Introduction
Lung cancer has the highest morbidity and mortality
of all malignancies in the USA in 2011 and 2013 (1,2).
Non-small cell lung carcinoma (NSCLC) is the most common form of
lung cancer, accounting for 70-85% of cases and is primarily
treated using systemic chemotherapy (3). However, NSCLC treatment has evolved
due to the development of therapy targeting the activation of
mutations in the epidermal growth factor receptor (EGFR) (4).
EGFR activation is caused by genetic mutations,
conferring susceptibility to EGFR tyrosine kinase inhibitor (TKI)
treatment, and was first reported in 2004 (5). EGFR mutations of pulmonary
adenocarcinoma are present in 5-15% of the Caucasian population and
40-55% of the East Asian population (6-8).
Clinical trials have shown that patients with pulmonary
adenocarcinoma with an EGFR mutation exhibit clinical responses to
orally administered EGFR inhibitors (5,7,9). In
2016, Lin et al (4)
reported that 20/137 (14.6%) patients with EGFR-mutant metastatic
lung adenocarcinoma had a survival time of 5-years (4). Therefore, detection of EGFR mutations
is an important step in the treatment-decision pathway for patients
with pulmonary adenocarcinoma.
Previously, direct DNA sequencing was the standard
method for detecting genetic mutations (10); however, at present, several
alternative methods for mutation testing have been developed
(11,12). For example, the Therascreen EGFR
PCR kit® (Qiagen, Inc.) is a commercial quantitative
(q)PCR kit and has been widely adopted for clinical practice;
however, this method is time-consuming and possesses certain
procedural complexities, for example, requiring several temperature
changes during DNA amplification (11). Next-generation sequencing has
improved the efficiency of oncogene testing by high-throughput
sequencing, which can detect dozen of mutations at the same time,
but the high-cost of this technique limits its clinical usage
(13,14). Therefore, detecting oncogenic
mutations using a simple, easy and highly reproducible method
remains a challenge.
Loop-mediated isothermal amplification (LAMP) is a
new PCR based method with high levels of specificity and
amplification efficiency and utilizes six primers (15). This method is performed under
isothermal conditions, thereby enabling rapid amplification. Due to
the high specificity and rapid detection quality of LAMP, this
method has been widely used in the fields of bacteriology (16) and virology (17,18).
However, to the best of our knowledge, there are very few studies
reporting the value of LAMP in determining EGFR mutations.
Therefore, the present study aimed to detect EGFR mutations in
surgically resected tumor tissues from patients with pulmonary
adenocarcinoma using this method, as detection of EGFR mutations is
one a key examination for patients with pulmonary adenocarcinoma
(4,5,7,9). In
addition, the sensitivity, specificity, positive predictive value,
negative predictive value and accuracy of LAMP was evaluated and
compared with the Therascreen EGFR PCR kit®.
Materials and methods
Tumor tissue samples
The tumor tissues were surgically resected from 189
consecutive patients diagnosed with pulmonary adenocarcinoma by the
expert pathologist at The Saitama Cardiovascular and Respiratory
Center (Kumagaya, Japan) between January 2016 and October 2017. All
pathological diagnosis was determined on the basis of the WHO
classification version 8 (19) by
an expert pathologist, who normally makes a diagnosis using
HE-stained slides using light microscope (Nikon Co., ECLIPSE Ni-u)
from a low magnification to a high magnification. The inclusion
criteria were as follows: i) Surgically resected tissue of primary
lung cancer; ii) pulmonary adenocarcinoma; iii) enough volume
materials for molecular testing; and iv) informed written consent
from patients. Conversely, cases with no informed consent or less
volume sample were excluded. Clinical characteristics of the 59
patients are presented in Table I.
The mean patient age was 69.6 years and included 28 males and 31
females. All samples were fixed with 10% buffer formalin at room
temperature (24-36 h) to create formalin-fixed, paraffin-embedded
(FFPE) tumor blocks at Department of Pathology in Saitama
Cardiovascular and Respiratory Center. Hematoxylineosin staining
was performed by the standard method using Tissue-Tek Prisma
(Sakura Finetek Japan Co., Ltd.) according to the manufacturer's
protocol. Prior to DNA extraction, the tumor content of each sample
was assessed using light microscopy at ×10 and ×100 magnification,
to ensure efficient PCR amplification. After sections were
deparaffinized with xylene and hydrated through a graded series of
ethanol (100, 100, 85 and 70% ethanol), DNA from the tissue blocks
was extracted using the QIAampTM DNA FFPE Tissue kit®
(Qiagen, Inc.) and analyzed using a QIAcube Robot®
(Qiagen, Inc.) according to the manufacturer's protocols (20).
 | Table IClinical characteristics of patients
with pulmonary adenocarcinoma. |
Table I
Clinical characteristics of patients
with pulmonary adenocarcinoma.
| Characteristic | n (%) |
|---|
| Age, years ±
standard deviation | 69.6±7.1 |
| <40 | 0 (0.00) |
| 41-50 | 1 (0.02) |
| 51-60 | 5 (0.08) |
| >60 | 53 (89.80) |
| Sex | |
| Male | 28 (47.5) |
| Female | 31 (52.5) |
| Smoking status | |
| Never smoker | 27 (45.8) |
| Current or former
smoker | 32 (55.2) |
| Tumor location | |
| Right lung | 34 (57.6) |
| Left lung | 25 (42.4) |
| Histology | |
| Papillary
predominant | 34 (57.6) |
| Solid
predominant | 7 (11.9) |
| Acinar
predominant | 3 (5.1) |
| Invasive mucinous
predominant | 7 (11.9) |
| Lepidic
predominant | 4 (6.8) |
| Minimally invasive
predominant | 4 (6.8) |
| Pathological
stage | |
| IA1 | 14 (23.7) |
| IA2 | 20 (33.9) |
| IA3 | 6 (10.2) |
| IB | 4 (6.8) |
| IIA | 1 (1.7) |
| IIB | 3 (5.1) |
| IIIB | 11 (18.6) |
Therascreen qPCR mutation analysis
The presence of EGFR mutations was determined using
a Therascreen EGFR PCR kit® (Qiagen, Inc.) according to
the manufacturer's protocols (21).
LAMP mutation analysis
Primer design
A primer set for LAMP amplification of the partial
sequence of the EGFR gene (NG_007726) was designed using Primer
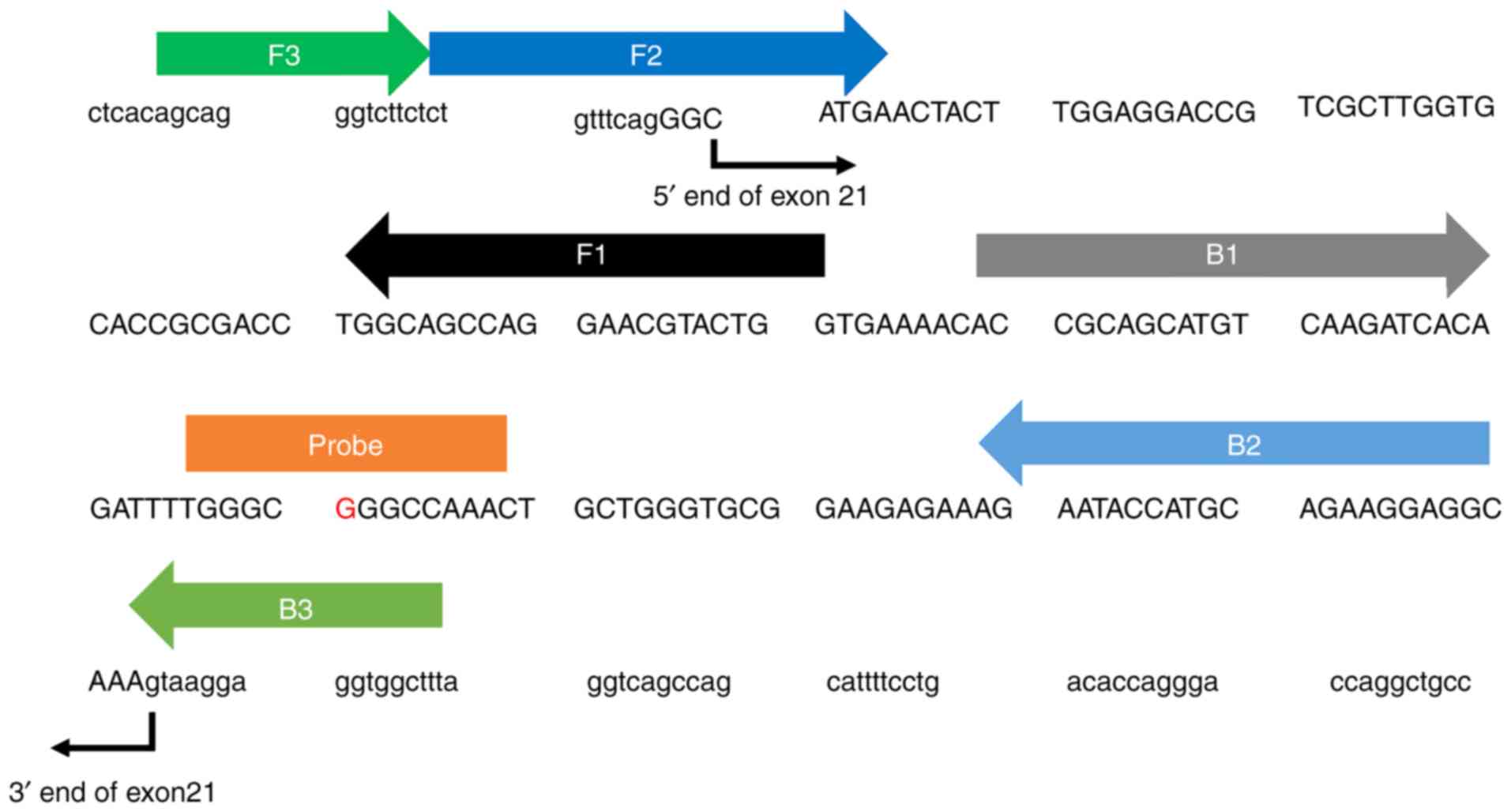
Explorer (primerexplorer.jp/e/). Fig.
1 presents the sequences of the primers used with forward and
backward outer primers (F3 and B3), forward and backward inner
primers (FIP and BIP) and forward and back-loop primers (LF and
LB). The primers were synthesized and purified by Eurofin Genomics.
Block oligo and fluorophore-labelled probes were synthesized and
purified by Japan Bio Services Co., Ltd., or Gene Design, Inc.
LAMP assay
LAMP assays were performed using the 25 reaction
volume with 30 mM KCl, 1.8% Dextran, 14 mM Tricine-NaOH (pH 8.8),
0.1% n-heptyl, 0.5% Tween-20, 0.3% Fish collagen peptide, 1 mM DTT,
1.7 mM each dNTPs, 8.2 mM MgSO4, 1.6 µM each of the forward
and backward inner primer, 0.8 µM each of the forward and
backward loop primer, 0.2 µM forward and backward outer
primer, 1.2 µM block oligo, 0.04 µM
fluorophore-labelled probe, 0.0375 x Gel green and 12.3 units Bst
DNA polymerase. KCl, Dextran, Trincine NaOH, n-heptyl, Tween-20,
Fish collagen peptide, DTT, and MgSO4 were obtained from FUJIFILM
Wako Pure Chemical Corporation (http://ffwk.fujifilm.co.jp/en/index.html). dNTPs were
obtained from NIPPON GENE CO., Ltd. (https://www.nippongene.com/english/index.html). Gel
green was obtained from Biotium (https://biotium.com/). Bst DNA polymerase was obtained
from New England Biolabs Inc. (https://international.neb.com). The LAMP reaction was
run for 120 min at 65°C using a LightCycler 480® (Roche
Diagnostics K.K.).
Detection of the LAMP products
Melting curve analysis was used to detect LAMP
products. The LAMP amplicons were denatured at 95°C for 5 min,
followed by hybridization at 37°C for 5 min. Subsequently, the
temperature was gradually raised to 80°C and the fluorescent
intensity was measured 7 times per 1°C increment. The obtained data
were analyzed by the LightCycler 480® software (version
1.5.1.62; https://lifescience.roche.com/global_en/products/lightcycler14301-480-software-version-15.html)
to calculate melting peak.
Sensitivity of LAMP and Therascreen
assays
In order to evaluate the analytical sensitivity, an
artificial gene harboring an EGFR L858R mutation (c.2573T>G) as
a plasmid DNA was obtained from Eurofin Genomics. The plasmid DNA
that had been linearized using EcoR V and Xho I were extracted and
purified from 1% agarose gel after 40 min electrophoresis with 100
V. The agarose gel was stained by 0.5 µg/ml ethidium bromide
(Tokyo Chemical Industry Co., Ltd.; https://www.tcichemicals.com/eshop/en/jp/commodity/E0370/)
for 30 min and observed by LuminoGraph 2 (ATTO Corporation;
https://www.atto.co.jp/eng). Then, this
DNA fragment was added to human pooled genomic DNA (Promega
Corporation) to prepare several spiked-in samples with different
concentrations for a validation of lower limit of detection. In the
present study, 1500.0, 150.0, 75.0, 15.0, 7.5, 3.0, and 1.5 copies
of DNA fragment harboring EGFR L858R mutation were added to 1500
copies of genomic DNA, resulting in mutation rates of 50.00, 9.09,
4.76, 0.99, 0.50, 0.20, 0.10 and 0.00%, respectively. Genetic
analysis of each of these samples was conducted three times using
PCR and LAMP.
Direct sequence method
Direct sequencing of EGFR exon 19, S7681 and G719X
regions were conducted by using BigDye® Direct Cycle
Sequencing Kit (Thermo Fisher Scientific, Inc.) and a ABI PRISM 310
Genetic Analyzer® (Applied Biosystems; Thermo Fisher
Scientific, Inc.) to confirm the EGFR mutation status in Case-X.
The data were analyzed using Genetyx (version 13.0; https://www.genetyx.co.jp/) and FinchTV (version
1.4.0; https://digitalworldbiology.com/FinchTV). The primers
had the following sequences: Exon 19, forward TCCCAGAAGGTGAGAAAGT,
reverse: AGA GCT AGA AAG GGA AAG ACA; S768I:, forward: CCT CCA GGA
AGC CTA CGT, reverse: GTC TTT GTG TTC CCG GAC AT; and G719X,
forward: CCC TTG TCT CTG TGT TCT TG, reverse: TAC ACC GTG CCG AAC
GC.
Statistical analysis
All statistical analysis performed using SPSS
version 24 for Windows® (IBM Corp). The clinical
sensitivity, specificity, positive predictive value (PPV), negative
predictive value (NPV) and accuracy of conventional PCR testing of
the LAMP method were calculated through comparison with the
Therascreen PCR, which was considered as the current gold standard
test for detecting EGFR mutations in the present study.
Results
Characteristics of patients with
pulmonary adenocarcinoma
Mean age of the 59 patients was 69.6 years and
included 28 males and 31 females. Of these, 27 were non-smokers
(45.8%). The number in pathological stage IA1, IA2, IA3, IB, IIA,
IIB, IIIB was 14 (23.7%), 20 (33.9%), 6 (10.2%), 4 (6.8%), 1
(1.7%), 3 (5.1%), and 11 (18.6%), respectively. All patients had
been diagnosed with pulmonary adenocarcinoma, including of 34
papillary predominant (57.6%), 7 solid predominant (11.9%), 3
acinar predominant (5.1%), 7 invasive mucinous (11.9%), 4 lepidic
predominant (6.8%) and 4 minimally invasive adenocarcinoma cases
(6.8%; all Table I).
Therascreen EGFR PCR mutation
analysis
Among 59 pulmonary tumors, there were 26 tumors with
EGFR wild type and 33 tumors with EGFR mutations (Table II). Overall, 18 exon 21 L858R
point mutations (54.5%), 12 exon 19 deletions (36.4%), 2
simultaneous exon 18 G719X point mutation/exon 20 S768I point
mutation (6.1%) and 1 exon 20 SS761I point mutation (3.0%) were
detected in the pulmonary tumors with EGFR mutations (Table II).
 | Table IIEGFR mutations possessed by patients
with pulmonary adenocarcinoma identified using Therascreen and LAMP
assays. |
Table II
EGFR mutations possessed by patients
with pulmonary adenocarcinoma identified using Therascreen and LAMP
assays.
| EGFR mutation | Therascreen | LAMP |
|---|
| Exon 21 L858R | 18 | 18 |
| Exon 19
deletions | 12 | 11 |
| Exon 20 S768I | 1 | 1 |
| Exon 18 G719X and
exon 20 S768I | 2 | 2 |
| Total | 33 | 32 |
LAMP EGFR mutation analysis
The LAMP assay detected 27 EGFR wild types and 32
EGFR mutations (Table II). Among
32 EGFR mutations, 18 exon 21 L858R point mutations (54.5%), 11
exon 19 deletions (33.3%), 2 simultaneous exon 18 G719X point
mutation/exon 20 S768I point mutations (6.1%) and 1 exon 20 SS761I
point mutation alone (3.0%) were identified (Table II).
Comparison of results of Therascreen PCR
and LAMP assays
Table II
demonstrates that 33 EGFR mutations in the LAMP assay (18 samples
with exon 21 L858R, 11 samples with exon 19 deletions, 1 sample
with exon 20 S768I, and 2 samples with exon 18 G719X and exon 20
S768I), and that 32 EGFR mutations in the Therascreen assay (18
samples with exon 21 L858R, 12 samples with exon 19 deletions, 1
sample with exon 20 S768I, and 2 samples with exon 18 G719X and
exon 20 S768I); i.e., there was one case showing EGFR wild type of
the LAMP but one deletion mutation in exon 19 of the Therascreen
PCR (Table III). As assuming
that the results of Therascreen PCR assay was true, qualitative
analysis was performed for calculating sensitivity, specificity,
PPV, NPV and accuracy of the LAMP assay. These values were 97.0%,
100.0, 100.0, 96.3 and 98.3%, respectively. Furthermore, a genetic
analysis was performed to evaluate the detection rate of EGFR
mutations in different concentrations. The minimum concentration
for EGFR mutation detection was 4.8% of DNA sample in Therascreen
assay (Table SI). On the other
hand, 0.1% was the minimum concentration in LAMP assay, since LAMP
assay demonstrated one success of the detection per 3 tests at the
level of 0.1, 0.5 and 1.0% concentrations and all positive per 3
tests at >4.8% concentration (Table SI).
 | Table IIIAssociation between the results
between the LAMP assay and the Therascreen assay. |
Table III
Association between the results
between the LAMP assay and the Therascreen assay.
| EGFR status | Therascreen PCR
|
|---|
| Positive | Negative |
|---|
| LAMP | Positive | 32 | 0 |
| Negative | 1 | 26 |
Direct sequencing of Case X
It was observed that a single case deviated in the
identification of the mutation in exon 19 between Therascreen PCR
and the LAMP assay. To confirm this result, direct sequencing of
the target site of exon 19 was performed. Direct sequencing results
demonstrated no mutation in exon 19 in Case X, which was concordant
with the results of the LAMP assay.
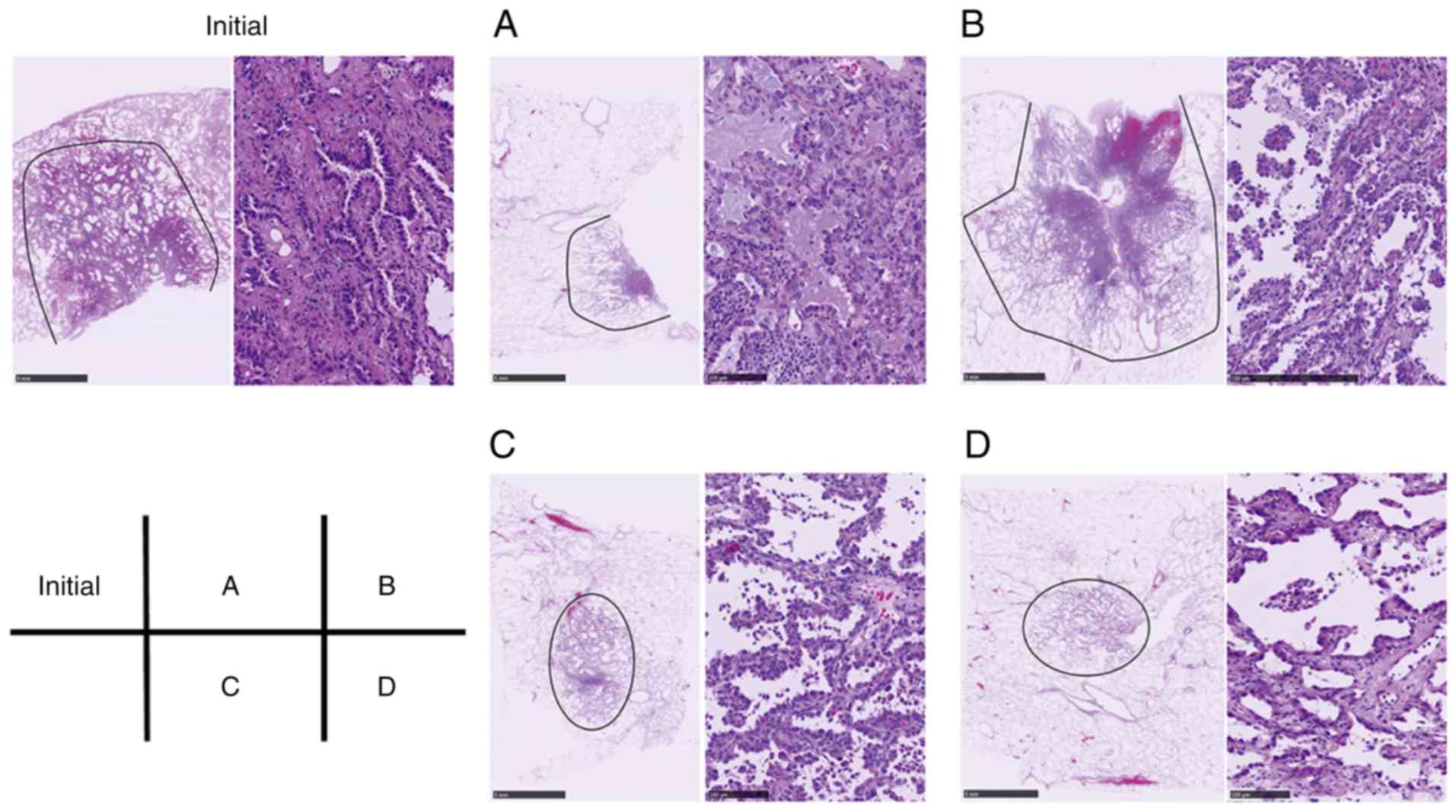
To reconfirm the status of EGFR mutation in Case X,
four additional FFPE tissue blocks in Case X were used to extract
further DNA samples. The hematoxylineosin (HE) images of these FFPE
blocks are presented in Fig. 2.
Following removal of normal lung tissues, DNA samples were
extracted as aforementioned, and investigated using Therascreen
EGFR PCR and a LAMP assay. In all the four samples, the deletion
mutation in exon 19 was identified using both Therascreen PCR and
LAMP assays. Furthermore, direct sequencing revealed a novel exon
19 EGFR deletion mutation in samples a and b; NG_007726.3:
g.160744_160761delinsGCA represented the deletion of nucleotides
g.160744 to g.160761 (ATTAAGAGAAGCAACATC, data not shown), which
were replaced by a GCA nucleotide triplet, changing
GGAATTAAGAGAAGCAACATCTCC to GGAGCATCC (data not shown), resulting
in shortening substation in the protein
(p.Leu747_Ser752delinsHis).
Discussion
EGFR mutations in pulmonary adenocarcinoma are
associated with sensitivity to TKI therapy. Hence the
identification of EGFR mutations has become a standard analysis in
the treatment pathway of patients with pulmonary adenocarcinoma.
Although there are a number of methods available, there is no
standardized approach to satisfy the practical clinical
requirements of simplicity, rapid and cheap. Several PCR based
methods have previously been used as a routine test for the
detection of EGFR status in United States, European Union, Japan
and China, including the Scorpion Amplification Refractory Mutation
System (ARMS)® (22),
such as Therascreen PCR assay. This method was approved by the FDA
as a standard approach for EGFR gene analysis in lung cancer
(fda.gov/medical-devices/recently-approved-devices/therascreenr-fgfr-rgq-pcr-kit-p180043);
however, although stable and reliable, this approach has procedural
complexities, including complex settings and controls for the
temperature at several times using a thermal cycler. Alternatives,
such as PCR-Invader® (23), peptide nucleic acid-locked nucleic
acid (PNA-LNA) PCR clamp (24) and
Cycleave PCR™ (25), have been
developed in Japan and are commercially used in centralized
laboratories. The sensitivity of the PNA-LNA PCR clamp is >97%
with 100% specificity (26) and
the accuracy of Cycleave PCR is 96.7% (27), so it was the same for our results
as it was those.
LAMP is a new PCR method and is considered to be a
robust approach for gene analysis as it does not require
sophisticated or expensive equipment, such as a thermal cycler
necessary for PCR (15).
Therefore, the LAMP method may have potential to decrease the costs
of gene analysis. Previous studies have demonstrated the value of
LAMP in field of bacteriology and virology (15,17,28,29);
however, few studies have reported the value of LAMP in oncology.
Ikeda et al (30) used LAMP
assays to detect EGFR mutations in NSCLC, demonstrating the value
of LAMP, but only the L858R mutation was studied. Therefore, the
present study aimed to investigate other EGFR mutations, including
those in exon 19, exon 21 and other minor mutations using LAMP
assays.
The sensitivity, specificity, PPV, NPV and accuracy
values of LAMP for EGFR mutations compared with the Therascreen
assay method were 97, 100, 100, 96.3 and 98.3%, respectively,
demonstrating the potential of LAMP as an efficient alternative
approach t oncogene mutation analyses. To evaluate the sensitivity
of these two assays, a genetic analysis was performed to
investigate the minimum concentration of EGFR mutation detection in
both methods. The minimum concentration for LAMP was 4.8% and there
were no detection under 1.0% in the Therascreen assay. Therefore,
the minimum concentration for the LAMP assay is ~50 times higher
than 4.8% in the Therascreen assay. With almost equal efficiency,
detection of the exon 19 mutation in Case X using the Therascreen
method suggested that LAMP, as well as direct sequence methods,
could identify false negatives. However, additional experiments
investigating Case X tumor tissues demonstrated the presence of
deletion mutations in exon 19. The direct sequence method has
reported low sensitivity (31,32),
whereas the Therascreen method was generally recognized as a
promising method (11,12). However, the identified mutation
should be simplified, and an improved, faster and cheaper method of
identification should be developed for its clinical
application.
The additional Case X experiments also identified a
novel EGFR mutation using direct sequencing which was not
identified using Therascreen or LAMP; however, this mutation may
have been detected as an exon 19 deletion by the primers of the
similarly targeted mutation. However, the details of the primer of
LAMP method could not be disclosed due to the policies of Eiken
Co., Ltd., and further information concerning the primers of
Therascreen EGFR PCR kit could not be obtained due to the patent.
Furthermore, the present study noted that this novel mutation was
not included in the COSMIC database and that no previous studies
had reported this mutation. Therefore, the present study is the
first report this EGFR mutation, to the best of our knowledge.
In conclusion, the LAMP method may be a valuable
alternative for the identification of oncogenic mutations in lung
cancer. Currently, the study group is developing a new method of
detecting oncogenes using liquid biopsies (data not shown). In
addition, a novel mutation, NG_007726.3:g.160744_16076 1delinsGCA,
was identified exon 19 of EGFR. However, this needs further
validation before clinical use.
Supplementary Data
Funding
The present study was funded by Eiken Chemical Co.,
Ltd.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author upon reasonable
request.
Authors' contributions
SH analyzed the data and wrote the initial draft of
the manuscript. YS designed the study, analyzed and interpreted the
data and assisted in preparing the manuscript. AM performed all
experiments and analyzed all results in the study. YS made
pathological diagnosis in all cases and provided all tissue
samples. NT, TI and EH contributed to data collection and
interpretation. MY performed some of the experiments, and
critically reviewed the manuscript, and organized research group in
this study. Besides AM, all authors approved the final version of
the manuscript and they agree to be accountable for all aspects of
the work.
Ethical approval and consent to
participate
The present study was approved by The Institutional
Review Board of the Saitama Cardiovascular and Respiratory Center
(approval no. 2016015). Written informed consent was provided by
all patients.
Patient consent for publication
Not applicable.
Competing interests
AM is an employee of Eiken Chemical Co., Ltd, and
was the only author who conducted all the experiments. Eiken
Chemical Co., Ltd, provided the research grant for the present
study, and provided the LAMP assay which is not currently
commercially available. Eiken Chemical Co., Ltd., had no control
over the interpretation, writing, or publication of the present
study.
Abbreviations:
|
EGFR
|
epidermal growth factor receptor
|
|
FFPE
|
formalin-fixed, paraffin-embedded
|
|
NSCLC
|
non-small cell lung cancer
|
|
LAMP
|
loop-mediated isothermal
amplification
|
|
PPV
|
positive predictive value
|
|
NPV
|
negative predictive value
|
|
PCR
|
polymerase chain reaction
|
|
TKI
|
tyrosine kinase inhibitors
|
Acknowledgments
The authors would like to thank Mr Satoru Michiyuki,
employee of Eiken Chemical Co., Ltd., Otawara, Japan, for his
valuable comments and suggestions concerning the LAMP assay; and
Mr. Yasuhito Kobayashi, Mr. Hidehiro Numagami and Ms. Mei Miyagawa
of the Saitama Cardiovascular and Respiratory Center, Kumagaya,
Japan, for their technical assistance.
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Siegel R, Naishadham D and Jemal A: Cancer
statics, 2013. CA Cancer J Clin. 63:11–30. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Travis WD, Brambilla E, Burke AP, Marx A
and Nicholson AG: Introduction to the 2015 world health
organization classification of tumors of the lung, pleura, thymus,
and heart. J Thorac Oncol. 10:1240–1242. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lin JJ, Cardarella S, Lydon CA, Dahlberg
SE, Jackman DM, Jänne PA and Johnson BE: Five-year survival in
EGFR-mutant metastatic lung adenocarcinoma treated with EGFR-TKIs.
J Thorac Oncol. 11:556–565. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lynch TJ, Bell DW, Sordella R,
Gurubhagavatula S, Okimoto RA, Brannigan BW, Harris PL, Haserlat
SM, Supko JG, Haluska FG, et al: Activating mutations in the
epidermal growth factor receptor underlying responsiveness of
non-small-cell lung cancer to gefitinib. N Engl J Med.
350:2129–2139. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kohno T, Tsuta K, Tsuchihara K, Nakaoku T,
Yoh K and Goto K: RET fusion gene: Translation to personalized lung
cancer therapy. Cancer Sci. 104:1396–1400. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Pao W and Hutchinson KE: Chipping away at
the lung cancer genome. Nat Med. 18:349–351. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Li T, Kung HJ, Mack PC and Gandara DR:
Genotyping and genomic profiling of non-small-cell lung cancer:
Implications for current and future therapies. J Clin Oncol.
31:1039–1049. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Paez JG, Janne PA, Lee JC, Tracy S,
Greulich H, Gabriel S, Herman P, Kaye FJ, Lindeman N, Boggon TJ, et
al: EGFR mutations in lung cancer: Correlation with clinical
response to gefitinib therapy. Science. 304:1497–1500. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
De Pas T, Toffalorio F, Manzotti M,
Fumagalli C, Spitaleri G, Catania C, Delmonte A, Giovannini M,
Spaggiari L, de Braud F and Barberis M: Activity of epidermal
growth factor receptor-tyrosine kinase inhibitors in patients with
non-small cell lung cancer harboring rare epidermal growth factor
receptor mutations. J Thorac Oncol. 6:1895–1901. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wu YL, Zhou C, Liam CK, Wu G, Liu X, Zhong
Z, Lu S, Cheng Y, Han B, Chen L, et al: First-line erlotinib versus
gemcitabine/cisplatin in patients with advanced EGFR
mutation-positive non-small-cell lung cancer: Analyses from the
phase III, randomized, open-label, ENSURE study. Ann Oncol.
26:1883–1889. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yang JC, Wu YL, Schuler M, Sebastian M,
Popat S, Yamamoto N, Zhou C, Hu CP, O'Byrne K, Feng J, et al:
Afatinib versus cisplatin-based chemotherapy for EGFR
mutation-positive lung adenocarcinoma (LUX-Lung 3 and LUX-Lung 6):
Analysis of overall survival data from two randomised, phase 3
trials. Lancet Oncol. 16:141–151. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Garinet S, Laurent-Puig P, Blons H and
Oudart JB: Current and future molecular testing in NSCLC, what can
we expect from new sequencing technologies? J Clin Med. 7:E1442018.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Marino P, Touzani R, Perrier L, Rouleau E,
Kossi DS, Zhaomin Z, Charrier N, Goardon N, Preudhomme C,
Durand-Zaleski I, et al: Cost of cancer diagnosis using
next-generation sequencing targeted gene panels in routine
practice: A nationwide French study. Eur J Hum Genet. 26:314–323.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Notomi T, Okayama H, Masubuchi H, Yonekawa
T, Watanabe K, Amino N and Hase T: Loop-mediated isothermal
amplification of DNA. Nucleic Acids Res. 28:E632000. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yoshino M, Annaka T, Kojima T and Ikedo M:
Sensitive and rapid detection of mycoplasma pneumoniae by
loop-mediated isothermal amplification. Kansenshogaku Zasshi.
82:168–176. 2008.In Japanese. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Poon LL, Leung CS, Tashiro M, Chan KH,
Wong BW, Yuen KY, Guan Y and Peiris JS: Rapid detection of the
severe acute respiratory syndrome (SARS) coronavirus by a
loop-mediated isothermal amplification assay. Clin Chem.
50:1050–1052. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Imai M, Ninomiya A, Minekawa H, Notomi T,
Ishizaki T, Van Tu P, Tien NT, Tashiro M and Odagiri T: Rapid
diagnosis of H5N1 avian influenza virus infection by newly
developed influenza H5 hemagglutinin gene-specific loop-mediated
isothermal amplification method. J Virol Methods. 141:173–180.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Goldstraw P, Chansky K, Crowley J,
Rami-Porta R, Asamura H, Eberhardt WE, Nicholson AG, Groome P,
Mitchell A, Bolejack V, et al: The IASLC lung cancer staging
project: Proposals for revision of the TNM stage groupings in the
forthcoming (eighth) edition of the TNM Classification for lung
cancer. J Thorac Oncol. 11:39–51. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Angulo B, García-García E, Martínez R,
Suárez-Gauthier A, Conde E, Hidalgo M and López-Ríos F: A
commercial real-time PCR kit provides greater sensitivity than
direct sequencing to detect KRAS mutations: A morphology-based
approach in colorectal carcinoma. J Mol Diagn. 12:292–299. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Vallée A, Le Loupp AG and Denis MG:
Efficiency of the Therascreen® RGQ PCR kit for the detection of
EGFR mutations in non-small cell lung carcinomas. Clin Chim Acta.
429:8–11. 2014. View Article : Google Scholar
|
|
22
|
Kimura H, Kasahara K, Kawaishi M, Kunitoh
H, Tamura T, Holloway B and Nishio K: Detection of epidermal growth
factor receptor mutations in serum as a predictor of the response
to gefitinib in patients with non-small-cell lung cancer. Clin
Cancer Res. 12:3915–3921. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hall JG, Eis PS, Law SM, Reynaldo LP,
Prudent JR, Marshall DJ, Allawi HT, Mast AL, Dahlberg JE,
Kwiatkowski RW, et al: Sensitive detection of DNA polymorphisms by
the serial invasive signal amplification reaction. Proc Natl Acad
Sci USA. 97:8272–8277. 2002. View Article : Google Scholar
|
|
24
|
Nagai Y, Miyazawa H, Huqun, Tanaka T,
Udagawa K, Kato M, Fukuyama S, Yokote A, Kobayashi K, Kanazawa M
and Hagiwara K: Genetic heterogeneity of the epidermal growth
factor receptor in non-small cell lung cancer cell lines revealed
by a rapid and sensitive detection system, the peptide nucleic
acid-locked nucleic acid PCR clamp. Cancer Res. 65:7276–7282. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yatabe Y, Hida T, Horio Y, Kosaka T,
Takahashi T and Mitsudomi T: A rapid, sensitive assay to detect
EGFR mutation in small biopsy specimens from lung cancer. J Mol
Diagn. 8:335–341. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Tanaka T, Nagai Y, Miyazawa H, Koyama N,
Matsuoka S, Sutani A, Huqun, Udagawa K, Murayama Y, Nagata M, et al
Reliability of the peptide nucleic acid-locked nucleic acid
polymerase chain reaction clamp-based test for epidermal growth
factor receptor mutations integrated into the clinical practice for
non-small cell lung cancers. Cancer Sci. 98:246–252. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Nakamura H, Koizumi H, Sakai H, Kimura H,
Miyazawa T, Marushima H, Saji H and Takagi M: Accuracy of the cobas
EGFR mutation assay in non-small-cell lung cancer compared with
three laboratory-developed tests. Clin Lung Cancer. 19:170–174.
2018. View Article : Google Scholar
|
|
28
|
Waites KB, Xiao L, Liu Y, Balish MF and
Atlinson TP: Mycoplasma pneumoniae from the respiratory tract and
beyond. Clin Microbiol Rev. 30:747–809. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Dinh DT, Le MT, Vuong CD, Hasebe F and
Morita K: An updated loop-mediated isothermal amplification method
for rapid diagnosis of H5N1 avian influenza viruses. Trop Med
Health. 39:3–7. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ikeda S, Takabe K, Inagaki M, Funakoshi N
and Suzuki K: Detection of gene point mutation in paraffin sections
using in situ loop-mediated isothermal amplification. Pathol Int.
57:594–599. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Fan X, Furnari FB, Cavenee WK and
Castresana JS: Non-isotopic silver-stained SSCP is more sensitive
than automated direct sequencing for the detection of PTEN
mutations in a mixture of DNA extracted from normal and tumor
cells. Int J Oncol. 18:1023–1026. 2001.PubMed/NCBI
|
|
32
|
Endo K, Konishi A, Sasaki H, Takada M,
Tanaka H, Okumura M, Kawahara M, Sugiura H, Kuwabara Y, Fukai I, et
al: Epidermal growth factor receptor gene mutation in non-small
cell lung cancer using highly sensitive and fast TaqMan PCR assay.
Lung Cancer. 50:375–384. 2005. View Article : Google Scholar : PubMed/NCBI
|