Introduction
Melanoma originates from melanocytes and is the most
aggressive type of skin cancer, accounting for the majority of skin
cancer-related deaths, despite only accounting for 1-2% of all skin
cancers (1). Melanomagenesis has
been attributed to melanocytic nevi (2), genetic factors (3) and ultraviolet light exposure
(4), although the underlying
molecular mechanisms have yet to be fully elucidated.
Uncoupling proteins (UCPs) are anion carriers
located in the mitochondrial inner membrane, where they facilitate
anions crossing the inner membrane, thereby allowing protons back
to the matrix and reducing the mitochondrial membrane potential
(5). In humans, the UCP family
includes five members: UCP1 is mainly expressed in brown adipose
tissue (6), UCP2 is ubiquitously
expressed (7), UCP3 is mainly
expressed in the heart and skeletal muscle (8), and UCP4 and UCP5 are only expressed
in the brain (9,10). The UCP2 protein has been studied in
human diseases and was found to be involved in diabetes,
cardioprotection, neuroprotection, carcinogenesis, and the immune
response (5). The tumor-promoting
effect of UCP2 is attributed to its regulation of the cellular
redox status, which allows it to promote cancer cell growth
(7) and a metabolic shift from
oxidative phosphor-ylation to glycolysis and glutaminolysis
(7,11). For example, an altered cellular
redox status can affect redox-sensitive kinase signaling, with UCP2
deficiency in progenitor cells decreasing cell proliferation via
inactivation of mitogen-activated protein kinase
(MAPK)/extracellular signal-regulated kinase (ERK) signaling
(12). Furthermore, inhibition of
UCP2 in human pancreatic cancer cells causes an increase in
reactive oxygen species (ROS), which activates the protein kinase B
(Akt)/mammalian target of rapamycin (mTOR) pathway (13).
In our previous study using UCP2 knockout mice, UCP2
was found to promote chemically induced skin carcinogenesis in
vivo (14). In the JB6 P+ skin
cell transformation model, over-expression of UCP2 promoted
glycolytic flux by activating the Akt pathway (15) and enhanced skin cell transformation
by activating PLC-γ1 signaling (16). However, it remains unclear whether
UCP2 plays a role in melanoma, which is the deadliest type of skin
cancer. Therefore, the aim of the present study was to evaluate
whether inhibition of UCP2 could be useful for treating melanoma
and, to the best of our knowledge, it is the first study to compare
UCP2 expression in specimens from normal skin, compound nevus and
melanoma. The hypothesis was that UCP2 would be relatively highly
expressed in melanoma tissues and that its levels would be
negatively associated with the patient's prognosis. In addition,
human melanoma cells with stable knockdown of UCP2 were generated
in order to investigate its potential mechanism(s) of action.
Materials and methods
Patients and tissue samples
The protocol of this retrospective study was
approved by the Institutional Review Board of China-Japan Union
Hospital, Jilin University. Specimens were collected from 81
consecutive patients who were diagnosed with skin and mucosal
melanoma at the Department of Pathology (China-Japan Union
Hospital) between September 2016 and December 2018. Informed
consent was obtained from patients at the time of sample
collection. The diagnosis had been established based on
pathological examination following complete surgical excision of
the lesion. The eligibility criteria included i) age 18-80 years
and ii) primary skin lesion without a history of radiotherapy or
chemotherapy. The exclusion criteria included overweight status
(body mass index >25 kg/m2), diabetes, and
concomitant tumors, as elevated UCP2 expression may be associated
with these conditions. Based on these criteria, the present study
included 65 patients (33 men and 32 women) with cutaneous (n=52)
and mucosal (n=13) melanoma. For comparison, control skin tissues
were obtained from 49 healthy individuals who had undergone
cosmetic surgery (control group) and surgical specimens were also
collected from 51 healthy individuals who had undergone excision of
compound nevus (compound nevus group). The clinical characteristics
of the patients are summarized in Tables I and II.
 | Table IImmunohistochemical expression of
UCP2 in normal skin tissue, compound nevus tissue and skin mucosal
melanoma tissues. |
Table I
Immunohistochemical expression of
UCP2 in normal skin tissue, compound nevus tissue and skin mucosal
melanoma tissues.
| Tissue | n | UCP2 expression (n)
| Positivity rate
(%) |
|---|
| Positive (n) | Negative (n) |
|---|
| Normal control | 49 | 1 | 48 | 2.0 |
| Compound nevus | 51 | 3 | 48 | 5.9 |
| Melanoma | 65 | 50 | 15 | 76.9 |
 | Table IIAssociation between UCP2 expression
and the clinicopathological characteristics of skin melanoma. |
Table II
Association between UCP2 expression
and the clinicopathological characteristics of skin melanoma.
|
Characteristics | n | UCP2 expression (n)
| χ2 | P-value |
|---|
| Negative | Mild | Strong |
|---|
| Clark level | | | | | 16.50 | <0.001 |
| I | 1 | 1 | 0 | 0 | | |
| II | 8 | 5 | | 3 | 0 | |
| III | 7 | 2 | 5 | 0 | | |
| IV | 18 | 3 | 10 | 5 | | |
| V | 18 | 1 | 8 | 9 | | |
| Lymphocyte
infiltration | | | | | 3.5687 | NS |
| No | 14 | 2 | 8 | 4 | | |
| Yes | 29 | 6 | 14 | 9 | | |
| Active | 9 | 4 | 4 | 1 | | |
| Breslow thickness,
cm | | | | | 7.5038 | 0.0235 |
| <0.5 | 25 | 9 | 11 | 5 | | |
| 0.5-1.0 | 26 | 2 | 15 | 9 | | |
| >1.0 | 1 | 1 | 0 | 0 | | |
| Ulceration | | | | | 3.6254 | NS |
| Yes | 33 | 9 | 18 | 6 | | |
| No | 19 | 3 | 8 | 8 | | |
Cell culture and reagents
Human melanoma A375 cells were purchased from the
American Type Culture Collection (CRL-1619). Human melanoma
SK-Mel-28 cells were kindly provided by Dr Stephan Witt from our
institution (originally purchased from the American Type Culture
Collection). A375 cells are more aggressive compared with SK-Mel-28
cells (17). The cells were grown
in RPMI-1640 medium supplemented with 10% fetal bovine serum
(Atlanta Biologicals, Inc.) and 1 mM sodium pyruvate, which was
maintained at 37̊C in a humidified incubator (95% air and 5%
CO2). Mycoplasma testing was routinely performed for the
cell lines.
UCP2 shRNA lentivirus (LVPi026640) and scramble
shRNA lentivirus (LVP015G) were purchased from Applied Biological
Materials. Both vectors contained a green fluorescent protein (GFP)
tag. The target sequences were as follows: 5′-CGG TTA CAG ATC CAA
GGA GAA-3′, 5′-GGC CTG TA TGA TTC TGT CA-3′, 5′-GCA CCG TCA ATG CCT
ACA A-3′ and 5′-CGT GGT CAA GAC GAG ATA CAT GAA CTC TG-3′. JC-1 dye
was purchased from Cayman Chemicals (cat. no. 15003).
Antibodies and reagents
All primary antibodies were diluted at a ratio of
1:1,000. Antibodies to β-actin (cat. no. sc-47778), ERK (cat. no.
sc-94), and phosphorylated ERK (p-ERK; cat. no. sc-7383) were
purchased from Santa Cruz Biotechnology, Inc. Antibodies to
p-4E-BP1 (cat. no. 13396), 4E-BP1 (cat. no. 9452), p-Akt (cat. no.
9275), Akt (cat. no. 2920), p-p70S6K (cat. no. 9205) and p70S6K
(cat. no. 9202) were purchased from Cell Signaling Technologies,
Inc.
Establishing the UCP2 KD melanoma
cells
A375 and SK-Mel-28 cells were seeded in 24-well
plates (20,000 cells/well). On the next day, a cell infection
mixture was prepared: 10 MOI viruses per 1 ml of culture medium
plus 2 µl of polybrene (4 µg/µl). The cell culture medium was then
removed and replaced with 500 µl of the cell infection mixture.
After 24 h, the infection mixture was replaced with fresh culture
medium and the cells were incubated at 37˚C for another 24 h before
the addition of medium with puromycin (1 µg/ml). Clonal selection
lasted for 12 days with the puro-mycin-containing medium replaced
once every 3 days. The scramble shRNA-infected clones were enriched
via sorting of GFP‑positive cells using flow cytometry and
collected in bulk, whereas the UCP2 knockdown (KD) clones were
collected as single cells via GFP sorting using flow cytometry. The
UCP2 KD clones were expanded and western blot analysis was
performed for selection.
MTT assay
The melanoma cells were seeded in 96-well plates
(6,000 cells/well) and cultured at 37˚C overnight. The cells were
then treated using various cisplatin concentrations (15, 5, 1.67,
0.56, 0.19 and 0 µM). Cisplatin has been approved for the treatment
of metastatic melanoma in the U.S. (18). Cisplatin (cat. no. 1134357,
Sigma-Aldrich; Merck KGaA) was dissolved in phosphate‑buffered
saline (PBS). After incubation at 37˚C for 48 h, cell viability was
determined using the MTT assay (M5655; Sigma-Aldrich; Merck KGaA),
with 10% MTT diluted in serum-free medium added to each well before
a 4-h incubation at 37˚C. The MTT solutions were then replaced with
dimethyl sulfoxide and the plates were shaken for 15 min at room
temperature. The absorbance at 595 nm was then measured using a
96-well plater reader (Bio-Rad Laboratories, Inc.). All experiments
were repeated at least three times.
Detection of mitochondrial membrane
potential based on JC‑1 staining
A total of 10,000 melanoma cells were seeded in
96-well plates. On the next day, the culture medium was replaced
with fresh medium containing the JC-1 dye (2 µg/ml) and incubated
at 37˚C for 30 min. The medium was then removed and the cells were
washed once using PBS. The fluorescence intensities were measured
immediately using a fluorescence spectrophotometer (JC‑1 green:
Ex=485 nm, Em=525 nm; JC-1 red: Ex=535 nm, Em=590 nm), and the
ratio of JC-1 red vs. JC-1 green was used to evaluate the
mitochondrial membrane potential. All experiments were repeated at
least three times.
Detection of hydrogen peroxide
levels
The Amplex Red Hydrogen Peroxide/Peroxidase Assay
Kit (A22188, Molecular Probes; Thermo Fisher Scientific, Inc.) was
used to measure intracellular hydrogen peroxide
(H2O2) levels based on the manufacturer's
instructions. The melanoma cells were grown in p100 dishes and
collected via centrifugation (600 x g for 5 min) at room
temperature. The cell pellets were then suspended in 400 µl PBS
containing proteinase inhibitors (cat. no. sc-29130, Santa Cruz
Biotechnology, Inc.). The cells were then sonicated and the lysates
collected via centrifugation at 4˚C (12,800 x g for 30 min).
Freshly prepared cell lysates were filtered through 10K cut‑off
columns (82031‑348, VWR) and the product's absorbance was measured
at 560 nm using a 96‑well plate reader. Background fluorescence was
corrected by subtracting the value derived from the
no-H2O2 control. All experiments were
repeated at least three times.
Detection of lactate and ATP levels
Freshly prepared cell lysates were prepared as for
the H2O2 assay and filtered through 10K
cut-off columns before being used for both assays. The Lactate
Colorimetric/Fluorometric Assay Kit (cat. no. K607-100, BioVision)
was used to evaluate intracellular lactate levels, based on the
absorbance at 570 nm measured using a 96-well plate reader. The ATP
Luminescence Detection Assay Kit (cat. no. 700410, Cayman
Chemicals) was used to evaluate intracellular ATP levels, based on
the luminescence measured using a BioTek Multi-Mode microplate
reader. All experiments were repeated at least three times.
Cell invasion assay
Invasion ability was evaluated using Transwell
inserts (cat. no. 3422, Corning, Inc.) that were coated with 50
µg/ml of Matrigel. A total of 50,000 melanoma cells were suspended
in serum-free medium and 100 µl of the cell suspension was
transferred into the inserts, whereas complete growth medium was
added to the bottom wells. The cells were then cultured for 12 h at
37˚C before the inserts were removed, fixed in 10% neutral‑buffered
formalin, and stained at room temperature using 0.5% crystal violet
solution. All experiments were repeated at least three times.
Spheroid growth assay
The liquid overlay technique was used to grow
melanoma spheroids (19). First,
96-well plates were coated with 50 µl of agar (1.25%) and 30 min
later 25,000 melanoma cells (200 µl) were added to the wells.
Spheroids were allowed to form and images were captured on the
following day. The volume of the spheroids was calculated as
follows: Volume=(4/3)π x b2 x c, where b is the longest
(semi-major) axis and c is the shortest (semi-minor) axis. All
experiments were repeated at least three times.
Western blot analysis
Whole-cell lysates were prepared using the RIPA
lysis buffer (cat. no. sc-24948A, Santa Cruz Biotechnology, Inc.)
and collected via centrifugation at 12,800 x g at 4˚C for 30 min.
Protein concentrations were determined using the Bradford method
and 50 µg of the samples were denatured and loaded onto a 10%
polyacryl-amide gel. After separation, the proteins were
transferred onto a polyvinylidene fluoride membrane, which was
blocked with 5% non-fat milk for 1 h followed by overnight
incubation with the primary antibody at 4˚C on a shaker. The
membrane was then washed three times using PBS/0.05% Tween 20 and
incubated with a horseradish peroxidase (HRP)-conjugated secondary
antibody (Jackson ImmunoResearch Laboratories, cat. no.
111-035-003, dilution 1:2,500) for 1 h. All bands were detected
using an ECL Western blot kit (Genesee, 20-302B). All experiments
were repeated at least three times.
Immunohistochemistry
Paraffin-embedded sections of normal skin, nevus, or
melanoma were dewaxed and gradually rehydrated before being
immersed in an EDTA solution (pH 8.0) and heated using a pressure
cooker for 2 min. After cooling to room temperature, the tissue
sections were rinsed with PBS and normal goat serum was used to
block non‑specific binding. The tissue sections were then incubated
with a mouse anti-human UCP2 monoclonal antibody (1:200 dilution)
overnight at 4̊C. After washing with PBS, the tissue sections were
incubated with an HRP-conjugated secondary antibody for 30 min at
room temperature. The Ultraview Red agent was added to distinguish
positive staining from skin pigmentation, and red particles
deposited in the cytoplasm were considered as a positive result.
Hematoxylin and eosin staining was performed separately to evaluate
the pathological characteristics.
Semi‑quantitative analysis of stained
tissue sections
The stained sections were evaluated via double-blind
scoring based on a slightly modified version of the procedure
described by Bosman et al (20). Five different fields were randomly
selected, and histochemical scores were calculated according to the
positive rate of tumor cells [P(i)] and the staining intensity
[S(i)] as follows:
In this equation, the P(i) is scored as 0 (no
positive cells), 1 (<10% positive cells), 2 (10-50% positive
cells), or 3 (>50% positive cells). The S(i) was scored as 0 (no
staining), 1 (light yellow staining), 2 (brownish yellow staining),
or 3 (red staining). The mean score for all 5 fields was
calculated, and the result was graded as negative (-, score 0-1),
mild (+, score 2‑3), or strong (++, score ≥4).
Statistical analysis
The statistical analyses were performed using the
χ2 test or analysis of variance as appropriate. One-way
analysis of variance followed by Tukey-Kramer adjustment was used
to examine differences between multiple groups. All statistical
analyses were performed using SPSS software, version 13.0 (SPSS
Inc.), and the results were considered statistically significant at
P‑values of <0.05.
Results
Associations between UCP2 expression and
clinical charac‑ teristics of melanoma
Immunohistochemistry was used to evaluate UCP2
expression in the tissue specimens from the melanoma group (n=65),
the compound nevus group (n=51) and the control group (n=49). As
shown in Fig. 1, the red particles
deposited in the cytoplasm indicate a positive result and the
quantified results are presented in Table I. The UCP2 positivity rates were
low in normal skin tissue (2.0%) and compound nevus tissue (5.9%),
but relatively high in melanoma tissue (76.9%).
It was also evaluated whether UCP2 expression was
correlated with Clark level and Breslow thickness (depth of
invasion), lymph node infiltration and presence of ulceration. As
summarized in Table II, UCP2
expression increased with the Clark level (P<0.001) and was
significantly correlated with Breslow thickness (P=0.0235), but was
not significantly correlated with lymph node infiltration status or
the presence of ulceration.
UCP2 KD suppresses melanoma cell growth
and induces cisplatin sensitivity
As UCP2 was more highly expressed in melanoma
tissues compared with nevus tissues, it was further evaluated
whether inhibiting UCP2 expression could suppress melanoma cell
growth and sensitize these cells to cisplatin. Two widely used
melanoma cell lines, A375 and SK-Mel-28, were infected using the
control or UCP2 shRNA-containing lentivirus. After antibiotic
selection, control lentivirus-infected cells (LC) and two stable
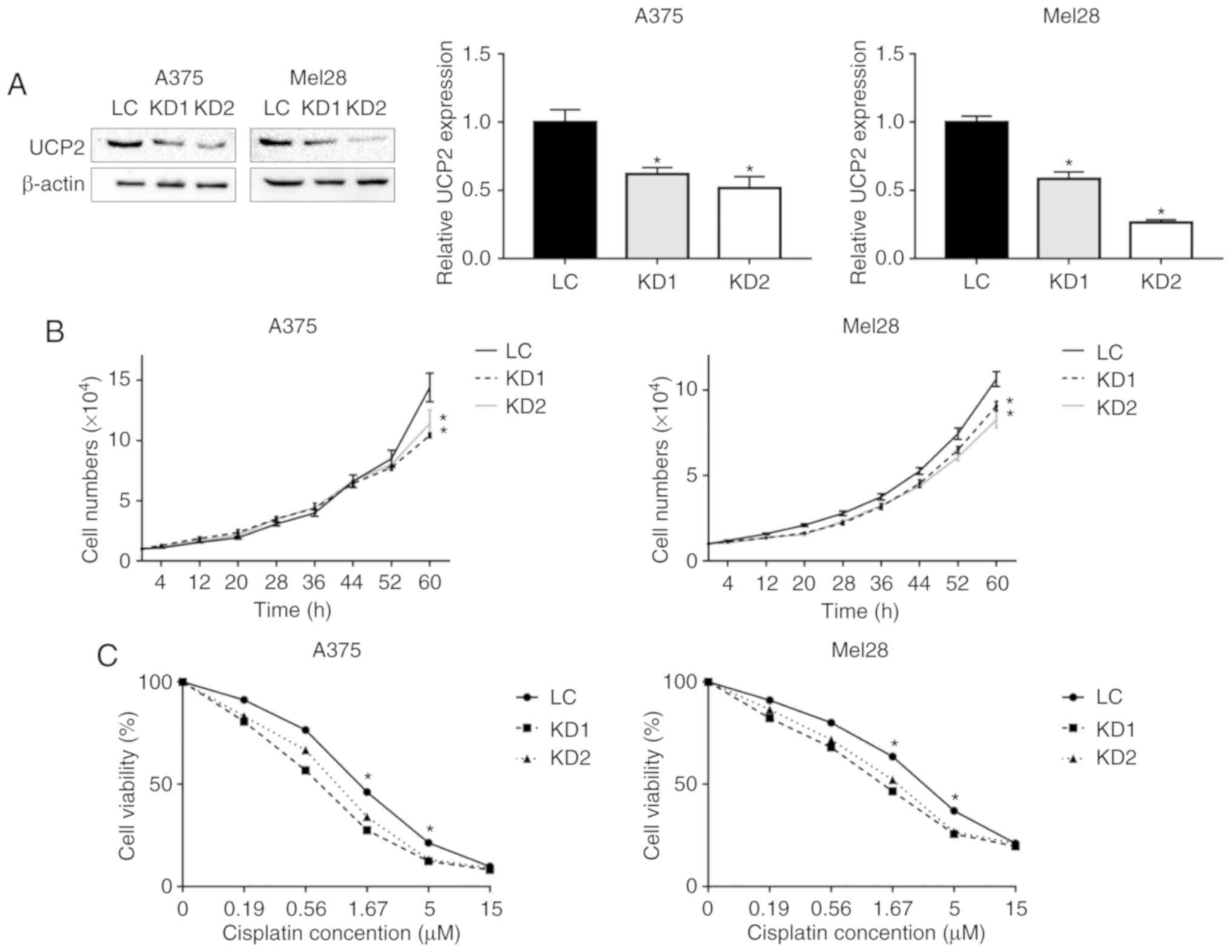
UCP2 KD clones were established in each cell line (Fig. 2A). The UCP2 protein levels were
reduced by 40-50% in the A375 KD clones and by 50-70% in the Mel-28
KD clones. As shown in Fig. 2B,
after growing for 60 h, the proportion of cell growth was 72.4 and
79.3% for the A375 clones, relative to the control cells. For the
SK-Mel-28 cells, the proportion of cell growth was 81.2 and 73.8%,
relative to the control cells.
The cell viability assay was used to test
sensitivity to cisplatin, which is a common chemotherapeutic agent
used for the treatment of melanoma (18). The UCP2 KD and control cells were
treated using different concentrations of cisplatin for 48 h, and
cell viability was measured using the MTT assay. As shown in
Fig. 2C, the UCP2 KD cells were
more sensitive to cisplatin at concentrations of 1.67 and 5 µM. For
example, at a concentration of 1.67 µM, the viability of A375 UCP2
KD clones was ~30% (vs. 46% for the control cells) and the
viability of SK-Mel-28 UCP2 KD cells was ~49% (vs. 64% for the
control cells). These results indicate that UCP2 inhibition
increased the sensitivity of melanoma cells to cisplatin.
UCP2 inhibition decreases the
mitochondrial membrane potential and the levels of ATP,
H2O2 and lactate
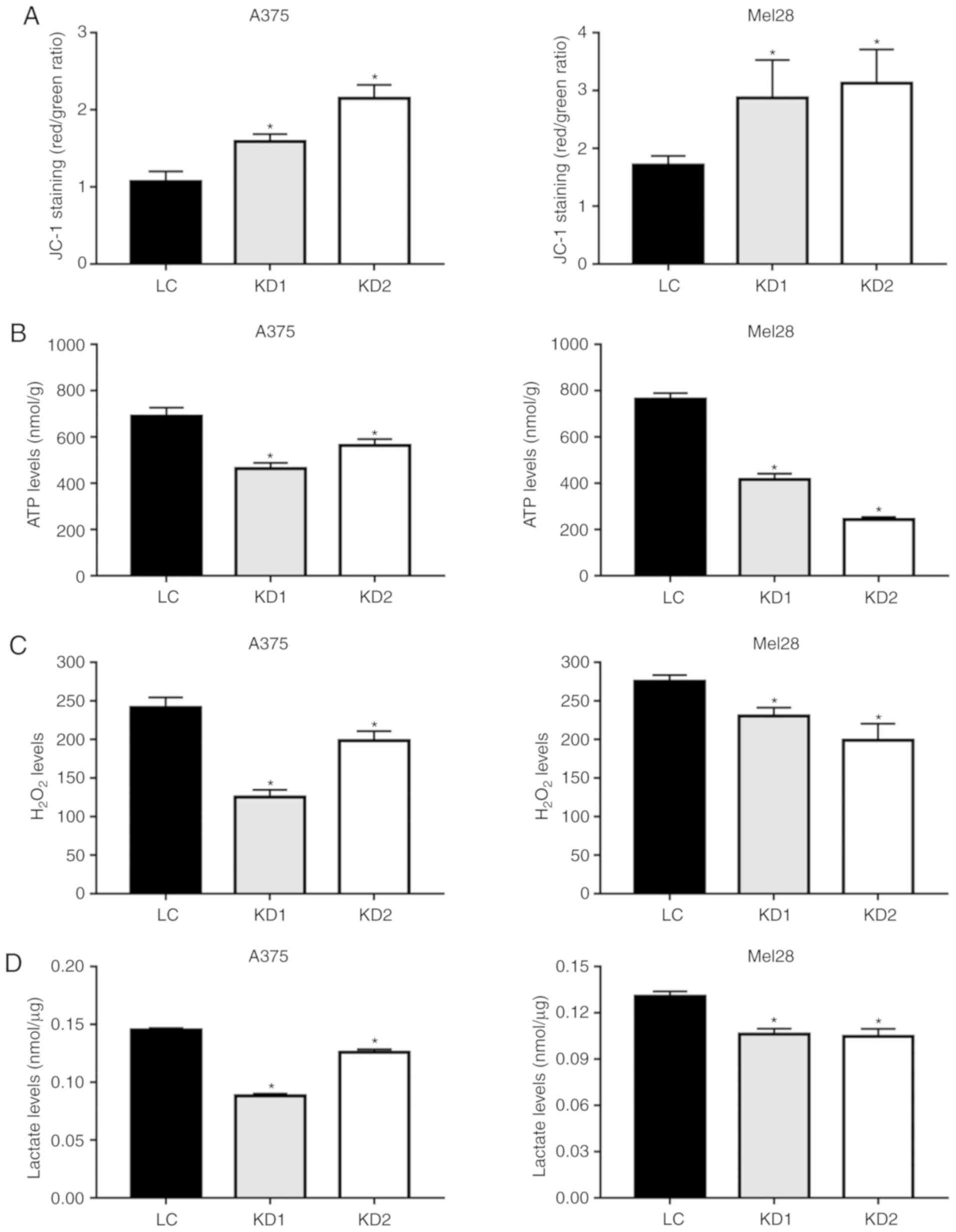
As an uncoupling protein, UCP2 regulates the
mitochondrial membrane potential, ATP synthesis and ROS generation
(21). The JC-1 dye was used to
examine the effects of UCP2 inhibition on the membrane potential
(ΔΨm), and the UCP2 KD clones exhibited increases in the membrane
potential of 40-65% for the A375 and SK-Mel-28 cells (Fig. 3A). However, the increased membrane
potential did not result in increased ATP generation, with ATP
levels decreasing by 30-60% in the A375 and SK-Mel-28 UCP2 KD cells
(Fig. 3B). As shown in Fig. 3C, the H2O2
levels were also decreased by 25-40% in the A375 and SK-Mel-28 UCP2
KD cells (Fig. 3C).
Glycolysis is often enhanced in tumor cells for ATP
production and anabolism, which leads to increased lactate levels
(22). However, in the A375 and
SK-Mel-28 UCP2 KD cells, the lactate levels were decreased by
20-30% (Fig. 3D). These results
suggested that inhibiting UCP2 reduced the rate of glycolysis and
ATP generation in melanoma cells.
UCP2 inhibition suppresses melanoma cell
invasion and three‑dimensional growth
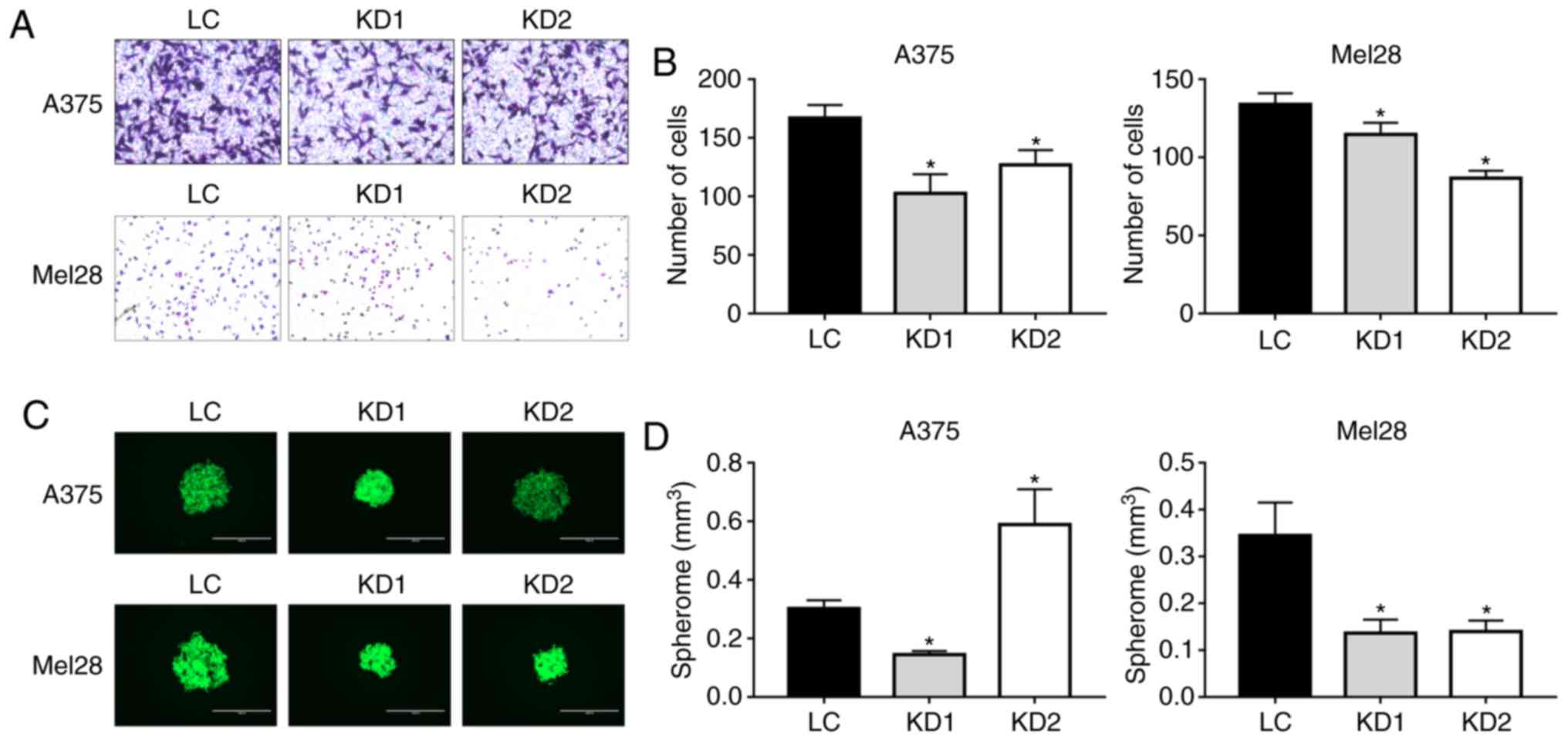
Malignant melanoma is an invasive tumor, and the
Matrigel invasion assay was used to examine whether UCP2 inhibition
affected cell migration and invasion. As shown in Fig. 4A and B, after a 12-h incubation,
only 60-75% of the A375 UCP2 KD cells had migrated through the
Matrigel (vs. the control A375 cells) and only 65-85% of the
SK-Mel-28 UCP2 KD cells had migrated (vs. the control SK-Mel-28
cells). The three-dimensional spheroid growth assay was performed
to evaluate the tumorigenicity of the UCP2 KD cells. For the
SK-Mel-28 cells, the UCP2 KD cells formed spheroids with ~40% of
the volume of the control cell spheroids (Fig. 4C and D). For the A375 cells, one
UCP2 KD clone formed smaller spheroids compared with the control,
while the other clone formed larger spheroids, but with markedly
lower density based on fluorescence intensity analysis. These
results suggested that inhibition of UCP2 expression reduced the
tumorigenicity of melanoma cells.
UCP2 inhibition suppresses Akt/mTOR and
ERK signaling in melanoma cells
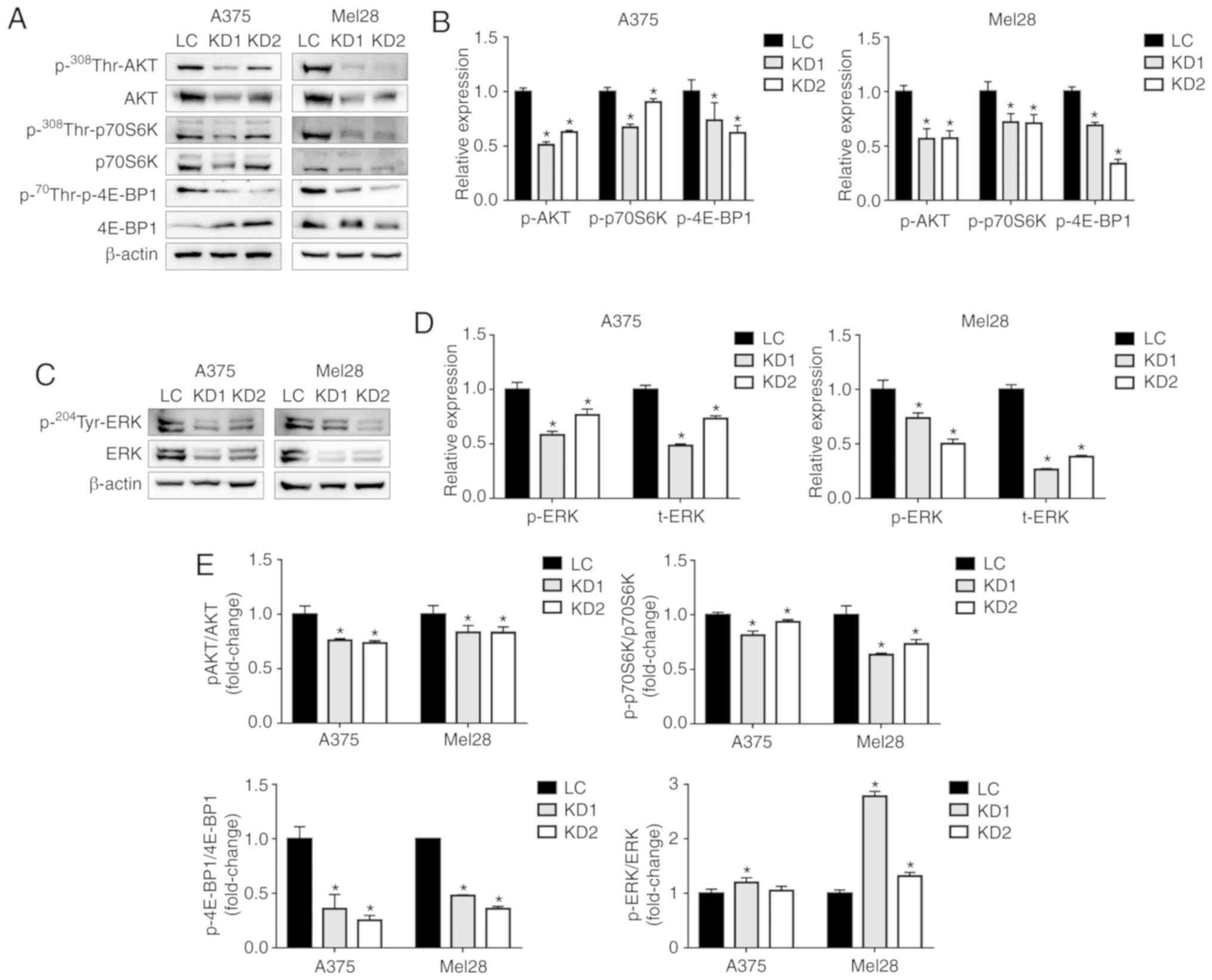
During melanomagenesis, Akt/mTOR signaling plays a
key role in the regulation of cell proliferation, growth and
apoptosis (23). Fig. 5A and B show the lower levels of
phosphorylated Akt (Thr308) in both lines of UCP2 KD cells. As
downstream effectors for mTOR, p70S6K and 4E-BP1 regulate cell
growth and proliferation, and their activation is often associated
with tumor development (24,25).
Inhibition of UCP2 expression was found to be associated with
significantly reduced levels of phosphorylated p70S6K (Thr389) and
4E-BP1 (Thr70) relative to the control cells (Fig. 5A, B and E). The expression of
p70S6K was not significantly altered, although the expression of
4E-BP1 was increased in the A375 UCP2 KD cells, but not in the
SK-Mel-28 UCP2 KD cells.
The ERK pathway also plays a crucial role in
regulating melanoma cell proliferation, differentiation and
apoptosis (26). The UCP2 KD
clones in both cell lines exhibited significantly reduced levels of
both phosphorylated ERK (Tyr204) and total ERK proteins, relative
to the control cells (Fig. 5C and
D); therefore, the p-ERK/ERK ratio was not decreased in the
UCP2 KD clones (Fig. 5E). These
results suggested that inhibition of UCP2 expression suppressed
Akt/mTOR and ERK signaling in melanoma cells.
Discussion
The incidence of melanoma has continued to rise in
recent years, which is a major cause of concern (27), as malignant melanoma is highly
invasive and is difficult to cure after metastasis has occurred
(28). The incidence of melanoma
is affected by age and sex, with women having a higher incidence at
younger ages and men having a higher incidence at older ages
(29). These differences suggest
that metabolic and hormone changes may affect the pathogenesis of
melanoma. As an uncoupling protein, UCP2 is an important regulator
of metabolism (7) and is often
highly expressed in human cancers, where it promotes the shift from
oxidative phosphorylation to glycolysis (11). Our earlier studies have
demonstrated that UCP2 is highly expressed in non-melanoma skin
cancers (30), although UCP2
knockout in a mouse model suppressed chemically-induced skin
carcinogenesis (14). Furthermore,
carcinogen treatment induced glycolysis, which was suppressed by
the UCP2 knockout (14).
Therefore, our earlier work was extended to focus on the role of
UCP2 in human melanoma, which is the deadliest type of skin
cancer.
To the best of our knowledge, this is the first
study to evaluate whether UCP2 expression was correlated with
melanoma's Clark level and Breslow thickness, which reflect the
depth of invasion. As melanoma is one of the most aggressive and
treatment-resistant cancers (3),
the results suggest that UCP2 may contribute to melanoma's
aggressiveness and that targeting UCP2 may suppress melanoma
progression. This hypothesis was tested in UCP2 KD melanoma cells,
and inhibition of UCP2 expression in human melanoma cells
suppressed cell migration, invasion and three-dimensional spheroid
growth (Fig. 4). Furthermore, the
inhibition of UCP2 expression sensitized melanoma cells to
cisplatin (Fig. 2C). The fact that
inhibition of UCP2 expression decreased the membrane potential is
consistent with its role as an uncoupling protein. Moreover, the
reduced lactate levels and ATP production in UCP2 KD cells suggest
that glycolysis was inhibited, which is also consistent with the
role of UCP2 in shifting metabolism towards glycolysis (11).
Several key signaling pathways contribute to
melanoma aggressiveness, including the MARK and Akt pathways. As
one of the main arms of the MAPK pathway, Ras/Raf/MEK/ERK play a
vital role in melanomagenesis (31), with the signal cascade culminating
in ERK1/2 and activating downstream transcription factors, thereby
contributing to melanoma cell proliferation and migration (31,32).
Inhibition of UCP2 expression inactivated ERK, suggesting that
tumor suppression may be achieved in melanoma cells by targeting
UCP2 (Fig. 5C).
The activation of Akt is another important pathway
in melanomagenesis (33), which
contributes to stimulating ROS generation and DNA mutation
(34), promoting drug resistance
(35), and promoting metastasis to
the lung and brain (36), as well
as being associated with poor survival (37). Activated Akt transduces signals
through a number of target proteins, including mTOR, and mTOR
stimulates protein synthesis via effectors p70S6K and p4E-BP1
(38). As a serine/threonine
protein kinase, mTOR also plays an oncogenic role in several human
cancers, including melanoma, where mTOR activation promotes
melanoma cell proliferation and invasiveness (39). Moreover, inhibition of Akt/mTOR
greatly increases the sensitivity of melanoma cells to chemotherapy
(e.g., cisplatin or temozolomide) (40). The present study also revealed that
inhibition of UCP2 resulted in lower levels of phosphorylated Akt,
phosphorylated p70S6K and phosphorylated 4E-BP1, which suggests
that UCP2 promotes the Akt/mTOR pathway in melanoma cells. Similar
results have been observed in breast cancer cells, where
upregulated UCP2 was shown to activate the PI3K/Akt/mTOR pathway
and lead to increased tumor autophagy, which is responsible for
drug resistance (41).
In remains unclear how UCP2 promotes ERK and
Akt/mTOR signaling in melanoma cells. However, the present study
revealed that UCP2 KD cells exhibited lower levels of ROS
(H2O2), which contrasts with the generally
elevated H2O2 levels in cancer cells
(42). There is a variety of
factors contributing to the production of
H2O2 in cancer cells, which promotes cancer
cell metabolism, proliferation and metastasis (43). Furthermore, both ERK and Akt/mTOR
are activated by elevated ROS levels (12,13).
Therefore, the decreased H2O2 levels in UCP2
KD cells may contribute to downregulation of Akt/mTOR and ERK
signaling, although the precise underlying mechanism remains
unclear.
There were several limitations to the present study.
First, paradoxical roles have been reported for UCP2 in
tumorigenesis and there is controversy regarding its role in
melanoma (44). Second,
ethnicity-related differences may help explain the differences in
certain clinical characteristics, and variations in cell lines and
genetic techniques (e.g., UCP2 overexpression or KD) may also
account for some of the differences observed during in vitro
studies. However, the findings of the present study suggest that
UCP2 KD in melanoma cells conferred a treatment benefit, which
raises the possibility that UCP2 may be a useful target for
adjuvant therapy.
To the best of our knowledge, this is the first
report of UCP2 being more highly expressed in human melanoma
tissues compared with compound nevus tissues. In addition, the
level of UCP2 expression was found to be correlated with tumor
grade and depth of invasion. Furthermore, inhibition of UCP2
expression inactivated the Akt/mTOR and ERK pathways, which may be
responsible for the observed decrease in cell proliferation and
invasion, as well as increase in sensitivity to cisplatin
treatment. Further studies are required to analyze the mRNA
expression of UCP2 in tissue samples, in order to evaluate the
effects of drugs that target UCP2 and Akt/mTOR/ERK, which may
represent a novel treatment strategy for melanoma.
Funding
The present study was supported by funds from the
Department of Pharmacology, Toxicology and Neuroscience, LSU Health
Sciences Center in Shreveport.
Availability of data and materials
All the datasets generated and analyzed during the
present study are available from the corresponding author on
reasonable request.
Authors' contributions
Study design: JL, CN, XC and YZ. Data collection and
analysis: JL, YJ, LA and CN. Manuscript preparation: JL, CN, XC and
YZ.
Ethics approval and consent to
participate
The protocol of this retrospective study was
approved by the Institutional Review Board of China-Japan Union
Hospital, Jilin University. Informed consent was obtained from
patients at the time of sample collection.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
The IncuCyte Live Cell Analysis system was provided
by the Feist-Weiller Cancer Center's Innovative North Louisiana
Experimental Therapeutics program (INLET), which is directed by Dr
Glenn Mills at LSUHSC-Shreveport and supported by the LSU Health
Shreveport Foundation. The authors would like to thank Dr Ana-Maria
Dragoi (Associate Director of INLET), Dr Jennifer Carroll (Director
of the In vivo, In vitro Efficacy Core), and Reneau
Youngblood (Research Associate) for their assistance with the
IncuCyte experiments. Flow cytometry experiments were performed by
David Custis at the institutional Research Core Facility.
References
|
1
|
Linares MA, Zakaria A and Nizran P: Skin
cancer. Prim Care. 42:645–659. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Haenssle HA, Mograby N, Ngassa A, Buhl T,
Emmert S, Schön MP, Rosenberger A and Bertsch HP: Association of
patient risk factors and frequency of nevus-associated cutaneous
melanomas. JAMA Dermatol. 152:291–298. 2016. View Article : Google Scholar
|
|
3
|
Tsao H, Chin L, Garraway LA and Fisher DE:
Melanoma: From mutations to medicine. Genes Dev. 26:1131–1155.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Moon H, Donahue LR, Choi E, Scumpia PO,
Lowry WE, Grenier JK, Zhu J and White AC: Melanocyte stem cell
activation and translocation initiate cutaneous melanoma in
response to UV exposure. Cell Stem Cell. 21:665–678. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ježek P, Holendová B, Garlid KD and
Jabůrek M: Mitochondrial uncoupling Proteins: Subtle regulators of
cellular redox signaling. Antioxid Redox Signal. 29:667–714. 2018.
View Article : Google Scholar
|
|
6
|
Rial E, González‑Barroso MM, Fleury C and
Bouillaud F: The structure and function of the brown fat uncoupling
protein UCP1: Current status. Biofactors. 8:209–219. 1998.
View Article : Google Scholar
|
|
7
|
Toda C and Diano S: Mitochondrial UCP2 in
the central regulation of metabolism. Best Pract Res Clin
Endocrinol Metab. 28:757–764. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Boss O, Samec S, Paoloni-Giacobino A,
Rossier C, Dulloo A, Seydoux J, Muzzin P and Giacobino JP:
Uncoupling protein-3: A new member of the mitochondrial carrier
family with tissue‑specific expression. FEBS Lett. 408:39421997.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Mao W, Yu XX, Zhong A, Li W, Brush J,
Sherwood SW, Adams SH and Pan G: UCP4, a novel brain‑specific
mitochondrial protein that reduces membrane potential in mammalian
cells. FEBS Lett. 443:326–330. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yu XX, Mao W, Zhong A, Schow P, Brush J,
Sherwood SW, Adams SH and Pan G: Characterization of novel
UCP5/BMCP1 isoforms and differential regulation of UCP4 and UCP5
expression through dietary or temperature manipulation. FASEB J.
14:1611–1618. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Brandi J, Cecconi D, Cordani M,
Torrens-Mas M, Pacchiana R, Dalla Pozza E, Butera G, Manfredi M,
Marengo E, Oliver J, et al: The antioxidant uncoupling protein 2
stimulates hnRNPA2/B1, GLUT1 and PKM2 expression and sensitizes
pancreas cancer cells to glycolysis inhibition. Free Radic Biol
Med. 101:305–316. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Elorza A, Hyde B, Mikkola HK, Collins S
and Shirihai OS: UCP2 modulates cell proliferation through the
MAPK/ERK pathway during erythropoiesis and has no effect on heme
biosynthesis. J Biol Chem. 283:30461–30470. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Dando I, Pacchiana R, Pozza ED, Cataldo I,
Bruno S, Conti P, Cordani M, Grimaldi A, Butera G, Caraglia M, et
al: UCP2 inhibition induces ROS/Akt/mTOR axis: Role of GAPDH
nuclear translocation in genipin/everolimus anticancer synergism.
Free Radic Biol Med. 113:176–189. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Li W, Zhang C, Jackson K, Shen X, Jin R,
Li G, Kevil CG, Gu X, Shi R and Zhao Y: UCP2 knockout suppresses
mouse skin carcinogenesis. Cancer Prev Res (Phila). 8:487–491.
2015. View Article : Google Scholar
|
|
15
|
Sreedhar A, Petruska P, Miriyala S,
Panchatcharam M and Zhao Y: UCP2 overexpression enhanced glycolysis
via activation of PFKFB2 during skin cell transformation.
Oncotarget. 8:95504– 95515. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sreedhar A, Lefort J, Petruska P, Gu X,
Shi R, Miriyala S, Panchatcharam M and Zhao Y: UCP2 upregulation
promotes Plcγ-1 signaling during skin cell transformation. Mol
Carcinog. 56:2290–2300. 2017. View
Article : Google Scholar : PubMed/NCBI
|
|
17
|
Rossi S, Cordella M, Tabolacci C, Nassa G,
D'Arcangelo D, Senatore C, Pagnotto P, Magliozzi R, Salvati A,
Weisz A, et al: TNF-alpha and metalloproteases as key players in
melanoma cells aggressiveness. J Exp Clin Cancer Res. 37:3262018.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Bhatia S, Tykodi SS and Thompson JA:
Treatment of metastatic melanoma: An overview. Oncology (Williston
Park). 23:488–496. 2009.
|
|
19
|
Rofstad EK, Wahl A, Davies Cde L and
Brustad T: Growth characteristics of human melanoma multicellular
spheroids in liquid-overlay culture: Comparisons with the parent
tumour xenografts. Cell Tissue Kinet. 19:205–216. 1986.PubMed/NCBI
|
|
20
|
Bosman FT, de Goeij AF and Rousch M:
Quality control in immunocytochemistry: Experiences with the
oestrogen receptor assay. J Clin Pathol. 45:120–124. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Baffy G, Derdak Z and Robson SC:
Mitochondrial recoupling: A novel therapeutic strategy for cancer?
Br J Cancer. 105:469–474. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Gill KS, Fernandes P, O'Donovan TR,
McKenna SL, Doddakula KK, Power DG, Soden DM and Forde PF:
Glycolysis inhibition as a cancer treatment and its role in an
anti-tumour immune response. Biochim Biophys Acta. 1866:87–105.
2016.PubMed/NCBI
|
|
23
|
Pópulo H, Lopes JM and Soares P: The mTOR
signalling pathway in human cancer. Int J Mol Sci. 13:1886–1918.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Calero R, Morchon E, Martinez-Argudo I and
Serrano R: Synergistic anti-tumor effect of 17AAG with the
PI3K/mTOR inhibitor NVP-BEZ235 on human melanoma. Cancer Lett.
406:1–11. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Babchia N, Calipel A, Mouriaux F, Faussat
AM and Mascarelli F: The PI3K/Akt and mTOR/P70S6K signaling
pathways in human uveal melanoma cells: Interaction with B-Raf/ERK.
Invest Ophthalmol Vis Sci. 51:421–429. 2010. View Article : Google Scholar
|
|
26
|
Mao XH, Chen M, Wang Y, Cui PG, Liu SB and
Xu ZY: MicroRNA-21 regulates the ERK/NF-κB signaling pathway to
affect the proliferation, migration, and apoptosis of human
melanoma A375 cells by targeting SPRY1, PDCD4, and PTEN. Mol
Carcinog. 56:886–894. 2017. View
Article : Google Scholar
|
|
27
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2017. CA Cancer J Clin. 67:7–30. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Eggermont AM, Spatz A and Robert C:
Cutaneous melanoma. Lancet. 383:816–827. 2014. View Article : Google Scholar
|
|
29
|
Rigel DS: Epidemiology of melanoma. Semin
Cutan Med Surg. 29:204–209. 2010. View Article : Google Scholar
|
|
30
|
Li W, Nichols K, Nathan CA and Zhao Y:
Mitochondrial uncoupling protein 2 is up-regulated in human head
and neck, skin, pancreatic, and prostate tumors. Cancer Biomark.
13:377–383. 2013. View Article : Google Scholar
|
|
31
|
Estrada Y, Dong J and Ossowski L: Positive
crosstalk between ERK and p38 in melanoma stimulates migration and
in vivo proliferation. Pigment Cell Melanoma Res. 22:66–76. 2009.
View Article : Google Scholar
|
|
32
|
Dhillon AS, Hagan S, Rath O and Kolch W:
MAP kinase signalling pathways in cancer. Oncogene. 26:3279–3290.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Altomare DA and Testa JR: Perturbations of
the AKT signaling pathway in human cancer. Oncogene. 24:7455–7464.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Govindarajan B, Sligh JE, Vincent BJ, Li
M, Canter JA, Nickoloff BJ, Rodenburg RJ, Smeitink JA, Oberley L,
Zhang Y, et al: Overexpression of Akt converts radial growth
melanoma to vertical growth melanoma. J Clin Invest. 117:719–729.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Niessner H, Forschner A, Klumpp B,
Honegger JB, Witte M, Bornemann A, Dummer R, Adam A, Bauer J,
Tabatabai G, et al: Targeting hyperactivation of the AKT survival
pathway to overcome therapy resistance of melanoma brain
metastases. Cancer Med. 2:76–85. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Cho JH, Robinson JP, Arave RA, Burnett WJ,
Kircher DA, Chen G, Davies MA, Grossmann AH, VanBrocklin MW,
McMahon M and Holmen SL: AKT1 activation promotes development of
melanoma metastases. Cell Rep. 13:898–905. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Dai DL, Martinka M and Li G: Prognostic
significance of activated Akt expression in melanoma: A
clinicopathologic study of 292 cases. J Clin Oncol. 23:1473–1482.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Polivka Jr J and Janku F: Molecular
targets for cancer therapy in the PI3K/AKT/mTOR pathway. Pharmacol
Ther. 142:164–175. 2014. View Article : Google Scholar
|
|
39
|
Yang Y, Luo Z, Hao Y, Ba W, Wang R, Wang
W, Ding X and Li C: mTOR-mediated Na+/Ca2+ exchange affects cell
proliferation and metastasis of melanoma cells. Biomed
Pharmacother. 92:744–749. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Sinnberg T, Lasithiotakis K, Niessner H,
Schittek B, Flaherty KT, Kulms D, Maczey E, Campos M, Gogel J,
Garbe C and Meier F: Inhibition of PI3K-AKT-mTOR signaling
sensitizes melanoma cells to cisplatin and temozolomide. J Invest
Dermatol. 129:1500–1515. 2009. View Article : Google Scholar
|
|
41
|
Yu X, Luo A, Liu Y, Wang S, Li Y, Shi W,
Liu Z and Qu X: MiR-214 increases the sensitivity of breast cancer
cells to tamoxifen and fulvestrant through inhibition of autophagy.
Mol Cancer. 14:2082015. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Szatrowski TP and Nathan CF: Production of
large amounts of hydrogen peroxide by human tumor cells. Cancer
Res. 51:794–798. 1991.PubMed/NCBI
|
|
43
|
Lisanti MP, Martinez-Outschoorn UE, Lin Z,
Pavlides S, Whitaker-Menezes D, Pestell RG, Howell A and Sotgia F:
Hydrogen peroxide fuels aging, inflammation, cancer metabolism and
metastasis: The seed and soil also needs 'fertilizer'. Cell Cycle.
10:2440–2449. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Cheng WC, Tsui YC, Ragusa S, Koelzer VH,
Mina M, Franco F, Läubli H, Tschumi B, Speiser D, Romero P, et al:
Uncoupling protein 2 reprograms the tumor microenvironment to
support the anti-tumor immune cycle. Nat Immunol. 20:206–217. 2019.
View Article : Google Scholar : PubMed/NCBI
|