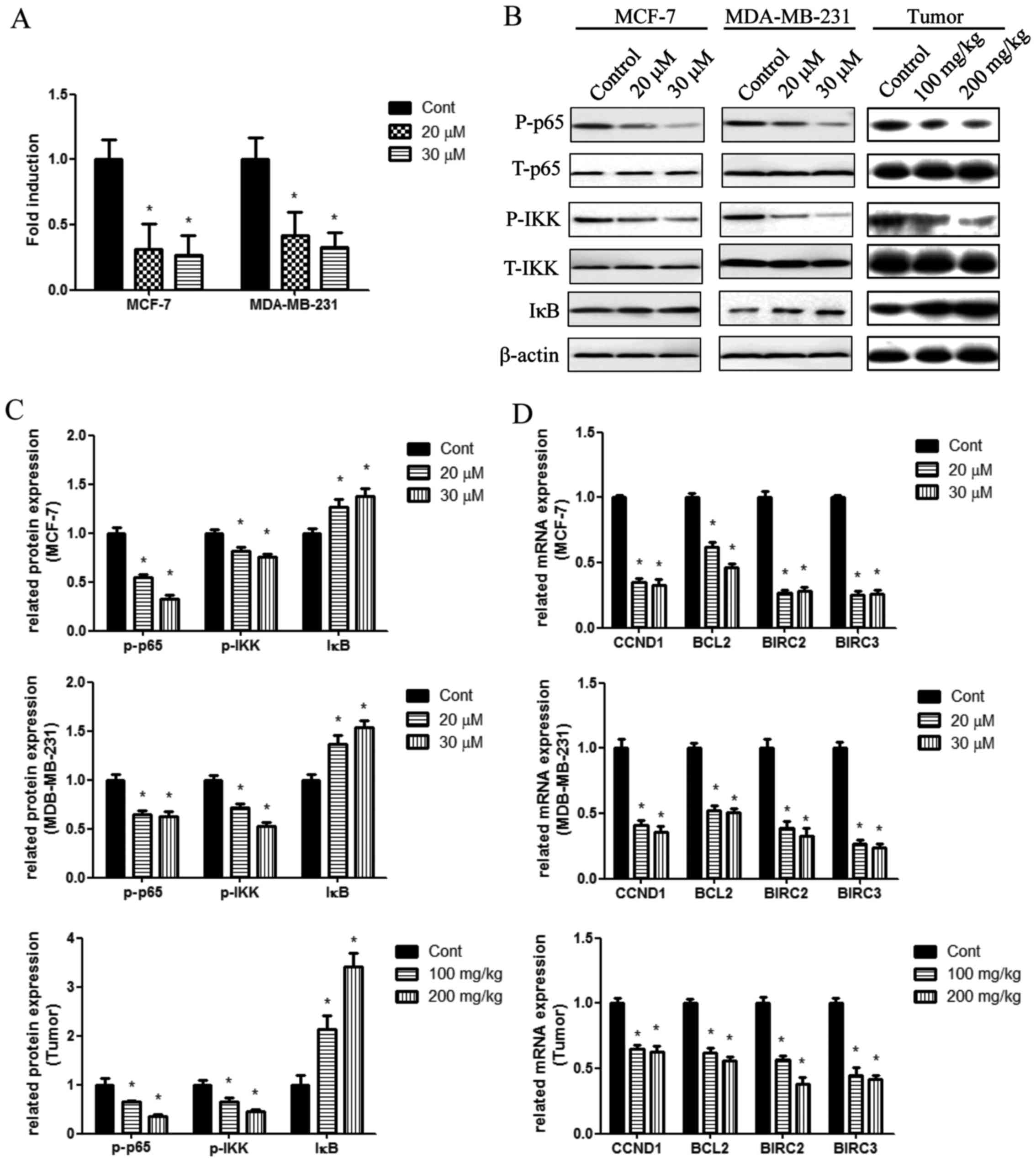
Baicalin suppresses the activation of
NF-ĸB
Based on the key role of NF-κB in the regulation of
tumor-associated inflammation and cancer progression, we next
investigated the association between baicalin and NF-κB. The cells
were manually counted in 6 wells per group, and the number of mice
per group was n=6. In a dual luciferase reporter assay, a 3-fold
lower luminescence intensity was observed in cells treated with 20
and 30 μM baicalin compared with the control group in
vitro, indicating that the activation of NF-κB was inhibited by
baicalin (Fig. 5A). Western blot
analysis further confirmed that NF-κB was inhibited by baicalin in
breast cancer cells in vivo and in vitro. The
phosphorylation levels of IκB kinase β (IKKβ) and NF-κB-p65 were
reduced, whereas the expression of IκBα, an essential NF-κB
inhibitor, was markedly upregulated in response to baicalin
treatment (Fig. 5B and C).
NF-κB also regulates oncological behavior. The
expression of cyclin D1 (CCND1), BCL2, BIRC2
(cIAP1) and BIRC3 (cIAP2) were examined by
RT-qPCR. The results demonstrated that baicalin reduced the
expression of these genes, indicating that baicalin may suppress
cell proliferation by inhibiting NF-ĸB-induced expression of
CCND1, and suppress cell invasion and migration by
inhibiting NF-ĸB-induced expression of BCL2, BIRC2
and BIRC3 (Fig. 5D).
Discussion
Breast cancer is one of the most commonly diagnosed
types of malignancy among women worldwide (1,2). The
current standard therapies for breast cancer are associated with
numerous side effects. Traditional Chinese herbal medicines have
been suggested as potential new therapeutic drugs with a more
tolerable toxicity profile (3,4).
NF-κB has been demonstrated to be a key factor enabling
precancerous and malignant cells to escape apoptosis (5-7).
Inhibition of NF-κB activity results in the partial release of
cells from the G2/M arrest after curcumin treatment of MCF-7 cells
(8,9). Baicalin is a chemical commonly used
to treat diseases of the CNS, hepatic disorders and inflammatory
conditions (10,11), and it inhibits
lipopolysaccharide-induced inflammation caused by endotoxic shock
(12,16). Baicalin also inhibits ROS
production in arteriosclerotic vascular disease by reducing the
activation of the NF-κB signaling pathway (17). Given its ability to inhibit the
activation of the NF-κB signaling pathway, it was hypothesized that
baicalin, a traditional Chinese herbal medicine with few known side
effects (18,19), may suppress breast cancer growth.
In the present study, the therapeutic efficacy of baicalin in
regulating the inflammatory reaction in breast cancer was
determined.
It was herein demonstrated that 20 and 30 μM
baicalin suppressed the invasion and migration of MCF-7 and
MDA-MB-231 breast cancer cells (Fig.
2A-D). Furthermore, the results indicated that the mechanism
through which baicalin suppresses breast cancer cell proliferation
involves induction of G1/S arrest (Fig. 1A-C). The results of in vivo
experiments demonstrated that 100 and 200 mg/kg baicalin suppressed
breast tumor growth in xenograft mice (Fig. 3A-D). Furthermore, baicalin mediated
growth arrest of breast cancer cell lines without affecting
non-tumorigenic normal breast epithelial cells (Fig. 1A-C). Treatment doses <20
μM did not induce significant effects in vitro,
whereas treatment doses <100 mg/kg also did not exert
significant effects in vivo (data not shown). Taken
together, these results indicate that baicalin is therapeutically
effective in breast cancer.
The development of breast cancer has been strongly
associated with inflammation (20). The conventional inflammatory
reaction is mediated by numerous cytokines, including TNF-α, IL-1β,
IL-6, TGF-β, IL-8 and IL-10, among others (20,21).
In the present study, it was demonstrated that baicalin
significantly decreased the secretion of TNF-α and IL-1β into
breast cancer cell medium in vitro (Fig. 4A and B). Furthermore, baicalin
significantly reduced the levels of TNF-α and IL-1β in the plasma
of xenograft mice (Fig. 4C).
Collectively, these data suggest that baicalin suppresses the
inflammatory response by reducing the secretion of key inflammatory
cytokines by MCF-7 and MDA-MB-231 breast cancer cells in
vitro, with similar effects in xenograft mice.
TNF-α and IL-1β are key pro-inflammatory cytokines
that exert their effects by regulating the NF-ĸB pathway through
interaction with specific receptors on cell membranes (22,23).
The NF-ĸB pathway is a key mediator of inflammatory response in
cancer (24). Several studies on
acute and chronic inflammation have demonstrated that the
anti-inflammatory activity of baicalin is associated with the
regulation of NF-ĸB. For example, in Staphylococcus aureus-induced
mastitis and cigarette smoke-induced inflammation, baicalin
effectively attenuated inflammation through inhibition of NF-ĸB
activation (25,26). In addition, NF-ĸB activation plays
a pathogenic role in cell proliferation and has an anti-apoptotic
effect in breast cancer (5-7). A
recent study also demonstrated that treatment with the NF-κB
inhibitor, BAY117082, suppressed breast cancer tumor growth in
xenograft mice (24). Consistent
with these reports, we herein observed that baicalin suppressed the
activation of NF-ĸB in vitro (Fig. 5A). Furthermore, the phosphorylation
levels of NF-ĸB-p65 were reduced by baicalin treatment in a
dose-dependent manner, not only in MCF-7 and MDA-MB-231 breast
cancer cells, but also in xenograft mice (Fig. 5B and C). Taken together, these data
suggest that the NF-ĸB pathway mediates the effect of baicalin
inhibition of breast cancer progression.
To the best of our knowledge, no previous studies
have investigated the regulatory mechanism baicalin on NF-ĸB to
date. In the present study, the effects of baicalin on NF-ĸB were
found to involve IĸB. In its inactive form, NF-ĸB is sequestered in
the cytoplasm and bound by members of the IĸB family of inhibitor
proteins. In tumors, the various stimuli that activate NF-ĸB cause
phosphorylation of IĸB, which is followed by its ubiquitination and
subsequent degradation (24,26).
IĸB proteins are phosphorylated by the IĸB kinase complex (IKK).
TNF-α and IL-1β can activate IKK and, thus, regulate the NF-ĸB
pathway (24,27). IKK-knockout mice have a
dysfunctional NF-ĸB pathway, leading to embryonic fatality
(28). The present study
demonstrated that the levels of phosphorylated IKKβ were lower in
the baicalin group, suggesting that baicalin suppresses the
activity of IKKβ in breast cancer cells and xenograft mice
(Fig. 5A-C). In addition,
activated IKK phosphorylates IĸBα (Ser32 and 36), and IĸBα is
ubiquitinated by Lys21 and Lys22, the ubiquitinated IĸBα is
degraded and, thus, activated NF-ĸB is released (29). In the present study, the protein
expression level of IĸBα was higher in the baicalin treatment
groups, suggesting that, through suppressing the activity of IKKβ,
baicalin inhibited the degradation of IĸBα, thus inhibiting the
activation of NF-ĸB in breast cancer cells and xenograft mice.
However, further investigation is required to elucidate the
underlying molecular mechanisms.
CCND1 is involved in regulation of the cell
cycle, and NF-ĸB promotes the expression of CCND1 and, thus,
cell proliferation (30,31). Therefore, baicalin treatment leads
to a reduction of CCND1 expression, which indicates that
baicalin likely inhibits cell proliferation via downregulation of
NF-ĸB-induced expression of CCND1. Inflammation-activated
NF-ĸB also induces expression of BCL2, BIRC2 and
BIRC3, the proteins encoded by which inhibit the activation
of various apoptosis-related enzymes, thereby reducing the
apoptosis of tumor cells (31,32).
The BCL2 family of proteins has anti-apoptotic members that are
expressed in several types of cancer, including lymphoma, lung,
prostate and breast cancer (33).
BIRC2 and BIRC3 are members of the inhibitor of
apoptosis protein family, which are expressed in several types of
cancer, and are associated with chemoresistance, disease
progression and poor prognosis (34). The present study demonstrated that
baicalin significantly inhibited the expression of these
apoptosis-inhibiting genes (Fig.
5D). NF-ĸB is crucial for cell invasion and migration in breast
cancer (35). Therefore, it may be
inferred that the ability of baicalin to inhibit invasion and
migration of MCF-7 and MDA-MB-231 breast cancer cells is likely to
be associated with inhibition of the activation of NF-ĸB. However,
the detailed mechanism of NF-ĸB-induced cell invasion and migration
remains unclear. Therefore, the detailed mechanism underlying
baicalin-induced inhibition of cell invasion and migration requires
further research.
In summary, the results of the present study clearly
demonstrated that baicalin, an important component isolated from
the dry root of S. baicalensis Georgi, has therapeutic
properties against breast cancer via inhibition of the inflammatory
response, through the suppression of inflammatory
cytokine-activated NF-ĸB-p65, and the expression of CCND1,
BCL2, BIRC2 and BIRC3. This process inhibits
breast cancer cell proliferation, invasion and migration, and
downregulates the expression of anti-apoptotic factors in
vitro and in vivo. Furthermore, baicalin did not affect
the non-tumorigenic normal breast epithelial cells.
Funding
The present study was supported by grants from the
National Natural Science Foundation of China (no. 31771601), the
Natural Science Foundation of Heilongjiang Province (no.
QC2014C016), the Innovation and Technology Special Fund for
Researchers of Harbin City (no. 2013RFXXJ010), and the China
Postdoctoral Science Foundation Special Financial Grant (no.
2013T60353).
Ethics approval
The present study was approved by the Ethics
Committee for Animal Experimentation of the School of Life Science
and Technology, Harbin Institute of Technology. All animal
experiments were performed according to the Guidelines for the Care
and Use of Experimental Animals and approved by the Heilongjiang
Province People’s Congress (http://www.nicpbp.org.cn/sydw/CL0249/2730.html).
Patient consent for publication
Not applicable.
Availability of data and materials
The datasets generated and analyzed in the present
study are all included in this published article.
Authors’ contributions
QW conceived the idea. YG, HL and HZW designed the
experiments. YG, HLH and HJH performed the in vitro
experiments. HZW, HJS and CJJ performed the in vivo
experiments. XH, DL and SC contributed to gene expression analysis.
NG and QG contributed to protein expression analysis. HL performed
statistical analysis. HJH and NG made figures. YG and HLH wrote the
manuscript. All authors have read and approved the final
manuscript.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
Not applicable.
References
|
1
|
Guo L, Liu S, Zhang S, Chen Q, Zhang M,
Quan P, Lu J and Sun X: C-reactive protein and risk of breast
cancer: A systematic review and meta-analysis. Sci Rep.
5:105082015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zhang HY, Liang F, Wang F, Zhang JW, Wang
L, Kang XG, Wang J and Duan QL: In vitro effects of HAS-2 gene
silencing on the proliferation and apoptosis of the MCF-7 human
breast cancer cell line. Cell Physiol Biochem. 40:807–817. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Vulcano E, Montesano M, Battista C, Carino
R, Perrone G, Vincenzi B and Altomare V: Urinary complications from
breast cancer metastasis: Case report and review of the literature.
G Chir. 31:243–245. 2010.PubMed/NCBI
|
|
4
|
Al-Sadoon MK, Abdel-Maksoud MA, Rabah DM
and Badr G: Induction of apoptosis and growth arrest in human
breast carcinoma cells by a snake (Walterinnesia aegyptia) venom
combined with silica nanoparticles: Crosstalk between Bcl2 and
caspase-3. Cell Physiol Biochem. 30:653–665. 2012. View Article : Google Scholar
|
|
5
|
Naugler WE and Karin M: NF-kappaB and
cancer-identifying targets and mechanisms. Curr Opin Genet Dev.
18:19–26. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Biswas DK, Martin KJ, McAlister C, Cruz
AP, Graner E, Dai SC and Pardee AB: Apoptosis caused by
chemotherapeutic inhibition of nuclear factor-kappaB activation.
Cancer Res. 63:290–295. 2003.PubMed/NCBI
|
|
7
|
Karin M: Nuclear factor-kappaB in cancer
development and progression. Nature. 441:431–436. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gutierrez CM, Lopez-Valdez R, Subramani R,
Arumugam A, Nandy S, Rajamanickam V, Ravichandran V and
Lakshmanaswamy R: A breast tissue protein expression profile
contributing to early parity-induced protection against breast
cancer. Cell Physiol Biochem. 37:1671–1685. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Berrak Ö, Akkoç Y, Arısan ED, Çoker-Gürkan
A, Obakan-Yerlikaya P and Palavan-Ünsal N: The inhibition of PI3K
and NFκB promoted curcumin-induced cell cycle arrest at G2/M via
altering polyamine metabolism in Bcl-2 overexpressing MCF-7 breast
cancer cells. Biomed Pharmacother. 77:150–160. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Xu XF, Cai BL, Guan SM, Li Y, Wu JZ, Wang
Y and Liu B: Baicalin induces human mucoepidermoid carcinoma Mc3
cells apoptosis in vitro and in vivo. Invest New Drugs. 29:637–645.
2011. View Article : Google Scholar
|
|
11
|
Hou J, Wang J, Zhang P, Li D, Zhang C,
Zhao H, Fu J, Wang B and Liu J: Baicalin attenuates proinflammatory
cytokine production in oxygen-glucose deprived challenged rat
microglial cells by inhibiting TLR4 signaling pathway. Int
Immunopharmacol. 14:749–757. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Liu LL, Gong LK, Wang H, Xiao Y, Wu XF,
Zhang YH, Xue X, Qi XM and Ren J: Baicalin inhibits macrophage
activation by lipopolysaccharide and protects mice from endotoxin
shock. Biochem Pharmacol. 75:914–922. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yin F, Liu J, Ji X, Wang Y, Zidichouski J
and Zhang J: Baicalin prevents the production of hydrogen peroxide
and oxidative stress induced by Aβ aggregation in SH-SY5Y cells.
Neurosci Lett. 492:76–79. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Taira Z, Yabe K, Hamaguchi Y, Hirayama K,
Kishimoto M, Ishida S and Ueda Y: Effects of Sho-saiko-to extract
and its components, Baicalin, baicalein, glycyrrhizin and
glycyrrhetic acid, on pharmacokinetic behavior of salicylamide in
carbon tetrachloride intoxicated rats. Food Chem Toxicol.
42:803–807. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Xu Y, Feng Y, Li H and Gao Z: Ferric
citrate CYP2E1-independently promotes alcohol-induced apoptosis in
HepG2 cells via oxidative/nitrative stress which is attenuated by
pretreatment with baicalin. Food Chem Toxicol. 50:3264–3272. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Cao Y, Mao X, Sun C, Zheng P, Gao J, Wang
X, Min D, Sun H, Xie N and Cai J: Baicalin attenuates global
cerebral ischemia/ reperfusion injury in gerbils via anti-oxidative
and anti-apoptotic pathways. Brain Res Bull. 85:396–402. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Dinda B, Dinda S, DasSharma S, Banik R,
Chakraborty A and Dinda M: Therapeutic potentials of baicalin and
its aglycone, baicalein against inflammatory disorders. Eur J Med
Chem. 131:68–80. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang HZ, Wang HH, Huang SS, Zhao H, Cao
YG, Wang GZ, Wang D, Wang ZG and Liu YH: Inhibitory effect of
baicalin on collagen-induced arthritis in rats through the nuclear
factor-κB pathway. J Pharmacol Exp Ther. 350:435–443. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yang X, Yang J and Zou H: Baicalin
inhibits IL-17-mediated joint inflammation in murine
adjuvant-induced arthritis. Clin Dev Immunol. 2013:2680652013.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Barham W, Chen L, Tikhomirov O, Onishko H,
Gleaves L, Stricker TP, Blackwell TS and Yull FE: Aberrant
activation of NF-κB signaling in mammary epithelium leads to
abnormal growth and ductal carcinoma in situ. BMC Cancer.
15:6472015. View Article : Google Scholar
|
|
21
|
Kim HJ, Hawke N and Baldwin AS: NF-kappaB
and IKK as therapeutic targets in cancer. Cell Death Differ.
13:738–747. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Bai HY, Mogi M, Nakaoka H, Kan-No H,
Tsukuda K, Wang XL, Shan BS, Kukida M, Yamauchi T, Higaki A, et al:
Synergistic inhibitory effect of rosuvastatin and angiotensin II
type 2 receptor agonist on vascular remodeling. J Pharmacol Exp
Ther. 358:352–358. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Gibson CJ, Hossain MM, Richardson JR and
Aleksunes LM: Inflammatory regulation of ATP binding cassette
efflux transporter expression and function in microglia. J
Pharmacol Exp Ther. 343:650–660. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Goswami S and Sharma-Walia N:
Osteoprotegerin rich tumor microenvironment: Implications in breast
cancer. Oncotarget. 7:42777–42791. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lixuan Z, Jingcheng D, Wenqin Y, Jianhua
H, Baojun L and Xiaotao F: Baicalin attenuates inflammation by
inhibiting NF-kappaB activation in cigarette smoke induced
inflammatory models. Pulm Pharmacol Ther. 23:411–419. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Guo M, Zhang N, Li D, Liang D, Liu Z, Li
F, Fu Y, Cao Y, Deng X and Yang Z: Baicalin plays an
anti-inflammatory role through reducing nuclear factor-κB and p38
phosphorylation in S aureus- induced mastitis. Int Immunopharmacol.
16:125–130. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Delhase M, Hayakawa M, Chen Y and Karin M:
Positive and negative regulation of IkappaB kinase activity through
IKKbeta subunit phosphorylation. Science. 284:309–313. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Shi C, Zhang N, Feng Y, Cao J, Chen X and
Liu B: Aspirin inhibits IKK-β-mediated prostate cancer cell
invasion by targeting matrix metalloproteinase-9 and urokinase-type
plasminogen activator. Cell Physiol Biochem. 41:1313–1324. 2017.
View Article : Google Scholar
|
|
29
|
Ingrassia R, Lanzillotta A, Sarnico I,
Benarese M, Blasi F, Borgese L, Bilo F, Depero L, Chiarugi A, Spano
PF, et al: 1B/ (-)IRE DMT1 expression during brain ischemia
contributes to cell death mediated by NF-κB/RelA acetylation at
Lys310. PLoS One. 7:e380192012. View Article : Google Scholar
|
|
30
|
Lee-Rivera I, López E, Parrales A,
Alvarez-Arce A and López-Colomé AM: Thrombin promotes the
expression of Ccnd1 gene in RPE cells through the activation of
converging signaling pathways. Exp Eye Res. 139:81–89. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wang J, Wang Q, Cui Y, Liu ZY, Zhao W,
Wang CL, Dong Y, Hou L, Hu G, Luo C, et al: Knockdown of cyclin D1
inhibits proliferation, induces apoptosis, and attenuates the
invasive capacity of human glioblastoma cells. J Neurooncol.
106:473–484. 2012. View Article : Google Scholar
|
|
32
|
Jin H, Dong YY, Zhang H, Cui Y, Xie K and
Lou G: shRNA depletion of cIAP1 sensitizes human ovarian cancer
cells to anticancer agent-induced apoptosis. Oncol Res. 22:167–176.
2014. View Article : Google Scholar
|
|
33
|
Badr G, Al-Sadoon MK and Rabah DM:
Therapeutic efficacy and molecular mechanisms of snake
(Walterinnesia aegyptia) venom-loaded silica nanoparticles in the
treatment of breast cancer- and prostate cancer-bearing
experimental mouse models. Free Radic Biol Med. 65:175–189. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Gyrd-Hansen M, Meier P and Ps IA: IAPs:
From caspase inhibitors to modulators of NF-kappaB, inflammation
and cancer. Nat Rev Cancer. 10:561–574. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Huber MA, Azoitei N, Baumann B, Grünert S,
Sommer A, Pehamberger H, Kraut N, Beug H and Wirth T: NF-kappaB is
essential for epithelial-mesenchymal transition and metastasis in a
model of breast cancer progression. J Clin Invest. 114:569–581.
2004. View Article : Google Scholar : PubMed/NCBI
|