Introduction
Gastrointestinal stromal tumours (GISTs) are the
most common type of soft tissue sarcomas that arise from the
mesenchymal cells of the gastrointestinal tract and have
characteristic histological and molecular features (1). According to the literature, most
GISTs are driven by activating mutations in the proto-oncogenes
KIT and PDGFRA (2).
In total, ~85% of malignant GISTs harbour activating mutations in
one of the genes, KIT or PDGFRA, while 10-15% of all
GISTs have no detectable KIT or PDGFRA mutations
(2-4).
Receptors KIT and platelet derived growth factor
receptor α (PDGFRA) have a similar structure and activation
mechanism, as they belong to the same sub-family, the type III
receptor tyrosine kinases (5).
Their typical structure is composed of an extracellular (EC)
domain, a transmembrane domain, an intracellular juxtamembrane (JM)
domain and a cytoplasmic kinase domain, which is divided by a
kinase insert into two catalytic parts, tyrosine kinase domain I
(TK I) and tyrosine kinase domain II (TK II) (6).
Gain-of-function mutations in KIT or
PDGFRA gene cause functional changes in KIT or PDGFRA
receptor, which lead to constitutive, ligand-independent
phosphorylation of the receptor and activation of downstream to
KIT/PDGFRA signalling pathways (RAS/RAF/MAPK, PI3K/AKT/mTOR and
JAK/STAT3), ultimately increasing cell proliferation and inhibiting
apoptosis (6,7).
In GIST, 67% of KIT mutations occur in exon
11 (JM domain). The mutation frequency in other regions of the KIT
receptor is markedly lower; mutations in exon 9 (EC domain) occur
in 8-10% of GISTs, whilst exons 13 and 17 (TK I and TK II) are
mutated in ~1% of all GISTs (8-10).
PDGFRA mutations occur in ~8% of GISTs, of which exon 14 (TK
I) and exon 18 (TK II) are mutated in 6-7% of the GISTs, and exon
12 (JM domain) in 1% (11,12).
GISTs without a mutation in the KIT or
PDGFRA genes are historically known as wild-type (WT) GIST;
however, their molecular biology is very heterogeneous.
KIT/PDGFRA WT GISTs can have alterations in genes of the
succinate dehydrogenase complex or in BRAF, PIK3CA, or
rarely RAS family genes (13,14).
BRAF V600E mutation has been reported in 7-15% of
KIT/PDGFRA WT GISTs, meanwhile RAS and PIK3CA mutant GISTs
are reported to be very rare (15-18).
Even more rare are recently reported NTRK fusions in these
patients, however they are important for treatment decisions
(19).
Before the discovery of tyrosine kinase inhibitor
(TKI) imatinib, no effective therapy was available for the
treatment of unresectable, metastatic or recurrent GIST. The
overall survival (OS) time of such patients was 9-20 months
(20,21). With imatinib, the median OS time of
patients with advanced GISTs is significantly longer and is
reported to be ~55-57 months (21). Although the majority of
KIT/PDGFRA-mutant GIST patients (90%) achieve clinical
benefit from imatinib, primary resistance frequently occurs in
GISTs that are KIT/PDGFRA WT, have KIT exon 9 mutation or
have a substitution D842V in gene PDGFRA (22,23).
Furthermore, secondary resistance develops in 40-50% of patients
with GIST within 2 years of successful treatment due to secondary
KIT/PDGFRA mutations or activating mutations of another
downstream signalling pathway, such as the RAS/RAF/MAPK or
PI3K/AKT/mTOR path-ways (18,22,23).
Imatinib efficacy greatly depends on tumour genotype; therefore,
molecular characterization of GIST is a very important step in
optimizing GIST clinical treatment (21,23).
The aim of the present study was to molecularly
characterize a cohort of 70 patients with metastatic GISTs from the
Slovenian Cancer Registry (National Cancer Registry) treated
between period 2002 and 2011. The spectrum of KIT and
PDGFRA mutations was reported, as well as the spectrum of
hot spot mutations in KRAS, NRAS, BRAF, PIK3CA and
AKT1 genes in patients with GIST.
Materials and methods
Patients
In total, 70 patients with metastatic KIT-positive
GIST, treated with TKIs at the Institute of Oncology Ljubljana
(Ljubljana, Slovenia) between January 2002 and December 2011, were
recruited and included in the present analysis.
Formalin-fixed, paraffin-embedded (FFPE) primary
tissue samples were mainly obtained from the Department of
Pathology, Institute of Oncology Ljubljana (72%) and also from
pathology departments from other hospitals and clinics in Slovenia
(University Medical Centre Ljubljana, Ljubljana; University Medical
Centre Maribor, Maribor; Izola General Hospital, Izola; and Celje
General Hospital, Celje). All patients were treated with TKIs at
the Institute of Oncology Ljubljana. In total, 12 of the 70 samples
were obtained from patients after the start of imatinib therapy.
Clinicopathological characteristics of the patients, as well as
prognostic risk groups according to Miettinen et al
(4), are presented in Table I.
 | Table IClinicopathological characteristics
of 70 patients with gastrointestinal stromal tumour. |
Table I
Clinicopathological characteristics
of 70 patients with gastrointestinal stromal tumour.
| Clinicopathological
characteristic | n (%) |
|---|
| Sex | |
| Male | 32 (45.7) |
| Female | 38 (54.3) |
| Age | |
| Median, years | 65 |
| Range, years | 34-89 |
| ≤60 years | 28 (40.0) |
| >60 years | 42 (60.0) |
| Curative
resection | |
| Yes | 13 (18.6) |
| No | 57 (81.4) |
| Imatinib
therapy | |
| Yes | 70 (100.0) |
| No | 0 (0.0) |
| Outcome | |
| Alive | 21 (30.0) |
| Dead | 49 (70.0) |
| Primary tumour
location | |
| Oesophagus | 2 (2.9) |
| Stomach | 33 (47.1) |
| Small
intestine | 25 (35.7) |
| Rectum | 6 (8.6) |
| Extraluminal | 4 (5.7) |
| Mitotic index,/50
HPF | |
| ≤5 | 22 (31.4) |
| 5.1-10 | 11 (15.7) |
| >10 | 37 (52.9) |
| Primary tumour
size, cm | |
| 0-5 | 6 (8.5) |
| 5.1-10 | 23 (32.9) |
| >10 | 37 (52.9) |
| Unknown | 4 (5.7) |
| Risk
classificationa | |
| High | 49 (70.0) |
| Intermediate | 11 (15.7) |
| Low | 9 (12.9) |
| Very low | 1 (1.4) |
| Morphology | |
| Spindle cells | 47 (67.1) |
| Epithelioid
cells | 8 (11.5) |
| Mixed cells | 15 (21.4) |
As controls for immunohistochemistry (IHC), normal
appendiceal tissue samples were obtained from 5 patients (3
females, 2 males; age, 57-74 years; median, 63 years) undergoing
various surgical procedures. In addition, tumour tissue samples
were obtained from 10 patients (6 females, 4 males; age, 42-83
years; meadian, 60 years) with histopathologically confirmed
CD117-positive non-metastatic GIST. All patients included as IHC
controls were treated at the Institute of Oncology Ljubljana
between January 2002 and December 2011.
All collected tumour samples were evaluated and were
histopathologically examined under a microscope by pathology
specialists, who confirmed the diagnosis using immunohistochemical
staining for CD117 antigen expression. IHC analysis of CD117 was
performed on 2-4 µm FFPE tissue sections, dried at 56°C for
2 h. Heat-mediated epitope retrieval was performed in a microwave
oven using home-brew pH 9.0 Tris-EDTA buffer for 10 min at 100°C at
high pressure. Detection was performed in the semi-automated IHC
system LabVision™ Autostainer AS480. Endogenous peroxidases were
blocked using Dako REAL™ Peroxidase blocking solution (cat. no.
S2023; Dako; Agilent Technologies, Inc.) for 8 min at room
temperature. CD117 was detected using a commercially available
mouse polyclonal antibody CD117 (cat. no. A4502; Dako; Agilent
Technologies, Inc.; 1:100), which was added for 30 min at room
temperature. The antibody was diluted using Dako REAL™ antibody
diluent (cat. no. S2022; Dako; Agilent Technologies, Inc.). Primary
antibody was visualised using 2-step polymer detection system Dako
REAL™ EnVision™ Detection system, Peroxidase/DAB+ Rabbit/Mouse
(cat. no. K5007; Dako; Agilent Technologies, Inc.). Ready to-use
secondary antibody Dako REAL™ EnVision™/HRP Rabbit/Mouse (cat. no.
K5007; Dako; Agilent Technologies, Inc.) was added for 30 min at
room temperature. Chromogene Dako REAL™ DAB+ Chromogen (cat. no.
K5007; Dako; Agilent Technologies, Inc.) was diluted at 1:50,
according to manufacturer's protocol and incubated twice for 7.5
min each at room temperature.
Tissue sections were viewed under a light microscope
(magnification, ×10). The section of the FFPE slides that contained
the highest number of tumour cells (always >70% in all GIST
samples) was marked and used for DNA extraction.
The present study was approved by the Institutional
Review Board of the Institute of Oncology Ljubljana (permission no.
OIRIKE00036) and by the National Medical Ethics Committee of
Republic of Slovenia (permission no. 1090216). Individual patient
consent was waived for this study as it was a retrospective study,
the research involved no risk to the subjects, and the
institutional informed consent forms for treatment included consent
for the use of patient's data, materials and/or test results for
research purposes. All procedures followed in the present study
were therefore in accordance with the ethical standards of the
responsible committees on human experimentation (institutional and
national) and the Helsinki Declaration of 1975, as revised in
2013.
Molecular analysis
Mutational analyses of KIT, PDGFRA, KRAS,
NRAS and BRAF genes were performed by the Department of
Molecular Diagnostic at the Institute of Oncology Ljubljana
(Ljubljana, Slovenia). Genomic DNA of 70 sporadic GISTs was
isolated from FFPE tissues, using macrodissection and standard
procedures, according to the manufacturer's protocol of the QIAmp
DNA FFPE Tissue kit (Qiagen GmbH). The quality and concentration of
DNA extracts were assessed using a NanoDrop spectrophotometer
(Thermo Fisher Scientific, Inc.).
All GISTs (n=70) were examined for somatic mutations
in KIT (exons 9, 11, 13 and 17) and PDGFRA (exons 12,
14 and 18) by polymerase chain reaction (PCR) amplification and
direct Sanger sequencing. For PCR amplification, Invitrogen™EXPRESS
SYBR™ GreenER™ qPCR Supermix, universal (Invitrogen; Thermo Fisher
Scientific, Inc.), which includes Platinum™ Taq DNA Polymerase, was
used. PCR reactions were run on an Applied Biosystems™ 7900HT Fast
Real-Time PCR system (Applied Biosystems; Thermo Fisher Scientific,
Inc.) with the following thermocycling conditions: Activation at
50°C for 2 min; followed by denaturation at 95°C for 10 min; 20
cycles of denaturation at 95°C for 35 sec, annealing at 64°C for 45
sec and elongation at 72°C for 1 min; followed by another 30 cycles
of denaturation at 95°C for 15 sec, annealing at 55°C for 45 sec
and elongation at 72°C for 1 min. Direct Sanger sequencing
reactions were performed using a Big Dye Terminator v3.1 (Applied
Biosystems; Thermo Fisher Scientific, Inc.) and run on an ABI 3500
Genetic Analyzer (Applied Biosystems; Thermo Fisher Scientific,
Inc.). Specific primers for target exon amplification and
sequencing reactions were designed using Primer3 web software
(version 0.1g; http://biotools.nubic.northwestern.edu/Primer3.html)
and are shown in Table SI.
Furthermore, all patients with KIT/PDGFRA WT
GIST were tested for the presence of hot spot mutations in KRAS,
NRAS, BRAF, PIK3CA and AKT1. Testing was performed using
Colorectal Cancer Mutation Detection Panel v1.3 (EntroGen, Inc.), a
quantitative PCR-based assay, according to the manufacturer's
protocol. A list of all hot spot mutations that are included in the
Colorectal Cancer Mutation Detection Panel are presented in
Table SII.
Sequence analysis
DNA sequences were analysed using Sequencing
Analysis software v5.4 software (Applied Biosystems; Thermo Fisher
Scientific, Inc.), ChromasLite software v2.1.1 (Technelysium Pty
Ltd.) and GeneRunner software (http://www.softpedia.com/get/Science-CAD/Gene-Runner.shtml).
Mutation nomenclature followed the recommendations of the Human
Genome Variation Society v19.01 (http://varnomen.hgvs.org/). Reference sequences used
in the present study were taken from the National Centre for
Biotechnology Information data-base (https://www.ncbi.nlm.nih.gov/): KIT
(NM_000222.2), PDGFRA (NM_006206), KRAS (NM_004985),
NRAS (NM_002524), BRAF (NM_004333), PIK3CA
(NM_006218.2) and AKT1 (NM_005163.2).
Literature search of detected
variants
A literature search for all detected variants found
in the present study was performed using Alamut Visual v2.11
software (SOPHiA GENETICS). To perform a search for the references
of every variant detected, a gene name and a nucleotide change
(c.notation) were typed into Alamut Visual browser, which is linked
to Google.
Online in silico analysis and
bioinformatics tools
In silico mutation prediction analyses were
performed using the online bioinformatics tools: Align GVGD
(http://agvgd.hci.utah.edu/)
MutationTaster2 (http://www.muta-tiontaster.org), and Cancer Genome
Interpreter (https://www.cancergenomeinterpreter.org/analysis).
Their pathogenicity was assessed according to The American College
of Medical Genetics and Genomics/Association for Molecular
Pathology (ACMG/AMP) criterions using Genetic Variant
Interpretation Tool (http://www.medschool.umaryland.edu/Genetic_Variant_Interpretation_Tool1.html/).
Results
Genotype analysis KIT/PDGFRA mutant
GISTs
Among 70 metastatic GIST tumours, 62 (88.6%)
KIT/PDGFRA mutant GISTs were identified. Of the analysed
GISTs, 60 (85.7%) had a mutation in KIT and only 2 (2.9%)
had a mutation in PDGFRA. Of the 60 patients with KIT
mutant GISTs, 6 (10%) developed a secondary mutation in exon 13 or
17 of the KIT gene. These secondary mutations were found in
the patients whose tissue samples were obtained following imatinib
treatment.
Among the 60 patients with KIT mutation,
KIT exon 11 was mutated in 52 patients (86.7%), harbouring
40 different variants. KIT exon 9 was mutated in 6 cases
(10%) and all carried a frequent six nucleotides duplication,
c.1504_1509dup p.(Ala502_ Tyr503dup). KIT exon 13 was
mutated in 5 cases (8.3%); 2 patients had a primary mutation and 3
patients had a secondary mutation. KIT exon 17 was mutated
in 3 cases (5%), where all 3 patients had a secondary mutation
(Table II).
 | Table IIMutational status and mutation
location of all 70 patients with GIST from the Slovenian Cancer
Registry. |
Table II
Mutational status and mutation
location of all 70 patients with GIST from the Slovenian Cancer
Registry.
| Mutation
genotype | Number of
GISTs |
|---|
|
KIT/PDGFRA-mutant GIST | 62 |
| KIT (exon
9) | 6 |
| KIT (exon
11) | 52 |
| KIT (exon
13) | 5a |
| KIT (exon
17) | 3b |
| PDGFRA (exon
12) | 0 |
| PDGFRA (exon
14) | 1c |
| PDGFRA (exon
18) | 1 |
| Wild-typed GIST | 8 |
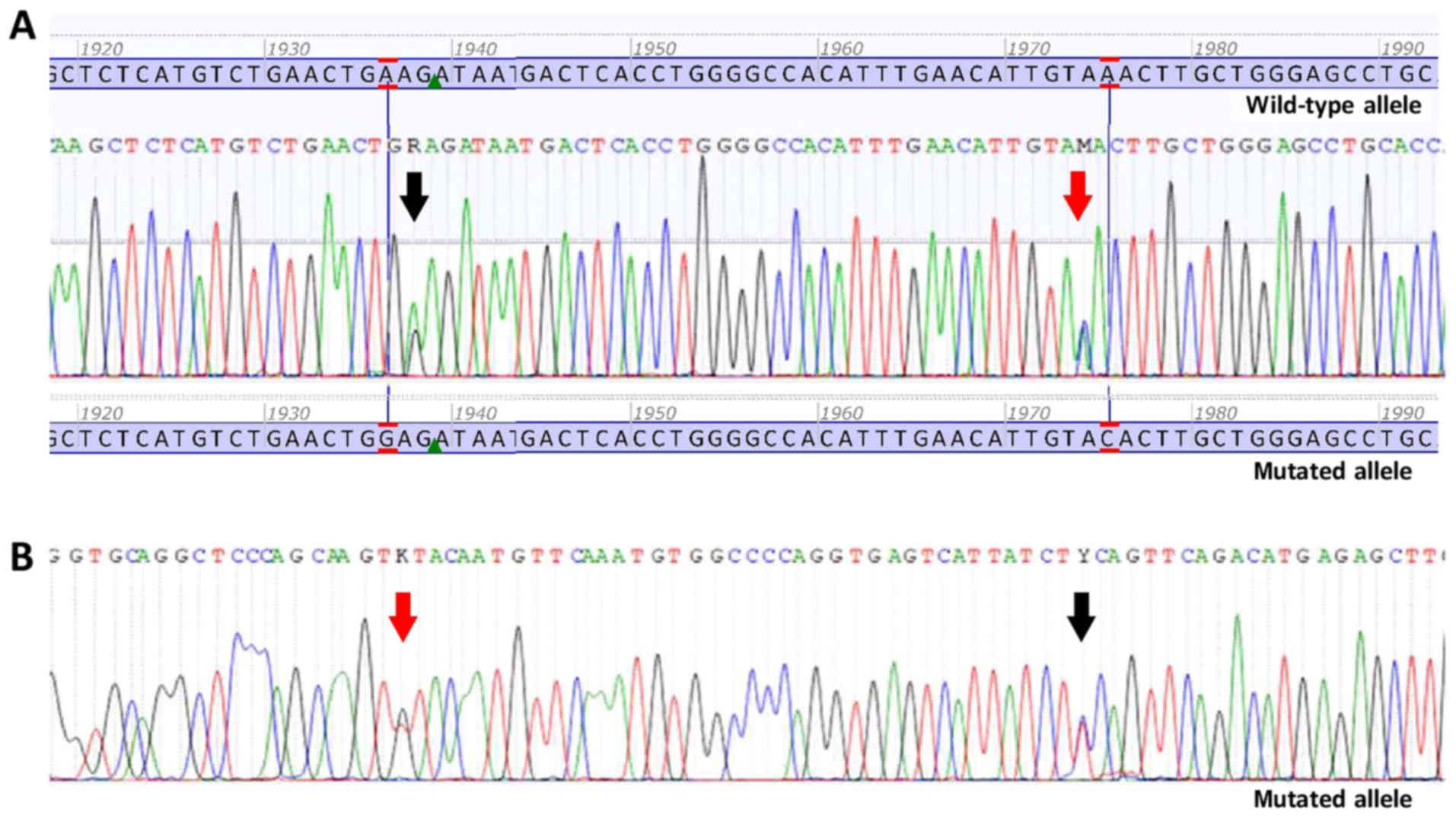
As aforementioned, mutations in PDGFRA were
found in 2 cases (2.9%). The first patient had a single nucleotide
change in exon 18, c.2525A>T p.(Asp842Val), while the second
patient had two different single nucleotide changes, c.1936A>G
p.(Lys627Glu) and c.1975A>C p.(Asn659His), both in exon 14. The
latter case was a rare example of double mutant GIST (Fig. 1). Mutational status and mutation
location of all 70 GISTs are presented in Table II. All detected alterations and
their status of pathogenicity in 62 KIT/PDGFRA mutant GISTs
from the Slovenian Cancer Registry are presented in Table SIII.
KIT/PDGFRA wild-type GIST
All eight KIT/PDGFRA WT GISTs had no
mutations in hot spot regions of KRAS (codons
12/13/59/61/117/146), NRAS (codons 12/13/59/61/117/146),
BRAF (codon 600), PIK3CA (codons 542/545/1047) or
AKT1 (codon 17).
Novel variants
The present study detected 49 different variants in
total, of which eight were novel. A reference search for all
detected variants (n=49) was performed using Google browser trough
Alamut Visual software, on the 19th of January 2019.
All eight novel heterozygous variants, which had not
been reported previously, were detected in exon 11 of gene
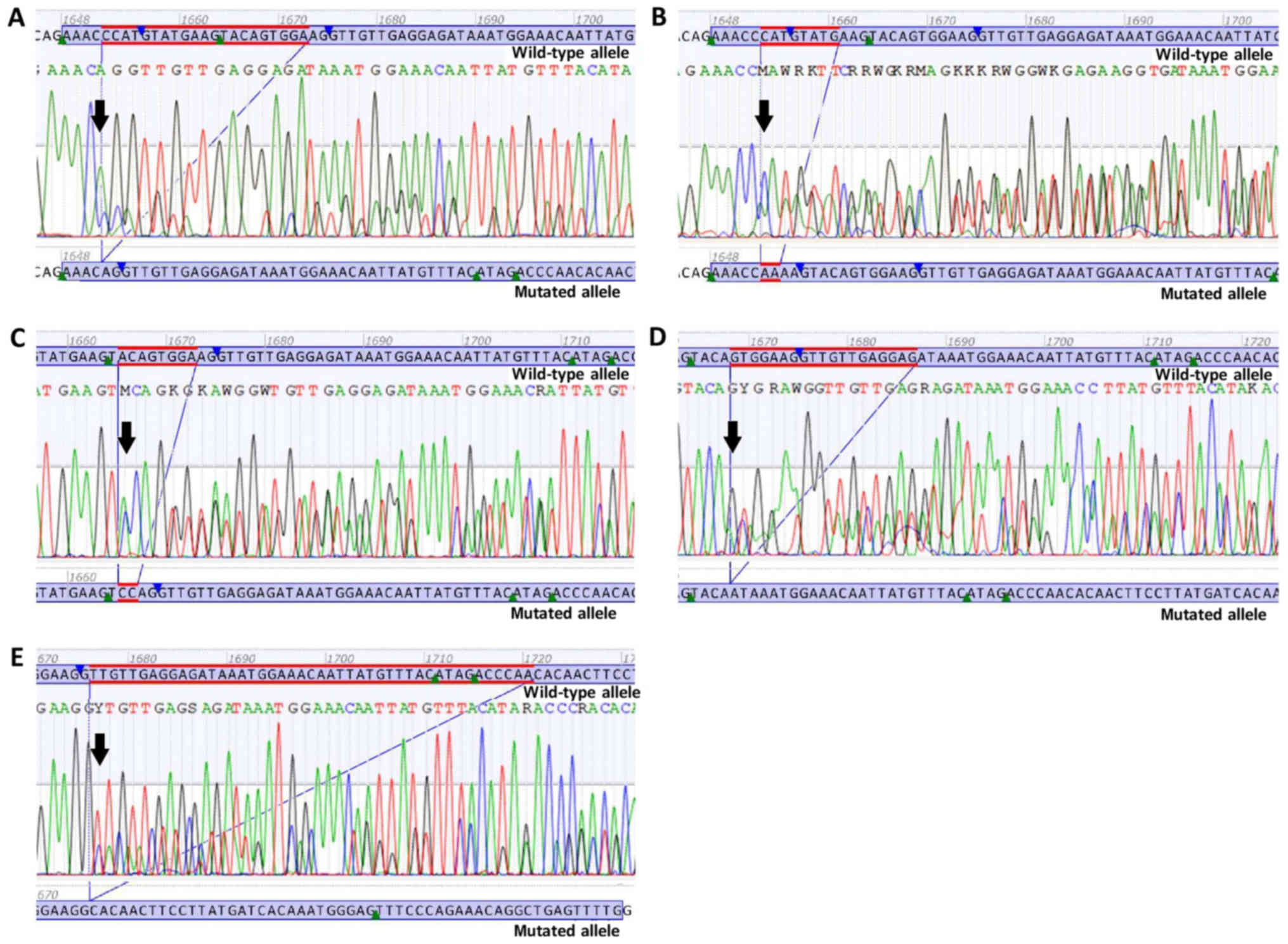
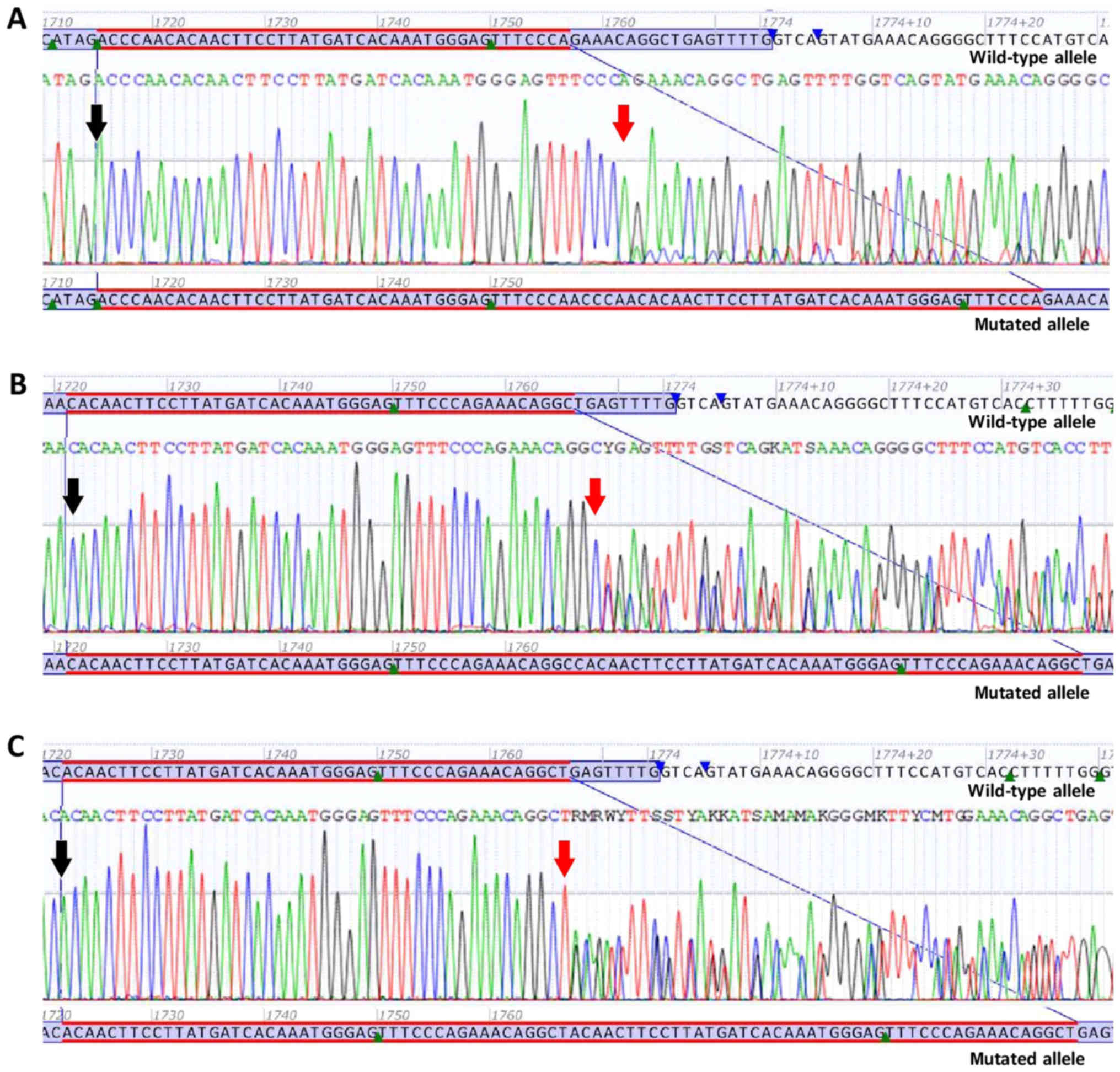
KIT (Figs. 2 and 3). The precise effect of these eight
novel variants on KIT protein function is unknown; however, based
on Cancer Genome Interpreter and ACMG/AMP criterions, all of them
were classified as likely pathogenic. The classification of all
eight novel variants found in KIT exon 11 is presented in
Table III and their descriptions
are provided in the following paragraphs.
 | Table IIIClassification of eight novel
heterozygous variants in KIT exon 11. |
Table III
Classification of eight novel
heterozygous variants in KIT exon 11.
Location of die
alteration
| In silico
prediction
| ACMG/AMP
Classification by Genetic Variant Interpretation Tool
| Cancer Genome
Interpreter
|
|---|
| Nucleotide change
(c .notation) | Amino ccid change
(p.notation) | Align GVGD | MutadonTaster2 | Criterion | Pathogenicity | Oncogenic
prediction |
|---|
| c.l652_1672del | p.(Pro55
l_Lys558dehnsGln) | Class C65 (GV: 0.00
- GD: 75.14) | Prediction disease
causing - long InDel. Model: complex_aae. prob:
0.999999999999935 | PM1.PM2. PM4.
PP3 | Likely pathogenic
(IV) | Predicted driver:
tier 1 |
|
c.l653_16bOdelinsAA |
p.(Met552_Ghi554delinsLys) | Class C65 (GV: 0.00
- GD: 94.49) | Prediction disease
causing. Model: complex_aae. prob: 0.895033008598755 | PM1.PM2. PM4.
PP3 | Likely pathogenic
(IV) | Predicted driver:
tier 1 |
|
c.l665_1672delinsCC |
p.(Trp557_Lys558del) | N/A | Prediction
polymorphism. Model: complex_aae. prob: 0.981440878256269 | PM1.PM2. PM4 | Likely pathogenic
(IV) | Predicted driver:
tier 2 |
| c.l66S_16S6del | p.(Trp557') | N/A | Prediction disease
causing. Model: complex_aae. prob. 1 | PM1.PM2. PM4.
PP3 | Likely pathogenic
(IV) | Predicted driver:
tier 2 |
| c.l676_1720del | p
.(Val559_Thr574dehnsAla) | Class C55 (GV: 0.00
- GD: 64.43) | Prediction disease
causing - long InDel. Model: complex_aae. prob: 0.
99999994804898S | PM1.PM2. PM4.
PP3 | Likely pathogenic
(IV) | Predicted driver:
tier 1 |
| c.l715_175odup |
p.(Pro5S5_Arg5Soinsl4) | N/A | Prediction
polymorphism Model: complex_aae. prob: 0.999999947411599 | PM1.PM2. PM4 | Likely pathogenic
(IV) | Predicted
passenger |
| c,1721_1765dup |
p.(Arg5S8_Leu5S9insl5) | N/A | Prediction
polymorphism. Model: complex_aae. prob: 0.999999998406041 | PM1.PM2. PM4 | Likely pathogenic
(IV) | Predicted
passenger |
| c,1722_1766dup | p .(Gln575_Leu5
89dup) | Class C65 (GV: 0.00
- GD: 112.44) | Prediction
polymorphism Model: complex_aae. prob: 0.999999998406041 | PM1.PM2. PM4 | Likely pathogenic
(IV) | Predicted driver:
tier 2 |
c.1652_1672del p.(Pro551_Lys558delinsGln) is a
deletion of 21 nucleotides in the exon 11 of the KIT gene,
from position c.1652 to position c.1672. At the protein level,
p.(Pro551_ Lys558delinsGln) results in the deletion of eight amino
acids in the JM domain of the KIT protein from amino acids 551 to
558, combined with the insertion of a glutamine (Gln) at the same
site (Fig. 2A).
c.1653_1660delinsAA p.(Met552_Glu554delinsLys) is a
deletion of eight nucleotides in exon 11 of the KIT gene,
from position c.1653 to position c.1660, and an insertion of two
adenine bases at the same position. At the protein level,
p.(Met552_Glu554delinsLys) results in the deletion of three amino
acids in the JM domain of the KIT protein from amino acids 552 to
554, combined with the insertion of a lysine (Lys) at the same site
(Fig. 2B).
c.1665_1672delinsCC p.(Trp557_Lys558del) is a
deletion of eight nucleotides in exon 11 of the KIT gene,
from position c.1665 to position c.1672, and an insertion of two
cytosine bases at the same position. At the protein level,
p.(Trp557_ Lys558del) results in the deletion of two amino acids in
the JM domain of the KIT protein from amino acids 557 to 558
(Fig. 2C).
c.1668_1686del p.(Trp557*) is a deletion
of 19 nucleotides in exon 11 of the KIT gene, from position
c.1668 to position c.1686. At the protein level,
p.(Trp557*) results in the premature termination of the
KIT protein at amino acid 557. Due to the loss of the protein
kinase domain, the mutation c.1668_1686del p.(Trp557*)
is predicted to lead to a loss of KIT protein function (Fig. 2D).
c.1676_1720del p.(Val559_Thr574delinsAla) is a
deletion of 45 nucleotides in exon 11 of the KIT gene, from
position c.1676 to position c.1720. At the protein level,
p.(Val559_Thr574delinsAla) results in the deletion of 16 amino
acids in the JM domain of the KIT protein from amino acids 559 to
574, combined with the insertion of an Alanine (Ala) at the same
site (Fig. 2E).
c.1715_1756dup p.(Pro585_Arg586ins14) is a
duplication of 42 nucleotides in exon 11 of the KIT gene,
from position c.1715 to position c.1756. At the protein level,
p.(Pro585_Arg586ins14) indicates the insertion of 14 amino acids
(Asn, Pro, Thr, Gln, Leu, Pro, Tyr, Asp, His, Lys, Trp, Glu, Phe
and Pro) in the JM domain of the KIT protein, starting with
Asparagine (Asn) at position 586 (Fig.
3A).
c.1721_1765dup p.(Arg588_Leu589ins15) is a
duplication of 45 nucleotides in exon 11 of the KIT gene,
from position c.1721 to position c.1765. At the protein level,
p.(Arg588_Leu589ins15) indicates the insertion of 15 amino acids
(Pro, Gln, Leu, Pro, Tyr, Asp, His, Lys, Trp, Glu, Phe, Pro, Arg,
Asn and Arg) in the JM domain of the KIT protein, starting with
Proline (Pro) at position 589 (Fig.
3B).
c.1722_1766dup p.(Gln575_Leu589dup) is a duplication
of 45 nucleotides in exon 11 of the KIT gene, from position
c.1722 to position c.1766. At the protein level,
p.(Gln575_Leu589dup) indicates the duplication of 15 amino acids
(Gln 575 through Leu 589) in the JM domain of the KIT protein,
starting with Gln at position 590 (Fig. 3C).
Discussion
Genotype analysis of 70 patients from the Slovenian
Cancer Registry was performed in the present study to determine the
spectrum and frequency of KIT and PDGFRA alterations
in Slovenian patients with metastatic GISTs, as well as the
frequency of hot spot mutations in KRAS, NRAS, BRAF, PIK3CA
and AKT1 in KIT/PDGFRA WT GISTs. Known mutation
status of the aforementioned genes has been proven to be an
important prognostic and predictive factor in the management and
treatment of patients with GIST with TKIs (21,23).
Mutation analysis performed on Slovenian patients
with GIST has confirmed a high degree of mutation heterogeneity in
KIT and PDGFRA genes, as reported in previous studies
(24-29). With direct Sanger sequencing of
exons 9, 11, 13 and 17 of KIT gene and exons 12, 14 and 18
of PDGFRA gene, a total of 49 different alterations were
detected. In the present study, the overall mutation rate was
88.5%, which was slightly higher than in a study from Italy (80%),
yet comparable to frequencies observed in studies from Germany
(86.1%) and Poland (85.1%), a French population-based study
(KIT, 82.8%; PDGFRA, 2.1%), and in two phase III
clinical trials that enrolled patients with metastatic GIST from
the European Organisation for Research and Treatment of Cancer
(KIT, 86.2%; PDGFRA, 1.6%) and Cancer and Leukemia
Group B (KIT, 84.6%; PDGFRA, 2.65%) (24-29).
The mutation frequency in the patients analysed in
the present study, was expectedly the highest in the KIT
gene, with most mutations in exon 11 (86.7%). The highest mutation
frequency in KIT exon 11 has also been reported in other
studies (57, 66.1 and 87.3%, respectively) (14,24,30).
In 8 patients with GIST in the present study (11.4%), no
alterations in KIT/PDGFRA genes were found. According to the
literature, ~10-15% of adult GISTs are KIT/PDGFRA WT
(13,31). All 8 patients with no alterations
in KIT or PDGFRA genes were also negative for hot
spot mutations in KRAS, NRAS, BRAF, PIK3CA and AKT1
genes, which are included in the RAS/RAF/MAPK and PI3K/AKT/mTOR
signalling pathways, suggesting that the frequency of these
mutations is low (32). As
reported by Shi et al (33), patients with KIT/PDGFRA WT
GIST can harbour mutations in EGFR. Since in the present
cohort, only 8 patients had KIT/PDGFRA WT GIST, and since
the likelihood of EGFR mutations in these patients is
<1%, the EGFR gene was not tested.
As aforementioned, the majority of identified
KIT alterations in the present study were located in exon
11, between codons 550 and 580, which is similar to results from
other studies (2,10,34-37).
The most common type of KIT exon 11 alterations identified
was an in-frame deletion of one to several codons. This is in line
with the previously described frequencies of this change,
representing 60-70% of all alterations in exon 11 (30,38,39).
Furthermore, it was observed that a high presence of deletions or
deletion/insertions in the present study was detected between
codons 556 and 561 (38.5%). Of these, a specific and commonly
reported deletion, p.(Trp557_Lys558), was identified in 9.6%, which
is the same as that reported by Emile et al (27). Similar with the reports in the
literature, the appearance of single nucleotide changes located
around the same region of KIT exon 11, at codons 557, 559
and 560, such as p.(Trp557Arg), p.(Val559Asp) and p.(Val560Asp),
was lower in the present study (5.8, 3.8 and 5.8%, respectively)
(27,40).
In the present cohort of patients with metastatic
GIST, there was a relatively high frequency (11.5%) of specific
KIT exon 9 duplication, p.(Ala502_Tyr503dup), which was
reported to be more frequent in metastatic GISTs (27,40).
In KIT exon 13, two single nucleotide
changes, c.1924A>G p.(Lys642Glu) and c.1961T>C p.(Val654Ala),
were identified. The substitution of amino acids Lys to Glu at
codon 642, has been rarely described in literature and it has been
suggested that this alteration disrupts normal auto-inhibitory
function of the JM domain (13).
Single nucleotide changes in PDGFRA were
identified in exon 18, c.2525A>T p.(Asp842Val), which is the
most common PDGFRA mutation and accounts for 60-65% of all
PDGFRA mutations seen in GIST (known to be resistant to the
imatinib treatment) (12,41). Notably, 1 patient was identified
with a rare example of double mutant GIST, which had two different
primary mutations in the PDGFRA gene, c.1936A>G
p.(Lys646Glu) and c.1975A>C p.(Asn659His), both located in exon
14. These patient samples were collected prior to the initiation of
TKI treatment. According to the literature, double mutant GISTs,
harbouring two primary mutations in the PDGFRA gene, found
prior to TKI therapy, are rare (42,43).
Most of the patients who initially respond to
imatinib develop resistance after a long period of treatment.
Resistance to imatinib has been associated with secondary
heterogeneous KIT receptor mutations in up to 90% of the patients.
Secondary mutations most commonly arise in exons 13 and 14
(ATP-binding domain) or exons 17 and 18 (the activation loop),
whereas primary KIT mutations predominantly affect the JM domain
encoded by exon 11 (44). The
present study identified 6 patients with KIT exon 13 or 17
secondary mutation. Primary tumour samples from these patients were
obtained after imatinib treatment, suggesting secondary mutations
most probably occurred as a response to the treatment, which has
been reported in previous studies (10,41,45).
In 3 out of 4 patients with secondary mutation in KIT exon
13 the same mutation was detected, a single nucleotide change
c.1961T>C p.(Val654Ala), confirming that this is the most
commonly described secondary mutation (10). Due to the existence of intra-tumour
heterogeneity in GIST, with different clones being
sensitive/resistant to different drugs, using a combination of
different drugs could be a better approach for maximal clinical
benefit (44). The most relevant
drug combination in GIST aims to circumvent upstream KIT
reactivation following imatinib failure by blocking KIT-downstream
effectors, or bypass oncoproteins or KIT-mutated receptors
themselves. If two or more not cross-resistant TKIs are used
simultaneously in patients with KIT-receptor dependant secondary
mutations, their cumulative toxic side effects are very high.
Therefore, for clinical use their simultaneous combination is
hardly feasible. However, their rapid alternation could improve the
modest clinical benefit seen when they are used sequentially as
second or third line treatment (44).
Eight novel likely pathogenic somatic variants were
found in KIT exon 11 and they have been submitted to ClinVar
database (https://www.ncbi.nlm.nih.gov/clinvar/), accession
numbers SCV000902496 - SCV000902503. In order to characterize and
classify the novel variants, the present study performed online
in silico mutation prediction analysis using Align GVGD,
MutationTaster2 and Cancer Genome Interpreter. Cancer Genome
Interpreter categorises variants using OncodriveMUT bioinformatics
method, which classifies variants into four classes: i) Predicted
driver tier 1; ii) predicted driver tier 2 (where tier 1 and 2
represent higher and lower level of stringency of the driver
prediction, respectively); iii) predicted passenger; and iv)
polymorphism. Variants pathogenicity was determined according to
ACMG/AMP guidelines using Genetic Variant Interpretation Tool
(criterion selection PM1, PM2, PM4, PP3).
Exon 11 encodes the JM domain of KIT protein that
prevents the kinase activation loop from shifting into the active
state. Alterations in exon 11 predominantly disrupt the
auto-inhibitory function and trigger ligand-independent receptor
activation (46,47). Since all eight novel variants
detected in the present study were located in a well-established
functional domain and mutational hot spot, ACMG/AMP criterion PM1
was applied. None of the novel variants are described in public
databases of human variation GnomAD (https://gnomad.broadinstitute.org/) or ExAC
(https://www.re3data.org/repository/r3d100012122),
hence ACMG/AMP criterion PM2 was applied. All of the novel variants
cause protein length change due to deletion, insertion or
duplication, therefore ACMG/AMP criterion PM4 was applied.
Five of the novel variants in KIT gene
detected in the present study were deletions or
deletions/insertions, [c.1652_1672del p.(Pro551_Lys558delinsGln),
c.1653_1660delinsAA p.(Met552_Glu554delinsLys), c.1665_1672delinsCC
p.(Trp557_Lys558del), c.1668_1686del p.(Trp557*), and
c.1676_1720del p.(Val559_Thr574delinsAla)], localized within the
most commonly mutated region of exon 11, between codons 550 and
580. Occurrence of deletions/insertions in this region of KIT
receptor are described as activating alterations, which are
according to literature, associated with malignant tumour behaviour
(2,8,48,49).
Therefore it could be predicted that newly identified variants in
the present study, [c.1652_1672del p.(Pro551_Lys558delinsGln),
c.1653_1660delinsAA p.(Met552_Glu554delinsLys), c.1676_1720del
p.(Val559_Thr574delinsAla), and c.1665_1672delinsCC
p.(Trp557_Lys558del)], are a gain-of-function mutations, leading to
protein activation. Variant c.1665_1672delinsCC
p.(Trp557_Lys558del) results in deletion of two amino acids Trp557
and Lys558. Deletion of codons 557-558 is frequently reported in
other studies and has been described to cause constitutive
activation of KIT and increased ERK phosphorylation
(38,50,51).
However, deletion of codons 557-558 as a result of this specific
deletion of eight nucleotides from c.1665 to c.1672 coupled with
insertion of two cytosines is firstly described in the present
study. Deletion of codon 557 was detected also as a result of a
novel deletion causing premature stop codon c.1668_1686del
p.(Trp557*). Because of the premature termination of the
KIT protein at amino acid 557, it was predicted that
p.(Trp557*) leads to the loss of KIT protein function.
Using Cancer Genome Interpreter, an evidence-based somatic variant
classification tool, these five novel somatic variants were
classified as predicted driver (tier 1 or tier 2), with high level
of pathogenicity. Additionally, MutationTaster2 analysis predicted
that four out of these five novel variants were disease-causing;
based on their predicted oncogenic effect the ACMG/AMP criterion
PP3 was applied.
Three of the novel variants were duplications of 17
to 45 nucleotides at the 3′-end of exon 11: c.1715_1756dup
p.(Pro585_Arg586ins14), c.1721_1765dup p.(Arg588_ Leu589ins15) and
c.1722_1766dup p.(Gln575_Leu589dup). Duplications in GISTs are
known to almost exclusively appear at the 3′-end of KIT exon
11, they range from 3 to 57 nucleotides and they are reported to
have a relatively good clinical outcome (52,53).
In conclusion, by identifying and classifying eight
novel variants in KIT exon 11, and by detecting one
double-mutant GIST with two mutations in PDGFRA exon 14, the
present study contributes to broadening the spectrum of known
mutations in GIST tumours. Mutation frequencies of KIT and
PDGFRA gene observed in the present cohort (85.7 and 2.9%,
respectively) are in accordance with previous literature. The
current results also confirm the incidence of identified
alterations in the previously reported and most frequently altered
regions of KIT or PDGFRA, and suggest that the
occurrence of alterations in other genes underlying GIST
development, including KRAS, NRAS, BRAF, PIK3CA and
AKT1, are very rare.
The present study is the first from Slovenia where
a detailed characterization of gene abnormalities in patients with
metastatic GIST from the Slovenian Cancer Registry has been
performed. Knowing the accurate mutation status of patients with
GIST is of great value for understanding GIST molecular biology,
oncogenesis and GIST subtype classification, as well for the
appropriate use of specific targeted therapy with KIT/PDGFRA
TKIs.
Supplementary Data
Acknowledgements
Not applicable.
Funding
This study received funding from the Slovenian
Research Agency (grant nos. P3-0352 and P3-0321).
Availability of data and materials
All data generated or analyzed during this study
are included in this published article.
Authors' contributions
AB wrote the manuscript, collected the related
literature, performed the majority of wet lab work, and contributed
to the analysis and interpretation of sequencing data,
bioinformatics analysis and classification of novel variants. BZ
contributed to the conception of the study, acquired tumour samples
and patient clinical data, and contributed to the revision of the
manuscript. MB contributed to the wet lab experiments and
contributed to the analysis of sequencing data. VSD contributed to
the bioinformatics analysis, and interpretation and classification
of novel variants. BG performed the pathological evaluation of
tumour samples. VS contributed to the interpretation and
classification of novel variants. GK created figures and
contributed to the interpretation and classification of novel
variants. SN contributed to the conception of this study and
designed, reviewed, wrote and revised the manuscript. All authors
read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Institute of
Oncology Ljubljana Ethic's Committee (approval no. OIRIKE00036) and
the Republic of Slovenia National Medical Ethics Committee
(approval no. 1090216). Individual patient consent was waived for
this study as it was a retrospective study, the research involved
no risk to the subjects, and the institutional informed consent
forms for treatment included consent for the use of
patient's data, materials and/or test results for research
purposes.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Miettinen M and Lasota J: Gastrointestinal
stromal tumors - definition, clinical, histological,
immunohistochemical, and molecular genetic features and
differential diagnosis. Virchows Arch. 438:1–12. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hirota S, Isozaki K, Moriyama Y, Hashimoto
K, Nishida T, Ishiguro S, Kawano K, Hanada M, Kurata A, Takeda M,
et al: Gain-of-Function Mutations of c-kit in Human
Gastrointestinal Stromal Tumors. Science. 279:577–580. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Corless CL and Heinrich MC: Molecular
Pathobiology of Gastrointestinal Stromal Sarcomas. Annu Rev Pathol
Mech Dis. 3:557–586. 2008. View Article : Google Scholar
|
|
4
|
Miettinen M and Lasota J: Gastrointestinal
stromal tumors: Pathology and prognosis at different sites. Semin
Diagn Pathol. 23:70–83. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Duensing A, Medeiros F, McConarty B,
Joseph NE, Panigrahy D, Singer S, Fletcher CDM, Demetri GD and
Fletcher JA: Mechanisms of oncogenic KIT signal transduction in
primary gastrointestinal stromal tumors (GISTs). Oncogene.
23:3999–4006. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Miettinen M and Lasota J: Gastrointestinal
Stromal Tumors Review on Morphology, Molecular Pathology,
Prognosis, and Differential Diagnosis. Arch Pathol Lab Med.
130:1466–1478. 2006.PubMed/NCBI
|
|
7
|
Heinrich MC, Rubin BP, Longley BJ and
Fletcher JA: Biology and genetic aspects of gastrointestinal
stromal tumors: KIT activation and cytogenetic alterations. Hum
Pathol. 33:484–495. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Corless CL, Fletcher JA and Heinrich MC:
Biology of gastrointestinal stromal tumors. J Clin Oncol.
22:3813–3825. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Miettinen M, El-Rifai W, H L Sobin L and
Lasota J: Evaluation of malignancy and prognosis of
gastrointestinal stromal tumors: A review. Hum Pathol. 33:478–483.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lasota J and Miettinen M: Clinical
significance of oncogenic KIT and PDGFRA mutations in
gastrointestinal stromal tumours. Histopathology. 53:245–266. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Heinrich MC, Corless CL, Duensing A,
McGreevey L, Chen CJ, Joseph N, Singer S, Griffith DJ, Haley A,
Town A, et al: PDGFRA activating mutations in gastrointestinal
stromal tumors. Science. 299:708–710. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Corless CL, Schroeder A, Griffith D, Town
A, McGreevey L, Harrell P, Shiraga S, Bainbridge T, Morich J and
Heinrich MC: PDGFRA mutations in gastrointestinal stromal tumors:
Frequency, spectrum and in vitro sensitivity to imatinib. J Clin
Oncol. 23:5357–5364. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Corless CL, Barnett CM and Heinrich MC:
Gastrointestinal stromal tumours: Origin and molecular oncology.
Nat Rev Cancer. 11:865–878. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Joensuu H, Hohenberger P and Corless CL:
Gastrointestinal stromal tumour. Lancet. 382:973–983. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Daniels M, Lurkin I, Pauli R, Erbstösser
E, Hildebrandt U, Hellwig K, Zschille U, Lüders P, Krüger G, Knolle
J, et al: Spectr um of KIT/PDGF RA/BRAF mutations and
Phosphatidylinositol-3-Kinase pathway gene alterations in
gastro-intestinal stromal tumors (GIST). Cancer Lett. 312:43–54.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Miranda C, Nucifora M, Molinari F, Conca
E, Anania MC, Bordoni A, Saletti P, Mazzucchelli L, Pilotti S,
Pierotti MA, et al: KRAS and BRAF mutations predict primary
resistance to imatinib in gastrointestinal stromal tumors. Clin
Cancer Res. 18:1769–1776. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Pantaleo MA, Nannini M, Corless CL and
Heinrich MC: Quadruple wild-type (WT) GIST: Defining the subset of
GIST that lacks abnormalities of KIT, PDGFRA, SDH, or RAS signaling
pathways. Cancer Med. 4:101–103. 2015. View Article : Google Scholar :
|
|
18
|
Lasota J, Felisiak-Golabek A, Wasag B,
Kowalik A, Zięba S, Chłopek M, Wang ZF, Coates T, Kopczynski J,
Gozdz S, et al: Frequency and clinicopathologic profile of PIK3CA
mutant GISTs: Molecular genetic study of 529 cases. Mod Pathol.
29:275–282. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Drilon A, Laetsch TW, Kummar S, DuBois SG,
Lassen UN, Demetri GD, Nathenson M, Doebele RC, Farago AF, Pappo
AS, et al: Efficacy of larotrectinib in TRK fusion-positive cancers
in adults and children. N Engl J Med. 378:731–739. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Isozaki K and Hirota S: Gain-of-Function
Mutations of Receptor Tyrosine Kinases in Gastrointestinal Stromal
Tumors. Curr Genomics. 7:469–475. 2006. View Article : Google Scholar
|
|
21
|
Nishida T, Takahashi T and Miyazaki Y:
Gastrointestinal stromal tumor: A bridge between bench and bedside.
Gastric Cancer. 12:175–188. 2009. View Article : Google Scholar
|
|
22
|
Fletcher JA and Rubin BP: KIT mutations in
GIST. Curr Opin Genet Dev. 17:3–7. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Gramza AW, Corless CL and Heinrich MC:
Resistance to tyrosine kinase inhibitors in gastrointestinal
stromal tumors. Clin Cancer Res. 15:7510–7518. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Origone P, Gargiulo S, Mastracci L,
Ballestrero A, Battistuzzi L, Casella C, Comandini D, Cusano R, Dei
Tos AP, Fiocca R, et al Liguria GIST Unit: Molecular
characterization of an Italian series of sporadic GISTs. Gastric
Cancer. 16:596–601. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Penzel R, Aulmann S, Moock M, Schwarzbach
M, Rieker RJ and Mechtersheimer G: The location of KIT and PDGFRA
gene mutations in gastrointestinal stromal tumours is site and
phenotype associated. J Clin Pathol. 58:634–639. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wozniak A, Rutkowski P, Schöffski P,
Ray-Coquard I, Hostein I, Schildhaus HU, Le Cesne A, Bylina E,
Limon J, Blay JY, et al: Tumor genotype is an independent
prognostic factor in primary gastrointestinal stromal tumors of
gastric origin: A european multicenter analysis based on
ConticaGIST. Clin Cancer Res. 20:6105–6116. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Emile JF, Brahimi S, Coindre JM, Bringuier
PP, Monges G, Samb P, Doucet L, Hostein I, Landi B, Buisine MP, et
al: Frequencies of KIT and PDGFRA mutations in the MolecGIST
prospective population-based study differ from those of advanced
GISTs. Med Oncol. 29:1765–1772. 2012. View Article : Google Scholar
|
|
28
|
Debiec-Rychter M, Sciot R, Le Cesne A,
Schlemmer M, Hohenberger P, van Oosterom AT, Blay JY, Leyvraz S,
Stul M, Casali PG, et al EORTC Soft Tissue and Bone Sarcoma Group;
Italian Sarcoma Group; Australasian GastroIntestinal Trials Group:
KIT mutations and dose selection for imatinib in patients with
advanced gastrointestinal stromal tumours. Eur J Cancer.
42:1093–1103. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Heinrich MC, Owzar K, Corless CL, Hollis
D, Borden EC, Fletcher CDM, Ryan CW, Von Mehren M, Blanke CD,
Rankin C, et al: Correlation of kinase genotype and clinical
outcome in the North American intergroup phase III trial of
imatinib mesylate for treatment of advanced gastrointestinal
stromal tumor: CALGB 150105 study by cancer and leukemia group B
and southwest oncology gr. J Clin Oncol. 26:5360–5367. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Andersson J, Bümming P, Meis-Kindblom JM,
Sihto H, Nupponen N, Joensuu H, Odén A, Gustavsson B, Kindblom L-G
and Nilsson B: Gastrointestinal stromal tumors with KIT exon 11
deletions are associated with poor prognosis. Gastroenterology.
130:1573–1581. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Boikos SA, Pappo AS, Killian JK, LaQuaglia
MP, Weldon CB, George S, Trent JC, von Mehren M, Wright JA,
Schiffman JD, et al: Molecular Subtypes of KIT/PDGFRAWild-Type
Gastrointestinal Stromal Tumors. JAMA Oncol. 2:9222016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Huss S, Pasternack H, Ihle MA,
Merkelbach-Bruse S, Heitkötter B, Hartmann W, Trautmann M,
Gevensleben H, Büttner R, Schildhaus HU, et al: Clinicopathological
and molecular features of a large cohort of gastrointestinal
stromal tumors (GISTs) and review of the literature: BRAF mutations
in KIT/PDGFRA wild-type GISTs are rare events. Hum Pathol.
62:206–214. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Shi SS, Wu N, He Y, Wei X, Xia QY, Wang X,
Ye S, Li R, Rao Q and Zhou X-J: Bin, Li R, Rao Q and Zhou XJ: EGFR
gene mutation in gastrointestinal stromal tumours. Histopathology.
71:553–561. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Nakahara M, Isozaki K, Hirota S, Miyagawa
J, Hase-Sawada N, Taniguchi M, Nishida T, Kanayama S, Kitamura Y
and Shinomura Y: A novel gain-of-function mutation of c-kit gene in
gastrointestinal stromal tumors. Gastroenterology. 115:1090–1095.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Lasota J, Jasinski M, Sarlomo-Rikala M and
Miettinen M: Mutations in exon 11 of c-Kit occur preferentially in
malignant versus benign gastrointestinal stromal tumors and do not
occur in leiomyomas or leiomyosarcomas. Am J Pathol. 154:53–60.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Taniguchi M, Nishida T, Hirota S, Isozaki
K, Ito T, Nomura T, Matsuda H and Kitamura Y: Effect of c-kit
mutation on prognosis of gastrointestinal stromal tumors. Cancer
Res. 59:4297–4300. 1999.PubMed/NCBI
|
|
37
|
Moskaluk CA, Tian Q, Marshall CR, Rumpel
CA, Franquemont DW and Frierson HF Jr: Mutations of c-kit JM domain
are found in a minority of human gastrointestinal stromal tumors.
Oncogene. 18:1897–1902. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Wardelmann E, Losen I, Hans V, Neidt I,
Speidel N, Bierhoff E, Heinicke T, Pietsch T, Büttner R and
Merkelbach-Bruse S: Deletion of Trp-557 and Lys-558 in the
juxtamembrane domain of the c-kit protooncogene is associated with
metastatic behavior of gastrointestinal stromal tumors. Int J
Cancer. 106:887–895. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Martín-Broto J, Rubio L, Alemany R and
López-Guerrero JA: Clinical implications of KIT and PDGFRA
genotyping in GIST. Clin Transl Oncol. 12:670–676. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Van Glabbeke M; Gastrointestinal Stromal
Tumor Meta-Analysis Group (MetaGIST): Comparison of two doses of
imatinib for the treatment of unresectable or metastatic
gastrointestinal stromal tumors: A meta-analysis of 1,640 patients.
J Clin Oncol. 28:1247–1253. 2010. View Article : Google Scholar
|
|
41
|
Lasota J and Miettinen M: KIT and PDGFRA
mutations in gastrointestinal stromal tumors (GISTs). Semin Diagn
Pathol. 23:91–102. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Conca E, Miranda C, Dal Col V, Fumagalli
E, Pelosi G, Mazzoni M, Fermeglia M, Laurini E, Pierotti MA,
Pilotti S, et al: Are two better than one? A novel double-mutant
KIT in GIST that responds to Imatinib. Mol Oncol. 7:756–762. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Pai T, Bal M, Shetty O, Gurav M, Ostwal V,
Ramaswamy A, Ramadwar M and Desai S: Unraveling the spectrum of KIT
mutations in gastrointestinal stromal tumors: An Indian Tertiary
Cancer Center Experience. South Asian J Cancer. 6:113–117. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Serrano C, Mariño-Enríquez A, Tao DL,
Ketzer J, Eilers G, Zhu M, Yu C, Mannan AM, Rubin BP, Demetri GD,
et al: Complementary activity of tyrosine kinase inhibitors against
secondary kit mutations in imatinib-resistant gastrointestinal
stromal tumours. Br J Cancer. 120:612–620. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Wang CM, Huang K, Zhou Y, Du CY, Ye YW, Fu
H, Zhou XY and Shi YQ: Molecular mechanisms of secondary imatinib
resistance in patients with gastrointestinal stromal tumors. J
Cancer Res Clin Oncol. 136:1065–1071. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Gajiwala KS, Wu JC, Christensen J,
Deshmukh GD, Diehl W, Dinitto JP, English JM, Greig MJ, He Y-A,
Jacques SL, et al: KIT kinase mutants show unique mechanisms of
drug resistance to imatinib and sunitinib in gastrointestinal
stromal tumor patients. Proc Natl Acad Sci U S A. 106:1542–1547.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Mol CD, Dougan DR, Schneider TR, Skene RJ,
Kraus ML, Scheibe DN, Snell GP, Zou H, Sang BC and Wilson KP:
Structural basis for the autoinhibition and STI-571 inhibition of
c-Kit tyrosine kinase. J Biol Chem. 279:31655–31663. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Roskoski R Jr: Structure and regulation of
Kit protein-tyrosine kinase - the stem cell factor receptor.
Biochem Biophys Res Commun. 338:1307–1315. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Bachet JB, Hostein I, Le Cesne A, Brahimi
S, Beauchet A, Tabone-Eglinger S, Subra F, Bui B, Duffaud F,
Terrier P, et al: Prognosis and predictive value of KIT exon 11
deletion in GISTs. Br J Cancer. 101:7–11. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Garner AP, Gozgit JM, Anjum R, Vodala S,
Schrock A, Zhou T, Serrano C, Eilers G, Zhu M, Ketzer J, et al:
Ponatinib inhibits polyclonal drug-resistant KIT oncoproteins and
shows thera-peutic potential in heavily pretreated gastrointestinal
stromal tumor (GIST) patients. Clin Cancer Res. 20:5745–5755. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Wang HC, Li TY, Chao YJ, Hou YC, Hsueh YS,
Hsu KH and Shan YS: Kit exon 11 codons 557-558 deletion mutation
promotes liver metastasis through the CXCL12/CXCR4 Axis in
gastroin-/CXCR4 Axis in gastroin CXCR4 Axis in gastroin--testinal
stromal tumors. Clin Cancer Res. 22:3477–3487. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Wozniak A, Rutkowski P, Piskorz A,
Ciwoniuk M, Osuch C, Bylina E, Sygut J, Chosia M, Rys J, Urbanczyk
K, et al Polish Clinical GIST Registry: Prognostic value of
KIT/PDGFRA mutations in gastrointestinal stromal tumours (GIST):
Polish Clinical GIST Registry experience. Ann Oncol. 23:353–360.
2012. View Article : Google Scholar
|
|
53
|
Lasota J, Wasag B, Steigen SE, Limon J and
Miettinen M: Improved detection of KIT exon 11 duplications in
formalin-fixed, paraffin-embedded gastrointestinal stromal tumors.
J Mol Diagn. 9:89–94. 2007. View Article : Google Scholar : PubMed/NCBI
|