Introduction
Lung cancer is the leading cause of cancer-related
mortality worldwide, resulting in 1.8 million deaths annually
(1). Lung cancer is mainly
subdivided into non-small cell lung cancer (NSCLC) and small-cell
lung cancer, whereas ~85% of lung cancers are NSCLC, which includes
three major histological subtypes: Lung adenocarcinoma (LUAD), lung
squamous cell carcinoma (LUSC), and large-cell lung cancer
(2). Over the past two decades,
the use of targeted therapies and immuno-therapies has achieved
survival benefits in a proportion of the patients (3,4).
However, the 5-year survival rate for lung cancer of all stages
combined was only 18.6% in 2019 (5,6);
therefore, there is an urgent need to identify new molecular
targets in order to develop novel therapies and improve patient
outcomes.
RNA-binding proteins (RBPs) are crucial regulators
of RNA stability, splicing and translation, and play key roles in
the pathological processes underlying multiple diseases, including
cancer (7,8). The Musashi family of RBPs comprises
Musashi-1 (MSI1) and Musashi-2 (MSI2), which play complementary as
well as independent roles in stem cells (9,10).
MSI2 belongs to the class A/B heterogeneous nuclear
ribonucleoproteins (hnRNPs) and contains two tandem RNA recognition
motifs and a carboxyl-terminal poly-A-binding protein association
domain (10,11). Recently, MSI2 was suggested to be a
potential oncoprotein regulating cancer initiation, development,
and resistance to treatment (12).
MSI2 has been found to be increased in chronic myeloid leukemia
(CML) (13), acute myeloid
leukemia (AML) (14) and several
types of solid tumors, including colorectal (15), lung (16,17),
breast (18), cervical (11,19),
and pancreatic cancer (20), as
well as several other cancers (21-23).
Overexpression of MSI2 promotes the proliferation, invasion and
metastasis of pancreatic (20),
cervical (19) and esophageal
squamous cell carcinoma cells (21), and induces resistance to paclitaxel
in ovarian cancer cells (24).
Knockdown of MSI2 inhibits cell proliferation, invasion and
metastasis in NSCLC (16,17) and leukemia (25,26),
and sensitizes AML cells to treatment with daunorubicin (26). These findings suggest that MSI2 may
be a promising therapeutic target in cancer. Two small compounds,
gossypolone and Ro 08-2750, were shown to be able to inhibit MSI1
and MSI2 (27,28). However, the effects of MSI2
inhibition on lung cancer cells remain to be investigated.
The aim of the present study was to investigate the
potential of MSI2 as a drug target for lung cancer therapy, and
identify natural compounds targeting MSI2 and evaluate their
antitumor activity. The expression of MSI2 was examined in NSCLC
cell lines and human lung cancer specimens, and it was investigated
whether its expression is associated with prognosis. Furthermore,
the effects of largazole, a marine natural cyclic depsipeptide
extracted from a cyanobacterium of the genus Symploca
(29), on the expression of MSI2
and its downstream mammalian target of rapamycin (mTOR) signaling
pathway, cancer cell proliferation and apoptosis were investigated,
in order to determine whether largazole may achieve clinical
benefits through MSI2 inhibition.
Materials and methods
Patient samples
The present study was approved by the Institutional
Review Board of the Institute of Zoology, Chinese Academy of
Sciences and the Third Affiliated Hospital of Kunming Medical
University; all tissue samples were obtained with written informed
consent from the patients or their families. Tumor and adjacent
normal lung tissues were obtained between June and November 2011
from 6 patients with previously untreated NSCLC. These included two
men and four women, with a mean age of 58 years (range, 41-68
years). The samples were immediately frozen in liquid nitrogen
following surgical resection. The expression of MSI2 at the
mRNA level was assayed in The Cancer Genome Atlas (TCGA)
transcrip-tome database containing 1,053 NSCLC tumor samples and
109 normal lung specimens. The survival curve was estimated by the
Kaplan-Meier method and log-rank test using the Online Survival
Analysis Software (30)
(http://kmplot.com/analysis/index.php?p=service&cancer=lung).
Reagents and antibodies
Largazole was synthesized by our chemistry
laboratory (31). The purity of
largazole was 98% (determined by reverse-phase high-performance
liquid chromatography). PS-341 was obtained from Millennium
Pharmaceuticals and chloroquine (CQ) was purchased from
Sigma-Aldrich; Merck KGaA. The antibodies used included rabbit
anti-human MSI2 (cat. no. ab76148, Abcam; 1:1,000 for western
blotting), rabbit anti-human S6K (cat. no. 9202, 1:1,000 for
western blotting), rabbit anti-human p-S6K (Thr389) (cat. no. 9205,
1:1,000 for western blotting), rabbit anti-human p-S6 ribosomal
protein (Ser240/244) (cat. no. 5364, 1:1,000 for western blotting),
rabbit anti-human PARP (cat. no. 9542, 1:1,000 for western
blotting), rabbit anti-human p-4E-BP1 (Ser65) (cat. no. 9451,
1:1,000 for western blotting) from Cell Signaling Technology, Inc.,
mouse anti-human actin (cat. no. A5441, Sigma-Aldrich; Merck KGaA;
1:5,000 for western blotting), rabbit anti-human p-Akt (Ser473)
(cat. no. sc-7985-R, 1:500 for western blotting) and rabbit
anti-human Akt (cat. no. sc-8312, 1:500 for western blotting) from
Santa Cruz Biotechnology, Inc.
Cell culture
The human normal lung epithelial cell line 16HBE was
obtained from Clonetics. The C57BL/6 murine Lewis lung carcinoma
(LLC) cell line, the human leukemic cell line K562, and the NSCLC
cell lines A549, H460, H520 and H1975 (harboring EGFR-L858R/T790M
mutations) were obtained from the American Type Culture Collection.
The 32Dcl3-Bcr-Abl-T315I (32D-BA-T315I) cell line was obtained by
stably infecting 32Dcl3 cells with the MSCV-Bcr-Abl-T315I-IRES/GFP
(Bcr-Abl-T315I/GFP) retroviral transducing vector, which was kindly
provided by Dr Warren Pear (University of Pennsylvania). The
pSRalpha plasmid constructs containing the wild-type and T315I
mutant cDNAs of the Bcr-Abl tyrosine kinase were provided by Dr
Brian Druker (Oregon Health & Science University). Cells
expressing 32Dcl3-Bcr-Abl (32D-BA), 32D-BA-T315I and the
imatinib-resistant cell line K562R were established and kept in our
laboratory (32). All cell lines
were cultured according to the recommended protocols.
Cell viability and apoptosis assay
Cell viability was determined by the Cell Counting
Kit-8 (CCK-8; Dojindo Molecular Technologies, Inc.) assay according
to the manufacturer's protocol: Cell suspensions (100 µl) were
added into each well of 96-well plates at a density of 5,000
cells/well, the cells were then exposed to the indicated
concentrations of largazole for 24 or 48 h, 10 µl of CCK-8 reagent
was added to each well, and the cells were incubated at 37˚C for
1‑2 h. The absorbance was measured at 450 nm using a microplate
reader (Bio-Tek Instruments, Inc.). Cell apoptosis was analyzed
using the Annexin V-FITC Apoptosis Detection kit (BD Biosciences)
according to the manufacturer's instructions. The cells were
treated with the indicated concentrations of largazole for 48 h and
collected, resuspended in Annexin V binding buffer, incubated with
Annexin V-FITC and propidium iodide (PI) for 15 min at room
temperature in the dark, and tested by flow cytometry using a FACS
Calibur flow cytometer (Becton, Dickinson and Company). FACS data
were analyzed by BD CellQuest™ Pro 6.0 software (Becton, Dickinson
and Company).
Soft agar colony formation assay
Cells were treated with the indicated concentrations
of largazole for 12 h, then counted and resuspended in RPMI-1640
containing 10% fetal bovine serum (Gibco; Thermo Fisher Scientific,
Inc.) and 0.3% low-melting-point agarose (Amresco), and plated on
the bottom layer in a 35-mm plate containing 0.6% agarose; the
experiment was performed in triplicate. After 12 days of culture,
the colonies were stained with 0.005% crystal violet solution for
30 min at room temperature (Sigma-Aldrich; Merck KGaA) and
counted.
Western blot analysis
For western blot analysis in cultured cells, NSCLC
and CML cells were treated with the indicated protocols, then
harvested and lysed in RIPA buffer containing 50 mM Tris (pH 7.4),
150 mM NaCl, 0.1% sodium dodecyl sulfate, 1% sodium deoxycholate,
1% Triton X-100, 1 mM EDTA, 1 mM Na3VO4, 1 mM
NaF, 1 mM PMSF and protease inhibitor cocktail. For western blot
analysis in tissue specimens, frozen tissues were ground in liquid
nitrogen-cooled mortar, tissue powder was lysed on ice in RIPA
buffer. The lysates were centrifuged at 12,000 x g for 10 min at
4˚C, the supernatant was dissolved with 5X sample loading buffer
containing 250 mM Tris-HCl (pH 6.8), 10% sodium dodecyl sulfate,
50% glycerol, 2.5% bromophenol blue, and 5% β-mercaptoethanol, and
boiled for 5 min. Equivalent amounts of protein (30 µg/lane) were
separated by 10-15% sodium dodecyl sulfate polyacrylamide gel
electrophoresis and transferred to nitrocellulose membranes. The
membranes were blocked with 5% non-fat milk and incubated with the
indicated primary and corresponding secondary antibodies, i.e.,
horseradish peroxidase-conjugated anti-mouse (cat. no. 115-035-003,
Jackson ImmunoResearch Laboratories, Inc.; 1:10,000) or anti-rabbit
(cat. no. 111-035-003, Jackson ImmunoResearch Laboratories, Inc.;
1:10,000). Immunoreactive bands were visualized by using
Luminescent Image Analyzer LSA 4000 (GE Healthcare). Densitometry
analysis was performed using ImageJ software (version 1.4.3.67;
National Institutes of Health).
Reverse transcription‑quantitative PCR
(RT‑qPCR) analysis
Total RNA was prepared with TRIzol reagent
(Invitrogen; Thermo Fisher Scientific, Inc.) according to the
manufacturer's instructions. RT was carried out using a First
Strand cDNA Synthesis kit (Fermentas; Thermo Fisher Scientific,
Inc.). The RT steps were as follows: 25˚C for 10 min, 42˚C for 60
min and 70˚C for 10 min. qPCR was performed in CFX™96 Real Time
System (Bio-Rad Laboratories, Inc.) using SYBR Premix Ex Taq™
(Takara Biotechnology, Inc.) according to the manufacturer's
protocol. The thermocycling conditions of the qPCR step were as
follows: 95˚C for 5 min followed by 40 cycles of 95˚C for 15 sec
and 60˚C for 30 sec. The primer sequences were as follows: Human
GAPDH, forward 5′-GGAGCGAGA TCCCTCCAAAA-3′ and reverse
5′-GGCTGTTGTCATACT TCTCATGG-3′; human MSI2, forward
5′-GTTATCTGCGAA CACAGTAGTG-3′ and reverse 5′-ACCCTCTGTGCCTGT
TGGTAG-3′.
Molecular docking analysis
The structure of largazole was prepared by
ChemBioDraw Ultra 14.0 (PerkinElmer, Inc.) and was converted to 3D
model via the Ligprep module in Schrödinger Maestro 10.5
(Schrödinger LLC). We performed docking studies with a reported
human MSI2 structure (28). The
docking procedure was performed by glide docking in Maestro
with a precision level of SP (Standard Precision). The docking
results were analyzed by Biovia Discovery Studio 2016 client
(Dassault Systèmes Biovia Cc).
Statistical analysis
The results are expressed as mean ± standard
deviation. Two-tailed Student's t-test was used to determine the
statistical significance between two groups. One-way ANOVA with
Dunnett's or Bonferroni's post hoc test was used for the comparison
of multiple groups. P<0.05 was considered to indicate
statistically significant differences.
Results
MSI2 is upregulated in lung cancer and is
inversely associated with prognosis
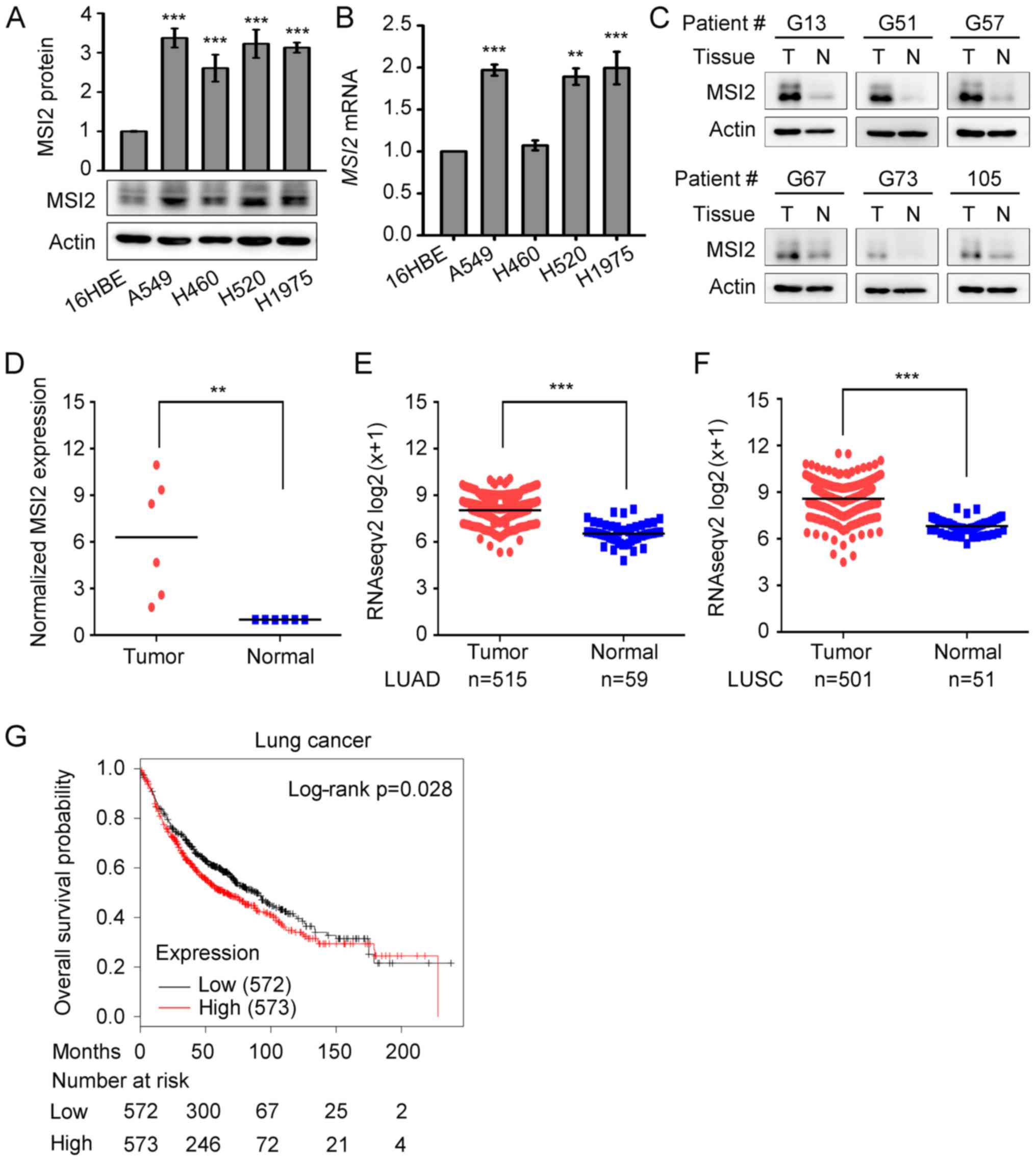
To assess the MSI2 expression in lung cancer, the
expression levels of MSI2 in normal human lung epithelial 16HBE
cells and four lung cancer cell lines (A549, H460, H520 and H1975)
were first analyzed by western blotting and qPCR. Compared with the
normal lung epithelial 16HBE cells, the lung cancer cell lines
exhibited elevated protein and mRNA levels of MSI2 (Fig. 1A and B). The expression of MSI2 was
further examined in lung cancer tissues from 6 patients with NSCLC.
As shown in Fig. 1C and D, the
protein levels of MSI2 were significantly increased in lung cancer
tissues compared with those in adjacent normal lung tissues. Since
the sample size of this study was small, we explored the expression
levels of MSI2 in TCGA level 3 IlluminaHiseq RNAseqV2
tran-scriptome database containing 1,053 NSCLC tumor samples and
109 normal lung specimens. This analysis revealed that MSI2
expression was significantly higher in LUADs (n=515) and LUSCs
(n=501) compared with that in normal lung tissues (Fig. 1E and F). To further investigate the
correlation between MSI2 expression and the survival of lung
cancer patients, Kaplan-Meier analysis was performed using the
online cancer survival analysis database (http://kmplot.com/analysis/) (30). As shown in Fig. 1G, lung cancer patients with higher
expression of MSI2 had poorer overall survival (OS) compared
with those with lower expression of MSI2. The data mentioned
above suggest that MSI2 is involved in the pathogenesis of lung
cancer and may be a useful therapeutic target.
MSI2 is targeted by the small compound
largazole
Largazole is a natural macrocyclic depsipeptide
isolated from the marine cyanobacterium Symploca sp.
(33). Our team has successfully
synthesized this compound, as well as some analogues, and
demonstrated its potent antitumor activity in certain cancers
(including lung cancer) at 0.01-1 µM (31,34).
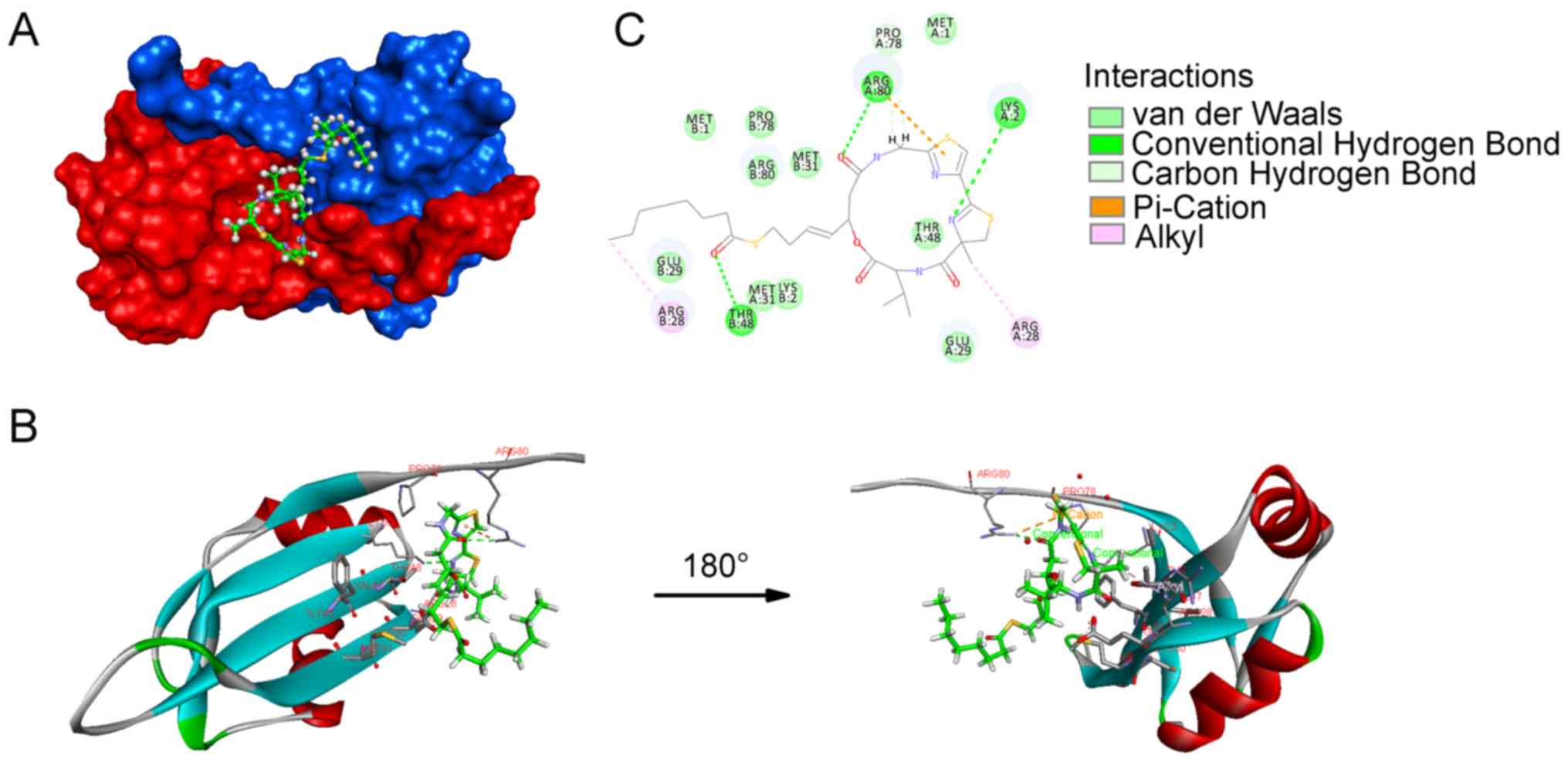
By molecular docking analysis, we herein demonstrated that
largazole was able to bind to MSI2. A crystal structure of the apo
human MSI2 RNA-recognition motif 1 (RRM1) at 1.7 Å resolution was
recently obtained (28). Docking
studies with this structure were performed and it was reported that
largazole can be docked into the dimer structure of MSI2 with most
of the compound interacting with one of the MSI2 chains in 6DBP
(chain A, red), while the thioester residue falls into the cave
formed by residues in chain B (blue; Fig. 2A). The backbone part of largazole
mainly interacts with the β-sheets (Arg28-Met31, Phe46-Phe49) of
MSI2 (Fig. 2B). As shown in
Fig. 2B and C, the valine residue
forms an alkyl-alkyl bond with the side chain of Glu29. The methyl
group attached at 4,5-dihydrothia-zole forms an alkyl bond with
Arg28. A hydrogen bond forms between Lys2 and nitrogen in
4,5-dihydrothiazole. As regards the thiazole ring, a pi-cation
interaction forms with the side chain of Arg80. Furthermore, the
guanidine group of Arg80 forms a hydrogen bond with the carbonyl
group of the HoSer residue. The predicted binding energy of this
docking model is -30.2752 kcal/mol.
Largazole inhibits the expression of
MSI2
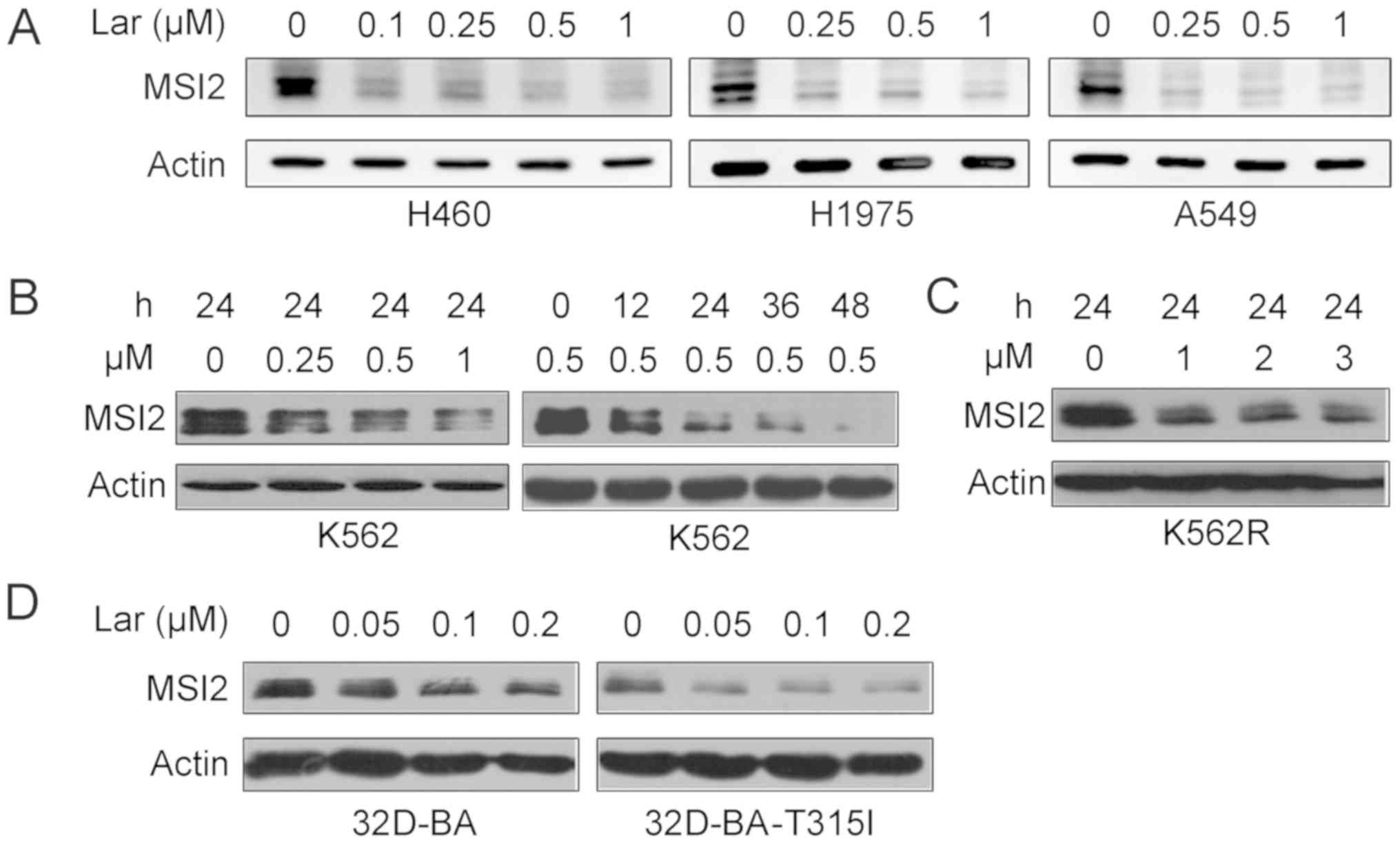
The data presented above prompted us to determine
whether largazole inhibits the expression of MSI2. Western blot
analysis demonstrated that treatment with indicated concentrations
of largazole for 24 h drastically inhibited the expression of MSI2
in human lung cancer H460, H1975 and A549 cells (Fig. 3A). MSI2 plays a critical role in
CML progression (13,14). The effects of this compound were
tested on CML cells, and it was found that largazole also repressed
the protein expression of MSI2 in K562 CML cells in a
concentration- and time-dependent manner (Fig. 3B). Largazole decreased the
expression of MSI2 in the K562R cell line, which is resistant to
the Bcr-Abl tyrosine kinase inhibitor imatinib (Fig. 3C). In a pair of 32Dcl3 murine cell
lines stably expressing wild-type or T315I mutant Bcr-Abl, namely
32D-BA and 32D-BA-T315I cells, treatment with largazole at 0.05-0.2
µM for 24 h inhibited MSI2 expression (Fig. 3D).
Largazole decreases MSI2 at the mRNA
level
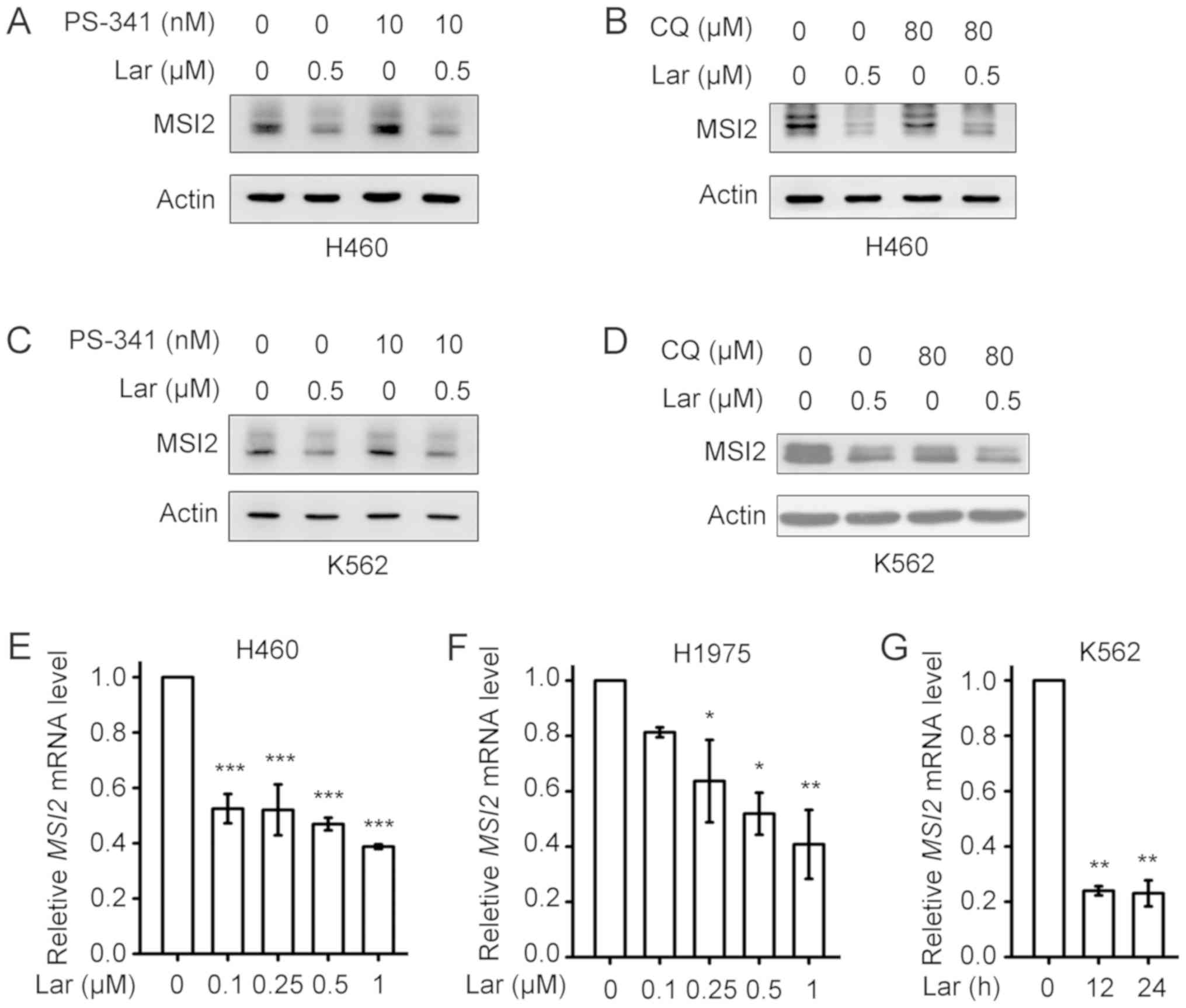
To further understand the mechanism underlying
largazole-induced downregulation of MSI2, the proteasome inhibitor
PS-341 and lysosome inhibitor CQ were used. As shown in Fig. 4A-D, pretreatment with PS-341 or CQ
could not block largazole-induced downregulation of MSI2 in H460
and K562 cells. Furthermore, treatment of H460 and H1975 cells with
largazole for 24 h also decreased the mRNA levels of MSI2 in
a concentration-dependent manner (Fig.
4E and F). Treatment of K562 cells with 0.5 µM largazole for
12-24 h markedly reduced the mRNA levels of MSI2 (Fig. 4G).
Largazole inhibits proliferation and
induces apoptosis of cancer cells
Our previous study has demonstrated that largazole
markedly inhibits the proliferation of multiple lung cancer cell
lines and induces apoptosis of lung cancer A549 cells (34). The present study demonstrated that
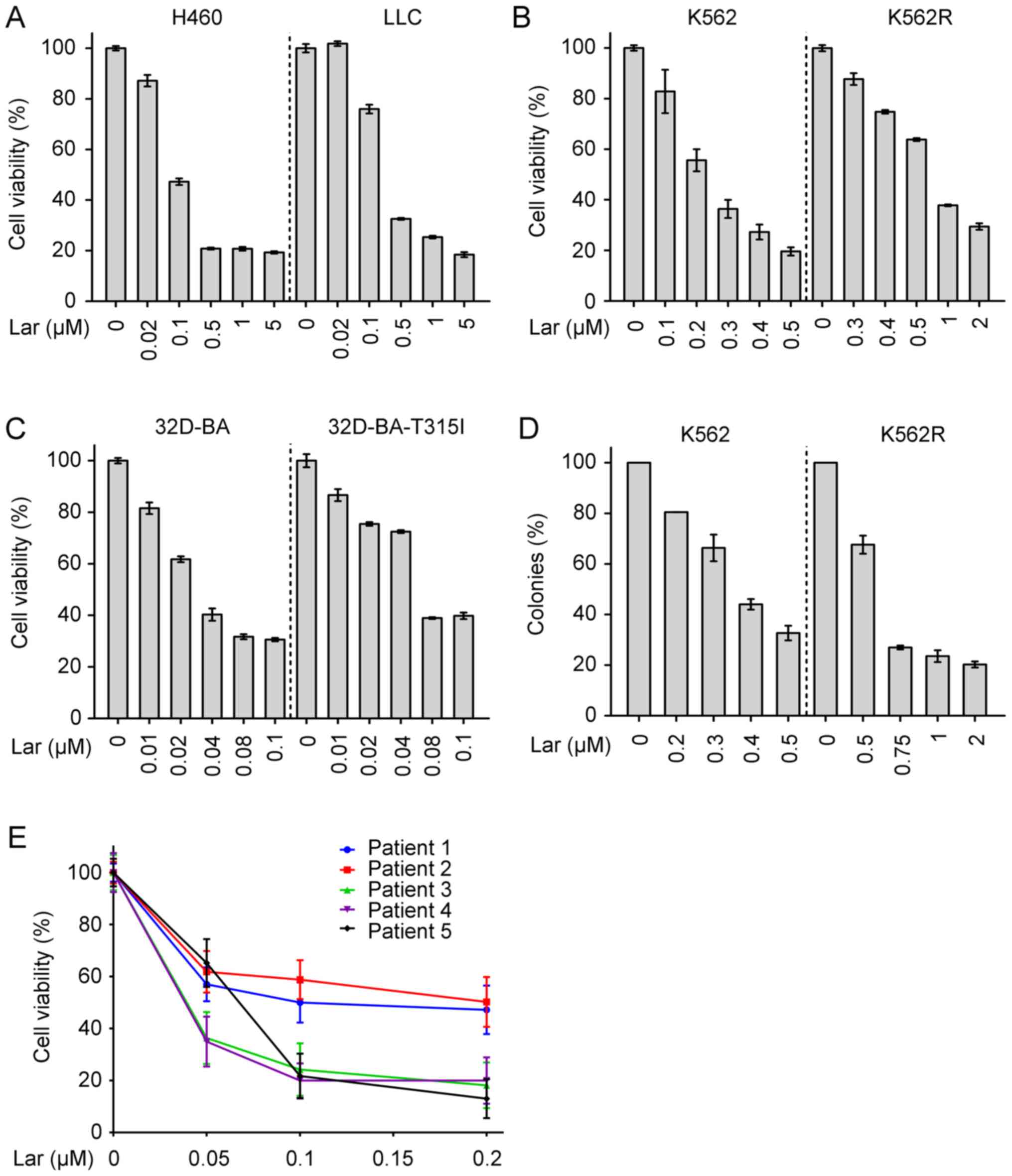
treatment with largazole significantly repressed the proliferation
of H460, LLC, K562, K562R, 32D-BA and 32D-BA-T315I cells in a
dose‑dependent manner (Fig. 5A‑C).
Largazole significantly suppressed the colony-forming ability of
K562 and K562R cells, as assessed by a soft agar colony formation
assay (Fig. 5D). Of note,
largazole markedly inhibited the proliferation of mononuclear cells
that were isolated from the bone marrow of 5 patients with CML
(Fig. 5E).
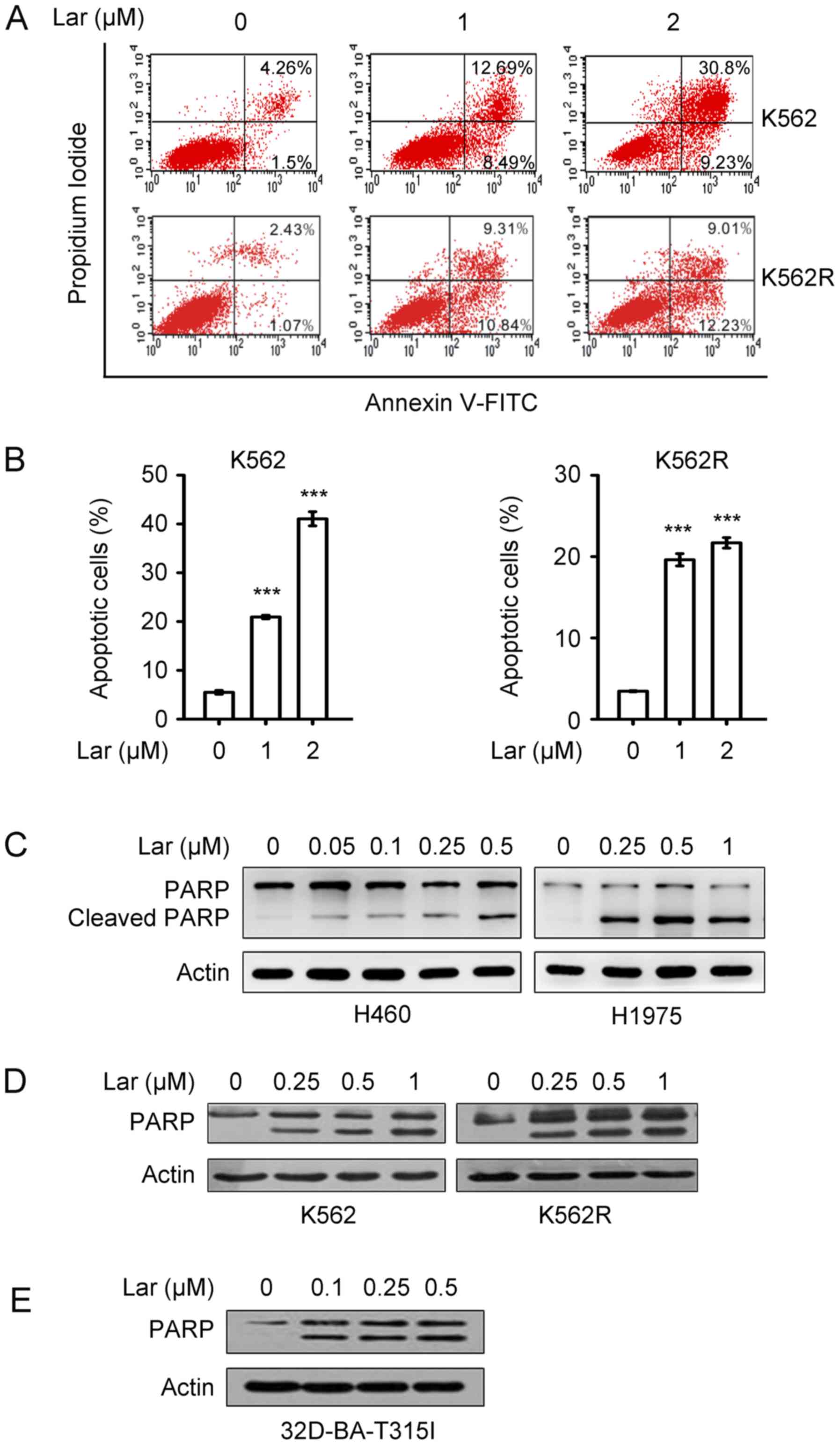
Largazole-induced apoptosis was investigated by
Annexin V-FITC/PI double staining, and the results demonstrated
that treatment of K562 and K562R cells with largazole at 1 and 2 µM
for 48 h significantly increased the percentage of Annexin
V+ apoptotic cells (Fig. 6A
and B). Western blot analysis revealed that largazole caused
the cleavage of poly(ADP-ribose) polymerase (PARP) in H460, H1975
(Fig. 6C), K562, K562R (Fig. 6D) and 32D-BA-T315I cells (Fig. 6E), indicating activation of the
apoptosis effector caspase-3.
Largazole inhibits the downstream mTOR
signaling pathway of MSI2
One of the main oncogenic pathways downstream of
MSI2 is mTOR complex 1 (mTORC1), which becomes activated upon MSI2
binding to the tumor suppressor phosphatase and tensin homolog
(15,35). Accumulating evidence indicates that
the mTOR signaling pathway plays a key role in regulating cell
metabolism, growth, proliferation and survival (36,37).
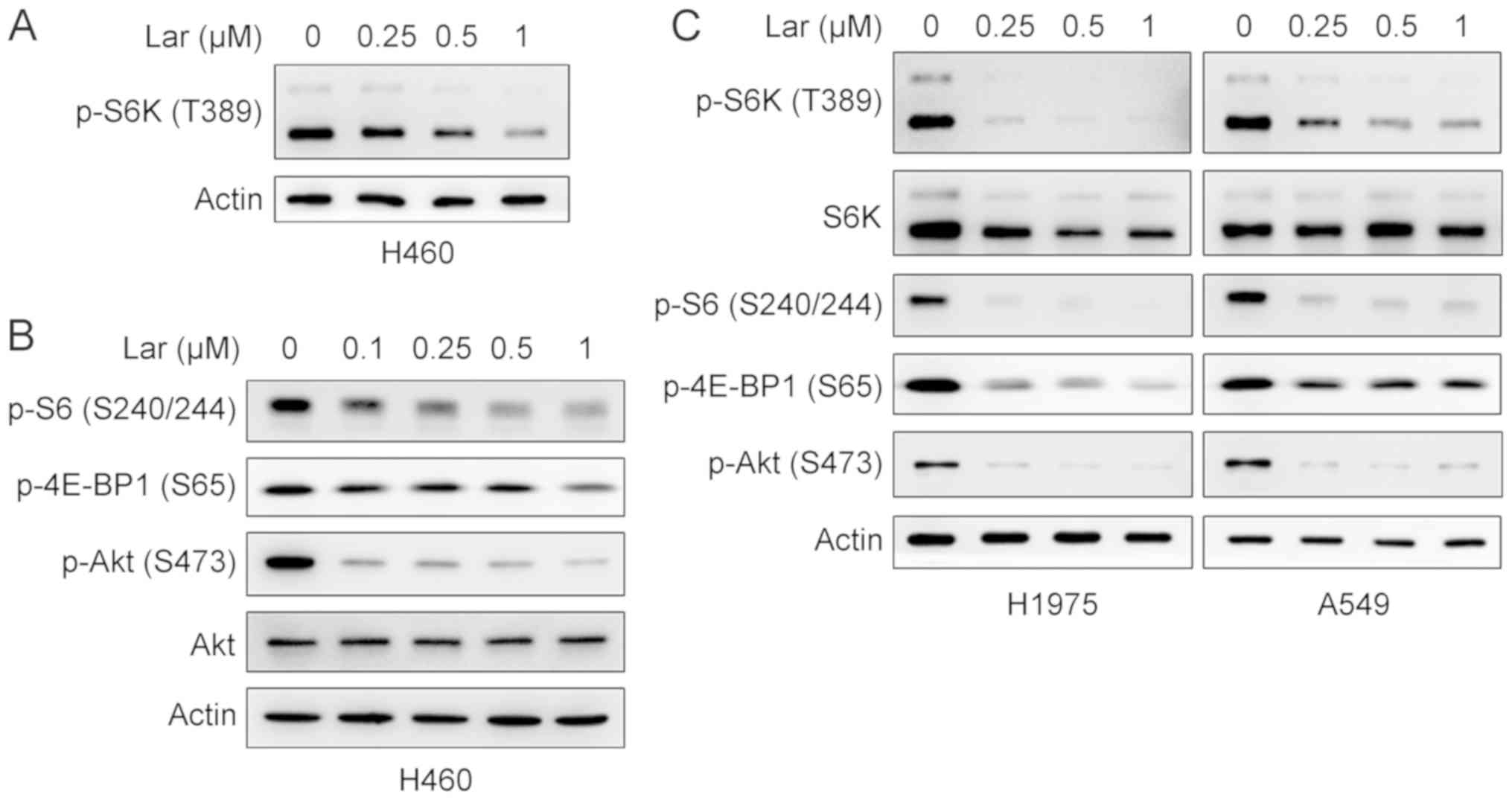
The effects of largazole on the mTOR signaling pathway were
examined. Western blot analysis demonstrated that largazole
repressed the phosphorylation of S6K and 4E-BP1, the two best
characterized downstream effector molecules of mTORC1 (38), in a concentration-dependent manner
in H460, H1975 and A549 cells (Fig.
7A-C). Largazole also inhibited the phosphorylation of S6, a
substrate of S6K (Fig. 7B and C).
These results indicated that largazole suppresses mTORC1 signaling.
mTOR forms two structurally and functionally distinct multi-protein
complexes, mTORC1 and mTORC2 (39). mTORC2 phosphorylates Akt on S473
(40). It was then investigated
whether largazole exerted an inhibitory effect on the mTORC2
signaling pathway. In all tested cells, largazole markedly
suppressed the phosphorylation of Akt (S473), indicating that
largazole inhibits mTORC2 signaling (Fig. 7B and C). The aforementioned results
suggest that largazole represses cell proliferation, at least in
part, by targeting MSI2 to suppress mTOR signaling pathway.
Discussion
Accumulating evidence indicates that MSI2 plays a
key role in cell proliferation, cancer stemness,
epithelial-to-mesenchymal transition, invasion, migration and drug
resistance in multiple types of cancer (12,41).
The MSI2 level is increased in various types of tumors, and its
overexpression is closely associated with aggressive
characteristics and poor prognosis (12-14,20).
We herein demonstrated that the protein levels of MSI2 were
significantly increased in lung cancer tissues from 6 Chinese
patients compared with those in adjacent normal lung tissues
(Fig. 1C and D). An
immunohistochemistry analysis of matched NSCLC specimens containing
normal lung tissue, primary tumor and tumor-positive lymph nodes
from 14 American patients demonstrated a significant increase of
MSI2 levels in the primary tumor (2.4-fold) and in the lymph nodes
(4.5-fold) compared with normal lung tissue (16). At the mRNA level, the expression of
MSI2 was inversely associated with clinical outcome
(Fig. 1G). These data suggest a
crucial role of MSI2 in lung carcinogenesis.
The key role of MSI2 in diverse cancers has prompted
an attempt to develop small-molecule inhibitors of this protein.
Using molecular docking analysis, largazole was identified as a
novel compound that was able to bind to human MSI2 (Fig. 2). The homology between human MSI2
and mus musculus MSI2 is 94.22%, while the key binding sites of
human MSI2 for largazole are identical to those in mouse MSI2,
suggesting that largazole may also bind and inhibit mus
musculus MSI2. Furthermore, the homology between human MSI2 and
MSI1 is 75% (12), and the amino
acids of the key sites displayed by the docking analysis are also
found in MSI1; thus, largazole may also bind MSI1. This possibility
warrants further investigation. Largazole drastically decreased the
protein levels of MSI2 in multiple types of cancer cells, including
H460, H1975, A549, K562 and K562R, among others (Fig. 3), indicating that largazole-induced
downregulation of MSI2 is not a cell type-dependent event. It has
been reported that MSI2 directly interacts with the tumor
suppressor deleted in breast cancer-2, and this interaction
promotes polyubiquitination-mediated proteasomal degradation of
MSI2 in breast cancer (42).
Ubiquitin‑specific protease 10 can interact with MSI2 and regulate
MSI2 stability via deubiquitination in colon cancer (43). MSI2 is a direct transcriptional
target of Kruppel‑like factor 4, a zinc finger transcription
factor, in pancreatic ductal adenocarcinomas (20). Transcription factors USF2 and PLAG1
bind the promoter of MSI2 and promote its transcription thus
collectively playing a key role in hematopoietic stem and
progenitor cell function (44).
These findings suggest that the MSI2 protein level may be regulated
at the transcriptional and/or post-translational level. Of note,
pretreatment with the proteasome inhibitor PS-341 or the lysosome
inhibitor CQ were unable to block largazole-induced downregulation
of MSI2 in H460 and K562 cells (Fig.
4A-D). Moreover, largazole markedly reduced the mRNA levels of
MSI2 in H460, H1975 and K562 cells (Fig. 4E-G). The results mentioned above
suggest that largazole did not induce protein degradation of MSI2
by the ubiquitin-proteasome pathway or by the lysosome-dependent
pathway, but markedly reduced MSI2 mRNA. Largazole may also
inhibit MSI2 function by direct binding via hydrogen bonds, thereby
inhibiting interactions between MSI2 and its cofactors. This
possibility should be tested in future studies.
Although the targets of the MSI2 protein have not
been fully identified, studies have shown that MSI2 regulates
numerous oncogenic pathways involved in cell cycle progression,
proliferation and metabolism, among others (12,45).
For example, MSI2 was found to provide essential support for
transforming growth factor-β signaling and inhibit tight
junction-associated claudins to promote NSCLC metastasis (16). MSI2 has been shown to act as an
oncoprotein through mTORC1 activation in colorectal cancer
(15,35). MSI2 promotes invasion and migration
through activation of the JAK2/STAT3 signaling pathway in bladder
cancer (23). Thus, it was
inferred that blocking MSI2 function with small-molecule inhibitors
may be of therapeutic value in various malignancies. Indeed, the
MSI2-targeting compound largazole significantly suppressed the
proliferation of cancer cells and induced apoptosis (Figs. 5A-D and 6). Of note, largazole markedly inhibited
the proliferation of mononuclear cells that were isolated from the
bone marrow of 5 patients with CML (Fig. 5E). Largazole exerted potent
growth-inhibitory effects in multiple cancer cell models, whereas
non-transformed epithelial cells survived at higher doses (34,46,47).
In a human colon cancer HCT116 xenograft mouse model, largazole
significantly inhibited tumor growth with no obvious toxicity
(29,48), indicating that largazole has high
bioavailability and low toxicity. Further studies demonstrated that
largazole drastically suppressed mTORC1, which was found to be
downstream of MSI2, and repressed mTORC2 signaling in NSCLC cell
lines (Fig. 7). Taken together,
these findings suggest that largazole inhibits cell proliferation,
at least in part, by targeting MSI2 to suppress mTOR signaling, and
may hold promise as a candidate for cancer therapy.
Funding
The present study was supported by grants from the
National Key Research and Development Program of China (no.
2016YFC0905501), the National Natural Science Funds for
Distinguished Young Scientists (no. 81425025), the Key Project of
the National Natural Science Foundation of China (no. 81830093),
the CAMS Innovation Fund for Medical Sciences (CIFMS; no.
2019-I2M-1-003), and the National Natural Science Foundation of
China (nos. 81672765 and 81802796). The study sponsors had no role
in the design of the study, the data collection, analysis, or
interpretation, the writing of the article, or the decision to
submit for publication.
Availability of data and materials
The data generated or analyzed during the present
study are included in this published article or are available from
the corresponding author on reasonable request.
Authors' contributions
The project was conceived and designed by GBZ. The
experiments were conducted by MW, XYS, KJZ and GZW. Biospecimens
were harvested/provided by YCZ, YCH and SJ. Data were analyzed by
GBZ, JL and YZL. The manuscript was written by GBZ. All the authors
have read and approved the final version of the manuscript.
Ethics approval and consent to
participate
The present study was approved by the Institutional
Review Board of the Institute of Zoology, Chinese Academy of
Sciences and the Third Affiliated Hospital of Kunming Medical
University; all tissue samples were obtained with written informed
consent from the patients or their families.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
The authors would like to thank Professor Warren
Pear of University of Pennsylvania for kindly providing the
MSCV-Bcr-Abl-T315I-IRES/GFP retroviral transducing vector, and
Professor Brian Druker of Oregon Health & Science University
for providing the pSRalpha plasmids containing the wild-type and
T315I mutant Bcr‑Abl.
References
|
1
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Herbst RS, Heymach JV and Lippman SM: Lung
cancer. N Engl J Med. 359:1367–1380. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Herbst RS, Morgensztern D and Boshoff C:
The biology and management of non-small cell lung cancer. Nature.
553:446–454. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hirsch FR, Scagliotti GV, Mulshine JL,
Kwon R, Curran WJ Jr, Wu YL and Paz-Ares L: Lung cancer: Current
therapies and new targeted treatments. Lancet. 389:299–311. 2017.
View Article : Google Scholar
|
|
5
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2019. CA Cancer J Clin. 69:7–34. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhou G: Tobacco, air pollution,
environmental carcinogenesis, and thoughts on conquering strategies
of lung cancer. Cancer Biol Med. 16:700–713. 2019.
|
|
7
|
Anji A and Kumari M: Guardian of Genetic
Messenger-RNA-Binding Proteins. Biomolecules. 6:42016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Pereira B, Billaud M and Almeida R:
RNA-Binding Proteins in Cancer: Old Players and New Actors. Trends
Cancer. 3:506–528. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Fox RG, Park FD, Koechlein CS, Kritzik M
and Reya T: Musashi signaling in stem cells and cancer. Annu Rev
Cell Dev Biol. 31:249–267. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lan L, Xing M, Douglas JT, Gao P, Hanzlik
RP and Xu L: Human oncoprotein Musashi-2 N-terminal RNA recognition
motif backbone assignment and identification of RNA‑binding pocket.
Oncotarget. 8:106587–106597. 2017. View Article : Google Scholar :
|
|
11
|
Liu Y, Fan Y, Wang X, Huang Z, Shi K and
Zhou B: Musashi-2 is a prognostic marker for the survival of
patients with cervical cancer. Oncol Lett. 15:5425–5432.
2018.PubMed/NCBI
|
|
12
|
Kudinov AE, Karanicolas J, Golemis EA and
Boumber Y: Musashi RNA-Binding Proteins as Cancer Drivers and Novel
Therapeutic Targets. Clin Cancer Res. 23:2143–2153. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ito T, Kwon HY, Zimdahl B, Congdon KL,
Blum J, Lento WE, Zhao C, Lagoo A, Gerrard G, Foroni L, et al:
Regulation of myeloid leukaemia by the cell-fate determinant
Musashi. Nature. 466:765–768. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kharas MG, Lengner CJ, Al-Shahrour F,
Bullinger L, Ball B, Zaidi S, Morgan K, Tam W, Paktinat M, Okabe R,
et al: Musashi-2 regulates normal hematopoiesis and promotes
aggressive myeloid leukemia. Nat Med. 16:903–908. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wang S, Li N, Yousefi M, Nakauka‑Ddamba A,
Li F, Parada K, Rao S, Minuesa G, Katz Y, Gregory BD, et al:
Transformation of the intestinal epithelium by the MSI2 RNA-binding
protein. Nat Commun. 6:65172015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kudinov AE, Deneka A, Nikonova AS, Beck
TN, Ahn YH, Liu X, Martinez CF, Schultz FA, Reynolds S, Yang DH, et
al: Musashi-2 (MSI2) supports TGF-β signaling and inhibits claudins
to promote non-small cell lung cancer (NSCLC) metastasis. Proc Natl
Acad Sci USA. 113:6955–6960. 2016. View Article : Google Scholar
|
|
17
|
Zhang MR, Xi S, Shukla V, Hong JA, Chen H,
Xiong Y, Ripley RT, Hoang CD and Schrump DS: The Pluripotency
Factor Musashi-2 Is a Novel Target for Lung Cancer Therapy. Ann Am
Thorac Soc. 15(Suppl 2): S1242018. View Article : Google Scholar
|
|
18
|
Kang MH, Jeong KJ, Kim WY, Lee HJ, Gong G,
Suh N, Győrffy B, Kim S, Jeong SY, Mills GB, et al: Musashi
RNA-binding protein 2 regulates estrogen receptor 1 function in
breast cancer. Oncogene. 36:1745–1752. 2017. View Article : Google Scholar
|
|
19
|
Dong P, Xiong Y, Hanley SJB, Yue J and
Watari H: Musashi-2, a novel oncoprotein promoting cervical cancer
cell growth and invasion, is negatively regulated by p53-induced
miR-143 and miR-107 activation. J Exp Clin Cancer Res. 36:1502017.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Guo K, Cui J, Quan M, Xie D, Jia Z, Wei D,
Wang L, Gao Y, Ma Q and Xie K: The Novel KLF4/MSI2 Signaling
Pathway Regulates Growth and Metastasis of Pancreatic Cancer. Clin
Cancer Res. 23:687–696. 2017. View Article : Google Scholar :
|
|
21
|
Li Z, Jin H, Mao G, Wu L and Guo Q: Msi2
plays a carcinogenic role in esophageal squamous cell carcinoma via
regulation of the Wnt/β-catenin and Hedgehog signaling pathways.
Exp Cell Res. 361:170–177. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
He L, Zhou X, Qu C, Hu L, Tang Y, Zhang Q,
Liang M and Hong J: Musashi2 predicts poor prognosis and invasion
in hepatocellular carcinoma by driving epithelial-mesenchymal
transition. J Cell Mol Med. 18:49–58. 2014. View Article : Google Scholar
|
|
23
|
Yang C, Zhang W, Wang L, Kazobinka G, Han
X, Li B and Hou T: Musashi-2 promotes migration and invasion in
bladder cancer via activation of the JAK2/STAT3 pathway. Lab
Invest. 96:950–958. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lee J, An S, Choi YM, Lee J, Ahn KJ, Lee
JH, Kim TJ, An IS and Bae S: Musashi-2 is a novel regulator of
paclitaxel sensitivity in ovarian cancer cells. Int J Oncol.
49:1945–1952. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhang H, Tan S, Wang J, Chen S, Quan J,
Xian J, Zhang S, He J and Zhang L: Musashi2 modulates K562 leukemic
cell proliferation and apoptosis involving the MAPK pathway. Exp
Cell Res. 320:119–127. 2014. View Article : Google Scholar
|
|
26
|
Han Y, Ye A, Zhang Y, Cai Z, Wang W, Sun
L, Jiang S, Wu J, Yu K and Zhang S: Musashi-2 Silencing Exerts
Potent Activity against Acute Myeloid Leukemia and Enhances
Chemosensitivity to Daunorubicin. PLoS One. 10:e01364842015.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lan L, Liu H, Smith AR, Appelman C, Yu J,
Larsen S, Marquez RT, Wu X, Liu FY, Gao P, et al: Natural product
derivative Gossypolone inhibits Musashi family of RNA-binding
proteins. BMC Cancer. 18:8092018. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Minuesa G, Albanese SK, Xie W, Kazansky Y,
Worroll D, Chow A, Schurer A, Park SM, Rotsides CZ, Taggart J, et
al: Small-molecule targeting of MUSASHI RNA-binding activity in
acute myeloid leukemia. Nat Commun. 10:26912019. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hong J and Luesch H: Largazole: From
discovery to broad-spectrum therapy. Nat Prod Rep. 29:449–456.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Győrffy B, Surowiak P, Budczies J and
Lánczky A: Online survival analysis software to assess the
prognostic value of biomarkers using transcriptomic data in
non-small-cell lung cancer. PLoS One. 8:e822412013. View Article : Google Scholar
|
|
31
|
Zeng X, Yin B, Hu Z, Liao C, Liu J, Li S,
Li Z, Nicklaus MC, Zhou G and Jiang S: Total synthesis and
biological evaluation of largazole and derivatives with promising
selectivity for cancers cells. Org Lett. 12:1368–1371. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Hu Z, Pan XF, Wu FQ, Ma LY, Liu DP, Liu Y,
Feng TT, Meng FY, Liu XL, Jiang QL, et al: Synergy between
proteasome inhibitors and imatinib mesylate in chronic myeloid
leukemia. PLoS One. 4:e62572009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Taori K, Paul VJ and Luesch H: Structure
and activity of largazole, a potent antiproliferative agent from
the Floridian marine cyanobacterium Symploca sp. J Am Chem Soc.
130:1806–1807. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wu LC, Wen ZS, Qiu YT, Chen XQ, Chen HB,
Wei MM, Liu Z, Jiang S and Zhou GB: Largazole Arrests Cell Cycle at
G1 Phase and Triggers Proteasomal Degradation of E2F1 in Lung
Cancer Cells. ACS Med Chem Lett. 4:921–926. 2013. View Article : Google Scholar
|
|
35
|
Li N, Yousefi M, Nakauka-Ddamba A, Li F,
Vandivier L, Parada K, Woo DH, Wang S, Naqvi AS, Rao S, et al: The
Msi Family of RNA-Binding Proteins Function Redundantly as
Intestinal Oncoproteins. Cell Rep. 13:2440–2455. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Laplante M and Sabatini DM: mTOR signaling
in growth control and disease. Cell. 149:274–293. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Saxton RA and Sabatini DM: mTOR Signaling
in Growth, Metabolism, and Disease. Cell. 169:361–371. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Shimobayashi M and Hall MN: Making new
contacts: The mTOR network in metabolism and signalling crosstalk.
Nat Rev Mol Cell Biol. 15:155–162. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Zoncu R, Efeyan A and Sabatini DM: mTOR:
From growth signal integration to cancer, diabetes and ageing. Nat
Rev Mol Cell Biol. 12:21–35. 2011. View Article : Google Scholar
|
|
40
|
Sarbassov DD, Guertin DA, Ali SM and
Sabatini DM: Phosphorylation and regulation of Akt/PKB by the
rictor-mTOR complex. Science. 307:1098–1101. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Fang T, Lv H, Wu F, Wang C, Li T, Lv G,
Tang L, Guo L, Tang S, Cao D, et al: Musashi 2 contributes to the
stemness and chemore-sistance of liver cancer stem cells via LIN28A
activation. Cancer Lett. 384:50–59. 2017. View Article : Google Scholar
|
|
42
|
Choi YM, Kim KB, Lee JH, Chun YK, An IS,
An S and Bae S: DBC2/RhoBTB2 functions as a tumor suppressor
protein via Musashi-2 ubiquitination in breast cancer. Oncogene.
36:2802–2812. 2017. View Article : Google Scholar :
|
|
43
|
Ouyang SW, Liu TT, Liu XS, Zhu FX, Zhu FM,
Liu XN and Peng ZH: USP10 regulates Musashi-2 stability via
deubiquiti-nation and promotes tumour proliferation in colon
cancer. FEBS Lett. 593:406–413. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Belew MS, Bhatia S, Keyvani Chahi A,
Rentas S, Draper JS and Hope KJ: PLAG1 and USF2 Co-regulate
Expression of Musashi-2 in Human Hematopoietic Stem and Progenitor
Cells. Stem Cell Reports. 10:1384–1397. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Minuesa G, Antczak C, Shum D, Radu C,
Bhinder B, Li Y, Djaballah H and Kharas MG: A 1536‑well
fluorescence polarization assay to screen for modulators of the
MUSASHI family of RNA-binding proteins. Comb Chem High Throughput
Screen. 17:596–609. 2014. View Article : Google Scholar
|
|
46
|
Schnekenburger M, Dicato M and Diederich
M: Epigenetic modulators from "The Big Blue": A treasure to fight
against cancer. Cancer Lett. 351:182–197. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Pilon JL, Clausen DJ, Hansen RJ, Lunghofer
PJ, Charles B, Rose BJ, Thamm DH, Gustafson DL, Bradner JE and
Williams RM: Comparative pharmacokinetic properties and antitumor
activity of the marine HDACi Largazole and Largazole peptide
isostere. Cancer Chemother Pharmacol. 75:671–682. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Liu Y, Salvador LA, Byeon S, Ying Y, Kwan
JC, Law BK, Hong J and Luesch H: Anticolon cancer activity of
largazole, a marine-derived tunable histone deacetylase inhibitor.
J Pharmacol Exp Ther. 335:351–361. 2010. View Article : Google Scholar : PubMed/NCBI
|