Introduction
Breast cancer (BC) is a malignant tumor that occurs
in the epithelial tissue of the mammary gland and was the most
common cancer type among women globally (1). In China, in 2015, 272,400 novel BC
cases were identified with a mortality toll of 70,700 in 2015
(2). In the USA, in 2016, the
number of novel cases and mortalities of BC were 249,260 and
40,890, respectively (3). Although
the combined treatment of surgery, radiotherapy, chemotherapy,
endocrine therapy and targeted therapy has achieved good clinical
results, recurrence and metastasis remain the primary factors
affecting survival (4). Non-coding
RNAs, which do not encode protein, may be classified as short
non-coding RNAs, including microRNAs (miRNAs), and long non-coding
RNAs (lncRNAs), for example, HOTAIR, H19 and MALAT1 have been
widely studied (5). These RNAs
bind to specific sequences to degrade the transcription of target
genes (5). Previous studies
confirmed that lncRNAs serve important functions in numerous
biological processes, including guiding chromosome remodeling and
regulating miRNAs (6-9). Compared with normal tissues, cancer
tissues exhibit abnormal expression of lncRNAs (10). Increasing evidence demonstrated
that a high level of the lncRNA nuclear paraspeckle assembly
transcript 1 (NEAT1) is associated with unfavorable prognosis in BC
(11,12).
NEAT1 is located at nuclear paraspeckles to form and
maintain their structure (13,14).
The stabilization of paraspeckles can interrupt mRNA, which is
retained in para-speckles and translated into protein, regulating
the expression of target genes accurately and rapidly (15,16).
NEAT1 is upregulated prominently in hypoxia-regulated BC cells
(17). The transcription of NEAT1
is primarily regulated by hypoxia-inducible factor-2, and the
overexpression of NEAT1 can accelerate cell proliferation and
inhibit apoptosis (18). The
prognosis of patients with BC with high expression of NEAT1 is
significantly decreased, compared with patients with low NEAT1
expression (19). Further studies
indicated that NEAT1 combines with miR-107 and downregulates its
expression level to promote the progression of numerous human
cancer types, including glioma and laryngeal squamous cell cancer
(20,21). A number of studies have identified
that miR-107 serves a significant function in the development and
progression of tumors. This miRNA acts as a tumor suppressor
molecule whose expression is inhibited in the majority of cancer
types, including glioma (20),
laryngeal squamous cell cancer (21), pancreatic carcinoma (22), colon carcinoma (23) and BC (24).
Carnitine palmitoyltransferase-1 (CPT1) is a key
regulatory and rate-limiting enzyme of long-chain fatty acids for
β-oxidation (25,26). This protein is located on the outer
membrane of mitochondria through the transmembrane domain and
catalyzes the formation of acylcarnitine, which is then transferred
to the mitochondrial inner membrane and β-oxidized by CPT2
(27). CPT1A, a subtype of the
CPT1 gene family, is expressed in all tissue types except skeletal
muscle cells and brown adipose cells (27). The proliferation of tumors requires
a large amount of raw material and energy, and the abnormality of
lipid synthesis and catabolism are classical features of tumor
cells (28). CPT1A is highly
expressed in ovarian cancer, prostate cancer and other types of
cancer, and it serves a key function in promoting tumor cell
proliferation and metastasis (29,30).
In BC, CPT1A is associated with histone deacetylase
activity to regulate cell survival, cell death escape and invasion
of MCF-7, SK-BR3 and MDA-MB-231 cell lines (31). miR-107, an inhibitor of β-oxidation
by downregulating the expression of CTP1A and other associated
genes (32), can promote lipid
accumulation (33) and inhibit
tumor progression (20-24). However, the inhibitory mechanism of
miR-107 to CPT1A is unclear. Previous studies demonstrated that
NEAT1 downregulates the expression level of miR-107 to promote the
progression of numerous human cancer types, including glioma and
laryngeal squamous cell cancer (20,21),
but whether this inhibitory mechanism is applicable to BC requires
confirmation.
In the present study, first, the expression of NEAT1
and miR-107 in BC cells was determined using the reverse
transcription-quantitative polymerase chain reaction (RT-qPCR), and
it was identified that the lncRNA NEAT1 was upregulated and that
miR-107 was downregulated and associated with the progression of
BC. Subsequently, the function of NEAT1 and miR-107 in BC cells was
investigated. It was identified that knockdown of NEAT1 or
transfection of mimics-miR-107 inhibited the proliferation and
invasion of BC cells. Furthermore, it was identified that NEAT1
upregulated CPT1A through inhibiting the expression of miR-107.
These results provide evidence that BC is promoted by the
NEAT1-miR-107-CPT1A regulatory network.
Materials and methods
Cell and reagents
Human mammary epithelial cell lines MCF-10A (cat.
no. ATCC® CRL-10317™), BC lines MCF-7 (cat. no.
ATCC® HTB-22™), MDA-MB-231 (cat. no. ATCC®
HTB-26™) were and 293 (cat. no. ATCC® CRL-1573™)
purchased from the American Type Culture Collection (Manassas, VA,
USA). These cells were cultured in Dulbecco's modified Eagle's
medium (DMEM; cat. no. 11965-084; Gibco; Thermo Fisher Scientific,
Inc., Waltham, MA, USA) at 37°C in humidified atmosphere of 5%
CO2. Lentivirus vector pLKO.1 (cat. no. E365), and
packaging plasmids psPAX2 (cat. no. E366) and pMD2G (cat. no. E367)
were purchased from AtaGenix Laboratories (Wuhan, China). The
pcDNA3.1 plasmid (cat. no. V00454) was purchased from Fenghui Bio
(Changsha, China). TRIzol® (cat. no. 15596018),
ThermoScript™ RT-PCR System (cat. no. K1691),
Lipofectamine® 2000 (cat. no. 11668019), RPMI-1640 (cat.
no. 11875085) and fetal bovine serum (FBS; cat. no. 10099141) were
purchased from Invitrogen (Thermo Fisher Scientific, Inc.). iTaq™
Universal SYBR® Green supermix (cat. no. 172-5121) and
LumiPico® Enhanced Chemiluminescent (ECL) reagent (cat.
no. 1705060) were purchased from Bio-Rad Laboratories, Inc.
(Hercules, CA, USA). Cell Proliferation kit I (MTT; cat. no.
11465007001) was purchased from Sigma-Aldrich; Merck KGaA
(Darmstadt, Germany). Anti-B-cell lymphoma 2-associated agonist of
cell death (BAD; cat. no. ab32445), anti-caspase 9 (CASP9; cat. no.
ab32539), anti-collagen type XVII a 1 (COL18A1; cat. no. ab64569),
anti-metallopeptidase inhibitor 1 (TIMP-1; cat. no. ab61224),
anti-platelet-derived growth factor subunit A (PDGF-A; cat. no.
ab38562), anti-serpin family B member 2 (SERPINB2; cat. no.
ab47742), anti-cyclin D1 (cat. no. ab134175), anti-cyclin-dependent
kinase 4 (CDK4; cat. no. ab108357) and horseradish
peroxidase-conjugated goat anti-rabbit immunoglobulin G (cat.
ab205718) antibodies were purchased from Abcam (Cambridge, MA,
USA). Short hairpin RNA against NEAT1 (sh-NEAT1), sh-negative
control (NC), mimics-miR-107 (sense: 5′-ACU AUC GGG ACA UGU UAC GAC
GA-3′; antisense: 5′-UCG UCG UAA CAU GUC CCG AUA GUU U-3′) and
inhibitor-miR-107 (5′-UCG UCG UAA CAU GUC CCG AUA GU-3′) were
synthetized by Shanghai GenePharma Co., Ltd. (Shanghai, China). The
Dual Luciferase Reporter assay system (cat. no. E1910) was
purchased from Promega Corporation (Madison, WI, USA). Cells were
analyzed for DNA content by flow cytometry (FACSCalibur; BD
Biosciences, Franklin Lakes, NJ, USA).
Transient transfection
Lipofectamine 2000 (2 µl) was diluted with
100 µl Opti-MEM at 1:50 (both Gibco; Thermo Fisher
Scientific, Inc.) and incubated for 5 min at room temperature.
pcDNA3.1-NEAT1 plasmid (1 µg; ratio, 1:50), 2 µg
mimics-miRNA or inhibitor-miRNA (both final concentration, 100 nM)
was diluted in 50 µl Opti-MEM. The diluted Lipofectamine
2000 and pcDNA3.1-NEAT1 plasmid, mimics-miRNA or inhibitor-miRNA
were mixed (1:1) and incubated at room temperature for 20 min.
Finally, the mixture was added to MCF-7 and MDA-MB-231 for culture
for between 24 and 48 h at 37°C.
sh-NEAT1 was cloned into the lentivirus vector
pLKO.1 and termed pLKO.1-shNEAT1. MCF-7 and MDA-MB-231 cells were
inoculated into the 6-well plate until the density reached 80%
confluence. pLKO.1-shNEAT1, psPAX2 and pMD2G were mixed at a ratio
of 5:3:2 in Opti-MEM.
293 cells were inoculated in six-well plates using
DMEM supplemented with 10% FBS, 1% penicillin (100 U/ml) and
streptomycin (100 U/ml) at 37°C until the density reached 90%
confluence. The pLKO.1-shNEAT1 plasmid or pLKO.1-shNC was then
transfected into 293 cells with psPAX2 packaging plasmid and pMD2G
envelope plasmid by Lipofectamine 2000 to produce shRNA-containing
lentivirus. The infection efficiency was detected under a
fluorescence microscope (at a magnification of ×200) according to
the green fluorescent protein expression level. Following
incubation for 20 min with Lipofectamine 2000 at room temperature,
the mixture was added to the cells and cultured for 8 h at 37°C.
Culture medium was replaced with fresh DMEM supplemented with 10%
FBS, 1% penicillin and streptomycin for virus packaging. After 24
h, the lentiviral particles in the supernatant were harvested and
filtered by centrifugation at 500 × g for 10 min at 4°C.
MCF-7 and MDA-MB-231 cells were cultured with 1.5 ml
RPMI-1640 to 50% confluence in 6-well plates at 37°C. Subsequently,
0.5 ml virus was added into each well for infection. After 24 h,
the medium containing the virus was replaced with a fresh RPMI-1640
medium and the cells were cultured for another 48 h at 37°C. The
infection efficiency was detected under a fluorescence microscope
(at a magnification of ×200) according to the green fluorescent
protein expression level.
RNA extraction and RT-PCR
MCF-7 and MDA-MB-231 cells were collected and lysed
with TRIzol reagent. Total RNA was extracted with TRIzol and was
precipitated using 0.5 ml of 95% isopropanol, followed by washing
once with 75% ethanol. Finally, the total RNA was dissolved in
RNase-free water. Following RNA extraction, cDNA was synthetized
using the ThermoScript™ RT-PCR system according to the
manufacturer's protocol. iTaq Universal SYBR Green supermix was
used for qPCR according to the manufacturer's protocol. cDNA was
then amplified using the following cycling conditions: One initial
PCR activation step at 95°C for 15 min followed by 40 cycles of
denaturation at 94°C for 15 sec, annealing at 53°C for 30 sec, and
elongation at 72°C for 30 sec. The U6 (for miR-107) and GAPDH (for
NEAT1 and CPT1A) were used as internal controls. Cq values were
used for quantification using a previously described protocol
(34). Primers were as follows:
NEAT1 forward, 5′-CTT CCT CCC TTT AAC TTA TCC ATT CAC-3′; reverse,
5′-CTC TTC CTC CAC CAT TAC CAA CAA TAC-3′; miR-107 forward, 5′-ATG
ATG AGC AGC ATT GTA CAG G-3′; reverse, 5′-GCA GGG TCC GAG GTA
TTC-3′; CPT1A forward, 5′-TTC AGT TCA CGG TCA CTC CG-3′; reverse
5′-TGA CCA CGT TCT TCG TCT GG-3′; GAPDH forward, 5′-TGC ACC ACC AAC
TGC TTA GC-3′; reverse, 5′-GGC ATG GAC TGT GGT CAT GAG-3′; U6
forward, 5′-CTC GCT TCG GCA GCA CA-3′; reverse, 5′-AAC GCT TCA CGA
ATT TGC GT-3′.
MTT assay
MCF-7 and MDA-MB-231 cells were plated in 96-well
plates at the density of 10,000 cells per well, and cultured at
37°C for 72 h in an atmosphere containing 5% CO2. Cells
that were cultured for 0, 24, 48 and 72 h were analyzed using a
Cell Proliferation kit I according to the manufacturer's protocol.
Following culture, MTT solution was added to cells and incubated
for another 4 h at 37°C. Subsequently, the medium was removed, and
200 µl dimethyl sulfoxide (Beyotime Institute of
Biotechnology, Haimen, China) was added to each well to dissolve
the formazan crystals. The proliferation of cells was measured
using a Universal microplate spectrophotometer at 550 nm.
Wound-healing assay
MCF-7 and MDA-MB-231 cells were plated on a 6-well
plate and were cultured in RPMI-1640 supplemented with 10% FBS, 1%
penicillin and streptomycin at 37°C until reaching 90% confluence.
Cells were scraped off each well at a width of 1 mm using 1-ml
Labtip pipette tips and debris was washed with PBS three times.
Subsequently, the cells were cultured with fresh RPMI-1640 at 37°C
in an atmosphere containing 5% CO2 for 24 h. The scraped
line of each well was viewed under a light microscope (at a
magnification of ×16) to assay the migration of cells. Furthermore,
wound-healing graphs were produced.
Matrigel assay
Transwell chambers were pre-coated with Matrigel at
a concentration of 8 µg/µl and set on a 24-well
plate. The sublayer of the Transwell chamber was filled with
RPMI-1640 containing 10% FBS. The MCF-7 and MDA-MB-231 cells were
digested and suspended in RPMI-1640 medium containing 0.1% FBS.
Subsequently, the suspended cells were inoculated in chambers at a
density of 1×105 cells/ml at 37°C. After 36 h, the cells
and Matrigel that did not pass through the chamber were removed
with cotton swabs. The remaining cells were fixed with pre-cold 4%
paraformaldehyde for 30 min and stained with 0.1% crystal violet
for 30 min at room temperature. The cells passing through the
sublayer of the chamber were observed and enumerated under a light
phase-contrast microscope (at a magnification of ×100).
Western blotting
Total protein was extracted from MCF-7 and
MDA-MB-231 cells with radioimmunoprecipitation assay lysis buffer
(Beyotime Institute of Biotechnology) and quantified with a
Bicinchoninic Acid kit. Protein samples (30 µg) were
subjected to SDS-PAGE on 10% gels. The resolved proteins were
transferred onto polyvinylidene fluoride membranes, which were then
blocked with 5% bovine serum albumin (Beyotime Institute of
Biotechnology) in TBST at 25°C for 1 h. Following incubation with
the primary antibodies (1:1,000) at 4°C for overnight and secondary
antibodies (1:5,000) at room temperature for 1 h, membranes were
subjected to chromogenic analysis using LumiPico ECL reagent.
Cell cycle analysis by flow
cytometry
Cell cycle analysis was determined by Keygen Cell
Cycle detection kit (Nanjing KeyGen Biotech Co., Ltd., Nanjing,
China). Briefly, MCF-7 and MDA-MB-231 cells were collected and
washed with ice-cold PBS three times. Following centrifugation for
5 min at 1,000 × g and room temperature, the cells were suspended
in ice-cold PBS and fixed in absolute ethanol for 30 min at 4°C.
Following fixing, the ethanol was removed, and the cells were
washed with PBS once to remove residual ethanol. Subsequently, the
cells were resuspended in PBS with RNase A and incubated at 37°C
for 30 min. Finally, the cells were stained with prop-idium iodide
for 30 min at 37°C, and the cell cycle distribution was detected by
flow cytometry (FACSCalibur) using Cell Quest Pro software version
5.1 (both BD Biosciences).
Luciferase reporter assay
Starbase tool (version 2.0; http://starbase.sysu.edu.cn) and Targetscan tool
(version 7.1; http://www.targetscan.org) were used to predict the
binding sites of NEAT1 and CPT1A on miR-107. A total of four
luciferase reporter plasmids, wild-type (wt)-NEAT1, mutated
(mut)-NEAT1, wt-CPT1A and mut-CPT1A, were constructed by Shanghai
GenePharma Co., Ltd. pRL-Tk, wt-NEAT1 or mut-NEAT1, and
mimics-miR-107 or inhibitor-miR-107 were co-transfected into 293
cells using Lipofectamine 2000 trans-fection reagent to investigate
the interaction between NEAT1 and miR-107. pRL-Tk, wt-CPT1A or
mut-CPT1A, and mimics-miR-107 or inhibitor-miR-107 were
co-transfected into 293 cells to investigate the interaction
between CPT1A and miR-107. After 48 h of transfection, the activity
of firefly luciferase, quantified as luminescence, was detected
using the Dual-Luciferase Reporter system, and the fluorescence
value of Renilla luciferase was set as the internal
reference.
Statistics
Data from at least three independent experiments are
presented as the mean ± standard deviation. Statistical analysis
was performed using SPSS software 16.0 (SPSS, Inc., Chicago, IL,
USA). Statistical significance was measured using Student's t-test
or one-way analysis of variance followed by Tukey's post-hoc test.
P<0.05 was considered to indicate a statistically significant
difference.
Results
NEAT1 inhibits the expression of miR-107
in BC cells
A total of two BC cell lines, MCF-7 and MDA-MB-231,
were selected to analyze the expression levels of NEAT1 and miR-107
as they are commonly used cell lines for the study of BC. MCF-10A
cells, a mammary epithelial cell line, was used as the control. The
total RNA of these three cell lines was extracted and
reverse-transcribed into cDNA. RT-qPCR indicated that NEAT1
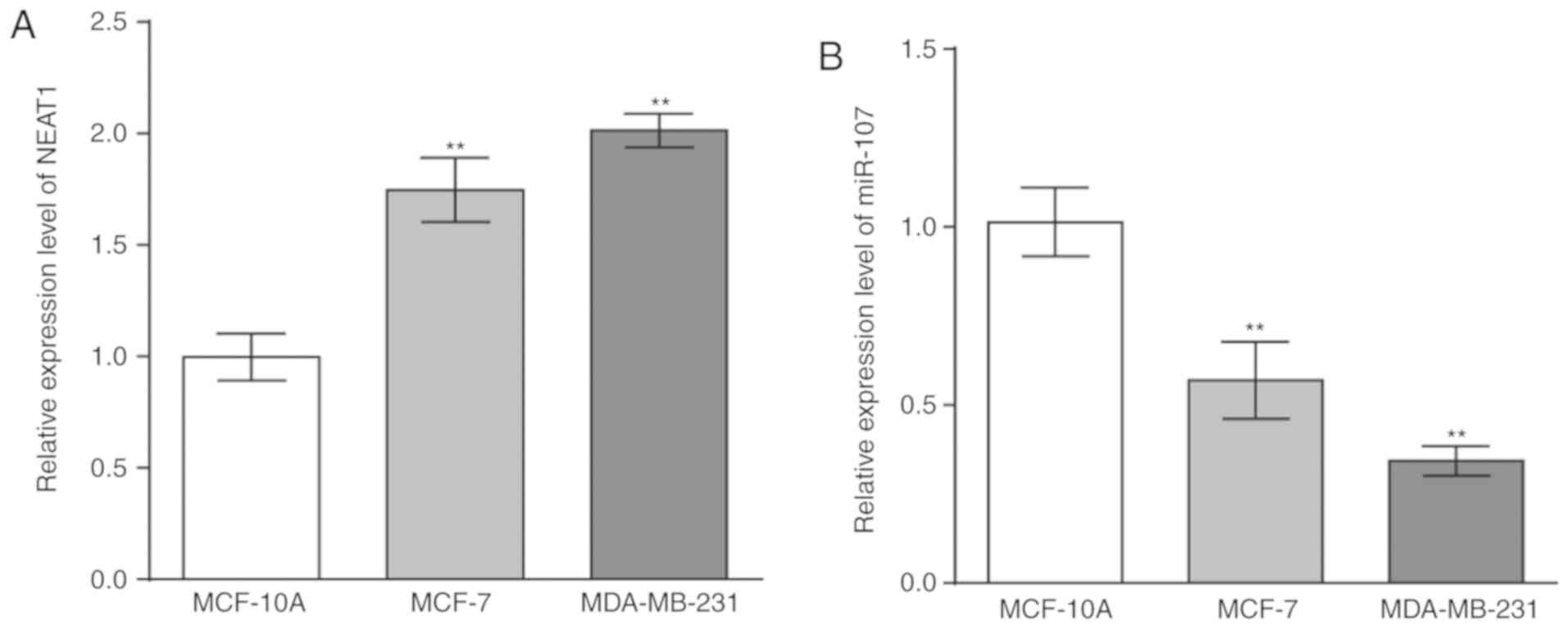
expression was significantly increased (Fig. 1A) and that miR-107 expression was
significantly decreased in BC cells, compared with the MCF-10A
cells (Fig. 1B). To investigate
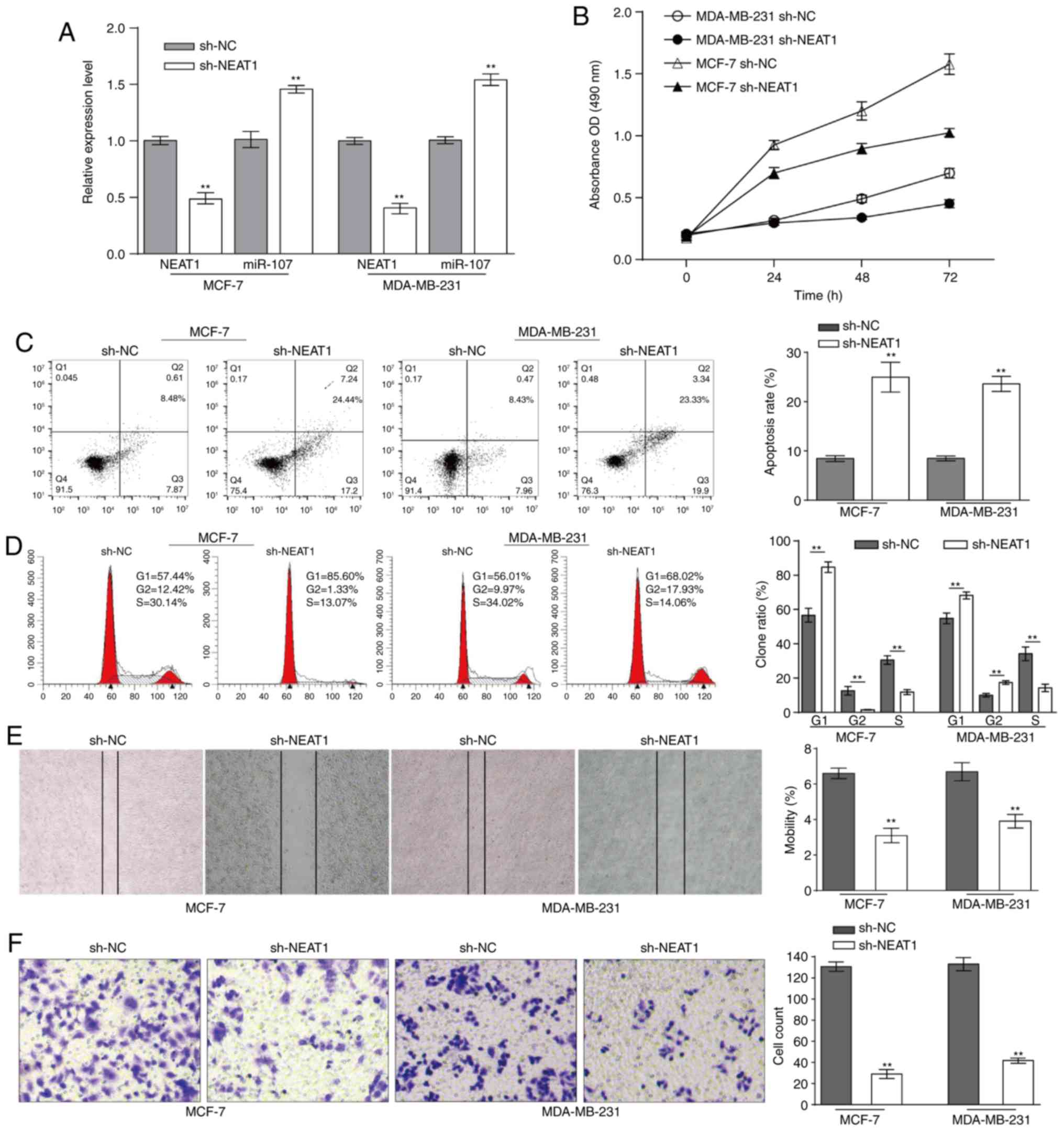
the association between NEAT1 and miR-107 in BC cells, sh-NEAT1 was
transfected into MCF-7 and MDA-MB-231 cells to significantly
downregulate the expression of NEAT1, compared with the negative
control (Fig. 2A). In contrast
with NEAT1 expression, miR-107 expression was significantly
enhanced in MCF-7 and MDA-MB-231 cells, compared with the negative
control (Fig. 2A). In conclusion,
NEAT1 inhibits the expression of miR-107 in BC cells.
NEAT1 knockdown inhibits the progression
of BC cells
NEAT1-downregulated MCF-7 and MDA-MB-231 cells were
cultured for 72 h, and cell proliferation was determined using an
MTT assay at 0, 24, 48, 72 h. Accompanied by downregulated NEAT1
and upregulated miR-107, cell proliferation was decreased (Fig. 2B). Subsequently, the effect of
NEAT1 on the BC cell cycle was investigated using flow cytometric
analysis. Fig. 2C indicates that
NEAT1 knockdown significantly promoted apoptosis in BC cells,
compared with the negative control. Compared with the proportion of
cells in G1 phase in the negative control that was transfected with
sh-NC, the proportion of MCF-7 or MDA-MB-231 cells in G1 phase
transfected with sh-NEAT1 was significantly increased (Fig. 2D). The wound healing and Matrigel
assays demonstrated that the migratory and invasive abilities of BC
cells were significantly inhibited when NEAT1 was downregulated
(Fig. 2E and F). Collectively,
NEAT1 knockdown inhibits the proliferation, cell cycle, migration
and invasion of BC cells.
NEAT1 knockdown affects tumor
development-associated genes in BC cells
To further detect the effect of NEAT1 on the
progression of BC cells, the expression of genes that were
associated with the proliferation, cell cycle, migration and
invasion of BC cells were determined in sh-NEAT1-trans-fected MCF-7
and MDA-MB-231 cells. RT-qPCR and western blotting data
demonstrated that the mRNA and protein expression levels of
apoptotic genes (BAD, CASP9 and COL18A1) were significantly
upregulated in sh-NEAT1-transfected BC cells, whereas those of the
invasion-associated genes (TIMP-1, PDGF-A and SERPINB2) were
significantly downregulated in sh-NEAT1-transfected BC cells
(Fig. 3A). The expression of the
BC cell cycle-associated gene cyclin D1 was also significantly
downregulated in sh-NEAT1-transfected BC cells, indicating that
cells remained at G1 phase. Furthermore, significant downregulation
of CDK4 indicated that the cells did not enter the S division phase
(Fig. 3B). Additionally, CPT1A, a
novel biomarker of BC, was significantly downregulated at the mRNA
and protein levels in sh-NEAT1-transfected BC cells (Fig. 3B).
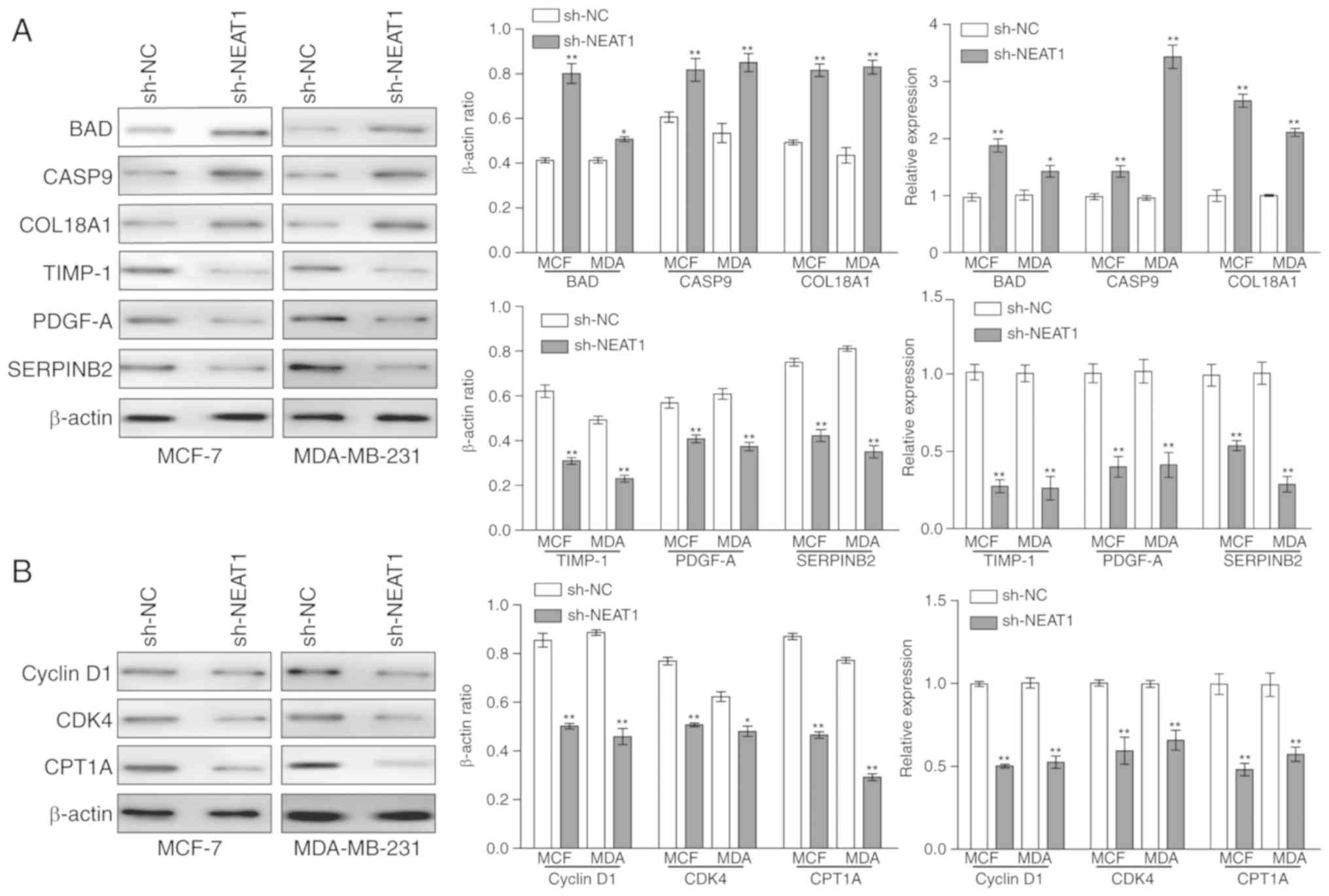 | Figure 3Detection of tumor
development-associated genes in NEAT1-knockdown breast cancer
cells. (A) Western blot analysis and reverse
transcription-quantitative polymerase chain reaction assays were
used to determine the protein and mRNA expression levels of BAD,
CASP9, COL18A1, TIMP-1, PDGF-A and SERPINB2 in MCF-7 and MDA-MB-231
cells following NEAT1 knockdown. (B) A western blot assay was used
to detect the protein expression levels of cyclin D1, CDK4 and
CPT1A in MCF-7 and MDA-MB-231 following NEAT1 knockdown. Data was
obtained from at least three independent experiments. *P<0.05,
**P<0.01 vs. sh-NC. BAD, B-cell lymphoma 2-associated
agonist of cell death; CASP9, caspase 9; COL18A1, collagen type
XVII a 1; TIMP-1, TIMP metallopeptidase inhibitor 1; PDGF-A,
platelet-derived growth factor subunit A; SERPINB2, serpin family B
member 2; CDK4, cyclin-dependent kinase 4; CPT1A, carnitine
palmitoyltransferase-1; NEAT1, nuclear paraspeckle assembly
transcript 1; miR-107, microRNA-107; sh-NC, short hairpin-negative
control; MCF, MCF-7; MDA, MDA-MB-231. |
miR-107 reversely regulates the
expression of NEAT1 and negatively regulates the progression of BC
cells
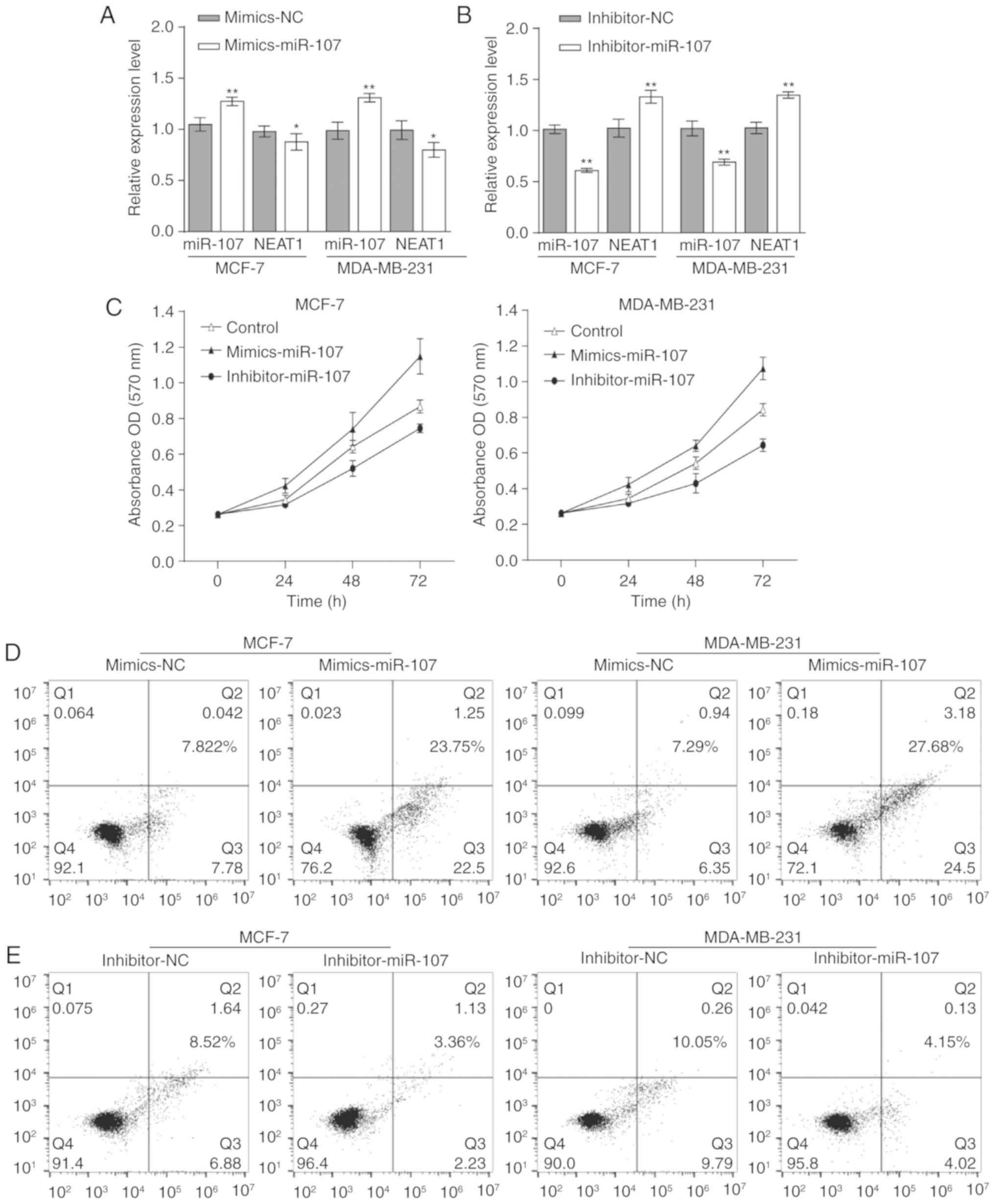
To investigate the function of miR-107 in BC cells,
mimics-miR-107 was transfected into MCF-7 and MDA-MB-231 cells to
significantly upregulate its expression, and NEAT1 was demonstrated
to be significantly downregulated (Fig. 4A). Furthermore, miR-107 was
significantly downregulated with the significant overexpression of
NEAT1 when MCF-7 and MDA-MB-231 cells were transfected with
inhibitor-miR-107 (Fig. 4B). These
results indicated that miR-107 can reversely regulate the
expression of NEAT1.
The progression of these cells was investigated.
Following transfection with mimics-miR-107, an MTT assay revealed
that the proliferation rates of MCF-7 and MDA-MB-231 cells were
decreased, the rates of apoptosis in BC cells were increased, and
the cell cycle distribution of the majority of the cells remained
at G1 phase, compared with the negative control cells. However,
when miR-107 was downregulated by inhibitor-miR-107, the
proliferation of BC cells was increased, the rates of apoptosis in
BC cells were decreased, and the majority of the cells were not
held at G1 phase (Fig. 4C-I).
Thus, miR-107 inhibits the proliferation and cell cycle of BC
cells. In the migration and invasion experiments, miR-107 knockdown
significantly promoted the migration and invasion of MCF-7 and
MDA-MB-231 cells. By contrast, overexpression of miR-107
significantly inhibited the migration and invasion of these BC
cells (Fig. 4J and K). In summary,
miR-107 negatively regulates the progression of BC cells.
miR-107 regulates the progression of BC
cells through tumor development-associated genes
To identify how miR-107 negatively regulates the
progression of BC cells, the expression of tumor
development-associated genes was detected in mimics-miR-107 or
inhibitor-miR-107-transfected BC cells. To determine the β-actin
ratio and relative expression of each protein and gene, western
blotting and RT-qPCR were used. With the upregulation of miR-107 by
mimics-miR-107, the expression levels of apoptosis-associated genes
BAD, CASP9 and COL18A1 were significantly increased, whereas the
expression levels of invasion-associated genes TIMP-1, PDGF-A and
SERPINB2 were significantly decreased (Fig. 5A). Consistent with the flow
cytometry results, cyclin D1 and CDK4, which regulate the cell
cycle, were significantly downregulated. CPT1A was also
significantly downregulated (Fig.
5B). The identical analysis was performed on miR-107-knockdown
MCF-7 and MDA-MB-231 cells that were transfected with
inhibitor-miR-107. In contrast with the aforementioned results, the
expression levels of BAD, CASP9 and COL18A1 were significantly
decreased (Fig. 5C), and the
expression levels of TIMP-1, PDGF-A, SERPINB2, cyclin D1, CDK4 and
CPA1A were significantly increased (Fig. 5C and D). Collectively, these
results indicate that miR-107 regulates the progression of BC cells
through tumor development-associated genes.
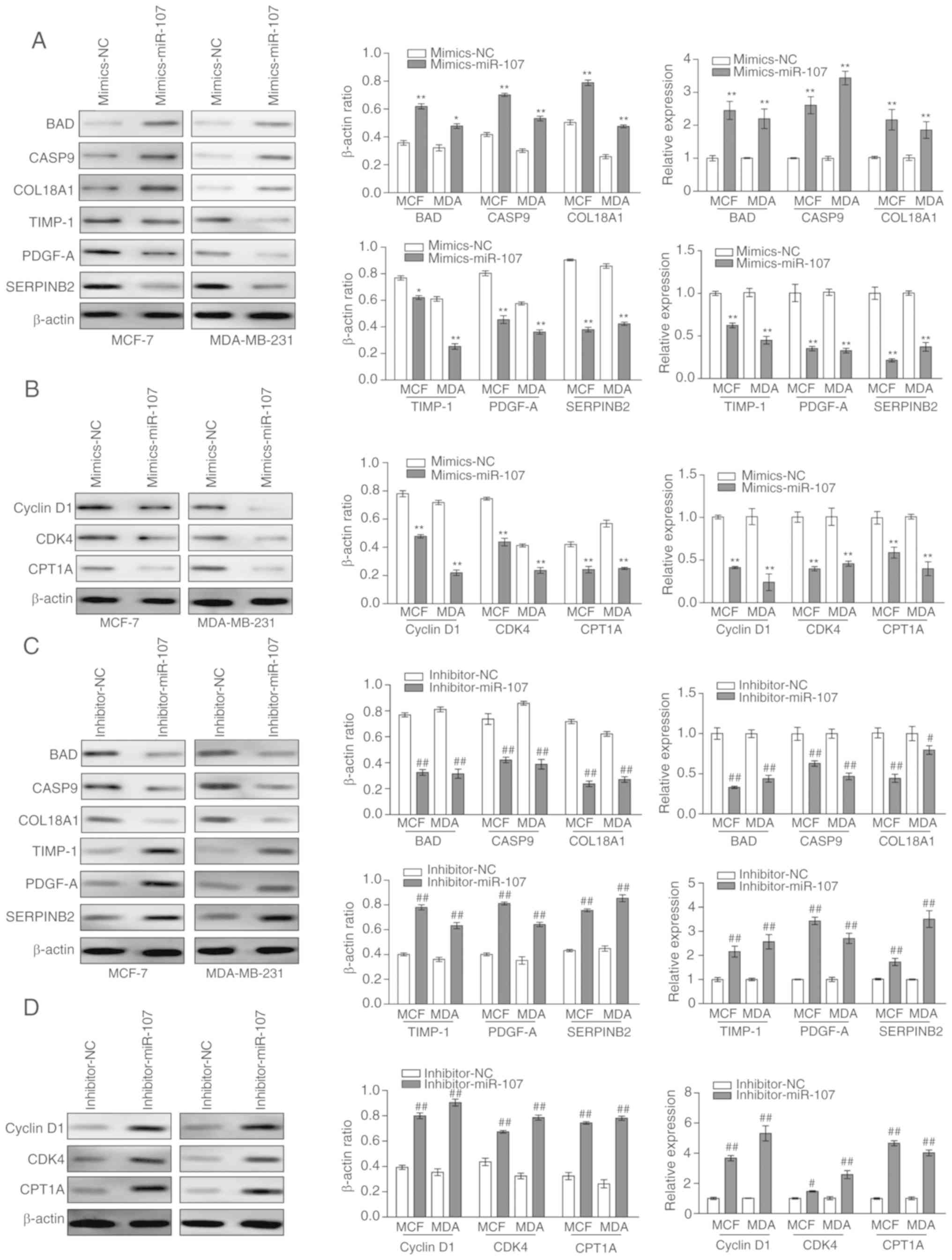 | Figure 5Detection of tumor
development-associated genes in miR-107 increased or decreased
breast cancer cells. The western blot lanes were translated into
corresponding greyscale values to present the β-actin ratio and
relative expression of each protein. (A) Western blot analysis and
RT-qPCR assays were used to determine the protein and mRNA
expression levels of BAD, CASP9, COL18A1, TIMP-1, PDGF-A and
SERPINB2 in MCF-7 and MDA-MB-231 cells that were transfected with
mimics-miR-107. (B) Western blot analysis and RT-qPCR assays were
used to detect the levels of cyclin D1, CDK4 and CPT1A. (C) Western
blot analysis and RT-qPCR assays were used to determine the protein
and mRNA expression levels of BAD, CASP9, COL18A1, TIMP-1, PDGF-A
and SERPINB2 in MCF-7 and MDA-MB-231 cells that were transfected
with inhibitor-miR-107. (D) Western blot analysis and RT-qPCR
assays were used to determine the protein and mRNA expression
levels of cyclin D1, CDK4 and CPT1A in MCF-7 and MDA-MB-231 cells
that were transfected with inhibitor-miR-107. Data was obtained
from at least three independent experiments. *P<0.05
and **P<0.01 vs. mimics-NC; #P<0.05 and
##P<0.01 vs. inhibitor-NC. RT-qPCR, reverse
transcription-quantitative polymerase chain reaction; BAD, B-cell
lymphoma 2-associated agonist of cell death; CASP9, caspase 9;
COL18A1, collagen type XVII a 1; TIMP-1, TIMP metallopeptidase
inhibitor 1; PDGF-A, platelet-derived growth factor subunit A;
SERPINB2, serpin family B member 2; CDK4, cyclin-dependent kinase
4; CPT1A, carnitine palmitoyltransferase-1; NEAT1, nuclear
paraspeckle assembly transcript 1; miR-107, microRNA-107; NC,
negative control. |
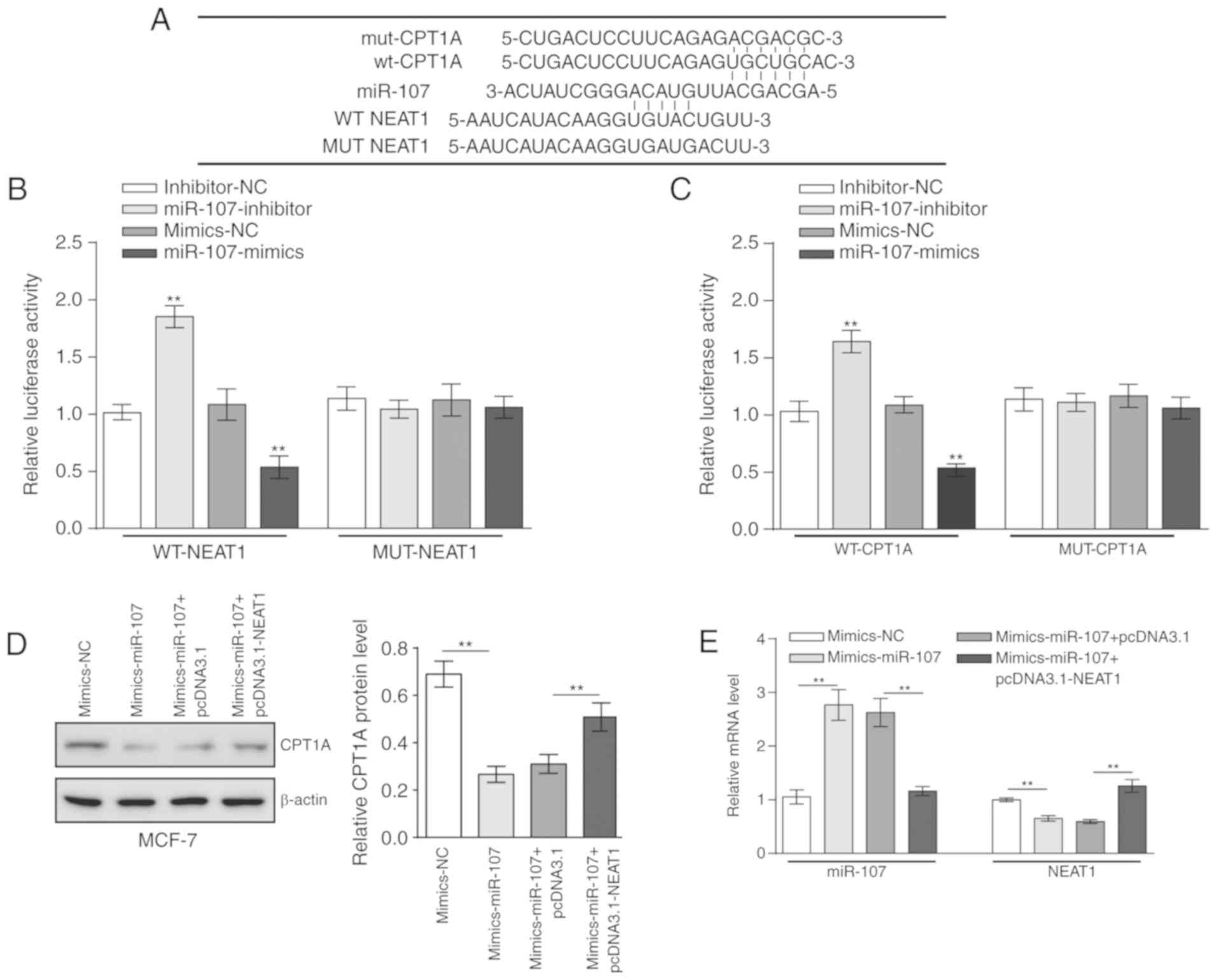
miR-107 regulates the expression of NEAT1
and CPT1A by direct targeting
As aforementioned, NEAT1 negatively regulates the
expression of miR-107, and miR-107 inhibits the downstream gene
CPT1A. Additionally, miR-107 feedback regulates the expression of
NEAT1. The binding sites of NEAT1 and CPT1A on miR-107 were
predicted (Fig. 6A). To
investigate the regulatory mechanism between miR-107 and NEAT1 or
CPT1A, wt-NEAT1, mut-NEAT1, wt-CPT1A and mut-CPT1A luciferase
reporter vectors were constructed. wt-NEAT1 and mimics-miR-107 or
inhibitor-miR-107 were co-transfected into 293 cells. The activity
of luciferase was significantly increased in the
miR-107-downregulated 293 cells, compared with the negative control
(Fig. 6B). Conversely, in
miR-107-upregulated 293 cells, the activity of luciferase was
significantly decreased, compared with the negative control
(Fig. 6B). However, in the
mut-NEAT1 groups, there were no significant changes in luciferase
activity in the miR-107-overexpression and the miR-107-knockdown
293 cells (Fig. 6B). Similar
results were observed for CPT1A (Fig.
6C). The expression levels of CPT1A and NEAT1 were
significantly downregulated by mimics-miR-107, and transfection
with pcDNA3.1-NEAT1 rescued the inhibitory effect of mimics-miR-107
on MCF-7 cells, with the expression level of miR-107 presenting the
opposite trend (Fig. 6D and E).
These results indicated that miR-107 can target NEAT1 and CPT1A
directly to regulate their expression. Therefore, NEAT1 and CPT1A
can compete in conjunction with miR-107.
Discussion
LncRNAs are a class of transcription factors with a
length >200 bp that do not encode proteins, but regulate the
expression levels of genes at various levels in the form of RNA
(35). Studies have demonstrated
that lncRNA is associated with cancer, and the expression of lncRNA
in normal tissues and corresponding tumor tissues demonstrated
significant differences (21,23,24).
Abnormal lncRNA expression serves a substantial function in tumor
progression and can be a predictor of tumor progression (36); however, the function of lncRNAs in
BC is not well-characterized.
The lncRNA NEAT1 has been reported to be upregulated
in various tumor types. High levels of NEAT1 were significantly
associated with clinicopathological features of hepatocellular
carcinoma (37). Additionally,
upregulated NEAT1 was associated with aggressive prostate cancer
(38). In BC, a previous study
indicated that the expression of NEAT1 was increased significantly
under hypoxia and promoted the proliferation of BC cells (18). In the present study, it was
affirmed that NEAT1 was overexpressed in MCF-7 and MDA-MB-231
cells. Furthermore, the knockdown of NEAT1 by sh-NEAT1 resulted in
the inhibition of proliferation, cell cycle, migration and invasion
of BC cells. Additionally, the apoptotic genes BAD, CASP9 and
COL18A were upregulated, but the invasion-associated genes TIMP-1,
PDGF-A, SERPINB2 and cell cycle-associated genes cyclin D1 and CDK4
were downregulated. However, the mechanism by which NEAT1 affects
BC remains unclear.
The disorder of lipid metabolism can induce the
development of a number of tumor types, such as glioma and breast
cancer. Enhancing the expression of CPT1A in BC cells, which can
promote the oxidation of fatty acids and decrease its accumulation,
can significantly improve BC symptoms (39). In the present study, it was
identified that NEAT1 could promote the progression of BC by
regulating its proliferation. NEAT1 knockdown downregulated the
expression of CPT1A and inhibits the development of BC cells.
In recent years, an increasing number of studies
have focused on miRNA, and a normal expression of miRNA requires a
balanced physiological environment (40). These miRNAs, such as miR-15 and
miR-16, participate in the majority of genetic pathways, including
cell cycle checkpoint, cell proliferation and apoptosis (40). Deregulation of miRNA expression
causes widespread changes in gene expression and is associated with
various cancer types, such as lung cancer and pancreatic cancer
(40). miR-107 has been reported
to be a tumor suppressor molecule in numerous cancer types, such as
BC, glioma and cervical cancer (20,21).
The results of the present study indicated that the upregulation of
miR-107 inhibited the proliferation and migration of BC cells and
the expression of CPT1A. However, the knockdown of miR-107 promoted
the proliferation and invasion of BC cells, and the expression of
CPT1A was increased. These results indicated that miR-107
negatively regulates CPT1A.
Emerging evidence has revealed the association of
the interaction between lncRNAs and miRNAs, and cancer metastasis
(41,42). miRNA can negatively regulate lncRNA
by a mechanism similar to that of mRNA (43) or positively regulate lncRNA through
epigenetic regulation (44).
lncRNA can competitively combine with the miRNA to inhibit the
negative regulation of the miRNA on target genes indirectly
(45). Another regulatory
mechanism between lncRNA and miRNA is that lncRNA acts as competing
endogenous RNA to suppress miRNA expression by acting as a miRNA
sponge (46). Additionally, lncRNA
acts as a potential primary miRNA to produce mature miRNA, thereby
indirectly regulating the expression of target genes (47). In the present study, two different
combined sites on miR-107 that exhibited reverse complementarity to
NEAT1 and CPT1A separately were predicted. Luciferase assays
demonstrated that NEAT1 and CPT1A could bind to miR-107. These
results indicated that NEAT1 and CPT1A could compete in combination
with miR-107.
In summary, downregulation of NEAT1 result in
decreased expression of miR-107 and increased expression of CPT1A.
Furthermore, miR-107 knockdown triggers the upregulation of NEAT1
and CPT1A. Combined with the results of proliferation experiments
of BC cells and luciferase assays, a potential regulation mechanism
involving NEAT1, miR-107 and CPT1A in BC cells, in which miR-107
inhibits the expression of CPT1A and NEAT1 downregulates the
expression of miR-107 through the competitive combination of
miR-107 and CPT1A, were determined. Thus, NEAT1 promotes the
expression of CPT1A by inhibiting miR-107 to improve the
progression of BC cells.
Funding
No funding received.
Availability of data and materials
The datasets used and analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YX designed the study and prepared the manuscript.
ZeL did literature research and performed experimental studies. ZhL
and SW analyzed the data and statistics. NS performed the
experimental studies. YX acquired and analyzed the data. TH made
substantial contributions to the conception of the work, edited and
reviewed the manuscript, and agreed to be accountable for all
aspects of the work in ensuring that questions related to the
accuracy. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
Not applicable.
References
|
1
|
DeSantis C, Ma J, Bryan L and Jemal A:
Breast cancer statistics, 2013. CA Cancer J Clin. 64:52–62. 2014.
View Article : Google Scholar
|
|
2
|
Chen W, Chen W, Zheng R, Baade PD, Zhang
S, Zeng H, Bray F, Jemal A, Yu XQ and He J: Cancer statistics in
China, 2015. CA Cancer J Clin. 66:115–132. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2016. CA Cancer J Clin. 66:7–30. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Samuel SM, Varghese E, Varghese S and
Büsselberg D: Challenges and perspectives in the treatment of
diabetes associated breast cancer. Cancer Treat Rev. 70:98–111.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Malhotra A, Jain M, Prakash H, Vasquez KM
and Jain A: The regulatory roles of long non-coding RNAs in the
development of chemoresistance in breast cancer. Oncotarget.
8:110671–110684. 2017. View Article : Google Scholar
|
|
6
|
Mercer TR, Dinger ME and Mattick JS: Long
non-coding RNAs: Insights into functions. Nat Revi Genet.
10:155–159. 2009. View
Article : Google Scholar
|
|
7
|
Costa FF: Non-coding RNAs: New players in
eukaryotic biology. Gene. 357:83–94. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Shi XF, Sun M, Liu H, Yao Y and Song Y:
Long non-coding RNAs: A new frontier in the study of human
diseases. Cancer Lett. 339:159–166. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhang Z, Li Z, Li Y and Zang A: MicroRNA
and signaling pathways in gastric cancer. Cancer Gene Ther.
21:305–316. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Gibb EA, Vucic EA, Enfield KS, Stewart GL,
Lonergan KM, Kennett JY, Becker-Santos DD, MacAulay CE, Lam S,
Brown CJ and Lam WL: Human cancer long non-coding RNA
transcrip-tomes. PLoS One. 6:e259152011. View Article : Google Scholar
|
|
11
|
Ke H, Zhao L, Feng X, Xu H, Zou L, Yang Q,
Su X, Peng L and Jiao B: NEAT1 is required for survival of breast
cancer cells through FUS and miR-548. Gene Regul Syst Bio. 10(Suppl
1): S11–S17. 2016.
|
|
12
|
Jiang X, Zhou Y, Sun AJ and Xue JL: NEAT1
contributes to breast cancer progression through modulating miR-448
and ZEB1. J Cell Physiol. 233:8558–8566. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Clemson CM, Hutchinson JN, Sara SA,
Ensminger AW, Fox AH, Chess A and Lawrence JB: An architectural
role for a nuclear noncoding RNA: NEAT1 RNA is essential for the
structure of paraspeckles. Mol Cell. 33:717–726. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Souquere S, Beauclair G, Harper F, Fox A
and Pierron G: Highly ordered spatial organization of the
structural long noncoding NEAT1 RNAs within paraspeckle nuclear
bodies. Mol Biol Cell. 21:4020–4027. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Iseli C, Stevenson BJ, de Souza SJ, Samaia
HB, Camargo AA, Buetow KH, Strausberg RL, Simpson AJ, Bucher P and
Jongeneel CV: Long-range heterogeneity at the 3′ends of human
mRNAs. Genome Res. 12:1068–1074. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chen LL and Carmichael GG: Gene regulation
by SINES and inosines Biological consequences of A-to-I editing of
Alu element inverted repeats. Cell Cycle. 7:3294–3301. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Choudhry H, Schödel J, Oikonomopoulos S,
Camps C, Grampp S, Harris AL, Ratcliffe PJ, Ragoussis J and Mole
DR: Extensive regulation of the non-coding transcriptome by
hypoxia: Role of HIF in releasing paused RNApol2. EMBO Rep.
15:70–76. 2014. View Article : Google Scholar :
|
|
18
|
Choudhry H, Albukhari A, Morotti M, Haider
S, Moralli D, Smythies J, Schödel J, Green CM, Camps C, Buffa F, et
al: Tumor hypoxia induces nuclear paraspeckle formation through
HIF-2α dependent transcriptional activation of NEAT1 leading to
cancer cell survival. 34:pp. 4482–4490. 2015
|
|
19
|
Curtis C, Shah SP, Chin SF, Turashvili G,
Rueda OM, Dunning MJ, Speed D, Lynch AG, Samarajiwa S, Yuan Y, et
al: The genomic and transcriptomic architecture of 2,000 breast
tumours reveals novel subgroups. Nature. 486:346–352. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yang XL, Xiao Z, Du X, Huang L and Du G:
Silencing of the long non-coding RNA NEAT1 suppresses glioma
stem-like properties through modulation of the miR-107/CDK6
pathway. Oncol Rep. 37:555–562. 2017. View Article : Google Scholar
|
|
21
|
Wan P, Wu T, Zhou H, Jin Q, He G, Yu H,
Xuan L, Wang X, Tian L, Sun Y, et al: Long noncoding RNA NEAT1
promotes laryngeal squamous cell cancer through regulating
miR-107/CDK6 pathway. J Exp Clin Cancer Res. 35:222016. View Article : Google Scholar
|
|
22
|
Lee KH, Lotterman C, Karikari C, Omura N,
Feldmann G, Habbe N, Goggins MG, Mendell JT and Maitra A:
Epigenetic silencing of MicroRNA miR-107 regulates cyclin-dependent
kinase 6 expression in pancreatic cancer. Pancreatology. 9:293–301.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Davidson LA, Wang N, Shah MS, Lupton JR,
Ivanov I and Chapkin RS: n-3 Polyunsaturated fatty acids modulate
carcinogen-directed non-coding microRNA signatures in rat colon.
Carcinogenesis. 30:2077–2084. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Li XY, Luo QF, Wei CK, Li DF, Li J and
Fang L: MiRNA-107 inhibits proliferation and migration by targeting
CDK8 in breast cancer. Int J Clin Exp Med. 7:32–40. 2014.PubMed/NCBI
|
|
25
|
Fritz IB and Yue KT: Long-chain carnitine
acyltransferase and the role of acylcarnitine derivatives in the
catalytic increase of fatty acid oxidation induced by carnitine. J
Lipid Res. 4:279–288. 1963.PubMed/NCBI
|
|
26
|
Britton CH, Schultz RA, Zhang B, Esser V,
Foster DW and McGarry JD: Human liver mitochondrial carnitine
palmitoyltransferase I: Characterization of its cDNA and
chromosomal localization and partial analysis of the gene. Proc
Natl Acad Sci USA. 92:1984–1988. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
McGarry JD and Brown NF: The mitochondrial
carnitine palmitoyltransferase system. From concept to molecular
analysis. Eur J Biochem. 244:1–14. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Swierczynski J, Hebanowska A and
Sledzinski T: Role of abnormal lipid metabolism in development,
progression, diagnosis and therapy of pancreatic cancer. World J
Gastroenterol. 20:2279–2303. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kroemer G and Pouyssegur J: Tumor cell
metabolism: cancer's Achilles' heel. Cancer Cell. 13:472–482. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lin H, Lu JP, Laflamme P, Qiao S, Shayegan
B, Bryskin I, Monardo L, Wilson BC, Singh G and Pinthus JH:
Inter-related in vitro effects of androgens, fatty acids and
oxidative stress in prostate cancer: A mechanistic model supporting
prevention strategies. Int J Oncol. 37:761–766. 2010.PubMed/NCBI
|
|
31
|
Pucci S, Zonetti MJ, Fisco T, Polidoro C,
Bocchinfuso G, Palleschi A, Novelli G, Spagnoli LG and Mazzarelli
P: Carnitine palmitoyl transferase-1A (CPT1A): A new tumor specific
target in human breast cancer. Oncotarget. 7:19982–19996. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Trajkovski M, Hausser J, Soutschek J, Bhat
B, Akin A, Zavolan M, Heim MH and Stoffel M: MicroRNAs 103 and 107
regulate insulin sensitivity. Nature. 474:649–653. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Bhatia H, Pattnaik BR and Datta M:
Inhibition of mitochondrial β-oxidation by miR-107 promotes hepatic
lipid accumulation and impairs glucose tolerance in vivo. Int J
Obes (Lond). 40:861–869. 2016. View Article : Google Scholar
|
|
34
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
35
|
Caley DP, Pink RC, Trujillano D and Carter
DR: Long noncoding RNAs, chromatin, and development. Scientific
World Journal. 10:90–102. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Gibb EA, Enfield KS, Stewart GL, Lonergan
KM, Chari R, Ng RT, Zhang L, MacAulay CE, Rosin MP and Lam WL: Long
non-coding RNAs are expressed in oral mucosa and altered in oral
premalignant lesions. Oral Oncol. 47:1055–1061. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Guo S, Chen W, Luo Y, Ren F, Zhong T, Rong
M, Dang Y, Feng Z and Chen G: Clinical implication of long
non-coding RNA NEAT1 expression in hepatocellular carcinoma
patients. Int J Clin Exp Pathol. 8:5395–5402. 2015.PubMed/NCBI
|
|
38
|
Chakravarty D, Sboner A, Nair SS,
Giannopoulou E, Li R, Hennig S, Mosquera JM, Pauwels J, Park K,
Kossai M, et al: The oestrogen receptor alpha-regulated lncRNA
NEAT1 is a critical modulator of prostate cancer. Nat Commun.
5:53832014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Linher-Melville K, Zantinge S, Sanli T,
Gerstein H, Tsakiridis T and Singh G: Establishing a relationship
between prolactin and altered fatty acid β-Oxidation via carnitine
palmitoyl transferase 1 in breast cancer cells. BMC Cancer.
11:562011. View Article : Google Scholar
|
|
40
|
Mishra S, Yadav T and Rani V: Exploring
miRNA based approaches in cancer diagnostics and therapeutics. Crit
Rev Oncol Hematol. 98:12–23. 2016. View Article : Google Scholar
|
|
41
|
Wu Q, Guo L, Jiang F, Li L, Li Z and Chen
F: Analysis of the miRNA-mRNA-lncRNA networks in ER plus and
ER-breast cancer cell lines. J Cell Mol Med. 19:2874–2887. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Ye S, Yang L, Zhao X, Song W, Wang W and
Zheng S: Bioinformatics method to predict two regulation mechanism:
TF-miRNA-mRNA and lncRNA-miRNA-mRNA in Pancreatic Cancer. Cell
Biochem Biophys. 70:1849–1858. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Chiyomaru T, Fukuhara S, Saini S, Majid S,
Deng G, Shahryari V, Chang I, Tanaka Y, Enokida H, Nakagawa M, et
al: Long Non-coding RNA HOTAIR is targeted and regulated by miR-141
in human cancer cells. J Biol Chem. 289:12550–12565. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Braconi C, Kogure T, Valeri N, Huang N,
Nuovo G, Costinean S, Negrini M, Miotto E, Croce CM and Patel T:
microRNA-29 can regulate expression of the long non-coding RNA gene
MEG3 in hepatocellular cancer. Oncogene. 30:4750–4756. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Faghihi MA, Modarresi F, Khalil AM, Wood
DE, Sahagan BG, Morgan TE, Finch CE, St Laurent G III, Kenny PJ and
Wahlestedt C: Expression of a noncoding RNA is elevated in
Alzheimer's disease and drives rapid feed-forward regulation of
beta-secretase. Nat Med. 14:723–730. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Salmena L, Poliseno L, Tay Y, Kats L and
Pandolfi PP: A ceRNA Hypothesis: The Rosetta stone of a hidden RNA
language? Cell. 146:353–358. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Keniry A, Oxley D, Monnier P, Kyba M,
Dandolo L, Smits G and Reik W: The H19 lincRNA is a developmental
reservoir of miR-675 that suppresses growth and lgf1r. Nat Cell
Biol. 14:659–665. 2012. View Article : Google Scholar : PubMed/NCBI
|