Introduction
Malignant mesothelioma (MM) is an aggressive and
fatal tumor, frequently related to the prolonged exposure to
asbestos. Although it is a rare disease, its incidence has been
increasing in recent years due to the use of asbestos for many
years in the past (1,2). Better survival is achieved with
multimodal approaches, in particular when surgery, radiotherapy and
chemotherapy (platinum compounds-pemetrexed) are used.
Nevertheless, treatment has a moderate impact on survival; thus,
novel therapeutic strategies are urgently required to improve
prognosis, for example the use of immunotherapy (3). According to the recent pathological
classification proposed by the World Health Organization,
mesotheliomas can be divided into three main subtypes: Epithelioid,
sarcomatoid and biphasic (4).
Epithelioid MM is the most common subtype and has better prognosis,
while the sarcomatoid one is the rarest type with the poorest
prognosis (4). MM has been
reported as an immunogenic tumor that induces infiltration of
immune effector cells, regulatory T cells and cytokines (5,6); the
immunological aspects of the disease require further
investigation.
Is well known that tumor immune microenvironment
(TME) serves a major role in neoplastic progression, facili tating
tumor cell evasion from adaptive immunity and T-cell checkpoint
pathways (7). Among the factors
implicated in immune response inhibition by tumor cells, programmed
death-1 ligand 1 (PD-1) and its main ligand PD-L1 play a key role
(7). PD-L1 acts as a negative
regulator of T lympho cytes (8-10):
It is expressed by T-cells, B cells, monocytes and several
tumor-infiltrating lymphocytes (TILs) and it has an important role
in attenuating antitumor immune response. In addition, PD-L1
expression has been widely reported in several malignant tumors
(11). Cancer cells expressing
PD-L1 promote the apoptosis of antigen-specific human T-cell clones
(12) and inhibit CD4+
and CD8+ T-cell activation, thus decreasing the immune
action against tumor cells (12).
Immune check point inhibitors (ICIs) have already shown promising
results in other tumors (13), and
are also under investigation in MM in which the expression of PD-L1
has been detected, regard less of the predictive value in this
neoplasia (3,14). ≥1% PD-L1 expression is reported
with different percentages in patients with MM (15–21),
with higher positivity rates in non-epithelioid subtypes (18,19).
Given the interaction between immune infiltrate and
neoplastic cells, the characterization of TME could be of great
value not only to understand the immune response, but also to
determine its possible prognostic value.
Additionally, the value of the combined immunoscore
(PD-L1 expression in both tumor and immune cells) is increasing due
to recent data in other tumor types (e.g. head and neck cancer). In
particular the combined immunoscore seems to better predict
efficacy to immunotherapy (22).
Nevertheless, many studies into this field are lacking conclusive
data (23).
An important role in various malignancies is played
by mismatch repair (MMR) genes, such as mutL homolog 1
(MLH1), mutS homolog 2 (MSH2), mutS homolog 6
(MSH6) and PMS1 homolog 2, mismatch repair system component
(PMS2). Some mismatch repair-deficient tumors are sensitive
to immune check point blockade, as of the increased number of
neoantigens encoded by cancers, which enhance the anti-tumor
response (24-26). Several studies have shown that the
number of mutations in MMR genes correlates with the response to
PD-1 blockade, providing further support for a relationship between
mutational burden and treatment response (25,27).
MMR-deficiency may be considered in the identification of patients
who may benefit from PD-1 pathway blockade.
MM has a lower tumor mutational burden when compared
with other cancers (28); however,
some patients may benefit from ICIs; this response may be related
to the presence of immune cells in tumor microenvironment.
The aim of this retrospective study was to evaluate
PD-L1 expression in tumor cells and TILs in different histological
subtypes of MM, and to characterize the immune microenvironment.
MMR protein expression was been also investigated. Additionally,
these parameters were evaluated in association with
clinicopathological features to explore their possible prognostic
value.
Materials and methods
Patient selection and samples
Fifty-eight consecutive cases of MM were collected
from January 2014 to December 2017 at the Division of Medical
Oncology of Azienda Ospedaliero-Universitaria Policlinico of
Modena. Out of the initial cohort, 55 surgical formalin fixed and
paraffin embedded specimens were available from the Unit of
Pathology of Modena and were histologically identified with a light
microscope as MM after staining with hematoxylin and eosin. The
cases included 10 samples from females and 45 from males. The
median age at diagnosis was 74 years old (range 45–88 years). All
the samples were obtained through surgical biopsies. The study was
approved by the Ethical Committee of Azienda
Ospedaliero-Universitaria of Modena (393/17).
Immunohistochemistry
All immunostainings were performed on the automated
system Ventana BenchMark XT (Roche Diagnostics) (29) using the detection kit UltraView
DAB; in the case of double staining for CD4 and CD8, the UltraView
universal alkaline phosphatase red detection kit (Roche
Diagnostics) according to the manufacturer's instructions. The
following primary antibodies were used: Anti PD-L1 (clone 22C3,
cat. no. M3653, Dako; Agilent Technologies, Inc.) diluted 1:100,
anti human CD4 (cat. no. 790-4423) and anti-human CD8 (cat. no.
790-4460) for TILs (Roche Diagnostics), and antibodies against MLH1
(cat. no. 790-5091), MSH2 (cat. no. 790–5093), MSH6 (cat. no.
790-5092) and PMS2 (cat. no. 790-5094) for MMR-related protein
expres sion (Roche Diagnostics) prediluted. The use of 22C3 PD-L1
staining on Ventana's platform was supported by the assuring
results reported in a recent paper (30). Positive controls were included in
each staining run, human placenta for PD-L1, human tonsil for the
immune markers, obtained from the Unit of Pathology of Modena. For
MMR protein expression, a positive internal control was represented
by non neoplastic adjacent cells present in each slide. All
sections were scored by two observers including one pathologist.
PD-L1 expression was retained when tumor cells or TILs displayed a
membranous staining, partial or complete of any intensity. The
percentage of PD-L1 positive tumor cells relative to all viable
tumor cells present in the specimen was recorded. PD-L1 positivity
was defined as ≥1% of tumor cells or TILs staining by
immunohistochemistry (31). Tissue
sections were analyzed for the presence of tumor lymphocytic
infiltration scored according to Marcq et al (15). Therefore, we analyzed the presence
of lymphocytes and lymphoid aggregates (score 0=0 aggregates; score
1=1-5 aggregates; score 2=5-10 aggregates; score 3=>10
aggregates) (15). A lymphoid
aggregate was defined as ≥50 lymphocytes clustered together
(15). Then, cases were classified
in negative when absence of tumor lymphocytic infiltration was
observed; immunoscore 1+ (score 1); immunoscore 2+ (score 2);
immunoscore 3+ (score 3). To analyze the CD4+ and
CD8+ components, a percentage of expression of each
marker was obtained via the evaluation of the total TILs.
Statistical analysis
Descriptive statistics of the study patients were
reported. Continuous variables were expressed as mean, median,
standard deviation and range, while categorical variables as
absolute and percentage frequencies. Associations between
categorical variables were assessed by means of Fisher exact tests.
The Mann-Whitney test was performed to evaluate the relationship
between PD-L1 expression and histological subtype (epithelioid vs.
others), stage at diagnosis, smoking history, professional asbestos
exposure and gender. Overall survival times from diagnosis were
graphically reported as Kaplan-Meier survival curves which were
compared using a log-rank test. The survival analysis compared
PD-L1 expression using different systems: In the first analysis,
PD-L1 expression evaluated on tumor cells, TILs, and on both cells
types was stratified into <1, 1-49 and ≥50%. In the second
analysis, PD-1 expression was stratified into <50 and ≥50%, and
in the third analysis into <1 and ≥1%. Comparisons between
survival curves were expressed as hazard ratios (HR) with 95%
confidence interval from a Cox regression model. Statistical
analyses were performed with R 3.4.3 (The R Foundation for statis
tical computing, Wien). P<0.05 was considered to indicate a
statistically significant difference.
Results
Patient population
Samples comprised 44 epithelioid, 3 sarcomatoid, 7
biphasic and 1 desmoplastic tumors; 51 involved the pleura and 4
the peritoneum. According to the American Joint Committee on Cancer
(32), 18 tumors were of stage I,
13 of stage II, 15 of stage III and 5 of stage IV; for 4 cases, the
stage could not be evaluated. None of the patients received
immunotherapy. The clinicopathological features of our MM series
are summarized in Table I.
 | Table IClinicopathological features of
mesothelioma patients. |
Table I
Clinicopathological features of
mesothelioma patients.
| Clinicopathological
features | Patients n=55 |
|---|
| Age (mean ± SD,
years) | 73.7±8.2 |
| Median
(range) | 74 (43-88) |
| Sex | |
| Male | 45 (82%) |
| Female | 10 (18%) |
| Histological
subtype | |
| Epithelioid | 44 (80%) |
| Sarcomatoid | 3 (5%) |
| Biphasic | 7 (13%) |
| Desmoplastic | 1 (2%) |
| Smoking
history | |
| Never smoker | 78 (14%) |
| Current/former
smoker | 28 (51%) |
| Unknown | 19 (35%) |
| Professional
asbestos exposure | |
| Yes | 30 (55%) |
| No | 25 (45%) |
| Site | |
| Peritoneum | 4 (7%) |
| Pleural | 51 (93%) |
| Stage | |
| I | 18 (33%) |
| II | 13 (24%) |
| III | 15 (27%) |
| IV | 5 (9%) |
| Unknown | 4 (7%) |
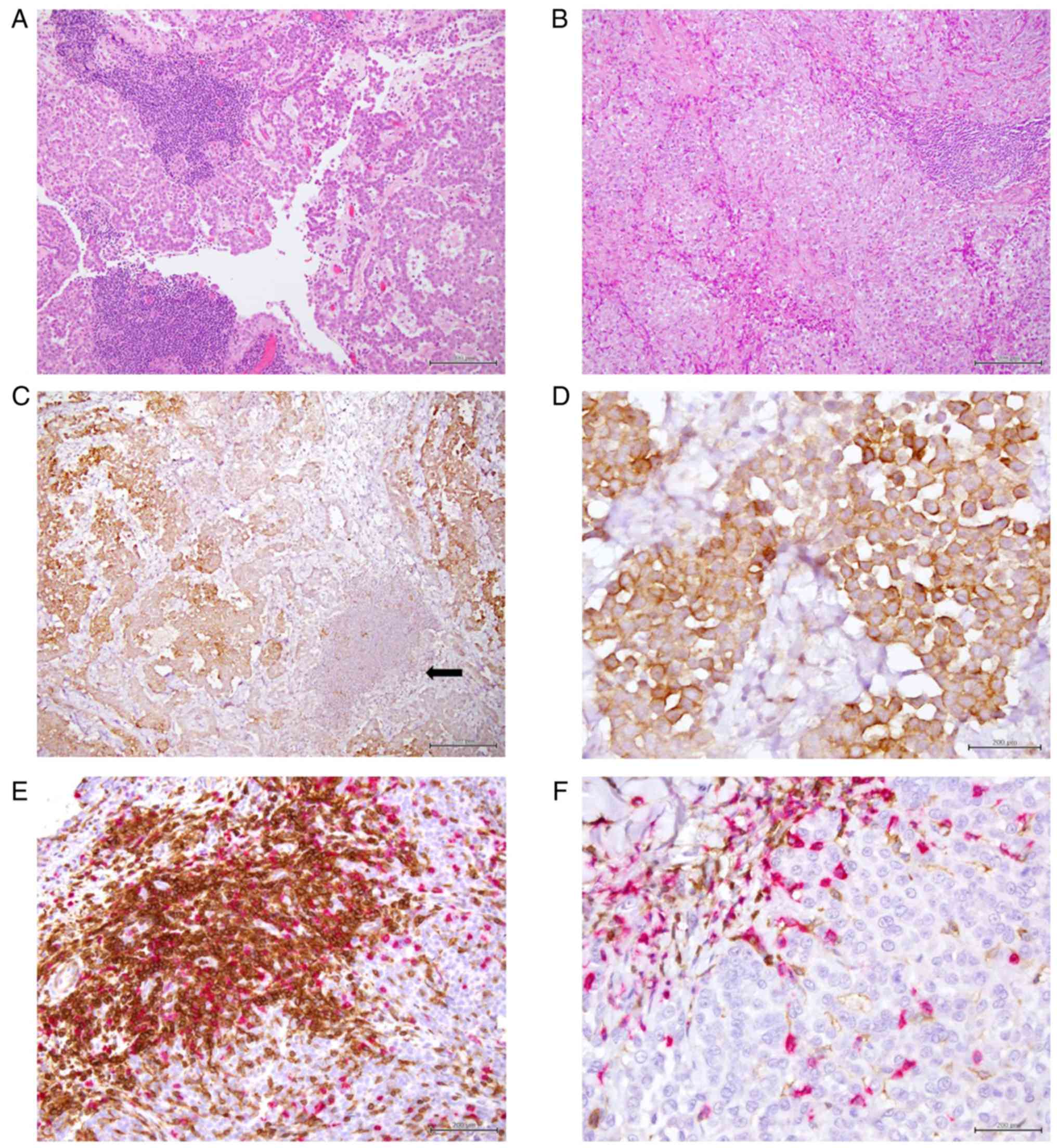
Immune composition of MM tissue
samples
TILs were observed in all but two samples (53/55).
The majority of cases had an immunoscore 1+ (30/53), while an
immunoscore 2+ or 3+ was observed in 15/53 and 8/53 tissue samples,
respectively. Tissue sections were stained for two immune cell
markers. CD4+ and CD8+ TILs were present in all 53
samples. The CD8+/CD4+ ratio was calculated
dividing the percentage of CD8 positive cells on CD4 positive
cells; the predominance of CD8+ compared with
CD4+ was defined as a ratio >1. A predominance of
CD8+ expression was highlighted in 8 cases (15%).
Representative examples of different immunoscore and
CD4+ and CD8+ predominance are presented in
Fig. 1.
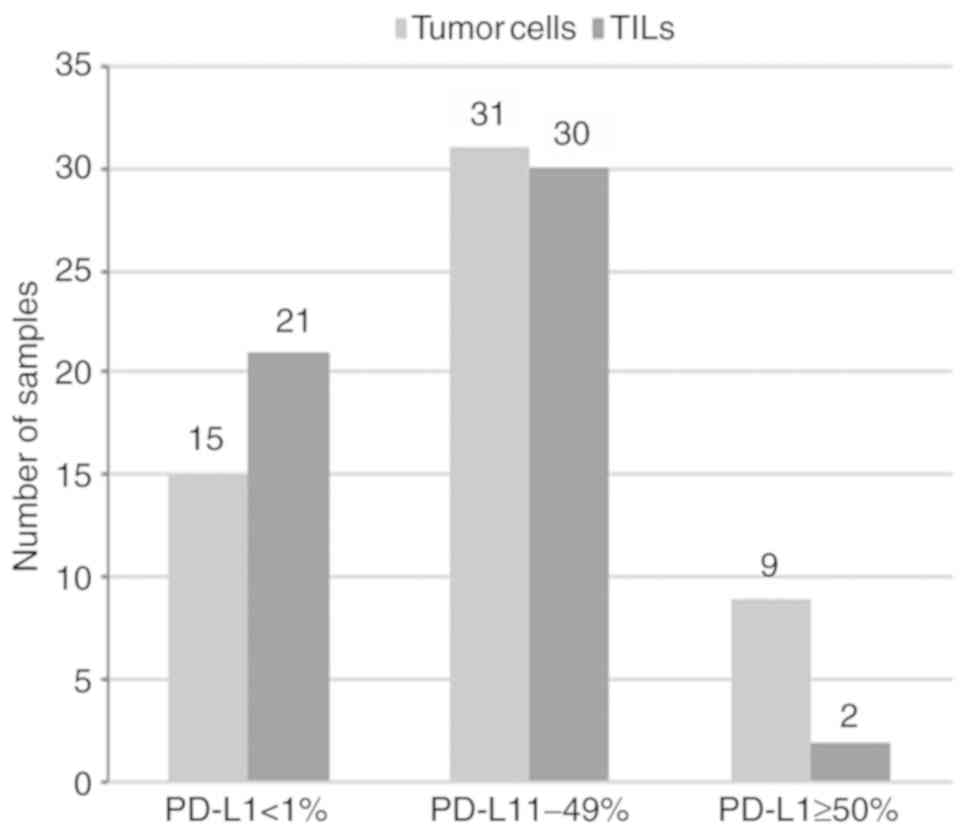
PD-L1 expression on tumor cells and TILs,
and its association with tumor lymphocyte infiltration
The immu nohistochemical analysis for PD-L1, using a
threshold of ≥1% of tumor cells, showed membranous expression of
PD-L1 in 40 tumors (73%) whereas 15 (27%) were negative (Fig. 2). According to Herbst et al
(31), we added a third
stratification variable considering PD-L1 expression ≥50% of cells:
Among the 40 positive cases, it was observed in 9 cases (5
epithelioid, 2 sarcomatoid, 1 biphasic and 1 desmoplastic). In two
of them (one biphasic and one epithelioid), this high positivity
was present both on tumor cells and on TILs. The expression of
PD-L1 on TILs was detected in 32 cases (60%), of which two samples
exhibited >50% of PD-L1 staining (Fig. 2). PD-L1 positivity was evaluated
both on tumor cells and TILs. In 11 cases negative expression was
detected in both cell types, while 28 cases were positive both on
tumor cells and TILs. In 23 of these, the results were concordant
with PD-L1 between 1-49% and in two samples with PD-L1 ≥50%; while
in three cases PD-L1 ≥50% was found only on tumor cells (Table II). In 10 cases PD-L1 positivity
was detected only on tumor cells, 7 with PD-L1 between 1-49% and 3
with ≥50% (Table II). On the
contrary, in 4 cases, PD-L1 was positive only on TILs, yet to a low
degree (1%) (Table II).
 | Table IICombined expression of PD-L1 on TC
and TILs. |
Table II
Combined expression of PD-L1 on TC
and TILs.
| Cells | PD-L1 ≥1%
| PD-L1 <1%
|
|---|
| 1-49% | ≥50% | |
|---|
| TC and TILs | 28/53 (54%) | 11/53 (20%) |
| 23/53 | 2/53; 3/53
(TC) | |
| TC only | 10/53 (19%) | |
| 7/53 | 3/53 | |
| TILs only | 4/53 (7%) | |
| 4/53 | | |
The association between PD-L1 expression and
CD4+ or CD8+ predominance was reported in
Table III (P=0.058). A
predominance of CD4+ infiltrate was observed in the majority of
tumors independently of PD-L1 expression. The correlation of PD-L1
and immunoscore is displayed in Table III. Higher immunoscore (2+/3+)
was significantly associated to an inter mediate PD-L1 expression
(1-49%) (P=0.019). Representative examples of PD-L1 immunostaining
are shown in Fig. 1.
 | Table IIIAssociation between of PD-L1
expression on tumor cells and CD4+ or CD8+
predominance and immunoscore. |
Table III
Association between of PD-L1
expression on tumor cells and CD4+ or CD8+
predominance and immunoscore.
| PD-L1 expression on
tumor cells | Number of
samplesa |
CD8+/CD4+
| P-value | Immunoscore
| P-value |
|---|
| Predominance of
CD8+ (%) | Predominance of
CD4+ (%) | 1+ (%) | 2+/3+ (%) |
|---|
| ≥50% | 8 | 3 (37.5) | 5 (62.5) | P=0.058b | 5 (62.5) | 3 (37.5) | P=0.019b |
| 1–49% | 31 | 2 (6.5) | 29 (93.5) | | 13 (41.9) | 18 (58.1) | |
| <1% | 14 | 3 (21.4) | 11 (78.6) | | 12 (85.7) | 2 (14.3) | |
| Total | 53 | 8 (15.1) | 45 (84.9) | | 30 (56.7) | 23 (43.3) | |
MMR protein expression
In all the analyzed MM samples, no alteration in MMR
staining was found.
Association between PD-L1 expression and
clinicopathological features
≥50% PD-L1 expression on tumor cells was
significantly less common in the epithelioid subtype than in the
other types (P=0.047). The absence of an association between PD-L1
expression was found with smoking history, asbestos exposure and
stage. Furthermore, the link between gender with immune checkpoints
showed no statistically significant differences. The association
with PD-L1 expression on tumor cells was evaluated with three
systems: <1 and ≥1%; <1, 1-49, ≥50; <50 and ≥50% (P=0.282;
P=0.572; P=0.202). Also, the CD8+/CD4+ ratio
(P=0.097) and immunoscore 2+/3+ vs. 1+ (P=0.164) exhibited no
association with gender (data not shown).
Association of PD-L1 expression and tumor
immune infiltrate with survival
After an average follow up of 14.4 months, 22
(40.0%) patients were still alive; all but two had epithelioid
mesothelioma, and the majority of them had PD-L1 expression
<50%. The median overall survival time was 13.3 months. Median
survival for patients for negative (<1%), moderate (from 1-49%)
and strong PD-L1 staining (≥50%) was 13.0, 20.6 and 5.1 months,
respectively. Median survival for epithelioid subtype was 17.3
months, while for other subtypes it was 5.9 months. According to
the CD8+/CD4+ ratio, median survival in
patients with CD8+ predominance was 3.3 months, while
for CD4+ predominance was 17.3 months. Furthermore,
patients with an immunoscore 1+ had 13.0 months of overall median
survival, whereas it was equal to 20.6 months in patients with an
immunoscore 2+/3+.
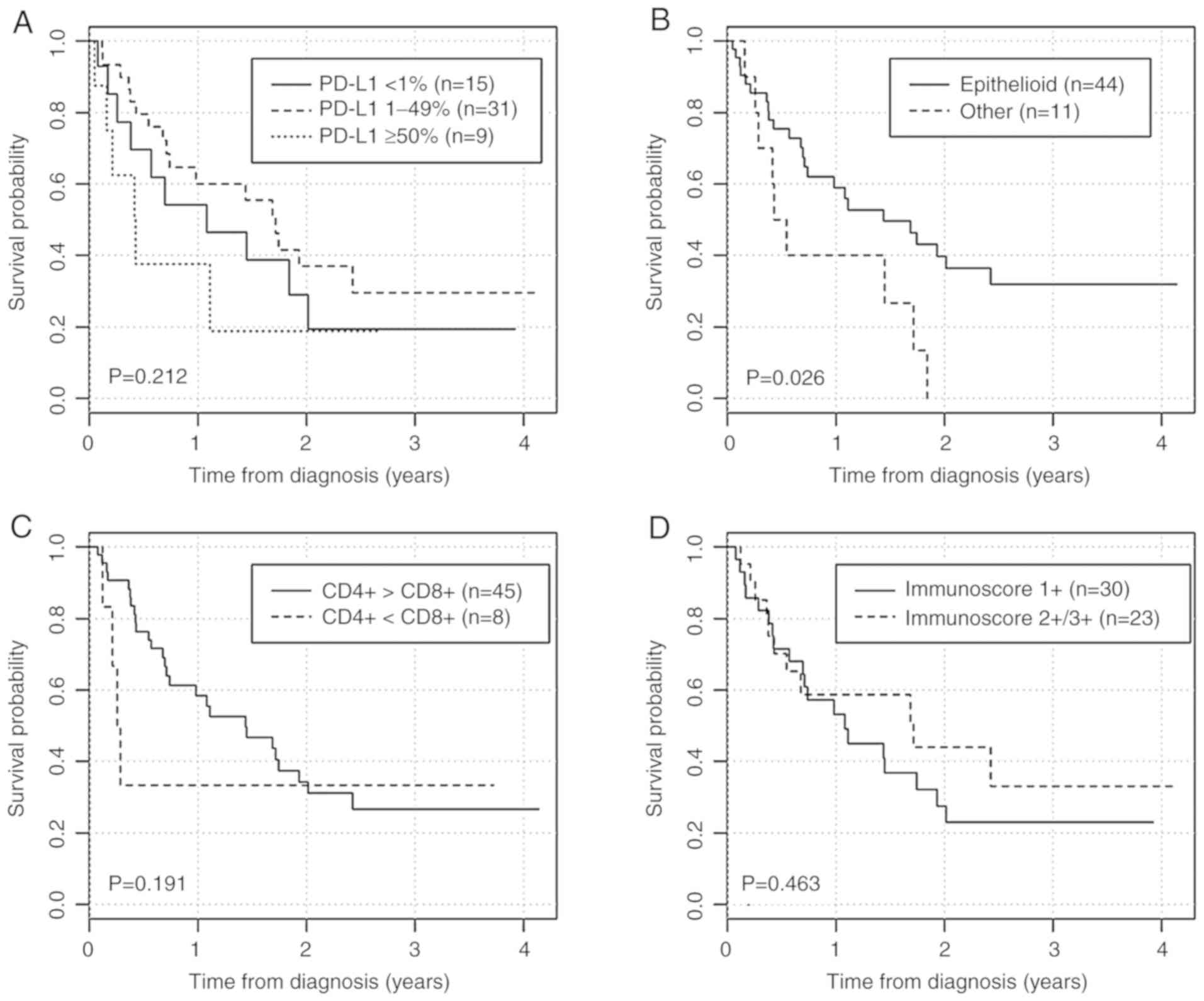
Survival curves based on the PD-L1 expression
percentage (<1, 1-49, ≥50%), histological subtype,
CD4+ or CD8+ predominance (expressed as
CD8+/CD4+ ratio; Table IV) and immunoscore (2+/3+ vs. 1+)
are presented in Fig. 3 and HR
values are listed in Table IV.
Additionally, survival curves based on PD-L1 expression on tumor
cells (<1 and ≥1%) exhibited no statistically significant
differences (Fig. S1).
 | Table IVUnivariate analysis of pathological
variables on overall survival. |
Table IV
Univariate analysis of pathological
variables on overall survival.
| Variables | Univariate Cox
analysis
|
|---|
| HR | 95 CI% | P-value |
|---|
| PD-L1 expression on
tumor cells | | | |
| ≥50 vs.
<1% | 1.58 | 0.57-4.39 | 0.376 |
| 1-49 vs.
<1% | 0.70 | 0.32-1.53 | 0.367 |
| Histological
subtype | | | |
| Epithelioid vs.
Other | 0.42 | 0.19-0.92 | 0.19-0.92 |
|
CD8+/CD4+ Ratio | | | |
| ≥1 vs. <1 | 1.96 | 0.68-5.67 | 0.215 |
| Immunoscore | | | |
| 2+/3+ vs. 1+ | 0.76 | 0.37-1.60 | 0.476 |
Epithelioid subtype was associated with lower risk
of death during the follow-up period (HR=0.42-95% CI=0.19-0.92;
P=0.031). Moreover, we observed a trend toward a poorer prognosis
for PD-L1 expression on tumor cells ≥50%, lower immunoscore (1+)
and CD8+ tumor-infiltrating lymphocytes predominance.
When we consider only two groups of PD-L1 expression (<50 and
≥50%), the association with survival was more apparent; however,
statistical significance was not observed (P=0.116). Furthermore,
the association between PD-L1 expression on TILs (P=0.906 for
<1, 1-49 and ≥50%; P=0.657 for <1 and ≥1%) and the combined
expression on tumor cells and TILs (P=0.285) with survival were not
statistically significant.
As the extent of PD-L1 positivity was less observed
in the epithelioid subtype, we applied the survival analysis only
on these cases. Under these conditions, no statistically
significant association was reported between PD-L1 expression and
survival (P=0.362 for <1, 1-49 and ≥50%; P=0.974 for <1 and
≥1%; P=0.168 for <50 and ≥50%). Additionally,
CD8+/CD4+ ratio (P=0.474) and immunoscore
2+/3+ vs. 1+ (P=0.183) were not linked to survival.
Discussion
The present study aimed to evaluate PD-L1 expression
on tumor cells and on TILs, to characterize the immune
microenvironment and investigate MMR protein expression in MM. We
also explored the possible prognostic value of these
parameters.
In our series of MM, PD-L1 expression on tumor cells
was observed in 73% of cases using a cut-off value of ≥1%. This
finding is in accordance with several studies (13,16,17),
which reported percentage from 54-68%, but differs from others
(18–21), which revealed a lower percentage of
positivity (from 20.7-41.8%). This is may be due to differences in
sample size, antibodies and cut-off values, as well as patient
heterogeneity among different studies. Of note, we reported an
association between PD-L1 positivity and histotype. Furthermore,
some studies have found lower PD-L1 expression in epithelioid
subtype compared with other subtypes (15-18).
PD-L1 on tumor cells was not always expressed simultaneously with
PD-L1 on TILs as already reported (15,16,21)
and in concordance with the findings in other tumor types (33).
PD-L1 expression has been correlated with worse
prognosis but better response to anti PD-1-antibodies in many
cancer types, such as NSCLC (34-37).
In MM, several studies have demonstrated that PD-L1 tumor cells
expression was an independent factor for worse overall survival
(16,18-21).
Our results showed that PD-L1 expression does not appear to be
significantly associated with survival despite a trend toward a
poorer prognosis with PD-L1 expression in ≥50% on tumor cells. The
absence of a significant association with survival is in agreement
with two recent studies, in which a specific antibody (SP142)
(38) and four different
antibodies were employed (17).
This finding was observed even when we compared survival with PD-L1
expression on TILs, and with the combined expression of tumor cells
and TILs. Therefore, the value of PD-L1 requires further
investigation and standardization of its assessment is needed to
improve clinical practice.
The predictive value of immunohistochemical
screening concerning the response to anti PD-L1 treatment for MM
needs to be studied. Moreover, clinical practice may demand access
to different PD-L1 antibodies matched with specific staining plat
forms and score criteria are required to apply a novel diagnostic
tool. In addition, PD-L1 expression is a heterogeneous continuum in
tumor cells that cannot be stated only as ‘present' or ‘absent'.
The identification of a cut-off value for expression positivity
based on immunohistochemistry remains a challenge at present. Also,
the location of PD-L1 expression should be considered; it is
presumed that membranous expression of PD-L1 may be the most
relevant as PD-1 mediates downstream signaling cascades only when
it has been ligated (39).
Pathologists, oncologists and scientists should work together in
order to develop guidelines to apply in this new field of cancer
treatment.
The role of the tumor immune microenvironment in
neoplastic progression is supported by a large body of evidence
(40). In our series of MM, we
found in less than half of cases (23/53), a higher degree of
lymphocytic infiltration (expressed as immunoscore 2+/3+) which was
significantly associated to an intermediate level of PD-L1
expression. In the majority of cases (85%), immune cell
infiltration was characterized by CD4+ predominance
independently of PD-L1 expression, while a predominance of
CD8+ was detected only in 15% of mesotheliomas. Our data
showed a trend toward a poorer prognosis with lower immunoscore
(1+) and CD8+ TIL predominance. These findings are in line with
recent literature data: CD4+ TILs are associated with better
prognosis; MM cases characterized by higher CD8+ T lymphocyte
expression and a lower CD4/CD8-positive ratio achieve worse
survival (13,16,20).
In contrast, a favorable prognostic value of high levels of TILs in
several tumors have been reported (41-43)
and also in MM (44,45). However, as their true biological
role in suppressing and promoting tumor growth is governed by a
variety of positive and negative T-cell factors, as well as the
PD/PD-L1 pathway, further investigation is needed.
The role of MMR in the neoplastic process is well
established for several tumors (24). MMR-deficiency might be taken into
consideration in order to identify those patients who may benefit
from PD-1 pathway blockade. Some studies have reported a
contradictory relationship between mutational burden and treatment
response (25,27), but further study is required. A
recent report examined this in MM (46), and revealed a very low frequency
(1.8%) of MMR deficiency. In our study, none of the examined MM
samples presented loss of expression of the proteins codified by
the four genes (MLH1, MSH2, MSH6 and PMS2). Taken
together, these data suggest that the MMR pathway is less involved
in the pathogenesis of mesothelioma. Other mechanisms should be
investigated to understand the response to ICI.
In conclusion, in our series of 55 MM samples, we
reported that the epithelioid subtype is an independent factor of
better prognosis as previously reported in the literature. The
other immunological parameters, including PD-L1, immunoscore and
predominance of CD8+ TIL, were not significantly
associated with survival, although we observed a trend toward a
poorer prognosis for increased PD-L1 expression on tumor cells,
lower immunoscore (1+) and CD8+ TIL predominance.
However, we highlight the importance of the tumor immune
microenvironment and the standardization of immunohistochemical
techniques for PD-L1 assessment in order to obtain comparable
results to improve understanding of the pathogenesis of this very
aggressive neoplasia.
We reported a case series in which the immunological
parameters analyzed may provide insight into the prognostic aspects
of the disease. Investigation should also be conducted in a
clinical setting with patients receiving immunotherapy to validate
the predictive value of these parameters. Firstly, it would be
important for pathologists and oncologists to work together to
develop homogeneous guidelines for this new field of cancer
treatment.
Supplementary Data
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
LL and AM contributed to the conception of the
study. FBer, GG, CB, FBar, SC obtained the clinical data. LL and GG
wrote the manuscript and analyzed the data. FBan performed
statistical analysis. LF performed immunohistochemical analysis. LB
and AA obtained histological samples. LL, AA and LS interpreted the
immunohisto chemical results. All authors read and approved the
final version of the manuscript.
Ethical approval and consent to
participate
This study was approved by the Ethical Committee of
the Azienda Ospedaliero-Universitaria of Modena (393/17).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
Not applicable.
References
|
1
|
Merlo DF, Bruzzone M, Bruzzi P, Garrone E,
Puntoni R, Maiorana L and Ceppi M: Mortality among workers exposed
to asbestos at the shipyard of Genoa, Italy: A 55 years follow-up.
Environ Health. 17:942018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ferrante D, Chellini E, Merler E, Pavone
V, Silvestri S, Miligi L, Gorini G, Bressan V, Girardi P, Ancona L,
et al: Italian pool of asbestos workers cohorts: Mortality trends
of asbestos-related neoplasms after long time since first exposure.
Occup Environ Med. 74:887–898. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Alley EW, Lopez J, Santoro A, Morosky A,
Saraf S, Piperdi B and van Brummelen E: Clinical safety and
activity of pembrolizumab in patients with malignant pleural meso
thelioma (KEYNOTE-028): Preliminary results from a non-randomised,
open-label, phase 1b trial. Lancet Oncol. 18:623–630. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Travis WD, Brambilla E, Burke AP, Marx A
and Nicholson AG: WHO classification of tumours of the Lung,
Pleura, Thymus and Heart. WHO Press WHO Classification of Tumors.
7. 4th edition. Lyon; France: 2015
|
|
5
|
Hegmans JP, Hemmes A, Hammad H, Boon L,
Hoogsteden HC and Lambrecht BN: Mesothelioma environment comprises
cyto kines and T-regulatory cells that suppress immune responses.
Eur Respir J. 27:1086–1095. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Anraku M, Cunningham KS, Yun Z, Tsao MS,
Zhang L, Keshavjee S, Johnston MR and de Perrot M: Impact of
tumor-infiltrating T cells on survival in patients with malignant
pleural mesothelioma. J Thorac Cardiovasc Surg. 135:823–829. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chen DS and Mellman I: Oncology meets
immunology: The cancer-immunity cycle. Immunity. 39:1–10. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Freeman GJ, Long AJ, Iwai Y, Bourque K,
Chernova T, Nishimura H, Fitz LJ, Malenkovich N, Okazaki T, Byrne
MC, et al: Engagement of the PD-1 immunoinhibitory receptor by a
novel B7 family member leads to negative regu lation of lymphocyte
activation. J Exp Med. 192:1027–1034. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Keir ME, Francisco LM and Sharpe AH: PD-1
and its ligands in T-cell immunity. Curr Opin Immunol. 19:309–314.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Francisco LM, Salinas VH, Brown KE,
Vanguri VK, Freeman GJ, Kuchroo VK and Sharpe AH: PD-L1 regulates
the development, maintenance, and function of induced regulatory T
cells. J Exp Med. 206:3015–3029. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Teng F, Meng X, Kong L and Yu J: Progress
and challenges of predictive biomarkers of anti PD-1/PD-L1
immunotherapy: A systematic review. Cancer Lett. 414:166–173. 2018.
View Article : Google Scholar
|
|
12
|
Dong H, Strome SE, Salomao DR, Tamura H,
Hirano F, Files DB, Roche PC, Lu J, Zhu G, Tamada K, et al:
Tumor-associated B7-H1 promotes T-cell apoptosis: A potential
mechanism of immune evasion. Nat Med. 8:793–800. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Marcq E, Pauwels P, Van Meerbeeck JP and
Smits EL: Targeting immune checkpoints: New opportunity for
mesothelioma treat ment? Cancer Treat Rev. 41:914–924. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hassan R, Thomas A, Patel MR, Nemunaitis
JJ, Bennouna J, Powderly JD, Taylor MH, Dowlati A, Chen F, Leach J,
et al: Avelumab (MSB0010718C; anti-PD-L1) in patients with advanced
unresectable mesothelioma from the JAVELIN solid tumor phase Ib
trial: Safety, clinical activity, and PD-L1 expres sion. J Clin
Oncol. 34(15 Suppl): S8503. 2016. View Article : Google Scholar
|
|
15
|
Marcq E, Siozopoulou V, de Waele J, van
Audenaerde J, Zwaenepoel K, Santemans E, Heins N, Pauwels P, van
Meerbeeck JP and Smits EL: Prognostic and predic tive aspects of
the tumor immune microenvironment and immune checkpoints in
malignant pleural mesothelioma. Oncoimmunology. 6:e12612412016.
View Article : Google Scholar
|
|
16
|
Pasello G, Zago G, Lunardi F, Urso L, Kern
I, Vlacic G, Grosso F, Mencoboni M, Ceresoli GL, Schiavon M, et al:
Malignant pleural mesothelioma immune microenvironment and
checkpoint expres sion: Correlation with clinical-pathological
features and intratumor heterogeneity over time. Ann Oncol.
29:1258–1265. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Watanabe T, Okuda K, Murase T, Moriyama S,
Haneda H, Kawano O, Yokota K, Sakane T, Oda R, Inagaki H and
Nakanishi R: Four immunohistochemical assays to measure the PD-L1
expression in malignant pleural mesothelioma. Oncotarget.
9:20769–20780. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Mansfield AS, Roden AC, Peikert T, Sheinin
YM, Harrington SM, Krco CJ, Dong H and Kwon EC: B7-H1 expression in
malignant pleural mesothelioma is associated with sarcomatoid
histology and poor prognosis. J Thorac Oncol. 9:1036–1040. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Cedrés S, Ponce Aix S, Zugazagoitia J,
Sansano I, Enguita A, Navarro-Mendivii A, Martinez-Marti A,
Martinez P and Felip E: Analysis of expression of programmed cell
death 1 ligand 1 (PD-L1) in malignant pleural mesothelioma (MPM).
PLoS One. 10:e01210712015. View Article : Google Scholar
|
|
20
|
Thapa B, Salcedo A, Lin X, Walkiewicz M,
Murone C, Ameratunga M, Asadi K, Deb S, Barnett SA, Knight S, et
al: The immune microenvironment, genome-wide copy number aberra
tions, and survival in mesothelioma. J Thorac Oncol. 12:850–859.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Combaz-Lair C, Galateau-Sallé F,
McLeer-Florin A, Le Stang N, David-Boudet L, Duruisseaux M,
Ferretti GR, Brambilla E, Lebecque S and Lantuejoul S: Immune
biomarkers PD-1/PD-L1 and TLR3 in malignant pleural mesotheliomas.
Hum Pathol. 52:9–18. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Burtness B, Harrington KJ, Greil R,
Soulières D, Tahara M, De Castro G Jr, Psyrri A, Baste Rotllan N,
Neupane PC, Bratland A, et al: KEYNOTE-048: Phase 3 study of
first-line pembrolizumab (P) for recurrent/metastatic head and neck
squamous cell carcinoma (R/M HNSCC). In: Presented at ESMO
Congress; Munich. 2018
|
|
23
|
Minnema-Luiting J, Vroman H, Aerts J and
Cornelissen R: Heterogeneity in immune cell content in malignant
pleural mesothelioma. Int J Mol Sci. 19:–pii: E1041. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Baretti M and Le DT: DNA mismatch repair
in cancer. Pharmacol Ther. 189:45–62. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Dudley JC, Lin MT, Le DT and Eshleman JR:
Microsatellite instability as a biomarker for PD-1 blockade. Clin
Cancer Res. 22:813–820. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Cohen R, Pellat A, Boussion H, Svrcek M,
Lopez-Trabada D, Troudilloud I, Afchain P and André T:
Immunotherapy and metastatic colorectal cancers with microsatellite
instability or mismatch repair deficiency. Bull Cancer.
106:137–142. 2019. View Article : Google Scholar
|
|
27
|
Le DT, Durham JN, Smith KN, Wang H,
Bartlett BR, Aulakh LK, Lu S, Kemberling H, Wilt C, Luber BS, et
al: Mismatch repair deficiency predicts response of solid tumors to
PD-1 blockade. Science. 357:409–413. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chalmers ZR, Connelly CF, Fabrizio D, Gay
L, Ali SM, Ennis R, Schrock A, Campbell B, Shlien A, Chmielecki J,
et al: Analysis of 100,000 human cancer genomes reveals the
landscape of tumor mutational burden. Genome Med. 9:342017.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ponti G, Losi L, Martorana D, Priola M,
Boni E, Pollio A, Neri TM and Seidenari S: Clinicopathological and
biomolecular findings in Italian patients with multiple cutaneous
neurofibromas. Hered Cancer Clin Pract. 9:62011. View Article : Google Scholar
|
|
30
|
Neuman T, London M, Kania-Almog J, Litvin
A, Zohar Y, Fridel L, Sandbank J, Barshak I and Vainer GW: A
harmonization study for the use of 22C3 PD-L1 immunohistochemical
staining on ventana's platform. J Thorac Oncol. 11:1863–1868. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Herbst RS, Baas P, Kim DW, Felip E,
Pérez-Gracia JL, Han JY, Molina J, Kim JH, Arvis CD, Ahn MJ, et al:
Pembrolizumab versus docetaxel for previously treated,
PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE 010):
A randomised controlled trial. Lancet. 387:1540–1550. 2016.
View Article : Google Scholar
|
|
32
|
Rusch VW, Chansky K, Nowak AK, Rice D,
Kindler H, Gill RR and Travis WD: Malignant pleural mesothelioma.
Amin B: AJCC Cancer staging Manual. 8th edition. Switzerland:
Springer; pp. 457–468. 2017, View Article : Google Scholar
|
|
33
|
Taube JM: Unleashing the immune system:
PD-1 and PD-Ls in the pre-treatment tumor microenvironment and
correlation with response to PD-1/PD-L1 blockade. Oncoimmunology.
3:e9634132014. View Article : Google Scholar
|
|
34
|
Topalian SL, Sznol M, McDermott DF, Kluger
HM, Carvajal RD, Sharfman WH, Brahmer JR, Lawrence DP, Atkins MB,
Powderly JD, et al: Survival, durable tumor remission, and
long-term safety in patients with advanced melanoma receiving
nivolumab. J Clin Oncol. 32:1020–1030. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Garon EB, Rizvi NA, Hui R, Leighl N,
Balmanoukian AS, Eder JP, Patnaik A, Aggarwai C, Gubens M, Horn L,
et al: Pembrolizumab for the treatment of non-small-cell lung
cancer. N Engl J Med. 372:2018–2028. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Borghaei H, Paz-Ares L, Horn L, Spigel DR,
Steins M, Ready NE, Chow LQ, Vokes EE, Felip E, Holgado E, et al:
Nivolumab versus docetaxel in advanced nonsquamous non-small-cell
lung cancer. N Engl J Med. 373:1627–1639. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Brahmer J, Reckamp KL, Baas P, Crinò L,
Eberhardt WE, Poddubskaya E, Antonia S, Pluzanski A, Vokes EE,
Holgado E, et al: Nivolumab versus docetaxel in advanced
squamous-cell non-small-cell lung cancer. N Engl J Med.
373:123–135. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Valmary Degano S, Colpart P, Villeneuve L,
Monnien F, M'Hamndi L, Lang Averous G, Capovilla M, Bibeau F,
Laverriere MH, Verriele-Beurrier V, et al: Immunohistochemical
evaluation of two antibodies against PD-L1 and prognostic
significance of PD-L1 expression in epithelioid peritoneal malig
nant mesothelioma: A RENAPE study. Eur J Surg Oncol. 43:1915–1923.
2017. View Article : Google Scholar
|
|
39
|
Hamid O and Carvajal RD: Anti-programmed
death-1 and anti-programmed death-ligand 1 antibodies in cancer
therapy. Expert Opin Biol Ther. 13:847–861. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Mittal D, Gubin MM, Schreiber RD and Smyth
MJ: New insights into cancer immunoediting and its three component
phases-elimination, equilibrium and escape. Curr Opin Immunol.
27:16–25. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Al-Shibli KI, Donnem T, Al-Saad S, Persson
M, Bremnes RM and Busund LT: Prognostic effect of epithelial and
stromal lymphocyte infiltration in non small cell lung cancer. Clin
Cancer Res. 14:5220–5227. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Galon J, Costes A, Sanchez-Cabo F,
Kirilovsky A, Mlecnik B, Lagorce-Pagès C, Tosolini M, Camus M,
Berger A, Wind P, et al: Type, density, and location of immune
cells within human colorectal tumors predict clinical outcome.
Science. 313:1960–1964. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Mahmoud SM, Paish EC, Powe DG, Macmillan
RD, Grainge MJ, Lee AH, Ellis IO and Green AR: Tumor-infiltrating
CD8+ lymphocytes predict clinical outcome in breast cancer. J Clin
Oncol. 29:1949–1955. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Yamada N, Oizumi S, Kikuchi E, Shinagawa
N, Konishi-Sakakibara J, Ishimine A, Aoe K, Gemba K, Kishimoto T,
Torigoe T and Nishimura M: CD8+ tumor-infiltrating lymphocytes
predict favorable prognosis in malignant pleural mesothelioma after
resection. Cancer Immunol Immunother. 59:1543–1549. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Burt BM, Rodig SJ, Tilleman TR, Elbardissi
AW, Bueno R and Sugarbaker DJ: Circulating and tumor-infiltrating
myeloid cells predict survival in human pleural mesothelioma.
Cancer. 117:5234–5244. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Arulananda S, Thapa B, Walkiewicz M,
Zapparoli GV, Williams DS, Dobrovic A and John T: Mismatch repair
protein defects and microsatellite instability in malignant pleural
meso thelioma. J Thorac Oncol. 13:1588–1594. 2018. View Article : Google Scholar : PubMed/NCBI
|