Introduction
Acute myeloid leukemia (AML) is the most common
acute leukemia in adults, with an yearly incidence of 5.5 per
100,000 population, and a mortality of 55.5% in the United States
(1). The incidence of AML
increases with age, with a 5-year survival rate of 26.6% in the
United States (1-3). The conventional chemotherapy regimen
for AML is the daunorubicin (D) + cytarabine (A) ′7+3′ modality (A
treatment for 7 days combined with D treatment for 3 days; 3 days
of combined treatment followed by a further 4 days of A), which can
achieve complete remission (CR) in 60-80% of adult patients
(4); however, most patients
inevitably experience recurrence. Furthermore, 20-30% of newly
diagnosed patients do not achieve CR (4-6).
Therefore, there is an urgent requirement to introduce a novel
effective induction modality into the clinic to increase the CR
rate (CRR) and reduce the recurrence rate to improve long-term
survival.
Previous studies have reported that AML is caused by
pre-leukemia hematopoietic stem cells (pre-HSCs) that do not
respond to chemotherapy and lead to disease relapse (7-9). In
addition, pre-HSCs carry mutations in DNA methylation genes,
including DNA methyltransferase 3α (DNMT3A), tet methyl-cytosine
dioxygenase 2 (TET2) and isocitrate dehydrogenase isozyme (IDH)1/2,
which can persist from AML initiation to relapse (7-9).
Whole-genome hypermethylation is associated with poor clinical
outcome, and gene promoter-specific hypermethylation is an
important step in tumor development during AML (10). However, DNMT3A is associated with
hypomethylation, whereas TET2 and IDH1/2 display global and
gene-specific hypermethylation during AML (11,12).
Therefore, studying the efficacy of demethylation drugs in patients
with AML is crucial.
The demethylating drug decitabine, a natural
adenosine analogue of 2′-deoxycytidine, reduces DNA methylation by
inhibiting DNA methyltransferase (6). Decitabine inhibits tumor cell
proliferation and prevents drug resistance. As mutations in DNA
methylation genes persist from initiation to relapse, decitabine
may improve the clinical efficacy against AML by reducing the
frequency of DNA methylation (3).
Recent studies have suggested that decitabine can improve the
remission rate of elderly patients with AML (3,4,13).
However, it has not been reported whether decitabine is beneficial
in younger patients or reduces the recurrence rate of AML.
Therefore, in the present study, the therapeutic effects of
decitabine in combination with the DA induction modality in de
novo non-elderly patients with AML were investigated.
Materials and methods
Patients
A total of 81 patients with de novo AML
(non-M3) who received D + A or equivalent modalities [D + A,
deme-thoxydaunorubicin (I) + A or homoharringtonine (H) + A] with
or without decitabine as induction chemotherapy were recruited
retrospectively between January 2017 and December 2018 at Huazhong
University of Science and Technology Affiliated Tongji Hospital.
AML was diagnosed according to the criteria of the World Health
Organization (WHO) (14) and
French-American-British (FAB) classification (15). Inclusion criteria were as follows:
i) Aged 14-65 years; ii) patients with AML (non-M3) with clear
diagnosis meeting the WHO 2008/2016 standard and FAB
classification; iii) newly diagnosed AML after January 1st 2017;
iv) received ≥2 courses of D + A, I + A or H + A modalities with or
without decitabine (20 mg/m2/d) for 5 days; and v) an
Eastern Cooperative Oncology Group (ECOG) score ≤2 points.
Exclusion criteria were as follows: i) Allergic to decitabine; ii)
patients who had a previous AML diagnosis; iii) transformation of
myelodysplastic syndrome (MDS) or other hematological diseases,
including bone marrow fibrosis; iv) central nervous system
invasion; v) other serious diseases, including myocardial
infarction, severe or unstable angina, severe arrhythmia or
cerebrovascular events (including transient cerebral ischemia); and
vi) <2 courses of treatment, or treatment efficacy not assessed.
The present study was approved by the Ethics Committee of Tongji
Hospital, Tongji Medical College, Huazhong University of Science
and Technology and was conducted in accordance with the Declaration
of Helsinki. Written informed consent was not obtained from the
patients, as the Ethics Committee approved the application for
exemption of informed consent. All patients received follow-up at
the clinic or via a telephone call. The span of follow-up time was
24 months (median, 10 months; range, 1-24 months).
The present study recruited 43 male and 38 female
patients (male:female, 1.13:1; age, 14-71 years). The observation
group (decitabine with D + A, I + A or H + A modalities) consisted
of 35 patients, and the control group (D + A, I + A or H + A
modalities without decitabine) consisted of 46 patients. The ECOG
performance status score ranged from 0 to 2. No significant
differences in sex, age, blood cell count or organ function were
identified between the two groups.
Study design and treatment
Patients with leukocytosis were treated with
hydroxyurea or A to reduce leukocyte counts to ≤50×109/l
prior to initiation of induction therapy. Risk stratification was
determined by cytogenetics and molecular aberrations according to
the National Comprehensive Cancer Network and European Leukemia Net
criteria (16,17). After 1-2 cycles of treatment, the
initial response was evaluated. Post-remission treatment included
consolidation of intensive therapy and/or HSC transplantation.
Patients who achieved CR or partial remission (PR) after 1-2
courses of induction chemotherapy were treated with the original
regimen, medium dose A as consolidation therapy or transplantation.
The efficacy of consolidation therapy was comparable between the
two groups. Follow-up was continued until relapse. The specific
induction schemes were as follows: i) The observation group
received decitabine with D + A (14 patients), I + A (16 patients)
or H + A (5 patients) modalities, and the control group received D
+ A (17 patients), I + A (20 patients) or H + A (9 patients)
modalities. The specific protocol for the observation group was as
follows: Decitabine (20 mg/m2/day) for 5 days + A
(100-200 mg/m2/day) for 7 days + D (60
mg/m2/day) for 3 days, I (10 mg/m2/day) for 3
days or H (3 mg/m2/day) for 7 days. The specific
protocol for the control group was as follows: A (100-200
mg/m2/day) for 7 days + D (60 mg/m2/day) for
3 days, I (10 mg/m2/day) for 3 days or H (3
mg/m2/day) for 7 days.
Response criteria and outcome
evaluation
The following conditions were defined according to
the International Working Group criteria (18). CR was defined as <5% bone marrow
blasts, no blasts with Auer rods, the absence of extramedullary
disease, an absolute neutrophil count >1.0×109/l or a
platelet count ≥100×109/l. PR was defined as a ≥50%
decrease (5-25%) in the frequency of blasts detected in bone marrow
aspirates and normalized blood counts. Overall remission rate (ORR)
was calculated as the sum of CR + PR. Non-remission was defined as
a response that had achieved neither CR nor PR. Relapse was defined
as the reappearance of leukemia cells in the peripheral blood or
>5% blasts in the bone marrow. Overall survival (OS) was defined
as the time from diagnosis to death or the follow-up deadline.
Recurrence-free survival (RFS) was defined as the period between
remission by induction chemotherapy and relapse or death. The
follow-up deadline was December 30th 2018.
Statistical analysis
Statistical analyses were performed using SPSS
(version 20.0; IBM Corp.) and GraphPad Prism (version 7.0; GraphPad
Prism, Inc.) software. Data are expressed as the mean ± standard
deviation. Comparisons were performed using Student′s t-test.
Categorical data were compared using χ2 test,
χ2 test with correction for continuity or Fisher′s exact
test as applicable. The Kaplan-Meier method was used to plot
survival curves and survival data (RFS and OS probability) were
analyzed by the log-rank test. P<0.05 were considered to
indicate statistically significant differences.
Results
Patient characteristics
A total of 81 patients were included in the present
study, including 43 male and 38 female patients. The median age of
the patients was 58.5 years (age range, 14-71 years). The
observation group consisted of 35 patients and the control group
consisted of 46 patients. There were no significant differences in
sex, age or peripheral blood cell count between the two groups
(P>0.05). The adverse effects of each group were compared, and
no significant differences were observed. Patient characteristics
are summarized in Tables I and
II.
 | Table IClinical characteristics of patients
with acute myeloid leukemia. |
Table I
Clinical characteristics of patients
with acute myeloid leukemia.
A, Favorable risk
|
|---|
| Characteristics | Control group | Observation
group | P-value |
|---|
| Sex | | | >0.999 |
| Male | 2 | 2 | |
| Female | 4 | 2 | |
| Age, years, median
(range) | 37 (22-46) | 37 (22-70) | 0.519 |
| WBC count,
x109/l | | | |
| Mean ± SD | 20.23±11.23 | 14.81±10.30 | 0.462 |
| Median (range) | 22.06
(2.32-26.15) | 15.66
(1.63-26.29) | |
| Hb, g/l | | | |
| Mean ± SD | 71.33±18.80 | 56.75±7.19 | 0.183 |
| Median
(range) | 62.50
(57.00-106.00) | 57.50
(48.00-64.00) | |
| PLT,
×109/l | | | |
| Mean ± SD | 36.00±13.27 | 28.50±11.45 | 0.384 |
| Median
(range) | 36.50
(15.00-54.00) | 30.50
(13.00-40.00) | |
|
| B, Intermediate
risk |
|
|
Characteristics | Control group | Observation
group | P-value |
|
| Sex | | | 0.949 |
| Male | 18 | 14 | |
| Female | 12 | 9 | |
| Age, years, median
(range) | 39 (14-66) | 43 (15-63) | 0.791 |
| WBC count,
×109/l | | | |
| Mean ± SD | 48.22±62.47 | 52.41±104.77 | 0.861 |
| Median
(range) | 17.085
(1.22-232.83) | 10.09
(0.38-486.69) | |
| Hb, g/l | | | |
| Mean ± SD | 82.89±20.41 | 76.91±16.16 | 0.266 |
| Median
(range) | 84.00
(43.00-127.00) | 75.50
(51.00-105.00) | |
| PLT,
×109/l | | | |
| Mean ± SD | 52.64±41.09 | 68.27±60.50 | 0.306 |
| Median
(range) | 38.5
(4.00-205.00) | 39.00
(2.00-219.00) | |
|
| C, Unfavorable
risk |
|
|
Characteristics | Control group | Observation
group | P-value |
|
| Sex | | | >0.999 |
| Male | 4 | 3 | |
| Female | 6 | 5 | |
| Age, years, median
(range) | 39 (30-57) | 55.5 (28-71) | 0.081 |
| WBC count,
×109/l | | | |
| Mean ± SD | 25.81±30.82 | 32.65±46.48 | 0.713 |
| Median
(range) | 17.48
(4.43-104.55) | 9.51
(0.45-133.19) | |
| Hb, g/l | | | |
| Mean ± SD | 80.30±20.68 | 93.00±34.45 | 0.356 |
| Median
(range) | 79.50
(49.00-110.00) | 76.00
(67.00-153.00) | |
| PLT,
×109/l | | | |
| Mean ± SD | 59.20±40.93 | 41.00±23.62 | 0.309 |
| Median
(range) | 46.50
(16.00-136.00) | 35.00
(16.00-82.00) | |
 | Table IIClinical characteristics of patients
with acute myeloid leukemia. |
Table II
Clinical characteristics of patients
with acute myeloid leukemia.
|
Characteristics | Observational
group, n (%) | Control group, n
(%) |
|---|
| Sex | | |
| Male | 19 (54) | 24 (52) |
| Female | 16 (46) | 22 (48) |
| Age | | |
| Median
(range) | 55 (15-71) | 49 (14-66) |
| FAB
classification | | |
| M0 | 1 (3) | 0 (0) |
| M1 | 2 (6) | 5 (11) |
| M2 | 11 (30) | 13 (28) |
| M4 | 4 (11) | 8 (17) |
| M5 | 16 (47) | 20 (44) |
| M7 | 1 (3) | 0 (0) |
| Gene mutation | | |
| Methylation | | |
| DNMT3A | 4 (11) | 2 (4) |
| TET2 | 1 (3) | 1 (2) |
| IDH2 | 2 (6) | 3 (7) |
|
Non-methylation | | |
| NPM1 | 2 (6) | 3 (7) |
| CEBPA | 7 (20) | 12 (26) |
| FLT3-TKD | 1 (3) | 2 (4) |
| FLT3-ITD | 6 (17) | 7 (15) |
| CBFβ-MYH11 | 4 (11) | 5 (11) |
| AML1-ETO | 4 (11) | 7 (15) |
| C-kit | 2 (6) | 10 (22) |
| NUP98-HOX11 | 1 (3) | 0 (0) |
| ASXL1 | 0 (0) | 1 (2) |
| EZH2 | 0 (0) | 1 (2) |
| DEK/CAN | 0 (0) | 1 (2) |
| MLL | 3 (9) | 4 (9) |
| Cytogenetics | | |
| t(8;21) | 3 (9) | 8 (17) |
| inv(16) | 4 (11) | 5 (11) |
| Complex
karyotype | 1 (3) | 1 (2) |
| Risk
stratificationa | | |
| Favorable | 4 (11) | 6 (13) |
| Intermediate | 23 (66) | 30 (65) |
| Unfavorable | 8 (23) | 10 (22) |
| Allo-HSCT | 4 (11) | 6 (13) |
| Adverse
eventb | | |
| Febrile
neutropenia (grade 3-4) | 15 (43) | 19 (1) |
| Abdominal pain
(grade 2) | 0 (0) | 1 (2) |
| Oral pain (grade
2) | 0 (0) | 1 (2) |
| Vomiting (grade
1-2) | 10 (29) | 14 (30) |
| Multi-organ
failure (grade 3) | 0 (0) | 1 (2) |
| Gallbladder
infection (grade 3) | 1 (3) | 0 (0) |
| Lung infection
(grade 3-4) | 8 (23) | 14 (30) |
| Skin infection
(grade 2-3) | 2 (6) | 2 (4) |
| Sepsis (grade
4) | 1 (3) | 1 (2) |
Response to induction therapy
In the observation group, 32 patients achieved CR
and 3 patients achieved PR. The observation group displayed a 91.4%
CRR (95% CI, 81.7-100%) and 100% ORR (95% CI, 100-100%). In the
control group, 32 patients achieved CR and 7 patients achieved PR;
therefore, the CRR was 69.6% (95% CI, 55.8-83.4%) and the ORR was
84.8% (95% CI, 74-95.6%). Significant differences in CRR and ORR
were identified between the observation and control groups (P=0.017
and P=0.044, respectively; Table
III).
 | Table IIIComparison of efficacy between
control and observation groups. |
Table III
Comparison of efficacy between
control and observation groups.
| Group | N | CR | PR | NR | CR, % | ORR, % |
|---|
| Observation
group | 35 | 32 | 3 | 0 | 91.4 | 100 |
| Control group | 46 | 32 | 7 | 7 | 69.6 | 84.8 |
Based on cytogenetic abnormalities and molecular
mutations, 81 de novo patients with AML were stratified into
favorable risk, intermediate risk and unfavorable risk groups
(Table IV). For patients with
intermediate and unfavorable genetic aberrations, the observation
group displayed an improved CRR compared with the control group
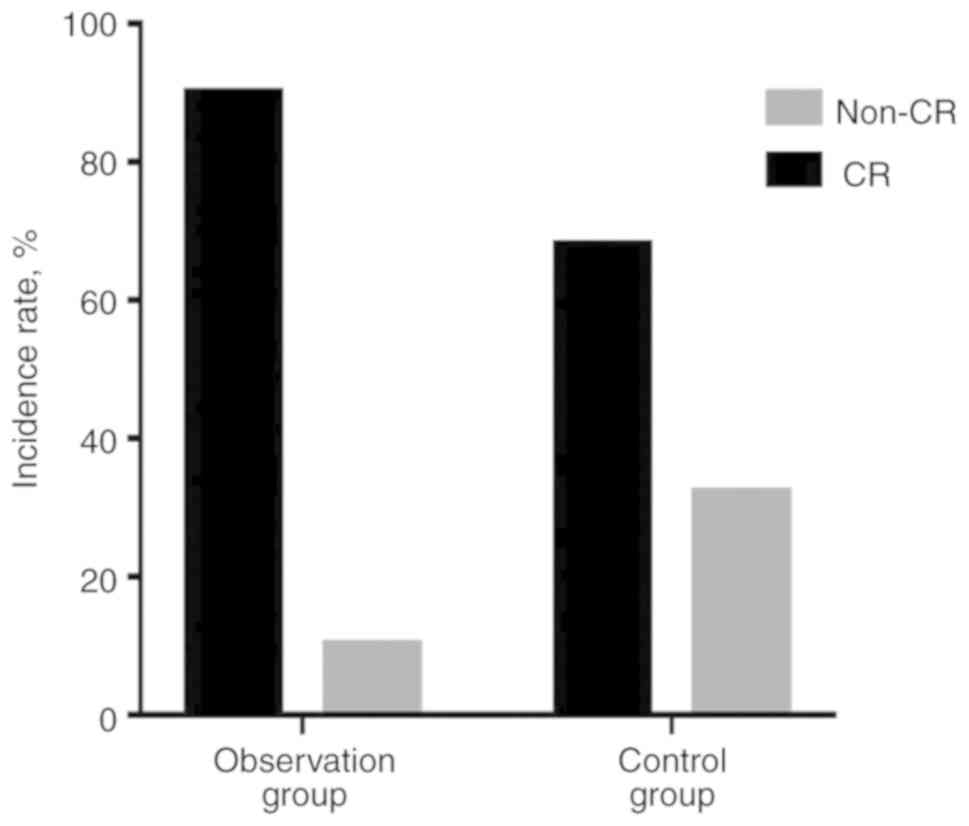
(90.3 vs. 67.5%, respectively; P=0.022; Fig. 1). In patients without methylation
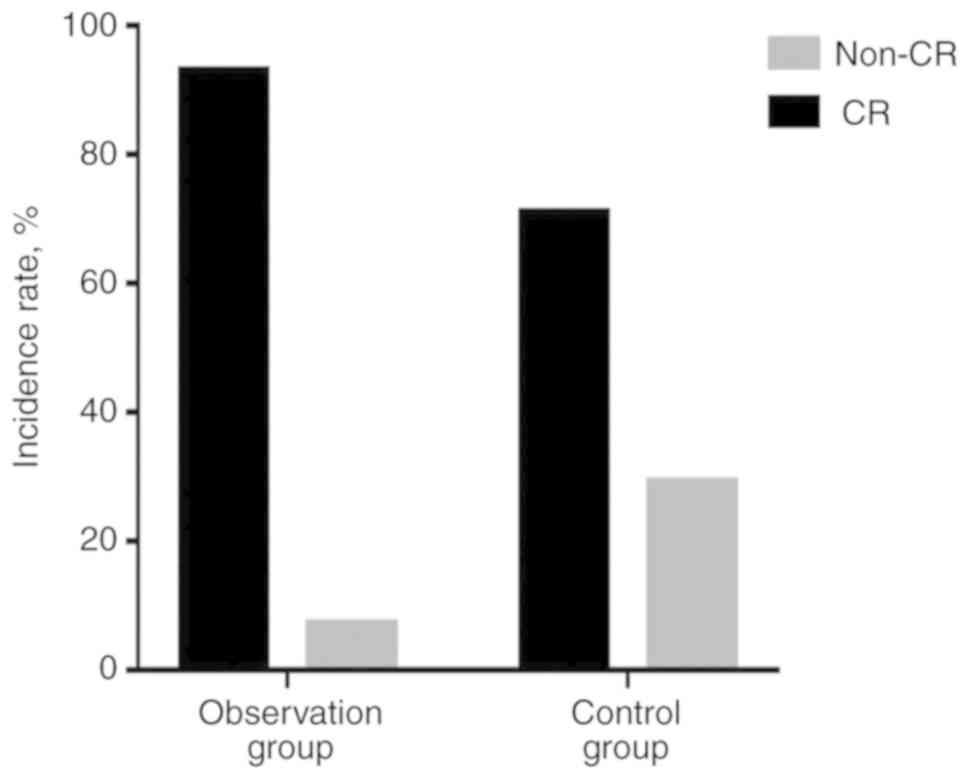
gene mutations, the CRR was improved in the observation group
compared with the control group (92.9 vs. 71.4%, respectively;
P=0.028; Fig. 2).
 | Table IVComparison of efficacy between the
two groups stratified by favorable risk, intermediate risk and
unfavorable risk. |
Table IV
Comparison of efficacy between the
two groups stratified by favorable risk, intermediate risk and
unfavorable risk.
| Risk group | CR (%) | Non-CR (%) |
|---|
| Favorable risk | | |
| Observation
group | 4 (100) | 0 (0) |
| Control group | 5 (83.3) | 1 (16.7) |
| Intermediate
risk | | |
| Observation
group | 21 (91.3) | 2 (8.7) |
| Control group | 21 (70) | 9 (30) |
| Unfavorable
risk | | |
| Observation
group | 7 (87.5) | 1 (12.5) |
| Control group | 6 (60) | 4 (40) |
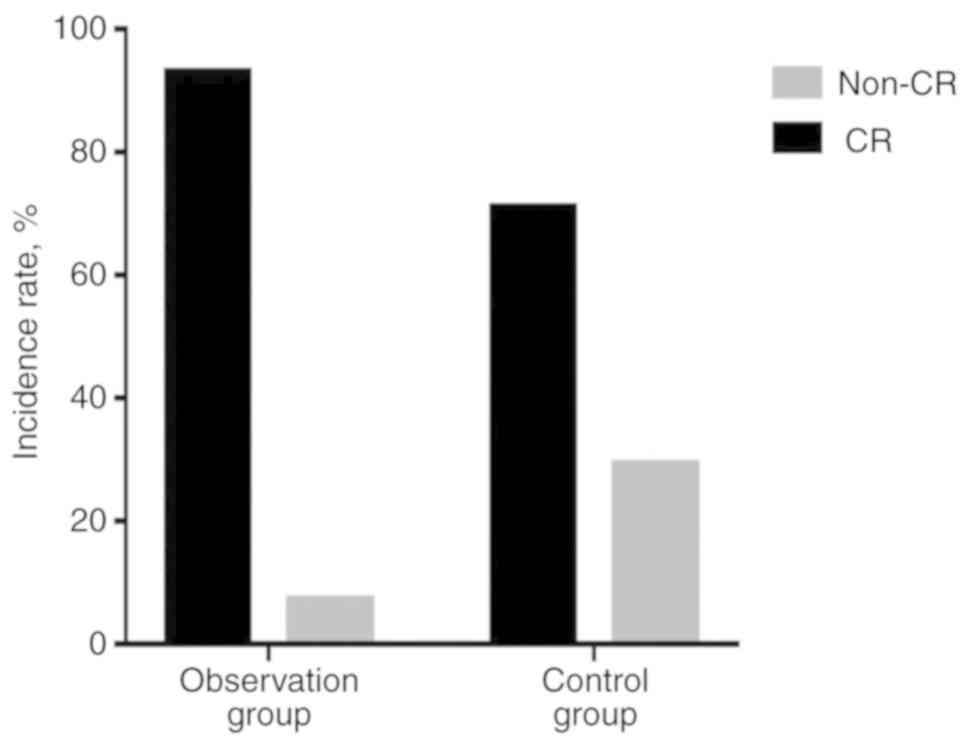
According to patient age, patients were grouped into
<60 and ≥60 years groups. The observation group consisted of 28
patients aged <60 years and the control group consisted of 45
patients aged <60 years. The ORR did not differ significantly
between the observation and the control groups (100 vs. 84.4%,
respectively; P=0.074); however, the CRR was improved in the
observation group compared with that in the control group (92.9 vs.
71.1%, respectively; P=0.025; Fig.
3).
OS and RFS
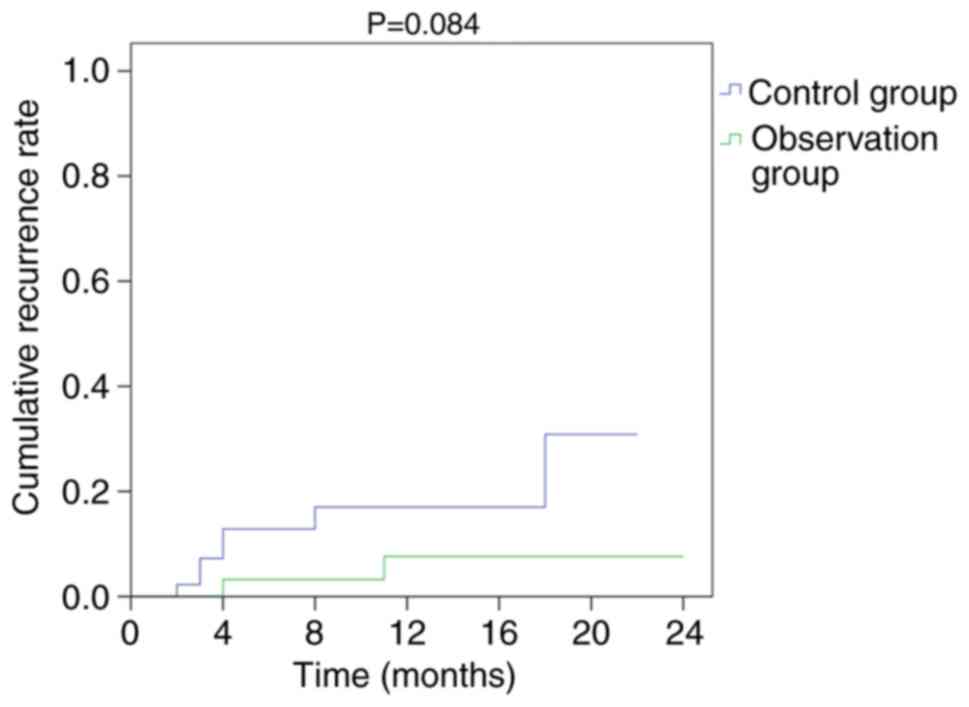
Among the 81 patients, 9 developed recurrence during
follow-up, including 2 patients in the observation group (5.7%) and
7 patients in the control group (15.2%). The recurrence rate curves
of the two groups are presented in Fig. 4. Although the 2-year recurrence
rate was similar between the two groups (P>0.05), the 2-year OS
rate was improved in the observation group compared with that in
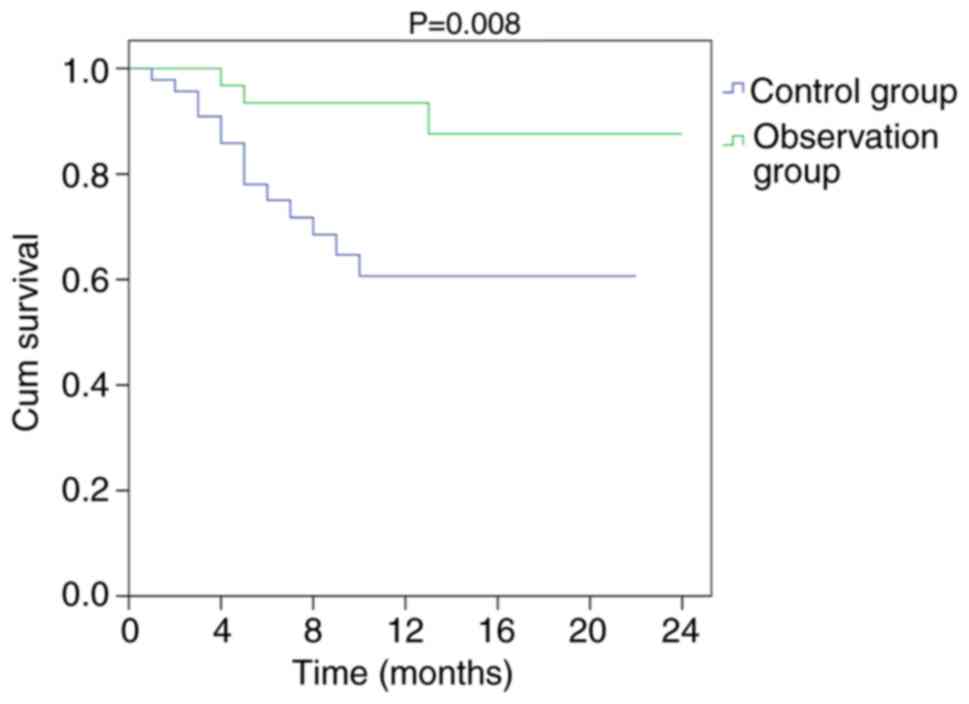
the control group (P=0.008). The OS curves of the two groups are
presented in Fig. 5. The 2-year OS
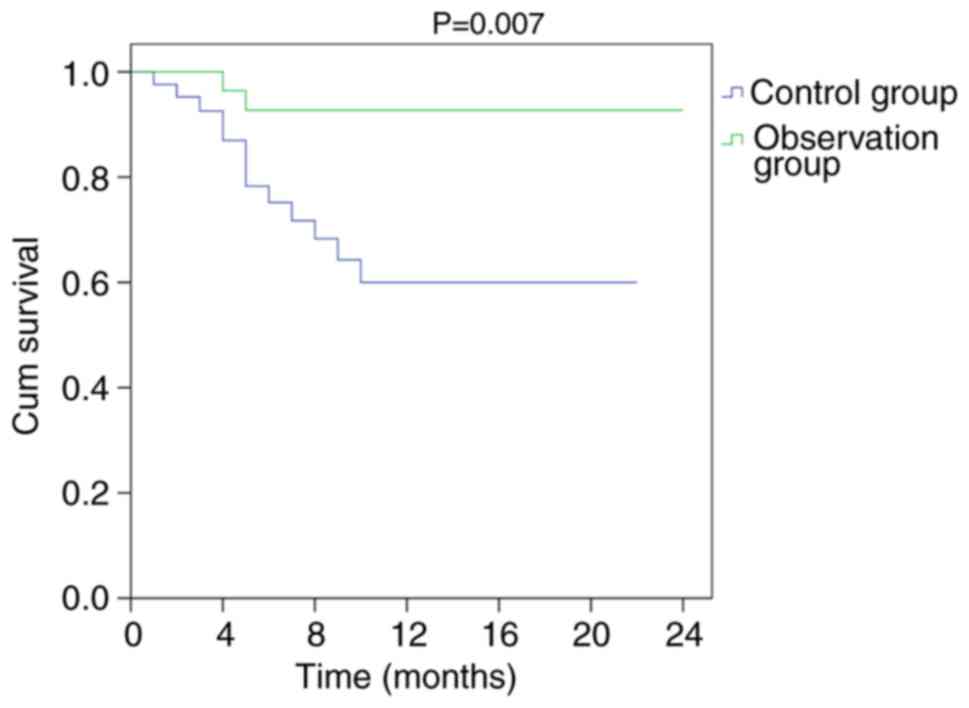
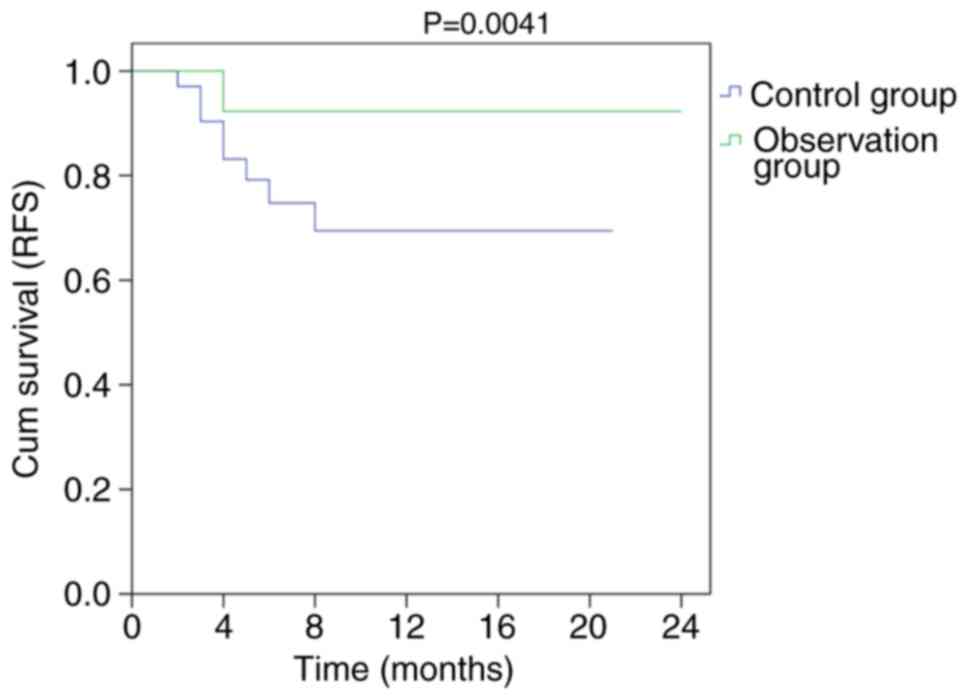
and RFS in patients with undetected methylation gene mutations were
compared between the two groups. OS (P=0.007) and RFS (P=0.041)
were significantly improved in the observation group compared with
those in the control group (Figs.
6 and 7).
Discussion
AML is a heterogenous malignant disease, and its
pathogenesis remains elusive. Although the conventional
chemotherapy regimen achieves a 60-80% CRR in adult patients, the
remission time is short and the majority of the patients eventually
develop disease relapse (4-6).
Therefore, there is an urgent need to improve the remission rate
and reduce the recurrence rate in patients with AML. Although
recent studies have suggested that decitabine treatment can improve
the remission rate of patients with AML (3,6,13),
it has not been reported whether demethylation drugs are beneficial
for reducing the recurrence rate of AML. In addition, patients with
AML with methylation gene mutations (including DNMT3A and TET2)
have a poor prognosis (8);
however, certain patients with AML may have undetected methylation
gene mutations. It has not been reported whether or not decitabine
is beneficial for the treatment of these cases and for patients
with AML without methylation gene mutations.
With the rapid development of epigenetics, the role
of DNA methylation abnormalities in the occurrence and
transformation of neoplasms has gradually been recognized (11,12).
Previous studies have reported that high levels of DNA methylation
are present in the genome of patients with AML, which can decrease
the transcription of or completely silence genes related to
reducing cell self-monitoring, and inhibiting abnormal
differentiation and proliferation (10,11).
Gene promoter-specific hypermethylation contributes to tumor
development during AML (10,11);
therefore, the use of demethylation drugs, which can reverse the
hypermethylation status, may serve as a promising strategy for
treating leukemia and reducing the risk of recurrence.
The demethylating drug decitabine was originally
approved for the treatment of MDS, and was later approved for the
first-line treatment of AML patients aged >65 years (19). Several studies have reported that
decitabine improves the efficacy of treatment for AML (3,6);
however, the majority of studies have focused on elderly patients
with AML and administrated decitabine as a monotherapy, low-dose
chemotherapy, or combined with A or aclarubicin (C) + A +
granulocyte colony-stimulating factor (G) regimen (6,13).
The present study compared the efficacy of D + A, I + A and H + A
modalities with or without decitabine, analyzing the efficacy of
decitabine in the treatment of newly diagnosed non-elderly AML. A
total of 81 patients were enrolled, with 35 patients in the
observation group and 46 patients in the control group. The median
age of the enrolled patients was 58.5 years, and there was no
significant difference in age between the two groups. In the
control group, the CRR was 69.6% and the ORR was 84.8%, which was
consistent with the literature (4-6).
Furthermore, the CRR and ORR of the induction regimen with
decitabine were significantly higher compared with those in the
control group (P=0.017 and P=0.044, respectively). In addition, it
has been reported that the ′7+3′ conventional chemotherapy modality
has a CRR of 60-85% in patients with AML aged <60 years
(20,21). In the present study, 73 patients
aged <60 years were examined. The CRR in patients <60 years
was increased in the observation group (92.9%) compared with the
control group (71.1%; P=0.025). The results suggested that, when
combined with the conventional induction therapy used for AML,
decitabine significantly improves the CRR and ORR of patients, and
it plays a particularly important role in the treatment of
non-elderly patients with AML.
Welch et al (18) recruited 116 patients with AML/MDS
who were treated with decitabine monotherapy, identifying a higher
response rate in patients with an unfavorable-risk cytogenetic
profile (67%; P<0.001). Bai et al (19) compared the curative effect of the C
+ A + G regimen with that of the C + A + G regimen combined with
decitabine in elderly patients with AML, reporting that the CRR and
ORR of patients receiving the C + A + G regimen combined with
decitabine were increased compared with those in the C + A + G
group (57.1 vs. 15% and 71.4 vs. 25%, respectively; P<0.05).
Although the number of favorable-risk patients was not suffi-cient
to perform statistical analyses in the present study, the
observation group displayed a higher CRR (90.3 vs. 67.5%) in
intermediate- and unfavorable-risk patients compared with the
control group (P=0.022).
Additionally, a number of studies have reported that
decitabine has been effective in the treatment of AML with DNA
methylation gene mutations. A study investigating the use of
decitabine alone or in combination with bortezomib indicated that
the CRR and OS were increased in patients with AML with DNMT3A
mutations compared with patients with AML with wild-type DNMT3A
(22). Certain patients with AML
displayed undetected methylation gene mutations; therefore, whether
individuals with undetected methylation gene mutations benefited
from the administration of decitabine has not been reported.
Whole-genome hyper-methylation is widespread in AML and is
associated with poor clinical outcome (10); therefore, the majority of AML cases
with undetected methylation gene mutations display a low frequency
of DNA methylation (10,12). DNA methylation is reversible;
therefore, it was hypothesized that demethylating drugs may reduce
the frequency of methylation to reduce the occurrence and
recurrence of AML, and improve treatment efficacy. The CRR of
patients with undetected methylation mutation genes was increased
in the observation group compared with that in the control group
(P=0.028), which was consistent with this hypothesis.
In addition, a comparison of the survival analysis
of the two groups was performed. The recurrence rate in the
observation group was reduced compared with that in the control
group (5.7 vs. 15.2%, respectively; P>0.05); however, this
decrease was not statistically significant. If the follow-up period
was prolonged, significant differences may have been observed.
Furthermore, the 2-year OS in the two groups was assessed. The
results indicated that induction therapy with decitabine
significantly prolonged the 2-year OS of patients with AML compared
with induction therapy without decitabine (P=0.008). In 2012, a
phase III clinical trial of decitabine monotherapy with A or
supportive therapy in elderly patients with AML was conducted
(23). The results indicated that
the decitabine monotherapy group displayed an improved CR; although
the OS was not significantly different, the difference exhibited a
trend in favor of decitabine (24,25).
In the present study, the differences in the 2-year
OS and RFS between the two groups in patients with undetected
meth-ylation gene mutations were statistically significant (P=0.007
and P=0.041, respectively). The results further supported the
hypothesis that decitabine may improve the efficacy of AML
treatment strategies and reduce recurrence. However, the present
study had a number of limitations, including a small sample size,
limited follow-up and the lack of a prospective study; therefore,
large-scale and prospective clinical trials are required to verify
the results of the present study.
The combination of decitabine and D + A, I + A or H
+ A regimens as induction chemotherapy displayed improved induction
responses, reduced recurrence and prolonged survival in patients
with de novo non-elderly AML (non-M3). Additionally, OS and
RFS were prolonged in patients with undetected methylation gene
mutations.
Acknowledgments
Not applicable.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during the present
study are included in the published article.
Authors' contributions
DZ, LZ and LH conceived and designed the study. LZ,
DZ, LH, YH, LH, YL, ZS, JW, ZW, XM, YW and MX collected and
analyzed the data. LZ, DZ and YL wrote the manuscript. All authors
were responsible for data collection and analysis, and read and
approved the final version of the manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of Tongji Hospital, Tongji Medical College, Huazhong
University of Science and Technology (approval no. TJ-IRB20190913).
Patient consent was not obtained, as the Ethics Committee approved
the application for exemption of informed consent.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel R, Ma J, Zou Z and Jemal A: Cancer
statistics, 2014. CA Cancer J Clin. 64:9–29. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Juliusson G, Lazarevic V, Hörstedt AS,
Hagberg O and Höglund M; Swedish Acute Leukemia Registry Group:
Acute myeloid leukemia in the real world: Why population-based
registries are needed. Blood. 119:3890–3899. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Li WY, Wang Y, Chen SN, Qiu HY, Fu ZZ, Wu
DP and Sun AN: Consolidation therapy with decitabine and
intermediate-dose cytarabine followed by HLA-mismatched peripheral
blood stem cells infusion for older patients with acute myeloid
leukemia in first remission. Leuk Lymphoma. 59:1652–1658. 2018.
View Article : Google Scholar
|
|
4
|
Thol F, Schlenk RF, Heuser M and Ganser A:
How I treat refractory and early relapsed acute myeloid leukemia.
Blood. 126:319–327. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sasine JP and Schiller GJ: Emerging
strategies for high-risk and relapsed/refractory acute myeloid
leukemia: Novel agents and approaches currently in clinical trials.
Blood Rev. 29:1–9. 2015. View Article : Google Scholar
|
|
6
|
Li G, Ren L, Li G, et al: The
effectiveness comparison between the CLAG regimens and MEA regimens
for the treatment of patients with relapsed or refractory acute
myeloid leukemia. J Mod Oncol. 26:264–268. 2018.In Chinese.
View Article : Google Scholar
|
|
7
|
Corces-Zimmerman MR, Hong WJ, Weissman IL,
Medeiros BC and Majeti R: Preleukemic mutations in human acute
myeloid leukemia affect epigenetic regulators and persist in
remission. Proc Natl Acad Sci USA. 111:2548–2553. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tan Y, Liu H and Chen S: Mutant DNA
methylation regulators endow hematopoietic stem cells with the
preleukemic stem cell property, a requisite of leukemia initiation
and relapse. Front Med. 9:412–420. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Arber DA, Orazi A, Hasserjian R, Thiele J,
Borowitz MJ, Le Beau MM, Bloomfield CD, Cazzola M and Vardiman JW:
The 2016 revision to the World Health Organization classification
of myeloid neoplasms and acute leukemia. Blood. 127:2391–2405.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bennett JM, Catovsky D, Daniel MT,
Flandrin G, Galton DA, Gralnick HR and Sultan C: Proposals for the
classification of the acute leukemias. French-American-British
(FAB) co-operative group. Br J Haematol. 33:451–458. 1976.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
NCCN: Acute Myeloid Leukemia, NCCN
Clinical Practice Guidelines in Oncology. Accessed April 4,
2016.
|
|
12
|
Döhner H, Estey E, Grimwade D, Amadori S,
Appelbaum FR, Büchner T, Dombret H, Ebert BL, Fenaux P, Larson RA,
et al: Diagnosis and management of AML in adults: 2017 ELN
recommendations from an international expert panel. Blood.
129:424–447. 2017. View Article : Google Scholar :
|
|
13
|
Cheson BD, Bennett JM, Kopecky KJ, Büchner
T, Willman CL, Estey EH, Schiffer CA, Doehner H, Tallman MS, Lister
TA, et al: Revised recommendations of the International Working
Group for Diagnosis, Standardization of Response Criteria,
Treatment Outcomes, and Reporting Standards for Therapeutic Trials
in Acute Myeloid Leukemia. J Clin Oncol. 21:4642–4649. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Spencer DH, Russler-Germain DA, Ketkar S,
Helton NM, Lamprecht TL, Fulton RS, Fronick CC, O′Laughlin M, Heath
SE, Shinawi M, et al: CpG island hypermethylation mediated by
DNMT3A is a consequence of AML progression. Cell. 168:801–816.e13.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Deneberg S, Grövdal M, Karimi M, Jansson
M, Nahi H, Corbacioglu A, Gaidzik V, Döhner K, Paul C, Ekström TJ,
et al: Gene-specific and global methylation patterns predict
outcome in patients with acute myeloid leukemia. Leukemia.
24:932–941. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Döhner H, Weisdorf DJ and Bloomfield CD:
Acute myeloid leukemia. N Engl J Med. 373:1136–1152. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Short NJ, Rytting ME and Cortes JE: Acute
myeloid leukaemia. Lancet. 392:593–606. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Welch JS, Petti AA, Miller CA, Fronick CC,
O′Laughlin M, Fulton RS, Wilson RK, Baty JD, Duncavage EJ, Tandon
B, et al: TP53 and decitabine in acute myeloid leukemia and
myelodys-plastic syndromes. N Engl J Med. 375:2023–2036. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bai X, Xiao X, Li Y, Zhao M and Deng Qi:
Curative effect of CAG regimen compared with CAG combined with
decitabine regimen in elderly patients with acute myeloid leukemia.
Clin Focus. 33:240–243. 2018.In Chinese.
|
|
20
|
Metzeler KH, Walker A, Geyer S, Garzon R,
Klisovic RB, Bloomfield CD, Blum W and Marcucci G: DNMT3A mutations
and response to the hypomethylating agent decitabine in acute
myeloid leukemia. Leukemia. 26:1106–1107. 2012. View Article : Google Scholar
|
|
21
|
Kantarjian HM, Thomas XG, Dmoszynska A,
Wierzbowska A, Mazur G, Mayer J, Gau JP, Chou WC, Buckstein R,
Cermak J, et al: Multicenter, randomized, open-label, phase III
trial of decitabine versus patient choice, with physician advice,
of either supportive care or low-dose cytarabine for the treatment
of older patients with newly diagnosed acute myeloid leukemia. J
Clin Oncol. 30:2670–2677. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Thomas XG, Arthur C, Delaunay J, Jones M,
Berrak K and Kantarjian HM: A post hoc sensitivity analysis of
survival probabilities in a multinational phase III trial of
decitabine in older patients with newly diagnosed acute myeloid
leukemia. Clin Lymphoma Myeloma Leuk. 14:68–72. 2014. View Article : Google Scholar
|
|
23
|
Nieto M, Demolis P, Béhanzin E, Moreau A,
Hudson I, Flores B, Stemplewski H, Salmonson T, Gisselbrecht C,
Bowen D and Pignatti F: The European medicines agency review of
decitabine (Dacogen) for the treatment of adult patients with acute
myeloid leukemia: Summary of the scientific assessment of the
committee for medicinal products for human use. Oncologist.
21:692–700. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Figueroa ME, Abdel-Wahab O, Lu C, Ward PS,
Patel J, Shih A, Li Y, Bhagwat N, Vasanthakumar A, Fernandez HF, et
al: Leukemic IDH1 and IDH2 mutations result in a hypermethylation
phenotype, disrupt TET2 function, and impair hematopoietic
differentiation. Cancer Cell. 18:553–567. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Rothenberg-Thurley M, Amler S, Goerlich D,
Köhnke T, Konstandin NP, Schneider S, Sauerland MC, Herold T,
Hubmann M, Ksienzyk B, et al: Persistence of pre-leukemic clones
during first remission and risk of relapse in acute myeloid
leukemia. Leukemia. 32:1598–1608. 2018. View Article : Google Scholar : PubMed/NCBI
|