Introduction
Positron emission tomography (PET) provides
functional information of regions of interest and is widely used in
oncology, for staging, prognosis and evaluating therapeutic
efficacy (1). PET can reveal a
therapeutic response before a change in tumor size is detected by
morphological imaging modes, such as computed tomography and
magnetic resonance imaging (2).
18F-labeled fluorodeoxyglucose ([18F]FDG) PET
is most commonly used to assess therapeutic efficacy in clinical
practice, despite having several limitations; e.g.,
[18F]FDG also accumulates in areas of inflammation
induced by anticancer therapy (3).
Several attempts have been made to develop a new PET probe to
compensate for the limitations of [18F]FDG (3). An amino acid-based PET probe is a
promising candidate as amino acid transporters are upregulated in
many types of cancer, but are limited in inflammatory cells
(3). 11C-labeled
methyl-l-methionine
([11C]Met) is used most often among amino acid-based PET
probes and accumulates less in areas of inflammation than
[18F]FDG (3).
Therefore, [11C]Met is useful for accurately evaluating
therapeutic efficacy. In clinical practice, the application of
[11C]Met is limited to brain tumor imaging, however, due
to its instability in vivo and its high accumulation in
several healthy organs (4).
Therefore, there is a strong need to develop novel amino acid-based
PET tracers that are stable in vivo.
Non-natural amino acids, 2-aminoisobutyric acid
(AIB) and its derivative 2-(methylamino)isobutyric acid (MeAIB),
are metabolically stable in vivo and their
14C-labeled analogs accumulate in tumors (5,6). AIB
and MeAIB are transported mainly by amino acid transport system A,
in contrast to [11C] Met, which is transported via
system L (7). Therefore, these
positron-labeled analogs are expected to play a different role than
[11C]Met in oncology imaging.
11C-labeled AIB was synthesized as
[1-11C]AIB (8), and its
usefulness has been demonstrated in patients with melanoma and soft
tissue sarcomas (5,9,10).
The clinical applications of [1-11C]AIB are quite
limited, however, as the labeling procedure for
[1-11C]AIB is complex and inefficient (11). Recently, a simple and efficient
synthetic method for [3-11C]AIB was established in our
previous study, in which C-11 is located at the 3-position instead
of the 1-position (12). Although
[3-11C]AIB has not yet been clinically evaluated,
several preclinical studies have demonstrated that
[3-11C]AIB highly accumulates in tumors, but much less
so in inflammatory lesions, and is useful for evaluating early
metabolic changes following radiation therapy in mouse models
(13-15). The low uptake of
[3-11C]AIB in the lung (13-15)
suggests the potential usefulness of [3-11C]AIB for
clinical application in lung cancer diagnostic imaging.
A synthetic method for [11C]MeAIB was
also established, and several clinical studies have reported that a
high uptake of [11C]MeAIB is observed in patients with
lung cancer, head and neck cancer and lymphoma (6,16,17,18).
[11C]MeAIB has higher specificity than
[18F]FDG for differentiating between benign and
malignant chest diseases (18).
Both AIB and MeAIB are transported into cells by the
amino acid transport system A and have similar affinities for
system A (19). In contrast to
MeAIB, which is a highly selective substrate of system A, AIB is
also partially transported by systems L and ASC (7). These findings suggest that the
additional contribution of systems L and ASC may increase the tumor
uptake of [3-11C]AIB compared with
[11C]MeAIB, but no direct comparisons of tumor uptake
between these two PET probes have been reported. In the present
study, the tumor uptake of [3-11C]AIB and
[11C]MeAIB in eight lung cancer models was compared, and
possible correlations of tumor uptake with several factors, such as
the expression of amino acid transporters associated with systems
A, L and ASC, and the contribution of the amino acid transport
systems to AIB uptake and tumor proliferation indices, were
explored.
Materials and methods
Cell culture
Six human lung cancer cell lines A549 (CCL-185), H82
(HTB-175), H441 (HTB-174), H460 (HTB-177), H1299 (CRL-5803) and
H1650 (CRL-5883) were obtained from ATCC (Manassas, VA, USA). SY
was obtained from Immuno-Biological Laboratories (Takasaki, Japan)
and PC14 (RCB0446) was obtained from RIKEN Cell Bank (Tsukuba,
Japan). The cells were maintained in RPMI-1640 medium (Wako Pure
Chemical Industries, Osaka, Japan) supplemented with 10% fetal
bovine serum (JRH Biosciences, Lenexa, KS, USA) in a humidified
incubator maintained at 37°C with 5% CO2.
Tumor models
The protocols used for the animal experiments were
approved by the Institutional Animal Care and Use Committee of the
National Institute of Radiological Sciences (Chiba, Japan), and all
animal experiments were conducted in accordance with the
institutional guidelines regarding animal care and handling. Mice
(BALB/c-nu/nu, 6 weeks old) were obtained from CLEA Japan (Tokyo,
Japan) and weighed 18-20 g. The animals were maintained in an
environment with a controlled temperature and humidity, under a 12
h-light/dark cycle. The animals were provided with food and water
ad libitum. The human lung cancer cells were subcutaneously
inoculated into both shoulders of male nude mice (12 mice/each
model) under isoflurane anesthesia (1.5%). We employed mice in
which subcutaneous tumors reached a diameter of approximately 9 mm,
and the maximum tumor diameter was 12 mm.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA from subcutaneous tumors was purified
using an RNeasy Plus Universal Mini kit (Qiagen, Hilden, Germany).
Human lung total RNA was purchased from Clontech (Mountain View,
CA, USA). First-strand cDNA was synthesized using a SuperScript
First-Strand Synthesis system for RT-PCR (Life Technologies,
Carlsbad, CA, USA). Real-time (quantitative) PCR (qPCR) was
conducted in triplicate with TaqMan probes to detect amino acid
transporters (SLC38A1, Hs01562168_m1; SLC38A2, Hs00255854_m1;
SLC38A4, Hs00215989_m1; SLC1A5, Hs01056542_m1 and SLC7A5,
Hs00185826_m1) and 18S rRNA (4319413E, Thermo Fisher Scientific,
Waltham, MA, USA) using Premix Ex Taq reagent (Takara Bio, Otsu,
Japan) and Mx3000P qPCR systems (Agilent Technologies, Santa Clara,
CA, USA). Gene expression levels of the amino acid transporters
were normalized to 18S rRNA expression in each sample.
PET imaging
[3-11C]AIB was synthesized by combining
iodo[11C] methane and methyl
N-(diphenylmethylene)-d,l-alaninate in the presence of
tetrabutylammonium fluoride, followed by alkaline hydrolysis and
acidic deprotection, as described previously (12). [11C]MeAIB was
synthesized by methylating AIB methyl ester hydrochloride using
[11C]methyl triflate in the presence of
1,2,2,6,6-pentamethyl-piperidine, followed by alkaline hydrolysis,
as previously described (16). The
10-min PET scans of tumor-bearing mice (n=1 per tumor model and per
tracer) began at 30 min after the intravenous injection of
approximately 12 MBq of [3-11C]AIB or
[11C]MeAIB in saline using a small-animal PET system
(Inveon, Siemens Medical Solutions, Malvern, PA, USA) under
isoflurane anesthesia (1.5%). Body temperature was maintained at
~37°C with a heat lamp and a heated bed during scans. Images were
reconstructed using a 3D maximum a posteriori (18 iterations
with 16 subsets, β=0.2) without attenuation correction.
Biodistribution
Approximately 12 MBq of [11C]AIB or
[11C]MeAIB in saline were administered to tumor-bearing
mice via the tail vein. At 30 min post-injection, the mice were
euthanized by isoflurane inhalation, and tumors, blood and major
organs were removed and weighed. The radioactivity was measured
with an auto-well gamma counter (Perkin-Elmer, Waltham, MA, USA).
Each mouse had two tumors (one in each shoulder), and two mice were
injected for each tumor model (two with each PET tracer); i.e., 4
data, tumor uptake of each tracer for each tumor model; and 16
data, organ uptake of each tracer, were collected. Data are
expressed as the percentage of injected dose per gram wet tissue
weight (% ID/g).
Contribution of amino acid transport
systems to AIB uptake in vitro
The cultured cells were rinsed three times with
Dulbecco’s phosphate-buffered saline (PBS) containing 5 mM
MgCl2 and 9 mM CaCl2 (PBS) or choline
phosphate buffer (137 mM choline-Cl, 2.7 mM KCl, 8.1 mM
K2HPO4, 1.47 mM KH2PO4, 5 mM
MgCl2 and 9 mM CaCl2; pH 7.4). As competitive
inhibitors, 10 mM 2-amino-2-norbornanecarboxylic acid (BCH) and 10
mM MeAIB were used. The cells washed with PBS or choline phosphate
buffer were incubated with 92.5 kBq of [14C]AIB
(American Radiolabeled Chemicals, St. Louis, MO, USA) in 500
μl PBS or choline phosphate buffer and incubated for 15 min
at 37°C. Following incubation, the cells were washed three times
with ice-cold PBS or ice-cold choline phosphate buffer and lysed in
50 mM NaOH aqueous solution. The radioactivity was measured with a
liquid scintillation counter (Perkin-Elmer). The total protein
concentration in the cell lysate was determined using the Bradford
protein assay (Bio-Rad, Hercules, CA, USA). Each experiment was
conducted in triplicate. The contribution of amino acid transport
systems A, L and ASC to [14C]AIB uptake was calculated
as previously described (20).
Briefly, the total cell uptake of [14C]AIB was divided
into the contribution of each system as follows: System A, the
portion of the sodium-dependent uptake that was inhibited by MeAIB;
system ASC, the portion of the sodium-dependent uptake that was not
inhibited by MeAIB; system L, sodium-independent uptake that was
inhibited by BCH; and the non-saturable fraction, the remaining
uptake. Data are expressed as a percentage of the total uptake and
three independent experiments were conducted.
Ki-67 immunohistochemical staining
As a separate experiment, mice were subcutaneously
injected with human lung cancer cells (3 tumors/each tumor model).
Subcutaneous tumors were resected from mice and fixed in 10%
neutral-buffered formalin and embedded in paraffin. The tumor
sections (1-μm-thick) were deparaffinized and stained with
mouse anti-Ki-67 antibody (MIB-1, Dako Cytomation, Glostrup,
Denmark) at a dilution of 1:100, as previously described (21). The Ki-67 index (%) was calculated
by counting the percentage of Ki-67-positive cell nuclei per
2,500-6,000 cells in 5 regions with a high density of stained
cells.
Tumor doubling time
Subcutaneous tumor size (n=8/tumor model) was
measured twice a week using a caliper. Tumor volume was calculated
according to the following formula: Tumor volume (mm3) =
(length x width2)/2. Tumor volume doubling time during
the logarithmic growth phase was calculated according to the
following formula: Doubling time (days) =
tln2/ln(V1/V0), where t is the
time interval between the initial and second measurement,
V0 is the tumor volume at the initial measurement and
V1 is the tumor volume at the second measurement
(22).
Statistical analysis
The uptake of [3-11C]AIB and
[11C] MeAIB was analyzed by a Student’s t-test. The mRNA
expression levels in normal lung and tumors were analyzed by ANOVA
with Dunnett’s multiple comparison test. The correlation among
tumor uptake and potential factors was examined using Pearson’s
correlation coefficient (r). A value of P<0.05 was
considered to indicate a statistically significant difference.
Results
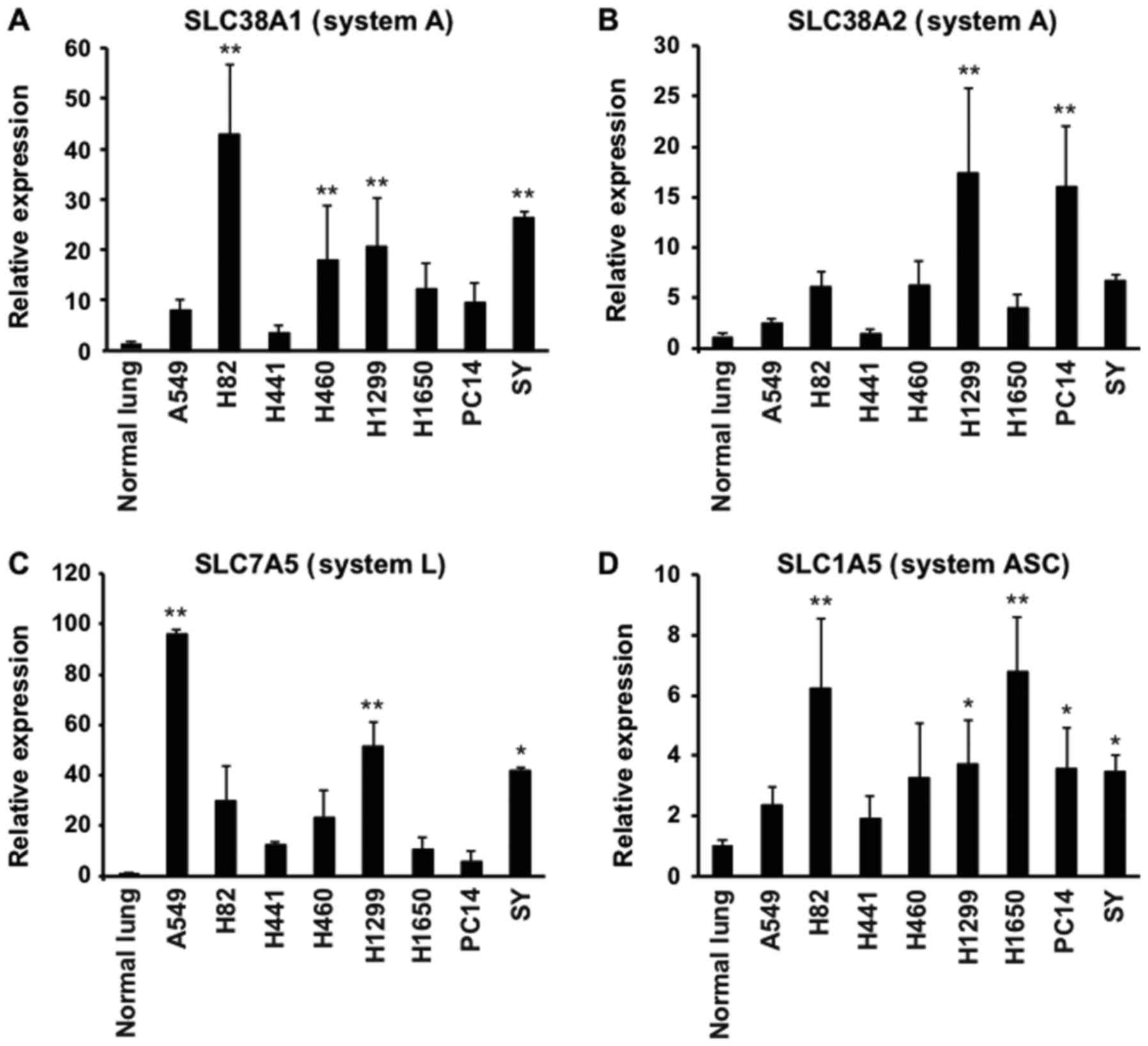
mRNA expression of amino acid
transporters in xenograft tumors
The mRNA expression levels of amino acid
transporters of system A (SLC38A1, SLC38A2 and SLC38A4), system L
(SLC7A5) and system ASC (SLC1A5) in xenograft tumors were
determined by RT-qPCR. The SLC38A1, SLC38A2, SLC7A5 and SLC1A5 mRNA
levels were detected and upregulated in all eight types of tumors
(Fig. 1A-D), whereas SLC38A4 mRNA
was not detected in normal lung or in any of the tumors (data not
shown). The SLC38A1 and SLC38A2 expression levels in the tumors
were 3.6- to 42.8-fold and 1.4- to 17.3-fold higher than those in
the normal lung, respectively (Fig. 1A
and B). The expression of SLC38A1 in the H82, H460, H1299 and
SY tumors, and that of SLC38A2 in the H1299 and PC14 tumors was
significantly higher than that in the normal lung (P<0.01,
Fig. 1A and B). The expression
levels of SLC7A5 were 5.8- to 95.9-fold higher in the tumors than
in the normal lung, with significant differences between A549,
H1299 or SY and the normal lung (P<0.01 in the A549 and H1299
tumors, and P<0.05 in the SY tumors, Fig. 1C). The expression levels of SLC1A5
were 1.9- to 6.8-fold higher than those in the normal lung, with
significant differences obtained between the H82, H1299, H1650,
PC14 or SY tumors and the normal lung (P<0.01 in H82 and H1650
tumors; P<0.05 in H1299, PC14 and SY tumors; Fig. 1D). The eight xenograft tumors
exhibited various expression patterns of the amino acid
transporters involved in AIB and MeAIB uptake.
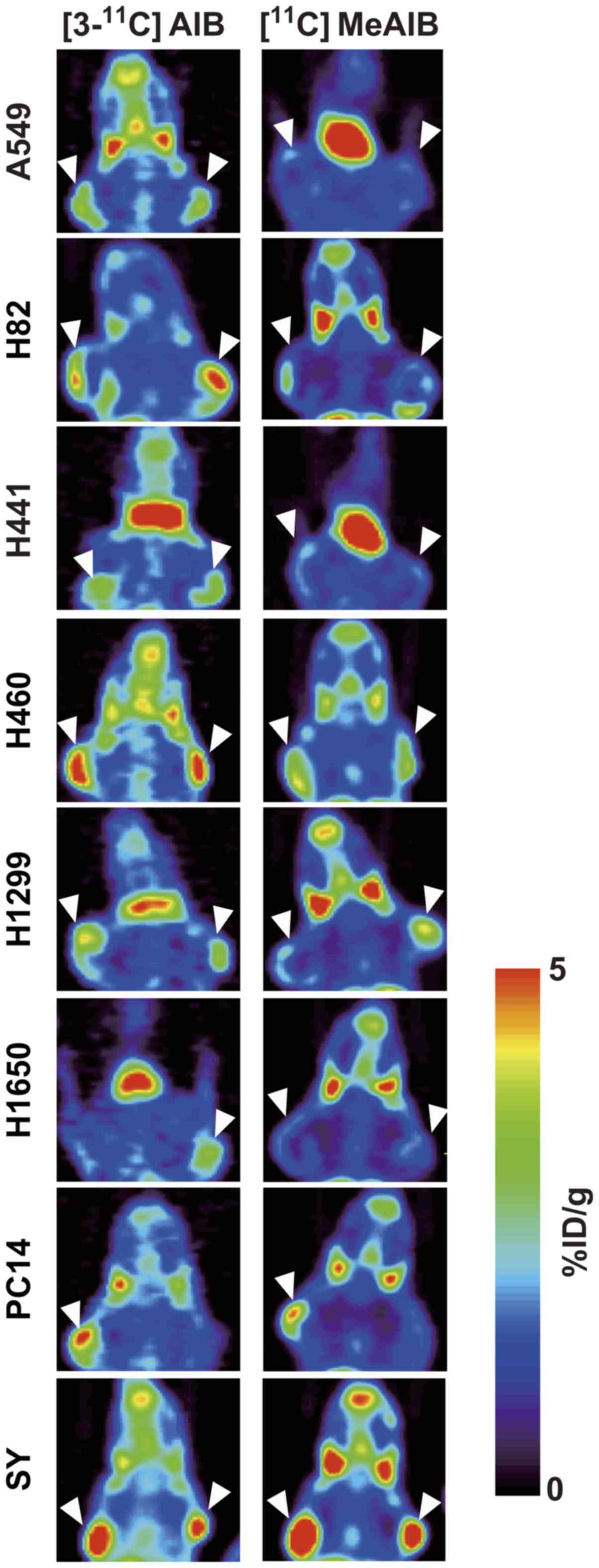
PET imaging with [3-11C]AIB
and [11C]MeAIB in tumor-bearing mice
Coronal images revealed a higher uptake of
[3-11C]AIB than of [11C]MeAIB in the eight
tumors (Fig. 2). Both tracers
accumulated at low levels in the lungs and at high levels in the
salivary glands, consistent with previous reports (6,13,23).
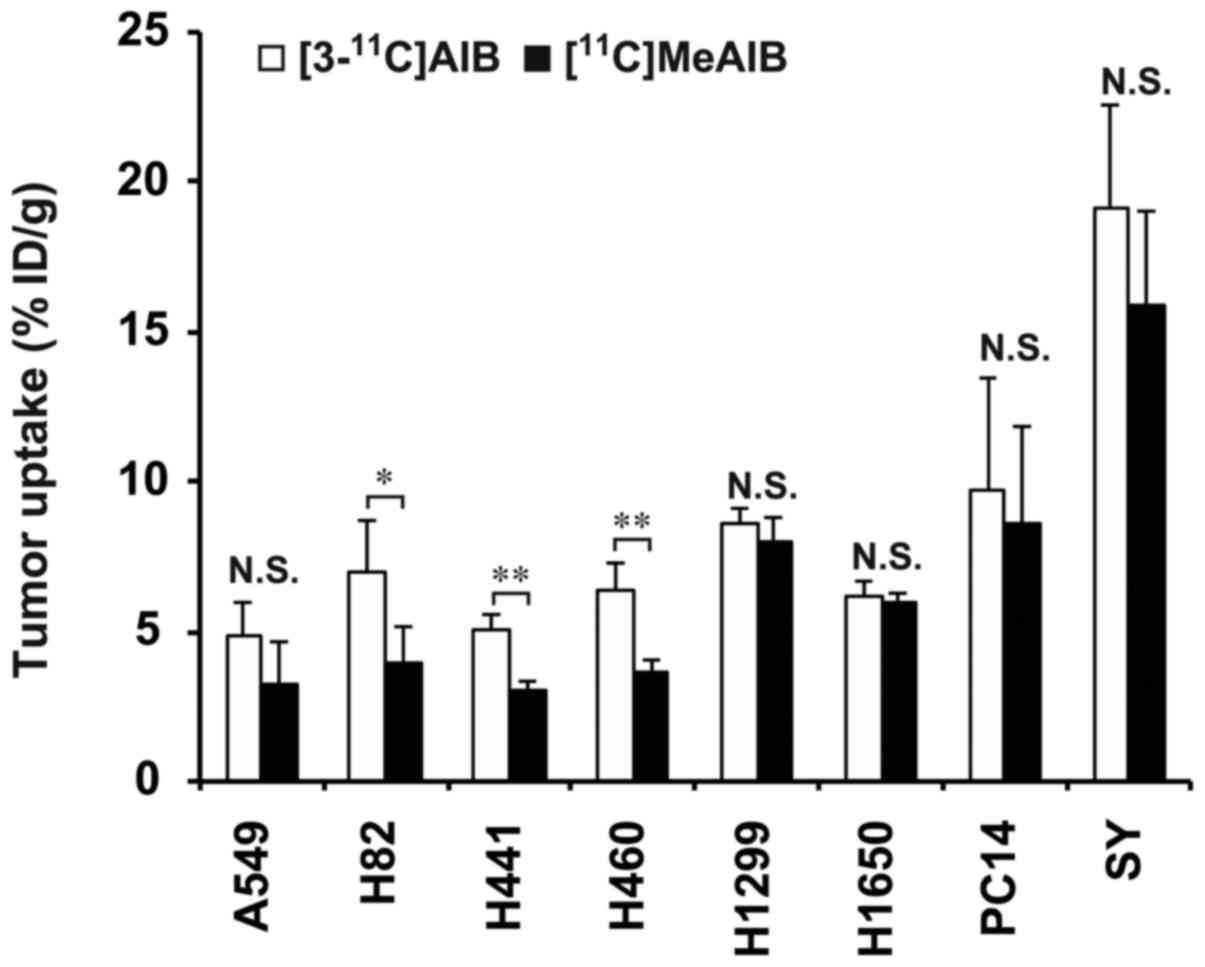
Biodistribution of [3-11C]AIB
and [11C]MeAIB in tumor-bearing mice
The mean tumor uptake of [3-11C]AIB at 30
min after injection ranged from 4.9 to 19.2% ID/g, and that of
[11C] MeAIB ranged from 3.1 to 15.9% ID/g in the eight
lung cancer mouse models (n=4/tracer, Fig. 3). The tumor uptake of
[3-11C] AIB in the H82, H441 and H460 tumors was
significantly higher than that of [11C]MeAIB (P<0.05
in the H82 tumors, and P<0.01 in the H441 and H460 tumors,
Fig. 3). Although the difference
was not statistically significant, [3-11C]AIB uptake in
the other tumors was higher than that of [11C]MeAIB in
the corresponding tumors (Fig. 3).
[3-11C]AIB uptake in the blood, lung, pancreas, kidney
and muscle was significantly higher than that of
[11C]MeAIB (n=16/tracer, Table I).
 | Table INormal organ uptake (% ID/g) of
[3-11C]AIB and [11C]MeAIB at 30 min after
intravenous injection in tumor-bearing mice. |
Table I
Normal organ uptake (% ID/g) of
[3-11C]AIB and [11C]MeAIB at 30 min after
intravenous injection in tumor-bearing mice.
| Organ |
[3-11C]AIB (n=16) |
[11C]MeAIB (n=16) |
|---|
| Blood | 2.27±0.32 | 1.48±0.18a |
| Lung | 4.38±0.39 | 3.19±0.52a |
| Liver | 12.76±2.42 | 11.97±2.05 |
| Spleen | 11.23±1.18 | 12.05±2.50 |
| Pancreas | 62.87±7.10 | 43.85±6.29a |
| Intestine | 7.77±1.61 | 6.74±1.64 |
| Kidney | 19.62±4.48 | 14.88±3.07a |
| Muscle | 1.26±0.20 | 1.01±0.28a |
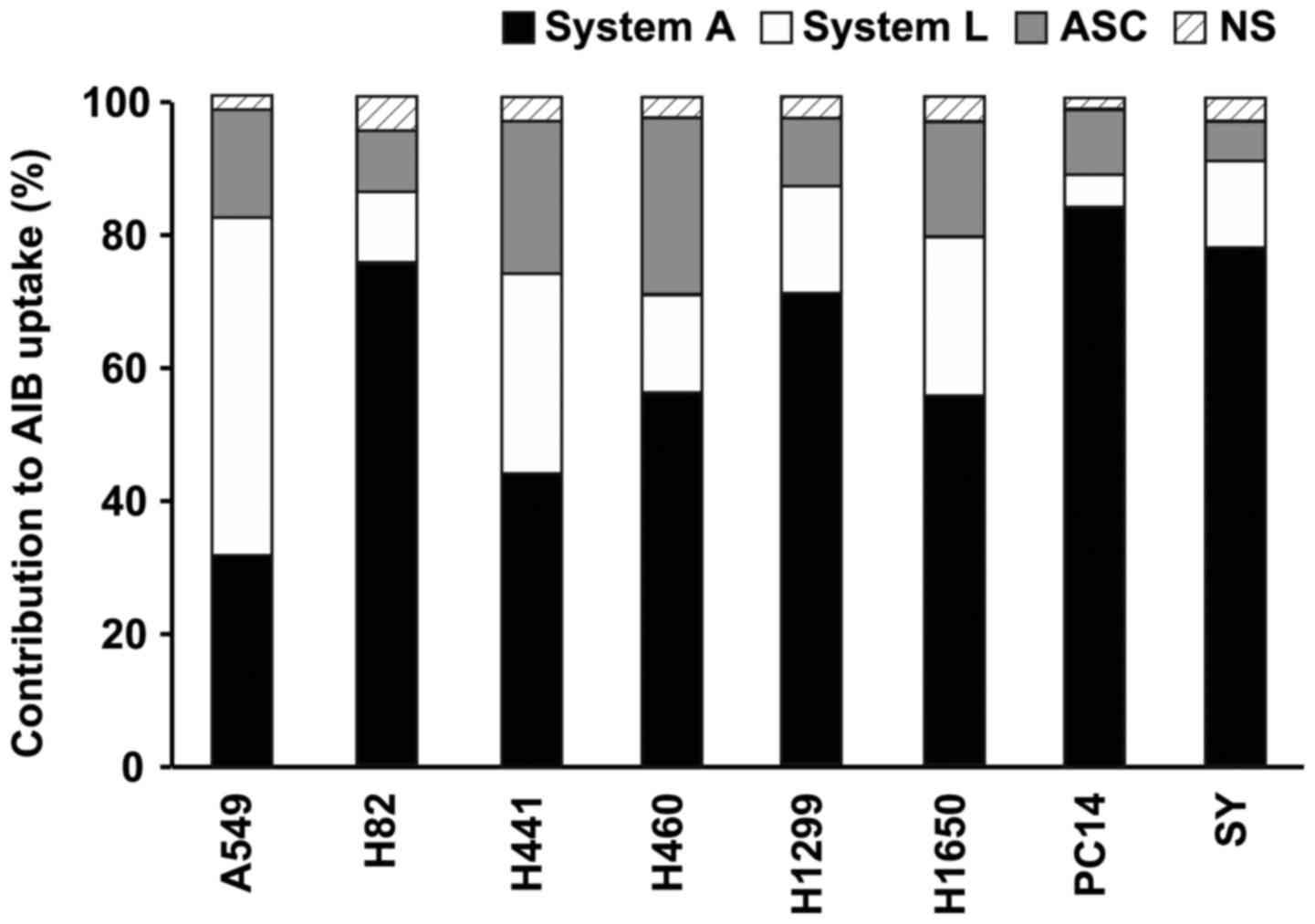
Contribution of amino acid transport
systems to AIB cell uptake
As the transport of AIB into cells has been reported
to occur via amino acid transport systems A, L and ASC (7), the contribution of each system to
uptake in the eight different types of lung cancer cells was
evaluated. The contributions ranged from 31 to 84% for system A,
from 5 to 50% for system L and from 9 to 27% for system ASC
(Fig. 4). In the seven cell lines
other than A549, the contribution of system A was higher than that
of system L, whereas in the A549 cells, the contribution of system
L was 1.6-fold higher than that of system A (Fig. 4).
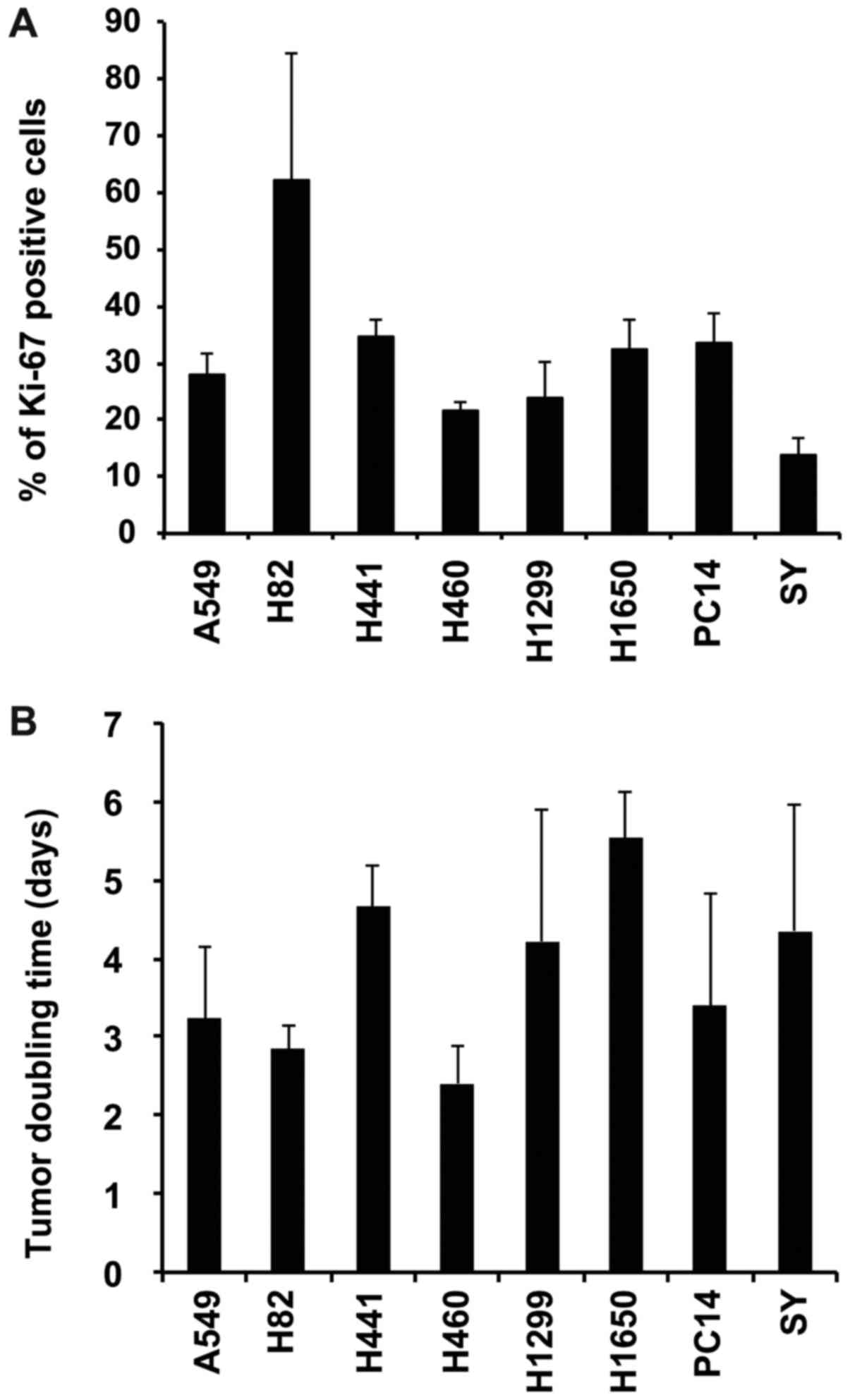
Tumor proliferation
To evaluate the association between tumor
proliferation and the tumor uptake of each tracer, the Ki-67 index
based on tumor sections immunostained with the anti-Ki-67 antibody
and tumor doubling time by measuring tumor size in the eight tumor
models were determined. The Ki-67 index was 14.0 to 62.1% (Fig. 5A), and the doubling time during the
logarithmic growth phase was 2.4 to 5.5 days (Fig. 5B).
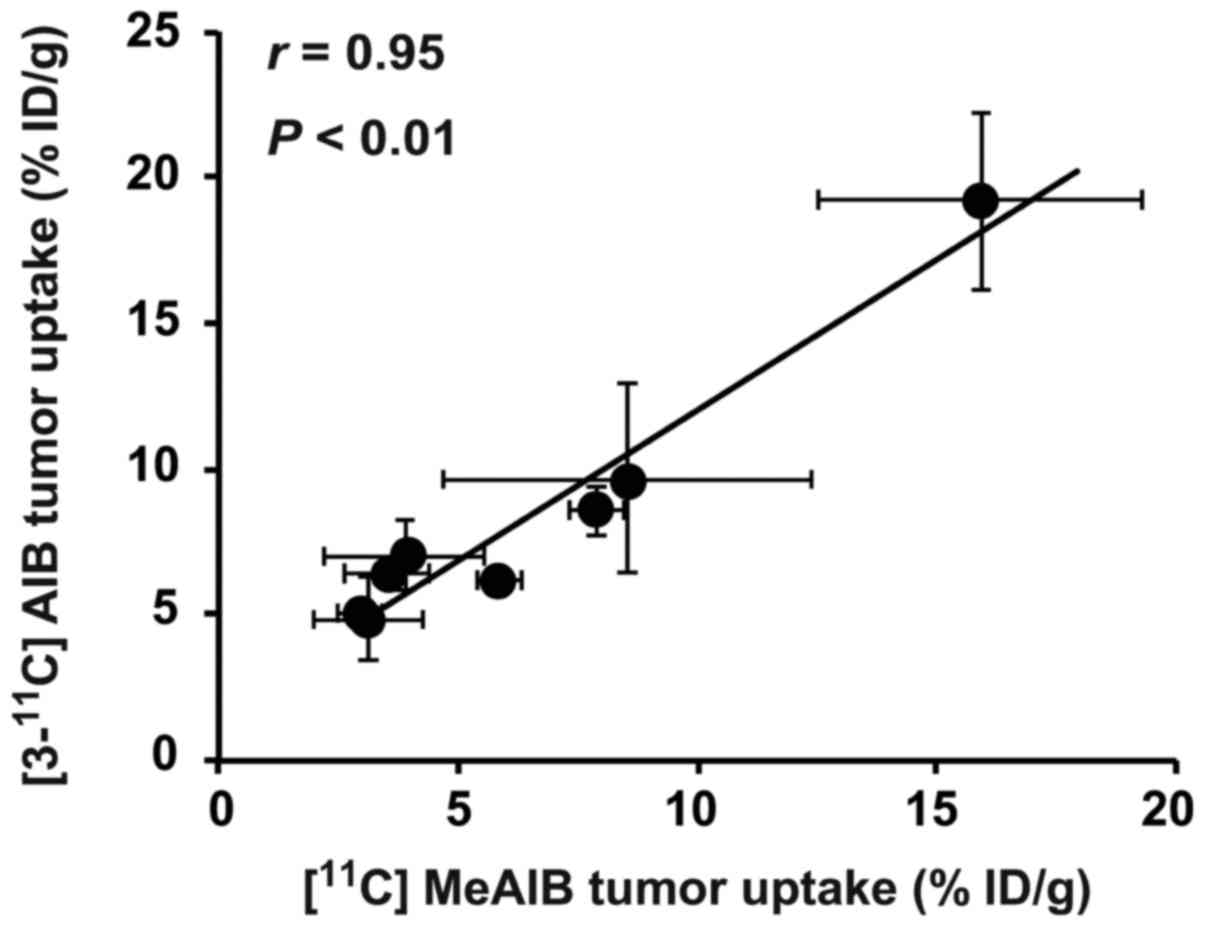
Correlation analysis
The tumor uptake of [3-11C]AIB
significantly and positively correlated with that of
[11C]MeAIB in the eight tumor models (r=0.95,
P<0.01, Fig. 6 and Table II). The tumor uptakes of
[3-11C]AIB and [11C]MeAIB were quantified in
PET images, and there were significant correlations of tumor uptake
in the PET analyses with that in the biodistribution analyses
(r= 0.69, P<0.05 for [3-11C]AIB; r=
0.81, P<0.05 for [11C]MeAIB). The expression of the
individual amino acid transporter mRNAs did not correlate with the
uptake of [3-11C] AIB or that of [11C]MeAIB
(Table II). The contribution of
system A to uptake significantly and positively correlated with the
expression of SLC38A2 mRNA (r=0.71, P<0.05, Table II), whereas there no significant
correlation was observed between the contributions of the other
systems to uptake and the mRNA expression of the other amino acid
transporters, or between the contributions of the other systems and
tumor uptake (Table II). The
proliferation indices (Ki-67 index and the tumor doubling time) did
not correlate with tumor uptake (Table II).
 | Table IIPearson’s correlation coefficients
(r) among tumor uptake ([3-11C]AIB and
[11C]MeAIB) and potential factors. |
Table II
Pearson’s correlation coefficients
(r) among tumor uptake ([3-11C]AIB and
[11C]MeAIB) and potential factors.
| Tumor uptake
| Amino acid
transporter expression
| Contribution of
amino acid transport system
| Proliferation
indices
|
|---|
| [3-11C]
AIB | [11C]
MeAIB | SLC 38A1 | SLC 38A2 | SLC 7A5 | SLC 1A5 | System A | System L | System ASC | Ki-67 index | Tumor doubling
time |
|---|
|
[3-11C]AIB | 1.00 |
0.95a | 0.35 | 0.28 | -0.01 | -0.02 | 0.63 | -0.46 | -0.69 | -0.45 | 0.14 |
|
[11C]MeAIB | | 1.00 | 0.21 | 0.36 | -0.04 | 0.03 | 0.65 | -0.44 | -0.69 | -0.50 | 0.29 |
| SLC38A1 | | | 1.00 | NA | NA | NA | 0.55 | NA | NA | 0.44 | -0.34 |
| SLC38A2 | | | | 1.00 | NA | NA |
0.71b | NA | NA | -0.13 - | 0.15 |
| SLC7A5 | | | | | 1.00 | NA | NA | 0.67 | NA | -0.22 | -0.24 |
| SLC1A5 | | | | | | 1.00 | NA | NA | -0.25 | 0.51 | 0.21 |
| System A | | | | | | | 1.00 | NA | NA | NA | NA |
| System L | | | | | | | | 1.00 | NA | NA | NA |
| System ASC | | | | | | | | | 1.00 | NA | NA |
| Ki-67 index | | | | | | | | | | 1.00 | 0.22 |
| Tumor doubling
time | | | | | | | | | | | 1.00 |
Discussion
Non-natural amino acid PET tracers
[3-11C]AIB and [11C] MeAIB are expected to
play a new role in oncology imaging that differs from that of
[11C]Met, which is currently the most widely used amino
acid PET tracer in clinical practice (5-7,13).
MeAIB is transported by amino acid transport system A, whereas AIB
is transported by systems L and ASC in addition to system A. How
the difference in transport affects tumor uptake of these tracers
is unclear, however, as there have been no direct comparisons of
the uptake of the two PET probes. In the present study, tumor
uptake of the two tracers was directly compared in eight lung
cancer models, and possible correlations of the tumor uptake with
several factors were analyzed.
First, the mRNA expression levels of amino acid
transporters associated with uptake of AIB and MeAIB in xenograft
tumors derived from eight lung cancer cell lines (A549, H82, H441,
H460, H1299, H1650, PC14, and SY) were determined. The mRNAs
involved in system A (SLC38A1 and SLC38A2), system L (SLC7A5) and
system ASC (SLC1A5) were overexpressed in all the tumors compared
with normal lung, with the amplitude of overexpression varying
among the eight tumors, indicating that these tumor models are
suitable for evaluating differences in tumor uptake of
[3-11C]AIB and [11C]MeAIB.
Next, PET and biodistribution analyses with
[3-11C]AIB and [11C]MeAIB were conducted in
the eight tumor models. PET images revealed a high accumulation of
[3-11C]AIB in all eight tumors. Moreover, the tumor
uptake of [3-11C]AIB was higher than that of
[11C]MeAIB. The biodistribution experiments of
[3-11C]AIB and [11C]MeAIB confirmed these
findings, i.e., the tumor uptake of [3-11C]AIB was
higher than that of [11C]MeAIB, and the differences in
uptake were statistically significant in three tumors (P<0.01 in
H441 and H460 tumors, and P<0.05 in H82 tumors). Several
clinical studies of [11C]MeAIB PET have reported high
tumor uptake in patients (6,17)
and high specificity in the differential diagnosis between benign
and malignant disease (18). In
the present study, tumor uptake between the two tracers was
significantly correlated (r= 0.94, P<0.01). Taken
together, [3-11C]AIB PET is expected to visualize tumors
in patients more efficiently than [11C]MeAIB PET and to
be useful for imaging malignancies.
We hypothesized that additional contributions of
systems L and ASC to the uptake of AIB may increase AIB uptake in
tumors expressing systems A, L and ASC. Therefore, several
correlation analyses were conducted to find factor(s) associated
with the observed difference in tumor uptake between
[3-11C]AIB and [11C]MeAIB. There is no
correlation between tumor uptake and the expression of amino acid
transporters. Kagawa et al reported that MeAIB cell uptake
correlated with the expression of amino acid transport system A
(24). This discrepancy could be
due to the different cell sets used [they used A431, LS180, H441
and PC14 (24)] and the difference
between the in vitro cellular uptake (24) and in vivo tumor uptake (the
present study). Next, a correlation analysis was performed between
tumor uptake and the contribution of each amino acid transport
system (A, L, and ASC) to AIB uptake. As mentioned above, AIB is
transported via systems L and ASC in addition to system A, and the
contributions to uptake by these systems in the eight tumor cell
lines were determined and compared with the in vivo tumor
uptake. There was no correlation between the contribution of each
system and tumor uptake. Of note, the contribution of each
transport system substantially varied among cell lines, and the
contribution of system A was not large in several tumors,
particularly in A549 tumors, in which the contribution of system A
was only 31.4% and that of system L was 50.0%. To our knowledge,
although there are many reports that AIB is transported mainly via
system A (20,25,26),
there is no report that some tumor cells transport it mainly via
the other amino acid transport system(s). On the other hand, tumor
uptake was significantly correlated between [3-11C]AIB
and [11C]MeAIB, as shown in the present biodistribution
study. These findings suggest that the injected amount of
[3-11C]AIB for the in vivo study (PET and
biodistribution) was very small and the affinity of AIB to system A
is higher than that to the other systems; therefore, AIB would be
transported into tumors in vivo mainly via system A. In
other words, the difference in the contributions of the transport
systems would produce no prominent differences in tumor uptake
in vivo. Finally, a correlation analysis of tumor uptake and
proliferation indices was conducted as some studies have reported
that upregulation of amino acid transport system A is associated
with prognosis in several tumors (27,28)
and expression of the system A transporter SLC38A1 correlates with
the cell proliferation index Ki-67 in breast cancer (28). Although the Ki-67 index and tumor
doubling time in the eight xenograft tumors were determined in the
eight models, these proliferation indices were not correlated with
the tumor uptake of the two PET tracers. Unfortunately, we cannot
provide direct evidence to address the difference of AIB and MeAIB
tumor uptake in the present study. There are several potential
reasons: i) Blood flow and/or blood volume (29,30);
ii) variations in amino acid metabolism (31-33);
iii) alterations in transporter activity (31-33);
and iv) differences in the expression levels between mRNA and
protein (34). In general,
xenograft tumor models cannot completely reproduce the
characteristics of human cancer, and system A activity is reported
to change with the levels of intracellular amino acid pools, cell
density, cell division and hormonal stimulation (31-33).
These potential explanations could account for the lack of
correlation detected, but clinical PET studies are needed to
clarify these points.
Although the present study showed the potential of
PET with [3-11C]AIB for lung cancer diagnosis, there are
several limitations as follows: First, the PET analyses were
conducted only a mouse per tumor model. However, there are
significant correlations of tumor uptake in the PET analyses with
that in the biodistribution studies (n=4/tumor model), supporting
the results of PET analyses. To provide additional evidence of our
results, there is a need for further PET analyses with a large
number. Second, the present study employed only male mice since
lung cancer is more common in men compared with women (35). However, lung cancer develops also
in women (35), and further
studies with female animals are required before clinical
studies.
In conclusion, the direct comparison of the tumor
uptake of [3-11C]AIB and [11C]MeAIB in eight
lung cancer xenograft models revealed greater accumulation of
[3-11C]AIB than [11C] MeAIB in all eight
tumors. Although the amino acid transporter expression levels, the
contribution of transport systems to AIB uptake, and proliferation
indices showed substantial variations among the eight models, tumor
uptake was not correlated with those factors. The higher tumor
uptake of [3-11C]AIB and the correlation of tumor uptake
between [3-11C]AIB and [11C]MeAIB warrant
further investigation in clinical studies to clarify a role of
[3-11C]AIB PET in oncology imaging.
Funding
This study was supported by KAKENHI (15K09976 and
16K10304), AMED-CREST, and the National Institutes for Quantum and
Radiological Science and Technology.
Availability of data and materials
All data generated or analyzed during this study
are included in this published article.
Authors’ contributions
HS was involved in the data design, data
collection, data analysis and interpretation, and in the writing of
the manuscript. ABT was involved in research design, data design,
data collection, data analysis and interpretation, and in the
writing of the manuscript. AS was involved in data collection. MO
was involved in data interpretation and PET tracer production. KK
was involved in data interpretation and in the writing of the
manuscript. MRZ was involved in data interpretation. TS was
involved in data interpretation and in the writing of the
manuscript. TH was involved in data interpretation and in the
writing of the manuscript. All authors have read and approved the
final manuscript.
Ethics approval and consent to
participate
The protocols used for the animal experiments were
approved by the Institutional Animal Care and Use Committee of the
National Institute of Radiological Sciences (Chiba, Japan), and all
animal experiments were conducted in accordance with the
institutional guidelines regarding animal care and handling.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
The authors would like to thank the staff of the
Cyclotron Operation section for producing the radioisotope; Mr.
Yusuke Kurihara, Mr. Masanao Ogawa and Mr. Nobuki Nengaki for the
technical support for the PET tracer synthesis; Mr. Hidekatsu
Wakizaka for operation and quality control of the animal PET
system; and Ms. Yuriko Ogawa and Ms. Naoko Kuroda for technical
assistance with the animal experiments.
References
|
1
|
Juweid ME and Cheson BD: Positron-emission
tomography and assessment of cancer therapy. N Engl J Med.
354:496–507. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bading JR and Shields AF: Imaging of cell
proliferation: Status and prospects. J Nucl Med. 49(Suppl 2):
S64–S80. 2008. View Article : Google Scholar
|
|
3
|
Jager PL, Vaalburg W, Pruim J, de Vries
EG, Langen KJ and Piers DA: Radiolabeled amino acids: Basic aspects
and clinical applications in oncology. J Nucl Med. 42:432–445.
2001.PubMed/NCBI
|
|
4
|
Leskinen-Kallio S, Någren K, Lehikoinen P,
Ruotsalainen U, Teräs M and Joensuu H: Carbon-11-methionine and PET
is an effective method to image head and neck cancer. J Nucl Med.
33:691–695. 1992.PubMed/NCBI
|
|
5
|
Sordillo PP, DiResta GR, Fissekis J, Conti
P, Benua RS, Yeh SD and Laughlin JS: Tumor imaging with carbon-11
labeled alpha-aminoisobutyric acid (AIB) in patients with malignant
melanoma. Am J Physiol Imaging. 6:172–175. 1991.PubMed/NCBI
|
|
6
|
Sutinen E, Jyrkkiö S, Grönroos T,
Haaparanta M, Lehikoinen P and Någren K: Biodistribution of
[11C] methylaminoisobutyric acid, a tracer for PET
studies on system A amino acid transport in vivo. Eur J Nucl Med.
28:847–854. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Christensen HN: Role of amino acid
transport and counter-transport in nutrition and metabolism.
Physiol Rev. 70:43–77. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Schmall B, Conti PS, Bigler RE, Zanzonico
PB, Dahl JR, Sundoro-Wu BM, Jacobsen JK and Lee R: Synthesis and
quality assurance of [11C]alpha-aminoisobutyric acid
(AIB), a potential radiotracer for imaging and amino acid transport
studies in normal and malignant tissues. Int J Nucl Med Biol.
11:209–214. 1984. View Article : Google Scholar
|
|
9
|
Conti PS, Sordillo PP, Schmall B, Benua
RS, Bading JR, Bigler RE and Laughlin JS: Tumor imaging with
carbon-11 labeled alpha-aminoisobutyric acid (AIB) in a patient
with advanced malignant melanoma. Eur J Nucl Med. 12:353–356. 1986.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Schmall B, Conti PS, Bigler RE, Zanzonico
PB, Reiman RE, Benua RS, Yeh SD, Dahl JR, Lee R and Laughlin JS:
Imaging studies of patients with malignant fibrous histiocytoma
using C-11-alpha-aminoisobutyric acid (AIB). Clin Nucl Med.
12:22–26. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Schmall B, Conti PS and Alauddin MM:
Synthesis of [11C-methyl]-alpha-aminoisobutyric acid
(AIB). Nucl Med Biol. 23:263–266. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kato K, Tsuji AB, Saga T and Zhang M-R: An
efficient and expedient method for the synthesis of
11C-labeled α-aminoisobutyric acid: A tumor imaging
agent potentially useful for cancer diagnosis. Bioorg Med Chem
Lett. 21:2437–2440. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Tsuji AB, Kato K, Sugyo A, Okada M, Sudo
H, Yoshida C, Wakizaka H, Zhang MR and Saga T: Comparison of
2-amino-[3-¹¹C]isobutyric acid and 2-deoxy-2-[18F]
fluoro-D-glucose in nude mice with xenografted tumors and acute
inflammation. Nucl Med Commun. 33:1058–1064. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tsuji AB, Sugyo A, Sudo H, Suzuki C,
Wakizaka H, Zhang MR, Kato K and Saga T: Preclinical assessment of
early tumor response after irradiation by positron emission
tomography with 2-amino-[3-¹¹C]isobutyric acid. Oncol Rep.
33:2361–2367. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Okada M, Kikuchi T, Okamura T, Ikoma Y,
Tsuji AB, Wakizaka H, Kamakura T, Aoki I, Zhang MR and Kato K:
In-vivo imaging of blood-brain barrier permeability using positron
emission tomography with 2-amino-[3-11C]isobutyric acid.
Nucl Med Commun. 36:1239–1248. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Någren K, Sutinen E and Jyrkkiö S:
[N-methyl-11C]MeAIB, a tracer for system A amino acid
transport: Preparation from [11C] methyl triflate and
HPLC metabolite analysis of plasma samples after intravenous
administration in man. J Labelled Comp Radiopharm. 43:1013–1021.
2000. View Article : Google Scholar
|
|
17
|
Sutinen E, Jyrkkiö S, Alanen K, Någren K
and Minn H: Uptake of
[N-methyl-11C]alpha-methylaminoisobutyric acid in
untreated head and neck cancer studied by PET. Eur J Nucl Med Mol
Imaging. 30:72–77. 2003. View Article : Google Scholar
|
|
18
|
Nishii R, Higashi T, Kagawa S, Kishibe Y,
Takahashi M, Yamauchi H, Motoyama H, Kawakami K, Nakaoku T, Nohara
J, et al: Diagnostic usefulness of an amino acid tracer,
α-[N-methyl-(11)C]-methylaminoisobutyric acid ((11) C-MeAIB), in
the PET diagnosis of chest malignancies. Ann Nucl Med. 27:808–821.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kilberg MS and Häussinger D: Mammalian
Amino Acid Tansport. Springer Science & Business Media; New
York, NY: 1992, View Article : Google Scholar
|
|
20
|
Shotwell MA, Jayme DW, Kilberg MS and
Oxender DL: Neutral amino acid transport systems in Chinese hamster
ovary cells. J Biol Chem. 256:5422–5427. 1981.PubMed/NCBI
|
|
21
|
Sudo H, Tsuji AB, Sugyo A, Ogawa Y, Sagara
M and Saga T: ZDHHC8 knockdown enhances radiosensitivity and
suppresses tumor growth in a mesothelioma mouse model. Cancer Sci.
103:203–209. 2012. View Article : Google Scholar
|
|
22
|
Mehrara E, Forssell-Aronsson E, Ahlman H
and Bernhardt P: Specific growth rate versus doubling time for
quantitative char-acterization of tumor growth rate. Cancer Res.
67:3970–3975. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Bigler RE, Zanzonico PB, Schmall B, Conti
PS, Dahl JR, Rothman L, Sgouros G and MacEwen EG: Evaluation of
[1-11C]-alpha-aminoisobutyric acid for tumor detection
and amino acid transport measurement: Spontaneous canine tumor
studies. Eur J Nucl Med. 10:48–55. 1985. View Article : Google Scholar
|
|
24
|
Kagawa S, Nishii R, Higashi T, Yamauchi H,
Ogawa E, Okudaira H, Kobayashi M, Yoshimoto M, Shikano N and Kawai
K: Relationship between [14C]MeAIB uptake and amino acid
transporter family gene expression levels or proliferative activity
in a pilot study in human carcinoma cells: Comparison with
[3H]methionine uptake. Nucl Med Biol. 49:8–15. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Oxender DL and Christensen HN: Distinct
mediating systems for the transport of neutral amino acids by the
Ehrlich cell. J Biol Chem. 238:3686–3699. 1963.PubMed/NCBI
|
|
26
|
Anderson LC and Mixson E:
Alpha-aminoisobutyric acid transport in isolated rat submandibular
salivary acinar cells. Arch Oral Biol. 34:131–136. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yu W-L, Cong WM, Zhang Y, Chen Y, Wang F
and Yu G: Overexpression of ATA1/SLC38A1 predicts future recurrence
and death in Chinese patients with hilar cholangiocarcinoma. J Surg
Res. 171:663–668. 2011. View Article : Google Scholar
|
|
28
|
Wang K, Cao F, Fang W, Hu Y, Chen Y, Ding
H and Yu G: Activation of SNAT1/SLC38A1 in human breast cancer:
Correlation with p-Akt overexpression. BMC Cancer. 13:3432013.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Nesteruk M, Lang S, Veit-Haibach P, Studer
G, Stieb S, Glatz S, Hemmatazad H, Ikenberg K, Huber G, Pruschy M,
et al: Tumor stage, tumor site and HPV dependent correlation of
perfusion CT parameters and [18F]-FDG uptake in head and
neck squamous cell carcinoma. Radiother Oncol. 117:125–131. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Tixier F, Groves AM, Goh V, Hatt M,
Ingrand P, Le Rest CC and Visvikis D: Correlation of intra-tumor
18F-FDG uptake heterogeneity indices with perfusion CT
derived parameters in colorectal cancer. PLoS ONE. 9:e995672014.
View Article : Google Scholar
|
|
31
|
Hyde R, Taylor PM and Hundal HS: Amino
acid transporters: Roles in amino acid sensing and signalling in
animal cells. Biochem J. 373:1–18. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Franchi-Gazzola R, Gaccioli F, Bevilacqua
E, Visigalli R, Dall’Asta V, Sala R, Varoqui H, Erickson JD,
Gazzola GC and Bussolati O: The synthesis of SNAT2 transporters is
required for the hypertonic stimulation of system A transport
activity. Biochim Biophys Acta. 1667:157–166. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Gaccioli F, Huang CC, Wang C, Bevilacqua
E, Franchi-Gazzola R, Gazzola GC, Bussolati O, Snider MD and
Hatzoglou M: Amino acid starvation induces the SNAT2 neutral amino
acid transporter by a mechanism that involves eukaryotic initiation
factor 2alpha phosphorylation and cap-independent translation. J
Biol Chem. 281:17929–17940. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
de Sousa Abreu R, Penalva LO, Marcotte EM
and Vogel C: Global signatures of protein and mRNA expression
levels. Mol Biosyst. 5:1512–1526. 2009.PubMed/NCBI
|
|
35
|
Ferlay J, Soerjomataram I, Dikshit R, Eser
S, Mathers C, Rebelo M, Parkin DM, Forman D and Bray F: Cancer
incidence and mortality worldwide: Sources, methods and major
patterns in GLOBOCAN 2012. Int J Cancer. 136:E359–E386. 2015.
View Article : Google Scholar
|