Introduction
Lung cancer is one of the most common malignancies.
In 2012, an estimated 1.8 million new lung cancer cases occurred,
accounting for ~13% of total cancer diagnoses cases worldwide
(1). Non-small cell lung cancer
(NSCLC) accounts for >80% of lung cancer cases and its 5-year
survival rate is <15% (2). For
these reasons, the elucidation of the molecular mechanisms involved
in NSCLC carcinogenesis is important for the identification and
development of potential therapeutic targets for NSCLC. At present,
increasing evidence has shown that noncoding small RNAs participate
in the pathogenesis of NSCLC, providing new perspectives for
disease biology.
microRNAs (miRNAs or miRs) are 21-24 nucleotide long
small noncoding regulatory RNAs that or miRs) are 21-24 nucleotide
long small noncoding regulatory RNAs that negatively regulate gene
expression at the post-transcriptional and/or translational level
by binding to a complementary sequence within the 3′-untranslated
regions (UTRs) of target mRNAs (3,4).
Approximately one-third to half of human genes are directly
regulated by miRNAs and thus, they are associated to a variety of
biological processes, including cell proliferation, metastasis,
differentiation and apoptosis, which are important in the
development of cancer (5).
Abnormal changes in miRNA expression have been reported to be
closely associated with cancer initiation and progression, and
therefore, these molecules are key oncogenes or tumor suppressor
genes in NSCLC (6-8).
miR-20b is located on human chromosome X, encoded by
the miR-106a-363 cluster and divided into miR-17 family members
based on the degree of homology of the seed sequence (9). Numerous studies have shown the
different roles of miR-20b in various types of cancer. For example,
miR-20b was reduced in papillary thyroid carcinoma (10) and renal cell carcinoma (11), showing its tumor-suppressor
function. In contrast, the carcinogenic potential of miR-20b has
been demonstrated in breast cancer (12), esophageal cancer (13), colorectal cancer (14) and T-cell leukemia (15). However, the biological functions of
miR-20b in NSCLC remain poorly understood.
The Wnt/β-catenin signaling pathway is involved in
various biological processes, such as cell proliferation, movement,
differentiation and cell death, which are necessary for cell
development and morphology (16-18).
When β-catenin enters the cell nucleus, it can regulate target gene
transcription by interacting with the transcription factor lymphoid
enhancer-binding factor 1/T cell-specific transcription factor.
These target genes, such as cyclin D1 and c-myc, are signifi-cant
for cell differentiation, proliferation and apoptosis (19-21)
The purpose of this study was to clarify the
biological function of miR-20b and its role in regulating Wnt
signaling in NSCLC. The findings revealed that miR-20b regulates
the canonical Wnt signaling pathway in NSCLC cells to promote
proliferation. In addition, we observed that miR-20b activated Wnt
signaling by reducing adenomatous polyposis coli (APC) expression.
These results elucidated a new mechanism for excessive activation
of the Wnt/β-catenin signaling pathway in NSCLC and miR-20b may be
a potential therapeutic target for lung cancer.
Materials and methods
Cell culture and transfection
Lung adenocarcinoma cell lines (PC-9, H1975 and
A549), a non-small cell lung cancer line (H1299), a normal lung
epithelial cell line (BEAS-2B) were purchased from the American
Type Culture Collection. PC-9, H1975, A549 and H1299 were cultured
in RPMI-1640 (Gibco; Thermo Fisher Scientific, Inc.). BEAS-2B cells
were cultivated in Bronchial Epithelial Cell Basal medium (BEBM;
Lonza Group, Ltd.). All culture media were supplemented with 10%
fetal bovine serum (FBS; Invitrogen; Thermo Fisher Scientific,
Inc.) with 100 U/ml penicillin and 100 µg/ml streptomycin
(Invitrogen; Thermo Fisher Scientific, Inc.). All cells were
cultivated at 37°C in a 5% CO2 incubator.
Information of the cell lines is as detailed by the
supplier as follows: i) BEAS-2B cells were derived from normal
bronchial epithelium obtained from autopsy of noncancerous
individuals. Cells were infected with a replication-defective
SV40/adenovirus 12 hybrid and cloned; ii) PC-9 was originally
deposited with the Riken BioResource Center as a cell line derived
from human lung adenocarcinoma (undifferentiated type) in 1989;
iii) H-1975 is an adenocarcinoma, non-small cell lung cancer cell
line; iv) A-549 are adenocarcinoma human alveolar basal epithelial
cells and the cell line was developed in 1972 through the removal
and culturing of cancerous lung tissue in the explanted tumor of a
58-year-old Caucasian male; and v) H1299 are carcinoma, non-small
cell lung cancer cells.
Transfection
miRNA mimics, inhibitors and negative controls were
synthesized by Shanghai GenePharma Co., Ltd. and transfection was
performed with Lipofectamine™ LTX Reagent (Invitrogen; Thermo
Fisher Scientific, Inc.). A total of 1.5x105 PC-9 or
H1975 cells per well were seeded in 6-well plates 24 h prior to
transfection. The transfection reaction contained 100 µM
miRNA diluted in 100 µl Opti-MEM® I Reduced Serum
Medium (Invitrogen; Thermo Fisher Scientific, Inc.). Next, 4
µl LTX reagent was added to each sample and incubated for
5-15 min at room temperature. Finally, the transfection mixture was
added to the cells and mixed gently. After 48 h, expression levels
were detected by reverse transcription-quantitative (RT-q) PCR.
Cell viability assay
Cells were seeded at 5,000 cells per well in 96-well
plates at 24 h after transfection. The MTT assay was used to
determine cell viability at 24, 48, 72 and 96 h after the cells
were seeded. Briefly, 20 µl of MTT dye solution (5 mg/ml)
was added to each well and continually incubated for 4 h. Then, the
supernatant was removed and 150 µl of DMSO was added to stop
the reaction. The absorbance at 490 nm was measured.
The results were confirmed by manual cell counts.
Here, transfected cells were seeded in 24-well plates at 10,000
cells per well, collected at 24, 48, 72 and 96 h and counted using
a hemocytometer.
Colony formation assay
After transfection with miR-20b mimics and
inhibitors for 48 h, cells were plated in 6-well plates at 5,000
per well and grown for 2 weeks. After 2 weeks, the colonies were
washed 3 times with cold PBS, fixed with 4% paraformaldehyde for 20
min and then cells were washed twice with PBS and fixed with
methanol/acetic acid (3:1, v/v) for 15 min at room temperature.
Then, they were stained with 0.5% crystal violet for 30 min at room
temperature. The number of colonies was counted under a light
microscope (magnification, x4).
Wound healing assay
Scratch wound assays were performed to evaluate the
motility of the cells at 24 h after transfection. A total of
2×105 cells per well were seeded in 6-well plates. At
90-95% confluence, the monolayer of cells was scratched with a 10
µl micropipette tip. After removal of cellular debris, the
cultures were incubated in RPMI 1640 for 24 h, and recovery of the
wound was observed and photographed with a light microscope
(magnification, ×10).
Migration and invasion assays
For the Transwell assays, 8-µm pore size
chambers (Corning, Inc.) were used with an insert without
(migration) or with (invasion) Matrigel coating (BD Biosciences).
At 24 h after transfection, 1x105 cells in serum-free
medium were added to the upper chamber. The lower chamber was
filled with 10% FBS RPMI 1640. After 24-h incubation, cells
remaining on the upper surface of the membrane were removed,
whereas cells that had invaded through the membrane were fixed with
0.1% paraformaldehyde for 20 min at room temperature, stained with
0.1% crystal violet for 30 min at room temperature, imaged and
counted under a light microscope (magnification, x10).
Nuclear isolation
The nuclear fraction was extracted with the nuclear
extraction kit (Thermo Fisher Scientific, Inc.). Briefly,
5×106 transfected cells were gently suspended in 500
µl Hypotonic Buffer by pipetting up and down and incubated
on ice for 15 min. Then, 25 µl detergent (10% NP40) was
added and vortexed for 10 sec at the highest setting at room
temperature. The homogenate was centrifuged for 10 min at 3,000 × g
at 4°C. The supernatant was the cytoplasmic and the pellet the
nuclear fraction.
Immunofluorescence
After treatment, cells were fixed with 0.1%
paraformaldehyde for 20 min at room temperature. The fixed cells
were blocked with 5% BSA (Sigma-Aldrich; Merck KGaA) at room
temperature for 2 h and then incubated with anti-β-catenin primary
antibody (1:1,000; cat. no. 8480; Cell Signaling Technology, Inc.)
overnight at 4°C. After washing with PBS at room temperature three
times (5 min/each), Goat anti-rabbit fluorescence conjugated second
antibody (1:10,000; cat. no. A21245; Invitrogen; Thermo Fisher
Scientific, Inc.) was added for 1 h at room temperature and imaged
using a confocal microscope (magnification, ×60).
Western blot analysis
Transfected cells were lysed in RIPA lysis buffer
with protease and phosphatase inhibitors (Roche Applied Science.)
to extract the total protein. The concentration of the total
protein extract was determined with a DCTM Protein Assay kit
(Bio-Rad Laboratories, Inc.). Then, 40 µg total protein
lysate were separated on 10% SDS-PAGE gels and transferred to a
nitrocellulose membrane. Membranes were blocked with 5% non-fat
milk in TBST (5% Tween-20) for 1 h at room temperature. Primary
antibodies, including anti-APC (cat. no. 2504), anti-β-catenin
(cat. no. 8480), anti-c-Myc (cat. no. 18583), anti-cyclin D1(cat.
no. 2978), GAPDH (cat. no. 5174) and lamin B (cat. no. 12586),
which were all purchased from Cell Signaling Technology, Inc, were
incubated with the membranes at 1:1,000 dilution overnight at 4°C.
After the membrane was washed with TBST (3x5 min),
fluorescent-conjugated goat anti-rabbit (cat. no. 926-32211;
1:10,000; LI-COR Biosciences) or goat anti-mouse secondary
antibodies (cat. no. 926-32210; 1:10,000; LI-COR Biosciences) was
added to the membrane at room temperature for 1 h. GAPDH and lamin
B were used as loading controls for cellular and nuclear proteins,
respectively. The signal intensity of the membranes was detected
with an Odyssey Scanner (LI-COR, Inc.).
Isolation of human plasma
Human healthy and lung cancer plasma specimens (20
each) were obtained following the guidelines approved by the
institutional review board at Taihe Hospital of Hubei University of
Medicine, and written informed consent was obtained from patients
in all cases. A total of 40 subjects were enrolled in this study,
including 20 NSCLC patients (age, 57.3±9.1 years; male, 14; female,
6) between January 2018 and July 2018, of which 11 patients had
tumor resection (stages I, II and IIIA) and 9 patients did not
(stages IIIB and IV), and 20 age- and sex-matched healthy
volunteers as controls (age, 54.1±9.2 years; male, 15; female, 5;
P= 0.081). NSCLC patients were recruited at Department of
Respiratory and Critical Care Medicine, Taihe Hospital of Hubei
University of Medicine, China. Blood samples were taken before
chemotherapy in both operable and non-operable patients. There were
no other inclusion or exclusion criteria for this study. Tumors
were staged according to the tumor-node-metastasis (TNM) staging
system of the American Joint Committee on Cancer (1). None of the patients had received
adjuvant chemotherapy or radiotherapy before admission. Informed
consents were obtained from all enrolled subjects and the local
Ethics Committee approved the protocol.
RNA extraction
miRNA was extracted with mirVana™ miRNA extraction
kit (Invitrogen; Thermo Fisher Scientific, Inc.) according to the
manufacturer's instructions. Briefly, after the medium was removed,
0.6 ml lysis buffer with 1% 2-mercap-toethanol was added to the
1×106 cells. The total cell lysate was transferred to an
RNase-free tube and the cell pellet was dispersed. One volume of
70% ethanol was added to the cell lysate and mixed thoroughly to
disperse visible precipitate. The mixture was transferred to a spin
cartridge, centrifuged at 12,000 × g for 15 sec at room temperature
and the flow-through was discarded. The spin cartridge was washed
with wash buffer I and buffer II, and RNA was eluted with 50
µl of RNase-free water. The RNA concentration was determined
and RNA quality was assessed by agarose (1%) electrophoresis.
cDNA synthesis
The synthesis of first-strand cDNA was carried out
following the instructions of the Transcriptor First Strand cDNA
synthesis kit (Roche Diagnostics). DNase-treated RNA (1 µg)
was used in the synthesis reaction. The RNA sample was incubated
with 2 µl random and 1 µl Oligo (dT) primers (with
kit) at 65°C for 10 min and then cooled on ice for 2 min. The
reaction mixture, containing reaction buffer, RNase inhibitor and
reverse transcriptase, was added to the tube and incubated at 25°C
for 10 min followed by 55°C for 30 min. The reaction was terminated
by heating to 70°C for 15 min. cDNA was stored at −80°C until
further use. Primers were designed as follows: U6 for reverse
transcription (RT), 5′-GTC GTA TCC AGT GCA GGG TCC GAG GTG CAC TGG
ATA CGA CAA AAT ATG GAA C-3′; and miR-20b for RT, 5′-GTC GTA TCC
AGT GCA GGG TCC GAG GTG CAC TGG ATA CGA CCT ACC TG-3′.
Quantitative (q) PCR
qPCR was performed using FastStart Universal SYBR
Green Master Mix (Roche Applied Science). The reaction mixture
contained 10 µl SYBR Master mix, 1 µl forward and
reverse primers, 0.2 µl template and water to 20 µl.
The PCR protocol was 94°C for 10 min, followed by 40 cycles of 94°C
for 10 sec and 60°C for 30 sec. U6 and β-actin were used as
references for normalization of the expression of miRNA and mRNAs,
respectively, and the 2−ΔΔCq method was used to
determine the relative expression of each transcript (22). Experiments were repeated at least
three times. The RT-qPCR primers were as follows: U6, forward,
5′-TGC GGG TGC TCG CTT CGG CAG C-3 and reverse, 5′-CCA GTG CAG GGT
CCG AGG T-3′; miR-20b, forward, 5′-GCC CGC CAA AGT GCT CAT AGT G-3′
and reverse, 5′-CCA GTG CAG GGT CCG AGG T-3′; β-actin, forward,
5′-TCA CCC ACA CTG TGC CCA TCT-3′ and reverse, 5′-GTG AGG ATC TTC
ATG AGG TAG TCA GTC-3′; and APC, forward, 5′-AAG CGT ATT GAG TGC
CTT ATG G-3′ and reverse, 5′-GGT AAG TAA GAG TGC CAA CCA A-3′.
A549β-catenin(−/−)CRISPR-Cas9
sgRNA design and sgRNA cloning
The study was based on Cas9, which was used to
target the PAM sequence on β-catenin. The two targeting sequences
used to knock out β-catenin were GGA CTC TGG AAT CCA TTC TG and ACC
ACA GCT CCT TCT CTG AGA G. DNA oligos were synthe-sized and cloned
into pX335-U6-Chimeric_BB-CBh-hSpCas9n (D10A) (cat. no. 42335;
Addgene, Inc.). The vector was transfected into A549 cells using
Lipofectamine™ 2000 (Invitrogen; Thermo Fisher Scientific, Inc.).
The β-catenin knockout cell line comes from our latest publication
(23).
Tumor xenograft model
Animal studies were approved by the Ethical
Committee of Macau University of Science and Technology. BALB/c
Nude mice (n=16; age, 6-8 weeks) were maintained under specific
pathogen-free conditions and housed in plastic cages in groups of
four. Each group contained 8 mice. The housing conditions of the
animals were as follows: Temperature 22±1°C, humidity 40-60%, 12-h
dark/light cycles and free access to food and water.
miR-20b-overexpressing H1975 cells and empty vector control cells
cultured in RPMI-1640 were harvested, washed with PBS and
re-suspended in medium. A total of 1×106 cells/100
µl were mixed with 50 µl Matrigel and injected
subcutaneously into the right forelimb of each nude mouse. Tumor
volume was measured using a caliper every 7 days and calculated
using the following equation: Volume = (width2 ×
length)/2. At 28 days after inoculation, mice were sacrificed and
tumor weights were assessed.
Statistical analyses
All data are expressed as the mean ± SEM of three
individual experiments. Differences between groups were determined
using one-way ANOVA followed by Bonferroni's test using GraphPad
Prism 5 (GraphPad Software, Inc.) or by paired Student's t-test was
used to compare two groups. The level of significance was set at
P<0.05.
Results
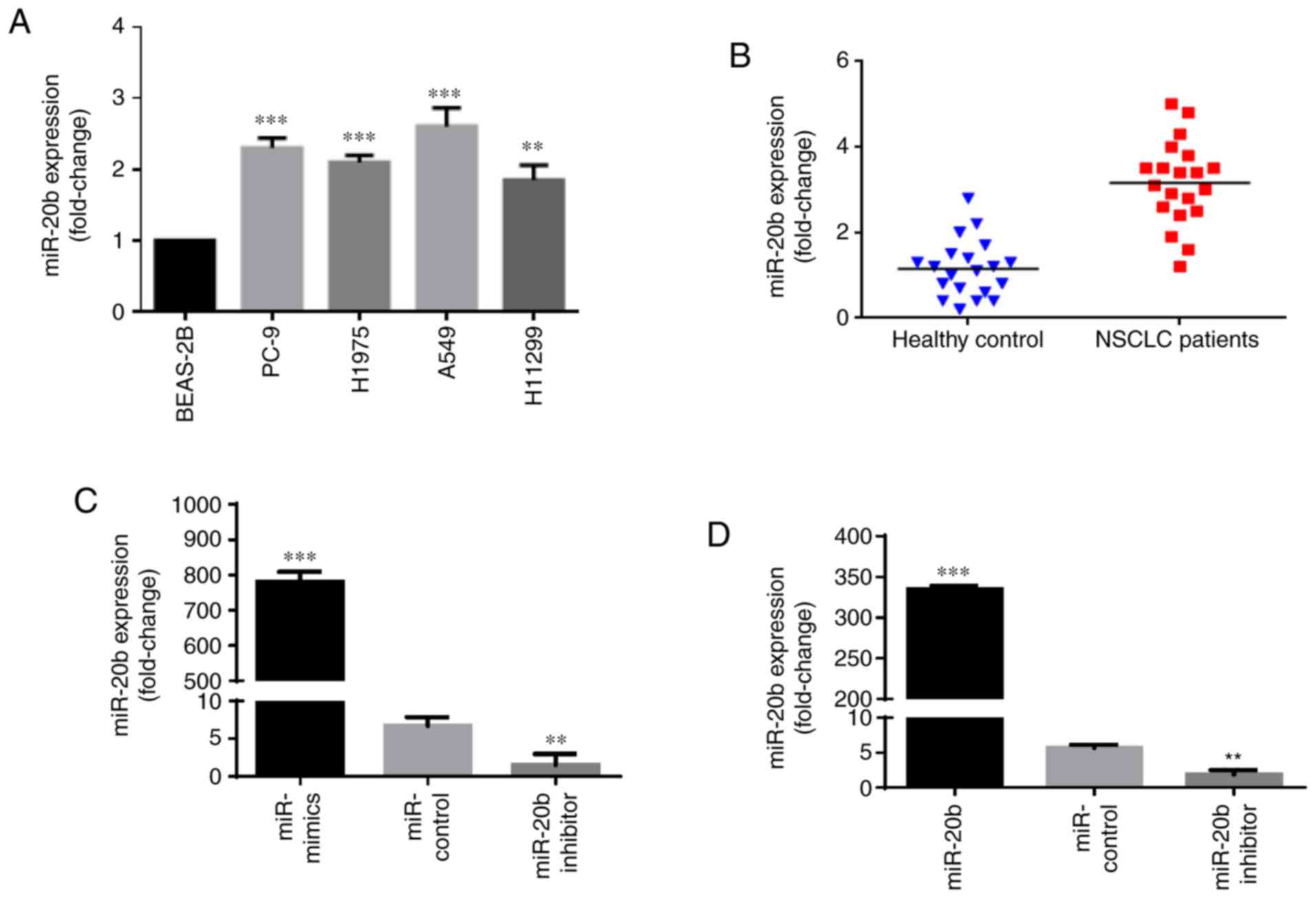
miR-20b is significantly upregulated in
NSCLC cell lines
To determine the role of miR-20b in NSCLC, we first
investigated and compared its expression in a normal lung
epithelial cell line, BEAS-2B, and different types of NSCLC cell
lines, namely PC-9, H1975, A549 and H1299. The results showed that
the levels of miR-20b in PC-9, H1975, A549 and H1299 were
significantly higher than that in BEAS-2B (Fig. 1A). Moreover, we compared miR-20b
expression in clinical NSCLC samples and healthy donor samples. By
analyzing the miR-20b expression levels in the plasma of 20 healthy
donors and 20 NSCLC patients, we found that the plasma level of
miR-20b was increased in the NSCLC patients (Fig. 1B), which is consistent with the
results of the cell lines. Taken together, these data indicated
that miR-20b was increased in NSCLC cells and tissues and may act
as an oncogene.
miR-20b enhances proliferation, migration
and invasion of NSCLC cells
Since the EGFR mutation is a dominant mutation in
NSCLC (24), we chose PC-9
(EGFRexon19del E746–A750) and H1975
(EGFRL858R+T790M) for further experiments. miR-20b
inhibitors, mimics and negative control were transfected into PC-9
and H1975. Transfection efficiency was measured by RT-qPCR and
compared with the miR-NC, miR-20b expression was significantly
increased in the presence of the mimics and significantly
downregulated in the presence of miR-20b inhibitors in PC-9 and
H1975 (Fig. 1C and D).
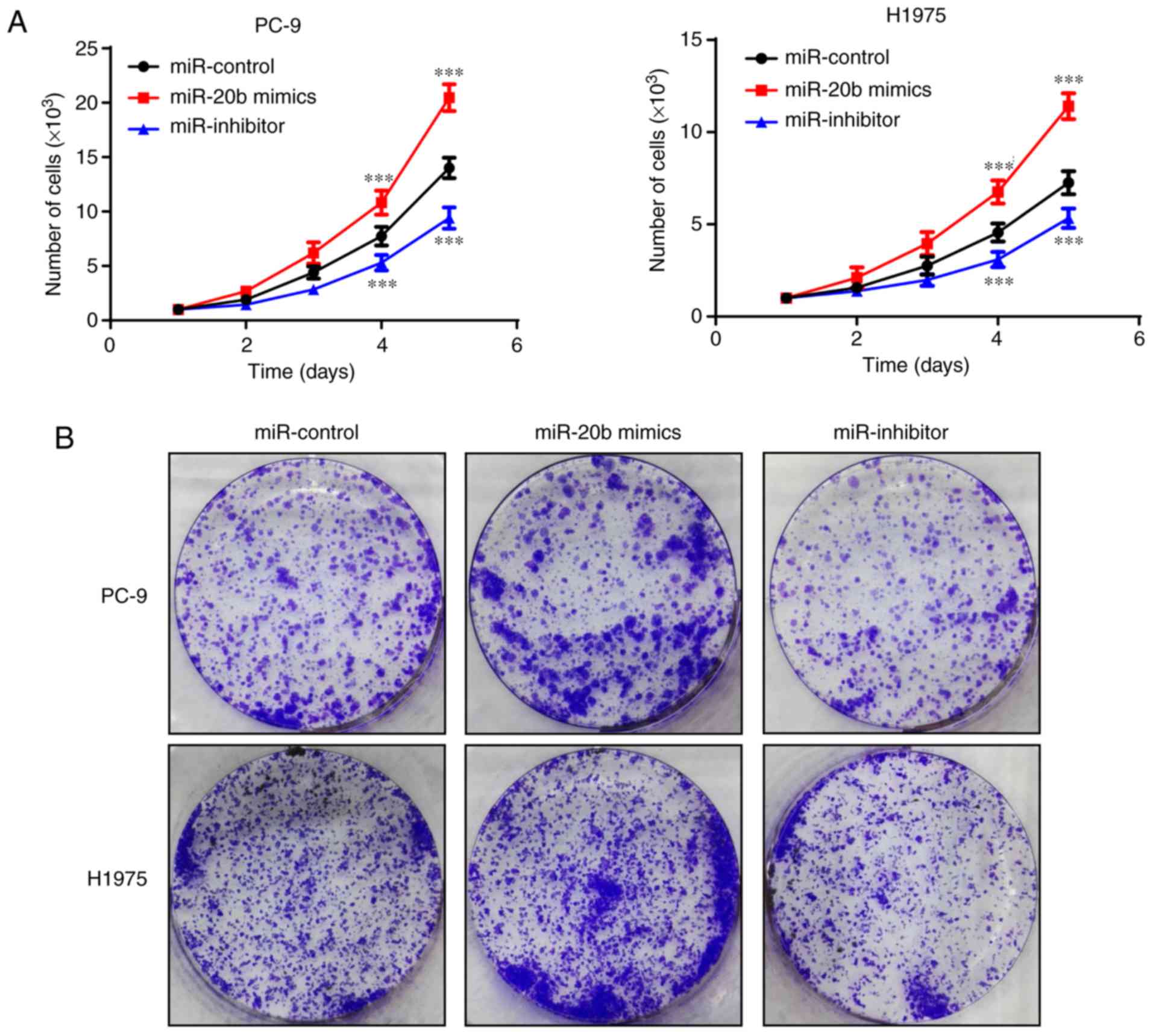
To investigate whether miR-20b acted as an oncogene
in NSCLC, cellular function assays were performed after
transfection. First, growth curves and colony formation assays were
applied to assess cell proliferation. As shown in Fig. 2, an increasing level of miR-20b was
associated with the cell proliferation rate. Increased miR-20b
levels in PC-9 and H1975 significantly enhanced the proliferative
capacity, and decreased miR-20b levels significantly suppressed
cell growth in PC-9 and H1975.
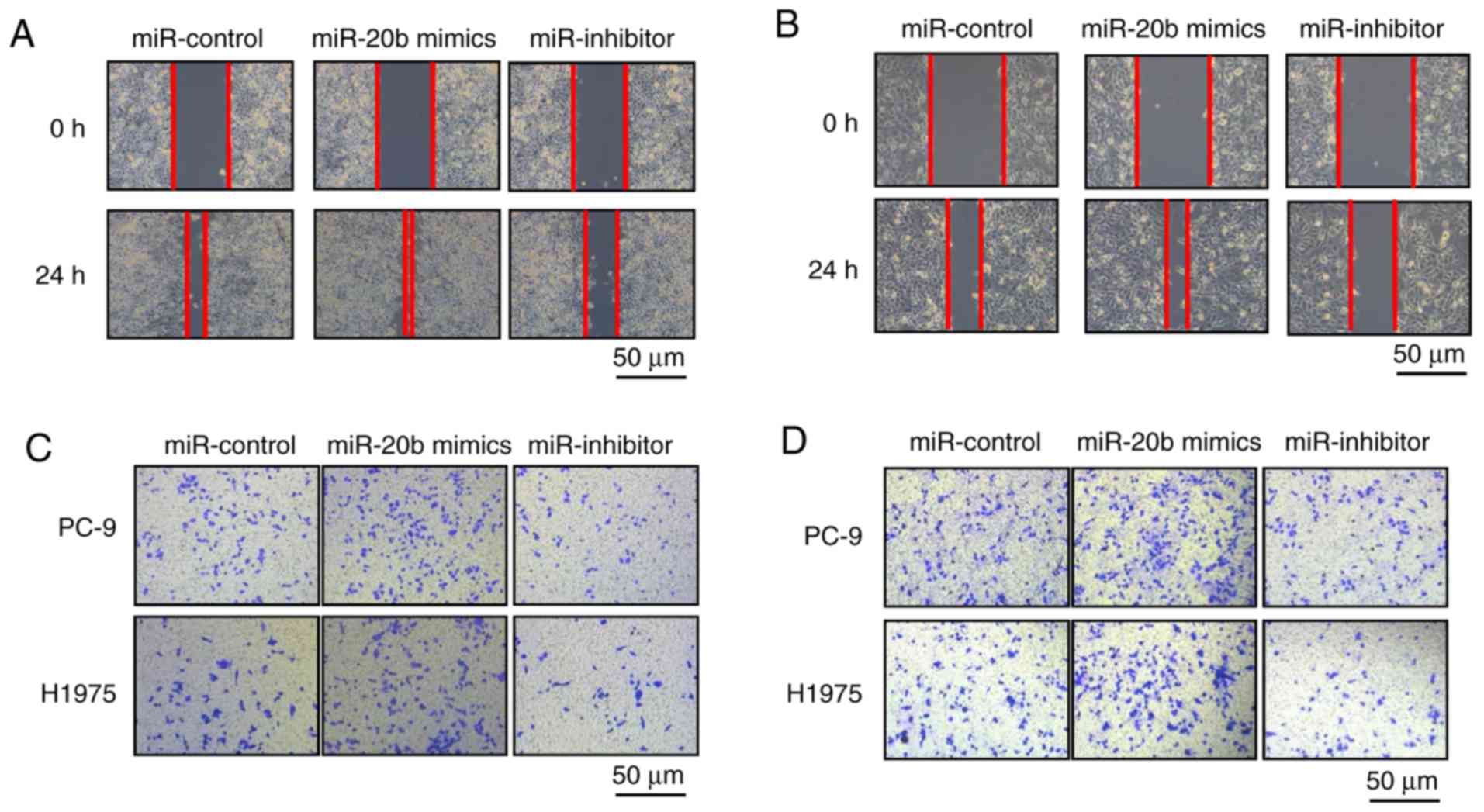
Next, we used wound healing and Transwell assays to
investigate the effect of miR-20b on the migration and invasion of
NSCLC cells. Overexpression of miR-20b markedly promoted wound
closure compared with the control in PC-9 and H1975 cells, whereas
miR-20b inhibitor treatment reduced cell migration (Fig. 3A and B). Similar results were
observed in the migration and invasion assays. Using Transwell
assays with or without Matrigel, we found that in cells with
increased miR-20b expression, the number of invasive and migrated
cells increased, while miR-20b inhibitors had the opposite effect
on NSCLC cells (Fig. 3C and
D).
In summary, the above data suggested that
overexpression of miR-20b promoted cell proliferation, migration
and invasion in NSCLC cells, whereas miR-20b inhibitors reduced
these functions.
miR-20b promotes the Wnt signaling
pathway through regulating APC
As miR-20b was demonstrated to be beneficial in
promoting NSCLC growth in vitro, we further explored its
role as an oncogene. Previous reports on other types of cancer
showed that APC is a downstream target of miR-20b (25). We detected and compared APC
expression in normal lung epithelial cells and NSCLC cell lines. As
shown in Fig. 4A, APC mRNA in
PC-9, H1975, A549 and H1299 was significantly lower than in normal
lung BEAS-2B cells and APC protein levels were also changed.
Additionally, we determined APC levels in cells transfected with
miR-20b mimics to increase and miR-20b inhibitors to decrease
miR-20b expression. Protein levels of APC were decreased in
mimic-treated and increased in inhibitor-treated cells compared
with the respective controls (Fig.
4B). These data suggested that miR-20b affected the expression
of APC.
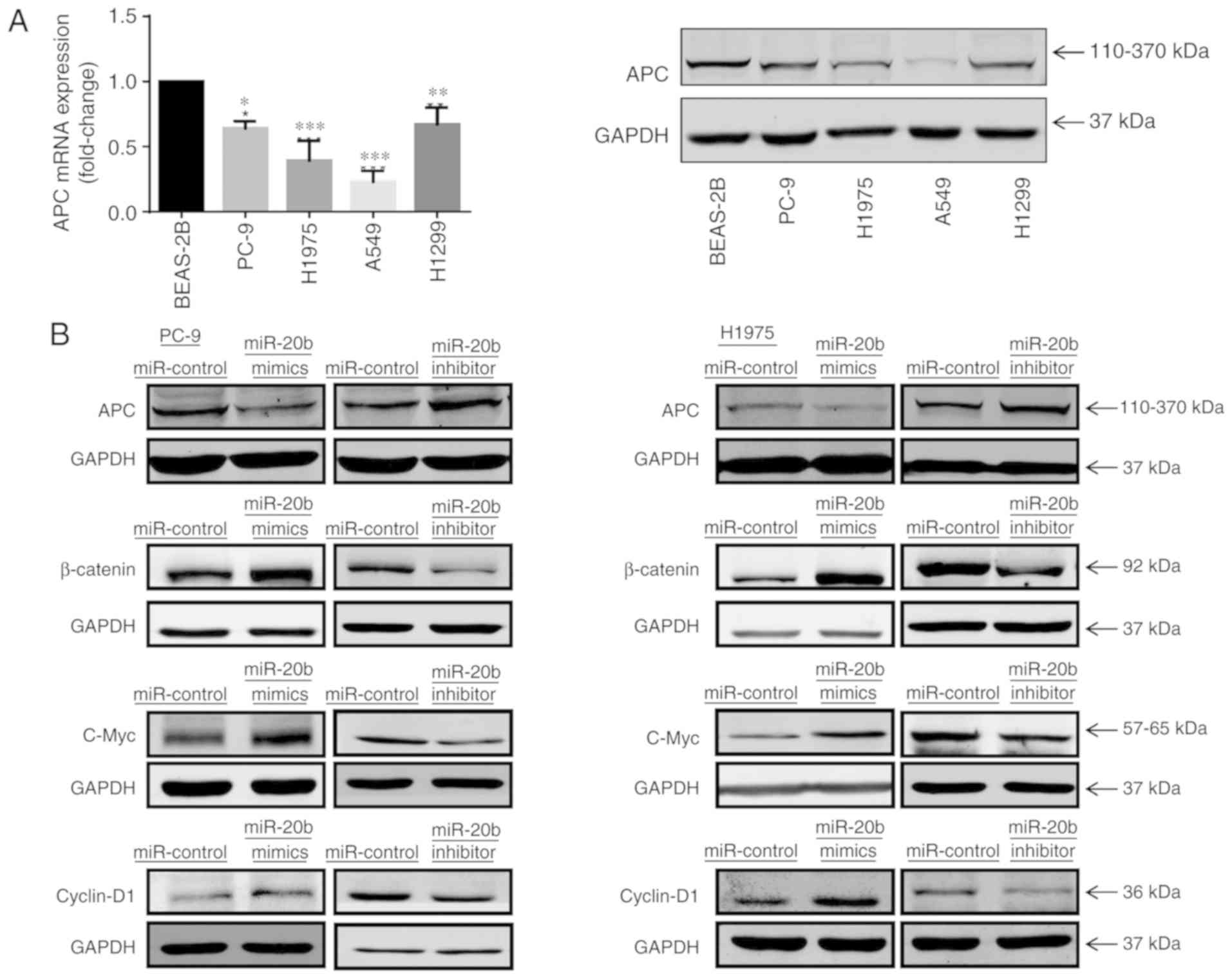 | Figure 4miR-20b activates the Wnt/β-catenin
signaling pathway. (A) Relative mRNA and protein expression levels
of APC in non-small cell lung cancer cell lines, PC-9, H1975, A549
and H1299, and BEAS-2B cells. *P<0.05,
**P<0.01 and ***P<0.001 vs. BEAS-2A.
(B) Western blot analysis of APC, β-catenin, C-Myc and cyclin D1
expression in PC-9 and H1975 cells transfected with miR-20b mimics,
miR-20b inhibitor or miR-NC. miR, microRNA; NC, negative control;
APC, adenomatous polyposis coli. |
APC is a negative regulator of the Wnt/β-catenin
signaling pathway, which is an essential factor in cancer growth
and metastasis (7). To identify
whether miR-20b promotes cancer through Wnt signaling transduction,
we detected the levels of β-catenin in NSCLC cells transfected with
miR-20b mimics and inhibitors. As depicted in Fig. 4B, a marked increase of β-catenin
was detected in PC-9 and H1975 cells trans-fected with miR-20b
mimic, whereas the miR-20b inhibitor decreased β-catenin compared
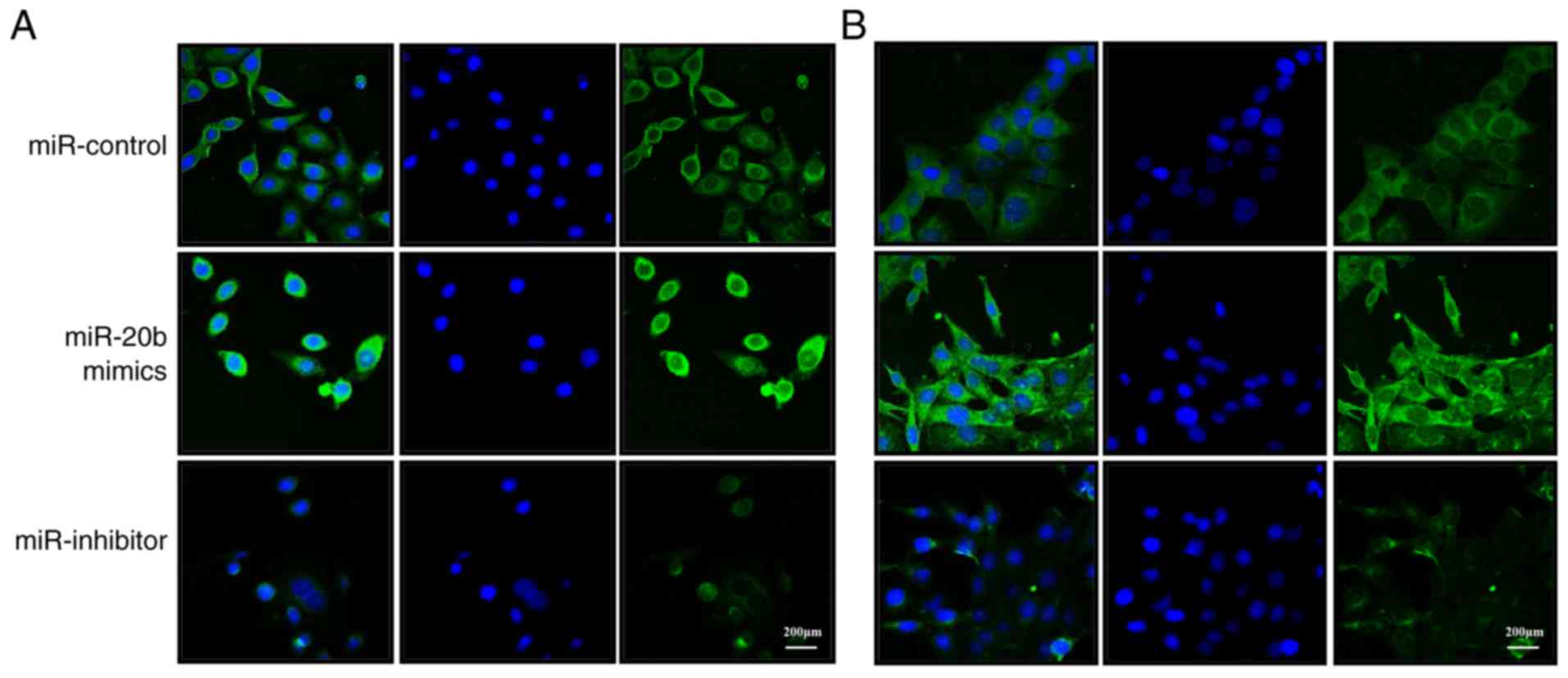
with the respective controls. Immunofluorescence confocal imaging
showed an increase in the β-catenin protein expression in PC-9 and
H1975 cells overexpressing miR-20b, while transfection with miR-20b
inhibitors decreased expression compared with the control (Fig. 5). The translocation of β-catenin to
the nucleus was enhanced by miR-20b overexpression (Fig. S1).
In addition, we explored the effects of miR-20b
over-expression and knockdown on c-Myc and cyclin D1, which are
downstream target genes of the Wnt/β-catenin signaling pathway in
cancer (26,27). As shown in Fig. 4B, protein levels of these genes
increased in PC-9 and H1975 overex-pressing miR-20b and decreased
in inhibitor-treated cells compared with the controls. These
findings suggested that miR-20b enhanced the expression of
β-catenin and activated Wnt/β-catenin downstream signaling in NSCLC
cells.
miR-20b modulates the Wnt/β-catenin
signaling pathway in tumorigenesis through a positive feedback
loop
To investigate the association between β-catenin and
miR-20b, we examined miR-20b expression in three different samples,
namely A549WT, A549β-catenin(−/−) and A549
treated with Wnt inhibitor. The results demonstrated a
significantly higher expression of miR-20b and a significantly
increased level of APC in A549WT cells compared with
A549β-catenin(−/−) and A549 cells treated with Wnt
inhibitor (Fig. 6A). These assays
demonstrated that β-catenin induced miR-20b transcription, and in
turn, miR-20b activated the Wnt/β-catenin signaling pathway. Thus,
it was suggested that miR-20b and Wnt signaling may be coupled
through a forward-positive feedback loop and form a biological
regulatory circuit.
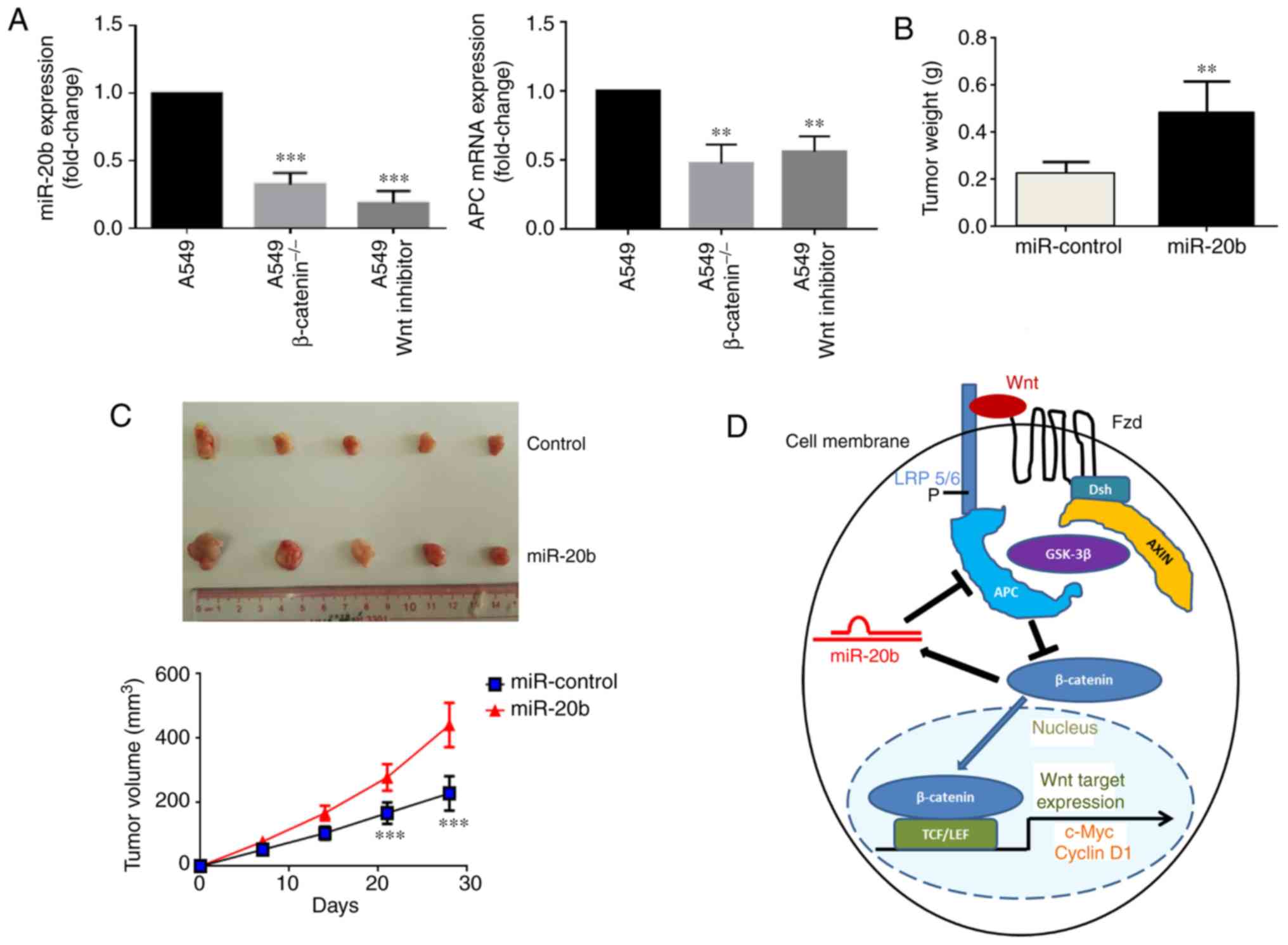 | Figure 6miR-20b promotes tumor growth of
H1975 in vivo. (A) Relative expression levels of miR-20b and
APC in A549 (wild type), A549β-catenin(−/−) and A549 Wnt
inhibitor cells determined by reverse transcription-quantitative
PCR. **P<0.01 and ***P<0.001 vs. A549.
Tumor (B) weight and (C) volume of xenograft tumors derived from
nude mice (n=8 per group) injected with control or
miR-20b-overexpressing H1975 cells after 28 days. As some of the
samples were damaged during the excision process, 5 specimens from
each group were presented. **P<0.01 and
***P<0.001 vs. miR-control. (D) Cell model of the
miR-20b mechanism of action. miR, microRNA; APC, adenomatous
polyposis coli; P, phosphate; Fzd, frizzled; Dsh, dishevelled;
AXIN, axis inhibition protein; GSK-3β, glycogen synthase kinase 3β;
TCF/LEF, T-cell factor/lymphoid enhancer-binding factor; LRP,
lipoprotein receptor-related proteins. |
miR-20b promotes tumor growth in
vivo
To further classify the function of miR-20b in NSCLC
tumor growth, we used established an in vivo xenograft mouse
model. H1975 overexpressing miR-20b and control cells were injected
subcutaneously into the right forelimb of the mice. As shown in
Fig. 6B and C, the tumor weight
and volume for animals injected with miR-20b-overexpressing cells
were significantly higher than those of the control group. These
results confirmed that miR-20b promoted tumor growth of H1975 cells
transfected with miR-20b in vivo.
Discussion
miR-20b levels were reported to be significantly
higher in serum exosomes from patients with NSCLC at more advanced
stages of disease and the presence of lymph node metastases than in
healthy controls, suggesting that miR-20b might be a promising
biomarker for the diagnosis of lung cancer (28). In our study, evidence for a new
mechanistic link between miR-20b and the Wnt/β-catenin signaling
pathway was suggested for NSCLC cells (Fig. 6D). We discovered that miR-20b was
markedly upregulated in NSCLC cell lines and in clinical NSCLC
samples. In vitro and in vivo, miR-20b overexpression
accelerated cancer progression, but miR-20b downregulation
suppressed the growth and metastasis of NSCLC cells in
vitro. In addition, we confirmed that miR-20b regulated the
expression of APC, a key negative regulator of the Wnt/β-catenin
signaling pathway, leading to activation of the Wnt/β-catenin
signaling pathway. Taken together, our findings suggested that
miR-20b may function as an onco-miR and may be considered a key
target for clinical treatment in NSCLC.
Activation of the Wnt/β-catenin signaling pathway is
commonly observed in many types of human malignancy and is
considered to promote cancer progression (29-31).
APC has been found to be inhibited in cancer, which helps to
promote cancer progression via regulation of the oncogenic
Wnt/β-catenin signaling pathway. For instance, in human hepatoma
cells, miR-106b activated canonical Wnt signaling to enhance cancer
progression by directly targeting APC (32). Downregulation of miR-129-5p
inhibits growth and induces apoptosis in laryngeal squamous cell
carcinoma by targeting APC (33).
In addition, the loss of APC function in a mouse model leads to
hyper-activation of Wnt/β-catenin signaling and causes colorectal
cancer (34). Mice with a
heterozygous truncated APC mutant exhibit enhanced Wnt/β-catenin
signaling activity and develop mammary adenocarcinomas and
subsequent pulmonary metastases (35). Here, we showed that miR-20b
inhibited APC expression, which was consistent with the tumor
suppressor effect of APC. Overexpression of miR-20b in NSCLC cell
lines significantly promoted the proliferation of NSCLC in
vitro and in vivo. Our research revealed a potentially
novel mechanism of the miR-20b/APC axis in NSCLC.
Based on the impact of Wnt/β-catenin signaling on
cancer progression, anticancer drugs targeting the Wnt/β-catenin
signaling pathway have attracted much attention (36). However, most Wnt signaling genes
mutated in colorectal cancer, including APC, are tumor suppressors
and cannot be directly targeted for therapeutic purposes (37). β-catenin is a proto-oncogene that
is a ubiquitously expressed cell adhesion molecule and cannot be
used as a drug target (37).
Therefore, finding new molecules that play an important role in the
inactivation of the Wnt/β-catenin signaling pathway has clinical
application potential.
In summary, the results of the present study
indicated for the first time that miR-20b and Wnt signaling were
coupled through a feed-forward positive feedback loop, forming a
biological regulatory circuit. Our results provided evidence that
miR-20b promoted NSCLC partially by inhibiting APC and the findings
uncover a novel mechanism of Wnt/β-catenin signaling pathway hyper
activation in NSCLC. However, there are limitations to this study,
including the status of miR-20b and APC in tumor tissue remains
unknown. To validate this potential target in the future, the
difference between primary lung tumor tissues and adjacent
non-tumor tissues could be examined.
Supplementary Data
Funding
This work was supported by FDCT grants from the
Science and Technology Development Fund of Macao (grant nos.
003/2018/A1, 130/2017/A3 and 046/2016/A2) and the Scientific and
Technological Project of Shiyan City of Hubei Province of China
(grant no. ZD2013014).
Availability of data and materials
All the datasets generated and analyzed in the
present study are included in this published article.
Authors' contributions
ELHL, YJT and MWC conceived the study. ELHL and YJT
designed the experiments and supervised all research. TR, XXF and
MFW carried out the experiments and prepared the draft of the
manuscript. FGD, CLW and RZL performed the animal study. ZBJ, YWW
and XJY analyzed the data. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
Human lung cancer tissue specimens were obtained
following the guidelines approved by the institutional review board
at Taihe Hospital of Hubei University of Medicine, and written
informed consent was obtained from patients in all cases. Animal
studies were approved by the Ethical Committee of Macau University
of Science and Technology.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
Not applicable.
References
|
1
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Fassina A, Cappellesso R and Fassan M:
Classification of non-small cell lung carcinoma in transthoracic
needle specimens using microRNA expression profiling. Chest.
140:1305–1311. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bartel DP: MicroRNAs: Genomics,
biogenesis, mechanism, and function. Cell. 116:281–297. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lewis BP, Burge CB and Bartel DP:
Conserved seed pairing, often flanked by adenosines, indicates that
thousands of human genes are microRNA targets. Cell. 120:15–20.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Schepeler T: Emerging roles of microRNAs
in the Wnt signaling network. Crit Rev Oncog. 18:357–371. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhang T, Hu Y, Ju J, Hou L, Li Z, Xiao D,
Li Y, Yao J, Wang C, Zhang Y and Zhang L: Downregulation of miR-522
suppresses proliferation and metastasis of non-small cell lung
cancer cells by directly targeting DENN/MADD domain containing 2D.
Sci Rep. 6:193462016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Stoddart A, Wang J, Hu C, Fernald AA,
Davis EM, Cheng JX and Le Beau MM: Inhibition of WNT signaling in
the bone marrow niche prevents the development of MDS in the
Apc(del/+) MDS mouse model. Blood. 129:2959–2970. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Iqbal MA, Arora S, Prakasam G, Calin GA
and Syed MA: MicroRNA in lung cancer: Role, mechanisms, pathways
and therapeutic relevance. Mol Aspects Med. 70:3–20. 2019.
View Article : Google Scholar
|
|
9
|
Mendell JT: miRiad roles for the miR-17-92
cluster in development and disease. Cell. 133:217–222. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hong S, Yu S, Li J, Yin Y, Liu Y, Zhang Q,
Guan H, Li Y and Xiao H: miR-20b displays tumor-suppressor
functions in papillary thyroid carcinoma by regulating the MAPK/ERK
signaling pathway. Thyroid. 26:1733–1743. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Li Y, Chen D, Jin L, Liu J, Su Z, Li Y,
Gui Y and Lai Y: MicroRNA-20b-5p functions as a tumor suppressor in
renal cell carcinoma by regulating cellular proliferation,
migration and apoptosis. Mol Med Rep. 13:1895–1901. 2016.
View Article : Google Scholar
|
|
12
|
Li D, Ilnytskyy Y, Kovalchuk A, Khachigian
LM, Bronson RT, Wang B and Kovalchuk O: Crucial role for early
growth response-1 in the transcriptional regulation of miR-20b in
breast cancer. Oncotarget. 4:1373–1387. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang B, Yang J and Xiao B: MicroRNA-20b
(miR-20b) promotes the proliferation, migration, invasion, and
tumorigenicity in esophageal cancer cells via the regulation of
phosphatase and tensin homologue expression. PLoS One.
11:e01641052016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhu J, Chen L, Zou L, Yang P, Wu R, Mao Y,
Zhou H, Li R, Wang K, Wang W, et al: miR-20b, -21, and -130b
inhibit PTEN expression resulting in B7-H1 over-expression in
advanced colorectal cancer. Hum Immunol. 75:348–353. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Landais S, Landry S, Legault P and Rassart
E: Oncogenic potential of the miR-106-363 cluster and its
implication in human T-cell leukemia. Cancer Res. 67:5699–5707.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Clevers H: Wnt/beta-catenin signaling in
development and disease. Cell. 127:469–480. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hoppler S and Kavanagh CL: Wnt signalling:
Variety at the core. J Cell Sci. 120:385–393. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
MacDonald BT, Tamai K and He X:
Wnt/beta-catenin signaling: Components, mechanisms, and diseases.
Dev Cell. 17:9–26. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yang Z, Shah K, Busby T, Giles K,
Khodadadi-Jamayran A, Li W and Jiang H: Hijacking a key chromatin
modulator creates epigenetic vulnerability for MYC-driven cancer. J
Clin Invest. 128:3605–3618. 2018. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
Dejure FR and Eilers M: MYC and tumor
metabolism: Chicken and egg. EMBO J. 36:3409–3420. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Beà S: Cyclin D1 transcriptional
activation in MCL. Blood. 123:1979–1980. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
23
|
Duan FG, Wang MF, Cao YB, Dan Li, Li RZ,
Fan XX, Khan I, Lai HL, Zhang YZ, Hsiao WW, et al MicroRNA-421
confers paclitaxel resistance by binding to the KEAP1 3′UTR and
predicts poor survival in non-small cell lung cancer. Cell Death
Dis. 10:8212019. View Article : Google Scholar
|
|
24
|
Ou SI, Cui J, Schrock AB, Goldberg ME, Zhu
VW, Albacker L, Stephens PJ, Miller VA and Ali SM: Emergence of
novel and dominant acquired EGFR solvent-front mutations at Gly796
(G796S/R) together with C797S/R and L792F/H mutations in one EGFR
(L858R/T790M) NSCLC patient who progressed on osimertinib. Lung
Cancer. 108:228–231. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Huang T, Alvarez AA, Pangeni RP, Horbinski
CM, Lu S, Kim SH, James CD, J Raizer JA, Kessler J, Brenann CW, et
al: A regulatory circuit of miR-125b/miR-20b and Wnt signalling
controls glioblastoma phenotypes through FZD6-modulated pathways.
Nat Commun. 7:128852016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yamada N, Noguchi S, Mori T, Naoe T, Maruo
K and Akao Y: Tumor-suppressive microRNA-145 targets catenin δ-1 to
regulate Wnt/β-catenin signaling in human colon cancer cells.
Cancer Lett. 335:332–342. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Trautmann M, Sievers E, Aretz S, Kindler
D, Michels S, Friedrichs N, Renner M, Kirfel J, Steiner S, Huss S,
et al: SS18-SSX fusion protein-induced Wnt/β-catenin signaling is a
therapeutic target in synovial sarcoma. Oncogene. 33:5006–5016.
2014. View Article : Google Scholar
|
|
28
|
Silva J, Garcia V, Zaballos Á, Provencio
M, Lombardía L, Almonacid L, García JM, Domínguez G, Peña C, Diaz
R, et al Vesicle-related microRNAs in plasma of nonsmall cell lung
cancer patients and correlation with survival. Eur Respir J.
37:617–623. 2011. View Article : Google Scholar
|
|
29
|
Clevers H and Nusse R: Wnt/β-catenin
signaling and disease. Cell. 149:1192–1205. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Nusse R and Clevers H: Wnt/β-catenin
signaling, disease, and emerging therapeutic modalities. Cell.
169:985–999. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Chen Q, Cao HZ and Zheng PS: LGR5 promotes
the proliferation and tumor formation of cervical cancer cells
through the Wnt/β-catenin signaling pathway. Oncotarget.
5:9092–9105. 2014.PubMed/NCBI
|
|
32
|
Shen G, Jia H, Tai Q, Li Y and Chen D:
miR-106b downregulates adenomatous polyposis coli and promotes cell
proliferation in human hepatocellular carcinoma. Carcinogenesis.
34:211–219. 2013. View Article : Google Scholar
|
|
33
|
Li M, Tian L, Wang L, Yao H, Zhang J, Lu
J, Sun Y, Gao X, Xiao H and Liu M: Down-regulation of miR-129-5p
inhibits growth and induces apoptosis in laryngeal squamous cell
carcinoma by targeting APC. PLoS One. 8:e778292013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Korinek V, Barker N, Morin PJ, van Wichen
D, de Weger R, Kinzler KW, Vogelstein B and Clevers H: Constitutive
transcriptional activation by a beta-catenin-Tcf complex in
APC-/- colon carcinoma. Science. 275:1784–1787. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Gaspar C, Franken P, Molenaar L, Breukel
C, van der Valk M, Smits R and Fodde R: A targeted constitutive
mutation in the APC tumor suppressor gene underlies mammary but not
intestinal tumorigenesis. PLoS Genet. 5:e10005472009. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Janssens N, Janicot M and Perera T: The
Wnt-dependent signaling pathways as target in oncology drug
discovery. Invest New Drugs. 24:263–280. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Yamada T and Masuda M: Emergence of TNIK
inhibitors in cancer therapeutics. Cancer Sci. 108:818–823. 2017.
View Article : Google Scholar : PubMed/NCBI
|