Introduction
In recent years, dietary polyphenols have attracted
increasing scientific attention as potential chemopreventive and
chemotherapeutic agents, due to their putative low toxicity
profiles, their common availability, low cost and a wide range of
biological activities (1-4). Despite the large variety of
structural variants of dietary polyphenols, not all of these share
the same antitumor activity; hence, a considerable focus of
researchers has been concentrated on selected groups, including
flavonoids. One of the most intensely studied compound of this
class has become fisetin (3,3′,4′,7-tetrahydroxyflavone; FIS),
whose anticancer properties have relatively recently been
discovered, and earlier mainly its antimicrobial, as well as its
neuroprotective activities had been known. FIS belongs to a
flavonol subgroup of flavonoids and constitutes common ingredient
of the human diet, as it can be found in several fruits, vegetables
and nuts, including strawberries, apples, kiwi fruit, grapes,
persimmons, onions and cucumbers (5). There is accumulating evidence that
FIS may exert anti-proliferative, anti-inflammatory, pro-death,
anti-invasive, anti-migratory and anti-angiogenic effects in a
large panel of human cancer cell lines and several animal models
(6-8). At the cellular and molecular level,
these anti-carcinogenic effects of FIS have been shown to be
associated with the modulation of multiple signal transduction
pathways, including the phosphatidylinositol 3-kinase/AKT/mammalian
target of rapamycin (PI3K/AKT/mTOR), cyclooxygenase 2 (COX-2),
Janus kinase/signal transducer and activator of
transcription (JAK/STAT), extracellular-signal regulated kinase 1/2
(ERK1/2), c-Jun N-terminal kinase (JNK) and p38 mitogen-activated
protein kinase (p38 MAPK) pathways, AMP-activated protein kinase
(AMPK) signaling, KIT receptor signaling, growth hormone receptor
signaling, as well as Wnt/β-catenin, epidermal growth factor
receptor (EGFR) and nuclear factor κB (NF-κB) pathways.
Furthermore, FIS has been found to bind and inhibit the activity of
cyclin-dependent kinases (CDKs) 2, 4, 6, decrease the levels of
cyclin B1, D1 and cyclin E, suppress retinoblastoma protein (pRb)
phosphorylation, increase the levels of p21, p27 and p53, as well
as to function as an antagonist of DNA topoisomerases I, II and as
a ligand for the androgen receptor (AR) (5,7,9). At
the cellular level, FIS possesses a microtubule stabilizing
property and binds to β-tubulin, leading to the disruption of
microtubule dynamics (10). It has
been demonstrated that FIS-induced cancer cell death occurs
predominantly through apoptosis, although its ability to trigger
autophagy and mitotic catastrophe has also been documented
(11-13). FIS-induced apoptosis has been found
to be associated with endoplasmic reticulum (ER) stress (14,15),
the activation of the caspase cascade (16-19),
the inhibition of heat shock factor 1 (HSF1) (20), the induction (19) or the suppression (17) of reactive oxygen species (ROS)
production, the downregulation of anti-apoptotic Bcl-2,
Bcl-XL, Mcl-1L, cIAP-1/2, XIAP, survivin and TRAF1
proteins, and the upregulation of pro-apoptotic Bcl-2 family
members, such as Bax, Bim and Bad (16,19,21,22).
The occurrence of apoptosis following treatment with FIS has been
demonstrated in a wide variety of cancer types, including chronic
myelogenous leukemia (23,24), promyelocytic leukemia (25), melanoma (26), hepatocellular carcinoma (18), multiple myeloma (19), breast (27), bladder (22), liver (6), pancreatic (28), cervical (29), prostate (16), colon (20,30,31),
epidermoid (32), laryngeal
(33) and oral squamous cell
carcinoma (34,35). However, the therapeutic application
of FIS, similar to other flavonoids, has been hampered due to
insufficient oral bioavailability as a result of its poor aqueous
solubility, poor absorption, as well as extensive and rapid
metabolism. Therefore, considerable efforts have been made to
improve the bioavailability of FIS through e.g., co-crystallization
with caffeine, nicotinamide and isonicotinamide, complexation with
cyclodextrins and encapsulation with nanoparticles (36-39).
Although numerous in vitro studies have been
devoted to investigating the antitumor efficacy, as well as the
mechanisms of action of FIS, only few of these have used low, in
vivo achievable concentrations of this agent. To limit a lack
of reproducibility of the in vitro studies in clinical
trials, the use of clinically relevant concentrations in the in
vitro testing of agents is currently strongly recommended
(40). Therefore, in this study,
we aimed to investigate the cellular and molecular effects of in
vivo attainable concentrations of FIS on K562 human chronic
myeloid leukemia (CML) cells. Furthermore, since we, as well as
others have previously reported that FIS can act synergistically
with certain anticancer drugs (27,41-43),
thereby establishing its potential as a possible candidate for
combination therapy, herein we also aimed to assess whether this
flavonoid may enhance the anticancer effects exerted by arsenic
trioxide (ATO) against K562 leukemic cells.
Materials and methods
Cell culture and treatment
The K562 human chronic myeloid leukemia cells (ATCC)
were maintained in Roswell Park Memorial 1640 medium (RPMI-1640;
BioWhittaker, Lonza) supplemented with 10% fetal bovine serum (FBS,
Sigma-Aldrich) and 50 µg/ml gentamycin (Sigma-Aldrich). A
humidified atmosphere of 95% air and 5% CO2 at a
constant temperature of 37°C was provided by the CO2
incubator. After reaching approximately 70-80% confluency, the
cells were passaged and seeded on 12- or 6-well plates (BD Falcon)
for further assays. Concentrated stock solutions of FIS (100 mM;
Abcam) were prepared in dimethyl sulfoxide (DMSO; Sigma-Aldrich),
stored at -25°C and serially diluted in complete growth medium
right before use. ATO (Sigma-Aldrich) was dissolved in 1 M of NaOH
to generate 100 mM stock solution, which was then stored at 4°C and
serially diluted in complete growth medium immediately prior to
cell treatment. The control cells were cultured under identical
conditions, although without the addition of the test agents. The
final concentrations of DMSO or NaOH (0.02 and 0.0001%,
respectively) did not influence the experimental results (data not
shown). The cells were evaluated for mycoplasma contamination by
DAPI staining methods. Cells at low passage were used for all the
experiments (≤5). The range of concentrations for cell viability
assays (10-50 µM) were selected to enable the construction
of the dose-response curves for the estimation of the half-maximal
inhibitory concentration values and drug-drug interaction analysis.
Since in vivo achievable levels of FIS are only up to 20
µM (44), 10 and 20
µM concentrations of this flavonoid were employed for all
subsequent experiments. Likewise, clinically-relevant (1.5-2.5
µM) and sub-therapeutic (0.5-1.0 µM) concentrations
of ATO were used in drug-drug interaction experiments, as such an
approach has previously been recommended to limit its toxicity and
to reduce unwanted side-effects (45,46).
Given the stability and cellular uptake of several flavonoid
compounds (47,48), the incubation time was 24 and/or 48
h, unless otherwise stated.
Cell viability assays
The cytotoxic effects of the test agents on the
viability of the K562 cells was evaluated by the thiazolyl blue
tetrazolium bromide (MTT) colorimetric assay, as well as Trypan
blue dye exclusion test. For both experiments, the cells were
seeded into 12-well plates and treated with FIS at concentrations
of 10, 20, 30, 40, 50 µM for 24 or 48 h. After the indicated
treatment period, the cells were incubated in the MTT working
solution, which was prepared by diluting the MTT stock solution
[made by dissolving 5 mg of MTT (Sigma-Aldrich) in 1 ml of PBS]
with the culture medium without phenol red (HyClone, Thermo Fisher
Scientific) at a ratio of 1:9. Following 3 h of incubation in the
CO2 incubator at a standard condition, the resulting
formazan crystals were dissolved in isopropanol (POCH, Poland) and
the absorbance was read at 570 nm using a spectrophotometer
(Spectra Academy, K-MAC). Following Trypan blue staining (3 min at
room temperature), at least 100 cells per data point were counted
under an Eclipse E800 light microscope (Nikon Corp.) and blue
stained cells were considered dead. The cell viability was
calculated assuming the absorbance of control cells as 100%.
Drug interaction analysis
To determine the combined effects of FIS and ATO on
K562 cells, the data of MTT assay were analyzed with CompuSyn
software (ComboSyn Inc.) based on the combination index method of
Chou and Talalay (49). This
method is entirely based on the physical, chemical and mathematical
principles of the mass-action law, i.e., unified median-effect
equation and the combination index equation, and it is the most
cited and the broadest cited method for drug combination studies
(50). For MTT assays, ATO (at
concentrations of 0.5, 1.0, 1.5, 2.0, 2.5 µM) or FIS (10,
20, 30, 40, 50 µM) were added to the cells for 24 h as
either single or combined agents at a fixed concentration ratio of
1:20. The combination index (CI) values for each concentration and
the corresponding effect level, referred to as the fraction
affected (fa; the fraction of cells inhibited after the
drug exposure, e.g., 0.5 when cell growth is inhibited by 50%),
were determined. The resulting combination index offers a
quantitative determination of drug interaction, where CI <1, = 1
and >1 indicates synergism, an additive effect and antagonism,
respectively. For a graphical illustration of drug interaction, the
Fa-CI plot was constructed by computerized simulation of
serial CI values over a range of fa levels from 0.001
(IC0.1) to 0.90 (IC90). The dose reduction
index (DRI) at fa=0.5 was also calculated to indicate
the fold of dose reduction allowed for each agent in the
combination treatment compared to the single-agent treatment that
is required to achieve the same effect level. DRI = 1 signifies no
dose reduction, whereas DRI >1 and <1 represent favorable and
unfavorable dose-reduction, respectively (51).
Cell apoptosis assay
To quantify cell apoptosis, the Annexin V-Alexa
Fluor 488 and Propidium Iodide kit (Thermo Fisher Scientific) was
used according to the manufacturer's instructions with some
modifications. In brief, following 24 and 48 h of incubation with
10 and 20 µM FIS, the cells were collected from 6-well
plates, centrifuged (300 × g, 5 min, room temperature), resuspended
in Annexin V Binding Buffer (ABB) and incubated with Annexin
V-Alexa Fluor 488 at room temperature in the dark. Following 20 min
of incubation, the cells were once more centrifuged (300 × g, 5
min, room temperature) and resuspended in ABB. Finally, 1 µl
of propidium iodide (PI) was added for 5 min at room temperature in
the dark. The data were acquired with the Tali image-based
cytometer (Thermo Fisher Scientific) and analyzed using the FCS
express Research Edition Software (version 4.03; De Novo Software).
The sum of the early (Annexin V+/PI−) and
late (Annexin V+/PI+) apoptotic cells
represented the total apoptosis.
Cell cycle assay
To examine cell populations in various phases of the
cell cycle, the measurement of the DNA content was performed using
the Guava Cell Cycle reagent (Merck KGaA) according to the
manufacturer's protocol. Briefly, the cells treated with 10 and 20
µM FIS for 24 and 48 h were harvested from 6-well plates,
centrifuged (300 × g, 5 min, room temperature) and then fixed in
ice-cold 70% ethanol at 4°C followed by overnight incubation at
−25°C. The cells were then centrifuged at 650 × g for 5 min at room
temperature and washed with PBS. Following centrifugation at 500 ×
g for 7 min at room temperature, the cells were resuspended in
Guava Cell Cycle reagent, and incubated for 30 min at room
temperature in the dark. The cells were then analyzed with the
Guava easyCyte 6HT-2L Benchtop Flow Cytometer (Merck KGaA), and the
percentage of cells in each phase of the cell cycle was determined
using InCyte software (version 4.03; De Novo Software).
Transwell invasion and migration
assays
The invasion and migration of the K562 cells was
assessed using the Transwell chamber system. The upper side of
24-well polycarbonate filter inserts (8 µm pore size;
Corning, Inc.) were pre-coated for 24 h at 37°C with 40 µl
of Matrigel (2 mg/ml in serum-free medium). The cells treated or
untreated with the flavonoid were placed in the upper compartment
of the chamber at a density of 3.0×105. Transwell
inserts were then placed in the lower chamber filled with 750
µl of medium containing 10% FBS as a chemoattractant and
allowed to invade for 40 h under standard culture conditions
provided by a CO2 incubator. After the indicated period
of time, invaded or migrated cells recovered from the lower chamber
were counted with the Tali image-based cytometer (Thermo Fisher
Scientific), and the results were expressed relative to control
cells, set as 1. Transwell migration assay was performed in a
manner similar to the above-described invasion assay with the
exception that Matrigel was omitted and the cells were counted
following 20 h of incubation.
Fluorescence staining of β-catenin and
F-actin
β-catenin and filamentous actin (F-actin)
fluorescence staining was performed according to a previously
described protocol (52). Briefly,
control and FIS-treated K562 cells were collected from 12-well
plates and centrifuged onto glass coverslips (768 × g, 10 min, room
temperature), and then fixed with 4% paraformaldehyde (Serva), as
well as permeabilized with 0.25% Triton X-100. F-actin was labeled
with Alexa Fluor 488-conjugated phalloidin (dilution 1:40 in PBS,
20 min; Life Technologies). Non-specific background was blocked
with 1% bovine serum albumin (BSA). Rabbit polyclonal
anti-β-catenin antibody was used as a primary antibody to label
β-catenin (dilution 1:150 in BSA, 60 min; cat. no. SAB4500541,
Sigma-Aldrich), followed by incubation with a secondary goat
anti-rabbit antibody conjugated to Alexa Fluor 594 (dilution 1:200
in BSA, 60 min; cat. no. R37117, Thermo Fisher Scientific). Cell
nuclei (DNA) were stained with 4′,6-diamidino-2-phenylindole (DAPI,
dilution 1:20,000, 10 min; Sigma-Aldrich). All incubations were
carried out at room temperature, whereas fluorescence staining was
performed in the dark. Finally, the slides were mounted in
Aqua-Poly/Mount (Polysciences) and analyzed using the C1
laser-scanning confocal microscope (with a 100X oil immersion
objective; Nikon) and Nikon EZ-C1 software version 3.80 (both from
Nikon). The acquisition parameters, including laser power, pixel
dwell time and gains, were maintained at the same level for all
fluorescence images captured with the confocal microscope. The
fluorescence intensity of β-catenin was normalized to DAPI signal
and measured using ImageJ software (version 1.52k; National
Institutes of Health).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was isolated from the K562 cells using the
Total RNA Mini Plus kit (A&A Biotechnology) according to the
provided manufacturer's instructions, followed by the
spectrophotometric determination of RNA concentration and purity
(BioSpectrometer basic; Eppendorf). A one-step RT-qPCR was carried
out with LightCycler RNA Master SYBR-Green I kit (Roche Applied
Science) on a LightCycler 2.0 Instrument (Roche Applied Science).
The total reaction mixture (20 µl per single LightCycler
capillary) contained 100 ng of RNA and 0.2 µM of each primer
(oligo.pl) in addition to the LightCycler RNA Master SYBR-Green I
kit components. The following thermocycling conditions were used:
One cycle of reverse transcription for 20 min at 61°C, one cycle of
denaturation for 1 min at 95°C, and 45 cycles of denaturation for 5
sec at 95°C, followed by annealing and extension for 20 sec at
55-60°C (depending on the melting temperature of the primers) and 5
sec at 72°C, respectively. The results obtained from at least 3
independent experiments were analyzed with LightCycler Software
version 4.0. The expression of each target mRNA relative to the
glyceraldehyde-3-phosphate dehydrogenase (GAPDH) internal
control was calculated based on the ΔΔCq method (2−ΔΔCq
method) (53). The primers used
for the PCR reactions are listed in Table I.
 | Table IDetails of the investigated and
reference genes. |
Table I
Details of the investigated and
reference genes.
| Gene | Primer
sequence |
|---|
| AIF | Forward:
5′-CTGAAAGACGGCAGGAAGGTAG-3′ |
| Reverse:
5′-CTCCAGCCAATCTTCCACTCAC-3′ |
| AKT | Forward:
5′-GGCTATTGTGAAGGAGGGTTG-3′ |
| Reverse
5′-TCCTTGTAGCCAATGAAGGTG-3′ |
| BAX | Forward:
5′-AGATGTGGTCTATAATGCGTTTTCC-3′ |
| Reverse:
5′-CAGAAGGCACTAATCAAGTCAAGGT-3′ |
| BCL2 | Forward:
5′-AACATCGCCCTGTGGATGAC-3′, |
| Reverse:
5′-AGAGTCTTCAGAGACAGCCAGGAG-3 |
| BECN1 | Forward:
5′-AGCTGCCGTTATACTGTTCTG-3′ |
| Reverse:
5′-ACTGCCTCCTGTGTCTTCAATCTT-3′ |
| CASP3 | Forward:
5-TGGTTCATCCAGTCGCTTTG-3′ |
| Reverse:
5′-CATTCTGTTGCCACCTTTCG-3′ |
| CD44 | Forward:
5′-ACCCCAACTCCATCTGTGC-3′ |
| Reverse:
5′-TTCTGGACATAGCGGGTG-3′ |
| GAPDH | Forward:
5′-ACAACTTTGGTATCGTGGAAGG-3′ |
| Reverse:
5′-GCCATCACGCCACAGTTTC-3′ |
|
MAP1LC3B | Forward:
5′-CGGTGATAATAGAACGATACAAGG-3′ |
| Reverse:
5′-CTGAGATTGGTGTGGAGACG-3′ |
| MMP2 | Forward:
5′-GATACCCCTTTGACGGTAAGGA-3′ |
| Reverse:
5′-CCTTCTCCCAAGGTCCATAGC-3′ |
| MMP9 | Forward:
5′-CCCTGGAGACCTGAGAACCA-3′ |
| Reverse:
5′-CCCGAGTGTAACCATAGCGG-3′ |
| MTOR | Forward:
5′-TCACATTACCCCCTTCACCA-3′ |
| Reverse:
5′-TCAGCGAGTTCTTGCTATTCC-3′ |
| PI3K | Forward:
5′-AGTAGGCAACCGTGAAGAAAAG-3′ |
| Reverse:
5′-GAGGTGAATTGAGGTCCCTAAGA-3′ |
| PYK2 | Forward:
5′-GAGACCTACCGCTGTGAAC-3′ |
| Reverse:
5′-CTGCTAGGGATGAGGTTTTG-3′ |
| RHOA | Forward:
5′-CCAAATGTGCCCATCATCCTAGTTG-3′ |
| Reverse:
5′-TCCGTCTTTGGTCTTTGCTGAACAC-3′ |
| RIP3 | Forward:
5′-AATTCGTGCTGCGCCTAGAAG-3′ |
| Reverse:
5′-TCGTGCAGGTAAAACATCCCA-3′ |
| ROCK1 | Forward:
5′-GGTGCTGGTAAGAGGGCATT-3′ |
| Reverse:
5′-AGCATCCAATCCATCCAGCAA-3′ |
| SNAI1 | Forward:
5′-TTCAACTGCAAATACTGCAACAAG-3′ |
| Reverse:
5′-CGTGTGGCTTCGGATGTG-3′ |
| SNAI2 | Forward:
5′-TGTGACAAGGAATATGTGAGCC-3′ |
| Reverse:
5′-TGAGCCCTCAGATTTGACCTG-3′ |
|
SQSTM1/P62 | Forward:
5′-GGGGACTTGGTTGCCTTTT-3′ |
| Reverse:
5′-CAGCCATCGCAGATCACATT-3′ |
| TWIST | Forward:
5′-GTCCGCAGTCTTACGAGGAG-3′ |
| reverse:
5′-GCTTGAGGGTCTGAATCTTGCT-3′ |
| VIM | Forward: 5′-
AGTCCACTGAGTACCGGAGAC-3′ |
| Reverse:
5′-CATTTCACGCATCTGGCGTTC-3′ |
Statistical analysis
Statistical analysis was performed with the Prism
software package (version 7.01, GraphPad Software). The evaluation
of data normality was carried out with the Shapiro-Wilk test.
One-way or two-way ANOVA with Tukey's or Sidak's post hoc
comparisons, Kruskal-Wallis with Dunn's post hoc tests or unpaired
t-test with Welch's correction were used for data comparison where
appropriate. All values are expressed as the means ± standard
deviation and all statistical assessments were two-sided.
Probability (P)-values <0.05 was considered to indicate
statistically significant differences.
Results
Effects of FIS treatment on the viability
of the K562 human chronic myeloid leukemia cells
To examine the cytotoxic effects of FIS towards the
K562 cells, we first performed an MTT assay, which is a sensitive
and reliable indicator of metabolically active mitochondria. As
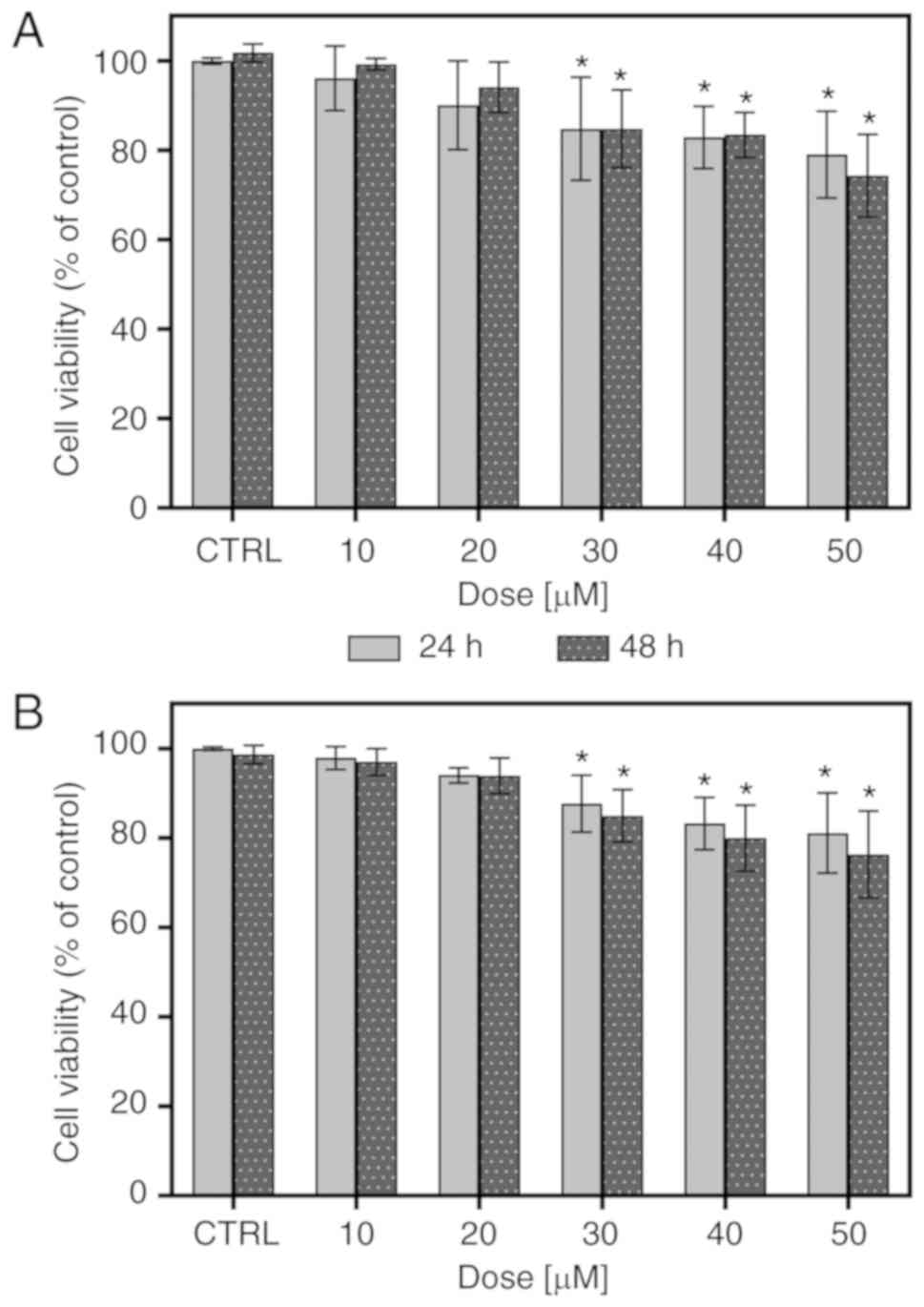
depicted in Fig. 1A, FIS at
concentrations ranging from 10 to 50 µM was not highly toxic
to the K562 cells, as approximately 80% of the cells managed to
survive following treatment with the highest concentration of this
compound, regardless of the incubation time. At low concentrations
(10 and 20 µM), FIS did not cause any apparent changes in
the viability of the K562 cells; however, at higher concentrations
(30-50 µM), it led to a statistically significant, although
slight decrease in the survival rate of these cells. Furthermore,
all tested concentrations resulted in no significant differences in
cell viability between the two incubation periods (24 vs. 48 h).
The results of MTT assays were confirmed by Trypan blue exclusion
assay (Fig. 1B).
Effects of FIS on apoptosis induction and
cell cycle distribution in the K562 human chronic myeloid leukemia
cells
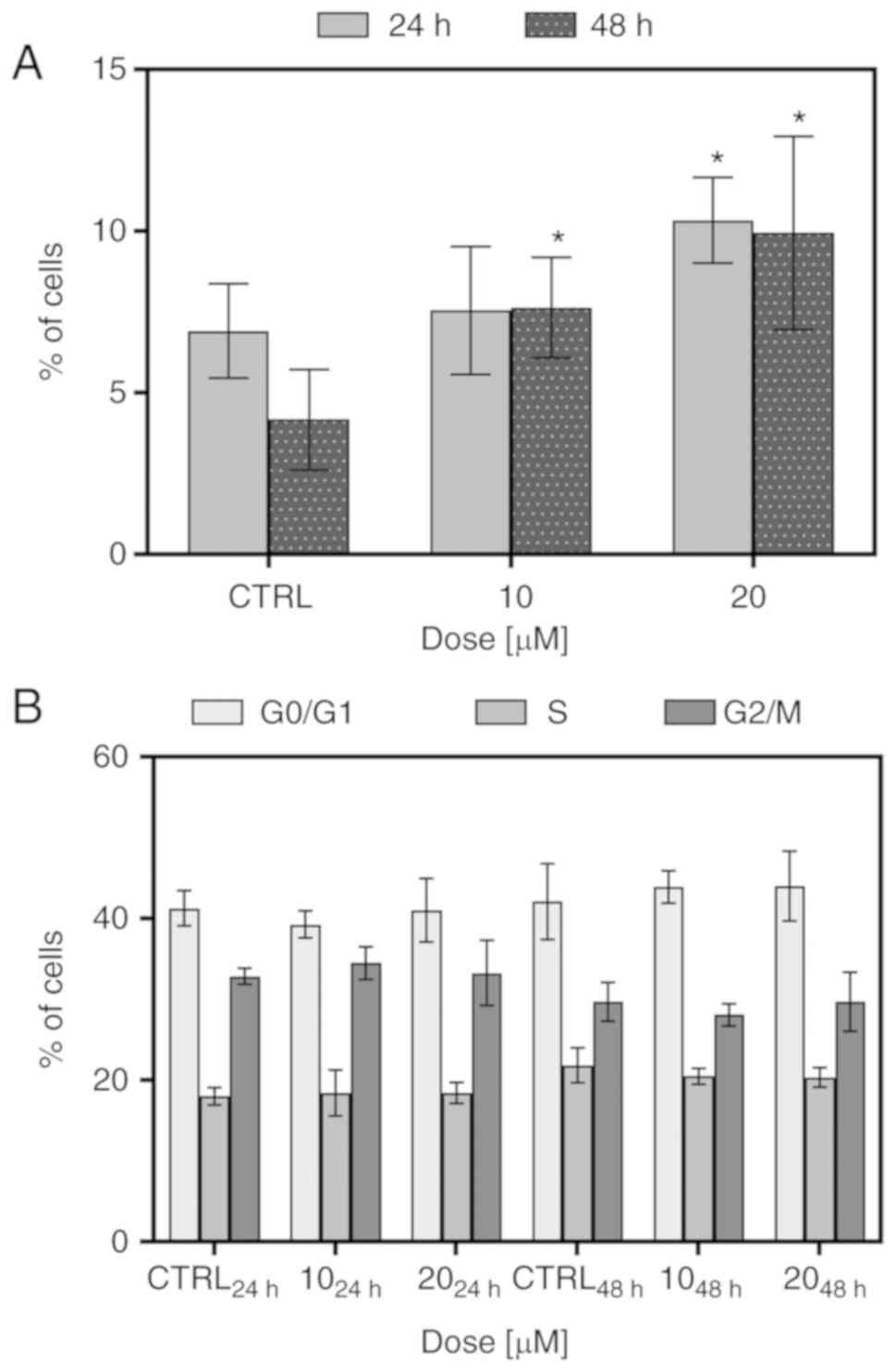
To determine whether FIS at low concentrations can
induce the apoptosis and cell cycle arrest of the K562 cells,
Annexin V/PI double staining and PI single staining were performed,
respectively, following 24 and 48 h of exposure to the 10 and 20
µM concentrations of this flavonoid. As shown in Fig. 2A, FIS was not found to be a potent
inducer of apoptosis and triggered only low cell death in the K562
cells. At the 10 µM concentration of the agent, the increase
in the number of apoptotic cells was significant only following the
longer exposure time; however, simultaneously, there were no
statistically significant differences between the two incubation
times (24 vs. 48 h). At the 20 µM concentration of FIS, the
increase in apoptosis was found to be significant in response to
both exposure time periods in comparison to the control; however,
in this case as well, the longer incubation time did not result in
a marked change in apoptosis when compared to the shorter
incubation time. As depicted in Fig.
2B, treatment of the K562 cells with FIS at both concentrations
did not cause any significant changes in cell cycle distribution
when compared to the untreated cells.
Effects of FIS on the migratory and
invasive behaviors of the K562 human chronic myeloid leukemia
cells
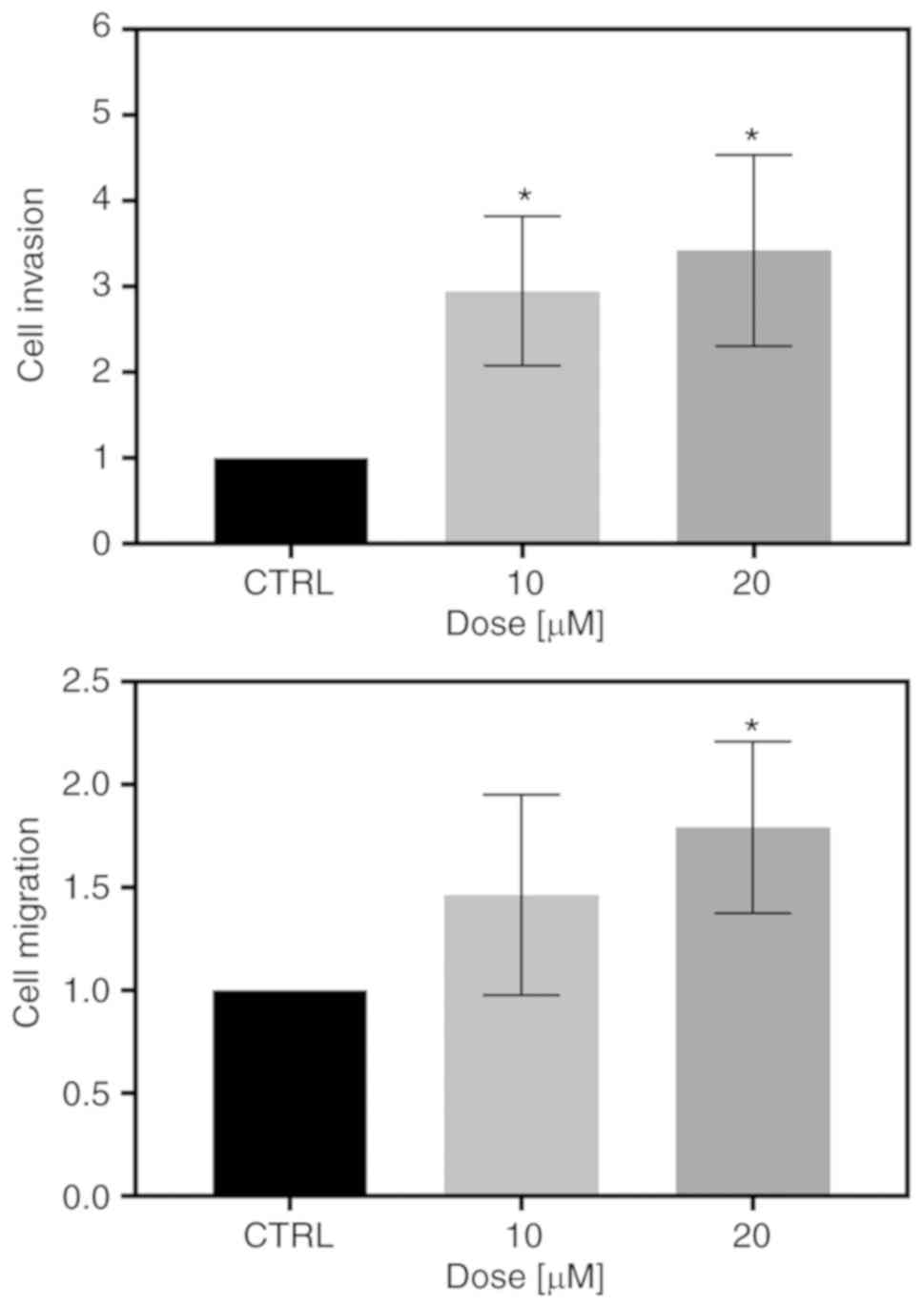
To examine whether FIS at low concentrations can
affect the invasive and migratory ability of the K562 cells,
Transwell invasion and migration assays were conducted. As shown in
Fig. 3A, the FIS-treated K562
cells exhibited a significantly greater capacity to invade through
the Matrigel matrix than the untreated ones. Likewise, treatment of
the K562 cells with both tested concentrations of this flavonoid
visibly increased the migratory capability through the Transwell
membranes compared with that of the control cells; however,
statistical significance was reached only at the concentration of
20 µM (Fig. 3B).
Effects of FIS on F-actin and β-catenin
in the K562 human chronic myeloid leukemia cells
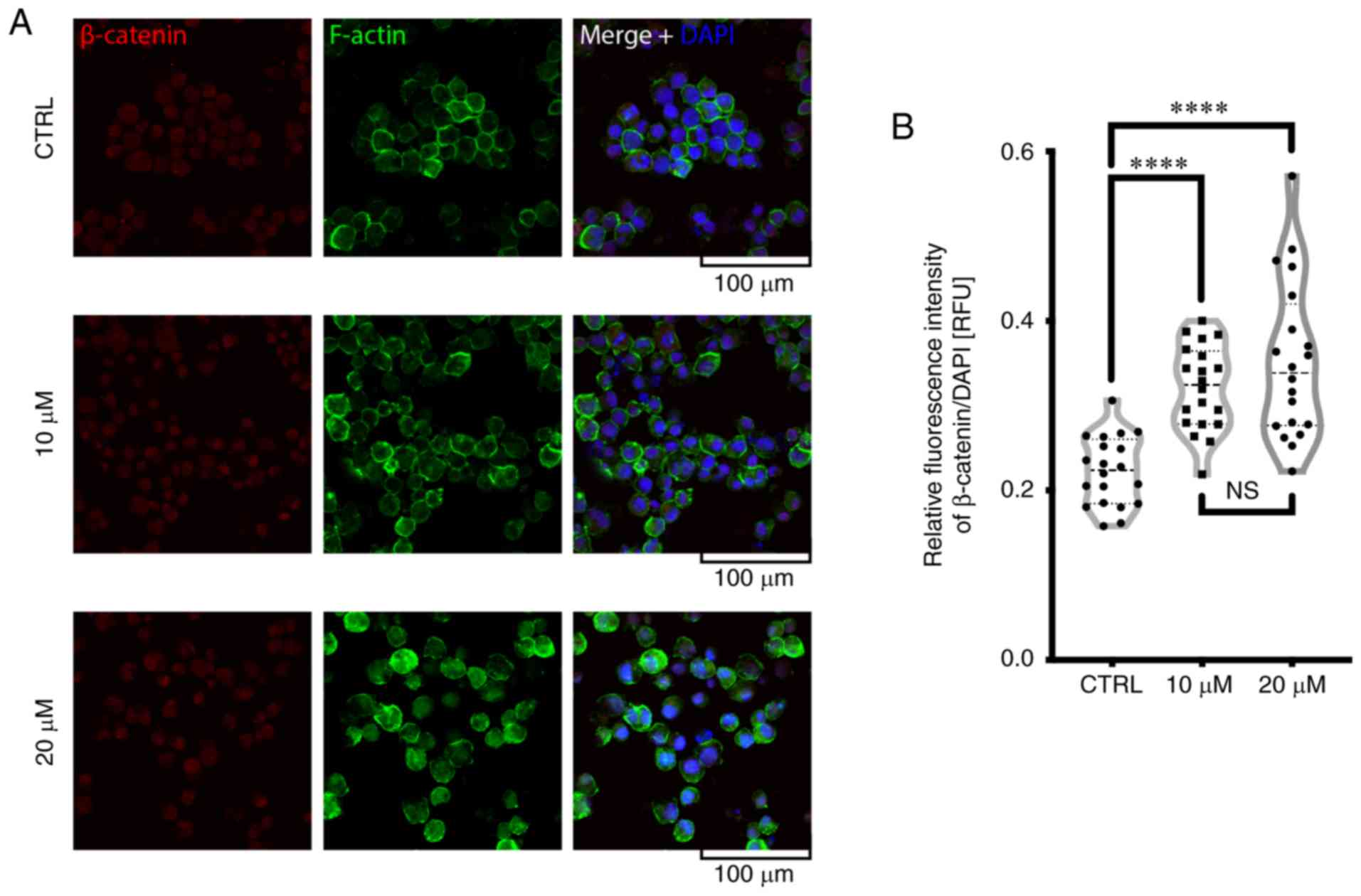
As shown in Fig.
4A, treatment of the K562 cells with FIS resulted in an
increase in F-actin staining and its more visible redistribution
toward the plasma membrane when compared to the untreated cells.
Furthermore, FIS treatment enhanced the nuclear localization of
β-catenin (Fig. 4).
Effects of FIS on the expression of
pro-survival, cell death-related and metastasis-related markers in
the K562 human chronic myeloid leukemia cells
To assess the effects of FIS at low concentrations
on the K562 cells at a molecular level, the mRNA expression of a
range of cell death-related and metastasis-related markers was
investigated by RT-qPCR. As depicted in Fig. 5A-C, the flavonoid modulated the
transcript level of apoptosis-associated genes. It upregulated the
expression of BAX and BCL2, resulting in the ratio of
BCL2 to BAX being marginally higher than 1 (1.06 and
1.19 for 10 and 20 µM FIS, respectively; Fig. 4A). Furthermore, this compound
increased the mRNA levels of caspase-3 and AIF; however, in the
case of the former, statistical significance was reached only at
the concentration of 20 µM of the agent (similar to the
results obtained for BCL2; Fig.
5A-C). In addition to apoptosis, the expression of two markers
of regulated necrosis, RIP3 and PARP1, was also
markedly upregulated by FIS (Fig. 5D
and E). Furthermore, since the autophagy-associated gene,
BECN1 was significantly overexpressed upon treatment of the
K562 cells with 20 µM FIS (Fig.
5F), the mRNA levels of two other autophagy markers, LC3B and
p62, were also measured in these cells. However, the expression
levels of these markers did not indicate the activation of
autophagy in response to FIS exposure, as the expression of the
former was unaltered and that of the latter was markedly elevated
(Fig. 5G and H). Likewise, the
mRNA level of the master negative regulator of autophagy,
MTOR, increased upon FIS treatment (Fig. 5I). The agent also upregulated the
expression of two other critical nodes in the pro-survival PI3K/AKT
and mTOR signaling pathways, whereby PI3K mRNA was
significantly increased only at the higher concentration of FIS,
and AKT mRNA only at the lower one (Fig. 5J and K).
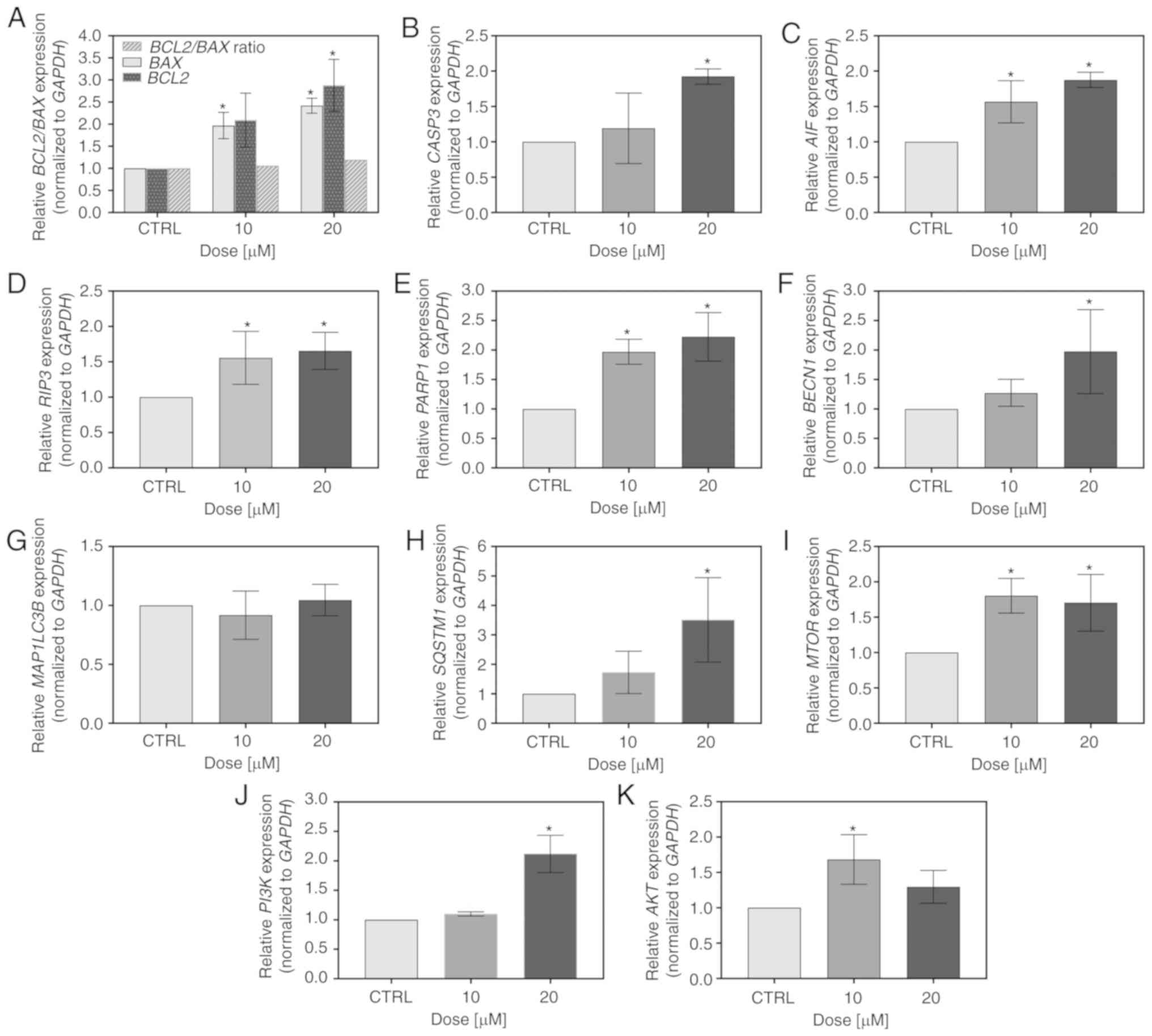 | Figure 5Measurement of the expression level
of pro-survival and death-associated markers by RT-qPCR. The K562
cells were treated for 24 h with 10 and 20 µM FIS or left
untreated (CTRL). RT-qPCR measurement of the (A) BCL2/BAX,
(B) CASP3, (C) AIF, (D) RIP3, (E)
PARP1, (F) BECN1, (G) MAP1LC3B, (H)
SQSTM1/P62, (I) MTOR, (J) PI3K and (K)
AKT mRNA expression level. Relative gene expression was
normalized to the GAPDH housekeeping gene and expressed as a
fold difference relative to a calibrator sample (untreated cells;
assumed as 1). An asterisk denotes statistically significant
differences in comparison to the control (*P<0.05;
one-way ANOVA with Tukey's post hoc test). Data represent the means
± standard deviation of at least 3 independent experiments.
BCL2, B-cell lymphoma 2; BAX, BCL2 associated X;
CASP3, caspase-3; AIF, apoptosis-inducing factor;
RIP3, receptor interacting serine/threonine-protein kinase
3; PARP1, poly(ADP-ribose) polymerase 1; BECN1,
Beclin 1; MAP1LC3B, microtubule associated protein 1 light
chain 3 beta; SQSTM1/P62, sequestosome 1/PP62; MTOR,
mammalian target of rapamycin; PI3K, phosphatidylinositol
3-kinase; AKT, protein kinase B. |
FIS also influenced the expression of
metastasis-related genes in the K562 cells. At both the tested
concentrations, it significantly increased the CD44,
MMP2, SNAIL, SLUG, VIM and ROCK1 mRNA levels;
however, it simultaneously had no effect on RHOA expression
(Fig. 6A-G). Furthermore, there
was a significant upregulation of MMP9, TWIST,
PYK2 and CTNNB1 expression following treatment with
20 µM FIS. The 10-fold lower concentration of the flavonoid
also upregulated the mRNA level of MMP9, TWIST and
PYK2; however, in these cases, statistical significance was
not reached (Fig. 6H-K).
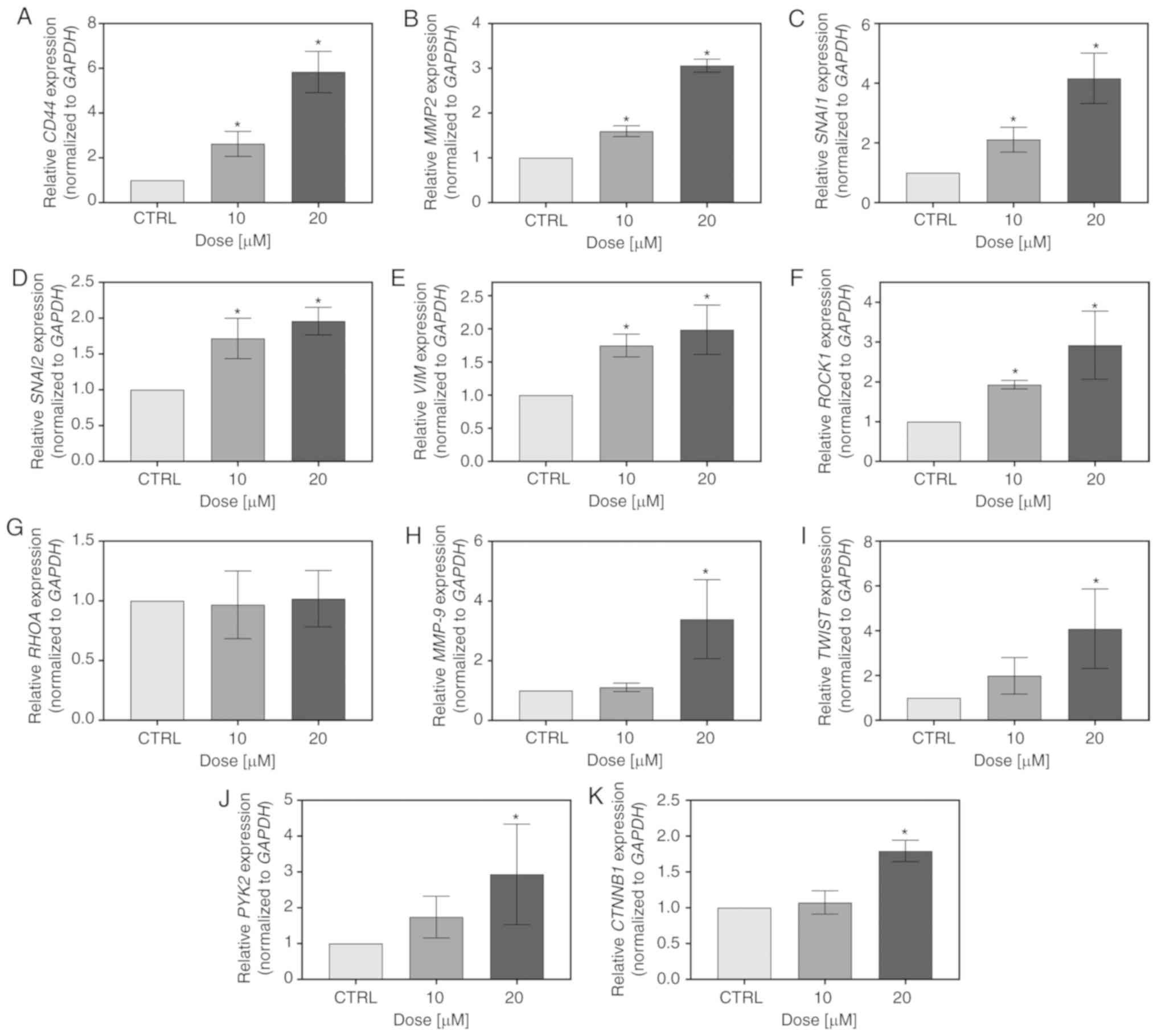 | Figure 6Measurement of the expression level
of metastasis-related markers by RT-qPCR. The K562 cells were
treated for 24 h with 10 and 20 µM FIS or left untreated
(CTRL). RT-qPCR measurement of the (A) CD44 (B) MMP2,
(C) SNAI1, (D) SNAI2, (E) VIM, (F)
ROCK1, (G) RHOA, (H) MMP9, (I) TWIST,
(J) PYK2, (K) CTNNB1 mRNA expression level. Relative
gene expression was normalized to the GAPDH housekeeping
gene and expressed as a fold difference relative to a calibrator
sample (untreated cells; assumed as 1). An asterisk denotes
statistically significant differences in comparison to control
(*P<0.05; one-way ANOVA with Tukey's post hoc test).
Data represent the means ± standard deviation of at least 3
independent experiments. CD44, cluster of differentiation
44; MMP2, matrix metalloproteinase 2; SNAI1, snail
family transcriptional repressor 1; SNAI2, snail family
transcriptional repressor 2; VIM, vimentin; ROCK1,
Rho associated coiled-coil containing protein kinase 1;
RHOA, Ras homolog family member A; MMP9, matrix
metalloproteinase 9; TWIST, Twist family BHLH transcription
factor 1; PYK2, proline-rich tyrosine kinase 2;
CTNNB1, catenin beta 1. |
Combined effects of FIS and ATO on the
K562 human chronic myeloid leukemia cells
The interaction between FIS and ATO was analyzed
based on the results of MTT assays and the median-effect principle
of Chou and Talalay (49). As
shown in Fig. 7A, a single
treatment with ATO, at concentrations ranging from 0.5 to 2.5
µM, had little if any effect on the viability of the K562
cells, the results of which are consistent with those of previous
studies on this cell line (54,55).
In turn, the combination of FIS and ATO produced a highly variable
effect on cell survival in dependence of the concentrations of both
agents. Co-treatment with 10 µM FIS and 0.5 µM ATO
resulted in a slight increase in the viability of the K562 cells;
however, the results did not achieve statistical significance
compared to the control or either agent alone (Fig. 7A). The calculated CI value
indicated a very potent antagonism between FIS and ATO at this
experimental point (dose combination) (Table II). However, when the cells were
simultaneously treated with higher concentrations of FIS (20 or 30
µM) and ATO (1 or 1.5 µM), a significant decrease in
cell viability compared to the control cell population and
ATO-treated cells, but not to the FIS-treated cells was observed
(Fig. 7A). The computed CI values
indicated the occurrence of a slight antagonism and synergism
between the former (20 µM FIS + 1 µM ATO) and the
latter (30 µM FIS + 1.5 µM ATO) dose-pair,
respectively (Table II). At the
two highest concentrations (40 µM FIS + 2 µM ATO and
50 µM FIS + 2.5 µM ATO), the combined cytotoxic
effects of FIS and ATO against the K562 cells were greater than
those induced by each agent individually (Fig. 7A). At these experimental points,
the interactions of the two agents were moderately synergistic, as
indicated by calculating the CI values (Table II). Apart from the determination
of the CI values for actual experimental points, the CI values for
a wide range of effect levels were generated by the computerized
simulation, and depicted graphically as combination index curve
(Fa-CI plot). As demonstrated in Fig. 7B, this analysis revealed that the
interactions between FIS and ATO varied from antagonism at lower
concentrations (i.e., the lowest fa values) to synergism
at higher ones (i.e., higher fa values). The dose
requirement to achieve 50% growth inhibition (fa=0.5)
decreased to 48.3 and 2.4 µM for FIS, respectively, thus
reducing the dose by 3.1- and 11.5-fold (Fig. 7B). Importantly, according to the
previous reports (44), this
concentration of the flavonoid is still outside physiologically
attainable levels (≤20 µM). Likewise, the therapeutic
concentrations of ATO have been reported to be approximately 1-2
µM (56).
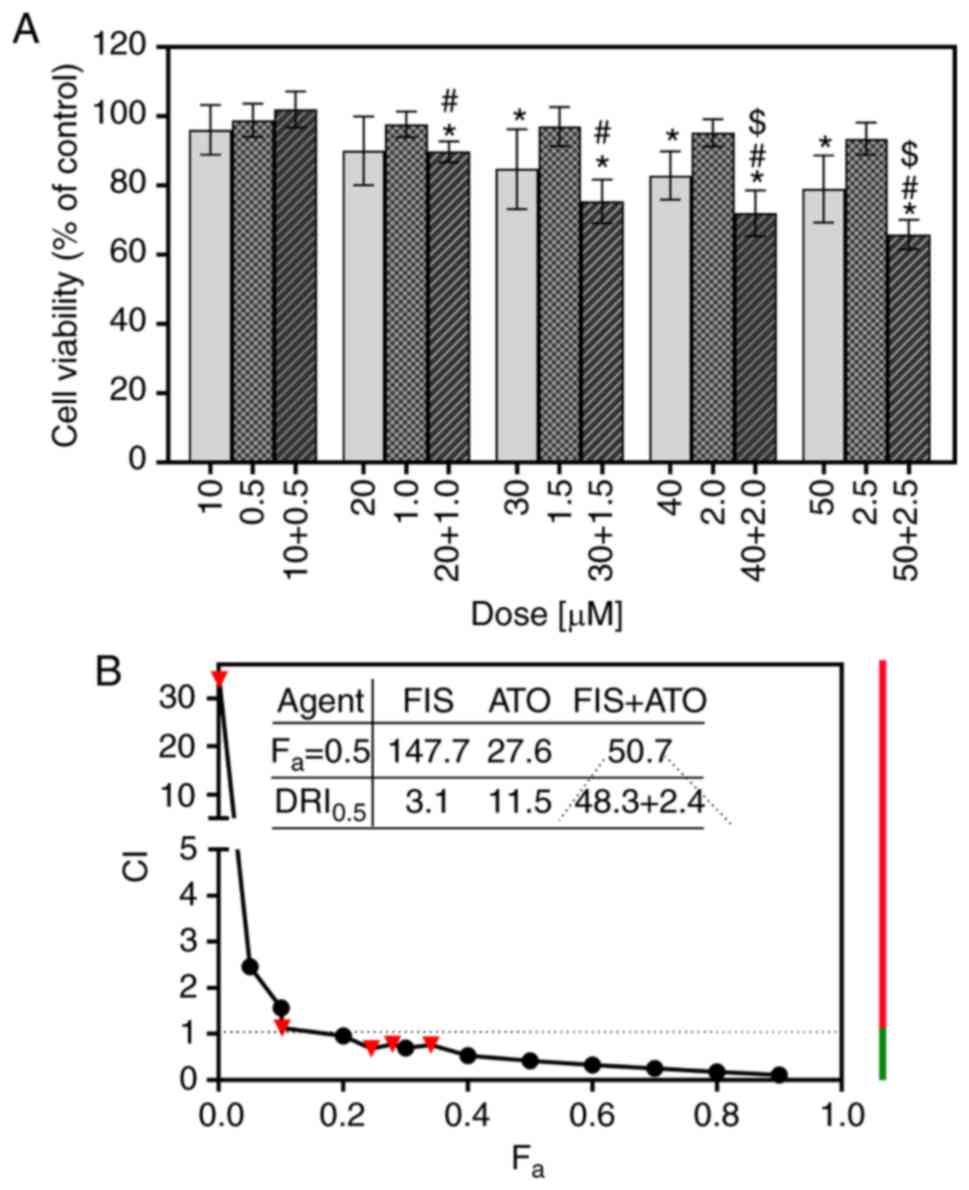 | Figure 7Combined effects of fisetin (FIS) and
arsenic trioxide (ATO) on the K562 cells. The cells were treated
with various concentrations of ATO (0.5, 1.0, 1.5, 2.0, 2.5
µM) and FIS (10, 20, 30, 40, 50 µM), either alone or
in a fixed ratio of 1:20, for 24 h. (A) Cell viability was
determined by MTT colorimetric assay. Data are expressed as a
percentage of the control. The symbols $ and # indicate
statistically significant differences compared with FIS or ATO
treatment alone, respectively (P<0.05; unpaired t-test with
Welch's correction). The asterisk (*) indicates statistically
significant differences compared with the control. All values
represent the means ± standard deviation of 5 independent
experiments. (B) Combination index plot (Fa-CI plot) for
FIS and ATO co-treatment in K562 cells. CI values are plotted as a
function of the fractional inhibition (fa) of cell
viability by computer simulation (CompuSyn software) from 0.001 to
0.90. Triangles represent CI values derived from the actual data
points. CI <1 indicates synergism (denoted by green line), CI=1
designates additivity (denoted by a dashed line), and CI >1
represents antagonism (denoted by red line). The dose reduction
index (DRI) =1 signifies no dose reduction, whereas DRI >1 and
<1 represent a favorable and unfavorable dose-reduction,
respectively. |
 | Table IIThe combination index values at
actual experimental points for the combination of fisetin and
arsenic trioxide in K562 cells. |
Table II
The combination index values at
actual experimental points for the combination of fisetin and
arsenic trioxide in K562 cells.
Drug combination
| CI | fa | Interaction
type |
|---|
| FIS
(µM) | ATO
(µM) |
|---|
| 10 | 0.5 | 33.91 | 0.001 | Very potent
antagonism |
| 20 | 1.0 | 1.13 | 0.102 | Slight
antagonism |
| 30 | 1.5 | 0.68 | 0.245 | Synergism |
| 40 | 2.0 | 0.78 | 0.279 | Moderate
synergism |
| 50 | 2.5 | 0.76 | 0.341 | Moderate
synergism |
Discussion
Advances achieved over the past two decades in the
treatment of CML, associated mainly with the development of
BCR/ABL1 tyrosine kinase inhibitors (TKIs), have markedly improved
the prognosis and survival of Philadelphia chromosome-positive
(Ph+) CML patients (57). However,
resistance to TKIs and their toxicity have become an increasingly
important cause of treatment failure (58). Therefore, the identification and
development of novel agents with therapeutic potential against CML
is still an important subject for research. With this aim, in the
present study, we investigated whether the flavonoid, FIS, at
achievable serum concentrations, exhibits anticancer activity
against K562 human chronic myeloid leukemia cells, in the context
of both potential anti-proliferative and anti-metastatic effects.
We decided to focus mainly on these concentrations of the flavonoid
that have previously been reported to correspond with its in
vivo attainable levels (≤20 µM) (44). The concentration issue is of
particular importance in the field of both in vitro and
flavonoid research since there is currently the conception that to
limit a lack of reproducibility of the cell line studies in
clinical trials, it is essential to employ only low, clinically
relevant concentrations of tested agents. In the case of dietary
flavonoids, this issue is mostly related to the fact that an
increasing number of studies have been demonstrating a dual,
dose-dependent functional effect of these compounds on cancer cell
behavior, with a desired anticancer effect at high doses (usually
>60 µM) and no or unfavorable response at lower ones
(59-61). Our previous studies (41,42)
and the current study seem to confirm such observations.
In this study, we found that low concentrations (10
and 20 µM) of FIS only negligibly affected the
viability of the K562 cells through the induction of apoptosis,
accompanied by the increase in the migratory and invasive
properties of these leukemia cells. Some markers of cell death were
significantly elevated in response to FIS treatment; however, they
were counterbalanced through anti-apoptotic and pro-survival
signals. These results are consistent with the current
understanding that in response to either death or non-lethal stress
from the treatment, apoptotic signals may have functions other than
cell death, including the promotion of a rapid proliferation of
neighboring surviving tumor cells, and the increase in their
metastatic capacity. This process is referred to as
apoptosis-induced proliferation (AiP) and its direct crosstalk with
signaling networks linked to migration and invasion has recently
been proposed (62-67). Diverse mechanisms of AiP, such as
the 'Phoenix Rising' (PR) pathway have been more recently
identified and the role of the latter in tissue regeneration, as
well as tumor repopulation and resistance following cytotoxic
therapies (either ionizing radiation or chemotherapy) has also been
reported (68-70). Therefore, it is possible that
pro-apoptotic proteins, mostly caspase 3 confer a pro-survival and
pro-invasive phenotype to FIS-treated K562 cells through the PR
pathway. However, the assessment of other key molecular players of
the PR pathway [e.g., caspase-7, prostaglandin E2 (PGE2), protein
kinase Cδ (PKCδ), calcium-independent phospholipase A2 (iPLA2),
COX-2, NF-κB] should be performed, among other assays (71-72),
to confirm this assumption.
There are currently a few studies on the effects of
FIS on human CML cells and their authors suggest that this
flavonoid has a promising activity against these cells (23,24).
Indeed, in K562 cells, FIS has been shown to arrest the cell cycle
at both the S and G2/M phases with the subsequent induction of
mitochondrial- and caspase-dependent apoptosis. Simultaneously,
wide genome microarray analysis has revealed that this flavonoid
dose-dependently modulates hundreds of genes implicated in
proliferation, differentiation, cell cycle, growth arrest,
apoptosis, DNA repair, invasion and metastasis, including those
being well-recognized oncogenes and tumor suppressor genes, e.g.,
NFKBIA (also known as IκBα), metallothionin (MT) family genes,
PMAIP1/NOXA, CDKN1A (p21/WAF1/Cip1), GADD45B, MYC (v-myc avian
myelocytomatosis viral oncogenehomolog), MYB (v-myb avian
myeloblastosis viral oncogene homolog), C-KIT (v-kit
Hardy-Zuckerman 4 feline sarcoma viral oncogene homolog), tubulin
family members, such as TUBA1A and TUBA1C, serpin family members,
STAT proteins and the downstream targets of JAK/STAT pathway, such
as PIM1/2 kinases (24). The
above-mentioned effects required the concentrations of FIS far
outside those reported as physiologically achievable levels (up to
200 µM) as well as the prolonged incubation time
(IC50 values at 48 and 72 h were estimated as 163 and
120 µM, respectively) (24). Simultaneously, it should be
emphasized that the IC50 values reported by the cited
authors for FIS-treated K562 cells were similar to those obtained
by us, whereas in the studies presented by another research group,
50-µM FIS promoted a robust cell death of nearly 50% of K562
cells after 24 h of treatment (23). Following the same concentration and
incubation period, we found that FIS induced apoptosis in nearly
14% of the K562 cells (data not shown). Such discrepancies between
the in vitro drug effects can be quite often found in the
literature and usually result from differences in culture
conditions, which are rather difficult to discuss, when there is a
lack of methodological details provided in the study. Furthermore,
the cited authors have demonstrated that the FIS-induced apoptosis
of CML cells was mediated by nitric oxide (NO), although the exact
underlying mechanism was investigated in THP-1 acute monocytic
leukemia cells, which were much more sensitive to this flavonoid
than K562 cells (23). Therefore,
even if, in contrast to our results, 50 µM of FIS is able to
induce a high level of apoptosis of K562 CML cells, it is currently
considered that the concentrations of FIS (as well as most other
dietary polyphenols) >20 µM are unachievable in
vivo, and some researchers set this limit even at half lower
dose (10 µM) (73-75).
Our efforts towards assessing the effects of FIS on
some markers of both cancer cell migration/invasion and cancer cell
growth/death were related to emerging evidence demonstrating that
some chemotherapeutic drugs, alongside their cytotoxic activity,
may promote invasive behaviors of cancer cells, resulting in the
induction or acceleration of metastasis formation (76,77).
With regard to CML, a recent example of such action has been
derived from imatinib studies. For instance, Ovcharenko et
al reported an increase in the adhesion, migratory and invasive
capabilities of K562 cells following imatinib exposure and
identified proline-rich tyrosine kinase 2 (PYK2) as one of the
major mediator of these processes (78). Herein, we found that of treatment
the K562 cells with FIS also resulted in the upregulation of the
PYK2 transcript level, in addition to its ability to
increase the expression of ROCK1, although not the
expression of upstream RHOA, both of which have been
implicated in the proliferation, migration and invasion of CML
cells and are currently considered as therapeutic targets for CML
patients (79,80). Moreover, the PI3K/AKT/mTOR
signaling pathway enhances not only the growth and survival of
cancer cells, including CML, but also cell invasion, migration and
drug resistance (81,82). Likewise, a high expression of CD44
has been linked to tumor invasion, metastasis, proliferation,
recurrence and chemoresistance (83), and with regard to the K562 cell
line, it has been suggested that the proliferation of these cells
depends to a greater extent on CD44 (84). Therefore, it is not without
significance that FIS led to the increase in the transcript levels
of these above-mentioned markers in CML cells. Furthermore,
although epithelial-mesenchymal transition (EMT)-related proteins
are well recognized for playing a pivotal role in the invasiveness,
migration capability, stemness, and drug resistance of epithelial
tumors, evidence of their similar importance in the hematological
malignancies (despite their mostly mesenchymal status, as blood
cells originate from the embryonic mesoderm) has been increasingly
provided in recent years (85-87).
EMT-related transcription factors, including TWIST, SLUG and SNAIL
have been linked with the enhanced aggressiveness of CML cells
(88-90), and we found that FIS at in
vivo relevant concentrations increased their mRNA level in K562
CML cells. Likewise, this flavonoid promoted the overexpression of
the EMT-associated markers, vimentin and MMP2/9, as well as the
nuclear localization of β-catenin. Furthermore, FIS treatment
resulted in enriched F-actin staining and its visible remodeling
toward the plasma membrane. These results were in agreement with
our in vitro migration and invasion assays, and therefore,
it can be hypothesized that all these cellular and molecular
changes underlie the increased migration and invasion of K562 cells
in the presence of this flavonoid and constitute a clear
undesirable outcome of its in vitro testing in this cancer
model. Although further studies are required, particularly those
evaluating the effects of low-dose FIS on a larger panel of
cancer-related markers at both mRNA and protein level, as well as
the phosphorylation status of selected proteins, it can be
concluded that a particular caution should be taken when
considering FIS as a potential candidate for CML treatment.
Given the lack of utility of FIS in the monotherapy
of CML, as well as our (41,42)
and other (27,43,91)
recent findings on its considerable efficacy in the combination
with standard chemotherapeutic drugs in some cancer models, herein
we also decided to assess whether this flavonoid may synergize with
ATO in K562 cells. ATO is known for its high in vitro and
clinical activity against acute promyelocytic leukemia (APL), and
numerous preclinical data have also supported its role as a therapy
for CML (92,93). ATO treatment has been shown to
present similar cellular effects on CML cell lines as on APL ones,
although higher and clinically unachievable concentrations are
required to promote the cell cycle arrest and apoptosis of the
former leukemia cells (54,55,94,95).
Therefore, there has been a rationale for the development of
optimized ATO-based combination regimens to maintain or potentiate
a treatment response, while reducing its dosage to
sub-pharmacological, non-toxic levels (95-98).
In this study, we found that the interactions between FIS and ATO
varied from potent antagonism at lower concentrations (i.e., the
lowest fa values) to potent synergism at higher ones
(i.e., higher fa values). This raises two important
concerns. Firstly, the dose-dependence of the in vitro
interaction patterns between FIS and ATO make it difficult to
predict the nature of their interaction in vivo, where the
drug concentrations vary depending on their pharmacokinetic profile
and intra-tumoral distribution. Secondly, it was the low,
physiologically achievable concentrations of FIS, which acted
antagonistically with clinically relevant doses of ATO, what again
implies that the utility of FIS in the combination of ATO as a
chemotherapy for CML is rather limited. This also suggests that the
supplementation of FIS during the treatment of CML patients with
ATO may detrimentally affect therapeutic response. This seems of
particular importance when taking into consideration that FIS is
widely available in the form of dietary supplements (at the
recommended dose of 100-200 mg per day), which are usually taken
without medical supervision. However, since the interaction
patterns between FIS and ATO were examined only based on the
results of MTT assays and at a particular concentration range, it
is of importance to confirm our findings using various
concentration ratios of both agents and additional assays, e.g.,
Annexin V/PI assay.
In conclusion, the present study revealed that
low-dose FIS had not only negligible effect on the viability and
apoptosis of K562 cells, but also modulated the mRNA level of
selected metastatic-related markers, accompanied by the increase in
the migratory and invasive properties of these cancer cells.
Although the levels of some markers of cell death were
significantly elevated in response to FIS treatment, they were
counterbalanced through anti-apoptotic and pro-survival signals.
FIS dose-dependently interacts with ATO; however, it was the low,
in vivo achievable concentrations of the flavonoid, which
acted antagonistically with clinically relevant doses of cytostatic
drug. All these results suggest that careful consideration should
be taken when advising FIS as dietary supplement in cancer patients
and when considering this flavonoid as a potential candidate for
the treatment of CML. However, it should be emphasized that our
results were obtained under specific in vitro culture
conditions, hence their relevance to human health is currently
limited, and only in vivo and particularly clinical studies
will be able to verify the potential advantages and disadvantages
of using FIS in patients with CML.
Acknowledgments
Not applicable.
Funding
The study was supported by research tasks no. 142
within the frame-work of basal research activity (Nicolaus
Copernicus University in Toruń, Faculty of Medicine, Collegium
Medicum in Bydgoszcz).
Availability of data and materials
The datasets used and/or analyzed during the
current study are available from the corresponding author on
reasonable request.
Authors' contributions
AKW and DG conceived and designed the experiments,
performed some of the experiments, analyzed the data, performed the
statistical analysis and wrote the manuscript; PC, MHW, JD and PA
performed experiments and helped to draft the manuscript; MG
performed some of the experiments; AG revised the manuscript and
was involved in the conception of the study. All authors have read
and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have not competing
interests.
References
|
1
|
Thomasset SC, Berry DP, Garcea G, Marczylo
T, Steward WP and Gescher AJ: Dietary polyphenolic
phytochemicals-promising cancer chemopreventive agents in humans? A
review of their clinical properties. Int J Cancer. 120:451–458.
2007. View Article : Google Scholar
|
|
2
|
Ramos S: Cancer chemoprevention and
chemotherapy: Dietary polyphenols and signalling pathways. Mol Nutr
Food Res. 52:507–526. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sak K: Chemotherapy and dietary
phytochemical agents. Chemother Res Pract. 2012:2825702012.
|
|
4
|
Surh YJ: Cancer chemoprevention with
dietary phytochemicals. Nat Rev Cancer. 3:768–780. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Jash SK and Mondal S: Bioactive flavonoid
fisetin-a molecule of pharmacological interest. J Org Biomol Chem.
2:89–128. 2014.
|
|
6
|
Liu XF, Long HJ, Miao XY, Liu GL and Yao
HL: Fisetin inhibits liver cancer growth in a mouse model: Relation
to dopamine receptor. Oncol Rep. 38:53–62. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Khan N, Asim M, Afaq F, Abu Zaid M and
Mukhtar H: A novel dietary flavonoid fisetin inhibits androgen
receptor signaling and tumor growth in athymic nude mice. Cancer
Res. 68:8555–8563. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Khan N, Syed DN, Ahmad N and Mukhtar H:
Fisetin: A dietary antioxidant for health promotion. Antioxid Redox
Signal. 19:151–162. 2013. View Article : Google Scholar :
|
|
9
|
Syed DN, Adhami VM, Khan N, Khan MI and
Mukhtar H: Exploring the molecular targets of dietary flavonoid
fisetin in cancer. Semin Cancer Biol. 40-41:130–140. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Mukhtar E, Adhami VM, Sechi M and Mukhtar
H: Dietary flavonoid fisetin binds to β-tubulin and disrupts
microtubule dynamics in prostate cancer cells. Cancer Lett.
367:173–183. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Suh Y, Afaq F, Khan N, Johnson JJ, Khusro
FH and Mukhtar H: Fisetin induces autophagic cell death through
suppression of mTOR signaling pathway in prostate cancer cells.
Carcinogenesis. 31:1424–1433. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Jia S, Xu X, Zhou S, Chen Y, Ding G and
Cao L: Fisetin induces autophagy in pancreatic cancer cells via
endoplasmic reticulum stress-and mitochondrial stress-dependent
pathways. Cell Death Dis. 10:1422019. View Article : Google Scholar
|
|
13
|
Salmela AL, Pouwels J, Varis A, Kukkonen
AM, Toivonen P, Halonen PK, Perälä M, Kallioniemi O, Gorbsky GJ and
Kallio MJ: Dietary flavonoid fisetin induces a forced exit from
mitosis by targeting the mitotic spindle checkpoint.
Carcinogenesis. 30:1032–1040. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kang KA, Piao MJ, Madduma Hewage SR, Ryu
YS, Oh MC, Kwon TK, Chae S and Hyun JW: Fisetin induces apoptosis
and endoplasmic reticulum stress in human non-small cell lung
cancer through inhibition of the MAPK signaling pathway. Tumor
Biol. 37:9615–9624. 2016. View Article : Google Scholar
|
|
15
|
Su CH, Kuo CL, Lu KW, Yu FS, Ma YS, Yang
JL, Chu YL, Chueh FS, Liu KC and Chung JG: Fisetin-induced
apoptosis of human oral cancer SCC-4 cells through reactive oxygen
species production, endoplasmic reticulum stress, caspase-, and
mitochondria-dependent signaling pathways. Environ Toxicol.
32:1725–1741. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Khan N, Afaq F, Syed DN and Mukhtar H:
Fisetin, a novel dietary flavonoid, causes apoptosis and cell cycle
arrest in human prostate cancer LNCaP cells. Carcinogenesis.
29:1049–1056. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lee WR, Shen SC, Lin HY, Hou WC, Yang LL
and Chen YC: Wogonin and fisetin induce apoptosis in human
promyelo-leukemic cells, accompanied by a decrease of reactive
oxygen species, and activation of caspase 3 and Ca(2+)-dependent
endo-nuclease. Biochem Pharmacol. 63:225–236. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chen YC, Shen SC, Lee WR, Lin HY, Ko CH,
Shih CM and Yang LL: Wogonin and fisetin induction of apoptosis
through activation of caspase 3 cascade and alternative expression
of p21 protein in hepatocellular carcinoma cells SK-HEP-1. Arch
Toxicol. 76:351–359. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Jang KY, Jeong SJ and Kim SH, Jung JH, Kim
JH, Koh W, Chen CY and Kim SH: Activation of reactive oxygen
species/AMP activated protein kinase signaling mediates
fisetin-induced apoptosis in multiple myeloma U266 cells. Cancer
Lett. 319:197–202. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kim JA, Lee S, Kim DE, Kim M, Kwon BM and
Han DC: Fisetin, a dietary flavonoid, induces apoptosis of cancer
cells by inhibiting HSF1 activity through blocking its binding to
the hsp70 promoter. Carcinogenesis. 36:696–706. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sung B, Pandey MK and Aggarwal BB:
Fisetin, an inhibitor of cyclin-dependent kinase 6, down-regulates
nuclear factor-kappaB-regulated cell proliferation, antiapoptotic
and metastatic gene products through the suppression of TAK-1 and
receptor-interacting protein-regulated IkappaBalpha kinase
activation. Mol Pharmacol. 71:1703–1714. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Li J, Cheng Y, Qu W, Sun Y, Wang Z, Wang H
and Tian B: Fisetin, a dietary flavonoid, induces cell cycle arrest
and apoptosis through activation of p53 and inhibition of NF-kappa
B pathways in bladder cancer cells. Basic Clin Pharmacol Toxicol.
108:84–93. 2011. View Article : Google Scholar
|
|
23
|
Ash D, Subramanian M, Surolia A and Shaha
C: Nitric oxide is the key mediator of death induced by fisetin in
human acute monocytic leukemia cells. Am J Cancer Res. 5:481–497.
2015.PubMed/NCBI
|
|
24
|
Adan A and Baran Y: Fisetin and hesperetin
induced apoptosis and cell cycle arrest in chronic myeloid leukemia
cells accompanied by modulation of cellular signaling. Tumor Biol.
37:5781–5795. 2016. View Article : Google Scholar
|
|
25
|
Adan A and Baran Y: The pleiotropic
effects of fisetin and hesperetin on human acute promyelocytic
leukemia cells are mediated through apoptosis, cell cycle arrest,
and alterations in signaling networks. Tumor Biol. 36:8973–8984.
2015. View Article : Google Scholar
|
|
26
|
Syed DN, Lall RK, Chamcheu JC, Haidar O
and Mukhtar H: Involvement of ER stress and activation of apoptotic
pathways in fisetin induced cytotoxicity in human melanoma. Arch
Biochem Biophys. 563:108–117. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Smith ML, Murphy K, Doucette CD,
Greenshields AL and Hoskin DW: The dietary flavonoid fisetin causes
cell cycle arrest, caspase-dependent apoptosis, and enhanced
cytotoxicity of chemotherapeutic drugs in triple-negative breast
cancer cells. J Cell Biochem. 117:1913–1925. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Murtaza I, Adhami VM, Hafeez BB, Saleem M
and Mukhtar H: Fisetin, a natural flavonoid, targets chemoresistant
human pancreatic cancer AsPC-1 cells through DR3-mediated
inhibition of NF-kappaB. Int J Cancer. 125:2465–2473. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ying TH, Yang SF, Tsai SJ, Hsieh SC, Huang
YC, Bau DT and Hsieh YH: Fisetin induces apoptosis in human
cervical cancer HeLa cells through ERK1/2-mediated activation of
caspase-8-/caspase-3-dependent pathway. Arch Toxicol. 86:263–273.
2012. View Article : Google Scholar
|
|
30
|
Lim DY and Park JH: Induction of p53
contributes to apoptosis of HCT-116 human colon cancer cells
induced by the dietary compound fisetin. Am J Physiol Gastrointest
Liver Physiol. 296:G1060–G1068. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Suh Y, Afaq F, Johnson JJ and Mukhtar H: A
plant flavonoid fisetin induces apoptosis in colon cancer cells by
inhibition of COX2 and Wnt/EGFR/NF-kappaB-signaling pathways.
Carcinogenesis. 30:300–307. 2009. View Article : Google Scholar
|
|
32
|
Pal HC, Sharma S, Elmets CA, Athar M and
Afaq F: Fisetin inhibits growth, induces G2/M arrest and
apoptosis of human epidermoid carcinoma A431 cells: Role of
mitochondrial membrane potential disruption and consequent caspases
activation. Exp Dermatol. 22:470–475. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhang XJ and Jia SS: Fisetin inhibits
laryngeal carcinoma through regulation of AKT/NF-κB/mTOR and ERK1/2
signaling pathways. Biomed Pharmacother. 83:1164–1174. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Li YS, Qin XJ and Dai W: Fisetin
suppresses malignant proliferation in human oral squamous cell
carcinoma through inhibition of Met/Src signaling pathways. Am J
Transl Res. 9:5678–5683. 2017.
|
|
35
|
Shih YL, Hung FM, Lee CH, Yeh MY, Lee MH,
Lu HF, Chen YL, Liu JY and Chung JG: Fisetin induces apoptosis of
HSC3 human oral cancer cells through endoplasmic reticulum stress
and dysfunction of mitochondria-mediated signaling pathways. In
Vivo. 31:1103–1114. 2017.PubMed/NCBI
|
|
36
|
Sowa M, Slepokura K and Matczak-Jon E: A
1:2 cocrystal of genistein with isonicotinamide: Crystal structure
and Hirshfeld surface analysis. Acta Crystallogr C. 69:1267–1272.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Sowa M, Slepokura K and Matczak-Jon E:
Improving solubility of fisetin by cocrystallization. Cryst Eng
Comm. 16:105922014. View Article : Google Scholar
|
|
38
|
Kadari A, Gudem S, Kulhari H, Bhandi MM,
Borkar RM, Kolapalli VR and Sistla R: Enhanced oral bioavailability
and anticancer efficacy of fisetin by encapsulating as inclusion
complex with HPβCD in polymeric nanoparticles. Drug Deliv.
24:224–232. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Ragelle H, Crauste-Manciet S, Seguin J,
Brossard D, Scherman D, Arnaud P and Chabot GG: Nanoemulsion
formulation of fisetin improves bioavailability and antitumour
activity in mice. Int J Pharm. 427:452–459. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Smith MA and Houghton P: A proposal
regarding reporting of in vitro testing results. Clin Cancer Res.
19:2828–2833. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Klimaszewska-Wisniewska A,
Halas-Wisniewska M, Tadrowski T, Gagat M, Grzanka D and Grzanka A:
Paclitaxel and the dietary flavonoid fisetin: A synergistic
combination that induces mitotic catastrophe and autophagic cell
death in A549 non-small cell lung cancer cells. Cancer Cell Int.
16:102016. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Klimaszewska-Wiśniewska A,
Hałas-Wiśniewska M, Grzanka A and Grzanka D: Evaluation of
anti-metastatic potential of the combination of fisetin with
paclitaxel on A549 non-small cell lung cancer cells. Int J Mol Sci.
19:E6612018. View Article : Google Scholar
|
|
43
|
Tripathi R, Samadder T, Gupta S, Surolia A
and Shaha C: Anticancer activity of a combination of cisplatin and
fisetin in embryonal carcinoma cells and xenograft tumors. Mol
Cancer Ther. 10:255–268. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Khan N, Afaq F, Khusro FH, Mustafa Adhami
V, Suh Y and Mukhtar H: Dual inhibition of phosphatidylinositol
3-kinase/Akt and mammalian target of rapamycin signaling in human
nonsmall cell lung cancer cells by a dietary flavonoid fisetin. Int
J Cancer. 130:1695–1705. 2012. View Article : Google Scholar
|
|
45
|
Atashrazm F, Lowenthal RM, Dickinson JL,
Holloway AF and Woods GM: Fucoidan enhances the therapeutic
potential of arsenic trioxide and all-trans retinoic acid in acute
promyelocytic leukemia, in vitro and in vivo. Oncotarget.
19:46028–46041. 2016.
|
|
46
|
Moloudi K, Neshasteriz A, Hosseini A,
Eyvazzadeh N, Shomali M, Eynali S, Mirzaei E and Azarnezhad A:
Synergistic effects of arsenic trioxide and radiation: Triggering
the intrinsic pathway of apoptosis. Iran Biomed J. 21:330–337.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Bandaruk Y, Mukai R and Terao J: Cellular
uptake of quercetin and luteolin and their effects on monoamine
oxidase-A in human neuroblastoma SH-SY5Y cells. Toxicol Rep.
1:639–649. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Shia CS, Tsai SY, Kuo SC, Hou YC and Chao
PD: Metabolism and pharmacokinetics of
3,3′,4′,7-tetrahydroxyflavone (fisetin), 5-hydroxyflavone, and
7-hydroxyflavone and antihemolysis effects of fisetin and its serum
metabolites. J Agric Food Chem. 57:83–89. 2009. View Article : Google Scholar
|
|
49
|
Chou TC and Talalay P: Quantitative
analysis of dose-effect relationships: The combined effects of
multiple drugs or enzyme inhibitors. Adv Enzyme Regul. 22:27–55.
1984. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Chou TC: Theoretical basis, experimental
design, and comput-erized simulation of synergism and antagonism in
drug combination studies. Pharmacol Rev. 58:621–681. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Chou TC: Preclinical versus clinical drug
combination studies. Leuk Lymphoma. 49:2059–2080. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Gagat M, Grzanka D, Izdebska M and Grzanka
A: Effect of L-homocysteine on endothelial cell-cell junctions
following F-actin stabilization through tropomyosin-1
overexpression. Int J Mol Med. 32:115–129. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
54
|
Shim MJ, Kim HJ, Yang SJ, Lee IS, Choi HI
and Kim T: Arsenic trioxide induces apoptosis in chronic
myelogenous leukemia K562 cells: Possible involvement of p38 MAP
kinase. J Biochem Mol Biol. 35:377–383. 2011.
|
|
55
|
Song LL, Tu YY, Xia L, Wang WW, Wei W, Ma
CM, Wen DH, Lei H, Xu HZ and Wu YL: Targeting catalase but not
peroxiredoxins enhances arsenic trioxide-induced apoptosis in K562
cells. PLoS One. 9:e1049852014. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Zhu XH, Shen YL, Jing YK, Cai X, Jia PM,
Huang Y, Tang W, Shi GY, Sun YP, Dai J, et al: Apoptosis and growth
inhibition in malignant lymphocytes after treatment with arsenic
trioxide at clinically achievable concentrations. J Natl Cancer
Inst. 91:772–778. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Larson RA: Is there a best TKI for chronic
phase CML? Blood. 126:2370–2375. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Santos FP and Ravandi F: Advances in
treatment of chronic myelogenous leukemia new treatment options
with tyrosine kinase inhibitors. Leuk Lymphoma. 50(Suppl 2):
S16–S26. 2009. View Article : Google Scholar
|
|
59
|
Cui S, Wang J, Wu Q, Qian J, Yang C and Bo
P: Genistein inhibits the growth and regulates the migration and
invasion abilities of melanoma cells via the FAK/paxillin and MAPK
pathways. Oncotarget. 8:21674–21691. 2017.PubMed/NCBI
|
|
60
|
Chen HH, Chen SP, Zheng QL, Nie SP, Li WJ,
Hu XJ and Xie MY: Genistein promotes proliferation of human
cervical cancer cells through estrogen receptor-mediated
PI3K/Akt-NF-κB pathway. J Cancer. 9:288–295. 2018. View Article : Google Scholar :
|
|
61
|
Guo JM, Xiao BX, Liu DH, Grant M, Zhang S,
Lai YF, Guo YB and Liu Q: Biphasic effect of daidzein on cell
growth of human colon cancer cells. Food Chem Toxicol.
42:1641–1646. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Fogarty CE and Bergmann A: Killers
creating new life: Caspases drive apoptosis-induced proliferation
in tissue repair and disease. Cell Death Differ. 24:1390–1400.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Rudrapatna VA, Bangi E and Cagan RL:
Caspase signalling in the absence of apoptosis drives JNK-dependent
invasion. EMBO Rep. 14:172–177. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Ryoo HD and Bergmann A: The role of
apoptosis-induced proliferation for regeneration and cancer. Cold
Spring Harb Perspect Biol. 4:a0087972012. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Gdynia G, Grund K, Eckert A, Böck BC,
Funke B, Macher-Goeppinger S, Sieber S, Herold-Mende C, Wiestler B,
Wiestler OD and Roth W: Basal caspase activity promotes migration
and invasiveness in glioblastomacells. Mol Cancer Res. 5:1232–1240.
2007. View Article : Google Scholar
|
|
66
|
Mukai M, Kusama T, Hamanaka Y, Koga T,
Endo H, Tatsuta M and Inoue M: Cross talk between apoptosis and
invasion signaling in cancer cells through caspase-3 activation.
Cancer Res. 65:9121–9125. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Portela M and Richardson HE: Death takes a
holiday-non-apoptotic role for caspases in cell migration and
invasion. EMBO Rep. 14:107–108. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Li F, Huang Q, Chen J, Peng Y, Roop DR,
Bedford JS and Li CY: Apoptotic cells activate the 'phoenix rising'
pathway to promote wound healing and tissue regeneration. Sci
Signal. 3:ra132010. View Article : Google Scholar
|
|
69
|
Huang Q, Li F, Liu X, Li W, Shi W, Liu F,
O'Sullivan B, He Z, Peng Y, Tan AC, et al: Caspase 3-mediated
stimulation of tumor cell repopulation during cancer radiotherapy.
Nat Med. 17:860–866. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Donato AL, Huang Q, Liu X, Li F, Zimmerman
MA and Li CY: Caspase 3 promotes surviving melanoma tumor cell
growth after cytotoxic therapy. J Invest Dermatol. 134:1686–1692.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Cheng J, Tian L, Ma J, Gong Y, Zhang Z,
Chen Z, Xu B, Xiong H, Li C and Huang Q: Dying tumor cells
stimulate proliferation of living tumor cells via caspase-dependent
protein kinase Cδ activation in pancreatic ductal adenocarcinoma.
Mol Oncol. 9:105–114. 2015. View Article : Google Scholar
|
|
72
|
Cheng J, He S, Wang M, Zhou L, Zhang Z,
Feng X, Yu Y, Ma J, Dai C, Zhang S, et al: The
caspase-3/PKCδ/Akt/VEGF-A signaling pathway mediates tumor
repopulation during radiotherapy. Clin Cancer Res. 25:3732–3743.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Stevenson DE, Cooney JM, Jensen DJ,
Wibisono R, Adaim A, Skinner MA and Zhang J: Comparison of
enzymically gluc-uronidated flavonoids with flavonoid aglycones in
an in vitro cellular model of oxidative stress protection. In Vitro
Cell Dev Biol Anim. 44:73–80. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Zeng M, Sun R, Basu S, Ma Y, Ge S, Yin T,
Gao S, Zhang J and Hu M: Disposition of flavonoids via recycling:
Direct biliary excretion of enterically or extrahepatically derived
flavonoid glucuronides. Mol Nutr Food Res. 60:1006–1019. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Sak K: In vitro cytotoxic activity of
flavonoids on human ovarian cancer cell lines. Cancer Sci Res.
2:1–13. 2015. View Article : Google Scholar
|
|
76
|
Volk-Draper L, Hall K, Griggs C, Rajput S,
Kohio P, DeNardo D and Ran S: Paclitaxel therapy promotes breast
cancer metastasis in a TLR4-dependent manner. Cancer Res.
74:5421–5434. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Daenen LG, Roodhart JM, van Amersfoort M,
Dehnad M, Roessingh W, Ulfman LH, Derksen PW and Voest EE:
Chemotherapy enhances metastasis formation via VEGFR-1-expressing
endothelial cells. Cancer Res. 71:6976–6985. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Ovcharenko A, Granot G, Rokah OH, Park J,
Shpilberg O and Raanani P: Enhanced adhesion/migration and
induction of Pyk2 expression in K562 cells following imatinib
exposure. Leuk Res. 37:1729–1736. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Molli PR, Pradhan MB, Advani SH and Naik
NR: RhoA: A therapeutic target for chronic myeloid leukemia. Mol
Cancer. 11:162012. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Qi P, Tang R, Liu F and Zhou J:
MicroRNA-186 regulates cell malignancy by targeting ROCK1 in
chronic myeloid leukemia. Int J Clin Exp Pathol. 10:6290–6298.
2017.
|
|
81
|
Martini M, DeSantis MC, Braccini L,
Gulluni F and Hirsch E: PI3K/AKT signaling pathway and cancer: An
updated review. Ann Med. 46:372–383. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Mirabilii S, Ricciardi MR, Piedimonte M,
Gianfelici V, Bianchi MP and Tafuri A: Biological aspects of mTOR
in leukemia. Int J Mol Sci. 19:E23962018. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Xu H, Tian Y, Yuan X, Wu H, Liu Q, Pestell
RG and Wu K: The role of CD44 in epithelial-mesenchymal transition
and cancer development. Onco Targets Ther. 8:3783–3792. 2015.
|
|
84
|
Chang G, Zhang H, Wang J, Zhang Y, Xu H,
Wang C, Zhang H, Ma L, Li Q and Pang T: CD44 targets Wnt/β-catenin
pathway to mediate the proliferation of K562 cells. Cancer Cell
Int. 13:1172013. View Article : Google Scholar
|
|
85
|
Chou YS and Yang MH:
Epithelial-mesenchymal transition-related factors in solid tumor
and hematological malignancy. J Chinese Med Assoc. 78:438–445.
2015. View Article : Google Scholar
|
|
86
|
Chen SC, Liao TT and Yang MH: Emerging
roles of epithelial-mesenchymal transition in hematological
malignancies. J Biomed Sci. 25:372018. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Kahlert UD, Joseph JV and Kruyt FAE: EMT-
and MET-related processes in nonepithelial tumors: Importance for
disease progression, prognosis, and therapeutic opportunities. Mol
Oncol. 11:860–877. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Wang N, Guo D, Zhao YY, Dong CY, Liu XY,
Yang BX, Wang SW, Wang L, Liu QG, Ren Q, et al: TWIST-1 promotes
cell growth, drug resistance and progenitor clonogenic capacities
in myeloid leukemia and is a novel poor prognostic factor in acute
myeloid leukemia. Oncotarget. 6:20977–20992. 2015.PubMed/NCBI
|
|
89
|
Cosset E, Hamdan G, Jeanpierre S, Voeltzel
T, Sagorny K, Hayette S, Mahon FX, Dumontet C, Puisieux A, Nicolini
FE and Maguer-Satta V: Deregulation of TWIST-1 in the CD34+
compartment represents a novel prognostic factor in chronic myeloid
leukemia. Blood. 117:1673–1676. 2011. View Article : Google Scholar
|
|
90
|
Kidan N, Khamaisie H, Ruimi N, Roitman S,
Eshel E, Dally N, Ruthardt M and Mahajna J: Ectopic expression of
Snail and Twist in Ph+ Leukemia cells upregulates CD44 expression
and alters their differentiation potential. J Cancer. 8:3952–3968.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Touil YS, Seguin J, Scherman D and Chabot
GG: Improved antiangiogenic and antitumour activity of the
combination of the natural flavonoid fisetin and cyclophosphamide
in Lewis lung carcinoma-bearing mice. Cancer Chemother Pharmacol.
68:445–455. 2011. View Article : Google Scholar
|
|
92
|
O'Dwyer ME, La Rosée P, Nimmanapalli R,
Bhalla KN and Druker BJ: Recent advances in Philadelphia
chromosome-positive malignancies: The potential role of arsenic
trioxide. Semin Hematol. 39(2 Suppl): S18–S21. 2002. View Article : Google Scholar
|
|
93
|
O'Dwyer M: Multifaceted approach to the
treatment of bcrabl-positive leukemias. Oncologist. 7(Suppl 1):
S30–S38. 2002. View Article : Google Scholar
|
|
94
|
Potin S, Bertoglio J and Bréard J:
Involvement of a Rho-ROCK-JNK pathway in arsenic trioxide-induced
apoptosis in chronic myelogenous leukemia cells. FEBS Lett.
581:118–124. 2007. View Article : Google Scholar
|
|
95
|
Yan H, Wang YC, Li D, Wang Y, Liu W, Wu YL
and Chen GQ: Arsenic trioxide and proteasome inhibitor bortezomib
synergis-tically induce apoptosis in leukemic cells: The role of
protein kinase Cdelta. Leukemia. 27:1488–1495. 2007. View Article : Google Scholar
|
|
96
|
El Eit RM, Iskandarani AN, Saliba JL,
Jabbour MN, Mahfouz RA, Bitar NM, Ayoubi HR, Zaatari GS, Mahon FX,
De Thé HB, et al: Effective targeting of chronic myeloid leukemia
initiating activity with the combination of arsenic trioxide and
interferon alpha. Int J Cancer. 134:988–996. 2014. View Article : Google Scholar
|
|
97
|
Xu W, Wei W, Yu Q, Wu C, Ye C, Wu Y and
Yan H: Arsenic trioxide and bortezomib interact synergistically to
induce apoptosis in chronic myelogenous leukemia cells resistant to
imatinibmesylate through Bcr/Abl-dependent mechanisms. Mol Med Rep.
10:1519–1524. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
La Rosée P, Johnson K, O'Dwyer ME and
Druker BJ: In vitro studies of the combination of imatinib mesylate
(Gleevec) and arsenic trioxide (Trisenox) in chronic myelogenous
leukemia. Exp Hematol. 30:729–737. 2002. View Article : Google Scholar : PubMed/NCBI
|