Introduction
Peptide receptor radionuclide therapy (PRRT) with
radiola-beled somatostatin analogs has for >25 years been used
for the delivery of therapeutic radionuclides to somatostatin
receptor (SSTR)-positive neuroendocrine tumors (NETs) (1). As a result of the comprehensive
NETTER-1 study, PRRT with the soma-tostatin analog,
177Lu-DOTATATE (Lutathera), was recently approved for
the treatment of gastroenteropancreatic NETs (2). The study concluded that PRRT
significantly increased disease-free survival and prolonged overall
survival compared to standard care; however, complete response was
rare (2).
Therefore, ongoing research focuses on improving
PRRT with 177Lu-DOTATATE, for example by enhancing the
affinity of peptides, the use of intra-arterial injection, or
various combinations of peptides and radionuclides (3-5). In
this respect, the possibility to use alpha emitters, such as
213Bi and 225Ac is currently being explored
(6-8). Another strategy with which to enhance
the treatment efficacy is the use of radiosensitizers, e.g. drugs
that can potentiate the effects of radiation (9). One promising approach is the
inhibition of molecular chaperones of the heat shock protein (HSP)
family, such as HSP90, that regulate and stabilize a large number
of oncogenic client proteins (10), of which several are involved in DNA
damage response (DDR) (11).
Apart from its promising effects on
radiosensitization, HSP90 inhibition has also been suggested as a
suitable anti-tumorigenic strategy in NETs. Histopathological
analyses have revealed a high expression of HSP90 in primary tumors
and metastases (12,13), and the inhibition of HSP90 has been
demonstrated to exert anti-proliferative effects on NET cells
(12-15). HSP90 is upstream of e.g., both
mammalian target of rapamycin (mTOR) and mitogen-activated protein
kinase (MAPK)/extracellular-signal-regulated kinase (ERK) pathways,
controlling the expression of growth factor receptors, such as
epidermal growth factor (EGFR) and insulin-like growth factor 1
receptor (IGF1R), which initiate signaling (16). Mechanistic analyses using NET cell
lines have concluded that the extensive crosstalk between the mTOR
and MAPK/ERK pathways can be problematic when both pathways are
inhibited separately and may lead to a poor drug response (16). Consequently, as HSP90 inhibition
suppresses the signaling of proteins within both pathways
simultaneously (15), this may be
a more effective approach than e.g. the mTOR inhibitor, everolimus,
that was approved for the treatment of NETs in 2016 following the
RADIANT trials (17-19).
Several HSP90 inhibitors have been assessed
preclinically and in clinical trials (20). However, treatment success has often
been limited due to poor solubility and hepatotoxicity. This has
led to the development of second-generation HSP90 inhibitors
(21). One such promising agent is
onalespib, a second-generation HSP90 inhibitor currently being
tested in clinical trials. It is administered both as a monotherapy
and in combination with other chemotherapeutic drugs, in various
types of solid tumors, including NETs (22). In a recent preclinical study,
onalespib displayed potent radiosensitizing properties when used in
combination with external beam irradiation (23). Although the mechanisms behind the
radio-sensitizing properties of onalespib are not yet fully
understood, there are several indications for the involvement of
DDR (23).
Consequently, the dual role of onalespib, as both a
NET antitumorigenic agent and a radiotherapy potentiator, may
render it an optimal candidate for use in combination with
177Lu-DOTATATE in NETs. 177Lu-DOTATATE is
already established as a therapeutic option with the local delivery
of radiation to tumor lesions. The addition of onalespib could
affect proliferation and survival and may act as a radiosensitizer
through the regulation of DDR. Therefore, the aim of this study was
to investigate whether the HSP90 inhibitor onalespib, can reduce
NET growth and act as a radiosensitizer when used in combination
with 177Lu-DOTATATE. This was assessed in vitro,
measuring the therapeutic effects of both treatments as
monotherapies and in combination, as well as by investigating the
molecular effects of the treatments.
Materials and methods
Cell lines
The human cell line, BON (24), established from a lymph node
metastasis of a carcinoid tumor of the pancreas, was kindly
provided by Professor Townsend (The University of Texas Medical
Branch, Texas University) and grown in DMEM/Ham's F12 1:1
(Biochrome) supplemented with 10% fetal bovine serum
(Sigma-Aldrich), L-glutamine and antibiotics (100 IU penicillin and
100 µg/ml streptomycin) from (Biochrome). The NCI-H727
(CRL-5815) cells (25), a well
differentiated neuroendocrine cell line derived from a human lung
carcinoid, and the NCI-H460 (HTB-177) cells (25,26),
a large cell lung carcinoma human cell line with neuroendocrine
features, were purchased from ATCC and grown in RPMI-1640
(Biochrome) with above-mentioned supplements. All cells were grown
in an incubator with 37°C and 5% CO2 and cultivated
according to good cell culture practice.
Drug and radioconjugate preparation
Onalespib (Selleckchem) was stored at −20°C as a
lyophilized powder. Prior to drug treatment, it was dissolved in
DMSO to a concentration of 122.1 mM and subsequently diluted in
cell media.
For DOTATATE labeling, 1.5 µg DOTATATE
(Bachem) dissolved in water was mixed with reaction buffer (25 mM
sodium ascorbate/50 mM sodium acetate, pH 5) and 60 MBq
177Lu (ITG GmbH). The reaction vial was incubated at
80°C for 30 min. The labeling yield was assessed with instant thin
layer chromatography (Biodex Medical Systems) and sodium citrate
(0.1 M, pH 5.5) as mobile phase, with subsequent quantification in
a phosphoimager (BAS-1800II, Fujifilm).
XTT cell viability assay
The cells (5×103 BON, 12×103
NCI-H727 or 0.7×103 NCI-H460 cells) were seeded in
96-well plates and incubated at 37°C for 1-2 days. The medium was
then replaced and onalespib was added with concentrations ranging
from 1-104 nM. After 72 h, an XTT assay (301011K, LGC
Standards) was performed according to the manufacturer's
instructions. Briefly, 80 µl XTT activation reagent and 4 ml
XTT reagent were mixed and added to 8 ml cell medium. The old
medium was removed and 150 µl were added to each well. The
plates were then incubated for 4 h at 37°C and analyzed using a
plate reader (Bio-Rad Laboratories).
Cellular uptake of
177Lu-DOTATATE
The cells (4×104 BON, 4×104
NCI-H727 or 1.5×104 NCI-H460 cells) were seeded in
24-well plates and incubated at 37°C for 48 h.
177Lu-DOTATATE was added at a final concentration of 20
nM. Following 24 h of incubation at 37°C, unbound
177Lu-DOTATATE was removed and the cells were
trypsinized. Cell-associated activity was counted in a gamma
counter (1480 Wizard 3′, Wallace).
Multicellular tumor spheroids
Agarose-coated 96-well plates were prepared as
previously described (27). A
total of 5×103 BON, 3×103 NCI-H727 or
1.5×103 NCI-H460 cells were then seeded. Following
spheroid formation (3-4 days), onalespib (25-100 nM) and/or
177Lu-DOTATATE (1-50 kBq) was added daily for 3 days.
Onalespib was added 3 h prior to 177Lu-DOTATATE. The
spheroids were photographed every 2-3 days using a Canon EOS 700D
(Canon, Inc.) mounted on a Nikon Diaphot-TMD microscope (Nikon) and
media was added or exchanged 1-2 times per week. The cross-section
area was measured using Fiji and the volume of each spheroid was
calculated (28). All spheroids
were followed individually and normalized to the size at the start
of treatment.
Western blot analysis
The BON cell spheroids were treated with 25 nM
onalespib and/or 5 kBq 177Lu-DOTATATE. At 24 h
post-treatment, whole cell lysates were prepared with lysis buffer:
1% NP-40 Alternative, 20 mM Tris (pH 8.0), 137 mM NaCl, 10%
glycerol, 2 mM EDTA, 1 mM heat activated sodium orthovanadate
Na3VO4, (95 C for 10 min) and protease inhibitor cocktail
(Sigma-Aldrich) for 30 min on ice. The protein concentration was
measured using a Nanophotometer P-Class (Implen). The samples were
equalized to a relative protein amount and equal volumes of protein
lysates were separated by SDS-PAGE [Tris-Acetate 3-8% gel
(ThermoFisher Scientific)] and transferred onto a nitrocellulose
membrane (Immobilon-P Transfer membrane, Millipore) by wet blotting
for 24 h at 4°C followed by blocking of the membrane for 1 h in PBS
with 5% BSA. The membrane was divided according to the size of the
expected proteins and the slices were incubated with the according
rabbit monoclonal anti-EGFR (1:5,000, ab52894, Abcam), mouse
monoclonal anti-SSTR5 (1:1,000, ab109495, Abcam), rabbit polyclonal
anti-HSP90 (1:20,000, ab13495, Abcam), mouse monoclonal
anti-β-actin (1:10,000, A5441, Sigma-Aldrich), mouse monoclonal
anti-γ H2A histone family member X (γH2AX; 1:1,000, 05-636,
Merck/Millipore), rabbit monoclonal anti-IGF1-R (1:1,000, ab182408,
Abcam), poly-clonal mTOR (1:2,000, ab2732, Abcam), mouse monoclonal
anti-ERK1 + ERK2 (1:2,000, ab54230, Abcam), rabbit monoclonal
anti-AKT1/2/3 (1:10,000, ab179463, Abcam), rabbit monoclonal
anti-sodium potassium ATPase (1:2,000, ab76020, Abcam), rabbit
polyclonal anti-phosphatase and tensin homolog (PTEN; 1:1,000,
#9552, Cell Signaling Technologies) rabbit polyclonal H2AX
(1:10,000, ab11175, Abcam) antibodies at 4°C overnight. The
membrane was rinsed in PBS-Tween (0.1%) and incubated with
species-specific horseradish peroxidase-labeled secondary
antibodies (anti-mouse 1:3,000, #65-6520, ThermoFisher Scientific;
anti-rabbit 1:5,000, #65-6120, ThermoFisher Scientific) for 1 h at
room temperature. Electro-chemiluminescent solution (Immobilon,
Millipore) was applied and immunoreactive bands were visualized
with a CCD camera (SuperCCD HR, Fujifilm). Image analysis and
quantification of the bands was evaluated using ImageJ 1.48v
software (NIH). The total protein expression levels were adjusted
to the corresponding loading control (β-actin or sodium potassium
ATPase) according to the following formula: Total protein
expression=band intensity protein/band intensity loading control.
The total protein expression level was then normalized to the
intensity level of the adjusted untreated control sample (untreated
control level=1). To further evaluate the expression of yH2AX
compared to unphosphorylated protein (H2AX) the following formula
was used: Total yH2AX expression (z)=the band intensity yH2AX/band
intensity of H2AX. The error analysis of yH2AX (Dz) was performed
with the equation Dz/z=Sum[(Da/a)2+(Db/b)2+(Dc/c)2], where each
value (a, b, c) has an associated standard deviation (Da, Db or Dc,
respectively).
Flow cytometry
BON spheroids were seeded and treated as described
above with 25 nM onalespib and/or 5 kBq 177Lu-DOTATATE.
At 24 h after final treatment, the spheroids were trypsinized and
resuspended in PBS. The cells were then incubated with 2 µM
CellEventTM Caspase 3/7 Green Detection Reagent (Thermo
Fisher Scientific) for 15 min at room temperature, protected from
light. Sytox Red Dead Cell Stain (Thermo Fisher Scientific) was
added at a final concentration of 5 nM and the cells were incubated
at room temperature for an additional 15 min. The cells were then
filtered through a CellTrics 50 µm filter (Sysmex) and
analyzed using a flow cytometer (CyFlow Space, Sysmex). Data
analysis was performed with the FCGUI Toolbox for Matlab 2016b
(Mathworks).
Statistical analysis
Data are presented as the means ± standard deviation
if not otherwise stated. Statistical analysis was performed using
Graphpad Prism 7 software (GraphPad Software), where a P-value
≤0.05 was considered to indicate a statistically significant
difference. In viability assays, IC50 calculations (50% of maximal
inhibition) were made in Graphpad by fitting the data to a
dose-response curve (standard slope) according to the following
equation:
where y is viability in %, a is minimum response and
x is log10(concentration) of onalespib. For
spheroid assays, one-way ANOVA with Dunnett's (dose-response of
monotherapies) or Tukey's (combination assays) multiple comparison.
Synergy calculations was performed according to the Chou-Talalay
(
29) method with Compusyn
(Combosyn). Western blot data was analyzed using one-way ANOVA and
Tukey's multiple comparison.
Results
Characterization of cell lines
Onalespib reduced viability in
monolayer NET cells
The effects of onalespib on monolayer cell cultures
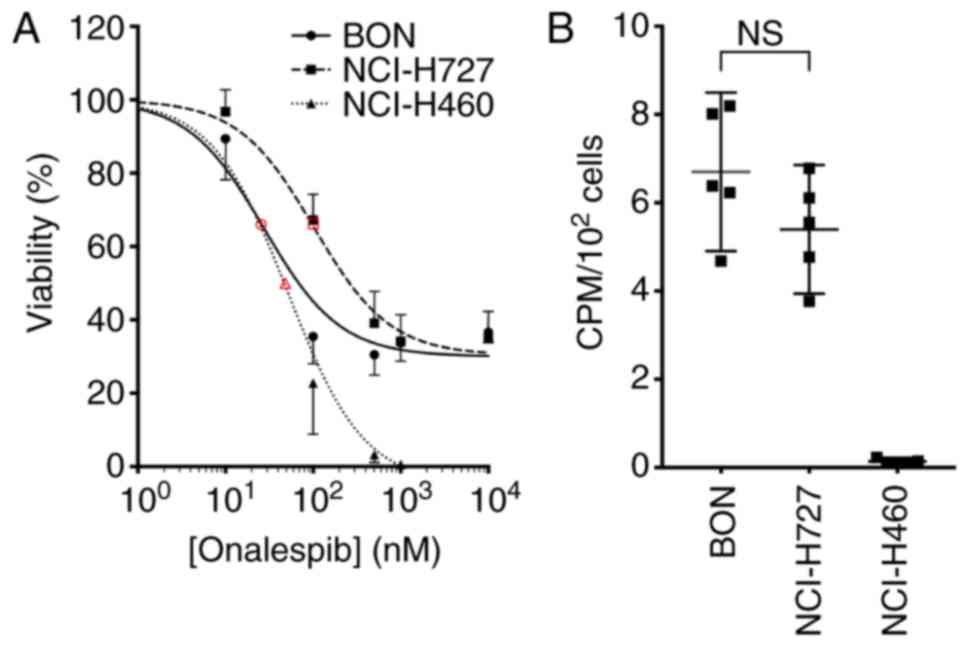
were assessed by XTT cell viability assays (Fig. 1A). All cell lines were found to be
sensitive to onalespib treatment and the calculated IC50 values
were 27 nM (95% CI 19-39 nM) for the BON, 102 nM (95% CI 78-132 nM)
for the NCI-H727 and 51 nM (95% CI 38-69 nM) for the NCI-H460
cells.
177Lu-DOTATATE uptake in NET
cell lines
The uptake of 177Lu-DOTATATE was assessed
by measuring the cell-associated radioactivity 24 h following the
addition of the compound. The uptake of 177Lu-DOTATATE
was observed in the BON and NCI-H727 cells at similar levels, while
the NCI-H460 cells exhibited no detectable uptake (Fig. 1B). From these results, the BON and
NCI-H727 cells were defined in this study as SSTR-positive and the
NCI-H460 cells were defined as SSTR-negative. The
177Lu-DOTATATE uptake in the SSTR-positive cell lines
was not affected by treatment with onalespib (Fig. S1).
Multicellular tumor spheroids
Multicellular tumor spheroids mimic in
vivo-like conditions, with nutrient and oxygen gradients within
the spheroid similar to those in tumors. Moreover, it is a relevant
model for PRRT due to the three-dimensional distribution of the
emitted radiation. In the current study, spheroids were treated
with daily concentrations of onalespib, ranging from 25-100 nM and
1-50 kBq 177Lu-DOTATATE on 3 consecutive days. Spheroid
size was measured at 14 (NCI-H460) or 20 (BON, NCI-H727) days
following the start of treatment.
Growth inhibitory effect of onalespib and
177Lu- DOTATATE as monotherapies
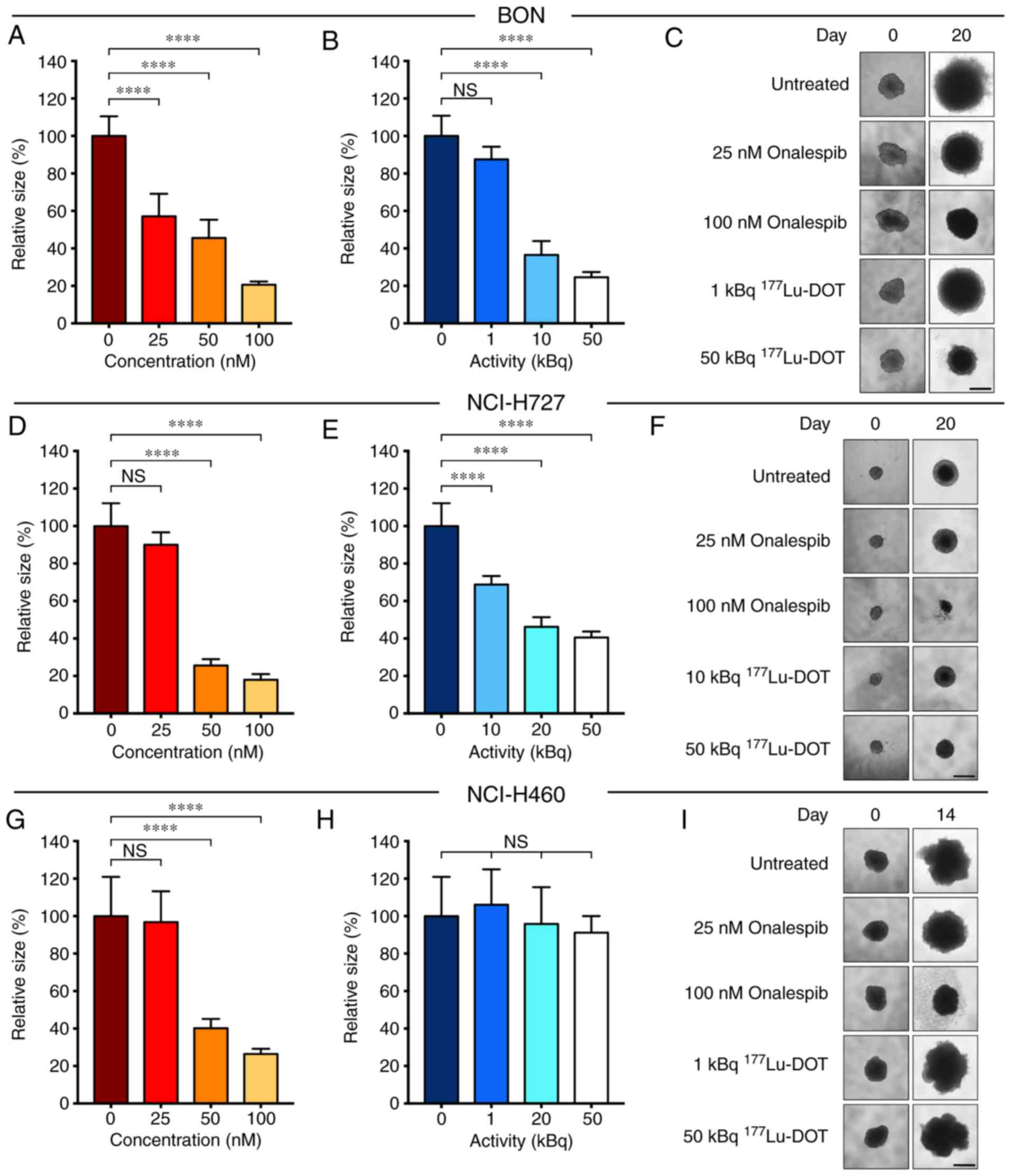
Onalespib exerted growth inhibitory effects on all
cell lines (Fig. 2, A, D and G).
With a concentration of 50 nM, the spheroid size compared to the
untreated cells was 46±10, 26±3 and 40±5% for the BON (Fig. 2A), NCI-H727 (Fig. 2D) and NCI-H460 (Fig. 2G) cells, respectively. DMSO had no
marked effect on spheroid growth (Fig. S2). It was found that
177Lu-DOTATATE significantly inhibited the growth of the
BON (Fig. 2B) and NCI-H727
(Fig. 2E) cell-derived spheroids.
However, 177Lu-DOTATATE had no effect on the spheroid
growth of SSTR-negative NCI-H460 cells (Fig. 2H). These results were in accordance
with the SSTR status. Following treatment with 50 kBq
177Lu-DOTATATE, the spheroid size compared to the
untreated cells was 25±3, 41±3 and 91±9% in the BON, NCI-H727 and
NCI-H460 cells, respectively. Unlabeled DOTATATE did not have a
marked effect on spheroid growth (Fig. S2).
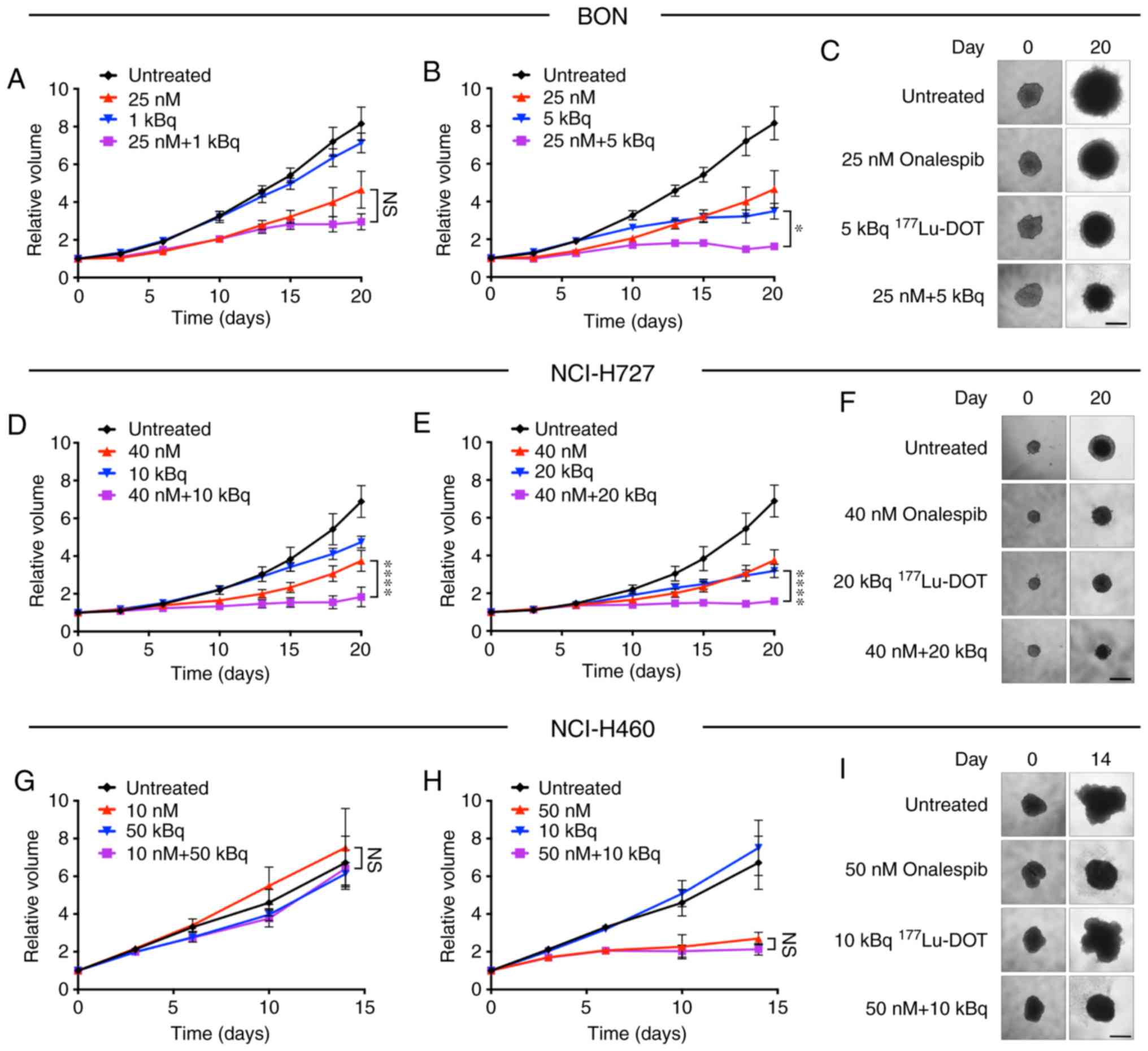
Specific and synergistic effects in the
spheroids treated with the combined treatment
The combined treatment of the spheroids with
onalespib and 177Lu-DOTATATE (Fig. 3) resulted in additional growth
inhibitory effects compared to monotherapies in the SSTR-positive
cell lines, BON and NCI-H727. In the NCI-H460 cells, no treatment
combinations led to any significantly differences compared to
onalespib monotherapy. Additional combinations were tested, with
significant differences already observed at day 14 for the BON and
NCI-H727 cell-derived spheroids (Fig.
S3).
Synergy calculations were performed according to the
Chou-Talalay method for the BON and NCI-H727 cells, where a
combination index (CI) of <1 indicated synergy. Table I displays results of the synergy
calculations for various combinations on days 14 and 20. All tested
combinations were synergistic in the BON cell-derived spheroids,
while in the NCI-H727 cells, synergy was present following
treatment with 40 nM onalespib.
 | Table ISynergistic analysis of BON and
NCI-H727 cells. |
Table I
Synergistic analysis of BON and
NCI-H727 cells.
| Onalespib (nM) |
177Lu-DOT (kBq) | CI day 14 | CI day 20 |
|---|
| BON | 10 | 10 | 0.40a | n.a. |
| 25 | 1 | n.a. | 0.50 |
| 25 | 5 | 0.46 | 0.39a |
| 25 | 10 | 0.45a | 0.65 |
| 25 | 20 | 0.62a | n.a. |
| NCI-H727 | 25 | 50 | 1.45 | n.a. |
| 40 | 10 | 1.02 | 0.67a |
| 40 | 20 | 0.65a | 0.62a |
| 40 | 50 | 0.69a | n.a. |
Combination treatment downregulates EGFR
expression and increases apoptosis
To further determine the molecular effects of
onalespib and 177Lu-DOTATATE, western blot analysis and
flow cytometry were performed on the BON cell-derived spheroids
(Fig. 4). EGFR, a client protein
of HSP90, mediates the signaling of cell proliferation and survival
e.g., by initiating the mTOR and MAPK/ERK signaling pathways
(20). In this study, onalespib
treatment resulted in the downregulation of EGFR, while
177Lu-DOTATATE had no effect (Fig. 4A and B). The spheroids treated with
the combination treatments exhibited the lowest EGFR expression.
Compared to the untreated control, EGFR expression was 78±7, 109±12
and 64±5% in the spheroids treated with onalespib,
177Lu-DOTATATE and their combination, respectively. No
significant differences in HSP90 expression were detected (Fig. 4A and C).
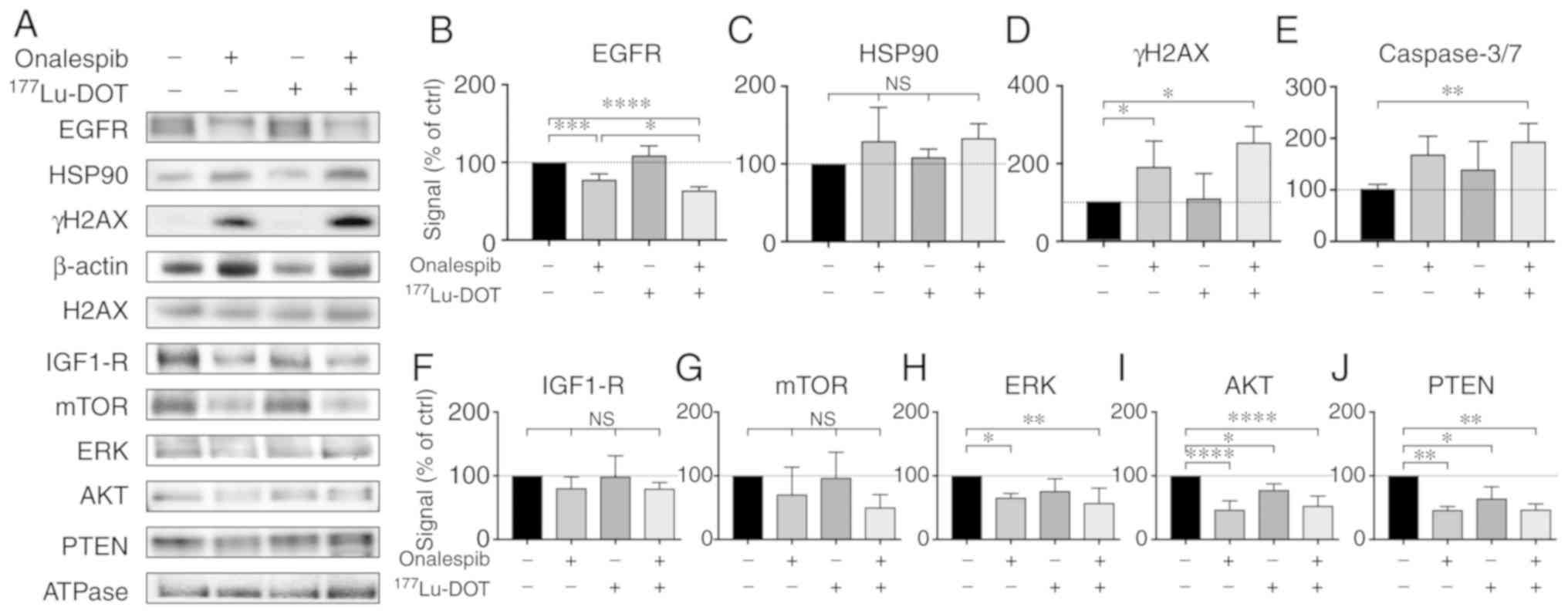 | Figure 4Western blot analysis and flow
cytometry of the treated spheroids. (A) Western blot analysis of
EGFR, HSP90, IGF1-R, mTOR, ERK, AKT, PTEN and γH2AX 24 h after
final treatment. β-actin and Na/K-ATPase were used as loading
controls. Representative blots are shown. (B-D and F-J) Western
blot analysis quantification of EGFR, HSP90, IGF1-R, mTOR, ERK,
AKT, PTEN and γH2AX. Graphs display signal normalized to loading
control and to untreated control samples (means ± standard
deviation, γH2AX expression was normalized to unphosphorylated
protein expression (H2AX; n=3-6). (E) Flow cytometric assessment of
caspase 3 and 7 activity in the treated spheroids.
*P≤0.05, **P≤0.01, ***P≤0.001 and
****P≤0.0001; n.s, not significant. EGFR, epidermal
growth factor receptor; HSP90, heat shock protein 90; IGF1-R,
insulin-like growth factor 1; mTOR, mammalian target of rapamycin;
ERK, extracellular-signal-regulated kinase; PTEN, phosphatase and
tensin homolog; γH2AX, γ H2A histone family member X. |
γH2AX expression can be used to indicate the level
of DNA double-strand breaks. The spheroids treated with onalespib
or the combination treatment both exhibited significantly higher
levels of γH2AX (Fig. 4A and D)
compared to the untreated spheroids, whereas
177Lu-DOTATATE alone did not markedly affect γH2AX
expression compared the untreated spheroids. Combined treatment did
not lead to significant differences in γH2AX expression compared to
treatment with onalespib alone. γH2AX expression in the spheroids
normalized to the untreated cell was 181±64, 104±39 and 216±167%
for the cells treated with onalespib, 177Lu-DOTATATE and
the combination treatment, respectively.
Caspase 3 and 7 are commonly used as markers of
apoptosis. In this study, flow cytometry was used to quantify the
amount of caspase 3/7 in the cells. There was a 2-fold increase in
caspase 3/7 activity in the combination treated spheroids, while
treatment with onalespib or 177Lu-DOTATATE alone had no
effect on caspase activity (Figs.
4E and S4).
To further investigate the involvement of the mTOR
and MAPK/ERK signaling pathways, the levels of IGF1-R, as well as a
number of downstream targets were assessed by western blot analysis
(Fig. 4A and F-J). In general, the
levels of the assessed proteins followed the same pattern as that
of EGFR, with decreased levels in the onalespib and
combination-treated samples. For IGF1-R and mTOR, these changes
were however, not statistically significant. ERK expression was
downregulated following treatment with onalespib and combination
treatment, with the lowest levels observed in the spheroids treated
with the combination treatment (57±23% of the untreated control).
AKT and PTEN expression levels were downregulated by onalespib
(47±14 and 46±5% of the untreated control, respectively); however,
a significant effect was also observed following
177Lu-DOTATATE monotherapy (77±14 and 64±18%,
respectively). Combination treatment resulted in AKT and PTEN
levels similar to those from onalespib treatment.
Discussion
PRRT with 177Lu-DOTATATE has
revolutionized the treatment of SSTR-positive NETs (2). However, despite a marked increase in
disease-free survival, a complete response is rare. The combined
use of PRRT with radiosensitizers may be a promising strategy with
which to potentiate the treatment efficacy, enabling a systemic
dual targeting treatment. HSP90 was recently proposed as a target
for NET therapy, and the HSP90 inhibitor onalespib has previously
demonstrated radiosensitizing properties in combination with
external radiotherapy (23).
In the present study, we examined efficacy of the
HSP90 inhibitor, onalespib, on a panel of NET cell lines in
monolayer cultures (Fig. 1), as
well as in multicellular tumor spheroids (Fig. 2), demonstrating antitumorigenic
activity in all the cell lines examined. These findings are in line
with those of previous studies validating the high potential of
HSP90 inhibitors for NETs (12,14,15).
The effect of 177Lu-DOTATATE on spheroid
growth was then assessed on the NET cell lines, BON (pancreatic
carcinoid), NCI-H727 (bronchial carcinoid) and NCI-H460 (large cell
lung carcinoma). As expected, treatment resulted in the growth
inhibition of SSTR-positive cell lines, while the SSTR-negative
NCI-H460 cell line was unaffected. As the NCI-H460 cell line is a
large cell carcinoma with different biological and pathological
features from the carcinoids, it is not surprising that this cell
line differed from the other two in SSTR expression (25). Moreover, unlabeled DOTATATE in
equivalent concentrations had no effect on spheroid growth
(Fig. S2). These results confirm
the specificity of 177Lu-DOTATATE treatment.
Furthermore, when onalespib and
177Lu-DOTATATE (Fig. 3)
were used in combination, the growth inhibitory effects on the BON
and NCI-H727 cell-derived spheroids were even more pronounced than
the corresponding monotherapies. Following combined treatment, the
BON cells exhibited synergistic effects for all tested
concentrations and time points. Synergy was also found in the
NCI-H727 cells and was most pronounced at 20 days following
treatment. Low concentrations of onalespib led to an additive
effect on NCI-H727 cell-derived spheroids. As the Chou-Talalay
method requires both drugs to demonstrate a significant effect and
177Lu-DOTATATE had no effect on NCI-H460 cells, no
synergy calculations were performed for this cell line. However, as
none of the combination treatments led to significant differences
compared to onalespib monotherapy, 177Lu-DOTATATE was
not able to enhance the effects of onalespib or vice versa in the
NCI-H460 cells. Consequently, it was concluded that the combination
of onalespib and 177Lu-DOTATATE is synergistic and
specific for SSTR-positive NETs.
After the growth inhibitory effects of monotherapy
and the combination treatments had been verified, the responses at
the molecular level were investigated. One way to validate the
molecular effects of onalespib is by assessing EGFR expression.
EGFR, an HSP90 client protein, is an activator of the mTOR and
MAPK/ERK pathways, which are highly relevant in NET tumorigenesis
(20,30,31).
Blocking these pathways by the downregulation of growth factor
receptors, such as EGFR can enhance the therapeutic response. The
results from western blot analysis of EGFR expression (Fig. 4A and B) demonstrated the
downregulation in the onalespib-treated BON spheroids, consistent
with the findings of previous studies (23,32).
177Lu-DOTATATE treatment did not affect EGFR expression.
Notably, the lowest level of EGFR expression was observed in the
combination treatment group. The reason for this is not clear. One
potential explanation could be radiotherapy-induced HSP90 cleavage.
It is known that radiotherapy increases the level of reactive
oxygen species (ROS). Several studies have investigated the
association between ROS and HSP90, finding that ROS can cleave
HSP90 at the N-terminal, leading to the loss of function and
additional client protein degradation (33,34).
However, this could not be confirmed in HSP90 expression analyses
(Fig. 4A and C), where no
differences in HSP90 expression were observed between the treatment
groups. Nonetheless, the clear decrease in EGFR expression in the
onalespib-treated samples verify the growth inhibitory effects of
the drug, and point to an even more effective outcome when used in
combination with 177Lu-DOTATATE. This was further
supported by the results from western blot analysis of additional
proteins involved in the mTOR and MAPK/ERK signaling pathways
(Fig. 4A and F-J).
HSP90 plays an important role in DDR (11). In this study, we investigated DDR
by investigating the DNA double-strand break marker, γH2AX, 24 h
following treatment (Fig. 4A and
D). The nuclear expression of γH2AX is a sensitive marker for
DNA double-strand breaks, where γH2AX accumulates in foci within
seconds after the occurrence of the DNA double-strand breaks, and
the level usually peaks within 1 h, followed by decreasing levels
proportional to the repair rate. Moreover, pan-nuclear γH2AX
staining represents apoptotic signaling, triggered by ATM and
DNA-PKcs activation (35).
Consequently, as western blot analysis of total γH2AX cannot
distinguish between the nuclear and pan-nuclear expression of the
protein, γH2AX expression indicates either an insufficient DNA
damage repair machinery or induction of apoptosis in this assay. In
this study, at 24 h following treatment with
177Lu-DOTATATE, the BON cells were able to repair the
induced DNA damage. However, a significant increase in γH2AX
expression was observed in the spheroids treated with onalespib and
the combination treatment. This suggests that the enhanced growth
inhibition in the spheroids treated with onalespib and the
combination treatment is in part due to an increased number of
double-strand breaks and apoptosis.
To further investigate the involvement of apoptosis,
flow cytometry was used to measure caspase 3 and 7 activity at 24 h
after final treatment. While no significant increase in caspase 3/7
activity was observed in the onalespib- or
177Lu-DOTATATE-treated spheroids, combination treatment
led to a 2-fold increase in caspase activity at this time point.
The somewhat different staining profiles of γH2AX, and caspase 3
and 7 indicate different kinetic profiles and/or mechanisms of the
investigated events. However, the results suggest that the enhanced
growth inhibition in the spheroids treated with the combination
treatment is in part due to increased apoptosis.
In conclusion, the present study demonstrates that
HSP90 inhibition with onalespib is a feasible treatment option for
NETs. Moreover, the combination of onalespib and
177Lu-DOTATATE is particularly promising due to
SSTR-specific synergistic effects. The potent synergistic effects
observed in the present study are most likely due to several
contributing factors, involving the suppression of EGFR signaling
and the induction of apoptosis. The combination of onalespib and
177Lu-DOTATATE may improve the therapeutic response for
patients with inoperable and metastatic NETs, leading to increased
cure rates. Further in vivo studies investigating this
strategy are required to confirm these promising findings.
Supplementary Data
Acknowledgments
The authors would like to thank Dr Andris
Abramenkovs, Uppsala University, for assisting with the image
analysis, Mrs. Anna Mäkiniemi and Ms. Martina Nilsson, Uppsala
University, for assisting in XTT assays and Ms. Noelle Chung,
Uppsala University, for performing Western blots.
Funding
This study was supported by grants from the Swedish
Cancer Society (CAN 2018/494, CAN2016/649, CAN 2015/1080 and CAN
2015/385), the Swedish Research Council (2013-30876-104113-30),
Torsten and Ragnar Söderbergs Foundation for Oncological
Endocrinology, and Lennart Glans Foundation.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
SL contributed to the design of the study and
contributed to the major part of the experimental studies (with
focus on XTT, spheroid and flow cytometric assays), analyzed and
interpreted the data, and drafted and revised the manuscript. DS
contributed to the design of the study, contributed to experimental
studies (with focus on the western blot analysis experiments),
analyzed and interpreted data and revised the manuscript. BS
contributed to the design of the study and data interpretation, and
revised the manuscript. MN initiated and designed the study,
contributed to data analysis and interpretation and revised the
manuscript. All authors have read and approved the final
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Krenning EP, Kooij PP, Bakker WH, Breeman
WA, Postema PT, Kwekkeboom DJ, Oei HY, de Jong M, Visser TJ, Reijs
AE, et al: Radiotherapy with a radiolabeled somatostatin analogue,
[111In-DTPA-D-Phe1]-octreotide. A case history. Ann N Y Acad Sci.
733:496–506. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Strosberg J, El-Haddad G, Wolin E,
Hendifar A, Yao J, Chasen B, Mittra E, Kunz PL, Kulke MH, Jacene H,
et al: Phase 3 trial of 177Lu-dotatate for midgut
neuroendocrine tumors. N Engl J Med. 376:125–135. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kratochwil C, Lopez-Benitez R, Mier W,
Haufe S, Isermann B, Kauczor HU, Choyke PL, Haberkorn U and Giesel
FL: Hepatic arterial infusion enhances DOTATOC radiopeptide therapy
in patients with neuroendocrine liver metastases. Endocr Relat
Cancer. 18:595–602. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Virgolini I, Patri P, Novotny C, Traub T,
Leimer M, Füger B, Li SR, Angelberger P, Raderer M, Wogritsch S, et
al: Comparative somatostatin receptor scintigraphy using
in-111-DOTA-lanreo-tide and in-111-DOTA-Tyr3-octreotide versus
F-18-FDG-PET for evaluation of somatostatin receptor-mediated
radionuclide therapy. Ann Oncol. 12(Suppl 2): S41–S45. 2001.
View Article : Google Scholar
|
|
5
|
Claringbold PG, Price RA and Turner JH:
Phase I-II study of radiopeptide 177Lu-octreotate in combination
with capecitabine and temozolomide in advanced low-grade
neuroendocrine tumors. Cancer Biother Radiopharm. 27:561–569. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kratochwil C, Giesel FL, Bruchertseifer F,
Mier W, Apostolidis C, Boll R, Murphy K, Haberkorn U and
Morgenstern A: 213Bi-DOTATOC receptor-targeted
alpha-radionuclide therapy induces remission in neuroendocrine
tumours refractory to beta radiation: A first-in-human experience.
Eur J Nucl Med Mol Imaging. 41:2106–2119. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chan HS, Konijnenberg MW, Daniels T, Nysus
M, Makvandi M, de Blois E, Breeman WA, Atcher RW, de Jong M and
Norenberg JP: Improved safety and efficacy of
213Bi-DOTATATE-targeted alpha therapy of somatostatin
receptor-expressing neuroendo-crine tumors in mice pre-treated with
L-lysine. EJNMMI Res. 6:832016. View Article : Google Scholar
|
|
8
|
Miederer M, Henriksen G, Alke A,
Mossbrugger I, Quintanilla-Martinez L, Senekowitsch-Schmidtke R and
Essler M: Preclinical evaluation of the alpha-particle generator
nuclide 225Ac for somatostatin receptor radiotherapy of
neuro-endocrine tumors. Clin Cancer Res. 14:3555–3561. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Maier P, Hartmann L, Wenz F and Herskind
C: Cellular pathways in response to ionizing radiation and their
targetability for tumor radiosensitization. Int J Mol Sci. 17:pii:
E102. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Den RB and Lu B: Heat shock protein 90
inhibition: Rationale and clinical potential. Ther Adv Med Oncol.
4:211–218. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Pennisi R, Ascenzi P and di Masi A:
Correction: Pennisi, R., et al. Hsp90: A new player in DNA repair?
Biomolecules. 2015.5:2589–2618, Biomolecules 6: pii: E40, 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Gilbert JA, Adhikari LJ, Lloyd RV,
Halfdanarson TR, Muders MH and Ames MM: Molecular markers for novel
therapeutic strategies in pancreatic endocrine tumors. Pancreas.
42:411–421. 2013. View Article : Google Scholar :
|
|
13
|
Gilbert JA, Adhikari LJ, Lloyd RV, Rubin
J, Haluska P, Carboni JM, Gottardis MM and Ames MM: Molecular
markers for novel therapies in neuroendocrine (carcinoid) tumors.
Endocr Relat Cancer. 17:623–636. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Gloesenkamp C, Nitzsche B, Lim AR, Normant
E, Vosburgh E, Schrader M, Ocker M, Scherübl H and Höpfner M: Heat
shock protein 90 is a promising target for effective growth
inhibition of gastrointestinal neuroendocrine tumors. Int J Oncol.
40:1659–1667. 2012.PubMed/NCBI
|
|
15
|
Zitzmann K, Ailer G, Vlotides G, Spoettl
G, Maurer J, Göke B, Beuschlein F and Auernhammer CJ: Potent
antitumor activity of the novel HSP90 inhibitors AUY922 and HSP990
in neuroendocrine carcinoid cells. Int J Oncol. 43:1824–1832. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Mendoza MC, Er EE and Blenis J: The
Ras-ERK and PI3K-mTOR pathways: Cross-talk and compensation. Trends
Biochem Sci. 36:320–328. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yao JC, Shah MH, Ito T, Bohas CL, Wolin
EM, Van Cutsem E, Hobday TJ, Okusaka T, Capdevila J, de Vries EG,
et al: Everolimus for advanced pancreatic neuroendocrine tumors. N
Engl J Med. 364:514–523. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yao JC, Fazio N, Singh S, Buzzoni R,
Carnaghi C, Wolin E, Tomasek J, Raderer M, Lahner H, Voi M, et al:
Everolimus for the treatment of advanced, non-functional
neuroendocrine tumours of the lung or gastrointestinal tract
(RADIANT-4): A randomised, placebo-controlled, phase 3 study.
Lancet. 387:968–977. 2016. View Article : Google Scholar
|
|
19
|
Fazio N, Granberg D, Grossman A, Saletan
S, Klimovsky J, Panneerselvam A and Wolin EM: Everolimus plus
octreo-tide long-acting repeatable in patients with advanced lung
neuroendocrine tumors: Analysis of the phase 3, randomized,
placebo-controlled RADIANT-2 study. Chest. 143:955–962. 2013.
View Article : Google Scholar
|
|
20
|
Hong DS, Banerji U, Tavana B, George GC,
Aaron J and Kurzrock R: Targeting the molecular chaperone heat
shock protein 90 (HSP90): Lessons learned and future directions.
Cancer Treat Rev. 39:375–387. 2013. View Article : Google Scholar
|
|
21
|
Shapiro GI, Kwak E, Dezube BJ, Yule M,
Ayrton J, Lyons J and Mahadevan D: First-in-human phase I dose
escalation study of a second-generation non-ansamycin HSP90
inhibitor, AT13387, in patients with advanced solid tumors. Clin
Cancer Res. 21:87–97. 2015. View Article : Google Scholar
|
|
22
|
National Library of Medicine National
Institutes of Health: Onalespib, Dabrafenib, and trametinib in
treating patients with BRAF-mutant melanoma or solid tumors that
are metastatic or cannot Be removed by surgery. 2019
|
|
23
|
Spiegelberg D, Dascalu A, Mortensen AC,
Abramenkovs A, Kuku G, Nestor M and Stenerlöw B: The novel HSP90
inhibitor AT 13387 potentiates radiation effects in squamous cell
carcinoma and adenocarcinoma cells. Oncotarget. 6:35652–35666.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Townsend CM Jr, Ishizuka J and Thompson
JC: Studies of growth regulation in a neuroendocrine cell line.
Acta Oncol. 32:125–130. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Gazdar AF, Helman LJ, Israel MA, Russell
EK, Linnoila RI, Mulshine JL, Schuller HM and Park JG: Expression
of neuro-endocrine cell markers L-dopa decarboxylase, chromogranin
A, and dense core granules in human tumors of endocrine and
nonendocrine origin. Cancer Res. 48:4078–4082. 1988.PubMed/NCBI
|
|
26
|
Banks-Schlegel SP, Gazdar AF and Harris
CC: Intermediate filament and cross-linked envelope expression in
human lung tumor cell lines. Cancer Res. 45:1187–1197.
1985.PubMed/NCBI
|
|
27
|
Friedrich J, Seidel C, Ebner R and
Kunz-Schughart LA: Spheroid-based drug screen: Considerations and
practical approach. Nat Protoc. 4:309–324. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Schindelin J, Arganda-Carreras I, Frise E,
Kaynig V, Longair M, Pietzsch T, Preibisch S, Rueden C, Saalfeld S,
Schmid B, et al: Fiji: An open-source platform for biological-image
analysis. Nat Methods. 9:676–682. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chou TC and Talalay P: Quantitative
analysis of dose-effect relationships: The combined effects of
multiple drugs or enzyme inhibitors. Adv Enzyme Regul. 22:27–55.
1984. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zitzmann K, Ruden J, Brand S, Göke B,
Lichtl J, Spöttl G and Auernhammer CJ: Compensatory activation of
Akt in response to mTOR and Raf inhibitors-a rationale for
dual-targeted therapy approaches in neuroendocrine tumor disease.
Cancer Lett. 295:100–109. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Briest F and Grabowski P:
PI3K-AKT-mTOR-signaling and beyond: The complex network in
gastroenteropancreatic neuro-endocrine neoplasms. Theranostics.
4:336–365. 2014. View Article : Google Scholar :
|
|
32
|
Spiegelberg D, Mortensen AC, Selvaraju RK,
Eriksson O, Stenerlöw B and Nestor M: Molecular imaging of EGFR and
CD44v6 for prediction and response monitoring of HSP90 inhibition
in an in vivo squamous cell carcinoma model. Eur J Nucl Med Mol
Imaging. 43:974–982. 2016. View Article : Google Scholar :
|
|
33
|
Beck R, Verrax J, Gonze T, Zappone M,
Pedrosa RC, Taper H, Feron O and Calderon PB: Hsp90 cleavage by an
oxidative stress leads to its client proteins degradation and
cancer cell death. Biochem Pharmacol. 77:375–383. 2009. View Article : Google Scholar
|
|
34
|
Beck R, Dejeans N, Glorieux C, Creton M,
Delaive E, Dieu M, Raes M, Levêque P, Gallez B, Depuydt M, et al:
Hsp90 is cleaved by reactive oxygen species at a highly conserved
N-terminal amino acid motif. PLoS One. 7:e407952012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ding D, Zhang Y, Wang J, Zhang X, Gao Y,
Yin L, Li Q, Li J and Chen H: Induction and inhibition of the
pan-nuclear gamma-H2AX response in resting human peripheral blood
lymphocytes after X-ray irradiation. Cell Death Discov.
2:160112016. View Article : Google Scholar : PubMed/NCBI
|